Long-term outcomes of hepatocellular carcinoma that underwent chemoembolization for bridgi
肝癌疗效评价mresist标准

肝癌疗效评价mresist标准Liver cancer, also known as hepatocellular carcinoma,is a serious and potentially life-threatening disease that requires effective evaluation and treatment. The evaluation of treatment effectiveness for liver cancer is crucial in order to provide the best possible care for patients and to improve their chances of survival. One of the commonly used standards for evaluating the effectiveness of liver cancer treatment is the mresist standard.The mresist standard is a comprehensive evaluation system that takes into account various factors such as tumor size, tumor response, and overall survival rates. It provides a standardized framework for assessing the effectiveness of different treatment modalities for liver cancer, including surgery, chemotherapy, and radiation therapy. By using the mresist standard, healthcare professionals can make more informed decisions about the most appropriate treatment options for their patients.One of the key aspects of the mresist standard is its focus on tumor response evaluation. This involves assessing the changes in the size and characteristics of the tumor following treatment. By carefully monitoring the tumor response, healthcare professionals can determine whether the treatment is effectively shrinking or controlling the tumor, which is a critical factor in evaluating treatment effectiveness.In addition to tumor response evaluation, the mresist standard also considers overall survival rates as an important measure of treatment effectiveness. By analyzing the survival rates of patients who have undergone different treatment modalities, healthcare professionals can gain valuable insights into the long-term outcomes of these treatments and make more informed recommendations forfuture patients.Furthermore, the mresist standard takes into account the potential side effects and complications associated with different treatment modalities. This is important because it allows healthcare professionals to weigh thepotential benefits of a treatment against the potential risks and make more personalized and patient-centered decisions.Overall, the mresist standard provides a comprehensive and systematic approach to evaluating the effectiveness of liver cancer treatment. By considering factors such as tumor response, overall survival rates, and potential side effects, healthcare professionals can make more informed decisions about the most appropriate treatment options for their patients. This not only improves the quality of care for patients with liver cancer but also contributes to the ongoing advancement of treatment strategies for this challenging disease.。
原发性肝癌诊治要点

有一个SBRTⅡ期试验入组了50例不能手术的 TACE失败的病人,随访2年的局部控制率、 OS、和PFS分别为94.6%,68.7%,33.8%
Kang JK1, Kim MS, Cho CK, et al. Stereotactic body radiation therapy for inoperable hepatocellular carcinoma as a local salvage treatment after incomplete transarterial chemoembolization. Cancer. 2012 Nov 1;118(21):5424-31.
放射治疗 (Radiation therapy)
放疗是恶性肿瘤的基本治疗手段之一,但在20世纪 90年代以前,由于放疗的效果较差,且对肝脏损伤 较大,因此对HCC患者较少进行放疗。
全肝移动条照射
RA
全肝大野照射
RA
同中心放疗
放射治疗 (Radiation therapy)
主要技术:三维适型放疗(3-D conformal RT, 3D-CRT) 调强放疗(intensity-modulated radiation therapy,IMRT) 立体定向放疗(stereotactic body radiation therapy,SBRT) 质子放疗(proton beam therapy)
2.
射频消融(RFA)
peng报道了用RFA一线治疗小于2cm的小肝癌病 人, 共入组了218例,结果CR 98%,中位随访31 个月CR 97%
5年OS:中央型小于2cm的HCC,RFA优于手术 切除,80%VS62% RFS分别为67%和40%
1. Peng ZW1, Lin XJ, Zhang YJ, et al. Radiofrequency ablation versus hepatic resection for the treatment of hepatocellular carcinomas 2 cm or smaller: a retrospective comparative study. Radiology. 2012 Mar;262(3):1022-33.
腹腔镜肝切除术治疗肝癌疗效观察

腹腔镜肝切除术治疗肝癌的疗效观察[摘要] 目的比较腹腔镜肝切除术与开腹肝切除术治疗原发性肝癌的疗效。
方法回顾性分析我科2009年1~12月收治的68例原发性肝癌患者,其中28例行腹腔镜肝切除术(腔镜组),40例行开腹肝切除术(开腹组)。
比较两组患者围手术期情况及术后三年生存率。
结果两组患者均无住院期间死亡。
腔镜组的手术时间高于开腹组,但肛门排气时间、引流管留置时间和住院时间明显低于开腹组(p 0.05)。
结论腹腔镜肝切除术安全可行,较传统开腹肝切除术微创、经济,且远期疗效无明显差异。
[关键词] 腹腔镜;肝切除术;肝细胞肝癌[中图分类号] r735.7 [文献标识码] b [文章编号] 1673-9701(2013)17-0157-02腹腔镜肝切除术一直被看作是高风险手术,但近年随着腔镜技术的进步及切肝设备的革新,腹腔镜肝切除术也随之不断发展和普及。
与传统开腹肝切除术比较,腹腔镜肝切除术在患者围手术期恢复方面存在较大优势,但关于其术后患者长期疗效的报道仍然较少。
为此,我们回顾性比较腹腔镜肝切除术与开腹肝切除术治疗肝癌的长期疗效,评价腹腔镜肝切除术治疗肝癌的应用价值。
1 资料与方法1.1 临床资料从本科2009年1~12月收治的101例肝癌患者中筛选出68例肝癌患者,入选标准:术后病理确诊为肝细胞癌;child-pugh a或b级;身体功能状况karnofsky评分60分以上;肿瘤于肝脏ⅱ、ⅲ、ⅳ、ⅴ、ⅵ段,结节单发且直径小于8 cm;无肝内主血管侵犯及远处转移。
无肝癌手术及tace治疗或化疗史。
其中40例患者拒绝腹腔镜术式而行开腹手术,余28例患者行腹腔镜肝切除术。
两组患者在性别、年龄及肿瘤大小等方面均无统计学差异(p > 0.05),见表1。
1.2 手术方式所有患者采用全身麻醉。
左半肝肿瘤行规则性左肝切除,肝脏边缘或右肝肿瘤行肝脏局部切除术。
腔镜组患者取分腿仰卧位,脐下缘小切口建立co2气腹,气腹压12 mm hg左右,置10 mm trocar 导入30°腹腔镜探查以确定术式。
精细肝脏外科联合快速康复外科理念在肝癌围手术期中的应用

应用 精细 肝脏外 科 理念治 疗肝 癌可 获得精 确 的 肝切 面 ,不仅使 得肝 切缘 满足要 求 ,且避 免 了不 必要 的肝 组织 及血 管 、胆 管损 伤 ,最 大程 度保 护有 功能 的 肝组 织 。我科 应用精 细 肝脏外 科理 念治 疗肝癌 的结 果发 现 :精细 肝切 除组 较 传 统 肝切 除组 标 本 切缘 癌 残 留率更 低 ,在根治 层 面上更具 有优 势 ;且术后 并 发 症发 生率 较低 ,亦 未 增加 患者 住 院 费 用 ,安全 有 效 , 值得 推广 。 2.4 残 余肝 脏功 能的保 护
肝胆 外科 杂志 2012年 2月第 20卷第 1期 Journal ofHepatobiliary Surgery,Vol,20,No.1,Feb.2012
9
精细肝脏外科联合快速 康复外科理念在肝癌 围手术期 中的应用
许 戈 良,荚卫 东
【关键词 】 肝脏外科 ;肝癌 ;手术 【中图分类 号】 R 735.7 【文献标识码 】 C 【文章编号 】 1006-4761(2012)01-0009-03
2024年原发性肝癌的诊断和治疗新策略英文版

2024年原发性肝癌的诊断和治疗新策略英文版New Strategies for the Diagnosis and Treatment of Primary Liver Cancer in 2024 Primary liver cancer, also known as hepatocellular carcinoma (HCC), is a leading cause of cancer-related deaths worldwide. In recent years, advancements in technology and research have led to the development of new strategies for the diagnosis and treatment of HCC.1. Early Detection:Early detection of HCC is crucial for improving patient outcomes. Screening programs for individuals at high risk, such as those with chronic liver disease or hepatitis B and C infections, have been shown to increase early diagnosis rates. Imaging techniques like ultrasound and MRI can help detect tumors at an early stage.2. Biomarker Testing:Biomarker testing plays a key role in the diagnosis of HCC. Alpha-fetoprotein (AFP) is a commonly used biomarker for HCC, but new biomarkers like glypican-3 and osteopontin are being researched for their potential to improve early detection and monitoring of the disease.3. Precision Medicine:Advances in genetic testing and molecular profiling have paved the way for precision medicine in HCC treatment. Targeted therapies, such as sorafenib and lenvatinib, can selectively target cancer cells while minimizing damage to healthy tissue. Personalized treatment plans based on a patient's genetic profile are showing promise in improving treatment outcomes.4. Immunotherapy:Immunotherapy has emerged as a promising approach for the treatment of HCC. Checkpoint inhibitors, such as nivolumab and pembrolizumab, work by enhancing the immune system's ability to recognize and attack cancer cells. Combination therapies involving immunotherapy and targeted agents are being studied for their potential synergistic effects.5. Minimally Invasive Procedures:Minimally invasive procedures, such as radiofrequency ablation and transarterial chemoembolization, are becoming increasingly popular for the treatment of HCC. These procedures offer fewer complications and faster recovery times compared to traditional surgery, making them a preferred option for many patients.6. Multidisciplinary Care:Multidisciplinary care involving a team of specialists, including hepatologists, oncologists, radiologists, and surgeons, is essential for providing comprehensive care to HCC patients. Collaborative decision-making and personalized treatment plans tailored to each patient's unique needs can lead to better outcomes.In conclusion, the field of HCC diagnosis and treatment is rapidly evolving, with new strategies focusing on early detection, precision medicine, immunotherapy, minimally invasive procedures, and multidisciplinary care. These advancements offer hope for improvedoutcomes and quality of life for patients with primary liver cancer in 2024.。
