High-throughput genotyping by whole-genome resequencing
Ion Torrent

Ion Torrent : 个人化基因组测序仪张远涛Ph.DSr Technical Sales SpecialistYuantao.zhang@主要内容:Ion TorrentIon Torrent技术原理Nature文章Ion Torrent: 个人化基因组测序仪Main FrameSanger Sequencing Next‐Gen Sequencing Ion Semiconductor Sequencingcan use them.使用方便的桌上型仪器芯片既是测序仪革命性的创新技术:可升级的半导体技术+简单的化学反应原理Semiconductor ManufacturingH+∆pHSensing LayerSensor PlateDrain SourceSilicon Substrate可升级的半导体技术–100X in the First YearIon 314™Chip Ion 316™ChipIon 318™Chip**Some products have not yet been officially released and information about those products is subject to change without noticeSept 2011 data for 314 and 316 chips based on internal R&D runs▲▲▲Specification简单的化学反应原理简单的化学反应原理Ion torrent :整体工作流程Clonal AmplificationDNA / RNALoad Chip and SequenceIon Paired-end Sequencing(双向测序)Innovative automated template preparation for PGM sequencer matches •Complete end-to-end workflow within 1 day or multiple samples per day文库制备模板制备测序3+hr3 hr1.5 hr0.5 hr快速:整个工作流程仅需1天时间featuring low input material and fast and streamlined workflowAnalysis 3+ hours 2 hours1 hourGenetic diversity and population structure of the endangered marsupial Sarcophilus harrisiiMiller et al., PNAS (2011)/animals/mammals/tasmanian-devil/Rapid sequencing, de novo assembly & identification of novel microbial strains.Rapid sequencing, de novo assembly & identification of novel microbial strains.德国大肠杆菌爆发: 用Ion torrent 三天内得到变异的大肠杆菌基因组序列E coli 55989 complete genomeE coli LB226692 draft assemblyMonday May 30*Library preparationO104:H4 and HUSC41 samples (reference) strain libraries preparedTuesday May 31Sequencing runs0104:H4 amplified and sequenced 2 x 2 runs (Ion 314)Wednesday June 01 Sequencing runs0104:H4 sequenced 3 x 2 runs (Ion 314)Thursday June 02 AssemblyDraft Genome identified, Assembled, Submitted and Released from NCBI Friday June 03Assay DesignTaqMan Assays Designed*May 22 CEDC reports significant increase in patients with hemolytic uremic syndrome“The biggest advantage [of the PGM] from my point of view as a public health official is that it's speedy, and speed is what is needed at the moment,”Prof. Dr. Med Dag Harmsen, University Hospital Muenster“[The PGM] takes the shortest time to generate genomic data.”Junjie Qin, BGI荷兰肺炎杆菌爆发:Ion torrent再次大显身手strain_1191100241the RIVMEnterohemorrhagic E. coli & Klebsiella Pneumoniae Outbreaks NEJMMark Pallen MD, Ph.D., Holger Rohde, M.D., Junjie Qin, Ph.D et al PLoS oneAlexander Mellmann., DagHarmsen*., Craig A. Cummings.,et alScienceAlexander Mellmann., DagHarmsen*., Craig A.Cummings.金黄色葡萄球菌抗药基因进化研究美国马赛诸塞州综合医院:石蜡切片的体细胞突变检测(KRAS )Demonstrated the ability to detect somatic mutations to ~5% with accuracy of ~99.5%Single day workflow using fusion primer approach Ion DNA Barcoding Kit (Up to 16) available in Q3 for simultaneous analysis of multiple samplesKRAS exon 2Mean per base error: 0.43%26% freq. variant detection 575X CoverageDr. Long Le, Mass General HospitalPrepare library via fusion primers or post-PCR ligation Fragment via mechanical or enzymatic shearingTemplate Sequence AnalysisLibrary Amplicon sequencing using the fusion primer bidirectional sequencing approach for FFPE tumor tissue samples. A series of 10 amplicons were created to demonstrate concordance with Mass Gen’s current gold standard SNaPshot capillary sequencing based assayAmplicon sequencing using the fusion primer bidirectional sequencing approach for FFPE tumor tissue samples. A series of 10 amplicons were created to demonstrate concordance with Mass Gen’s current gold standard SNaPshot capillary sequencing based assay美国亚利桑那州肿瘤中心:体细胞突变验证(BRAF&NRAS )Demonstrated sequencing is possible from 6 year old FFPE tissue.53 samples sequenced representing 47 unique samples on four 314 chipsPer 314 Chip: 14 barcoded samples x 5 amplicons/sample + 2 controlsPositive controls 100% accurate and Mutations found in 47% of samples10 BRAF (V600E,K) and 12 NRAS codon 61 (Q61R,K,H)No sample had more than one mutationTemplate Sequence AnalysisLibrary Barcoded amplicon sequencing for detection of BRAF & NRAS mutations in melanomasBarcoded amplicon sequencing for detection of BRAF & NRAS mutations in melanomas 2 rounds of PCR using bar-coded primer sets allowed 16 samples x 5 amplicons per 314 chip (14 samples plus 2 controls)Melanoma Cell Line A375Positive ControlMelanoma SampleGenBank SampleDr. George WattsDeep sequence for heteroplasmydetection ( > 1000x coverage on Ion 314)Ability to do 16 samples per run with barcodingAccurate variant calling, especially in hypervariable regions of mitochondria美国宾州州立大学:人类线粒体基因组测序(遗传疾病风险评估)Mutation detected on position 15450Prof/. Stefan Schuster Penn. State UniversityAmplify mtDNA via two overlapping long range PCR Fragment via mechanical or enzymatic shearingTemplateSequenceAnalysisLibraryRapid resequencing of mitochondrial genomes for disease research, biodiversity assessmentRapid resequencing of mitochondrial genomes for disease research, biodiversity assessment澳大利亚西澳大学医学中心:人类线粒体基因组测序服务AmpliSeq™肿瘤基因筛查芯片AmpliSeq™肿瘤基因筛查芯片: 肿瘤基因组成美国贝勒医学院推出AmpliSeq™肿瘤基因筛查芯片的检测服务客户端:200bp 读长测序结果• University of Münster • Broad InstituteAligned Q20 (Mb) 380200Q17 reads 1,912,390Percent reads >200 bp 61%Aligned Q20 (Mb) 323 200Q17 reads 1,822,145Percent reads >200 bp 62.2%31318 Chip :1.27G测序数据 和 391 bp平均读长525 bp 读长CGCTAAGTAATATTCGCCCCGTTCACACGATTCCTCTGTAGTTCAGTCGGTAGAACGGCGGACTGTTAATCCGTATGTCACTGGTTCGAGTCCAGTCAGA |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||| CGCTAAGTAATATTCGCCCCGTTCACACGATTCCTCTGTAGTTCAGTCGGTAGAACGGCGGACTGTTAATCCGTATGTCACTGGTTCGAGTCCAGTCAGA 1 100 GGAGCCAAATTCTAAAAATTCGCTTTTTTAGCGCAATGTCACTGACCTTAGTTGAACATTGTTTTTTAACGGATAGCGGGTTTTTAACATCTTAAGCGCC |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||| GGAGCCAAATTCTAAAAATTCGCTTTTTTAGCGCAATGTCACTGACCTTAGTTGAACATTGTTTTTTAACGGATAGCGGGTTTTTAACATCTTAAGCGCC 101 200 CTCGACCTTTATGGTTGAGGGCGTTTTGCTATGAACGCCATCACCATTTTCCCCTCGATTATAAAACTTGAGTTATTCAGTAGTCTCCCCTCTTGCAACT |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||| CTCGACCTTTATGGTTGAGGGCGTTTTGCTATGAACGCCATCACCATTTTCCCCTCGATTATAAAACTTGAGTTATTCAGTAGTCTCCCCTCTTGCAACT 201 300 CACACCCAAAACTGCCTAACGAAAAGTTATTAATTTTCAATCATATTGCTATCAGTATTTACATTTTTTCGCTGTGCTAGAAAGGGCGCATTTATGTTAG |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||| CACACCCAAAACTGCCTAACGAAAAGTTATTAATTTTCAATCATATTGCTATCAGTATTTACATTTTTTCGCTGTGCTAGAAAGGGCGCATTTATGTTAG 301 400 CTCGTTCAGGGAAGGTAAGCATGGCTACGAAGAAGAGAAGTGGAGAAGAAATAAATGACCGACAAATATTATGCGGGATGGGAATTAAACTACGCCGCTT |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||| CTCGTTCAGGGAAGGTAAGCATGGCTACGAAGAAGAGAAGTGGAGAAGAAATAAATGACCGACAAATATTATGCGGGATGGGAATTAAACTACGCCGCTT 401 500 AACTGCGGGTATCTGTCTGATAACT ||||||||||||||||||||||||| AACTGCGGGTATCTGTCTGATAACT 50160033Ion Torrent 产品34Personal Genome Machine™ (PGM™) ProductsThe Chip is the Machine™Barcode Kit Library Kits (RNA and DNA) Control Kit TaqMan® Library Quant Kit Ion Sphere Control Kit Ion OneTouch™ Template KitBioruptorIon Personal Genome Machine (PGM) Ion 314™ Chip (1M wells) Ion 316™ Chip (6M wells) Ion 318™ Chip (12M wells) Sequencing KitIKATorrent Server & Torrent SuiteQuBit® FluorimeterArgon Gas & 18MOhm WaterSome products have not yet been officially released and information about those products is subject to change without notice35Ion Core Kits-100 and 200bp测序试剂盒to-use reagents for quality control of every stepIon对照试剂盒 for PGM™ WorkflowReady-Ion Control Kit • Library Control • Template Control • Sequencing Control• •Ion Sphere Quality Control Kit• Pre- and post-enrichment quality control of Ion Spheres Ready-to-use reagents contain Fam™ and Cy-5® Dye labeled primers targeting Ion adaptors Uses Qubit® 2.0 FluorometerE.coli DH10b Control gDNAE.coli DH10b Control LibraryControl Ion Sphere™ ParticlesLibraryTemplateSequenceAnalysisLibraryTemplateSequenceAnalysis37Ion Community :Ion用户论坛Community Access • User Videos • User Wiki • Application notes • FAQsCustomer Access • Site prep • User guides • Torrent Suite Guides • Release notes • File formats • Database schema38PGM & the Ion OneTouch System• Speed, Scalability and Simplicity • More reads • Longer reads • Higher quality • Higher throughput • Shorter time to result39Thank For Your Time!!Questions?。
HIVID技术检测HBV病毒整合

HIVID:An ef ficient method to detect HBV integration using low coverage sequencing ☆Weiyang Li a ,b ,1,Xi Zeng a ,1,Nikki P.Lee c ,1,Xiao Liu a ,Shengpei Chen a ,Bing Guo a ,Shang Yi a ,Xuehan Zhuang a ,Fang Chen a ,Guan Wang a ,Ronnie T.Poon c ,Sheung Tat Fan c ,Mao Mao d ,Yingrui Li a ,Songgang Li a ,Jun Wang a ,JianWang a ,Xun Xu a ,Hui Jiang a ,Xiuqing Zhang e ,⁎aBGI-Shenzhen,Shenzhen,518083,ChinabSchool of Bioscience and Bioengineering,South China University of Technology,China cDepartment of Surgery,University of Hong Kong,Hong Kong,China dP fizer Oncology,San Diego,CA 92121,USA eThe Guangdong Enterprise Key Laboratory of Human Disease Genomics,BGI-Shenzhen,Shenzhen,Chinaa b s t r a c ta r t i c l e i n f o Article history:Received 18January 2013Accepted 8July 2013Available online xxxx Keywords:Hepatocellular carcinoma Integration CaptureHigh-throughput Cost-effectiveWe reported HIVID (high-throughput Viral Integration Detection),a novel experimental and computational method to detect the location of Hepatitis B Virus (HBV)integration breakpoints in Hepatocellular Carcinoma (HCC)genome.In this method,the fragments with HBV sequence were enriched by a set of HBV probes and then processed to high-throughput sequencing.In order to evaluate the performance of HIVID,we compared the results of HIVID with that of whole genome sequencing method (WGS)in 28HCC tumors.We detected a total of 246HBV integration breakpoints in HCC genome,113out of which were within 400bp upstream or downstream of 125breakpoints identi fied by WGS method,covering 89.3%(125/140)of total breakpoints.The integration was located in the gene TERT,MLL4,and CCNE1.In addition,we discovered 133novel breakpoints missed by WGS method,with 66.7%(10/15)of validation rate.Our study shows HIVID is a cost-effective methodol-ogy with high speci ficity and sensitivity to identify viral integration in human genome.©2013The Authors.Published by Elsevier Inc.All rights reserved.1.IntroductionMore than 350million people are infected by hepatitis B virus (HBV)all over the world [1,2],and approximately 30%–50%of the estimated 320,000annual HBV-related deaths are consequences of hepatocellular carcinoma (HCC)[3].Previous studies have discovered evidence of the involvement of HBV in the tumorigenesis [4,5],indi-cating HBV integration in host genome is suspected to be one of the most important etiological events in HBV-induced HCC [6].The recur-rent HBV integration event was first reported to be located at the TERT gene in two HCC tumor samples [7,8],and subsequent studies also identi fied HBV integration breakpoints in FAR2,ITPR1(IP3R1),IRAK2,MAPK1,MLL2and MLL4genes [9,10].Previous reports have suggested that a number of cellular genes such as hTERT and FN1were frequently targeted by HBV in HCC tissue [7,9,10].Although HBV integration into the host genome has been reported in tumors from HBV-infected individuals,the mechanism of viral-host interactionremains elusive.However,previous studies indicated integration of HBV can change the expression level and function of endogenous parative analysis of gene expression levels indicated that samples with HBV integration demonstrated higher expression of TERT,MLL4and CCNE1than those samples not harboring HBV DNA integration [11].It is intriguing that the overall MLL4transcription output is much higher in the affected genome,but the resulting fusion transcript lacks the AT-hook DNA-binding domain of MLL4[12].Moreover,HBV integration was also reported to relate with somatic copy number variations [11].DNA copy number analysis in this region revealed that this viral integra-tion colocalized precisely with the junction of a large DNA copy number loss [12].Traditionally,most of the HBV integration breakpoints are detect-ed by PCR-based methods,such as Alu-HBV PCR.In the process of Alu-HBV PCR,signi ficant bias happens towards Alu regions;in other words,only the integration near Alu region can be ef ficiently detected [9,13].It is still a challenge to study integrations at genome-wide scale using PCR-based methods.With the rapid development of mas-sive parallel sequencing technology,whole genome sequencing (WGS)brings new insight to detect HBV integration in HCC genome [11,12,14].However,this approach is also limited by the high cost to perform population-scale studies.Thus,a sensitive low cost method is urgently needed to study the relationship between virus integration and tumorigenesis.Genomics xxx (2013)xxx –xxx☆This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-No Derivative Works License,which permits non-commercial use,distribution,and reproduction in any medium,provided the original author and source are credited.