美国药典溶出介质缓冲液的配制
溶出度检查法美国药典USP-711

<711> DISSOLUTION溶出度(USP39-NF34 Page 540) General chapter Dissolution <711> is being harmonized with the corresponding texts of the European Pharmacopoeia and/or the Japanese Pharmacopoeia. These pharmacopeias have undertaken to not make any unilateral change to this harmonized chapter.通则<711>溶出度与欧盟药典和日本药典中的相应部分相统一。
这三部药典承诺不做单方面的修改。
Portions of the present general chapter text that are national USP text, and therefore not part of the harmonized text, are marked with symbols to specify this fact.本章中的部分文字为本国USP内容,并没有与其他药典统一。
此部分以()标注。
This test is provided to determine compliance with the dissolution requirements where stated in the individual monograph for dosage forms administered orally. In this general chapter, a dosage unit is defined as 1 tablet or 1 capsule or the amount specified. Of the types of apparatus designs described herein, use the one specified in the individual monograph. Where the label states that an article is enteric coated and a dissolution or disintegration test does not specifically state that it is to be applied to delayed-release articles and is included in the individual monograph, the procedure and interpretation given for Delayed-Release Dosage Forms are applied, unless otherwise specified in the individual monograph.本测试用于检测药品口服制剂的溶出度是否符合各论中的规定。
溶出介质的选用与配

溶出介质的选用与配No.4 ——溶出介质的选用与配制】1. 溶出介质的选用建议采用多条溶出曲线对产品的内在品质进行评价。
选取原则建议如下:【普通制剂】(1)酸性药物制剂pH值分别为1.0或1.2、5.5~6.5、6.8~7.5和水;(2)中性或碱性药物/包衣制剂 pH值分别为1.0或1.2、3.0~5.0、6.8和水;(3)难溶性药物制剂 pH值分别为1.0或1.2、4.0~4.5、6.8和水;(4)肠溶制剂 pH值分别为1.0或1.2、6.0、6.8和水;【调释制剂】pH值分别为1.0或1.2、3.0~5.0、6.8~7.5和水。
2. 其他事项(1) 以上含有pH值范围的,可分别按0.5或1.0间隔测试,如差异较大,应分别予以关注;如无明显差异,酌情选择即可。
pH值1.0或1.2,系因各国要求不同。
(2)在了解了该药物pKa值之后,如pKa±1.0值未能涵盖于以上各pH值中,建议测定pKa±1.0值溶出曲线,以更好地把握该药物的溶出特性。
(3)如能测定更多pH值曲线,自然可得到更多关于该制剂内在品质信息;当然工作量亦会增加。
(4)无论何种制剂都不建议采用pH8.0以上的介质进行表达。
如确有必要,应提供充足的理由。
(5)某些品种还可根据临床使用情况,考虑在“含有胃蛋白酶的模拟胃液”和“含有胰酶的模拟肠液”中的体外溶出情况。
(6)含有胃蛋白酶的模拟胃液,英文全称为Simulated Gastric Fluid,简写为SGF。
有时亦可附有下标“sp”,缩写为SGF[sp]。
其配制方法——【中国药典】取稀盐酸16.4ml(相当于盐酸3.84ml),加水约800ml与胃蛋白酶10g,摇匀后,加水稀释成1000ml,即得。
【美国药典】取2.0氯化钠和3.2g胃蛋白酶(标识应为每mg中含800~2500个活度单位),加7.0ml盐酸和水使溶解至1000ml,即得。
该溶液pH值应为1.2。
阿奇霉素溶出条件及液相测定方法

阿奇霉素溶出条件及液相测定方法
一、溶出条件
1、溶出介质1:PH6.0磷酸盐缓冲液(0.1mol/L磷酸氢二钠1000ml,加盐酸约6.6ml,
调至PH6.0±0.5)
溶出介质2:PH4.5醋酸盐缓冲液(取醋酸钠18g加水溶解,加冰醋酸9.8ml,加水稀释至1000ml)
溶出介质3:PH2.0盐酸溶液(取盐酸溶液9.0ml,加水至1000ml)
溶出介质4:0.5%SDS水溶液(取十二烷基硫酸钠18g,加水溶解并稀释至1000ml)
2、溶出方法:第二法浆法
3、溶出介质体积:900ml
4、取样体积:2ml
5、转速:75r/min
6、温度:37℃±0.5℃
7、取样时间:5、10、20、30、45min
二、液相方法
1、色谱条件:
色谱柱:kromasil 4.6*150mm 5µm
流动相:0.025mol/L磷酸氢二钾(加磷酸调PH至7.10):乙腈=55:45
检测波长:210nm
柱温:40℃
进样量:20µl
流速:1.0ml/min
2、对照品溶液:精密量取阿奇霉素对照品0.028g置100ml量瓶中,加溶出介质溶解,
并稀释至刻度,摇匀,作为对照品溶液。
3、样品:直接进样。
(注:PH4.5醋酸盐缓冲液介质的中样品,取样后需做处理,即往
2ml样品中加入32%氢氧化钠溶液,摇匀后进样。
)
4、数据处理(注:PH2.0盐酸中样品,因其稳定性较差,易产生降解峰,数据处理时
应取降解峰与主峰之和)。
欧洲及美国日本药典不同pH溶出介质(缓冲盐溶液)配制
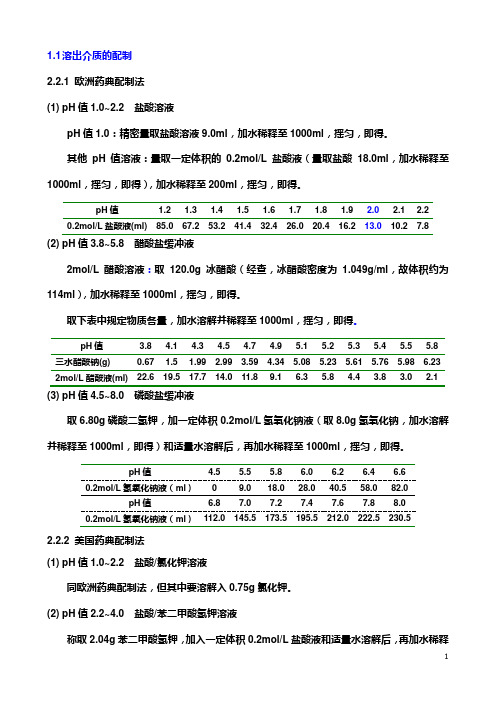
1.1 溶出介质的配制2.2.1 欧洲药典配制法(1) pH值1.0~2.2 盐酸溶液pH值1.0:精密量取盐酸溶液9.0ml,加水稀释至1000ml,摇匀,即得。
其他pH值溶液:量取一定体积的0.2mol/L盐酸液(量取盐酸18.0ml,加水稀释至1000ml,摇匀,即得),加水稀释至200ml,摇匀,即得。
pH值 1.2 1.3 1.4 1.5 1.6 1.7 1.8 1.9 2.0 2.1 2.20.2mol/L盐酸液(ml) 85.0 67.2 53.2 41.4 32.4 26.0 20.4 16.2 13.0 10.2 7.8 (2) pH值3.8~5.8 醋酸盐缓冲液2mol/L醋酸溶液:取120.0g冰醋酸(经查,冰醋酸密度为1.049g/ml,故体积约为114ml),加水稀释至1000ml,摇匀,即得。
取下表中规定物质各量,加水溶解并稀释至1000ml,摇匀,即得。
pH值 3.8 4.1 4.3 4.5 4.7 4.9 5.1 5.2 5.3 5.4 5.5 5.8 三水醋酸钠(g) 0.67 1.5 1.99 2.99 3.59 4.34 5.08 5.23 5.61 5.76 5.98 6.23 2mol/L醋酸液(ml) 22.6 19.5 17.7 14.0 11.8 9.1 6.3 5.8 4.4 3.8 3.0 2.1 (3) pH值4.5~8.0 磷酸盐缓冲液取6.80g磷酸二氢钾,加一定体积0.2mol/L氢氧化钠液(取8.0g氢氧化钠,加水溶解并稀释至1000ml,即得)和适量水溶解后,再加水稀释至1000ml,摇匀,即得。
pH值 4.5 5.5 5.8 6.0 6.2 6.4 6.60.2mol/L氢氧化钠液(ml)0 9.0 18.0 28.0 40.5 58.0 82.0pH值 6.8 7.0 7.2 7.4 7.6 7.8 8.00.2mol/L氢氧化钠液(ml)112.0 145.5 173.5 195.5 212.0 222.5 230.52.2.2 美国药典配制法(1) pH值1.0~2.2 盐酸/氯化钾溶液同欧洲药典配制法,但其中要溶解入0.75g氯化钾。
美国药典25版收载的溶出度品种概况

美国药典25版收载的溶出度品种概况美国药典25版收载的溶出度品种概况溶出度系指药物从片剂或胶囊剂等固体制剂在规定溶剂中溶出的速度和程度,溶出限度以标示量的百分数表示,是一种模拟口服固体制剂在胃肠道中崩解和溶出体外试验,是控制药物制剂质量的检测方法,是研究固体及半固体制剂所含主药的晶型、粒度、处方组成、辅料品种和性质、生产工艺等对制剂质量统一性的新方法。
因此溶出度已迅速发展成为广泛应用于质量标准中的检验方法。
美、英、日、中国药典已把溶出度列为保证药物制剂安全有效的重要检测项目。
现将美国药典25版收载溶出度品种内容概述如下:1、美国药典25版共收载的片剂和胶囊有698个品种,其中进行溶出度品种有535个,约占77%。
缓释制剂一般测定释放度,如碳酸锂延迟释放片、盐酸多西环素延迟释放胶囊、吲哚美辛持续释放片、阿斯匹林持续释放片。
阴道片和溶液片一般采用崩解时限,如克霉唑阴道片、盐酸可卡因溶液片。
无机盐类制剂如硅镁铝片、氧化镁片、碳酸钙镁鍶复方片,溶出量测定方法繁琐,需用效价测定或溶出量难以测定的品种,如维生素D2片剂或胶囊、酯化雌激素片、已烯雌酚片、颠茄提取片、洋地黄片、依托红霉素胶囊及片、三乙酰竹桃霉素胶囊、胡箩卜素片等均采用崩解时限。
2、采用仪器装置有1法(转篮法)211个品种,2法(桨法)约332个品种,采用释放度装置3法(往复瓶法)3个品种,如卡马西平片、盐酸羟嗪片、碘甲腺氨酸钠片。
有的品种设2~4种测定法按标签中规定选择测定。
3、转速 I法转速100rpm 185个品种II法转速50rpm 228个品种(也有采用转速35rpm、75rpm、120rpm、150rpm)。
4、溶出介质以水(245个品种),0.01mol/L~0.1mol/L盐酸溶液(约146个品种)为主,其他介质有:醋酸盐缓冲液(pH4.5),磷酸盐缓冲液(pH4.0~ 8.6),三羟基甲基氨基甲烷溶液(pH7.2~9.0),不含酶的人工胃液或人工肠液等。
usp中缓冲溶液和标准溶液的详细配制方法

