美国BC ESU-2050 ESU-2050P高频电刀分析仪
高频电刀检测仪操作规程

高频电刀检测仪操作规程一、设备配置:美国FLUKE公司QA-ES Ⅱ型高频电刀检测仪二、工作原理1.实际输出功率测量通过直接测量被检设备输出波的高频电压(均方根),再根据测量时设置的负载阻抗计算出被检设备的实际输出功率。
2.负载/功率曲线测试测量被检设备(设定于一定输出功率时),在不同负载电阻(10Ω~1000Ω)下的输出功率,从而得到被检设备的负载/功率曲线。
3.波峰因子(CF)测量测量手术电极上输出的峰值电压,检测装置可自动计算其与高频电压均方根的比值,即波峰因子(CF)的大小,用来评定被检设备输出波形的稳定性及电切、电凝的效果。
三、操作规程:1.检测前检查(1)查看设备接地情况,电源接地端子与机器外壳短接,辅助接地良好。
(2)打开设备开关,查看设备电源开关有无损坏或接触不良现象,设备自检是否通过,各项声光指示是否正常,关机。
(3)激发手术电极、脚踏开关各个控制钮,是否控制正常。
(4)在开机、各种控制开关操作过程中注意观察被检设备的各个颜色指示灯,绿色代表电源通过,黄色代表切割输出激励,蓝色代表凝血输出激励,红色代表设备故障。
重复(2)-(3)检查三次。
2.输出功率(1)额定负载下,不同功率时实际输出功率测量连接方法: 单极输出功率测量:按图(1)连接被检测设备与测试仪,单极有电切和电凝两种模式双极输出功率测量,按图(2)连接被检测设备与测试仪。
(2)不同负载时实际输出功率测量连接方法: 单极输出功率测量:按图(1)连接被检测设备与测试仪。
双极输出功率测量,按图(2)连接被检测设备与测试仪。
图(1) 输出功率检测图(2) 输出功率检测3.波峰因子:连接方式分别与功率测量方式相同。
4.高频漏电检测:直接测量高频加载、空载时手术电极、中性电极(或双极电极)的高频漏电流。
连接无感电阻200Ω,调至最大输出额定功率,记录每个模式中最大输出高频漏电流。
(1)加载时手术电极对地高频漏电流,按图(3)连接被检测设备与测试仪。
高频电刀校准中的问题及解决方法

维修工程
险性要比绝缘输出系统大,故目前所用的高频电刀均作者简介杨莎,女,(1990- ),本科学历,助理工程
师。
新疆医科大学附属
肿瘤医院医学工程科,从事医疗设备质量控制工作。
中国医学装备2017年3月第14卷第3期 China Medical Equipment 2017 March V ol.14 No.3①新疆医科大学附属肿瘤医院医学工程部 新疆 乌鲁木齐 830011
中性电极以地为基准的高频漏电流电路图2 中性电极对地隔离的高频电流电路图高频时以地为基准示图高频时与地隔离示图
在进行HFSE高频漏电流检测时,首先要注意确
定HFSE高频时是以地为基准还是与地隔离,从而选择正确的线路连接图。
被校仪器处于开机状态,输出功率设定为最大,测量自中性电极流经200 Ω无感电阻
到地的高频漏电流,连续测量3次,取其最大值为中性
电极正常工作状态下的高频电流。
手术电极高频漏电流
维修工程中国医学装备2017年3月第14卷第3期高频电刀校准中的问题及解决方法-杨莎等。
ESU-2000电刀测试仪

ESU-2000 Series Product OverviewA Paradigm Shift In Electrosurgery Testing Technology and Capability Is HereYour current ESU Analyzer just became obsolete – no matter how new it is!1Copyright June, 2007 by BC Group International, Inc. Author: Michael R. Erwine Revision 1 – June 13, 2007INDEXThe Next Generation in ESU Testing is Finally Here.................................................................................................................... 3 Electrosurgery 101 – A Basic Review of Electrosurgery .............................................................................................................. 3 ESU Testing 101 – Some Testing History .................................................................................................................................... 7 ESU-2050: Truly Unique in the Market......................................................................................................................................... 9 ESU-2050: A Replacement for the Discontinued Fluke 8920A Instrument................................................................................. 11 ESU-2050: Unprecedented 1% Accuracy in ESU Testing.......................................................................................................... 11 ESU-2300: A More Conventional Approach ............................................................................................................................... 11 ESU-2400: More High-End Technology to Come....................................................................................................................... 11 Common Element: Patent Pending DFA Technology................................................................................................................. 11 Up To 32,768 Data Points! ......................................................................................................................................................... 12 ESU-2050: Precision Load Resistors Are Where Accuracy Starts ............................................................................................. 13 Product Development In Cooperation with ESU Manufacturers................................................................................................. 14 Industry Standard Current Sensing Technology......................................................................................................................... 13 Working With The Best In Current Sensing: Pearson Electronics .............................................................................................. 13 Current Sensing vs. Voltage Measurement................................................................................................................................ 13 Ensuring Quality By Taking Care Of The Details ....................................................................................................................... 