AROMATIC AND HETEROCYCLIC DINITRILES AND THEIR POLYMERS. Ⅺ. STUDY ON THE POLYMERIZATION OF
有机化学芳香化合物-Aromatic-compounds中英文课件

C2H5OH
H
H
H
H
H
HH
9.5.2 Addition Reaction 加成反应
Cl2, hv
Cl H Cl H
H Cl
H Cl
Cl H H Cl
Cl Cl Cl
Cl Cl
Cl
-异构体 杀虫效果最好
Cl Cl C l
Cl
C l Cl
最稳定的异构体
六六六 C6H6Cl6
9.5.3 Reactions of the side chain 侧链的反应
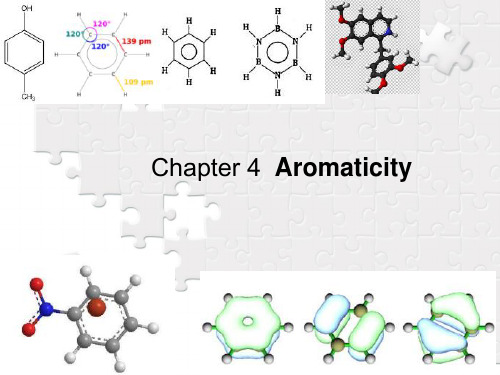
9.1 Structure
9.1.1 Molecular structure 9.1.2 Aromaticlty 9.1.3 Explanation
9.1.1 Molecular structure 分子结构
1.09 Å
H
H
H
120o
1.39 Å
120o
H
H
H
A planer and symmetric molecule
9.2.1 Classification 9.2.2 Isomerization 9.2.3 Nomenclature
9.2.1 Classification 分类
根据芳环的个数
单环芳烃
多环芳烃
根据芳环连接的方式
H
联苯
多苯代脂肪烃
8
1
9
7
2
6
3
10
5
4
稠环芳烃
9.2.2 Isomerization 异构
取代产物有芳香性
E
Nu E E+
H Nu E
Nu
加成产物无芳香性
amine胺 大学有机化学

•(七)對人要好,但不能期待人家對你好。 你怎樣對人,並不付表人家就會怎樣對你, 如果看不透這一點,你只會徒添不必要的煩 惱。 •(八)我買了十多二十年六合彩,還是一窮 二白,連三獎也沒有中,這證明人要發達, 還是要努力工作才可以,世界上並沒有免費 午餐。 •(九)親人只有一次的緣份,無論這輩子我 和你會相處多久,也請好好珍惜共聚的時光, 下輩子,無論愛與不愛,都不會再見。
26
• RNH2 + H2O
• •
RNH3+ + OH-
Kb = [RNH3+][OH-] [RNH2]
•
pKb = -log Kb
• Alkylamine pKb = 3 ~ 4
27
28
29
Basicity of amines
(a) Ammonia pKb = 4.74 (b) Alkyl amines are usually stronger bases than ammonia. (c) Increasing the number of alkyl groups decreases solvation of ion, so 2 and 3 amines are similar to 1 amines in basicity. (d) The N lone-pair electrons in arylamines are delocalized by interaction with the aromatic ring electron system and are less able to accept H+ than are alkylamines (lower 30 basicity)
15
16
小学上册第十二次英语第一单元综合卷

小学上册英语第一单元综合卷英语试题一、综合题(本题有100小题,每小题1分,共100分.每小题不选、错误,均不给分)1.I like to watch ________ in the summer.2.My favorite holiday is ________ (圣诞节). I like to decorate the ________ (圣诞树).3.My favorite book is ________.4.What do we call the place where you can buy groceries?A. StoreB. MarketC. MallD. Supermarket5.The _______ of a balloon can be affected by altitude.6.The _______ (兔子) hops around quickly when it is excited.7.What is the name of the game where you shoot hoops?A. SoccerB. BasketballC. BaseballD. TennisB8. A thermochemical reaction involves heat and chemical ______.9. (85) is a famous park in New York City. The ____10.The _______ (Apollo 11) mission successfully landed humans on the Moon.11.What is 100 - 25?A. 65B. 70C. 75D. 8012.What is the main ingredient in sushi?A. RiceB. NoodlesC. BreadD. PotatoesA13.The bear roams in the _____ woods.14.__________ are important for environmental sustainability.15.The chemical formula for table salt is ______.16.What is the capital of Honduras?A. TegucigalpaB. San Pedro SulaC. La CeibaD. CholutecaA17. A ______ (狗) has a keen sense of smell.18.The ancient Greeks created _______ to explain natural phenomena. (神话)19.The teacher, ______ (老师), guides us in our studies.20.The cake is _______ (刚出炉).21.The _____ (first) man-made satellite was Sputnik, launched by the USSR.22.The capital of Faroe Islands is __________.23.The __________ can provide critical insights into environmental health and stability.24.What do you call the place where we see many books?A. SchoolB. LibraryC. StoreD. Park25.What do you call the study of the Earth's atmosphere?A. MeteorologyB. GeologyC. AstronomyD. Ecology26.What is the term for the distance around a circle?A. AreaB. DiameterC. CircumferenceD. RadiusC27. A ___ (小蝴蝶) flutters gently in the air.28.My ________ (玩具) is made of plush material.29.What do we call the act of cleaning a room?A. TidyingB. OrganizingC. DeclutteringD. CleaningA30.What do we call the tool we use to write on paper?A. MarkerB. PenC. PencilD. All of the above31.The teacher gives _____ (作业) every week.32.The _______ of matter refers to whether it is a solid, liquid, or gas.33.What is the opposite of short?A. TallB. WideC. NarrowD. ThickA34.I like to play ___ (video games).35.I like to play ________ with my friends after school.36.My _____ (表妹) is visiting this weekend.37.The ________ was a famous treaty that settled disputes in Europe.38.What do you call the action of planting flowers in a garden?A. GardeningB. LandscapingC. CultivatingD. SowingA39.ts can live for ______ (数十年). Some pla40.My family lives near a __________ (水库).41.What is the opposite of right?A. WrongB. CorrectC. TrueD. AccurateA42.The _____ (羊) eats grass in the field.43.What is the term for a person who collects stamps?A. PhilatelistB. NumismatistC. CollectorD. HobbyistA44.Every year, we celebrate ______ (感恩节) with a big feast and share what we are thankful for.45.The ancient Egyptians created vast ________ (陵墓) for their pharaohs.46.I have a _____ (遥控车) that can go super fast. 我有一辆可以跑得非常快的遥控车。
腈水解酶在羧酸合成中的研究进展

早在20世纪30年代,就有人提出用某些植物 组织能将腈化物转化成酸的假说来解释一些化学
收稿日期:2008-05-26 基金项目:国家自然科学基金资助项目(20506037,20672037);华东理工大学生物反应器工程国家重点实验室开放课题资助项目(2008004) 作者简介:何玉财(1979一),男,辽宁开原人,博士,研究方向:生物催化与生物加工;许建和(联系人),教授,博士生导师,E-mail:jianhexu@∞-
Aci,地£06口c把,AK 226
alkyInitriles,arylnitriles. dinitriles,hetemcyclic mononitriles
Agrobacterium sP.DSM 6336 Alcaligenes sp.ECU0401
heterocyelic nitriles
Comamonas testosteroni
Cryptococcus∥倒淞UFMG—Y61
alkylnitriles isobutyronitrile
Exophiala oligosperma R1 Fusarium solani 01 Gordona terrae FERM BP-4535
phenylacetonitrile 3-Cyanopyrid合成中的重要中间体,它可以通过 氰基的化学水解来获得,通常需要强酸、强碱和高 温回流等苛刻条件,而且常伴随有大量盐类形成, 给产品的分离纯化带来困难,也造成一定程度的环 境污染¨]。用腈水解酶实现氰基的水解,其优势不 仅在于反应条件温和、污染少和处理容易的环境友
好过程,而且更重要的是可以实现一般化学转化所 不具有的优良的化学、区域和立体选择性。
第7卷第1期 2009年1月
化学基础英文4_aromaticity芳香族化合物
For comparison, reactions of cyclohexene, a typical alkene, with these reagents are also shown. As experimental evidence for a wide assortment of compounds was acquired, those incorporating this exceptionally stable six-carbon core came to be called "aromatic".
If benzene is forced to react by increasing the temperature and/or by addition of a catalyst, It undergoes substitution reactions rather than the addition reactions that are typical of alkenes. This further confirms the previous indication that the sixcarbon benzene core is unusually stable to chemical modification.
In practice, 1,3-cyclohexadiene is slightly more stable than expected, by about 2 kcal, presumably due to conjugation of the double bonds. Benzene, however, is an extraordinary 36 kcal/mole more stable than expected. This sort of stability enhancement is now accepted as a characteristic of all aromatic compounds.
