对氨基水杨酸
药合实验2讲义
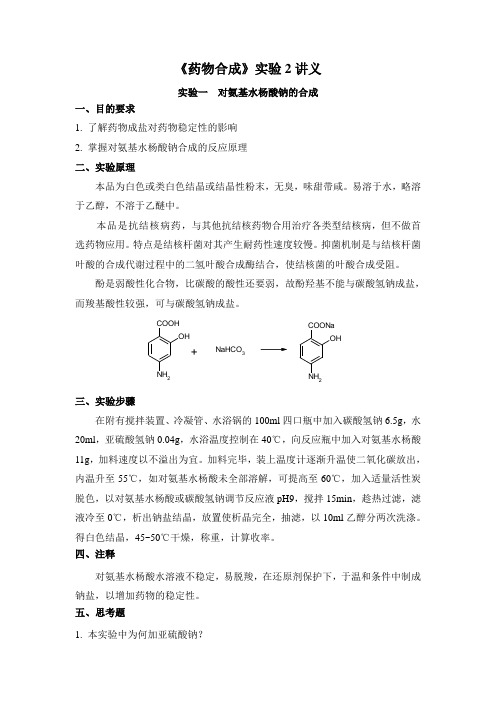
《药物合成》实验2讲义实验一 对氨基水杨酸钠的合成一、目的要求1. 了解药物成盐对药物稳定性的影响2. 掌握对氨基水杨酸钠合成的反应原理二、实验原理本品为白色或类白色结晶或结晶性粉末,无臭,味甜带咸。
易溶于水,略溶于乙醇,不溶于乙醚中。
本品是抗结核病药,与其他抗结核药物合用治疗各类型结核病,但不做首选药物应用。
特点是结核杆菌对其产生耐药性速度较慢。
抑菌机制是与结核杆菌叶酸的合成代谢过程中的二氢叶酸合成酶结合,使结核菌的叶酸合成受阻。
酚是弱酸性化合物,比碳酸的酸性还要弱,故酚羟基不能与碳酸氢钠成盐,而羧基酸性较强,可与碳酸氢钠成盐。
OH NH 2COOH NaHCO 3OHNH2COONa三、实验步骤在附有搅拌装置、冷凝管、水浴锅的100ml 四口瓶中加入碳酸氢钠6.5g ,水20ml ,亚硫酸氢钠0.04g ,水浴温度控制在40℃,向反应瓶中加入对氨基水杨酸11g ,加料速度以不溢出为宜。
加料完毕,装上温度计逐渐升温使二氧化碳放出,内温升至55℃,如对氨基水杨酸未全部溶解,可提高至60℃,加入适量活性炭脱色,以对氨基水杨酸或碳酸氢钠调节反应液pH9,搅拌15min ,趁热过滤,滤液冷至0℃,析出钠盐结晶,放置使析晶完全,抽滤,以10ml 乙醇分两次洗涤。
得白色结晶,45~50℃干燥,称重,计算收率。
四、注释对氨基水杨酸水溶液不稳定,易脱羧,在还原剂保护下,于温和条件中制成钠盐,以增加药物的稳定性。
五、思考题1. 本实验中为何加亚硫酸钠?2. 本实验中的碳酸氢钠能否改为氢氧化钠?3. 试比较对氨基水杨酸和对氨基水杨酸钠的稳定性。
实验二扑热息痛的制备一、目的要求1. 了解选择性乙酰化对氨基酚的氨基而保留酚羟基的方法。
2. 掌握易被氧化产品的重结晶精制方法。
二、实验原理对乙酰氨基酚(APAP)又名醋氨酚,也称扑热息痛,它是—种白色、无臭单斜行结晶。
味微苦,溶于甲醇、乙醇、丙酮和乙酸乙酯,易溶于热水。
最新用药常识对氨基水杨酸钠

用药常识对氨基水杨酸钠
世界防治结核病日
药剂名称:对氨基水杨酸钠
别名:SodiumPara-aminosalicylateAminox、PAS-Na
主要成分和含量:
性状:为白色结晶性粉末,无臭、味甜带咸。
易溶于水,略溶于乙醇。
药理和应用:本品的抗结核作用虽比异烟肼、链霉素弱,但结核菌对它产生耐药性速度很慢,一般在服药4~12个月后才逐渐产生,停药后又可恢复其敏感性。
本品一般不单独使用,常与异烟肼或链霉素合用,这不仅可延缓耐药性的产生,又可,全国公务员共同的天地增强抗结核作用。
临床主要用于各型活动性结核病,亦用于甲状腺功能亢进等。
用法和用量:静滴:从每日3~4g开始,逐日增加至每日8~12g,用5%葡萄糖液稀释后,经3~5小时滴完,每日或隔日1次,溶液变色不能再用。
对结核性脓胸可作胸腔内注射:每次用生理盐水配成10~20%溶液10~20ml,每周2次。
一般应先用生理盐水洗涤胸腔后,再注入本品。
体内过程:
不良反应和注意:本品口服除可有胃肠道反应外,并可引起皮疹等过敏反应及影响血象,并因其乙酰化产物水溶较低,易损于肾脏。
静滴如长期,全国公务员共同的天地用药可致静脉硬化及阻塞等。
配伍禁忌:
制剂和规格:。
对氨基水杨酸肠溶颗粒介绍-欧盟委员会

ANNEX ISUMMARY OF PRODUCT CHARACTERISTICS1. NAME OF THE MEDICINAL PRODUCTGRANUPAS4 g gastro-resistant granules2. QUALITATIVE AND QUANTITATIVE COMPOSITIONEach sachet contains 4 g of para-aminosalicylic acid.For the full list of excipients, see section 6.1.3. PHARMACEUTICAL FORMGastro-resistant granulesThe granules are small off white/ light brown coloured approximately 1.5mm diameter.4. CLINICAL PARTICULARS4.1 Therapeutic indicationsGRANUPAS is indicated for use as part of an appropriate combination regimen for multi-drug resistant tuberculosis in adults and paediatric patients from 28 days of age and older when an effective treatment regimen cannot otherwise be composed for reasons of resistance or tolerability (see section 4.4).Consideration should be given to official guidance on the appropriate use of antibacterial agents.4.2 Posology and method of administrationPosologyAdults4 g (one sachet) three times per day.The recommended schedule is 4 g every 8 hours. GRANUPAS can be taken with food.Maximum daily dose is 12 g. Usual duration of treatment is 24 months.Paediatric populationThe optimal dose regimen in children is uncertain. Limited pharmacokinetic data suggest no substantial difference between adults and children.For infants, children and adolescents the dosage will be adapted to the patient’s weight at 150 mg/kg per day, divided in two intakes. A dosing spoon is provided to measure small doses below 4g for young children.The safety and efficacy of GRANUPAS in neonates have not been established.No data are available. DesensitizationDesensitization can be accomplished by starting with 10 mg para-aminosalicylic acid given as a single dose. The dosage is doubled every 2 days until reaching a total of 1 gram after which the dosage is divided to follow the regular schedule of administration. If a mild temperature rise or skin reaction develops, the increment is to be dropped back one level or the progression held for one cycle. Reactions are rare after a total dosage of 1.5 g.Method of administrationOral use.The contents of the sachet should be added to a glass of orange or tomato juice. They will not dissolve, but swirling the juice in the glass will help re-suspend the granules if they sink. It should be drunk at once ensuring that the granules are not left in the glass. Any granules left-over at the bottom of the glass should be swallowed immediately by adding a small quantity of liquid. Smaller doses in children should be measured using the dosing spoon and given by sprinkling on apple sauce or yogurt.The medicinal product should be swallowed immediately after mixing with orange juice, tomato juice, apple sauce and yogurt whilst the granules are intact.The granules should not be crushed or chewed.4.3 ContraindicationsHypersensitivity to the active substance or to any of the excipients listed in section 6.1.Severe renal disease. Patients with severe renal