Belbin Interpretation sheet (new)
贝尔蒙报告

贝尔蒙报告贝尔蒙报告: 保护参加科研的人体实验对象的道德原则和方针(The Belmont Report, Ethical Principles and Guidelines for the Protection of Human Subjects of Research.,1979)保护参加科研的人体实验对象的道德原则和方针保护参加生物医学和行为学研究人体实验对象全国委员会的报告目录I. 概要II. 委员会成员III. Belmont 报告: 引言A.行医和科研之间的分界线B.基本道德原则1.尊重个人2.善行3.平等公正C.应用1.知情同意2.对风险及益处的评估3. 人体实验对象的选择概要1974年7月12日,国家科研法案(公共法则93348)立法,由此成立了保护参加生物医学和行为学研究人体实验对象的全国委员会。
委员会的主要任务之一就是为涉及人体实验对象的生物医学和行为学研究确定基本的道德原则,制定方针以监督有关科研按这些原则进行。
在执行以上任务的同时,委员会还需考虑:(i) 生物医学和行为学研究与所认可的常规行医之间的分界,(ii) 对危险及利益标准的评估在决定涉及人体实验对象科研的适当性中所起的作用, (iii) 选择参与科研的人体实验对象的准则,以及 (iv) 各种情况下知情同意的性质和定义。
Belmont报告总结了委员会审议后确定的基本道德原则。
它是1976年2月在Smithsonian机构Belmont会议中心举行的4天会议的产物以及4年来委员会每月审议的结果。
它是对基本的道德原则及方针的陈述,应用来帮助解决涉及人体实验对象的科研中所产生的道德问题。
通过由联邦注册出版以及应需提供单版本,部长意在让科学家、单位评审会成员和政府雇员容易得到这份报告。
两册附录包括协助委员会完成这一任务的专家写的长篇报告,由政府印刷办公室文件主管处出售(华盛顿,哥伦比亚特区,20402),出版号为DHEW No. (OS) 780013及78-0014.Belmont报告与委员会的其他报告不同,它没有向卫生,教育,福利部长推荐具体的行政措施。
Sun Professional All In 1 Tablets 安全数据表单说明书

Sun Professional All In 1 TabletsRevision: 2022-01-23Version:07.2Safety Data SheetAccording to Regulation (EC) No 1907/2006SECTION 1: Identification of the substance/mixture and of the company/undertaking1.1 Product identifierTrade name: Sun Professional All In 1 TabletsSun is a registered trade mark and is used under licence of Unilever UFI: PT26-S0D0-4005-92VN1.2 Relevant identified uses of the substance or mixture and uses advised againstSWED - Sector-specific worker exposure description :AISE_SWED_PW_8a_2AISE_SWED_PW_8b_2PC35-Washing and cleaning products AISE_SWED_PW_1_1AISE_SWED_PW_4_1PC35-Washing and cleaning products1.3 Details of the supplier of the safety data sheetDiversey Europe Operations BV, Maarssenbroeksedijk 2, 3542DN Utrecht, The NetherlandsContact details Diversey LtdWeston Favell Centre, Northampton NN3 8PD, United Kingdom Tel: 01604 405311, Fax: 01604 4068091.4 Emergency telephone numberSeek medical advice (show the label or safety data sheet where possible)For medical or environmental emergency only: call 0800 052 0185SECTION 2: Hazards identification2.1 Classification of the substance or mixture Eye Irrit. 2 (H319) 2.2 Label elementsSignal word: Warning. Contains subtilisin (Subtilisin)Hazard statements:H319 - Causes serious eye irritation.EUH208 - May produce an allergic reaction.Precautionary statements:P102 - Keep out of reach of children.P305 + P351 + P338 - IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing.P337 + P313 - If eye irritation persists: Get medical advice or attention.Product use:Dish wash product. Uses advised against:Uses other than those identified are not recommended.2.3 Other hazardsNo other hazards known.SECTION 3: Composition/information on ingredients3.2 MixturesSpecific concentration limitssodium percarbonate:• Eye Dam. 1 (H318) >= 25% > Eye Irrit. 2 (H319) >= 7.5%Workplace exposure limit(s), if available, are listed in subsection 8.1.ATE, if available, are listed in section 11.For the full text of the H and EUH phrases mentioned in this Section, see Section 16..SECTION 4: First aid measures4.1 Description of first aid measuresInhalation: Get medical attention or advice if you feel unwell.Skin contact: Wash skin with plenty of lukewarm, gently flowing water. If skin irritation occurs: Get medical adviceor attention.Eye contact: Hold eyelids apart and flush eyes with plenty of lukewarm water for at least 15 minutes. Removecontact lenses, if present and easy to do. Continue rinsing. If irritation occurs and persists, getmedical attention.Ingestion: Rinse mouth. Immediately drink 1 glass of water. Never give anything by mouth to an unconsciousperson. Get medical attention or advice if you feel unwell.Self-protection of first aider: Consider personal protective equipment as indicated in subsection 8.2.4.2 Most important symptoms and effects, both acute and delayedInhalation: No known effects or symptoms in normal use.Skin contact: No known effects or symptoms in normal use.Eye contact: Causes severe irritation.Ingestion: No known effects or symptoms in normal use.4.3 Indication of any immediate medical attention and special treatment neededNo information available on clinical testing and medical monitoring. Specific toxicological information on substances, if available, can be found in section 11.SECTION 5: Firefighting measures5.1 Extinguishing mediaCarbon dioxide. Dry powder. Water spray jet. Fight larger fires with water spray jet or alcohol-resistant foam.5.2 Special hazards arising from the substance or mixtureNo special hazards known.5.3 Advice for firefightersAs in any fire, wear self contained breathing apparatus and suitable protective clothing including gloves and eye/face protection. SECTION 6: Accidental release measures6.1 Personal precautions, protective equipment and emergency proceduresNo special measures required.6.2 Environmental precautionsDo not allow to enter drainage system, surface or ground water.6.3 Methods and material for containment and cleaning upCollect mechanically. Do not place spilled materials back into the original container. Collect in closed and suitable containers for disposal.6.4 Reference to other sectionsFor personal protective equipment see subsection 8.2. For disposal considerations see section 13.SECTION 7: Handling and storage7.1 Precautions for safe handlingMeasures to prevent fire and explosions:No special precautions required.Measures required to protect the environment:For environmental exposure controls see subsection 8.2.Advices on general occupational hygiene:Follow general hygiene considerations recognised as common good workplace practices. Keep away from food, drink and animal feeding stuffs. Keep out of reach of children. Do not mix with other products unless adviced by Diversey. Wash face, hands and any exposed skin thoroughly after handling. Avoid contact with eyes. Use only with adequate ventilation. See chapter 8.2, Exposure controls / Personal protection.7.2 Conditions for safe storage, including any incompatibilitiesStore in accordance with local and national regulations. Store in a closed container. Keep only in original packaging. Keep out of reach of children.For conditions to avoid see subsection 10.4. For incompatible materials see subsection 10.5.7.3 Specific end use(s)No specific advice for end use available.SECTION 8: Exposure controls/personal protection8.1 Control parametersWorkplace exposure limitsAir limit values, if available:Biological limit values, if available:Recommended monitoring procedures, if available:Additional exposure limits under the conditions of use, if available:DNEL/DMEL and PNEC valuesHuman exposureDNEL/DMEL oral exposure - Consumer (mg/kg bw)DNEL/DMEL dermal exposure - WorkerDNEL/DMEL dermal exposure - ConsumerDNEL/DMEL inhalatory exposure - Worker (mg/m3)DNEL/DMEL inhalatory exposure - Consumer (mg/m3)Environmental exposureEnvironmental exposure - PNECEnvironmental exposure - PNEC, continued8.2 Exposure controlsThe following information applies for the uses indicated in subsection 1.2 of the Safety Data Sheet.If available, please refer to the product information sheet for application and handling instructions.Normal use conditions are assumed for this section.Recommended safety measures for handling the undiluted product:Appropriate engineering controls: No special requirements under normal use conditions.Appropriate organisational controls: Avoid direct contact and/or splashes where possible. Train personnel.REACH use scenarios considered for the undiluted product:SWED - Sector-specific worker exposuredescription LCS PROC Duration(min)ERCPC35-Washing and cleaning products PC35-Washing andcleaning productsC - - ERC8a Manual transfer and dilution AISE_SWED_PW_8a_2 PW PROC 8a 60 ERC8a Manual transfer and dilution AISE_SWED_PW_8b_2 PW PROC 8b 60 ERC8bPersonal protective equipmentEye / face protection: No special requirements under normal use conditions.Hand protection: No special requirements under normal use conditions.Body protection: No special requirements under normal use conditions.Respiratory protection: If exposure to dust cannot be avoided use: full-face mask (EN 136) with filter type HEPA (N100,Class H14) (EN 1822) or self-contained or compressed air breathing apparatus (EN 137 / EN 138)Consider specific local use conditions. In consultation with the supplier of respiratory protectionequipment a different type providing similar protection may be chosen.Environmental exposure controls: No special requirements under normal use conditions.Recommended safety measures for handling the diluted product:Recommended maximum concentration (% w/w): 0.04Appropriate engineering controls: No special requirements under normal use conditions.Appropriate organisational controls: No special requirements under normal use conditions.REACH use scenarios considered for the diluted product:SWED LCS PROC Duration(min)ERCPC35-Washing and cleaning products PC35-Washing andcleaning productsC - - ERC8a Automatic application in a dedicated closed system AISE_SWED_PW_1_1 PW PROC 1 480 ERC8a Automatic application in a dedicated system AISE_SWED_PW_4_1 PW PROC 4 480 ERC8a Personal protective equipmentEye / face protection: No special requirements under normal use conditions.Hand protection: No special requirements under normal use conditions.Body protection: No special requirements under normal use conditions.Respiratory protection: No special requirements under normal use conditions.Environmental exposure controls: No special requirements under normal use conditions.SECTION 9: Physical and chemical properties9.1 Information on basic physical and chemical propertiesInformation in this section refers to the product, unless it is specifically stated that substance data is listedPhysical state: SolidAppearance: TabletsColour: Speckles , WhiteOdour: Product specificOdour threshold: Not applicableSubstance data, boiling pointFlammability (liquid): Not applicable.