Regulator of G Protein Signaling 5
调节阿片受体mor胞内信号转导的分子机制研究进展

元对神经递质信号的转导。MOR 通过腺苷酸环化 酶抑制 G 蛋白家族 (G i/o) 发出信号,其通过激活 G 蛋白偶联内流钾通道,并抑制电压门控钙通道来 介导下游信号的转导。
一、调节 MOR 信号转导的蛋白质 与 其 他 GPCR 一 样,MOR 的 信 号 转 导 主 要 受 G 蛋 白 信 号 转 导 调 节 蛋 白 (Regulators of G protein signaling, RGS)、G 蛋 白 偶 联 受 体 激 酶 (G protein-coupled receptor kinase, GRK)、β- 抑 制 蛋 白 (βarrestin) 等影响。 1. G 蛋白信号转导调节蛋白 RGS 是 MOR 信号转导中最重要的调节蛋白。 RGS 中由 120 个氨基酸组成的结构域可以作用于与 GTP 结合的 Gα 亚基,加快其 GTP 的水解速率。哺 乳动物 RGS 家族由大约四十种蛋白质组成,分为九
阿片类药物成瘾性的产生密切相关。虽然 DOR 和 KOR 也与阿片类药物的镇痛作用相关,但阿片类药 物的镇痛作用主要通过 MOR 介导 [1]。
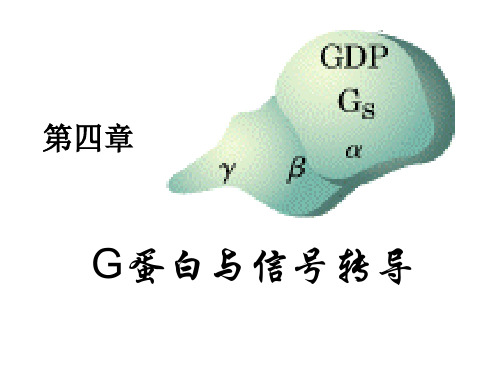
MOR 的镇痛作用主要通过 G 蛋白跨膜运输,G 蛋白通常是指细胞膜上的 G 蛋白异源三聚体,这种 蛋白质由 α、β、γ 三种亚基构成,后两种被称为 β-γ 复合物。目前,已经在哺乳动物中发现了 16 个不 同的 α 亚基家族成员,5 个不同的 β 亚基家族成员 和 11 个不同的 γ 亚基家族成员 [2]。
肿瘤微环境中周细胞参与肿瘤转移的研究进展

网络出版时间:2023-09-2715:42:37 网络出版地址:https://link.cnki.net/urlid/34.1086.R.20230926.1428.012肿瘤微环境中周细胞参与肿瘤转移的研究进展章 腾1,宋梦瑶1,钱 程1,赵 杨1,2,陆 茵1,3,4(南京中医药大学1.药学院,江苏省中药药效与安全性评价重点实验室,2.医学院·整合医学学院生物化学与分子生物学系,3.江苏省中医药防治肿瘤协同创新中心,4.江苏省中医药与再生医学研究国际合作联合实验室,江苏南京 210023)收稿日期:2023-05-11,修回日期:2023-07-22基金项目:国家自然科学基金资助项目(No82003991);南京中医药大学国家自然科学基金配套资助项目(NoXPT82003991);江苏省特聘教授资助项目(No013038021001);江苏省研究生科研创新项目(NoKYCX 1794)作者简介:章 腾(1999-),男,硕士生,研究方向:中药抗肿瘤药理,E mail:ZT1480283412qq@163.com;陆 茵(1963-),女,博士,教授,博士生导师,研究方向:中药抗肿瘤药理,通信作者,E mail:luyingreen@njutcm.edu.cndoi:10.12360/CPB202208008文献标志码:A文章编号:1001-1978(2023)10-1819-05中国图书分类号:R329 24;R364 3;R730 2;R73 37摘要:周细胞是血管壁细胞的重要组成部分,具有调控血流量、清除或吞噬细胞碎屑等作用,是一种包裹全身毛细血管的收缩细胞。
它控制血管通透性并参与血管的发育,是血管生成和血管功能的重要调节者和潜在药物靶点。
周细胞也被认为在肿瘤微环境中发挥着关键作用,尤其是在肿瘤生长和远端转移过程中。
因此,该文对参与肿瘤血管生成的周细胞与肿瘤转移之间的关系以及靶向周细胞治疗肿瘤进行综述,以期能为后续的研究提供依据。
G蛋白调节信号5在肿瘤微环境中对血管成熟度的研究进展

G蛋白调节信号5在肿瘤微环境中对血管成熟度的研究进展石雨卉;李爽;文世民;李光明【期刊名称】《四川生理科学杂志》【年(卷),期】2015(037)001【摘要】本文拟从分子水平探讨G蛋白调节信号5(Regulator of G-protein signaling 5,RGS5)、肿瘤血管周细胞及肿瘤微环境之间相互作用的关系.RGS5是肿瘤组织及血管周细胞高度表达的标记性产物,负性调节G蛋白信号传导途径.肿瘤血管周细胞形态结构异常,缺乏RGS5的肿瘤血管形态恢复正常且血流丰富.根据研究,肿瘤血管对于肿瘤微环境中的炎症细胞产生的炎症因子(血管生成因子VEGF、肿瘤生长因子EGF,放大炎症状态的细胞因子、其他血管原因子如FGF2,炎症趋化因子等)有着较高敏感性,这些炎症因子可以诱导和帮助肿瘤血管生成,而正常脉管却对这种反应不敏感.因此我们可在恶性肿瘤中降低RGS5的表达和抑制炎症因子的释放,使肿瘤周细胞逆转为成熟周细胞,肿瘤血管变得正常化,再采取抗肿瘤治疗可增加药物到达病灶的浓度,提高疗效.为恶性肿瘤的临床治疗、预后评价开辟新的途径.【总页数】4页(P37-40)【作者】石雨卉;李爽;文世民;李光明【作者单位】川北医学院第二附属医院-南充市中心医院,四川南充 637000;川北医学院第二附属医院-南充市中心医院,四川南充 637000;川北医学院第二附属医院-南充市中心医院,四川南充 637000;川北医学院第二附属医院-南充市中心医院,四川南充 637000【正文语种】中文【相关文献】1.G蛋白信号调节蛋白5在肿瘤研究中的进展 [J], 徐绘;黄桂春;陈龙邦2.G蛋白信号调节蛋白5在肿瘤血管正常化中的作用 [J], 黄维荪3.外泌体对肿瘤微环境的免疫调节作用及在肿瘤诊疗中的应用研究进展 [J], 何鑫;任伟宏;桑锋;邓博文;李文博;荣昊;刘朝阳4.外泌体对肿瘤微环境的免疫调节作用及在肿瘤诊疗中的应用研究进展 [J], 孙稚超5.外泌体对肿瘤微环境的免疫调节作用及在肿瘤诊疗中的应用研究进展 [J], 孙稚超因版权原因,仅展示原文概要,查看原文内容请购买。
G蛋白信号调节因子在心血管系统中的作用

G蛋白信号调节因子在心血管系统中的作用[摘要] g蛋白偶联受体(g-protein-coupled-receptors, gpcrs)在体内分布广泛,几乎参与所有生理活动的调节。
g蛋白调节因子(regulator of g protein signaling, rgs)参与了g蛋白失活的调节。
目前研究已证明,参与心血管系统生理和病理活动的很多递质和激素都是通过gpcr信号转导通路发挥作用的。
rgs 蛋白通过调节gpcr通路信号转导和非gpcr依赖性途径影响多种心血管疾病的发生,其在心脏血管结构和功能中的地位已逐渐引起重视,有望成为相关疾病治疗的新靶点。
本文将就rgs 蛋白及其在心血管系统中的作用作一综述。
[关键词] g蛋白信号调节因子;g蛋白偶联受体;心血管系统;作用[中图分类号] r331.3+6[文献标识码] a[文章编号] 1674-4721(2012)04(c)-0015-03g蛋白偶联受体(g-protein-coupled-receptors, gpcrs)在体内分布广泛,几乎参与所有生理活动的调节。
心血管系统中有100多种不同的gpcrs[1]。
gpcrs与g蛋白结合,后者由α、β和γ三个亚基组成。
g蛋白根据α亚基结构上的相似性可分为四个亚家族:gs,gi,gq和g12[2]。
哺乳动物细胞中至少有20种α亚基。
激动剂与gpcrs结合后可以促进g蛋白的活化。
这个过程是通过催化α亚基上gdp-gtp的交换实现的。
静息状态下,α亚基与gtp结合,受刺激后其结构发生改变,与βγ亚基分离。
与gtp结合的α亚基、βγ亚基二聚体继续调节下游效应物。
这个信号转导过程是由gα亚基的内源性的gtp酶活性所终止的,即gα亚基把gtp水解为gdp,使α与βγ亚基重新聚合。
通常认为,α亚基内源性的gtp酶活性不足以使g蛋白如此快地失活。
因此,人们设想存在加快g蛋白失活的辅助因子。
g蛋白调节因子(regulator of g protein signaling, rgs)就参与了g蛋白失活的调节。
G蛋白信号调节因子2与心血管疾病的关系

G蛋白信号调节因子2与心血管疾病的关系G蛋白信号转导的调节是当前分子生物学研究的热点内容之一。
G蛋白信号转导的持续时间和强度受G蛋白信号调节因子的调节。
作为一个不断增加的新的蛋白家族,RGS都有一个高度保守的具有GAP活性的RGS结构域。
这些RGS蛋白的功能也正在逐渐被认识。
目前在心血管疾病中研究和认识较多的是RGS2。
本文将对RGS2与高血压等心血管疾病的关系作一综述。
标签:G蛋白信号转导;信号调节因子2;心血管疾病;分子生物学G蛋白信号转导的调节是当前分子生物学研究的热点内容之一。
G蛋白藕联受体(G protein-coupled receptors,GPCRs)和特异性的配体结合后,选择性地激活不同的G蛋白,将信号通过不同途径传递到细胞内。
GPCRs在高血压和心血管疾病中的作用已经得到公认[1]。
GPCRs例如β肾上腺素能受体和血管紧张素受体的拮抗剂已成为心血管疾病治疗的基石。
目前发现G蛋白信号转导虽然形式多样,但其共同过程均为鸟苷酸酶循环(GTPase cycle),包括GTP/GDP交换和GTP水解两个环节。
G蛋白信号转导的持续时间和强度受G蛋白信号调节因子(regulator of G protein signalling,RGS)的调节。
RGS主要通过促进GTP 酶活性,数千倍地加速GTP水解,以终止G蛋白信号转导。
因此,RGS曾被称为GTP酶激活蛋白(GTPase-activating protein,GAP)。
除了表现GAP活性以外,RGS还可以通过拮抗G蛋白通路的下游信号分子起负性调控的作用。
到现在已经发现了数十种RGS蛋白。