⁎Corresponding author.Fax:+8675525273884.E-mail address:zhangxq@ (X.Zhang).1These authors contributed equally to this work.YGENO-08536;No.of pages:7;4C:0888-7543/$–see front matter ©2013The Authors.Published by Elsevier Inc.All rights reserved./10.1016/j.ygeno.2013.07.002Contents lists available at ScienceDirectGenomicsj o u r n a l h o m e p a ge :w ww.e l s e v i e r.c o m/l o c a t e /y g e n oHere,we present a novel experimental and computational meth-od,HIVID (high-throughput viral integration detection),to effectively detect hbv integration at a single base pair resolution using HBV cap-ture sequencing (Fig.1).Probes were prepared to capture the HBV inserted fragments in host genome and these fragments were further sequenced on Illumina HiSeq 2000platform.Based on the sequence data of enriched DNA,we recruited a pair-read assemble strategy to locate the integration breakpoints.To examine the performance of HIVID,this method was tested in 28HCC samples that were previously sequenced at the whole-genome level for identifying HBV integrations [11].The integration hotspots of human genome were located in the genes TERT,MLL4and CCNE1.Most of the breakpoints were signi ficantly enriched in a 500bp region on HBV genome from 1500bp to 2000bp.We compared the results of HIVID and WGS method,and validated the novel HBV integration using PCR and Sanger sequencing.Our study provided an accurate and cost-effective method for HBV integration re-search,which can also be adapted to other virus integration studies.2.Materials and methodsBased on our previous study [11],28Chinese HCC samples from Queen Mary Hospital,Hong Kong were selected.All patients had been diagnosed with HCC with concurrent HBV rmed written consents were obtained from each patient.Approvals were acquired from the BGI Ethics Committee and the Institutional Review Board of The University of Hong Kong/Hospital Authority Hong Kong West Cluster.All samples had been previously used to discover HBV integration breakpoints.The results from 30×WGS data were used to evaluate sensitivity and speci ficity of HIVID.2.1.HBV probes preparationFull-length HBV genome of type B and type C were ampli fied by PCR using biotin-labeled dNTPs.PCR mix was prepared as follows:5μl of HBV DNA (1ng/μl);5μl of 10*LA Taq Buffer;26.5μl of H 2O;4μl of 2.5mM dNTPs (1/4dNTPs biotin-labeled);2.5μl of 10pmol P1(TTT TTC ACC TCT GCC TAA TCA);2.5μl of 10pmol P2(AAA AAGTTG CAT GGT GCT GG);and 0.5μl of LA Taq enzyme (Takara Bio,Inc.).PCR was subjected to the following cycling condition:initial denaturation for 3min at 94°C;32cycles of denaturation for 30s at 94°C,annealing for 50s at 56°C,and extension for 2.5min at 68°C;final extension for 10min at 72°C.Amplicons were puri fied by Ampure beads (Beckman Coulter,Inc.)and were then fragmented by Covaris E-210(Covaris,Inc.,Woburn,MA)to 250bp –500bp through the 2%agarose electrophoresis.Single-stranded HBV probes were generated by high temperature denaturation at 94°C for 5min.2.2.HBV-inserted fragments enrichment and sequencingSequencing libraries of 170bp insert size were constructed for 28samples following the instruction of Illumina.Genomic DNA was sheared to around 150bp –200bp DNA fragments by Covaris E-210(Covaris,Inc.,Woburn,MA).These fragments were puri fied,end blunted,“A ”tailed,and adaptor ligated.10cycles of PCR were performed after size selection in 2%agarose gel.The concentration of libraries was quanti fied by Bioanalyzer 2100(Agilent Technologies,Santa Clara,CA).Libraries were hybridized with HBV probes at 47°C for 24hours and then washed to remove un-captured fragments.The eluted fragments were ampli fied by 16cycles of PCR to generate libraries for sequencing (Fig.2).Libraries were quanti fied and proceeded to 101cycles paired-end index sequencing in the Illumina HiSeq 2000sequencer according to manufacturer's instructions (Illumina Inc.,San Diego,CA).2.3.HBV integration breakpoints detectionThe whole pipeline for data analysis in HIVID consists of six steps (Fig.1).After we isolated the paired-end reads which may contain the signals of HBV integration,paired-end read assembly was conducted to obtain reconstructed inserted fragment and detected HBV integra-tionbreakpoints.Fig.1.Overall work flow of HIVID.The pipeline includes the work flow of experiment and bioinformatics process.In the process,we do Raw data filter first,then perform Raw-mapping with filtered raw data.After that we pick out the chimeric paired-end reads to do Paired-end read assembly.PE-assembled reads are conducted to go through Re-mapping to locate the HBV integrations sites.Finally,Signal merging and Signal filter are performed to obtain the final results.To do the step of Paired-end reads assembly,we used a program developed by ourselves.2W.Li et al./Genomics xxx (2013)xxx –xxx2.3.1.Raw data filterLow quality reads and duplication reads,as well as adaptor contami-nation reads,was first removed to obtain clean reads for subsequent analysis.If a read whose bases with quality value (b =5)occupy 50%of the read length,the read is considered as a low quality read.We then had the clean reads for detecting the HBV integration after the low qual-ity reads and duplication reads removal.2.3.2.Raw-mappingClean reads were mapped to human (NCBI build 37,HG19)and HBV genome using SOAP2(-l 40-v 5-r 1)[15].We removed reads that perfectly paired-end aligned to human or HBV genome and reserved chimeric paired-end reads (partial read sequence aligned to human genome and partial aligned to HBV genome)for assembly.This might help identify HBV integration breakpoint.2.3.3.Paired-end reads assemblyPaired-end reads assembly was used to reconstruct around 170bp of fragment sequences,assembly which might increase the potential to locate the exact position of breakpoints.The paired-end reads were first changed to the same strand.In case that the tail of an upstream end (read1)and the head of a downstream end (read2)overlap by more than 5bp where the mismatch rate was less than 0.2,the two ends would be spliced into one continuous sequence,called PE-assembled read,which was reconstructed inserted fragment (Fig.3A).2.3.4.Re-mappingTo determine the exact location of HBV integration breakpoint,the PE-assembled reads were re-mapped to human and HBV genome using BWA 0.5.9-r16(-a 1,-b 2,-q,5,-r,2)[16].If the match length on both HBV genome reference and human genome reference for one PE-assembled read was larger than 30bp,the PE-assembled read alignment result was reported to detect a precise breakpoint.The joint position of human and HBV sequence was the breakpoints for HBV inte-gration (Fig.3B).2.3.5.Signal mergingWe also calculated the total number of support PE-assembled reads (NSS)of each HBV integration breakpoint to examine its reliability.Considering errors in the procedure of experiment and bioinformatics analysis and highly heterogeneous nature of tumors,we merged breakpoints within 20bp to select the breakpoint with largest NSS as the representative,and reset its NSS as sum of NSS of all breakpoints in this