CODEHOP使用说明
在线商城系统说明书

在线商城系统说明书一、引言在线商城系统是一种基于互联网的电子商务系统,旨在提供一个便捷、安全、高效的购物平台。
本说明书旨在向用户介绍在线商城系统的特点、功能和使用方法,使用户能够更好地了解和使用该系统。
二、系统概述在线商城系统提供了以下功能:1. 商品浏览和搜索:用户可以通过系统浏览和搜索各类商品,查看商品详细信息和图片。
2. 购物车管理:用户可以将感兴趣的商品加入购物车,并随时查看购物车中的商品数量和总金额。
3. 订单管理:用户可以提交订单,选择支付方式并填写配送地址,系统会自动生成订单号并跟踪订单状态。
4. 会员中心:用户可以注册成为系统会员,享受会员特权,如积分累积、优惠券领取等。
5. 支付与配送:用户可以选择合适的支付方式,并填写收货地址,系统会安排商品配送并提供快递跟踪服务。
三、系统安装与设置1. 硬件要求:在线商城系统可以在各类主流计算机硬件上运行,建议使用稳定的网络环境以确保系统的流畅运行。
2. 软件要求:系统运行需要安装相应的服务器软件,如Apache、MySQL等,并兼容常用的浏览器。
3. 数据库配置:在数据库中创建相应的表格和字段,确保系统能够正确存储和读取相关数据。
4. 网站域名与主机配置:将系统部署到互联网上的特定域名下,配置合适的主机环境。
四、系统登录与权限管理1. 用户注册与登录:用户需要注册账号才能登录系统,并提供必要的个人信息,如用户名、密码、手机号码等。
2. 密码保护:系统应使用加密算法对用户密码进行保护,确保用户信息的安全性。
3. 权限管理:系统应对用户权限进行管理,根据用户身份赋予不同的操作权限,以确保系统的安全性和合法性。
五、商品管理1. 商品分类:系统应提供商品分类功能,方便用户查找具体商品。
2. 商品发布:商家可以通过系统发布新的商品,包括商品名称、价格、库存、详细描述、产品图片等信息。
3. 商品推荐:系统应根据用户的购买记录和浏览行为,智能地推荐相关商品,提高用户购买的几率。
CODEHOP使用流程

1.输入以下网址:/codehop.html
2.找到该行:
The input should be a set of local multiple alignments (blocks) of a group of related protein sequences. The alignments must be in Blocks Database format, such as in Block Maker output.
点击“block maker”进入格式制作。
3.向该页的以下框内输入保守区域的多个序列
输入格式为:
>名字1
……(同源蛋白保守区域序列1)
>名字2
……(同源蛋白保守区域序列2)
>名字3
……(同源蛋白保守区域序列3)
然后点击方框下发的“Make Blocks”
4.新的对话框页面上显示如下:
Making your blocks, please wait...
Your results will be available for approximately 24 hours here.
点击“here”.
5.在新的页面的底部点击[BLOCKS format]。
6.新的对话框中显示CODEHOP的格式,将该页面内的全部内容复制,粘贴至CODEHOP主页的方框(如下所示)中。
Paste your block(s) below:
7.调整方框下方的各个参数,点击方框右上方的“look for primers”即可。
科脉商业管理软件说明书
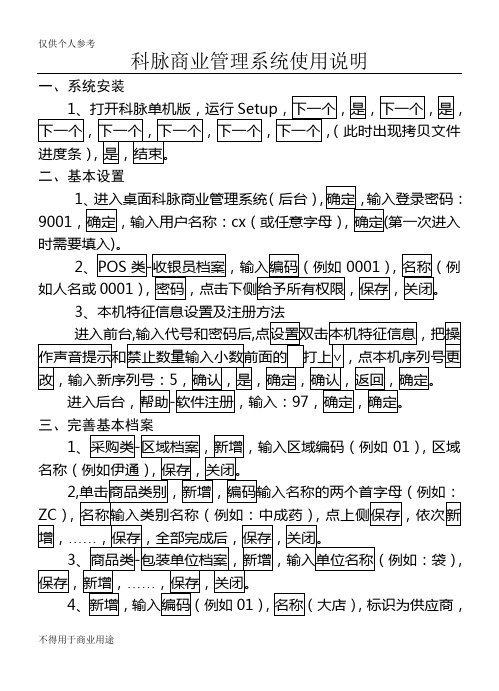
科脉商业管理系统使用说明一、系统安装二、基本设置1(后台)9001cx第一次进入2如人名或0001)3三、完善基本档案1),区域),4四、业务工作(一)库存管理12345、查实时库存输入编码(或直接在后侧选择相应类别)。
6。
78即自动计算出现差错(二)销售相关1734查找,(三)前台POS端设置1、前台零售操作步骤1POS,按的数字,完成一次交易。
(2,再按入条码,按,再按。
)会员卡收款:先输入所购买商品,按,输入卡号,按,完成退货。
(7)退出POS在当前收款状态下,按号即为小票号。
销售状态,按,条码,店内码作废,退货结算,人民币,前台日结,会员卡上面提到的设置,都是指第一次进入时需要设置,再次进入时是指在键盘上对应的按键。
在收银界面按某键时,提示XXX键没有定义,只要按一下,就好了。
仅供个人参考仅供个人用于学习、研究;不得用于商业用途。
For personal use only in study and research; not for commercial use.Nur für den persönlichen für Studien, Forsc hung, zu kommerziellen Zwecken verwendet werden.Pour l 'étude et la recherche uniquement à des fins personnelles; pas à des fins commerciales.толькодля людей, которые используются для обучения, исследований и не должны использоваться в коммерческих целях.以下无正文。
CODEHOP使用说明

CODEHOP使用说明CODEHOP是一种用于设计引子扩展PCR引物的方法,用于在没有已知序列信息的情况下扩增目标DNA片段。
它是一种基于保守区域的引物设计方法,可以在短序列的基础上快速设计出高度特异性的引物。
CODEHOP是由Michael A. Innis等人在1990年首次提出的,是一种被广泛应用于分子生物学和基因工程研究中的技术。
2.识别保守区域:在比对结果中,可以识别出具有高度保守性的区域。
这些保守区域即为CODEHOP引物设计的基础,因为这些区域相对稳定,可以用于设计特异性高的引物。
3.设计CODEHOP引物:通过使用一系列特殊的引物序列,在保守区域周围设计具有高度特异性的CODEHOP引物。
这些引物设计需要考虑以下几个因素:引物长度应为20-30个碱基,GC含量应在40-60%范围内,引物的末端应为CPG三个氨基酸,引物的内部序列应具有高度特异性。
此外,引物序列之间应有一定的相似性,以确保其在PCR反应中协同工作。
4.测试引物特异性:设计完成后,使用引物进行PCR反应,使用目标物种的基因组DNA作为模板,进行反应。
通过观察PCR产物的大小和特异性,评估CODEHOP引物的特异性。
CODEHOP引物设计方法的优点是快速、简单且高效。
它不需要已知的序列信息,仅通过保守区域的比对就能够设计出高度特异性的引物。
此外,CODEHOP引物可以广泛应用于各种分子生物学研究中,如基因克隆、基因表达和突变分析等。
然而,CODEHOP方法也存在一些限制。
