美国环保局 EPA 试验 方法 8440
美国国家环保局(EPA)温室气体清单编制机制

美国国家环保局(EPA)温室气体清单编制机制
吴伟强;吴安琪
【期刊名称】《科学与管理》
【年(卷),期】2012(032)002
【摘要】美国虽拒签《京都议定书》,但仍积极编制本国的温室气体清单。
本文基于对IPCC和EPA温室气体清单方法学的比较研究,并通过对EPA温室气体清单编制的演变过程、流程和特点的系统分析,揭示其内在机制。
以期借鉴较为成熟的温室气体清单编制方法,完善我国温室气体排放的核算体系。
【总页数】11页(P11-21)
【作者】吴伟强;吴安琪
【作者单位】浙江工业大学经贸管理学院,杭州市310023;浙江工业大学经贸管理学院,杭州市310023
【正文语种】中文
【中图分类】X16
【相关文献】
1.美国国家环保局(EPA)关于在美国禁用和逐步淘汰石棉制品的通知书 [J],
2.美国国家环保局(EPA)对毒物控制的新设想 [J], 吕伟
3.离子色谱法检测饮用水中的无机阴离子——美国国家环保局(U.S.EPA)方法300.1简介(下) [J],
4.离子色谱法检测饮用水中的无机阴离子——美国国家环保局(U.S.EPA)方法300.1简介(上) [J],
5.离子色谱法检测饮用水中的无机阴离子——美国国家环保局(U.S.EPA)方法300.1简介(中) [J], 美国DIONEX(戴安)公司中国服务中心
因版权原因,仅展示原文概要,查看原文内容请购买。
美国环境监测五大标准分析方法系列特点

美国环境监测五大标准分析方法系列特点
王炳华
【期刊名称】《干旱环境监测》
【年(卷),期】1993(007)004
【摘要】着重介绍了美国环保局(EPA)开发的五套环境监测标准分析方法系列:EPA600,EPA500,CLP,EPASW-846,EPA200的要点,并简要评述了其特点,这些代表美国环境科学技术最新成就的方法系列应当为我们参考和借鉴.
【总页数】9页(P246-253,F004)
【作者】王炳华
【作者单位】无
【正文语种】中文
【中图分类】X830.2
【相关文献】
1.毒品及滥用药物的HPLC、GC分析方法——美国新泽西州法医局部分毒物标准化分析方法简介(续) [J], 高利生
2.美国环境监测分析方法的技术体系 [J], 黄俊;周申范
3.《环境监测分析方法标准制修订技术导则》 ( HJ 168—2010 )对土壤环境监测标准制修订适用性探讨 [J], 夏新;姜晓旭;邹家素;王静;于勇;杨楠
4.美国国家标准和技术研究院信息安全标准化系列研究(十三) 美国NIST云计算安全标准跟踪及研究 [J], 王惠莅;杨晨;杨建军
5.美国国家标准和技术研究院信息安全标准化系列研究(三) SP 800系列信息安全标准研究 [J], 王惠莅;杨晨;张明天;杨建军
因版权原因,仅展示原文概要,查看原文内容请购买。
美国饮用水水质标准(EPA)

《美国饮用水水质标准》(EPA)
[标题]:《美国饮用水水质标准》
[颁布者]:美国
[编号]:
[颁布日期]:
[实施日期]:
[有效性]:有效
国家一级饮用水规程(NPDWRs或一级标准),是法定强制性的标准,它适用于公用给水系统。
一级标准限制了那些有害公众健康的及已知的或在公用给水系统中出现的有害污染物浓度,从
注:
①、污染物最高浓度目标MCLG-对人体健康无影响或预期无不良影响的水中污染物浓度。
它规定了确当的安全限量,MCLGs是非强制性公共健康目标。
②、污染物最高浓度-它是供给用户的水中污染物最高允许浓度,MCLGs它是强制性标准,MCLG 是安全限量,确保略微超过MCL限量时对公众健康不产生显着风险。
③、TT处理技术-公共给水系统必须遵循的强制性步骤或技术水平以确保对污染物的控制。
④、除非有特别注释,一般单位为mg/L。
⑤、
度:
病毒
HPC每毫升不超过500细菌数。
⑨、每月总大肠杆菌阳性水样不超过5%,于每月例行检测总大肠杆菌的样品少于40只的给水系统,总大肠菌阳性水样不得超过1个。
含有总大肠菌水样,要分析粪型大肠杆菌,粪型大肠杆菌不容许存在。
⑩、粪型及艾氏大肠杆菌的存在表明水体受到人类和动物排泄物的污染,这些排泄物中的微生物可引起腹泻,痉挛,恶心,头痛或其它症状。
岳宇明译岳舜琳校。
美国环保署 EPA 方法 8440

METHOD 8440TOTAL RECOVERABLE PETROLEUM HYDROCARBONS BY INFRAREDSPECTROPHOTOMETRY1.0SCOPE AND APPLICATION1.1Method 8440 (formerly Draft Method 9073) is used for the measurement of total recoverable petroleum hydrocarbons (TRPHs) extracted with supercritical carbon dioxide from sediment, soil and sludge samples using Method 3560.1.2Method 8440 is not applicable to the measurement of gasoline and other volatile petroleum fractions, because of evaporative losses.1.3Method 8440 can detect TRPHs at concentrations of 10 mg/L in extracts. This translates to 10 mg/Kg in soils when a 3 g sample is extracted by SFE (assuming 100 percent extraction efficiency), and the final extract volume is 3 mL.1.4This method is restricted to use by or under the supervision of trained analysts. Each analyst must demonstrate the ability to generate acceptable results with this method.2.0SUMMARY OF METHOD2.1Soil samples are extracted with supercritical carbon dioxide using Method 3560. Interferences are removed with silica gel, either by shaking the extract with loose silica gel, or by passing it through a silica gel solid-phase extraction cartridge. After infrared (IR) analysis of the extract, TRPHs are quantitated by direct comparison with standards.3.0INTERFERENCES3.1The analyte class being measured (TRPHs) is defined within the context of this method. The measurement may be subject to interferences, and the results should be interpreted accordingly.3.2Determination of TRPHs is a measure of mineral oils only, and does not include the biodegradable animal greases and vegetable oils captured in oil and grease measurements. These non-mineral-oil contaminants may cause positive interferences with IR analysis, if they are not completely removed by the silica gel cleanup.3.3Method 8440 is not appropriate for use in the analysis of gasoline and other volatile petroleum fractions because these fractions evaporate during sample preparation.4.0APPARATUS AND MATERIALS4.1Infrared spectrophotometer - Scanning or fixed wavelength, for measurement around 2950 cm.