133 Unit 8 Advanced user administration (高级的用户管理)
使用 IBM Rational Quality Manager(RQM)V2.0 来优化测试管理

使用IBM Rational Quality Manager(RQM)V2.0 来优化测试管理简介: IBM® Rational® Quality Manager 解决方案是IBM Rational 最新的质量管理环境。
构建在Jazz 平台上,Rational Quality Manager 工具提供了大量的新特性,以帮助您创建测试计划、测试脚本、测试执行功能,并检测日志与报告。
引言IBM® Rational® Quality Manager 解决方案是IBM Rational 最新的质量管理环境。
构建在Jazz 平台上,Rational Quality Manager 是一种能够提供大量选项的灵活工具。
本文还展示了怎样实施IBM® Rational Quality Manager 和IBM® Rational® Functional Tester 环境之间的集成。
另外,您可以从更高层次上查看Quality Manager 解决方案的一些特性,其中包含创建测试计划、测试用例、手动测试脚本、测试执行,以及缺陷报告并生成测试报告。
(尽管本文主要关注与Rational Functional Tester 的集成,Rational Quality Manager 解决方案还提供了与其他IBM 产品环境的集成方案,例如以下这些产品:∙IBM® Rational® Performance Tester∙服务测试∙IBM® Rational® Robot∙IBM® Rational® AppScan® Tester Edition∙其他Rational 测试产品除了IBM Rational 产品,Rational Quality Manager 解决方案还提供了与其他测试自动化方案集成的功能。
Sun Java Enterprise System 产品文档说明书

Sun Java Enterprise System文档汇总信息本指导性文件列出了有助于您的企业安装和使用 Sun Java™ Enterprise System 的相关文档。
本文件根据 Java Enterprise System 生命周期进行组织。
它将 Java Enterprise System 生命周期分成三个主要阶段:分析和计划、部署 Java Enterprise System,以及运行部署的 Java Enterprise System。
针对 Java Enterprise System 生命周期的主要阶段,本资料包括以下各节:• 第 2 页的“Java Enterprise System 分析和计划”包括您在分析业务需求和计划Java Enterprise System 部署时需要执行的任务。
• 第 5 页的“Java Enterprise System 部署”包括您在实现部署设计时需要执行的任务,包括制定详细的部署计划、安装 Java Enterprise System 组件产品以及配置安装的组件产品。
• 第 10 页的“Java Enterprise System 运行”包括您在运行 Java Enterprise System 时定期执行的任务。
• 第 12 页的“Java Enterprise System 文档集”列出所有可用的 Java Enterprise System 文档。
要使用本文件,请转至包含您感兴趣的生命周期阶段的相应章节。
在相应章节中,您将找到一个涉及该生命周期阶段的 Java Enterprise System 文档列表。
Java Enterprise System 分析和计划Java Enterprise System 分析和计划Java Enterprise System 由若干软件组件组成,如 Sun Java System Directory Server5 2004Q2 和 Sun Java System Identity Server 2004Q2。
Cognos 权限管理

Cognos 权限管理李光涛4/17/20131.1.编写目的 (3)1.2.开发工具 (3)2.报表权限设置 (3)2.1.第三方目录管理器 (3)2.1.1.NTML (3)2.1.2.Sun ONE LDAP (5)2.1.3.IBM Cognos Service (13)2.1.4.Java Project (24)2.2.报表服务器权限管理 (25)2.2.1.cognos用户权限控制 (25)2.2.2.设置文件夹访问权限 (28)2.2.3.设置报表访问权限 (30)2.2.4.设置报表服务器功能权限 (31)2.2.5.cognos中增加新的分组 (32)2.2.6.cognos中增加新的联系人 (33)2.3.FrameWork权限管理 (34)2.3.1.FM控制报表发布包权限 (34)2.3.2.FM控制报表数据展示权限 (36)2.3.1.手动安全数据控制 (37)2.3.2.FM中查询项权限控制 (38)引言1.1.编写目的本文档是结合以往的开发经验,从实际报表开发出发,详细介绍了ReportNet报表设计流程、开发技巧,报表性能调优、FM建模规范及技巧讲解。
总结的文档希望对大家在日后的开发中带来帮助。
1.2.开发工具●FrameWork Manager10.1.0模型●Business Intelligence Server 32-bit 10.1.0 Windows Multilingual)报表服务器。
2.报表权限设置2.1.第三方目录管理器众所周知,cognos自身并不带用户管理模块,需要嵌入第三方的用户管理,可以使用sunone 的ldap服务器作为用户管理模块的较为常见,采用第三方管理报表服务器权限,首先要先将报表服务器的匿名访问设置为false,之后介绍我所用过的几种第三方软件。
2.1.1.NTMLNTML在众多方法中最简单,也最实用。
使用操作系统的用户账户来管理Cognos报表服务器的权限设置,即登录Cognos服务器的用户,也就是登录计算系统的用户。
Dell PowerConnect M8024-k 交换机入门指南说明书

Contents
3
4 Starting and Configuring the Switch . . . 10
Regulatory Model PCM8024-k
March 2011 P/N 7XG64 Rev. A00
Contents
1 Introduction . . . . . . . . . . . . . . . . . . . . . . . . 5
PowerConnect M8024-k Overview . . . . . . . . . . . . 5
Expansion Slot
The 10G expansion slot supports the following modules: • SFP+ (four ports) • CX-4 (three ports) • 10GBASE-T (two ports) The modules are sold separately.