首先,引物设计仅仅依赖于保守区域,可能导致引物的特异性不够高。
其次,CODEHOP方法需要在实验过程中进行引物的优化和测试,以确保其特异性和扩增效率。
总之,CODEHOP是一种快速、简单且高效的引物设计方法,可以用于扩增目标DNA片段。
通过比对已知序列,识别保守区域,并设计具有高度特异性的CODEHOP引物,研究人员能够在没有已知序列信息的情况下成功扩增目标DNA,为基因工程和分子生物学研究提供了强有力的工具。
网上购物商城使用说明书

网上购物商城使用说明书主要功能网上购物商城主要由前台会员模块和后台管理模块两部分组成。
❑前台功能模块前台模块主要包括会员注册、登录、修改个人信息、购物、查询购物情况和查看各种服务条款等功能。
❑后台管理模块后台模块主要包括后台管理员对会员、商品、仓库、订单和管理员的管理等功能。
会员在登录进入该网上购物商城后,不仅可以查看其各种服务条款,还可以选择查看各种商品的详细信息并购买。
管理员登录后,可以查看商品销售情况,及管理会员、商品、仓库和其自身的信息。
管理员还可以根据实际情况添加其他管理员以维护该网上购物商城的购物环境和安全。
操作注意事项用户在使用《网上购物商城》之前,应注意以下事项:(1)本系统后台管理员用户名为:51aspx,密码为:51aspx。
(2)单击前台首页的“后台入口”按钮,弹出登录页面,添加正确的用户名和密码进入后台。
(3)本系统后台的“仓库管理”模块虽然实现了添加、修改、删除等功能,但与前台并没有建立连接,前台的仓库类型是固定的。
(4)本系统后台的“订单管理”模块是按订货人统计而不是订单号。
业务流程在使用本系统时,请按照以下流程进行操作:1.前台前台中所有的功能模块只需用户单击相关超链接,便可进入信息展示页面。
(1)通过【会员管理】页面可以进行会员注册。
(2)通过【首页】页面可以查看商品信息及购买商品。
(3)通过【购物信息查询】页面可以查看购物记录。
注意:在“购物车”和“购物信息查询”模块中,用户需先通过首页进行“注册”,成为本站的会员后才能进行购物及查看购物记录。
下面给出商品的购买过程。
(1)单击导航区上的【首页】菜单按钮,进入如图1.1所示的界面。
– 1 –图1.1 首页页面(2)在此页面中单击“详细信息”按钮,进入商品详细信息页面,如图1.2所示。
图1.2 商品详细信息(3)如果您已经注册为会员,可以直接单击“购买”按钮进入购物车页面如图1.3所示,否则提示“您还没有登录,请登录后再购买,谢谢合作!”。
网上商城系统操作手册

网上商城系统操作手册目录1 系统描述 (1)1.1总体介绍 (1)1.2软件需求 (1)2系统后台管理介绍和操作说明 (1)2.1后台登陆 (1)2.2管理员信息管理 (1)2.2.1添加管理员 (1)2.2.2管理员列表 (1)2.2.3修改密码 (2)2.3 用户信息管理 (3)2.4 销售量查询 (3)2.5 商品信息管理 (3)2.5.1显示、修改、删除商品信息 (3)2.5.2添加商品信息 (4)2.5.3添加、删除商品类别 (4)3系统前台管理介绍和操作说明 (5)3.1商品浏览 (5)3.2用户管理 (5)3.2.1用户登录 (5)3.2.2修改密码 (5)3.2.3修改个人资料 (6)3.3购物记录查询 (6)3.4购物车管理 (6)1 系统描述1.1总体介绍整个项目分前台和后台两个项目开发。
前台用户可以在网上商城按类别浏览商品的信息,也可以搜索自己所需要的商品的内容,放入购物车以便购买,下订单,也可以修改自己的注册信息,察看自己的订单。
同时可以注册和编辑个人信息。
后台包括用户管理和商品管理和用户权限管理。
前台功能描述:➢商品浏览:热门商品浏览(首页显示点击率最高的商品列表)、新到商品浏览(首页显示最新添加的商品列表)、商品分类浏览,按商品名称搜索、商品详细信息;注册账户才可以购买商品,用户登录对用户身份进行验证,未注册用户只能浏览商品,不能完成结账;➢用户管理:注册新用户、登录、用户修改密码、用户个人资料管理;➢购物车管理:用户可以将感兴趣的商品加入购物车,便于商品购买,如果用户未登录则提示先登录后购买;➢订单管理:用户下订单后,可以对订单删除和更改商品,以及修改联系方式,系统判断客户账户里有没有足够的资金,如果没有足够的资金则给出提示,如有则可完成订单。
后台管理功能描述:➢管理员信息管理:登录;添加新管理员、删除管理员;修改密码;管理员日志(记录管理员的每个操作,由超级管理员进行查询);➢商品信息管理:添加、删除商品类别;添加、修改、删除商品信息;➢用户信息管理:查询用户信息、修改账户金额;➢销售量查询:查询某月的销售情况(包括每种商品的售出数量、相关订单数、销售收入)。
秘奥商务管理软件超市版的前台使用说明书1

系统简介为满足众多的中小型零售企业对销售管理的需要,秘奥软件有限公司开发出了一套产品简洁直观、易学易用的特点,将店面销售业务与整个系统无缝地链接起来,使店面的销售情况,随时可以传递、汇总到后台,为您解决了每日处理大量销售单据的烦恼,帮助您在最短的时间内准确无误地计算出各种商品的销售数量、销售金额和利润等数据,使您可以及时掌握仓库库存信息,安排采购计划。
系统由后台进销存系统与前台的POS销售组成。
后台可以录入收银员、货品、班次等基础资料等信息,前台主要作为零售的操作并把数据传到后台。
这些零售数据提交到服务器后,后台可查看这些单据,并通过零售日结功能来实现冲减库存、计算利润的过程,此外还可以通过各种报表查看商品销售情况,收银员收款、交款情况。
●可在局域网和互联网中应用,由总部(配送中心)系统和门店系统组成,即前台POS+后台MIS;●连网/断网自由切换的工作方式使您在任何网络环境下可工作;●集团化统一采购和门店独立采购相结合,统一配送、统一结算、统一定价、统一促销;●门店可以分多个柜组、多个导购员销售管理;●支持各种POS硬件设备:小票打印、顾显、钱箱、条码枪等;●全面消费卡支持:会员、积分、储值、折扣;●灵活多样的促销支持:按限时、按限量、按限时限量;●智能化错误提醒功能(错误条码、商品价格、数量等)●严格权限控制,对各个收银员可设置不同权限;●前台交接班日志,详细记录收银员的所有操作,方便核对;●强大报表系统,与灵活的自定义报表工具——强大的报表系统,保证了总部、门店之间信息的通畅;前台基本业务流程前台零售业务流程入下图:登录模式选择软件启动后将出现如下图登陆模式选择的对话框:连网模式:当选此模式时,在系统后台实时查看到前台的销售情况,前台并可实时取得后台更新的商品、柜组、导购等基本资料。
无需做上传与下载操作。
此工作模式缺点是前台一直与服务器保持连接,在服务器或网络较忙时会影响前台销售速度。
断网模式:当选此模式时,前台首先需要下载基础资料,然后就可以与服务器断开连接,需要上传销售单据时,再进行连接,后台才可以查看到有关的数据。
codesoft 6.0中文使用手册2

