罗氏电化学发光免疫分析
罗氏电化学发光免疫分析仪测试项目定标物保存方法
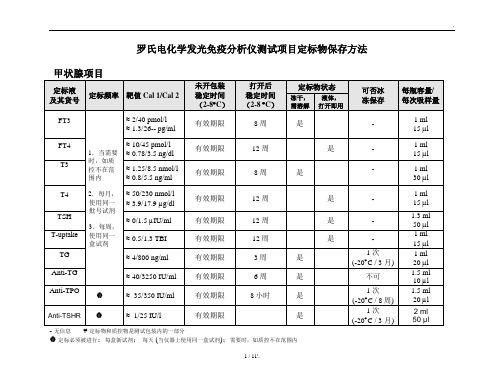
罗氏电化学发光免疫分析仪测试项目定标物保存方法无信息定标物和质控物是测试包装内的一部分定标必须被进行:每盒新试剂;每天 (当仪器上使用同一盒试剂);需要时,如质控不在范围内心肌标志物性激素项目肿瘤标志物贫血诊断指标、骨标志物传染病项目其它唐氏筛查先兆子痫罗氏电化学发光免疫分析仪定标物保存方法(简版)定标物不需要分装冰冻保存的项目:使用时从2-8︒C取出,摇均后吸200μl(此数据为国内实验室试验所得,不代表罗氏官方)到日立杯,马上将剩余定标品放回2-8︒CFT3、FT4、T3、T4、TSH、T-uptake、Anti-TG、HCG+β、HCG STAT、ProgesteroneCEA、CA 125、CA 153、CA 724、Cyfra21-1、NSE、Free PSA、HE4Myoglobin、Myoglobin - STAT 、Digoxin、Digitoxin、Ferritin、IgE、β-CrossLapsAnti-HAV、Anti-HAV IgM、HBsAg、HBsAg II quant、 Anti-HBs、HBeAg、Anti-HBe、Anti-HBc、 Anti-HBc IgM、 HIV combi、HIV Ag 、HIV combi PTToxo IgG、 Toxo IgM、Rubella IgG、Rubella IgM、CMV IgG、CMV IgM定标物需要分装冰冻保存的项目:定标物加水复溶,用子弹头进行分装(分装量为200μl(此数据为国内实验室试验所得,不代表罗氏官方)),然后放在-20︒C保存,使用时取出并平衡到室温TG、Anti-TPO、 Anti-TSHR、CK-MB、CK-MB STAT、ProBNP、Troponin T HS、Troponin T HS STAT free βHCG、PAPP-A、 Insulin、C-peptide、IL-6 、PCT、Anti-CCP.Cortisol、ACTH、Estradiol II、FSH、LH、Prolactin、Testosterone、DHEA-S、SHBG、PIGF、 sFlt-1、hGHAFP、CA 199、TPSA、S100Vitamin B12、Folate III 、N-MID、Total P1NP、PTH、PTH STAT、PTH(1-84)Vitamin D3、Vitamin D totalHSV-1 IgG、 HSV-2 IgG11 / 11'.。
罗氏 cobas e 801 电化学发光免疫分析仪使用说明书 - 检测抗 HBs 抗体的试剂盒

Elecsys Anti-HBs IIREFSYSTEM********************** 300cobas e 801EnglishSystem information Short name ACN (application code number) AHBS 2 10138 Immunoassay for the in vitro quantitative determination of human antibodies to the hepatitis B surface antigen (HBsAg) in human serum and plasma.Anti-HBs assays are used within the scope of hepatitis B vaccination to check the necessity and success of vaccination. In addition, anti-HBs assays are used to monitor the course of disease following acute hepatitis B infection. This test is not intended for diagnosis.The e lectro c hemi l uminescence i mmuno a ssay “ECLIA” is intended for use on the cobas e 801 immunoassay analyzer.Note: Please note that the catalogue number appearing on the package insert retains only the first 8 digits of the licensed 11-digit Catalogue Number: 07026854190 for the Elecsys Anti-HBs II assay. The last 3 digits -190 have been replaced by -119 for logistic purposes. SummaryAnti-HBs is a specific (generally IgG) antibody that is directed against the hepatitis B surface antigen (HBsAg).1,2 Anti ‑HBs can be detected several weeks after the disappearance of hepatitis B surface antigen.3,4 Anti ‑HBs can be formed following a hepatitis B infection or after hepatitis Bvaccination.3,4 Antibodies are formed against the HBsAg determinant a, which is common to all subtypes, and against subtype-specific determinants.1,5,6Anti ‑HBs assays are used within the scope of hepatitis B vaccination to check the necessity and success of vaccination.2,4,7 In addition, anti ‑HBs assays are used to monitor the course of disease following acute hepatitis B infection.3The Elecsys Anti ‑HBs II assay uses a mixture of purified antigens fromhuman serum (HBsAg subtype ad), and recombinant HBsAg subtype ay from CHO (Chinese Hamster Ovary) cells. Test principleSandwich principle. Total duration of assay: 18 minutes.▪ 1st incubation: Anti ‑HBs in the sample (24 μL), biotinylated HBsAg(ad/ay), and HBsAg (ad/ay) labeled with a ruthenium complex a) react to form a sandwich complex.▪ 2nd incubation: After addition of streptavidin-coated microparticles, thecomplex becomes bound to the solid phase via interaction of biotin and streptavidin.