-14.2IR cells - 10 mm, 50 mm, and 100 mm pathlength, sodium chloride or IR-grade glass. CD-ROM8440 - 1Revision 0December 1996CD-ROM 8440 - 2Revision 0December 19964.3Magnetic stirrer with polytetrafluoroethylene (PTFE)-coated stirring bars.4.4Optional - A vacuum manifold consisting of glass vacuum basin, collection rack and funnel, collection vials, replaceable stainless steel delivery tips, built-in vacuum bleed valve and gauge is recommended for use when silica gel cartridges are used. The system is connected to a vacuum pump or water aspirator through a vacuum trap made from a 500 mL sidearm flask fitted with a one-hole stopper and glass tubing.5.0REAGENTS5.1Reagent-grade chemicals shall be used in all tests. Unless otherwise indicated, it is intended that all reagents shall conform to the specifications of the Committee on Analytical Reagents of the American Chemical Society, where such specifications are available. Other grades may be used, provided it is first ascertained that the reagent is of sufficiently high purity to permit its use without lessening the accuracy of the determination.5.2Tetrachloroethylene, C Cl - spectrophotometric grade, or equivalent.245.3Raw materials for reference oil mixture - spectrophotometric grade, or equivalent.5.3.1n-Hexadecane, CH (CH )CH 321435.3.2Isooctane, (CH )CCH CH(CH )332325.3.3Chlorobenzene, C H Cl 655.4Silica gel.5.4.1Silica gel solid-phase extraction cartridges (40 µm particles, 60 A pores), 0.5 g,Supelco, J.T. Baker, or equivalent.5.4.2Silica gel, 60 to 200 mesh, Davidson Grade 950 or equivalent (deactivated with1 to2 percent water).5.5Calibration mixtures:5.5.1The material of interest, if available, or the same type of petroleum fraction, if itis known and original sample is unavailable, shall be used for preparation of calibration standards. Reference oil is to be used only for unknowns. Whenever possible, a GC fingerprint should be run on unknowns to determine the petroleum fraction type.5.5.2Reference oil - Pipet 15.0 mL n-hexadecane, 15.0 mL isooctane, and 10.0 mLchlorobenzene into a 50 mL glass-stoppered bottle. Maintain the integrity of the mixture by keeping stoppered except when withdrawing aliquots. Refrigerate at 4E C when not in use.5.5.3Stock standard - Pipet 0.5 mL calibration standard (Section 5.5.1 or 5.5.2) intoa tared 100 mL volumetric flask and stopper immediately. Weigh and dilute to volume with tetrachloroethylene.5.5.4Working standards - Pipet appropriate volumes of stock standard (Sec. 5.5.3) into100 mL volumetric flasks according to the cell size to be used. Dilute to volume withCD-ROM 8440 - 3Revision 0December 1996tetrachloroethylene. Calculate the concentrations of the standards from the stock standard concentrations.5.6Calibration of silica gel cleanup5.6.1Prepare a stock solution of corn oil and mineral oil by placing about 1 mL each(0.5 to 1 g) of corn oil and mineral oil into a tared 100 mL volumetric flask. Stopper the flask and weigh to the nearest milligram. Dilute to the mark with tetrachloroethylene, and shake the contents to effect dissolution.5.6.2Prepare additional dilutions to cover the range of interest.5.6.3Transfer 2 mL (or other appropriate volume) of the diluted corn oil/mineral oilsamples to vials.5.6.4Add 0.3 g of loose silica gel to the vials of diluted corn oil/mineral oil samples andshake the mixture for 5 minutes, or pass the extract through a 0.5 g silica gel solid-phase extraction cartridge (conditioned with 5 mL of tetrachloroethylene). Elute with tetrachloroethylene. Collect three 3 mL (or other appropriate volume) fractions of eluant.When using loose silica gel, filter the extract through a plug of precleaned silanized