usp中缓冲溶液和标准溶液的详细配制⽅法BUFFER SOLUTIONSThe successful completion of many Pharmacopeial tests and assays requires adjustment to or maintenance of a specified pH by the addition of buffer solutions. In pH measurements, standard buffer solutions are required for reference purposes. For convenience, the preparation of these solutions is in some instances described in the sections in which their use is specified;i.e., five separate phosphate buffers are described under Antibiotics -- Microbial Assays <81>, and several miscellaneous single-purpose solutions are described in the individual monographs.A solution is said to be buffered if it resists changes in theactivity of an ion on the addition of substances that are expected to change the activity of that ion. Buffers are substances or combinations of substances that impart this resistance to a solution. Buffered solutions are systems in which the ion is in equilibrium with substances capable of removing or releasing the ion.Buffer capacity refers to the amount of material that may be added to a solution without causing a significant change in ion activity. It is defined as the ratio of acid or base added (in gram-equivalents per liter) to the change in pH (in pH units). The capacity of a buffered solution is adjusted to the conditions of use, usually by adjustment of the concentrations of buffer substances.Buffers are used to establish and maintain an ion activity withinnarrow limits. The most common systems are used to establishhydrogen-ion activity for the calibration of pH meters, in thepreparation of dosage forms that approach isotonicity, in analyticalprocedures, and to maintain stability of various dosage forms. Buffers used in physiological systems are carefully chosen so as not to interfere with pharmacological activity of the medicament or normal function of the organism. It is essential that buffers used in chemical analysis be compatible with the substance determined and the reagents used.STANDARD BUFFER SOLUTIONS -- Standard solutions of definite pH are readily available in buffer solutions prepared from the appropriate reagents. In addition, buffer solutions, buffer tablets, and buffersolids may be obtained from commercial sources in convenient prepackaged form. Such preparations are available for the entire working range in pharmaceutical analysis, but are not recommended for pH meter standardization (see pH <791>. The required reagents are described in the section, Reagents.Previously dry the crystalline reagents, except the boric acid, at 110 degrees to 120 degrees for 1 hour.NOTE -- Where water is specified for solution or dilution of test substances in pH determinations, use carbon dioxide-free water.Store the prepared solutions in chemically resistant, tight containerssuch as Type I glass bottles. Use the solutions within 3 months.Standard buffer solutions for various ranges between pH 1.2 and 10.0may be prepared by appropriate combinations of 0.2 M solutions described herein, used in the proportions shown in the accompanying table. The volumes shown in the table are for 200 mL of buffer solution.1. Hydrochloric Acid, 0.2 M, and Sodium Hydroxide, 0.2 M -- Prepareand standardize as directed under Volumetric Solutions.2. Potassium Biphthalate, 0.2 M -- Dissolve 40.85 g of potassium biphthalate [KHC[6]H[4](COO)[2]] in water, and dilute with water to 1000 mL.3. Potassium Phosphate, Monobasic 0.2 M -- Dissolve 27.22 g of monobasic potassium phosphate (KH[2]PO[4]) in water, and dilute with water to 1000 mL.4. Boric Acid and Potassium Chloride, 0.2 M -- Dissolve 12.37 g ofboric acid (H[3]BO[3]) and 14.91 g of potassium chloride (KCl) in water, and dilute with water to 1000 mL.5. Potassium Chloride, 0.2 M -- Dissolve 14.91 g of potassiumchloride (KCl) in water, and dilute with water to 1000 mL.6. Acetic Acid, 2 N -- Prepare and standardize as directed under Volumetric Solutions.Composition of Standard Buffer SolutionsSee Graphic G-1097------------------------------------------------------------------------ Hydrochloric Acid BufferPlace 50 mL of the potassium chloride solution in a 200-mL volumetric flask, add the specified volume of the hydrochloric acid solution, thenadd water to volume.pH 1.2 1.3 1.4 1.5 1.6 1.7 1.8 1.9 2.0 2.1 2.2HCl, mL 85.0 67.2 53.2 41.4 32.4 26.0 20.4 16.2 13.0 10.2 7.8Acid Phthalate BufferPlace 50 mL of the potassium biphthalate solution in a 200-mL volumetric flask, add the specified volume of the hydrochloric acid solution, then add water to volume.pH 2.2 2.4 2.6 2.8 3.0 3.2 3.4 3.6 3.8 4.0HCl, mL 49.5 42.2 35.4 28.9 22.3 15.7 10.4 6.3 2.9 0.1Neutralized Phthalate BufferPlace 50 mL of the potassium biphthalate solution in a 200-mL volumetric flask, add the specified volume of the sodium hydroxide solution, then add water to volume.pH 4.2 4.4 4.6 4.8 5.0 5.2 5.4 5.6 5.8NaOH, mL 3.0 6.6 11.1 16.5 22.6 28.8 34.1 38.8 42.3Phosphate BufferPlace 50 mL of the monobasic potassium phosphate solution in a 200-mL volumetric flask, add the specified volume of the sodium hydroxide solution, then add water to volume.pH 5.8 6.0 