化学工程与工艺专业英语试卷
济南大学成人教育《化学工程与工艺专业英语(224201)》期末考试复习题及参考答案

9.钠:( )
A、sodium,Na B、iron,Fe C、gold,Au D、iodine,I E、nitrogen,N F、tin,Sn
答案: A
10.氧:( )
A、calcium,Ca B、carbon,C C、oxygen,O D、silver,Ag E、hydrogen,H F、chlorine,Cl
氧 答案: 二 化硫
13. aluminum oxide:( )
氧 铝 答案: 化
四、 句式改写
请 两 简单 为 导 1. 将 个 句合并 which或that引 的从句
The peak of graphene oxide was shifted to 22.5°. This is due to partial reduction of graphene oxide to graphene caused by coprecipitation reaction of iron ions.
苯 答案: 三甲基
3. calcium hypochlorite:( )
氯 钙 答案: 次 酸
4. sodium perchlorate:( )
氯 钠 答案: 高 酸
5. copper sulphate:( )
铜 答案: 硫酸
6. 2-hexene:( )
烯 答案: 2-己
7. dichloromethane:( )
单词 两 答案: therefore、hence、consequently、thus,在表示“因此”的 任意 个
4. replace:( )、( )
单词 两 答案: displace、substitute,在表示“替代”的 任意 个
5. in addition to:( )、( )
济南大学成人教育《化学工程与工艺专业英语(224201)》期末考试复习题及参考答案全文

一、单项选择题1.硫:()A、zinc,ZnB、carbon,CC、aluminum,AlD、mercury,HgE、chlorine,ClF、sulfur,S答案: F2.氯:()A、calcium,CaB、carbon,CC、oxygen,OD、silver,AgE、hydrogen,HF、chlorine,Cl答案: F3.铝:()A、zinc,ZnB、carbon,CC、aluminum,AlD、mercury,HgE、chlorine,ClF、sulfur,S答案: C4.钙:()A、calcium,CaB、carbon,CC、oxygen,OD、silver,AgE、hydrogen,HF、chlorine,Cl答案: A5.磷:()A、copper,CuB、aluminum,AlC、gold,AuD、tin,SnE、phosphorus,PF、magnesium,Mg 答案: E6.铁:()A、sodium,NaB、iron,FeC、gold,AuD、iodine,IE、nitrogen,NF、tin,Sn答案: B7.镁:()A、copper,CuB、aluminum,AlC、gold,AuD、tin,SnE、phosphorus,PF、magnesium,Mg 答案: F8.碘:()A、sodium,NaB、iron,FeC、gold,AuD、iodine,IE、nitrogen,NF、tin,Sn答案: D9.钠:()A、sodium,NaB、iron,FeC、gold,AuD、iodine,IE、nitrogen,NF、tin,Sn答案: A10.氧:()A、calcium,CaB、carbon,CC、oxygen,OD、silver,AgE、hydrogen,HF、chlorine,Cl答案: C11.铜:()A、copper,CuB、aluminum,AlC、gold,AuD、tin,SnE、phosphorus,PF、magnesium,Mg 答案: A12.汞:()A、zinc,ZnB、carbon,CC、aluminum,AlD、mercury,HgE、chlorine,ClF、sulfur,S答案: D13.银:()A、calcium,CaB、carbon,CC、oxygen,OD、silver,AgE、hydrogen,HF、chlorine,Cl答案: D14.碳:()A、zinc,ZnB、carbon,CC、aluminum,AlD、mercury,HgE、chlorine,ClF、sulfur,S答案: B15.氮:()A、sodium,NaB、iron,FeC、gold,AuD、iodine,IE、nitrogen,NF、tin,Sn答案: E16.锌:()A、zinc,ZnB、carbon,CC、aluminum,AlD、mercury,HgE、chlorine,ClF、sulfur,S答案: A二、填空题1.be named as:()、()答案: be called、be known as,在表示“命名、称作”的词组任意两个2.but:()、()答案: however、nevertheless、yet,在表示“转折”的单词任意两个3.so:()、()答案: therefore、hence、consequently、thus,在表示“因此”的单词任意两个4.replace:()、()答案: displace、substitute,在表示“替代”的单词任意两个5.in addition to:()、()答案: other than、in addition、as well as、except for,在表示“除了”的词组任意两个6.main:()、()答案: predominant、chief、major、principal,在表示“主要”的单词任意两个7.only:()、()答案: just、merely、barely、simply,在表示“仅是”的单词任意两个8.many:()、()答案: numerous、a great deal of、plenty of、a great many of、many a、lots of,在表示“许多”的单词任意两个三、化合物名称翻译1.sodium hydroxide:()答案:氢氧化钠2.trimethylpentane:()答案:三甲基苯3.calcium hypochlorite:()答案:次氯酸钙4.sodium perchlorate:()答案:高氯酸钠5.copper sulphate:()答案:硫酸铜6.2-hexene:()答案: 2-己烯7.dichloromethane:()答案:二氯甲烷8.1-hexene:()答案: 1-己烯9.sodium chloride:()答案:氯化钠10.butyl chloride:()答案:丁基氯11.calcium carbonate:()答案:碳酸钙12.sulfur dioxide:()答案:二氧化硫13.aluminum oxide:()答案:氧化铝四、句式改写1.请将两个简单句合并为which或that引导的从句The peak of graphene oxide was shifted to 22.5°.This is due to partial reduction of graphene oxide to graphene caused by co-precipitation reaction of iron ions.答案: The peak of graphene oxide was shifted to 22.5°, which is due to partial reduction of graphene oxide to graphene caused by co-precipitation reaction of iron ions.五、英译汉1.With the rapid development of industrialization, heavy metal contaminationhas become an important environmental problem, leading to serious healthproblems for human beings.答案:随着工业化的快速发展,重金属污染已成为一个重要的环境问题,给人类带来了严重的健康问题。