region.2.3.6.Signal filterTo minimize the impact of total sequencing data for each breakpoint frequency and remove the noise signals,we normalized NSS to Normal-ized number of support PE-assembled reads (NNSS)using its number of effective paired-reads (EFR)as following:NNSS ¼NSS Â106=EFREffective paired-reads are de fined as reads used to search breakpoints except contaminated data.NNSS could be considered as the number of supporting reads of each HBV integration breakpoint out of every million read-pairs and represents the reliability and data utilization with no bias.In this study,HBV breakpoints with NNSS N =1was regarded as true signal.2.4.PCR and Sanger sequencing validationPCR and Sanger sequencing was used to verify the selected HBV integration breakpoints from HIVID.PCR primers were designed based on the PE-assembled fragment,in which one primer located in human genome and the other in HBV genome.PCR were performed by GeneAmp®PCR System 9700thermal cycler and then preceded to Sanger sequencing on Applied Biosystems 3730x DNA analyzer (Life Technologies,Inc).3.Results3.1.HBV integration breakpoints detectionTo get the overall evaluation of the accuracy of breakpoint detection using HIVID approach,we compared our results to that of previously re-ported ~30×WGS approach [11].Sung et al.reported a total of 399HBV integration breakpoints in 81HBV-positive and 7HBV-negative HCC and adjacent non-tumor samples,with a validation rate of 82.0%[11].We randomly selected 28samples for HIVID to conduct HBV capture sequencing on Illumina HiSeq 2000,generating ~1.43Gb on average (Table 1).The effect of paired-end reads for the following analysis were about 6millions,among them around 83.72%and 0.08%can be aligned to human genome and HBV genome,respectively.After paired-end assembly and re-mapping,we finally obtained an average of 1654reads supporting HBV integration breakpoints for each sample.A total of 246HBV integration breakpoints were detected within these 28sam-ples,in average 8.8breakpoints per sample.The number of integration breakpoints varied from 1to 27among samples (Table S1).3.2.HBV integration breakpoint detection comparisonWe firstly compared HBV integration breakpoints between HIVID and WGS approach in 28selected samples.To minimize the impact of different work flows we de fined a shared breakpoint in HIVID and WGS method when the distance of two breakpoints from the same sam-ple was less than 400bp.There were 140HBV integration breakpoints from Sung's study with at least two paired-end reads supporting and 89.3%of them (125/140)was shared with HIVID test (Tables S2and S3),suggesting that the current approach has high speci ficity when com-pared with WGS approach.In contrast,only 45.9%of HBV integration breakpoints (113/246)from HIVID approach were shared with WGS approach (Fig.4).Fourteen of 113HBV integration sites that were select-ed for validation were all validated in previous WGS study,showing 100%validation rate [11].Further analysis for the average NNSSvalue,Fig.2.HBV capture work flow.The different colored images marked represented differ-ent meaning.The dark yellow color represented the HBV fragments,while the glaucous color represented the human genome fragments.DNA libraries were hybridized with HBV probes at 47°C for 24hours and then washed to remove un-captured fragments.The eluted fragments were ampli fied by 16cycles of PCR to generate libraries for se-quencing.(For interpretation of the references to color in this figure legend,the reader is referred to the web version of this article).3W.Li et al./Genomics xxx (2013)xxx –xxxwhich was 13.6for 113WGS-shared HBV integration breakpoints and 8.5for all breakpoints,indicated WGS approach might tend to identify high frequency HBV integration breakpoints due to a relatively limited sequencing depth.The comparative analysis indicated HIVID test could detect almost the same HBV integration breakpoints as WGS approach with much less sequencing data.In the 133novel breakpoints we randomly picked 15novel HBV integration breakpoints from six samples for PCR and Sanger sequencing validation test.66.7%(10/15)of novel HBV integration sites were suc-cessfully con firmed (Table S4),which is signi ficantly lower than the validation rate for WGS-shared breakpoints.We then investigated the average NNSS value of the 133novel breakpoints,which were only 4.2,compared to 13.6for WGS-shared breakpoints and 8.5for the total ones.It was obvious that HIVID showed a notable advantage on breakpoints enrichment ef ficiency.On average,the HIVID approach generated 6mil-lion paired-end reads,compared to 600million read pairs for WGS meth-od.Moreover,the average NNSS values of two methods were 8.5and 0.0142,representing that HIVID approach could enrich HBV integration fragments close to 600times than WGS approach.This high ef ficiency for HBV integration sites enrichment would be bene fit for large-scale virus-related tumor development studies.3.3.Characteristics of HBV integration breakpointsWe also investigated the characteristics of HBV integration breakpoints,which might be related with HCC development.Integra-tion events were detected in HBV subtype C (AB014381.1).Among them,33.5%(68/203;P =2.7×10−5)were signi ficantly enriched in a 500bp region on HBV genome from 1500bp to 2000bp (Supplemen-tary Fig.S1),which was consistent with the results of the WGS study [11,12].Additionally,we also surveyed the frequency of integration breakpoints around HBV genes,showing 38.9%(79/203)breakpoints were located in the region of pre-S1(Table S5).The analysis for recurrent genes with HBV integration breakpoints in those 28samples was also consistent with previous studies (Fig.5)[10,17–19].Using HIVID approach,HBV integration breakpoints were detected in TERT ,MLL4and CCNE1genes,affecting 25%,17.9%,and 7.1%of HCC samples (Table 2).Notably,we identi fied HBV integration breakpoint in TERT gene for sample 90T and 41T,which was missed in previous WGS approach.Besides the frequent bias of HBV integration sites in HBV and human genome,we also noticed that HBV integration events with the deletion of human genome.For example,a 49bp human genome sequence in sample 43T was replaced by a 41bp HBV sequence on ChrX:20,156,148(Table S4).In sample 49T the human genome fragment of 728,975bp in chromosome 2were replaced by 127bp HBV sequence (Fig.6).The abundant supporting reads for breakpoints provided by HIVID may be helpful to explore the complex relation.4.DiscussionIn this study,we developed a novel approach for HBV integration breakpoint detection and employed 28HCC samples to estimate the performance on integration sites with high