presentation evaluation criteria
FMEA评分标准
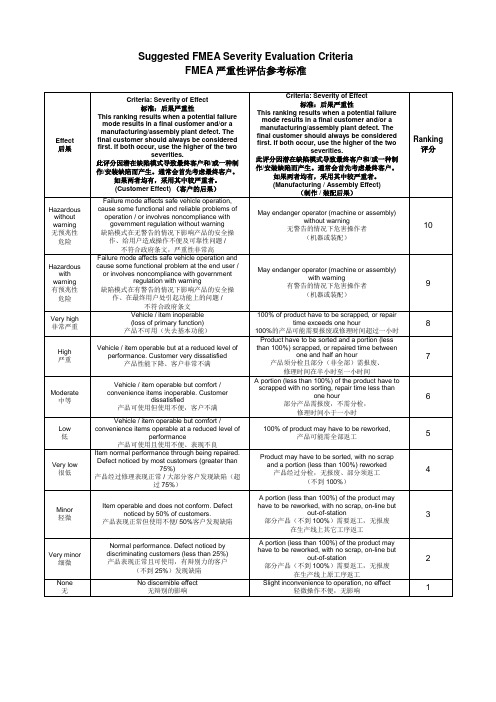
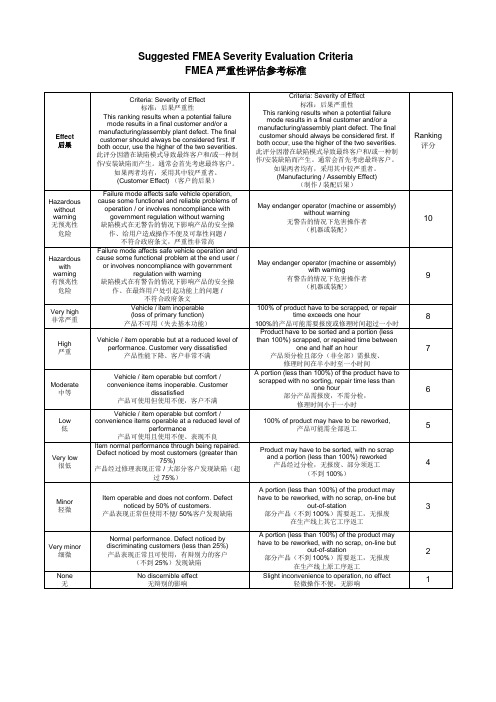
Product may have to be sorted, with no scrap and a portion (less than 100%) reworked 产品经过分检,无报废、部分须返工 (不到 100%)
Vehicle / item operable but at a reduced level of performance. Customer very dissatisfied 产品性能下降、客户非常不满
Vehicle / item operable but comfort / convenience items inoperable. Customer
final customer should always be considered first. If both occur, use the higher of the two
severities. 此评分因潜在缺陷模式导致最终客户和/或一种制 作/安装缺陷而产生。通常会首先考虑最终客户。
如果两者均有,采用其中较严重者。 (Customer Effect) (客户的后果)
or involves noncompliance with government regulation with warning
缺陷模式在有警告的情况下影响产品的安全操 作、在最终用户处引起功能上的问题 / 不符合政府条文 Vehicle / item inoperable (loss of primary function) 产品不可用(失去基本功能)
out-of-station 部分产品(不到 100%)需要返工,无报废
presentation_evaluation
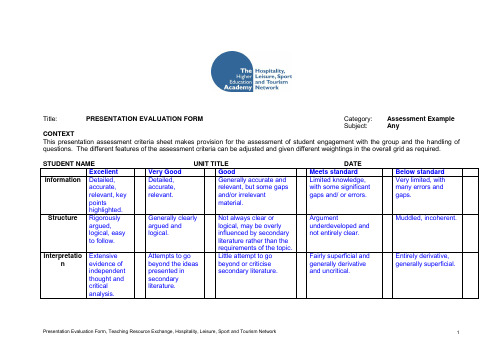
Engaged well with group, encouraged discussion, and responded well to questions.
Attempts to engage with group and responded reasonably well to questions.
Argument underdeveloped and not entirely clear.
Muddled, incoherent.
Interpretation
Extensive evidence of independent thought and critical analysis.
Attempts to go beyond the ideas presented in secondary literature.