化学工程与工艺专业英语课后习题参考答案
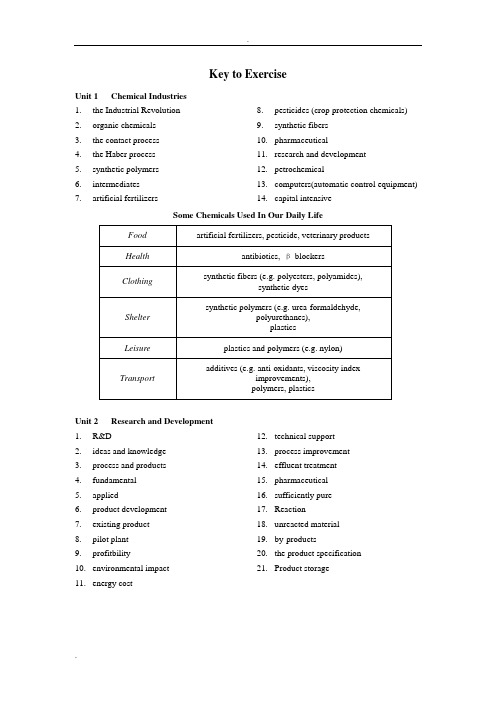
Key to Exercise Unit 1 Chemical Industries1.the Industrial Revolutionanic chemicals3.the contact process4.the Haber process5.synthetic polymers6.intermediates7.artificial fertilizers 8.pesticides (crop protection chemicals)9.synthetic fibers10.pharmaceutical11.research and development12.petrochemicalputers(automatic control equipment)14.capital intensiveSome Chemicals Used In Our Daily LifeUnit 2 Research and Development1.R&D2.ideas and knowledge3.process and products4.fundamental5.applied6.product development7.existing product8.pilot plant9.profitbility10.environmental impact11.energy cost 12.technical support13.process improvement14.effluent treatment15.pharmaceutical16.sufficiently pure17.Reaction18.unreacted material19.by-products20.the product specification21.Product storageUnit 3 Typical Activities of Chemical Engineers1.Mechanical2.electrical3.civil4.scale-upmercial-size6.reactors7.distillation columns8.pumps9.control and instrumentation10.mathematics11.industry12.academia13.steam 14.cooling water15.an economical16.to improve17.P&I Drawings18.Equipment Specification Sheets19.Construction20.capacity and performance21.bottlenecks22.Technical Sales23.new or improved24.engineering methods25.configurationsUnit 4 Sources of Chemicals1.inorganic chemicals2.derive from (originate from)3.petrochemical processes4.Metallic ores5.extraction process6.non-renewable resource7.renewable sources8.energy source9.fermentation process10.selective 11.raw material12.separation and purification13.food industry14.to be wetted15.Key to success16.Crushing and grinding17.Sieving18.Stirring and bubbling19.Surface active agents20.OverflowingUnit 5 Basic Chemicals 1. Ethylene 2. acetic acid 3.4. Polyvinyl acetate5. Emulsion paintUnit 6 Chlor-Alkali and Related Processes 1. Ammonia 2. ammonia absorber 3. NaCl & NH 4OH 4.5. NH 4Cl6. Rotary drier7. Light Na 2CO 3Unit 7 Ammonia, Nitric Acid and Urea 1. kinetically inert 2. some iron compounds 3. exothermic 4. conversion 5. a reasonable speed 6. lower pressures 7. higher temperatures 8.9. energy 10. steam reforming 11. carbon monoxide 12. secondary reformer 13. the shift reaction 14. methane 15. 3:1Unit 8 Petroleum Processing 1. organic chemicals 2. H:C ratios3. high temperature carbonization4. crude tar5. pyrolysis6. poor selectivity7. consumption of hydrogen8. the pilot stage9. surface and underground 10.fluidized bed 11. Biotechnology 12. sulfur speciesUnit 9 PolymersUnit 10 What Is Chemical EngineeringMicroscale (≤10-3m)●Atomic and molecular studies of catalysts●Chemical processing in the manufacture of integrated circuits●Studies of the dynamics of suspensions and microstructured fluidsMesoscale (10-3-102m)●Improving the rate and capacity of separations equipment●Design of injection molding equipment to produce car bumpers madefrom polymers●Designing feedback control systems for bioreactorsMacroscale (>10m)●Operability analysis and control system synthesis for an entire chemicalplant●Mathematical modeling of transport and chemical reactions ofcombustion-generated air