程序文件ISO9001-2015
ISO9001:2015全套程序文件英文版

ISO9001:2015全套程序文件英文版(本人辛苦原创)Code QM-COP-01Date2018.10.24Date2018.10.241.0 PurposeAll the documents required by the Company’s quality management system should be controlled to ensure the version applied by all the relevant departments is valid.2.0 ScopeIt is applicable to all the documents pertaining to the quality management system including external documents.3.0 Definition3.1 Controlled document: The document applied in and out of the Company is controlled in modifications, identities, versions, version numbers, formats, fonts, etc.3.2 DCC: Document Controlling Center3.3 External document: It refers to the document that has been handled by outside individuals like national/international standards, laws and regulations, documents provided by customers or suppliers, material certificates, amendment advice, etc.3.3.1 Administrative documents on quality management system or product, released from local government authorities and regulatory agencies such as the notices from Guangdong Food and Drug Administration.3.3.2 National laws and regulations such as Product Quality Law of the People’s Republic of China, Regulation on the Supervision and Administration of Medical Devices, 93/42/EEC, etc.3.3.3 International standards such as Medical devices—Quality management systems—Requirements for regulatory purposes.3.3.4 National standards such as Medical electrical equipment – Part 1: General requirements for safety.3.3.5 Regulations and standards provided by customers such as agreements and commitments signed with customers.3.3.6 Drawings provided by customers such as drawings, mold drawings provided by a certain customer.3.3.7 Other important external documents relating to the product, including official materials like customer’s notice.4.0 Duties4.1 General Manager: Responsible for approval of the Company’s quality manual.4.2 Management Representative: Responsible for the Company’s procedure files, quality plans and cross-department three-order files and approval of external documents.4.3 Principals of each department: Responsible for approval of three-order files and all kinds of tables as well as department-related external documents.4.4 Department: Responsible for compilation, number and review of the documents dominated by the department.Code QM-COP-01Date2018.10.24Date2018.10.244.5 Quality Management Department: Responsible for all the controlled documents of the Company to ensure the electronic document is the latest version, and responsible for the updating of the controlled document list of all the departments.5.0 Procedures5.1 Document classification: The management system documents includes four layers and external documents5.1.1The Quality Manual (including policies and goals) is a principle-based and master document guiding the implementation of the quality management system. As the first level document, it does not just explain the scope of application but also describe the interaction among all the procedures in the quality management system.5.1.2 