Week 4 Homework Solutions
U4-homework-answer

Unit 4PassageA:Translate the following phrases实际的practical学士学位bachelor’s degree文理学院College of Arts and Science胜任qualify for英语专业的学生English major证明某事不实put the lie to….法律、医学、商业和公职本科生undergraduate直接联系的directly relevant to选修….课程take courses in….政治科学、历史、会计、商业管理political science, history, accounting, and business administration引导到多种课程be steered into multiple courses入学到医学院entrance into medical school 批判性的思考critical thinking自我表达self-expression合格院校(官方认证的院校)accredited institution平均学分grade point average (GPA)法学院入取考试L.S.A.T评价evaluate自然和社会科学natural and social sciences 最低分数minimum score 医学院入学考试M.C.A T全面发展well-rounded大学文科学生liberal arts students基础知识basic grounding人类行为的见解insight into human behaviors 文科教育liberal education资格qualification占据工作位置hold a job流动drift年轻有为的promisingsufficiently literate文字能力强保险insurance制造manufacture专门培训special training营销marketing系统工程system engineering人事管理personnel management编程programming工程设计project design劳资关系labor relation在很大程度上by a wide margin劳动大军work force申请者applicant毫不奇怪not surprisinglyPassage B公认的法则the accepted rules推崇champion引发了一场激烈的争论lead to a lively spat 创造性的语法结构creative syntax自然的思路natural flow of thoughts对…有偏见feel/have prejudice against从最基础的东西开始from the ground up耽误set back留居日本16年 a resident of Japan for 16 years漂亮地道纯正的口音high-prestige accents 与…无关do not touch客座教师visiting instructor专家小组a panel of experts晋级机会promotion prospects 西方对日本文化的腐蚀western erosion of Japanese culture迫使oblige媒介medium在某种程度上,稍微in a way首相prime minister做比较compare to落后lag behind写出下列词的相关词性cautious--- n. & v. cautionclaim--- v.claimcommerce--- mercialconverse--- n. conversationeffective--- n. Effect, effectiveness interpret--- n. interpretationnegotiation--- v.negotiate oblige--- n.obligation propose--- n.proposal responsible--- n.& responsibilityAntonym irresponsible assure--- n.assurance。
week 4 EMP sample 讲解

…labial humorouslabile humoral substances…an SP…an MA a master of arts…a SARS patient…or…, i.e.…, apposition…, which means……, namely…acronym…vessels // vehicles // craft///crafts craftsman …studies research…upon activation ……so…that…, thus doing …, where……stimulus stimuli …distinguished alumnus alumni …locus loci…focus foci…fungus fungi…germ…, thus suggesting that.…, which suggests that ……stimulus…stimulant …stimulation…subsequently=thereafter …consequently=therefore …chunk…lead to the production of ……rise…grow the increased level///the increasing level…increase…be increased…be raised…be elevated…surge hike burgeonThere has been wide agreement that ……thereby…the increase of ……the improvement of ……energy-consuming process…may be ///maybe…fall//drop///decline///decrease///be decreased///be reduced considerably dramatically…spark concerns over ……pose a serious threat to ……trigger the response of sth to sth else …sugar rush…base control…in agreement with…in consistence with…written consent…permit…maximum luggage allowance …approval ofA total of 500 patients were admitted to JPH, 50% of whom were females, ranging in age from 0.5 to 66.5.A total of 500 patients were admitted to JPH, and 50% of them were females, ranging in age from 0.5 to 66.5.A total of 500 patients were admitted to JPH, 50% of them being females, ranging in age from 0.5 to 66.5.It is generally agreed upon that …There has been wide agreement=consensus that …It has been established that …It is well known that……has been exposed under limelight. These results suggest that ……long-lived friendship…long-awaited book …longstanding problem……enduring support…protracted diarrhea…the Economist…the increase of CAMP …Our improved methodsA ,B and C…via…enzyme…toxic substances…toxin…poisonous poisonIt has been reported that……be reported to beReportedly, …According to the report by…, …personal hygiene ///sanitary conditions…sanitation…manifestation…be manifested as…be characterized byThe purpose of this study was to characterize the gene……be located in info gap …feature vt…locate vt=try to find onset …presentationsThe patient presented with high fever. High fever was present in patients with …presence…met a bolism…anabolism…catabolism…cation…anionPlagiarism falsification fabrication It is reported that …1.…be reported///known///found/// thought to be2.Reportedly, ……peace-loving people…time-consuming…money-consuming…ABC-stimulated …H2S-generating enzyme facilitator H2S-induced///evoked///facilitated/// offered dilation…aorta…approximatelyUsing…technique, direct evidence was obtained that …(dangling) Using…technique, we obtained direct evidence that ……advice…fall into…fascinating…by by using using via ..with…fall into…be divided into…amazing brilliant fabulous …the idea that H2S is a new …isintriguing ///fascinating ///amazing …the idea of H2S being a new…Mounting evidence exists that …There is no evidence to suggest that ……advice。