乙型肝炎相关的英语作文

乙型肝炎相关的英语作文Hepatitis B: A Global Health ConcernHepatitis B is a serious viral infection that affects the liver, causing significant health problems worldwide. This infectious disease is a major public health concern, with an estimated 296 million people living with chronic hepatitis B globally. The hepatitis B virus (HBV) can be transmitted through various means, including exposure to infected blood, unprotected sexual contact, and from mother to child during pregnancy or childbirth. The impact of hepatitis B extends beyond the individual, as it can lead to long-term complications such as cirrhosis, liver failure, and hepatocellular carcinoma, which can be debilitating and even life-threatening.The prevalence of hepatitis B varies greatly across different regions of the world. Certain parts of the world, such as sub-Saharan Africa and parts of Asia, have a high prevalence of chronic hepatitis B infection, with more than 8% of the population affected. In contrast, other regions, such as North America and Western Europe, have a lower prevalence, with less than 2% of the population affected. This disparity in disease burden highlights the need for targeted and comprehensive public health interventions to address the globalhepatitis B pandemic.One of the key challenges in the fight against hepatitis B is the lack of universal access to effective prevention, diagnosis, and treatment strategies. Vaccination is a highly effective means of preventing hepatitis B, and the World Health Organization (WHO) recommends that all infants receive the hepatitis B vaccine as part of their routine immunization schedule. However, in many parts of the world, vaccine coverage remains suboptimal, leaving a significant portion of the population vulnerable to infection.In addition to vaccination, early diagnosis and appropriate treatment are crucial in managing hepatitis B. Timely diagnosis allows for the initiation of antiviral therapy, which can suppress viral replication, reduce the risk of liver disease progression, and improve overall health outcomes. However, access to diagnostic testing and treatment is often limited, particularly in resource-limited settings, where the burden of hepatitis B is the highest.Addressing the global hepatitis B epidemic requires a multifaceted approach that encompasses prevention, diagnosis, and treatment. Governments, healthcare systems, and international organizations must work collaboratively to implement comprehensive strategies that address the various barriers to effective hepatitis B control.One such initiative is the WHO's Global Health Sector Strategy on Viral Hepatitis, which aims to eliminate viral hepatitis as a public health threat by 2030. This strategy outlines a set of ambitious goals, including the reduction of new hepatitis B infections by 90% and the provision of treatment to 80% of eligible individuals. To achieve these goals, the strategy emphasizes the importance of strengthening health systems, improving access to vaccines and treatment, and enhancing surveillance and monitoring efforts.At the national level, many countries have developed and implemented their own hepatitis B control strategies, tailored to their specific epidemiological and socioeconomic contexts. These strategies often involve a combination of vaccination programs, screening and diagnostic services, linkage to care, and the provision of antiviral medications. By addressing the unique challenges faced by different populations, these national initiatives can contribute to the global effort to combat hepatitis B.Beyond the realm of public health, the scientific community has played a crucial role in advancing our understanding of hepatitis B and driving the development of new prevention and treatment options. Ongoing research has led to the discovery of novel antiviral agents, improved diagnostic tools, and a better understanding of the virus's biology and pathogenesis. These advancements have the potential to significantly impact the management and control ofhepatitis B in the years to come.In conclusion, hepatitis B remains a significant global health challenge, with millions of people affected worldwide. Addressing this pandemic requires a comprehensive and collaborative approach that encompasses prevention, diagnosis, and treatment. By strengthening health systems, improving access to essential services, and fostering scientific innovation, we can work towards the goal of eliminating hepatitis B as a public health threat. Through concerted efforts at the global, national, and local levels, we can strive to improve the health and well-being of those affected by this devastating disease.。
肝细胞癌伴门静脉左支癌栓形成多学科综合治疗1例报告
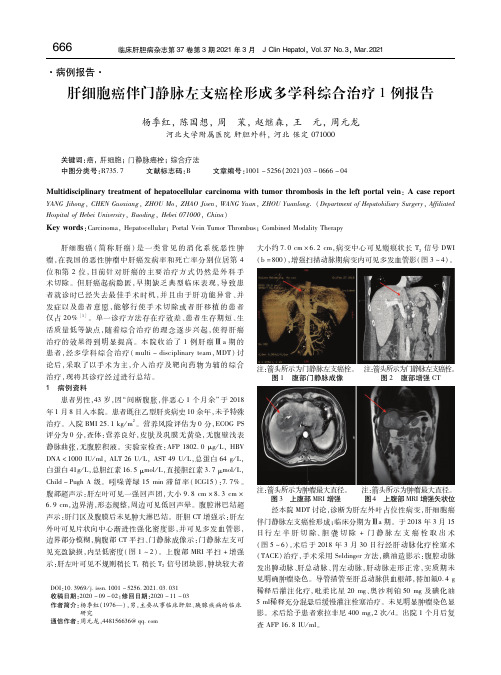
见充盈缺损,内呈低密度(图1 ~ 2)。上腹部MRI 平扫+ 增强
示:肝左叶可见不规则稍长T1 稍长T2 信号团块影,肿块较大者
注: 箭图头3所 示上为腹肿部瘤M最RI大增直强径 。 注图:箭4 头上所腹示部为M肿R瘤I 增最强大矢直状径位。 经本院MDT 讨论,诊断为肝左外叶占位性病变,肝细胞癌 伴门静脉左支癌栓形成;临床分期为Ⅲa 期。于2018 年3 月15 日行左半肝切除、胆囊切除+ 门静脉左支癌栓取出术 (图5 ~6)。术后于2018 年3 月30 日行经肝动脉化疗栓塞术 (TACE)治疗,手术采用Seldinger 方法,碘油造影示:腹腔动脉 发出脾动脉、肝总动脉、胃左动脉,肝动脉走形正常,实质期未
稀释后灌注化疗,吡柔比星20 mg、奥沙利铂50 mg 及碘化油 5 ml稀释充分混悬后缓慢灌注栓塞治疗。未见明显肿瘤染色显 影。术后给予患者索拉非尼400 , mg 2 次/ d。出院1 个月后复 查 。 AFP 16. 8 IU / ml
杨季红,等. 肝细胞癌伴门静脉左支癌栓形成多学科综合治疗1 例报告
续口服瑞戈非尼。2019 年4 月23 日患者主因进行性皮肤及巩
膜黄染7 d 入院。入院总胆红素410. 1 μmol/ L,直接胆红素
315. 3 , μmol / L AFP 454. 2 ng/ ml。上腹部核磁示:肝门处、肝
胃、脾胃间隙多发结节,考虑转移、肝门处胆总管受侵可能,胆总
管下端截断伴胆道梗阻(图11)。拟行经内镜逆行性胰胆管造影
治疗的效果得到明显提高。本院收治了1 例肝癌Ⅲa 期的
患者,经多学科综合治疗(multi - disciplinary , )讨 team MDT
论后,采取了以手术为主,介入治疗及靶向药物为辅的综合 治疗,现将其诊疗经过进行总结。
ALBI和PALBI分级在乙肝相关性肝细胞癌病人预后评估中的价值

