工程热力学第三版电子教案第4章自我测验题
工程热力学第三版电子教案第3章自我测验题(五篇)

工程热力学第三版电子教案第3章自我测验题(五篇)第一篇:工程热力学第三版电子教案第3章自我测验题第三章自我测验题1、填空题(1)气体常数Rg与气体种类_____关,与状态_____关。
通用气体常数R与气体种类______关,与状态_____关。
在SI制中R的数值是_____,单位是______。
(2)质量热容c,摩尔热容Cm与容积热容C'之间的换算关系为_________。
(3)理想气体的Cp及Cv与气体种类______关,与温度_________关。
它们的差值与气体种类_______关,与温度_______关。
它们的比值与气体种类_________关,与温度_______关。
(4)对于理想气体,dU =CvdT,dh=CpdT。
它们的适用条件分别是________。
(5)2kg氮气经定压加热过程从67℃升到237℃。
用定值比热容计算其热力学能约变化为________,吸热量为________。
接着又经定容过程降到27℃,其焓变化为______,放热量为_______。
2、利用的计算公式。
3、公式(1),以及(2),这两组导出多变过程膨胀功的计算公式,利用导出多变过程技术功公式对于理想气体的不可逆过程是否适用?对于实际气体的可逆过程是否适用?怎么样修改才适用于菲理想气体的可逆过程?4、绝热过程中气体与外界无热量交换,为什么还能对外作功?是否违反热力学第一定律?5、试将满足以下要求的理想气体多变过程在p-v图和T-s图上表示出来。
(1)工质又膨胀,又放热。
(2)工质又膨胀、又升压。
(3)工质又受压缩、又升温,又吸热。
(4)工质又受压缩、又降温,又将压。
(5)工质又放热、又降温、又升压。
6、理想气体的3个热力过程如图所示,试将3种热力过程定性地画在p-v图上;分析3个过程多变指数的范围,井将每个过程的功量、热量及热力学能变化的正负号填在表中。
7、试将图示的p-v图上的2个循环分别表示在T-s图上。
工程热力学第三版答案【英文】第4章
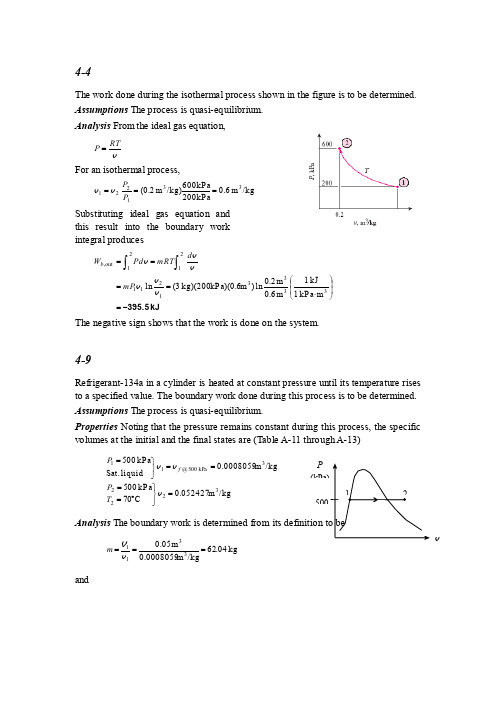
4-4The work done during the isothermal process shown in the figure is to be determined. Assumptions The process is quasi-equilibrium. Analysis From the ideal gas equation,vRTP =For an isothermal process,/kg m 0.6kP a200kP a 600/kg)m (0.2331221===P P v v Substituting ideal gas equation andthis result into the boundary work integral produceskJ395.5-=⎪⎪⎭⎫⎝⎛⋅====⎰⎰333312112121out ,m kP a 1kJ 1m 0.6m 0.2ln )m kP a)(0.6 kg)(200 (3lnv v v vvv mP d mRTd P W b The negative sign shows that the work is done on the system.4-9Refrigerant-134a in a cylinder is heated at constant pressure until its temperature risesto a specified value. The boundary work done during this process is to be determined. Assumptions The process is quasi-equilibrium.Properties Noting that the pressure remains constant during this process, the specific volumes at the initial and the final states are (Table A-11 through A-13)/kg m 0.052427C 07kP a 005/kg m 0.0008059liquid Sat.kP a 00532223kPa 005@11=⎭⎬⎫︒====⎭⎬⎫=v v v T P P fAnalysiskg 04.62/kgm 0.0008059m 0.053311===v V m andvPkJ1600=⎪⎪⎭⎫⎝⎛⋅-=-=-==⎰33121221out ,m kPa 1kJ1/kg m 0.0008059)427kPa)(0.052 kg)(500 (62.04)()( v v V VV mP P d P W b Discussion The positive sign indicates that work is done by the system (work output).4-23A saturated water mixture contained in a spring-loaded piston-cylinder device is heated until the pressure and temperature rises to specified values. The work done during this process is to be determined. Assumptions The process is quasi-equilibrium. Analysis The initial state is saturated mixture at 90︒C. The pressure and the specific volume at this state are (Table A-4),/kgm 23686.0)001036.03593.2)(10.0(001036.0kP a183.70311=-+=+==fgf x P v v vThe final specific volume at 800 kPa and 250°C is (Table A-6)/kg m 29321.032=vSince this is a linear process, the work done is equal to the area under the process line1-2:kJ24.52=⎪⎭⎫ ⎝⎛⋅-+=-+==331221out ,m kPa 1kJ 1)m 23686.01kg)(0.2932 (12)kPa 800(70.183)(2Area v v m P P W b4-29An insulated rigid tank is initially filled with a saturated liquid-vapor mixture of water. An electric heater in the tank is turned on, and the entire liquid in the tank is vaporized. The length of time the heater was kept on is to be determined, and the process is to be shown on a P-v diagram.Assumptions 1 The tank is stationary and thus the kinetic and potential energy changes are zero. 2 The device is well-insulated and thus heat transfer is negligible. 3The energy stored in the resistance wires, and the heat transferred to the tank itself is negligible.Analysis We take the contents of the tank as the system. This is a closed system since no mass enters or leaves. Noting that the volume of the system is constant and thus there is no boundary work, the energy balance for this stationary closed system can be expressed as)(V 0)=PE =KE (since )(1212in ,energiesetc. potential, kinetic,internal,in Change systemmassand work,heat,by nsfer energy tra Net out in u u m t I Q u u m U W E E E e -=∆=-=∆=∆=-The properties of water are (Tables A-4 through A-6)()[]()k J /k g 2569.7v a p o rs a t ./k g m 0.29065k J /k g980.032052.30.25466.97/km 0.290650.0010531.15940.250.001053k J/k g 3.2052,97.466/kgm 1.1594,001053.025.0kP a 150/kg m 0.29065@2312113113113==⎪⎭⎪⎬⎫===⨯+=+==-⨯+=+=====⎭⎬⎫==g fg f fg f fg f g f u u u x u u x u u x P v v v v v v v Substituting,min60.2==∆⎪⎪⎭⎫⎝⎛-=∆s 33613kJ/s 1VA 1000.03)kJ/kg 980kg)(2569.7 (2)A 8)(V 110(t t4-39A saturated water mixture contained in a spring-loaded piston-cylinder device isheated until the pressure and volume rise to specified values. The heat transfer and the work done are to be determined.Assumptions 1 The cylinder is stationary and thus the kinetic and potential energy changes are zero. 2 There are no work interactions involved other than the boundary work. 3 The thermal energy stored in the cylinder itself is negligible. 