Homework 4
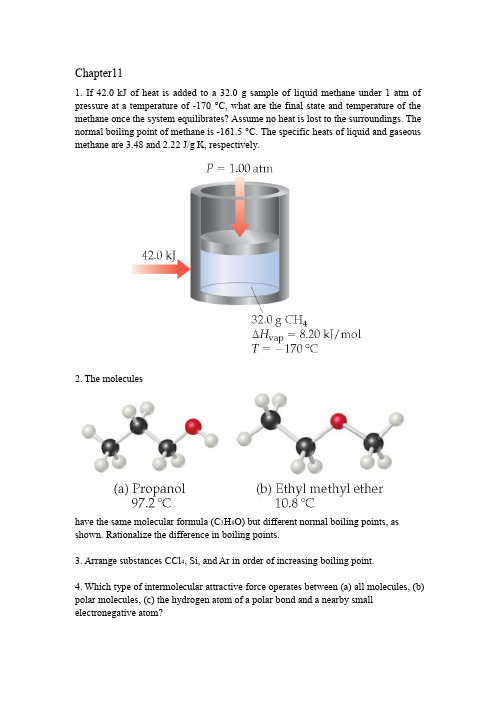
Chapter111.If 42.0 kJ of heat is added to a 32.0g sample of liquid methaneunder 1 atm of pressure at a temperature of -170 °C, what arethe final state and temperature of the methane once the systemequilibrates? Assume no heat is lost to the surroundings.The normal boiling point of methane is-161.5 °C. Thespecific heats of liquid and gaseous methane are3.48 and2.22 J/gK, respectively.2.The moleculeshave the same molecular formula (C3H8O) but different normalboiling points, as shown. Rationalize the difference inboiling points.3.Arrange substances CCl4, Si, and Ar in order of increasingboiling point.4.Which type of intermolecular attractive force operates between(a) all molecules, (b) polar molecules, (c) the hydrogenatom of a polar bond and a nearby smallelectronegativeatom?5.Describe the intermolecular forces that must be overcome toconvert these substances from a liquid to a gas: (a) SO2,(b) CH3COOH, (c) H2S.6.Which member in each pair has the larger dispersion forces:(a) H2O or H2S, (b) CO2 or CO, (c) SiH4 or GeH4?7.Ethylene glycol (HOCH2CH2OH), the major substance in antifreeze,has a normal boiling point of 198 °C. By comparison,ethyl alcohol (CH3CH2OH) boils at78 °C at atmospheric pressure.Ethylene glycol dimethyl ether (CH3OCH2CH2OCH3)has a normal boiling point of 83 °C, and ethyl methyl ether(CH3CH2OCH3) has a normal boiling point of 11 °C. (a) Explainwhy replacement of a hydrogen on the oxygen by a CH3group generally results in a lower boiling point. (b) What arethe major factors responsible for the difference in boilingpoints of the two ethers?8.Explain the following observations: (a) The surface tension ofCHBr3 is greater than that of CHCl3. (b) As temperature increases,oil flows faster through a narrow tube. (c) Raindropsthat collect on a waxed automobile hood take on a nearlyspherical shape. (d) Oil droplets that collect on a waxed automobilehood take on a flat shape. the phase transition in each of the following situationsand indicate whether it is exothermic or endothermic:(a)When ice is heated, it turns to water. (b)Wet clothes dry ona warm summer day. (c) Frost appears on a window on a coldwinter day. (d) Droplets of water appear on a cold glass ofbeer.10.For many years drinking water has been cooled in hot climatesby evaporating it from the surfaces of canvas bags or porousclay pots.How many grams of water can be cooled from35 °Cto20 °C by the evaporation of 60 g of water? (The heat of vaporizationof water in this temperature range is 2.4 kJ/g. Thespecific heat of water is 4.18 J/gK.)11.Ethanol (C2H5OH) melts at-114 °C and boils at 78 °C. Theenthalpy of fusion of ethanol is 5.02 kJ/mol, and its enthalpyof vaporization is 38.56 kJ/mol. The specific heats of solid andliquid ethanol are 0.97 J/gKand 2.3 J/gK, respectively.(a) How much heat is required to convert 42.0 g of ethanol at35 °C to the vapor phase at 78 °C? (b) How much heat isrequired to convert the same amount of ethanol at -155 °Ctothe vapor phase at 78 °C?12.(a) Place the following substances in order of increasing volatility:CH4, CBr4,CH2Cl2, CH3Cl, CHBr3, and CH2Br2. Explain.(b) How do the boiling points vary through this series?13.The phase diagram for neon isUse the phase diagram to answer the following questions. (a)What is the approximate value of the normal melting point?(b) Over what pressure range will solid neon sublime? (c) Atroom temperature (T = 25 °C) can neon be liquefied by compressingit?14.Chapter 131.The structures of vitamins E and B6 are shown below. Predictwhich is largely water soluble and which is largely fat soluble.Explain.2.The following diagram shows the vapor-pressure curves of avolatile solvent and a solution of that solvent containing anonvolatile solute. (a) Which line represents the solution? (b)What are the normal boiling points of the solvent and thesolution?3.Indicate the type of solute–solvent interaction that should be most important in each of the following solutions:(a) CCl4 in benzene (C6H6), (b) methanol (CH3OH) inwater,(c) KBr in water, (d) HCl in acetonitrile (CH3CN).mon laboratory solvents include acetone (CH3COCH3),methanol (CH3OH), toluene (C6H5CH3), and water.Which ofthese is the best solvent for nonpolar solutes? Explain.5.