pollutants●Manipulating a petroleum reservoir during enhanced oil recoverythrough remote sensing of process data, development and use of dynamicmodels of underground interactions, and selective injection of chemicalsto improve efficiency of recoveryUnit 12 What Do We Mean by Transport Phenomena?1.density2.viscosity3.tube diameter4.Reynolds5.eddiesminar flow7.turbulent flow 8.velocity fluctuations9.solid surface10.ideal fluids11.viscosity12.Prandtl13.fluid dynamicsUnit 13 Unit Operations in Chemical Engineering 1. physical 2. unit operations 3. identical 4. A. D. Little 5. fluid flow6. membrane separation7. crystallization8. filtration9. material balance 10. equilibrium stage model 11. Hydrocyclones 12. Filtration 13. Gravity 14. VaccumUnit 14 Distillation Operations 1. relative volatilities 2. contacting trays 3. reboiler4. an overhead condenser5. reflux6. plates7. packing8.9. rectifying section 10. energy-input requirement 11. overall thermodynamic efficiency 12. tray efficiencies 13. Batch operation 14. composition 15. a rectifying batch 1 < 2 < 3Unit 15 Solvent Extraction, Leaching and Adsorption 1. a liquid solvent 2. solubilities 3. leaching 4. distillation 5. extract 6. raffinate 7. countercurrent 8. a fluid 9. adsorbed phase 10. 400,000 11. original condition 12. total pressure 13. equivalent numbers 14. H + or OH –15. regenerant 16. process flow rates17. deterioration of performance 18. closely similar 19. stationary phase 20. mobile phase21. distribution coefficients 22. selective membranes 23. synthetic24. ambient temperature 25. ultrafiltration26. reverse osmosis (RO).Unit 16 Evaporation, Crystallization and Drying 1. concentrate solutions 2. solids 3. circulation 4. viscosity 5. heat sensitivity 6. heat transfer surfaces 7. the long tube8. multiple-effect evaporators 9.10. condensers 11. supersaturation 12. circulation pump 13. heat exchanger 14. swirl breaker 15. circulating pipe 16. Product17. non-condensable gasUnit 17 Chemical Reaction Engineering1.design2.optimization3.control4.unit operations (UO)5.many disciplines6.kinetics7.thermodynamics,8.fluid mechanics9.microscopic10.chemical reactions 11.more valuable products12.harmless products13.serves the needs14.the chemical reactors15.flowchart16.necessarily17.tail18.each reaction19.temperature and concentrations20.linearUnit 18 Chemical Engineering Modeling1.optimization2.mathematical equations3.time4.experiments5.greater understanding6.empirical approach7.experimental design8.differing process condition9.control systems 10.feeding strategies11.training and education12.definition of problem13.mathematical model14.numerical methods15.tabulated or graphical16.experimental datarmation1.the preliminary economics2.technological changes3.pilot-plant data4.process alternatives5.trade-offs6.Off-design7.Feedstocks 8.optimize9.plant operations10.energy11.bottlenecking12.yield and throughput13.Revamping14.new catalystUnit 19 Introduction to Process Design1. a flowsheet2.control scheme3.process manuals4.profit5.sustainable industrial activities6.waste7.health8.safety9. a reactor10.tradeoffs11.optimizations12.hierarchyUnit 20 Materials Science and Chemical Engineering1.the producing species2.nutrient medium3.fermentation step4.biomass5.biomass separation6.drying agent7.product8.water9.biological purificationUnit 21 Chemical Industry and Environment1.Atmospheric chemistry2.stratospheric ozone depletion3.acid rain4.environmentally friendly products5.biodegradable6.harmful by-product7.efficiently8.power plant emissions 9.different plastics10.recycled or disposed11.acidic waste solutionsanic components13.membrane technology14.biotechnology15.microorganisms。