作为一个不断增加的新的蛋白家族,RGS都有一个高度保守的具有GAP活性的RGS结构域。
这些RGS蛋白的功能也正在逐渐被认识。
目前在心血管疾病中研究和认识较多的是RGS2[2]。
本文将对RGS2与高血压等心血管疾病的关系作一综述。
1 RGS2的结构与分布RGS2最早在1994年被发现。
DEP结构域的功能研究进展

DEP结构域的功能研究进展王晓江【摘要】DEP结构域首先是在三种蛋白(鼠蓬乱蛋白、产卵缺陷10蛋白、血小板-白细胞C激酶底物2蛋白)中发现的,是一个由接近100个氨基酸组成的蛋白模序.人类基因组中至少有67种基因编码含DEP同源结构域蛋白.目前所了解的DEP结构域具有细胞膜定位、信号转导等多种功能.因此,它在细胞的生物学功能方面发挥着重要作用.可通过发掘DEP结构域与复杂的生物系统中多种分子和基因的相互作用,进一步研究DEP结构域的功能.%DEP domain was firstly found in three proteins( Dishevelled, Egl-lO and Pleckstrin ). It's a motif composed of nearly 100 amino acid. There are at least 67 genes in human genome encoding proteins with DEP homeodomain. Up to now it is known that DEP domain has membrane positioning, signal transduction and other functions. Thus, it plays a significant role in the cellular biological function. By exploring the interactions between DEP domain and the various molecules and genes in complex biological system, the function of DEP domain will be further understood.【期刊名称】《医学综述》【年(卷),期】2011(017)012【总页数】3页(P1772-1774)【关键词】DEP结构域;Wnt;尿苷三磷酸酶;非经典的细胞极性【作者】王晓江【作者单位】福建医科大学基础医学院生物化学与分子生物学系,福州,350004【正文语种】中文【中图分类】R34结构域是蛋白质结构和功能的基本组成单位。
第四章G蛋白
•
内啡肽既能增强又能抑制免疫功能,T 细胞和巨噬细胞膜上内胞的延迟凋亡是组织操作的主 要原因,而该细胞的延迟凋亡与G蛋白表 达改变有关。
重要的炎症介质受体均与G蛋白偶联。 血小板活化因子的大量释放,可活化血 小板,促进多种炎症介质的级联反应。其 受体与Gq偶联。
一、G 蛋白概况
(一)G 蛋白的发现和意义
Rodbell 等在 20 世纪 70 年代发现跨 膜信号转导需要 GTP 存在。 1977年,Gilman发现了G蛋白。
1981年,Gilman纯化了G蛋白。
1994年,Gilman 和 Rodbell 获医学 和生理学诺贝尔奖。
Alfred Goodman Gilman (1941- ) 吉尔曼(Alfred G. Gilman)
第四章
G蛋白与信号转导
【目的要求】
1.掌握G蛋白、小G蛋白的概念;G蛋白基 本结构。 2.熟悉G蛋白的种类;G蛋白通过AC和PLC 的信号转导机制。
3.了解G蛋白的发现和意义;G蛋白通过某 些受体门控离子通道和cGMP-PDE的信号转 导机制;小G蛋白的特点。
G 蛋白:
是鸟苷酸结合蛋白(guanosine nucleotide-binding protein)的简称,是 指能与 GTP 或 GDP 结合,与膜受体偶联 而具有信号转导作用的蛋白质。
二、G 蛋白信号转导机制
(一)G 蛋白通过 AC 的信号转导机制 1. 信号分子结合改变受体构象; 2. 受体与 G 蛋白结合并使其激活; 3. GTP 取代 GDP,G 游离; 4. G 结合并激活 AC,产生 cAMP; 5. GTP 水解, G 与 再结合。
Gs型G蛋白的亚基通过与受体和AC 的结合和解离达到信号跨膜传递的介导和 终止,GDP与GTP的互变起触媒作用。
生物信息学分析GNG5在肝癌中的表达及临床意义

生物信息学分析GNG5在肝癌中的表达及临床意义G蛋白是一类蛋白质家族,通过与G蛋白偶联受体结合,参与了多种细胞功能,如细胞分裂、分化及转移等[1]。
GNG 5,作为G蛋白的γ亚基,调控多种疾病的发生和发展。
在肿瘤方面,GNG5在胶质瘤中高表达,并可作为脑胶质瘤临床诊断及判断预后的新型生物标志物[2]。
在乳腺浸润性导管癌中通过Wnt信号通路调节E-cadherin的分泌[3]。
然而,GNG5在肝癌中的表达及预后中的功能作用知之甚少。
本研究旨在评价GNG5在肝癌中的生物学功能和预后作用。
1.材料与方法1.1.TIMER数据库:通过TIMER数据库分析GNG5与肝癌免疫细胞浸润之间的关系。
1.2.UALCAN数据库:通过UALCAN数据库分析GNG5在肝癌中的表达情况并分析GNG5表达水平与肝癌临床病理特征之间的关系。
1.3.Human Protein Atlas数据库:通过HPA数据库可以得到免疫组化图像直接比较了正常肝脏组织和肝癌组织中GNG5的蛋白表达。
1.4.Kaplan-Meier plotter数据库:通过该数据库,分析了GNG5 mRNA表达与总生存期和无病生存期之间的关系。
2结果2.1.GNG5在肝癌中的表达为了探究GNG5在肝癌中的差异表达,我们利用了UALCAN数据库验证了肝癌中GNG5的mRNA表达,结果显示,GNG5在肝癌中的表达水平显著升高(p=1.62e-12)。
在确定转录水平后,利用HPA数据库分析了GNG5在肝癌中的蛋白水平,相比于正常组织,GNG5在肝癌中的表达明显升高(图1)。
综上所述,GNG5在肝癌中的mRNA和蛋白质表达水平均增高。
图1:GNG5在肝癌中的mRNA及蛋白质表达水平。
2.2.肝癌患者GNG5mRNA水平与临床病理参数的分析我们探讨了GNG5的表达水平与肝癌患者临床特征之间的关系。
结果显示(图2),GNG5 mRNA表达增高与肿瘤的临床分期显著相关,且随着肿瘤分期的增高,表达水平也随之增高。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Manis JP:2007.Knock out,knock in,knock downâgenetically manipulated mice and the Nobel Prize.N Engl J Med357: 2426–2429.Marchant D.,Cheung C.,Carthy J.,et al.2009.MatrixMetalloproteinase-12expression is a critical mediator of the innate antiviral immune response.In review.Mastroianni CM&Liuzzi GM:2007.Matrix metalloproteinase dysregulation in HIV infection:implications for therapeutic stra-tegies.Trends Mol Med13:449–459. McQuibban GA,Gong JH,Tam EM,et al: 2000.Inflammation dampened by gelati-nase A cleavage of monocyte chemoattrac-tant protein-3.Science289:1202–1206. Nelissen I,Martens E,Van den Steen PE,et al: 2003.Gelatinase B/matrix metalloprotei-nase-9cleaves interferon-beta and is a target for immunotherapy.Brain126: 1371–1381.Opavsky MA,Penninger J,Aitken K,et al: 1999.Susceptibility to myocarditis is depen-dent on the response of alphabeta T lym-phocytes to coxsackieviral infection.Circ Res85:551–558.Parks WC,Wilson CL&Lopez-Boado YS: 2004.Matrix metalloproteinases as modu-lators of inflammation and innate immu-nity.Nat Rev Immunol4:617–629. Pauschinger M,Rutschow S,Chandrase-kharan K,et al:2005.Carvedilol improves left ventricular function in murine coxsack-ievirus-induced acute myocarditis associa-tion with reduced myocardial interleukin-1beta and MMP-8expression and a modu-lated immune response.Eur J