glass wool in a disposable glass pipette.5.6.5Fill a clean IR cell with the solution. Determine the fraction(s) in which thehydrocarbons will elute without corn oil being present using the absorbances of each fraction of the extract at 2800-3000 cm (hydrocarbon range) and 1600-1800 cm(ester range). If the -1 -1absorbance indicates that the absorptive capacity of the silica gel has been exceeded or that the silica gel is not absorbing the corn oil (corn oil is present in the extract), select new silica gel or solid phase cartridges.6.0SAMPLE COLLECTION, PRESERVATION, AND HANDLING6.1Solid samples should be collected and stored as any other solid sample containing semivolatile analytes. See the introductory material to this Chapter, Organic Analytes, Sec. 4.1.6.2Samples should be analyzed with minimum delay, upon receipt in the laboratory, and must be kept refrigerated prior to analysis.7.0PROCEDURE7.1Prepare samples according to Method 3560.7.2Add 0.3 g of loose silica gel to the extract and shake the mixture for 5 minutes, or pass the extract through a 0.5 g silica gel solid-phase extraction cartridge (conditioned with 5 mL of tetrachloroethylene). When using loose silica gel, filter the extract through a plug of precleaned silanized glass wool in a disposable glass pipette.7.3After the silica gel cleanup, fill a clean IR cell with the solution and determine the absorbance of the extract. If the absorbance exceeds the linear range of the IR spectrophotometer,prepare an appropriate dilution and reanalyze. The possibility that the absorptive capacity of thesilica gel has been exceeded can be tested at this point by repeating the cleanup and determinative steps.7.4Select appropriate working standard concentrations and cell pathlengths according to the following ranges:Concentration rangePathlength (mm)(µg/mL of extract)Volume (mL)10 5 to 500 350 1 to 100151000.5 to 5030Calibrate the instrument for the appropriate cells using a series of working standards. Determine absorbance directly for each solution at the absorbance maximum at about 2950 cm.-1 Prepare a calibration plot of absorbance versus concentration of petroleum hydrocarbons in the working standards.7.5Determine the concentration of TRPHs in the extract by comparing the response against the calibration plot.7.6Calculate the concentration of TRPHs in the sample using the formula:R x D x VConcentration (mg/Kg) =Wwhere:R=mg/mL of TRPHs as determined from the calibration plotV=volume of extract, in millilitersD=extract dilution factor, if usedW=weight of solid sample, in kilograms.7.7Recover the tetrachloroethylene used in this method by distillation or other appropriate technique.8.0QUALITY CONTROL8.1Reagent blanks or matrix-spiked samples must be subjected to the same analytical procedures as those used with actual samples.8.2Refer to Chapter One for specific Quality Control procedures and to Method 3500 for sample preparation procedures.8.3Based on manufacturer's recommendation, each laboratory should establish quality control practices necessary to evaluate the scanning or fixed wavelength infrared spectrophotometer.CD-ROM8440 - 4Revision 0December 19969.0METHOD PERFORMANCE9.1Table 1 presents a comparison of certified values and the values obtained using Methods 3560 and 8440. Data are presented for both Freon-113 and tetrachloroethylene, since both solvents were found to be an acceptable collection solvent. However, only tetrachloroethylene is recommended as a collection solvent for TRPHs in Method 3560.9.2Table 2 presents precision and accuracy data from the single-laboratory evaluation of Methods 3560 and 8440 for the determination of petroleum hydrocarbons from spiked soil samples. These data were obtained by extracting samples at 340 atm/80E C/60 minutes (dynamic).10.0REFERENCES1.Rohrbough, W. G.; et al. Reagent Chemicals, American Chemical Society Specifications, 7thed.; American Chemical Society, Washington, DC, 1986.2.Methods for Chemical Analysis of Water and Wastes; U.S. Environmental Protection Agency.Office of Research and Development, Environmental Monitoring and Support Laboratory. ORD Publication Offices of Center for Environmental Research Information, Cincinnati, OH, 1983;EPA-600/4-79-020.CD-ROM8440 - 5Revision 0December 1996CD-ROM 8440 - 6Revision 0December 1996CERTIFIED AND SPIKE VALUES COMPARED TO RESULTSOBTAINED BY METHODS 3560/8440Spike conc. or Methods certified conc.3560/8440Reference Material(mg/kg)(mg/kg)Environmental Resource Assoc.TPH-1 (lot 91012)1,8301,920±126a Environmental Resource Assoc.2,2302,150±380a TPH-2 (lot 91012)Clay spiked with kerosene 10086.0; 93.0bClay spiked with light gas oil10084.0; 98.0c Clay spiked with heavy gas oil 100103; 108dEnvironmental Resource Assoc.TPH-1 (lot 91017)614562; 447eEnvironmental Resource Assoc.TPH-2 (lot 91017)2,0501,780; 1,780e Three 60 minute extractions. The extracted material was collected in Freon-113; thea concentrations were determined against the reference oil standard.Duplicate 30 minute extractions. The extracted material was collected in tetrachloroethylene;b the concentrations were determined against standard made from the spiking material.Six 30 minute extractions. The extracted material was collected in tetrachloroethylene; the c concentrations were determined against a standard made from the spiking material.Four 30 minute extractions. The extracted material was collected in tetrachloroethylene; the d concentrations were determined against a standard made from the spiking material.Three 30 minute extractions. The extracted material was collected in tetrachloroethylene; the econcentrations were determined against the reference oil standard.CD-ROM 8440 - 7Revision 0December 1996SINGLE-LABORATORY METHOD ACCURACY AND PRECISION FORMETHODS 3560/8440 FOR SELECTED MATRICESSpike conc. or Method Method certified conc.Spike accuracy precision Matrix(mg/kg)Material (% recovery)(% RSD)Clay soil 2,500Motor oil 1048.5aERA TPH-12,350Vacuum oil 80.319.7a(lot 91016)ERA TPH-21,450Vacuum oil 88.619.6a(lot 91016)SRS103-10032,600c 94.2 4.0bEight determinations were made using two different supercritical fluid extraction systems.a The extracted material was collected in Freon-113.Ten determinations were made using three different supercritical fluid extraction systems.b The extracted material was collected in Freon-113.cThis is a standard reference soil certified for polynuclear aromatic hydrocarbons. No spike was added.CD-ROM 8440 - 8Revision 0December 1996METHOD 8440TOTAL RECOVERABLE PETROLEUM HYDROCARBONSBY INFRARED SPECTROPHOTOMETRY。
EPA方法索引

EPA方法索引和相关标准品EPA 是美国国家环境保护局(U.S Environmental Protection Agency) 的英文缩写。
它的主要任务是保护人类健康和自然环境。
EPA 制定了一系列标准分析方法用于环境监测领域。
主要包括:EPA T01~T14 系列标准分析方法——空气中有毒有机物分析方法EPA IP1~IP10 系列标准分析方法——室内空气污染物的分析测定方法EPA 200 系列标准分析方法———金属的分析方法EPA 500 系列标准分析方法——饮用水中有机物的分析方法EPA 600 系列标准分析方法——城市和工业废水中有机化合物的分析方法SW -846 系列标准分析方法——固体废弃物试验分析评价手册1300 系列是毒性试验方法3000 系列是金属元素的提取方法3500 系列是半(非) 挥发性有机物的提取方法3600 系列是净化、分离方法5000 系列是挥发性有机物的提取方法6000 系列是测定金属的新方法7000 系列是原子吸收法测定金属元素8000 系列是有机物分析方法9000 系列是常规项目分析方法其中,500系列,600系列和8000系列是环境种有机物分析最常用的方法。
EPA 600系列方法是美国为贯彻“净水法”(CW A) 、“全国水体污染物排放消除制度”(NPDES) 和“许可证制度”,严格控制点源排放,保护地表水,使其免受城市和工业废水中有机物的污染而制定的。
EPA 500 系列方法是为执行“安全饮用水法”(SDW A) 和“国家一级饮用水法案”(National Primary Drinking Water Regulations) ,确保饮用水及饮用水源的质量而制订的。
EPA 500 系列是针对比较洁净的水样(饮用水、地下水、地表水) 开发的,有些方法仅用试剂水和饮用水验证过SW-846 系列集中贯彻了“资源保护回收法”和“陆地处置限制法规”的精神,包含了固体废弃物采样和分析试验的全部方法, 是在EPA200 ~EPA 600 系列的基础上发展起来的。
美国EPA 关于空气自动监测系统性能指标的规定和测试方法
美国EPA关于大气自动监测系统性能指标的规定和测试方法引言环境空气污染的自动监测方法有多种,一般采用湿法和干法两种。