6.2 6.4 6.6 6.8 7.0 7.2 7.4 7.6 7.8 8.0NaOH, mL 3.6 5.6 8.1 11.6 16.4 22.4 29.1 34.7 39.1 42.4 44.5 46.1 Alkaline Borate BufferPlace 50 mL of the boric acid and potassium chloride solution in a200-mL volumetric flask, add the specified volume of the sodium hydroxide solution, then add water to volume.pH 8.0 8.2 8.4 8.6 8.8 9.0 9.2 9.4 9.6 9.8 10.0NaOH, mL 3.9 6.0 8.6 11.8 15.8 20.8 26.4 32.1 36.9 40.6 43.7Acetate BufferPlace the specified amount of sodium acetate (NaC[2]H[3]O[2].3H[2]O) in a 1000-mL volumetric flask, add the specifiedvolume of the acetic acid solution, then add water to volume, and mix.pH 4.1 4.3 4.5 4.7 4.9 5.1 5.2 5.3 5.4 5.5pH (measured) 4.10 4.29 4.51 4.70 4.90 5.11 5.18 5.30 5.40 5.48NaC[2]H[3]O[2]. 1.50 1.99 2.99 3.59 4.34 5.08 5.23 5.61 5.76 5.98 3H[2]O, g CH[3]COOH, mL 19.5 17.7 14.0 11.8 9.1 6.3 5.8 4.4 3.8 3.0。
No.4 —— 溶出介质的选用与配制

上海市药品检验所谢沐风撰写【No.4 ——溶出介质的选用与配制】—— 上海市药品检验所 谢沐风 撰写1. 溶出介质的选用 建议采用多条溶出曲线对产品的内在品质进行评价。
选取原则建议如下: 【普通制剂】 (1)酸性药物制剂 pH 值分别为 1.0 或 1.2、5.5~6.5、6.8~7.5 和水; pH 值分别为 1.0 或 1.2、3.0~5.0、6.8 和水;(2)中性或碱性药物/包衣制剂 (3)难溶性药物制剂 (4)肠溶制剂 【调释制剂】pH 值分别为 1.0 或 1.2、4.0~4.5、6.8 和水;pH 值分别为 1.0 或 1.2、6.0、6.8 和水;pH 值分别为 1.0 或 1.2、3.0~5.0、6.8~7.5 和水。
2. 其他事项 (1) 以上含有 pH 值范围的,可分别按 0.5 或 1.0 间隔测试,如差异较大,应分别予以关注; 如无明显差异,酌情选择即可。
pH 值 1.0 或 1.2,系因各国要求不同。
(2) 在了解了该药物 pKa 值之后,如 pKa±1.0 值未能涵盖于以上各 pH 值中,建议测定 pKa±1.0 值溶出曲线,以更好地把握该药物的溶出特性。
(3) 如能测定更多 pH 值曲线,自然可得到更多关于该制剂内在品质信息;当然工作量亦会 增加。
(4) 无论何种制剂都不建议采用 pH8.0 以上的介质进行表达。
如确有必要,应提供充足的理 由。
(5) 某些品种还可根据临床使用情况, 考虑在“含有胃蛋白酶的模拟胃液”和“含有胰酶的模拟 肠液”中的体外溶出情况。
含有胃蛋白酶的模拟胃液,英文全称为 Simulated Gastric Fluid,简写为 SGF。
有时 亦可附有下标“sp” ,缩写为 SGF[sp]。
其配制方法—— 【中国药典】 取稀盐酸 16.4ml(相当于盐酸 3.84ml) ,加水约 800ml 与胃蛋白酶 10g,上海市药品检验所 谢沐风 撰写1上海市药品检验所谢沐风撰写摇匀后,加水稀释成 1000ml,即得。
溶出度检查法美国药典USP-711

<711> DISSOLUTION溶出度<USP39-NF34 Page540> General chapter Dissolution <711> is being harmonized with the corresponding texts of the European Pharmacopoeia and/or the Japanese Pharmacopoeia. These pharmacopeias have undertaken to not make any unilateral change to this harmonized chapter.通则<711>溶出度与欧盟药典和日本药典中的相应部分相统一.这三部药典承诺不做单方面的修改.Portions of the present general chapter text that are national USP text, and therefore not part of the harmonized text, are marked with symbols to specify this fact.本章中的部分文字为本国USP内容,并没有与其他药典统一.此部分以〔〕标注.This test is provided to determine pliance with the dissolution requirements where stated in the individual monograph for dosage forms administered orally. In this general chapter, a dosage unit is defined as 1 tablet or 1 capsule or the amount specified. Of the types of apparatus designs described herein, use the one specified in the individual monograph. Where the label states that an article is enteric coated and a dissolution or disintegration test does not specifically state that it is to be applied to delayed-release articles and is included in the individual monograph, the procedure and interpretation given for Delayed-Release Dosage Forms are applied, unless otherwise specified in the individual monograph.本测试用于检测药品口服制剂的溶出度是否符合各论中的规定.本章中,除另有规定外,单位制剂定义为1片或1粒胶囊.对于本章中所述多种仪器,使用各论中规定的种类.除各论中另有规定外,如果检品是肠溶衣片且各论中的溶出度或崩解时限检查项下没有特别指出适用迟释剂的,使用本章中适用于迟释剂的流程和解释.FOR DOSAGE FORMS CONTAINING OR COATED WITH GELATIN涂有或包含明胶的剂型If the dosage form containing gelatin does not meet the criteria in the appropriate Acceptance Table <see Interpretation, Immediate-Release Dosage Forms, Extended-Release Dosage Forms, or Delayed-Release Dosage Forms> because of evidence of the presence of cross-linking, the dissolution procedure should be repeated with the addition of enzymes to the medium, as described below, and the dissolution results should be evaluated starting at the first stage of the appropriate Acceptance Table. It is not necessary to continue testing through the last stage <up to 24 units> when criteria are not met during the first stage testing, and evidence of cross-linking is observed.如果剂型中含有明胶,其不符合验收表中的标准〔见判断,速释制剂,延释制剂,缓释制剂〕,因为存在明胶交联结合作用,它的溶解过程与外加的媒介酶是重复的,见下面的描述,并且溶解结果可以通过适当的验收表的开始的第一阶段标准进行评估.如果溶出结果不满足第一阶段的测试标准,那么就没有必要继续测试到最后阶段,并且也证明了明胶交联结合作用的存在.Gelatin, in the presence of certain pounds and/or in certain storage conditions, including but not restricted to high humidity and temperature, may present cross-linking.A pellicle may form on the external and/or internal surface of the gelatin capsule shell or on the dosage form that prevents the drug from being released during dissolution testing <see more information in Capsules—Dissolution Testing and Related Quality Attributes <1094>>.明胶,存在于某一处方和/或某一储存条件下,如:高温高湿,可能存在明胶交联结合作用.在胶囊壳或其他剂型的外表面和/或内表面形成一层膜阻止溶出试验过程中药物的释放〔见胶囊-溶出度检测和相关质量属性<1094>〕.N OTE— All references to a chapter above <1000> are for information purposes only, for use as a helpful resource. These chapters are not mandatory unless explicitly called out for this application.注-超过<1000>章节的所有引用应用的目的仅为提供参考信息.