impairment should not receive GRANUPAS. Patients with severe renal disease will accumulate the inactive acetyl metabolite of para-aminosalicylic acid. 4.4 Special warnings and precautions for useMild to moderate renal impairmentGiven that the metabolites of para-aminosalicylic acid are largely excreted via glomerular filtration, caution is warranted in patients with mild to moderate renal impairment (see also section 4.3). Gastric ulcerGRANUPAS should be used with caution in patients with peptic ulcer.Hepatic impairmentGRANUPAS should be used with caution in patients with hepatic impairment.Hepatic toxicityPara-aminosalicylic acid may cause hepatitis. The first symptoms usually appear within three months of the start of therapy with a rash as the most common adverse reaction followed by fever and much less frequently by gastrointestinal disturbances of anorexia, nausea or diarrhoea. Treatment should be stopped immediately in this case.HypersensitivityThe patient must be monitored carefully during the first three months of therapy and treatment must be discontinued immediately at the first sign of a rash, fever or other premonitory signs of intolerance. See section 4.2 for posology adjustements for desensitization.Patients should be advised that the skeletons of the granules may be seen in the stools.4.5 Interaction with other medicinal products and other forms of interactionNo interaction studies have been performed with GRANUPAS.Results from literature suggest the following:Vitamin B12Vitamin B12 absorption may be reduced by para-aminosalicylic acid with clinically significant erythrocyte abnormalities developing after depletion; patients on therapy of more than one month should be considered for maintenance of vitamin B12.Malabsorption syndromeA malabsorption syndrome can develop in patients on para-aminosalicylic acid, but is usually not complete. The complete syndrome includes steatorrhoea, an abnormal small bowel pattern on x-ray, villus atrophy, depressed cholesterol, reduced D-xylose and iron absorption. Triglyceride absorption is always normal.DigoxinPara-aminosalicylic acid may decrease the gastrointestinal absorption of digoxin, by inhibiting the absorption function of intestinal cells. Serum digoxin levels should be monitored in patients on concomitant therapy.EthionamideCo-administration of para- aminosalicylic acid and ethionamide may intensify adverse reactions of para-aminosalicylic acid, mainly the gastrointestinal effects, including jaundice, hepatitis, nausea, vomiting, diarrhoea, abdominal pain or anorexia. Ethionamide should be withdrawn if these effects are significant.DiphenylhydramineThis medicinal product decreases the gastrointestinal absorption of para-aminosalicylic acid, and should not be administered concomitantly.AntiretroviralsNo drug interaction studies have been conducted in patients with HIV infection taking antiretroviral agents and para-aminosalicylic acid.Given the metabolic pathway of GRANUPAS no significant drug interaction is anticipated.4.6 Fertility, pregnancy and lactationPregnancyThere are no or limited data from the use of para-aminosalicylic acid in pregnant women. Studies in animals have shown some embryologic toxicity (see section 5.3).Literature reports on para- aminosalicylic acid in pregnant women always report co-administration of other medicinal products. As there are no adequate and well controlled studies of para- aminosalicylic acid in humans, GRANUPAS should be given to a pregnant woman only if clearly needed. BreastfeedingPara-aminosalicylic acid is excreted into breast milk, therefore breastfeeding mothers should not breastfeed during treatment.FertilityThere is no evidence available on the effect of para-aminosalicylic acid on fertility.4.7 Effects on ability to drive and use machinesPara-aminosalicylic acid has negligeable influence on the ability to drive and use machines.4.8 Undesirable effectsSummary of the safety profileMost frequent adverse reactions were related to the gastrointestinal system. Cutaneous hypersensitivity reactions were also frequent as well as adverse reactions related to the nervous system.Tabulated list of adverse reactionsIn the table below all adverse reactions are listed by system organ class and by frequency. Frequency is defined as very common (≥1/10), common (≥1/100 to <1/10), uncommon (≥1/1,000 