▪ The reaction mixture is aspirated into the measuring cell where themicroparticles are magnetically captured onto the surface of the electrode. Unbound substances are then removed with ProCell II M. Application of a voltage to the electrode then induces chemiluminescent emission which is measured by a photomultiplier.▪ Results are determined via a calibration curve which is instrument-specifically generated by 2‑point calibration and a master curve provided via the cobas link.a) Tris(2,2'-bipyridyl)ruthenium(II)-complex (Ru(bpy)32+)Reagents – working solutionsThe cobas e pack (M, R1, R2) is labeled as AHBS 2. M Streptavidin-coated microparticles, 1 bottle, 13.2 mL:Streptavidin-coated microparticles 0.72 mg/mL; preservative. R1 HBsAg~biotin, 1 bottle, 16.7 mL:Biotinylated HBsAg (ad/ay) human/recombinant, > 0.5 mg/L; MES b) buffer 85 mmol/L, pH 6.5; preservative. R2 HBsAg~Ru(bpy)32+, 1 bottle, 15.8 mL:HBsAg (ad/ay) human/recombinant, labeled with ruthenium complex > 0.3 mg/L; MES buffer 85 mmol/L, pH 6.5; preservative.b) MES = 2-morpholino-ethane sulfonic acidAHBS 2 Cal1 Calibrator 1, 1 bottle of 1.3 mL:Anti ‑HBs (human) in human serum; preservative.AHBS 2 Cal2 Calibrator 2, 1 bottle of 1.3 mL:Anti ‑HBs (human) in human serum; preservative.Precautions and warnings For in vitro diagnostic use.Exercise the normal precautions required for handling all laboratory reagents. Disposal of all waste material should be in accordance with local guidelines. Safety data sheet available for professional user on request.This kit contains components classified as follows in accordance with the Regulation (EC) No. 1272/2008:n ‑Octyl ‑N,N ‑dimethyl ‑3‑ammonio ‑1‑propanesulfonateEUH 208 May produce an allergic reaction.Product safety labeling primarily follows EU GHS guidance. All human material should be considered potentially infectious.The calibrators (AHBS 2 Cal1 and AHBS 2 Cal2) have been preparedexclusively from the blood of donors tested individually and shown to be free from HBsAg and antibodies to HCV and HIV. The testing methods applied were FDA ‑approved or cleared in compliance with the European Directive 98/79/EC, Annex II, List A.The HBsAg starting material used was inactivated prior to labeling with biotin or ruthenium by heating to 60 °C for 15 hours. In addition, any virus particles remaining were removed by ultracentrifugation.However, as no inactivation or testing method can rule out the potential risk of infection with absolute certainty, the material should be handled with the same level of care as a patient specimen. In the event of exposure, the directives of the responsible health authorities should be followed.8,9 Avoid foam formation in all reagents and sample types (specimens, calibrators and controls). Reagent handlingThe reagents (M, R1, R2) in the kit are ready-for-use and are supplied in cobas e packs. CalibratorsThe calibrators are supplied ready ‑for ‑use in bottles compatible with the system.Unless the entire volume is necessary for calibration on the analyzer, transfer aliquots of the ready ‑for ‑use calibrators into empty snap ‑cap bottles (CalSet Vials). Attach the supplied labels to these additional bottles. Store the aliquots at 2‑8 °C for later use.Perform only one calibration procedure per aliquot.All information required for correct operation is available via the cobas link. Storage and stability Store at 2‑8 °C. Do not freeze.Store the cobas e pack upright in order to ensure complete availability of the microparticles during automatic mixing