这些章节是非强制的,除非另有规定.Dissolution Medium with pH ≤4.0 pH≤4.0的溶出介质Enzyme: Pepsin, activity determined by the procedure in purified pepsin, in the Reagent Specifications section酶:胃蛋白酶,活性视试剂规格部分中的胃蛋白酶提纯过程而定.Amount: A quantity of pepsin that results in an activity of NMT 750,000 Units/L of dissolution medium数量:一些胃蛋白酶对溶出介质提供NMT 750,000 单位/L的生物活性. Dissolution Medium with pH >4.0 and <6.8pH >4.0 和<6.8的溶出介质Enzyme: Papain, activity determined by the Assay test in the monograph for Papain; or bromelain, activity determined by the procedure in bromelain, in the Reagent Specifications section酶:木瓜蛋白酶,活性视木瓜蛋白酶专论中的分析测试而定;或菠萝蛋白酶,活性视试剂规格部分中的菠萝蛋白酶生产过程而定.Amount: A quantity of papain that results in an activity of NMT 550,000 Units/L of dissolution medium, or a quantity of bromelain that results in an activity of NMT 30 gelatin-digesting units <GDU>/L of dissolution medium数量:一些木瓜蛋白酶对溶出介质提供NMT 550,000 单位/L的生物活性;一些菠萝蛋白酶对溶出介质提供NMT 30明胶消化单位/L的生物活性.Dissolution Medium with pH ≥6.8pH≥6.8的溶出介质Enzyme: Pancreatin, protease activity determined by the procedure in Assay for protease activity <Casein digestive power> in the monograph for Pancreatin酶:胰液素,蛋白酶活性视胰液素专论中的蛋白酶活性〔酪蛋白消化能力〕分析中的生产过程而定.Amount: A quantity of pancreatin that results in a protease activity of NMT 2000 Units/L of dissolution medium数量:一些胰液素对溶出介质提供NMT 550,000 单位/L的蛋白酶活性. Dissolution Medium Containing Surfactant or Other Ingredients Known to Denature the Enzyme含有表面活性剂或其他已知成分变性酶的溶出介质If the dissolution medium contains surfactant or other ingredients that are known to denature the enzyme used, a pretreatment step in the dissolution testing of the dosage form may be applied. This pretreatment step is done using the specified dissolutionmedium without the surfactant or the ingredient and with the addition of the appropriate amount of enzyme according to the medium pH. The amount of enzyme added is appropriate to the volume of dissolution medium used in the pretreatment. To achieve the specified medium volume for the final dissolution testing, the pretreatment step may be conducted with a smaller volume of medium without the ingredient such that the final volume is obtained when the ingredient is added at the end of the pretreatment step. All of the other conditions of the test <apparatus, rotation, or flow rate> should remain as described in the method or monograph. Typically, the duration of the pretreatment step is NMT 15 min. The required pretreatment time should be evaluated on a case-by-case basis and should be scientifically justified. This time should be included in the total time of the test. As an example, if the total time of the test is 45 min and 15 min are used in the pretreatment step, the test will continue for 30 min after the addition of the ingredient.如果溶出介质中添加了表面活性剂或其他已知成分的变性酶,那么此溶出实验就要把预处理步骤考虑进去.预处理过程就是是根据溶出介质的pH来确定加入酶的量,此处的溶出介质不含有表面活性剂和原料.酶加入的量要适合预处理所用的溶出介质的体积.为了达到最终溶出试验所需要的特定的溶出介质的体积,预处理阶段所用的溶出介质〔不含原料〕的体积要稍微小点,如此在预处理最后阶段加入原料的时候方可获得最终的溶出介质体积.其他所有的测试条件〔如:设备、转速、流速〕应该与方法或专论中描述的一致.通常预处理阶段的持续时间为NMT 15 min.所需的预处理时间应该根据具体案例具体分析,且应该科学、合理.预处理时间应该包含在实验的总时间里.例如,如果实验的总时间为45min,预处理时间为15min,那么加入原料后实验还要继续进行30min.USP Reference Standards 〈11〉—USP Prednisone Tablets RS.USP参考标准<11>-USP强的松片RS.APPARATUS仪器Apparatus 1 <Basket Apparatus>第1法〔篮法〕The assembly consists of the following: a vessel, which may be covered, and made of glass or other inert, transparent material;1 a motor; a metallic drive shaft; and a cylindrical basket. The vessel is partially immersed in a suitable water bath of any convenient size or heated by a suitable device, such as a heating jacket. The water bath or heating device permits holding the temperature inside the vessel at 37 ± 0.5° during the test and keeps the bath fluid in constant, smooth motion. No part of the assembly, including the environment in which the assembly is placed, contributes significant motion, agitation, or vibration beyond that due to the smoothly rotating, stirring element. An apparatus that permits observation of the specimen and of the stirring element during the test is preferable. The vessel is cylindrical, with a hemispherical bottom and with one of the following dimensions and capacities: for a nominal capacity of 1 L, the height is 160–210 mm, and its inside diameter is 98–106 mm; for a nominal capacity of 2 L, the height is 280–300 mm, and its inside diameter is 98–106 mm; and for a nominal capacity of 4 L, the height is 280–300 mm, and its inside diameter is 145–155 mm. Its sides are flanged at the top. A fitted cover may be used to retardevaporation.2 The shaft is positioned so that its axis is NMT 2 mm at any point from the vertical axis of the vessel and rotates smoothly and without significant wobble that could affect the results. A speed-regulating device is used that allows the shaft rotation speed to be selected and maintained at the specified rate given in the individual monograph within ±4%.设备由下列部分组成:有盖或无盖的溶出杯,由玻璃或其他惰性的透明材料1制成;马达;转轴;转篮.溶出杯部分浸没在合适大小的水浴中,或者由合适的装置加热,例如电热套.