STUDENT NAMEUNIT TITLEDATE
Excellent
Very Good
Good
Meets standard
Below standard
Information
Detailed, accurate, relevant, key points highlighted.
Detailed, accurate, relevant.
Generally accurate and relevant, but some gaps and/or irrelevant material.
Limited knowledge, with some significant gaps and/ or errors.
Very limited, with many errors and gaps.
肿瘤大小评分标准简介

•疾病稳定(SD) – 靶病灶最大径之和缩小未达PR,或增 大未达PD。
•疾病进展(PD) – 靶病灶最大径之和至少增加≥20%, 及其绝对值增加至少5mm,出现新病变也视为PD。
注:如仅一个靶病灶的最长径增大≥20%,而记录到的 所有靶病灶的最长径之和增大未达20%,则不应评价为 “PD”。
PD
疗效的确认
•在首要指标为有效率的临床试验中尤其重要 •评价为CR或PR的患者必须在至少4周后重复评 价确认 •评价为SD的患者应在方案规定的间隔时间后 重复评价确认( 一般不低于6-8 周)
瘤转移 直径≧10mm但<15mm的结节不应该视为靶病灶;而<10mm
的结节则不属于病理结节范畴,不必予以记录和进一步观 察。
治疗后如出现坏死、液化,则需重新划定经线, 尽量避开坏死区域。
肿瘤疗效评价-靶病灶
•完全缓解(CR ) – 所有靶病灶消失,无新病灶出现, 且肿瘤标志物正常,至少维持4周。
在整个研究过程中,建议由同一位医师进行肿瘤的测量。
应测量肿瘤病灶的数目 :应代表所有累及器官,每个脏 器最多2个,如果有几个脏器同时受累,应选择至少2个至 多5个作为评价对象。
除了不能用影像学检查,而仅能用临床检查评价的病灶外,所有病灶 必须用影像学检查评价。
胸部X片:仅用于清晰明确病灶,且肺部通气良好。
可测量病灶
至少单径可精确测量, 并记录最大径(LD)
病灶最长径符合以下条件 常规技术(体格检查,传
统CT、X片,MRI)≥20 mm 螺旋CT ≥10 mm
不可测量病灶
除可测量病灶外的所有病灶 病灶最大径小于可测量病灶
规定的大小 骨病灶 膀胱、胆囊病灶 脑脊膜病灶 胸、腹腔/心包积液/盆腔积
FMEA严重性评估参考标准(中英文)
Level 级别
Probability 可能性
Very high 非常高
High 高
Persistent failures 缺陷持续不断
Frequent failures 缺陷频繁出现
Moderate 中等
Occasional failures 缺陷偶尔出现
Low 低
Remote 几乎不会
Relatively few failures 缺陷相对较少
or involves noncompliance with government regulation with warning
缺陷模式在有警告的情况下影响产品的安全操 作、在最终用户处引起功能上的问题 / 不符合政府条文 Vehicle / item inoperable (loss of primary function) 产品不可用(失去基本功能)
than 100%) scrapped, or repaired time between one and half an hour
产品须分检且部分(非全部)需报废、 修理时间在半小时至一小时间
A portion (less than 100%) of the product have to scrapped with no sorting, repair time less than one hour 部分产品需报废,不需分检, 修理时间小于一小时
11返工无报废在生产线上原工序返工2none无nodiscernibleeffect无辩别的影响slightinconveniencetooperationnoeffect轻微操作不便无影响1fmeaoccurrenceevaluationcriteriafmea机率性评估参考标准level级别probability可能性likelyfailurerates可能出现的缺陷机率dppm以一百万块板里的缺陷数计dppmppkranking评分veryhigh非常高persistentfailures缺陷持续不断100perthousandpieces5
评估和面向结果管理的关键术语

EVALUATION AND AID EFFECTIVENESSGlossary of Key Terms in Evaluationand Results Based Managemen t评估和面向结果管理的关键术语 DEVELOPMENT ASSISTANCE COMMITTEE Array经济合作发展组织发展援助委员会(OECD/DAC)授权 国家科技评估中心翻译Glossaire 关键术语GLOSSARY OF KEY TERMS IN EVALUATIONAND RESULTS BASED MANAGEMENT评估和面向结果管理的关键术语1Glossaire 关键术语FOREWORDThe DAC Working Party on Aid Evaluation (WP-EV) has developed this glossary of key terms in evaluation and results-based management because of the need to clarify concepts and to reduce the terminological confusion frequently encountered in these areas. Evaluation is a field where development partners – often with widely differing linguistic backgrounds – work together and need to use a common vocabulary. Over the years, however, definitions evolved in such a way that they bristled with faux ami s, ambivalence and ambiguity. It had become urgent to clarify and refine the language employed and to give it a harmonious, common basis. With this publication, the WP-EV hopes to facilitate and improve dialogue and understanding amo ng all those who are involved in development activities and their evaluation, whether in partner countries, development agencies and banks, or non-governmental organisations. It should serve as a valuable reference guide in evaluation training and in practical development work.The selection of terms and their definitions in the attached glossary have been carefully discussed and analysed and have benefited from advice and inputs, notably from DAC Members and the academic evaluation community. A WP-EV Task Force, chaired by the World Bank, led the overall project, in collaboration with the Secretariat. France took the lead on the French version, whilst the Inter-American Development Bank produced the Spanish translation. Denmark, the Netherlands, Norway, and UNDP provided financial support for the initial collection and review work, and Switzerland contributed financial support for producing this free distribution publication.The process has been guided by the highest considerations of clarity and conciseness and a spirit of collaboration and compromise in terms of the willingness of major development agencies and banks not to impose their specific vocabulary on others. Although terminology will continue to evolve alongside changing development practices and management instruments, this glossary is a “state-of- the-art” of key terms in use today.Niels DabelsteinChair of the Working Party on Aid Evaluation.2Glossaire 关键术语前言 这份术语表包含了关于评估和面向结果的管理的关键术语。
Academic Presentation-Unit1听力答案

His famous PowerPoint presentation has attracted the public, with its meticulously researched content and clear style.