prior to use. Stability of the cobas e pack: unopened at 2‑8 °Cup to the stated expiration date on the cobas e 801 analyzer 16 weeksStability of the calibrators: unopened at 2‑8 °C up to the stated expiration date after opening at 2‑8 °C 16 weeks on the cobas e 801 analyzer at 20‑25 °Cuse only onceadhering to the snap ‑cap.Specimen collection and preparationOnly the specimens listed below were tested and found acceptable.Serum collected using standard sampling tubes or tubes containing separating gel.K2‑EDTA and K3‑EDTA plasma.Criterion: Slope 1.00 ± 0.15 + intercept 0 ± 2 IU/L + bias at 10 IU/L: ≤ 30 %. Stable for 3 days at 20‑25 °C, 6 days at 2‑8 °C, 3 months at ‑20 °C(± 5 °C). The samples may be frozen 5 times.For plasma treated with lithium heparin, lithium heparin with gel or sodium heparin, the values found were on average up to 20 % lower than those obtained in serum. For plasma treated with sodium citrate, the values found were on average up to 30 % lower than those obtained with serum.The sample types listed were tested with a selection of sample collection tubes or systems that were commercially available at the time of testing, i.e. not all available tubes of all manufacturers were tested. Sample collection systems from various manufacturers may contain differing materials which could affect the test results in some cases. When processing samples in primary tubes (sample collection systems), follow the instructions of the tube manufacturer.Centrifuge samples containing precipitates and thawed samples before performing the assay.Do not use heat‑inactivated samples.Do not use samples and controls stabilized with azide.Ensure the samples and calibrators are at 20‑25 °C prior to measurement. Due to possible evaporation effects, samples and calibrators on the analyzers should be analyzed/measured within 2 hours.The performance of the Elecsys Anti‑HBs II assay has not been established with cadaveric samples or body fluids other than serum and plasma. Materials providedSee “Reagents –working solutions” section for reagents.▪ 2 x 6 bottle labelsMaterials required (but not provided)▪REF 11876317122, PreciControl Anti‑HBs, 16 x 1.3 mL▪REF 11776576322, CalSet Vials, 2 x 56 empty snap-cap bottles▪REF***********,DiluentUniversal,45.2mLsamplediluent▪▪cobas e 801 analyzerAccessories for the cobas e 801 analyzer:▪REF***********,ProCellIIM,2x2Lsystemsolution▪REF 04880293190, CleanCell M, 2 x 2 L measuring cell cleaning solution ▪REF***********,ReservoirCups,8cupstosupplyProCellIIMand CleanCell M▪REF***********,PreCleanIIM,2x2Lwashsolution▪REF***********,AssayTip/AssayCuptray,6magazinesx6magazine stacks x 105 assay tips and 105 assay cups, 3 wasteliners▪REF***********,LiquidFlowCleaningCup,2adaptorcupstosupply ISE Cleaning Solution/Elecsys SysClean for Liquid Flow CleaningDetection Unit▪REF***********,PreWashLiquidFlowCleaningCup,1adaptorcupto supply ISE Cleaning Solution/Elecsys SysClean for Liquid Flow Cleaning PreWash Unit▪REF 11298500316, ISE Cleaning Solution/Elecsys SysClean,5 x 100 mL system cleaning solutionAssayFor optimum performance of the assay follow the directions given in this document for the analyzer concerned. Refer to the appropriate operator’s manual for analyzer‑specific assay instructions.Resuspension of the microparticles takes place automatically prior to use. Place the cooled (stored at 2‑8 °C) cobas e pack on the reagent manager. Avoid foam formation. The system