AOAC Starch(total)in Cereal Products amyloglucosidase-a-amylase method
AOAC脂肪提取标准

39.1.08AOAC Official Method 991.36 Fat (Crude) in Meat and Meat ProductsSolvent Extraction (Submersion) MethodFirst Action 1991Final Action 1996[Applicable to meat and meat food products that can be analyzed using 960.39 (see 39.1.05), 976.21 (see 39.1.06), and 985.15 (see39.1.07).]Results of the interlaboratory study supporting acceptance of the method:x—, 4.34% fat: s r= 0.106; s R = 0.112; RSD r= 2.44%; RSD R = 2.59%x—, 27.29% fat: s r= 0.534; s R = 0.637; RSD r= 1.95%; RSD R = 2.33%x—, 27.95% fat: s r= 0.648; s R = 0.739; RSD r= 2.32%; RSD R = 2.84%x—, 34.51% fat: s r= 0.764; s R = 0.799; RSD r= 2.21%; RSD R = 2.31%x—, 33.57% fat: s r= 0.340; s R = 0.516; RSD r= 1.01%; RSD R = 1.53%x—, 26.20% fat: s r= 0.406; s R = 0.631; RSD r= 1.55%; RSD R = 2.34%A. Apparatus装置(a) Extraction system.—Capable of simultaneous extraction of 6 test portions. Extraction unit for solvent addition to cups, 2-stage extraction process, and solvent recovery cycle. Service unit to supply hot oil through insulated tubing to extraction unit and to pump air for evaporation of last traces of solvent from cups (Soxtec System meets these specifications).(b) Thimbles and stand.—26 *60 mm, cellulose thimbles, and stand to hold 6 thimbles.(c) Extraction cups.—Al, 44 id, 60 mm height.(d) Glass beads.—3–4 mm diameter.(e) Mechanical convection oven. — Maintaining 125° ± 1°C.Items (a)–(c) are available as Soxtec sys tem from Perstorp Analytical/Tecator, Inc. (2875 C Towerview Rd, Herndon, V A 22071, USA).B. Reagents(a) Petroleum ether. — To meet specifications in 945.16A (see 27.4.04).(b) Sand.—<0.004 g extractables/5 g.(c) Cotton.—Defatted.C. DeterminationAccurately weigh ca 3 g test portion into thimble. Add sand to test portion and mix with glass rod. Place thimble in thimble stand and dry 1 h in 125°C oven. Remove from oven and let cool. Loosen test portion/sand mixture using glass rod. Wipe glass rod with small amount of cotton and place cotton in top of thimble. Transfer thimble to extraction unit. Accurately weigh extraction cup containing a few glass beads.Extract thimble with dried mixture with 40 mL petroleum ether in boiling position for 25 min and in rinsing position for 30 min. Adjust temperature of extraction unit to ensure condensation rate≥5 drops/s. At completion of extraction, close condenser valves and recover ether.Dry cup and contents 30 min in 125°C oven. Cool and weigh.D. CalculationsCalculate percent fat in test sample as follows:Fat content, % = (B -C) *100Awhere A = g test portion weight, B = g weight of extraction cup afterdrying, and C = g weight of extraction cup prior to extraction.Reference: J. AOAC Int. 75, 289(1992).39.1.07AOAC Official Method 985.15Fat (Crude) in Meat and Poultry 家禽Products Rapid Microwave-Solvent Extraction Method First Action 1985Final Action 1991A. Reagents and Apparatus(a) Automated solvent extractor.—Enclosed, self-contained, thermostatically controlled 恒温控制fat extraction and solvent recovery system with 0.5 mg fat sensitivity and 0–100% fat measurement range (CEM Corp., PO Box 200, Matthews, NC 28106, USA), or equivalent.(b) Methylene chloride.二氯甲烷—Reagent grade (Fisher Scientific Co., No. D-37) , or equivalent.(c) Glass fiber pads.玻璃纤维垫子—9.8 *10.2 cm rectangular 矩形and 11 cm round (CEM Corp.), or equivalent.(d) Microwave moisture analyzer.—0.2 mg H2O sensitivity, moisture/solids range of 0.1–99.9%,0.01% resolution分辨率. Includes automatic tare electronic balance, microwave drying system,and microprocessor digital computer control. Electronic balance pan is located inside drying chamber. (Balance sensitivity: 0.2 mg at 15 g capacity or 1.0 mg at 40 g capacity [CEM Corp., or equivalent].)B. DeterminationPrepare test samples as in 983.18 (see 39.1.01). Place 2 rectangular and one round glass fiber pad on balance pan inside microwave moisture analyzer, and tare. Remove rectangular pads and evenly spread ca 4 g well-mixed test portion onto rough side of one pad, cover with second pad, and place together with round pad on balance pan. Dry 3–5 min at 80–100% power, depending on product type. At end of drying cycle, remove from balance pan. Fold rectangular pads, with dried test portion, in half and place in automated solvent extractor chamber. Place round pad in recessed area at top of extractor chamber, close and latch lid. Start extraction cycle (test portion and rectangular pads are blended at this time with sufficient CH2Cl2to extract fat). After completion of extraction cycle, remove round pad with residue, and place on balance pan in microwave moisture analyzer. Redry pad and residue to constant weight (ca 30 s at 80–100% power) to re move residual solvent or moisture. Weight loss due to solvent extraction is converted to % fat by microprocessor and displayed on digital read out panel.Certain product classes require addition of adjustment factors to read out for accurate results, as follows: fresh meats, pre-blends, emulsions, cured/cooked meats, factor = 0.40; cooked sausages, factor = 0.80.Reference: JAOAC 68, 876(1985).39.1.06AOAC Official Method 976.21Fat (Crude) in Meat Rapid Specific Gravity MethodFirst Action 1976Final Action 1979A. Apparatus and Reagents(a) Foss-let fat analyzer.—Includes orbital shaker, specific gravity readout unit, solvent dispenser, reference standard oil (specific gravity at 23°C = 0.915; for periodic check of potentiometer calibration), stainless steel cup with cover and 8 mm bore brass hammer, pressure filtration device, and conversion chart (Foss Food Technology Corp.).(b) Drying agent.—Plaster of Paris (available locally through paint, hardware, or building supply dealers), 8 mesh Drierite, or an hydrous CaSO4.(c) Tetrachloroethylene.—Technical grade C2Cl4 (distributed locally through dry cleaning sup pliers or Fisher Scientific Co.,No. C-182).B. Deter mi na tionPrepare test sam ples as in 983.18 (see 39.1.01). Check calibration of Foss-let potentiometer daily by us ing C2Cl4 alone to set zero point.Use mixture of 22.5 g reference standard oil and 120 mL C2Cl4(specific gravity of mixture at 37° = 1.4763) to set 50% fat point at 850.0.Using either top-load or triple-beam balance with 0.1 g sensitivity, tare Foss-let cup after setting brass ham mer on itsspin dle. To an a lyze prod ucts con tain ing £60% fat, weigh 45.0 gtest sam ple into cup; for prod ucts con tain ing >60% fat, weigh22.5 g. Add ca 80 g Plas ter of Paris (or ca 60 g an hy drous CaSO4).Dis pense 120 mL C2Cl4 into cup. Press cover onto cup and in stall inor bital shaker. Set shaker timer for 2 min and turn unit on. Whileex trac tion pro ceeds, as sem ble pres sure fil tra tion de vice by plac inginto per fo rated base 7 cm fil ter pa per. To pro duce clear fil trate freeof mois ture drop lets (for very wet test sam ples), first place highre ten tion pa per, Whatman No. 50, or equiv a lent, and then phasesep a rat ing pa per, Whatman No. 1PS, or equiv a lent. Af ter 2 minex trac tion, re move cup from shaker, lift cover, and re move brassham mer from cup. Im merse cup in ice-water bath ca 0.4 min whilestir ring con tents with ther mom e ter to cool con tents from 47°–52°Cto ca 40°C. Wipe H2O from outer sur face of cup and pour con tentsinto as sem bled fil ter. Place pis ton at top of fil tra tion de vice andslowly press ex tract through mea sur ing sys tem. De press drain valvebut ton when ex tract ap pears in over flow tube and let cham ber drain;then re lease valve but ton. Re peat fill ing and drain ing 2 more timesun til 40–50 mL ex tract has flowed through, re tain ing fi nal 10 mLex tract in mea sur ing cham ber. Re move fil tra tion de vice, slideview ing lens into po si tion, ro tate con trol of read out po ten ti om e terclock wise un til hy drom e ter rises, and re cord read ing. Es tab lish thatex tract is at cham ber tem per a ture by re peat ing read ing 3–4 times.Av er age read ings and con vert into % fat by means of con ver sion chart. (Mul ti ply chart % fat by 2 if a 22.5 g por tion of high-fat product was used.)Ref er ences: JAOAC 58, 1182(1975); 60, 853(1977);68, 240(1985).。
关于AOAC简介

AOAC婴幼儿食品营养素检测 国际标准研讨会
受国际配方食品咨询委员会委托,AOAC(国际官定分析检测协会) 计划在今后 两年半时间内,对婴幼儿配方食品及成人营养品中优先考虑的至少20种营养素制 定AOAC国际标准。为此,在2010年9月和11月,AOAC分别举行了两次SPIFAN会议 (Stakeholder Panel on Infant Formula and Adult Nutritional),讨论了最 初的五个营养素检测方法,并指定了标准主持实验室和协同验证实验室。为推动 中国对婴幼儿食品营养检测国家标准的制修订能力建设,部分中国专家和企业代 表赴美国参加了工作会议。
分部是由AOAC总部批准成立的专门设在中国的分部组织, 旨在帮助来自分析化学、微生物及其他专业领域的中国会员更 多地了解AOAC;积极参加AOAC的各项活动;协助AOAC总部联系 中国会员;组织各项专业培训及教育活动等。
AOAC Official Methods SM
AOAC 逐步认可新的检测技术作为官方方法,如 LC-MS(液 质联用技术) 和 SPR(表面等离子共振技术)。 2007-至今,SPADA (PCR 检测微生物会议)
液相色谱-质谱联用仪:它结合了液相色谱仪有效分离热不稳性及高沸点 化合物的分离能力与质谱仪很强的组分鉴定能力。是一种分离分析复杂有 机混合物的有效手段。实现对复杂混合物更准确的定量和定性分析。而且 也简化了样品的前处理过程,使样品分析更简便。
质谱仪由以下几部分组成
数据控制和采集及供电系统 ┏━━━━┳━━━━━╋━━━━━━┓ 进样系统 离子源 质量分析器 检测接收器
于250 mL三角瓶中,固体试样需用约50 mL 45 ℃~50 ℃水使其溶解,加 入维生素D3内标1 mL(1 ug/mL) ,混合均匀。
AOAC 官方方法 997.02 食品中酵母菌和霉菌的计数 再水化干膜法(英文版)