化工专业英语试题及答案

2014~2015学年秋季学期化工专业英语期末考试一、简单词汇翻译(每题1分,共20分)1、Alkali ( )2、sulphuric ( )3、ammonia ( )4、polymer ( )5、polyethylene( )6、polyurethane ( )7、cyclohexane ( ) 8、hydrogen( )9、nitric ( ) 10、profitability( )11、Seale-up ( ) 12、leaching( )13、corriosion ( ) 14、distillation( )15、gradient ( ) 16、exothermic( ) 17、polycarbonate( )18、isothermal( )19、cybernetics ( ) 20、filtration( )二、句子翻译(每题5分,共30分)1、Once the pilot plant is operational,performance and optimization data can be obtained in order to evaluate the process from an economic point of view.___________________________________________________________2、By contrast,the chemical engineer typically works with much larger quantities of material and with very large equipment.___________________________________________________________3、pressure drives the equilibrium forward ,as four molecules of gas are being transformed into two.___________________________________________________________4、What industry needs to achieve in the process is an acceptable combination of reaction speed and reaction yield.___________________________________________________________5、The ammonia and air mixture can be oxidized to dinitrogen and water.___________________________________________________________6、The important point to keep in mind is that all energy of all kinds must be included,although it may be converted to a single equivalent.___________________________________________________________三、化工专业名词书写(每题一分,共24分)1、加热()2、焙烧()3、吸收()4、冷凝()5、沉降()6、结晶()7、粉碎()8、电解()9、搅动()10、离心()11、平衡()12、体积()13、催化剂()14、一()15、二()16、三()17、四()18、五()19、六()20、七()21、八()22、九、()23、十()24、氮基化合物()四、表达方式运用,用括号里的单词翻译下列句子(每题5分,共20分)1、化学工程师经典的角色是把化学家在实验室里的发现拿来并发展成为能赚钱的、商业规模的化学过程。
化工专业英语试卷参考答案

化学工程与工艺专业英语试题卷参考答案一.Put the following into English or Chinese.1.石油化学制品2. alkali3. sodium carbonate4. 聚合作用5. ammonia6. 药物7. antioxidant8. 聚四氟乙烯9. 环己烷10..carbonmonoxide 11. 乙醇胺12. thermodynamics 13. 光谱学14. refinery 15. 多相的16. isothermal17. 聚氧化亚甲基18. chloride 19. ethanol 20. 聚氯乙烯二.Translation.1. 4 generations under one roof2. to be a winner of the family3. 教务处4.国家助学金5.Principles of chemistry(unit operations)6.生产实习7.graduation thesis8.妇产医院9.transport phenomena10.金窝,银窝,不如自己的狗窝11.normal university 12.综合性大学13.应试教育14.master of business administrator 15.分析化学16.税务局17.party committee 18.专卖店19.chain store(multiple shop) 20.主任医生三、Put the following sentences underlined into ChineseA在20世纪六、七十年代,由于聚乙烯、聚丙烯、尼龙、聚酯环氧树脂等聚合物合成需求量的大量增加,石油化工产品产量呈现爆炸式的增长。
B单一的化工厂产量有从精细化工领域的每年几吨到肥料、石油领域的化工巨头的每年500,000吨。
C一方面,化学生产工业的扩张,另一方面,化学工程与工艺科学的先进,这些使为化工生产奠定了理论基础成为了可能。
化学化工英语试题及答案