[收稿日期]2020-06-25 [修回日期]2020-12-28[基金项目]安徽省高校自然科学研究重点项目(KJ2018ZD022);蚌埠医学院自然科学研究重点项目(BYKY1858ZD);国家级大学生创新创业训练项目(201910367053)[作者单位]蚌埠医学院第一附属医院肝胆外科,安徽蚌埠233004[作者简介]范龙飞(1994-),男,硕士研究生,住院医师.[通信作者]谈 燚,博士,主任医师,教授.E⁃mail:doctortanyi2007@[文章编号]1000⁃2200(2023)03⁃0330⁃05㊃临床医学㊃ALBI 和PALBI 分级在乙肝相关性肝细胞癌病人预后评估中的价值范龙飞,吴斌全,庞 青,汪鹿瑶,潘华东,王春芳,刘会春,谈 燚[摘要]目的:探讨白蛋白-胆红素(ALBI)和血小板-白蛋白-胆红素(PALBI)分级在Child⁃Pugh A 级肝细胞癌(HCC)病人预后评估中的价值㊂方法:行根治性手术的Child⁃Pugh A 级乙肝相关性HCC 病人134例,根据术前血清学检查结果计算ALBI 和PALBI 分级,采用Kaplan⁃Meier 曲线和Cox 回归分析评估病人总生存期(OS)和无复发生存期(RFS)㊂结果:经随访,病死37例,复发35例㊂Kaplan⁃Meier 分析结果显示,ALBI㊁PALBI 分级与OS 和RFS 均具有相关关系(P <0.05)㊂多因素分析显示,ALBI 和PALBI 分级均为病人独立的预后模型㊂不同评分系统间,PLABI +肿瘤直径㊁ALBI +肿瘤直径㊁ALBI +意大利肝癌小组评分(CLIP)较单独的CLIP㊁PLABI㊁ALBI㊁终末期肝癌模型评分㊁巴塞罗那分期在预后预测能力方面更具有意义㊂结论:ALBI 和PALBI 分级是评估Child⁃Pugh A 级HCC 病人预后有价值的模型,如结合肿瘤直径,PALBI 较ALBI 分级有更好的预后价值㊂[关键词]肝细胞肿瘤;白蛋白-胆红素分级;血小板-白蛋白-胆红素分级;预后[中图法分类号]R 735.7 [文献标志码]A DOI :10.13898/ki.issn.1000⁃2200.2023.03.012The value of ALBI and PALBI grades in the prognostic assessmentof hepatitis B virus associated HCC patientsFAN Long⁃fei,WU Bin⁃quan,PANG Qing,WANG Lu⁃yao,PANG Hua⁃dong,WANG Chun⁃fang,LIU Hui⁃chun,TAN Yi(Department of Hepatobiliary Surgery ,The First Affiliated Hospital of Bengbu Medical College ,Bengbu Anhui 233004,China )[Abstract ]Objective :To explore the value of albumin⁃bilirubin (ALBI)and platelet⁃albumin⁃bilirubin (PALBI)grades in the prognostic evaluation of Child⁃Pugh grade A HCC patients.Methods :Clinical data of 134Child⁃Pugh grade A hepatitis B virus associated HCC patients undergoing radical resection were retrospectively analyzed.ALBI and PALBI grades were calculated based onpreoperative serologic examination.Overall survival (OS)and recurrence⁃free survival (RFS)were estimated using Kaplan⁃Meier curves and Cox regression.Results :After follow⁃up,37patients died and 35patients relapsed.Kaplan⁃Meier analysis showed that ALBI and PALBI grades were significantly correlated with OS andRFS (P <0.05).Multivariate analysis indicated that bothALBI and PALBI grades were independent prognostic models for patients.Among different scoring systems,PLABI +tumor diameter,ALBI +tumor diameter,and ALBI +Italian liver cancer group score (CLIP)were more significant in predictingprognosis than CLIP,PLABI,ALBI,end⁃stage liver cancer model score,and Barcelona staging alone.Conclusions :ALBI[6] NAZARPOUR S,RAMEZANI TF,SIMBAR M,et al .Thyroiddysfunction and pregnancy outcomes [J].Iran J Reprod Med,2015,13(7):387.[7] 周玮,黄婷.妊娠期甲状腺疾病监测与处理[J].中国实用妇科与产科杂志,2017,33(3):263.[8] 黄璐,罗丹,王利民,等.成都地区妊娠期特异性甲状腺激素水平参考值范围的探讨及临床分析[J].现代妇产科进展,2016,25(4):269.[9] 卫蔷,张力,刘兴会,等.妊娠不同时期甲状腺功能指标参考值的临床分析[J].中华妇产科杂志,2018,53(5):299.[10] 彭晶晶,陶峰,陈红波,等.妊娠期甲状腺生理及特异性参考值范围建立的现状[J].临床与病理杂志,2020,40(4):1023.[11] 贺淑巍,张晶晶,王旭,等.妊娠期甲状腺激素相关指标的参考区间研究[J].现代妇产科进展,2018,27(8):608.[12] 陈瑶,韩艳,黄锟,等.马鞍山育龄妇女孕早期和孕中期甲状腺激素参考值队列研究[J].中国公共卫生,2018,34(4):469.[13] 杜静,蔺莉,李智,等.不同促甲状腺激素切点值对妊娠早期亚临床甲状腺功能减退症诊断的影响[J].中华医学杂志,2019,99(2):120.[14] 陈赟,马维青,陶存武.合肥市城区居民尿碘水平与甲状腺功能相关性的临床分析[J].中华地方病学杂志,2018,37(4):334.[15] 唐胜利,吴君霞.246例合肥市妊娠中期妇女尿碘水平的分析[J].临床输血与检验,2017,19(5):467.(本文编辑 卢玉清)and PALBI grades are valuable models for evaluating the prognosis of Child⁃Pugh grade A HCC patients,if when they combine with tumor diameter,PALBI grade has better prognostic value for HCC patients than ALBI grade.[Key words]hepatocellular carcinoma;albumin⁃bilirubin grade;platelet⁃albumin⁃bilirubin grade;survival 在世界范围内,肝细胞癌(HCC)是导致癌症死亡的第四大原因,每年约造成782000例病人死亡[1]㊂在HCC高发地区,如中国和撒哈拉以南非洲地区,慢性乙型肝炎病毒(HBV)仍是其主要病因㊂手术切除是目前早期HCC的重要治疗手段,术后病人5年生存率可达70%[2]㊂研究[3]表明,HCC治疗方案的选择与预后和肝功能密切相关㊂Child⁃Pugh 分级A级是HCC根治性手术的重要指征,然而仍有部分病人预后较差[4-5]㊂有学者[6]建立了一种新的㊁简单的肝功能评估模型,称为白蛋白-胆红素(albumin⁃bilirubin,ALBI)分级㊂有研究[7-8]发现, ALBI分级在评估HCC病人肝功能㊁肝切除术后肝衰竭风险及术后生存方面的价值均优于传统的Child⁃Pugh分级㊂然而,ALBI分级却不包括反映肝硬化程度的指标,肝硬化合并门脉高压被认为是影响肝癌病人长期生存的不利因素㊂血小板-白蛋白-胆红素(platelet⁃albumin⁃bilirubin,PALBI)分级系统则把血小板计数作为门脉高压的替代品用来反映肝硬化的程度,其已被证实是一种更理想的肝功能评估模型[9-10]㊂目前有关ALBI和PALBI分级在Child⁃Pugh分级A级HCC病人中的应用尚无报道,本文就2种分级模型在HBV相关性HCC病人预后评估中的应用价值作一探讨㊂现作报道㊂1 对象与方法1.1 研究对象 选取2014年1月至2018年6月入住我院行根治性手术的HCC病人134例,其中男102例,女32例,年龄50~64岁㊂纳入标准:(1)经组织病理学证实为HCC;(2)乙肝表面抗原均呈阳性;(3)首次行根治性肝切除术;(4)Child⁃Pugh分级A级;(5)均予以抗病毒治疗,病毒计数控制在正常范围内㊂排除标准:(1)再次肝切除;(2)合并HCV感染;(3)混合型HCC;(4)肝外转移;(5)术前ALBI㊁PALBI评分无法计算;(6)合并血液病㊁营养不良或其他严重疾病而影响ALBI㊁PALBI评分;(7)术前选择介入㊁化疗㊁靶向药物等治疗方式㊂本研究经我院伦理委员会批准通过㊂1.2 方法 利用电子病历提取病人一般资料,包括年龄㊁性别㊁肿瘤特征(肿瘤数目㊁直径和血管侵犯情况)㊁腹水㊁肝硬化状况㊁意大利肝癌小组评分(CLIP)㊁终末期肝癌模型评分(MELD)㊁巴塞罗那分期(BCLC)㊁病理结果和术前血清学检查,其中血清学检查包括丙氨酸氨基转移酶(ALT)㊁天门冬氨酸氨基转移酶(AST)㊁血清总胆红素(TBIL)㊁白蛋白(ALB)㊁血小板计数(PLT)㊁凝血酶原时间-国际标准化比值(PT⁃INR)和甲胎蛋白(AFP)㊂按照公式计算得出病人术前ALBI和PALBI值㊂(1)ALBI= 0.66×lnTBIL(μmol/L)-0.085×ALB(g/L)㊂ALBI分级:1级,≤-2.60;2级,>-2.60~ -1.39;3级,>-1.39[8]㊂(2)PALBI=2.02×lnTBIL(μmol/L)-0.37×(lnTBIL)2-0.04×ALB (g/L)-3.48×lnPLT(109/L)+1.01×(lnPLT)2㊂PALBI分级:1级,≤-2.53;2级:>-2.53~ -2.09;3级,>-2.09[12]㊂1.3 随访 病人出院后定期随访至2018年12月或死亡㊂随访内容包括CT㊁MRI㊁腹部超声㊁血清学检查,记录术后复发和死亡情况㊂复发病人视情况给予补救治疗,包括再次肝切除㊁消融或经导管动脉化疗栓塞术㊂评估病人总生存期(OS)和无复发生存期(RFS)㊂1.4 统计学方法 采用χ2检验㊁t检验㊁秩和检验和Log⁃rank检验㊁Kaplan⁃Meier生存分析及Cox回归模型分析㊂2 结果2.1 不同临床特征病人ALBI、PALBI分级比较 134例病人中,ALBI分级1级86例(64.2%),2级48例(35.8%);PALBI分级1级83例(61.9%),2级44例(32.8%),3级7例(5.2%)㊂ALT㊁TBIL㊁ALB在不同ALBI分级病人间差异均有统计学意义,AST㊁TBIL㊁ALB㊁PLT和肿瘤直径在不同PALBI 分级病人间差异均有统计学意义(P<0.05~P< 0.01)(见表1)㊂2.2 HCC病人生存分析 经27个月的中位随访后,病死37例(27.6%),术后复发35例(26.1%)㊂HCC病人3年OS中位数为22个月,3年RFS中位数为21个月㊂依据ALBI分级(1级和2级)生存分析,其分级下OS中位数及四分位数间距分别为23 (21)㊁19(20)个月(u c=6.07,P<0.05);RFS中位数及四分位数间距分别为22(21)㊁18(18)个月(u c=8.60,P<0.01),差异均有统计学意义㊂依据PALBI分级(1级和2/3级)生存分析,其OS中位数及四分位数间距分别为24(20)㊁20(20)个月(u c=7.88,P<0.01);其RFS中位数及四分位数间距分别为23(19)和19(16)个月(u c=3.36,P<0.01),差异均有统计学意义㊂表1 相关指标在不同ALBI㊁PALBI分级病人中比较(n)变量ALBI分级 1级(n=86) 2级(n=48) χ2 P PALBI分级 1级(n=83) 2/3级(n=51) χ2 P 性别 男 女662012360.05>0.0567163516 2.54>0.05年龄/岁51.5±10.952.3±11.50.40*>0.0551.5±10.552.2±12.00.33*>0.05 ALT/(U/L)30.7(20.0~47.9)38.7(26.9~91.3) 1.82#<0.0533.0(23.2~47.5)36.0(25.5~82.1)0.94#>0.05 AST/(U/L)32.5(25.4~45.2)39.3(25.6~76.3) 1.15#>0.0532.4(24.4~44.6)42.0(27.8~79.7) 3.17#<0.05 TBIL/(μmol/L)13.1(10.0~16.1)15.7(11.6~20.3) 2.64#<0.0511.8(9.6~14.3)18.5(14.9~24.3) 6.17#<0.01 ALB/(g/L)42.2(40.7~45.2)36.2(34.0~37.2)13.04#<0.0141.7(39.0~44.4)38.1(35.9~41.7) 4.54#<0.01 PT⁃INR 1.06(1.03~1.12)1.09(1.02-1.19) 1.46#>0.051.06(1.02~1.13)1.09(1.03~1.18) 1.57#>0.05 PLT/(109/L) 146(111~190) 120(95-183) 1.15#>0.05 130(96~169) 173(105~235) 3.25#<0.01AFP/(ng/mL) ≥400 <400285012320.95>0.05264814340.47>0.05腹水 是 否10768400.67>0.0510738430.36>0.05肿瘤直径/cm >5 ≤537492721 2.16>0.05325132517.41<0.01肿瘤数目 多发 单发6805430.14>0.056775460.04>0.05血管侵犯 是 否14725430.46>0.051568447 1.94>0.05肝硬化 是 否464029190.60>0.05483527240.31>0.05 *示t值;#示u c值2.3 病人OS和RFS的相关危险因素分析 随访结果显示,HCC病人1㊁2㊁3年OS率分别为83.5%㊁72.9%㊁66.4%,RFS率分别为78.4%㊁65.4%㊁62.1%㊂单因素分析表明,ALBI分级(OR=2.197, 95%CI1.152~4.191)㊁PALBI分级(OR=2.452, 95%CI1.279~4.702)㊁AST(OR=1.967,95%CI 1.025~3.773)㊁AFP(OR=3.187,95%CI1.650~ 60.156)㊁肿瘤直径(OR=6.158,95%CI2.699~ 14.047)㊁肿瘤数目(OR=3.078,95%CI1.351~ 7.014)与HCC病人OS均具有相关性(P<0.01)㊂ALBI(OR=2.261,95%CI1.258~4.062)㊁PALBI (OR=2.194,95%CI1.220~3.945)㊁AST(OR=1.961,95%CI1.084~3.548)㊁TBIL(OR=1.826, 95%CI1.010~3.301)㊁AFP(OR=3.193,95%CI1.760~5.791)㊁肿瘤直径(OR=5.426,95%CI2.678~10.991)㊁肿瘤数目(OR=2.432,95%CI 1.085~5.451)㊁肝硬化(OR=2.056,95%CI 1.079~3.919)与HCC病人RFS均具有相关关系(P<0.05~P<0.01)㊂多因素分析显示,ALBI分级(OR=2.332,95% CI1.171~4.633)㊁PALBI分级(OR=2.343,95%CI 1.191~4.624)和AFP(OR=2.011,95%CI1.001~ 4.012)㊁肿瘤直径(OR=4.892,95%CI2.073~ 11.542)㊁肿瘤数目(OR=2.783,95%CI1.202~6.473)均为OS的独立影响因素(P<0.05~P< 0.01);ALBI分级(OR=2.392,95%CI1.231~4.624)㊁PALBI分级(OR=2.460,95%CI1.131~5.382)㊁AFP(OR=2.401,95%CI1.272~4.521)㊁肿瘤直径(OR=4.391,95%CI2.112~9.113)㊁肿瘤数目(OR=2.931,95%CI1.193~7.241)均为RFS的独立影响因素(P<0.05~P<0.01)㊂2.4 ALBI㊁PALBI分级与BCLC㊁CLIP分期和MELD对预后评价能力比较 基于C指数评价生存模型依次为:CLIP(C指数0.699,95%CI0.594~ 0.804)㊁PALBI(C指数0.671,95%CI0.565~ 0.776)㊁BCLC(C指数0.670,95%CI0.565~ 0.775)㊁ALBI(C指数0.650,95%CI0.542~ 0.758)㊁MELD(C指数0.477,95%CI0.366~ 0.588)㊂本研究中病人均为ALBI分级1~2级,无BCLC0期病人,因此将ALBI分级纳入原BCLC分期(BCLC⁃ALBI),得出其与BCLC分期的C指数相同㊂而与CLIP评分相比,将ALBI分级纳入原CLIP 评分(CLIP⁃ALBI)在预测生存率方面具有更高的准确性㊂结合肿瘤直径后,ALBI+肿瘤直径(C指数0.754,95%CI0.664~0.844)和PALBI+肿瘤直径(C指数0.762,95%CI0.675~0.849)在评估预后价值方面较BCLC㊁BCLC⁃ALBI㊁CLIP及CLIP⁃ALBI 等分期系统更佳㊂3 讨论 