CodeSoft 中文说明简明手册·软件的安装 2 ·基本界面和操作步骤 3 ·设置打印机 4 ·标签的设置 5 ·条码的输入 6 ·数据库的输入 8 ·计数器的的设置 14·将数据库和计数器等变量导入条形码 15软件的安装步骤安装步骤:1.开机后启动 Windows95/98/2000.2.点击“开始”按钮,选择“程序”下的资源管理器,或在“开始”按钮处单击右键并选择资源管理器单击后,进入资源管理器。
3.将系统安装光盘安装盘插入对应的驱动器。
4.·运行光盘根目录下的“setup.exe”文件,·选择语言为“间体中文” ,·选择安装目录, 建议选择默认安装路径即可, 第一次安装会提示重新启动计算机 , 按确定即可, ·然后按“下一步”直到“结束”完成安装;5. 安装完毕请拷贝软件目录下的 CsUtil.dll,将安装路径目录下的此文件覆盖粘贴,如果第一步安装过程选择默认 C 盘安装路径,则只需找到 C:\program files\cs6下覆盖粘贴即可;如果上步的安装选择了新路径,则需找到和第一步相同的路径;6.安装完成以上两步,即可运行执行文件 Cs6.exe,运行 CODESOFT6.0条码软件,她可为您带来丰富的而强大的中文条码编辑打印功能。
7.严禁翻录或者拷贝用作其它商业用途,违者追究法律责任。
安装结果(默认路径 C:\Program Files\CS6 ,在安装目录下执行以下文件:Cs.exe 主执行文件基本界面和操作步骤基本界面:操作步骤:选择打印机:选择或添加打印机,设置打印机参数标签设置:设置标签的大小,边距插入对象:条码:条码种类,高度等信息数据库的导入计数器的设置选择打印机按 F5键或在工具栏上点击如图按钮,弹出对话框:如果打印列表中没有您对应的打印机,点击在右边的“添加”按钮,弹出对话框:在左边“安装”列表中选择对应打印机类型, (下面以 Zebra Z4M打印机为例接口选择 LPT1(即为串口接口 :如图所示:选择完毕后,点击“完成”按钮。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
CODEHOP : COnsensus-DEgenerate HybridOligonucleotide PrimersCODEHOP helpOn this page:Other pages: •General information • Getting started •Obtaining input alignments • CODEHOP program algorithm • Terms and parameters • CODEHOP manuscript • Strictness parameter details • Basic tips• PublicationGeneral informationThe CODEHOP program designs PCR (Polymerase Chain Reaction) primers from protein multiple-sequence alignments. The program is intended for cases where the protein sequences are distant from each other and degenerate primers are needed.The multiple-sequence alignments should be of amino acid sequences of the proteins and be in the Blocks Database format Proper alignments can be obtained by different methods .The result of the CODEHOP program are suggested degenerate sequences of DNA primers that you can use for PCR. You have to choose appropriate primer pairs, get them synthesized and perform the PCR.A CODEHOP primer is degenerate at the 3' core region, with a length of 11-12 bp across four codons of highly conserved amino acids, and is non-degenerate at the 5' consensus clamp region, with a length which depends on its desired annealing temperature, typically between 20 and 30bp:5' 3'--------------------===========non-degenerate degenerateconsensus clamp core#bases from temp 11-12 basesThe hybrid structure (5' consensus and 3' degenerate) of CODEHOP primers allow the PCR amplification to be specific during the early cycles from the original source DNA and selective during the late cycles from the PCR synthesized products:Schematic comparison of standard degenerate PCR (left) with the CODEHOP (right), illustrating regions of mismatch in primer-to-template annealing during early PCR cycles and in primer-to-product annealing during subsequent cycles. Vertical lines indicate nucleotide matches between primer (arrow) and template or synthesized product. The overall degeneracy is the product of degeneracies at each nucleotide position, so that the fraction of precisely hybridizing primers is 1/degeneracy.Obtaining input alignmentsInput alignments for the CODEHOP program must be in the Blocks Database