"The only vice president ever to mock his stiff image by (imitating) a wax-museum figure, Gore turns out to be the best professor you never had -- easygoing, knowledgeable and funny." —Rolling Stone
How to Make A Presentation Part3
Keys
• • • • • • • • • • • (1) introduce yourself (2) your name (3) give the talk (4) title (5) be talking about (6) how long (7) ask questions (8) yourself (9) outlined (10) transition (11) moving on to • (12) you help your audience to understand you • (13) to remember that you have your are having a conversation with them • (14) giving a lecture • (15) reading from the script • (16) eye contact • (17) scratching your head • (18) blowing your nose • (19) sticking your hands in the pocket
肿瘤疗效评价标准中英文

Response Evaluation Criteria in Solid Tumors (RECIST) Quick Reference:Eligibility· Only patients with measurable disease at baseline should be included in protocols where objective tumor response is the primary endpoint.Measurable disease - the presence of at least one measurable lesion. If the measurable disease is restricted to a solitary lesion, its neoplastic nature should be confirmed by cytology/histology. Measurable lesions - lesions that can be accurately measured in at least one dimension with longest diameter ³20 mm using conventional techniques or ³10 mm with spiral CT scan.Non-measurable lesions - all other lesions, including small lesions (longest diameter <20 mm with conventional techniques or <10 mm with spiral CT scan), i.e., bone lesions, leptomeningeal disease, ascites, pleural/pericardial effusion, inflammatory breast disease, lymphangitis cutis/pulmonis, cystic lesions, and also abdominal masses that are not confirmed and followed by imaging techniques; and.· All measurements should be taken and recorded in metric notation, using a ruler or calipers. All baseline evaluations should be performed as closely as possible to the beginning of treatment and never more than 4 weeks before the beginning of the treatment.· The same method of assessment and the same technique should be used to characterize each identified and reported lesion at baseline and during follow-up.· Clinical lesions will only be considered measurable when they are superficial (e.g., skin nodules and palpable lymph nodes). For the case of skin lesions, documentation by color photography, including a ruler to estimate the size of the lesion, is recommended.Methods of Measurement –· CT and MRI are the best currently available and reproducible methods to measure target lesions selected for response assessment. Conventional CT and MRI should be performed with cuts of 10 mm or less in slice thickness contiguously. Spiral CT should be performed using a 5 mm contiguous reconstruction algorithm. This applies to tumors of the chest, abdomen and pelvis. Head and neck tumors and those of extremities usually require specific protocols.· Lesions on chest X-ray are acceptable as measurable lesions when they are clearly defined and surrounded by aerated lung. However, CT is preferable.· When the primary endpoint of the study is objective response evaluation, ultrasound (US) should not be used to measure tumor lesions. It is, however, a possible alternative to clinical measurements of superficial palpable lymph nodes, subcutaneous lesions and thyroid nodules. US might also be useful to confirm the complete disappearance of superficial lesions usually assessed by clinical examination.· The utilization of endoscopy and laparoscopy for objective tumor evaluation has not yet been fully and widely validated. Their uses in this specific context require sophisticated equipment and a high level of expertise that may only be available in some centers. Therefore, the utilization of such techniques for objective tumor response should be restricted to validation purposes in specialized centers. However, such techniques can be useful in confirmingcomplete pathological response when biopsies are obtained.· Tumor markers alone cannot be used to assess response. If markers are initially above the upper normal limit, they must normalize for a patient to be considered in complete clinical response when all lesions have disappeared.· Cytology and histology can be used to differentiate between PR and CR in rare cases (e.g., after treatment to differentiate between residual benign lesions and residual malignant lesions in tumor types such as germ cell tumors).Baseline documentation of “Target” and “Non-Target” lesions· All measurable lesions up to a maximum of five lesions per organ and 10 lesions in total, representative of all involved organs should be identified as target lesions and recorded and measured at baseline.