化学化工英语试题及答案一、选择题(每题2分,共20分)1. Which of the following is a chemical element?A. WaterB. OxygenC. HydrogenD. Carbon答案:B, C, D2. The chemical formula for table salt is:A. NaOHB. NaClC. HClD. NaHCO3答案:B3. What is the process called when a substance changes from a solid to a liquid?A. SublimationB. VaporizationC. MeltingD. Condensation答案:C4. In the periodic table, which group contains alkali metals?A. Group 1B. Group 2C. Group 17D. Group 18答案:A5. What is the name of the process where a substance decomposes into two or more substances due to heat?A. CombustionB. OxidationC. ReductionD. Decomposition答案:D6. Which of the following is a physical property of a substance?A. ColorB. TasteC. SolubilityD. Reactivity答案:A7. What is the term for a compound that releases hydrogen ions (H+) when dissolved in water?A. BaseB. AcidC. SaltD. Neutral答案:B8. The law of conservation of mass states that in a chemical reaction:A. Mass is lostB. Mass is gainedC. Mass remains constantD. Mass can be converted into energy答案:C9. Which of the following is a type of chemical bond?A. Ionic bondB. Covalent bondC. Hydrogen bondD. All of the above答案:D10. What is the name of the process where a substance absorbs energy and changes from a liquid to a gas?A. MeltingB. VaporizationC. SublimationD. Condensation答案:B二、填空题(每题2分,共20分)1. The symbol for the element iron is ________.答案:Fe2. The pH scale ranges from ________ to ________.答案:0 to 143. A compound that produces a basic solution when dissolvedin water is called a ________.答案:base4. The smallest particle of an element that retains its chemical properties is called a ________.答案:atom5. The process of separating a mixture into its individual components is known as ________.答案:separation6. The study of the composition, structure, and properties of matter is called ________.答案:chemistry7. The process of a substance changing from a gas to a liquid is called ________.答案:condensation8. A(n) ________ reaction is a type of chemical reactionwhere two or more substances combine to form a single product. 答案:synthesis9. The volume of a gas at constant temperature and pressureis directly proportional to the number of ________.答案:moles10. The process of converting a solid directly into a gas without passing through the liquid phase is known as ________. 答案:sublimation三、简答题(每题10分,共30分)1. Explain what is meant by the term "stoichiometry" in chemistry.答案:Stoichiometry is the calculation of the relative quantities of reactants and products in a chemical reaction.It is based on the law of conservation of mass and involvesthe use of balanced chemical equations and the molar massesof substances to determine the amounts of reactants needed to produce a certain amount of product or the amounts ofproducts formed from a given amount of reactant.2. Describe the difference between a physical change and a chemical change.答案:A physical change is a change in the state or form of a substance without altering its chemical composition. Examples include melting, freezing, and boiling. A chemical change, on the other hand, involves a change in the chemical composition of a substance, resulting in the formation of new substances. Examples include combustion and rusting.3. What are the three main types of chemical bonds, and givean example of each.答案:The three main types of chemical bonds are ionic bonds, covalent bonds, and metallic bonds. An ionic bond is formed when electrons are transferred from one atom to another, resulting in the formation of oppositely charged ions. An example is the bond between sodium (Na) and chloride (Cl) in table salt (NaCl). A covalent bond is formed when two atoms share electrons, as seen in water (H2O) where hydrogen atoms share electrons with oxygen. Metallic bonds occur in metals, where a "sea" of delocalized electrons is shared among positively charged metal ions, as in sodium metal。