( UN Manual of Tests and Criteria, section 32, L.2 )Substance data, flammability or explosive limits, if available:Decomposition temperature: Not applicable.Solubility in / Miscibility with Water: SolubleSubstance data, solubility in waterSubstance data, partition coefficient n-octanol/water (log Kow): see subsection 12.3 Method / remarkMelting point/freezing point (°C): Not determined Not relevant to classification of this product Initial boiling point and boiling range (°C): Not determined Not applicable to solids or gasesMethod / remarkFlammability (solid, gas): Not determinedFlash point (°C): Not applicable.Sustained combustion: Not applicable.Lower and upper explosion limit/flammability limit (%): Not determined See substance dataMethod / remarkAutoignition temperature: Not determinedpH: Not applicableDilution pH: ≈ 11 (0.04 %) ISO 4316Kinematic viscosity: Not determined Not applicable to solids or gasesSubstance data, vapour pressure9.2 Other information9.2.1 Information with regard to physical hazard classes9.2.2 Other safety characteristicsNo other relevant information available.SECTION 10: Stability and reactivity10.1 ReactivityNo reactivity hazards known under normal storage and use conditions. 10.2 Chemical stabilityStable under normal storage and use conditions.10.3 Possibility of hazardous reactionsNo hazardous reactions known under normal storage and use conditions.10.4 Conditions to avoidNone known under normal storage and use conditions.10.5 Incompatible materialsNone known under normal use conditions.10.6 Hazardous decomposition productsNone known under normal storage and use conditions.SECTION 11: Toxicological information11.1 Information on toxicological effectsMixture data:.Relevant calculated ATE(s):ATE - Oral (mg/kg): >2000Substance data, where relevant and available, are listed below:.Acute toxicityAcute oral toxicityAcute dermal toxicityAcute inhalative toxicityAcute inhalative toxicity, continuedIrritation and corrosivitySkin irritation and corrosivityEye irritation and corrosivityRespiratory tract irritation and corrosivity Method / remarkVapour pressure: Not determined See substance dataMethod / remarkRelative density: ≈ 0.93 (20 °C) OECD 109 (EU A.3)Relative vapour density: No data available. Not applicable to solidsParticle characteristics: Not determined. Not relevant to classification of this product. Explosive properties: Not explosive.Oxidising properties: Not oxidising.Corrosion to metals: Not determined Not applicable to solids or gasesSensitisationSensitisation by skin contactSensitisation by inhalationCMR effects (carcinogenicity, mutagenicity and toxicity for reproduction) MutagenicityCarcinogenicityToxicity for reproductionRepeated dose toxicitySub-acute or sub-chronic oral toxicitySub-chronic dermal toxicitySub-chronic inhalation toxicityChronic toxicitySTOT-single exposureSTOT-repeated exposureAspiration hazardSubstances with an aspiration hazard (H304), if any, are listed in section 3.Potential adverse health effects and symptomsEffects and symptoms related to the product, if any, are listed in subsection 4.2.11.2 Information on other hazards11.2.1 Endocrine disrupting propertiesEndocrine disrupting properties - Human data, if available:11.2.2 Other informationNo other relevant information available.SECTION 12: Ecological information12.1 ToxicityNo data is available on the mixture.Substance data, where relevant and available, are listed below:Aquatic short-term toxicityAquatic short-term toxicity - fishAquatic short-term toxicity - crustaceaAquatic short-term toxicity - algaeAquatic short-term toxicity - marine speciesImpact on sewage plants - toxicity to bacteriaAquatic long-term toxicityAquatic long-term toxicity - fishAquatic long-term toxicity - crustaceaAquatic toxicity to other aquatic benthic organisms, including sediment-dwelling organisms, if available:Terrestrial toxicityTerrestrial toxicity - soil invertebrates, including earthworms, if available:Terrestrial toxicity - plants, if available:Terrestrial toxicity - birds, if available:Terrestrial toxicity - beneficial insects, if available:Terrestrial toxicity - soil bacteria, if available:12.2 Persistence and degradabilityAbiotic degradationAbiotic degradation - photodegradation in air, if available:Abiotic degradation - hydrolysis, if available:Abiotic degradation - other processes, if available:BiodegradationReady biodegradability - aerobic conditionsReady biodegradability - anaerobic and marine conditions, if available:Degradation in relevant environmental compartments, if available:12.3 Bioaccumulative potentialPartition coefficient n-octanol/water (log Kow)Bioconcentration factor (BCF)12.4 Mobility in soilAdsorption/Desorption to soil or sediment12.5 Results of PBT and vPvB assessmentSubstances that fulfill the criteria for PBT/vPvB, if any, are listed in section 3.12.6 Endocrine disrupting propertiesEndocrine disrupting properties - Environmental effects, if available:12.7 Other adverse effectsNo other adverse effects known.SECTION 13: Disposal considerations13.1 Waste treatment methodsWaste from residues / unused products: The concentrated contents or contaminated packaging should be disposed of by a certified handler or according to the site permit. Release of waste to sewers is discouraged. The cleaned packaging material is suitable for energy recovery or recycling in line with local legislation.European Waste Catalogue: 20 01 29* - detergents containing dangerous substances. SECTION 14: Transport informationLand transport (ADR/RID), Sea transport (IMDG), Air transport (ICAO-TI / IATA-DGR)14.1 UN number: Non-dangerous goods14.2 UN proper shipping name: Non-dangerous goods14.3 Transport hazard class(es): Non-dangerous goods14.4 Packing group: Non-dangerous goods14.5 Environmental hazards: Non-dangerous goods14.6 Special precautions for user: Non-dangerous goods14.7 Transport in bulk according to Annex II of MARPOL and the IBC Code: Non-dangerous goodsSECTION 15: Regulatory information15.1 Safety, health and environmental regulations/legislation specific for the substance or mixtureNational regulations :• Regulation (EC) 1907/2006 - REACH (UK amended)• Regulation (EC) 1272/2008 - CLP (UK amended)• Regulation (EC) 648/2004 - Detergents regulation (UK amended)• Delegated Regulation (EU) 2017/2100 and Regulation (EU) 2018/605 (UK amended)• Agreement concerning the International Carriage of Dangerous Goods by Road (ADR)• International Maritime Dangerous Goods (IMDG) CodeAuthorisations or restrictions (Regulation (EC) No 1907/2006, Title VII respectively Title VIII): Not applicable.Ingredients according to Detergents Regulationoxygen-based bleaching agents 5 - 15 %phosphonates, polycarboxylates, non-ionic surfactants < 5 %perfumes , enzymesComah - classification: Not classified15.2 Chemical safety assessmentA chemical safety assessment has not been carried out on the mixtureSECTION 16: Other informationThe information in this document is based on our best present knowledge. However, it does not constitute a guarantee for any specific product features and does not establish a legally binding contractSDS code: MSDS6574 Version: 07.2 Revision: 2022-01-23 Reason for revision:Overall design adjusted in accordance with Amendment 2020/878, Annex II of Regulation (EC) No 1907/2006, This data sheet contains changes from the previous version in section(s):, 3, 8, 9, 11, 12, 16Classification procedureThe classification of the mixture is in general based on calculation methods using substance data, as required by Regulation (EC) No1272/2008. If for certain classifications data on the mixture is available or for example bridging principles or weight of evidence can be used for classification, this will be indicated in the relevant sections of the Safety Data Sheet. See section 9 for physical chemical properties, section 11 for toxicological information and section 12 for ecological information.Full text of the H and EUH phrases mentioned in section 3:• H272 - May intensify fire; oxidiser.• H302 - Harmful if swallowed.• H315 - Causes skin irritation.• H318 - Causes serious eye damage.• H319 - Causes serious eye irritation.• H334 - May cause allergy or asthma symptoms or breathing difficulties if inhaled.• H335 - May cause respiratory irritation.• H400 - Very toxic to aquatic life.• H411 - Toxic to aquatic life with long lasting effects.• H412 - Harmful to aquatic life with long lasting effects.Abbreviations and acronyms:• AISE - The international Association for Soaps, Detergents and Maintenance Products• ATE - Acute Toxicity Estimate• DNEL - Derived No Effect Limit• EC50 - effective concentration, 50%• ERC - Environmental release categories• EUH - CLP Specific hazard statement• LC50 - Lethal Concentration, 50% / Median Lethal Concentration• LCS - Life cycle stage• LD50 - Lethal Dose, 50% / Median Lethal dose• NOAEL - No observed adverse effect level• NOEL - No observed effect level• OECD - Organization for Economic Cooperation and Development• PBT - Persistent, Bioaccumulative and Toxic• PNEC - Predicted No Effect Concentration• PROC - Process categories• REACH number - REACH registration number, without supplier specific part• vPvB - very Persistent and very BioaccumulativeEnd of Safety Data Sheet。