Regulatory Model: PCM8024-k
Dell PowerConnect M8024-k Switch
Getting Started Guide
Regulatory Model: PCM8024-k
Notes
NOTE: A NOTE indicates important information that helps you make better use of your computer.
诺基亚LTE后台网管操作详解

诺基亚网管系统NetAct 8系列基本操作使用说明目录第一章 NetAct 8简介 (3)1.1 网管系统NetAct的主要功能 (3)1.2 NetAct 8系统架构 (3)第二章网管功能调用 (5)2.1网管功能的WEB调用 (5)2.2主要功能介绍 (9)第三章 Topology Manager拓扑管理 (10)3.1 进入拓扑管理 (10)3.2 网管应用的跳转 (11)第四章 Monitoring告警相关应用 (14)4.1 Monitor模块使用 (14)4.1.1 活动告警 (14)4.1.2 历史告警 (15)4.1.3 对象管理 (16)4.1.4 告警更新 (18)4.2 告警统计 (18)第五章 Configuration 配置管理 (20)5.1 配置编辑管理 (20)5.1.1 参数查询修改 (20)5.1.2 Site信息 (21)5.1.3 CM History (22)5.2 配置操作管理 (22)5.2.1 数据导出 (22)5.2.2数据导入 (23)5.2.3参数更新 (24)第六章 License 许可证管理 (25)6.1许可证管理 (26)第七章 Reporting性能管理 (26)8.1 创建KPI公式 (26)8.2 创建KPI报告 (28)8.3 报告定时生成 (32)8.4报表的生成与删除 (35)第八章工程模式查看操作 (38)11.1 查找网元对象 (38)11.2 维护模式的使用以及状态查找[工程模式] (39)第九章、N8网管垃圾数据删除 (40)第十章 LTE批量修改参数操作步骤 (43)第十一章 BTS LOG抓取 (46)第十二章 LTE告警提取方法 (48)第十三章如何修改天线顺序 (53)第一章 NetAct 8简介1.1 网管系统NetAct的主要功能×管理网元的告警数据。
×管理网元的性能数据(即测量数据)×对网元进行配置管理,软件管理。
汽车行业专用英语词汇及常用缩写【范本模板】
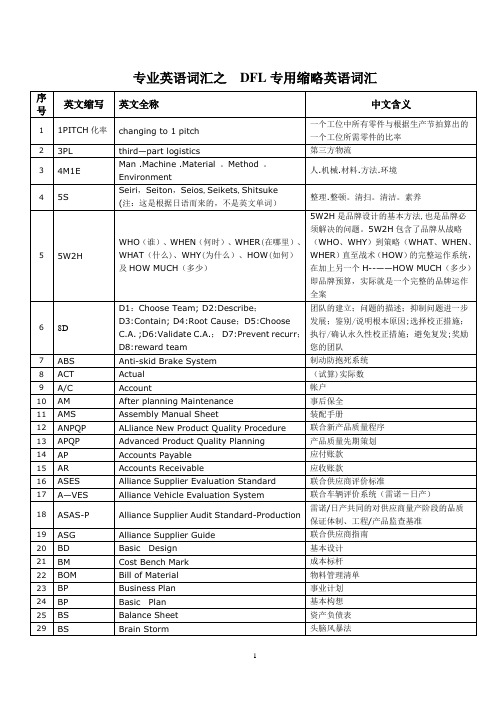
专业英语词汇之DFL专用缩略英语词汇汽车行业常用缩写AAR: Appearance Approval Report 外观批准报告A/D/V: Analysis/Development/Validation 分析/开发/验证A/D/V–DV: ADV Design Validation ADV设计验证A/D/V P&R: Analysis/Development/Validation Plan and Report。
This form is used to summarize the plan and results for validation testing. Additional information can be found in the GP—11 procedure.分析/开发/验证计划和报告A/D/V–PV: ADV Product Validation ADV产品验证AIAG: Automotive Industries Action Group, an organization formed by General Motors,Ford and Daimler—Chrysler to develop common standards and expectations for automotive suppliers. 汽车工业行动集团AP: Advance Purchasing 先期采购APQP: Advanced Product Quality Planning 产品质量先期策划APQP Project Plan:A one-page summary of the SGM APQP process that describes the tasks and the timeframe in which they occur. APQP项目策划AQC:Attribute Quality Characteristic 属性质量特性ASQE: Advanced Supplier Quality Engineer 先期供应商质量工程师BIW: Body in White。
中小学校园网网络操作系统的配置与应用(140单选+30多选+30判断)全
1.一个用户帐户可以加入()个组 DA1 B2 C3 D多2.在windows Server 2008中基本磁盘类型中,一块磁盘最多可以创建多少( )个主分区? CA2 B3 C4 D53.工作组模式中计算机数量最好不要超过多少台() BA5 B10 C15 D204.在Windows Server 2008中,要创建隐藏共享文件夹,只需要在共享名后加()符号。
BA% B$ C& D*5.根据文件的命名规则,下列字符串中是合法文件名的是() BA *ASDF.FNTB AB_F@!.C2MC CON.PRGD CD?.TXT6.手动清除本机DNS缓存,可使用ipconfig命令,加上参数() BA /displaydnsB /flushdnsC /registerdnslD /showclassid7.每个Web站点必须有一个主目录来发布信息,iis默认的主目录为,除了主目录以外还可以采用虚拟目录作为发布目录()。
BA \WebsiteB \Inetpub\wwwrootC \InternetD \internet\website8.活动目录中的逻辑单元包括:()①域②域名③组织单元(Organizational Unit,OU)④域树⑤域控制器⑥域森林 CA①②③④⑤B①③④⑤C①③④⑥D①②④⑤9.在Windows Server 2008中基本磁盘类型中,一块磁盘最多可以创建多少()个主分区? CA2 B3 C4 D510.如果您提议引入DHCP服务器以自动分配IP地址,那么下列哪一组网络ID将是您最好的选择()BA24.X.X.X B172.16.X.X C194.150.X.X D206.100.X.X11.在Windows Server 2008的密码策略中,缺省的密码最长使用期限是()天? CA30 B35 C42 D4812.在Windows Server 2008平台上,FAT32文件系统支持的最大单个文件为()。
ADManager Plus用户指南说明书