4 The compression or expansion process is quasi-equilibrium. Analysis We take the contents of the cylinder as the system. This is a closed system since no mass enters or leaves. The energy balance for this stationary closed system can be expressed asv)(0)=PE =KE (since )(12ou ,in 12ou ,in energiesetc. potential, kinetic,internal,in Change systemmassand work,heat,by nsfer energy tra Net out in u u m W Q u u m U W Q E E E t b t b -+=-=∆=-∆=-The initial state is saturated mixture at 75 kPa.The specific volume and internal energy at this state are (Table A-5),kJ/kg30.553)8.2111)(08.0(36.384/kgm 1783.0)001037.02172.2)(08.0(001037.0131=+=+==-+=+=fg f fg f xu u u x v v vThe mass of water iskg 22.11/kgm 1783.0m 23311===v V m The final specific volume is/kg m 4458.0kg22.11m 53322===m V vThe final state is now fixed. The internal energy at this specific volume and 225 kPa pressure is (Table A-6) kJ/kg 4.16502=u Since this is a linear process, the work done is equal to the area under the process line 1-2:kJ 450=⎪⎭⎫⎝⎛⋅-+=-+==331221out ,m kP a 1kJ 1)m 2(52)kP a 225(75)(2Area V V P P W b Substituting into energy balance equation giveskJ 12,750=-+=-+=kJ/kg )30.553kg)(1650.4 22.11(kJ 450)(12out ,in u u m W Q b4-43Two tanks initially separated by a partition contain steam at different states. Now thepartition is removed and they are allowed to mix until equilibrium is established. The temperature and quality of the steam at the final state and the amount of heat lost from the tanks are to be determined.Assumptions 1 The tank is stationary and thus the kinetic and potential energy changes are zero. 2 There are no work interactions.Analysis (a ) We take the contents of both tanks as the system. This is a closed system since no massenters or leaves. Noting that the volume of the system is constant and thus there is no boundary work, the energy balance for this stationary closed system can be expressed as[][]0)=PE =KE (since )()(1212out energiesetc. potential, kinetic,internal,in Change systemmassand work,heat,by nsfer energy tra Net out in =-+-=∆+∆=-∆=-W u u m u u m U U Q E E E B A B AThe properties of steam in both tanks at the initial state are (Tables A-4 through A-6)kJ/kg 7.2793/kg m 25799.0C 300kPa 1000,13,1,1,1==⎪⎭⎪⎬⎫︒==A A A A u T P v ()[]()kJ/kg4.15954.19270.50.66631/kg m 0.196790.0010910.392480.500.001091kJ/kg 4.1927,66.631/kgm .392480,001091.050.0C 1501,131,131,1=⨯+=+==-⨯+=+=====⎭⎬⎫=︒=fg f B fg f B fg f g f B u x u u x u u x T v v v v vThe total volume and total mass of the system arekg523m 106.1/kg)m 19679.0kg)( 3(/kg)m 25799.0kg)( 2(333,1,1=+=+==+=+=+=B A B B A A B A m m m m m v v V V VNow, the specific volume at the final state may be determined/kg m 22127.0kg5m 106.1332===m Vvwhich fixes the final state and we can determine other properties()kJ/kg8.12821.19820.3641.11561001073.060582.0001073.022127.0/kg m 22127.0kPa 0032222kPa 300 @sat 2322=⨯+=+==--=--=︒==⎪⎭⎪⎬⎫==fg f fg f u x u u x T T P 0.3641C 133.5v v v v v (b ) Substituting,[][]kJ 3959kJ/kg )4.15958.1282(kg) 3(kJ/kg )7.27938.1282(kg) 2()()(1212out -=-+-=-+-=∆+∆=-BA B A u u m u u m U U QorkJ 3959=out Q4-60The air in a rigid tank is heated until its pressure doubles. The volume of the tank and the amount of heat transfer are to be determined.Assumptions 1 Air is an ideal gas since it is at a high temperature and low pressure relative to its critical point values of -221︒F and 547 psia. 2 The kinetic and potential energy changes are negligible, ∆∆pe ke ≅≅0. 3 Constant specific heats at room temperature can be used for air. This assumption results in negligible error in heating and air-conditioning applications. Properties The gas constant of air is R = 0.3704 psia.ft 3/lbm.R (Table A-1E).Analysis (a3ft 80.0=⋅⋅==psia50R) R)(540/lbm ft psia 4lbm)(0.370 (20311P mRT V(b) We take the air in the tank as our system. The energy balance for this stationary closed system can be expressed as)()(1212in in energiesetc. potential, kinetic, internal,in Change systemmassand work,heat,by nsferenergy tra Net out in T T mc u u m Q UQ E E E -≅-=∆=∆=-vThe final temperature of air isR 1080R) (540211222211=⨯==−→−=T P P T T P T P V V The internal energies are (Table A-17E)u u u u 12====@@540R 1080R 92.04Btu /lbm 186.93Btu /lbmSubstituting, Q in = (20 lbm)(186.93 - 92.04)Btu/lbm = 1898 BtuAlternative solutions The specific heat of air at the average temperature of T avg = (540+1080)/2= 810 R = 350︒F is, from Table A-2Eb, c v ,avg = 0.175 Btu/lbm.R. Substituting,Q in = (20 lbm)( 0.175 Btu/lbm.R)(1080 - 540) R = 1890 BtuDiscussion Both approaches resulted in almost the same solution in this case.4-64A student living in a room turns her 150-W fan on in the morning. The temperature in the room when she comes back 10 h later is to be determined.Assumptions 1 Air is an ideal gas since it is at a high temperature and low pressure relative to its critical point values of -141︒C and 3.77 MPa. 2 The kinetic andQpotential energy changes are negligible, ∆∆ke pe ≅≅0. 