to <1/100), rare (≥1/10,000 to <1/1,000), very rare (<1/10,000), not known (cannot be estimated from the available data). Within each frequency grouping, adverse reactions are presented in order of decreasing seriousness.System Organ Class Frequency Adverse reactionBlood and lymphatic systemdisorders Very rareThrombocytopenia, purpura, leukopenia,anemia, methemoglobinemia, agranulocytosisMetabolism and nutritiondisordersRare hypothyroidism Very rare HypoglycemiaNervous system disorders Very rareTendon pain, headache, visual abnormalities,peripheral neuropathy, dizziness Common Giddiness, vestibular syndromeGastrointestinal disordersCommonabdominal pain, vomiting, nausea, bloating,diarrhea, soft stools, Uncommon anorexia,RareMalabsorption syndrome, peptic ulcer,gastrointestinal bleeding, jaundice, metallictasteSkin and subcutaneous tissuedisorders Common Cutaneous hypersensitivity, skin rash Rare urticariaRenal and urinary disorders Very rare crystalluriaInvestigations Very rare Decreased prothrombine level, hepatocytolysis.Increased blood alkaline phosphatase,transaminases. weight lossPaediatric populationFrequency, type and severity of adverse reactions in children are expected to be the same as in adults. Reporting of suspected adverse reactionsReporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via the national reporting system listed in Appendix V.4.9 OverdoseNo case of overdose in adults or paediatrics has been reported. Treatment is symptomatic and supportive.5. PHARMACOLOGICAL PROPERTIES5.1 Pharmacodynamic propertiesPharmacotherapeutic group: Antimycobacterials, drugs for treatment of tuberculosis ATC code: J04AA01Mechanism of actionAminosalicylic acid is bacteriostatic against Mycobacterium tuberculosis. It inhibits the onset of bacterial resistance to streptomycin and isoniazid.The mechanism of action of para-aminosalicylic acid resembles the sulfonamides, competing with paraminobenzoic acid (PABA) for dihydropteroate synthetase (DHP), a key enzyme in the biosynthesis of folates. However, para-aminosalicylic acid appears to be a weak inhibitor of DHP invitro, raising the possibility that it may have a different target. Para-aminosalicylic acid is acetylated in the liver and converted into the inactive metabolite, N-acetyl-para-aminosalicylic acid which is devoid of bacteriostatic activity. The plasma half-life of this agent is about 1 hour, the concentration is not substantially altered in hepatic dysfunction. The concentration of the metabolite may be increased in cases of renal failure.5.2 Pharmacokinetic propertiesAbsorptionGRANUPAS is a gastro-resistant preparation and, therefore, the acid-resistant coating of the granules protects against degradation in the stomach therefore preventing the formation of meta-aminophenol (a known hepatotoxin). The small granules are designed to escape the restriction on gastric emptying of large particles. Under neutral conditions as are found in the small intestine or in neutral foods, the acid-resistant coating is dissolved within one minute.Care must be taken in the administration of these granules to protect the acid-resistant coating by maintaining the granules in an acidic food during dosage administration.Because the granules are protected by an enteric coating, absorption does not commence until they leave the stomach. The soft skeletons of the granules remain and may be seen in the stools.In a single dose (4 grams) pharmacokinetic study in healthy adult volunteers (N=11) the initial time to a 2 µg/mL serum level of aminosalicylic acid was 2 hours with a range of 45 minutes to 24 hours; the median time to peak was 6 hours with a range of 1.5 to 24 hours; the mean peak level was 20 µg/mL with a range of 9 to 35µg/mL: a level of 2µg/mL was maintained for an average of 8 hours with a range of 5 to 9.5 a level of 1 µg/mL was maintained for an average of 8.8 hours with a range of 6 to 11.5 hours.DistributionPara-aminosalicylic acid is distributed in various tissues and fluids including the lungs, kidneys, liver and peritoneal fluid. Pleural or synovial fluid concentrations are approximately equal to plasma. The drug does not cross the blood brain barrier in patients unless the meninges are inflamed, when the concentration of para-aminosalicylic acid in cerebrospinal fluid is about 10 to 50% of the plasma. It is unknown