05-JES-表面掺F
JES详细说明

表格编码:Q/RM-A450015A0检查安全D2符号序号S 签名S 1班S2班标记处数3班日期/编制/日期审核/日期批准/日期非增值时间操作工班组长文件编号Q/RM-BJZ-Z-01步行时间工段主任产品编码工作要素单(JES)工位名称浇注工位号Z010工厂铸造工厂版本号修订号关键操作工具推拉防错班组浇注班组要素名称浇注前准备要素编号0工段铸造四工段主要步骤(What)作业要点(How)原因(Why)领取工具干冰清理机、清理刀、涂料测厚仪、气枪、锤子、3#钢印、过滤网(20目,300×300mm)、刷子(每班前清理浇口杯)、煤气、喷火枪、燃烧室及基准点防护罩、型腔涂料、喷壶等;可参见《辅助工具清单》生产待用2操作前要穿戴好防护用品(手套、袖套、劳保鞋、工作服);操作工准备防止烫伤、砸伤1为喷涂做准备燃烧室底模定位点使用:140ESS涂料与水按1:2比例进行配比,侧面使用:140ESS涂料与水按1:1比例进行配比,先向洗刷干净的配料容器中加入水,然后用电子天平称取相对比例的140ESS涂料倒入配料容器中,用小木棒边加边搅拌,搅拌均匀.(也是浇包使用的涂料)『专人配制涂料』金属型型腔涂料配制3产品名称B系列缸盖增值时间更改编号签字CTTC表格编码:Q/RM-A450015A0检查安全D2符号序号S 签名S 1班S2班标记处数3班更改编号签字日期产品编码/非增值时间编制/日期审核/日期批准/日期保证铸件质量版本号修订号模具安装前需五点进行检测① 模具大平面平面度② 模具三个基准面平面度③ 模具三个基准面角度④ 燃烧室高度⑤ 三个基准面分别与底平面的角度模具如有刮伤、破损、凹陷等缺陷,必须先修整好,检查顶杆及排气塞是否通畅,排气塞垂直,水道脚定位处顶杆无凸出,无异物,如有异常,立即排故或更换.排气不畅,产品内部产生气孔,水道脚定位处顶杆凸出,砂芯易断裂,引起产品报废模具大平面平面度模具三个基准面角度工作要素单(JES)工位名称浇注工位号Z010工厂铸造工厂关键操作工具推拉防错班组浇注班组浇注前准备要素编号0工段铸造四工段文件编号Q/RM-BJZ-Z-01步行时间产品名称B系列缸盖增值时间操作工班组长工段主任将模具安装在浇注机上,并调整分型面的间隙,安装水管(1#燃烧室蓝色、2#燃烧室红色、3#燃烧室黄色、4#燃烧室白色)及负压抽气装置(气管黑色).123保证铸件尺寸符合要求要素名称主要步骤(What)作业要点(How)模具检测安装前模具检查模具安装原因(Why)GM-B12D缸盖B15D缸盖B470缸盖模具安装前需检测项目表缸盖名称三个基准面分别与底平面的角度≤2°≤2°≤2°燃烧室高度差≤0.01mm≤0.01mm ≤0.01mm 燃烧室高度根据修模单要求调整尺寸根据修模单要求调整尺寸根据修模单要求调整尺寸≤0.05mm≤0.05mm ≤0.05mm 模具三个基准面平面度≤0.015mm≤0.15mm ≤0.025mm ≤0.5°≤0.5°≤0.5°CTT表格编码:Q/RM-A450015A0检查安全D2符号序号1S 签名S 1班S2班标记处数3班领取干冰机,接上气管把干冰装入干冰机内,将气管阀打开,将喷头对准模具,打开开关清理,必须把模具上的涂料和留在上面的垃圾清理干净,每三班清理一次【具体要求按模具清理作业指导书操作】班组浇注工位号Z010工厂铸造工厂推拉编制/日期审核/日期版本号修订号产品编码/非增值时间更改编号签字日期Q/RM-BJZ-Z-01步行时间批准/日期文件编号产品名称工作要素单(JES)工位名称B系列缸盖增值时间操作工浇注前准备0铸造四工段模具清理防错浇注班组要素名称要素编号工段关键操作工具工段主任保证铸件外观质量班组长主要步骤(What)作业要点(How)原因(Why)要求每周更换一次。
译林版小学3-6年级英语单词表(带音标)
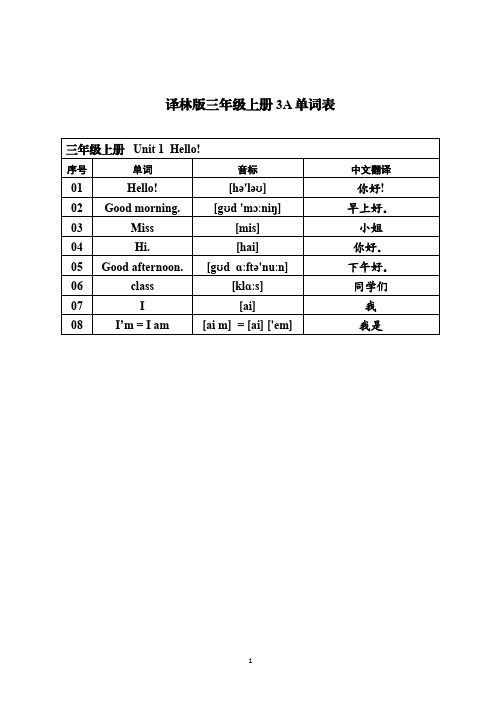
译林版三年级上册3A单词表三年级上册Unit1Hello!序号单词音标中文翻译01Hello![hə'ləʊ]你好! 02Good morning.[gʊd'mɔːniŋ]早上好。
03Miss[mis]小姐04Hi.[hai]你好。
05Good afternoon.[gʊdɑːftə'nuːn]下午好。
06class[klɑːs]同学们07I[ai]我08I’m=I am[ai m]=[ai]['em]我是三年级上册Unit2I’m Liu Tao序号单词音标中文翻译01are[ɑː]是02you[juː]你03yes[jes]是;对04am['em]是05no[nəʊ]不;不是;没有06not[nɒt]不;没有07Goodbye.[gʊd'bai]再见。
三年级上册Unit3My friends序号单词音标中文翻译01my[mai]我的02friend[frend]朋友03she[ʃiː]她04she’s=she is[ʃiːz]=[ʃiː][iz]她是……05he[hiː]他06he’s=he is[hiːz]=[hiː][iz]他是……07too[tuː]也08this[ðis]这;这个09is[iz]是10sister['sistə]姐姐;妹妹三年级上册Unit4My family序号单词音标中文翻译01family['fæməli]家;家庭02father['fɑːðə]父亲;爸爸03mother['mʌðə]母亲;妈妈04brother['brʌðə]哥哥;弟弟05me[miː]我06grandpa['græn(d)pɑː]祖父;外祖父07grandma['græn(d)mɑː]祖母;外祖母08Good evening.[gʊd'iːvniŋ]晚上好。
官能团改性CNT_增强过硫酸盐活化降解染料AO7

收稿日期:2022-05-31基金项目:国家自然科学基金资助项目(21906117,52170008);中国博士后科学基金(2018T110201,2017M620092);天津市高等学校大学生创新创业训练计划项目(202110058123)第一作者:王骏(1990—),男,博士,副教授,主要研究方向为环境功能材料与污染控制化学。
E-mail :*********************.cn 通信作者:于子钧(1988—),男,博士研究生,讲师,主要研究方向为多孔碳和纤维材料的制备及其环境功能应用。
E-mail :********************.cn官能团改性CNT 增强过硫酸盐活化降解染料AO7王骏1,2,佟显东1,2,叶布天1,2,3,尚雨佳1,2,于子钧1,2,4,张阳1,2(1.天津工业大学省部共建分离膜与膜过程国家重点实验室,天津300387;2.天津工业大学环境科学与工程学院,天津300387;3.天津工业大学材料科学与工程学院,天津300387;4.天津工业大学纺织科学与工程学院,天津300387)摘要:为了提升碳纳米管对过硫酸盐的催化活性,通过化学处理分别得到表面氨基化、羟基化和羧基化改性的碳纳米管材料(CNT-NH 2、CNT-OH 、CNT-COOH )。
采用SEM 和XPS 对材料结构进行了表征,并测试了其激活过硫酸盐(PDS )降解偶氮染料酸性橙(AO7)的性能。
结果表明:氨基和羟基改性能够显著增强CNT 的催化活性,其中,CNT-NH 2在60min 内能够降解超过95%的AO7溶液(0.1mmol/L ,100mL ),反应速率远高于原始CNT 以及CNT-OH 和CNT-COOH ,这是由于氨基改性提高了CNT 表面的正电性,从而促进PDS 和AO7在CNT-NH 2上的吸附和反应;机理研究表明,非自由基电子转移是CNT-NH 2/PDS 体系的主要氧化途径,因此,该催化体系在pH 值为3~11的范围内保持稳定的催化活性且对于实际水体具有较好的适应能力。
菲尔斯特 pH 值传感器使用手册说明书

菲尔斯特PH值传感器使用手册1.技术参数参数指标测量范围0-14pH温度测量范围0-80.0℃,0-60℃斜率≥96%零点电位7.00±0.25壳体材质PC,PBT防腐液接界聚四氟乙烯连接螺纹NPT3/4,M39*1.5信号线长度5M(可定制)耐压范围0-4bar膜电阻<500MΩ防护等级IP68输出4-2mA和RS485信号线信号线防护套防护套2.使用前说明2.1使用之前请仔细研读本说明2.2本说明适用于菲尔斯特品牌的智慧型pH系列电极2.3传感器敏感膜球泡属于易损品,一旦损坏将无法修复2.4打开包装之前请检查包装是否有损坏,如果外包装已破损,请不要继续打开包装,请立即与菲尔斯特传感器品牌最近的授权代理商或直接与我们取系,运输方代表到场后共同打开包装检验电极是否损坏,建议拍照取证。
2.5如外包装完好但电极损坏请立即与菲尔斯特传感器品牌最近的授权代理商或直接与我们联系,并将电极原包装寄回。
2.6不要将电极放在蒸馏水或去离子内存储。
2.7测量过程中,电极敏感膜球泡处若有污垢、黏着物或结垢,将会导致测量值不准确或波动,应及清洗和校准。
2.8传感器球泡内若有空气,将会导致测量值不准或波动,可以轻轻甩动电极将气泡甩去。
2.9该说明书所阐述的内容将随产品不断改进而改变,本公司在说明书中将不另行通知,并且不承担由此带来的后果。
3.电极的接线3.1请仔细按照说明书接线,错误的接线将导致产品完全损坏。
3.2严禁在所有线缆连接完成之前送电,发免发生危险,在送电之前请务必仔细检查系统所有接线,确认完成正确后方可送电。
电极出线M12接口注:具体接线方式以产品标识为准4.电极的活化4.1电极需在3MKCL溶液中活化4.2干放的电极需活化后才能使用。
5电极的标定5.1仪表出厂前一般已做标定,用户可直接投入使用。
5.2标定时建议使用两点法标定,通常先用pH6.86或7.00缓冲液标定位,然后用pH4.01或9.18缓冲液确定斜率。
瑞士制造 系列 混凝土耐久性电阻率检测一体化解决方案 高级 Resipod 系列说明书

混凝土耐久性电阻率检测一体化解决方案可在不同的天数重复检测,如 28 天、56天同一圆柱还可用抗压强度检测带有 1.5” (38mm) 探头间距的 Resipod 完全符合上述标准Resipod 几何体 (SR)AASHTO T 358标准仅限用于最大聚体粒径为何体。
Resipod 几何体设计符合最新研究,旨在打破现行AASHTO标准的局限。
Resipod 几何体附带提供有可用于更大聚体粒径的可变间距探头。
这还可让用户通过 ResipodLink 软件输入几何校正系数,以直接在仪器上给出正确的电阻率读数。