Heart Fail7: 444–452.Renckens R,Roelofs JJ,Florquin S,et al:2006.Matrix metalloproteinase-9deficiency impairs host defense against abdominal sepsis.J Immunol176:3735–3741. Schnurr DP,Cao Y&Schmidt NJ:1984.Coxsackievirus B3persistence and myocar-ditis in N:NIH(S)II nu/nu and+/nu mice.J Gen Virol65(Pt7):1197–1201.Schonbeck U,Mach F&Libby P:1998.Generation of biologically active IL-1beta by matrix metalloproteinases:a novel cas-pase-1-independent pathway of IL-1beta processing.J Immunol161:3340–3346. Van Den Steen PE,Wuyts A,Husson SJ,et al: 2003.Gelatinase B/MMP-9and neutrophil collagenase/MMP-8process the chemo-kines human GCP-2/CXCL6,ENA-78/ CXCL5and mouse GCP-2/LIX and modu-late their physiological activities.Eur J Biochem270:3739–3749.Visse R&Nagase H:2003.Matrix metallopro-teinases and tissue inhibitors of metallopro-teinases:structure,function,and biochemistry.Circ Res92:827–839.S1050-1738(09)00053-X TCM Regulator of G Protein Signaling5:A New Player in Vascular Remodeling Mitali Manzur,Ruth Ganss⁎Regulators of G protein signaling(RGS)proteins are important modulators of G protein-coupled receptors and,therefore,critical for cardiovascular functions.One family member,RGS5,has recently been identified as a key regulator of vascular remodeling and pericyte maturation in tumors.Here,we discuss a potential role for RGS5and its relatives,RGS2and4,within the cardiovascular system.Insights into RGS5signaling are likely to be highly significant for vascular pathologies such as hypertension,atherosclerosis,and angiogenesis. (Trends Cardiovasc Med2009;19:26–30)n2009,Elsevier Inc.A Critical Role of Regulators ofG Protein Signaling Proteins inG Protein-Coupled ReceptorSignalingG protein-coupled receptors(GPCRs)areessential regulators of biologic processes.In the cardiovascular system alone,sev-eral hundred GPCRs are expressed andregulate key functions such as vasculartone and heart rate(Tang and Insel2004).Coupled to GPCRs are heterotri-meric G proteins that act as signaltransducers and are classified into fourcategories based on the functional andstructural similarities of theαsubunit;the Gαs subfamily stimulates adenylylcyclase(AC)activity;Gαi/o proteinsinhibit AC,as well as voltage-dependentcalcium channels;Gαq proteins stimu-lateβisoforms of phospholipase C;andG12/13signaling leads to GTPase Rhoactivation.In their active forms,bothGTP-bound Gαand the Gβγdimer dis-associate and signal to various intracel-lular effectors.Subsequently,G-proteindeactivation occurs by the intrinsicGTPase activity(GAP)of the Gαsubunit,which involves the hydrolysis of the Gα-bound GTP back to GDP,resulting in aconformational change that promotesthe reassociation of the Gβγsubunitand,thus,terminates the initial trigger.G protein-coupled receptor signalingis rapidly activated and deactivated inresponse to environmental cues.Although activation of G proteins israpid,self-catalyzed hydrolysis of GαGTPto GαGDP is slow and relies on externalGAP activity to sustain physiologicallymeaningful rates.Such GAP activity isprovided by the family of regulators of Gprotein signaling(RGS)molecules.Although RGS proteins were originallyidentified as negative regulators of Gproteins,it is now understood that theyare multifunctional proteins that gener-ally modulate G protein signaling.Todate,there are more than30RGSproteins that have been identified inmammals(Willars2006).All contain ahighly conserved carboxyl-terminaldomain of approximately125aminoacids(RGS domain or RGS box),whichconfers the unifying catalytic function ofRGS rger RGS moleculeshave additional domains enabling themto form multiprotein complexes.Severalreviews have excellently detailed thestructure,expression,and classificationof RGS proteins(Hendriks-Balk et al. Mitali Manzur and Ruth Ganss are at theWestern Australian Institute for MedicalResearch,The University of Western AustraliaCentre for Medical Research,Perth,WesternAustralia6000,Australia.⁎Address correspondence to:Ruth Ganss,Western Australian Institute for MedicalResearch,Rear,50Murray St.,MRF Building,Level5,Perth,WA6000,Australia.Tel.:(+61)892240354;fax:(+61)892240322.;e-mail:ganss@.au.