whether it passes through the placental barrier. Small amounts of this agent are distributed in the milk and bile.Plasma protein binding is about 50 to 60%, the kinetic distribution has a half-life of 0.94 hours and a volume of distribution of 1.001 L/kg.BiotransformationThe major metabolites of PAS are produced by conjugation to glycine in para-aminosalicyluric acid (PASU) for up to 25% of the dose and to N-acetyl in N-acetyl para-aminosalicylic acid (Ac-PAS) for up to 70% of the dose. Together they constitute more than 90% of the total metabolites of PAS found in urine.EliminationIn a single dose study the plasma half-life of para-aminosalicylic acid administered as GRANUPAS was 1.62±0.85 h.Para-aminosalicylic acid and its metabolites are excreted by glomerular filtration and tubular secretion. The cumulative excretion of para-aminosalicylic after 24 hours is 84% of an oral dose of 4 g, 21% as para-aminosalicylic acid and 63% as the acetylated form. The acetylation process is not genetically determined as is the case for isoniazid.5.3 Preclinical safety dataNon-clinical data reveal no special hazard for humans based on conventional studies of safety pharmacology and repeated dose toxicity.The data available from a rat embrofetal development study, where animals were given sodium aminosalicylate (3.85 to 385 mg/kg) were limited. Bone defects were observed at 77 mg/kg only.and increased fetal weight was noted at the other doses. Other malformations were observed; however, the exact nature of these findings is unknown. The lack of a dose-response relationship suggests that the findings are not of clinical relevance, but it is noted that the findings were observed at doses below those proposed clinically. In the rabbit, sodium aminosalicylate had no effects on embryofetal development; however, the doses evaluated were below those proposed clinically.Sodium aminosalicylic acid was not mutagenic in Ames test strain TA 100. In human lymphocyte cultures in-vitro clastogenic effects of achromatic, chromatid, isochromatic breaks or chromatid translocations were not seen at 153 or 600 µg /mL but at 1500 and 3000 µg/mL there was a dose related increase in chromatid aberrations. No in vivo genotoxicity study has been conducted with GRANUPAS.6. PHARMACEUTICAL PARTICULARS6.1 List of excipientsColloidal silicon dioxideDibutyl sebacateMethacrylic acid – Ethyl acrylate Copolymer (1:1) Dispersion 30%HypromelloseMicrocrystalline celluloseTalc6.2 IncompatibilitiesNot applicable.6.3 Shelf life2 years.6.4 Special precautions for storageStore in a refrigerator (2°C – 8°C).The sachets can be stored below 25°C up to 24 hours after first opening.6.5 Nature and contents of containerSachets consisting of paper/low density polyethylene/aluminium foil/primer/low density polyethylene. Pack size of 30 sachets. A calibrated measuring spoon is provided.6.6 Special precautions for disposal and other handlingThe granules should not be crushed or chewed.DO NOT USE if sachet is swollen or if the granules have lost their light brown colour, and are turning dark brown or purple.Any unused product or waste material should be disposed in accordance with local requirements.7. MARKETING AUTHORISATION HOLDERLucane Pharma,172 rue de Charonne75011 ParisFrance8. MARKETING AUTHORISATION NUMBER(S)EU/1/13/896/0019. DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATIONDate of first authorisation: 07 April 2014.10. DATE OF REVISION OF THE TEXTDetailed information on this medicinal product is available on the website of the European Medicines Agency: http://www.ema.europa.eu.ANNEX IIA. MANUFACTURER RESPONSIBLE FOR BATCH RELEASEB. CONDITIONS OR RESTRICTIONS REGARDING SUPPLY AND USEC. OTHER CONDITIONS AND REQUIREMENTS OF THE MARKETINGAUTHORISATIOND. CONDITIONS OR RESTRICTIONS WITH REGARD TO THE SAFE ANDEFFECTIVE USE OF THE MEDICINAL PRODUCTA. MANUFACTURER RESPONSIBLE FOR BATCH RELEASEName and address of the manufacturer responsible for batch releaseLucane Pharma,172 rue de Charonne75011 ParisFranceB. CONDITIONS OR RESTRICTIONS REGARDING SUPPLY AND USEMedicinal product subject to medical prescription.C. OTHER CONDITIONS AND REQUIREMENTS OF THE MARKETINGAUTHORISATION•Periodic safety update reportsThe marketing authorisation holder shall submit the first periodic safety update report for this product within 6 months following