cep电子版纸版递交指南中英文cep_guidance_for_cep_applications

201306 PA/PH/CEP(09)108 2R CEP电子提交和纸质提交指南(中英文)官网原文下载:.edqm.eu/en/Guidance-for-electronic-and-paper-submissions-1585.html?mbID=107新版本生效日期:2013年8月1日Certification of Substances DivisionFK/CBPUBLIC DOCUMENT公开文件(LEVEL 1)第一层次English only/Anglais seulement仅英文版PA/PH/CEP(09)108, 2RStrasbourg, June 2013 Certification of suitability to Monographs of the European PharmacopoeiaGuidance for electronic and paper submissionsFor Certificates of Suitability (CEP) applicationsCEP电子和纸质申报指南Date of implementation: 1 August 2013Address: 7, sllee Kastner, CS 30026F-67081 Strasbourg (France)Telephone: 33(0)3 88 41 30 30 – Fax: 33 (0) 3 88 41 27 71 – :cepedqm.euInternet:.edqm.euTable of content目录1Introduction介绍2Scope and general requirements围和一般要求3Electronic submission formats电子申报格式3.1eCTD submission format eCTD申报格式3.2NeeS submission format NeeS申报格式3.3PDF format PDF格式4Submission of paper dossiers纸质文件提交5Content and structure of an application申报的容和结构6Lifecycle management of applications申报的生命周期管理6.1Granularity and updated sections章节分类和更新部分6.2When to submit a baseline Module 3?什么时候提交基准模块37Validation by the EDQM EDQM验证8Routes (or pathways) of submission递交方式(途径)9Security安全性1Introduction介绍This document is guidance for electronic and paper submissions for Certificates of Suitability (CEPs) applications. Information and requirements described in this document are intended to facilitate the handling and assessment of submissions for certificates of suitability (CEPs) and to maintain their lifecycle even if the submission is not an eCTD.本文件意在指导CEP的电子和纸质申报。
新西兰1027表格下载

新西兰1027表格下载篇一:新西兰签证新西兰签证仅适用于旅游目的访问签证所需材料1. 申请表:访问申请表-INZ1188,仅适用于中国公民,并且作为旅游人仕在新西兰逗留时间少于6个月2. 补充申请表:① 仅适用于中国公民以及在中国出生的申请人的访问、学生及工作申请(INZ1027)② 補充表適用於香港居民的訪問、學生及工作申請(INZ 1220) 僅香港特別行政區和香港英國國民海外護照持有人及香港簽證身份書持有人3. 有效护照(原件):申请中包括的所有申请人的护照个人资料页复印件4. 申请人近期护照照片2 张5. 申请人户口簿(仅适用于中国公民)① 中国身份证副本(仅中国公民)② 香港/澳门身份证复印件(仅适用于香港和澳门居民)6. 如配偶或未成年子女随行,请递交关系证明,例如户口簿,结婚证书,子女出生证(显示父母名字)。
7. 如有未成年子女随行,但您的配偶不随行,必须递交不随行的父母一方的书面同意函,表示同意该子女随您出行。
8. 财务状况的材料:需提供资金证明,表明有能力负担在新西兰访问期间的费用及返程机票。
① 银行存折,显示存款历史② 最近6个月的银行对账单或工资单③ 信用卡对账单,显示信用额度④ 其他资金证明9.工作证明:若有工作,须提供雇主证明信,须包含以下信息:① 申请人的职位及收入② 工作年限,准假证明。
③ 雇主证明及有效10.如为私营业主,需提供以下材料:① 营业执照以及验资报告② 情况说明一份,说明业务范围及员工人数③ 近期企业收入所得税税单④ 近期企业银行对账单,显示每日交易内容11.如退休,需提供退休证。
注:8.9.10.三点中的可选项只要其中一项即可; Candice深蓝篇二:新西兰送检材料列表CHINA UNIONPAY PLATINUM AND DIAMOND CREDIT CARDHOLDER VISITOR VISA LICANTS持有中国银联白金信用卡、钻石信用卡的访问签证申请人需要提供的文件Immigration New Zealand reserves the right to request additional information in the course of assessing an application and to retain information and documents on file.新西兰移民局有权在审理申请过程中要求申请人提交补充材料,并将申请人提交的信息及材料存档保留。
指纹图谱 外文

Journal of Ethnopharmacology 140 (2012) 482–491Contents lists available at SciVerse ScienceDirectJournal ofEthnopharmacologyj o u r n a l h o m e p a g e :w w w.e l s e v i e r.c o m /l o c a t e /j e t h p h a rmThe potential of metabolic fingerprinting as a tool for the modernisation of TCM preparationsHelen Sheridan a ,Liselotte Krenn b ,Renwang Jiang c ,Ian Sutherland d ,Svetlana Ignatova d ,Andreas Marmann e ,Xinmiao Liang f ,Jandirk Sendker g ,∗aTrinity College,Dublin,School of Pharmacy and Pharmaceutical Sciences,East End Development 4/5,Dublin 2,Ireland bUniversity of Vienna,Department of Pharmacognosy,Althanstrasse 14,A-1090Vienna,Austria cGuangdong Province Key Laboratory of Pharmacodynamic Constituents of Traditional Chinese Medicine and New Drugs Research,College of Pharmacy,Jinan University,Guangzhou 510632,People’s Republic of China dBrunel University,Brunel Institute for Bioengineering,Uxbridge,Middlesex UB83PH,United Kingdom eUniversity of Düsseldorf,Institute of Pharmaceutical Biology and Biotechnology,Universitätsstr.1,40225Düsseldorf,Germany fChinese Academy of Sciences,Dalian Institute of Chemical Physics,Bio-technology Department,457Zhongshan Road,Dalian 116023,People’s Republic of China gUniversity of Münster,Institute of Pharmaceutical Biology and Phytochemistry,Hittorfstrasse 56,48149Münster,Germanya r t i c l ei n f oArticle history:Received 31October 2011Received in revised form 30January 2012Accepted 31January 2012Available online 7 February 2012Keywords:Chinese herbal medicines ExtractionCompound analysis MetabolomicsFingerprint analysisa b s t r a c tA vast majority Chinese herbal medicines (CHM)are traditionally administered as individually prepared water decoctions (tang )which are rather complicated in practice and their dry extracts show techno-logical problems that hamper straight production of more convenient application forms.Modernised extraction procedures may overcome these difficulties but there is lack of clinical evidence supporting their therapeutic equivalence to traditional decoctions and their quality can often not solely be attributed to the single marker compounds that are usually used for chemical extract optimisation.As demonstrated by the example of the rather simple traditional TCM formula Danggui Buxue Tang,both the chemical com-position and the biological activity of extracts resulting from traditional water decoction are influenced by details of the extraction procedure and especially involve pharmacokinetic synergism based on co-extraction.Hence,a more detailed knowledge about the traditional extracts’chemical profiles and their impact on biological activity is desirable in order to allow the development of modernised extracts that factually contain the whole range of compounds relevant for the efficacy of the traditional application.We propose that these compounds can be identified by metabolomics based on comprehensive finger-print analysis of different extracts with known biological activity.TCM offers a huge variety of traditional products of the same botanical origin but with distinct therapeutic properties,like differentially pro-cessed drugs and special daodi qualities.Through this variety,TCM gives an ideal field for the application of metabolomic techniques aiming at the identification of active constituents.© 2012 Elsevier Ireland Ltd. All rights reserved.1.IntroductionThere is an increasing interest in the use and application of CHM throughout the Western hemisphere.Notwithstanding the intake of powdered herbal drugs in different delivery forms,the typical preparation of CHM involves some kind of extraction of herbal drug material.Traditionally,the most important delivery form is theAbbreviations:AR,Astragali Radix;ASR,Angelicae sinensis Radix;CHM,Chi-nese herbal medicine;CMM,Chinese Materia Medica;DBT,Danggui Buxue Tang;FP,fingerprint analysis;PCA,principal component analysis;PLS-DA,partial least square discriminant analysis;SCO,supercritical carbon dioxide;SFE,Supercritical Fluid Extraction;TCM,traditional Chinese medicine.∗Corresponding author.Tel.:+492518333379;fax:+492528338341.E-mail address:Jandirk.sendker@uni-muenster.de (J.Sendker).water decoction of a mixture containing typically 2–12different herbal materials (Yi and Chang,2004).It should be explicitly stated that the item which is finally administered to a patient is an extract which is not just represented by the botanical origin of its herbal ingredients but also influenced by any procedure that is applied to the herbal material.Any such procedure may severely impact upon the extract’s chemical composition and hence the products’qual-ity with regard to its therapeutic efficacy.This is especially true for complex TCM formulae where we still lack a comprehensive understanding of the interactions between the thousands of chem-ical compounds that constitute the herbal metabolome(s).Besides showing synergistic effects on a pharmacodynamic level,chem-ical compounds of a TCM formula or even an individual herbal material can also interact with each other (i)on a pharmacoki-netic level,influencing the solubility,stability or resorption of compounds or (ii)on a biochemical level,where residual herbal0378-8741/$–see front matter © 2012 Elsevier Ireland Ltd. All rights reserved.doi:10.1016/j.jep.2012.01.050H.Sheridan et al./Journal of Ethnopharmacology140 (2012) 482–491483enzymes may impact the chemical composition;(iii)a mixture of herbal drugs could even be shown to contain metabolites that none of the individual herbal ingredients displayed(Nüsslein et al.,2000; Spinella,2002;Gu et al.,2004;Nualkaew et al.,2004;Ma et al., 2009;Nahrstedt and Butterweck,2010).Facing this complexity, the recent approaches for modernisation of CHM,aiming at the development of more convenient and better standardised prod-ucts,bear the risk that such features are lost by turning away from the traditional products and their traditional drug processing and extraction procedures.Extract optimisation is frequently guided by the analysis of single compounds which