Chapter_16_Heterocyclic_Compounds
CH3CH2CH2CH3 + S 550 ~ 600 oC S
WBQ
CH C CHO OH HO O 糠醛 CHO
WBQ
(C5H10O5)n
CH2=CHCH2CH3 + SO2 CH2=CHCH=CH2 + SO2
聚戊醛糖 (稻糠、玉米芯)
NH3
O Zn-Cr2O3-MnO2 CHO 400 ~ 500 oC O + CO
O
Al2O3/430 oC
CuCl
N H
HC CH + 2 HCHO
NH3 HOCH2C CCH2OH pressure
N H
24
23
4
2.3 Synthesis of furan, thiophene, pyrrole
(2) In Lab P2O5
2.3 Synthesis of furan, thiophene, pyrrole
O O+ O
(1) ClCH2Ph O (2) O O
O H O H O O
WBQ
H N NH +
N H
H2/Ni O
H2/MoS2 S S
O
THF
NH
O N CH2Ph H O H O O
CO2R S +
19
CO2R S CO2R
-S
CO2R CO2R
CO2R
20
2.2.3 Basicity and Acidity of Pyrrole
+
WBQ
R H H+
2 3
N H
N
R 2' NH2 1 N 1' H R' R N
萨特勒标准红外谱图
Go to: home • ir • proton nmr • carbon nmr• mass specTable of Contents - IRI. HydrocarbonsII. Halogenated HydrocarbonsIII. Nitrogen Containing CompoundsIV. Silicon Containing Compounds (Except Si-O)V. Phosphorus Containing Compounds (Except P-O And P(=O)-O) VI. Sulfur Containing CompoundsVII. Oxygen Containing Compounds (Except -C(=O)-)VIII. Compounds Containing Carbon To Oxygen Double BondsI. HydrocarbonsA. Saturated Hydrocarbons1. Normal Alkanes2. Branched Alkanes3. Cyclic AlkanesB. Unsaturated Hydrocarbons1. Acyclic Alkenes2. Cyclic Alkenes3. AlkynesC. Aromatic Hydrocarbons1. Monocyclic (Benzenes)2. PolycyclicII. Halogenated HydrocarbonsA. Fluorinated Hydrocarbons1. Aliphatic2. AromaticB. Chlorinated Hydrocarbons1. Aliphatic2. Olefinic3. AromaticC. Brominated Hydrocarbons1. Aliphatic2. Olefinic3. AromaticD. Iodinated Hydrocarbons1. Aliphatic and Olefinic2. AromaticIII. Nitrogen Containing CompoundsA. Amines1. Primarya. Aliphatic and Olefinicb. Aromatic2. Secondarya. Aliphatic and Olefinicb. Aromatic3. Tertiarya. Aliphatic and Olefinicb. AromaticB. PyridinesC. QuinolinesD. Miscellaneous Nitrogen HeteroaromaticsE. HydrazinesF. Amine SaltsG. Oximes (-CH=N-OH)H. Hydrazones (-CH=N-NH2)I. Azines (-CH=N-N=CH-)J. Amidines (-N=CH-N)K. Hydroxamic AcidsL. Azo Compounds (-N=N-)M. Triazenes (-N=N-NH-)N. Isocyanates (-N=C=O)O. Carbodiimides (-N=C=N-)P. Isothiocyanates (-N=C=S)Q. Nitriles (-C≡N)1. Aliphatic2. Olefinic3. AromaticR. Cyanamides (=N-C≡N)S. Thiocyanates (-S-C≡N)T. Nitroso Compounds (-N=O)U. N-Nitroso Compounds (=N-N=O)V. Nitrites (-O-N=O)W. Nitro Compounds (-NO2)1. Aliphatic2. AromaticX. N-Nitro-Compounds (=N-NO2)IV. Silicon Containing Compounds (Except Si-O)V. Phosphorus Containing Compounds (Except P-O and P(=O)-O) VI. Sulfur Containing CompoundsA. Sulfides (R-S-R)1. Aliphatic2. Heterocyclic3. AromaticB. Disulfides (R-S-S-R)C. Thiols1. Aliphatic2. AromaticD. Sulfoxides (R-S(=O)-R)E. Sulfones (R-SO2-R)F. Sulfonyl Halides (R-SO2-X)G. Sulfonic Acids (R-SO2-OH)1. Sulfonic Acid Salts (R-SO2-O-M)2. Sulfonic Acid Esters (R-SO2-O-R)3. Sulfuric Acid Esters (R-O-S(=O)-O-R)H. Thioamides (R-C(=S)-NH2)I. Thioureas (R-NH-C(=S)-NH2)J. Sulfonamides (R-SO2-NH2)K. Sulfamides (R-NH-SO2-NH-R)VII. Oxygen Containing Compounds (Except -C(=O)-)A. Ethers1. Aliphatic Ethers (R-O-R)2. Acetals (R-CH-(-O-R)2)3. Alicyclic Ethers4. Aromatic Ethers5. Furans6. Silicon Ethers (R3-Si-O-R)7. Phosphorus Ethers ((R-O)3-P)8. Peroxides (R-O-O-R)B. Alcohols (R-OH)1. Primarya. Aliphatic and Alicyclicb. Olefinicc. Aromaticd. Heterocyclic2. Secondarya. Aliphatic and Alicyclicb. Olefinicc. Aromatic3. Tertiarya. Aliphaticb. Olefinicc. Aromatic4. Diols5. Carbohydrates6. PhenolsVIII. Compounds Containing Carbon To Oxygen Double BondsA. Ketones (R-C(=O)-R)1. Aliphatic and Alicyclic2. Olefinic3. Aromatic4. α-Diketones and β-DiketonesB. Aldehydes (R-C(=O)-H)C. Acid Halides (R-C(=O)-X)D. Anhydrides (R-C(=O)-O-C(=O)-R)E. Amides1. Primary (R-C(=O)-NH2)2. Secondary (R-C(=O)-NH-R)3. Tertiary (R-C(=O)-N-R2)F. Imides (R-C(=O)-NH-C(=O)-R)G. Hydrazides (R-C(=O)-NH-NH2)H. Ureas (R-NH-C(=O)-NH2)I. Hydantoins, Uracils, BarbituratesJ. Carboxylic Acids (R-C(=O)-OH)1. Aliphatic and Alicyclic2. Olefinic3. Aromatic4. Amino Acids5. Salts of Carboxylic AcidsK. Esters1. Aliphatic Esters of Aliphatic Acids2. Olefinic Esters of Aliphatic Acids3. Aliphatic Esters of Olefinic Acids4. Aromatic Esters of Aliphatic Acids5. Esters of Aromatic Acids6. Cyclic Esters (Lactones)7. Chloroformates8. Esters of Thio-Acids9. Carbamates10. Esters of Phosphorus AcidsPublished by Bio-Rad Laboratories, Inc., Informatics Division. © 1978-2004 Bio-Rad Laboratories, Inc. All Rights Reserved.Go to: home • ir • proton nmr • carbon nmr• mass specTable of Contents - Proton NMRI. HydrocarbonsII. Halogenated HydrocarbonsIII. Nitrogen Containing CompoundsIV. Silicon Containing Compounds (Except Si-O)V. Phosphorus Containing Compounds (Except P-O and P(=O)-O) VI. Sulfur Containing CompoundsVII. Oxygen Containing Compounds (Except -C(=O)-)VIII. Compounds Containing Carbon To Oxygen Double BondsI. HydrocarbonsA. Saturated Hydrocarbons1. Normal Alkanes2. Branched Alkanes3. Cyclic AlkanesB. Unsaturated Hydrocarbons1. Acyclic Alkenes2. Cyclic Alkenes3. AlkynesC. Aromatic Hydrocarbons1. Monocyclic (Benzenes)2. PolycyclicII. Halogenated HydrocarbonsA. Fluorinated Hydrocarbons1. Aliphatic2. AromaticB. Chlorinated Hydrocarbons1. Aliphatic2. AromaticC. Brominated Hydrocarbons1. Aliphatic2. AromaticD. Iodinated Hydrocarbons1. Aliphatic2. AromaticIII. Nitrogen Containing CompoundsA. Amines1. Primarya. Aliphaticb. Aromatic2. Secondarya. Aliphaticb. Aromatic3. Tertiarya. Aliphaticb. AromaticB. PyridinesC. Quaternary Ammonium SaltsD. HydrazinesE. Amine SaltsF. Ylidene Compounds (-CH=N-)G. Oximes (-CH=N-OH)H. Hydrazones (-CH=N-NH2)I. Azines (-CH=N-N=CH-)J. Amidines (-N=CH-N)K. Hydroxamic AcidsL. Azo Compounds (-N=N-)M. Isocyanates (-N=C=O)N. Carbodiimides (-N=C=N-)O. Isothiocyanates (-N=C=S)P. Nitriles (-C≡N)1. Aliphatic2. Olefinic3. AromaticQ. Cyanamides (=N-C≡N)R. Isocyanides (-N≡C )S. Thiocyanates (-S-C≡N)T. Nitroso Compounds (-N=O)U. N-Nitroso Compounds (=N-N=O)V. Nitrates (-O-NO2)W. Nitrites (-O-N=O)X. Nitro Compounds (-NO2)1. Aliphatic2. AromaticY. N-Nitro-Compounds (=N-NO2)IV. Silicon Containing Compounds (Except Si-O)V. Phosphorus Containing Compounds (Except P-O and P(=O)-O) VI. Sulfur Containing CompoundsA. Sulfides (R-S-R)1. Aliphatic2. AromaticB. Disulfides (R-S-S-R)C. Thiols1. Aliphatic2. AromaticD. Sulfoxides (R-S(=O)-R)E. Sulfones (R-SO2-R)F. Sulfonyl Halides (R-SO2-X)G. Sulfonic Acids (R-SO2-OH)1. Sulfonic Acid Salts (R-SO2-O-M)2. Sulfonic Acid Esters (R-SO2-O-R)3. Sulfuric Acid Esters (R-O-S(=O)-O-R)4. Sulfuric Acid Salts (R-O-SO2-O-M)H. Thioamides (R-C(=S)-NH2)I. Thioureas (R-NH-C(=S)-NH2)J. Sulfonamides (R-SO2-NH2)VII. Oxygen Containing Compounds (Except -C(=O)-)A. Ethers1. Aliphatic Ethers (R-O-R)2. Alicyclic Ethers3. Aromatic Ethers4. Furans5. Silicon Ethers (R3-Si-O-R)6. Phosphorus Ethers ((R-O)3-P)B. Alcohols (R-OH)1. Primarya. Aliphaticb. Olefinicc. Aromatic2. Secondarya. Aliphaticb. Aromatic3. Tertiarya. Aliphaticb. Aromatic4. Diols and Polyols5. Carbohydrates6. PhenolsVIII. Compounds Containing Carbon To Oxygen Double BondsA. Ketones (R-C(=O)-R)1. Aliphatic and Alicyclic2. Olefinic3. Aromatic4. a-Diketones and b-DiketonesB. Aldehydes (R-C(=O)-H)C. Acid Halides (R-C(=O)-X)D. Anhydrides (R-C(=O)-O-C(=O)-R)E. Amides1. Primary (R-C(=O)-NH2)2. Secondary (R-C(=O)-NH-R)3. Tertiary (R-C(=O)-N-R2)F. Imides (R-C(=O)-NH-C(=O)-R)G. Hydrazides (R-C(=O)-NH-NH2)H. Ureas (R-NH-C(=O)-NH2)I. Hydantoins, Uracils, BarbituratesJ. Carboxylic Acids (R-C(=O)-OH)1. Aliphatic and Alicyclic2. Olefinic3. Aromatic4. Amino Acids5. Salts of Carboxylic AcidsK. Esters1. Aliphatic Esters of Aliphatic Acids2. Olefinic Esters of Aliphatic Acids3. Aromatic Esters of Aliphatic Acids4. Cyclic Esters (Lactones)5. Chloroformates6. Carbamates7. Esters of Phosphorus AcidsPublished by Bio-Rad Laboratories, Inc., Informatics Division. © 1978-2004 Bio-Rad Laboratories, Inc. All Rights Reserved.Go to: home • ir • proton nmr • carbon nmr• mass specTable of Contents - Carbon NMRI. HydrocarbonsII. Halogenated HydrocarbonsIII. Nitrogen Containing CompoundsIV. Silicon Containing Compounds (Except Si-O)V. Phosphorus Containing Compounds (Except P-O And P(=O)-O) VI. Sulfur Containing CompoundsVII. Oxygen Containing Compounds (Except -C(=O)-)VIII. Compounds Containing Carbon To Oxygen Double BondsI. HydrocarbonsA. Saturated Hydrocarbons1. Normal Alkanes2. Branched Alkanes3. Cyclic AlkanesB. Unsaturated Hydrocarbons1. Acyclic Alkenes2. AlkynesC. Aromatic Hydrocarbons1. Monocyclic (Benzenes) and PolycyclicII. Halogenated HydrocarbonsA. Fluorinated Hydrocarbons1. Aliphatic2. AromaticB. Chlorinated Hydrocarbons1. Aliphatic2. AromaticC. Brominated Hydrocarbons1. Aliphatic2. AromaticD. Iodinated Hydrocarbons1. Aliphatic2. AromaticIII. Nitrogen Containing CompoundsA. Amines1. Primarya. Aliphaticb. Aromatic2. Secondarya. Aliphaticb. Aromatic3. Tertiarya. Aliphaticb. AromaticB. PyridinesC. Amine SaltsD. Oximes (-CH=N-OH)E. Quaternary Ammonium SaltsF. Nitriles (-C≡N)1. Aliphatic2. Olefinic3. AromaticG. Thiocyanates (-S-C≡N)H. Nitro Compounds (-NO2)1. Aliphatic2. AromaticIV. Silicon Containing Compounds (Except Si-O)V. Phosphorus Containing Compounds (Except P-O and P(=O)-O) VI. Sulfur Containing CompoundsA. Sulfides (R-S-R)1. Aliphatic2. AromaticB. Disulfides (R-S-S-R)C. Thiols1. Aliphatic2. AromaticD. Sulfones (R-SO2-R)VII. Oxygen Containing Compounds (Except -C(=O)-)A. Ethers1. Aliphatic Ethers (R-O-R)2. Alicyclic Ethers3. Aromatic EthersB. Alcohols (R-OH)1. Primarya. Aliphatic and Alicyclicb. Aromatic2. Secondarya. Aliphatic and Alicyclic3. Tertiarya. Aliphatic4. PhenolsVIII. Compounds Containing Carbon To Oxygen Double BondsA. Ketones (R-C(=O)-R)1. Aliphatic and Alicyclic2. AromaticB. Aldehydes (R-C(=O)-H)C. Acid Halides (R-C(=O)-X)D. Anhydrides (R-C(=O)-O-C(=O)-R)E. Amides1. Primary (R-C(=O)-NH2)2. Secondary (R-C(=O)-NH-R)3. Tertiary (R-C(=O)-N-R2)F. Carboxylic Acids (R-C(=O)-OH)1. Aliphatic and Alicyclic2. AromaticG. Esters1. Aliphatic Esters of Aliphatic Acids2. Olefinic Esters of Aliphatic Acids3. Aromatic Esters of Aliphatic AcidsPublished by Bio-Rad Laboratories, Inc., Informatics Division. © 1978-2004 Bio-Rad Laboratories, Inc. All Rights Reserved.Go to: home • ir • proton nmr • carbon nmr• mass specTable of Contents - MSComing SoonI. HydrocarbonsII. Halogenated HydrocarbonsIII. Nitrogen Containing CompoundsIV. Silicon Containing Compounds (Except Si-O)V. Phosphorus Containing Compounds (Except P-O And P(=O)-O) VI. Sulfur Containing CompoundsVII. Oxygen Containing Compounds (Except -C(=O)-)VIII. Compounds Containing Carbon To Oxygen Double BondsI. HydrocarbonsA. Saturated Hydrocarbons1. Normal Alkanes2. Branched Alkanes3. Cyclic AlkanesB. Unsaturated Hydrocarbons1. Acyclic Alkenes2. Cyclic Alkenes3. AlkynesC. Aromatic Hydrocarbons1. Monocyclic (Benzenes)2. PolycyclicII. Halogenated HydrocarbonsA. Fluorinated Hydrocarbons1. Aliphatic2. AromaticB. Chlorinated Hydrocarbons1. Aliphatic2. Olefinic3. AromaticC. Brominated Hydrocarbons1. Aliphatic2. Olefinic3. AromaticD. Iodinated Hydrocarbons1. Aliphatic and Olefinic2. AromaticIII. Nitrogen Containing CompoundsA. Amines1. Primarya. Aliphatic and Olefinicb. Aromatic2. Secondarya. Aliphatic and Olefinicb. Aromatic3. Tertiarya. Aliphatic and Olefinicb. AromaticB. PyridinesC. QuinolinesD. Miscellaneous Nitrogen HeteroaromaticsE. HydrazinesF. Amine SaltsG. Oximes (-CH=N-OH)H. Hydrazones (-CH=N-NH2)I. Azines (-CH=N-N=CH-)J. Amidines (-N=CH-N)K. Hydroxamic AcidsL. Azo Compounds (-N=N-)M. Triazenes (-N=N-NH-)N. Isocyanates (-N=C=O)O. Carbodiimides (-N=C=N-)P. Isothiocyanates (-N=C=S)Q. Nitriles (-C≡N)1. Aliphatic2. Olefinic3. AromaticR. Cyanamides (=N-C≡N)S. Thiocyanates (-S-C≡N)T. Nitroso Compounds (-N=O)U. N-Nitroso Compounds (=N-N=O)V. Nitrites (-O-N=O)W. Nitro Compounds (-NO2)1. Aliphatic2. AromaticX. N-Nitro-Compounds (=N-NO2)IV. Silicon Containing Compounds (Except Si-O)V. Phosphorus Containing Compounds (Except P-O and P(=O)-O) VI. Sulfur Containing CompoundsA. Sulfides (R-S-R)1. Aliphatic2. Heterocyclic3. AromaticB. Disulfides (R-S-S-R)C. Thiols1. Aliphatic2. AromaticD. Sulfoxides (R-S(=O)-R)E. Sulfones (R-SO2-R)F. Sulfonyl Halides (R-SO2-X)G. Sulfonic Acids (R-SO2-OH)1. Sulfonic Acid Salts (R-SO2-O-M)2. Sulfonic Acid Esters (R-SO2-O-R)3. Sulfuric Acid Esters (R-O-S(=O)-O-R)H. Thioamides (R-C(=S)-NH2)I. Thioureas (R-NH-C(=S)-NH2)J. Sulfonamides (R-SO2-NH2)K. Sulfamides (R-NH-SO2-NH-R)VII. Oxygen Containing Compounds (Except -C(=O)-)A. Ethers1. Aliphatic Ethers (R-O-R)2. Acetals (R-CH-(-O-R)2)3. Alicyclic Ethers4. Aromatic Ethers5. Furans6. Silicon Ethers (R3-Si-O-R)7. Phosphorus Ethers ((R-O)3-P)8. Peroxides (R-O-O-R)B. Alcohols (R-OH)1. Primarya. Aliphatic and Alicyclicb. Olefinicc. Aromaticd. Heterocyclic2. Secondarya. Aliphatic and Alicyclicb. Olefinicc. Aromatic3. Tertiarya. Aliphaticb. Olefinicc. Aromatic4. Diols5. Carbohydrates6. PhenolsVIII. Compounds Containing Carbon To Oxygen Double BondsA. Ketones (R-C(=O)-R)1. Aliphatic and Alicyclic2. Olefinic3. Aromatic4. α-Diketones and