format. Block multiple-sequence alignments are ungapped and usually local alignments. Local alignments cover only parts of the protein sequences. The regions between the blocks are the ones with no sequence similarity or where gaps must be inserted to align the sequences.You can get a multiple alignment from a group of related protein sequences using the Block Maker or other automated methods(such as ClustalW). The alignments can also be manually made or modified according to your knowledge of the proteins (position of the active sites orpost-translational modifications etc.). In any case the alignments passed to the CODEHOP program must be in the Blocks format. BlockMaker blocks need no reformatting. Appropriate parts of Clustal- or FASTA-formatted multiple alignments can be automatically made into blocks by the Blocks multiple alignment processor. Other types of multiple alignment can be semi-manually reformatted with the Blocks formatter. All of the above Blocks programs have links to send the resulting blocks directly to theCODEHOP program and also provide other information for evaluating the blocks (logos, trees, searches).More information on multiple alignments can be found in the notes for the ISMB97 tutorial on Introduction to making and using protein multiple alignments.Terms and parameters•Degeneracy measures how many different sequences are specified by the primer. The degeneracy of each position depends on the sum of the nucleotides appearing in it (1 to 4). The degeneracy of the primer is the product of the degeneracies of all of its positions.The degenerate core region is scored by its degeneracy, with lower degeneracy scored higher. A standard degenerate alphabet is used in the core region.•Degeneracy strictness specifies how to count nucleotide(s) with low occurrences. It is a parameter between 0 and 1. Strictness of 0 means that all nucleotides that actually appear in the position arecounted to calculate the degeneracy. Strictness of 1 means that only the nucleotides with the highest value in the position are counted.Intermediate strictness values give behavior in between. You can look at a detailed explanation for more information on how thestrictness is calculated.•Temperature is computed for the clamp region plus any adjacent non-degenerate positions in the core region. Differenttemperatures affect only the length of the clamp; highertemperatures result in longer clamps. Temperature is computed using the nearest-neighbor method of Rychlik, et al ( NAR 1990 18:21, 6409-6412). This method takes into consideration the primer and K+ (potassium ions, taken as 50 mM) concentrations.•Nucleotide runs in the clamp region can be limited (the default is5 in a row). If longer runs are encountered, they are broken up bytaking the next highest- scoring nucleotide in the position with the lowest-scoring run nucleotide.