14 Those Crazy & Exotic Pulsed Waveforms.................................................................................................................................. 15 ESU-2300: External Load Capabilities – Built In Non-Obsolescence ......................................................................................... 16 ESU-2000 Series PC Utility Software......................................................................................................................................... 16 One Picture is Worth a Thousand Words – Or Up To 32,768 Data Points ................................................................................. 16 See The Data You Want – The Way You Want ......................................................................................................................... 17 Easy Setup and Operation ......................................................................................................................................................... 18 ESU-2050: Graphical Mode ....................................................................................................................................................... 18 Product Comparison Overview................................................................................................................................................... 18 One-Stop-Source: We Make It Easy for You.............................................................................................................................. 19 Conclusion ................................................................................................................................................................................. 20 ESU-2050 Product Specifications .............................................................................................................................................. 21 ESU-2300 Product Specifications .............................................................................................................................................. 22 Technical References................................................................................................................................................................. 23 About the Author ........................................................................................................................................................................ 24APPENDICESAPPENDIX A Fluke Biomedical 454A Instrument Specifications ............................................................................................... 26 APPENDIX B Fluke Biomedical RF-303RS Instrument Specifications ........................................................................................ 27 APPENDIX C Dale Technology DALE3000 Instrument Specifications....................................................................................... 31 APPENDIX D Metron QA-ES Instrument Specifications ............................................................................................................ 33 APPENDIX E Valleylab ForceFX Generator Output waveforms ................................................................................................ 34 APPENDIX F Valleylab Force 2 Generator Output Waveforms ................................................................................................. 36 APPENDIX G Conmed System 5000 Output Waveforms .......................................................................................................... 38 APPENDIX H (Pearson Electronics Model 411 Data Sheet)...................................................................................................... 41 APPENDIX I (Pearson Electronics Model 4100 Data Sheet) ..................................................................................................... 42 APPENDIX J (Vishay Dale NH-250 Data Sheet)........................................................................................................................ 43 ® APPENDIX K (Sample Microsoft Excel Data Export Workbook) .............................................................................................. 45 APPENDIX L (Tyco Healthcare / Valleylab Recommended Test Procedures)........................................................................... 46 APPENDIX M (ESU-2000 Series PC Utility Software Screen Shots) ......................................................................................... 