The Active Directory Management ChallengeEvery IT Administrator faces the challenge of managing user accounts in Active Directory almost everyday. Configuring user properties manually is extremely time consuming, tiresome, and error-prone, especially in a large, complex Windows network. Moreover, accomplishing these tasks demands a deep knowledge in Active Directory and related technologies.A software that can automate these cumbersome tasks, simplify AD man-agement and provide exhaustive reports on tasks done and their status, is the need of the hour.Introducing ADMtanager PlusADManager Plus is a comprehensive web-based Active Directory Man-agement software that simplifies User provisioning and Active Directory administration. The solution features a single console from which IT man-agement can view and manage Active Directory users, computers, contacts, groups and reports on Active Directory environment, ADManager Plus avoids manual, error prone administrative activities in Active Directory and save time and cost.We evaluated ScriptLogicActive Administrator,Quest AD management tooland ADManager Plus tosolve our day-to-day ADmanagement & Reportingneeds. ADManager Pluswith its high-end featuresand low-end costwas an obvious choice “Meraz Nasir,Manager of Infrastructure (ITS),Interfaith Medical Center.- Automate mundane, repetitive tasks in AD- Do away with command line tools or scripts- Delegate tasks to help desk technicians- Comply with regulatory standards like SOX, HIPAA - Ease through audits with over 100 in-built AD reportsEnjoy hassle-free AD Management and Reporting!More than 100 out-of-the-box granular reports of Active Directtory infrastructure resources.Web-based ManagementSystem RequirementsDashboard ScreenshotPentium IV 1.0 Ghz, 512 MB RAM on Windows 2000, XP , 2003, Vista, Windows 7, Windows 2008, Windows Server 2008 R2 . Browsers: IE 5.5 and above & Firefox 1.5 and above.Create/modify thousands of user accounts on the fly Manage user mailboxes in Exchange servers Manage passwords for user accounts in AD Template-based approachClean-up Active Directory - delete/disable/move accounts“v Web-based Managementv Get access to user activity, computer status, Exchange server properties, file system & printersExport to a number of formats like HTML, PDF, XLS, and CSV.Schedule reports to run daily, weekly, monthly or during off-peak hours.Flexible custom report development.More granular delegation of administrative authorityDelegation: Role-based Access Management v Delegate tasks like reset passwords, create/delete/disable accounts, unlock user accounts; to help desk techsExercise control over every attribute modified by help desk techs OU-based administrationRetrieve and utilize AD data to assist in organizational policy complianceCompliancev Compliance reports to meet regulatory standards like SOX, HIPAA and moreFor more informationWebsite : Live Demo : ForSalesQueries:**********************ForTechSupport:*************************Toll Free : 1-888-720-9500。
医疗器械说明书和标签管理规定(国食药监第6号令2014.10)
Provisions on the Administration of Instructions for Use and Labels of Medical Devices(CFDA Decree No. 6)Issued on July 30, 2014China Food and Drug Administration DecreeNo. 6The Provisions on the Administration of Instructions for Use and Labels of Medical Devices have been examined and passed on the CFDA General Meeting held on June 27, 2014 and now is for publication. The Provisions shall come into effect as of October 1, 2014.General Director: Zhang YongJuly 30, 2014Provisions on the Administration ofInstructions for Use and Labels of Medical DevicesArticle 1.These Provisions are established in accordance with the Regulation on Supervision and Administration of Medical Devices for the purpose of regulating the instructions for use andlabels of medical devices, and ensuring the safe use of medical devices.Article 2.Medical devices sold or used within the territory