authorisation. Subsequently, the marketing authorisation holder shall submit periodic safety update reports for this product in accordance with the requirements set out in the list of Union reference dates (EURD list) provided for under Article 107c(7) of Directive 2001/83/EC and published on the European medicines web-portal.D. CONDITIONS OR RESTRICTIONS WITH REGARD TO THE SAFE ANDEFFECTIVE USE OF THE MEDICINAL PRODUCT•Risk Management Plan (RMP)The MAH shall perform the required pharmacovigilance activities and interventions detailed in the agreed RMP presented in Module 1.8.2 of the Marketing Authorisation and any agreed subsequent updates of the RMP.An updated RMP should be submitted:•At the request of the European Medicines Agency;•Whenever the risk management system is modified, especially as the result of new information being received that may lead to a significant change to the benefit/risk profile or as the result of an important (pharmacovigilance or risk minimisation) milestone beingreached.If the submission of a PSUR and the update of a RMP coincide, they can be submitted at the same time.•Additional risk minimisation measuresNone•Obligation to conduct post-authorisation measuresNoneANNEX IIILABELLING AND PACKAGE LEAFLETA. LABELLINGPARTICULARS TO APPEAR ON THE OUTER PACKAGINGCARTON BOX1. NAME OF THE MEDICINAL PRODUCTGRANUPAS 4 g gastro-resistant granulesPara-aminosalicylic acid2. STATEMENT OF ACTIVE SUBSTANCE(S)Each sachet contains 4 g of para-aminosalicylic acid3. LIST OF EXCIPIENTS4. PHARMACEUTICAL FORM AND CONTENTSGastro-resistant granules30 sachetsCalibrated measuring spoon5. METHOD AND ROUTE(S) OF ADMINISTRATIONRead the package leaflet before use.Oral use.Do not chew or crush.Warning: Do not use if sachet is swollen or the granules have lost their light brown color and are dark brown or purple6. SPECIAL WARNING THAT THE MEDICINAL PRODUCT MUST BE STORED OUT OF THE SIGHT AND REACH OF CHILDRENKeep out of the sight and reach of children.7. OTHER SPECIAL WARNING(S), IF NECESSARY8. EXPIRY DATEEXP9. SPECIAL STORAGE CONDITIONSStore in a refrigerator.10. SPECIAL PRECAUTIONS FOR DISPOSAL OF UNUSED MEDICINAL PRODUCTS OR WASTE MATERIALS DERIVED FROM SUCH MEDICINAL PRODUCTS, IF APPROPRIATE11. NAME AND ADDRESS OF THE MARKETING AUTHORISATION HOLDER Lucane Pharma172 rue de Charonne75011 ParisFrance12. MARKETING AUTHORISATION NUMBER(S)EU/1/13/896/00113. BATCH NUMBERLot14. GENERAL CLASSIFICATION FOR SUPPLYMedicinal product subject to medical prescription.15. INSTRUCTIONS ON USE16. INFORMATION IN BRAILLEGRANUPAS 4gMINIMUM PARTICULARS TO APPEAR ON SMALL IMMEDIATE PACKAGING UNITS SACHET1. NAME OF THE MEDICINAL PRODUCT AND ROUTE(S) OF ADMINISTRATION GRANUPAS 4g gastro-resistant granulesPara aminosalicylic acidOral use2. METHOD OF ADMINISTRATION3. EXPIRY DATEEXP4. BATCH NUMBERLot5. CONTENTS BY WEIGHT, BY VOLUME OR BY UNIT4 g6. OTHERWarning: Do not use if sachet is swollen or the granules have lost their light brown color and are dark brown or purple.Do not chew or crush.Read the package leaflet before use.B. PACKAGE LEAFLETPackage leaflet: Information for the patientGRANUPAS 4 g gastro-resistant granulesPara-aminosalicylic acidRead all of this leaflet carefully before you start taking this medicine because it contains important information for you.•Keep this leaflet, you may need to read it again•If you have any further questions, ask your doctor or pharmacist.•This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.•If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.What is in this leaflet1. What GRANUPAS is and what it is used for2. What you need to know before you take GRANUPAS3. How to take GRANUPAS4. Possible side effects5.How to store GRANUPAS6. Contents of the pack and other information1.What GRANUPAS is and what it is used forGRANUPAS contains para-aminosalicylic acid which is used in adults and children aged 28 days and older to treat resistant tuberculosis in combination with other medicines, in cases of resistance or intolerability with other treatments.2.What you need to know before you take GRANUPASDo not take GRANUPAS if•you are allergic to para-aminosalicylic acid or any of the other ingredients of this medicine (listed in section 6)•you have severe kidney disease.If you are not sure, talk to your doctor or pharmacist before taking GRANUPAS.Warnings and