水浴或加热装置需能在测试过程中将杯内温度保持在37±0.5℃,并且容许杯内液体持续、平缓的流动.整个仪器包括周围的环境,除了平稳转动的搅拌部件,不得有明显的运动,搅动或振动.仪器最好能允许在检测过程中能够观察到检品和搅拌部件.溶出杯为圆柱形,底部为半球形,尺寸和容积如下:名义容积1L的,高160-210mm,内径98-106mm;名义容积2L的,高280-300mm,内径98-106mm;名义容积4L的,高280-300mm,内径145-155mm.内壁顶部有缘.可以使用合适的盖子减缓溶剂蒸发2.转轴与溶出杯的纵轴在任意部位不得相差差过2mm,转动平滑,无明显摇晃以至于影响检测结果.速度调节装置控制转轴的转速,并可维持在各论中规定值的±4%X围内.Shaft and basket ponents of the stirring element are fabricated of stainless steel, type 316, or other inert material, to the specifications shown in Figure 1. A basket having a gold coating of about 0.0001 inch <2.5 µm> thick may be used. A dosage unit is placed in a dry basket at the beginning of each test. The distance between the inside bottom of the vessel and the bottom of the basket is maintained at 25 ± 2 mm during the test.转轴和篮筐组件由316号不锈钢或者其他惰性材料制成,尺寸如图1所示.可使用镀金厚度0.0001英寸〔2.5μm〕的篮筐.开始检测时,将一剂药品至于干燥的篮筐中.在测试过程中,溶出杯底部到篮筐底部的距离应保持在25±2mm.Figure 1. Basket stirring element.图1. 转篮组成Apparatus 2 <Paddle Apparatus>第2法〔桨法〕Use the assembly from Apparatus 1, except that a paddle formed from a blade and a shaft is used as the stirring element. The shaft is positioned so that its axis is NMT 2 mm from the vertical axis of the vessel at any point and rotates smoothly without significant wobble that could affect the results. The vertical center line of the blade passes through the axis of the shaft so that the bottom of the blade is flush with the bottom of the shaft. The paddle conforms to the specifications shown in Figure 2. The distance of 25 ± 2 mm between the bottom of the blade and the inside bottom of the vessel is maintained during the test. The metallic or suitably inert, rigid blade and shaft pose a single entity. A suitable two-part, detachable design may be used, provided that the assembly remains firmly engaged during the test. The paddle blade and shaft may be coated with a suitable coating so as to make both of them inert. The dosage unit is allowed to sink to the bottom of the vessel before rotation of the blade is started. A small, loose piece of nonreactive material, such as NMT a few turns of wire helix, may be attached to dosage units that would otherwise float. An alternative sinker device is shown in Figure 2a. Other validated sinker devices may be used.使用第1法中的设备,除了使用一个由叶片和转轴组成的桨作为搅拌单元.转轴与溶出杯的纵轴在任意部位不得相差差过2mm,转动平滑,无明显摇晃以至于影响检测结果.叶片的垂直中性线穿过转轴的轴线,叶片的下缘与转轴底部平齐.桨的尺寸应符合图2中的规定.在测试过程中,叶片底部与溶出杯底部的距离应保持在25±2mm.金属或硬质的叶片和转轴应是一个整体.两部分组合的设计也可以使用,只要组件在检测过程中牢固固定在一起.可以在桨叶和转轴上涂布合适的涂层以使其为惰性.在桨叶开始旋转前,将一剂药品沉至溶出杯底.如果药剂浮在页面上,可以在其上附着一个惰性,松弛的小部件,例如几圈线圈,使其沉没.图2是一种可替代使用的沉子.其他经验证的沉子也可以使用.Figure 2. Paddle stirring element.图2. 搅拌桨组成Figure 2a. Alternative sinker. All dimensions are expressed in mm.图2a. 可选的沉降篮〔单位均为mm〕Apparatus 3 <Reciprocating Cylinder>第3法〔往复圆筒法〕NOT ACCEPTED BY THE JAPANESE PHARMACOPOEIA日本药典未收录The assembly consists of a set of cylindrical, flat-bottomed glass vessels; a set of glass reciprocating cylinders; inert fittings <stainless steel type 316 or other suitable material>, and screens that are made of suitable nonsorbing and nonreactive material and that are designed to fit the tops and bottoms of the reciprocating cylinders; and a motor and drive assembly to reciprocate the cylinders vertically inside the vessels; if desired, index the reciprocating cylinders horizontally to a different row of vessels. The vessels are partially immersed in a suitable water bath of any convenient size that permits holding the temperature at 37 ± 0.5° during the test. No part of the assembly, including the environment in which the assembly is placed, contributes significant motion, agitation, or vibration beyond that due to the smooth, vertically reciprocating cylinder. A device is used that allows the reciprocation rate to be selected and maintained at the specified dip rate given in the individual monograph within ±5%. An apparatus that permits observation of the specimens and reciprocating cylinders is preferable. The vessels are provided with evaporation caps that remain in place for the duration of the test. The ponents conform to the dimensions shown in Figure 3, unless otherwise specified in the individual monograph.所用设备包含一套圆柱形平底玻璃杯;一套玻璃往复圆筒;惰性配件〔316号不锈钢或其他合适的材质〕;由合适的非吸附,不反应材料制成的筛网,挡在往复圆筒的上下两端;一套马达和传动装置,将圆筒在玻璃杯中垂直往复运动,如果需要,也可以将往复圆筒平行移至另一行玻璃杯中.玻璃杯部分浸没在合适尺寸的水浴中,水浴温度保持在37±0.5℃.仪器的任何部件,以与仪器所处的环境,都不应当引起明显的移动,搅动,振动,除了平滑的垂直往复运动的圆筒.使用设备维持往复速度在各论中所规定值的±5%X围内.仪器最好可以在检测过程中观察到样品和往复圆筒.玻璃杯配有蒸发帽,在检测中一直盖在玻璃杯上.除另有规定外,各部分的尺寸如图3所示.Figure 3. Apparatus 3 <reciprocating cylinder>.图3. 图3 第3法〔往复圆筒法〕设备Apparatus 4 <Flow-Through Cell>第4法〔流通池法〕The assembly consists of a reservoir and a pump for the Dissolution medium; a flow-through cell; and a water bath that maintains the Dissolution medium at 37 ± 0.5°. Use the specified cell size as given in the individual monograph.所用设备包含一个溶出介质的容器和相应的泵,一个流通池和水浴.水浴将溶出介质保持在37±0.5℃.