ResipodLink 软件技术信息系统需求: Windows XP、Windows Vista、Windows 7、Windows 8、USB 连接器。
自动软更新需要用)。
固件更新(使用 PqUpgrade)需要 Internet 连接(如可用)。
需要 PDF Reader 显示“帮助手册”。
81038102C ver 12 2017 © Proceq SA ,瑞士。
版权所有。
总部 Proceq SA Ringstrasse 2 CH-8603 Schwerzenbach瑞士电话: +41 (0)43 355 38 00传真: +41 (0)43 355 38 12info@ 如有更改,恕不另行通知。
Proceq SA 出于善意提供本文档的所有信息,并相信这些信息正确无误。
对于信息的完整性和准确性,Proceq SA 不做任何担保,也不承担任何责任。
对于 Proceq SA 所生产和(或)销售的任何产品的使用和应用,我们已对特定的适用操作给予了明确的参考指引。
Resipod 技术信息电阻率测量测程1 – 约 1000 k Ωcm (取决于探头间距)分辨率(额定电流 200µA )±0.2 k Ωcm 或 ±1%(取二者中的较大值)分辨率(额定电流 50µA )±0.3 k Ωcm 或 ±2%(取二者中的较大值)分辨率(额定电流小于 50µA )±2 k Ωcm 或 ±5%(取二者中的较大值)显示3½ 数显频率40 Hz AC 内存非易失,约 500 个测量值电源>50 小时续航时间充电器连接B 型 USB 类型 (5V , 100mA)尺寸197 x 53 x 69.7 mm (7.8 x 2.1 x 2.7 inch )重量318g (11.2 oz)操作温度0° 到 50°C (32° 到 122°F )存储温度-10° 到 70°C (14° 到 158°F )订购信息组件描述381 10 000Resipod ,50mm 探头间距,检测板,泡沫垫,充电器和 USB 线缆,软件,背带,文档和盒子。
有机配体对铁电絮凝体系中羟基自由基氧化降解苯胺的影响

收 稿 日 期 : 2019-06-05; 录 用 日 期 : 2019-09-15 基金项目:国家自然科学基金资助项目 (51808415)
2 结果与讨论
2.1 不同有机配体对电絮凝降解苯胺的影响
自然界中溶解态铁多以络合配体的形式存在,为研究有机配体在铁电絮凝中对有机污染降解
的影响,实验中选取了几种水体中常见的有机酸,观察其在铁电絮凝中对有机污染物降解情况。
图 2 反映了溶液中存在不同有机配体时苯胺在铁电絮凝体系中的降解情况。在铁电絮凝中分别加
图 1 实验装置示意图 Fig. 1 Schematic diagram of experimental device
第3期
符文晶等:有机配体对铁电絮凝体系中羟基自由基氧化降解苯胺的影响
607
环境工程学报版权所有
定量水样,并用 0.22 μm尼龙滤膜过滤,用于溶液中苯胺或对羟基苯甲酸浓度分析。所有实验至少 重复 2 次。
606
环 境 工 程 学 报
第 14 卷
环境工程学报版权所有
态 铁 多 以 络 合 配 体 的 形 式 存 在 , 是 水 体 净 化 中 重 要 的 氧 化 剂 和 还 原 剂 [8]。 近 几 年 , 铁 -有 机 络 合 物 在水处理中的应用也越来越多,Fe(Ⅱ)-有机络合物 (如草酸盐,柠檬酸盐和丙酮酸盐) 可以在有氧 条件下还原氧气来产生活性氧物质 (ROS),如·OH、H2O2 和O−2,且产生的 ROS 对多种有机物 (如阿 特拉津、4-氯苯酚和普萘洛尔) 的氧化作用已经得到了证实[9]。YI 等[10] 也通过电子顺磁共振实验证 明,EDTA 有助于 Fe(Ⅲ)/H2O2 体系中产生·OH。因此,在使用铁电絮凝处理含有有机配体的有机污 染废水或自然水体时,有机配体的存在可能会促进体系中·OH 的产生,从而影响目标污染物的降 解或转化。苯胺是化工生产中的原料和中间产物,在污废水中广泛存在。本研究采用苯胺作为目 标污染物,在有机配体存在的铁电絮凝体系中证明这一作用机制的存在,继而以苯甲酸与·OH 反 应,生成对羟基苯甲酸为体系中·OH 的定量手段[11],研究了有机配体对体系产·OH 效率的影响以 及不同有机配体含量时铁电絮凝体系中目标污染物的降解规律,研究结果为人们进一步了解铁电 絮凝中污染物去除机制提供参考。
Built In Quality

LH Shown, RH Similar
PQS Document # :
No. No.
1 015 Product Line :
C.1.015.01
8/8/05
GMS Orientation 2-Day (BIQ) 21
Developing A Product Quality Standard
前大灯与翼子板的配合要求 我们需要一个产品质量标准 前大灯与翼子板(386)
间隙: 小于2 mm 阶差: 大灯比翼子板高2mm至 比翼子板低1mm
这个标准是哪里来的?
5/31/09
GMS Orientation 2-Day (BIQ) 9
客户的质量感知程度 vs. 时间
感知质量
购买 外观&款式 内饰 可靠性&持久性
如果产品质量在10分钟内不能唤起顾客的热情, 我们将失去在24个月后达到期望目标的机会!
Perceptual Quality
Hard Quality
拿到产品的 时间 10 sec 5/31/09 10 min
GMS Orientation 2-Day (BIQ)
10 weeks
24 months
10
将质量渗入到制造过程中去
产品工程 制造工程 工厂 客户
产品
•设计质量 •预防 •低生命周期成本 •结果-售后索赔
工艺
•过程控制 •防错 •过程能力 •结果 – 售后索赔, 工厂 一次通过率
18
产品质量标准的应用
•代表客户的呼声
类普鲁士蓝的制备及其活化PMS降解双酚S

化工进展Chemical Industry and Engineering Progress2023 年第 42 卷第 12 期类普鲁士蓝的制备及其活化PMS 降解双酚S杨有威1,2,3,曾亦婷1,2,郭昌胜3,罗玉霞1,2,高艳1,2,王春英1,2(1 矿冶环境污染防控江西省重点实验室,江西 赣州 341000;2 江西理工大学资源与环境工程学院, 江西 赣州341000;3 中国环境科学研究院环境基准与风险评估国家重点实验室, 北京 100012)摘要:通过简单共沉淀法合成了类普鲁士蓝化合物(CoFe-PBA ),用于活化过一硫酸盐(PMS )降解有机污染物双酚S (BPS )。
使用扫描电镜、X 射线衍射、X 射线光电子能谱等手段对CoFe-PBA 进行表征,结果表明CoFe-PBA 由紧密结合的Co 3[Fe(CN)6]2构成,为纳米级,表面均匀分布着C 、Fe 、Co 、O 元素,具有丰富的活性位点。
催化剂投加量300mg/L 、PMS 投加量400mg/L 、pH=5.89条件下,CoFe-PBA/PMS 降解体系40min 内去除73.77%的BPS ,对酸性和共存离子(SO 42−、NO 3−和Cl −)敏感,碱性环境能促进PMS 快速活化,重复实验显示该体系具有良好稳定性,使用4次后仅下降26.70%,活化性能优于其他材料。
机理分析表明,CoFe-PBA 与PMS 相互作用,作用过程中改变了金属位点价态,发生电子转移,产生各种活性物质降解BPS ,其主要作用活性物种为1O 2;产物分析表明,在CoFe-PBA 活化PMS 系统中,BPS 可历经三种路径最终转化为开环产物及CO 2和H 2O 。
本研究通过低耗能、低成本、快速简易的方法制备CoFe-PBA ,可为活化PMS 绿色降解BPS 提供思路。
关键词:双酚S ;过硫酸盐活化;类普鲁士蓝;过渡金属中图分类号:X703 文献标志码:A 文章编号:1000-6613(2023)12-6676-11Preparation of Prussian blue and its activation of PMS fordegrading bisphenol SYANG Youwei 1,2,3,4,ZENG Yiting 1,2,GUO Changsheng 3,LUO Yuxia 1,2,GAO Yan 1,2,WANG Chunying 1,2(1 Jiangxi Provincial Key Laboratory of Environmental Pollution Prevention and Control in Mining and Metallurgy,Ganzhou 341000, Jiangxi, China; 2 School of Resources and Environmental Engineering, Jiangxi University of Science and Technology, Ganzhou 341000, Jiangxi, China; 3 State Key Laboratory of Environmental Criteria and Risk Assessment,Chinese Research Academy of Environmental Sciences, Beijing 100012, China)Abstract: A Prussian blue-like compound (CoFe-PBA) was synthesized by a simple co -precipitation method for activating permonosulfate (PMS) to degrade organic pollutant bisphenol S (BPS). CoFe-PBA showed high activity on the removal of bisphenol S from activated PMS. Scanning electron microscopy, X-ray diffraction, and X-ray photoelectron spectroscopy were used to characterize CoFe-PBA. The results showed that CoFe-PBA was composed of Co 3[Fe(CN)6]2, which was in nanometer scale. The surface was evenly distributed with C, Fe, Co, O elements, with abundant active sites. Under the conditions of catalyst研究开发DOI :10.16085/j.issn.1000-6613.2023-0803收稿日期:2023-05-15;修改稿日期:2023-07-21。