化学工程与工艺专业英语

the development. 2. Types of Industrial Research and Development The applied or more targeted type of research and
development commonly carried out in industry can be of several type and we will briefly consider each. They are: (i) product development, (ii) process development, (iii) process improvement and (iv) applications development. Even under these headings there are a multitude of aspect so only a typical example can be quoted in each case. The emphasis on each of these will vary considerably within the different sectors of the chemical industry. Product development. Product development
化学工程与工艺专业英语

Commodity chemicals日用化学品specialty chemicals专用化学品fine chemicals精细化学品raw material原料sodium chloride氯化钠unit operation单元操作flow sheet工艺流程图chemical processes化学工艺size reduction粉碎RD研究开发nanotechnology纳米技术micro reaction微量反应end of pipe treatment末端处理macromolecule大分子bio engineering生物工程pharmaceuticals制药lab on a chip芯片实验室chlor alkali氯碱end product终端品sulfur Dioxide二氧化硫sodium carbonate碳酸钠soda ash 苏达灰diammonium hydrogen phosphate磷酸氢二铵dyestuff染料 silicon tetrafluoride四氧化硅petroleum refining石油炼制coal gasification煤气化alkylation烷基化solvent extraction 溶剂萃取catalytic hydrocracking催化加氢裂解 butylene丁烯BTX苯甲苯二甲苯modern refinery现代炼油厂Feedstock原料hydrocarbon碳氢paraffin石蜡fused benzene ring酬和苯环carboxylic acid ester 羧酸脂catalyst deacitivation催化剂失活acetylene乙炔pyridine吡啶natural gas 天然气Liquefied petroleum gas(LPG)液化石油气straight rungasoline 直馏汽油coexisting zone 共存区dumped packing 乱堆填料 ordered packing规整填料rectifhing section经六段stripping sectiong提馏段flash drunt闪蒸段equilibrium stage平衡级batch distillation间歇精馏acetic acid 醋酸dimethylformamide二甲基甲酰胺mixer settler混合沉降器 sieveplate筛板water immiscible水不溶mechanical agitation机械搅拌molecular sieves分子筛ion exchange离子交换activeted carbon活性炭single effect evaporator单板蒸发器multiple effectevaporaion多效蒸发器force circulation强制循环condenser冷凝器reboiler 再沸器conserve energy能量守恒Reflux pump回流泵reversible process可逆过程dynamic equilibrium 动力学平衡entropy熵In bulk 大剂量汉翻英氢氧化钠Sodium hydroxide 硫酸Sulfuric acid 有机合成Organic synthesis 表面活性剂surfactant离子交换Ion exchange热传递Heat transfer工艺流程图Process flow diagram副产物by-products盐酸Hydrochloric acid无机化学品Inorganic chemicals硝酸Nitric acid氢氧化钙Calcium hydroxide磷酸phosphate硅胶Silica gel煤气厂gasworks水处理Water treatment石油化学品Petroleum chemical原油Crude oil精馏distillation沸点Boiling point催化重整Catalytic reforming异构化 isomerization 生物柴油biodiesel 燃油fuel环烷烃cycloparaffin 甲烷methane丙烷propane 乙烯ethylene树脂Resin五元环Five member ring苯benzene杂原子化合物Heteroatom compound相态Phase state气相gas phase液相liquid phase相对挥发度Relative volatility流速flow rate冷凝器condenser多组分混合物Multicomponent mixture回流比reflux ratio萃取器extractor结晶crystallization吸收absorption吸附adsorption 解吸desorption溶质solute溶解性solubility沉淀precipitation1. We define industrial chemistry as the branch of chemistry which applies physical chemical procedures towards the transformation of natural raw materials and their derivatives to products that are of benefit to humanity. 我们定义的工业化学是化学的一个分支,应用物理化学程序对改造天然原材料及其衍生物产品,造福人类。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
南华大学2014 –2015学年度第一学期
化学工程与工艺专业英语课程试卷(A卷12级化学工程与工艺专业)考试日期:2014年11月28日考试类别:考试考试时间:120分钟命题人:审阅人:
一、将下列句子翻译成汉语(20分)
1. Since 1940 the chemical industry has grown at a remarkable rate, although this has slowed sign ificantly in recent years.