17.2.09AOAC Official Method997.02Yeast and Mold Counts in FoodsDry Rehydratable Film Method(Petrifilm™Method)First Action1997Final Action2000(Applicable to enumeration of total yeasts and molds in foods.) See Tables997.02A and B for the results of the interlaboratory study supporting the acceptance of the method.A.PrincipleMethod uses culture plates of dry medium supplemented with an-tibiotics,dye to enhance visualization of growth,and cold H2O-soluble gelling agent.Undiluted or diluted suspensions are added to plates at a rate of1mL/plate.Suspension is spread over ca 30cm2growth area.Gelling agent is allowed to solidify,plates are incubated,and yeasts and molds are counted.B.Apparatus and Reagent(a)Yeast and mold count plates.—Contain nutrients supple-mented with chlortetracycline,chloramphenicol,cold H2O-soluble gelling agent,and dye sensitive to presence of phosphatase (5-bromo-4-chloro-3-indolyl phosphate)that enhances visualiza-tion of yeast and mold growth.Circular growth area of single plate contains thirty1×1cm squares outlined on film base.(Available as 3M™Petrifilm™Yeast and Mold Count plates from3M Microbiol-ogy Products,3M Center,Bldg.275-5W-05,St.Paul, MN55144-1000,USA.)(b)Plastic spreader.—Provided with Petrifilm plates,designed to spread suspension evenly over plate growth area.(c)Pipets.—Serological pipet or pipetting syringe accurately de-livering1.0mL.(d)Colony counter.—Standard apparatus,Quebec model preferred, or one providing equivalent magnification(1.5×)and visibility. (e)Blender.—High speed mechanical blender rotating at 10000–12000rpm,or stomacher.(f)Dilution water.—Butterfield’s phosphate-buffered dilution water.Place34g KH2PO4into1L volumetric flask and dissolve in 500mL H2O.Adjust pH to7.2with1M NaOH(40g/L)and dilute to volume with H2O.Autoclave15min at121°C.Store stock solution in refrigerator.Prepare dilution blanks by pipetting1.25mL stock solution into1L volumetric flask and dilute to volume with H2O. Dispense90or99±1mL into bottles.Autoclave15min at121°C. C.General InstructionsStore unopened yeast and mold count plate foil pouches at≤8°C. After opening,return unused plates to foil pouch.Seal pouch by folding and taping the open end.Store resealed foil pouch at≤8°C in a dry e plates within1month after opening.Exposure of yeast and mold count plates to temperatures>25°C and/or humidities>50%RH can affect performance of plates.After use,plates contain viable yeast and/or mold cultures.Auto-clave used plates15min at121°C prior to discarding.D.Preparation of Test SuspensionAseptically prepare1:10or greater dilution of food product with dilution H2O.Blend or stomach2min and plate.Prepare additional dilutions as required.E.AnalysisPlace yeast and mold count plate on flat surface.Lift top film,hold pipet perpendicular to plate,and carefully inoculate1mL test sus-pension onto center of film base.Place top film down onto inoculum. Lift plastic spreader using circular handle.Align center of spreader with approximate center of plate.Distribute suspension evenly using gentle downward pressure on center of spreader.Do not slide spreader across film.Remove spreader and leave plate un-disturbed1min to let gel solidify.Place plates in incubator in horizontal position,clear side up,in stacks not exceeding20units.Incubate plates5days at20–25°C. Count plates promptly after incubation period.Yeasts appear as blue-green or off-white in color and form small defined colonies. Mold colonies are usually blue but may also assume their natural pigmentation(e.g.,black,yellow,green).They tend to be larger and more diffuse than yeast colonies.To calculate yeast and mold count,multiply total number of yeast and mold colonies/plate(or average number of colonies/plate,if counting duplicate plates of same dilution)by appropriate dilution factor.When counting colonies on duplicate plates of consecutive dilutions,calculate mean number of colonies for each dilution be-fore determining average yeast and mold count.Estimated counts can be made on plates with>150colonies and should be reported as estimated counts.In making such counts,de-termine average count/1cm2and multiply by30(circular growth area is ca30cm2).High numbers of yeast colonies may cause the entire growth area to turn blue.High numbers of mold colonies may cause growth area to turn blue,black,yellow,etc.When this occurs,do not make estimated counts, but further dilute and plate test suspension to obtain more accurate count. Reference:J.AOAC Int.80,806(1997).Revised:March2002©2002AOAC INTERNATIONAL。
AOAC 991.43
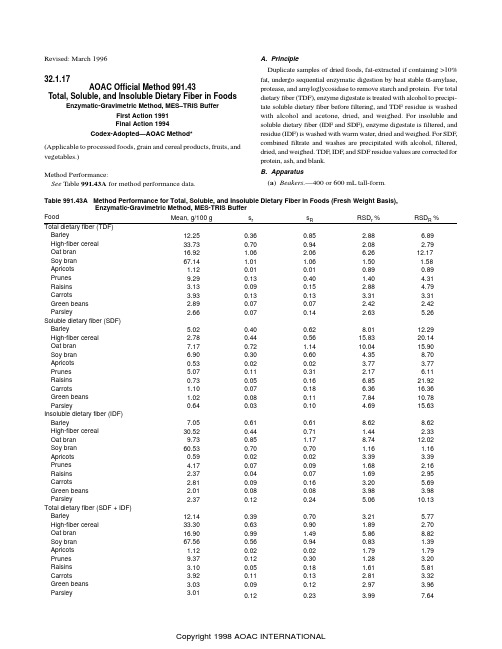
Revised: March 1996 32.1.17AOAC Official Method 991.43Total, Soluble, and Insoluble Dietary Fiber in FoodsEnzymatic-Gravimetric Method, MES–TRIS BufferFirst Action 1991Final Action 1994Codex-Adopted—AOAC Method*(Applicable to processed foods, grain and cereal products, fruits, and vegetables.)Method Performance:See Table 991.43A for method performance data.A. PrincipleDuplicate samples of dried foods, fat-extracted if containing >10%fat, undergo sequential enzymatic digestion by heat stable α-amylase,protease, and amyloglycosidase to remove starch and protein. For total dietary fiber (TDF), enzyme digestate is treated with alcohol to precipi-tate soluble dietary fiber before filtering, and TDF residue is washed with alcohol and acetone, dried, and weighed. For insoluble and soluble dietary fiber (IDF and SDF), enzyme digestate is filtered, and residue (IDF) is washed with warm water, dried and weighed. For SDF,combined filtrate and washes are precipitated with alcohol, filtered,dried, and weighed. TDF, IDF, and SDF residue values are corrected for protein, ash, and blank.B. Apparatus(a ) Beakers.—400 or 600 mL tall-form.Table 991.43A Method Performance for Total, Soluble, and Insoluble Dietary Fiber in Foods (Fresh Weight Basis),Enzymatic-Gravimetric Method, MES-TRIS BufferFood Mean, g/100 g s r s R RSD r%RSD R % Barley 12.250.360.85 2.88 6.89High-fiber cereal 33.730.700.94 2.08 2.79 Oat bran 16.92 1.06 2.06 6.2612.17 Soy bran 67.14 1.01 1.06 1.50 1.58 Apricots 1.120.010.010.890.89 Prunes 9.290.130.40 1.404.31 Raisins 3.130.090.15 2.88 4.79 Carrots 3.930.130.13 3.31 3.31 Green beans 2.890.070.07 2.42 2.42 Parsley 2.660.070.14 2.635.26Soluble dietary fiber (SDF) Barley 5.020.400.628.0112.29 High-fiber cereal 2.780.440.5615.83 20.14 Oat bran 7.170.72 1.1410.04 15.90 Soy bran6.900.300.60 4.358.70 Apricots 0.530.020.02 3.77 3.77 Prunes 5.070.110.31 2.17 6.11 Raisins 0.730.050.16 6.8521.92 Carrots 1.100.070.18 6.3616.36 Green beans 1.020.080.117.8410.78 Parsley 0.640.030.10 4.6915.63 Insoluble dietary fiber (IDF) Barley 7.050.610.618.628.62 High-fiber cereal 30.520.440.71 1.44 2.33 Oat bran9.730.85 1.178.7412.02 Soy bran 60.530.700.70 1.16 1.16 Apricots 0.590.020.02 3.39 3.39 Prunes 4.170.070.09 1.68 2.16 Raisins 2.370.040.07 1.69 2.95 Carrots 2.810.090.16 3.20 5.69 Green beans 2.010.080.08 3.98 3.98 Parsley 2.370.120.24 5.0610.13 Total dietary fiber (SDF + IDF) Barley 12.140.390.70 3.21 5.77 High-fiber cereal 33.300.630.90 1.89 2.70 Oat bran 16.900.99 1.49 5.868.82 Soy bran 67.560.560.940.83 1.39 Apricots 1.120.020.02 1.79 1.79 Prunes 9.370.120.30 1.28 3.20 Raisins 3.100.050.18 1.61 5.81 Carrots 3.920.110.13 2.81 3.32 Green beans 3.030.090.12 2.97 3.96 Parsley 3.010.120.23 3.997.64(b ) Filtering crucible.—With fritted disk, coarse, ASTM 40–60µm pore size, Pyrex 60 mL (Corning No. 36060 Bchner, Corning,Inc., Science Products, Corning, NY 14831, USA, or equivalent).Prepare as follows. Ash overnight at 525° in muffle furnace. Let furnace temperature fall below 130° before removing crucibles.Soak crucibles 1 h in 2% cleaning solution at room temperature.Rinse crucibles with H 2O and then deionized H 2O; for final rinse,use 15 mL acetone and then air-dry. Add ca 1.0 g Celite to dry crucibles, and dry at 130° to constant weight. Cool crucible ca 1h in desiccator, and record weight, to nearest 0.1 mg, of crucible plus Celite.(c ) V acuum system.—V acuum pump or aspirator with regulating device. Heavy walled filtering flask, 1 L, with side arm. Rubber ring adaptors, for use with filtering flasks.(d ) Shaking water baths.—(1) Capable of maintaining 98 2°,with automatic on-and-off timer. (2) Constant temperature, adjust-able to 60°.(e ) Balance.—Analytical, sensitivity 0.1 mg.(f ) Muffle furnace.—Capable of maintaining 525 5°.(g ) Oven.—Capable of maintaining 105 and 130 3°.(h ) Desiccator .—With SiO 2 or equivalent desiccant. Biweekly,dry desiccant overnight at 130°.(i ) pH meter .—Temperature compensated, standardized with pH 4.0, 7.0, and 10.0 buffer solutions.(j ) Pipetters .—With disposable tips, 100–300 µL and 5 mL capacity.(k ) Dispensers.—Capable of dispensing 15 0.5 mL for 78%ethanol, 95% ethanol, and acetone; 40 0.5 mL for buffer.(l ) Magnetic stirrers and stir bars.C. ReagentsUse deionized water throughout.(a ) Ethanol solutions.—(1) 85%. Place 895 mL 95% ethanol into 1 L volumetric flask, dilute to volume with H 2O. (2) 78%. Place 821 mL 95% ethanol into 1 L volumetric flask, dilute to volume with H 2O.(b ) Heat-stable α-amylase solution.—Catalog Number A 3306,Sigma Chemical Co., St. Louis, MO 63178, USA, or Termamyl 300L, Catalog Number 361-6282, Novo-Nordisk, Bagsvaerd, Den-mark, or equivalent.(c ) Protease.—Catalog Number P 3910, Sigma Chemical Co, or equivalent. Prepare 50 mg/mL enzyme solution in MES/TRIS buff-er fresh daily.(d ) Amyloglucosidase solution .—Catalog Number AMG A9913,Sigma Chemical Co, or equivalent. Store at 0–5°.(e ) Diatomaceous earth.—Acid washed (Celite 545 AW, No.C8656, Sigma Chemical Co. or equivalent).(f ) Cleaning solution .