frequency and low frequen-cy.With only 1.5Gb HBV capture sequencing data,HIVID approach can identify about 90%of HBV integration breakpoints identi fied from ~30×WGS data,and over 50%of novel low frequent integration sites missed by WGS approach.HIVID approach shows its strength on several aspects compared with previously reported approaches.Unlike the widely used HBV-Alu PCR method,HIVID can be uti-lized to detect integration sites at the genome-wide level instead of the regions close to Alu repeats.In fact,83.3%(205/246)of the discov-ered HBV integration breakpoints were more than 10,000bp away from Alu repeats (Table S2),signi ficantly larger than 7.8%of WGS ap-proach [11].Although both HIVID and WGS approach are based on mas-sively parallel sequencing platform,HIVID approach has dramatically higher ef ficiency on virus integration detection.First,HIVID approach requires much less sequencing data due to the process of HBVfragmentFig.3.Insight of computational process of HIVID.A,principle of pair-end reads assembly.The figure shows that the tail of an upstream end (read1)and the head of a downstream end (read2)were spliced into one continuous sequence,called PE-assembled read;B,determination of breakpoint.The figure shows that one PE-assembled read consists of the part of human sequence and part of HBV sequence.The joint position was the breakpoint for HBV integration.4W.Li et al./Genomics xxx (2013)xxx –xxxcapture,but with an enrichment close to 600times compared with WGS approach.HIVID could also be suitable for detecting the breakpoints of other types of viruses,such as human papillomavirus,human immuno-de ficiency virus.Moreover,it also could decrease the cost and shorten the time to generate the results.Secondly,HIVID can detect 89.3%(125/140)integration breakpoints which had been identi fied by WGS method,and those integration sites are also characterized by higher NNSS and validation rate.These results demonstrate the HIVID method has a high speci ficity of detecting viral integration breakpoints.Thirdly,HIVID method is more sensitive for detecting low frequency HBV inte-gration breakpoints.Although the validation rate was 66.7%(10/15),HIVID methods could identify 50%more HBV integration sites.The reduced average NNSS value of the novel sites (4.2)compared with the WGS shared ones (13.6)indicates HBV integration in these sites may be less frequent among the tumor cells.The other cause for the lower veri fication rate of new breakpoints could be attributed to the primer design dif ficulty for the shorter support reads.The validationrate might be improved if we adopt the 454long reads sequencing.The long reads also will help us to get more information about the HBV insertion sequence.The major limitation of this approach com-pared with WGS is that we can only target speci fically one or more virus that have genome references,and identify their integrations.This is a hypothesis driven approach.Another technical limitation is that human genome reads and free virus reads take up a high propor-tion of the data.The method had a low capture speci ficity,although it had an obvious enrichment.We will get more integration breakpoints and need less sequencing data if the capture speci ficity could get im-proved.The single cell study for virus integration breakpoints could fig-ure out monoclonal evolution and the roles of high and low frequency integration events.Since we used part of samples from our previous study,the similar hotspot regions in human and HBV genome were de-tected with these two methods.However,with more supporting reads we annotated the complicated types of HBV integration events,which were omitted in our previous study.Herein,we found a trend of dele-tion in human genome accompanying with HBV fragments insertion,which might induce chromosomal instability and further carcinogene-sis.Genetic instability triggered by HBV integration has been considered in some reports to be an important contributing factor in the pathogen-esis of HCC [20,21].HBV insertions are commonly associated with large genetic alterations:deletions,duplications and chromosomal transloca-tions,which might re flect the abrogation of control mechanisms that safeguard chromosomal integrity [22].However,the impact of those human structure variations on molecular mechanism of HCC still re-mains unclear.In summary we reported the HIVID approach to detect HBV inte-gration breakpoints with high speci ficity and sensitivity using only 1.5Gb sequencing data,for which this approach can be used to screen virus integration in a large cohort of samples and leads to a systematic study for its relationship with disease etiology and tumorigenesis in a comprehensive and unbiased way.Supplementary data to this article can be found online at /10.1016/j.ygeno.2013.07.002.Table 1Data production of 28tested samples.Sample Total Bases Reads number EFR (M paired-reads)Human alignment (pair end)HBV alignment (pair end)Integration rate 101T 1.50G 7.51M;7.51M 5.8895.58%0.03%738(0.01%)43T 1.32G 6.58M;6.58M 5.6686.30%0.04%2520(0.02%)71T 1.49G 7.45M;7.45M 6.4685.05%0.11%1858(0.01%)58T 1.47G 7.33M;7.33M 6.1684.35%0.05%1230(0.01%)34T 1.39G 6.93M;6.93M 5.9884.35%0.02%480(0.00%)55T 1.53G 7.63M;7.63M 6.4782.91%0.10%602(0.00%)35T 1.64G 8.22M;8.22M 7.0585.13%0.02%188(0.00%)41T 1.15G 5.77M;5.77M 4.9784.23%0.12%1042(0.01%)32T 0.70G 3.52M;3.52M 3.1287.08%0.08%424(0.01%)186T 1.99G 9.94M;9.94M 8.5986.70%0.01%374(0.00%)49T 1.17G 5.83M;5.83M 4.9582.61%0.11%2026(0.02%)26T 1.40G 7.01M;7.01M 5.9481.78%0.03%442(0.00%)90T 1.34G 6.70M;6.70M 5.8188.05%0.20%1212(0.01%)182T 1.55G 7.74M;7.74M 6.6887.93%0.03%474(0.00%)266T 1.36G 6.80M;6.80M 5.7786.27%0.03%860(0.01%)145T 1.41G 7.03M;7.03M 5.7486.09%0.08%1398(0.01%)174T 1.35G 6.75M;6.75M 5.7887.22%0.03%290(0.00%)122T 1.52G 7.59M;7.59M 6.4885.11%0.15%1086(0.01%)114T 1.85G 9.25M;9.25M 7.5986.53%0.01%176(0.00%)17T 1.47G 7.36M;7.36M 6.4386.35%0.23%584(0.00%)200T 1.45G 7.25M;7.25M 5.6274.33%0.18%1546(0.01%)95T 1.24G 6.21M;6.21M 5.1877.77%0.04%1546(0.01%)23T 1.17G 5.83M;5.83M 4.8879.94%0.08%2634(0.03%)64T 1.17G 5.83M;5.83M 4.8777.04%0.01%136(0.00%)30T 1.66G 8.28M;8.28M 6.9380.35%0.05%572(0.00%)65T 1.80G 9.01M;9.01M 7.5677.03%0.05%482(0.00%)70T 1.04G 5.19M;5.19M 4.3479.30%0.30%1752(0.02%)82T1.90G9.51M;9.51M8.0278.64%0.03%288(0.00%)This table shows the data production of 28samples.Including human alignment ratio of pair-end reads,HBV alignment ratio of pair-end reads,raw data quantity and integration rate,etc.Fig.4.Overview of breakpoints detected by HIVID.The figure shows that HIVID detect-ed 246breakpoints on human genome in total.There were 113novel breakpoints and 