(a) Would you expect stearic acid, CH3(CH2)16COOH, to bemore soluble in water or in carbon tetrachloride? Explain.(b) Which would you expect to be more soluble in water,cyclohexane or dioxane? Explain.6.(a) Explain why carbonated beverages must be stored in sealedcontainers. (b) Once the beverage has been opened, why doesit maintain more carbonation when refrigerated than at roomtemperature?7.The Henry’s law constant for helium gas in water at30 °C is3.7 * 10-4 M/atm and the constant for N2 at30 °C is 6.0 * 10-4 M/atm. If the two gases are each present at1.5 atm pressure, calculate the solubility of each gas.8.(a) Calculate the mass percentage of Na2SO4 in a solutioncontaining 10.6 g Na2SO4 in 483 g water. (b) An ore contains2.86 g of silver per ton of ore. What is the concentration ofsilver in ppm?9.A solution is made containing 14.6 g of CH3OH in 184 g H2O.Calculate (a) the mole fraction of CH3OH, (b) the mass percentof CH3OH, (c) the molality of CH3OH.mercial aqueous nitric acid has a density of 1.42 g/mL andis 16 M. Calculate the percent HNO3 by mass in the solution.11.Consider two solutions, one formed by adding 10 g of glucose(C6H12O6) to 1 L of water and the other formed by adding10 g of sucrose (C12H22O11) to 1 L of water. Are the vaporpressures over the two solutions the same? Why or why not?12.At 63.5 °C the vapor pressure of H2O is 175 torr, and that ofethanol (C2H5OH) is 400 torr. A solution is made by mixingequal masses of H2O and C2H5OH. (a) What isthe mole fractionof ethanol in the solution? (b) Assuming ideal-solutionbehavior, what is the vapor pressure of the solution at 63.5 °C? (c)What is the mole fraction of ethanol in the vapor above thesolution?13.At20 °C the vapor pressure of benzene (C6H6) is 75 torr, andthat of toluene (C7H8) is 22 torr. Assume that benzene andtoluene form an ideal solution. (a)What is the composition inmole fractions of a solution that has a vapor pressure of35 torr at 20 °C?(b) What is the mole fraction of benzene inthe vapor above the solution described in part (a)?14.List the following aqueous solutions in order of increasingboiling point: 0.120 m glucose, 0.050 m LiBr, 0.050 mZn(NO3)2.15.What is the osmotic pressure formed by dissolving 44.2 mg ofaspirin (C9H8O4) in 0.358 L of water at 25 °C?16.Two beakers are placed in a sealed box at 25 °C. One beakercontains 30.0 mL of a 0.050 M aqueous solution of a nonvolatilenonelectrolyte. The other beaker contains 30.0 mL ofa 0.035 M aqueous solution of NaCl. The water vapor fromthe two solutions reaches equilibrium. (a) In which beakerdoes the solution level rise, and in which one does it fall? (b)What are the volumes in the two beakers when equilibriumis attained, assuming ideal behavior?17.At ordinary body temperature (37 °C) the solubility of N2 inwater in contact with air at ordinary atmospheric pressure(1.0 atm) is 0.015 g/L. Air is approximately 78 mol % N2.Calculate the number of moles of N2 dissolved per liter ofblood, which is essentially an aqueous solution.At a depth of100 ft in water, the pressure is 4.0 atm.What is the solubilityof N2from air in blood at this pressure? If a scuba diver suddenlysurfaces from this depth, how many milliliters of N2gas, in the form of tiny bubbles, are released into the bloodstreamfrom each liter of blood?。
Homework 4