of the People's Republic of China shall be accompanied by instructions for use and labels according to these Provisions.Article 3.Medical device instructions for use is a technical document prepared by registration applicant or filing registrant and provided to user along with the product, which covers basicinformation of product's safety and effectiveness and is used as guidance for correct installation, commissioning, operation, use, maintenance and care of the medical device.Medical device labels refer to text descriptions, graphics and symbols attached to the medicaldevice or its packaging for identification of product features and providing safety warnings. Article 4.Contents of instructions for use and labels of medical devices shall be scientific, true, complete and accurate, and consistent with the product features.Contents of instructions for use and labels of medical devices shall be consistent with thecontents that have been registered or filed.Contents of medical device labels shall be consistent with contents of the instructions for use. Article 5.Instructions for use and labels of medical devices shall adopt and use nationwide universal and standard technical terminology and measurement units in their descriptions of diseasenames, specialty terminology, diagnosis and treatment process and results.Article 6.Symbols or color identifications used in the instructions for use and labels of medical devices shall be in conformity with requirements of applicable national standards. Symbols orcolor identifications not included in existing standards shall be clearly described in theinstructions for use.Article 7.The minimum sales unit of medical devices shall be accompanied by the instructions for use.Users of medical devices shall follow the instructions for use when using the medical devices. Article 8.Generic name shall be used for the medical device product name, which shall be consistent with the medical device naming rules established by China Food and DrugAdministration. Product name of Class II or Class III medical devices shall be consistent with the product name indicated in the Medical Device Registration Certificate.Product name shall be clearly indicated at conspicuous position of the instructions for use andlabels.Article 9.Text information of instructions for use and labels shall be in Chinese. The use of Chinese language shall conform to national uniform language norms. Other languages may beaccompanied in the instructions for use and labels of the medical devices; however the Chinese text shall prevail.Texts, symbols, tables, numbers, and graphics of the instructions for use and labels of medicaldevices shall be accurate, clear and standardized.Article 10.Instructions for use of medical devices shall generally include the following:(1) Name, model and specification of product;(2) Name, address, contact details and after-sale service providers of registration applicant orfiled registrant, and name, address and contact information of agent for imported medicaldevices;(3) Manufacturer's name, address, manufacturing site, contact, production license number orproduction filing number; if under contracted production, the entrusted company's name,address, manufacturing site, production license number or production filing number shall bealso indicated;(4) The medical device's registration certificate number or filing certificate number;(5) Serial