· Target lesions should be selected on the basis of their size (lesions with the longest diameter) and their suitability for accurate repeated measurements (either by imaging techniques or clinically).· A sum of the longest diameter (LD) for all target lesions will be calculated and reported as the baseline sum LD. The baseline sum LD will be used as reference by which to characterize the objective tumor.· All other lesions (or sites of disease) should be identified as non-target lesions and should also be recorded at baseline. Measurements of these lesions are not required, but the presence or absence of each should be noted throughout follow-up.Response CriteriaEvaluation of target lesions* Complete Response (CR): Disappearance of all target lesions* Partial Response (PR): At least a 30% decrease in the sum of the LD of target lesions, taking as reference the baseline sum LD* Progressive Disease (PD): At least a 20% increase in the sum of the LD of target lesions, taking as reference the smallest sum LD recorded since the treatment started or the appearance of one or more new lesions* Stable Disease (SD): Neither sufficient shrinkage to qualify for PR nor sufficient increase to qualify for PD, taking as reference the smallest sum LD since the treatment started Evaluation of non-target lesions* Complete Response (CR): Disappearance of all non-target lesions and normalization of tumor marker level* Incomplete Response/ Stable Disease (SD): Persistence of one or more non-targetlesion or/and maintenance of tumor marker level above the normal limits* Progressive Disease (PD): Appearance of one or more new lesions and/or unequivocal progression of existing non-target lesions (1)(1) Although a clear progression of “non target” lesions only is exception al, in such circumstances, the opinion of the treating physician should prevail and the progression status should be confirmed later on by the review panel (or study chair).Evaluation of best overall responseThe best overall response is the best response recorded from the start of the treatment until disease progression/recurrence (taking as reference for PD the smallest measurements recorded since the treatment started). In general, the patient's best response assignment will depend on the achievement of both measurement and confirmation criteriaTarget lesions Non-Target lesions New Lesions Overall responseCR CR No CRCR Incomplete response/SD No PRPR Non-PD No PRSD Non-PD No SDPD Any Yes or No PDAny PD Yes or No PDAny Any Yes PD· Patients with a global deterioration of health status requiring discontinuation of treatment without objective evidence of disease progression at that time should be classified as having “symptomatic deterioration”. Every effort should be made to document the objective progression even after discontinuation of treatment.· In some circumstances it may be difficult to distinguish residual disease from normal tissue. When the evaluation of complete response depends on this determination, it is recommended that the residual lesion be investigated (fine needle aspirate/biopsy) to confirm the complete response status.Confirmation· The main goal of confirmation of objective response is to avoid overestimating the response rate observed. In cases where confirmation of response is not feasible, it should be made clear when reporting the outcome of such studies that the responses are not confirmed.· To be assigned a status of PR or CR, changes in tumor measurements must be confirmed by repeat assessments that should be performed no less than 4 weeks after the criteria for response are first met. Longer intervals as determined by the study protocol may also be appropriate.· In the case of SD, follow-up measurements must have met the SD criteria at least once after study entry at a minimum interval (in general, not less than 6-8 weeks) that is defined in the study protocolDuration of overall response· The duration of overall response is measured from the time measurement criteria are met for CR or PR (whichever status is recorded first) until the first date that recurrence or PD is objectively documented, taking as reference for PD the smallest measurements recorded since the treatment started.Duration of stable disease· SD is measured from the start of the treatment until the criteria for disease progression aremet, taking as reference the smallest measurements recorded since the treatment started. · The clinical relevance of the duration of SD varies for different tumor types and grades. Therefore, it