Homework for Grade 5 (4)Name________1.Read, tick or cross.(判断划线部分发音是否相同)( ) 1. class April ( ) 2. kite climb ( ) 3. season breakfast( ) 4. get bed ( ) 5. make have ( ) 6. pear bear( ) 7. sport work ( ) 8. brown black2.Read and fill in the blanks.(选词填空)( warm, happy, cold, colourful, food , trees, sky, river, picnic, visit, Festival, wear ) I’m Sandy. I like spring best. It’s ___.We can see many______flowers and gr een ___ everywhere. The birds can freely fly in the ___ . I usually have a ___ in spring. We have Spring ___ in spring. We ___our new clothes. We ___ our friends. We have much ___to eat. We are ___ .3.Choose.()1、What is the ______ today?- It’s April 29th.A、weather B 、day C 、date()2、Is ____ birthday in February?A、youB、yourC、me()3、.When ____ you get up?A、doesB、doC、are()4、They are my family _____ .A、photoB、photoesC、photos()5、I usually eat dinner ____7:00 ____ the evening.A、in: atB、at ;inC、at ;at()6、What ____ the children doing?A、canB、isC、are()7、-Why do you like winter? -______ Christmas.A、It’sB、BecauseC、Because of()8、I _____ plant trees in springA、amB、canC、like()9、_____ is in June and July.A、Summer vacationB、Winter vacationC、Children’s Day()10、Amy is playing ______.A、pianoB、the pianoC、a piano4.翻译中文。
Course Outline

Course Meeting Time and Location:Mondays and Wednesdays, 11:15am - 12:40pmClass meets in room 220 of the Stuart Building (220 SB)Textbook:Chi-Tsong Chen, Linear System Theory and Design, 3rd ed., Oxford University Press, 1999. ISBN: 0-19-511777-8Course Objectives:After completing this course, the student should be able to do the following things correctly: ∙formulate state space descriptions of linear dynamical systems, in both time-invariant and time-varying cases, and for both continuous-time and discrete-time systems;∙find the analytic solution of state equations and give a geometric interpretation of the state space in terms of the system dynamics;∙apply the concepts of stability, controllability and observability in interpreting and analyzing system behavior;∙formulate input-output descriptions of linear dynamical systems, in both time-invariant and time-varying cases, and for both continuous-time and discrete-time systems;∙construct realizations of input-output system descriptions via state space system dynamics;∙use state feedback to reshape system dynamics;∙use observers to infer knowledge of the system states given input and output measurements;∙use computer-based analysis and design tools (such as {\sc Matlab} software) in the analysis of linear, time-invariant systems.Course Documents:These documents are available in pdf or html format.∙Course syllabuso Syllabus as an html documento Syllabus as an Excel document∙Suggested additional references∙The Kalman Decomposition: a document describing how to find the coordinate transformation leading to the Kalman decomposition and giving an example of theapproach.Homework Policy and Assignments:Please follow these rules when submitting homework papers. Any exceptions to these basic policies should be confirmed with the instructor before submitted your paper.∙Use 8 1/2 x 11 inch or A4 paper (other sizes are not accepted), with multiple pages stapled together in the upper left corner. Do not use any means other than stapling tohold pages together (in that event, the grader has full authority to discard all but the first page).∙Your homework paper should present answers to the questions in the same order that they appear in the assignment. At the grader's discretion, zero credit may be given forwork the appears out of order.∙No email submissions are accepted unless prior arrangements have been made due to special circumstances, or unless the assignment specifically requests email submission.∙No late homeworks will be accepted without prior approval by the instructor. (Generally, approval of late homework submission requires there to be very extenuatingcircumstances. I drop the lowest homework score in part to accommodate the commonsituations that prevent persons from completing the assignments on time.)Homework assignments, when available, will be posted here in pdf format.∙Assignment #1 [posted 26 Jan 2007] (due 7 February 2007) [Note the new (later) due date!]∙Assignment #2 [posted 7 Feb 2007] (due 14 February 2007)∙Assignment #3 [posted 14 Feb 2007] (due 28 February 2007)∙Assignment #4 [posted 13 Mar 2007] (due 28 March 2007) [Problem #1 typo corrected15 Mar 2007]o Here are a couple of supplementary problems for controllable and Kalman decompositions that are non-degenerative (unlike problems 4 and 5 on HomeworkAssignment \#4). For your convenience the various system matrices are stored inthe file hw4_supp_data.mat. To access the variables, save this file to yourcomputer, and change the working directory of Matlab to the directory where yousaved the file (or alternatively add that directory to Matlab's path). You then loadin the system matrices by typing "load hw4_supp_data" at the Matlab prompt.