β-DiketonesB. Aldehydes (R-C(=O)-H)C. Acid Halides (R-C(=O)-X)D. Anhydrides (R-C(=O)-O-C(=O)-R)E. Amides1. Primary (R-C(=O)-NH2)2. Secondary (R-C(=O)-NH-R)3. Tertiary (R-C(=O)-N-R2)F. Imides (R-C(=O)-NH-C(=O)-R)G. Hydrazides (R-C(=O)-NH-NH2)H. Ureas (R-NH-C(=O)-NH2)I. Hydantoins, Uracils, BarbituratesJ. Carboxylic Acids (R-C(=O)-OH)1. Aliphatic and Alicyclic2. Olefinic3. Aromatic4. Amino Acids5. Salts of Carboxylic AcidsK. Esters1. Aliphatic Esters of Aliphatic Acids2. Olefinic Esters of Aliphatic Acids3. Aliphatic Esters of Olefinic Acids4. Aromatic Esters of Aliphatic Acids5. Esters of Aromatic Acids6. Cyclic Esters (Lactones)7. Chloroformates8. Esters of Thio-Acids9. Carbamates10. Esters of Phosphorus AcidsPublished by Bio-Rad Laboratories, Inc., Informatics Division. © 1978-2004 Bio-Rad Laboratories, Inc. All Rights Reserved.Go to: home • ir • proton nmr • carbon nmr• mass specSaturated HydrocarbonsNormal Alkanes1. C-H stretching vibration:CH3 asymmetric stretching, 2972-2952 cm-1CH3 symmetric stretching, 2882-2862 cm-1CH2 asymmetric stretching, 2936-2916 cm-1CH2 symmetric stretching, 2863-2843 cm-12. C-H bending vibration:CH3 asymmetric bending, 1470-1430 cm-1CH2 asymmetric bending, 1485-1445 cm-1(overlaps band due to CH3 asymmetricbending)3. C-H bending vibration:CH3 symmetric bending, 1380-1365 cm-1(when CH3 is attached to a C atom)4. C-H wagging vibration:CH2 out-of-plane deformations wagging, 1307-1303 cm-1 (weak) 5. CH2 rocking vibration:(CH2)2 in-plane deformations rocking, 750-740 cm-1(CH2)3 in-plane deformations rocking, 740-730 cm-1(CH2)4 in-plane deformations rocking, 730-725 cm-1(CH2) ≥ 6 in-plane deformations rocking, 722 cm-1Splitting of the absorption band occurs in most cases (730 and 720 cm-1) when the long carbon-chain alkane is in the crystalline state (orthorombic or monoclinic form).Coming Soon!Click on a vibrational mode link in the table to the leftor the spectrum above to visualize the vibrational mode here.Published by Bio-Rad Laboratories, Inc., Informatics Division. © 1978-2004 Bio-Rad Laboratories, Inc. All Rights Reserved.Saturated HydrocarbonsBranched Alkanes1. C-H stretching vibration:CH3 asymmetric stretching, 2972-2952 cm-1CH3 symmetric stretching, 2882-2862 cm-1CH2 asymmetric stretching, 2936-2916 cm-1CH2 symmetric stretching, 2863-2843 cm-12. C-H bending vibration:CH3 asymmetric bending, 1470-1430 cm-1CH2 asymmetric bending, 1485-1445 cm-1(overlaps band due to CH3 symmetric bending)3. C-H bending vibration:-C-C(CH3)-C-C- symmetric bending, 1380-1365 cm-1(when CH3 is attached to a C atom)-C-C(CH3)-C(CH3)-C-C- symmetric bending, 1380-1365 cm-1(when CH3 is attached to a C atom)(CH3)2CH- symmetric bending, 1385-1380 cm-1and 1365 cm-1(two bands of about equal intensity)-C-C(CH3)2-C- symmetric bending,1385-1380 cm-1and 1365 cm-1 (two bands of about equal intensity).(CH3)3C- symmetric bending, 1395-1385 cm-1and 1365 cm-1(two bands of unequal intensity with the 1365 cm-1 band as the much stronger component of the doublet).4. Skeletal vibration:-C-C(CH3)-C-C-,1159-1151cm-1-C-C(CH3)-C(CH3)-C-C-,1130-1116 cm-1(CH3)CH-,1175-1165 cm-1 and 1170-1140 cm-1-C-C(CH3)2-C-,1192-1185 cm-1(CH3)3C-, 1255-1245 cm-1 and 1250-1200 cm-15. C-H rocking vibration:(CH2)2 in-plane deformations rocking, 750-740 cm-1(CH2)3 in-plane deformations rocking, 740-730 cm-1(CH2)4 in-plane deformations rocking, 730-725 cm-1(CH2) ≥ 6 in-plane deformations rocking, 722 cm-1Coming Soon!Click on a vibrational mode link in the table to the left or the spectrum above to visualize the vibrational modehere.Published by Bio-Rad Laboratories, Inc., Informatics Division. © 1978-2004 Bio-Rad Laboratories, Inc. All Rights Reserved.Saturated HydrocarbonsCyclic AlkanesCyclopropanes1. C-H stretching vibration:ring CH2 asymmetric stretching, 3100-3072 cm-1 ring CH2 symmetric stretching, 3030-2995 cm-1 2. Ring deformation vibration:ring deformation, 1050-1000 cm-13. C-H deformation vibration:CH2 wagging, 860-790 cm-1 Cyclobutanes1. C-H stretching vibration:ring CH2 asymmetric stretching, 3000-2974 cm-1ring CH2 symmetric stretching, 2925-2875 cm-12. C-H deformation vibration:ring CH2 asymmetric bending, ca 1444 cm-13. Ring deformation vibration:ring deformation, 1000-960 cm-1888-838 cm-14. C-H deformation vibration:ring CH2 rocking, 950-900 cm-1Cyclopentanes1. C-H stretching vibration:ring CH2 asymmetric stretching, 2960-2952 cm-1ring CH2 symmetric stretching, 2866-2853 cm-12. C-H deformation vibration:ring CH2 asymmetric bending, ca 1455 cm-13. Ring deformation vibration:ring deformation, 1000-960 cm-14. C-H deformation vibration:ring CH2rocking, 930-890 cm-1Cyclohexanes1. C-H stretching vibration:ring CH2 asymmetric stretching, ca 2927 cm-1ring CH2 symmetric stretching, ca 2854 cm-12. C-H deformation vibration:ring CH2 asymmetric bending, ca 1462 cm-13. C-H deformation vibration:ring CH2 wagging, ca 1260 cm-14. Ring deformation vibration:ring deformation, 1055-1000 cm-11000- 952 cm-15. C-H deformation vibration:ring CH2 rocking, 890-860 cm-16. The spectra of cyclic alkanes of five or more ring carbons showring CH2 stretching frequencies which overlap those of CH3 and CH2groups of their alkyl substituents. These frequencies also overlap thoseof the CH3 and CH2 stretching frequencies of acylic alkanes. When samplesof unknown composition are examined for the presence of such ring structures, the absorption bands of their spectra at the C-H stretching region should have the best possible resolution.Coming Soon!Click on a vibrational mode link in the table to the left or the spectrum above to visualize the vibrational modehere.Numerous references cite the spectral region of 2800-2600 cm-1 for obtainingconfirmatory evidence of the presence of saturated simple ring structures. Absorptionat this region consists of a weak band or bands whose pattern and band locations arehelpful in confirming or indicating the presence of these rings. Although such absorptionfeatures have a limited diagnostic value, it is most reliable when the absorption occursin the spectra of simple saturated aliphatic hydrocarbons.Cycloalkanes (8, 9, and 10 C atoms)1 C-H stretching vibration:ring CH2 asymmetric stretching, ca 2930 cm-1ring CH2 symmetric stretching, ca 2850 cm-12. C-H deformation vibration:ring CH2 asymmetric bending, 2 or 3 absorption bands,1487-1443 cm-1Published by Bio-Rad Laboratories, Inc., Informatics Division. © 1978-2004 Bio-Rad Laboratories, Inc. All