—Liquid surfactant-type laboratory cleaner, designed for critical cleaning (Micro ®, International Prod-ucts Corp., Burlington, NJ 08601, USA, or equivalent). Prepare 2%solution in H 2O.(g ) MES .—2-(N -Morpholino)ethanesulfonic acid (No. M-8250,Sigma Chemical Co., or equivalent.)(h ) TRIS.—Tris(hydroxymethyl)aminomethane (No. T-1503,Sigma Chemical Co., or equivalent).(i ) MES–TRIS buffer solution.—0.05M MES, 0.05M TRIS, pH 8.2 at 24°. Dissolve 19.52 g MES and 12.2 g TRIS in 1.7 L H 2O.Adjust pH to 8.2 with 6N NaOH, and dilute to 2 L with H 2O.(Note: It is important to adjust pH to 8.2 at 24°. However, if buffer temperature is 20°, adjust pH to 8.3; if temperature is 28°,adjust pH to 8.1. For deviations between 20 and 28°, adjust by interpolation.)(j ) Hydrochloric acid solution.—0.561N . Add 93.5 mL 6N HCl to ca 700 mL H 2O in 1 L volumetric flask. Dilute to 1 L with H 2O.D. Enzyme PurityTo ensure absence of undesirable enzymatic activities and pres-ence of desirable enzymatic activities, run standards listed in Table 991.43B each time enzyme lot changes or at maximum interval of 6months.E. Sample Preparation and DigestionPrepare samples as in 985.29E (see 45.4.07) (if fat content of sample is unknown, defat before determining dietary fiber). For high sugar samples, desugar before determining dietary fiber by extract-ing 2–3 times with 85% ethanol, 10 mL/g, decanting, and then drying overnight at 40°.Run 2 blanks/assay with samples to measure any contribution from reagents to residue.Weigh duplicate 1.000 0.005 g samples (M 1 and M 2), accurate to 0.1 mg, into 400 mL (or 600 mL) tall-form beakers. Add 40 mL MES–TRIS buffer solution, pH 8.2, to each. Stir on magnetic stirrer until sample is completely dispersed (to prevent lump formation,which would make test material inaccessible to enzymes).Add 50 µL heat-stable α-amylase solution, stirring at low speed.Cover beakers with Al foil, and incubate in 95–100° H 2O bath 15min with continuous agitation. Start timing once bath temperature reaches 95° (total of 35 min is normally sufficient).Remove all beakers from bath, and cool to 60°. Remove foil.Scrape any ring from inside of beaker and disperse any gels in bottom of beaker with spatula. Rinse beaker walls and spatula with 10 mL H 2O.Add 100 µL protease solution to each beaker. Cover with Al foil,and incubate 30 min at 60 1° with continuous agitation. Start timing when bath temperature reaches 60°.Remove foil. Dispense 5 mL 0.561N HCl into beakers while stirring. Adjust pH to 4.0–4.7 at 60°, by adding 1N NaOH solution or 1N HCl solution. (Note: It is important to check and adjust pH while solutions are 60° because pH will increase at lower tempera-tures.) (Most cereal, grain, and vegetable products do not require pH adjustment. Once verified for each laboratory, pH checking procedure can be omitted. As a precaution, check pH of blank routinely; if outside desirable range, check samples also.)Add 300 µL amyloglucosidase solution while stirring. Cover with Al foil, and incubate 30 min at 60 1° with constant agitation. StartTable 991.43B Standards for Testing Enzyme Activity Standard Activity Tested Weight of Standard, gExpected Recovery, (%)Citrus pectin Pectinase 0.1–0.295–100 Arabinogalactan Hemicellulase 0.1–0.295–100β-Glucan β-Glucanase 0.1–0.295–100Wheat starch α-Amylase + AMG 1.0 0–1 Corn starch α-Amylase + AMG 1.00–1Casein Protease0.30–1timing once bath reaches 60°.F. Determination of Total Dietary FiberTo each digested sample, add 225 mL (measured after heating) 95% ethanol at 60°. Ratio of ethanol to sample volume should be 4:1. Remove from bath, and cover beakers with large sheets of Al foil. Let precipitate form 1 h at room temperature.Wet and redistribute Celite bed in previously tared crucible B(b), using 15 mL 78% ethanol from wash bottle. Apply suction to crucible to draw Celite onto fritted glass as even mat.Filter alcohol-treated enzyme digestate through crucible. Using wash bottle with 78% ethanol and rubber spatula, quantitatively transfer all remaining particles to crucible. (Note: If some samples form a gum, trapping the liquid, break film with spatula.)Using vacuum, wash residue 2 times each with 15 mL portions of 78% ethanol, 95% ethanol, and acetone. Dry crucible containing residue overnight in 105° oven. Cool crucible in desiccator ca 1 h. Weigh crucible, containing dietary fiber residue and Celite, to near-est 0.1 mg, and calculate residue weight by subtracting weight of dry crucible with Celite, B(b).Use one duplicate from each sample to determine protein, by method 960.52 (see 12.1.07), using N× 6.25 as conversion factor. For ash analysis, incinerate second duplicate 5 h at 525°. Cool in desiccator, and weigh to nearest 0.1 mg. Subtract weight of crucible and Celite, B(b), to determine ash weight.G. Determination of Insoluble Dietary FiberWet and redistribute Celite bed in previously tared crucible, B(b), using ca 3 mL H2O. Apply suction to crucible to draw Celite into even mat. Filter enzyme digestate, from E, through crucible into filtration flask. Rinse beaker, and then wash residue 2 times with 10 mL 70° H2O. Combine filtrate and water washings, transfer to pretared 600 mL tall-form beaker, and reserve for determination of soluble dietary fiber, H. Using vacuum, wash residue 2 times each with 15 mL portions of 78% ethanol, 95% ethanol, and acetone. (Note: Delay in washing IDF residues with 78% ethanol, 95% ethanol, and acetone may cause inflated IDF values.)Use duplicates to determine protein and ash as in F.H. Determination of Soluble Dietary FiberProceed as for insoluble dietary fiber determination through in-struction to combine the filtrate and water washings in pretared 600 mL tall-form beakers. Weigh beakers with combined solution of filtrate and water washings, and estimate volumes.Add 4 volumes of 95% ethanol preheated to 60°. Use portion of 60° ethanol to rinse filtering flask from IDF determination. Alterna-tively, adjust weight of combined solution of filtrate and water washings to 80 g by addition of H2O, and add 320 mL 60° 95% ethanol. Let precipitate form at room temperature 1 h.Follow TDF determination, F, from “Wet and redistribute Celite bed . . . .”I. CalculationsBlank (B, mg) determination:B = [(BR1 + BR2)/2] – P B – A Bwhere BR1 and BR2 = residue weights (mg) for duplicate blank determinations; and P B and A B = weights (mg) of protein and ash, respectively, determined on first and second blank residues. Dietary fiber (DF, g/100 g) determination:DF = {[(R1 + R2)/2] – P – A – B}/[(M1 + M2)/2] × 100 where R1 and R2 = residue weights (mg) for duplicate samples; P and A = weights (mg) of protein and ash, respectively, determined on first and second residues; B = blank weight (mg); and M1 and M2 = weights (mg) for samples.Total dietary fiber determination: Determine either by independent analysis, as in F, or by summing IDF and SDF, as in G and H. Reference: J. AOAC Int. 75, 395(1992).*Adopted as a Codex Defining Method for gravimetry/enzymatic di-gestion of total dietary fiber in infant formula and follow-up for-mula.。
AOAC 2001.03 测定特定食品中的总膳食纤维 包含抗性麦芽糊精 酶重量法和液相色谱法

45.4.13AOAC Official Method2001.03Total Dietary Fiber in FoodsContaining Resistant MaltodextrinEnzymatic-Gravimetric Methodand Liquid Chromatography DeterminationFirst Action2001[This method is applicable to resistant maltodextrin(RMD)and to foods containing RMD listed in Table2001.03at 1.4%RMD.] A.PrincipleThis method determines total dietary fiber(TDF)value of pro-cessed foods containing insoluble dietary fiber(IDF)and high mo-lecular weight soluble dietary fiber(HMWSDF),which are precipitated in ethanol and low molecular weight resistant maltodextrin(LMWRMD),which is soluble in ethanol.This method defines dietary fiber(DF)as consisting of nondigestible car-bohydrates having a degree of polymerization with3sugar moieties (DP3)or higher after enzymatic hydrolysis.All the starches con-tained in food are converted to glucose after this enzymatic hydroly-sis.This method to determine TDF content in processed foods containing RMD is a combination of985.29(see45.4.07)for DF and a LC method for LMWRMD.A food is first analyzed for the to-tal quantity of IDF and HMWSDF,precipitated in ethanol,accord-ing to985.29(see45.4.07).Then an LC determination is conducted on the desalted filtrate to obtain the quantity of LMWRMD not pre-cipitated in the78%alcohol preparation.These2values[(IDF+ HMWSDF)+LMWRMD]are summed to obtain the TDF value in the food.B.Apparatus(a)Balance.—Analytical,weighing to0.1mg.(b)Beakers.—Tall-form,500mL.(c)Water baths.—To maintain a temperature of95–100C and 60C with ability to shake the containers.(d)Filtering crucibles.—Coarse,ASTM,40–60m pore size, Pyrex,50mL.(e)Glass or plastic columns.—To hold ion exchange resins, 75cm15mm id;a shorter(40–75cm15mm id)column can also be used.(f)Liquid chromatograph(LC).—With oven to maintain a col-umn temperature of80C and a20L injection loop.Column oper-ating conditions are:Temperature,80C;mobile phase,distilled water,C(d);flow rate,0.5mL/min.(g)Guard column(or precolumn).—TSK®guard column PWXL,6.0mm id4cm(Tosoh Corp.,distributed by TosoHaas, Montgomeryville,PA,USA;)or equivalent. (h)LC columns.—Two LC columns connected in series, TSK-GEL®G2500PWXL,7.8mm id30cm(Tosoh Corp.),or equivalent.(i)Detector.