can often be regarded as the active substance(Yan et al.,2010).Yet,lacking the appro-priate knowledge,the activity of many herbal preparations cannot be clearly linked to single chemical compounds(Vlietinck et al., 2009).Even very well established herbal products like extracts of Salicis cortex have shown efficacy beyond that which is explicable by their content of salicin derivatives which have been consid-ered to solely constitute the extract’s active substance for decades (Schmid et al.,2001).With regard to CHM,it seems desirable that the traditional procedures like the unique pàozhìdrug processing and the extraction of specific combinations of herbal drugs are sys-tematically investigated for their impact on the herbal extracts’therapeutic qualities and metabolomes(Yi and Chang,2004;Zhao et al.,2010).In this review we will describe the current situation and problems connected to the extraction and compound anal-ysis of CHM and show possible research approaches to gain the chemical information required for a rational extract modernisation that gives consideration to the complex composition of traditional preparations.2.Traditional extraction of CHMThe majority of CHM for oral use is applied as water decoc-tions(tang).Other oral preparations include macerates in aqueous ethanol,the intake of powdered drugs suspended in water or pre-pared in pills,e.g.with honey,water or rice gruel as excipients(Li et al.,2007;Martin and Stöger,2008).Decoctions,macerates and suspensions are very simple to prepare and allow a highflexibility of the recipes.A factor which fundamentally influences the chem-ical composition of extracts is the pàozhìprocessing of the crude drug prior to extraction.This is of special importance for the detox-ification of toxic materials like Aconitum drugs(Singhuber et al., 2010)but also for the preservation of active constituents,ease of administration,flavour correction or cleansing(Zhu,1998).Many drugs can be processed by various methods like stir-frying,steam-ing or calcining in order to gain a product with altered therapeutic properties and–implicitly–altered chemical composition(Zhao et al.,2010;Zhan et al.,2011).To prepare a typical traditional decoction,a complex TCM for-mula is macerated with water for a period of time before afirst boiling step follows.Afterfiltration,the herbal material is re-extracted a second time(usually with less water)and the combined extracts are ingested and/or kept for further use.The time spans for soaking and cooking depend on the drugs as well as on the indica-tion.As a rule of thumb,decoctions for acute conditions are cooked for a shorter period of time as compared to preparations for chronic diseases.Over thousands of years numerous special instructions for the boiling process have been developed in the use of CHM.These methods seem to be related to the different physical,chemical and pharmacological characteristics of the active compounds(Martin and Stöger,2008;Körfers and Sun,2009).Herbal drugs containing volatile or temperature-sensitive sub-stances are added only a few minutes before the end of thefirst boiling period to avoid losses or decomposition(hòuxià).Expen-sive drugs,e.g.Ginseng Radix or Panacis quinquefolii Radix might be cooked separately to optimise the yield and avoid adsorption of active compounds to other ingredients within the prescription (lìngji¯an,lìngd¯un).In some instances drugs have to be added to the mixture wrapped in a thin cloth(b¯aoji¯an),this occurs with drugs like Typhae Pollen,which might lead to turbid decoctions or Inulae Flos which contains drug particles which could cause intestinal irri-tations.For toxic drugs such as Aconiti radix,which are only used orally after pàozhìprocessing,additional cooking steps or longer boiling times for the preparation are recommended.In prepara-tions containing Acori Rhizoma,an extended decoction process(in summary3h)has been proven to be very efficient for the reduction of genotoxicß-asarone(Chen et al.,2009).3.The example of Danggui Buxue Tang(DBT)In order to evaluate the significance of traditional procedures for the therapeutic value of a CHM,the rather simple two-herb for-mula DBT has been chosen as a model to investigate the influence of numerous extraction parameters on the chemical composition and pharmacological efficacies of a water decoction.The traditional composition of DBT as established over the centuries is reported as five parts Astragali Radix(AR)and one part Angelicae sinensis Radix (ASR).An investigation of several mixtures with different ratios of the two drugs has shown the best pharmacological effects as well as the highest yields of the(active)markers astragaloside IV,caly-cosin,formononetin,ferulic acid,total saponins,totalflavonoids and total polysaccharides for the traditional composition,while the undesired compound ligustilide was found to be least con-centrated with this ratio.The concentrations of these compounds varied over the examined drug ratios(AR:ASR from1:1to10:1) by a factor of∼2,demonstrating a significant impact of the drug ratio on the extraction yield(Dong et al.,2006;Po et al.,2007). Systematic investigations compared different durations of boiling, drug-solvent-ratios and numbers of extractions for DBT and also examined the chemical properties of both herbal ingredients’indi-vidual extracts.It could be shown that the treatment with DBT was up to twice as effective as individual extracts of its herbal ingredients at the same concentration.Interestingly,the extraction corresponding most closely to the traditional prescription(twofold extraction of the drug mixture with the eightfold amount of water and an entire boiling time of2h)resulted in the best extraction of active markers and the best effect in two bio-assays(Song et al., 2004;Gao et al.,2006).A mixture of individually prepared extracts of AR and ASR showed an inferior pharmacological effect when compared to the traditional co-extraction of the drugs as well as a lower concentration of active markers.It is interesting to note that the investigated markers from ASR(ligustilide and ferulic acid) showed an astonishing increase of concentration of more than25 fold when co-extracted with ASR,indicating a massive influence of AR on the extraction of these compounds(Mak et al.,2006).Another study of DBT yielded similar results with respect to biological activ-ity,however,the chromatographicfingerprints did not show such a huge influence of co-extraction on the ASR markers.The authors concluded that other,non-observed compounds influenced by co-extraction would impact the biological activity(Choi et al.,2011). The pàozhìprocessing of ASR with rice wine prior to extraction was also examined and resulted in higher extraction yields of astraga-loside IV,isoflavones and polysaccharides(Dong et al.,2006).As the processing of ASR with rice wine results in a decreased con-centration of ligustilide in the processed product,it was examined if the co-extraction of AR with pure ligustilide would impact the extraction yields of AR markers;in fact,ligustilide lowered the yields of these markers in a dose-dependent manner,indicating that the pàozhìprocessing of ASR influences the activity of DBT by altering its properties with regard to co-extraction with AR(Zheng484H.Sheridan et al./Journal of Ethnopharmacology140 (2012) 482–491et al.,2010).Further,the exchange of ASR with another drug,Radix Chuanxiong,which shares the occurrence of ligustilide and ferulic acid with ASR,was examined,resulting in both an inferior pharma-cological efficacy and lower concentrations of the AR markers when compared to a DBT extract,indicating that other,non-observed compounds of ASR may impact the quality of DBT(Li et al.,2009).It must be stated that the activities in the above mentioned stud-ies were assessed by completely different methods.Nevertheless, the example of DBT impressively demonstrates that the subject of extraction is not a trivial one and that the chemical composition and pharmacological efficacies of the traditional water decoctions are influenced to an astonishing degree by a multitude of factors which apparently have found their optimum in the traditional DBT prescription.4.Modernisation of CHM extractsThe development of modernised extracts and application forms is desired for numerous reasons:(i)individually prepared water decoctions are more likely to entail quality shortcomings caused by improper herbal drugs when compared to herbal medicines produced in industrial scale under best controllable conditions. (ii)Water decoctions are probably the worst possible preparation in terms of stability and may give rise to microbial contamina-tions,decomposition of constituents by hydrolytic or oxidative reactions or precipitations that may impact the product’s qual-ity.(iii)TCM water decoctions are infamous for their unpleasant organoleptic properties.(iv)The rather complicated and time con-suming preparation and storage of water decoctions may cause compliance problems while modernised application forms based on dry extracts(granules,capsules,tablets,etc.)are easily man-ageable for both patients and practitioners.(v)The possibility for standardisation of large-scale extracts is a prerequisite for future evidence-based research.