湿法是基于化学量理论的库仑法和电导法等测量原理,需使用大量试剂,存在试剂调整和废液处理等问题,操作比较繁琐,故障率较高,维护工作量较大;干法是基于物理光谱测量理论,使样品始终保持在气体状态,没有试剂的损耗,维护工作量较小。
比如SO2测量采用紫外荧光法,NOx测量采用化学发光法,O3测量采用紫外光度法,CO测量采用气体过滤相关分析法等,目前我国绝大部分空气自动监测采用的是该方法。
干法测量以欧美为主。
美国开展空气自动监测已有30年的历史,在空气自动监测方面积累了丰富的经验,并制定了详细的规范。
其中物理光谱法作为美国EPA的推荐方法,得到了广泛的应用。
湿法测量以日本为主,但自1996年起日本在法定的测量方法中增加了干式测量法。
利用物质的光谱特性进行污染物的分析已成为自动监测仪器发展的必然趋势。
我国在环境空气质量监测和质量保证方面的规定都参考了美国国家环保署(EPA)的规定。
目前,大气自动监测和空气质量日报工作在我国大部分省市已广泛开展,自动监测仪器监测数据的准确可靠是日报工作中的基础。
为使监测人员了解美国EPA关于空气自动监测的相关规定,特将其有关SO2、NO2、O3、CO自动监测仪器的性能指标规定和测试方法作简要说明,以供参考。
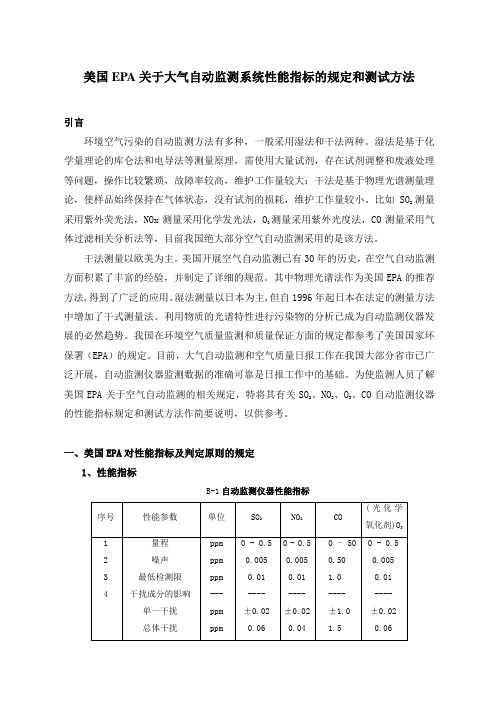
一、美国EPA对性能指标及判定原则的规定1、性能指标B-1自动监测仪器性能指标M/0.02447,M是该气体的摩尔质量。
2、判定原则对于每个性能指标(量程除外),测试程序从开始起要重复7次,得到7组测试结果。
每组结果要和表B-1中的规定指标相比较,高于或超出规定指标的值是一个超标值。
每个参数的7个结果说明如下:(1)0次超标:被测的参数合格;(2)3次或更多次超标:该参数不合格;(3)1次或2次超标:再重复测试该参数 8次,得到共15个测试结果。
将此15个测试结果说明如下:a:1次或2次超标:通过测试;b:3次以上:该参数不合格。
EPA测试方法-目录

目录---------------------------第一卷A 部分---------------------------免责声明摘要目录方法索引和换算表前言鸣谢第一部分分析测定方法第一章—质量控制1.0 引言2.0 质量保证计划3.0 室外操作4.0 实验室操作5.0 定义6.0 参考文献第二章—选择正确的步骤2.1 目的2.2 需要的信息2.3 使用指南2.4 特性2.5 地表水2.6 参考文献第三章—无机物分析3.1 采样注意事项3.2 样品准备方法方法3005A 用于火焰原子吸收光谱(FLAA)或电感偶合等离子发射光谱(ICP)进行总可回收或可溶性金属离子检测水样的酸性消化方法。
方法3010A 用于火焰原子吸收光谱(FLAA)或电感偶合等离子体原子发射光谱(ICP)进行全金属分析的水溶液或萃取物的酸性消化方法。
方法3015 水溶液或萃取物的微波辅助酸性消化方法。
方法3020A 用于无火焰原子吸收光谱法(GFAA)进行全金属分析的水溶液或萃取物的酸性消化方法。
方法3031 用于原子吸收光谱(AAS)或电感偶合等离子体原子发射光谱(ICP进行油脂中金属分析的油样的酸性消化方法。
方法3040A 油、脂和石蜡的溶解方法。
方法3050B 沉积物、污泥和土壤的酸性消化方法。
方法3051 沉积物、污泥和土壤的微波辅助酸性消化方法。
方法3052 硅酸盐和有机物质的微波酸性消化方法。
方法3060A 六价铬的碱性消化。
3.3 无机物的测定方法6010B 电感耦合等离子发射光谱(ICP-AES)方法6020 电感耦合等离子体质谱(ICP-MASS)方法7000A 原子吸收法方法7020 铝(原子吸收,直接进样)方法7040 锑(原子吸收,直接进样)方法7041 锑(原子吸收,石墨炉法)方法7060A 砷(原子吸收,石墨炉法)方法7061A 砷(原子吸收,氢火焰法)方法7062 锑和砷(原子吸收,氢硼化钠还原法)方法7063 砷水溶液和萃取物的阳极溶出伏安法(ASV)方法7080A 钡(原子吸收,直接进样)方法7081 钡(原子吸收,石墨炉法)方法7090 铍(原子吸收,直接进样)方法7091 铍(原子吸收,石墨炉法)方法7130 镉(原子吸收,直接进样)方法7131A 镉(原子吸收,石墨炉法)方法7140 钙(原子吸收,直接进样)方法7190 铬(原子吸收,直接进样)方法7191 铬(原子吸收,石墨炉法)方法7195 铬,六价(共沉淀法)方法7196A 铬,六价(比色法)方法7197 铬,六价(螯合/萃取)方法7198 铬,六价(微分脉冲极谱法DPP)方法7199 饮用水、地表水和工业排放废水中六价铬的测定-离子色谱法方法7200 钴(原子吸收,直接进样)方法7201 钴(原子吸收,石墨炉法)方法7210 铜(原子吸收,直接进样)方法7211 铜(原子吸收,石墨炉法)方法7380 铁(原子吸收,直接进样)方法7381 铁(原子吸收,石墨炉法)方法7420 铅(原子吸收,直接进样)方法7421 铅(原子吸收,石墨炉法)方法7430 锂(原子吸收,直接进样)方法7450 镁(原子吸收,直接进样)方法7460 锰(原子吸收,直接进样)方法7461 锰(原子吸收,石墨炉法)方法7470A 废液中的汞(冷蒸汽原子吸收光谱法)方法7471A 固体或半固体废弃物中的汞(冷蒸汽原子吸收光谱法)方法7472 阳极溶出伏安法测定水溶液和萃取物中的汞方法7480 钼(原子吸收,直接进样)方法7481 钼(原子吸收,石墨炉法)方法7520 镍(原子吸收,直接进样)方法7521 镍(原子吸收,石墨炉法)方法7550 锇(原子吸收,直接进样)方法7580 白磷(P4)的溶剂萃取-气相色谱法(GC)方法7610 钾(原子吸收,直接进样)方法7740 硒(原子吸收,石墨炉法)方法7741A 硒(原子吸收,氢火焰法)方法7742 硒(原子吸收,氢硼化钠还原法)方法7760A 硅(原子吸收,直接进样)方法7761 硅(原子吸收,石墨炉法)方法7770 钠(原子吸收,直接进样)方法7780 锶(原子吸收,直接进样)方法7840 铊(原子吸收,直接进样)方法7841 铊(原子吸收,石墨炉法)方法7870 锡(原子吸收,直接进样)方法7910 钒(原子吸收,直接进样)方法7911 钒(原子吸收,石墨炉法)方法7950 锌(原子吸收,直接进样)方法7951 锌(原子吸收,石墨炉法)附件—公司推荐---------------------------第一卷B 部分---------------------------免责声明摘要目录方法索引和换算表前言鸣谢第一章—质量控制1.0 引言2.0 质量保证计划3.0 室外操作4.0 实验室操作5.0 定义6.0 参考文献第四章—有机物分析4.1 采样注意事项4.2 样品准备方法4.2.1 萃取和准备方法3500B 有机物的萃取和样品准备方法3510C 分液漏斗液--液萃取方法3520C 连续液--液萃取方法3535 固相萃取(SPE)方法3540C 索氏萃取方法3541 自动索氏萃取方法3542 使用方法0010(改进后的采样教程方法5)萃取半挥发性有机物方法3545 加压液体萃取(PFE)方法3550B 超声波萃取方法3560 超临界流体萃取总可回收石油烃方法3561 超临界流体萃取多环芳烃方法3580A 固废分解方法3585 挥发性有机物固废样品的分解方法5000 挥发性有机物样品准备方法5021 用顶空法准备土壤中或其他固体物质中的挥发性有机物方法5030B 水溶液样品的吹扫捕集方法5031 共沸蒸馏法提取挥发性、不可吹出、水溶性有机化合物方法5032 挥发性有机物的真空蒸馏方法5035 封闭体系的吹扫捕集和萃取提取土壤和固废样品中的挥发性有机物方法5041A 挥发性有机物采样系统(VOST)吸附柱解吸物的分析4.2.2 净化方法3600C 净化方法3610B 铝氧土净化方法3611B 铝氧土柱的净化以及石油类废物的分离方法3620B Florisil柱的净化方法3630C 硅胶净化方法3640A 凝胶色谱柱净化方法3650B 酸性层析柱的净化方法3660B 硫的净化方法3665A 硫酸/高锰酸钾净化4.3 有机物的测定4.3.1 气相色谱法方法8000B 分析性的色谱分离方法8011 微萃取-气相色谱分析1,2-二溴乙烷和1,2-二溴-3-氯丙烷方法8015B 气相色谱-氢火焰离子检测器(GC-FID)测定非卤代有机物方法8021B 气相色谱法分析芳香族化合物和卤代挥发性有机物—使用光电离检测器(PID)和/或电导检测器(ELCD)方法8031 气相色谱分析丙烯腈方法8032A 气相色谱分析丙烯酰胺方法8033 气相色-氮磷检测器(GC-NPD)分析乙腈方法8041 气相色谱分析酚类方法8061A 气相色谱-电子捕获检测器(GC-ECD)分析邻苯二甲酸酯类方法8070A 气相色谱分析亚硝胺方法8081A 气相色谱分析有机氯杀虫剂方法8082 气相色谱分析多氯联苯方法8091 气相色谱分析硝基芳香烃和环酮方法8100 