precautionsTalk to your doctor or pharmacist before taking GRANUPAS•if you have liver problems or mild or moderate kidney disease•if you have a stomach ulcerChildrenUse of GRANUPAS is not recommended in newborn babies (under 28 days of age).Other medicines and GRANUPASTell your doctor or pharmacist if you are taking, have recently taken or are planning to take any other medicines.It is especially important to tell your doctor if you are taking any of the following: •Antituberculosis medicines or ethionamide (other treatments against tuberculosis) •Vitamin B12•Digoxin (for heart disease)•Diphenylhydramine (for allergic reactions)Pregnancy and breast-feedingIf you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine.•GRANUPAS should only be used during pregnancy if advised by your physician•Do not breastfeed whilst taking GRANUPAS. This is because small amounts of the medicine can pass into mother’s milk.Driving and using machinesGRANUPAS is unlikely to affect your ability to drive and use machines.3.How to take GRANUPASAlways take this medicine exactly as your doctor has told you. Check with your doctor or pharmacist if you are not sure.AdultsThe recommended dose for adults is 1 sachet three times a day, with a schedule of 1 sachet every 8 hours. Your physician may need to start with a lower dose to prevent possible side effects.Do not take more than 3 sachets per day. Treatment is usually given for two years (24 months). •Add the contents of the sachet to a drink of tomato or orange juice.•Drink straight away•If some granules are left in the glass, add some more juice and drink straight away.Infants, children and adolescentsThe dose in infants, children and adolescents will be calculated by your doctor based on the patient’s body weight. The recommended total dose per day is 150 mg for each kg of body weight. This daily amount is divided into two doses spread out through the day.•Use the spoon that comes with the medicine to measure the dose.•To measure the dose:o Lines on the spoon indicate the amount (in milligrams of para-aminosalicylic acid).Take the correct amount as prescribed by your doctor.o Put granules directly into the spoon.o Tap the spoon once on a table to give a horizontal level of granules and continue filling if necessary.•Sprinkle the granules onto apple sauce or yogurt.•Make your child eat it straight away.Taking this medicine•Do not crush or chew the granules.•Do not use the sachet if it is swollen or the granules have lost their light brown colour. •You may notice granules appearing in your stools; this is normal.If you take more GRANUPAS than you shouldSpeak to a doctor or pharmacist.If you forget to take a dose of GRANUPASDo not take a double dose to make up for a forgotten dose. Wait until the next dose is due, then take your normal dose.If you have any further questions on the use of this medicine, ask your doctor or pharmacist.4. Possible side effectsLike all medicines, this medicine can cause side effects, although not everybody gets them.During the first 3 months of your treatment with GRANUPAS, you must be attentive to any sign of allergic reaction or hepatitis, like skin eruption and/or fever. If you experience any of these symptoms, you must talk to your doctor immediately.Common side effects (may affect more than 1 in 100 people): giddiness, stomach ache (abdominal pain), vomiting, nausea, bloating, diarrhoea, soft stools, skin redness or rash, disturbance of gait and equilibrium.Uncommon side effects (may affect more than 1 in 1,000 people): loss of appetite (anorexia)Rare side effects (may affect more than 1 in 10,000 people): thyroid gland problems, reduced ability to absorb nutrients from food ulcer, bleeding in the gut, yellowing of skin or eyes (jaundice), metallic taste, itchy rash.Very rare side effects (may affect less than 1 in 10,000 people): reduction in numbers of red or white blood cells, reduction in blood platelets, red spots on the skin, low levels of blood sugar, tendon pain, headache, visual abnormalities, nerve damage in the hands and feet, dizziness, prolonged bleeding time, elevated liver enzymes, weight loss, crystals in urine.Reporting of side effectsIf you get any side effects, talk to your doctor or pharmacist.This includes any possible side effects not listed in this leaflet.You can also report side effects directly via the national reporting system listed in Appendix V. By reporting side effects you can help provide more information on the safetyof this medicine.5. How to store GRANUPASKeep out of the sight and reach of children.Do not use after the expiry date which is stated on the carton and sachet after EXP. The expiry date refers to the last day of the month.Store in a refrigerator (2°C – 8°C). The sachets can be stored below 25°C up to 24 hours after opening.Do not use GRANUPAS if you notice the sachets are swollen or if the granules are dark brown or purple.Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help to protect the environment.6. Contents of the pack and other informationWhat GRANUPAS containsThe active substance is para-aminosalicylic acid.Each sachet of gastroresistant granules contains 4 g of para-aminosalicylic acid.The other ingredients are colloidal silicon dioxide, dibutyl sebacate, methacrylic acid – ethyl acrylate copolymer (1:1) dispersion 30%, hypromellose, microcrystalline cellulose, talc.What GRANUPAS looks like and contents of the packThis medicine is presented as light brown gastro-resistant granules in sachets.Each box contains 30 sachets. A calibrated measuring spoon is provided.Marketing Authorisation HolderLucane Pharma172 rue de Charonne75011 Paris - FranceManufacturerLucane Pharma,172 rue de Charonne75011 ParisFranceFor any information about this medicine, please contact the local representative of the Marketing Authorisation Holder:België/Belgique/Belgien Lucane PharmaTél/Tel: + 33 153 868 750 info@ LietuvaMedical Need Europe AB Tel: + 46 8 533 39 500 info@БългарияLucane PharmaTeл.: + 33 153 868 750 info@ Luxembourg/Luxemburg Lucane PharmaTél/Tel: + 33 153 868 750 info@Česká republika Medical Need Europe AB Tel: + 46 8 533 39 500 info@ MagyarországMedical Need Europe AB Tel.: + 46 8 533 39 500 info@DanmarkMedical Need Europe AB Tlf: + 46 8 533 39 500 info@ MaltaLucane PharmaTel: + 33 153 868 750 info@DeutschlandLucane PharmaTel: + 33 153 868 750 info@ NederlandLucane PharmaTel: + 33 153 868 750 info@EestiMedical Need Europe AB Tel: + 46 8 533 39 500 info@ NorgeMedical Need Europe AB Tlf: + 46 8 533 39 500 info@ΕλλάδαLucane PharmaΤηλ: + 33 153 868 750 info@ ÖsterreichLucane PharmaTel: + 33 153 868 750 info@。
注射用对氨基水杨酸钠

注射用对氨基水杨酸钠【药品名称】通用名称:注射用对氨基水杨酸钠英文名称:Sodium Aminosalicylate for Injection【成份】本品主要成份为:对氨基水杨酸钠。
【适应症】适用于结核分枝杆菌所致的肺及肺外结核病,静滴可用于治疗结核性脑膜炎及急性扩散性结核病。
本品仅对分枝杆菌有效。
单独应用时结核杆菌能迅速产生耐药性,因此本...【用法用量】静脉滴注一日4~12g,临用前加灭菌注射用水适量使溶解后再用5%葡萄糖注射液500ml稀释,2~3小时滴完。
小儿每日0.2~0.3g/kg。
【不良反应】1.发生率较多者:瘙痒皮疹、关节酸痛与发热、极度度疲乏或软弱,嗜酸性粒细胞增多(较常见的原因为过敏)。
2.发生率较少者:下背部疼痛、尿痛或排尿烧灼感(结晶尿)、血尿;月经失调、发冷、男性性欲减低、皮肤干燥、颈前部肿胀、体重加重(甲状腺肿,粘液水肿);眼或皮肤黄染(黄疸、肝炎);腹痛、背痛、苍白(溶血性贫血,由于G6PD缺乏);发热、头痛、皮疹、咽痛、乏力(传染性单核细胞增多样综合症)。
【禁忌】尚不明确。
【注意事项】1.交叉过敏反应,对其他水杨酸类包括水杨酸甲酯(冬青油)或其他含对氨基苯基团(如某些磺胺药或染料)过敏的患者对本品亦可呈过敏;2.对诊断的干扰:使硫酸铜法测定尿糖出现假阳性;使尿液中尿胆原测定呈假阳性反应(氨基水杨酸类与Ehrlich试剂发生反应,产生橘红色混浊或黄色,某些根据上述原理做成的市售试验纸条的结果也可受影响);使丙氨酸氨基转移酶(ALT)和门冬氨酸氨基转移酶(AST)的正常值增高;3.下列情况应慎用:充血性心力衰竭、胃溃疡、葡萄糖-6-磷酸脱氢酶(G6PD)缺乏症、严重肝功能损害、严重肾功能损害;4.静脉滴注的溶液需新配,滴注时应避光,溶液变色即不得使用。
静脉滴注久用易致静脉炎。
【孕妇及哺乳期妇女用药】对孕妇未证实有问题,同时联合疗法对于胎儿的影响目前尚不清楚,但必须权衡利弊后选用。
对氨基水杨酸钠word版

对氨基水杨酸钠片【药品名称】通用名称:对氨基水杨酸钠片英文名称:Sodium Aminosalicylate Tablets【成份】其化学名称为:4-氨基-2-羟基苯甲酸钠盐二水合物。
分子式:C7H6NNaO3·2H2O分子量:211.14【适应症】适用于结核分枝杆菌所致的肺及肺外结核病。
本品仅对分枝杆菌有效,单独应用时结核杆菌对本品能迅速产生耐药性,因此必须与其他抗结核药合用。
链霉素和异烟肼与本...【用法用量】口服成人一次4~6片,一日16~24片,一日4次;小儿按体重每日0.2~0.3g/kg,分3~4次,儿童每日剂量不超过12g。
【不良反应】1.发生率较多者:胃肠道反应有食欲不振、恶心、呕吐、腹痛、腹泻;过敏反应有瘙痒、皮疹、药物热、哮喘、嗜酸性粒细胞增多。
2.发生率较少者:引起胃溃疡及其出血、血尿、蛋白尿、肝功损害及粒细胞减少。
【禁忌】对本品过敏者禁用【注意事项】1.交叉过敏反应,对其他水杨酸类包括水杨酸甲酯(冬青油)或其他含对氨基苯基团(如某些磺胺药和染料)过敏的患者对本品亦可呈过敏。
2.对诊断的干扰:使硫酸铜法测定尿糖出现假阳性;使尿液中尿胆原测定呈假阳性反应(氨基水杨酸类与Ehrlich试剂发生反应,产生橘红色混浊或黄色,某些根据上述原理做成的市售试验纸条的结果也可受影响);使丙氨酸氨基转移酶(ALT)和门冬氨酸氨基转移酶(AST)的正常值增高。
3.下列情况应慎用:充血性心力衰竭、胃溃疡、葡萄糖-6-磷酸脱氢酶(G6PD)缺乏症、严重肝功能损害、严重肾功能损害。
【药物相互作用】1 对氨基苯甲酸与本品有拮抗作用,两者不宜合用。
2 本品可增强抗凝药(香豆素或茚满二酮衍生物)的作用,因此在用对氨基水杨酸类时或用后,口服抗凝药的剂量应适当调整。
3 与乙硫异烟胺合用时可增加不良反应。
4 丙磺舒或苯磺唑酮与氨基水杨酸类合用可减少后者从肾小管的分泌量,导致血药浓度增高和持续时间延长及毒性反应发生。
对氨基水杨酸钠的缩写

对氨基水杨酸钠的缩写
氨基水杨酸钠(Ammonium salicylate)是一种化学物质,其化学式为C7H7NO3Na,属于水杨酸盐类化合物。
它是以水杨酸为原料经过反应合成得到的。
氨基水杨酸钠常用于药物制备、化妆品和个人护理产品中。
氨基水杨酸钠具有抗炎、消热、镇痛、抗菌等药理作用,因此被广泛应用于医药领域。
它可以用于治疗风湿病、关节炎、头痛、发烧等疾病。
其抗炎作用是通过抑制炎症介质的合成来实现的,可以减轻疼痛和红肿等症状。
氨基水杨酸钠还可以用于化妆品和个人护理产品中,常见于护肤品、洗发水、牙膏等产品中。
它可以起到清洁、舒缓、消炎等作用,有助于改善皮肤问题和保护牙齿健康。
此外,氨基水杨酸钠还可以用于染发产品中,起到固色和保持发色的作用。
氨基水杨酸钠作为一种化学物质,在使用过程中需要注意一些安全事项。
首先,应避免与眼睛接触,如不慎溅入眼中,应立即用大量清水冲洗,并及时就医。