HCC预后不仅取决于肿瘤负荷,还取决于肝脏的功能储备[5]㊂世界范围内,Child⁃Pugh分级被广泛用于评估HCC病人的肝功能损害程度㊁治疗方案的选择和预后的评估[2,4,11]㊂近年有学者[12-13]提出Child⁃Pugh分级的应用缺陷㊂首先,Child⁃Pugh分级中所包括的腹水和血清白蛋白两个因素是相互关联的;其次,腹水和肝性脑病评分的主观性较强,可能影响肝功能评估的准确性[14];此外,在Child⁃Pugh 分级中,5个变量具有相同的权重㊂根据HCC临床诊治指南,推荐Child⁃Pugh A级的病人行肝切除术[2,4],而部分病人预后仍然不佳[5]㊂JOHNSON 等[6]总结了1313例HCC病人,建立了肝功能ALBI 分级㊂ALBI分级只包含血清TBIL和ALB两个因素,有研究[7-8,15]表明ALBI分级比Child⁃Pugh分级具有更高的预后判断价值㊂ALBI分级也被推荐替代BCLC分期㊁CLIP分期在肝癌的应用,另外, PALBI分级在评价HCC肝脏储备功能和预后方面也优于Child⁃Pugh分级[9-10,16]㊂根据ALBI分级,Child⁃Pugh A级的HCC病人可分为预后明显不同的2组[6]㊂本研究将Child⁃Pugh A级HCC病人分为ALBI1级组和2级组以及PALBI1级组和PALBI2~3级组㊂我们发现, Child⁃Pugh A级HCC病人行根治性切除,术前较高ALBI分级㊁PALBI分级病人的生存率明显低于较低分级的病人;COX单因素及多因素分析显示,不仅ALBI分级㊁PALBI分级与HCC病人的预后有关, AFP㊁肿瘤直径㊁肿瘤数目也可以影响HCC病人的OS及RFS㊂提示其可以作为Child A级HCC病人预后的预测模型,当然其潜在的机制尚不清楚㊂目前关于比较ALBI㊁PALBI㊁MELD㊁CLIP和BCLC分期评价预后能力的研究较少,目前研究中,肿瘤直径与ALBI,特别是与PALBI结合,在生存预测方面较MELD㊁BCLC和CLIP评分系统有更好表现[23]㊂ALBI或PALBI分级较低㊁肿瘤体积较小的病人预后明显较好㊂本研究中,与BCLC⁃ALBI和CLIP⁃ALBI相比,肿瘤直径与ALBI和PALBI的简单组合显示出相似的C指数,这表明它们都可以用于评估Child⁃Pugh A级HCC病人的预后㊂HCC病人的ALBI分级和PALBI分级越高,提示OS及RFS越差㊂肿瘤的生长需要消耗机体的营养,而白蛋白作为机体的一项营养指标,临床上常被用来评估机体的营养状况㊂近来有研究[17]表明,低白蛋白血症与HCC等多种肿瘤的进展和预后密切相关㊂血小板作为参与凝血机制过程中的一份子,研究[20-22]发现,PLT与HCC的预后密切相关㊂血小板可以通过产生多种炎症因子(血管内皮生长因子㊁血小板衍生生长因子等)来刺激肿瘤的生长,血小板也可通过释放血小板衍生的介质促进HCC的进展㊁血管生成和转移[19]㊂这与本研究发现PALBI分级+肿瘤直径(C指数0.762)㊁ALBI分级+肿瘤直径(C指数0.752)较单独的PALBI分级(C指数0.671)㊁ALBI分级(C指数0.650)在预后预测方面更具价值㊂血清TBIL作为反映肝细胞损伤的敏感指标之一㊂有研究[18]系统比较了肝功能各指标的预后价值,发现血清ALB和TBIL是较理想的两个预后因素,这些与本研究发现2个分级中的ALB㊁TBIL和血小板,均与HCC的发生㊁发展和预后密切相关㊂本研究具有一定局限性,首先,本研究是一项单中心回顾性研究,样本量相对较小,需要多中心前瞻性研究的进一步验证;其次,本研究仅纳入了接受根治性肝切除的HCC病人,需进一步研究探讨ALBI 和PALBI分级在接受其他治疗的HCC病人中的预后指导价值㊂最后,ALBI分级㊁ALBI分级仅依赖血小板㊁白蛋白㊁胆红素3种临床指标,其他可能影响这3种指标变化的因素都可能造成ALBI等级和PALBI等级的偏差㊂最近也有报道[24]指出术后ALBI分级可能比术前分级更有意义,我们纳入的大多数病人尚缺少动态监测的数据㊂总之,对Child⁃Pugh分级A级HCC病人,ALBI和PALBI分级是简单㊁有价值的预后模型㊂[参考文献][1] BRAY F,FERLAY J,SOERJOMATARAM I,et al.Global cancerstatistics2018:GLOBOCAN estimates of incidence and mortalityworldwide for36cancers in185countries[J].CA Cancer J Clin,2018,68(6):394.[2] European Association for the Study of the Liver.EASL ClinicalPractice Guidelines:Management of hepatocellular carcinoma[J].J Hepatol,2018,69(1):182.[3] KULIK L,EL⁃SERAG HB.Epidemiology and management ofhepatocellular carcinoma[J].Gastroenterology,2019,156(2):477.[4] 中华人民共和国卫生和计划生育委员会医政医管局.原发性肝癌诊疗规范(2017年版)[J].中华肝脏病杂志,2017,25(12):886.[5] RAHBARI NN,MEHRABI A,MOLLBERG NM,et al.Hepatocellular carcinoma:current management and perspectivesfor the future[J].Ann Surg,2011,253(3):453. [6] JOHNSON PJ,BERHANE S,KAGEBAYASHI C,et al.Assessment of liver function in patients with hepatocellularcarcinoma:a new evidence⁃based approach⁃the ALBI grade[J].JClin Oncol,2015,33(6):550.[7] CHAN AW,CHONG CC,MO FK,et al.Applicability of albumin⁃bilirubin⁃based Japan integrated staging score in hepatitis B⁃associated hepatocellular carcinoma[J].J Gastroenterol Hepatol,2016,31(10):1766.[8] ZOU H,YANG X,LI QL,et al.A comparative study of albumin⁃bilirubin score with child⁃pugh score,model for end⁃stage liverdisease score and indocyanine green R15in predictingposthepatectomy liver failure for hepatocellular carcinoma patients[J].Dig Dis,2018,36(3):236.[9] LIU PH,HSU CY,HSIA CY,et al.ALBI and PALBI gradepredict survival for HCC across treatment modalities and BCLCstages in the MELD Era[J].J Gastroenterol Hepatol,2017,32(4):879.[10] LUO HM,ZHAO SZ,LI C,et al.Preoperative platelet⁃albumin⁃bilirubin grades predict the prognosis of patients with hepatitis Bvirus⁃related hepatocellular carcinoma after liver resection:aretrospective study[J].Medicine(Baltimore),2018,97(12):e0226.[11] REBONATO A,GRAZIOSI L,MAIETTINI D,et al.Inflammatorymarkers as prognostic factors of survival in patients affected byhepatocellular carcinoma undergoing transarterialchemoembolization[J].Gastroenterol Res Pract,2017,2017:4164130.[12] APLAN DE,DAI F,AYTAMAN A,et al.Development andperformance of an algorithm to estimate the child⁃turcotte⁃pughscore from a national electronic healthcare database[J].ClinGastroenterol Hepatol,2015,13(13):2333.[13] XAVIER SA,VILAS⁃BOAS R,BOAL CARVALHO P,et al.Assessment of prognostic performance of Albumin⁃Bilirubin,Child⁃Pugh,and Model for End⁃stage Liver Disease scores inpatients with liver cirrhosis complicated with acute uppergastrointestinal bleeding[J].Eur J Gastroenterol Hepatol,2018,30(6):652.[14] DURAND F,VALLA D.Assessment of prognosis of cirrhosis[J].Semin Liver Dis,2008,28(1):110.[15] WANG YY,ZHONG JH,SU ZY,et al.Albumin⁃bilirubin versusChild⁃Pugh score as a predictor of outcome after liver resection forhepatocellular carcinoma[J].Br J Surg,2016,103(6):725.[16] HO CHM,CHIANG CL,LEE FAS,et parison of platelet⁃albumin⁃bilirubin(PALBI),albumin⁃bilirubin(ALBI),andchild⁃pugh(CP)score for predicting of survival in advanced hccpatients receiving radiotherapy(RT)[J].Oncotarget,2018,9(48):28818.[17] GUPTA D,LIS CG.Pretreatment serum albumin as a predictor ofcancer survival:a systematic review of the epidemiologicalliterature[J].Nutr J,2010,9:69.[18] D′AMICO G,GARCIA⁃TSAO G,PAGLIARO L.Natural historyand prognostic indicators of survival in cirrhosis:a systematicreview of118studies[J].J Hepatol,2006,44(1):217. [19] GAY LJ,FELDING⁃HABERMANN B.Contribution of platelets totumour metastasis[J].Nat Rev Cancer,2011,11(2):123. [20] AU KP,CHAN SC,CHOK KS,et al.Child⁃Pugh parameters andplatelet count as an alternative to ICG test for assessing liverfunction for major hepatectomy[J].Hpb Surg,2017,2017:2948030.[21] BIHARI C,RASTOGI A,SHASTHRY SM,et al.Plateletscontribute to growth and metastasis in hepatocellular carcinoma[J].Apmis,2016,124(9):776.[22] PANG Q,QU K,ZHANG JY,et al.The prognostic value ofplatelet count in patients with hepatocellular carcinoma:asystematic review and meta⁃analysis[J].Medicine(Baltimore),2015,94(37):e1431.[23] HO SY,HSU CY,LIU PH,et al.Albumin⁃bilirubin(ALBI)grade⁃based nomogram to predict tumor recurrence in patientswith hepatocellular carcinoma[J].Eur J Surg Oncol,2019,45(5):776.[24] AMISAKI M,UCHINAKA E,MORIMOTO M,et al.Post⁃operativealbumin⁃bilirubin grade predicts long⁃term outcomes among Child⁃Pugh grade A patients with hepatocellular carcinoma after curativeresection[J].Hepatobiliary Pancreat Dis Int,2018,17(6):502.[25] FAITOT F,ALLARD MA,PITTAU G,et al.Impact of clinicallyevident portal hypertension on the course of hepatocellularcarcinoma in patients listed for liver transplantation[J].Hepatology,2015,62(1):179.(本文编辑 姚仁斌)。