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Improvement of High-Voltage Cycling Behavior ofSurface-Modified Li†Ni1/3Co1/3Mn1/3‡O2Cathodes by Fluorine Substitution for Li-Ion BatteriesG.-H.Kim,a J.-H.Kim,a S.-T.Myung,b,*C.S.Yoon,a and Y.-K.Sun a,*,za Department of Chemical Engineering,Center for Information and Communication Materials,Hanyang University,Seoul133-791,Koreab VK Corporation,Pyongtaek-City,Kyonggi-do450-090,KoreaTo improve the electrochemical property of Li͓Ni1/3Co1/3Mn1/3͔O2at high upper voltage limit of4.6V,fluorine was partly substituted for oxygen.Variation of lattice parameters and X-ray photoelectron spectroscopy analysis suggest thatfluorine is both substituted in bulk and coated on the surface of Li͓Ni1/3Co1/3Mn1/3͔O2.Li͓Ni1/3Co1/3Mn1/3͔O2−z F z͑z=0.05and0.1͒showed stable cycling performance and improvement of high rate capability compared to bare Li͓Ni1/3Co1/3Mn1/3͔O2.In addition,fluorine substitution catalyzes the growth of the primary particles,which in turn resulted in high tap density as well as high volumetric capacity compared to Li͓Ni1/3Co1/3Mn1/3͔O2.Differential scanning calorimetry at4.6V clearly shows thatfluorine substitution markedly improves the thermal stability of Li͓Ni1/3Co1/3Mn1/3͔O2−z F z.©2005The Electrochemical Society.͓DOI:10.1149/1.1952747͔All rights reserved.Manuscript submitted January5,2005;revised manuscript received March21,2005.Available electronically July21,2005.During the past decade,the lithium transition-metal oxides, Li͓Ni x Co1−2x Mn x͔O2,have received a great deal of interest as a cathode material for Li-ion secondary batteries.1-3Among them, Li͓Ni1/3Co1/3Mn1/3͔O2has been studied extensively as a promising cathode material for lithium secondary batteries.In attempts to in-crease the reversible capacity of the cathode material,the upper cutoff voltage limit has been gradually increased.The increased up-per voltage limit resulted in a moderate increase in the specific dis-charge capacity as expected.However,the improved discharge ca-pacity was accompanied by unstable cycling performance when cycled up to4.6V.4,5Moreover,even at upper voltage limits of 4.4–4.5V,capacity fading was still observed upon cycling.The origin of this capacity fading was mainly attributed to gradual de-caying of electroactive Co as reported by Shaju et al.4 LiCoO2,a commercialized cathode material,also has shown poor electrochemical properties when the upper voltage limits were higher than4.2V because of its phase transition from hexagonal to monoclinic.Moreover,it has been reported that the poor electro-chemical properties of LiCoO2at high voltage are due to the disso-lution of Co ions into electrolyte6.To solve this problem,various metal oxides were coated onto or substituted for LiCoO2.7-15It was proposed that the metal oxide coating or substitution effectively re-duced the Co dissolution,which accordingly led to the improvement in capacity retention at the high-voltage region.16,17Among them, 10%of Al-doped LiAl y Co1−y O2had less electrolyte decomposition with good electrochemical performances.Also,the use of aluminum ions as a dopant in transition-metal layered structure has a beneficial effect on suppressing the anisotropic structural change,because the Al3+ions together with lithium ions kept the interlayer distance during delithiation.18In addition,anion substitution for O has been also studied in LiCoO2coated withfluorine,which substantially suppressed the Co dissolution even at the high upper voltage limit of 4.3V.19To suppress Co dissolution,and thereby to improve the electro-chemical property at the high-voltage region,we substitutedfluorine for oxygen in the Li͓Ni1/3Co1/3Mn1/3͔O2.In this paper,we report the effects offluorine substitution on the structure,electrochemical behavior,and thermal stability of Li͓Ni1/3Co1/3Mn1/3͔O2−z F z͑where 0ϽzϽ0.5͒.ExperimentalLiOH·H2O,CoSO4·7H2O,NiSO4·6H2O,and MnSO4·H2O were used as starting materials.