automatically regulates the temperature of the reagents and the opening/closing of the cobas e pack. Calibrators:Place the calibrators in the sample zone.Read in all the information necessary for calibrating the assay.CalibrationTraceability: This method has been standardized against the 1st WHO Reference Standard 1977.The predefined master curve is adapted to the analyzer using AHBS 2 Cal1 and AHBS 2 Cal2.Calibration frequency: Calibration must be performed once per reagent lot using AHBS 2 Cal1, AHBS 2 Cal2 and fresh reagent (i.e. not more than24 hours since the reagent kit was registered on the analyzer).Renewed calibration is recommended as follows:▪after 12 weeks when using the same reagent lot▪after 28 days when using the same cobas e pack on the analyzer▪as required: e.g. quality control findings with PreciControl Anti‑HBs outside the defined limitsQuality controlFor quality control, use PreciControl Anti‑HBs.Controls for the various concentration ranges should be run individually at least once every 24 hours when the test is in use, once per cobas e pack, and following each calibration.The control intervals and limits should be adapted to each laboratory’s individual requirements. Values obtained should fall within the defined limits. Each laboratory should establish corrective measures to be taken if values fall outside the defined limits.If necessary, repeat the measurement of the samples concerned.Follow the applicable government regulations and local guidelines for quality control.CalculationThe analyzer automatically calculates the analyte concentration of each sample in IU/L.Interpretation of the resultsNumeric result Result message Interpretation< 10 IU/L Non-reactive Negative for anti-HBs≥ 10 IU/L Reactive Positive for anti-HBsvary depending on the testing procedure used. Results obtained from a single sample using tests from different manufacturers can therefore differ by up to a factor of 4 (or even a factor of 10 in rare cases). If there is a change in the assay procedure used during the monitoring of vaccination protection, then the anti‑HBs values obtained upon changing over to the new method must be confirmed by parallel measurements by both methods. Vaccination strategies in certain risk groups are based on the measured anti‑HBs concentration. Respective recommendations are given by national or regional guidelines. Limitations - interferenceThe effect of the following endogenous substances and pharmaceutical compounds on assay performance was tested. Interferences were tested up to the listed concentrations and no impact on results was observed. Endogenous substancesCompound Concentration testedBilirubin ≤ 513 μmol/L or ≤ 30 mg/dL Hemoglobin ≤ 0.621 mmol/L or ≤ 1000 mg/dL Intralipid ≤ 1500 mg/dLBiotin ≤ 41 nmol/L or ≤ 10 ng/mL Rheumatoid factors ≤ 1200 IU/mLAlbumin ≤ 7.0 g/dLIgG ≤ 7.0 g/dLIgA ≤ 1.6 g/dLIgM ≤ 1.0 g/dL2 / 42017-09, V 1.0 Can EnglishCriterion: Recovery for samples from Limit of Detection to 10 IU/L:≤ ± 2 IU/L, and samples > 10 IU/L: ≤ ± 20 % of initial value.Samples should not be taken from patients receiving therapy with high biotin doses (i.e. > 5 mg/day) until at least 8 hours following the last biotin administration.Pharmaceutical substancesIn vitro tests were performed on 16 commonly used pharmaceuticals. No interference with the assay was found.In addition, the following special drugs used in hepatitis B therapy were tested. No interference with the