The procedure document is the expansion and specification of the Quality Manual, providing the process, methods and controlling means for carrying out quality management. It belongs to the second level document.5.1.3 Supporting documents (operation/technical specifications, process/inspection standards, technical guidance and position description) specify the quality management goals, duties of the posts of all levels and specific operation methods. It belongs to the third level document.5.1.4 The table is applied to record the state and result of activities, belonging to the fourth level document.5.1.5 External document: It refers to the document directly obtained from outside and cited by the Company, including national/international standards, laws and regulations, documents provided by customers or suppliers, material certificates and amendment advice.5.1.6 The document is drawn up mainly in written or electronic form, and both shall be under control.5.2 Document compilation and approval5.2.1 The formats of the second and third level documents are the same as that of the document.5.2.2 The date of the document must be written in the form of “year month day”.5.2.3 Limits for examination and approving authority for documentsS/N Order Type ofdocumentPrepared byReviewedbyJointreviewed byApprovedbyRemark1 First ManagementManualQualityManagementDepartmentManagementRepresentativeSupervisorof eachdepartmentTopmanagement2 SecondProceduredocumentAlldepartmentDepartmentRelevantdepartmentManagementCodeQM-COP-01Date2018.10.24 Date2018.10.245.3 Document’s number and version/version number5.3.1 Number: The document compiler numbers the newly compiled documents according to the Basic Rules for Numbering the Controlled Documents and the document list of the department, and confirms the uniqueness of the numbers with the controlling center.5.3.2 Version/Version number: The version or version number of the controlled document is compiled insmanagermanagerRepresen tative3ThirdManagement documentAll department sDepartme nt managerRelevant department manager and Managemen tRepresentat iveManage ment Represen tativeJob Description of the personnel below the manager level is reviewed by the department manager and approved by the manager of HR Department.4ThirdProcess, inspection standarddocument and specification (including external document)All department sQuality Manageme nt Departme ntDepartm ent manager5Fourt hTablesAlldepartment sQualityManageme nt Departme ntDepartm entmanagerAdditional remarks: 1) The document can be compiled by the compilers or above the compiler level but must be approved by the personnel upper than the compiler.2) The relevant department refers to the departments having ties with others involved in this system. 