Rights Reserved.Go to: home • ir • proton nmr • carbon nmr• mass specUnsaturated HydrocarbonsAcyclic AlkenesMonosubstituted Alkenes (vinyl)1. C=C stretching vibration:C=C stretching, 1648-1638 cm-12. C-H deformation vibration:trans CH wagging, 995-985 cm-1CH2 wagging, 910-905 cm-13. C-H stretching vibration:CH2 asymmetric stretching, 3092-3077 cm-1CH2 symmetric stretching and CH stretching, 3025-3012 cm-1 4. C-H deformation vibration:CH2 asymmetric bending, 1420-1412 cm-15. C-H deformation vibration overtone:overtone of CH2 wagging, 1840-1805 cm-1Asymmetric Disubstituted Alkenes (vinylidine) 1. C=C stretching vibration:C=C stretching, 1661-1639 cm-12. C-H deformation vibration:CH2 wagging, 895-885 cm-13. C-H stretching vibration:CH2 stretching asymmetric, 3100-3077 cm-14. C-H deformation vibration overtone:overtone of CH2 wagging, 1792- 1775 cm-1 Symmetric Disubstituted Alkenes (cis)1. C=C stretching vibration:C=C stretching, 1662- 1631 cm-12. C-H deformation vibration:cis CH wagging, 730- 650 cm-13. C-H stretching vibration:CH stretching, 3050-3000 cm-1Symmetric Disubstituted Alkenes (trans)1. C=C stretching vibration:C=C stretching, ca 1673 cm-1, very weak or absent2. C-H deformation vibration:trans CH wagging, 980-965 cm-13. C-H stretching vibration:CH stretching, 3050-3000 cm-1Trisubstituted Alkenes1. C=C stretching vibration:C=C stretching, 1692-1667 cm-12. C—H deformation vibration:C-H wagging, 840-790 cm-13. C-H stretching vibration:C-H stretching, 3050-2990 cm -1Coming Soon!Click on a vibrational mode link in the table to the left or the spectrum above to visualize the vibrational modehere.Tetrasubstituted Alkenes1. C=C stretching vibration:C=C stretching, 1680-1665 cm-1, very weak or absentNOTES: The C=C stretching vibration of molecules which maintain acenter of symmetry absorbs very weakly, if at all, in the infrared region and,usually, is difficult to detect. This is true of the trans isomers and thetetrasubstitutedC=C linkages.When two or more olefinic groups occur in the hydrocarbon molecule, the infraredabsorption spectrum shows the additive and combined absorption of theunsaturatedgroups. However, if the unsaturated groups are subject to conjugation, the C=Cstretchingfrequency, usually, is lowered and a splitting of the C=C stretching frequencyband occurs.Conjugation also intensifies the C=C stretching frequency of trans unsaturatedgroups.Published by Bio-Rad Laboratories, Inc., Informatics Division. © 1978-2004 Bio-Rad Laboratories, Inc. All Rights Reserved.Go to: home • ir • proton nmr • carbon nmr• mass specUnsaturated HydrocarbonsCyclic AlkenesEndocyclic C=CEndocyclic C=C corresponds to cis symmetrically disubstituted C=C of acyclic alkenes.1. C=C stretching, vibration:C=C stretching, near 1650 cm-1(except cyclobutene, 1560 cm-1 and cyclopentene, 1611 cm-1)2. C-H deformation vibration:CH wagging, 730- 650 cm-13. C-H stretching vibration:CH stretching, 3075- 3010 cm-1(usually two bands, asymmetric stretching and symmetric stretchingfor 4, 6, 7, and 8 membered rings)1- substituted endocyclic C=C1- substituted endocyclic C=C corresponds to trisubstituted acyclic alkenes.1. C=C stretching vibration:C=C stretching, near 1650 cm-1 (frequency raised)2. C-H deformation vibration:CH wagging, 840-790 cm-13. C-H stretching vibration:CH stretching, near 3000 cm-11.2- disubstituted endocyclic C=C1. C=C stretching vibration:C=C stretching, 1690-1670 cm-1 (4, 5, and 6 membered rings)Exocyclic C=CH2Exocyclic C=CH2 corresponds to the asymmetrically disubstituted C=C of acyclic alkenes (vinylidine).1. C=C stretching,1678-1650 cm-1 (4, 5, and 6 membered rings)2. C-H deformation vibration:=CH2 wagging, 895-885 cm-13. C-H stretching vibration:=CH2 stretching, near 3050 cm-1NOTES: The C=C stretching frequency of both the endocyclic HC=CH and the exocyclicC=CH2 is sensitive to ring strain. As the ring size decreases from 6 to 4 members, the C=C stretching frequency of the endocyclic HC=CH is lowered. However, for the C=C stretching frequency of exocyclic C=CH2, a gradual increase in the C=C stretching frequency occurs as the ring gets smaller. Substitution of methyl groups for the hydrogens of the endocyclic HC=CH and the exocyclic C=CH2 cause an increase in the C=C stretching frequency. When two or more C=C groups occur in the hydrocarbon molecule, the infrared absorption spectrum shows the additive and combined absorption effects of the unsaturated groups. If such groups are subject to conjugation, the C=C stretching frequency is lowered and a splitting of the C=C stretching frequency band occurs.Coming Soon!Click on a vibrational mode link in the table to the left or the spectrum above to visualize the vibrational modehere.Published by Bio-Rad Laboratories, Inc., Informatics Division. © 1978-2004 Bio-Rad Laboratories, Inc. All Rights Reserved.Unsaturated Hydrocarbons AlkynesMonosubstituted Alkynes (RC≡CH) 1. C≡C stretching vibration:C≡C stretching, 2140-2100 cm-12. C-H stretching vibration:≡CH bending, ca 3300 cm-13. C-H deformation vibration:≡CH bending, 642-615 cm-14. C-H deformation vibration overtone:overtone of ≡CH deformation, 1260-1245 cm-1 Disubstituted Alkynes (RC≡CR')1. C≡C stretching vibration:C≡C stretching, 2260-2190 cm-1 (unconjugated)NOTES: Although the intensity of the absorption band caused bythe C≡C stretching vibration is variable, it is strongest when the alkynegroup is monosubstituted. When this group is disubstituted in open chain compounds, the intensity of the C≡C stretching vibration band diminishesas its position in the molecule tends to establish a pseudo center of symmetry. In some instances this band is too weak to be detected and, thus, its absence in the spectrum does not, necessarily, establish proof of the absence of this linkage.Occasionally, the spectra of disubstituted alkynes show two or more bandsat the C≡C stretching region.Conjugation with olefinic double bonds or aromatic rings tend to slightly increase the intensity of the C≡C stretching vibration band and shift it toa lower frequency.Coming Soon!Click on a vibrational mode link in the table to the left or the spectrum above to visualize the vibrational modehere.Published by Bio-Rad Laboratories, Inc., Informatics Division. © 1978-2004 Bio-Rad Laboratories, Inc. All Rights Reserved.。
化学竞赛PPT-第八章 芳烃-杂环化合物
★ 吡咯中氮原子为sp2杂化状态;形成三个σ键,一对孤
对电子所在p轨道与四个碳原子的p轨道平行重叠。 ★ 孤对电子因为p-π共轭,而呈给电子效应。
亲电取代反应的活性大于苯, 环上电子云密度比苯大.