—Refractive index(RI);maintained at40C. (j)Data integrator or computer.—For peak area measurement. (k)Filters for disposable syringe.—0.2m membrane,13mm. (l)Filters for water.—0.2m,47mm.(m)Filter apparatus.—To hold47mm,0.2m filter,(l);to filter larger volumes of water,C(d).(n)Glass rods.—With fire-polished ends,ca20cm long. (o)Syringes.—10mL,plastic disposable.(p)Pasteur pipet.(q)Volumetric pipet.—10mL.(r)Volumetric flasks.—10,50,250,and1000mL.(s)Top loading balance.—4000g capacity.(t)Tubing.—PVC,2.79mm id(for ion exchange columns). (u)Glass LC syringe.—50L.(v)Teflon scraping rod.—Use in place of glass stirring rod to scrape precipitate from tall-form beaker.(w)Rotary evaporator.—R-3000VW“Student”(Büchi,Swit-zerland;)or equivalent.C.Reagents(a)Ethanol.—95%.Technical grade,used at60C.(b)Ethanol.—78%.Place207mL water in1L volumetric flask and dilute to volume with95%ethanol,(a).(c)Acetone.—Reagent grade.(d)Distilled water.(e)Sodium phosphate dibasic.(f)Sodium phosphate monobasic.(g)Phosphate buffer.—0.08M,pH6.0.Dissolve1.400g Na2HPO4(or1.753g dihydrate)and9.68g NaH2PO4H2O(or10.94g dihydrate)in ca700mL water,(d).Dilute to1L with water,(d),and verify pH with pH meter.(h)Heat stable a-amylase solution(Termamyl).—No.120L(ac-tivity:12units/mg protein;Novo Laboratories,Inc.,59Danbury Rd, Wilton,CT06897,USA),or equivalent(should not contain glycerol).(i)LC retention time standard.—Standard source of the distribu-tion of oligosaccharides(DP$3)in the LMWRMD fraction of RMD, corn syrup solids(DE25;Matsutani Chemical Industry Co.,Ltd.,ItamiTable2001.03Interlaboratory results for the determination of total dietary fiber in selected foods containing resistant maltodextrin by enzymatic-gravimetric method and liquid chromatographyFood x,%bs a(b)s r RSD r,%s R RSD R,% Resistant maltodextrin95.368(0) 1.63 1.71 2.37 2.48Hard candy37.997(1)0.58 1.530.68 1.79 Chicken and vegetable soup25.418(0)0.74 2.89 1.18 4.65 Grapefruit juice 1.388(0)0.02 1.330.04 3.20 White bread9.608(0)0.33 3.410.64 6.66 Strawberry Jell-O9.918(0)0.60 6.100.939.39a(b)a=Number of laboratories retained after eliminating outliers;b=number of laboratories removed as outliers.City,Hyogo,Japan;),analyzed by LC(Fig-ure2001.03A)as in D.(j)Protease.—No.P-3910or P-5380(activity:7–15units/mg pro-tein;Sigma Chemical Co.,St.Louis,MO,USA),or equivalent(should not contain glycerol).Prepare protease stock solution just before use by adding100mg protease enzyme to a10mL volumetric flask and bring-ing to volume with water,(d),(amount is sufficient for9test portions in duplicate).(k)Amyloglucosidase.—No.A-9913(activity:400units/mg pro-tein;Sigma Chemical Co.),or equivalent(should not contain glycerol). (l)Celite.—No.C-8656(Sigma Chemical Co.)or No.C-211,acid washed(Fisher Scientific Co.,Fair Lawn,NJ,USA),or equivalent. (m)Mixed-bed ion exchange resins for each test por-tion.—(1)m-1.—25g Amberlite IRA-67(OH-type;Organo Corp., Tokyo,Japan,/organo_corp.htm),or equivalent.(2)m-2.—25g Amberlite200CT(HG)H(H-type;Organo Corp.),or equivalent,are mixed and packed in column for analysis of each test portion.The converted resin should satisfy the following specifications:(a)Total ion exchange capacity:1.74meq/mL (min);(b)Effective ion exchange capacity(R-H exchange capac-ity):1.6meq/mL(min);(c)pH:4–7.Before mixing and packing the 2resins into a column,wash each resin with H2O to obtain a pH value of7–8.8for m-1and4–7for m-2.If Amberlite200CT(HG)H cannot be obtained,Amberlite200(Na-type;Sigma Chemical Co.) or Amberlite200CT(Organo Corp.)can be used by converting it to “H-type”by the following procedure:Fill column(100cm40mm id),B(e),with600mL(500g) Amberlite200“Na-type”resin and determine approximate resin volume.Wash resin with2volumes of water,(d),at the rate of 60mL/min.Pass2volumes of10%HCl(1+3,w/w)through the resin at the rate of60mL/min.Remove HCl with3volumes of water, (d),passed through the resin at the rate of60mL/min.Add3–6vol-umes of additional water,(d),at the rate of120mL/min.The columnis adequately washed of HCl when a pH value of4–7is obtained.(It takes2–3h to charge and rinse these resins.)(n)Sodium hydroxide.—0.275M;reagent grade.Dissolve11.0g NaOH in ca700mL water,(d),in a1L volumetric flask.Dilute to volume with water,(d).(o)Hydrochloric acid.—0.325M;reagent grade.Dilute stock so-lution of known titer,e.g.,325mL1M HCl,to1L with water. (p)Glycerol(LC standard).—10mg/mL.For stock solution: weigh10g glycerol>99.5%purity into a small beaker.Quantita-tively transfer to1L volumetric flask with repeated washes with wa-ter,(d),and dilute to volume.It is important to measure and record the exact weight of the glycerol,weighing as close to10g as possi-ble.Take purity and weight of glycerol into consideration when cal-culating concentration of final glycerol LC standard.(q)Glycerol(for dextrose–glycerol standard).—100mg/mL.Weigh 10g high purity glycerol into a small beaker,transfer to a100mL volu-metric flask with water,(d),and dilute to volume.(r)Ammonium sulfate.—Reagent grade;standard to test mi-cro-Kjeldahl procedure.(s)Dextrose.—LC grade,high purity>99.5%.(t)Silver nitrate solution.—0.1M.Dissolve1.70g AgNO3in ca 70mL water,(d),in a100mL volmetric flask,and dilute to volume with water,(d).D.Determination(a)Enzymatic hydrolysis and filtration.—Weigh1.0g test por-tion(crushed,sieved to10mesh,fat extracted if>10%fat,and dried) into a500mL previously weighed tall-form beaker,B(b).Prepare induplicate with2blank digestion determinations.Disperse in50mL 0.08M phosphate buffer,C(g),and sonicated to ensure complete hydration.Add100L heat stable-amylase,C(h),and cover beaker with aluminum foil.Place beaker in shaker water bath and hold at95C for30min with shaking.Cool to room temperature,and adjust the pH of the solution to pH7.50.1with0.275M NaOH, C(n).Add0.5mL protease solution,C(j),and digest solution for 30min at60C.Cool solution to room temperature(25C),and ad-just pH to4.50.2with0.325M HCl,C(o).Add0.3mL amyloglucosidase,C(k),and digest at60C for30min.Upon com-pletion of the3enzyme digestion sequence,add4volumes of95% ethanol,C(a),by weight,previously heated e the top loading balance to weigh beaker with digestion mixture when add-ing ethanol(obtain tare weight of beaker before adding test portion). Assay the2blank digestions(i.e.,2beakers and2crucibles)in an identical manner.Let solutions stand overnight to form a precipitate.Filter by suc-tion,using a water aspirator or vacuum pump,through1.0g Celite layered on a Pyrex glass crucible filter that previously has been dried to constant weight.Wash the500mL tall-form beaker and the resi-due3times with20mL78%ethanol,C(b),2times with10mL95% ethanol,C(a),and2times with10mL acetone,C(c). Quantitatively transfer filtrate and washings to a1L round bottom flask.Dry residue in an air oven at105C overnight and record weight.This residue weight,minus the protein,ash,and blank resi-due weights represents the weight of the dietary fiber(IDF+ HMWSDF)recovered by the AOAC method.(b)Filtrate recovery,desalting,and LC analysis.—Evaporate with a rotary evaporator to near dryness.Dissolve the residue with a minimum amount of water,C(d),and transfer quantitatively to a 50mL volumetric flask.Add10mL of10mg/mL glycerol LC stan-dard and dilute to volume with water,C(d).Transfer contents of the 50mL volumetric flask to a column(75cm15mm id)containing 25g each,thoroughly mixed,of Amberlite IRA-67(m-1)and Amberlite200CT(HG)H(m-2)prepared just before use.Wash ex-tract through the column with250mL water,C(d),at the rate of 0.8mL/min.Collect250mL eluant from the ion exchange column and quanti-tatively transfer into a500mL round bottom flask.Evaporate to near dryness and quantitatively transfer to a10mL volumetric flask and dilute to volume with water,C(d).Transfer the contents of the 10mL volumetric flask to a10mL disposable syringe,B(o),and fil-ter through a0.2m filter,B(k).Use a50L LC glass syringe,B(u), to fill the20L injection loop on the LC,B(f).(c)Determining the response factor for dextrose;dextrose is equivalent to RMD in LC response.—Each chromatogram must be evaluated or standardized for the RI response of RMD.This is ac-complished using glycerol standard,C(q).The peak areas,represent-ing concentration,obtained by LC analysis of equal amounts of RMD and dextrose are equivalent.Glycerol is used as the internal standard but its peak area compared to the peak area for an equal amount of dextrose or RMD is not equivalent.A glycerol standard curve is there-fore prepared to obtain a“response factor”to calculate the exact amount of RMD in a chromatogram of each test portion.Prepare3solutions in individual100mL volumetric flasks con-taining the same amount of glycerol and3levels of dextrose.It is im-portant to know and use the reported content(i.e.,>99.5%purity)of both glycerol and dextrose standards as reported by suppliers.Accu-rately weigh0.5,1.0,and2.0g dextrose into3separate100mL volu-metric flasks,respectively.To each flask add10mL of the 100mg/mL glycerol standard,C(q).Dilute each flask to volume with water,C(d).These3flasks represent the standard solutions to calculate the“response factor”for dextrose that is used to determine the amount of RMD as displayed in LC chromatograms.Use a50L LC syringe,B(u),to fill the20L injection loop for each standard glycerol–dextrose solution.Obtain the values for the peak areas of dextrose and glycerol from the3chromatograms.The reciprocal of the slope obtained by comparing the ratio of peak area of dextrose/peak area of glycerol(y-axis)to the ratio of the weight of dextrose/weight of glycerol(x-axis)is the“response factor.”The av-erage“response factor”among laboratories is0.82,varying slightly in each laboratory.Response factor=1/(PA-dex/PA-gly)(Wt-gly/Wt-dex) where PA-dex=peak area dextrose;PA-gly=peak area glycerol; Wt-dex=weight of dextrose in standard;Wt-gly=weight of glyc-erol in standard.A flow diagram for a combined enzymatic-gravimetric method and LC determination is shown in Figure2001.03B.E.CalculationAll values used in calculations are in mg,except for percent(%) values.Assay each test portion in duplicate,resulting in2test portion weight values,test portion weight and test portion weight(prime);2cruciblesfor eachblankandtestportion,blankandblank(prime);and test portion and test portion(prime).