多核(多环)芳烃方法8111 气相色谱分析卤代醚类方法8121 气相色谱检测氯代烃:毛细管法方法8131 气相色谱法检测苯胺及其衍生物方法8141A 气相色谱分析有机磷化合物:毛细管法方法8151A 甲基化和氟代醇苯甲基化气相色谱法检测氯代除草剂4.3.2 气质联用法(GC-MASS)方法8260B 气质联用法测定挥发性有机物(VOCs)方法8270C 气质联用法测定半挥发性有机物方法8275A 热提取-气质联用法(TE/GC/MS)测定土壤、污泥和固废中的半挥发性有机物(多环芳烃PAHs和多氯联苯PCBs)方法8280A 高分辨率色谱/低分辨率质谱(HRGC/LRMS)法测定多氯二苯并对二英和多氯二苯并呋喃方法8290 高分辨率色谱/高分辨率质谱(HRGC/HRMS)法测定多氯二苯并二恶英(PCDDs)和多氯二苯并呋喃(PCDFs)附件A:实验室进行的擦拭实验的采集、处理、分析和报告步骤4.3.3 高效液相色谱法(HPLC)方法8310 多环芳烃方法8315A 高效液相色谱法测定碳酰(羰基)化合物附录A:2,4-二硝基苯肼的重结晶方法8316 丙烯酰胺、丙烯腈和丙烯醛的测定—高效液相色谱法方法8318 高效液相色谱分析N-氨基甲酸甲酯方法8321A 高效液相色谱/热喷雾/质谱法(HPLC/TS/MS)测定非挥发性有机物的可溶性提取物方法8325 高效液相色谱/粒子束/质谱法(HPLC/PB/MS)测定非挥发性有机物的可溶性提取物方法8330 硝基芳烃和硝胺的测定—高效液相色谱法方法8331 反相高效液相色谱法测定四氮烯方法8332 硝化甘油的测定—高效液相色谱法4.3.4 红外法方法8410 半挥发性有机物的测定—气相色谱/傅立叶变换红外光谱法(GC/FT-IR):毛细管柱方法8430 水溶液直接进样气相色谱/傅立叶变换红外光谱法(GC/FT-IR)分析双氯甲醚及其水解产物方法8440 红外光谱法分析总可回收石油烃4.3.5 多光谱分析法方法8520 环境空气中甲醛的连续免疫测定4.4 免疫测定法方法4000 免疫测定方法4010A 用免疫测定法进行五氯苯酚的筛检方法4015 用免疫测定法进行2,4-二氯苯氧基乙酸的筛检方法4020 用免疫测定法进行多氯联苯的筛检方法4030 用免疫测定法对土壤中石油烃进行筛检方法4035 用免疫测定法对土壤中多环芳烃进行筛检方法4040 用免疫测定法对土壤中毒杀芬进行筛检方法4041 用免疫测定法对土壤中氯丹进行筛检方法4042 用免疫测定法对土壤中DDT进行筛检方法4050 用免疫测定法对土壤中TNT爆炸性物质进行筛检方法4051 用免疫测定法对土壤中黑索今(环三亚甲基三硝胺RDX)进行筛检4.5 多种筛选法方法3810 顶空法方法3820 可清除有机物的十六烷提取和筛检法方法8515 土壤中TNT物质的比色筛检法方法9078 土壤中多氯联苯的筛选法方法9079 变压器油中多氯联苯的筛选法附件—公司推荐---------------------------第一卷C 部分---------------------------免责声明摘要目录方法索引和换算表前言鸣谢第一章—质量控制1.0 引言2.0 质量保证计划3.0 室外操作4.0 实验室操作5.0 定义6.0 参考文献第五章—其它各种测试方法方法5050 固废爆炸的预防方法方法9010C 总可测定氰化物:蒸馏法方法9012B 总可测定氰化物(手动蒸馏,自动比色)方法9013 固废和土壤中氰化物的萃取方法方法9014 滴定和手动分光光度法测定氰化物方法9020B 总有机卤素(TOX)方法9021 挥发性有机卤素(POX)方法9022 中子活法分析法测定总有机卤素(TOX)方法9023 土壤中的可萃取有机卤素(EOX)方法9030B 酸溶性和非酸溶性硫化物:蒸馏法方法9031 可萃取硫化物方法9034 滴定法测定酸溶性和非酸溶性硫化物方法9035 硫酸盐(比色法,自动,氯冉酸盐)方法9036 硫酸盐(比色法,自动,甲基百里酚兰,)方法9038 硫酸盐(浊度计)方法9056 离子色谱法测定无机阴离子方法9057 阴离子色谱法测定HCl/Cl2排放源采集样品中的氯化物方法9060A 总有机碳(TOC)方法9065 酚醛塑料(分光光度法,手动4-氨基安替比林蒸馏)方法9066 酚醛塑料(比色法,自动4-氨基安替比林蒸馏)方法9067 酚醛塑料(分光光度法,酚试剂蒸馏)方法9070A 用于水溶液样品的正己烷可提取物方法9071B 用于污泥、沉积物、土壤样品的正己烷可提取物方法9075 X射线荧光光谱法分析新的或使用过的石油产品中的总氯方法9076 燃烧氧化和微库仑法分析新的或使用过的石油产品中的总氯方法9077 新的或使用过的石油产品中的总氯(现场测试套件)方法A:固定终点套件法方法B:反向滴定终点定量套件法方法C:直接滴定终点定量套件法方法9131 大肠杆菌:多管发酵法方法9132 大肠杆菌:膜过滤法方法9210 离子选择电极点位滴定法测定水溶液样品中的硝酸盐方法9211 离子选择电极点位滴定法测定水溶液样品中的溴化物方法9212 离子选择电极点位滴定法测定水溶液样品中的氯化物方法9213 蒸馏后离子选择电极点位滴定法测定水溶液样品中的氰化物方法9214 离子选择电极点位滴定法测定水溶液样品中的氟化物方法9215 蒸馏后离子选择电极点位滴定法测定水溶液样品中的硫化物方法9250 氯化物(比色法,自动铁氰化物,AAI)方法9251 氯化物(比色法,自动铁氰化物,AAII)方法9253 氯化物(滴定法,硝酸银)方法9320 镭-288第六章—性质分析方法1030 固体的可燃性方法1120 皮肤腐蚀性方法1312 人工凝结浸出方法方法1320 多次萃取法方法1330A 含油废弃物的提取方法9041A pH试纸制作方法方法9045D 土壤和废弃物pH方法9050A 电导率方法9080 土壤的阳离子交换性能(醋酸铵)方法9081 土壤的阳离子交换性能(醋酸钠)方法9090A 废弃物和膜沉淀的兼容性试验方法9095B 滤芯液体试验方法9096 液体泄露试验方法附件A:液体泄露试验预试验方法9100 饱和水溶液电导,饱和滤出液电导以及固有磁导率方法9310 总α、总β放射性水平方法9315 α放射性同位素第二部分性状第七章—性状说明和定义7.1 可燃性7.2 腐蚀性7.3 反应性7.4 毒性物质提取步骤第八章—性状测定方法8.1 可燃性方法1010A 潘-马氏闭杯分析仪测定闪点方法1020B 闪点的标准测定方法Setaflash闭杯分析仪法8.2 腐蚀性方法9040C pH电极测量方法1110A 钢铁腐蚀性测验8.3 毒性方法1310B 提取过程毒性测试方法和结构完整性试验方法1311 毒性物质提取步骤附件—公司推荐---------------------------第二卷---------------------------免责声明摘要目录方法索引和换算表前言鸣谢第一章—质量控制1.0 引言2.0 质量保证计划3.0 室外操作4.0 实验室操作5.0 定义6.0 参考文献第三部分采样第九章—采样计划9.1 规划和设计9.2 执行第十章—采样方法方法0010 改进后的Method 5 Sampling Train附件A:XAD-2吸附树脂的准备附件B:所有可用色谱分析物质的分析方法0011 固定醛酮类排放源的采样方法方法0020 源头评价采样方法方法0023A 固定多氯联苯并二恶英和多氯联苯并呋喃源的采样方法方法0030 挥发性有机物采样教程方法0031 挥发性有机化合物采样方法方法0040 塑料袋燃烧源头主要持久性危险有机物的采样方法方法0050 HCl/Cl2排放源的等动力采样教程方法0051 HCl/Cl2排放源的小型冲击吸收瓶采样教程方法0060 烟囱排放源的金属测定方法0061 固定排放源六价铬的测定方法0100 室内甲醛和其它羰基化合物的采样第四部分监测第十一章—地表水监测11.1 背景和目标11.2 与标准的关系和与其他文件的关系11.3 修订本和附录11.4 可以接受的设计和措施11.5 不可接受的设计和措施第十二章—土地处理(有害废物的)监测12.1 背景12.2 处理区域12.3 定义12.4 监测和采样策略12.5 分析12.6 参考文献和内容提要第十三章—灰化13.1 说明13.2 定义13.3 废物定性策略13.4 烟囱气体排放物定性策略13.5 附加排放物定性策略13.6 采样和分析方法的选择13.7 参考文献附件—公司推荐。
美国EPA农药登记残留试验样品储藏稳定性资料要求概述

美国EPA农药登记残留试验样品储藏稳定性资料要求概述
美国EPA农药登记残留试验样品储藏稳定性资料要求概述张宏军;李富根;姜文议
【期刊名称】《农药科学与管理》
【年(卷),期】2016(037)003
【摘要】本文概述了美国环保局关于农药登记残留储藏稳定性试验的有关要求,包括基本要求、残留储藏稳定性试验要求、试验结果的使用、试验报告的编写等.希望对我国制定相关技术规范有所借鉴.