3) When the approver of the above documents is absent, his agent or Management Representative can sign it up instead to make the document effective.Code QM-COP-01Date2018.10.24Date2018.10.24the form of 26 alphabets from A to Z. The initial version number is “A/0”, the next revised version is “A/1” and so on. Changing Arabic numbers is enough for minor revisions while changing alphabets, for instance, from “A” to “B”, is necessary in case of major revisions.5.4 Document distribution and storage5.4.1 The document compiler sends the copy of the approved document and its electronic version to the Quality Management Department where the document will be checked whether it has been approved by designated personnel. After that, the document will be registered, controlled with the controlled document list updated.5.4.2 The document controller determines the scope of distribution, makes copies of the electronic file ina required number according to the List of distributed controlled documents, add the watermarks of correspondent departments on these copies, save them to the folder for controlled documents of each department and notify the departments for making and using the documents by email.5.4.3 All the department are responsible for checking if the controlled document is correct or not.5.4.4 The authority for the controlled document folder of each department shall be set as follows:①Document controller is permitted to modify, delete the content or add new content to the document.②Each department can only read but cannot delete, modify or add the content of controlled documents.5.4.5 The document controller must copy the electronic document as a backup.5.4.6 Visual management of documentsAs for the documents which are frequently applied at production site, all the departments should take correspondent measures such as hanging them on the wall, beside the equipment or enveloping them with plastic so to make it easy for operators to use.5.5 Document reading5.5.1 In case of reading the documents, the relevant personnel can open the PDF file which are saved in the Company’s share disk.5.6 Document review, modification, recovery, invalidation and destruction5.6.1 Review①The documents of the quality management system should be reviewed once a year by the Quality Management Department and internal review team organized by the Management Representative along with the Company’s internal review and reviewed with the result put down in the internal review record.②In case of special circumstances, some documents should be reviewed by the relevant department.③The review must take into account the influence of both the internal factors like the Company’s organization and position changes and the external factors like laws, regulations, relevant standards and market demands upon the sufficiency and applicability of the documents with the Review Record filled in.5.6.2 Revision/alteration①The director and executor of each unit should check the effect after implementing the documents. If the documents are not applicable or in doubt in addition to the opinions on the content of the documents from other units, the documents can be revised or modified by the department which revised or compiledCode QM-COP-01Date2018.10.24Date2018.10.24them last time after the discussion among the relevant departments. Relevant approval process is the same as that in 5.2.2.