•The clamp score is a measure of how well the non-degenerate 5' clamp matches the block given a codon usage table. The score is a weighted average of the maximum scores in each column of the DNA PSSM. A perfect score of 100 is obtained if the clamp consists of invariant methionines and tryptophans, and a minimum score of 25 is obtained if A, C, G and T are equally likely at all positions. In computing the clamp score, positions are increasingly down-weighted asdistance from the degenerate 3' core increases. The clamp score is intended to estimate how well the clamp will stabilize the core inannealing to the target templates. All else being equal, the higher the clamp score the better. However, the clamp score is not relevant to stability of the clamp/product hybrid, which is estimated by the annealing temperature using the nearest neighbor method.•The core/clamp boundary can be restricted to always be on a codon boundary.•Genetic code tables are used to back-translate from protein to DNA.•Codon usage tables are derived from the Codon Usage Database.Tables currently available: request tables for other organisms here.The codon usage table is used to convert the amino acid PSSMconstructed from the input blocks into the DNA PSSM. It can also be used to determine the content of the clamp region.•Clamp residues can be selected as the most common codons of the consensus amino acids are used in the clamp. Else the clamp residues are the ones with maximum weight in the DNA PSSM, which may result in 'artificial' codons. If the clamp residues are taken from the DNA PSSM and more than one residue in a position have maximum weight, one of them is selected at random to keep the clamp non-degenerate.•The program output can show all possible primers or only the single most degenerate primer in each region.Basic tipsOnce you have a block(s) in the input window of the CODEHOP page you can "Look for primers" using the default parameters. You can adjust the setting according to your intended use of the primers and the results you got with the defaults. If you don't get predictions, or you don't like what you get we recommend to first raise the degeneracy to 256 or higher (if you dare ...) and retry. Next, you might try raising the strictness of the core region, for example to 0.1 or 0.25.Your target sequence(s) might be expected to be more similar to some specific sequence(s) in the input blocks. In this case you can bias the primers towards these sequences. Rather than raising the degeneracy or strictness, increase the weights of the specific sequences. The weights are the values following each sequence segment of the block. Usually the highest weight is 100. In the CODEHOP input window increase the weights of your specific sequences (say to 3 or 4 times the original weight or to 200 or 400). You can also remove individual sequences from the input blocks by down-weighing them to 0 (the minimal weight) if they are too divergent and prevent finding primers.For amplification, we recommend using AmpliTaq Gold with a 9' preheat (this provides an automatic hotstart - a hotstart of some kind isimportant). We have had success using the time-release feature with the addition of 15-20 extra cycles. The primer finding strategy of the CODEHOP program (Rose, et al, manuscript accepted by NAR) is different from the usual degenerate PCR strategies. It is desirable to keep annealing temperatures high - 60o C is OK if you have a 60o C clamp. We recommend trying the highest temperature that yields a clean PCR product. We have used "touchdown" PCR down to Tm-3o C or lower, say from 63o C down to a good clamp annealing temperature in -0.5 to -1o C increments, and the remaining cycles are carried out at the 53-57o C clamp annealing temperature for a 60o C clamp. The intent of the touchdown is to give the correct product a head start, because it is likely to anneal at a higher temperature than any failure product. Once the clamp 'takes over', then all primed products, whether correct or not, will be on an even footing, so we try to keep the stringency high in all cycles. With luck, it should not be necessary to gel-purify product, but may rather try cloning directly from the reaction mix if a single band of the expected size is obtained.We and a few other users were already successful in using the CODEHOP strategy and program to amplify various sequences from complicated and diverged genomes. Please let us know if you have any tips to pass on based on your experience using CODEHOP-predicted primers.PublicationResults obtained by this method should cite:"Consensus-degenerate hybrid oligonucleotide primers for amplification of distantly-related sequences" by T.M. Rose, E.R. Schultz, J.G. Henikoff, S. Pietrokovski, C.M. McCallum and S. Henikoff, Nucleic Acids Research, 26(7):1628-1635.Genes identified using CODEHOP.CODEHOP programThe CODEHOP program designs a pool of primers containing all possible 11- or 12-mers for the 3' degenerate core region and having the most probable nucleotide predicted for each position in the 5' non-degenerate clamp region.The program consists of the following steps: (note the scheme on the right)1) A set of blocks is input, where a block is an aligned array of amino acidsequence segments without gaps that represents a highly conserved region ofhomologous proteins. A weight is provided for each sequence segment, which can beincreased to favor the contribution of selected sequences in designing the primer.A codon usage table is chosen for the target genome.2) An amino acid position-specific scoring matrix (PSSM) is computed for each blockusing the odds ratio method.3) A consensus amino acid residue is selected for each position of the block as thehighest scoring amino acid in the matrix.4) For each position of the block, the most common codon corresponding to the aminoacid chosen in step 3 is selected utilizing the user-selected codon usage table.This selection is used for the default 5' consensus clamp in step 8.5) A DNA PSSM is calculated from the amino acid matrix (step 2), genetic