49 APPENDIX N (History of BC Group International, Inc.).............................................................................................................. 522Copyright June, 2007 by BC Group International, Inc. Author: Michael R. Erwine Revision 1 – June 13, 2007The Next Generation in ESU Testing is Finally HereWith the introduction of the new BC Biomedical ESU-2000 Series of Electrosurgery (ESU) Analyzers (the ESU2050 and ESU-2300 instruments)1, BC Group International, Inc. brings to market, the most exciting and technologically significant advances in electrosurgical testing to come about in well over a decade! The new BC Biomedical ESU-2050 represents an 18-month duration major product design effort in full cooperation with some of the leading electrosurgery generator manufacturers in the worldwide medical device market. The ESU-2050 is the very first instrument of its kind to be introduced, specifically designed for electrosurgery generator testing, with 1% of reading accuracy and a testing methodology that is exactly the same as the one that many medical device manufacturers currently use2. The new ESU-2300 is a more conventional “mid-range” ESU analyzer, offering features and functionality above and beyond competitive analyzers in this “mid-range” class. Both analyzers can be easily upgraded in the field via the BC Biomedical Flash Update PC Utility Software in the event of a needed firmware update. Together, these new ESU analyzers from BC Group represent an unprecedented paradigm shift in electrosurgery testing technology, and set a new baseline for the electrosurgery test device industry. The long-awaited next generation in ESU testing has finally arrived!Electrosurgery 101 – A Basic Review of Electrosurgery3The following basic review on electrosurgery is derived from technical information obtained from various sources in the public sector, including the Tyco Healthcare/ Valleylab document, Electrosurgery Self Study Guide4, Copyright September 1999, Tyco Healthcare / Valleylab. This information is intended for basic review purposes of some of the terminology and basic principles of electrosurgery technology. Electrosurgery generally deals with electrical signal frequencies in the range of approximately 200 kHz to 3.3 MHz (see Figure 1). This is well above the human body’s inherent frequency range of susceptibility to the hazards of microshock.Figure 1Frequency Spectrum Showing Range of Frequencies for ElectrosurgeryFigure 2Current Density Differences at Surgical Site vs. Return PathElectrosurgery works based upon heat generated by the density (see Figure 2) of the high frequency current being passed through human tissue. At the surgical site, the density is typically very high, resulting in high heat and a cutting or coagulating effect. The “return path” for the high frequency current is much larger and consequently much less current density exists at this area, which allows the high frequency energy to safely leave the body without any adverse effects. There are two basic modes of electrosurgery: bipolar and monopolar. Bipolar surgery (see Figure 3) is accomplished by using two parallel poles in close proximity, where the flow of high frequency current is restricted to the two poles, one being the “source” and the other being the “return path”. A patient return electrode is typically not needed in bipolar electrosurgery applications, and because these two poles are close together, theCommercial availability scheduled for July/August 2007. See Appendix L for specific information regarding Tyco Healthcare / Valleylab recommended test setup procedures and recommended test equipment. 3 Sincere appreciation to Tyco Healthcare / Valleylab for the use of the illustrations in this section. Images and information are based upon the Valleylab publication Electrosurgery Self-Study Guide, Copyright September, 1999, Authored by Brenda C. Ulmer, RN, MN, CNOR. 4 This Tyco Healthcare / Valleylab publication can be downloaded in PDF format at /pages/list-book.html Copyright June, 2007 by BC Group International, Inc. Author: Michael R. Erwine 3 Revision 1 – June 13, 20072 1voltage level and resulting applied power are lower than in monopolar electrosurgical applications. This results in less localized tissue heating and reduced “charring” of tissue. Bipolar electrosurgery