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Submit a Manuscript: https:// World J Gastroenterol 2019 October 7; 25(37): 5687-5701 DOI: 10.3748/wjg.v25.i37.5687ISSN 1007-9327 (print) ISSN 2219-2840 (online)ORIGINAL ARTICLE Prospective StudyLong-term outcomes of hepatocellular carcinoma that underwent chemoembolization for bridging or downstagingBreno Boueri Affonso, Francisco Leonardo Galastri, Joaquim Mauricio da Motta Leal Filho, Felipe Nasser, Priscila Mina Falsarella, Rafael Noronha Cavalcante, Marcio Dias de Almeida,Guilherme Eduardo Gonçalves Felga, Leonardo Guedes Moreira Valle, Nelson WoloskerORCID number: Breno Boueri Affonso (0000-0002-2940-9016); Francisco Leonardo Galastri(0000-0001-9599-3778); Joaquim Mauricio da Motta Leal Filho (0000-0001-9844-6833); Felipe Nasser (0000-0002-3259-7142); Priscila Mina Falsarella (0000-0003-3063-9174); Rafael Noronha Cavalcante(0000-0002-1000-7009); Marcio Dias de Almeida (0000-0002-5312-0191); Guilherme Eduardo Gonçalves Felga (0000-0002-0676-4867); Leonardo Guedes Moreira Valle(0000-0001-6255-340X); Nelson Wolosker (0000-0003-1991-3507). Author contributions: da Motta Leal Filho JM and Nasser F designed research/study; Affonso BB, Galastri FL, da Motta Leal Filho JM, Nasser F, Falsarella PM, Cavalcante RN, and Wolosker N performed research/study; Affonso BB, Galastri FL, Falsarella PM, de Almeida MD, Felga GEG, Valle LGM, and Wolosker N contributed important reagents; Affonso BB, Galastri FL, Nasser F, da Motta Leal Filho JM, Falsarella PM, de Almeida MD, and Valle LGM collected data; Affonso BB, Galastri FL, da Motta Leal Filho JM, Cavalcante RN and Falsarella PM analyzed data; Affonso BB and Valle LGM submit a manuscript; da Motta Leal Filho JM and Cavalcante RN wrote the paper. Institutional review board statement: Process CEP/Einstein nº11/1704.Informed consent statement: All Breno Boueri Affonso, Francisco Leonardo Galastri, Joaquim Mauricio da Motta Leal Filho, Felipe Nasser, Priscila Mina Falsarella, Rafael Noronha Cavalcante, Leonardo Guedes Moreira Valle, Department of Interventional Radiology, Hospital Israelita Albert Einstein, São Paulo 05651-901, São Paulo, BrazilMarcio Dias de Almeida, Guilherme Eduardo Gonçalves Felga, Department of Liver Transplant, Hospital Israelita Albert Einstein, São Paulo 05651-901, São Paulo, BrazilNelson Wolosker, Department of Vascular Surgery, Hospital Israelita Albert Einstein, São Paulo 05651-901, São Paulo, BrazilCorresponding author: Breno Boueri Affonso, PhD, Medical Assistant, Research Scientist, Staff Physician, Surgeon, Teacher, Interventional Radiologist, Department of Interventional Radiology, Hospital Israelita Albert Einstein, Av. Albert Einstein, 627/701, São Paulo 05651-901, São Paulo, Brazil. breno.affonso@einstein.brTelephone: +55-11-982625115Fax: +55-11-21510434AbstractBACKGROUNDProspective study of 200 patients with hepatocellular carcinoma (HCC) that underwent liver transplant (LT) after drug-eluting beads transarterial chemoembolization (DEB-TACE) for downstaging versus bridging. Overall survival and tumor recurrence rates were calculated, eligibility for LT, time on the waiting list and radiological response were compared. After TACE, only patients within Milan Criteria (MC) were transplanted. More patients underwent LT in bridging group. Five-year post-transplant overall survival, recurrence-free survival has no difference between the groups. Complete response was observed more frequently in bridging group. Patients in DS group can achieve post-transplant survival and HCC recurrence-free probability, at five years, just like patients within MC in patients undergoing DEB-TACE.AIMTo determine long-term outcomes of patients with HCC that underwent LT after DEB-TACE for downstaging vs bridging.METHODSProspective cohort study of 200 patients included from April 2011 through Junestudy participants, or their legal guardian, provided written consent prior to study enrollment. Conflict-of-interest statement: Breno Affonso has no conflict of interest.Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: /licen ses/by-nc/4.0/Manuscript source: Unsolicited manuscriptReceived: June 1, 2019Peer-review started: June 3, 2019 First decision: July 21, 2019 Revised: August 30, 2019 Accepted: September 11, 2019 Article in press: September 11, 2019 Published online: October 7, 2019 P-Reviewer: Allam N, Chiu KW, Cholongitas E, Makisalo HS-Editor: Yan JPL-Editor: AE-Editor: Li X 2014. Bridging group included patients within MC. Downstaging group (out of MC) was divided in 5 subgroups (G1 to G5). Total tumor diameter was ≤ 8 cm for G1, 2, 3, 4 (n = 42) and was > 8 cm for G5 (n = 22). Downstaging (n = 64) and bridging (n = 136) populations were not significantly different. Overall survival and tumor recurrence rates were calculated by the Kaplan-Meier method. Additionally, eligibility for LT, time on the waiting list until LT and radiological response were compared.RESULTSAfter TACE, only patients within MC were transplanted. More patients underwent LT in bridging group 65.9% (P = 0.001). Downstaging population presented: higher number of nodules 2.81 (P = 0.001); larger total tumor diameter 8.09 (P = 0.001); multifocal HCC 78% (P = 0.001); more post-transplantation recurrence 25% (P = 0.02). Patients with maximal tumor diameter up to 7.05 cm were more likely to receive LT (P = 0.005). Median time on the waiting list was significantly longer in downstaging group 10.6 mo (P = 0.028). Five-year post-transplant overall survival was 73.5% in downstaging and 72.3% bridging groups (P = 0.31), and recurrence-free survival was 62.1% in downstaging and 74.8% bridging groups (P = 0.93). Radiological response: complete response was observed more frequently in bridging group (P = 0.004).CONCLUSIONTumors initially exceeding the MC down-staged after DEB-TACE, can achieve post-transplant survival and HCC recurrence-free probability, at five years, just like patients within MC in patients undergoing DEB-TACE.Key words: Hepatocellular carcinoma; Down-staging; Liver transplantation; Local regional therapy; Bridging©The Author(s) 2019. Published by Baishideng Publishing Group Inc. All rights reserved. Core tip: The great finding of this work was that through a homogeneous technique of hepatic chemoembolization with drug eluting beads, it was possible to perform the procedures controlling the drug delivery and end point. In conclusion, as far as the degree of tumor necrosis as well as in relation to survival, there was no difference between the group within the Milan criteria (bridging) and the group outside the criteria (downstaging). Therefore, it is worth investing in the treatment of patients out of the Milan criteria so that they have a survival with the same expectations of the patients in criterion.Citation: Affonso BB, Galastri FL, da Motta Leal Filho JM, Nasser F, Falsarella PM, Cavalcante RN, de Almeida MD, Felga GEG, Valle LGM, Wolosker N. Long-term outcomes of hepatocellular carcinoma that underwent chemoembolization for bridging or downstaging. World J Gastroenterol 2019; 25(37): 5687-5701URL: https:///1007-9327/full/v25/i37/5687.htmDOI: https:///10.3748/wjg.v25.i37.5687INTRODUCTIONHepatocellular carcinoma (HCC) is the sixth leading cause of cancer and the third leading cause of cancer death worldwide. It is the number one oncologic cause of death in cirrhotic patients, with approximately one million deaths/year[1,2]. According to the Barcelona Clinic Liver Cancer (BCLC) staging classification, liver resection (LR), radiofrequency ablation (RFA), and liver transplantation (LT) are potentially curative treatments for HCC[3,4].LT is a well-established modality for curative treatment of HCC because it removes the tumor, while excluding the cirrhotic environment, which could lead to the emergence of new malignant lesions[5]. Success rates of LT as a curative treatment are attributed to improved candidate selection using