3 Constant specific heats at room temperature can be used for air. This assumption results in negligible error in heating and air-conditioning applications. 4 All the doors and windows are tightly closed, and heat transfer through the walls and the windows is disregarded.Properties The gas constant of air is R = 0.287 kPa.m 3/kg.K (Table A-1). Also, c v = 0.718 kJ/kg.K for air at room temperature (Table A-2).Analysis We take the room as the system. This is a closed system since the doors and the windows are said to be tightly closed, and thus no mass crosses the system boundary during the process. The energy balance for this system can be expressed as)()(1212,,energiesetc. potential, kinetic,internal,in Change systemmassand work,heat,by nsfer energy tra Net T T mc u u m W UW E E E in e in e out in -≅-=∆=∆=-vThe mass of air iskg174.2K) K)(288/kg m kP a (0.287)m kP a)(144 (100m 14466433113=⋅⋅===⨯⨯=RT P m V VThe electrical work done by the fan isW W t e e==⨯= ∆(0.15kJ /s)(103600s)5400kJ Substituting and using the c v value at room temperature, 5400 kJ = (174.2 kg)(0.718 kJ/kg ⋅︒C)(T 2 - 15)︒CT 2 = 58.2︒CDiscussion Note that a fan actually causes the internal temperature of a confinedspace to rise. In fact, a 100-W fan supplies a room with as much energy as a 100-W resistance heater.4-69Carbon dioxide contained in a spring-loaded piston-cylinder device is heated. The work done and the heat transfer are to be determined.Assumptions 1 CO 2 is an ideal gas since it is at a high temperature relative to its critical temperature of 304.2 K. 2 The kinetic and potential energy changes are negligible, 0pe ke ≅∆≅∆. Properties The properties of CO 2 are R = 0.1889 kJ/kg ⋅K and c v = 0.657 kJ/kg ⋅K (Table A-2a ).PAnalysis We take CO 2 as the system. This is a closed system since no mass crosses the boundaries of the system. The energy balance for this system can be expressed as)(12out ,in energiesetc. potential, kinetic,internal,in Change systemmassand work,heat,by nsfer energy tra Net out in T T mc U W Q E E E b -=∆=-∆=-vThe initial and final specific volumes are33111m 5629.0k P a 100K) K)(298/kg m kPa kg)(0.1889 (1=⋅⋅==P mRT V 33222m 1082.0k P a1000K)K)(573/kg m kPa kg)(0.1889 (1=⋅⋅==P mRT V Pressure changes linearly with volume and the work done is equal to the area underthe process line 1-2:kJ1.250m kPa 1kJ 1)m 5629.0(0.10822)kPa1000(100)(2Area 331221out ,-=⎪⎪⎭⎫⎝⎛⋅-+=-+==V V P P W b Thus,kJ 250.1=in ,b WUsing the energy balance equation,kJ 4.69K )25K)(300kJ/kg 657.0(kg) 1(kJ 1.250)(12out ,in -=-⋅+-=-+=T T mc W Q b vThus,kJ 69.4=out Q4-76Air at a specified state contained in a piston-cylinder device with a set of stops is heated until a final temperature. The amount of heat transfer is to be determined.Assumptions 1 Air is an ideal gas since it is at a high temperature relative to its critical temperature of 304.2 K. 2 The kinetic and potential energy changes are negligible, 0pe ke ≅∆≅∆. Properties The properties of air are R = 0.287 kJ/kg ⋅K and c v = 0.718 kJ/kg ⋅K (Table A-2a ). Analysis We take air as the system. This is a closedsystem since no mass crosses the boundaries of the system. The energy balance for this system can be expressed as)(12out ,in energiesetc. potential, kinetic, internal,in Change systemmassand work,heat,by nsferenergy tra Net out in T T mc U W Q E E E b -=∆=-∆=-vThe volume will be constant until the pressure is 300 kPa:K 900kP a100kP a 300K) (3001212===P P T T The mass of the air iskg 4646.0K)K)(300/kg m kPa (0.287)m kPa)(0.4 (10033111=⋅⋅==RT P m V The boundary work done during process 2-3 iskJ04900)K -K)(1200/kg m kP a (0.287)kg 4646.0()()(323232out ,=⋅⋅=-=-=T T mR P W b V V Substituting these values into energy balance equation,kJ 340=-⋅+=-+=K )300K)(1200kJ/kg 718.0(kg) 4646.0(kJ 40)(13out ,in T T mc W Q b v4-85An egg is dropped into boiling water. The amount of heat transfer to the egg by the time it is cooked is to be determined.Assumptions 1 The egg is spherical in shape with a radius of r 0 = 2.75 cm. 2 The thermal properties of the egg are constant. 3 Energy absorption or release associated with any chemical and/or phase changes within the egg is negligible. 4 There are no changes in kinetic and potential energies.Properties The density and specific heat of the egg are given to be ρ = 1020 kg/m 3 and c p = 3.32 kJ/kg.︒C.Analysis We take the egg as the system. This is a closes system since no mass enters or leaves the egg. The energy balance for this closed system can be expressed as)()(1212egg in energiesetc. potential, kinetic,internal,in Change systemmassand work,heat,by nsfer energy tra Net out in T T mc u u m U Q E E E -=-=∆=∆=-Then the mass of the egg and the amount of heat transfer becomeBoilingPV (m 3)kJ 21.2=︒-︒=-=====C )880)(C kJ/kg. 32.3)(kg 0889.0()(kg0889.06m ) 055.0()kg/m 1020(612in 333T T mc Q D m p ππρρV。