▪Assignment #4 supplement▪hw4_supp_data.mat∙Assignment #5 [posted 26 Mar 2007] (due 13 April 2007, on-campus students please submit homework by putting it in my mailbox in the ECE Department; off campusstudents may submit homework in the usual way)∙Assignment #6 [posted 12 Apr 2007] (due 25 April 2007)Homework Solutions:Homework solutions will be posted either here or at the Galvin Library's Electronic Reserves.∙Solutions to Assignment #1 [submitted for posting at the Galvin Library's Electronic Reserves on 7 Feb 2007 and posted there on 8 Feb 2007]∙Solutions to Assignment #2 [submitted for posting at the Galvin Library's Electronic Reserves on 14 Feb 2007 and posted there on 15 Feb 2007]∙Solutions to Assignment #3 [submitted for posting at the Galvin Library's Electronic Reserves on 28 Feb 2007 and posted there on 1 Mar 2007]o ECE531_07S_hw3_p3.m: Matlab m-file for problem 3 of Assignment #3 (save to your own computer and run from there)∙Solutions to Assignment #4 [submitted for posting at the Galvin Library's Electronic Reserves on 28 Mar 2007 and posted there on 28 Mar 2007]∙Solutions to Assignment #5 [submitted for posting at the Galvin Library's Electronic Reserves on 16 Apr 2007 and posted there on 17 Apr 2007]∙Solutions to Assignment #6 [submitted for posting at the Galvin Library's Electronic Reserves on 25 Apr 2007 and posted there on 26 Apr 2007]Examinations:There will be two take-home examinations during the course of the term, and one in-class final examination. The take-home examinations, when available, will be posted here in pdf format.∙Take-home Exam #1 (due 7 March 2007 at 11:25am) [Problem 8 corrected on 2 March 2007 to include a missing transpose.]o Solutions to Take-home Exam #1 [submitted for posting at the Galvin Library's Electronic Reserves on 22 Mar 2007 and posted there on 23 Mar 2007] ∙Take-home Exam #2 (due 2 May 2007 at 11:25am)o Solutions to Take-home Exam #2 [submitted for posting at the Galvin Library's Electronic Reserves on 7 May 2007 and posted there on 7 May 2007]. Note: theproblem 2 solutions shown here are for a problem not on the exam. The problem 3solutions solve problem 2 on the exam. The problem 4 solutions solve problem 3.Problem 4's solutions are missing, but are included in the supplement.o Supplement to solutions to Take-home Exam #2 [submitted for posting at the Galvin Library's Electronic Reserves on 7 May 2007 and posted there on 7 May2007]. These are the missing problem 4 solutions.∙In-class final examination: Wednesday, 9 May 2007, 2:00 - 4:00pm∙Sample in-class examinations are available as follows:o Final Exam, Spring 2001o Final Exam, Spring 2002o Final Exam, Spring 2004o Final Exam, Spring 2005o Final Exam, Spring 2006Grading:∙Homework: 20% (best 5 of 6 assignments)∙Take-home Exam #1: 30%∙Take-home Exam #2: 30%In-class Final: 20% (the final is comprehensive)Academic Honesty:It is your responsibility to be familiar with IIT's Code of Academic Honesty. (Consult the IIT Student Handbook for this code.)In particular, the work that you submit for homework and individual project assignments and your work on examination papers must be your own. You may consult with other students about homework and project assignments. In fact, discussion of the assignments is encouraged. However, the written material that is submitted must be your own. Such written material includes computer programs and the results of using computer programs (computed values, plots, etc.). If the above policy or any part of IIT's Code of Academic Honesty is violated in regard to a submitted homework assignment, a grade of zero will be assigned to the work. If the above policy or any part of IIT's Code of Academic Honesty is violated in regard to a submitted project assignment or an examination paper, a punitive failing grade will be given in the course. In both cases, the matter will be reported to the appropriate university officials and offices. In the case of a second offense (with the first offense in this or any other course), expulsion from the university will be advocated.。
陕旅版小学四年级英语下册 Unit 4 第二课时课件
fly a kite
see a film
climb the mountain play football
5
Thi>n>kLeaadn-ind circle What do you often do on the weekend?