restrictive criteria based on number and tumor size, among which the most frequently used is the Milan criteria (MC)[6]. InAffonso BB et al. DEB-TACE response before liver transplantationAffonso BB et al. DEB-TACE response before liver transplantation centers that frequently perform LT, the 5-year post-transplant patient survival can achieve 75%-80%[7].According to the BCLC, transarterial chemoembolization (TACE) is indicated as palliative treatment in patients with intermediate HCC (BCLC B). However, over the last several years, TACE has being indicated as downstaging (reduction in the size of tumor using locoregional therapies (LRT) in selected patients to meet acceptable criteria for LT)[7,8] and bridging (neo-adjuvant therapy attempt to avoid HCC growth while the patient is waiting for transplantation)[9]. Nevertheless, there is a lack of consistent data on radiological response, overall and recurrence-free survival after transplantation in this heterogeneous group of patients.Drug-eluting beads (DEB-TACE) is a technology that has been developed to enhance tumor drug delivery and reduce systemic availability and toxicity. DEB-TACE loaded with doxorubicin is a safe and effective palliative treatment for HCC and offers clinical benefit to patients with more advanced disease[10]. Another benefit of this technology is that it allows for standardization of the chemoembolization technique, since it is possible to estimate the amount of drug delivered to each tumor. The purpose of this study was to compare the long-term outcomes of patients that underwent LT after DEB-TACE for downstaging versus bridging. Also, we aimed to investigate radiological tumor response after the first DEB-TACE session in both groups.MATERIALS AND METHODSThis study was a single-institution, prospective, cohort study, conducted at the Department of Interventional Radiology and approved by the research ethics committee (SGPP155711/CEP11/1704–CAAE0199.0.028.000-11). All patients signed an informed consent form and the study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki.PatientsThe present study included 200 consecutive patients with HCC, from April 1, 2011 to June 30, 2014, who underwent DEB-TACE at our institution using the outpatient treatment protocol previously described[11]. These patients were part of the liver transplant program and were divided into two groups: Bridging and downstaging (Figure 1). At that time, precise criteria for HCC downstaging related to the sum of the maximal tumor diameters were unclear; therefore, we included in the downstaging group all patients out of the MC[6], without vascular invasion based on cross-sectional magnetic resonance imaging (MRI) or computed tomography (CT) and without lymph node involvement by tumor or extra-hepatic tumor spread. Consistent with Yao et al[8], we classified the downstaging patients into 5 groups, as summarized in Table 1. It was identified a subgroup never before described, Group 4 which has a low tumor volume (less than 8 cm) but with 2 or 3 lesions above 5 cm. Patients who were within MC[6] or T2 of the United Network for Organs Sharing were classified as bridging group (Table 1) and were divided into 3 groups, Group 1 = one tumor; Group 2 = two tumors; and Group 3 = three tumors.Diagnostic imagingAll patients underwent multiphasic abdomen CT (Aquilion One 320, Toshiba, Tokyo, Japan, Aquillon 64, Toshiba, Tokyo, Japan-Aquilion Vision 640 Toshiba, Tokyo, Japan) or MRI (GE 2 HDXT-1.5T, General Electric, Boston, Massachusetts, United States and Siemens Espree-1.5T, Siemens AG, Berlin, Germany). The overwhelming majority of the imaging examinations were performed with MRI. All images were acquired using the following parameters: 120kV voltage; tube current (sure exposure 3D SD 10.00, Max 500 Min 100 mAs, reconstruction slice thickness: 1 or 3 mm - depending on the acquired sequence). Patients submitted to abdominal CT received an intravenous bolus injection of 1.7 mL of contrast/kg body weight of the non-ionic iodinated contrast agent Henetix® 350mg I/mL, Guerbet-Rio de Janeiro, Brazil (350 mg I/mL Iobitridol). Patients who underwent abdominal MRI received intravenous bolus injection of 0.2 mL/kg patient weight of the paramagnetic contrast agent Magnevistam®, Bayer-Leverkusen, Germany (469 mg/mL Dimeglumine Gadopentetate), both with injection rate of 3 to 4 mL/s. HCC diagnosis for a lesion ≥ 1 cm was based on either CT or MRI demonstrating arterial phase enhancement and washout during the delayed images, according to the American Association for the Study of Liver Diseases guidelines[12,13]. Hepatic nodules < 1 cm were not counted as HCC. Percutaneous biopsy was not routinely performed.EligibilityAffonso BB et al. DEB-TACE response before liver transplantationTable 1 Downstaging and bridging protocolMC: Milan criteria; UNOS: United Network for Organs Sharing; HCC: Hepatocellular carcinoma; MRI: Magnetic resonance imaging; CT: Computed tomography.Criteria for downstaging failure and exclusion from liver transplant are summarizedin Table 1. There was no time limit or DEB-TACE session limit for completingdownstaging. Eligibility for LT and time on the waiting list until LT were comparedbetween the downstaging and bridging groups.DEB-TACE protocolDEB-TACE protocol was previously described by Nasser et al[11] and Cavalcante etal[14]. Briefly, DEB-TACE procedures were performed under local anesthesia withlidocaine 2%, sedation and analgesia, with venous administration of midazolam andfentanyl.Using a unilateral femoral artery approach, diagnostic angiograms of the superiormesenteric, celiac trunk, and common hepatic artery were performed with thepurpose of outlining the hepatic artery anatomy, delineate the tumor, identify itsfeeding vessels, and evaluate portal vein patency. In each DEB-TACE session, feedingvessels were catheterized with a 2.8F microcatheter (Progreat, Terumo, Japan), andembolization of the tumors was performed with injection of iodinated contrastmedium mixed with one vial of DC-BEAD 100-300μm (Biocompatibles, UnitedKingdom) or HepaSphere 50-100 μm (Merit Medical Systems, United States) loadedwith 50 mg of Doxorubicin. If the endpoint was not achieved after the injection ofloaded beads, additional bland beads (Beadblock, Biocompatibles, United Kingdom orContour, Boston, United States) were injected until the endpoint (complete stasis) wasreached. For patients with more than one tumor, DEB-TACE began by the largestnodule to reach the smallest tumor, regardless of how many sessions were required[11].Vascular lake phenomenon was defined as a localized pooling of contrast mediawithin the tumor, which persists in the venous phase of angiography, resemblingextravasation[14].Radiological responseTumor response was assessed through imaging studies (contrast-enhanced MRI ormultiphasic abdomen CT) and performed 30-45 d after DEB-TACE, according to themodified response evaluation criteria in solid tumors (mRECIST) guidelines[15], asfollows: (1) Target lesion response: response of the treated nodules was evaluated byFigure 1 Flowchart describing the outcome of the 200 patients