(a)Calculate average%(IDF+HMWSDF)as fol-lows.—(1)Blank ash(Ab)=(ash+Celite+blank crucible)–(Celite +blank crucible).(2)Blank residue weight(BRW)=((BR+BR)/2)–(Pb+Ab)where Pb=blank protein,determined by micro-Kjeldahl procedure;BR=weight of first blank crucible with residue;BR=weight of second blank cruci-ble with residue;Ab=weight of blank ash from step(a)(1). (3)Test portion residue weight(SR)=(residue+Celite+test portion crucible)–(Celite+test portion crucible).Duplicate test portion residue weight(SR)=(residue+Celite+test portion cru-cible)–(Celite+test portion crucible).(4)Test portion ash weight(As)=(ash+Celite+crucible)–(Celite+crucible).(5)Final test portion residue weight(FSR)=SR–Ps–As–BRW =FSR where Ps=protein,determined by micro-Kjeldahl procedure; SR=final test portion residue weight from step(a)(3);As=test por-tion ash weight from step(a)(4);BRW=blank residue weight from step(a)(2).Repeat this calculation for FSR using SR–Ps–As–BRW(using values from duplicate test portion weights).(6)Percent final test portion residue weight(%FSR)=(FSR/ SW)100=%FSR where FSR=final test portion residue weight from step(a)(5);SW=test portion weight.Repeat this calculation for%FSR using FSR and SW.(7)%(IDF+HMWSDF)=average%FSR=(%FSR+%FSR)/2where %FSR=percent final test portion residue weight;%FSR=percent final duplicate test portion residue weight.(b)C a l c u l a t e a v e r a g e%L M W R M D a s f o l-lows.—(1)LMWRMD=(peak area of LMWRMD/peak area of glycerol)(glycerol standard,mg response factor).(2)%LMWRMD=(LMWRMD/SW)100where LMWRMD =weight of LMWRMD from step(b)(1);SW=test portion weight. Repeat calculations for%LMWRMD using LMWRMD and SW.(3)%ALMWRMD=average%LMWRMD=(%LMWRMD+ %LMWRMD)/2where%LMWRMD=percent LMWRMD for test portion from step(b)(2);%LMWRMD=percent LMWRMD for duplicate test portion from step(b)(2).(c)Calculate average%total dietary fiber(TDF)as fol-lows.—Percent(%)TDF=%(IDF+HMWSDF)+%ALMWRMD where%(IDF+HMWSDF)=average percent IDF+HMWSDF from step(a)(7);%ALMWRMD=average percent LMWRMD from step(b)(3).F.Resistant MaltodextrinThe commercially available U.S.GRAS status RMD is a source of dietary fiber.Resistant maltodextrin is certified as an approved di-etary fiber ingredient for the Program for Foods for Specific Health Use(FOSHU)in Japan.Dietary fiber supplements prepared simply by packaging RMD(or agglomerated RMD)in sachet forms and la-beled as RMD have been on the market.Fibersol®-2,RMD,is manu-factured and was supplied by Matsutani Chemical Industry Co.,Ltd. (Itami City,Hyogo,Japan).The moisture content of the product is 2.7%and DE is10.5.The RMD is produced by the pyrolysis and subsequent enzyme treatment of corn starch.It is an aggregate of glucose polymers with the MW distribution of180(DP-1)to >10000(DP-62)daltons,but the average MW is2000daltons.It contains1–4and1–6glucosidic bonds,which originate from starch and1–2and1–3glucosidic bonds that are created by transglucosidation during pyrolysis.Internal utilization of RMD by in vitro and in vivo tests show that <10%is digested and absorbed in the small intestine.Approximately 50%of the products are fermented in the large intestine and ca40%of the products are excreted into the feces.In order to distinguish this substance from conventional maltodextrin(digestible),the term“re-sistant”is added and used to describe this compound.The sugar,oligosaccharide,and polysaccharide composition of the LMWRMD fraction of the RMD has been determined before and after hydrolytic enzyme treatments and is shown in Fig-ure2001.03A.The distribution of these oligosaccharides is not sig-nificantly changed when RMD is treated with hydrolytic enzymes. To assess the oligosaccharide moieties and their distribution in the LMWRMD of RMD,corn syrup solids were used as a standard source of these oligosaccharides(Figure2001.03A).The nondigestible portions of RMD consists of DP units of3(DP-3)and above(Figure2001.03A).These nondigestible oligosaccharides and polysaccharides constitute>90%of RMD.Approximately60% of RMD consist of polymers having>10DP.References:J.AOAC Int.83,1013(2000);(future issue).。
真胃灌注不同水平淀粉对泌乳后期奶牛采食量、 泌乳性能

动物营养学报2014,26(6):1467-1476ChineseJournalofAnimalNutritiondoi :10.3969/j.issn.1006-267x.2014.06.006真胃灌注不同水平淀粉对泌乳后期奶牛采食量、泌乳性能、胃肠道发酵和血液代谢的影响邹 杨 李胜利 曹志军* 杨占山(中国农业大学动物科技学院,动物营养国家重点实验室,北京100193)摘 要:为了研究真胃灌注不同水平淀粉对泌乳后期奶牛采食量、泌乳性能、胃肠道发酵和血液代谢的影响,采用4×4拉丁方设计将4头安装有永久性瘤胃瘘管的荷斯坦2胎奶牛分为4组,分别向真胃内灌注0(对照组)、800、1600、2400g/d淀粉。
共进行4期试验,每期7d(5d的预试期和2d的正试期)。
结果表明:1)真胃灌注不同水平淀粉对奶牛干物质采食量无显著影响(P>0.05);奶牛的消化能(P=0.022)和泌乳净能采食量(P=0.014)均随着真胃淀粉灌注量的增加而呈线性增加,而奶牛的淀粉表观消化率则呈线性降低(P=0.029)。
2)随着真胃淀粉灌注量的增加,乳脂率(P=0.024)和乳脂产量(P=0.022)均呈二次曲线上升,以1600g/d灌注量时最大。
3)800g/d组的粪液丁酸浓度显著低于1600和2400g/d组(P<0.05),粪液总挥发性脂肪酸浓度显著低于对照组和1600g/d组(P<0.05)。
4)真胃灌注不同水平淀粉对瘤胃发酵特性及血液代谢指标均无显著影响(P>0.05)。
综合以上,乳脂率随着真胃淀粉灌注量的增加呈二次曲线上升;泌乳后期奶牛能够利用的过瘤胃淀粉的最大量为1600g/d。
关键词:淀粉灌注;采食量;泌乳性能;血液代谢;泌乳奶牛中图分类号:S823 文献标识码:A 文章编号:1006-267X(2014)06-1467-10收稿日期:2013-12-01基金项目:国家自然科学基金项目(31372334)作者简介:邹 杨(1985—),女,山东泰安人,博士研究生,从事反刍动物营养研究。
AOAC985.29食品中总膳食纤维测定中文翻译

45.4.07AOAC官方的方法985.29食品中总膳食纤维酶- 重量法1985年首次发布的准则1986年最终准则AOAC–AACC方法法典采用AOAC分析法*A.原理干燥食品的替换测验部分,脂肪提取,如果含>10 %的脂肪,是通过淀粉酶(热稳定a-淀粉酶),然后用朊酶和淀粉葡萄糖苷酶进行酶促化,以除去蛋白质和淀粉。
(在分析混合饮食时,随时提取之前确定总膳食纤维脂肪。
)添加四倍体积的乙醇以便沉淀可溶性膳食纤维。
过滤总残留量,用78 %乙醇,95%乙醇和丙酮冲洗。
干燥后,称量残留物。
对一份进行蛋白质分析,另一份在525°C灰化,测定灰分。
总膳食纤维=残留物的重量- 重量(蛋白质+灰分)。
B.设备(a) 多孔坩埚——孔隙度,第2号(耐热号,32940 ,粗,ASTM 40-60 μm;或者第36060号布赫呐,烧结盘,派热克斯玻璃,60 mL, ASTM 40-60 μm)。
彻底清洗,525℃加热1小时,浸泡,然后水冲洗。
给风干坩埚加约0.5克硅藻土,在130℃干燥至恒重(≥1小时)1 h). 冷却,存储在干燥器中备用。
(b) 真空源——真空泵或吸气器,配备直列双真空瓶,以防止出现水阻塞时混合。
(c)真空炉——70°C. 可选用,105℃的空气烘箱。
(d)干燥器。
(e)高温烘炉。
(f) 水浴槽。
——(1)煮沸(2)恒温——可调至60℃,无论是多站振动筛还是多站磁力搅拌器,酶水解期间给消化瓶(煮解瓶)提供恒力搅拌。
(g)烧杯——高烧杯,400或600毫升。
(h)天平——分析天平,灵敏度到0.1毫克。
(i)氢离子计——pH7和pH4缓冲液定型。
C.试剂(a) 95% 乙醇.——v/v. 工业级。
(b) 78% 乙醇.——1升量瓶中放置207毫升H2O。
用95%乙醇稀释至刻度。
必要时用95%乙醇再次稀释至刻度。
混合。
一个容量的H2O用四倍容量的95%的乙醇混合,也会能给出78%乙醇的最终浓度。
AOAC法检测聚葡萄糖的方法

INTRODUCTION:Dietary fibre is a mixture of complex organic substances including, non-swellable,more or less hydrophobic compounds such as cutins, suberins and lignins;as well as a range of hydrophilic compounds such as soluble and insoluble polysaccharides and non digestable oligosaccharides.The procedures for the determination of total dietary fibre as outlined in this booklet are based on the methods of Lee et al.1and Prosky et al.2,3(AOAC 991.43,AOAC 985.29,AACC 32-07 and AACC 32-05).However,the enzymes in the MegazymeT otal Dietary Fibre Kit can also be used in other dietary fibre analytical methods such as AACC Method 32-21 and AACC method 32-06.PRINCIPLE (TOTAL DIETARY FIBRE):T otal dietary fibre (TDF) is determined on duplicate samples of dried and defatted (if fat content is >10%) material.Samples are cooked at ~100°C with heat stable α-amylase to give gelatinisation,hydrolysis and depolymerisation of starch;incubated at 60°C with protease (to solubilise and depolymerise proteins) and amyloglucosidase (to hydrolyse starch fragments to glucose);and treated with four volumes of ethanol to precipitate soluble fibre and remove depolymerised protein and glucose (from starch).The residue is filtered;washed with 78% ethanol,95% ethanol,and acetone;dried;and weighed.One duplicate is analysed for protein and the other is incubated at 525°C to determine ash.The TDF is the weight of the filtered and dried residue less the weight of the protein and ash.The major advantage of the Megazyme TDF T est Kit is that it contains high purity enzymes devoid of interfering activities,and the activities of the enzymes are standardised.The importance of standardisedα-amylase activity in the measurement of resistant starch is well recognised.Megazyme amyloglucosidase is essentially devoid of cellulase,whereas other commonly used preparations contain significant contamination with this activity,which leads to solubilisation and underestimation of β-glucan.All Megazyme TDF enzymes are supplied in a ready-to-use,stabilised,liquid form. SCOPE:Applicable to cereal grains,fruit and vegetables,cereal and fruit products and foods.ENZYME PURITY AND STANDARDISATION:The effectiveness and purity of Megazyme α-amylase,protease and amyloglucosidase have been evaluated using the standards recommended in AOAC Method 985.29 and 991.43,and AACC Method 32-05.Megazyme thermostable α-amylase (E-BLAAM) has an activity of 3,000 U/mL (Ceralpha method);protease is supplied at aAPPARATUS:1.Beakers,400 mL