2. The chemical industry is a very high technology industry which takes full advantage of the lates t advances in electronics and engineering.
3 Indeed the level of a country’s development may be judged by the production level and sophistic ation of its chemical industry.
4. chemical engineering is that branch of engineering which is concerned with the study of the des ign, manufacture, and operation of plant and machinery in industrial chemical processes.
5.In addition to processes that result in materials with specific high-performance properties, chemi cal engineers continue to design new processes for the low-cost manufacture of polymers.
第1页
二、将下列单词翻译成汉语(10分)
1.polyethylene
2. acetic acid
3.benzene
4. impurity
5. precipitate
6.filtration
7. spontaneous 8. solution
9. immiscible 10. reactor
三、将下列词语翻译成英语(10分)
11.扩散12. 碳
13. 连续的14. 强碱的
15. 盐酸16. 还原
17. 平衡18. 沉降
19. 硫酸盐20. 溶解度
四、选择正确的单词填入下文划线部分(20分)
machines machinery properties branches adapts equipment separated
design production chemical industrial increase foundation engineering tecahnicl
In a wider sense, engineering may be defined as a scientific presentation of the techniques and facilities used in a particular industry. For example, mechanical 1
refers to the techniques and facilities employed to make machines. It is predominantly based on mechanical forces which are used to change the appearance and/or physical properties of the materials being worked, while their chemical 2 are left unchanged. Chemical engineering encompasses the chemical processing of raw materials, based on 3
and physico-chemical phenomena of high complexity.
Thus, chemical engineering is that branch of engineering which is concerned with the study of the
4 , manufacture, and operation of plant and machinery in industrial chemical processes. Chemical engineering is above all based on the chemical sciences, such as physical chemistry, chemical thermodynamics, and chemical kinetics. In doing so, however, it does not simply copy their findings, but them to bulk chemical processing. The principal objectives that set chemical engineering apart from chemistry as a pure science, is to find the most economical route of operation and to design commercial 6 and accessories that suit it best of all. Therefore, chemical engineering is inconceivable without close ties with economics, physics, mathematics, cybernetics, applied mechanics, and other 7 sciences.
第2页
increase in the number of chemical manufactures. Today, petroleum for example serves as the source material for the of about 80 thousand chemicals. The expansion of the chemical process industries on the one hand and advances in the chemical and technical sciences on the other have made it possible to lay theoretical 9 for chemical processing.
As the chemical process industries forged ahead, new data, new relationships and new generalizations were added to the subject-matter of chemical engineering. Many 10
in their own right have separated from the main stream of chemical engineering, such as process and plant design, automation, chemical process simulation and modeling, etc.
五、根据你所学的关于聚合物的知识完成下面表格(20分)
六、根据你对化学工程与工艺的了解,用英语写一篇不少于200字的短文(20分)
1、化学工程与工艺的内容
2、化学工程与工艺的前景
3、你对化学工程与工艺的看法
第3页
化学化工学院化工班学号:姓名:
参考答案
一、1.1940年以来,化学工业一直以引人注目的速度飞速发展。
尽管这种发展的速度近年来已大大减慢。
2.化学工业是高技术工业,它需要利用电子学和工程学的最新成果。
3.事实上,一个国家的发展水平可以通过其化学工业的生产水平和精细程度来加以判断。
4.化学工程学是工程学的一个分支,它涉及工业化化学过程中工厂和机器的设计、制造、和操作的研究。
5.除了这些可以得到具有特别高性能的材料的加工过程,化学工程师们还设计一些新的工艺过程以生产低成本的聚合物。
二、1.聚乙烯 2. 醋酸 3..苯 4.杂质 5.沉淀
6.过滤
7.自发的
8.溶液
9. 不互溶的10.反应器
三、11.diffuse 12.carbon 13.consequentia 14.alkaline 15.hydrochloric
16.reduce 17.equilibrium 18.settling 19.sulphate 20.solubility
四、1.engineering 2.properties 3.chemical 4. design 5.adapts
6.equipment
7. tecahnicl
8.production
9.foundation 10.branches
五、
六、在保证语句流畅单词书写正确的前提下,根据自己所学和想法书写即可。
第4页。