assay was found.Special drugsDrug Concentration testedmg/LPeginterferon alfa‑2a ≤ 0.18Peginterferon alfa‑2b ≤ 1.6Lamivudine ≤ 300Adefovir ≤ 10Entecavir ≤ 10Tenofovir ≤ 600Telbivudine ≤ 245Due to high-dose hook effect c), results from anti‑HBs concentrations of> 200000 IU/L may be found below the upper limit of the measuring range of 1000 IU/L. In rare cases, a high-dose hook effect from anti HBs concentrations of < 20000 IU/L cannot be excluded. Therefore in case of any unexpected low result the sample should be diluted 1:100 (refer to chapter “Dilution”) and tested again.In rare cases, interference due to extremely high titers of antibodies to streptavidin and ruthenium can occur. The test contains additives which minimize these effects.c) High-dose hook effect: A sample with a true concentration clearly above the measuring range, but found within the measuring range.Limits and rangesMeasuring range2‑1000 IU/L (defined by the Limit of Detection and the maximum of the master curve). Values below the Limit of Detection are reported as< 2 IU/L.Values above the measuring range are reported as > 1000 IU/L (or up to 100000 IU/L for 100‑fold diluted samples).DilutionSamples with anti‑HBs concentrations above the measuring range can be diluted with Diluent Universal. The recommended dilution is 1:100 (either automatically by the analyzer or manually). The concentration of the diluted sample must be > 10 IU/L.After manual dilution, multiply the result by the dilution factor.After dilution by the analyzer, the software automatically takes the dilution into account when calculating the sample concentration.Manual dilution can also be made with negative human serum.Note: Antibodies to HBsAg are heterogeneous. In some isolated cases, this may lead to non-linear dilution behavior.Specific performance dataRepresentative performance data on the analyzer is given below. Results obtained in individual laboratories may differ.PrecisionPrecision was determined using Elecsys reagents, samples and controls in a protocol (EP05‑A3) of the CLSI (Clinical and Laboratory Standards Institute): 2 runs per day in duplicate each for 21 days (n = 84). The following results were obtained:cobas e 801 analyzerRepeatability d)Intermediateprecision e)Sample MeanIU/LSDIU/LCV%SDIU/LCV% Human serum 1 4.33 0.224 5.2 0.272 6.3 Human serum 2 12.0 0.237 2.0 0.277 2.3 Human serum 3 475 6.81 1.4 7.55 1.6 PC f) Anti-HBs 1 < 2.00 - - - -PC Anti-HBs 2 83.8 1.08 1.3 1.28 1.5d) Repeatability = within-run precisione) Intermediate precision = between-run precisionf) PC = PreciControlAnalytical specificityNo cross-reactions with HAV, HCV, HEV, CMV, EBV, HIV, Rubella, Toxoplasma gondii, Treponema pallidum, rheumatoid arthritis, autoimmune response or alcoholic liver disease were observed.Measurements were performed on each of the pathogens listed above using ≥ 8 serum or plasma samples which were positive for antibodies to the above-mentioned pathogens.Relative sensitivityPerformance of the Elecsys Anti‑HBs II assay has been assessed by testing a total of 669 samples at two different study sites. 