is typically used in neurosurgical and gynecological procedures, and in other procedures where there is concern due to implanted pacemakers and automatic defibrillators. In general, bipolar electrosurgery is safer that monopolar electrosurgery, and the subsequent risks of high frequency burns at the return electrode site are avoided.Figure 3Electrosurgery – Bipolar ModeFigure 4Electrosurgery – Monopolar ModeMonopolar electrosurgery (see Figure 4) is a more generalized and more frequently used mode. Monopolar electrosurgery utilizes higher voltage levels than bipolar, resulting in higher power delivered at the surgical site. The need for a well prepared and maintained patient electrode site is of paramount concern in monopolar electrosurgical applications, in order to prevent high frequency burns at the patient return electrode site. The high frequency waveform produced by the electrosurgical generator determines the physiological effect of the application of this energy to the tissue in the body. The Cut mode of an electrosurgical generator creates a continuous waveform, as shown in Figure 5. Different degrees of hemostasis (coagulation) can be achieved by utilizing varying degrees of “Blended” waveforms as shown in Figure 6.Figure 5Pure Cut - Pure Sinusoidal WaveformFigure 6Blended WaveformsThe Coag mode (see Figure 7) of an electrosurgical generator creates a waveform with large amplitude but short duration “spikes” to achieve hemostasis (coagulation). The surrounding tissue is heated when the waveform spikes and then cools down (between spikes), producing coagulation of the cells. Fulguration is achieved in the Coag mode of the electrosurgical generator, with the tip of the surgical “active electrode” held above (but not in contact with) the tissue. Electrosurgical Desiccation is achieved in either the Cut or Coag modes of the generator. The difference between Desiccation and Fulguration is the tip of the “active electrode” must contact the tissue as in Figure 8 in order to achieve Desiccation. The more desired mode to achieve tissue Desiccation through direct tissue contact is the Cut mode. Older electrosurgical generators (those produced prior to around 1968) are generally ground-referenced devices and must be used with extreme care to avoid unwanted “current division” and possible resulting high frequency burns at this site (or at multiple sites). This is illustrated in Figure 9 below. Current division can occur at any point of contact with an earth grounded point, such as the frame of the surgical table or the outer chassis of another medical device. For the most part, these types of devices are no longer used in surgical procedures, mainly due to advances in electrosurgical generator technology and concerns over safety.Copyright June, 2007 by BC Group International, Inc. Author: Michael R. Erwine Revision 1 – June 13, 20074Advances in electrosurgery generator technology brought about the “solid state” generator around 1968. Along with this more reliable and more condensed electronics technology came the introduction of the isolated-output electrosurgical generator (see Figure 10 below), thus eliminating the concern over unwanted current division and vastly improving patient safety. The outputs of these generators were no longer earth ground-referenced, so even the best electrical ground-referenced contact made to the patient would not present the risk of high frequency burns at alternate sites.Figure 7Coagulation WaveformFigure 8Tissue Penetration: Cut vs. CoagThe shift in concern now focused on the quality of the patient return electrode and electrode site, and over the succeeding years, many manufacturers introduced new monitoring techniques designed to constantly measure the integrity of the patient electrode site in order to minimize the possibility of high frequency burns at the patient electrode. The varying technologies introduced by the various electrosurgical generator manufacturers over the years have generically become know in today’s market as the Contact Quality Monitor (CQM) function (see Figure 11) of the electrosurgical generator.Figure 9Ground-Referenced Electrosurgical GeneratorFigure 10Isolated Output Electrosurgical GeneratorIn more recent years, there has been a steady stream of advances in electrosurgery generator technology, one of the most significant of which was the