【总页数】8页(16-23)
【关键词】农药;登记;残留;储藏稳定性;IR-4
【作者】张宏军;李富根;姜文议
【作者单位】农业部农药检定所,北京100125;农业部农药检定所,北京100125;美国密歇根州立大学昆虫学系,美国密歇根48824 【正文语种】中文
【中图分类】S482
【相关文献】
1.美国EPA农药登记残留试验样品储藏稳定性资料要求概述 [J],
2.美国EPA农药登记残留试验中样品储藏稳定性试验[J], 朱光艳; 张志勇
3.农药登记资料的审批程序、资料要求及注意事项 [C], 吴志凤
4.从农药登记资料新要求看农药发展趋势 [J], 沈迎春
5.美国修订对四种农药的残留限量要求 [J],
以上内容为文献基本信息,获取文献全文请下载。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
METHOD 8440TOTAL RECOVERABLE PETROLEUM HYDROCARBONS BY INFRAREDSPECTROPHOTOMETRY1.0SCOPE AND APPLICATION1.1Method 8440 (formerly Draft Method 9073) is used for the measurement of total recoverable petroleum hydrocarbons (TRPHs) extracted with supercritical carbon dioxide from sediment, soil and sludge samples using Method 3560.1.2Method 8440 is not applicable to the measurement of gasoline and other volatile petroleum fractions, because of evaporative losses.1.3Method 8440 can detect TRPHs at concentrations of 10 mg/L in extracts. This translates to 10 mg/Kg in soils when a 3 g sample is extracted by SFE (assuming 100 percent extraction efficiency), and the final extract volume is 3 mL.1.4This method is restricted to use by or under the supervision of trained analysts. Each analyst must demonstrate the ability to generate acceptable results with this method.2.0SUMMARY OF METHOD2.1Soil samples are extracted with supercritical carbon dioxide using Method 3560. Interferences are removed with silica gel, either by shaking the extract with loose silica gel, or by passing it through a silica gel solid-phase extraction cartridge. After infrared (IR) analysis of the extract, TRPHs are quantitated by direct comparison with standards.3.0INTERFERENCES3.1The analyte class being measured (TRPHs) is defined within the context of this method. The measurement may be subject to interferences, and the results should be interpreted accordingly.3.2Determination of TRPHs is a measure of mineral oils only, and does not include the biodegradable animal greases and vegetable oils captured in oil and grease measurements. These non-mineral-oil contaminants may cause positive interferences with IR analysis, if they are not completely removed by the silica gel cleanup.3.3Method 8440 is not appropriate for use in the analysis of gasoline and other volatile petroleum fractions because these fractions evaporate during sample preparation.4.0APPARATUS AND MATERIALS4.1Infrared spectrophotometer - Scanning or fixed wavelength, for measurement around 2950 cm.-14.2IR cells - 10 mm, 50 mm, and 100 mm pathlength, sodium chloride or IR-grade glass. CD-ROM8440 - 1Revision 0December 1996CD-ROM 8440 - 2Revision 0December 19964.3Magnetic stirrer with polytetrafluoroethylene (PTFE)-coated stirring bars.4.4Optional - A vacuum manifold consisting of glass vacuum basin, collection rack and funnel, collection vials, replaceable stainless steel delivery tips, built-in vacuum bleed valve and gauge is recommended for use when silica gel cartridges are used. The system is connected to a vacuum pump or water aspirator through a vacuum trap made from a 500 mL sidearm flask fitted with a one-hole stopper and glass tubing.5.0REAGENTS5.1Reagent-grade chemicals shall be used in all tests. Unless otherwise indicated, it is intended that all reagents shall conform to the specifications of the Committee on Analytical Reagents of the American Chemical Society, where such specifications are available. Other grades may be used, provided it is first ascertained that the reagent is of sufficiently high purity to permit its use without lessening the accuracy of the determination.5.2Tetrachloroethylene, C Cl - spectrophotometric grade, or equivalent.245.3Raw materials for reference oil mixture - spectrophotometric grade, or equivalent.5.3.1n-Hexadecane, CH (CH )CH 321435.3.2Isooctane, (CH )CCH CH(CH )332325.3.3Chlorobenzene, C H Cl 655.4Silica gel.5.4.1Silica gel solid-phase extraction cartridges (40 µm particles, 60 A pores), 0.5 g,Supelco, J.T. Baker, or equivalent.5.4.2Silica gel, 60 to 200 mesh, Davidson Grade 950 or equivalent (deactivated with1 to2 percent water).5.5Calibration mixtures:5.5.1The material of interest, if available, or the same type of petroleum fraction, if itis known and original sample is unavailable, shall be used for preparation of calibration standards. Reference oil is to be used only for unknowns. Whenever possible, a GC fingerprint should be run on unknowns to determine the petroleum fraction type.5.5.2Reference oil - Pipet 15.0 mL n-hexadecane, 15.0 mL isooctane, and 10.0 mLchlorobenzene into a 50 mL glass-stoppered bottle. Maintain the integrity of the mixture by keeping stoppered except when withdrawing aliquots. Refrigerate at 4E C when not in use.5.5.3Stock standard - Pipet 0.5 mL calibration standard (Section 5.5.1 or 5.5.2) intoa tared 100 mL volumetric flask and stopper immediately. Weigh and dilute to volume with tetrachloroethylene.5.5.4Working standards - Pipet appropriate volumes of stock standard (Sec. 5.5.3) into100 mL volumetric flasks according to the cell size to be used. Dilute to volume withCD-ROM 8440 - 3Revision 0December 1996tetrachloroethylene. Calculate the concentrations of the standards from the stock standard concentrations.5.6Calibration of silica gel cleanup5.6.1Prepare a stock solution of corn oil and mineral oil by placing about 1 mL each(0.5 to 1 g) of corn oil and mineral oil into a tared 100 mL volumetric flask. Stopper the flask and weigh to the nearest milligram. Dilute to the mark with tetrachloroethylene, and shake the contents to effect dissolution.5.6.2Prepare additional dilutions to cover the range of interest.5.6.3Transfer 2 mL (or other appropriate volume) of the diluted corn oil/mineral oilsamples to vials.5.6.4Add 0.3 g of loose silica gel to the vials of diluted corn oil/mineral oil samples andshake the mixture for 5 minutes, or pass the extract through a 0.5 g silica gel solid-phase extraction cartridge (conditioned with 5 mL of tetrachloroethylene). Elute with tetrachloroethylene. Collect three 3 mL (or other appropriate volume) fractions of eluant.When using loose silica gel, filter the extract through a plug of precleaned silanized glass wool in a disposable glass pipette.5.6.5Fill a clean IR cell with the solution. Determine the fraction(s) in which thehydrocarbons will elute without corn oil being present using the absorbances of each fraction of