芳香性顺序:
环上电子云分布不均,芳香性不如苯。
>
>物
O
O
非芳香性杂环化合物具有与相 O
应脂肪族化合物相类似的性质。 O
体现各自功能团的性质
O
Aromatic Heterocycles 芳香杂环化合物
N H
NO H
一 .Classification & Nomenclature:
按环的大小
Five-membered ring Six-membered ring
2、与NaNH2反应,往吡啶环上引入胺基。 将吡啶与氨基钠在N,N-二甲基苯胺溶液中加热到110℃,
吡啶环上2-位上的氢负离子被亲核性极强的氨基负离子取代, 同时有氢气放出。齐齐巴宾(Chichibabin)反应。
3、与苯基锂作用生成2-苯基吡啶,反应中间体已分离鉴 定,证明为1,2-加成产物:
五、氧化还原反应
N3 2
S 1
CH3 4 3 Br
5 O 2 Br 1
第一节 吡咯、呋喃和噻吩
它们都含有由五个原子和六个π-电子所组成的共轭体系,环 内原子上的电子云密度大于苯环。因此,是富电子的芳香杂环化 合物。如以苯环上碳原子的电荷密度为标准(作为0),则五元杂环化合物
的有效电荷分布为
一、结构
★ 分子呈平面型,氧原子为sp2杂化状态;形成两个σ键, 一对孤对电子所在p轨道与四个碳原子的p轨道平行重叠。
Aromatic chloride to nitrile
Aromatic chloride to nitrile transformation:medicinal and synthetic chemistry †Lyn H.Jones,*Nicholas W.Summerhill,*Nigel A.Swain and James lsReceived 17th August 2010,Accepted 13th September 2010DOI:10.1039/c0md00135jThis review highlights the medicinal and synthetic chemistry relevance of replacing an aromaticchloride motif with an aromatic nitrile.We explore the desirable features that this transformation can bring in a drug design sense and the recent synthetic chemistry advances that effect this replacement in a single step.IntroductionIt is rare to find examples of ‘design rules’in medicinal chemistry.We are often faced with situations where the incorporation of thematic learnings from previous medicinal chemistry pro-grammes can lead to successful outcomes,but where specific ‘medicinal chemistry transformations’often do not translate.This ambiguity adds to the challenges of effective drug discovery and suggests that a formulation of successful transformations,if possible,would increase our chances of success.This philosophy has been applied to the area of ‘bioisosteric’replacements,where the key features of molecular interaction are mimicked in alter-native structural motifs that can modify the overall medicinal chemistry profile of the molecule.1Aromatic chlorides are ubiquitous amongst high affinity protein binders and are often hits from high throughput screening efforts.There are presumably various reasons for this,including a high proportion of chloroaromatics in screening sets in the first place,possibly due to the synthetic expediency of their creation,and a variety of available binding modes,including strong hydrophobic interactions with the target protein driven by the lipophilicity of the residue (see below).Within Pfizer we have discovered through experience that replacing this moiety with an aromatic nitrile can often result in a compound with similar affinity/potency,although this transformation does not seem to have achieved widespread use,presumably in part due to the lack of publications that signpost this useful substitution.This review will explore the fundamental medicinal chemistry aspects of this switch and describe the aromatic nitrile containing drugs on the market.We will augment the analysis with data that support the generality of the conversion and the improvements seen in drug-type properties driven by modifications to the physicochemical properties of the resulting molecules.Moreover,the synthetic chemistry to effect this transformation in a single step has made significant progress in the last decade,making this a particularly apt time for review.Aromatic chlorides are in general inexpen-sive and much more readily available than bromides and iodides.Therefore,they are ideal starting materials for cross coupling reactions.Unfortunately,chlorides also constitute the mostchallenging substrates for these reactions,due to the high bond dissociation energy of the C(sp 2)–Cl bond.For this reason the transition metal-catalysed cyanation reaction of aryl chlorides is an emerging but valuable field when aligned with the medicinal chemistry relevance of the conversion,and this review is antici-pated to be of interest to a wide community of synthetic and medicinal chemists from both academia and industry.Medicinal chemistry relevance and physicochemical propertiesDrugs containing an aromatic nitrileA search of Prous Science Integrity Ò(Thomson Reuters)revealed 12launched drugs (10structurally distinct compounds)that contain the aromatic nitrile moiety (Fig.1),compared with 224aromatic chloride-containing drugs.All but one (lodoxamide)of the aromatic nitrile drugs was found to possess structure–activity relationships (SARs)supporting a potent chloride partner,although the exact matched pair is not always available for nitrile containing drugs.For the selective serotonin re-uptake inhibitor (SSRI)and antidepressant drug citalopram,there is a matching chloro-analogue with similar potency called LU-10-134-C (Fig.1).2Similarly,febuxostat,a non-purine selective xanthine oxidase inhibitor for the treatment of hyperuricemia,possesses a match-ing potent chlorinated derivative 1.3Interestingly,a co-crystal structure of febuxostat with xanthine oxidase shows there to be a hydrogen bond between the nitrile moiety and the carboxamide residue of Asn768.4In the case of the aromatase inhibitor and anticancer agent letrozole,both nitriles can derive from chlorine substituents (molecule 2).5Additionally,structure–activity rela-tionships map fadrozole to the chloro-equivalent molecule.6Periciazine and cyamemazine are antipsychotic drugs from the phenothiazine class and although the exact matching pair where the nitrile is replaced by chlorine,are not known,both drugs derive from the prototypical chlorpromazine.7The SAR of bicalutamide,an oral antiandrogen for the treatment of prostate cancer,suggests that a 4-CN group is superior to a chloro-substituent (compound 3).8The crystal structure of bicalutamide with the antiandrogen receptor ligand binding domain shows there to be a hydrogen bond between the nitrile moiety and the Arg752residue.9Sandwich Chemistry,World Wide Medicinal Chemistry,Pfizer Ltd.,Ramsgate Road,Sandwich,United Kingdom.E-mail:lyn.jones@pfi;Fax:+44-1304-651821;Tel:+44-1304-644256†Electronic supplementary information (ESI)available:Supplementary figures.See DOI:10.1039/c0md00135jREVIEW /medchemcomm |MedChemCommP u b l i s h e d o n 11 O c t o b e r 2010. D o w n l o a d e d o n 21/12/2015 11:12:02.View Article Online / Journal Homepage / Table of Contents for this issueSimilarly,the cyano-group in the dipeptidyl peptidase IV inhibitor alogliptin (for the oral treatment of type 2diabetes)is able to hydrogen bond to Arg125in the enzyme binding site.10A corresponding chloro-derivative has also been described.11The non-nucleoside reverse transcriptase inhibitor (NNRTI)and anti-HIV drug etravirine contains two aromatic nitrile moieties and structure–activity relationships suggest that at least one can be effectively replaced by a chlorine atom.12A key structural component for resilience to drug resistant mutations in this class is an effective edge-to-face p -interaction with an immutable tryptophan residue in the allosteric reverse tran-scriptase (RT)binding pocket.13Crystal structures of etravirine 14and capavirine 15with RT show that the hydrogen atom ortho -to the chlorine atoms in capravirine and nitrile moiety in etravirine is indeed interacting with the immutable Trp229and both molecules possess excellent mutant viral profiles.We also have a matching pair of imidazole NNRTIs with excellent mutant profiles from our own work 16that supports these observations (compounds 5and 6,Fig.2).Additionally,the nitrile moiety in these ligands points through a hole in the enzyme towards solvent,an arrangement that is ideally suited to the shape and electrostatics of this residue (see below).17High throughput screening hitsFor the drugs highlighted above,it is likely that the preparation of the aromatic chlorides preceded that of the nitrile-containingdrug in those corresponding chemotypes,judging from the relevant publications.This may result from the much higher likelihood of discovering an aryl chloride ‘hit’or lead that could then persist through a drug discovery programme.For example,an analysis of 56recent,diverse high throughput screens (HTS)at Pfizer (across enzyme,GPCR and ion channel classes)revealed that the proportion of actives (>40%activity at 10m M)that contain an aromatic chloride is significantly higher than that for an aromatic nitrile (Fig.3).Additionally,the plots of the propensity of an aromatic chloride to yield an active versus an aromatic nitrile show that the chloride consistently outperforms the nitrile across the three substitutions (Fig.4,also see Supporting Information for loga-rithmicplots).Fig.1Aromatic nitrile-containing drugs and their corresponding chloride congeners (launch date inbrackets).Fig.2Potency,lipophilic efficiency (LipE)and metabolic stability of NNRTIs.P u b l i s h e d o n 11 O c t o b e r 2010. D o w n l o a d e d o n 21/12/2015 11:12:02.Additionally,there is a significant difference in the number of commercially available functional building blocks for synthesis,that we refer to as monomers ,containing aromatic chlorides versus nitriles (Table 1).18It follows that an aromatic chloride hit or lead may later be converted in a medicinal chemistry sense to a nitrile residue through bespoke synthesis,but the lack of functional monomers,and large number of chloroaromatic monomers,may also help explain the greater number of drugs containing an aromatic chloride residue.Electrostatic potential and dipole momentFig.5shows the electrostatic potential maps for chlorobenzene and benzonitrile which illustrate the similarity in the electron density of the benzene rings.19The key differences reside as expected in the region of the substituent,with an overall negative potential at the –CN terminus,whilst an electroposi-tive s ‘hole’is present on the outermost portion of the chlorine atom surface.This positive potential (present on chlorine,bromine and iodine)can interact non-covalently with the negative potential present on other atoms –this is referred to as ‘halogen bonding’.It may therefore be expected that if binding energies for a particular template are dominated through interactions with the benzene ring directly (e.g.p -stacking arrangements)then there may be little to choose between these groups regarding their affinity or potency,but that significant changes may result from interactions with the chlorine or nitrile substituents directly (see