工程热力学第三版答案【英文】第4章

4-4The work done during the isothermal process shown in the figure is to be determined. Assumptions The process is quasi-equilibrium. Analysis From the ideal gas equation,vRTP =For an isothermal process,/kgm 0.6kPa200kPa 600/kg)m (0.2331221===P P v vSubstituting ideal gas equation and this result into the boundary work integral produceskJ395.5-=⎪⎪⎭⎫⎝⎛⋅====⎰⎰333312112121out ,m kPa 1kJ 1m0.6m 0.2ln)m kPa)(0.6 kg)(200 (3lnv v v vvv mP d mRTd P W bThe negative sign shows that the work is done on the system.4-9Refrigerant-134a in a cylinder is heated at constant pressure until its temperature rises to a specified value. The boundary work done during this process is to be determined. Assumptions The process is quasi-equilibrium.Properties Noting that the pressure remains constant during this process, the specific volumes at the initial and the final states are (Table A-11 through A-13)/kgm 0.052427C 07kPa 005/kgm 0.0008059liquid Sat.kPa 00532223k Pa005@11=⎭⎬⎫︒====⎭⎬⎫=v vv T P P fAnalysiskg04.62/kgm 0.0008059m0.053311===v V mandvPkJ1600=⎪⎪⎭⎫⎝⎛⋅-=-=-==⎰33121221out ,m kPa 1kJ 1/kg m 0.0008059)427kPa)(0.052 kg)(500 (62.04)()( v v V V V mP P d P W bDiscussion The positive sign indicates that work is done by the system (work output).4-23A saturated water mixture contained in a spring-loaded piston-cylinder device is heated until the pressure and temperature rises to specified values. The work done during this process is to be determined. Assumptions The process is quasi-equilibrium. Analysis The initial state is saturated mixture at 90︒C. The pressure and the specific volume at this state are (Table A-4),/kgm 23686.0)001036.03593.2)(10.0(001036.0kPa183.70311=-+=+==fgfx P vvvThe final specific volume at 800 kPa and 250°C is (Table A-6)/kgm 29321.032=vSince this is a linear process, the work done is equal to the area under the process line 1-2:kJ24.52=⎪⎭⎫ ⎝⎛⋅-+=-+==331221out ,m kPa 1kJ 1)m 23686.01kg)(0.2932 (12)kPa 800(70.183)(2Area v v m P P W b4-29An insulated rigid tank is initially filled with a saturated liquid-vapor mixture of water. An electric heater in the tank is turned on, and the entire liquid in the tank is vaporized. The length of time the heater was kept on is to be determined, and the process is to be shown on a P-v diagram.Assumptions 1 The tank is stationary and thus the kinetic and potential energy changes are zero. 2 The device is well-insulated and thus heat transfer is negligible. 3The energy stored in the resistance wires, and the heat transferred to the tank itself is negligible.Analysis We take the contents of the tank as the system. This is a closed system since no mass enters or leaves. Noting that the volume of the system is constant and thus there is no boundary work, the energy balance for this stationary closed system can be expressed as)(V 0)=PE =KE (since )(1212in ,energiesetc. potential, k inetic,internal,in Change system massand work ,heat,by nsfer energy tra Net out in u u m t I Q u u m U W E E E e -=∆=-=∆=∆=-The properties of water are (Tables A-4 through A-6)()[]()k J /k g2569.7v a p o rs a t ./k g m 0.29065k J /k g980.032052.30.25466.97/k g m 0.290650.0010531.15940.250.001053k J /k g3.2052,97.466/kg m 1.1594,001053.025.0kPa 150/k gm 0.29065@2312113113113==⎪⎭⎪⎬⎫===⨯+=+==-⨯+=+=====⎭⎬⎫==g fgffg f fgf g f u u ux uu x uu x P v v v vv vvSubstituting,min60.2==∆⎪⎪⎭⎫ ⎝⎛-=∆s 33613kJ/s1VA1000.03)kJ/kg 980kg)(2569.7(2)A8)(V 110(t t4-39A saturated water mixture contained in a spring-loaded piston-cylinder device is heated until the pressure and volume rise to specified values. The heat transfer and the work done are to be determined.Assumptions 1 The cylinder is stationary and thus the kinetic and potential energy changes are zero. 2 There are no work interactions involved other than the boundary work. 3 The thermal energy stored in the cylinder itself is negligible. 