②All the modifications or alterations must be underlined (“___”). In case of version change, the previous underline should be substituted by the latest one.③The revision record should be written on the first page of the documents, containing the content of the revision, identification of the affected documents, signature of the approver, date of approval and effective time.④The relevant departments shall be notified of review and confirmation of the alteration, and personnel training will be provided if necessary.⑤In the following circumstances that there is any alteration to the documents of the quality management system or the documents relating to the Company’s medical device products, the top management or Management Representative of the Company should be notified of deciding whether to inform the competent authority or notified bodies about it. If it is necessary, the notification should be implemented in accordance with the local laws and administrative regulations.a. Major alterations to the Quality Manual.b. Major alterations to the product’s functions, performance, safety, reliability and electromagnetic compatibility, caused by altering product standards.c. Major alterations to the product’s functions, performance, safety, reliability and electromagnetic compatibility, caused by changing key components of products.d. Stipulated by laws and regulations.5.6.3 Once the new version of controlled document is distributed, the old one becomes invalid automatically. The document controller should delete the copies of invalid controlled documents in the controlled document folder, upload the latest version and keep the original documents printed with an “invalid” stamp at the document controlling center till the expiry date (at least five years) before destruction.5.6.4 As for the invalid original documents, the document controlling center should destruct them uniformly after Document/Record Destruction Registration Form filled in by the center is approved by the Management Representative.5.7 The non-controlled document is identified as the “Reference”. If a Company’s customer or other personnel need it for their jobs, they must have the copies of the Company’s controlled documents and get its copies approved by the Management Representative and stamped with the ‘Reference’ seal by the Quality Management Department. The ‘Reference’ documents will not be withdrawn or changed to the latest version.5.8 Temporary documentIt is not yet official for some reasons but needed by each department. Such document should have a ‘Temporarily Controlled’ stamp as well as the time limit and distribution department on them. The temporary document cannot be valid for more than 3 months.Code QM-COP-01Date2018.10.24Date2018.10.245.9 Management of external documents5.9.1 Each department of the Company can collect external documents through the following channels.a. National, provincial, municipal governments and their relevant functional