below).The dipole moments of these groups are also substantially different:PhCl ¼1.67debye,PhCN ¼4.44debye and reflect the higher propensity for a dipole interaction with the aryl nitrile.20Indeed,the high dipole moment and electronic polarisability of benzonitrile 21imparts interesting applications for this motif in linear and non-linear optical materials.22These values would suggest that the preferred binding environments for the aromatic nitrile residue are likely to have a comple-mentary polar nature,compared to that of the aryl chloride (see below).The hydrogen bonding to the aromatic nitrile drugs alogliptin,bicalutamide and febuxostat described above would appear to support this.Binding site similarity and differencesAnalysis of the polarity of the protein binding sub-pockets of 134aromatic chloride and nitrile-containing paired ligands in the Protein Data Bank (PDB)23and Pfizer internal co-crystal struc-tures,and the degree to which these residues are buried in the protein,demonstrates some fundamental differences between these moieties (Fig.6).Although this data set is relatively small,making firm conclusions difficult,it would appear that the preferred binding site of an aromatic chloride is more hydro-phobic (highlighted by Fig.6a)and buried (Fig.6b)relative to that of an aromatic nitrile.This is in line with the propensity for the more lipophilic aromatic chloride motif to prefer hydro-phobic regions of the binding protein (see discussion of lip-ophilicity below),which are likely to be more ‘buried’due to the hydrophobic effect.24The nature of the sub-pocket in the immediate vicinity of the expected area of difference in electrostatic potential between the chlorine atom (positive electrostatic potential in this region)and nitrile residue (negative electrostatic potential in this region)was also explored.This was assessed by outputting the nature of the atom contributing the closest surface point to the vector passing through the C–Cl or C–CN bond)for para -substituted phenyl rings (for ortho -and meta -substituted groups,the precise loca-tion of the substituent in the pocket is ambiguous).Although the small numbers of observations makes it difficult to generate statistically significant results,it can be seen that sub-pockets containing H-bond acceptor,acid or p -system atoms in this position show a preference for Cl moieties and those sites that contain a donor or amphiprotic atom show more of a preference for CN moieties,again in line with the electrostatic potentials of these groups (Fig.7).Specifically relevant for the ArCl systems is halogen bonding (the non-covalent interaction between the electropositive s -crown on the halogen and a Lewis base)and this has been reviewed regarding its importance in biologically-relevant inter-actions.26Of particular interest in halogen bonding is the affinity of aromatic chlorides for p -systems,as illustrated by the serine protease factor Xa inhibitors that interact in an edge-to-face manner through the chlorine atom of the inhibitor and Tyr228.27Fig.3Proportion of HTS actives containing an active ArCl or ArCN,for a)ortho -b)meta -and c)para -derivatives (unity line shown).Each point represents anHTS.Fig.4Propensity for an ArCl or ArCN to yield an active hit in an HTS for a)ortho -b)meta -and c)para -derivatives (unity line shown).Each point represents an HTS.P u b l i s h e d o n 11 O c t o b e r 2010. D o w n l o a d e d o n 21/12/2015 11:12:02.Similarly,an analysis of the Cambridge Structural Database 28revealed 400possible interactions between the chlorine atom on an aromatic ring and the p -system of a nearby aromatic residue(4.5 Acut off).29Since halogen bonding requires specific interactions with the electropositive crown on the chlorine atom,it is possible that a nitrile switch would result in an affinity reduction,particularlyif a large component of the binding energy is derived from the halogen bond itself.27Lipophilic efficiencyAs we previously mentioned,the conversion of an aromatic chloride to a nitrile is a desirable modification of the drug molecule particularly when the potency is similar,or even improved (see analysis below).The main reason for this is that the lipophilicity of the molecule is significantly reduced,as reflected in the measured LogP (logarithm of 1-octanol/water partition coefficient)for chlorobenzene of 2.84,30but only 1.5631for benzonitrile (Rekker fragment 32for arylCN ¼À0.2;arylCl ¼0.9;D LogP ¼1.1).A reduction in lipophilicity can improve drug-type properties,such as improved metabolic stability,33higher aqueous solubility 34and reduced toxicological outcomes 35(by reducing target promiscuity,amphiphilicity and detergent effects).For example,the NNRTI compounds shown in Fig.2illustrate the significant improvement in metabolic stability that can be achieved,as measured by half-life in human liver micro-somes,on making the nitrile switch.Since lipophilicity can drive binding through non-specific hydrophobic collapse (a D LogP of 1could translate to a D G difference of 1.4kcal mol À1)we can normalise potency for lipophilicity by using the lipophilic effi-ciency parameter,LipE ¼ÀLog(IC 50)–LogP.36Calculating LipE for the NNRTIs in Fig.2shows the di-cyanobenzene derivative 6outperforms the chloro-derivative 5,thus imparting a ‘true’benefit to the molecule.37To further explore the LipE relevance of the aromatic chloride to nitrile switch,an analysis of the Targetbook database 38comparing the potency of 1074distinctly matched ArCl-ArCN pairs across a multitude of target classes was undertaken.As expected,a direct comparison of the potency for matched pairs showed a spread of data from across enzyme,GPCR and ion channel targets (Fig.8a).However upon normalising for lip-ophilicity,using calculated LogP,the plot showed a clear trend towards improved LipE for aromatic nitriles versus their corre-sponding aromatic chloride congener (Fig.8b).39Very similar results are obtained when the Pfizer data set is interrogated in this way (see ESI†).A pie chart analysis of these data (Fig.8c)clearly shows an exceptionally high probabability of aryl nitriles displaying better lipophilic efficiency relative to the corresponding aryl chloride.These statistics make a compelling case for replacing an aromatic chloride with the nitrile derivative to improve the lipophilic efficiency,and by inference,the medicinal chemistry properties of the molecule.An analysis of the Pfizer data set confirms the translation of improved LipE for the aromatic nitrile providing improved metabolic stability relative to the aromatic chloride.Fig.9shows that the aromatic nitrile out-performs the chloride congener as assessed by an improvement in metabolic stability in human liver microsomes.Advances in the ArCl to ArCN synthetic transformationSince the replacement of an aromatic chloride with an aromatic nitrile can impart desirable drug-type properties to the resultingTable 1Commercial availability of aromatic chloride and nitrile monomers 18Monomer Chloride Nitrile RCO 2H 657053690RNH 21340637805ArOH 365771538ArB(OR)2527RCO 2Me465061948Fig.5Electrostatic potential maps for chlorobenzene and benzonitrile (red ¼lowest,blue ¼highest electrostatic potential energy).19Fig.6Sub-pockets predicted to contain ArCl and ArCN moieties (on the grounds that a co-crystallised ligand in the same pocket is at least 75%similar)were analysed using an in-house method to assess burial and polarity.For each such group,a regular array of 642vectors is sprayed out from the centroid,25each vector terminating at the closest point at which it meets the protein surface.The proportion of such vectors witha length <5 Agives a measure of sub-pocket burial and the proportion of vectors terminating at a polar protein atom gives a measure of the sub-pocket polarity.Each sub-pocket was defined as preferring ArCl,ArCN or neither using a 3-fold potencywindow.Fig.7Relationship between preference and nature of binding pocket at the point (X)where the electrostatic potentials of ArCl and ArCN differ.Pockets were defined as either CN-preferring (green),Cl-preferring (red)or preferring neither (amber)using a 3-fold potency cutoff window.Pocket properties are described by the nature of the atom (A ¼acceptor,B ¼basic,C ¼acidic,D ¼donor,H ¼hydrophobic,M ¼amphiprotic,P ¼p -system atom,S ¼solvent-exposed i.e.no atom)closest to X.Number of observations giving rise to each pie chart is shown.P u b l i s h e d o n 11 O c t o b e r 2010. D o w n l o a d e d o n 21/12/2015 11:12:02.molecule,synthetic methods that effect aromatic nitrile synthesis would facilitate their incorporation into drug designs.Several methods are available for the preparation of aromatic nitriles,including the Rosemund-von Braun reaction 40and diazotisation of anilines followed by the Sandmeyer reaction.41On tonne scale the route of choice is often ammoxidation 42where very simple aryl nitriles are synthesised from the toluene derivative by reac-tion with oxygen and ammonia at 300–550 C in the presence of heterogeneous fixed-bed catalysts.A more synthetically attrac-tive method is the transition metal-catalysed cyanation of aryl halides,which has been reviewed in the literature.43However,to date these reviews have focused almost exclusively on aryl bromide or iodide starting points.Aryl chlorides are in general much more readily available and inexpensive than bromides and iodides,and are ideal starting materials for cross-coupling reactions.44Unfortunately,chlo-rides also constitute the most challenging substrates for these reactions,due to the high bond dissociation energy of the C(sp 2)–Cl bond (C–Cl ¼402,C–Br ¼339,C–I ¼272kJ mol À1).45For this reason the transition-metal catalysed cyanation reaction of aryl chlorides is an emerging field,and significant advances have been made in the last decade.The purpose of this synthetic review is to bring together the known methods of this trans-formation catalysed by palladium specifically (see below).The attractiveness of this synthetic transformation is highlighted by that of the medicinal chemistry described above,thus providing opportunities to improve drug-type properties in a single-step conversion.Zinc metal-mediated cyanationsPrior to 2000,reports of