4 The compression or expansion process is quasi-equilibrium. Analysis We take the contents of the cylinder as the system. This is a closed system since no mass enters or leaves. The energy balance for this stationary closed system can be expressed asv)(0)=PE =KE (since )(12ou ,in 12ou ,in energiesetc. potential, k inetic,internal,in Change system massand work ,heat,by nsfer energy tra Net out in u u m W Q u u m U W Q E E E t b t b -+=-=∆=-∆=-The initial state is saturated mixture at 75 kPa. The specific volume and internal energy at this state are (Table A-5),kJ/kg30.553)8.2111)(08.0(36.384/kg m 1783.0)001037.02172.2)(08.0(001037.0131=+=+==-+=+=fgffg f xuuu x v vvThe mass of water iskg22.11/kgm 1783.0m23311===v V mThe final specific volume is/kgm 4458.0kg22.11m53322===m V vThe final state is now fixed. The internal energy at this specific volume and 225 kPa pressure is (Table A-6) kJ/kg 4.16502=u Since this is a linear process, the work done is equal to the area under the process line 1-2:kJ450=⎪⎭⎫ ⎝⎛⋅-+=-+==331221out ,m kPa 1kJ 1)m 2(52)kPa225(75)(2Area V V P P W bSubstituting into energy balance equation giveskJ12,750=-+=-+=kJ/kg )30.553kg)(1650.422.11(kJ 450)(12out ,in u u m W Q b4-43Two tanks initially separated by a partition contain steam at different states. Now the partition is removed and they are allowed to mix until equilibrium is established. The temperature and quality of the steam at the final state and the amount of heat lost from the tanks are to be determined.Assumptions 1 The tank is stationary and thus the kinetic and potential energy changes are zero. 2 There are no work interactions.Analysis (a ) We take the contents of both tanks as the system. This is a closed system since no massenters or leaves. Noting that the volume of the system is constant and thus there is no boundary work, the energy balance for this stationary closed system can be expressed as[][]0)=PE =KE (since )()(1212out energiesetc. potential, k inetic,internal,in Change systemmass and work ,heat,by nsfer energy tra Net out in =-+-=∆+∆=-∆=-W u u m u u m UUQ E E E B A BAThe properties of steam in both tanks at the initial state are (Tables A-4 through A-6)kJ/kg7.2793/kgm 25799.0C 300kPa 1000,13,1,1,1==⎪⎭⎪⎬⎫︒==A A A A u T P v()[]()kJ/kg4.15954.19270.50.66631/kgm 0.196790.0010910.392480.500.001091kJ/kg4.1927,66.631/kgm .392480,001091.050.0C 1501,131,131,1=⨯+=+==-⨯+=+=====⎭⎬⎫=︒=fgfB fg f B fgf gf B ux uu x u u x T v vv vvThe total volume and total mass of the system arekg523m106.1/kg)m 19679.0kg)( 3(/kg)m 25799.0kg)( 2(333,1,1=+=+==+=+=+=B A B B A A B A m m m m m v v V V VNow, the specific volume at the final state may be determined/kgm 22127.0kg5m 106.1332===m Vvwhich fixes the final state and we can determine other properties()kJ/kg8.12821.19820.3641.11561001073.060582.0001073.022127.0/kg m 22127.0kPa0032222k Pa 300 @sat 2322=⨯+=+==--=--=︒==⎪⎭⎪⎬⎫==fg f f g f u x u u x T T P 0.3641C133.5v v v v v(b ) Substituting,[][]kJ 3959kJ/kg )4.15958.1282(kg) 3(kJ/kg )7.27938.1282(kg) 2()()(1212out -=-+-=-+-=∆+∆=-BA BAu u m u u m UUQorkJ3959=out Q4-60The air in a rigid tank is heated until its pressure doubles. The volume of the tank and the amount of heat transfer are to be determined.Assumptions 1 Air is an ideal gas since it is at a high temperature and low pressure relative to its critical point values of -221︒F and 547 psia. 2 The kinetic and potential energy changes are negligible, ∆∆pe ke ≅≅0. 3 Constant specific heats at room temperature can be used for air. This assumption results in negligible error in heating and air-conditioning applications. Properties The gas constant of air is R = 0.3704 psia.ft 3/lbm.R (Table A-1E).Analysis (a3ft80.0=⋅⋅==psia50R)R)(540/lbm ft psia 4lbm)(0.370 (20311P mRT V(b) We take the air in the tank as our system. The energy balance for this stationary closed system can be expressed as)()(1212in in energiesetc. potential, k inetic,internal,in Change system massand work ,heat,by nsfer energy tra Net out in T T mc u u m Q UQ E E E -≅-=∆=∆=-vThe final temperature of air isR1080R) (540211222211=⨯==−→−=T P P T T P T P V VThe internal energies are (Table A-17E)u u u u 12====@@540R 1080R 92.04Btu /lbm 186.93Btu /lbmSubstituting, Q in = (20 lbm)(186.93 - 92.04)Btu/lbm = 1898 BtuAlternative solutions The specific heat of air at the average temperature of T avg = (540+1080)/2= 810 R = 350︒F is, from Table A-2Eb, c v ,avg = 0.175 Btu/lbm.R. Substituting,Q in = (20 lbm)( 0.175 Btu/lbm.R)(1080 - 540) R = 1890 BtuDiscussion Both approaches resulted in almost the same solution in this case.4-64A student living in a room turns her 150-W fan on in the morning. The temperature inthe room when she comes back 10 h later is to be determined.Assumptions 1 Air is an ideal gas since it is at a high temperature and low pressure relative to its critical point values of -141︒C and 3.77 MPa. 2 The kinetic andQpotential energy changes are negligible,∆∆ke pe ≅≅0. 3 Constant specific heats atroom temperature can be used for air. This assumption results in negligible error in heating and air-conditioning applications. 