133breakpoints shared with WGS method.The shared 133breakpoints covered 89.3%of WGS result.In addition the figure also provides the information of NNSS value and validated rate.5W.Li et al./Genomics xxx (2013)xxx –xxxFig.5.Distribution of breakpoints on human genome.In this CIRCOS figure,we showed the human chromosomes,the integration frequency of each breakpoint on the level of supporting reads and on the level of samples.The outer circle represents 24chromosomes and mitochondria with different color-code and number labeled.In the middle circle,the integration frequency of each breakpoint one the level of supporting reads is displayed as height of each blue point.In the inner circle,the height of each red point represents the integration frequency of each breakpoint on the level of samples.To explore the distribution of frequency of all integration breakpoints on the level of supporting reads,we pooled all breakpoints from all samples.We merged those breakpoints which appeared in several different samples but in the same location and reset NNSS of the new merged breakpoint as sum of NNSS of all breakpoints in this location.The new NNSS could be seen as the integration frequency of each merged breakpoint on the level of supporting reads.The middle circle was figured based on the process above and the NNSS of each merged breakpoint is displayed as the height of each blue point.To explore the distribution of frequency of integration breakpoints on the level of samples,the human genome sequence was divided into about three million windows,each with length of 1000bp.The total number of samples that the breakpoints in each window belonged to,was calculated.Each window was considered as one new breakpoint and the number of samples in each win-dow calculated above was considered as supporting samples that supported the window as a new breakpoint.The number could be seen as the integration frequency of each breakpoint on the level of samples.The inner circle was figured based on the process above and the height of each red point represents the integration frequency of each breakpoint on the level of samples.(For interpretation of the references to color in this figure legend,the reader is referred to the web version of this article).Table 2The enriched region of HBV integration.Sample ID PositionNSS NNSS Gene266T chr5:1,295,4639716.82TERT:Promoter 34T chr5:1,295,3759616.05TERT:Promoter 58T chr5:1,295,3398012.98TERT:Promoter 65T chr5:1,295,4429712.82TERT:Promoter 90T chr5:1,295,6397212.38TERT:Promoter41T chr5:1,295,12119 3.82TERT:NM_001193376:5-UTR;TERT:NM_198253:5-UTR;64T chr5:1,295,2959 1.85TERT:Promoter49T chr19:36,212,74121242.83MLL4:NM_014727:Intron;71T chr19:36,213,142467.12MLL4:NM_014727:Intron;186T chr19:36,214,00716619.34MLL4:NM_014727:CDS;70T chr19:36,212,313465107.03MLL4:NM_014727:CDS;95T chr19:36,212,5665711.01MLL4:NM_014727:CDS;145T chr19:30,303,49923140.24CCNE1:NM_001238:Intron;200Tchr19:30,315,3669416.72CCNE1:DownstreamThis table shows the hotspots of HBV integration in the 28samples.The relative genes of these integration positions have been shown in the table.6W.Li et al./Genomics xxx (2013)xxx –xxx。
三代测序与靶向捕获技术联用进行高分辨HLA基因分型及MHC区域单倍体型精细鉴定

Hereditas (Beijing) 2019年4月, 41(4): 337―348 收稿日期: 2018-11-26; 修回日期: 2019-01-25基金项目:“万人计划”青年拔尖人才项目资助[Supported by the Young Talents Program of National High-level Personnel of Special SupportProgram (The “Ten Thousand Talent Program”)]作者简介: 陈佳,在读硕士研究生,专业方向:微生物与生化制药。
E-mail: chenjia19940216@ 通讯作者:李一荣,主任医师,研究方向:临床分子免疫学诊断。
E-mail: liyirong838@邓子新,教授,研究方向:合成生物学。
E-mail: zxdeng@ 刘天罡,教授,研究方向:合成生物学。
E-mail: liutg@DOI: 10.16288/j.yczz.18-282网络出版时间: 2019/2/25 17:19:18URI: /kcms/detail/11.1913.R.20190225.1719.008.html技术与方法三代测序与靶向捕获技术联用进行高分辨HLA 基因分型及MHC 区域单倍体型精细鉴定陈佳1,舒明月1,里进2,付爱思1,杨帆3,王邹3,李一荣2, 邓子新1,刘天罡11. 武汉大学药学院,组合生物合成与新药发现教育部重点实验室,武汉 4300712. 武汉大学中南医院检验医学中心,武汉 4300713. 武汉生物技术研究院公共技术服务平台,武汉 430071摘要: 人类白细胞抗原(human leukocyte antigen ,HLA )的高分辨率、精准分型对于组织配型以及HLA 相关疾病研究具有重要意义。
本研究以12位原发性肝细胞癌病人的外周血为供试样本,分析二、三代测序数据用于高分辨率HLA 分型的优劣势,同时结合探针捕获与三代测序技术对YH 、HeLa 标准细胞系以及一个原发性肝细胞癌病人的主要组织相容性复合体(major histocompatibility complex ,MHC)区域进行靶向分析,探究长读长测序技术对于整个MHC 区域精细分析的潜力。
CRISPR procedure
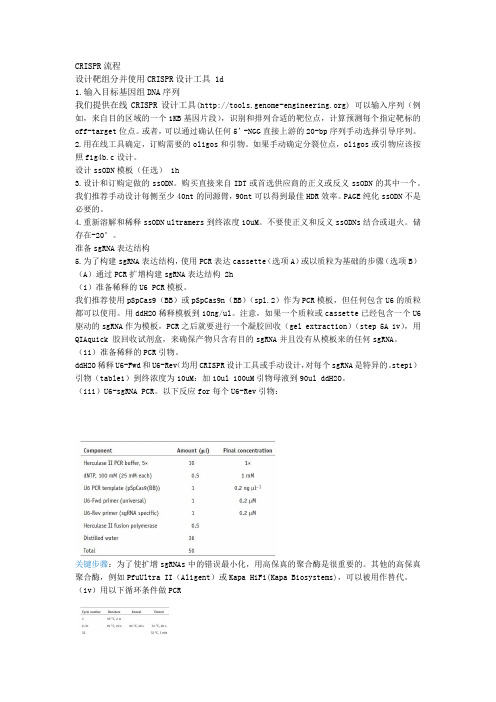
CRISPR流程设计靶组分并使用CRISPR设计工具 1d1.输入目标基因组DNA序列我们提供在线CRISPR设计工具() 可以输入序列(例如,来自目的区域的一个1KB基因片段),识别和排列合适的靶位点,计算预测每个指定靶标的off-target位点。
或者,可以通过确认任何5’-NGG直接上游的20-bp序列手动选择引导序列。
2.用在线工具确定,订购需要的oligos和引物。
如果手动确定分裂位点,oligos或引物应该按照fig4b.c设计。
设计ssODN模板(任选) 1h3.设计和订购定做的ssODN。
购买直接来自IDT或首选供应商的正义或反义ssODN的其中一个。
我们推荐手动设计每侧至少40nt的同源臂,90nt可以得到最佳HDR效率。
PAGE纯化ssODN不是必要的。
4.重新溶解和稀释ssODN ultramers到终浓度10uM。
不要使正义和反义ssODNs结合或退火。
储存在-20°。
准备sgRNA表达结构5.为了构建sgRNA表达结构,使用PCR表达cassette(选项A)或以质粒为基础的步骤(选项B)(A)通过PCR扩增构建sgRNA表达结构 2h(i)准备稀释的U6 PCR模板。
我们推荐使用pSpCas9(BB)或pSpCas9n(BB)(spl.2)作为PCR模板,但任何包含U6的质粒都可以使用。
用ddH2O稀释模板到10ng/ul。
注意,如果一个质粒或cassette已经包含一个U6驱动的sgRNA作为模板,PCR之后就要进行一个凝胶回收(gel extraction)(step 5A iv),用QIAquick 胶回收试剂盒,来确保产物只含有目的sgRNA并且没有从模板来的任何sgRNA。
(ii)准备稀释的PCR引物。
ddH2O稀释U6-Fwd和U6-Rev(均用CRISPR设计工具或手动设计,对每个sgRNA是特异的。
step1)引物(table1)到终浓度为10uM:加10ul 100uM引物母液到90ul ddH2O。
基于高通量测序开发玉米高效KASP分子标记

作物学报 ACTA AGRONOMICA SINICA 2019, 45(6): 872 878/ISSN 0496-3490; CN 11-1809/S; CODEN TSHPA9E-mail: zwxb301@本研究由国家重点研发计划项目(2017YFD0101205, 2017YFD0102005)和江苏省农业科技自主创新项目[CX(18)1001]资助。
This study was supported by the National Key Research and Development Program (2017YFD0101205, 2017YFD0102005) and the Jiangsu Agricultural Science and Technology Innovation Fund [CX(18)1001].*通信作者(Corresponding author): 赵涵, E-mail: zhaohan@, Tel: 025-********第一作者联系方式: E-mail: luhaiyan@, Tel: 025-********Received(收稿日期): 2018-10-07; Accepted(接受日期): 2019-01-19; Published online(网络出版日期): 2019-03-01. URL: /kcms/detail/11.1809.S.20190228.0935.002.htmlDOI: 10.3724/SP.J.1006.2019.83067基于高通量测序开发玉米高效KASP 分子标记陆海燕1 周 玲1 林 峰1 王 蕊2 王凤格2 赵 涵1,*1江苏省农业科学院 / 江苏省农业生物学重点实验室, 江苏南京 210014; 2北京市农林科学院玉米研究中心, 北京 100097摘 要: SNP (Single Nucleotide Polymorphism)在基因组中数量多、分布广, 适用于大规模、自动化基因型检测。
诺禾致源资料

CONTENTS
目录
建库测序
06 建库测序服务
基因组测序
08 动植物基因组测序 10 基因组特征评估 11 基因组de novo测序 14 泛基因组测序(pan-genome) 17 动植物重测序 18 变异检测(基于全基因组重测序) 20 变异检测(基于简化基因组测序) 22 单个性状定位 25 遗传图谱(基于全基因组重测序) 27 遗传图谱(基于RAD-seq简化基因组技术) 29 遗传图谱(基于GBS简化基因组技术) 31 群体进化(基于全基因组重测序) 33 群体进化(基于简化基因组测序)
测序策略
每台仪器数据产出
最低测序量
Q30
项目周期
建
PE300
13Gb data / 1 Run 13Gb data (1 Run)
微生物
55 16S/18S/ITS等扩增子测序 58 宏基因组测序 61 细菌基因组测序 64 真菌基因组测序 67 小基因组测序
1
诺禾致源 北京诺禾致源生物信息科技有限公司于2011年3月15日在北京中关村生命科学 园注册成立,以基因组学研究与应用开发为发展方向,致力于成为全球领先的基因组学研究解决方案提供者。专 注于开拓生物学、计算机科学和信息技术在动植物研究以及人类健康领域的应用。
[4] Li M, Tian S, Jin L, et al. Genomic analyses identify distinct patterns of selection in domesticated pigs and Tibetan wild boars[J]. Nature genetics, 2013, 45(12): 1431-1438.