296 samples from vaccinated persons and 373 samples from patients recovered from a hepatitis B infection have been measured with the Elecsys Anti‑HBs II assay and another commercially available fully automated anti‑HBs assay. Discrepant samples were tested with additional anti‑HBs assays to achieve a consensus.Characterization ofsamplesN ElecsysAnti‑HBs IIreactiveAnti‑HBscomparisontest reactiveSensitivity%Anti-HBs positive:vaccinees 296 296 296 100Anti-HBs positive:recovered from ahepatitis B infection373 373 373 100 Total 669 669 669 100 Relative specificityPerformance of the Elecsys Anti‑HBs II assay has been assessed by testing 2673 samples from blood donors negative for anti‑HBs at two different study sites and 1623 anti‑HBs negative samples from laboratory routine at three different study sites. Discrepant samples were tested with additional anti‑HBs assays to achieve a consensus.Characterization of samples N ElecsysAnti‑HBs IIfalsepositiveSpecificity%Anti-HBs negative: blood donors 2673 6 99.78 Anti-HBs negative: routinesamples1623 9 99.45 References1Seeger C, Zoulim F, Mason WS. Hepadnaviruses. In: Field’s Virology, Knipe DM, Howley RM (eds), 2007 5th edition, Lippincott Williams andWilkins, Philadelphia, USA. Chapter 76, pp2977-3029.2WHO. Hepatitis B vaccines. Wkly Epidemiol Rec 2009;84:405-420.3Liaw YF, Chu CM. Hepatitis B virus infection. Lancet2009;373:582-592.4Caspari G, Gerlich WH. The serologic markers of hepatitis B virus infection – proper selection and standardized interpretation. Clin Lab2007;53:335-343.5Kramvis A, Kew M, François G. Hepatitis B virus genotypes. Vaccine 2005;23:2409-2423.6Michel ML, Tiollais P. Hepatitis B vaccines: protective efficacy and therapeutic potential. Pathol Biol 2010;58:288-295.7Elgouhari HM, Abu-Rajab Tamimi TI, Carey WD. Hepatitis B virus infection: understanding its epidemiology, course, and diagnosis. Cleve Clin J Med 2008;75:881-889.8Occupational Safety and Health Standards: Bloodborne pathogens. (29 CFR Part 1910.1030). Fed. Register.9Directive 2000/54/EC of the European Parliament and Council of18 September 2000 on the protection of workers from risks related toexposure to biological agents at workFor further information, please refer to the appropriate operator’s manual for the analyzer concerned, the respective application sheets, the product information and the Method Sheets of all necessary components (if available in your country).A point (period/stop) is always used in this Method Sheet as the decimal separator to mark the border between the integral and the fractional parts of a decimal numeral. Separators for thousands are not used.SymbolsRoche Diagnostics uses the following symbols and signs in addition to those listed in the ISO 15223‑1 standard:CONTENT Contents of kitSYSTEM Analyzers/Instruments on which reagents can be used REAGENT ReagentCALIBRATOR CalibratorVolume after reconstitution or mixingGTIN Global Trade Item NumberCOBAS, COBAS E, ELECSYS and PRECICONTROL are trademarks of Roche. INTRALIPID is a trademark of Fresenius Kabi AB.All other product names and trademarks are the property of their respective owners. Additions, deletions or changes are indicated by a change bar in the margin.© 2016, Roche DiagnosticsRoche Diagnostics GmbH, Sandhofer Strasse 116, D-68305 Mannheim。
罗氏E411全自动电化学发光免疫分析仪常见问题及处理

罗氏E411全自动电化学发光免疫分析仪常见问题及处理发表时间:2015-10-23T14:00:43.717Z 来源:《医药前沿》2015年第24期供稿作者:陆绍科[导读] 贵州省独山县人民医院贵州独山处理方法:打开试剂盒盖子,检查试剂如加得太满,用移液器将试剂吸出后重新执行试剂扫描或手工注册即可。
陆绍科(贵州省独山县人民医院贵州独山 558200)【摘要】罗氏E411全自动电化学发光免疫分析仪它具有操作简便,自动化程度高,精密度高,重复性好样品用量少,节省试剂,检测速度快等优点。
是国际公认的“金标准”,而在使用过程中往往会出现一些小故障,需要自行尽快排除,以至于不影响日常工作。
【中图分类号】R197.3 【文献标识码】A 【文章编号】2095-1752(2015)24-0303-02 仪器在运行过程中,当出现报警信息时,在屏幕上方状态栏的颜色会发生改变,报警颜色呈红色或黄色,当报警颜色为红色时,仪器将发生停止工作,同时报警代码简要的描述以及报警类型及中文提示都会显示出来。
笔者就使用该仪器现场解决处理的一些经验报告如下。