4 All the doors and windows are tightly closed, and heat transfer through the walls and the windows is disregarded.Properties The gas constant of air is R = 0.287 kPa.m 3/kg.K (Table A-1). Also, c v = 0.718 kJ/kg.K for air at room temperature (Table A-2).Analysis We take the room as the system. This is a closed system since the doors and the windows are said to be tightly closed, and thus no mass crosses the system boundary during the process. The energy balance for this system can be expressed as)()(1212,,energiesetc. potential, k inetic,internal,in Change system massand work ,heat,by nsfer energy tra Net T T mc u u m W UW E E E in e in e out in -≅-=∆=∆=-vThe mass of air iskg174.2K)K)(288/kg m kPa (0.287)m kPa)(144 (100m14466433113=⋅⋅===⨯⨯=RT P m V VThe electrical work done by the fan isW W t e e==⨯= ∆(0.15kJ /s)(103600s)5400kJ Substituting and using the c v value at room temperature, 5400 kJ = (174.2 kg)(0.718 kJ/kg ⋅︒C)(T 2 - 15)︒CT 2 = 58.2︒CDiscussion Note that a fan actually causes the internal temperature of a confinedspace to rise. In fact, a 100-W fan supplies a room with as much energy as a 100-W resistance heater.4-69Carbon dioxide contained in a spring-loaded piston-cylinder device is heated. The work done and the heat transfer are to be determined.Assumptions 1 CO 2 is an ideal gas since it is at a high temperature relative to its critical temperature of 304.2 K. 2 The kinetic and potential energy changes are negligible,0pe ke ≅∆≅∆.Properties The properties of CO 2 are R = 0.1889 kJ/kg ⋅K and c v = 0.657 kJ/kg ⋅K (Table A-2a ).PAnalysis We take CO 2 as the system. This is a closed system since no mass crosses the boundaries of the system. The energy balance for this system can be expressed as)(12out ,in energiesetc. potential, k inetic,internal,in Change systemmass and work ,heat,by nsfer energy tra Net out in T T mc U W Q E E E b -=∆=-∆=-vThe initial and final specific volumes are 33111m5629.0k P a100K)K)(298/kg m kPa kg)(0.1889 (1=⋅⋅==P mRT V33222m1082.0kPa1000K)K)(573/kg m kPa kg)(0.1889 (1=⋅⋅==P mRT VPressure changes linearly with volume and the work done is equal to the area underthe process line 1-2:kJ1.250m kPa 1kJ 1)m5629.0(0.10822)kPa 1000(100)(2Area 331221out ,-=⎪⎪⎭⎫⎝⎛⋅-+=-+==V V P P W b Thus,kJ250.1=in ,b WUsing the energy balance equation,kJ4.69K )25K)(300kJ/kg 657.0(kg) 1(kJ 1.250)(12out ,in -=-⋅+-=-+=T T mc W Q b vThus, kJ 69.4=outQ4-76Air at a specified state contained in a piston-cylinder device with a set of stops is heated until a final temperature. The amount of heat transfer is to be determined.Assumptions 1 Air is an ideal gas since it is at a high temperature relative to its critical temperature of 304.2 K. 2 The kinetic and potential energy changes are negligible, 0pe ke ≅∆≅∆. Properties The properties of air are R = 0.287 kJ/kg ⋅K and c v = 0.718 kJ/kg ⋅K (Table A-2a ). Analysis We take air as the system. This is a closedsystem since no mass crosses the boundaries of the system. The energy balance for this system can be expressed as)(12out ,in energiesetc. potential, k inetic,internal,in Change systemmassand work ,heat,by nsfer energy tra Net out in T T mc U W Q E E E b -=∆=-∆=-vThe volume will be constant until the pressure is 300 kPa:K900kPa100kPa 300K)(3001212===P P T TThe mass of the air iskg4646.0K)K)(300/kg m kPa (0.287)m kPa)(0.4 (10033111=⋅⋅==RT P m VThe boundary work done during process 2-3 iskJ04900)K -K)(1200/kg m kPa (0.287)kg 4646.0()()(323232out ,=⋅⋅=-=-=T T mR P W b V VSubstituting these values into energy balance equation,kJ340=-⋅+=-+=K )300K)(1200kJ/kg 718.0(kg) 4646.0(kJ 40)(13out ,in T T mc W Q b v4-85An egg is dropped into boiling water. The amount of heat transfer to the egg by the time it is cooked is to be determined.Assumptions 1 The egg is spherical in shape with a radius of r 0 = 2.75 cm. 2 The thermal properties of the egg are constant. 3 Energy absorption or release associated with any chemical and/or phase changes within the egg is negligible. 4 There are no changes in kinetic and potential energies.Properties The density and specific heat of the egg are given to be ρ = 1020 kg/m 3 and c p = 3.32 kJ/kg.︒C.Analysis We take the egg as the system. This is a closes system since no mass enters or leaves the egg. The energy balance for this closed system can be expressed as)()(1212egg in energiesetc. potential, k inetic,internal,in Change system massand work ,heat,by nsfer energy tra Net out in T T mc u u m U Q E E E -=-=∆=∆=-Then the mass of the egg and the amount of heat transfer becomeBoiling PV (m 3)kJ 21.2=︒-︒=-=====C )880)(C kJ/kg. 32.3)(kg 0889.0()(kg0889.06m)055.0()kg/m1020(612in 333T T mc Q Dm p ππρρV。
工程热力学习题及答案1-5
第1章基本概念1.1 本章基本要求深刻理解热力系统、外界、热力平衡状态、准静态过程、可逆过程、热力循环的概念,掌握温度、压力、比容的物理意义,掌握状态参数的特点。
1.2 本章难点1.热力系统概念,它与环境的相互作用,三种分类方法及其特点,以及它们之间的相互关系。
2.引入准静态过程和可逆过程的必要性,以及它们在实际应用时的条件。
3.系统的选择取决于研究目的与任务,随边界而定,具有随意性。
选取不当将不便于分析。
选定系统后需要精心确定系统与外界之间的各种相互作用以及系统本身能量的变化,否则很难获得正确的结论。
4.稳定状态与平衡状态的区分:稳定状态时状态参数虽然不随时间改变,但是靠外界影响来的。
平衡状态是系统不受外界影响时,参数不随时间变化的状态。
二者既有所区别,又有联系。
平衡必稳定,稳定未必平衡。
5.状态参数的特性及状态参数与过程参数的区别。
1.3 例题例1:绝热刚性容器内的气体通过阀门向气缸充气。