and 600 mL tall-form.2.Fritted crucible,Corning No.36060 Büchner,fritted disk,Pyrex60mL,pore size,coarse,ASTM 40-60 µm,or equivalent.Prepare as follows:a.Ash overnight at 525°C in muffle furnaceb.Remove Celite and ash material by using a vacuumc.Soak in 2% Micro cleaning solution (reagent 7) at roomtemperature for 1 hr.d.Rinse crucibles with water and deionised water.e.For final rinse,use 15 mL acetone and air dry.f.Add approximately 1.0 g Celite to dried crucibles and dry at130°C to constant weight.g.Cool crucible in desiccator for approximately 1 hr andrecord weight of crucible containing Celite.3.Filtering flask,heavy-walled,with 1-L side arm.4.Rubber ring adaptors for use on filtering flasks.5.Vacuum source:vacuum pump or aspirator with regulatorcapable of regulating vacuum.6.Water bath,shaking,large-capacity (20-24 L) with covers;capableof maintaining temperature of 100°C;equipped with automatictimers for on-off operation.7.Balance,0.1 mg accuracy.8.Ovens,two,mechanical convection,set at 103±2°and 130±3°C.9.Timer.10.Desiccator,airtight,with SiO2or equivalent desiccant.Desiccantdried biweekly overnight in 130°C oven.11.pH meter.12.Pipettors and tips,50-200 µL and 5 mL capacity.13.Dispensersa.15±0.5 mL for 78% EtOH,95% ethanol,and acetone.b.40±0.5 mL for buffer14.Cylinder,500 mL.15.Magnetic stirrers and stirring bars.16.Rubber spatulas.17.Muffle furnace,525±5°C.REAGENTS:1.Ethanol,95% v/v.2.Ethanol,78%.Place 207 mL water into 1-L volumetric flask.Diluteto volume with 95% ethanol.Mix.3.Acetone,reagent grade.4.Enzymes for TDF assay(Megazyme International IrelandLimited).Store at 0-5°C.a.α-Amylase,heat-stable (E-BLAAM);3,000 Ceralpha Units/mL.b.Protease (E-BSPRT);50 mg/mL;350 T yrosine Units/mL.c.Amyloglucosidase (E-AMGDF);200 p-NP β-maltosideUnits/mL (or 3200 Units/mL on soluble starch).5.Deionised water.6.Celite,acid-washed,pre-ashed (Megazyme G-CEL100 orG-CEL500).7.Cleaning solution,Micro (International Products Corp.,T renton,NJ).Make 2% solution with deionised water.8.MES/TRIS buffer,0.05 M each,pH 8.2 at 24°C.Dissolve 19.52 g2(N-morpholino) ethanesulfonic acid (MES) (Sigma,M 8250) and14.2 g tris(hydroxymethyl)aminomethane (TRIS) (Sigma,T1503) in1.7 L deionised water.Adjust pH to 8.2 with 6.0 N NaOH.Diluteto 2 L with water.It is important to adjust pH of buffer toapproximately 8.3 at 20°C or approximately 8.1 at 27-28°C.9.Hydrochloric acid solution,0.561 N.Add 93.5 mL of 6 N HClto approximately 700 mL of water in 1-L volumetric flask.Dilute to 1-L with water.10.pH standards.Buffer solutions at pH 4.0,7.0 and 10.0.ENZYME PURITYT o ensure presence of appropriate enzyme activity and absence of undesirable enzyme activity,run materials listed below through entire procedure.Each new lot of enzymes should be tested,as should enzymes that have not been tested for previous 6 months. Alternatively,enzyme activity and purity can be determined using assay procedures as summarised on pages 17 and 18 of this booklet.e.Adjust temperature of water bath to 60°C by draining someof hot water from water bath and adding cold water.5.Incubation with proteasea.Add 100 µL protease solution to each sample.b.Re-cover with aluminium foil.c.Incubate in shaking water bath at 60±1°C,with continuousagitation for 30 min.Start timing when temperature of waterbath reaches 60°C.6.pH checka.Remove sample beakers from shaking water bath.b.Remove covers.c.Dispense 5 mL of 0.561 N HCl solution into sample whilestirring.d.Check pH,which should be 4.1- 4.8.Adjust pH,if necessary,with additional 5% NaOH solution or 5% HCl solution (SeeNote 2.)7.Incubation with amyloglucosidasea.Add 200 µL amyloglucosidase solution while stirring onmagnetic stirrer.b.Replace aluminium cover.c.Incubate in shaking water bath at 60°C for 30 min,withconstant agitation.Start timing when temperature of waterbath reaches 60°C.A.INSOLUBLE DIETARY FIBRE8.Filtration setupa.T are crucible containing Celite to nearest 0.1 mg.b.Wet and redistribute bed of Celite in crucible usingapproximately 3 mL distilled water.c.Apply suction to crucible to draw Celite onto fritted glass aseven mat.9.Filter enzyme mixture from Step 7 through crucible into afiltration flask.10.Wash residue twice with 10 mL distilled water preheated to70°e water to rinse beaker before washing residue incrucible.Save filtrate and water washings for determination ofSDF.T ransfer solution to a pretared 600 mL tall-form beaker.(For SDF determination,go to Step 11 of SDF procedure.)11.Wash residue twice with 10 mL of:a.95% EtOHb.Acetone12.Dry crucible containing residue overnight in 103°C oven.13.Cool crucible in desiccator for approximately 1 hr.Weighcrucible containing dietary fibre residue and Celite to nearest 0.1 mg.T o obtain residue weight,subtract tare weight,i.e.,weight of dried crucible and Celite.14.Protein and ash determination.One residue from each type of fibre is analysed for protein,and the second residue of the duplicate is analysed for ash.a.Perform protein analysis on residue using Kjeldahl method.Use 6.25 factor for all cases to calculate g of protein.b.For ash analysis,incinerate the second residue for 5 hr at525°C.Cool in desiccator and weigh to nearest 0.1 mg.Subtract crucible and Celite weight to determine ash.(SeeNote 3.)B.SOLUBLE DIETARY FIBRE1-10.Follow Steps 1-10 of IDF method.11.Weigh combined solution of filtrate and water washings inpretared beaker from Step 10 of IDF procedure.12.Precipitation of SDFa.Add 4 vols 95% EtOH preheated to 60°e a portion ofEtOH to rinse filtering flask from IDF procedure (Step 10).Alternatively,adjust weight of combined solution of filtrateand water washings to 80 g and add 320 mL of preheated(60°C) 95% EtOH.b.Allow the precipitate to form at room temperature for 60minutes.13.Filtration setupa.T are crucible containing Celite to nearest 0.1 mg.b.Wet and redistribute the bed of Celite in the crucible,using15 mL of 78% EtOH from wash bottle.c.Apply suction to crucible to draw Celite onto fritted glass asan even mat.14.Filtrationa.Filter precipitated enzyme digest from SDF Step 12 throughcrucible.ing a wash bottle with 78% EtOH and a rubber spatula,quantitatively transfer all remaining particles to crucible.15.WashUsing a vacuum,wash residue successively with two 15 mLportions of the following:(See Note 4.)a.78% EtOHb.95% EtOHc.Acetone16.Dry crucible containing residue overnight in 103°C oven.17.Proceed with Steps 13 and 14 of IDF method.The pH of MES-TRIS buffer (i.e.8.2 at 24°C) reaches 6.9-7.2 at85-90°C and 7.4-7.6 at 55-60°C.Note that pH optimum ofheat-stable α-amylase moves from pH 6.0 at 60°C toward pH7.0 at 90°C.b.The volume of thermostable α-amylase used has been reducedfrom 200 µL to 50 µL due to the higher activity of the enzymeemployed here.c.Any ring left around the beaker after heat-stable α-amylaseincubation is scraped,if necessary.With pipettor,10 mL ofwater is added to rinse spatula and side wall of beaker afterheat-stable α-amylase incubation.d.No pH adjustment is needed for protease action,thus noNaOH is added to the incubation mixture.e.For amyloglucosidase action,5 mL of 0.561 N HCl solution isadded.f.For TDF determination,the amount of 95% EtOH added forthe precipitation step is 225 mL instead of 280 mL.ForSDF/IDF determination,weight of filtrate and washing solutionis adjusted to 80 g instead of 100 g.Thus,320 mL of 95% EtOH at 60°C is added.Alternatively,weigh combined solution offiltrate and washing solution and add 4 vols.95% EtOH.T otalfiltration volume is reduced to 375-400 mL with thismodification.(See Figs.1 and 2.)2.It is important to leave beaker in 60°C water bath until it is readyfor pH adjustment,since pH of solution increases at a lowertemperature.Normally,additional pH adjustment (with 5% HCl or 5% NaOH) is not required for most oat,barley,wheat,and corn products.For such known products,one can skip the pH checking procedure after addition of 5 mL of 0.561 N HCl to sample.Routine checking of pH of blank is recommended as a disastercheck.If blank is not within desirable pH range,samples shouldalso be checked.3.There is some indication that delay in washing IDF residues with95% EtOH and acetone may cause inflated IDF values.Thus,it is suggested that IDF residues not be washed toward end ofSDF/IDF procedures.4.In some samples,a gum is formed,trapping liquid.If this occurs,break layer of film with spatula.REFERENCES:1.Lee,S.C.,Prosky,L.and DeVries,J.W.,(1992).Determination oftotal,soluble,and insoluble,dietary fiber in foods - enzymatic-gravimetric method,MES-TRIS buffer:Collaborative study.J.Assoc.Off.Anal.Chem.75:395-416.2.Prosky,L.,Asp.,N.-G.,Schweizer,T.F.,DeVries,J.W.and Furda,I.(1988).Determination of insoluble,soluble,and total dietary fibrein foods and food products.Interlaboratory study.J.Assoc.Off.Anal.Chem.71:1017-1023.3.Prosky,L.,Asp,N.