͓Ni1/3Co1/3Mn1/3͔͑OH͒x compounds were synthesized by the coprecipitation method,as reported previously.20 A mixture of the dehydrated͓Ni1/3Co1/3Mn1/3͔͑OH͒x and LiOH·H2O and LiF was heated at1000°C for10h,then subse-quently annealed at700°C for5h in air atmosphere.An excess of lithium was used to compensate for lithium loss during the calcina-tion.Chemical compositions of the resulting powders were analyzed by atomic absorption spectroscopy͑Vario6,Analyticjena͒and ion chromatography͑DX-320,Dionex,USA͒forfluorine.Powder X-ray diffraction͑XRD,Rint-2000,Rigaku,Japan͒mea-surement using Cu K␣radiation was employed to identify the crys-talline phase of the synthesized materials.XRD data were obtained 2=10–80°with a step size of0.03°and a count time of5s.From the XRD data,lattice parameters of Li͓Ni1/3Co1/3Mn1/3͔O2−z F z were calculated by the least-squares method.The morphology of as-prepared powders was observed using a scanning electron micro-scope͑SEM,JSM6400,JEOL,Japan͒.Pellet density͑PD͒of the oxides was obtained by making8mm diam pellets with approximately500mg powder under a pressure of*Electrochemical Society Active Member. z E-mail:yksun@hanyang.ac.krFigure1.Powder XRD profiles of Li͓Ni1/3Co1/3Mn1/3͔O2−z F z:͑a͒z=0,͑b͒z=0.05,͑c͒z=0.1,͑d͒z=0.15,͑e͒z=0.2,and͑f͒z=0.5.Journal of The Electrochemical Society,152͑9͒A1707-A1713͑2005͒0013-4651/2005/152͑9͒/A1707/7/$7.00©The Electrochemical Society,Inc.A170748,000psi.The thickness and diameter of the pellet after pressing was measured and the density was then calculated.The error is estimated to be ±0.08g/cm 3.The cathode was prepared by blending Li ͓Ni 1/3Co 1/3Mn 1/3͔O 2−z F z ,Super S carbon black,and polyvi-nylidene fluoride ͑80:10:10in weight ratio ͒in N -methyl-2-pyrrolidone.The slurry was then cast on an aluminum foil and dried at 110°C overnight in vacuum state.Disks were then punched out of the foil.Lithium foil was used as an anode.The long cycle-life tests were performed by employing mesocarbon microbead analysis ͑MCMB ͒as an anode.Cell tests were done using a 2032coin-type cell.The electrolyte solution was 1M LiPF 6in a mixture of ethyl-ene carbonate ͑EC ͒and diethyl carbonate ͑DEC ͒in a 1:1volumeratio.Then the coin-type cell was assembled in an argon-filled dry box and tested at a current density of 28mA g −1͑0.2C ͒at 30°C.For differential scanning calorimetry ͑DSC ͒experiments,the cells were fully charged to 4.6V and opened in the Ar-filled dry box.After the remaining electrolyte was carefully removed from the surface of the electrode,the cathode materials were recovered from the current collector.A stainless steel sealed pan with a gold-plated copper seal ͑which can withstand 150atm of pressure before rup-turing and has a capacity of 30L ͒was used to collect 3–5mg samples.The recovered composite cathode was infiltrated with about 0.5mg of electrolyte.The measurements were carried out in a DSC 200differential scanning calorimeter ͑NETZSH,Germany ͒us-ing a temperature scan rate of 1°C min −1.The weight of each sample ͑pan +sample ͒was measured before and after the experi-ment to verify that the system was hermetically sealed.The weight was constant in all cases,indicating that there were no leaks during the experiments.Results and DiscussionXRD analysis of Li ͓Ni 1/3Co 1/3Mn 1/3͔O 2−z F z .—Figure 1shows XRD patterns of Li ͓Ni 1/3Co 1/3Mn 1/3͔O 2−z F z with z =0,0.05,0.1,0.15,0.2,and 0.5.The XRD patterns could be indexed by a hexagonal ␣-NaFeO 2structure ͑space group R 3¯m ͒.The Li ͓Ni 1/3Co 1/3Mn 1/3͔O 2−z F z shows clear splits in the ͑006͒/͑102͒and ͑018͒/͑110͒peaks until z =0.2,which indicates the formation of a well-developed layered structure.Meanwhile,those peaks were merged into one and were hard to distinguish for z =0.5in Fig.1f.Also,with increasing fluorine content,z ,a small impurity peak was observed at around 31°in 2in Fig.1e and f.The XRD patterns clearly indicate that incorporation of F did not alter the crystal struc-ture of Li ͓Ni 1/3Co 1/3Mn 1/3͔O 2nor produce any secondary phases in the range of z =0−0.15.By the combination of atomic absorption and ion chromatography ͑IC ͒analyses for fluorine,the detected F amounts were 0.05,0.09,and 0.14for z =0.05,0.1,and 0.15,re-spectively.Figure 2.Variation of lattice parameters a and c as a function of F content in Li ͓Ni 1/3Co 1/3Mn 1/3͔O 2−z F z.Figure 3.SEM images of Li ͓Ni 1/3Co 1/3Mn 1/3͔O 2−z F z :͑a ͒z =0,͑b ͒z =0.05,͑c ͒z =0.1,and ͑d ͒z =0.15.A1708Journal of The Electrochemical Society ,152͑9͒A1707-A1713͑2005͒Lattice parameters of the Li ͓Ni 1/3Co 1/3Mn 1/3͔O 2−z F z ͑z =0,0.05,0.1,0.15,and 0.2͒were calculated from the XRD data by a least squares method.Figure 2shows the variation of the lattice parameters with increasing fluorine content,z .Substitution of fluo-rine resulted in the increase of the lattice