departments.b. All kinds of meetings, professional newspapers, magazines, publishers and suppliers.c. Internet, telephone and fax.5.9.2 The external document collected by each department should be selected timely and delivered to the relevant department to recognize its contents and decide whether make it a controlled document.a. The collected technical standards on our products should be delivered to the Technical Department to recognize its year, version and applicable articles.b. The laws, regulations and rules that are issued by the state on the quality and safety of the product should be delivered to the Quality Management Department to identify the required department and scope.c. Policy documents issued by the superior should be delivered to the administration department for recognition.d. The technical documents provided by suppliers or customers should be delivered to the Technical Department and Quality Management Department for recognition.f. The design input documents provided by customers should be delivered by the Market Department to the R&D Department for recognition. Saved in DHF format, they don’t have to be controlled by document controller.5.9.3 Numbering of external documentsAs for the external documents on technology and standards as well as other external documents, the Quality Management Department should number them in accordance with the Basic rules on numbering controlled documents.5.9.4 Distribution of external documentsa. After being recognized, the external documents should be kept on a file and put down on a list.b. The external documents should be distributed after the distribution scope is confirmed according to 5.4 of this procedure.5.9.5 Updating of external documentsAs for the external documents which need updating, the new version should be distributed with the invalid ones withdrawn immediately.5.9.6 Preservation and destruction of external documentsThe preservation and destruction should be implemented according to 5.6.3 of this procedure.5.10 The Quality Management Department should supervise and inspect irregularly the controlling process implemented by each department.6.0 Records and Tables6.1 Controlled Document Directory。
ISO9001:2015版标准的全套质量管理体系文件(范本)

ISO9001:2015版标准的全套质量管理体系⽂件(范本)⼀、遵循ISO9001:2015版标准的全套质量管理体系⽂件的整体思路与策划1、ISO9001:2015新版本不再要求编制《质量⼿册》和《程度⽂件》,对⽂件(记录)的数量也⼤⼤减少,⽽统⼀规定为“形成⽂件的信息”。
基于风险的考虑,原有的《质量⼿册》、《程度⽂件》、《作业指导书》、《记录》等进⾏重新整合。
2、原《质量⼿册》、《程度⽂件》、部分《体系三级⽂件》将合并为《预先防错·⼯作⼿册》,原《作业指导书》的重点将转向关注“经常犯错、最容易犯错的环节”,并针对这些环节,结合《应对风险与机遇的措施》,给出可以实施的⽅法对策,以最⼤限度减少⼈为错误(如失误、违章)导致的⾮预期的影响。
3、原《受控记录清单》将变更为“运⾏结果的证据性⽂件”最终的输出成果⽂件清单:《预先防错·⼯作⼿册》(内容包含:应对的风险、获得的机遇)《预误防错-防⽌错误·作业指导书(或操作规程)》《运⾏结果的证据性⽂件清单》⼆、应对风险和机遇的措施ISO9001:2015版新增“风险和机遇的应对措施”,其核⼼关键词是“措施”,即“⽅法和绝招”。
然⽽,我所见到的《风险和机遇的应对控制程序》,却⼤玩特玩“风险系数、风险频度、风险剩余”等⼀⼤堆枯燥乏味、晦涩难懂的“概念和理论”,唯独不见真正实质性的、能解决问题的“措施⽅法”。
中国的质量管理体系是否会再次⾛向“照本宣科、望⽂⽣义、断章取义”的死胡同?我看:是,⽽且⼀定是!其实,ISO9001:2015新版本的精髓在于预防。
我们经常讲的“预防为主”中的“预防”,其实是个简称,全称应该是“预先防错”、“预先防范(风险)”;其核⼼关键词是⼀个“先”字,因此应该把“预防为主”理解成“预防为先”更准确。
输出成果⽂件:《应对风险与机遇的措施》(关键词是“措施”)以下是《应对风险和机遇的措施之:防错法》的举例。