其次,应放置在儿童无法触及的地方,以免误食。
另外,应避免与火源接触,防止发生火灾。
在使用时,应佩戴适当的防护措施,如戴手套、穿长袖衣物等,以防止皮肤接触。
在药物和化妆品生产中,氨基水杨酸钠的质量和纯度是十分重要的。
合格的氨基水杨酸钠应该符合国家相关标准,如药典标准和化妆品
卫生标准等。
生产厂家应严格控制生产过程,确保产品的质量和安全性。
氨基水杨酸钠是一种常用于药物制备、化妆品和个人护理产品中的化学物质。
它具有抗炎、消热、镇痛、抗菌等药理作用,可以用于治疗疾病和改善皮肤问题。
在使用过程中,需要注意安全事项,并确保产品质量和纯度。
非抗生素类抗感染药:磺胺类-对氨基水杨酸钠
【摘要】对氨基⽔杨酸钠属于磺胺类及其增效剂栏⽬,主要讲述了药物名称对氨基⽔杨酸钠药物别名对氨柳酸钠,PAS-Na 英⽂名称 Sodium Aminosalicylate 说明⽚剂:每⽚0。
注射⽤对氨基⽔杨酸钠:每瓶2g。
功⽤作⽤对结核菌的对氨基苯甲酸合成起抑制作⽤因⽽可抑制其⽣长。
约有50%药物在体内⼄酰化,80%药物(包括代谢物)由尿排出、肾功不良时应...。
本⽂重点关注氨基药物注射作⽤等内容,您可以在本页对对氨基⽔杨酸钠进⾏讨论【关键字】磺胺类及其增效剂;氨基;药物;注射;作⽤;对氨基⽔杨酸钠【全⽂】药物名称对氨基⽔杨酸钠药物别名对氨柳酸钠,PAS-Na英⽂名称 Sodium Aminosalicylate说 明⽚剂:每⽚0.5g。
注射⽤对氨基⽔杨酸钠:每瓶2g;4g;6g。
功⽤作⽤对结核菌的对氨基苯甲酸合成起抑制作⽤因⽽可抑制其⽣长。
⼝服吸收良好,Vd为0.23L/kg。
约有50%药物在体内⼄酰化,80%药物(包括代谢物)由尿排出、肾功不良时应注意。
t1/2为0.5~1.5⼩时。
本品很少单独应⽤,常配合异烟肼、链霉素等应⽤,以增强疗效并避免细菌产⽣耐药性。
也可⽤于甲状腺功能亢进症。
对于甲亢合并结核患者较适⽤,在⽤碘剂⽆效⽽影响⼿术时,可短期服本品为⼿术创造条件。
本品尚有较强的降⾎脂作⽤。
⽤法⽤量(1)⼝服 每次2~3g,1⽇8~12g,饭后服。
⼩⼉每⽇200~300mg/kg,分4次服。
(2)静滴每⽇4~12g(先从⼩剂量开始),以等渗氯化钠注射液或5%葡萄糖液溶解后,配成3%~4%浓度滴注。
⼩⼉每⽇200~300mg/kg。
(3)胸腔内注射 每次10%~20%溶液10~20毫升(⽤等渗氯化钠注射液溶解)。
(4)甲亢⼿术前 1⽇8~12g,分4次服,同时服⽤维⽣素B、C。
服药时间不可过长,以防毒性反应出现。
注意事项(1)恶⼼、呕吐、⾷欲不振、腹泻、腹痛较多见,饭后服或与碳酸氢钠同服可减轻症状。
对氨基水杨酸钠结构式
对氨基水杨酸钠结构式
最近,对氨基水杨酸钠在多个领域中发挥着非常重要的作用,因此对其结构式的研究也变得越来越重要。
本文主要讨论对氨基水杨酸钠的结构,以及它的物理和化学特性。
氨基水杨酸钠的化学式为C7H6O3Na,它是一种无色结晶粉末,具有强烈的酸性味道。
它是氨基水杨酸的钠盐,它的分子氢键结构形式为C7H6O3Na的正八面体,由四个水杨酸分子和三个钠离子构成。
在可见光谱分析中,有三个强吸收峰在315nm,356nm和455nm,这些谱线表明其它官能团的存在。
氨基水杨酸钠是一种非常有用的溶剂,在药物合成和有机结晶中有广泛的应用。
由于它的特殊的酸性属性,它也用于抗过敏、抗炎、抗病毒和抗菌剂的研究。
此外,它还用于各种抗菌性防腐剂,可以防治食物中的细菌和霉菌。
氨基水杨酸钠也用于清洁剂、染料、油漆、油墨、植物激素和植物保护剂的生产。
同时,它也用于保护食品中的细菌,以及在植物中控制害虫和病毒的生长。
氨基水杨酸钠有许多物理和化学性质。
它的熔点为206℃,沸点为361℃,溶解度为每千克水溶液的46克,并且在水、乙醇和乙醚中都能很好的溶解。
它的熔点比氨基水杨酸要高,而其他物理性质基本上是一样的。
氨基水杨酸钠在各种领域中表现出了非常重要的作用。
它对于保护和洁净环境,控制害虫和病毒,以及抗炎、抗病毒和抗菌等疾病的
治疗都有重要的作用。
未来,它的应用将会发挥更多的作用,发挥更大的作用。
对氨基水杨酸钠的稳定性实验
药物化学实验讲义(8-7)项目名称对氨基水杨酸钠的稳定性试验实验学时4实验类型验证教学目的和要求:1.加强对实验中防止药物氧化重要性的认识。
药物氧化分解的结果,使药物失效、颜色变深、颜色变深、形成沉[淀或产生有毒物质(如新胂凡纳明暴露于空气中,易氧化变质,毒性显著增加而不能供药用)。
有些注射剂其中药物虽仅极少一部分氧化,但颜色变深,以致可能成为废品2.掌握药物抗氧化的措施。
实验原理:1946年发现对结核杆菌有选择性抑制作用的对氨基水杨酸,临床上应用其钠盐,对氨基水杨酸钠为常见的抗结核药,为白色结晶性粉末,无臭,味甜带咸,易溶于水,略溶于乙醇对氨基水杨酸钠盐水溶液很不稳定,易被氧化,遇光热颜色渐变深。
(主要是由于其发生脱羧反应生成褐色的间氨基酚再被继续氧化形成红棕色的二苯醌型化合物所致)在铜离子存在下,加速氧化。
如有抗氧剂或金属络合剂存在,可有效地防止氧化。
用光电比色计测定透光率(T)可看出其变化程度。
实验药品和器材:试剂:对氨基水杨酸钠,双氧水,硫酸铜,焦亚硫酸钠,乙二胺四乙酸(EDTA,它是一种能与Mg2+、Ca2+、Mn2+、Fe2+等二价金属离子结合的螯合剂)。
仪器:100 mL锥形瓶,试管,紫外可见分光光度计。
实验内容:取5支试管,编号,各加入0.025 % PAS-Na溶液10 mL。
除1号试管外,各试管分别加入双氧水(10 mL-50 mL)12滴。
在3号试管中加入Na2S2O5试液(10 g-30 mL)20滴。
在4、5号试管中分别加入Cu2+试液(2 mg - 10 mL)6滴。
在5号试管加入EDTA试液(10 mg-10 mL)20滴。
各试管用蒸馏水稀释至刻度一致。
将所有试管同时置入80-90℃水浴中,记录置入时间,维持此温度,间隔30 min取样,放置至室温,观察颜色深浅及变化。
用722型分光光度计在440 nm处测定样品的透光率。
A=Ig(1/T)颜色越深,吸光度越大,透光率越小1、双氧水拥有较强的腐蚀性、氧化性,所以浓度较高会产生较为严重的危害性;使用双氧水要现配现用。
注射用对氨基水杨酸钠
注射用对氨基水杨酸钠说明书【药品名称】通用名:注射用对氨基水杨酸钠曾用名:商品名:英文名:Sodium Aminosalicylate for Injection汉语拼音:Zhusheyong Dui'anji Shuiyangsuanna化学名称:4-氨基-2-羟基苯甲酸钠盐二水合物化学结构式:分子式:C7H6NNaO3 ·2H2O分子量:211.14【性状】本品为白色或类白色的结晶或结晶性粉末。
【药理毒理】只对结核杆菌有抑菌作用。
本品为对氨基苯甲酸(PABA)的同类物,通过对叶酸合成的竞争抑制作用而抑制结核分枝杆菌的生长繁殖。
【药代动力学】自胃肠道吸收良好。
较其他水杨酸类吸收更为迅速。
吸收后迅速分布至各种体液中,在胸水中达到很高浓度,但脑脊液中的浓度很低。
本品迅速弥散至肾、肺和肝组织,在干酪样组织中可达较高浓度。
蛋白结合率低(15%)。
口服后1~2小时血药浓度达峰值,持续时间约4小时,半衰期(T1/2)为45~60分钟,肾功能损害者可达23小时。
本品在肝中代谢,50%以上经乙酰化成为无活性代谢物。
给药后85%在7~10小时内经肾小球滤过和肾小管分泌迅速排出;14%~33%以原形经肾排出,50%为代谢物。
本品亦可经乳汁排泄。
血液透析能否清除本品不明。
【适应症】适用于结核分枝杆菌所致的肺及肺外结核病,静滴可用于治疗结核性脑膜炎及急性扩散性结核病。
本品仅对分枝杆菌有效。
单独应用时结核杆菌能迅速产生耐药性,因此本品必须与其他抗结核药合用。
链霉素和异烟肼与本品合用时能延缓结核杆菌对前二者耐药性的产生。
本品对不典型分枝杆菌无效。
主要用作二线抗结核药物。
【用法用量】静脉滴注一日4~12g,临用前加灭菌注射用水适量使溶解后再用5%葡萄糖注射液500ml稀释,2~3小时滴完。
小儿每日0.2~0.3g/kg。
【不良反应】1. 发生率较多者:瘙痒皮疹、关节酸痛与发热、极度度疲乏或软弱,嗜酸性粒细胞增多(较常见的原因为过敏)。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
对氨基水杨酸
氨基水杨酸(SalicylicAcid)是一种经常被用于护肤和治疗皮
肤问题的化合物,它也常常被称为“醋酸”。
氨基水杨酸原料大多来
自以药用植物为原材料的植物提取物,具有一系列良好的功能,可以帮助我们的皮肤保持健康。
氨基水杨酸可以用于清洁,净化和抑制皮肤内的细菌,同时能够减少皱纹和细纹,促进皮肤的新陈代谢,减少痤疮的出现。
氨基水杨酸的功能可以分为以下几种:
一、去角质清洁
氨基水杨酸具有很强的去角质、清洁作用,可以有效清除脸上的老废角质,让皮肤变得更加光滑,柔软,并且控油效果好,可以把脸部粗糙的角质去掉,促使皮肤新陈代谢,促进皮肤再生。
二、抗炎作用:
氨基水杨酸具有显著的抗炎作用,可以有效抑制及预防皮肤的炎症反应,减少痤疮的出现,预防痤疮继发感染,可以抗体增效作用,改善皮肤炎症嫩痕症状,及时减轻炎症反应。
三、抗衰老及保湿:
氨基水杨酸具有一定的抗衰老和保湿作用,它能够渗透皮肤深处,改善肌肤细胞细胞活力,促进皮肤细胞新陈代谢,妨碍皮肤老化及色斑现象,促进皮肤水分留存,使皮肤更加有弹性,提升皮肤活力,使肌肤更加健康。
四、祛痘:
氨基水杨酸有多种抗炎痘的作用,可以抑制皮肤炎症反应,减少痤疮的出现,加速皮肤恢复。
它可以抑制非调节性因素导致的内分泌紊乱,缓解皮肤痤疮,改善皮肤油腻,减少痤疮的发生。
五、祛斑:
氨基水杨酸有效抑制酪氨酸酶,抑制黑色素形成,改善皮肤炎症及色素沉着,使皮肤状态改善,肤色恢复晶莹有光。
这是关于氨基水杨酸的简单介绍,我们可以看到,氨基水杨酸的功效非常强大。
它可以帮助我们延缓皮肤老化,同时还可以有效抑制皮肤炎症,改善皮肤状态,减少痤疮和色斑的出现,让皮肤变得更加健康。
而且,氨基水杨酸可以从自然界中获取到,是一种安全可靠的保养成分。
不过,在使用氨基水杨酸之前,要对自己的皮肤状态进行检查,确保适合自己的使用,以免造成不良后果。