constants in the a axis.During delithiation,the c axis was found to gradually increase,go through a maximum at z =0.1,and then decrease with increasing the fluorine content.The increase in the a axis could be due to the partial reduction of transition metal ions for the charge compensa-tion of F anion.Because the a parameter is a measure of the average M–M ͑M =Li,Ni,Co,and Mn ͒distance in the basal plane of the hexagonal structure,the increase in a axis with increasing fluorine content could be attributed to larger Mn 3+͑0.645Å͒than Mn 4+͑0.53Å͒or larger Co 2+͑0.65Å͒than Co 3+͑0.545Å͒.21The in-crease in the c axis also reflects an increase of the ionic radii of partially reduced transition metal ions,which overrode the effect of the slightly smaller F −͑1.33Å͒than O 2−͑1.40Å͒.21Increasing fluorine contents of more than 0.1,however,resulted in the decrease of c axis due to the small ionic radius of F anion.XRD results clearly confirm that fluorine ions are successfully substituted for oxygen ions in Li ͓Ni 1/3Co 1/3Mn 1/3͔O 2structure.Morphology of Li ͓Ni 1/3Co 1/3Mn 1/3͔O 2−z F z .—Figure 3shows the SEM images of the Li ͓Ni 1/3Co 1/3Mn 1/3͔O 2−z F z ͑z =0,0.05,0.1,and 0.15͒powders.All powders consist of sphere-shaped particleswith average diameter of ϳ10m.Each sphere-shaped particle also consists of agglomerates of smaller primary particles.Li ͓Ni 1/3Co 1/3Mn 1/3͔O 2has the primary particles with average diam-eter of ϳ1m having round edges in Fig.3a.When fluorine con-tent increased,primary particles grew up and their shapes gradually changed from round-edged to well-developed polygonal forms.In fact,all the primary particles in Li ͓Ni 1/3Co 1/3Mn 1/3͔O 0.85F 0.15have well-developed facets in Fig.3d.It is notable that similar morpho-logical variation is prominent in anion-doped spinels.22When fluo-rine or sulfur was substituted for O in LiMn 2O 4,the primary par-ticles changed to well-developed octahedrons with the growth in the particle size.Morphological changes depending on the fluorine con-tent suggest that fluorine substitution not only effects the bulk struc-tures but alters the surface energy of the resulting powders,which accordingly leads to the changes in growth kinetics and final mor-phology of the powders.XPS analysis of Li ͓Ni 1/3Co 1/3Mn 1/3͔O 2−z F z .—In order to study the distribution of fluorine within a particle,X-ray photoelectron spectroscopy ͑XPS ͒analysis of Li ͓Ni 1/3Co 1/3Mn 1/3͔O 1.95F 0.05was carried out.F 1s and O 1s spectra were obtained from the sample before and after sputter-etching the particle surface and are shown in Fig.4.The binding energy for F 1s measured from Fig.4a was 684.9eV,which closely matches those of metal fluorides.In LiF,MnF 2,and NiF 2,the binding energy for F 1s lies between 648.5and 685.9eV;23-25hence,the fluorine in the compound exists as F −1.When the surface of the powder was sputter-etched using an Ar ion beam for 30min at an approximate rate of 1Å/min,the intensity of the F 1s peak was nearly halved after etching away the surface,which suggests that the majority of the F ions reside near the surface of the particle.When the O 1s peaks were examined before and after sputter-etching the surface,the O 1s intensity did not decrease as seen in Fig.4b,in contrast to the F 1s peaks.Although the relative intensity of the O 1s peak remained unchanged,there was a notice-able change in the peak shape before and after etching.Prior to sputtering,the O 1s spectrum contained a distinct shoulder near 532eV.After the top surface was etched away,however,the rising of intensity near 531eV made it hard to distinguish the shoulder.The binding energy of O 1s for most of the Co,Ni,and Mn oxides falls between 529and 531eV,in agreement with the main peak of O 1s spectra in Fig.4b.26,27Although the analogous binding energies of Co,Ni,and Mn oxides hinder calculation of their separated val-ues,the increased intensity of the shoulder peak in Fig.4b may indicate a change in the oxidation state of transition metals in re-sponse to the increased concentration of fluorine near the surface.Although detailed investigation is needed to substantiate this,XRDFigure 4.͑a ͒F 1s spectra of Li ͓Ni 1/3Co 1/3Mn 1/3͔O 1.95F 0.05before and after sputtering with argon ion for 30min.