©2009,Elsevier Inc.All rights reserved.1050-1738/09/$-see front matter2008,Willars2006).The following dis-cussion will focus on some members of the B/R4(RGS1-5,8,13,16,18,21) subfamily of RGS molecules,in particu-lar,RGS2,4,and5,and discuss their implications in cardiovascular function. An Emerging Role of RGS Mole-cules in Cardiovascular Function Recently,there has been an exponential increase in studies on RGS molecules and their signaling activities.However, these data are mainly derived from reconstituted in vitro systems,where RGS molecules are overexpressed along with GPCRs and very little is known of their physiologic relevance in vivo.In view of this,RGS knockout and trans-genic mouse models are now being developed and provide us with new insights into complex systems,including their role in cardiovascular function (Table1).For instance,knockout studies have revealed an important role for RGS2in vascular homeostasis in vivo. RGS2-deficient mice are hypertensive and exhibit prolonged vasoconstriction by attenuating signaling via Gαq-coupled receptors(Heximer et al.2003).Interest-ingly,RGS2itself is regulated by nitric oxide(NO)and,thus,is involved in a cross-talk between Gαq and NO signaling to mainly regulate peripheral vascular mechanisms(Sun et al.2005,Tang et al. 2003).Preliminary studies in humans further support an involvement of RGS2 in hypertension(Semplicini et al.2006, Yang et al.2005).Cardiac hypertrophy was not observed in RGS2knockout mice.However,decreased RGS2mRNA levels have been described in the hearts of Gαq-overexpressing mice before the development of cardiac hypertrophy (Zhang et al.2006).More recently,Takimoto et al.(2009)reported thatRGS2deficiency exacerbates hypertro-phy under chronic Gαq stimulation,thus,demonstrating a critical role of RGS2inearly cardiac stress in response to pres-sure overload.Another RGS molecule,RGS4,whoseexpression is enriched in the heart andcentral nervous system(Cifelli et al.2008,Erdely et al.2004,Rogers et al.2001),has been correlated to cardiachypertrophy in both mice(Rogers et al.1999)and humans(Owen et al.2001).RGS4is upregulated in failing humanhearts as a result of cardiomyopathy(Mittmann et al.2002,Owen et al.2001).Evidence is increasing that theenhanced RGS4expression counter-reg-ulates Gαq-induced signaling caused byhypertrophic stimuli(Rogers et al.2001,Tokudome et al.2008).Thus,RGS4,incontrast to RGS2,may have a protectiveeffect in late stages of heart failure.Moreover,RGS4is required for attenua-tion of parasympathetic-dependent Gprotein signaling and heart rate controlin correlation with its high expression inthe sinoatrial node(Cifelli et al.2008).These findings remarkably demonstratethe exquisite role of RGS modulators incardiac adaptation and the implicationsof their spatiotemporal expression.Changes in RGS expression have beenobserved in a variety of cardiovasculardiseases,and the relative importance andcell/organ specificity of different RGSsubtypes is only beginning to emerge(Hendriks-Balk et al.2008).A Newly Discovered Role of RGS5inVascular RemodelingRGS5,another member of the B/R4family,is expressed in most majororgans,including the heart,lungs,skele-tal muscle,brain,kidneys,and placenta,as well as in highly specialized cell typessuch as vascular smooth muscle cells,glomerular mesangial cells of the kidney,and cardiac myocytes(Seki et al.1998).Interestingly,gene expression studiesrevealed that RGS5expression is dyna-mically regulated in vivo,which is in linewith RGS5being a regulator of adaptiveprocesses and vascular remodeling.Forinstance,RGS5expression is downregu-lated in the brain capillaries and choroidplexus of stroke-prone hypertensive rats(Kirsch et al.2001).Gene profiling alsoidentified RGS5as a marker for arterialsmooth muscle cells over vein(Adams etal.2000,Li et al.2004).Interestingly,itsexpression is dramatically downregu-lated in atherosclerotic plaque-derivedsmooth muscle cells(SMCs)from pri-mates(Li et al.2004)and the fibrous capof advanced atherosclerotic plaques inhumans(Adams et al.2006).A gene profiling experiment is alsohow RGS5initially came to our