使用各论中规定的尺寸.The pump forces the Dissolution medium upward through the flow-through cell. The pump has a delivery range between 240 and 960 mL/h, with standard flow rates of 4, 8,and 16 mL/min. It must deliver a constant flow <±5% of the nominal flow rate>; the flow profile is sinusoidal with a pulsation of 120 ± 10 pulses/min. A pump without pulsation may also be used. Dissolution test procedures using a flow-through cell must be characterized with respect to rate and any pulsation.泵将溶出介质推动,向上通过流通池.泵的传输能力在240到960mL每小时之间,标准速率为4,8,16mL每分钟.泵的流速必须均匀〔名义流量的±5%以内〕.泵的流量特性曲线应为正弦波,脉冲为每分钟120 ± 10 冲.无脉冲泵也可以使用.使用流通池法的溶出度测试必须对应特定的流速和脉冲.The flow-through cell <see Figure 4 and Figure 5>, of transparent and inert material, is mounted vertically with a filter system <specified in the individual monograph> that prevents escape of undissolved particles from the top of the cell; standard cell diameters are 12 and 22.6 mm; the bottom cone is usually filled with small glass beads of about 1-mm diameter with one bead of about 5 mm, positioned at the apex to protect the fluid entry tube; and a tablet holder <see Figure 4 and Figure 5> is available for positioning of special dosage forms, e.g., inlay tablets. The cell is immersed in a water bath, and the temperature is maintained at 37 ± 0.5°.由透明且惰性材料制成的流通池〔见图4和图5〕垂直安放,配有过滤系统〔在各论中规定〕以防止未溶解的颗粒从流通池顶部逸出.标准的流通池直径为12和22.6mm.底部的锥形通常填有直径约1mm的小玻璃珠,其中一颗约5mm大的玻璃珠置于顶点处,以保护液体输入管.流通池配有药片架〔见图4和图5〕一满足特殊制剂的需要,如泡腾片.流通池浸没在37±0.5℃的水浴中.Figure 4. Apparatus 4: large cell fortablets and capsules <top>; tablet holderfor the large cell <bottom>. <Allmeasurements are expressed in mmunless noted otherwise.>图4.第4法设备,盛装片剂和胶囊的大流通池〔上〕,大药片架〔下〕.〔除另有说明,所有尺寸单位为mm.〕Figure 5. Apparatus 4: small cell fortablets and capsules <top>; tablet holderfor the small cell <bottom>. <Allmeasurements are expressed in mmunless noted otherwise.>图5 第4法设备,盛装片剂和胶囊的小流通池〔上〕,小药片架〔下〕.〔除另有说明,所有尺寸单位为mm.〕The apparatus uses a clamp mechanism and two O-rings to assemble the cell. The pump is separated from the dissolution unit to shield the latter against any vibrations originating from the pump. The position of the pump should not be on a level higher than the reservoir flasks. Tube connections are as short as possible. Use suitably inert tubing, such as polytef, with about a 1.6-mm inner diameter and chemically inert, flanged-end connections.流通池使用一个架子和2个O形圈固定.泵与溶出单元分开,以防止泵的振动干扰到后者.泵的水平位置不得高于溶出介质容器.管线连接尽可能短.使用合适的惰性管线,如聚四氟乙烯,内径1.6mm.法兰连接也应为化学惰性.APPARATUS SUITABILITY设备适用性The determination of suitability of a test assembly to perform dissolution testing must include conformance to the dimensions and tolerances of the apparatus as given above. In addition, critical test parameters that have to be monitored periodically during use include volume and temperature of the Dissolution medium, rotation speed <Apparatus 1 and Apparatus 2>, dip rate <Apparatus 3>, and flow rate of medium <Apparatus 4>. 溶出度测试仪器的适用性必须包括与上述各仪器在尺寸和限度上的一致性.另外,必须在使用过程中定期观测的关键测试参数包括:溶出介质的温度和体积,转速〔第1法和第2法〕,浸没频率〔第3法〕和溶出介质流速〔第4法〕. Determine the acceptable performance of the dissolution test assembly periodically.The suitability for the individual apparatus is demonstrated by the Performance verification test.定期检测溶出度测试设备的性能.单个设备的适用性由性能验证测试给出. Performance verification test, Apparatus 1 and Apparatus 2: Test USP Prednisone Tablets RS according to the operating conditions specified. The apparatus is suitable if the results obtained are within the acceptable range stated in the technical data sheet specific to the lot used and the apparatus tested.性能验证测试,第1法和第2法:根据规定的操作条件测试USP强的松片RS.如果结果在技术数据表上该批次和所用仪器的的可接受X围内,则设备是适用的. Performance verification test, Apparatus 3: [To e.]性能验证测试,第3法——[待续]Performance verification test, Apparatus 4: [To e.]性能验证测试,第4法——[待续]PROCEDURE测试方法Apparatus 1 and Apparatus 2第1法和第2法IMMEDIATE-RELEASE DOSAGE FORMS速释制剂Place the stated volume of the Dissolution medium <±1%> in the vessel of the specified apparatus given in the individual monograph, assemble the apparatus, equilibrate the Dissolution medium to 37 ± 0.5°, and remove the thermometer. Place 1 dosage unit in the apparatus, taking care to exclude air bubbles from the surface of the dosage unit, and immediately operate the apparatus at the specified rate given in the individual monograph. Within the time interval specified, or at each of the times stated, withdraw a specimen from a zone midway between the surface of the Dissolution medium and the top of the rotating basket or blade, NLT 1 cm from thevessel wall. [N OTE— Where multiple sampling times are specified, replace the aliquots withdrawn for analysis with equal volumes of fresh Dissolution medium at 37°or, where it can be shown that replacement of the medium is not necessary, correct for the volume change in the calculation. Keep the vessel covered for the duration of the test, and verify the temperature of the mixture under test at suitable times. ] Perform the analysis as directed in the individual monograph using a suitable assay method.3 Repeat the test with additional dosage form units.