number for the product's technical requirements;(6) Product performance, primary structural component or composition and indications for use;(7) Contents of contraindications, cautions, warnings and informative messages;(8) Installation and use instructions or diagrams; medical devices for self-use shall also havespecial safety instructions;(9) Product care and maintenance methods, special storage and transportation conditions andmethods;(10) Manufacturing date, service life or expiry date;(11) Part list, including the replacement cycles and replacement methods for spare parts,accessories, and consumables;(12) Explanation of graphics, symbols, abbreviations and others used in the labels of medicaldevices;(13) Preparation date or amendment date of the instructions for use;(14) Other contents to be indicated.Article 11.Cautions, warnings and informative messages of instructions for use of medical devices mainly include:(1) Population of the product use;(2) Potential safety hazard and use restrictions;(3) Protections for operators and users, as well as emergency response and corrective actionsin case of accident during normal use of the medical device;(4) Necessary monitoring, evaluation and control measures;(5) Single-use products shall be marked with "for single-use only" words or symbols; sterilizedproducts shall indicate sterilization method and handling methods in case of damagedsterile packaging; products requiring disinfection or sterilization before use shall explain themethod of disinfection or sterilization;(6) For products to be installed or used jointly with other medical devices, information for theother products such as product requirements, methods of use and points for attention shallbe provided;(7) Possible interference with each other and potential hazards during use with other products;(8) Potential adverse events during product use, or ingredients or additives in productcomposition that may potentially lead to side effects;(9) Matters requiring attention regarding medical device disposal, and appropriate disposalmethods for products that need to be disposed of after use;(10) Other reminders to operator and users based on product features/characteristics.Article 12.For reusable medical devices, their instructions for use shall clearly state treatment procedures for re-use, including methods of cleaning, disinfection, packaging and sterilizationand the number of repeated use allowed.Article bels of medical devices shall generally include the following:(1) Name, model and specification of product;(2) Name, address and contact details of registration applicant or filed registrant, and name,address and contact information of agent for imported medical devices;(3) The medical device's registration certificate number or filing certificate number;(4) Manufacturer's name, address, manufacturing site, contact, production license number orproduction filing number; if under contracted production, the entrusted company's name,address, manufacturing site, production license number or production filing number shall bealso indicated;(5) Manufacturing date, service life or expiry date;(6) Power connection conditions, input power;(7) Graphics, symbols and other relevant contents displayed based on product features;(8) Necessary warnings and precautions;(9) Special storage and handling conditions or explanation;(10) For medical devices that may damage