the extract at 2800-3000 cm (hydrocarbon range) and 1600-1800 cm(ester range). If the -1 -1absorbance indicates that the absorptive capacity of the silica gel has been exceeded or that the silica gel is not absorbing the corn oil (corn oil is present in the extract), select new silica gel or solid phase cartridges.6.0SAMPLE COLLECTION, PRESERVATION, AND HANDLING6.1Solid samples should be collected and stored as any other solid sample containing semivolatile analytes. See the introductory material to this Chapter, Organic Analytes, Sec. 4.1.6.2Samples should be analyzed with minimum delay, upon receipt in the laboratory, and must be kept refrigerated prior to analysis.7.0PROCEDURE7.1Prepare samples according to Method 3560.7.2Add 0.3 g of loose silica gel to the extract and shake the mixture for 5 minutes, or pass the extract through a 0.5 g silica gel solid-phase extraction cartridge (conditioned with 5 mL of tetrachloroethylene). When using loose silica gel, filter the extract through a plug of precleaned silanized glass wool in a disposable glass pipette.7.3After the silica gel cleanup, fill a clean IR cell with the solution and determine the absorbance of the extract. If the absorbance exceeds the linear range of the IR spectrophotometer,prepare an appropriate dilution and reanalyze. The possibility that the absorptive capacity of thesilica gel has been exceeded can be tested at this point by repeating the cleanup and determinative steps.7.4Select appropriate working standard concentrations and cell pathlengths according to the following ranges:Concentration rangePathlength (mm)(µg/mL of extract)Volume (mL)10 5 to 500 350 1 to 100151000.5 to 5030Calibrate the instrument for the appropriate cells using a series of working standards. Determine absorbance directly for each solution at the absorbance maximum at about 2950 cm.-1 Prepare a calibration plot of absorbance versus concentration of petroleum hydrocarbons in the working standards.7.5Determine the concentration of TRPHs in the extract by comparing the response against the calibration plot.7.6Calculate the concentration of TRPHs in the sample using the formula:R x D x VConcentration (mg/Kg) =Wwhere:R=mg/mL of TRPHs as determined from the calibration plotV=volume of extract, in millilitersD=extract dilution factor, if usedW=weight of solid sample, in kilograms.7.7Recover the tetrachloroethylene used in this method by distillation or other appropriate technique.8.0QUALITY CONTROL8.1Reagent blanks or matrix-spiked samples must be subjected to the same analytical procedures as those used with actual samples.8.2Refer to Chapter One for specific Quality Control procedures and to Method 3500 for sample preparation procedures.8.3Based on manufacturer's recommendation, each laboratory should establish quality control practices necessary to evaluate the scanning or fixed wavelength infrared spectrophotometer.CD-ROM8440 - 4Revision 0December 19969.0METHOD PERFORMANCE9.1Table 1 presents a comparison of certified values and the values obtained using Methods 3560 and 8440. Data are presented for both Freon-113 and tetrachloroethylene, since both solvents were found to be an acceptable collection solvent. However, only tetrachloroethylene is recommended as a collection solvent for TRPHs in Method 3560.9.2Table 2 presents precision and accuracy data from the single-laboratory evaluation of Methods 3560 and 8440 for the determination of petroleum hydrocarbons from spiked soil samples. These data were obtained by extracting samples at 340 atm/80E C/60 minutes (dynamic).10.0REFERENCES1.Rohrbough, W. G.; et al. Reagent Chemicals, American Chemical Society Specifications, 7thed.; American Chemical Society, Washington, DC, 1986.2.Methods for Chemical Analysis of Water and Wastes; U.S. Environmental Protection Agency.Office of Research and Development, Environmental Monitoring and Support Laboratory. ORD Publication Offices of Center for Environmental Research Information, Cincinnati, OH, 1983;EPA-600/4-79-020.CD-ROM8440 - 5Revision 0December 1996CD-ROM 8440 - 6Revision 0December 1996CERTIFIED AND SPIKE VALUES COMPARED TO RESULTSOBTAINED BY METHODS 3560/8440Spike conc. or Methods certified conc.3560/8440Reference Material(mg/kg)(mg/kg)Environmental Resource Assoc.TPH-1 (lot 91012)1,8301,920±126a Environmental Resource Assoc.2,2302,150±380a TPH-2 (lot 91012)Clay spiked with kerosene 10086.0; 93.0bClay spiked with light gas oil10084.0; 98.0c Clay spiked with heavy gas oil 100103; 108dEnvironmental Resource Assoc.TPH-1 (lot 91017)614562; 447eEnvironmental Resource Assoc.TPH-2 (lot 91017)2,0501,780; 1,780e Three 60 minute extractions. The extracted material was collected in Freon-113; thea concentrations were determined against the reference oil standard.Duplicate 30 minute extractions. The extracted material was collected in tetrachloroethylene;b the concentrations were determined against standard made from the spiking material.Six 30 minute extractions. The extracted material was collected in tetrachloroethylene; the c concentrations were determined against a standard made from the spiking material.Four 30 minute extractions. The extracted material was collected in tetrachloroethylene; the d concentrations were determined against a standard made from the spiking material.Three 30 minute extractions. The extracted material was collected in tetrachloroethylene; the econcentrations were determined against the reference oil standard.CD-ROM 8440 - 7Revision 0December 1996SINGLE-LABORATORY METHOD ACCURACY AND PRECISION FORMETHODS 3560/8440 FOR SELECTED MATRICESSpike conc. or Method Method certified conc.Spike accuracy precision Matrix(mg/kg)Material (% recovery)(% RSD)Clay soil 2,500Motor oil 1048.5aERA TPH-12,350Vacuum oil 80.319.7a(lot 91016)ERA TPH-21,450Vacuum oil 88.619.6a(lot 91016)SRS103-10032,600c 94.2 4.0bEight determinations were made using two different supercritical fluid extraction systems.a The extracted material was collected in Freon-113.Ten determinations were made using three different supercritical fluid extraction systems.b The extracted material was collected in Freon-113.cThis is a standard reference soil certified for polynuclear aromatic hydrocarbons. No spike was added.CD-ROM 8440 - 8Revision 0December 1996METHOD 8440TOTAL RECOVERABLE PETROLEUM HYDROCARBONSBY INFRARED SPECTROPHOTOMETRY。