transition-metal catalysed cyanations of aryl chlorides were infrequent.A few reports 46involved the use of Ni catalysts which have the disadvantage of being rather intol-erant of air,moisture and a range of functionalities.The reportsinvolving palladium catalysts were initially limited to those with strongly activated (heteroaryl)chlorides such as pyrazines and purines.47Therefore,there clearly remained a need for a more general procedure suitable for all chloroarenes.This need was first addressed by Jin and Confalone from the process group in DuPont,who published the first general set of conditions for the cyanation of aryl chlorides in 2000.484mol%of the Pd complex was generated from tris(dibenzylideneacetone)dipalladium(0)(Pd 2(dba)3)and 1,10-bis(diphenyl-phosphino)ferrocine (dppf),zinc cyanide as the CN source,and catalytic Zn powder at high temperature in dimethyl acetamide (DMA).The scope of the reaction was impressive as it was not limited to electron poor ‘‘activated’’aromatic chlorides (see Scheme 1)and could be applied to the preparation of 7,an intermediate en route to the factor Xa inhibitor razaxaban.49The role of the zinc powder is intriguing and worthy of further comment.Early mechanistic studies by Takagi et al.50showed that an excess of cyanide ions inhibits the catalytic cycle due to formation of inactive Pd(II )cyano compounds,that cannot be reduced to the catalytically active Pd(0)species;the Zn is key in reducing these compounds,thus allowing the cycle to continue.Cyanide ions can also react with Pd(0)giving species thatcannotFig.8a)Potency of ArCN versus ArCl;b)LipE of ArCN versus ArCl (line of unity in bold,Æ3-fold ‘error’IC 50lines either side,translating to a Æ0.5shift in LipE);c)Pie chart plot of data in b)(green/amber/red ¼ArCN better/same/worse potency thanArCl).Fig.9Assessment of metabolic stability in human liver microsomes for matched aromatic chloride-nitrile pairs.ArCN 2-fold more stable (green),ArCl 2-fold more stable (red)or the same stability (within 2-fold,amber).Number of observations giving rise to each pie chart isshown.Scheme 1Zinc-mediated cyanation (Jin and Confalone).48P u b l i s h e d o n 11 O c t o b e r 2010. D o w n l o a d e d o n 21/12/2015 11:12:02.undergo oxidative addition to Ar–Cl as illustrated in red in Fig.10.An analysis of the mechanisms by which palladium poisoning can occur in aromatic cyanations has been reported.51It is clear that the CN Àanion concentration in the reaction mixture is crucial;too much and the catalyst is deactivated as above,too little and the transmetallation (and hence reductive elimination step)is retarded.These effects also lead to solvents playing a key role in the reaction due to variation in cyanide solubility and show the importance of having a relatively insol-uble cyanide source.In the above case,zinc cyanide (originally introduced for cyanations by the Merck process group in 1994)is used,as its solubility in DMF (at 80 C)is only 1.8Â10À4g mL À1.52Using elegant NMR studies,Beller was able to isolate the oxidative addition complex and proved that this step is rate determining,the transmetallation and reductive elimination steps being much quicker.53In the same year Jiang et al.described dialkylcyanoboronates 8as a novel source of cyanide for the transformation.54However,these reagents required pre-synthesis from a diol precursor and only reacted in moderate yield with activated chlorides as shown in Scheme 2.Crown ether and amine additivesThe main issue related to the procedure described above is the generation of zinc chloride waste,the disposal of which is problematic and expensive.To avoid this,Beller and co-workers turned to KCN as the nitrile source,using toluene as a sparingly soluble solvent.55Using the knowledge that crown ethers have a positive effect on the reaction with other aryl halides,56Beller experimented using 18-crown-6with various bases and Pd ligands.This dramatically affected the yield on the model cyan-ation of 4-chlorobenzotriflbination of these prop-erties (chelation and base)into a single additive gave tetramethylethylenediamine (TMEDA)as the co-catalyst of choice,providing 91%yield for the model reaction compared with only 13%when this was absent (Scheme 3).55From a mechanistic perspective,the benefits of the crown ether or amine additive are two-fold:the concentration of CN Àanions is controlled and the exchange of cyanide with other ligands is facilitated.Also,crucial to the success of the reaction is the use of chelating phosphine ligands,with 1,5-bis(diphenylphosphino)-pentane (dpppe)being optimal.However,under these condi-tions,non-activated chlorides such as simple chlorobenzene give poor yields.Therefore,Beller investigated alternative amine co-catalysts such as those shown in Fig. 3.531-10-methyl-enediperidine (MDP)as an additive provided the best yields under these conditions (Fig.11,Scheme 4).Noteworthy here is the smooth reaction of 4-chlor-obenzaldehyde that may have been expected to undergo cyanide-catalysed benzoin condensation,reflecting the low cyanide concentration in the reaction.Also of interest are the cyanations of heteroaryl substrates,thus broadening the scope and relevance of this transformation.Cyanohydrin as the nitrile sourceBeller also investigated an alternative approach to controlling the concentration of CN Àanions in the cyanation reaction.57As previously mentioned,the main approach to this had been through judicious choice of solvent and/or additives such as crown ethers.This new approach instead used a liquid source of cyanide,acetone cyanohydrin which could be added in a controlled manner via a syringe pump.Although this method worked well for aryl bromides,allowing the reaction temperature to be dropped to 80 C,the yields and temperatures with chlo-rides (shown in Scheme 5)are quite similar to those achieved with Beller’s previous conditions.55The main advantage of this protocol is the reduction of catalyst loading (down to 0.5–1%)but this has to be weighed against the operationally more complex use of a syringe pump combined with the toxicity of a liquid cyanide source.In a further publication Beller also used TMS cyanide as a liquid CN Àsource but the yields of the aromatic nitriles were inferior to those obtained using acetone cyanohydrin.58Microwave cyanationsIn two papers published in 2006,microwave technology had its first impact on the aromatic chloride nitrile transformation.In the first,Pitts and co-workers built on earlier work by Hallberg (showing that microwave irradiation increased the rate of cyan-ation of various bromides)59and applied it to the last step oftheFig.10Proposed mechanism of the Pd-catalysed cyanation reaction.50,51Scheme 2Dialkylcyanoboronate cyanation (Jiang et al.)54Scheme 3TMEDA-catalysed cyanation (Beller et al.)55P u b l i s h e d o n 11 O c t o b e r 2010. D o w n l o a d e d o n 21/12/2015 11:12:02.synthesis of citalopram from the bromo precursor 9.60Several parameters were varied,including ligand,additives,temperature and time,to provide Pd 2(dba)3/Xantphos/TMEDA as the optimal conditions.These conditions also gave excellent yields for a narrow range of aryl chlorides (Scheme 6).In the second microwave paper,Chobanian and co-workers 61found that the key to a successful reaction in their case was the use of the Buchwald S-Phos ligand 62which was previously noted to possess ‘‘unprecedented activity’’in the coupling of aryl chlorides with boronic acids and esters.63Application of this ligand to cyanations under microwave conditions gave good results.For the first reaction shown below,use of dppf or dpppe gave no reaction at all,whereas S-Phos gave a 97%yield (Scheme 7).An important feature of this work is that the thermal condi-tions do not appear to give significantly different yields to the microwave under these conditions;even the length of reaction is comparable (30min for MW,1h for thermal reaction).Alsonoteworthy is the poor yield in the reaction of the relatively electron rich chloride 10,which,apart from an isolated example in the original Confalone work,48is a common theme in the papers described thus far.These conditions were recently used to convert the antitumour smoothened antagonists 11as a 1:1mixture of regioisomers into the nitrile containing derivatives 12(Scheme 8),64although X-Phos was the chosen ligand.65Interestingly,the nitrile con-taining compounds appeared to have improved LipE,although it should be noted the biological activity was measured on the regioisomeric mixture.Reducing reaction temperatureAnother issue with the conditions described above is the high reaction temperature required for the transformation (120–160 C),requiring the need for sealed tubes as solvents are used at temperatures exceeding their boiling points.The issues of high temperature and the lack of scope for electron rich chlorides were addressed by Littke and co-workers.66All possible variables with the reaction were optimised and a new catalyst system of Pd(TFA)2together with another Buchwald ligand (racemic 2-di-tert -butylphosphino-1,10-binaphthyl)67was proposed (catalyst system A).Catalytic Zn was used as the additive and it was noted that on large scale it was essential to use fine zinc particlestoFig.11Amine additives used by Beller et al.53Scheme 4MDP-catalysed cyanation (Beller et al.)53Scheme 5TMSCN cyanations (Beller et al.)57Scheme 6Microwave-assisted cyanations (Pitts et al.)60Scheme 7Microwave-assisted cyanations (Chobanion et al.)61P u b l i s h e d o n 11 O c t o b e r 2010. D o w n l o a d e d o n 21/12/2015 11:12:02.。
第十七章 胺
二甲基二苯基硝酸铵 硝酸二甲基二苯基铵
Dimethyldiphenyl ammonium nitrate
四乙基氢氧化铵 氢氧化四乙铵
Tetraethyl ammonium hydroxide
6
§2 Physical and Spectroscopic Properties
Physical Properties
R2NH
ArNH2
19
2 Alkylation of amines
RNH2 + RX R2NH
R3N R4NX
R4NX + AgOH R4NOH 季铵碱
PTC-Phase Transfer Catalysis
Hexane Hexane (n-Bu)4NCl
+ CHCl3
(n-Bu)4NCl OH-/H O 2
CH3 CH2C NH2 CH3
CH3 CH2C CONH2 CH3
CH3 CH2C CO2H CH3
PhCH2Br +
H H3C C2H5 CONH2 Br2/OHH3C C2H5 H NH2
(CH3)2CHCO2H
15
Related Reactions
Curtius Arrangement
O R C Cl (1) NaN3 (2) H2O O R C N3 RNH2
NH4X 卤化铵 R4NX 季铵盐
NH2 Aromatic amine
H N CH3
H2 CH3 C N CH3 Aliphatic amine
N
N H
Heterocyclic aromatic amine
N H
N H
Heterocyclic aliphatic amine