or have negative impacts on the environment duringuse, their labels shall contain warning signs or precautions in Chinese;(11) For radioactive or radiative medical devices, their labels shall contain warning signs orprecautions in Chinese;If labels of medical devices are unable to present all of above-mentioned information due torestrictions of position or size, they shall at least show product name, model, specification,manufacturing date and service life or expiry date, and clearly state in the labels "refer toinstructions for use for more information".Article 14.Instructions for use and labels of medical devices shall not contain the following:(1) Affirmation or guarantee for product effectiveness such as “best effectiveness”,“guaranteed cure”, “guaranteed healing”, “thorough treatment”, “instant effects”, “free ofany toxics or side-effects” and etc.;(2) Absolute wordings and expressions such as "most advanced technology", "most scientific","most advanced" or "best" and etc.;(3) Indicating cure rate or effective rate;(4) Making product effectiveness and safety comparison with products of other enterprises;(5) Carrying commitment words such as "insured by insurance company" or "refund if invalid";(6) Endorsements or recommendations using names or images of any organizations orindividuals;(7) Misleading statement making people feel they are already suffering from certain disease, orgiving them an impression that they would suffer from certain disease or their illness wouldaggravate if not using this medical device;(8) Other contents prohibited by laws and regulations.Article 15.Instructions for use of medical devices shall be submitted to food and drug administration authority for examination or filing when the registration applicant or filing registrant is applying for registration or filing for the medical devices, and the submitted instructions for use shall be consistent with other registration or filed information.Article 16.Upon registration examination by the food and drug administration authority, the instructions for use of medical devices shall be not changed arbitrarily.In case there is change of registration with the registered medical device, the applicant shall,upon obtaining the change document, amend the instructions for use and labels in accordancewith the change document.In case there are changes with the other contents of the instructions for use, the applicants shall notify the medical device registration examination and approval authority in writing and submit comparison matrix of amendments to the instructions for use as well as other supportingdocuments. If no denial notice regarding the instructions for use amendment is received within20 working days from the examination and approval authority after such amendmentnotification has been served, the amended instructions for use shall become effective.Article 17.For a filed medical device, if there are changes with the items of filing information form, filed product technical requirements or other contents of the instructions for use, the filingregistrant shall amend the instructions for use and labels accordingly.Article 18.In the circumstances where the instructions for use or labels are in violation of these Provisions, the food and drug administration departments above county level shall imposepenalties/fines in accordance with Article 67 of Regulation on Supervision and Administration of Medical Devices.Article 19.These Provisions shall come into effect as of the date of October 1, 2014. The Provisions on the Administration of Instructions for use and labels of Medical Devices published on July 8, 2004 (SFDA Decree No. 10) shall be abolished at the same time.。