(vi)The blinding of clinical studies employing traditional water extracts is hampered by their strong organoleptic properties(Martin and Stöger,2008;Flower et al., 2011).As evident from the studies on DBT,turning away from traditional procedures bears the risk of altering the product’s therapeutic properties.Therefore some modernisation approaches, directly based on water decoctions,have been developed to over-come the above mentioned problems.One such approach involves the delivery of water decoctions in sealed,separately packaged, daily dosages thus increasing the compliance and stability of the product.Also an unpleasantly tasting herbal placebo composed of nine culinary herbs was designed(Flower et al.,2011).4.1.Pressurised hot waterPressurised hot water extraction at high temperature is some-times applied and can be regarded as a kind of modernisation.This method has been shown to decrease the extractant’s polarity and thus provides the extraction of a wider range of compounds(Deng et al.,2007).Nevertheless,it is reported that the use of pressure cookers for the preparation of decoctions to shorten cooking times leads to less active preparations(Martin and Stöger,2008).4.2.Formation of granulesA major approach to modernise and facilitate the application of CHM was the introduction of granules,which are usually pre-pared from decoctions byfluid-bed granulation or spray-drying. As the technological properties of such extracts are impaired by high amounts of hydrophilic constituents,especially carbohydrates which result in hydroscopic,sticky and hence hardly processable extracts.Thus,excipients are added to the decoction of the herbal material(one step procedure)or mixed with the spray-dried prod-uct(two step procedure)(Martin and Stöger,2008;Ai et al.,2008). Rather high amounts of additives are required(Wang et al.,2011) and these additives further add on to the extract dose which is already quite large due to the presence of polar“bulk material”. Another method to improve the technological properties is the removal of highly polar compounds from the extract by ethanol precipitation prior to drying(Tan et al.,2006).Such preparations can be instantly applied by suspension in hot water or can be used to produce single dosage forms like capsules.An advantage is that these products can be applied quickly avoiding the time consuming preparation of a decoction.e of less polar extractantsOther modernisation approaches employ less polar extractants and hence can yield a dry extract with superior technologi-cal properties when compared to dried water extracts.These approaches comprise the extraction with(hydro)alcoholic or organic extractants performed by different extraction techniques like maceration,Soxhlet extraction,microwave-assisted extrac-tion,ultrasonic extraction or accelerated solvent extraction. Though these different extraction techniques show clear distinct properties(e.g.thermal decomposition during Soxhlet extraction, solvent limitations for microwave-assisted extraction),the dif-ferences can mainly be attributed to extraction speed while the chemical composition of the resulting extracts after establishment of equilibration is mainly influenced by the kind of extractant used and its temperature(Yan et al.,2010).From a general view, the differences in extract composition between these techniques can be regarded as negligible when compared to the differences between any of these techniques employing(hydro)alcoholic or organic solvents and a traditional water decoction.A study com-paring hydroalcoholic extracts of more than30drugs with their respective water decoctions clearly showed that decoction in most cases allows an extraction of phenolics which is similarly efficient as maceration with50%ethanol.The yields after extraction with 80%methanol,a very common method for analytical determina-tion of phenolics and their testing on antioxidant activity,was much lower.In this study,most of the decoctions,among them drugs from Rehmannia glutinosa Libosch,Astragalus membranaceus (Fisch.)Bge.or Atractylodes macrocephala Koidz.,showed better or similar antioxidant activity as compared to the hydroethanolic macerates.The activity of the80%methanolic extracts was worse for almost all tested drugs(Li et al.,2007).In a comparison of aque-ous and ethanolic extracts from different drugs a more pronounced effect on various CYP enzymes was proven for the water extracts (Tang et al.,2006).4.4.Supercritical Fluid Extraction(SFE)A completely different extraction technique that by its techni-cal properties is attractive for large scale extraction,is Supercritical Fluid Extraction(SFE).This is an extraction technique which takes advantage of the enhanced solvating and penetrating capacity of gases or liquids in their critical phases(ASTM,2006).The unique properties of supercriticalfluids were observed more than a cen-tury ago,but only in the last four decades SFE has emerged as an extraction technique(Khosravi-Darani,2010).Advantages of SFE in contrast to conventional extraction are(i)superior extraction efficiency and selectivity for low polar phytochemicals.The extrac-tion efficiency and selectivity can be tuned by optimising pressure and temperature(Wang et al.,2008;Liu et al.,2008a).(ii)Among the many applicable solvents for SFE,the most commonly used for extraction of CHM is supercritical carbon dioxide(SCO)which is inert,easily available,inexpensive,odourless,environmentalH.Sheridan et al./Journal of Ethnopharmacology140 (2012) 482–491485friendly and has mild critical point properties.To increase the sol-ubility of compounds from CHM in SCO,small amounts of polar modifiers,e.g.ethanol,may be added,usually not more than10%of the amount required for conventional extraction techniques(Chen and Ling,2000;Silva et al.,2009;Chen et al.,2011).(iii)SCO is eas-ily removable from the extract by depressurization,and thus no solvent residue is left in the extract(Vagi et al.,2002).(iv)Prefer-able product stability.The extraction is conducted at oxygen and light free operating conditions which prevent oxidation and light dependent changes,for example,a SCO extract of tomatoes could be stored for more than half a year at−20◦C without lycopene loss (Lenucci et al.,2010).Furthermore,the low temperatures minimize thermal degradation of sensitive materials,e.g.volatiles.As a green separation technique,SFE has a promising future in its application in thefields of TCM and natural products(Martinez,2008).However,there are limitations for any of the extraction method-ologies to be considered for the production of more convenient application forms.Because of the“Lipinski rule offive”(Lipinski et al.,2001),it is generally believed that less polar extractants like alcohols,acetone or SCO are capable of extracting pharmacologi-cally relevant analytes while excluding higher amounts of polar, technologically difficult“bulk material”like carbohydrates,pro-teins,amino acids etc.from the extract.However,this is a very reductionistic view which actually applies to a single active com-pound and especially excludes the possibility of pharmacokinetic synergism in herbal extracts,which has been shown to have a major impact on the chemical composition of CHM(e.g.Mak et al., 2006).It cannot be generally ruled out that the extraction of very polar constituents(needing water as extractant)can be essential for producing efficient extracts from specific drugs or for specific appli-cations,polymeric carbohydrates or compatible solutes like ectoine are examples for such highly polar bioactive compounds(Lentzen and Schwarz,2006).When relating any modernised CHM extract to the long experience of TCM it should be considered that its clinical efficacy should at least match the one of the traditional prepara-tion it relates to.It has been shown that the single active markers that are typically used for the quality control of herbal drugs may not be able to fully explain a product’s quality,especially when the drug is used as part of a co-extracted herbal mixture(Schmid et al.,2001;Li et al.,2009;Vlietinck et al.,2009),and frequently not even an active marker is known but analytical markers without known clinical relevance are used as a surrogate.As a consequence, such single markers cannot be generally recommended to guide the optimisation of an extract.Hence,for the rational development of new products it is essential to increase our knowledge about which chemical compounds define the quality of a traditional prepara-tion,so a production chain can be established thatfinally yields an improved product lacking technologically problematic“bulk mate-rial”while conserving any compound that has been shown to be relevant for the traditional product’s quality in terms of clinical efficacy.In summary,the rational modernisation of CHM requires further research to identify chemical compound that can be linked to an herbal preparation’s quality considering pharmacodynami-cally and pharmacokinetically relevant compounds.5.Fingerprint analysis in activity studies of herbal extractsA major challenge in the modernisation of CHM is the incorpo-ration of between one and twelve herbs in a given formula,thus conferring a high degree of complexity and the potential for vari-ation in composition and quality of a