ownattention(Berger et al.2005).RGS5wasfound to be highly expressed in angio-genic tumor vessels,whereas being sig-nificantly downregulated in a lessangiogenic vasculature in regressingtumors under therapy(Ganss et al.2002).Moreover,RGS5mRNA was upre-gulated during ovarian angiogenesis andin skin wounds during the formation ofgranulation tissue,before subsiding asthe wound closes(Berger et al.2005).Wesubsequently localized RGS5expressionto pericytes,close relatives of vascularSMCs(vSMC)that wrap aroundendothelial cells and are an integral partof the blood vessel wall(Armulik et al.2005).Thus,RGS5was identified as thefirst marker for angiogenic pericytes atsites of vessel remodeling in the tumormicroenvironment,during wound heal-ing and ovulation(Berger et al.2005).To functionally analyze RGS5intumors,we generated RGS5-deficientmice that were crossed with transgenic,tumor-prone RIP1-Tag5mice.Thesetransgenic mice express the oncogeneSV40large T antigen(Tag)under thecontrol of the rat insulin gene promoterinβcells of the pancreas and developpancreatic islet cell carcinomas.Bloodvessels in insulinomas are typicallydilated and tortuous,with an overallchaotic appearance(Ryschich et al.2002).However,in the absence ofTable1.Cardiovascular studies in mouse models in vivo involving RGS2, 4,and5RGS subtype Cardiovascular function ReferencesRGS2Blood pressure regulation Heximer et al.(2003)Tang et al.(2003)Cardiac adaptation Gross et al.(2005)Obst et al.(2006)Takimoto et al.(2009) RGS4Cardiac adaptation Rogers et al.(2001)Tokudome et al.(2008)Cifelli et al.(2008)RGS5Blood pressure regulation Cho et al.(2008)Nisancioglu et al.(2008)RGS5,tumors arise with a more homo-geneous vasculature that closely resem-bles vessels in normal organs(Hamzah et al.2008).Interestingly,wild-type tumor pericytes expressed high levels of plate-let-derived growth factor receptorβ(PDGFRβ)and low levels ofαsmooth muscle actin,an expression pattern typi-cal of immature pericytes(Song et al. 2005).In contrast,RGS5-deficient tumor pericytes displayed very low levels of PDGFRβand high levels ofαsmooth muscle actin expression,representing a mature pericyte population.This finding strongly implies that RGS5negatively impacts on pericyte maturation and is actively involved in vessel normalization. Importantly,these changes in vessel morphology correlated with alterations in the tumor environment such as reduced vascular leakiness and tumor hypoxia and significantly improved anti-tumor immunotherapy(reviewed in Manzur et al.(2008)).RGS5is also expressed in pericytes surrounding brain capillaries(Bondjers et al.2003), and deletion of RGS5reduced edema after transient cerebral ischemia,demon-strating reduced vascular permeability similar to our observation inânormali-zedâRGS5-deficient tumor vessels (Hamzah et al.2008).Currently,it is notclear how RGS5,via its intrinsic GAPactivity or functions outside the RGSbox,controls vascular maturation andvessel remodeling.These complex pro-cesses may involve communication withunderlying endothelial cells and GPCR-mediated adhesion and migration signals(Cho et al.2003).RGS5as a Regulator ofCardiovascular FunctionPericytes have been associated withhemodynamic functions,similar toSMC of larger vessels,and can controlvasoconstriction and vasodilation withincapillary beds(Bergers and Song2005).Thus,although it is possible that peri-cytes also play a role in blood pressurehomeostasis,they represent a heteroge-neous cell population,and a conclusivelink to RGS5is yet to be made.Interest-ingly,however,RGS5is expressed earlyduring arterial development(Bondjers etal.2003,Cho et al.2003)and is a markerof arterial SMC in adults(Adams et al.2000,Li et al.2004),suggesting a role ofRGS5in arterial contraction and regula-tion of vascular tone.This was supportedby a human genomewide linkage andcandidate gene-based screen that linkedRGS5to blood pressure regulation(Chang et al.2007).Recently,two inde-pendent studies demonstrated that bothsystolic and diastolic blood pressure wassignificantly lower in RGS5-deficientmice compared with wild type(Cho etal.2008,Nisancioglu et al.2008).Thisfinding