-G.,Schweizer,T.F.,DeVries,J.W.and Furda,I.,(1992).Determination of insoluble and soluble dietary fiber in foods and food products:Collaborative study.J.Assoc.Off.Anal.Chem.75:360-367.Figure 1.Analytical scheme for the total dietary fibre determination procedure.METHOD 2:DETERMINATION OF TOTAL DIETARY FIBRE Based on AACC method 32-05 and AOAC Method 985.29 APPARATUS:1.Dispensersa.280±2.0 mL for 95% ethanol.b.10±0.5 mL for 78% EtOH,95% ethanol,and acetone.c.50±0.5 mL for buffer.2.All other equipment is as described on page 4 of this booklet. REAGENTS:1.Phosphate buffer,0.08 M,pH 6.0.Dissolve 1.400 g Na phosphateanhydrate (Na2HPO4) (or 1.753 g dihydrate) and 9.68 g Naphosphate monobasic monohydrate (NaH2PO4) (or 10.94 gdihydrate) in approximately 700 mL distilled water.Dilute to 1 L with water.Check pH with pH meter.2.Sodium hydroxide solution,0.275 N.Dissolve 11.00 g ACS gradeNaOH in approximately 700 mL distilled water,using appropriate handling precautions,in 1 L volumetric flask.Cool and dilute to volume with water.3.Hydrochloric acid solution,0.325 N.Dilute stock solution ofknown titer (i.e.325 mL of 1.0 N HCl) to 1 L with water involumetric flask.PROCEDURE:Preparation of sampleT otal dietary fibre should be determined on an as-is basis on dried, low-fat or fat-free sample.Homogenise sample and dry overnight in 70°C vacuum oven.Cool in desiccator,reweigh,and record weight loss due to drying.Dry-mill portion of dried sample to 0.3-0.5 mm mesh.If sample cannot be heated,freeze-dry before milling.If high fat content (>10%) prevents proper milling,defat with petroleum ether three times with 25 mL portions (per g of sample) before milling. When analysing mixed diets,always extract fat before determining total dietary fibre.Record weight loss due to fat.Correct final % dietary fibre determination for both moisture and fat removed.Store dry-milled sample in capped jar in desiccator until analysis is run.MethodRun blank through entire procedure along with samples to measure any contribution from reagents to residue.1.Weigh duplicate 1 g samples,accurate to 0.1 mg,into 400 mLtall-form beakers.Sample weights should differ by less than 20mg from each other.Add 50 mL phosphate buffer (pH 6.0) toeach beaker and check pH with pH meter.Adjust if pH does not equal 6.0±0.1.2.Add 50 µL heat-stable α-amylase solution.3.Cover beaker with aluminium foil and place in boiling water bathfor 15 minutes.Shake gently at 5 min intervals.Note:Increase incubation time when number of beakers in bath makes it difficult for beaker contents to reach internaltemperature of 100°e thermometer to indicate that 15 min at 100°C is attained.T otal of 30 min in boiling water bath should be sufficient.4.Cool solutions to room temperature.5.Adjust to pH 7.5±0.1 by adding 10 mL 0.275 N NaOH solution.Check pH with pH meter.6.Add 100 µL of protease solution.7.Cover beaker with aluminium foil and incubate at 60°C withcontinuous agitation for 30 min.8.Cool and add 10 mL 0.325 N HCl solution to adjust pH to4.5±0.2.Check pH with pH meter.9.Add 200 µL amyloglucosidase,cover with aluminium foil,andincubate 20 min at 60°C with continuous agitation.10.Add 280 mL 95% EtOH preheated to 60°C (measure volumebefore heating).Let precipitate form at room temperature for60 min.11.Weigh crucible containing Celite to nearest 0.1 mg,then wet anddistribute bed of Celite in crucible by using stream of 78% EtOH from wash bottle.12.Apply suction to draw Celite onto fritted glass as even mat.Maintain suction and quantitatively transfer precipitate fromenzyme digest to crucible.Important Contaminating Activities:1.In Amyloglucosidase Preparations:An evaluation of amyloglucosidase preparations used in dietary fibre determinations,showed that many preparations (except very high purity and expensive preparations) contain significant levels of contaminating ß-glucanase (cellulase).In some preparations,this contamination was as high as 1% (on an activity basis) and resulted in an underestimation of ß-glucan by as much as 10-15%.Cellulase contamination in amyloglucosidase can best be demonstrated and estimated by viscometric studies using barley ß-glucan as substrate or by using the Megazyme Beta-Glucazyme test tablets (for the measurement of ß-glucanase and cellulase).The effect of cellulase contamination of amyloglucosidase on the viscosity (i.e.molecular size) of ß-glucan is demonstrated in Figure 4. Barley ß-glucan (10 mL,10 mg/mL) in sodium acetate buffer (50 mM, pH 4.5) was incubated with amyloglucosidase (0.2 mL of preparation as used in the TDF assay) at 40°C in a T ype C,U-tube viscometer. Viscosity measurements were taken at various time intervals,and the specific viscosity was calculated as (t-t o)/t o,where t o is the time of flow of the solvent and t is the time of flow of the digest.T wo enzyme preparations were compared:A.Megazyme amyloglucosidase (E-AMGDF) (at the same AMGconcentration as other commercial preparations used for TDFdeterminations).B.Another commercially available amyloglucosidase preparationrecommended for use in dietary fibre determinations.It is evident that Megazyme amyloglucosidase (E-AMGDF) is essentially devoid of contaminating ß-glucanase,whereas the other preparation contains significant levels of this contaminant.2.In Protease Preparations:The degree of contamination of protease with (1,3)(1,4) ß-glucanase can be determined using the procedure described for the measurement of cellulase in amyloglucosidase,with the modification that the assay pH is 7.5 (sodium phosphate buffer,50 mM).3.In Alpha-Amylase Preparations:No contaminating enzyme activities were detectable in thermostable α-amylase.Figure 4.Assay for cellulase contamination in amyloglucosidase preparations usinga viscometric assay with barley ß-glucan as substrate (refer to text).A.Megazyme amyloglucosidase (E-AMGDF);B.Another amyloglucosidase preparation which has been used indietary fibre determinations.WITHOUT GUARANTEEThe information contained in this booklet is,to the best of our knowledge,true and accurate,but since the conditions of use are beyond our control,no warranty is given or is implied in respect of any recommendation or suggestions which may be made or that any use will not infringe any patents.。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
32.2.05AAOAC Official Method996.11Starch(Total)in Cereal ProductsAmyloglucosidase–a-Amylase MethodFirst Action1996AOAC–AACC Method(Applicable to determination of total starch in cereal products.) See Table996.11for the results of the interlaboratory study sup-porting the acceptance of the method.Caution:See Appendix B,safety notes.Glucose oxidase-peroxidase-aminoantipyrine buffer mixture,MOPS,and acetate buffers contain sodium azide.Avoid con-tact with skin and eyes.In case of contact,immediatelyflush contact surfaces with plenty of water.Disposal ofthese reagents into sinks with copper or lead plumbingshould be followed immediately with large quantitiesof water to prevent potential explosive hazards.Dimethyl sulfoxide is a skin irritant and should be usedwith caution.A.PrincipleTest samples are hydrated and starch is hydrolyzed to maltodextrins with thermostableα-amylase at95–100°C.Tempera-ture and pH are adjusted and maltosaccharides are quantitatively hy-drolyzed to glucose with highly purified amyloglucosidase.Glucose is determined with high purity glucose oxidase–peroxidase reagent. Products containing high-amylose starches or resistant starch are pre-treated with dimethyl sulfoxide at95°C before treatment with α-amylase.Starch content is reported on“as is”basis.B.Apparatus(a)Grinding mill.—Centrifugal,with12-tooth rotor and0.5mm sieve,or similar device.Alternatively,cyclone mill can be used for small quantities.(b)Bench centrifuge.—Holding16×120mm glass test tubes, with rating of ca1000×g.(c)Water bath.—Maintaining50±0.1°C.(d)Boiling water bath.—Boiling H2O at95–100°C(e.g.,fryer filled with H2O).(e)Vortex mixer.(f)pH Meter.(g)Stopclock timer(digital).(h)Top-loading balance.(i)Analytical balance.(j)Laboratory oven.—With forced-convection;maintaining 103±1°C;used for determining dry weight of test sample.(k)Spectrophotometer.—Operating at510nm.(l)Pipets.—Delivering100and200µL;with disposable tips.Al-ternatively,motorized hand-held dispenser can be used.(m)Positive displacement pipetter.—With5.0mL tips accu-rately delivering100and200µL;and50mL tips delivering3.0mL. (n)Dispenser.—500mL,to ed for glucose oxidase–peroxidase–aminoantipyrine buffer mixture.(o)Glass test tubes.—16×120mm,17mL,for centrifugation at ca1000×g;and18×150mm.(p)Test tube racks.—48place,holding16×120and 18×150mm tubes.(q)Thermometer.—Reading103±1°C.(r)Filter paper.—Fast,ashless.C.Reagents(a)3-(N-morpholino)propanesulfonic acid(MOPS) buffer.—pH7.0.Contains50mM MOPS,5mM calcium chloride, and0.02%sodium azide.In1L volumetric flask dissolve11.55g MOPS in900mL H2O and adjust pH to7.0with1M HCl(ca17mL). Add0.74g CaCl2.2H2O and0.2g sodium azide.Dilute to volume with H2O.Buffer is stable at room temperature.(b)Thermostable a-amylase solution.—10mL;3000U/mL.Di-lute1mLα-amylase solution(in50%glycerol)to30mL with MOPS buffer,(a).Thermostable a-amylase solution is stable up toTable996.11Interlaboratory study results for determination of total starch in processed cereal products by amyloglucosidase–a-amylase methodSample Mean totalstarch a,%Moisture,%No.of labs s r a s R a RSD r,%RSD R,%r b R cChicken feed pellets44.911.432 1.4 2.1 3.1 4.7 3.9 5.9 White bread60.910.632 1.6 3.0 2.6 4.9 4.58.4 Green pea38.512.431 1.3 1.9 3.4 4.9 3.6 5.3 High amylose maize starch74.813.426 2.2 3.6 2.9 4.8 6.2White wheat flour68.012.831 2.0 2.9 2.9 4.3 5.68.1 Wheat starch85.312.226 2.8 3.3 3.3 3.97.89.2 Oat bran38.58.831 1.5 1.9 3.9 4.9 4.2 5.3 Spaghetti67.511.831 2.6 3.2 3.9 4.77.39.0 High amylose-maize starch(DMSO method)84.213.431 1.8 2.4 2.1 2.9 5.0 6.7 Wheat starch(DMSO method)84.712.231 2.6 3.9 3.1 4.67.310.9a Calculated on“as is”basis.b r=2.8×sr.c R=2.8×sR.3years when frozen.