植物生理习题整理-xujun
各位老师出的习题粗略整合韩斌1.如何完成高等植物基因组的精确测序?基因组测序的方法有两种:(1)克隆步移法(BAC-by-BAC strategy)克隆步移法的步骤主要如下:首先要构建遗传图,再利用几套高度覆盖的大片段基因组文库(现在主要采用的是BAC 和PAC构建文库)获得精细的物理图,选择合适的BAC或PAC克隆,再构建亚克隆库(sub-clone library)进行测序,最后利用计算机对序列进行装配。
要完成精确的测序,构建精细的物理图是基础。
这就要求构建高覆盖率的BAC或PAC 基因组文库。
将全基因组DNA部分酶切,电泳选取130Kb片段,建立BAC库,然后根据酶切指纹图等方法建立物理图谱。
根据BAC序列的重叠关系,将大片段的克隆在染色体上定位好,由相互关联,部分重叠的BAC克隆连成大的重叠群(contig)。
Contig中的每个BAC 克隆都分别用超声波破碎打断产生2~4kb的片断构建亚克隆库,使之产生10倍的测序冗余程度的亚克隆重叠群。
对此亚克隆库进行测序,序列组装以完成相应BAC克隆序列。
BAC 内部的空洞(gap)可以利用设计引物进行PCR等手段填补。
完成所有BAC克隆序列后,便可将重叠群中的BAC克隆组装起来,完成整个染色体的精细物理图。
应用最新发展的高通量测序方法包括454测序技术和solexa测序技术,可大大缩短测序周期。
测序的速度的较慢,要构建BAC库耗费大量的时间和资源。
优点是组装的准确度较高,基因组的比较完整空隙较少。
构建的克隆库可以反复使用。
也可以重复。
(2)全基因组鸟枪法(whole genome shotgun strategy)全基因组鸟枪法测序:主要步骤是,第一,建立高度随机、插入片段大小为2kb左右的基因组文库。
经末端测序的克隆片段的碱基总数应达到基因组5倍以上。
第二,高效、大规模的末端测序。
对文库中每一个克隆,进行两端测序,第三,序列集合,用计算机对序列进行装配。
四川农业大学最新nature genetics 文章,解密藏猪基因组
© 2013 Nature America, Inc. All rights reserved.
The genome had a high heterozygous SNP rate (1.82 × 10−3) and was assembled using SOAPdenovo (Supplementary Note), which yielded a contig N50 size (50% of the genome is in fragments of this length or longer) of 20.7 kb, a scaffold N50 size of 1.1 Mb and a total of 2.43 Gb of ungapped sequence that was very similar to the Duroc pig genome1 and had similar genomic features (for example, synteny of 2.34 Gb, or 93.41% of the Tibetan wild boar genome) (Table 1, Supplementary Figs. 3–11 and Supplementary Tables 2–11). We also carried out whole-genome resequencing of 30 Tibetan wild boars from six major distributed locations and 18 geographically related pigs in China (15 domestic pigs and 3 wild boars) with approximately fivefold coverage for each individual (~659 Gb in total) to study genetic diversity, effective population size and genomic regions under selection. RESULTS Evolution of gene families We predicted 21,806 protein-coding genes in the Tibetan wild boar genome (Table 1 and Supplementary Tables 12–16), with a core set of 10,190 genes (46.73%) being shared with Duroc pig and human
会议手册说明书
Oct. 16th-18th, 2020. Beijing89组织委员会副主任组织委员会委员(排名不分先后)曹志军 教授(中国农业大学) 刘剑锋 教授 (中国农业大学)王玉茂 研究员 (山东省滨州畜牧兽医研究院)余忠祥 研究员 (青海大学/青海畜牧科学院)姜 雨 教授 (西北农林科技大学)刘志红 副研究员(内蒙古农业大学)储明星 研究员(中国农业科学院北京畜牧兽医研究所)岳耀敬 副研究员(中国农业科学院兰州畜牧兽医研究所)魏彩虹 研究员 (中国农业科学院北京畜牧兽医研究所)凌英会 教授 (安徽农业大学)王建民 教授 (山东农业大学)王维民 副教授 (甘肃农业大学)刘武军 教授 (新疆农业大学)刘文忠 教授 (山西农业大学)姜怀志 教授 (吉林农业大学)菅复春 教授(河南农业大学)李 英 教授 (佛山科学技术学院) 赵永聚 教授 (西南农业大学)白文林 教授 (沈阳农业大学)马慧海 研究员(吉林省农业科学院)蒋永清 研究员(浙江农林科学院)张志东 研究员(中国农业科学院兰州兽医研究所)任艳玲 副研究员(山东省滨州畜牧兽医研究院)连正兴 教授 (中国农业大学) 张 微 教授 (中国农业大学)李文蓉 研究员(新疆畜牧科学院)沈 敏 研究员(新疆农垦科学院) 张艳丽 教授 (南京农业大学) 苏 蕊 教授 (内蒙古农业大学) 李 利 教授 (四川农业大学) 刘永刚 教授 (云南农业大学) 姜勋平 教授 (华中农业大学) 王小龙 教授 (西北农林科技大学) 陈 祥 教授 (贵州大学) 王惠娥 副教授(塔里木大学) 田发益 教授 (西藏大学) 曲绪仙 研究员(山东省畜牧总站) 孙 伟 教授 (扬州大学) 林亚秋 教授 (西南民族大学) 马 毅 研究员 (天津市农业科学院) 刘庆友 教授 (广西大学) 邓卫东 教授 (云南农业大学) 庞训胜 教授 (安徽科技学院) 许卫华 副教授 (龙岩学院) 会议日程Conference Program Day1 Online Conference Plenary talk Section talk Friday, October 16, 2020Session 1: Germplasm resources Chairs: Jianlin Han & Hans Lenstra Chairs: Menghua Li 8:00-8:10 (GMT)15:00-15:10 (BJS)8:10-8:20 (GMT)15:10-15:20 (BJS)8:20-8:30 (GMT)15:20-15:30 (BJS)8:30-8:40 (GMT)15:30-15:40 (BJS)8:40-8:50 (GMT)15:40-15:50 (BJS)8:50-9:30 (GMT)15:50-16:30 (BJS)9:30-10:10 (GMT)16:30-17:10 (BJS)10:10-10:20 (GMT)17:10-17:20 (BJS)10:20-10:45 (GMT)17:20-17:45 (BJS)10:45-11:05 (GMT)17:45-18:05 (BJS)11:05-11:30 (GMT)18:05-18:30 (BJS)11:30-12:30 (GMT)18:30-19:30 (BJS)Opening welcome Congratulatory letter from Chunli Bai (白春礼), the President of ANSO Welcome speech from the President of China Agricultural University Welcome speech from FAO, UN (联合国粮食及农业组织)Keynote speech: A Snap Shot at Alliance of International Science Organizations The state of global gene-banking of animal genetic resources Preliminary analysis of the molecular diversity of British sheep breeds: major types, origin and admixture Coffee time Synthesis of phenotypic and genomic architecture of indigenous African sheep genetic resources A genome-wide multidimensional selection signature analysis identifies novel genomic footprints for Merino-like phenotype in Italian sheep breeds Signatures of positive selection in several populations representing Balkan sheep Lunch break Menghua Li Jinghua Cao Qixin Sun Paul Boettcher Jinghua Cao Paul Boettcher Hans Lenstra Joram Mwacharo Elena Ciani Ino Curik CAU ANSO CAU FAO ANSO FAO, Italy UU,Netherland ICARDA,Ethiopia University of Bari,Italy University of Zagreb,Croatia 大会秘书处:吕锋骅 马佳莹 杨继 林宇娈 杨萌联系电话:010-******** 181******** 188********电子邮件:**********************1011Plenary talk Plenary talk Section talk Plenary talk Section talk Plenary talk Section talk 12:30-13:10 (GMT)19:30-20:10 (BJS)8:00-8:40 (GMT)15:00-15:40 (BJS)8:40-9:20 (GMT)15:40-16:20 (BJS)9:20-9:40 (GMT)16:20-16:40 (BJS)9:40-10:00 (GMT)16:40-17:00 (BJS)10:00-10:10 (GMT)17:00-17:10 (BJS)10:10-10:50 (GMT)17:10-17:50 (BJS)15:00-15:40 (GMT)22:00-22:40 (BJS)15:40-16:05 (GMT)22:40-23:05 (BJS)16:05-16:30 (GMT)23:05-23:30 (BJS)13:10-13:35 (GMT)20:10-20:35 (BJS)13:35-14:00 (GMT)20:35-21:00 (BJS)14:00-14:25 (GMT)21:00-21:25 (BJS)14:25-14:50 (GMT)21:25-21:50 (BJS)14:50-15:00 (GMT)21:50-22:00 (BJS)Historical introgression from wild relatives enhanced climatic adaptation and resistance to pneumonia in sheep The conservation of native and locally-adapted breeds: their potential to contribute to genetic diversity and future systems of livestock production Using genomic information in dairy sheep breeding in Spain Sheep breeding in Gansu sheep breeder company Ltd, China Feasibility of a genomic selection approach based on a female informative population in Sarda dairy sheep breed Coffee time The role of nutrition for meat quality of lamb Reading beneath the lines:parchment as a sheep genetic resource Retroviral insertions confirm the ancient origin of native sheep in Estonia and provide guidance for the conservation program I n s i g h t s i n to s h e e p d e m o g ra p h i c h i sto r y b y archaeogenetic analysis of Anatolian ancient sheep Deep genome resequencing reveals artificial and natural selection for visual deterioration, plateau adaptability and high prolificacy in Chinese domestic sheep Crossbred mapping populations as promising tool to reveal functional candidate genes: a study case in Russian sheep breeding Functional Genomics of early reproduction in domestic sheep Deciphering climate-mediated adaptation in European sheep Coffee time Menghua Li Lawrence Alderson Juan Jose Arranz David William Osborn Antonello Carta David Pethick