is somewhat unexpected becauseRGS5signaling has been mainly linkedto Gαi and Gαq proteins,both mediatorsof vasoconstriction in vSMC(Cho et al.2003,Wang et al.2002,2008).Currently,there is no evidence for a role of RGS5inendothelial cell-mediated vasorelaxationbecause of its specific expression inpericytes and vSMC.Indeed,furtherinsights into RGS-mediated cardiovascu-lar signaling and receptor selectivity arerequired to fully appreciate how the lackof RGS5induces hypotension,whereasRGS2-deficient mice are hypertensive(Heximer et al.2003).Regulatory Networks of RGS2and5in vSMCRGS5knockout mice have only recentlybeen generated,and therefore,mostsignaling studies have been conductedin cell culture.In contrast,the roleof Figure1.Potential roles of RGS2and RGS5in the regulation of vascular tone.Cellular messengers that modulate or are modulated by RGS2and RGS5either in vitro(segmented line)or in vivo(solid line).PLC indicates phospholipase C;GC,guanylate cyclase;PKA,protein kinase A;PKC, protein kinase C;PKG,protein kinase G;DAG,diacylglycerol;IP3,inositol phosphate3.RGS2in cardiovascular function has been analyzed in an elegant combination of in vivo and in vitro studies using gene knockout mice(Table1).RGS2prefer-entially interacts with Gαq(Heximer et al.1999)and attenuates signaling via Gαq-coupled GPCR receptors such as PAR1/thrombin,α-adrenergic receptor/ phenylephrine,and angiotensin II recep-tor type I/angiotensin,but not Gαi/ser-otonin(Heximer et al.2003,Tang et al. 2003).In addition,RGS2is phosphory-lated by cyclic guanosine monopho-sphate-dependent kinase I-α,which itself is activated by the NO–cyclic gua-nosine monophosphate pathway(Figure 1)(Takimoto et al.2009,Tang et al. 2003).The net effect of the cross-talk between Gαq and NO signaling is increased RGS2GAP activity,which terminates Gαq signaling.Loss of RGS2-mediated signaling induces hyper-tension in RGS2-deficient mice(Hexi-mer et al.2003).Moreover,RGS2also seems to interfere with Gαs-cyclic ade-nosine monophosphate(cAMP)signal-ing through direct inhibition of AC and Gαs proteins,which would be expected to enhance vasoconstriction(Roy et al. 2006,Sinnarajah et al.2001)(Figure1).RGS5is known to act as GAP for Gαq and Gαi proteins(Cho et al.2003,Wang et al.2008,Zhou et al.2001),and transient deletion of RGS5from vascular smooth muscle cells enhanced angio-tensin II signaling(Wang et al.2002) (Figure1).Protein kinase C phosphor-ylation of RGS5causes loss of inhibition of endothelin-1-Gαq signaling(Moroi et al.2007).This clearly contrasts with the in vivo finding of hypotension in RGS5-deficient mice.However,the in vivo scenario might be more complex.For instance,RGS5is a physiologic target for the NO-mediated N-end rule degradation pathway(Figure1)that functions through its ability to destroy specific regulatory proteins bearing an N-term-inal cysteine such as RGS4,5,and16(Hu et al.2005,Lee et al.2005).Moreover, RGS5was upregulated in cardiac tissue of mice that overexpressedβ2-adrenergic receptor(β2AR),as well as in hearts of rats that were chronically treated with theβAR agonist isoproterenol(Jean-Baptiste et al.2005).Interestingly,both β1AR andβ2AR signaling is usually coupled to Gαs proteins and increased cAMP levels,which ultimately lead to vessel relaxation.Therefore,RGS5may interfere with several regulatory path-ways,as does RGS2,however,with adifferent net outcome.More studies inphysiologically relevant disease modelswill be needed to determine dominantpathway(s)of RGS5in cardiovascularsignaling.Concluding RemarksCardiovascular diseases,includinghypertension,heart failure,and athero-sclerosis,remain one of the largestcauses of morbidity and mortality inWestern populations.GPCR-mediatedsignaling is critical for normal functionin the cardiovascular system and iscurrently the primary target for thepharmacologic treatment of disease.There is a growing interest in RGSmolecules because their role as specificGPCR modulators emerges.In particu-lar,RGS2and RGS4have been recog-nized as important regulators ofcardiovascular physiology and poten-tially novel drug targets.To date,animportant role for RGS5in