is highly recommended that the protocol specify the minimal time interval required between two measurements for determination of SD. This time interval should take into account the expected clinical benefit that such a status may bring to the population under study. Response review· For trials where the response rate is the primary endpoint it is strongly recommended that allresponses be reviewed by an expert independent of the study at the study’s completion.Simultaneous review of the patients’ files and radiological images is the best approach. Reporting of results· All patients included in the study must be assessed for response to treatment, even if there are major protocol treatment deviations or if they are ineligible. Each patient will be assigned one of the following categories: 1) complete response, 2) partial response, 3) stable disease, 4) progressive disease, 5) early death from malignant disease, 6) early death from toxicity, 7) early death because of other cause, or 9) unknown (not assessable, insufficient data).· All of the patients who met the eligibility criteria should be included in the main analysis of the response rate. Patients in response categories 4-9 should be considered as failing to respond to treatment (disease progression). Thus, an incorrect treatment schedule or drug administration does not result in exclusion from the analysis of the response rate. Precise definitions for categories 4-9 will be protocol specific.· All conclusions should be based on all eligible patients.· Subanalyses may then be performed on the basis of a subset of patients, excluding those for whom major protocol deviations have been identified (e.g., early death due to other reasons, early discontinuation of treatment, major protocol violations, etc.). However, these subanalyses may not serve as the basis for drawing conclusions concerning treatment efficacy, and the reasons for excluding patients from the analysis should be clearly reported.· The 95% confidence intervals should be provided.RECIST (肿瘤疗效评价标准) 快速参考筛选条件:l 当客观肿瘤疗效是评价的主要终点时,只有在基线有可测量疾病的人可以包含到试验方案中。
系统评价Meta分析方法学质量的评价工具AMSTAR

系统评价Meta分析方法学质量的评价工具AMSTAR一、本文概述Overview of this article本文旨在探讨和评价《系统评价Meta分析方法学质量的评价工具AMSTAR》这篇文章,深入解析AMSTAR(A Measurement Tool to Assess Systematic Reviews)这一评价工具在系统评价和Meta分析中的应用和重要性。
我们将从AMSTAR的背景、目的、方法、结果以及讨论等方面进行全面介绍,以便读者更好地理解和掌握这一评价工具。
This article aims to explore and evaluate the AMSTAR (A Measurement Tool to Assess Systematic Reviews) evaluation tool for the quality of meta-analysis methodology, and to provide an in-depth analysis of its application and importance in system evaluation and meta-analysis. We will provide a comprehensive introduction to AMSTAR from its background, purpose, methods, results, and discussion, in order for readers to better understand and master this evaluation tool.我们将简要介绍系统评价和Meta分析在医学研究中的重要性,以及为什么需要对这些方法学质量进行评价。
接着,我们将详细介绍AMSTAR的发展背景、理论基础和构建过程,以便读者了解该评价工具的起源和依据。
We will briefly introduce the importance of system evaluation and meta-analysis in medical research, as well as why it is necessary to evaluate the quality of these methodologies. Next, we will provide a detailed introduction to the development background, theoretical basis, and construction process of AMSTAR, so that readers can understand the origin and basis of this evaluation tool.在方法部分,我们将详细介绍AMSTAR的具体内容、评分标准和评价方法,包括各个条目的定义、评分依据以及如何运用AMSTAR对系统评价和Meta分析进行质量评价。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Professional StudiesPresentation Evaluation CriteriaThe numbers in the top of the boxes are points in a continuum. For example, you can assign 20 points for Organization. As long as you do not give more points than suggested in the leftmost box, the score will range between 0 and 100 when you add up the numbers.Organization (20%)20 Consistently clear, concise, well organized. Points were easy to follow because of the organization. Transitions between sections smooth and coordinated. 15Usually clear, concise,well organized. Most of thepresentation was easy tofollow. Transitions betweensections usually coordinated.10Not always clear orconcise. Organization wasadequate, but weak.Occasionally wandered andwas sometimes difficult tofollow. Transitions betweensections weak.5Often unclear anddisorganized, rambled toomuch. The presentation wasconfusing and difficult tofollow. Transitions betweensections awkward.Topic Knowledge (20%)20Displayed an excellent grasp of the material. Demonstrated excellent mastery of content, application and implications. Excellent research depth. 