ebs常用责任,菜单
RESPONSIBILITY_NAME RESPONSIBILITY_KEY1CH HRMS Manager CH_HRMS_MANAGER2Colombia Manufacturing COLOMBIA_MANUFACTURING3CRM HTML 管理JTF_ADMIN_USER4CRM HTML 开发员CRM_HTML_DEVELOPER5CRM 管理员JTF_ADMINISTRATOR6Desktop Integrator DESKTOP_INTEGRATOR7HRMS 配置工作台HRMS_RI_WORKBENCH8OA Framework 工具教程FWK_TBX_TUTORIAL9OA Framework 工具教程:练习FWK_TOOLBOX_TUTORIAL_LABS10Oracle Diagnostics Tool DIAG_TOOL_RESP11Oracle Web ADI WEBADI12Oracle XML Publisher 管理员XDO_ADMINISTRATION13US HR 管理员US_HR_MANAGER14采购管理超级用户PURCHASING_SUPER_USER15成本管理COST_MANAGEMENT16成本管理 - SLA COST_MANAGEMENT17订单管理系统超级用户ORDER_MGMT_SUPER_USER18法人主体管理器XLE_SUPER_USER19跟踪管理器AUDITING_MANAGER20工程管理ENGINEERING21工程管理员ENIENGMGR22功能管理员FND_FUNC_ADMIN23功能开发员FND_FUNC_DEV24工作流管理员 Web 应用FNDWF_ADMIN_WEB25工作流用户 Web 应用FNDWF_USER_WEB26集成 SOA 网关FND_REP_APP27交易社区管理员HZ_TCA_MANAGER28客户管理CUSTOMER_CARE29库存管理INVENTORY30全局 HR 管理员GLOBAL HR MANAGER31全局 HRMS 超级管理员GLB_SHRMS_MANAGER32全局 HRMS 管理员GLOBAL_HRMS_MANAGER33人力资源HR_FOUNDATION34首选项 SSWA PREFERENCES35税务管理员IDC_TEST36物料清单管理BILLS_OF_MATERIAL37物料计划员MATERIAL_PLANNER38系统管理SYSTEM_ADMINISTRATION39系统管理员SYSTEM_ADMINISTRATOR40销售管理员ASN_SALES_ADMINISTRATOR41销售用户ASN_SALES_USER42应付款系统管理员PAYABLES_MANAGER43应收帐款经理RECEIVABLES_MANAGER44应用开发员APPLICATION_DEVELOPER45用户管理UMX46预警系统管理器ALERT_MANAGER47在制品管理WORK_IN_PROCESS48中国 HRMS 管理员CNHRMS49桌面集成DESKTOP_INTEGRATION50桌面集成管理器DESKTOP_INTEGRATION_MANAGER 51总帐管理超级用户GENERAL_LEDGER_SUPERVISOR 52总帐管理超级用户(1)GENERAL_LEDGER_SUPER_USER 53Oracle Cash Management 超级用户CE_SUPERUSER54项目开单超级用户PROJECT_BILLING_SUPER_USER 55项目超级用户PA_PRM_PROJ_SU56项目实施超级用户PA_IMPLEMENTATION_SU_GUI57Oracle Approvals Management 业务分析专家AME_BUS_USER_RESP58Oracle Approvals Management 管理员AME_ADMIN_USER_RESP59工程管理ZB_ENG_SUPER60资产查询FA_WEB_ASSET_INQUIRY61企业资产管理EAM_ASSET_MANAGEMENT62质量管理系统QUALITY63维护超级用户EAM_ASSET_MANAGEMENT_JSP 64维护用户工作台EAM_MAINT_ENG_JSP65iSupplier Portal 完全访问ISUPPLIER_PORTAL_RESP66供应商管理管理员POS_SM_ADMIN67供应商配置文件经理POS_SUPPLIER_PROFILE_MNG 68来源补充供应商PON_SOURCING_SUPPLIER69来源超级用户PON_SRC_SUPER_USER7071APPLICATION_NAME MENU_NAME人力资源管理系统PER_F4_UK_HRMS_NAV成本管理系统COLOMBIA_MANUFACTURINGCRM 基础架构ETF_ADMINC_ROOT_HOMECRM 基础架构ETF_DEVELOPER_CONSOLE_HOME CRM 基础架构JTF_NAVIGATEWeb 应用产品桌面集成器DESKTOP INTEGRATION MENU人力资源管理系统PER_RI_WORKBENCH_ROOT_MENU 公用模块 - AK FWK_TBX_TUTORIAL公用模块 - AK FWK_TBX_TUTORIAL_LABS_TOP CRM 基础架构DIAG_TOOL_MENUWeb 应用产品桌面集成器WEBADI MENUXML Publisher XDO_TEMPLATE_MANAGER_ROOT人力资源管理系统US_HR_NAV采购管理系统PO_SUPERUSER_GUI物料清单管理系统CST_NAVIGATE成本管理系统CST_SLA_NAVIGATE订单管理ONT_SUPER_USERLegal Entity Configurator XLE_RESPONSIBILITY_MENU应用对象程序库AUDITING_MANAGER_MENU工程管理系统ENG_NAVIGATE_GUI产品智能系统ENI_DBI_ENGMGR_MAIN_MENU应用对象程序库FND_FUNC_ADMIN_ROOT应用对象程序库FND_FUNC_DEV_ROOT应用对象程序库FND_WFADMIN应用对象程序库FND_WFUSER应用对象程序库FND_REP_TOP_LEVEL_MENU应收帐款HZ_TCA_MAIN成本管理系统CSX_CC_NAVIGATE库存管理系统INV_NAVIGATE人力资源管理系统GLB_HR_NAV人力资源管理系统GLB_SHRMS_NAV人力资源管理系统GLB_HRMS_NAV人力资源管理系统HR_FOUNDATIONOracle iProcurement ICX_PREFERENCESE-Business Tax ZX_RESPONSIBILITY_MENU物料清单管理系统BOM_NAVIGATE_GUI主计划/MRP 管理系统MRP_NAVIGATE4.0Oracle iProcurement ICX_SYSTEM_ADMINISTRATION系统管理FND_NAVIGATE4.0销售管理系统ASN_ADMIN_MAIN_MENU销售管理系统ASN_MAIN_MENU应付帐款AP_NAVIGATE_GUI12应收帐款AR_NAVIGATE_GUI应用对象程序库FND_DEVNAVIGATE4.0应用对象程序库UMX_TOP_LEVEL_MENU预警系统ALR_OAM_NAV_GUI在制品管理系统WIP_NAVIGATE_10G人力资源管理系统CN