͑b ͒O 1s spectra of Li ͓Ni 1/3Co 1/3Mn 1/3͔O 1.95F 0.05before and after sputtering with argon ion for 30min.Figure 5.Initial charge and discharge curves of Li/Li ͓Ni 1/3Co 1/3Mn 1/3͔O 2−z F z ͑z =0–0.15͒cells at a current density of 28mA g −1͑0.2C rate ͒at 30°C.A1709Journal of The Electrochemical Society ,152͑9͒A1707-A1713͑2005͒and XPS analysis clearly demonstrated that fluorine resides in the bulk as well as near the surface of the particle,accompanied by possible changes in the oxidation state of the transition metal components.Electrochemical behavior of Li ͓Ni 1/3Co 1/3Mn 1/3͔O 2−z F z .—Fig-ure 5shows the initial voltage vs capacity profiles of Li/Li ͓Ni 1/3Co 1/3Mn 1/3͔O 2−z F z cells ͑x =0−0.1͒.At the first cycle,the cells were slowly charged and discharged between 2.8and 4.6V with a current density of 28mA g −1͑0.2C rate ͒at 30°C.The initial discharge capacity of the Li/Li ͓Ni 1/3Co 1/3Mn 1/3͔O 2−z F z cells de-creased as the fluorine content increased;their values were 182mAh g −1͑z =0͒,170mAh g −1͑z =0.05͒,and 165mAh g −1͑z =0.1͒,respectively.It is likely that a stronger Li–F bond ͑577kJ mol −1͒than Li–O ͑341kJ mol −1͒would hinder intercala-tion of Li +ions,which in turn lowers discharge capacities.21On charging,variation of lattice parameters was observed in the range of 2.8–4.6V for the Li 1−x ͓Ni 1/3Co 1/3Mn 1/3͔O 2−z F z ͑z =0and 0.1͒electrodes in Fig.6.In layered cathode materials,the c axis first elongates during Li +extraction due to the generation of coulombic repulsions between the MO 2͑M =transition metals ͒layers as the lithium ions which screen the oxygen-oxygen repulsions are re-moved.In Fig.6a,both electrodes showed increase in c axis at first,which maximized at x Ϸ0.5and then decreased again.Interestingly,the fluorine-doped sample showed less elongation in the c axis than did the fluorine-free sample at x =0.5.From this result,it can be speculated that electrostatic repulsion between oxide ions is partially offset by strong Li–F bonding during Li +extraction.Both samples exhibited similar values of a parameters on charging.Figure 7shows the differential capacity ͑dQ /dV ͒vs voltage pro-files of Li/Li ͓Ni 1/3Co 1/3Mn 1/3͔O 2−z F z ͑z =0,0.05,and 0.1͒cells during 50cycles.The cells were cycled between 2.8and 4.6V with a current density of 28mA g −1.At the first cycle,the Li/Li ͓Ni 1/3Co 1/3Mn 1/3͔O 2cell exhibited redox peaks at around 3.75V on charging and 3.74V on discharging.As the cycling pro-ceeded,however,the redox peaks became more polarized and then shifted to 3.8and 3.67V at the 50th cycle.In addition,decrease of initial discharge voltage ͑indicated with arrow ͒suggests the increase of internal resistance ͑IR ͒during cycling.Together with the increas-ing polarization between redox peaks,the voltage drop due to IR implies increasing cell resistance during cycling in the Li/Li ͓Ni 1/3Co 1/3Mn 1/3͔O 2cell.This redox voltage variation,how-ever,reduced for z =0.05and was finally negligible for z =0.1.Identical dQ /dV curves of the fluorine-substituted sample shows its stable redox reaction during cycling compared to the undoped one.Figure 8shows the variation of discharge capacities of the Li/Li ͓Ni 1/3Co 1/3Mn 1/3͔O 2−z F z ͑z =0–0.1͒cells during cycling in the range of 2.8–4.6V.Although the Li/Li ͓Ni 1/3Co 1/3Mn 1/3͔O 2samples had the highest discharge capacity at first,they showed abrupt decrease in capacity during cycling and exhibited 87%of capacity retention at the 50th cycle.The poor capacity retention of Li/Li ͓Ni 1/3Co 1/3Mn 1/3͔O 2is primarily due to its high voltage cutoff,because it exhibited good capacity retention with the capacity of 155mAh g −1in the range of 2.8–4.3V.Meanwhile,though fluorine-substituted samples had lower initial capacities,they showed far better capacity retentions during 50cycles in the same voltage range,96%for z =0.05and 97%for z =0.1at the 50th cycle,respectively.In order to observe long-term cycling properties,a carbon elec-trode was employed as the negative electrode.Figure 9exhibits discharge capacity vs.cycle number for C/Li ͓Ni 1/3Co 1/3Mn 1/3͔O 2and C/Li ͓Ni 1/3Co 1/3Mn 1/3͔O 1.95F 0.05cells by applying 1C͑140mA g −1͒between 2.8and 4.5V.The C/Li ͓Ni 1/3Co 1/3Mn 1/3͔O 2delivered somewhat higher capacity in the early stage of cycling compared to C/Li ͓Ni 1/3Co 1/3Mn 1/3͔O 1.95F 0.05.However,the higher capacity faded gradually as the cycling contin-ued.The capacity retention was about 75%at the 100th cycle.On the contrary,though C/Li ͓Ni 1/3Co 1/3Mn 1/3͔O 1.95F 0.05exhibited slightly smaller capacity,the capacity was greatly retained and ca-pacity retention was about 94%of its initial discharge capacity at the 100th