preparation.It has also been established that interactions between chemical components may take place during traditional co-extraction of complex herbal mix-tures and thus impact the extract’s chemical composition.Due to the lack of knowledge about the chemical compounds that completely constitute the quality of many CHM,the widespread practice of using single herb monographs and analysing of sin-gle compounds for the characterisation of CHM extracts tested in pharmacological or clinical studies seems to be insufficient.This is of particular issue when dealing with HM that contain multi-ple herbs,and can be illustrated by the fact that sometimes the same,rather unspecific metabolites are used for the quality assess-ment of herbal drugs with distinct clinical properties.Examples are chlorogenic acid,which is used as a marker compound for Caulis Lonicera,Flos Lonicera and Flos Chrysantemi,or berberine,which is used as a marker compound for Rhizoma Coptidis and Cortex Phel-lodendri(Zhou et al.,2008).Consequently,a more comprehensive view on the chemistry of extracts is desirable.It is obvious that the metabolomic techniques currently available forfingerprint analy-sis(FP)of complex biological and herbal samples and those with evolving applications in this area,have the potential to enhance the quality control of CHM and to assist with the correlation of bioactiv-ity with composition.FP can be defined in this context as an analysis aiming at the representation of an extract’s chemical composition to a maximum possible degree;while being largely untargeted,FP can also be used for or as an addition to the quantification of single compounds.Chromatographic FP and the simultaneous determination of multiple compounds is becoming an important trend(Liang et al., 2010).However an analysis of97original papers1assessing bio-logical effects of CHM extracts revealed that only16provided–exclusively chromatographic–FP data while24characterised the extracts by quantifying relevant single compounds and57did not chemically characterise the tested extract at all.We consider the presentation of FP data within activity studies of herbal extracts as fundamental information for chemical characterisation.Consider-ing the possible variability of an extract’s chemical profile that can be caused by differences in the herbal materials(growth conditions, post-harvest treatment,pàozhìprocessing,etc.)or manufacturing (extraction conditions,drying,etc.)it must be stated that the exam-ined item in such studies is literally unknown without at least some basic chemical characterisation.Different methods are used for FP which may be roughly cat-egorised as(i)low resolution techniques like TLC or IR-based methods which are typically applied for assessing the identity or origin of an herbal material by visual or–in case of NIR–computer aided comparison of signal patterns,mostly without(detailed) assignment of signals to chemical compounds(Xie et al.,2006;Sun et al.,2010).(ii)High resolution techniques like GC,HPLC,MS,NMR or hyphenated techniques allow for a detailed assignment of signals to the detected chemical compounds and are commonly accepted as well suited for FP aiming at a comprehensive extract charac-terisation as well as for metabolomic approaches.The advantages and disadvantages of these methods with regard to suitable tar-get metabolites,reproducibility of signal intensities and positions, sensitivity,resolution and sample preparation efforts have been extensively discussed(Verpoorte et al.,2008).It must be stated that no available analytical method is capable of fulfiling the demand for a complete qualitative and quantitative assessment of a biological sample’s whole chemical composition.1The literature research was performed with either Scopus or Pubmed using an OR-conjuncted combination of any botanical identifiers given by the consor-tium’s priority list of species(CP2005Latin binominal species name,taxonomically accepted Latin binominal species name,Latin drug names,Pinyin names,Latin binominal synonyms).The search result was then refined by limiting the hits to the topics“Extraction”and“Chemistry”or adding these terms to the general search term with“AND”conjunctions for Pubmed,respectively.The97papers mentioned here were chosen from the results by the fact that they assessed the biological activity of an herbal extract.。
比利时使馆探亲签证申请审核表
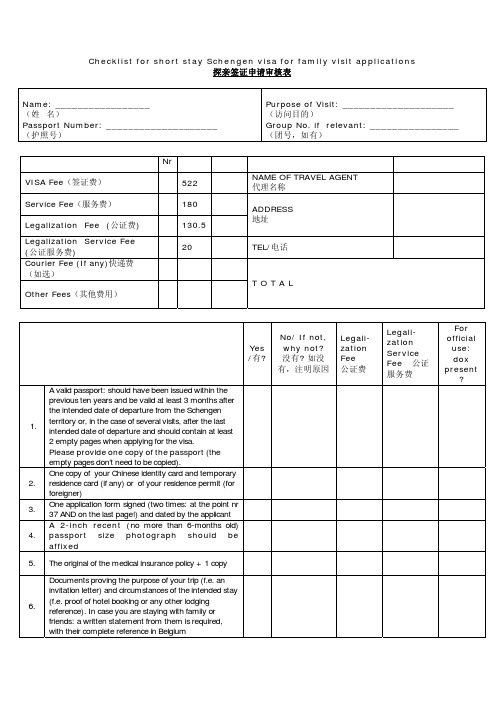
The applicant acknowledges that he has provided the documents here above marked as submitted and that he has been informed that some other documents might be requested by the Belgian Embassy / Consulate General. 申请人知道他已递交以上标记出的材料并已被通知比利时大使馆或领事馆可能还需要其他材料。
50€/day of stay if staying at a hotel)
OR
A letter of guarantee (in the prescribed form “Annexe
3bis”/“Bijlage 3bis” signed by an individual (the
sponsor) who has sufficient resources and which has
2 empty pages when applying for the visa.
Please provide one copy of the passport (the
empty pages don’t need to be copied).
One copy of your Chinese identity card and temporary
your employer concerning your monthly income/ tax
sheet / bank statement/..)(This should cover at least
38€/day of stay if staying with friends and at least
belstain
Beilstein 2000使用指南陈华祥编写一、 简介Beilstein 2000(贝尔斯塔)数据库是著名的化学合成数据库,对于有机合成工作者来说,其作用远远大于Chemical Abstract (CA)。
由于它的检索范围仅限于合成一块,避免了CA浩如烟海的检索,大大提高了文献检索效率,所以这是我们每个药明康德科研人员必须掌握的检索工具。
二、 登陆有机所图书馆数据库在公共图书馆电脑上打开IE,其主页设为有机所图书馆数据库,提示要登录,用户名密码输入后即可登录有机所数据库查询资料。
Scheme 1。
Scheme 1: 登陆有机所图书馆数据库三、 进入Beilstein登录后,您将进入到有机所数据库的界面,里面有许多实用的数据库,这里介绍Beilstein 2000。
在第一行您将看到“BC2000 or CommanderV6”(Scheme 2),您点击其中之一便可进入,后者是前者的升级版,功能大同小异。
进入后其中有两个功能您可选择使用:一是进入合成数据库查文献,点击工具栏红色交叉(CrossFire)。
二是结构命名,点击蓝色的正六边形(Name)。
不过我们的电脑多数是设置自动进入CrossFire。
进入后可单击“Structure”右边的图标或双击空白处进入结构编辑(Scheme 3)。
一般情况下,其默认的结构编辑器为“CrossFire Structure Editor”,其优点是可以定义所查化合物是产物(Product)或反应物(Reactant)来缩小检索范围。
不过,有时进入后结构编辑器会变成“ISIS/Draw”(不能定义产物或反应物),这可以通过依次点击主菜单“Options”“Structure Editors…”来更改,当然,如果您习惯使用“ISIS/Draw”,同样也可通过此法来调整结构编辑器。
(Scheme 4)Scheme 2:登录成功后的有机所图书馆页面Scheme 3:BC2000 CrossFire 结构编辑Scheme 4:BC2000结构编辑器的调整四、 编辑结构CrossFire Structure Editor的页面如Scheme 5:第一栏为主菜单,随后为两栏工具栏。
版本巴克莱银行备用证
AUTHENTICATED MT760 SBLC SPECIMEN VERBIAGE认证的MT760备用信用证样本ISSUING BANK: (NAME OF ISSUING BANK)开证行ADDRESS:地址PHONE:电话FAX:传真SWIFT CODE分行号BANK OFFICER:银行人员SBLC APPLICANT:备用信用证申请人RECEIVING BANK:收证行ADDRESS:地址PHONE:电话FAX:传真SWIFT CODE分行号BANK OFFICER:银行人员STANDBY LETTER OF CREDIT NUMBER:信用证号DATE OF ISSUE:开证日期DATE OF EXPIRATION:到期日BENEFICIARY:受益人ADDRESS:地址WE, HEREBY OPEN OURIRREVOCABLE,UNCONDITIONAL,TRANSFERABLE,DIVISIBLE,NEGOTIABLE, CASH BACKED STANDBY LETTER OF CREDIT IN YOUR FAVOR:XXXXXXXXXXXXXXXXXXXXXXXIN THE AMOUNT OF $500,000,000.00 (FIVE HUNDRED MILLION U.S DOLLARS) FOR THE ACCOUNT OF THE (LESSEE) COVERING: PERFORMANCE UNDER CONTRACT (REF NO:)兹开具不可撤销的,无条件的,可转让的,可分割,可承兑,现金支持的备用信用证以贵公司为受益人的:XXXXXXXXXXXXXXXXXXXXXXX总金额在$500,000,000.00为(承租人)账户的金额(五亿美元):合同下的(合同编号:)PAYMENT IS AVAILABLE UPON THE PRESENTATION OF BENEFICIARY'S FIRST WRITTEN DEMAND MARKED DRAWN UNDER STANDBY LETTER OF CREDIT ON XXXXXXXXXXX DATED ACCOMPANIED BY:由受益人第一书面要求标记的备用信用证在****日可支付附件BENEFICIARY'S STATEMENT THAT A PAYMENT DUE UNDER THE CONTRACT HAS NOT BEEN EFFECTED,THEREFORE THE AMOUNT DRAWN HEREUNDER IS DUE AND OWING.受益人陈述支付日期在合同未生效前,付款到期合同项下尚未实行,因此金额为本到期应付。
2023年全国高考英语试题及参考答案(全国乙卷)
2023年全国高考英语试题及参考答案(全国乙卷)(河南 江西 甘肃 陕西 宁夏 新疆 青海 内蒙古)第二部分阅读理解(共两节,满分40分)第一节(共15小题; 每小题2分,满分30分)阅读下列短文,从每题所给的A、B、C和D四个选项中,选出最佳选项。
APRACTITIONERSJacqueline Felic de (c. 1322) highlights the suspicion that women practicing medicine faced. Born to a Jewish family in Florence she moved to Paris where she worked as a physician and performed surgery. In 1322 she was tried for practicing unlawfully. In spite of the court hearing testimonials(证明)of her ability as a doctor, she was banned from medicine.Tan Yun (1461-1554) was a Chinese physician who learned her skills from her grandparents. Chinese women at the time could not serve a apprenticeships (学徒期)with doctors. However, Tan passed the official exam. Tan treated women from all walks of life. In 1511Tan wrote a book, sayings of Female Doctor, describing her life as physician.James Barry (c. 1789-1865) was born Margaret Bulkley in Ireland but, dressed as a man, she was accepted by Edinburgh University to study medicine She qualified as a surgeon in 1813, then joined the British Army,serving overseas. Barry retired in 1859, having practiced her entire medical profession living and working as a man.Rebecca Lee Crumpler (1831-1895) worked as a nurse for eight years before studying in medical college in Boston in 1860. Four years later, she was the first African American woman to receive a medical degree. She moved to Virginia in 1865, where she provided medical care to freed slaves.21. What did Jacqueline and James have in common?A. Doing teaching jobs.B. Being hired as physicians.C. Performing surgery.D. Being banned from medicine.22. How was Tan Yun different from the other practitioners?A. She wrote a book.B. She went through trials.C. She worked as a dentist.D. She had formal education.23.Who was the first African American with a medical degree?A. Jacqueline Felice de A.B. Tan Yun.C. James Barry.D. Rebcca Lee Crumpler.BLiving in Iowa and trying to become a photographer specializing in landscape(风景)can