如何结合组织的背景、应⽤到具体的实际⼯作当中去?既考验⽼师⽼师的⽔平,也考验管理者的智慧:。
ISO9001-2015批生产记录管理程序

批生产记录管理程序(ISO9001:2015)1.目的:规范生产过程中记录文件的编制、审核、批准、使用、保管的管理程序。
2.范围:适用于本公司的所有剂型、品种的批生产记录。
3.责任:3.1相关车间岗位操作人、岗位负责人严格执行本规程;3.2车间主任、生产技术部负责在实际工作贯彻落实本规程;3.3质量管理部对本规程的审核和实施过程中的监督。
4.内容:4.1批生产记录的编制原则:4.1.1批生产记录根据产品生产工艺规程、标准操作规程和技术参数等内容设计,并能体现剂型的特点。
4.1.2批生产记录需具有产品质量的可跟踪性,通过批生产记录能了解产品生产全过程的质量情况。
4.1.3批生产记录按产品生产先后顺序依次进行编制。
4.1.4批生产记录先由相关车间技术负责人制定初稿,然后交生产技术部部长审核,质量管理部部长批准后方可印刷、使用。
4.1.5批生产记录设计时应有足够的填写空间、但尽量无空格。
4.2批生产记录的编制要求:4.2.1反映生产品种的基本情况,如:品名、规格、批号、生产日期、岗位操作法或SOP名称等。
4.2.2反映产品生产过程中的各项卫生管理及清场管理结果。
4.2.3反映产品生产过程中的全部操作步骤。
如:指令、生产方法、作业顺序、生产结果等。
4.2.4反映原辅料的品名、规格数量、批号、中间产品、半成品、成品的检测结果、结论等。
4.2.5反映工艺规程执行情况及其采取的特殊措施情况。
4.2.6反映生产过程中出现偏差、质量事故的处理情况。
4.2.7反映出物料平衡情况。
4.2.8反映出操作人员、复核人员、检查人员及审核人员。
4.3批记录内容包括:4.3.1封面;4.3.2目录(项目、页数);4.3.3批生产指令单;4.3.4批包装指令单;4.3.5批生产记录;4.3.6批包装记录4.3.7各检验报告单(半成品、成品);4.3.8批监控记录(按工序);4.3.9批清场记录(按工序);4.3.10批审核放行单。
ISO9001-2015 文件清单-手册和程序文件

文件清单
文件类别:程序文件/质量手册
序号 文件编号 版本/版次 制订部门
文件名称
1
QM-01
A2
管理代表 品质手册
2
QE-001
3
QE-002
A0
管理代表 组织环境与相关方管理程序
A0
管理代表 风险和机遇管制程序
4
QE-003
B4
品保部 文件管理程序
5
QE-004
B4
品保部 记录管理程序
6
QP-005
B1
管理代表 管理责任程序
7
QP-006
B0
品保部 品质计划程序
8
QE-007
A3
管理代表 信息沟通管理程序
9
QE-008
B2
管理部 資源管理程序
10
QP-009
B4
品保部 檢驗與測試設備管理程序
11
QP-010
A0
管理代表 组织知识管理程序
12
QP-011
B5
采购部 采購管理程序
13
生效日期 2020.03.02
2020.03.02
2020.03.02 2020.03.02 2020.03.02 2020.03.02 2020.03.02 2020.03.02
东莞伸东电子有限公司
文件清单
文件类别:程序文件/质量手册
序号 文件编号 版本/版次 制订部门
文件名称
36 備 注:
QE-033
A0
家电部 生产运作控制程序
20
QE-020
B3
品保部 不合格品管理程序
21
QP-021
ISO9001-2015全套质量管理体系文件

**********有限公司企业标准QM/YS-2016质量手册按ISO9001:2015要求编制版本号: A/0受控号:2016-5-1发布 2016-5-1实施**********有限公司发布修订记录目录批准令本《质量手册》是依据ISO9001:2015质量管理体系标准要求,结合本公司产品生产特点、生产规模和体制实际情况,为确保和提高产品质量,健全质量管理体系而编制。
本手册规定了本公司的质量方针和目标,对产品实现过程的持续改进、质量管理体系的有效运行规定了准则和方法。
本手册是本公司质量管理体系运行开展各项质量活动的指导性文件、法规性文件,现予以发布。
本公司全体员工务必认真学习,严格遵照执行,确保本手册得以认真有效的实施。
本手册于二○一六年五月一日起正式实施。
凡于本手册不一致的质量文件一律以本手册为准。
总经理: ***二○一六年五月一日管理者代表任命书为了便于公司ISO9001质量管理体系的有效推行,由总经理任命***先生为本公司管理者代表,其职责和权限为:1、负责按ISO9001标准建立保持并经济有效地实施文件化质量体系,领导各职能部门开展质量活动;2、负责方针目标管理,及时向总经理汇报质量管理体系运行情况,负责质量管理体系内部审核的组织领导工作,并提供质量体系改进的依据和建议;3、负责组织贯彻实施企业经营管理决策、目标方针,完善各项管理制度,不断提高公司管理水平;4、领导内部质量审核活动,协调解决质量管理体系运行中的不一致等问题;5、负责做好对过程的监视和测量及数据分析的领导控制工作;6、负责质量管理体系有关事宜的外部联络工作;7、负责提高公司员工文化、生活水平,营造良好的作业环境和安全舒适的生活环境;8、负责公司重大纠正/预防措施的审批和组织实施。
总经理:***本公司宗旨:品质稳定----我们成功的基石公司的质量方针:开拓进取,群策群力;持续精进,客户满意。
释义:1、在当前的市场竞争中,保持质量管理体系运行的持续有效性是企业承诺的主题,其根本目的在于为社会和顾客提供满足要求的产品。
ISO9001-2015中文版(完整)

ISO9001-2015中文版(完整)ISO9001:2015标准目录1 范围2 规范性引用文件3 术语和定义4 组织的背景4.1 理解组织及其背景4.2 理解相关方的需求和期望4.3 质量管理体系范围的确定4.4 质量管理体系5 领导作用5.1 领导作用和承诺5.2 质量方针5.3 组织的作用、职责和权限6 策划6.1 风险和机遇的应对措施6.2 质量目标及其实施的策划6.3 变更的策划7 支持7.1 资源7.2 能力7.3 意识7.4 沟通7.5 形成文件的信息8 运行8.1 运行的策划和控制8.2 市场需求的确定和顾客沟通8.3 运行策划过程8.4 外部供应产品和服务的控制8.5 产品和服务开发8.6 产品生产和服务提供8.7 产品和服务放行8.8 不合格产品和服务9 绩效评价9.1 监视、测量、分析和评价9.2 内部审核9.3 管理评审10 持续改进10.1 不符合和纠正措施10.2 改进附录 A 质量管理原则文献1 范围本标准为有下列需求的组织规定了质量管理体系要求:a)需要证实其具有稳定地提供满足顾客要求和适用法律法规要求的产品和服务的能力;b)通过体系的的有效应用,包括体系持续改进的过程,以及保证符合顾客和适用的法律法规要求,旨在增强顾客满意。
注1:在本标准一中,术语“产品”仅适用于:a) 预期提供给顾客或顾客所要求的商品和服务;b) 运行过程所产生的任何预期输出。
注2:法律法规要求可称作为法定要求。
2 规范性引用文件下列文件中的条款通过本标准的引用而构成本标准的条款。