将各论中给出的溶出介质量〔±1%〕加入到规定的容器中,组装好设备,平衡溶出介质温度在37±0.5℃,移出温度计.将1单位剂量的药品小心加入设备中,注意避免表面产生气泡.立即按照各论中规定的速率开动设备.在规定的时间间隔或给定的时间点,从溶出介质液面以下和溶出篮或桨叶顶端之间,离杯壁至少1cm 的区域取出一份试样.[注:如果规定有多次取样,以等体积的37℃溶出介质补偿所取液体.或者,如果有证明不需要补偿介质,在计算中修正溶液体积的变化.在检测中保持容器加盖,并以适当的频率验证溶液的温度.]按照各论中规定的合适的方法进行分析3.重复试验以测试更多的剂量单元.If automated equipment is used for sampling or the apparatus is otherwise modified, verification that the modified apparatus will produce results equivalent to those obtained with the standard apparatus described in this general chapter is necessary.如果使用自动化装置取样或者设备在其他方面做出了更改,需要进行验证以显示修改后的设备可以给出与通用章节中的标准设备等效的结果.Dissolution medium: A suitable dissolution medium is used. Use the solvent specified in the individual monograph. The volume specified refers to measurements made between 20°and 25°. If the Dissolution medium is a buffered solution, adjust the solution so that its pH is within 0.05 unit of the specified pH given in the individual monograph. [ N OTE— Dissolved gases can cause bubbles to form, which may change the results of the test. If dissolved gases influence the dissolution results, dissolved gases should be removed before testing.4 ]溶出介质:使用合适的溶出介质.使用各论中规定的溶剂.所规定的体积指在20和25℃之间所测的值.如果溶出介质是缓冲液,调整缓冲液以保证缓冲液的pH值在各论中规定的pH值的0.05以内.[注:溶解的气体可以导致气泡的生成,从而改变测试结果.如果溶解的气体会影响溶出结果,在测试前除去溶解的气体4.] Time: Where a single time specification is given, the test may be concluded in a shorter period if the requirement for the minimum amount dissolved is met. Specimens are to be withdrawn only at the stated times, within a tolerance of ±2%.时间:当规定了单一的时间时,如果最小溶出量已达到,测试可以提前结束.试样必须在所述时间的±2%X围内取出.Procedure for a pooled sample for immediate-release dosage forms: Use this procedure where Procedure for a Pooled Sample is specified in the individual monograph. Proceed as directed for Immediate-Release Dosage Forms in Apparatus 1 and Apparatus 2 in the Procedure section. bine equal volumes of the filtered solutions of the six or twelve individual specimens withdrawn, and use the pooled sample as the test specimen. Determine the average amount of the active ingredient dissolved in the pooled sample.速释制剂集合样品测试方法:如果各论中有规定测试集合样品,使用本方法.按照测试方法章节中速释制剂第1法和第2法进行.集中全部所测的6或12个单独物种的等体积的溶剂,过滤,使用集合样品作为被测物种,测定集合样品中各活性成分的平均溶出量.EXTENDED-RELEASE DOSAGE FORMS缓释制剂Proceed as directed for Immediate-Release Dosage Forms.按照速释制剂的方法进行.Dissolution medium: Proceed as directed for Immediate-Release Dosage Forms.溶出介质:按照立即释放制剂的方法进行.Time: The test-time points, generally three, are expressed in hours.时间:测试时间点,通常是3个,以小时为单位.DELAYED-RELEASE DOSAGE FORMS NOT ACCEPTED BY THE JAPANESE PHARMACOPOEIA 日本药典未收录的迟释制剂Use Method A or Method B and the apparatus specified in the individual monograph. All test times stated are to be observed within a tolerance of ±2%, unless otherwise specified.按照各论中的规定,使用方法A或方法B.除另有规定外,所有测试时间与规定相差不得过±2%.Method A Procedure <unless otherwise directed in the individual monograph>方法A程序〔除各论中另有规定外〕ACID STAGE酸阶段Place 750 mL of 0.1 N hydrochloric acid in the vessel, and assemble the apparatus. Allow the medium to equilibrate to a temperature of 37 ± 0.5°. Place 1 dosage unit in the apparatus, cover the vessel, and operate the apparatus at the specified rate given in the monograph.向容器中加入0.1N的盐酸750mL,组装设备.将介质平衡在37±0.5℃.将1单位剂量的药品加入设备中,盖上容器,依照各论中规定的速率启动设备.After 2 h of operation in 0.1 N hydrochloric acid, withdraw an aliquot of the fluid, and proceed immediately as directed in the Buffer Stage.在0.1N的盐酸中搅拌2小时后,吸取一份试样溶液,然后立即按照缓冲液阶段的说明继续操作.Perform an analysis of the aliquot using a suitable assay method. The procedure is specified in the individual monograph.以适合的方法测试试样.测试方法在各论中给出.BUFFER STAGE缓冲液阶段[ N OTE— plete the operations of adding the buffer and adjusting the pH within 5 min. ] With the apparatus operating at the rate specified in the monograph, add to the fluid in the vessel 250 mL of 0.20 M tribasic sodium phosphate that has been equilibrated to 37 ± 0.5°. Adjust, if necessary, with 2 N hydrochloric acid or 2 N sodium hydroxide to a pH of 6.8 ± 0.05. Continue to operate the apparatus for 45 min, or for the specified time given in the individual monograph. At the end of the time period, withdraw an aliquot of the fluid, and perform the analysis using a suitable assay method. The procedure is specified in the individual monograph. The test may beconcluded in a shorter time period than that specified for the Buffer Stage if the requirement for the minimum amount dissolved is met at an earlier time.[注:加入缓冲液和调节pH的操作应在5分钟内完成.]设备在各论中规定的速率下运行,向容器中加入250mL预先平衡在37±0.5℃的0.20M的磷酸钠.必要时用2N的盐酸或2N的氢氧化钠调节pH至6.8±0.05.继续运转45分钟或各论中给定的时间.到时间后,吸取一份试样溶液,以适合的方法测试.测试方法在各论中给出.