cycle in Fig.9.Although more experimental works are required for clear expla-nation about the poor electrochemical properties of Li ͓Ni 1/3Co 1/3Mn 1/3͔O 2at high-voltage cutoff in detail,previous works reported the possible Co dissolution during cycling.Amatucci et al.6reported that the amount of Co dissolution became significant in LiCoO 2when voltage cutoff was higher than 4.2V.Furthermore,fluorine coating effectively reduced the dissolution of particles into electrolyte by passivation of particles.19From the viewpoint of Co dissolution,we could explain why the capacity of Li ͓Ni 1/3Co 1/3Mn 1/3͔O 2was deteriorated when the voltage cutoff was higher than 4.4V,especially at 4.6V.Hence,it is likely that the fluorine coating on the surface of Li ͓Ni 1/3Co 1/3Mn 1/3͔O 2−z F z pro-tects the active material from HF attack into electrolyte and makes it possible to endure high voltage cutoff.Rate capability testing also demonstrated the advantages of fluo-rine substitution.Figure 10shows voltage profiles of Li/Li ͓Ni 1/3Co 1/3Mn 1/3͔O 2−z F z cells at various C-rates in the range of 0.2–5.As observed in Fig.10a,the discharge capacity ofundopedFigure 6.Variation of lattice param-eters of Li ͓Ni 1/3Co 1/3Mn 1/3͔O 2−z F z ͑z =0and 0.1͒electrodes during delithiation:͑a ͒c axis and ͑b ͒a axis.A1710Journal of The Electrochemical Society ,152͑9͒A1707-A1713͑2005͒Li/Li ͓Ni 1/3Co 1/3Mn 1/3͔O 2dropped dramatically with increasing C-rate.Moreover,Li/Li ͓Ni 1/3Co 1/3Mn 1/3͔O 2had a large IR and in turn showed low operating voltages at high C-rates.However,a small amount of fluorine substitution gave rise to a noticeable im-provement in discharge capacity as well as IR drop at the high C-rates in Fig.10b and c.Together with the effect of fluorine on the Co dissolution,the small variation of c axis in Fig.6a could explain the superior rate capability of the fluorine-doped sample,because small c axis variation during cycling is advantageous to maintain structural stability,especially at high rates.Li/Li ͓Ni 1/3Co 1/3Mn 1/3͔O 1.9F 0.1exhibited the best rate capability in Fig.10c,showing 70%capacity retention at 5C rate compared to its capacity at 0.2C.Volumetric capacity of Li ͓Ni 1/3Co 1/3Mn 1/3͔O 2−z F z .—For practi-cal applications,PD of positive material is an important point with regard to volumetric capacity.Cathode material with high PD could provide high volumetric capacity within a limited inner space of practical battery.As the fluorine content increased,tap density also increased from 2.9g cm −3͑z =0͒to 3.21g cm −3͑z =0.05and 0.1͒because of growing primary particles in Fig.3.Specific gravimetric and volumetric capacities of the Li ͓Ni 1/3Co 1/3Mn 1/3͔O 2−z F z ͑z =0,0.05,and 0.1͒powders were compared in Fig.11.Each capacity was measured at a 0.2C rate.Although fluorine-doped samples had low gravimetric capacities,they exhibited high volumetric capacities compared to Li ͓Ni 1/3Co 1/3Mn 1/3͔O 2because of their high PD.Among them,Li ͓Ni 1/3Co 1/3Mn 1/3͔O 1.95F 0.05exhibited the highest volumetric ca-pacity of 547mAh cm −3,while Li ͓Ni 1/3Co 1/3Mn 1/3͔O 2showedFigure 7.DQ /dV curves of Li/Li ͓Ni 1/3Co 1/3Mn 1/3͔O 2−z F z ͑z =0–0.1͒cells during 50cycles at a current density of 28mAg −1͑0.2C rate ͒at30°C.Figure 8.Cycling performance of Li/Li ͓Ni 1/3Co 1/3Mn 1/3͔O 2−z F z ͑z =0–0.1͒cells during 50cycles at a current density of 28mA g −1͑0.2C rate ͒at30°C.Figure 9.Discharge capacity vs cycle number of C/Li ͓Ni 1/3Co 1/3Mn 1/3͔O 2and C/Li ͓Ni 1/3Co 1/3Mn 1/3͔O 1.95F 0.05cells during 100cycles by applying a current density of 1C ͑140mA g −1͒at 30°C.A1711Journal of The Electrochemical Society ,152͑9͒A1707-A1713͑2005͒531mAh cm −3.This result confirms that slightly low gravimetric capacities of fluorine-doped samples could be offset by their high volumetric capacities.Thermal stability of Li ͓Ni 1/3Co 1/3Mn 1/3͔O 2−z F z .