introduction by Tyco Healthcare / Valleylab in their Force FX Generator of “Tissue Response Technology” in the late 90’s. This technology utilizes a constant feedback loop to theFigure 11Contact Quality Monitor (CQM) FunctionFigure 12Tyco Healthcare / Valleylab Tissue Response Technology5Copyright June, 2007 by BC Group International, Inc. Author: Michael R. Erwine Revision 1 – June 13, 2007generator’s microprocessor and actually adjusts the power level output of the generator in order to provide relatively constant power delivery (and thus a consistent surgical effect) at the surgical site, regardless of tissue impedance. Electrosurgery generator improvements continue, with new introductions by leading manufacturers like Tyco Healthcare / Valleylab, Conmed (Electrosurgery Division), Erbe, Bovie, etc. on a regular basis The need for routine testing and performance verification of these generators has not deceased due to these introductions of new technologies. In fact, there are more features and safeguards to test for proper operation on today’s average electrosurgical generator than ever.Some Common Electrosurgery TerminologyActive Electrode: an electrosurgical instrument or accessory that concentrates the high frequency current at the surgical site, thus enabling the heating effect at the site and producing the desired electrosurgical effect Blend: an electrosurgical generator output waveform that combines the features of cut and coag waveforms, cutting with various degrees of hemostasis (coagulation) Contact Quality Monitor (CQM): a system that constantly monitors the impedance of the physical connection between the patient’s body and the patient return electrode and interrupts power form the electrosurgical generator is the quality of this connection is compromised electrically Current Density: the amount of electrical current flow per unit of surface area – as current density increases so does the heating of the tissue in the immediate location Current Division: high frequency electrical current leaving the intended electrosurgical patient circuit and following an alternate low impedance path of lesser resistance to earth ground, this introducing the possibility of high frequency burns at the alternate earth ground contact point – typically a concern in ground-reference generators and not isolated output generators. Coagulation: the clotting of blood or destruction of tissue with no cutting effect – electrosurgical fulguration and desiccation. Cut Mode: electrosurgical mode that produces a low voltage continuous waveform optimized for tissue cutting Desiccation: the effect of tissue dehydration and protein denaturation caused by direct contact between the electrosurgical “active electrode” and the tissue Fulguration: using electrical arcs (sparks) to coagulate tissue, whereby the sparks jump from the electrosurgical “active electrode” across an air gap to the tissue Ground-Referenced Output: an electrosurgical generator with an output that is electrically referenced to earth ground Isolated Output: an electrosurgical generator with an output that is not electrically referenced to earth ground Leakage Current: electrical current that flows along an undesired pathway, usually to earth ground – in an electrosurgical generator, RF leakage current is high frequency current that regains its ground reference and seeks earth ground. Patient Return Electrode: an electrically conductive plate or pad (also known as the dispersive electrode) that recovers the high frequency current introduced into the patient’s body by the “active electrode” during electrosurgery. This electrode minimizes the current density of this return current flow in order to minimize the possibility of high frequency burns at this electrode site. Radio Frequency (RF): frequencies above 100 kHz that transmit radio signals – the high frequency current utilized in electrosurgery Tissue Response Technology: the Tyco Healthcare / Valleylab electrosurgical generator technology that continuously measures the impedance/resistance of the tissue in contact with the patient return electrode and automatically adjusts the output of the generator accordingly to achieve a consistent tissue effect.6Copyright June, 2007 by BC Group International, Inc. Author: Michael R. Erwine Revision 1 – June 13, 2007ESU Testing 101 – Some Testing HistoryElectrosurgery generator