be quite a challenge, mainly because the corn state lacks geographical variation.Although landscapes in the Midwest tend to be quite similar either farm fields or highway, sometimes I find distinctive character in the hills or lakes. To make some of my landscape shots, I have travelled up to four hours away to shoot within 10-minture time for me, I tend to travel with a few of my friends to state parks or to the countryside to go on adventures and take photos along the way.Being at the right place at the right time is decisive in any style of photography. I often leave early to seek the right destinations so I can set up early to avoid missing the moment I am attempting to photograph. I have missed plenty of beautiful sun sets and rises due to being on the sport only five minutes before the best moment.One time my friends and I drove three hours t0Devil’s Lake, Wisconsin, to climb the purple quartz(石英) rock around the lake. After we found a crazy-looking road that hung over a bunch of rocks, we decided to photograph the scene at sunset. The position enabled us to look over the lake with the sunset in the background. We managed to leave this spot to climb higher because of the spare time until sunset.However, we did not mark the route(路线)so we ended up almost missing the sunset entirely. Once we found the place, it was stressful getting lights and cameras set up in the limited time. Still looking back on the photos, they are some of my best shots though they could have been so much better if I would have been prepared and managed my time wisely.24.How does the author deal with the challenge as a landscape photographer in the Midwest?A. By teaming up with other photographers.B. By shooting in the countryside or state parks.C. By studying the geographical conditions.D. By creating settings in the com fields.25. What is the key to successful landscape photography according to the author?A. Proper time management.B. Good shooting techniques.C. Adventurous spirit.D. Distinctive styles.26.What can we infer from the author’s trip with friends to Devil’s Lake?A. They went crazy with the purple quartz rockB. They felt stressed while waiting for the sunset.C. They reached the shooting spot later than expected.D. They had problems with their equipment.27. How does the author find his photos taken at Devil's Lake?A. Amusing.B. Satisfying.C. Encouraging.D. Comforting.CWhat comes into your mind when you think of British food? Probably fish and chips or a Sunday dinner of meat and two vegetables. But is British food really so uninteresting? Even though Britain has a reputation for less-than-impressive cuisine, it is producing more to class chefs who appear frequently on our televisionscreens and whose recipe books frequently top the best seller lists.It is thanks to these TV chefs rather than any advertising campaign that Britons are turning away from meat-and-two-veg and ready-made meals and becoming more adventurous in their cooking habits. It is recently reported that the number of those sticking to a traditional diet is slowly declining and around half of Britain's consumers would like to change or improve their cooking in some way. There has been a rise in the number of students applying for food courses at UK universities and colleges. It seems that TV have helped change what people thinking about cooking.According to a new study from market analysts, 1 in 5 Britons say that watching cookery on TV has encouraged them to try different food. Almost one third say they now use a wider variety of in- gradients(配料)than they used to, and just under 1 in 4 say they now buy better quality ingredients than before. One in four adults say that TV chefs have made them much more confident about expanding their cookery knowledge and skills, and young people are also getting more interested in cooking. The UK’sobsession(痴迷) with food is reflected through television scheduling. Cookery shows and documentaries about food are broadcast more often than before. With an increasing number of male chefs on TV, it’s no longer "uncool" for boys to like cooking.28. What do people usually think of British food?A. It is simple and plain.B. It is rich in nutrition.C. It lacks authentic tastes.D. It deserves a high reputation.29.Which best describes cookery on British TV?A. Authoritative.B. Creative.C. Profitable.D. Influential.30.Which is the percentage of the people using more diverse ingredients now?A.20%.B.24%.C.25%.D.33%.31.What might the author continue talking about?A. The art of cooking in other countries.B. Male chefs on TV.C. Table manners in the UK.D. Studies of big eaters.DIf you want to tell the history of the whole world, a history that does not privilege one part or humanity. you cannot do it through texts alone. Because only some of the world has ever had texts, while most of the world, for most of the time, has not. Writing is one of humanity’s later achievements, and until fairly recently even many literate(有文字的)societies recorded their concerns not only in writing but in tings.Ideally a history would bring together texts and objects, and some chapters of this book are able to do just that, but in many cases we simply can’t. The clearest example of this between literate and non-literate history is perhaps the first conflict at Botany Bay between Captain Cook's voyage and the Australian Aboriginals. From the English side, we have scientific reports and the captain’s record of that terrible day. From the Australian side, we have only a wooden shield(盾)dropped by a man in flight after his first experience of gunshot. If wewant to reconstruct what was actually going on that day, the shield must be questioned and interpreted as deeply and strictly as the written reports.In addition to the problem of miscomprehension from both sides, there are victories accidentally or deliberately twisted, especially when only the victors know how to write. Those who are on the losing side often have only their things to tell their stories. The Caribbean Taino. the Australian Aboriginals, the African people of Benin and the Incas all of whom appear in this book, can speak to us now of their past achievements most powerfully through the objects they made: a history told through things gives them back a voice. When we consider contact(联系)between literate and non-literate societies such as these, all our first-hand accounts are necessarily twisted, only one half of a dialogue. If we are to find the other half of that conversation, we have to read not just the texts, but the objects.32. What is the first paragraph mainly about?A. How past events should be presented.B. What humanity is concerned about.C. Whether facts speak louder than words.D. Why written language is reliable.33. What does the author indicate by mentioning Captain Cook in paragraph 2?A. His report was significantB. He represented the local people.C. He ruled over Botany Bay.D. His record was one-sided.34. What does the underlined word "conversation" in paragraph 3 refer to?A. Problem.B. History.C. Voice.D. Society.35. Which of the following books is the text most likely selected from?A. How Maps Tell Stories of the WorldB. A Short History of AustraliaC. A History of the World in 100 ObjectsD. How Art Works Tell Stories第二节(共5小题: 每小题2分满分10分)根据短文内容,从短文后的选项中选出能填入空白处的最佳选项。
BELBIN English Self-Perception Inventory-A4
ANSWER SHEETSURNAME (PRINT):Sex: M /FFIRST NAME (PRINT):Organization:Department:Date:Directions for Self-Perception completion:The BELBIN® Self-Perception Inventory (SPI) should be completed, preferably when you can arrange a quiet period free from interruptions. It usually takes about 15 minutes to complete. You should answer the questions after some serious thought, whilst avoiding spending too long on any given section. There are no right or wrong answers.For each section distribute a total of exactly 10 points between the sentences that you think most accurately describe your behaviour. These points may be distributed between several sentences.Try to avoid both extremes of giving one sentence all ten points or allocating one point to every sentence in each section.Please allocate whole numbers only - no fractions or decimals. If you have no points to allocate to a sentence, please leave the box blank.SECTIONI SECTIONIISECTIONIIISECTIONIVSECTIONVSECTIONVISECTIONVIIMARK MARK MARK MARK MARK MARK MARK1.02.03.04.05.06.07.01.12.13.14.15.16.17.11.22.23.24.25.26.27.21.32.33.34.35.36.37.31.42.43.44.45.46.47.41.52.53.54.55.56.57.51.62.63.64.65.66.67.61.72.73.74.75.76.77.71.82.83.84.85.86.87.81.92.93.94.95.96.97.9TOTAL1010101010101070For each section distribute a total of ten marks among the sentences which you think most accurately describe your behaviour. These marks may be distributed among several sentences; in extreme cases they might be spread among all the sentences or 10 marks may be given to a single sentence. However try and avoid either extreme. Enter the points in the INTERPLACE answer sheet provided.I. WHAT I BELIEVE I CANCONTRIBUTE TO A TEAM:1.0I think I can quickly see and take advantage ofnew opportunities.1.1My comments both on general and specificpoints are well received.1.2I can work well with a very wide range ofpeople.1.3Producing ideas is one of my natural assets. 