HRMS NAVWeb 应用产品桌面集成器DESKTOP INTEGRATION MENUWeb 应用产品桌面集成器DESKTOP_INTEGRATION_MGR_MENU 总帐管理系统GL_SUPERVISOR总帐管理系统GL_SUPERUSER现金管理系统CE_SUPERUSER_GUI项目PA_SUPERUSER_GUI (PB)项目PA_PRM_PROJ_SU项目PA_IMP_SUPERUSER_GUI人力资源管理系统AME_BUS_STANDARD_MENU人力资源管理系统AME_ADMIN_STANDARD_MENU工程管理系统ENG_NAVIGATE_GUI资产FA_WEB_INQUIRY企业资产管理系统EAM_MAIN质量管理系统QA_TOP企业资产管理系统EAM_MAIN_HOME_SS企业资产管理系统EAM_MAINT_ENG_MENU_SS网上供应商门户POS_NEW_APPLICATION_MENU网上供应商门户POS_SM_MAIN_MENU网上供应商门户POS_HT_SP_S_SUPP_VW物料补充管理系统PON_SOURCING_SUPPLIER_ROOT物料补充管理系统PON_SOURCING_SUPER_USER_ROOTUSER_MENU_NAMEF4 UK HRMS 浏览器哥伦比亚制造系统ETF_ADMINC_ROOT_HOMEETF_DEVELOPER_CONSOLE_HOME CRM 管理员主菜单桌面集成菜单配置工作台根菜单OA Framework 工具教程OA Framework 工具教程:练习 - 顶部Diagnostics Tool MenuOracle Web ADI MenuXDO:模板管理器根菜单US HR 浏览器采购管理系统超级用户 GUICST_NAVIGATE成本管理和子分类帐会计ONT_SUPER_USER法人主体配置器跟踪管理器菜单ENG_NAVIGATE_GUI工程管理员主菜单Fnd 功能管理员根Fnd 功能开发员根工作流管理员工作流管理系统用户集成信息库:顶层菜单TCA 主菜单客户管理INV_NAVIGATEGLB HR 浏览器GLB SHRMS 浏览器GLB HRMS 浏览器人力资源首选项Oracle E-Business Tax 责任菜单BOM_NAVIGATE_GUIMRP_NAVIGATE4.0系统管理浏览器菜单 - 系统管理员 GUIASN_ADMIN_MAIN_MENUASN_MAIN_MENUAP_NAVIGATE_GUI12AR_NAVIGATE_GUI浏览器菜单 - 应用开发员 GUI用户管理:顶层菜单ALR_OAM_NAV GUIWIP_NAVIGATE_10GCN HRMS 浏览器桌面集成菜单桌面集成管理器菜单GL_SUPERVISORGL_SUPERUSERCE_SUPERUSERPA_SUPERUSER_GUI (PB)项目超级用户责任主菜单PA_IMP_SUPERUSER_GUIAME 业务分析专家标准菜单AME 管理员标准菜单ENG_NAVIGATE_GUIFA_WEB_INQUIRY企业资产管理系统QA_TOP自助维护主页维护工程师工作台iSupplier Portal 新应用产品菜单供应商管理主菜单供应商视图:供应商信息管理器Oracle Sourcing 供应商用户根菜单Oracle Sourcing 超级用户主要根菜单。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
MADEZJJ
133Unit8Advanceduseradministration(高
级的用户管理
)
一、目标
1、网络用户的集中管理使用NIS和LDAP;
2、精确的访问控制列表(ACLs);
3、设置文件系统的磁盘配额;
4、理解SELinux的基本操作;
二、Selinux(基于安全的增强性的linux)
SELinux是美国政府责成美国安全局开发的一种新的安全机制,
用于防止越来越脆弱的互联网现状,应用在linux系统中称为SELinux,
在selinux中除非明确运行,否则拒绝所有;
三、SELinuxsercuritycontext(SELinux基于上下文的安全控制)
1、linux系统中的所有的文件和进程都对应的有安全的context
值;
2、一个context值都包含了数个元素,作为selinux安全的需要;
i.用户:角色:类型:敏感度:要素;
ii.最后两个栏位通常不显示;
3、ls-Z//查看文件的context值;
4、ps-Z//查看一个进程的context值;
四、SELinux:Targetedpolicy(达到的策略)
1、在linux系统安装时,如果选择selinux的状态为强制状态,那
么在安装linux系统的过程中,缺省都会为系统中的每个文件加入安
全标签的属性;
2、大部分的进程缺省的情况下是没有定义的;
3、系统在给每个文件定义context值时的,依据是此文件所在目
录的用途,我们发现相同的文件放在不同的目录时context值是不同
的;
使用system-config-selinux图形化界面下,可以查看到所有的目录
所对应的context;
3
4、使用chcon可以对context值进行更改;
chcon-ttmp_t/etc/hosts//改变/etc/hosts文件的context值为
tmp_t;
5、恢复目录的context值可以使用命令restorecon;
restorecon-v/etc/hosts//恢复/etc/hosts文件的context值,-v
显示context值恢复的过程;
五、SELinux:Management(selinux的管理)
1、使用system-config-securitylevel,图形化工具可以设置selinux
的模式,也可以直接编辑/etc/sysconfig/selinux文件;
vim/etc/sysconfig/selinux
2、selnux的模式有以下三种:
1)enforcing:强制状态;在rhce的考试中明确规定selinux的
模式要使用强制状态;强制模式最为严厉,除非明确允许否则
全部拒绝;
2)permissive:警告模式;通常用于排错使用,对于违反了selinux
的所有的操作,只做出警告的显示,而不会真正的阻挡拒绝;
3)disabled:关闭模式;相对于在系统中不存在selinux了;
3、查看当前的selinux模式命令:
4、无论我是通过system-config-securitylevel工具还是编辑
/etc/sysconfig/selinux文件,都必须重启之后才能够生效,而想改变当
前的selinux模式,可以使用以下命令,当时以下命令只能修改当前
状态,在系统重启之后还是以/etc/sysconfig/selinux配置文件为准;
5、当selinux设置不当导致系统不能正常启动,我们以下在系统
的启动过程中在grub下给kernel加入selinux=0参数,临时关闭
selinux;
6、在selinux的策略中除了context值为实施策略的依据外,还要
依据selinux的booleans(布尔值);布尔值更像一个阀门的开关;
7、getsebool命令可以查看系统中所有定义的布尔值及当前的开
启状态;
6
8、使用setsebool命令可以调整selinux的布尔值,0对应的为off
关;而1对应的on开;
例:设置httpd服务的布尔值,使得匿名用户写入具有写入的权限:
setseboolallow_httpd_anon_write=1
9、如果是在命令方式下,我们看不到五角星的提示信息,可以查
看tail/var/log/message|greprun关键字日志信息;
接着运行run后面的命令,就可以查看到和五角星下相同的信息。