enrolled in the study.comparing the baseline sum of diameters of target lesions before DEB-TACE with the sum of diameters of viable target lesions after DEB-TACE in each patient; (2)Complete response (CR) was defined as disappearance of any intra-tumoral enhancement in all target lesions; Partial response (PR) was defined as at least a 30%decrease in the sum of diameters of viable target lesions; Stable disease (SD) was defined as any case that does not qualify for either PR or progressive disease; Disease progression was defined as an increase of at least 20% in the sum of the diameters of viable target lesions; (3) Objective response (OR) rate was defined as the sum of CR and PR.SurvivalFive-year post-transplant overall survival and recurrence-free survival were evaluated and compared between the two groups.Statistical analysisStatistical analysis was performed with SPSS version 15.0 (IBM, Armonk, NY, United States). Quantitative characteristics were described by group (bridging and downstaging) before and after transplantation using summary measures (mean,standard deviation, median, minimum and maximum) and compared with Student's t -tests or Mann-Whitney tests. Qualitative characteristics were described by group (bridging and downstaging) before and after transplantation. Associations were tested with chi-square tests or exact tests (Fisher's exact test or likelihood ratio test).Overall survival and recurrence-free survival were estimated using bivariate Cox regression and multivariate Cox regression to verify the influence of significant characteristics on survival. Overall survival and recurrence-free survival were evaluated, by group, using the Kaplan-Meier method. Receiver operating characteristic (ROC) curve was generated to identify maximal tumor diameter most associated with liver transplant in the downstaging group. P value of 0.05 or less was considered significant.RESULTSBaseline characteristicsA total of 200 patients were enrolled during the inclusion period: 64 in downstaging group and 136 in bridging group. Three patients could not perform DEB-TACE and were excluded. Two patients were excluded as a result of hepatic artery dissection during the procedure. One patient was excluded because she presented withAffonso BB et al. DEB-TACE response before liver transplantationAffonso BB et al. DEB-TACE response before liver transplantationrespiratory failure after sedation and the procedure was interrupted beforeembolization (Figure 1).The groups did not significantly differ in terms of age, gender, etiology of liverdisease, Child score, or baseline alpha-fetoprotein levels. At presentation, thedownstaging population presented a greater number of nodules, median of 2.81nodules vs 1.47 (P = 0.001); increased total tumor diameter 8.09 vs 3.73 (P = 0.001);increased multifocal HCC 78% vs 34.6% of samples (P = 0.001); and increased vascularlake phenomenon 34.3% vs 12.5% (P = 0.001) (Table 2).Eligibility for transplantationSeveral variables that would increase the chance of the individual undergoingtransplant were evaluated to identify LT predictors. Patients with coagulopathy (RNI> 1.2) and thrombocytopenia (platelet count < 150.000/mm3)[16,17] were more likely tobe transplanted (Table 3). After TACE, only patients within MC were transplanted.More patients underwent LT in the bridging group than in downstaging (65.9% vs33.9%, P = 0.001) (Tables 3 and 4). Among the downstaging patients, G4 demonstratedthe best eligibility for orthotopic liver transplantation (OLT) (60%) and did not haveany cases of HCC recurrence (Table 4).Median time on the waiting list for LT (interval between the first DEB-TACE to LT)in the downstaging group was significantly longer 10.6 months (range, 1.7 to 20.1 mo)than in the bridging group 6.6 mo (range, 0.6 to 30.5 mo) (P = 0.028) (Table 5). ROCcurve analysis revealed that patients with maximal tumor diameter up to 7.05 cmwere more likely to receive LT during DEB-TACE (P = 0.005) than patients withmaximal tumor diameter more than 7.05, with sensitivity of 65.9% and specificity of71.4% (Figure 2).Radiological responseCR was observed more frequently in bridging than in downstaging group (P = 0.004).However, since PR occurred more often in the downstaging group, there was nostatistically significant difference in OR between groups (P = 0.105) (Table 6). Sixpatients from the bridging group were submitted to LT after the first DEB-TACEprocedure, thereby occurring before the imaging study (less than 30 d after DEB-TACE).Recurrence and survivalOverall survival and recurrence-free survival were estimated using bivariate Coxregression and multivariate Cox regression to verify the influence of baselinecharacteristics (age, gender, etiology, CHILD score, MELD, alpha-fetoprotein, numberof nodules, maximal tumor diameter) on survival. There was no influence of baselinecharacteristics (P > 0.05), and/or between groups (bridging versus downstaging) onoverall survival (P = 0.662) (Table 7) or recurrence-free survival (P = 0.874) (Table 8).In an intragroup analysis, there was no statistical difference between overallsurvival (P = 0.955) and recurrence-free survival (P = 0.955) observed in subgroups 1,2, and 3 of the bridging group. However, in the downstaging group, it seems thatsubgroup 3 had worse overall survival (P = 0.04) and worse recurrence-free survival(P = 0.027), compared to the other subgroups (Table 4). Post-transplantationrecurrence occurred more frequently in the downstaging group 25% (5/20) than in thebridging group 5.81% (5/86) (P = 0.020); however, these events did not significantlyaffect recurrence-free survival (P = 0.874).Kaplan-Meier’s 1, 3, and 5-year post-transplant overall survival probability were95%, 88.2%, 73.5% in the downstaging group, and 82.8%, 76.5%, 72.3% in the bridginggroup (P = 0.317), respectively (Figure 3). Median overall survival was 1150 d or 3.15years (SD = 1.33, range from 0.52 to 5.07) in the downstaging group, and 1083 d or2.97 years (SD = 1.66, range from 0 to 5.77) in bridging group. Kaplan-Meier’s 1, 3, and5-year post-transplant recurrence-free survival probability were 95%, 82.8%, 62.1% inthe downstaging group, and 80.2%, 76.5%, 74.8% in the bridging group (P = 0.935),respectively (Figure 4). Recurrence-free survival was 1104 d or 3.02 years (SD = 1.37,range from 0.34 to 5.07) in downstaging group, and 1070 days or 2.93 years (SD = 1.69,range from 0 to 5.77) in bridging group (Table 6).DISCUSSIONThe best approach to patients with HCC beyond MC is controversial given thescarcity of available organs for transplantation and the impreciseness of identifyingpatients who are most likely to benefit from LT. In 2010, downstaging of HCC hasbeen identified as a priority for research in the field of LT[18]. Since that time, we havenot found in the literature many published data evaluating this controversial subject.Table 2 Patients demographicsChi-squared test.1Student’s t test;2Likelihood ratio test;3Mann-Whitney test. AFP: Alpha-fetoprotein; HCC: Hepatocellular carcinoma; MELD: Model for End-Stage Liver Disease.The majority of papers is limited by small sample size, short