1. 吸样针检测到某项目试剂盒中有气泡1.1 报警此信息多数都是由于用户为了节约试剂,将多个试剂拼装后,拼装的试剂加得太满所致。
处理方法:打开试剂盒盖子,检查试剂如加得太满,用移液器将试剂吸出后重新执行试剂扫描或手工注册即可。
在不能确认是哪瓶试剂所致的报警时,可执行试剂扫描,当试剂针探测到某个试剂有问题时就会马上报警,此致即可确认是某个试剂的问题即可将其取出。
1.2 报此信息的另一个问题是:试剂在运输过程中受到振动可能的产生气泡。
处理方法:此时应将试剂静置30分钟后再用。
日常工作在取出试剂使用时应当避免人为晃动或颠倒试剂。
2.由于电导率升高导致某项目试剂的液面误检/运动过程中,样本/试剂针液面感应错误2.1 报警此信息多数是样本/试剂针表面有静电或有污物附着,处理方法:在关机状态下,手工移动样本/试剂针到方便擦拭的位置,用纱布沾上70%酒精,轻轻擦拭针的表面和针尖;再用蒸馏水清洁1次即可。
罗氏电化学发光免疫分析仪定标物保存方法

罗氏电化学发光免疫分析仪定标物保存方法罗氏电化学发光免疫分析仪是一种常见的实验仪器,用于检测和定量分析生物样本中的特定生物分子,如蛋白质、抗体、激素等。
定标是使用已知浓度的标准物质来建立分析仪器的检测曲线和定量范围。
定标物的保存方法对于保证分析仪器的准确性和稳定性非常重要。
本文将从两个方面介绍罗氏电化学发光免疫分析仪定标物的保存方法,包括标准物质的选择和保存条件。
选择标准物质:在进行定标前,首先需要选择与待测分析物相同或相似的标准物质进行定标。
标准物质应具有以下特点:1.纯度高:标准物质的纯度应尽可能接近100%,以减少不纯物质对分析结果的影响。
2.稳定性好:标准物质应具有良好的化学稳定性和物理稳定性,以确保在保存期间不发生分解、降解或溶解。
3.可溶性好:标准物质应易于溶解于使用的溶剂中,以便在定标过程中进行稀释。
4.经济适用:标准物质的价格应相对较低,以降低实验成本。
保存条件:标准物质的保存条件直接影响着其稳定性和可靠性,因此需要注意以下几个方面:1.存储温度:一般来说,标准物质的保存温度应根据其化学性质和稳定性来确定。
一些易降解的标准物质需在低温下保存,如-20℃或更低。
而一些稳定性较好的标准物质可以在常温下保存,但应避免暴露在光照、高温或高湿环境中。
2.容器选择:标准物质应存放在密封、不透光的容器中,以防止光和空气的影响。
常见的存储容器有玻璃瓶、塑料管或聚丙烯封口袋等。
3.避免冷冻解冻:对于需要在冷冻状态下保存的标准物质,应避免频繁的冷冻解冻过程,以免影响其稳定性。
可以根据实验需要,将标准物质分装成适量的小份,每次只取一小部分使用,并立即将剩余的封存起来。
4.防潮防湿:标准物质应避免接触水分和湿气,以免发生水解、分解或结晶现象。
可以在存放容器中加入干燥剂,如无水硫酸钠、碳酸钠或干燥的硅胶等,以吸湿保持干燥环境。
5.定期检测:定期对保存的标准物质进行检测,以确保其浓度和稳定性。
可以使用同一批次的标准物质进行再次定标,以验证保存的标准物质是否保持稳定。
罗氏电化学发光原理

罗氏电化学发光原理罗氏电化学发光原理是指在电化学发光(Electrochemiluminescence,ECL)过程中,通过电化学手段激发物质发光的原理。
这一原理在生物医学领域中有着广泛的应用,特别是在免疫分析和生物传感器领域。
本文将对罗氏电化学发光原理进行详细介绍,以便更好地理解其在实际应用中的作用。
首先,罗氏电化学发光原理的基本过程是通过电化学反应来产生激发态物质,从而激发发光。
这一过程通常涉及到两种基本的反应,氧化还原反应和化学发光反应。
在氧化还原反应中,通过施加电压或电流,使得电极上的物质发生氧化还原反应,产生激发态物质。
而在化学发光反应中,激发态物质通过与另一种物质发生化学反应,释放出光子,产生发光现象。
这两种反应的结合,构成了罗氏电化学发光的基本原理。
其次,罗氏电化学发光原理的应用主要集中在生物医学领域。
在免疫分析中,罗氏电化学发光被广泛应用于检测生物分子,如蛋白质、核酸和细胞等。
通过将待测物与特定的标记物结合,利用罗氏电化学发光技术来检测标记物的信号强度,从而实现对待测物的定量分析。
在生物传感器领域,罗氏电化学发光也被用于构建高灵敏度、高选择性的生物传感器,用于检测生物分子的存在和浓度。
此外,罗氏电化学发光原理还具有许多优势。
首先,它具有高灵敏度和高选择性,能够在复杂的生物样品中进行精准的分析。
其次,罗氏电化学发光技术还具有快速、简便的特点,适用于高通量的实验操作。
此外,由于其不需要外部光源的激发,可以减少背景信号的干扰,提高检测的准确性。
总的来说,罗氏电化学发光原理是一种重要的生物分析技术,具有广泛的应用前景。
通过对其原理和应用的深入理解,可以更好地推动生物医学领域的研究和应用,为人类健康事业做出更大的贡献。
在实际应用中,我们需要不断改进和优化罗氏电化学发光技术,提高其灵敏度、稳定性和成本效益,以满足不断增长的生物医学研究和临床检测需求。
相信随着技术的不断进步和创新,罗氏电化学发光原理将会发挥更加重要的作用,为人类健康事业带来更多的突破和进步。
罗氏ECL电化学免疫仪检测项目与参考范围
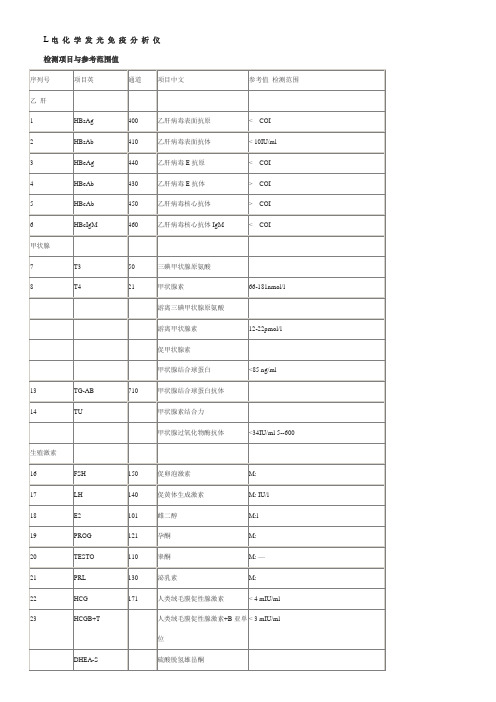
DHEA-S
硫酸脱氢雄甾酮
肿瘤
24
AFP
310
甲胎蛋白
≤ml
25
CEA
301
癌胚抗原
< ml
吸烟:< ml
26
FER
380
铁蛋白
M:30-400ng/ml
W13-150ng/ml 0500-2000
27
CA125
341
癌抗原125
< 35U/ml
28
CA15-3
332
癌抗原15-3
34
FPSA
391
游离前列腺特异性抗原
F:T>
贫血
35
FER
380
铁蛋白
M:30-400ng/ml
W13-150ng/ml 0500-2000
36
VitB12
600
维生素B12
197-866g/ml 145-637pmol/l
30-2000pg/ml22-1476pmol/l
37
FOL
610
叶酸
骨代谢
38
PTH
680
甲状旁腺素
15-65pg/ml
39
B-Crosslaps
670
B-胶原特殊序列
附pg/ml
40
N-MID
660
N-端骨钙素
附
糖尿病
41
INSULIN
650
胰岛素
药物
42
Cortisol
160
可的松
Am7-10:171-536nmol/ml
Pm 4-8: 64-340 nmol/ml
罗氏电化学发光免疫分析

罗氏电化学发光免疫分析技术是罗氏公司开发的,但全自动机械制造却由日本的日立公司承担,所以仪器上还有Hitachi的标志;这个仪器让大家吃惊的一大原因就在于一直在实验室研究的电致化学发光居然已经真正地产业化了,其中我们一直无法解决的诸多问题尤其是重现性均已得到解答,看来罗氏的确花了不少心血开发这款仪器;罗氏电化学发光免疫分析技术的性能特点——创新的技术,与众不同一、最先进的检测原理电化学发光免疫测定,是目前最先进的标记免疫测定技术,是继放射免疫、酶免疫、荧光免疫、化学发光免疫测定以后的新一代标记免疫测定技术,具有敏感、快速和稳定的特点,在固相标记免疫测定中技术上居领先地位;电化学发光ECL是一种在电极表面由电化学引发的特异性化学发光反应,实际上是电化学和化学发光两个过程的完美结合;电化学发光与普通化学发光的主要差异在于前者是电启动发光反应,循环及多次发光,后者是通过化合物混合启动发光反应,是单次瞬间发光;因此ECL反应易精确控制,重复性极好;电化学发光免疫测定是电化学发光ECL和免疫测定相结合的产物,直接以Rubpy32+标记抗体,反应时标记物直接发光;且Rubpy32+在电极表面的反应过程可以周而复始进行,产生许多光子,使光信号得以增强;二、专利的包被技术链霉亲和素streptoavidin,SA和生物素biotin,B是具有很强的非共价相互作用的一对化合物,特异性强且结合紧密;一分子SA可与四分子B相结合,增大了抗体结合量,达到放大效果;在ECL的试剂中,SA通过特殊的蛋白结合物均匀牢固地包被在磁性微粒上,形成通用的能与B结合的固相载体,另一试剂为活