凡是注日期的引用文件,只有引用的版本适用。
凡是不注日期的引用文件,其最新版本(包括任何修订)适用于本标准。
ISO9000:2015 质量管理体系基础和术语3 术语和定义本标准采用ISO9000:2015 中所确立的术语和定义。
4 组织的背景环境4.1 理解组织及其背景环境组织应确定外部和内部那些与组织的宗旨、战略方向有关、影响质量管理体系实现预期结果的能力的事务。
ISO 9001:2015版全套体系文件 进货检验和试验控制程序
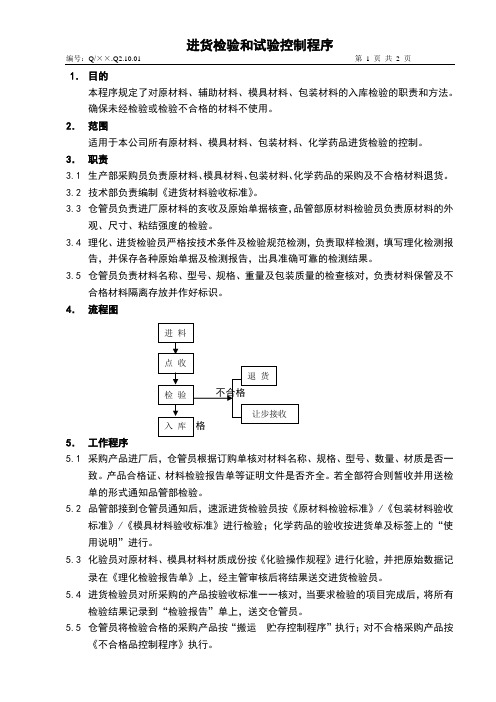
1.目的本程序规定了对原材料、辅助材料、模具材料、包装材料的入库检验的职责和方法。
确保未经检验或检验不合格的材料不使用。
2.范围适用于本公司所有原材料、模具材料、包装材料、化学药品进货检验的控制。
3.职责3.1生产部采购员负责原材料、模具材料、包装材料、化学药品的采购及不合格材料退货。
3.2技术部负责编制《进货材料验收标准》。
3.3仓管员负责进厂原材料的亥收及原始单据核查,品管部原材料检验员负责原材料的外观、尺寸、粘结强度的检验。
3.4理化、进货检验员严格按技术条件及检验规范检测,负责取样检测,填写理化检测报告,并保存各种原始单据及检测报告,出具准确可靠的检测结果。
3.5仓管员负责材料名称、型号、规格、重量及包装质量的检查核对,负责材料保管及不合格材料隔离存放并作好标识。
4.流程图5.工作程序5.1采购产品进厂后,仓管员根据订购单核对材料名称、规格、型号、数量、材质是否一致。
产品合格证、材料检验报告单等证明文件是否齐全。
若全部符合则暂收并用送检单的形式通知品管部检验。
5.2品管部接到仓管员通知后,速派进货检验员按《原材料检验标准》/《包装材料验收标准》/《模具材料验收标准》进行检验;化学药品的验收按进货单及标签上的“使用说明”进行。
5.3化验员对原材料、模具材料材质成份按《化验操作规程》进行化验,并把原始数据记录在《理化检验报告单》上,经主管审核后将结果送交进货检验员。
5.4进货检验员对所采购的产品按验收标准一一核对,当要求检验的项目完成后,将所有检验结果记录到“检验报告”单上,送交仓管员。
5.5仓管员将检验合格的采购产品按“搬运贮存控制程序”执行;对不合格采购产品按《不合格品控制程序》执行。
5.6进厂原材料的紧急放行5.6.1当客户急需产品,原材料来不及等检验结果出来就投产时,由生产车间填写《紧急放行例外转序申请审批表》,经品管部主管审核,管理者代表或总经理批准后,方可按“紧急放行”处理,原材料立即投入生产车间加工。
五金行业ISO9001:2015一整套文件(手册+程序文件+作业指导书+表单+内审管理评审)

五金行业ISO9001:2015一整套文件(手册+程序文件+作业指导书+表单+内审管理评审)1、管理手册2、程序文件3、作业指导书4、表单5、内审&管理评审6、内外部环境&风险机遇XXXXX有限公司质量管理体系管理手册GB/T19001-2016文件编号:QM-01修订版次:A/0第2页共33页0.0手册目录17章节质量管理体系及要求0.0目录0.1发布令0.2引言0.3公司简介0.4管理方针和目标0.5管理者代表任命书0.6职能分配表0.7手册管理规定1.0范围2.0引用标准3.0术语和定义4组织环境4.1理解组织及其环境4.2理解相关方的需求和期望4.3确定质量管理体系的范围4.4质量管理体系及其过程5领导作用5.1领导作用和承诺5.2质量方针5.3组织的岗位、职责和权限6策划6.1应对风险和机遇的措施6.2质量目标及其实现的策划6.3变更的策划7支持7.1资源7.2能力7.3意识7.4沟通7.5形成文件的信息8运行8.1运行策划和控制8.2产品和服务的要求8.3产品和服务的设计和开发(不适用)第3页共33页8.4外部提供的过程、产品和服务的控制8.5生产和服务提供8.6产品和服务的放行8.7不合格输出的控制9绩效评价9.1监视、测量、分析和评价9.2内部审核9.3管理评审10改进10.1总则10.2不合格和纠正措施10.3持续改进附一程序文件清单附二生产流程图第4页共33页0.1发布令本公司依据GB/T19001-2016质量管理体系标准编制完成了《管理手册》,准予2017年11月20颁布实施。
本手册是公司质量管理体系的法规性文件,是指导公司建立并实施质量管理体系的纲领和行动准则。
公司全体员工必须遵照执行。
总经理:日期: 2017年11月20第5页共33页0.2 引言0.2.l 引言为了适应后续市场竞争越演越烈的形势,不断满足顾客的要求和期望,提高产品质量、降低消耗、减少浪费、增加效益、增强社会信誉和市场竞争力,向社会、顾客证实我公司具备提供满足质量要求的产品的能力的决心,公司决定按照GB/T19001-2016标准建立质量管理系统。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
程序文件ISO9001-2015
引言
ISO 9001:2015是指国际标准化组织(ISO)于2015年发布的一种质量管理
体系标准,旨在确保制造商或服务商按照统一标准策划、制造和交付高质量产品或服务。
本文的目的是介绍ISO 9001:2015标准下的程序文件。
什么是程序文件
ISO 9001:2015鼓励组织对质量管理体系进行文件化,以确保质量管理体系
得到完整和准确的记录。
程序文件是质量体系文件的一个类型,它定义了质量管理的规划、实现和控制过程。
ISO 9001:2015标准下的程序文件类型
ISO 9001:2015标准要求制定一系列程序文件,包括但不限于以下几种:
质量手册
质量手册是组织的质量管理体系文件的核心。
它概述了组织的质量方针、目标
和质量管理体系的总体结构。
同时,它也定义了组织如何遵循质量管理体系的规定、监控和持续改进。
程序说明
程序说明是定义和记录如何执行某项任务的文件,它详细说明了实施该程序所
需的步骤、职责和控制措施。
工作指导书
工作指导书通常包括单个任务或步骤的详细说明。
这些文件由组织撰写,以确
保工作以正确方式进行,同时还确保工作符合标准。
它通常包括工作的实际步骤、工作要求和工作执行所需的图表、图像和文件。
表格和模板
表格和模板是帮助组织记录和维护重要信息的文件形式。
这些文件可能包括例
行审查报告、内部审核文件和评估文件等。
记录
记录是记录组织的质量管理体系执行情况的文件。
这些文件是质量管理体系的
实际执行情况的记录,包括管理评审、非符合性报告和内部审核等。
ISO 9001:2015标准下的程序文件的编写和维护
编写和维护ISO 9001:2015标准下的程序文件需要遵循以下流程:
1.确定流程:确定生产流程,以确定所需的过程文件类型。
2.编写:编写所需的文件。
3.内审:确保文件符合质量管理体系的要求。
4.审批:经过QA/QC程序后,仅当文件符合标准时才批准文件。
5.发布:发布文件。
6.维护:确保文件保持最新和最准确。
ISO 9001:2015标准下的程序文件帮助组织描述了质量管理体系的执行过程。
它们是为确保质量管理体系遵循统一的标准的重要文件。
组织必须将这些文件完整地编写、适时修订和维护,以确保一致性和持续性。