开始时气缸内没有气体,如图1.1所示。
气缸充气后,气体推动气缸内的活塞向上移动,如图1.2所示。
设管道阀门以及气缸均可认为是绝热的。
若分别选取开口系统与闭口系统,试说明它们的边界应该如何划定?这些系统与外界交换的功量与热量又如何?解:(1)若以容器内原有的气体作为分析对象,属于闭口系统。
容器放气前,边界如图1.1中的虚线所示。
放气后边界如图1.2中的虚线所示。
气体对活塞作的功W是闭口系统与外界交换的功量。
气体通过活塞与外界交换的热量Q是此闭口系统的传热量。
图1.1 图1.2图1.3 图1.4(2)若以容器放气后残留在容器内的气体作为分析对象,同样也是闭口系统。
这时放气前的边界如图1.3中的虚线所示。
放气后的边界如图1.4的虚线表示。
残留气体对离开容器的那部分放逸气体所作的功,是本闭口系统与外界交换的功,残留气体与放逸气体之间交换的热量是本系统的传热量。
(3)类似地若以放逸气体为分析对象,同样也是闭口系统。
其边界将如图1.3和图1.4中的点划线所示。
工程热力学04章习题提示与答案

习题提示与答案 第四章 理想气体的热力过程4-1 设气缸中有0.1 kg 二氧化碳,其压力为0.1 MPa 、温度为27 ℃。
如进行一个定压过程,气体对外作功3 kJ 。
设比热容为定值,试求过程中气体热力学能和熵的变化以及气体吸收的热量。
提示:理想气体;Q =ΔU +W ;ΔU =mc V 0ΔT ;12120ln lnp pR T T c s p g Δ−=。
答案:ΔU =10.5 kJ ,ΔS =0.036 11 kJ/K ,Q =13.5 kJ 。
4-2 有一气缸,其中氮气的压力为0.15 MPa 、温度为300 K 。
如果按两种不同的过程变化:(1)在定压下温度变化到450 K ;(2)在定温下压力下降到0.1 MPa 。
然后在定容下变化到0.15 MPa 及450 K 。
设比热容为定值,试求两种过程中热力学能和熵的变化以及从外界吸收的热量。
提示:略。
答案:(1)=111.15 kJ/kg ,=0.421 kJ/(kg ·K),q u Δs Δ1-2=155.7 kJ/kg 。
(2)=111.15 kJ/kg ,=0.421kJ/(kg ·K),q u Δs Δ1-3-2=147.25 kJ/kg 。
4-3 设气缸中空气的压力为0.5 MPa 、温度为600 K ,若经绝热过程膨胀到0.1 MPa ,试求膨胀终了的温度及比体积:(1)按定值比热容计算;(2)按空气的热力性质表进行计算。
提示:(2) 1200ln 12p p R S S g T T +=;依,由热力性质表确定T 02T S 2 及v r2。
答案:(1) T 2=378.8 K ,v 2=1.089 m 3/kg ;(2) T 2=382.6 K ,v 2=1.10 m 3/kg 。
4-4 柴油机吸气终了时气缸中空气的温度为60 ℃、压力为0.1 MPa 。
为使压缩终了时空气温度超过柴油的自燃温度以使其着火,故要求压缩终了的温度至少为720 ℃。
工程热力学第三版电子教案第1章自我测验题

第一章自我测验题1、引人热力平衡态解决了热力分析中的什么问题?准平衡过程如何处理“平衡状态”与“状态变化”的矛盾?准平衡过程的概念为什么不能完全表达可逆过程的概念?2、判断下列过程中,哪些是可逆的?哪些是不可逆的?哪些可以是可逆的?并扼要说明不可逆的原因。
(1)定质量的空气在无摩擦、不导热的气缸和活塞中被慢慢压缩。
(2)100℃的蒸汽流与25℃的水流绝热混合。
(3)在水冷摩托发动机气缸中的热燃气随活整迅速移动而膨胀。
(4)气缸中充有水,水上面有无摩擦的活塞,缓慢地对水加热使之蒸发。
3、如图所示的一圆筒容器,表A的读数为 360 kPa,表 B凌以为 170 kPa大气压力为101300Pa。
试求:①真空室以及 I室和Ⅱ室的绝对压力;②表C的读数;③圆筒顶面所受的作用力。
4、若用摄氏温度计和华氏温度计测量同一个物体的温度,有人认为这两种温度计的读数不可能出现数值相同的情况,对吗?若可能,读数相同的温度应是多少?5、气缸内的气体由容积0.4立方米压缩到0.1立方米,其内部压力和容积的关系p=0.3V+0.04,试求:①气缸作功量②若活塞与气缸间的摩擦力为1kN,活塞面积为O.2平方米时,实际耗功为多少?6、1kg气体经历如图所示的循环,A到B为直线变化过程,B到C为定容过程,C到A为定压过程。
试求循环的净功量。
如果循环为A-C-B-A,则净功量有何变化?第一章自测题答案2、(1)可逆过程;(2)不可逆过程;(3)不可逆过程;(4)可以是可逆的,也可以是不可逆的。
3、(1)P真空室=1.999kPa,P1=362kPa,P2=192kPa ;(2)P g,c=190kPa ;(3)F=15.8kN 。
4、不对,可能出现数值相同的情况,温度为t=-40℃,t=-40°F5、(a)(b)活塞移动的位移为-1.5m,克服摩擦力耗功为-1.5kJ,实际耗功为-36kJ6、循环A-B-C-A的净功量为w=0.5*(V2-V1)(P2-P1)循环A-C-B-A的净功量为w'=-0.5*(V2-V1)(P2-P1)。
工程热力学第三版电子教案教学大纲 (3)
教学大纲课程名称:工程热力学英文译名:Engineering Therodynamics (Architecture type)总学时数:54讲课学时:50(含习题课4)实验学时:8授课对象:建筑环境与设备专业、建材专业本科生课程要求:必修分类:技术基础课开课时间:第三学期主要先修课:高等数学、大学物理、理论力学、材料力学选用教材及参考书教材:采用由我校廉乐明主编,李力能、谭羽非参编的全国建筑暖通专业统编教材、全国高等学校教材《工程热力学》。
本书自1979年出版至今,历经第一版、第二版、第三版和第四版共四次修订,计十二次印刷,在全国发行量达12万余册。
本书曾获国家级教学成果奖教材二等奖、建设部部优教材奖。
主要参考教材:1、清华大学主编、高教出版社出版的《工程热力学》2、西安交通大学主编、高教出版社出版的《工程热力学》3、 Krle C.Potter Craig W .Somerton《Engineering Therodynamics》(1998年版)一、本课程的性质、教学目的及其在教学计划中的地位与作用本课程是研究物质的热力性质、热能与其他能量之间相互转换的一门工程基础理论学科,是建筑环境与设备专业的主要技术基础课之一。
本课程为专业基础课,主要用于提高学生热工基础理论水平,培养学生具备分析和处理热工问题的抽象能力和逻辑思维能力。
为学生今后的专业学习储备必要的基础知识,同时训练学生在实际工程中的理论联系实际的能力。
通过对本课程的学习,使学生掌握有关物质热力性质、热能有效利用以及热能与其它能量转换的基本规律,并能正确运用这些规律进行各种热工过程和热力循环的分析计算。
此外本课程在有关计算技能和实践技能方面也使学生得到一定的训练。
因此本课程不仅是学习后续课程,包括《供热工程》、《空调工程》、《锅炉及锅炉房设备》等主要专业的理论基础外,而且能广泛服务于机械工程、动力工程、冶金、石油、电力工程等各个研究领域。
工程热力学第三版课后习题答案
工程热力学第三版课后习题答案工程热力学第三版课后习题答案【篇一:工程热力学课后答案】章)第1章基本概念⒈闭口系与外界无物质交换,系统内质量将保持恒定,那么,系统内质量保持恒定的热力系一定是闭口系统吗? 答:否。
当一个控制质量的质量入流率与质量出流率相等时(如稳态稳流系统),系统内的质量将保持恒定不变。
⒉有人认为,开口系统中系统与外界有物质交换,而物质又与能量不可分割,所以开口系不可能是绝热系。
这种观点对不对,为什么?答:不对。
“绝热系”指的是过程中与外界无热量交换的系统。
热量是指过程中系统与外界间以热的方式交换的能量,是过程量,过程一旦结束就无所谓“热量”。
物质并不“拥有”热量。
一个系统能否绝热与其边界是否对物质流开放无关。
⒊平衡状态与稳定状态有何区别和联系,平衡状态与均匀状态有何区别和联系?答:“平衡状态”与“稳定状态”的概念均指系统的状态不随时间而变化,这是它们的共同点;但平衡状态要求的是在没有外界作用下保持不变;而平衡状态则一般指在外界作用下保持不变,这是它们的区别所在。
⒋倘使容器中气体的压力没有改变,试问安装在该容器上的压力表的读数会改变吗?在绝对压力计算公式p?pb?pe(p?pb); p?pb?pv(p?pb)中,当地大气压是否必定是环境大气压?答:可能会的。
因为压力表上的读数为表压力,是工质真实压力与环境介质压力之差。
环境介质压力,譬如大气压力,是地面以上空气柱的重量所造成的,它随着各地的纬度、高度和气候条件不同而有所变化,因此,即使工质的绝对压力不变,表压力和真空度仍有可能变化。
“当地大气压”并非就是环境大气压。
准确地说,计算式中的pb 应是“当地环境介质”的压力,而不是随便任何其它意义上的“大气压力”,或被视为不变的“环境大气压力”。
⒌温度计测温的基本原理是什么?答:温度计对温度的测量建立在热力学第零定律原理之上。
它利用了“温度是相互热平衡的系统所具有的一种同一热力性质”,这一性质就是“温度”的概念。
《工程热力学》第四章 习题
4
2)空气膨胀、升温,又吸热
q
n
n 1
cV
T2
T1
0, 且T2
T1
n
n 1
0
n
1或者n
p1v1n
p2v2n
RgT1 v1
v1n
RgT2 v2
v2n
T1v1n1
T2v2n1
T1 T2
vn1 2
vn1ห้องสมุดไป่ตู้1
1
vn1 2
vn1 1
,已知v2
v1
0 n1
5
3)n=1.6的膨胀过程,并判断q、w、△u的正负
n 1
cv
(T2
T1)
3.21kJ
W
mw
2
pdV
1
n
1 1
(
p1V1
p2V2
)
8.58kJ
④ U mcv (T2 T1) 5.36kJ
H mcp (T2 T1) 7.51kJ
S
m(cvIn
T2 T1
Rg In
v2 v1
)
0.0087kJ/K
9
3初态为p101mpat140的空气v10052立方米在气缸中被可逆多变地压缩到p20565mpav20013立方米试求该多变过程的多变指数n压缩后的温度t2过程中空气和外界交换的功量和热量压缩过程中气体热力学能焓和熵的变化
习题
1、在p-v图和T-s图上画出定比热容理想气体的(1) 可逆定容加热过程、可逆定压加热过程;(2)可逆 定温加热过程和可逆绝热膨胀过程。
此过程为放热,对外做功,内能减少
6
4) n=1.3的压缩过程,并判断q、w、△u的正负;
此过程为放热,外界对空气做功,内能增加。
工程热力学(第三版) (沈维道 著) 课后答案
3 3 斜角 α = 30° ,压力计中使用密度 ρ = 0.8 × 10 kg/m 的煤油,斜管中
液柱长度 l=200mm。当地大气压力 pv = 745mmHg 。求烟气的真空 。 度(以 mmH2O 表示)及绝对压力(以 Pa 表示) 解 :倾斜式压力计上读数即烟气的真空度
1-12 有一绝对真空的钢瓶, 当阀门的打开时, 在大气压 p0 = 1.013 × 10 Pa 的作用下有体积为
5
0.1m3 的空气被输入钢瓶,求大气对输入钢瓶的空气所作功为多少?