Matthew Teasdale Eve Rannamäe Fusun Ozer Weimin Wang Tatiana Deniskova Kisun PokharelMario Barbato CAU,ChinaChairman of Countrywide Livestock, UK Universidad de Leon,Spain Australia GRIS Sardinia, Bonassai,Italy Murdock University,Australia Cambridge University,UKTartu University,EstoniaHacettepe University Ankara, TurkeyCAU,ChinaL.K. Ernst Instituteof Animal Husbandry,RussiaNatural Resources Research Center,FinlandUniversità Cattolica del Sacro Cuore,ItalySession 3: Ancient DNA Chairs: Matthew Collins & Xingbo ZhaoSession 5: Nutrition and meat Chairs: David Pethick & Hailing Luo Day2 Online Conference Saturday, October 17, 2020Session 4: Molecular breeding Chairs: Lawrence Alderson & Caihong Wei Session 2: G3: genes, genomics and genetics Chairs: Rui Su & Ino Curik-18Oct.16th-18th3637 Tatiana DeniskovaPhD in Biological Sciences (Genetics), senior Researcher,head of the group for molecular genetics and genomics ofsmall ruminant, Department of Biotechnology and MolecularDiagnostics, L.K. Ernst Federal Science Center for AnimalHusbandry, Moscow region, RUSSIA. Her research topics arediversity and phylogenetic of the domestic and wild smallruminants based on various methods (STR and SNP-markers,mtDNA). Currently I focus on functional genomics of sheep,including mapping of QTLs and candidate genes associatedwith fat deposition in the sheep tails as well as with bodysizes and growth -relation traits, as well as on goat maternalorigins.E-mail:*****************AbstractSuccessful implementation of genome editing technologies creates possibilitiesfor target improving the economically important traits in livestock. Creatingcrossbred mapping populations by crossing farm animals with contrast variantsof desired traits provides high significant data on revealing of candidate genesof interest to be rapidly introduced in animal populations by genome editing.Although Russia has rich resources of domestic sheep, there are no specializedlocally adapted meat breeds were developed. To overcome this weakness ofnational sheep breeding, we established two crossbred mapping populationsin our experimental farm. The first one is set up to address growth, carcassand meat quality traits and includes the progeny of fast-growing Katahdinmeat breed and slow growing Romanov ewes. The second one comprises theoffspring of fat-tailed Karachaev and thin-tailed Romanov breeds to identifycandidate genes associated with fat deposition in the tails of local sheep. High-density SNP data were generated using Ovine Infinium HD SNP BeadChip.Phenotypic data including body weight, nine body measurements, and tailmeasurements, were recorded in the age of 6, 42, 90, 180, and 270 days. Thefirst genome-wide association studies have resulted in identification of thegenes, associated with skeletal muscle growth and meat quality related traits,and involved in carbohydrate and lipid metabolism. The detected genes can beintroduced into the populations of well-adapted local sheep breeds to improvetheir growth, carcass and meat quality traits. Genotyping of backcrosses fromKatahdin × Romanov population was financed by RFBR No. 17-29-08015. TheKarachaev × Romanov progeny was studied within RSF No. 19-16-00070. Thesamples of Romanov ewes were genotyped within theme No. 0445-2019-0026.Crossbred mapping populations as promising tool to reveal functionalcandidate genes: a study case in Russian sheep breedingDeniskova T., Petrov S., Sermyagin A., Dotsev A., Bagirov V., Brem G. and Zinovieva N.L.K. Ernst Federal Science Center for Animal Husbandry, Russia。
南京大学-杨荣武分子生物学课件4
One way for obtaining single-stranded DNA from a double stranded source--magnets
Reagents for sequencing: DNA polymerases • Should be highly processive, and incorporate ddNTPs efficiently • Should lack exonuclease activity
Animation of cycle sequencing: see / Click on: “manipulation” “techniques” “sorting and sequencing”
An automated sequencer
The output
• New recombinant DNA constructs must be sequenced to verify construction or positions of mutations • Etc.
History of DNA sequencing
History of DNA sequencing
How to visualize DNA fragments?
• Radioactivity – Radiolabeled primers (kinase with 32P) – Radiolabelled dNTPs (gamma 35S or 32P)
• Fluorescence – ddNTPs chemically synthesized to contain fluors – Each ddNTP fluoresces at a different wavelength allowing identification
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Supplementary figures, tables and software:
Supplementary Figure 1 Three sources of sequence errors contributing to SNP errors.
Supplementary Figure 2 Comparison between sequencing and PCR-based mapping resolution.
Supplementary Figure 3 Plant height QTL detected on chromosome 1 when using different window sizes.
Supplementary Table 1 Bin map of the 150 rice RILs.
Supplementary Table 2 Primer sequences of PCR markers in Figure 5. Supplementary Source Code High-throughput genotyping software for short reads
Additional online information:
Pseudomolecules harboring SNPs identified between indica cv. 9311 and japonica cv. Nipponbare are posted at our lab website: /english/edatabase.htm.
Supplementary Figure 1 Three sources of sequence errors contributing to SNP errors. (a ) RIL sequence errors occurred in the three-base indexes. (b ) RIL sequence errors occurred in 33-mers. (c ) Sequence errors in the genome sequences of the mapping parents.
Supplementary Figure 2 Comparison between sequencing and PCR-based mapping resolution. (a ) Resolution of recombination breakpoints in a randomly selected
region of chromosome 1 of RIL #76. The left breakpoint is mapped 19.9kb between SNPs with physical positions at 4978982 and 4998889 bp, and 0.8Mb between PCR markers with physical positions around 4635793 and 5424755 bp. The right
breakpoint is mapped 45.5 kb between SNPs with physical positions at 10925442 and 10970967 bp, and 1.7 Mb between the PCR markers with physical positions around 9390352 and 11116800 bp. (b ) Two randomly selected examples showing detected double crossovers by sequencing-based method but not by PCR-based mapping. Left : a region on chromosome 2 of RIL #38; Right : a region on chromosome 5 of RIL #10. Names, primer sequences, and physical locations of the PCR markers are given in Supplementary table 2. Red, indica genotype; Blue, japonica genotype.
Supplementary Figure 3 Plant height QTL detected on chromosome 1 when using
different window sizes. Tested window sizes in the number of SNPs include: (a ) 7. (b ) 11. (c ) 15. (d ) 19. (e ) 23.。