tumor peri-cytes and neovascularization has beendemonstrated.These findings reveal newperspectives on vascular remodeling andhave significant implications for antic-ancer therapy.Furthermore,there isincreasing evidence for RGS5involve-ment in vascular pathologies.How thesediverse biologic functions are connectedand whether they share common signal-ing cascades remains to be determined.As the unique and specific profile ofRGS5is being discovered,new effectorfunctions beyond GAP activity may berevealed.AcknowledgmentsThe authors acknowledge support fortheir original work summarized in thisarticle from the National Health andMedical Research Council,Australia,the Western Australian Institute for Med-ical Research,and the University ofWestern Australia,Perth,WesternAustralia.ReferencesAdams LD,Geary RL,McManus B&SchwartzSM:2000.A comparison of aorta and venacava medial message expression by cDNAarray analysis identifies a set of68consis-tently differentially expressed genes,all inaortic media.Circ Res87:623–631.Adams LD,Geary RL,Li J,et al:2006.Expression profiling identifies smooth mus-cle cell diversity within human intima andplaque fibrous cap:loss of RGS5distin-guishes the cap.Arterioscler Thromb VascBiol26:319–325.Armulik A,Abramsson A&Betsholtz C:2005.Endothelial/pericyte interactions.Circ Res97:512–523.Berger M,Bergers G,Arnold B,et al:2005.Regulator of G-protein signaling-5induc-tion in pericytes coincides with active vesselremodeling during neovascularization.Blood105:1094–1101.Bergers G&Song S:2005.The role ofpericytes in blood-vessel formation andmaintenance.Neuro Oncol7:452–464.Bondjers C,Kalen M,Hellstrom M,et al:2003.Transcription profiling of platelet-derivedgrowth factor-B-deficient mouse embryosidentifies RGS5as a novel marker forpericytes and vascular smooth musclecells.Am J Pathol162:721–729.Chang YP,Liu X,Kim JD,et al:2007.Multiplegenes for essential-hypertension suscept-ibility on chromosome1q.Am J HumGenet80:253–264.Cho H,Kozasa T,Bondjers C,et al:2003.Pericyte-specific expression of Rgs5:impli-cations for PDGF and EDG receptor signal-ing during vascular maturation.FASEB J17:440–442.Cho H,Park C,Hwang IY,et al:2008.Rgs5targeting leads to chronic low blood pres-sure and a lean body habitus.Mol Cell Biol28:2590–2597.Cifelli C,Rose RA,Zhang H,et al:2008.RGS4regulates parasympathetic signaling andheart rate control in the sinoatrial node.Circ Res103:527–535.Erdely HA,Lahti RA,Lopez MB,et al:2004.Regional expression of RGS4mRNA inhuman brain.Eur J Neurosci19:3125–3128.Ganss R,Ryschich E,Klar E,et al:2002.Combination of T-cell therapy and trigger ofinflammation induces remodeling of thevasculature and tumor eradication.CancerRes62:1462–1470.Gross V,Tank J,Obst M,et al:2005.Auto-nomic nervous system and blood pressureregulation in RGS2-deficient mice.Am JPhysiol Regul Integr Comp Physiol288:R1134–R1142.Hamzah J,Jugold M,Kiessling F,et al:2008.Vascular normalization in Rgs5-deficienttumours promotes immune destruction.Nature453:410–414.Hendriks-Balk MC,Peters SL,Michel MC&Alewijnse AE:2008.Regulation of G pro-tein-coupled receptor signalling:focus onthe cardiovascular system and regulator ofG protein signalling proteins.Eur J Phar-macol585:278–291.Heximer SP,Srinivasa SP,Bernstein LS,et al:1999.G protein selectivity is a determinantof RGS2function.J Biol Chem274: 34253–34259.Heximer SP,Knutsen RH,Sun X,et al:2003.Hypertension and prolonged vasoconstric-tor signaling in RGS2-deficient mice.J Clin Invest111:445–452.Hu RG,Sheng J,Qi X,et al:2005.The N-end rule pathway as a nitric oxide sensor controlling the levels of multiple regulators.Nature437:981–986.Jean-Baptiste G,Li X,Yang Z,et al:2005.Beta adrenergic receptor-mediated atrial specific up-regulation of RGS5.Life Sci76: 1533–1545.Kirsch T,Wellner M,Luft FC,et al:2001.Altered gene expression in cerebral capil-laries of stroke-prone spontaneously hyper-tensive rats.Brain Res910:106–115.Lee MJ,Tasaki T,Moroi K,et al:2005.RGS4 and RGS5are in vivo substrates of the N-end rule pathway.Proc Natl Acad Sci U S A 102:15030–15035.Li J,Adams LD,Wang X,et al:2004.Regulator of G protein signaling5marks peripheral