duration of follow-up,and absence of a comparison group [7-9,19-24]. Most studies have used MC as the endpoint for downstaging [9,19,22-24]. There appears to persist a doubt if patients beyond MC should undergo LT after successful downstaging. There is a lack of information in the literature regarding long-term overall and recurrence-free survival in these patients [25].The current understanding regarding neo-adjuvant treatment for HCC is that it would be most appropriate for: Controlling HCC progression for expected long waiting times (bridging), identifying patients with different probabilities of cancer progression (selection criterion), and reducing tumors sizes to meet acceptable criteria for LT (downstaging)[26]. However, there is no strong evidence that neo-adjuvant treatments should be applied if the expected waiting time for LT is shorter than 6mo [25-27].The present study included patients that have used neo-adjuvant therapy, DEB-TACE loaded with Doxorubicin, for HCC patients undergoing bridging and downstaging. Patients fulfilling the MC were immediately included on the waiting list for LT, whereas patients beyond MC were listed only after they met MC, regardless ofAffonso BB et al. DEB-TACE response before liver transplantationAffonso BB et al. DEB-TACE response before liver transplantationTable 3 Bivariate analysis of liver transplant predictorsChi-squared test.1Student’s t test;2Mann-Whitney test. AFP: Alpha-fetoprotein; HCC: Hepatocellular carcinoma; MELD: Model for End-StageLiver Disease.how many DEB-TACE sessions. Few studies followed the same protocol: noneexclusively using DEB-TACE and the majority using many different kinds ofLRT[19,20,22]. Graziadei et al[19], used conventional TACE (cTACE) for both groups:patients fulfilling the MC were started immediately after listing for OLT and patientsbeyond MC were included on the waiting list after showing response to the firstTACE. Ravaioli et al[20], used many kinds of LRT, such as LR, percutaneous ethanolinjection (PEI), RFA, and cTACE for both groups. Additionally, patients fulfilling theMC were listed immediately for LT and patients beyond MC were listed aftercompleting the pre-established downstaging protocol. De Luna et al., employedtranscatheter arterial chemoinfusion for both groups. Patients fulfilling the MC werelisted immediately for OLT and patients beyond MC were included on the waiting listafter reaching MC (downstaged)[22].Few studies have used LRT in patients beyond MC in order to achievedownstaging[7-9,21,23,24]. Some studies compared their results with other LRT, such astransarterial radioembolization[9]; some studies did not compare betweengroups[8,9,21,23,24]; and other studies used patients within the MC on the waiting list forLT as a comparative group[7]. Among these, the most significant study, highlighted bythe sample and methodology, was published recently by Yao et al[7]. In a prospectivecohort, the authors reported the outcomes of 118 patients (largest study group)exceeding the MC that underwent LRT (cTACE, RFA, and PEI) in a downstagingwell-established protocol with the intent for LT and compared to 488 patients withinthe MC on presentation[7].In the present study, at the presentation, the two populations did not havesignificantly different baseline demographic characteristics. Vascular lakephenomenon was much more frequent in downstaging population 34.3% (P = 0.001),perhaps since vascular lake phenomenon occurs more frequently in tumor of size ≥3.0 cm[14].More patients underwent LT in the bridging group (66%) compared to thedownstaging group (34%, P = 0.001). In the literature, eligibility for LT reported forthe bridging group ranges from 68% to 85.4%[19,20,22]. Yao et al[7], reported 68% eligibilityAffonso BB et al. DEB-TACE response before liver transplantation Table 4 Subgroup analysisCause of deaths: Bridging < 30 d: 3 Severe graft disfunction; 2 Sepsis; 2 Hemorrhagic shock; 1 Cardiogenic shock. Downstaging < 30 d: 0. Bridging > 30 d: 5 Sepsis; 4 Hepatocellular carcinoma recurrence; 1 Graft rejection; 1 Severe graft disfunction; 1 Pulmonary metastasis; 1 Stroke after brain biopsy; 1 not found. Downstaging > 30 d: 2 Hepatocellular carcinoma recurrence; 1 Pulmonary metastasis. HCC: Hepatocellular carcinoma; LT: Liver transplant.for LT in the MC group, similarly seen in our study. In a recent study, a dropout rateof 2.58% due to tumor progression was observed in patients who received bridgingLRT, while the rate among patients who did not receive LTR was 8.18% (P = 0.01)[28].Among downstaging patients, neo-adjuvant success rates regarding eligibility for LTwidely vary from 11% to more than 70%[29]. This large range reflects the heterogeneityof the criteria used to include patients in a downstaging protocol, differences in theLTR protocol itself, and several different criteria used worldwide on when to includea patient on a transplant list.In the downstaging protocol, it was included patients with maximal tumordiameter ≤ 8 cm (G1, G2, G3, and G4) and > 8 cm (G5). In this way, eligibility for LTwas 34%. However, if we closely look at eligibility for LT in subgroups with maximaltumor diameter up to 8 cm, eligibility for LT would be 47.5%, closer to Yao et al[7]’sreported eligibility of 54%. Maximal tumor diameter appears to influence success ofHCC downstaging due to eligibility for LT, so much so that Yao et al[7], limit maximaltumor diameter to 8 cm in their downstaging protocol.In the present study, G1, G2, and G3 used the same criteria described by Yao et al[7].Nevertheless, we found a new group of patients with maximal tumor diameter ≤ 8 cm(G4 = 2 or 3 lesions at least one > 5 cm with the sum of the maximal tumor diameters≤ 8 cm), not previously described. Among patients in the downstaging group, wesought to verify if there was a cutoff limit that related to maximal tumor diameter,and a better chance for the patient to be submitted to LT. We found that patients inthe downstaging group with maximal tumor diameter up to 7.05 cm had a greaterchance of LT (P = 0.005). Contrary to this finding, G4, with a mean maximal tumordiameter of 7.2 cm, had the best eligibility for LT (60%). On the other hand,corroborating Yao et al[7]’s impression, G5, where the maximal tumor diameter was10.9 cm on average, had the worst eligibility for LT (2 patients of 22; 9.1%). Othervariables that were implicated with a greater chance of LT were coagulopathy,thrombocytopenia, and belonging to the bridging group.Studies provided a cautionary note with an anticipated higher recurrence rate postLT: the further the tumor burden is beyond the MC (the Metro ticket concept)[25,30]. Inour study, post LT recurrence occurred more frequently in the downstaging group25% (5/20) compared to the bridging group 5.81% (5/86) (P = 0.02); however, thisfinding did not interfere with recurrence-free survival (P = 0.874). Group 4 did nothave a case of HCC recurrence. In the downstaging group, HCC recurrence rate washigher than Yao et al[7]’s rate (7.5%), but similar to Ravaioli et al[20]’s rate (18%).Our results suggest that patients, with tumors initially exceeding the MC down-。