(Note:One unit[U]ofα-amylase activity is amount of enzyme required to release1µmole p-nitrophenol from “end-blocked”p-nitrophenyl maltoheptaoside in presence of satu-rating levels ofα-glucosidase and amyloglucosidase[i.e.,Ceralpha α-amylase assay reagent]at40°C and pH6.0.)Thermostableα-am-ylase solution should be free of detectable levels of free glucose.(c)Amyloglucosidase solution.—10mL;200U/e directly without dilution.Solution is viscous;for dispensing,use positive displacement dispenser.Amyloglucosidase solution is stable up to 3years at4°C.(Note:One unit[U]of enzyme activity is amount of enzyme required to release1µmole p-nitrophenol from p-nitrophenylβ-maltoside in the presence of saturating levels of β-glucosidase[i.e.,amyloglucosidase assay reagent]at40°C and pH4.5.)Amyloglucosidase solution should be free of detectable levels of free glucose.(d)Glucose oxidase–peroxidase–aminoantipyrine buffer mix-ture.—Mixture of glucose oxidase,12000U/L;peroxidase, 650U/L;and4-aminoantipyrine,0.4mM.Prepare buffer concentrate by dissolving13.6g KH2PO4,4.2g NaOH,and3.0g4-hydroxybenzoic acid in90mL distilled H2O.Ad-just to pH7.4with either2M HCl(16.7mL HCl/100mL)or 2M NaOH(8.0g NaOH/100mL).Dilute solution to100mL,add 0.4g sodium azide,and mix until dissolved.Buffer concentrate is stable up to3years at4°C.To prepare glucose oxidase–peroxidase–aminoantipyrine buffer mixture,dilute50mL buffer concentrate e part of diluted buffer to dissolve the entire contents of vial containing freeze-dried glucose oxidase–peroxidase mixture.Transfer contents of vial to1L volumetric flask containing diluted buffer.Reagent is stable 2–3months at4°C and2–3years at–20°C.Color formed with glu-cose is stable several hours.(Note:Glucose oxidase must not be con-taminated withβ-and/orα-glucosidase and chromogen color complex must be stable at least60min.)C h e c k c o l o r f o r m a t i o n a n d s t a b i l i t y o f g l u c o s e oxidase–peroxidase–aminoantipyrine buffer mixture by incubating (in duplicate)3.0mL glucose oxidase-peroxidase-aminoantipyrine buffer mixture with glucose standard(100µg dried crystalline glu-cose in0.2mL0.2%sodium benzoate solution).After15,20,30, and60min incubation,read absorbance,A,of solution at510nm. Maximum color formation should be achieved within20min,and color should be stable at least60min at50°C.(e)Aqueous ethanol.—About80%(v/v).Dilute80mL95%etha-nol(laboratory grade)to95mL with H2O.(f)Sodium acetate buffer.—(1)200mM,pH4.5.—Pipet11.8mL glacial acetic acid(1.05g/mL)to900mL H2O.Adjust pH to4.5with 1M NaOH solution(ca60mL is required).Add0.2g sodium azide and dilute to1L with H2O.(Caution:Sodium azide should not be added until pH is adjusted.Acidification of sodium azide releases poisonous hydrazoic acid.)Buffer is stable12months at room tem-perature.(2)50mM,pH4.5.—Dilute200mM acetate buffer1+3 with H2O.(g)Dimethyl sulfoxide(DMSO).—Laboratory grade.(h)Glucose standard stock solution.—1mg/mL.Before prepar-ing solution,dry powdered crystalline glucose(purity≥97%)16h at 60°C under vacuum.Dissolve0.1g dried glucose,weighed to near-est mg,in100mL distilled water.(i)Corn starch.—Containing known content of starch(e.g.,ca 98%dry weight).Items(a)–(c),(h),and(i)are supplied in Total Starch Assay kit available from Megazyme International Ireland Ltd,Bray Business Park,Bray,Co.Wicklow,Ireland.D.Preparation of Test Samples,Standards,and Reagent Blank (a)Test sample.—Grind ca50g laboratory sample in grinding mill to pass0.5mm sieve.Transfer all material into wide-mouthed plastic jar and mix well by shaking and inversion.(b)D-Glucose standard working solutions.—50and100µg.Add 50and100µL D-glucose standard stock solution,C(h),to separate test tubes,and adjust volume in each tube to100µL with H2O.Pre-pare solutions immediately before use.(c)Reagent blank.—Transfer0.1mL H2O into test tubes and pro-ceed with total starch determination using standard assay procedure starting from step E(a)(7).E.Determination of Total Starch(a)Standard procedureα-amylase/amyloglucosidase (AA/AMG).—Run D-glucose working standard solutions(in qua-druplicate),reagent blank(in duplicate),and corn starch with each set of e reagent blank to zero spectrophotometer.(1)Ac-curately weigh90–100mg ground test portion directly into glass test tube.Tap tube gently on laboratory bench to ensure that all par-ticles drop to bottom of tube.(Note:When analyzing cereal prod-ucts containing high levels of glucose[processed cereal products {e.g.,breakfast cereals}and all products of unknown or uncertain composition{i.e.,products containing free glucose or maltodextrins}],pre-extract90–100mg of weighed,ground test sample2×with10mL80%aqueous ethanol,C(e),at ca80°C over 10min/extraction.Centrifuge slurry at1000×g and discard e sediment for analysis.)(2)Add0.2mL80% aqueous ethanol to tube and stir on Vortex mixer to ensure that test portion is wet.Add3.0mL thermostableα-amylase,C(b),and mix contents of tube on Vortex mixer to ensure complete dispersion.(3)Immediately place tube in boiling water bath for2min,remove from water bath,and mix vigorously on Vortex mixer.Return tube to boiling water bath for additional3min and then mix contents vigorously on Vortex mixer.(Note:Some solids will adhere to side of test tube;however,this will not affect analysis since tube con-tents are treated with enzyme in this step.)(4)Place tubes in water bath set at50°C and let equilibrate5min.Add4.0mL200mM so-dium acetate buffer,C(f)(1),and0.1mL amyloglucosidase solu-tion,C(c),and vigorously mix contents on Vortex mixer.Cap tube with marble and incubate30min at50°C.(5)Quantitatively trans-fer the entire contents of test tube to100mL volumetric e water wash bottle to rinse tube contents thoroughly.Dilute to100 mL with H2O.(Note:If product contains<10%starch,adjust vol-ume to10.0mL[instead of100mL].Make appropriate adjustments to calculations.)Thoroughly mix contents of flask.Centrifuge aliquot of suspension10min at1000×g.Alternatively,filter aliquot through filter paper.(6)Carefully and accurately transfer0.1 mL aliquot of each supernatant(or filtrate)to bottoms of separate test tubes;use2tubes/supernatant(or filtrate).(7)Add3.0mL glu-cose oxidase–peroxidase–aminoantipyrine buffer mixture,C(d),to each tube(reaction solutions from test portion and corn starch,re-agent blank,and D-glucose standard working solutions),and incu-bate20min at50°C.(8)Measure and record absorbance,A,of each test solution at510nm against reagent blank.Average A values for each test and use in Calculations,G.(b)Modified procedure(DMSO/AA/AMG).—For products con-taining enzyme-resistant starch.(1)Accurately weigh90–100mg ground test portions directly into glass test tube.Tap tube gently on laboratory bench to ensure that all particles drop to bottom of tube. (Note:When analyzing cereal products containing high levels of glucose,pre-extract90–100mg weighed,ground test sample2×with10mL aqueous ethanol,C(e),at ca80°C over10min/extrac-tion.Centrifuge slurry at1000×g and discard e sedi-ment for analysis.)(2)Add0.2mL80%aqueous ethanol to tube and stir on Vortex mixer to ensure that test portion is wet.(3)Immedi-ately add2mL DMSO solution,C(g),and stir tube on Vortex mixer. Place tube in vigorously boiling water bath and remove after5min. Add3.0mL thermostableα-amylase solution,C(b),and mix con-tents on Vortex mixer to ensure complete dispersion.(4)Immedi-ately proceed according to standard procedure(AA/AMG)starting from step E(a)(3).F.Determination of Enzyme-Resistant Starch(Optional) (Note:This part of method was not validated in collaborative study.) Level of enzyme-resistant starch in products depends on nature of original starch(e.g.,high amylose)and on processing conditions. This may vary from0.1–30%total starch in product.Determine level of enzyme resistant starch as follows:(1)Analyze product according to standard procedure, E(a)(1)–(4).(2)Centrifuge reaction solution10min at1000×g and carefully pour supernatant into100mL volumetric flask.(3)Resuspend pellet in5mL50mM sodium acetate buffer, C(f)(2),by vigorous stirring on Vortex mixer.Add additional5mL 50mM sodium acetate buffer,mix,and centrifuge10min at 1000×g.(4)Combine supernatant with that from step(2),and dilute to volume.Analyze for starch starting with step E(a)(6).(5)To pellet from(3)add2mL DMSO and analyze for starch by modified procedure E(b)starting from step E(b)(3)[proceed to pro-cedure E(a)as stated].(6)At step E(a)(5),adjust volume to10mL(instead of100mL).(7)Proceed with standard procedure,E(a),starting from step E(a)(6).Make appropriate adjustments to calculations.G.CalculationsCalculate total starch content(percent,on as is basis)in test sam-ple as follows:Total starch,%=A×F×1000×11000×100W×162180=A×FW×90where A=absorbance of reaction solutions read against reagent blank;F=factor to convert absorbance values toµg glucose= 100µg glucose/absorbance value for100µg glucose;1000=vol-ume correction,i.e.,0.1mL taken from100mL;1/1000=conver-sion fromµg to mg;100/W=conversion to100mg test portion; 162/180=factor to convert from free glucose,as determined,to anhydroglucose,as occurs in starch.Reference:J.AOAC Int.80,571(1997).Revised:March1998。