—Thermal sta-bility of positive materials,especially at delithiated state,is an im-portant factor in judging the suitability for practical application of lithium secondary batteries.Figure 12shows DSC profiles of wet electrodes of Li 1−x ͓Ni 1/3Co 1/3Mn 1/3͔O 2−z F z which were charged to 4.6V.The DSC experiments were made in welded scaled stainless steel tubes so that no leaking of pressurized electrolyte was possible.As the concentration of fluorine in Li 1−x ͓Ni 1/3Co 1/3Mn 1/3͔O 2−z F z in-creased,the thermal stability of the charged cathode material in the electrolyte was greatly improved.The Li 1−x ͓Ni 1/3Co 1/3Mn 1/3͔O 2had a large exothermic peak between 234and 283°C which produced 4227J g −1of heat.When fluorine content increased,Li 1−x ͓Ni 1/3Co 1/3Mn 1/3͔O 2−z F z showed higher onset temperature of exothermic peak and lower heat amount;280°C with 1733J g −1forz =0.05and 308°C with 839J g −1for z =0.1,respectively.Im-proved thermal stability of fluorine-doped samples shows the struc-tural stability of Li 1−x ͓Ni 1/3Co 1/3Mn 1/3͔O 2−z F z at highly charged state of 4.6V.ConclusionIn this paper we report the structure,morphology,electrochemi-cal properties,and thermal stability of Li ͓Ni 1/3Co 1/3Mn 1/3͔O 2−z F z .Variation of lattice parameters according to fluorine content,z ,sug-gests that fluorine is partly substituted in bulk structure.XPS analy-sis also shows that the majority of the fluorine ions reside near the surface of the particle.These results suggest that fluorine ions are both in bulk and on the surface of Li ͓Ni 1/3Co 1/3Mn 1/3͔O 2−z F z .Fluo-rine substitution catalyzes the growth of the primary particles as confirmed by SEM,which in turn results in high tap density as well as high volumetric capacity compared to Li ͓Ni 1/3Co 1/3Mn 1/3͔O 2.Although capacity fading was observed for Li ͓Ni 1/3Co 1/3Mn 1/3͔O 2at high voltage cutoff of 4.6V,Li ͓Ni 1/3Co 1/3Mn 1/3͔O 2−z F z shows good capacity retention.In addi-tion,Li ͓Ni 1/3Co 1/3Mn 1/3͔O 2−z F z exhibits superior rate capability compared to an F-free sample,probably due to the smaller c axis variation and fluorine coating effects.DSC measurement at 4.6V clearly shows that fluorine substitution markedly improves the ther-mal stability of Li ͓Ni 1/3Co 1/3Mn 1/3͔O 2−z F z .AcknowledgmentThis work was performed through the financial support of the National R&D programs of the Ministry of Science and Technology,Republic of Korea.Hanyang University assisted in meeting the publication costs of this ar-ticle.References1. D.D.MacNeil,Z.Lu,and J.R.Dahn,J.Electrochem.Soc.,149,A1332͑2002͒.2.T.Ohzuku and Y .Makimura,Chem.Lett.,30,642͑2001͒.3.S.-H.Kang,J.Kim,M.E.Stoll,D.Abraham,Y .K.Sun,and K.Amine,J.Power Sources ,112,41͑2002͒.4.K.M.Shaju,G.V .Subba Rao,and B.V .R.Chowdari,Electrochim.Acta ,48,145͑2002͒.5.G.-H.Kim,S.-T.Myung,H.J.Bang,J.Prakash,and Y .-K.Sun,Electrochem.Solid-State Lett.,7,A477͑2004͒.6.G.G.Amatucci,J.M.Tarascon,and L.C.Klein,Solid State Ionics ,83,167͑1996͒.7.H.Tukamoto and A.R.West,J.Electrochem.Soc.,144,3164͑1997͒.8.Y .-I.Jang,B.Huang,H.Wang,D.R.Sadoway,G.Ceder,Y .-M.Chiang,H.Liu,and H.Tamura,J.Electrochem.Soc.,146,862͑1999͒.9.M.Mladenov,R.S.Stoyanova,E.Zhecheva,and S.Vassilev,-mun.,3,410͑2001͒.10.H.Wang,Y .-I.Jang,B.Huang,D.R.Sadoway,and Y .-M.Chiang,J.PowerSources ,81–82,594͑1999͒.11.G.Ceder,Y .-M.Chiang,D.R.Sadoway,M.K.Aydinol,Y .-I.Jang,and B.Huang,Figure 10.Rate capability of Li/Li ͓Ni 1/3Co 1/3Mn 1/3͔O 2−z F z ͑z =0–0.1͒cells at 30°C:͑a ͒z =0,͑b ͒z =0.05,and ͑c ͒z =0.1.Figure parison of gravimetric and volumetric discharge capacities of Li/Li ͓Ni 1/3Co 1/3Mn 1/3͔O 2−z F z ͑z =0–0.1͒cells at a current density of 28mA g −1͑0.2C rate ͒at30°C.Figure parison of DSC traces of Li 1−x ͓Ni 1/3Co 1/3Mn 1/3͔O 2−z F z ͑z =0–0.1͒at 4.6V.A1712Journal of The Electrochemical Society ,152͑9͒A1707-A1713͑2005͒Nature(London),392,694͑1998͒.12.S.-T.Myung,N.Kumagai,S.Komaba,and H.-T.Chung,Solid State Ionics,139,47͑2001͒.13.J.Cho,Y.J.Kim,and B.Park,Chem.Mater.,12,3788͑2000͒.14.Z.Chen and J.R.Dahn,Electrochem.Solid-State Lett.,5,A213͑2002͒.15.M.C.Fredel and A.R.Boccaccini,J.Mater.Sci.,31,4375͑1996͒.16. A.M.Kannan,L.Rabenberg,and A.Manthiram,Electrochem.Solid-State Lett.,6,A16͑2003͒.17.Z.Chen and J.R.Dahn,Electrochem.Solid-State Lett.,5,A213͑2002͒.18.T.Ohsuku,A.Ueda,and M.Kouguchi,J.Electrochem.Soc.,142,4033͑1995͒.19.Y.Fumihiro,O.Hirokuni,and Y.Nobuyuki,Jpn.Pat.2003-221235͑2003.08.05͒.20.M.-H.Lee,Y.-J.Kang,S.-T.Myung,and Y.-K.Sun,Electrochim.Acta,50,939͑2004͒.21.J.A.Dean,Langes’s Handbook of Chemistry,15th ed.,pp.4.5.1–4.5.3,McGraw-Hill,New York͑1999͒.22.S.-H.Park,K.-S.Park,Y.-K.Sun,and K.-S.Nahm,J.Electrochem.Soc.,147,2116͑2000͒.23.G.E.Murch and R.J.Thorn,J.Phys.Chem.Solids,41,785͑1980͒.24. A.Aoki,Jpn.J.Appl.Phys.,15,305͑1976͒.25. B.P.Loechel and H.H.Strehblow,J.Electrochem.Soc.,131,713͑1984͒.26.J.Haber,J.Stoch,and L.Ungier,J.Electron Spectrosc.Relat.Phenom.,9,459͑1976͒.27.M.Oku,K.Hirokawa,and S.Ikeda,J.Electron Spectrosc.Relat.Phenom.,7,465͑1975͒.A1713Journal of The Electrochemical Society,152͑9͒A1707-A1713͑2005͒。