codetable and codon usage table. The DNA matrix has three positions for each position of the amino acid matrix. The score for each amino acid is divided amongits codons in proportion to their relative weights from the codon usage table, andthe scores for each of the four different nucleotides are combined in each DNAmatrix position. Nucleotide positions are treated independently when the scores arecombined. As an option, the highest scoring nucleotide residue from each positioncan replace the most common codons from step 4 that are used in the consensus clamp.6) The degeneracy is determined at each position of the DNA matrix based on thenumber of bases found there. As an option, a weight threshold can be specified suchthat bases that contribute less than a minumum weight are ignored in determiningdegeneracy.7) Possible degenerate core regions are identified by scanning the DNA matrix inthe 3' to 5' direction. A core region must start on an invariant 3' nucleotideposition, have length of 11 or 12 positions ending on a codon boundary, and have amaximum degeneracy of 128 (current default). The degeneracy of a region is theproduct of the number of possible bases in each position.8) Candidate degenerate core regions are extended by addition of a 5' consensus clampfrom step 4 or 5. The length of the clamp is controlled by a melting point temperaturecalculation (current default = 60o) and is usually ~20 nucleotides.9) Steps 7 and 8 are repeated on the reverse complement of the DNA matrix from step5 for primers corresponding to the opposite DNA strand.CODEHOP program scheme1) input - - - - - - - - - - - - - - - seq 1 Protein sequence block - - - - - - - - - - - - - - - seq 2- - - - - - - - - - - - - - - seq 3- - - - - - - - - - - - - - - seq 4- - - - - - - - - - - - - - - seq 5- - - - - - - - - - - - - - - etc.|| 2) transformation to AA PSSMV| | | | | | | | | | | | | | | Ala AA PSSM| | | | | | | | | | | | | | | Cys| | | | | | | | | | | | | | | Asp| | | | | | | | | | | | | | | Glu| | | | | | | | | | | | | | | Phe| | | | | | | | | | | | | | | Gly| | | | | | | | | | | | | | | His| | | | | | | | | | | | | | | Ile| | | | | | | | | | | | | | | Lys| | | | | | | | | | | | | | | etc.| || | 3) calculation of AA consensus sequence| V| - - - - - - - - - - - - - - - AA consensus sequence || || | 4) transformation to DNA consensus sequence| V| ------------------------------------------- DNA consensus sequence | || | 5) back-translation to DNA PSSM| V| ||||||||||||||||||||||||||||||||||||||||||| A DNA PSSM| ||||||||||||||||||||||||||||||||||||||||||| C| ||||||||||||||||||||||||||||||||||||||||||| G| ||||||||||||||||||||||||||||||||||||||||||| T| | || | | 6) calculation of degeneracies| | V| | ~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~ position degeneracy values | || | 7) identify degenerate regions ("===")| || 8) identify consensus regions for degenerate regions ("---")| |V V5' -------==== 3' CODEHOP primers output 3' ====--------- 5'。