technology has undergone tremendous technological advances over the past decade, but the technology base of ESU analyzers has remained relatively slow-moving over this same time period. The recently discontinued Fluke Biomedical Model 454A dates back to around 1992 or 1993, and until now, represents the culmination of research and development efforts on the behalf of competitive companies in the area of electrosurgery testing. Here is a brief history of ESU testing devices over the past 15 to 20 years. Analyzers are shown in the order of their introduction to the market.Bio-Tek Instruments RF-301: The very first offering in ESU analyzers by Bio-Tek Instruments. This 5 “passive” RF thermocouple ammeter type instrument got the job done. There are still quite a few RF301 instruments in use in the field today. The design was basic and rugged.No Picture AvailableNeurodyne Dempsey Model 403A: The Neurodyne Dempsey (which later became Dynatech Nevada Inc.) Model 403A was a very small-sized ESU tester with limited functionality. This was a passive technology device with an RF thermocouple type analog ammeter and a single fixed 500 internal load. Meter range was 0.2 A to 1.0 A / 20 watts to 500 watts. It was the company’s first dedicated ESU tester. There are very few of these units left in the market.Bio-Tek Instruments RF-302: The predecessor to the Bio-Tek RF-303, the RF-302 was a “passive” RF thermocouple ammeter type instrument. This gave an advantage to the RF-302 above other competitive “active” type ESU analyzers available at time. The RF-302 offered a better high frequency range than some competitive “active” units. Bio-Tek Instruments sold quite a few of these units in the market. This instrument is very similar to the BC Biomedical ESU2000A instrument that is still available today, for those customers who prefer a legacy type RF ammeter “passive” instrument approach to ESU generator testing.Dynatech Nevada Model 443: The Dynatech Nevada Model 443 was the company’s very first “active” type6 design in ESU analyzers. Despite it’s active internal circuitry and measurement technology, the Model 443 still utilized an analog meter. The Model 443 was discontinued shortly after the introduction of the Model 453A.Dynatech Nevada Model 453A: The predecessor to the 454A, the Dynatech Nevada Model 453A was probably the very first “Hi-Tech” ESU analyzer on the market. It utilized active technology. Introduced in the mid 1980’s, the Model 453A was in production until the introduction of the Dynatech Nevada Model 454A, starting in 1992 or 1993. The 453A had a small LED 7-segment display and was a fairly large instrument weighing well over 15 pounds. There are still many 453A ESU analyzers in use in biomedical departments across the U.S. today.56Passive technology in an ESU Analyzer refers to an instrument that does not require any external power source and simply meters the RF energy without any electronic signal processing. Active technology is an ESU Analyzer refers to an instrument that requires a power supply and has active electronic circuitry including components such as A/D converters. Operational amplifiers, thermal converters, etc. Copyright June, 2007 by BC Group International, Inc. Author: Michael R. Erwine 7 Revision 1 – June 13, 2007Dynatech Nevada Model 454A: Until it was recently discontinued by Fluke Biomedical (the Model 454A is no longer listed on the Fluke Biomedical web site and customers report having been informed that the 454A is no longer available from Fluke Biomedical) in favor of the more recent Metron QA-ES (re-branded as the Fluke Biomedical QA-ES effective March 18, 2007), the 454A was probably the most popular and successful ESU analyzer on the market. Originally designed by Dynatech Nevada Inc., the 454A utilized industry standard current sensing technology and offered accuracies of 5% of reading on RMS current and 10% of range on RMS power. For the the past decade, the 454A was considered to be an electrosurgery industry icon, but despite this status in the market, it never really attained any level of actual customer recommendation for any of the leading electrosurgery generator manufacturers. See Appendix A for full specifications on the discontinued Model 454A.Fluke Biomedical RF-303RS: Originally marketed as the Bio-Tek Instruments RF-303RS, this is the current “mid-range” ESU analyzer