如果缓冲液阶段的最小溶出量已提前达到,测试可以提前结束.Method B Procedure <unless otherwise directed in the individual monograph>方法B程序〔除各论中另有规定外〕ACID STAGE酸阶段Place 1000 mL of 0.1 N hydrochloric acid in the vessel, and assemble the apparatus. Allow the medium to equilibrate to a temperature of 37 ± 0.5°. Place 1 dosage unit in the apparatus, cover the vessel, and operate the apparatus at the rate specified in the monograph. After 2 h of operation in 0.1 N hydrochloric acid, withdraw an aliquot of the fluid, and proceed immediately as directed in the Buffer Stage.向容器中加入0.1N的盐酸1000mL,组装设备.将介质平衡在37±0.5℃.将1单位剂量的药品加入设备中,盖上容器,依照各论中规定的速率启动设备.在0.1N 的盐酸中搅拌2小时后,吸取一份试样溶液,然后立即按照缓冲液阶段的说明继续操作.Perform an analysis of the aliquot using a suitable assay method. The procedure is specified in the individual monograph.以适合的方法测试试样.测试方法在各论中给出.BUFFER STAGE缓冲液阶段[ N OTE— For this stage of the procedure, use buffer that previously has been equilibrated to a temperature of 37 ± 0.5°. ] Drain the acid from the vessel, and add to the vessel 1000 mL of pH 6.8 phosphate buffer, prepared by mixing 0.1 N hydrochloric acid with 0.20 M tribasic sodium phosphate <3:1> and adjusting, if necessary, with 2 N hydrochloric acid or 2 N sodium hydroxide to a pH of 6.8 ± 0.05. [N OTE— This may also be acplished by removing from the apparatus the vessel containing the acid, then replacing it with another vessel containing the buffer, and transferring the dosage unit to the vessel containing the buffer. ][注:此阶段使用预先平衡在37±0.5℃的缓冲液.]抽干容器中的酸液,加入1000mL pH6.8的磷酸盐缓冲液〔0.1N盐酸加0.20M磷酸钠,3:1〕.必要时用2N 的盐酸或2N的氢氧化钠调节pH至6.8±0.05.[注:也可以将设备中盛装酸液的容器移出,换以另一盛装缓冲液的容器,将药剂转移到缓冲液容器中.]Continue to operate the apparatus for 45 min, or for the specified time given in the individual monograph. At the end of the time period, withdraw an aliquot of the fluid, and perform the analysis using a suitable assay method. The procedure is specified in the individual monograph. The test may be concluded in a shorter time period than that specified for the Buffer Stage if the requirement for minimum amount dissolved is met at an earlier time.继续运转45分钟或各论中给定的时间.到时间后,吸取一份试样溶液,以适合的方法测试.测试方法在各论中给出.如果缓冲液阶段的最小溶出量已提前达到,测试可以提前结束.Apparatus 3 <Reciprocating Cylinder>第3法〔往复圆筒法〕NOT ACCEPTED BY THE JAPANESE PHARMACOPOEIA IMMEDIATE-RELEASE DOSAGE FORMS 日本药典未收录的速释制剂Place the stated volume of the Dissolution medium in each vessel of the apparatus, assemble the apparatus, equilibrate the Dissolution medium to 37 ± 0.5°, and remove the thermometer. Place 1 dosage form unit in each of the six reciprocating cylinders, taking care to exclude air bubbles from the surface of each dosage unit, and immediately operate the apparatus as specified in the individual monograph. During the upward and downward strokes, the reciprocating cylinder moves through a total distance of 9.9–10.1 cm. Within the time interval specified, or at each of the times stated, raise the reciprocating cylinders and withdraw a portion of the solution under test from a zone midway between the surface of the Dissolution medium and the bottom of each vessel. Perform the analysis as directed in the individual monograph. If necessary, repeat the test with additional dosage-form units.将指定量的溶出介质加入到设备的每个容器中,组装好设备,平衡溶出介质温度在37±0.5℃,移出温度计.在6个往复圆筒中分别加入1单位剂量的药品,注意避免表面产生气泡.立即按照各论中规定的速率开动设备.在上行和下行冲程中,往复圆筒移动的总距离为9.9到10.1cm.在规定的时间间隔或者每个给定的时间点,抬起往复圆筒,从溶出介质的表面到容器底部的中点区域取出一部分测试溶液.按照各论中的规定测试.必要时重复测试更多份的样品.Dissolution medium: Proceed as directed for Immediate-Release Dosage Forms in Apparatus 1 and Apparatus 2.溶出介质:同第1法和第2法下立即释放制剂项下处理.Time: Proceed as directed for Immediate-Release Dosage Forms in Apparatus 1 and Apparatus 2.时间:同第1法和第2法下立即释放制剂项下处理.EXTENDED-RELEASE DOSAGE FORMS缓释制剂Proceed as directed for Immediate-Release Dosage Forms in Apparatus 3.同第3法下速释制剂项下处理.Dissolution medium: Proceed as directed for Extended-Release Dosage Forms in Apparatus 1 and Apparatus 2.溶出介质:同第1法和第2法下缓释制剂项下处理.Time: Proceed as directed for Extended-Release Dosage Forms in Apparatus 1 and Apparatus 2.时间:同第1法和第2法下缓释制剂项下处理.DELAYED-RELEASE DOSAGE FORMS迟释制剂Proceed as directed for Delayed-Release Dosage Forms, Method B in Apparatus 1 and Apparatus 2, using one row of vessels for the acid stage media and the following row of vessels for the buffer stage media, and using the volume of medium specified <usually 300 mL>.同第1法和第2法下迟释制剂方法B项下处理.酸性阶段使用一排容器,缓冲液阶段使用另一排容器.使用规定体积的介质〔通常为300mL〕.Time: Proceed as directed for Immediate-Release Dosage Forms in Apparatus 1 and Apparatus 2.。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
美国药典溶出介质缓冲液的配制
美国药典配制法
1、在标准溶液配制标准指导下配制:0.2mol/L 盐酸溶液;0.2mol/L 氢氧化钠溶液
2、0.2mol/L邻苯二甲酸氢钾溶液:在水中溶解40.85g邻苯二甲酸氢钾,稀释制1000ml
3 、0.2mol/L磷酸二氢钾溶液:在水中溶解27.22g磷酸二氢钾,稀释制1000ml
4 、0.2mol/L硼酸氯化钾溶液:在水中溶解12.37g硼酸和14.91g氯化钾,稀释至1000ml
5、0.2mol/L氯化钾溶液:在水中溶解14.91g氯化钾,稀释至1000ml
6 、2N(当量浓度)的乙酸:在标准溶液配制标准指导下配制
标准缓冲液
盐酸缓冲液:将50ml氯化钾溶液置于200ml容量瓶中,加入一定量的盐酸溶液,用水定容至刻度。
酸性邻苯二甲酸缓冲液:将50ml邻苯二甲酸氢钾溶液置于200ml容量瓶中,加入一定量的盐酸溶液,用水定容至刻度。
中性邻苯二甲酸缓冲液:将50ml邻苯二甲酸氢钾溶液置于200ml容量瓶中,加入一定量的氢氧化钠溶液,用水定容至刻度。
磷酸二氢钾缓冲液:将50ml磷酸二氢钾溶液置于200ml容量瓶中,加入一定量的氢氧化钠溶液,用水定容至刻度。
碱性硼酸缓冲液:将50ml硼酸氯化钾溶液置于200ml容量瓶中,加入一定量的氢氧化钠溶液,用水定容至刻度。
醋酸缓冲液:将规定量的三水乙酸纳置于1000ml容量瓶中,加入规定量的乙酸溶液,用水定容至刻度,混匀。