6
weekend (名词)周末
【单词巧记】
week(周)+end(末尾)=weekend(周末)
【详解】“What about …?”意为“……怎么
样?”。“How about …?”用法同 “What about …?”。
【例句】— I’m a teacher. What/How about you? 我是一名老师。你呢?
— I’m a doctor. 我是一名医生。
Pract>>iPcracetice
【例句】He often wears a hat in winter. 他在冬天经常戴帽子。
重点句型二
He often climbs the mountains. 他经常爬山。
【详解】此句是一个一般现在时的句子,表达某
人经常做某事。句中的主语是第三人称 单数形式,故谓语动词也要用第三人称 单数形式。
表示经常性、习惯性的 动作或行为。
She often does some washing.
I often do my homework.
They often climb the mountain.
Let'>s>Ptreaslekntation Listen, then ask and answer
视 频
点击“Let's talk”,跟 我一起读吧!
Let's talk
Week 5 Homework

Week 5 HomeworkHomework for Week 5•发现和认识存在问题。
•小组:阅读彼此的作文•1. 文章有中心句(thesis statement)吗?写得好不好?•2. 文章分段写了吗?主题句(topic sentence)是什么?主题句体现段落的中心意思了吗?•3. 段落是紧扣文章中心展开的吗?•根据以上问题为所读文章撰写大纲(outline)•大纲写在作文试题纸的背面,并署上名字。
格式:原文作者:原文题目:大纲撰写人:Outline:套路:具体问题具体分析(blind)知识:联系哪些知识,如何用(sharpening)审题:如何做到紧扣题目(relevance)•Reading & summary: Which part is more relevant considering the possible point to be made?•development•知识运用:如何谈development?•话题相关:慈善、网络时代、消费主义、娱乐至死、营销、流行文化、乌合之众•切题:express your opinion towards the activity, especially whether the problems found with this kind of activity will finally undermine its original purpose.•*题目说明与阅读材料:缩小范围,限制话题Thinking & Writing•Examining the topic 审题•Determining a thesis 中心思想•Making an outline 结构大纲•development: summary•利弊与初衷•ArgumentationDetermining a thesis•Effective thesis statement: what & how?•What is an effective thesis statement?•1. A good thesis statement is specific and restricted.•2. A good thesis statement ties the whole essay together. It gives shape to the form of your essay.How:Formulating Thesis Statement•1. Neither too broad nor too narrow•2. A complete sentence•3. No announcement of the obvious•4. An opinion or attitude on a topic instead of the topic itself •5. Subtopics•Poor thesis:•Broad Noun + weak verb + vague, evaluative adjective•Better thesis•Specific noun + active verb +specific modifiers •Inner-city public schools grapple with some serious problems.•Some of the most serious problems in today’s inner-city public schools are the overcrowded classrooms, the low percentage of trained teachers, and the lack of resources such as textbooks for students.比较•1. Insects are fascinating creatures.•2. Ants are one of nature's most successful insects.•3. Strength, organization, and communication make ants one of nature's most successful insects.。
五年级春季《英语》(人教版)Unit 4 Last Weekend (Lesson 3)作业改一

Unit4 Last Weekend Lesson 3 Homework 一、读一读,为他们的周末活动找出单词或短语,并写在图片下面。 cleaned the window climbed a hill danced jumped rope listened to music played the chess played the piano rowed a boat visited grandparents washed clothes
1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 二、读短文,回答问题。 Hello. I’m Jessie. We had a lot of fun on weekends. Last Saturday, I helped my parents clean all the rooms and washed our cars. We were really tired. But we were happy to see all the tidy things. On Sunday morning, I walked in the park with my pet dog. Then I finished my homework. My parents cooked lunch for me. It’s really delicious. In the afternoon, we visited my grandparents. We cleaned the house for them. They were very happy. What a nice weekend!