1.4My ability rests in being able to draw peopleout whenever I detect they have something ofvalue to contribute to group objectives.1.5I can be relied upon to finish any task Iundertake.1.6My technical knowledge and experience areusually my major assets.1.7I am prepared to be blunt and outspoken in thecause of making the right things happen.1.8I can usually tell whether a plan or idea will fit aparticular situation.1.9I can offer a reasoned and unbiased case foralternative courses of action.II. IF I HAVE A POSSIBLE SHORTCOMING IN TEAM WORK, IT COULD BE THAT:2.0I am not at ease unless meetings are wellstructured and controlled and generally wellconducted.2.1I am inclined to be too generous towardsothers who have a valid viewpoint that has notbeen given a proper airing.2.2I am reluctant to contribute unless the subjectcontains an area I know well.2.3I have a tendency to talk a lot once the groupgets on to a new topic.2.4I am inclined to undervalue the importance ofmy own contributions.2.5My objective outlook makes it difficult for me tojoin in readily and enthusiastically withcolleagues.2.6I am sometimes seen as forceful andauthoritarian when dealing with importantissues.2.7I find it difficult to lead from the front, perhapsbecause I am over-responsive to groupatmosphere.2.8I am apt to get too caught up in ideas thatoccur to me and so lose track of what ishappening.2.9I am reluctant to express my opinions onproposals or plans that are incomplete orinsufficiently detailed.III. WHEN INVOLVED IN A PROJECT WITH OTHER PEOPLE: 3.0I have an aptitude for influencing peoplewithout pressurising them.3.1I am generally effective in preventing carelessmistakes or omissions from spoiling thesuccess of an operation.3.2I like to press for action to make sure that themeeting does not lose sight of the mainobjective.3.3I can be counted on to contribute somethingoriginal.3.4I am always ready to back a good suggestionin the common interest.3.5One can be sure I will just be my natural self. 3.6I am quick to see the possibilities in new ideasand developments.3.7I try to maintain my sense of professionalism.3.8I believe my capacity for judgement can help tobring about the right decisions.3.9I can be relied on to bring an organisedapproach to the demands of a job.IV. MY CHARACTERISTICAPPROACH TO GROUP WORKIS THAT:4.0I maintain a quiet interest in getting to knowcolleagues better.4.1I contribute where I know what I am talkingabout.4.2I am not reluctant to challenge the view ofothers or to hold a minority view myself.4.3I can usually find an argument to refuteunsound propositions.4.4I think I have a talent for making things workonce a plan has been put into operation.4.5I prefer to avoid the obvious and to open uplines that have not been explored.4.6I bring a touch of perfectionism to any job Iundertake.4.7I like to be the one who makes contactsoutside the group or firm.4.8I enjoy the social side of working relationships.4.9While I am interested in hearing all views Ihave no hesitation in making up my mind oncea decision has to be made.V. I GAIN SATISFACTIONIN A JOB BECAUSE:5.0I enjoy analysing situations and weighing up allthe possible choices.5.1I am interested in finding practical solutions to problems.5.2I like to feel I am fostering good workingrelationships.5.3I can have a strong influence on decisions.5.4I have a chance of meeting new people withdifferent ideas.5.5I can get people to agree on priorities.5.6I feel I am in my element where I can give atask my full attention.5.7I can find an opportunity to stretch myimagination.5.8I feel that I am using my special qualificationsand training to advantage.5.9I usually find a job gives me the chance toexpress myself.VI. IF I AM SUDDENLY GIVEN A DIFFICULT TASK WITH LIMITED TIME and UNFAMILIAR PEOPLE: 6.0I usually succeed in spite of thecircumstances.6.1I like to read up as much as I conveniently canon a subject.6.2I would feel like devising a solution of my ownand then trying to sell it to the group.6.3I would be ready to work with the person whoshowed the most positive approach.6.4I would find some way of reducing the size ofthe task by establishing how differentindividuals can contribute.6.5My natural sense of urgency would help toensure that we did not fall behind schedule. 6.6I believe I would keep my cool and maintainmy capacity to think straight.6.7In spite of conflicting pressures I would pressahead with whatever needed to be done.6.8I would take the lead if the group was makingno progress.6.9I would open up discussions with the view tostimulating new thoughts and gettingsomething moving.VII. WITH REFERENCE TO THE PROBLEMS I EXPERIENCE WHEN WORKING IN GROUPS:7.0I am apt to overreact when people hold upprogress.7.1Some people criticise me for being tooanalytical.7.2My desire to check that we get the importantdetails right is not always welcome.7.3I tend to show boredom unless I am activelyengaged with stimulating people.7.4I find it difficult to get started unless the goalsare clear.7.5I am sometimes poor at putting acrosscomplex points that occur to me.7.6I am conscious of demanding from others thethings I cannot do myself.7.7I find others do not give me enoughopportunity to say all I want to say.7.8I am inclined to feel I am wasting my time andwould do better on my own.7.9I hesitate to express my personal views infront of difficult or powerful people.。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
BELBIN ROLES INTERPRETATION SHEET
Dr Meredith designed this inventory to assist people to identify their preferred team roles. The usefulness of knowing ones preferred role is not so much so that one may adjust to other roles, but more that we can recognise our strengths and play to them, thus becoming more effective as a team member.
Here are the roles –the letters are those at the column heads in Table 1. Most people find that they have a predominant style and a secondary. Your tendency will be towards your primary role, but in certain circumstances and with certain groups you will feel it useful or comfortable to slip into subordinate style. The two lowest scores may indicate weaknesses, if they are very low compared with other scores.
OR Organiser–translates all the high-flown(夸张的,好高骛远的)stuff into practical, down-to-earth and gets on with them logically and loyally. Prefers stability but can adapt if it told why. Conservative, dutiful, stable.
+ Organiser, common sense, hard work, self-discipline.
- Lack of flexibility, unresponsiveness to unproven ideas.
CH The Chair–Stable, usually extrovert, rarely, brilliant, talks easily, asks questions, listens well. Not always the team leader, will have a quiet charisma(超凡魅力)which may well amount to authority. Can sum up th e team’s feelings, and when everyone has had their say, make a decision. Calm, self-–confident, controlled. judicious(明智而审慎的,有见地的)flattery. The vital spark. Individualistic, serious minded, unorthodox(非正统的, 非传统的, 非正规的).
+Genius, imagination, intellect, knowledge.
- Up in the clouds(脱离实际,不切实际), disregards practical details and protocol(惯例,习惯做法).
RI The Resource Investigator– is the dominant, extrovert, and very amiable. Either got six phones on the go(忙碌)or is out making contacts. The team’s PR, Big Ears, and diplomat, in outside world. Extroverted, enthusiastic, curious, communicative.
+ Makes contacts, and explores new avenues, responds well to challenge.
- Loses interest after initial fascination passes.
EV The Evaluator– is capable of
deep and dispassionate analysis of
huge quantities of data. Slow, stable, introvert, nor over imaginative and a
bit of a damper(压制者), also the team’s rock. Rarely faulty judgement. Sober, unemotional, prudent(谨慎
的,小心的).
+ Judgement, discretion, hard-
headedness(讲究实际,现实性;不
易动感情).
- Lacks inspiration or ability to
motivate others.
TW The Team Worker– is the most sensitive member, aware of all
+ Welcomes all contributes, strong sense of objectives.
- Ordinary in terms of intellect or creativity.
SH The Shaper– is the task leader Energetic, impatient argumentative, something of a bully, shapes the team’s efforts into a cohesive project. Extrovert, not always likeable. Highly strung(神经紧张的,易心烦的), dynamic, outgoing.
+ Drives and challenges inertia(无为;惯性,惰性)and complacency. - Provable, irritable and impatient.
IN The Innovator–is the team’s chief source of ideas, proposals, suggestions. Usually the most intelligent member, often a bit of a brat as well, the innovator is best handled with undercurrents(暗流, 掩盖着的倾向; 潜在势力), other’s troubles etc. Damps down(降低, 衰减)discord and promotes harmony. Seemingly a bit soft an d indecisive, this character’s value is most apparent at times of stress. Social, mild, sensitive.
+ Responds to people and to
situations and promotes team
spirit.
- Indecisive at moments of crisis.
FI The Finisher– is an anxious introvert, a stickler for detail, deadlines and schedules. A bit of a downer but as a professional worry-guts, the Finisher’s relentless follow-through is crucial.
Painstaking, orderly, conscientious, anxious.
+ Follows through to perfectionism. - Tendency to worry about little things, reluctant to let go
Please remember:
1. These are tendencies and not
written in tablets of stone
2. If you don’t like what you are, it is
possible to change.
3. The ‘-‘ are all OK if they counter
balance the ‘+’s。