arterial smooth muscle cells and is down-regulated in atherosclerotic plaque.J Vasc Surg40:519–528.Manzur M,Hamzah J&Ganss R:2008.Modulation of theâblood-tumorâbarrier improves immunotherapy.Cell Cycle7: 2452–2455.Mittmann C,Chung CH,Hoppner G,et al: 2002.Expression of ten RGS proteins in human myocardium:functional character-ization of an upregulation of RGS4in heart failure.Cardiovasc Res55:778–786.Moroi K,Nishiyama M,Kawabata S,et al: 2007.Phosphorylation of Ser166in RGS5 by protein kinase C causes loss of RGS function.Life Sci81:40–50.Nisancioglu MH,Mahoney WM,Jr.,Kimmel DD,et al:2008.Generation and character-ization of rgs5mutant mice.Mol Cell Biol 28:2324–2331.Obst M,Tank J,Plehm R,et al:2006.NO-dependent blood pressure regulation in RGS2-deficient mice.AM J Physiol Regul Integr Comp Physiol290:R1012–R1019.Owen VJ,Burton PB,Mullen AJ,et al:2001.Expression of RGS3,RGS4and Gi alpha2in acutely failing donor hearts and end-stage heart failure.Eur Heart J22:1015–1020.Rogers JH,Tamirisa P,Kovacs A,et al:1999.RGS4causes increased mortality andreduced cardiac hypertrophy in responseto pressure overload.J Clin Invest104:567–576.Rogers JH,Tsirka A,Kovacs A,et al:2001.RGS4reduces contractile dysfunction andhypertrophic gene induction in Galpha qoverexpressing mice.J Mol Cell Cardiol33:209–218.Roy AA,Baragli A,Bernstein LS,et al:2006.RGS2interacts with Gs and adenylyl cyclasein living cells.Cell Signal18:336–348.Ryschich E,Schmidt J,Hammerling GJ,et al:2002.Transformation of the microvascularsystem during multistage tumorigenesis.IntJ Cancer97:719–725.Seki N,Sugano S,Suzuki Y,et al:1998.Isolation,tissue expression,and chromoso-mal assignment of human RGS5,a novel G-protein signaling regulator gene.J HumGenet43:202–205.Semplicini A,Lenzini L,Sartori M,et al:2006.Reduced expression of regulator of G-protein signaling2(RGS2)in hypertensivepatients increases calcium mobilization andERK1/2phosphorylation induced by angio-tensin II.J Hypertens24:1115–1124.Sinnarajah S,Dessauer CW,Srikumar D,et al:2001.RGS2regulates signal transduction inolfactory neurons by attenuating activation ofadenylyl cyclase III.Nature409:1051–1055.Song S,Ewald AJ,Stallcup W,et al:2005.PDGFRβ+perivascular progenitor cells intumours regulate pericyte differentiation andvascular survival.Nat Cell Biol7:870–879.Sun X,Kaltenbronn KM,Steinberg TH&Blumer KJ:2005.RGS2is a mediator ofnitric oxide action on blood pressure andvasoconstrictor signaling.Mol Pharmacol67:631–639.Takimoto E,Koitabashi N,Hsu S,et al:2009.Regulator of G protein signaling2mediatescardiac compensation to pressure overloadand antihypertrophic effects of PDE5inhi-bition in mice.J Clin Invest119:408–420.Tang CM&Insel PA:2004.GPCR expressionin the heart;ânewâreceptors in myocytesand fibroblasts.Trends Cardiovasc Med14:94–99.Tang KM,Wang GR,Lu P,et al:2003.Regulator of G-protein signaling-2mediatesvascular smooth muscle relaxation andblood pressure.Nat Med9:1506–1512.Tokudome T,Kishimoto I,Horio T,et al:2008.Regulator of G-protein signaling subtype4mediates antihypertrophic effect of locallysecreted natriuretic peptides in the heart.Circulation117:2329–2339.Wang Q,Liu M,Mullah B,et al:2002.Receptor-selective effects of endogenousRGS3and RGS5to regulate mitogen-acti-vated protein kinase activation in rat vas-cular smooth muscle cells.J Biol Chem277:24949–24958.Wang X,Adams LD,Pabon LM,et al:2008.RGS5,RGS4,and RGS2expression andaortic contractibility are dynamically co-regulated during aortic banding-inducedhypertrophy.J Mol Cell Cardiol44:539–550.Willars GB:2006.Mammalian RGS proteins:multifunctional regulators of cellular sig-nalling.Semin Cell Dev Biol17:363–376.Yang J,Kamide K,Kokubo Y,et al:2005.Genetic variations of regulator of G-proteinsignaling2in hypertensive patients and inthe general population.J Hypertens23:1497–1505.Zhang W,Anger T,Su J,et al:2006.Selectiveloss of fine tuning of Gq/11signaling byRGS2protein exacerbates cardiomyocytehypertrophy.J Biol Chem281:5811–5820.Zhou J,Moroi K,Nishiyama M,et al:2001.Characterization of RGS5in regulation of Gprotein-coupled receptor signaling.Life Sci68:1457–1469.S1050-1738(09)0006-7TCM。