offering from Fluke Biomedical. The RF-303RS does not utilize industry standard current sensing technology, but uses simple voltage measurement instead. This product was designed during the period of time that Lionheart Technologies owned and operated Bio-Tek Instruments, DNI Nevada, and Dale Technology. A concurrent companion product to the RF-303RS was originally introduced in 1998 under the DNI Nevada (formerly Dynatech Nevada) brand as the Model 402A. The 402A was later re-branded as the Dale Technology DALE3000 following the acquisition of the biomedical holdings of Lionheart Technologies by Fluke Electronics (Fluke Biomedical) in 1993. Instrument specifications for the Fluke Biomedical (Bio-Tek Instruments) RF-303RS, the DNI Nevada 402A, and the Dale Technology DALE3000 are (were) essentially identical. Current Fluke Biomedical advertised specifications for the RF-303RS are + 5% of reading or + 3 watts (whichever is greater) on RMS power and + 2.5% of reading or + 15ma (whichever is greater) on RMS current. See Appendix B for full specifications on the RF-303RS. DNI Nevada Model 402A: The DNI Nevada Model 402A was the “sister product” to the Bio-Tek Model RF-303, introduced concurrently with the RF-303 (see information above under the RF303RS). Actual design, development, and manufacturing of the 402A and the RF-303 took place at the DNI Nevada Inc. facility in Carson City, NV, under the ownership and management of Lionheart Technologies, Inc. In order to make the two products look sufficiently different, and in order to somehow truly differentiate the two, the 402A was given an RS232 communications port and the RF-303 was given a battery for portable operation. Slightly different enclosures were also chosen, and the 402A was given an LED 7-segment display while the RF-303 was given an LCD 7-segment display. The instrument firmware that operated the 402A and RF-303 was common between the products, with firmware subroutines that recognized which instrument was being operated by the microprocessor. The RS232 communications port was added to the RF-303 much later in time, following the discontinuance of the 402A. When Fluke Electronics (Fluke Biomedical) acquired the biomedical holdings of Lionheart Technologies in 1993, the DNI Nevada Model 402A was soon after discontinued and re-branded under the Dale Technology brand as the DALE3000. Dale Technology DALE3000: The DALE3000 existed in the market for less than three-years before it was discontinued. The re-branding of the DNI Nevada Model 402A to the Dale Technology DALE3000 was concurrent with the relocation of the Dale Technology business from its original location in Thornwood, NY to Carson City, NV, in the then-existing Fluke Biomedical manufacturing facilities (the original Dynatech Nevada manufacturing facility and offices) in Carson City, NV. The discontinuance of the DALE3000 was actually fairly close in time to the Fluke acquisition of Metron AS of Trondheim, Norway, which brought the Metron QA-ES “highend” ESU analyzer to the Fluke Biomedical family of products.BC Biomedical ESU-2000A: The BC Biomedical ESU-2000A was originally introduced in the year 2000, based upon strong customer demand for a “simple but effective” legacy tester similar to the original Bio-Tek Instruments RF-302. With accuracy of + 2% of full scale on current and power, the ESU-2000A remains popular with customers today. It is still available from BC Group International. Full instrument specifications for the ESU-2000A ESU analyzer can be found on the BC Group International web site at: /acatalog/BCBiomedicalESU2000ADatasheet.pdf.8Copyright June, 2007 by BC Group International, Inc. Author: Michael R. Erwine Revision 1 – June 13, 2007。
高频电刀质量检测仪 HF-400、QA-ESII、377对比
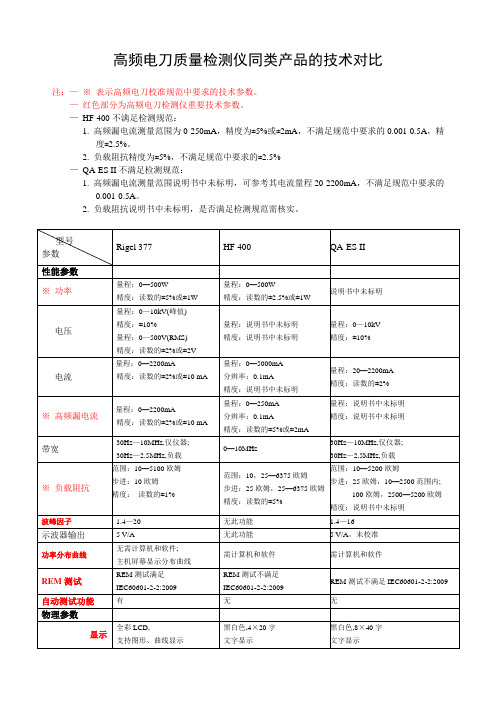
30Hz—10MHz,仅仪器;
30Hz—2.5MHz,负载
※负载阻抗
范围:10—5100欧姆
步进:10欧姆
精度:读数的±1%
范围:10,25—6375欧姆
步进:25欧姆,25—6375欧姆
精度:读数的±5%
范围:10—5200欧姆
步进:25欧姆,10—2500范围内;
100欧姆,2500—5200欧姆
精度:说明书中未标明
波峰因子
1.4—20
无此功能
1.4—16
示波器输出
5 V/A
无此功能
5 V/A,未校准
功率分布曲线
无需计算机和软件;
主机屏幕显示分布曲线
需计算机和软件
需计算机和软件
REM测试
REM测试满足IEC60601-2-2:2009
REM测试不满足IEC60601-2-2:2009
REM测试不满足IEC60601-2-2:2009
高频电刀质量检测仪同类产品的技术对比
注:—※表示高频电刀校准规范中要求的技术参数。
—红色部分为高频电刀检测仪重要技术参数。
—HF-400不满足检测规范:
1.高频漏电流测量范围为0-250mA,精度为±5%或±2mA,不满足规范中要求的0.001-0.5A,精度±2.5%。
2.负载阻抗精度为±5%,不满足规范中要求的±2.5%
—QA-ES II不满足检测规范:
1.高频漏电流测量范围说明书中未标明,可参考其电流量程20-2200mA,不满足规范中要求的0.001-0.5A。