1. Why was Jessie tired on Saturday? 2. What did Jessie do in her grandparents’ house? 3. How was Jessie’s weekend? 参考答案: 一、读一读,为他们的周末活动找出单词或短语,并写在图片下面。 1. climbed a hill 2. cleaned the window 3. played the piano 4. jumped rope 5. listened to music 6. danced 7. played the chess 8. visited grandparents 9. washed clothes 10. rowed a boat
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Chapter 08 - Cash and Internal Controls 8-26 Problem 8-4B (30 minutes) Part 1 STYLE CO. Bank Reconciliation December 31, 2011
Bank statement balance .............. $45,091.80 Book balance ................................$31,743.70 Add: Add: Deposit of Dec. 31 ....................... 7,666.10 Error (Ck 1267) .... $ 18.00 52,757.90 Proceeds of note less $20 fee........ 19,980.00 19,998.00 51,741.70 Deduct: Deduct: Checks No. 1242 ..... $ 370.50 NSF check ............ $ 749.50 1273 ..... 1,084.20 Printing fee ........... 79.00 1282 ..... 390.00 1,844.70 828.50 Adjusted bank balance ................. $50,913.20 Adjusted book balance ..............$50,913.20
Part 2 Dec. 31 Cash .....................................................................18.00 Office Supplies ............................................. 18.00 To correct an entry error.
31 Cash .....................................................................19,980.00 Collection Expense ............................................20.00 Note Receivable ............................................ 20,000.00 To record note collection less fees.
31 Accounts Receivable—Titus Industries ...........749.50 Cash ............................................................... 749.50 To charge account for NSF check plus fees.
31 Miscellaneous Expenses ...................................79.00 Cash ............................................................... 79.00 To record check printing charge.
Part 3 In a banking context, a debit memo is notification from the bank that it has debited the depositor's account. Since the depositor's account is a liability of the bank (a credit balance account), the debit notification means the bank has reduced the depositor's account balance. Conversely, a credit memo is a notification that the depositor's account has been credited, which means the bank has increased the depositor’s cash balance. Chapter 9 - Receivables 9-18 Problem 9-2A (35 minutes) 2014 a. Accounts Receivable ....................................... 1,803,750 Sales ............................................................ 1,803,750 To record sales on account.
Cost of Goods Sold ..........................................1,475,000 Merchandise Inventory .............................. 1,475,000 To record cost of sales.
b. Allowance for Doubtful Accounts ................... 20,300 Accounts Receivable ................................. 20,300 To write off accounts.
c. Cash ................................................................... 789,200 Accounts Receivable ................................. 789,200 To record cash received on account.
d. Bad Debts Expense .......................................... 35,214 Allowance for Doubtful Accounts ............ 35,214 To record estimated bad debts.*
*Beginning receivables ...................... $ 0
Credit sales ....................................... 1,803,750 Collections ........................................ (789,200) Write-offs ........................................... (20,300) Ending receivables ........................... 994,250
Percent uncollectible ........................ x 1.5% Required ending allowance .............. 14,914** Cr.
Unadjusted balance .......................... 20,300 Dr. Adjustment to the allowance ........... $ 35,214 Cr.
** rounded to nearest dollarChapter 09 - Receivables
9-19 Problem 9-2A (Concluded) 2015 e. Accounts Receivable ........................................... 1,825,700 Sales ................................................................ 1,825,700 To record sales on account.
Cost of Goods Sold ..............................................1,450,000 Merchandise Inventory .................................. 1,450,000 To record cost of sales.
f. Allowance for Doubtful Accounts ...................... 28,800 Accounts Receivable ..................................... 28,800 To record write-off of accounts.
g. Cash ...................................................................... 1,304,800 Accounts Receivable ..................................... 1,304,800 To record cash received on account.
h. Bad Debts Expense .............................................. 36,181 Allowance for Doubtful Accounts ................ 36,181 To record estimated bad debts.*
*Beginning receivables ..................... $ 994,250
Credit sales ....................................... 1,825,700 Collections ........................................ (1,304,800) Write-offs .......................................... (28,800) Ending receivables .......................... 1,486,350