15Displayed a generalgrasp of the material.Demonstrated good masteryof content, application andimplications. Good researchdepth.10Displayed some graspof the material.Demonstrated adequatemastery of content,application and implications.Research not very deep.5Displayed a poor graspof the material.Demonstrated a superficialhandling of content,application and implications.Little depth of research.Creativity (10%)10Very creative and original. Imaginative design and use of materials. Novel handouts, visual aids, or methods. 8 Exhibitedsomeoriginality and creativity.5 Routinetreatment,minimal thought given tooriginality or creativity.3Lacked creativity. Veryordinary and mundane.Visual Aids (15%)15Simple, clear, easy to interpret, easy to read. Well coordinated with content, well designed, used very effectively. Excellent example of how to prepare and use good visual aids 11Usually clear, easy tointerpret, easy to read.Generally well coordinatedwith content, design wasokay, generally usedeffectively. Demonstratedsome understanding of howto use visual aids.8 Marginallyacceptable,too complex, crowded,difficult to read or interpret.Adequate coordination withcontent. Used onlyadequately. Showed littleunderstanding of how toprepare and use visual aids.4Poor quality visual aids(or none), hard to read,technically inaccurate, poorlyconstructed. Poorcoordination with content.Used poorly. The presenterdid not seem to know how toprepare or use visual aidseffectively.Summary (15%)15Clear, concise, major points emphasized, clear recommendations, strong conclusion or call for action. 11Referred to main points,recommendations weak ormissing, weak conclusion orcall for action.8Vague mention of majorpoints, no recommendations,weak conclusion, weak or nocall for action.4No summary, norecommendations, noconclusions, no call foraction.Stage Presence (20%)20 Excellent stage presence. Confident, used notes well, at ease, excellent gestures, good audience attention, good eye contact. 15Good stage presence.Fairly confident, used notesfairly well, good gestures,acceptable audienceattention and eye contact.10 Adequate stagepresence. Read parts,fumbled with notes, severaldistracting mannerisms,minimal gestures, minimaleye contact, too many um=s.5Poor stage presence.Unprepared, awkward,shuffled papers, poor eyecontact, lots of um=s, turnedfrom audience to readoverheads, shuffled feet,fidgeted. Poor gestures.TOTAL POINTS COMMENTS:PRESENTATION EVALUATION CRITERIA1.Shorter College – Professional Studies is committed to helping students develop superiorpresentation skills. One method for accomplishing this is to evaluate each presentation in every class. This evaluation rubric was developed by the faculty to help assureconsistency in evaluating student presentations.2.This rubric must be used in every class in which students make presentations. No otherevaluation forms are to be used by any instructor.3.Give each student a copy of the Presentation Evaluation Criteria form at the beginning ofthe class. This will indicate how their presentations will be evaluated. If any of thecriteria are not clear, discuss what they mean.plete an evaluation for each individual or group presentation. It would be mosthelpful if students could receive the completed evaluation form on the same evening asthe presentation. If that is not possible, evaluation forms should be returned to students no later than the next class.5.OPTIONAL You may want to have students participate in the evaluations. Forinstance, you might ask three different students to evaluate each presentation. Studentevaluations can be averaged together and used to allocate some percentage of the grade.For example, some instructors use the average student ratings for 50 percent of thepresentation grade.6.During the individual or group presentation:a. Remind students that you will be using the standard presentation evaluation rubric.This rubric indicates how their presentations will be evaluated.b. Simply place a check mark in the appropriate box for each of the six categories.Write any additional comments at the bottom.c. Add up all the values in the upper left hand corner of each box checked, and write thetotal points in the space at the bottom of the form. Letter grades are matched to therange of possible scores.7.As soon as the presentation ends (if possible), it is beneficial to spend a few minutes onimmediate feedback. Ask the class to comment on what they thought was most positive about the presentation. Then ask what might have been improved to make thepresentation better. This kind of immediate feedback should be reassuring to thepresenter, as well as indicating how to improve future presentations.。