2.负载阻抗说明书中未标明,是否满足检测规范需核实。
型号
参数
Rigel 377
HF 400
电刀检测仪技术参数

电刀检测仪技术参数电刀检测仪是一种在手术中用于实时检测电刀电流参数的设备。
电刀是一种常见的医疗工具,用于手术过程中切割和凝固组织。
因此,电刀电流参数的准确测量对手术的成功和患者的安全至关重要。
以下是一些常见的电刀检测仪技术参数。
1.测量范围:电刀检测仪应具有广泛的测量范围,以覆盖不同手术过程中可能出现的不同电流强度。
通常,电刀检测仪的测量范围为10mA至5000mA。
2.分辨率:电刀检测仪应具有高分辨率,以确保对不同电流强度的准确测量。
通常,分辨率在1mA至10mA之间。
3.准确性:电刀检测仪应具有高精度,以确保对电刀电流参数的准确测量。
通常,准确度在±5%至±10%之间,具体取决于测量范围和分辨率。
4.响应时间:电刀检测仪应具有快速的响应时间,以实时监测电刀电流参数的变化。
通常,响应时间在1毫秒至100毫秒之间,取决于检测仪的设计和测量算法。
5.显示和报警:电刀检测仪应具有清晰的数字显示屏,以显示当前的电刀电流参数。
同时,它还应该具有报警功能,一旦电刀电流超出设定的安全范围,就能发出声音或光信号警报。
6.数据记录和存储:电刀检测仪应具有数据记录和存储功能,以便后续的回顾和分析。
这可以帮助医生了解手术过程中的电刀使用情况,并为质量控制和培训提供依据。
7.电源:电刀检测仪通常使用可充电电池或交流电源。
充电电池可以提供便携性,并在没有电源插座的情况下使用。
交流电源则可以持续供电,无需担心电池电量耗尽。
8.安全性:电刀检测仪应具有良好的电气隔离和绝缘,以防止电流泄漏和电击风险。
它还应具有防护功能,以防止外界干扰和损坏。
除了上述技术参数外,一些电刀检测仪还可能具有其他附加功能,如声音记录、网络连接和远程监控。
这些功能可以进一步提高电刀检测仪的功能和实用性。
总之,电刀检测仪是一种重要的医疗设备,用于实时监测手术中的电刀电流参数。
准确的测量和监测可以帮助医生确保手术过程的安全和成功。
正确选择和使用电刀检测仪对于手术医生和患者来说都是至关重要的。
关注高频电刀(一)

关注高频电刀(一)正文:电外科装置(ESU)即国内所称高频电刀,即可列入手术器械类,也可归于手术设备类。
它象手术刀一样具有切割功能,是一种取代原始切割的现代科技化的设备性的手术器械。
设备性手术器械目前已蔚为大观,有冷刀,超声刀,激光刀,微波刀,等离子刀,分子刀,水刀,红外凝固刀,立体定向放射外科刀(X刀,质子刀,自动控制刀)以及本文所述的高频电刀等。
这些刀都是运用各种先进科学技术达到切割目的的设备,虽然还不能完全取代常规使用的传统手术刀,但是在某些外科手术方面,却可取得比运用传统手术刀更好的效果,如不用切开,失血少,省时省力等。
高频电刀主要用在手术室中对组织进行切割和凝血,从这一使用角度来说,又和麻醉机,呼吸机,无影灯,手术床,吸引器等构成手术设备类产品。
1. 用途高频电刀主要用在控制失血,其方法是对手术部位进行凝血(止血),但它也可用于外科切割。
这类装置通过作用电极端释放出高频电流,对目标组织进行加热,以产生干燥,汽化和炭化作用。
高频电刀也可用于腹腔导管结扎和经尿道切除前列腺(TURP)之类的手术,由于其止血效果好,这类装置还可以具有渗出毛细血管床的脏器进行手术,如肝,脾,甲状腺和肺;以及用于需要大量抗凝剂的开胸手术中。
采用氩气可增强电外科凝血作用。
这类装置可对血管丰富的脏器的出血表面进行快速止血,氩气增强系统也可用来控制其它组织如骨髓,肺和肌肉出血。
有的高频电刀组有氩气增强系统;氩气增强系统也有单独式的,可和某些高频电刀配合使用。
与器械手术相比,高频电刀的优点是可对某些手术部位同时进行切割和凝血。
2. 工作原理\r2.1单极高频电刀单频高频电刀采用电路对组织进行切割和凝血,这一电路由高频电刀内的高频振荡器和放大器,病人,连接导线和电极组成。
在大多数应用中,采用有功导线和电极将电流输出到手术部位。
然后,电流通过手术病人由与之相连的回路电极及其导线发散到高频功率发生器的中性一端,回路电极通常置于手术病人手术部位另一端。
高频电刀的检测方法

高频电刀的检测方法江玉柱;李东;刘祥富【摘要】介绍了高频电刀检测仪的基本原理和检测方法,采用定期或阶段性地对高频电刀的重要参数进行检测,特别是对检测中应注意的问题作了说明,以达到其工作性能稳定,临床使用安全的目的.【期刊名称】《医疗卫生装备》【年(卷),期】2010(031)006【总页数】2页(P113-114)【关键词】高频电刀;检测;输出功率;高频漏电流;波峰因子【作者】江玉柱;李东;刘祥富【作者单位】250022,济南,济南军区联勤部药品仪器检验所;250022,济南,济南军区联勤部药品仪器检验所;250022,济南,济南军区联勤部药品仪器检验所【正文语种】中文【中图分类】TH777高频电刀是现代手术的必备设备,是一种取代机械手术刀进行组织切割的电外科器械。
它是利用高频电流对人体组织直接进行切割、止血或烧灼的一种高频大功率电气设备,其具有切口整洁、止血彻底、节省时间等特点,可以进行多种外科手术,如在手术中进行单极切割、凝血和双极凝血。
由于电刀频率高、有效面积小、电流密度大,假如使用不当,则会引起意外损伤,给患者带来痛苦[1],故对其安全性要求极为严格。
一旦设备的安全保障功能丧失、临床使用不当,患者会被灼(烧)伤、电击,严重危害患者的生命安全,会引发医疗纠纷。
目前,高频电刀在我国的应用仍处于推广普及过程之中,许多医护人员和设备技术人员对高频电刀还不十分熟悉,加上高频电刀的长期工作会造成输出功率下降,安全保障性能变差,因此安全问题是困扰我国高频电刀使用者的一个重大因素。
而我们采取定期或间断性地对高频电刀的输出功率、高频漏电流、波峰因子3个重要参数进行检测,达到了高频电刀的工作性能稳定,临床使用安全的目的。
QA-ES测试仪是通过测量内部设置的负载的输入值来测量高频电刀的输出值。
使用的内置负载范围为10~5 200 Ω,可自动进行功率分布曲线测量。
自动测量功能包括峰值系数的测量,测量带宽30 Hz~10 MHz,确保测试结果的真实性和一致性。
高频电刀技术参数

保修
1
保修期:二年(人为损坏除外)
7
供电电源:电源连接:220V∕, 50HZ,输出电流:4. 0A/220V;
8
工作模式:单极纯切、混切1、混切2、混切3、单极凝、双极凝
8. 1
单极纯切:工作频率512KHZ定额输出350W定额负载500 C
8.2
单极混1:工作频率512KHZ定额输出250W定额负载500。
8.3
单极混2:工作频率512KHZ定额输出200W定额负载500 Q
8.4
单极混3:工作频率512KHZ定额输出120W定额负载500 Q
8.5
单极凝:工作工作频率512KHZ定额输出50W定额负载IooC
9
输出方式:单极手控输出、单极脚控输出和双极脚控凝输出。
10
保护功能:本机具有开路、短路、过功率、过电流自动保护功能。 允许连续使用,允许长时间开路和短路。
高频电刀技术参数
N
技术参数
投标响应
1
适用范围:本机适用于需要切割和/或凝血的各类外科手术。
2
本机具有手控和脚控两路控制输出(只能交叉使用),可使用单 片中性电极和双片中性电极。配备的连续性接触质量检测器可靠 性高,能随时监测中性电极状态。若接触质量低于设定值,会有 声光报警并切断输出,确保安全。
3
输出特性:CF等级低频电流隔离、全悬浮式输出。
11
安全指标符合国家标准《GB9706. 1T995医用电气设备第一部分: 安全通用要求》及≪GB9706. 4-1999医用电气设备高频手术设备 专用安全要求》。
12
主机尺寸:456mm (D) ×382mm (W) × 186mm (H)
主机净重:11. 5kg
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
ESU-2050/ESU-2050P高频电刀分析仪是一款外置负载的高精度高频电刀分析仪。
符合GB9706.4-2009,IEC60601-2-2.2009等标准测试需求。
行业第一个使用DFA电流采集技术,使设备具有10MHz的频率响应,500W的测量范围,小巧轻便。
深圳市一测医疗测试技术有限公司是一家专注于医疗器械测试产品和技术的研发、销售与服务为一体的“国家高新技术企业”,我们拥有自主研发的国家发明专利技术并且代理了众多国外先进专业
测试产品,如无创血压(NIBP)寿命测试系统、液压式有创压测试系统、高频电刀分析仪等。
ESU-2050/ESU-2050P高频电刀分析仪是一个完全新标准的全功能的电外科分析仪,拥有前所未有的新功能。
行业标准的射频电流测量,在业内是一款全面而又准确的高频电刀全功能质量检测仪。
性能参数:
1、采用 DFA 的技术和严格遵守电外科业界的射频电流测量标准;
2、使用外置负载0 欧到9999欧范围的高精度非感性测试负载;
3、标准量程使用 0.1:1 RF 电流互感器;
4、RS232 或 USB数据输出;
5、内部数据储存3个全部的ESU波形数据
6、可创建定制负载电阻表
7、使用可选的数字显示屏
8、可进行连续波和脉冲波形测试
技术参数:
1、功率测量量程为 0—500W;分辨率为0.1W,精度为 1%;
2、高频漏电流测量量程为 2 mA-7000mA,分辨率为 0.1 mA,精度为 1%;
3、外置负载阻抗范围为 0-9999 欧姆,精度为±1%;
4、带宽:10k-10MHz ;
5、波峰因子:1.4-500;
6、无需计算机和软件,主机屏幕显示分布曲线;
7、采用可擦除编程,触摸屏,支持图形和曲线;
以上就是深圳一测医疗给大家介绍美国BC ESU-2050/ESU-2050P 高频电刀分析仪相关信息,如果您还想了解更多的相关事项可以拨打我们的热线电话,可以点击我们的官网在线实时咨询我们,或者关注我们的官方微信公众号,我们会有专业的工作人员为您解答。
我们通过与国际优秀的医疗器械测试仪器制造商和专业实验室的广泛深入合作以及国内行业专家的紧密交流与协作,并严格按照ISO9001:2015质量管理体系要求为医疗器械产业在研发、生产,监督、检验,在用售后、培训,教学与研究等各领域客户提供完善的医疗器械测试整体解决方案和专业的技术服务。