3
第一章 基本概念
解
W = p0V = 1.013 × 105 Pa × 0.1m3 = 1.013 × 104 J = 10.13kJ
1-8 容器被分隔成 AB 两室,如图 1-19 所示,已知当场大气压 pb = 0.1013MPa ,气压表 2 读为 peB 2 = 0.04MPa ,气压表 1 的读数 peA1 = 0.294MPa , 求气压表 3 的读数(用 MPa 表示) 。 解:
p A = pb + peA1 = 0.1013MPa + 0.294MPa = 0.3953MPa
pb = 755mm ,求容器中的绝对压力(以 MPa 表示) 。如果容器 ′ 中的绝对压力不变,而气压计上水银柱高度为 pb = 770mm ,求此时真空表上的读数(以
mmHg 表示)是多少? 解 :容器中气体压力低于当地大气压力,故绝对压力
p = pb − pv = (755 − 600)mmHg = 155mmHg = 0.0207MPa ′ 若容器中绝对压力不变,而大气压力变为 pb = 770mmHg 。则此时真空表上的读数为 ′ ′ pv = pb − p = (770 − 155)mmHg = 615mmHg
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
第四章自我测验题
1、是非题
(1)在任何情况下,向气体加热,熵一定增加;气体放热,熵总减少。
()
(2)熵增大的过程必为不可逆过程。
()
(3)熵减小的过程是不可能实现的。
()
(4)卡诺循环是理想循环,一切循环的热效率都比卡诺循环的热效率低。
()
(5)把热量全部变为功是不可能的。
(、
(6)若从某一初态经可逆与不可逆两条途径到达同一终态,则不可逆途径的△S必大于可逆途径的△S。
()
2、填充题
(1)大气温度为 300 K,从温度为 1000K的热源放出热量 1O0kJ,此热量的有效能为_________。
(2)度量能量品质的标准是______,据此,机械能的品质________热能的品质________;热量的品质_________功的品质;高温热量的品质___________低温热量的品质。
(3)卡诺循环的热效率=___________;卡诺制冷循环的制冷系数=________。
(4)任意可逆循环的热效率可用平均温度表示,其通式为__________。
(5)请在图上画出可逆过程l-2所吸收热量的热量拥和熵。
(6)图中1-2过程是不可逆绝热膨胀过程,过程中的有效能损失用哪块面积表示
3、一汽车发动机的热效率是18%,燃气温度为950℃,周围环境温度为25℃,这个发动机的工作有没有违反热力学第二定律?
4、两个绝热喷嘴,效率均为95%,喷嘴入口处氮气的压力均为2MPa,入口速度均可忽略,都膨胀到200kPa,每个喷嘴的氮气的质量流率是相同的,但是喷嘴A的进口温度为300℃,喷嘴B的进口温度为400℃。
以热力学角度着哪个喷嘴过程更好?证明并解释你的结论。
5、现有初温分别为T A、T B的两种不可压缩流体,它们的质量与比热容乘积分别为C A、C B,用它们分别作可逆机的有限热源和有限冷源,可逆热机工作到两流体温度相等时为止。
求(1)平衡的的温度;(2)热机作出的最大功量?
6、初态为47℃、200kPa的空气经历一过程达到267℃和800kPa的终态。
假定空气是热物性不变的理想气体,计算下列过程中每单位质量工质熵的变化:①此过程为淮平衡过程;②此过程为不可逆过程;③此过程为可逆过程。
7、用家用电冰箱将1kg、25℃的水制成0℃的冰,试问需要的最少电费应是多少?已知水的Cm =75.5J/(mol*K);冰0℃时的熔解热为6013.5J/mol;电费为0.16元/(kw*h);室温为25℃8、一座功率为 P=750 MW的核动力站,反应堆的温度为 586 K可以利用的河水温度为 293 K,求;
(1)动力站的最大热效率是多少?排放到河水中去的最小热流量是多少?
(2)如果动力站实际热效率为最大值的50%,排放到河水里去的热流量是多少?
(3)如果河水的流量为q V=165 立方米/s,河水的温升是多少?已知河水的比热容为4 180 J /(kg·K),河水密度为 1000 kg/立方米。
9、流率为3kg/s的稳定氮气流(1.4MPa、400℃),在等压可逆流动中向环境(0.1MPa、15℃)放热 8O0 kJ/s,试确定过程中有效能的损失?
10、容量为3立方米的A容器中装有80kPa,27℃的空气,B容器为真空。
若用空气压缩机对 A容器抽真空并向 B容器充气,直到 B容器中空气压力为 640 kPa、温度为 27℃时为止。
假定环境温度为27℃。
求
(1)空压机输入的最小功为多少7
(2)A容器抽真空后,将旁通阀打行使两容器内的气体压力平衡,气体的温度仍保持20℃,该不可逆过程造成气体作功能力损失为多少
11、10kg空气在气轮机中绝热膨胀,p1=600kPa,t1=800℃,p2=100kPa。
若气轮机作功W t=3 980 kJ,大气温度T o=300 K,试求有效能损失。
12、某锅炉用空气预热器吸收排出烟气中的热量来加热进入燃烧室的空气,若烟气的流量为50
000 kg/h,经空气预热器后由315℃降到205℃;空气的流量为46500kg/h,初始温度为37℃。
假定烟气和空气的比热容均为定值,,,环境状态为P o=0.1 MPa、t o=30℃。
求:
(l)烟气的初、终状态的拥参数各为多少?
(2)该传热过程中气体作功能力损失为多少?(分别用有效能法和熵法计算)
(3)假定从烟气向外传热是通过可逆热机实现的,空气的终温为多少?热机发出的功率又为多少?
第四章自测题答案
1、
(1)-(6)题均为“错”
2、
(1)70kJ;(2)能量得拥,高于,低于,高于;
(3);(4);(5)略
3、△S iso=0.001934Q1 ,所以发动机得工作没有违反热力学第二定律
4、在B喷嘴中得过程更好些,因为其有效能损失比A喷嘴小。
6、熵是状态参数,初态一定,则不论什么过程,熵增都相同△s=0.128kJ/(kg*K)
7、最少电费为0.0016元
8、(1)50%,750000kJ/s;(2)1750MJ/s;(3)2.54K
9、有效能损失为368.6kW
10、(1)-499kJ;(2)52944kJ
11、154.2kJ
12、(1)86.8kJ/kg,36.7kJ/kg;(2)49.9kJ/kg;(3)133.5℃,693.1kW。