化的B衍生物化合的抗原或抗体;两种试剂混合时,抗原或抗体即包被在磁性微粒上;三、独特的载体ECL中采用的固相载体是带有磁性的直径约m的聚苯乙烯微粒;其特点是反应面积极大,比板式扩大20-30倍,使反应在近乎液相中进行,反应速度大大加快,利用氧化铁的磁性,使用电磁场分离结合态和游离态,方便迅速,实现了精确的全自动化;四、独到的磁分离技术实现了结合相和游离相的完全自动化分离,且检测池在无电场时彻底清洗,避免了交叉污染;五、超高的测定灵敏度和测定线性发光信号检测的宽线性加上电化学发光独特的标记物本身发光底物循环发光和专利的链霉亲和素-生物素包被技术的信号放大作用,使电化学发光测定的检测下限可达10-12和10-18级,线性范围最大超越7个数量级,在待测抗原抗体极微量或达到病理期极限时,均能准确测定,避免了样本稀释重测定,既节约时间,又节省试剂;六、稳定的试剂电化学发光标记物三联吡啶钌在无电场和递电子体三丙胺存在的自然环境下非常稳定,保证了用它标记的抗体抗原试剂也非常稳定,2-8℃可稳定一年以上,批内和批间变异系数分别为<4%和<7%,在首日使用之后也可以稳定3个月;七、简便创新的定标概念每个测定项目的基本定标曲线已由罗氏公司完成,并已存入试剂的二维条形码,自动读入仪器,用户只需进行二点重定标即可;八、简便稳妥的二维条形码——当代最先进的全自动信息处理技术二维条形码是电化学发光分析仪的“信息高速公路”,可以存储超过一千字节的信息,使同批试剂仅做一次2点定标,亦使仪器自动化程度升高,不必做大量的定标、质控等前期工作,厂家已做12点标准曲线,既节约时间,又保障结果的准确;九、先进的闪烁存储技术断电时软件数据永不丢失,避免硬盘损坏,导致系统崩溃,启动速度比硬盘快10倍;十、广泛的应用范围和广阔的开发前景检测项目广泛应用于甲状腺、性激素、骨代谢、心肌梗塞、肿瘤标志物、传染病抗原抗体等的定量测定;ECL分析原理中采用三联吡啶钌作为标记物,其活化衍生物是三联吡啶钌+N羟基琥珀酸胺酯NHS;该衍生物性能稳定,且分子量很小,产生的空间位阻小,与免疫球蛋白的分子比超过20仍不会影响抗体的可溶性和免疫活性,因此它可与抗体、半抗原、激素、核酸等各种生物分子结合形成稳定的标记物,使检测的菜单大大丰富,为其检测菜单的开发前景提供了广阔空间;罗氏收购宝灵曼后,设有专职的研发部门为电化学发光分析仪开发配套试剂,所以每年都会有5-10个新项目问世,为用户的项目拓展奠定了基础;。
Cobase电化学发光免疫分析仪用户操作手册

cobas e411 (Disk )操作手册罗氏诊断产品(上海)有限公司广州分公司目 录第一章系统概述 • 一. e411主要部件 ---------------------- 二. 控制单元一 三. 样品/试剂区 ------------------------四.测试区 ----------------------------五. 消耗品区 ---------------------------A :操作开关C :测试区 位于仪器右侧面2 2 33 4 4666 6 67 9 9 10 10 10 12 12 12 13 14 15 16 第二章基本操作第一章系统概述e411王要部件B :样本(30个位置)/试剂区(18个位置,具自动开关盖装置)D :消耗品区E :显示及控制单元位于仪器左侧面A :主电源开关B :电源线 A : USB 接口C : HOST 接口控制单元A :系统试剂保护门B : Sipple 针C : CleanCell (黑盖)D : ProCell (白盖)三.样品/试剂区A :磁珠搅拌针 C :样本/试剂针A :定标/质控条形码阅读口四带开测试区试剂仓A B™ 和片FI 廿rn|A :触摸屏B :虚拟键盘(触摸屏幕 上需输入内容区域 时,自动在屏幕下方 I 鱼七跳岀)C :数字键盘A :孵育池(共183个位个孵育位)B :吸样位BAA :取样区B :样品盘保护盖 -CAB :冲洗站A:系统试剂保护门B: Sipple针C: CleanCell (黑盖)D: ProCell (白盖)五.消耗品区用水Db. 清空废液桶c.打开ProCell 和CleanCell 盖子,关好系统试剂保护门。
注意:运行过程中不能打开系统试剂保护门,否则仪器将停止运行。
d. 打开仪器右面主电源开关,再打开仪器前面操作开关,等界面岀现后,录入用户名和密码,仪器自动初始 化后进入待机状态注意:仪器分不同级别及权限使用,可根据实际情况设定;添加用户名后,第 一次输入的密码即为以后的密码。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
罗氏电化学发光免疫分析
技术是罗氏公司开发的,但全自动机械制造却由日本的日立公司承担,所以仪器上还有Hitachi的标志。
这个仪器让大家吃惊的一大原因就在于一直在实验室研究的电致化学发光居然已经真正地产业化了,其中我们一直无法解决的诸多问题(尤其是重现性)均已得到解答,看来罗氏的确花了不少心血开发这款仪器。
罗氏电化学发光免疫分析技术的性能特点——创新的技术,与众不同
一、最先进的检测原理
电化学发光免疫测定,是目前最先进的标记免疫测定技术,是继放射免疫、酶免疫、荧光免疫、化学发光免疫测定以后的新一代标记免疫测定技术,具有敏感、快速和稳定的特点,在固相标记免疫测定中技术上居领先地位。
电化学发光(ECL)是一种在电极表面由电化学引发的特异性化学发光反应,实际上是电化学和化学发光两个过程的完美结合。
电化学发光与普通化学发光的主要差异在于前者是电启动发光反应,循环及多次发光,后者是通过化合物混合启动发光反应,是单次瞬间发光。
因此ECL反应易精确控制,重复性极好。
电化学发光免疫测定是电化学发光(ECL)和免疫测定相结合的产物,直接以[Ru(bpy)3]2+标记抗体,反应时标记物直接发光。
且[Ru(bpy)3]2+在电极表面的反应过程可以周而复始进行,产生许多光子,使光信号得以增强。
二、专利的包被技术
链霉亲和素(streptoavidin,SA)和生物素(biotin,B)是具有很强的非共价相互作用的一对化合物,特异性强且结合紧密。
一分子SA可与四分子B 相结合,增大了抗体结合量,达到放大效果。
在ECL的试剂中,SA通过特殊的蛋白结合物均匀牢固地包被在磁性微粒上,形成通用的能与B结合的固相载体,另一试剂为活化的B衍生物化合的抗原或抗体。
两种试剂混合时,抗原或抗体即包被在磁性微粒上。
三、独特的载体
ECL中采用的固相载体是带有磁性的直径约2.8 m的聚苯乙烯微粒。
其特点是反应面积极大,比板式扩大20-30倍,使反应在近乎液相中进行,反应速度大大加快,利用氧化铁的磁性,使用电磁场分离结合态和游离态,方便迅速,实现了精确的全自动化。
四、独到的磁分离技术
实现了结合相和游离相的完全自动化分离,且检测池在无电场时彻底清洗,避免了交叉污染。
五、超高的测定灵敏度和测定线性
发光信号检测的宽线性加上电化学发光独特的标记物本身(发光底物)循环发光和专利的链霉亲和素-生物素包被技术的信号放大作用,使电化学发光测定的检测下限可达10-12和10-18级,线性范围最大超越7个数量级,在待测抗原(抗体)极微量或达到病理期极限时,均能准确测定,避免了样本稀释重测定,既节约时间,又节省试剂。
六、稳定的试剂
电化学发光标记物三联吡啶钌在无电场和递电子体(三丙胺)存在的自然环境下非常稳定,保证了用它标记的抗体(抗原)试剂也非常稳定,2-8℃可稳定一年以上,批内和批间变异系数分别为<4%和<7%,在首日使用之后也可以稳定3个月。
七、简便创新的定标概念
每个测定项目的基本定标曲线已由罗氏公司完成,并已存入试剂的二维条形码,自动读入仪器,用户只需进行二点重定标即可。
八、简便稳妥的二维条形码——当代最先进的全自动信息处理技术
二维条形码是电化学发光分析仪的“信息高速公路”,可以存储超过一千字节的信息,使同批试剂仅做一次2点定标,亦使仪器自动化程度升高,不必做大量的定标、质控等前期工作,厂家已做12点标准曲线,既节约时间,又保障结果的准确。
九、先进的闪烁存储技术
断电时软件数据永不丢失,避免硬盘损坏,导致系统崩溃,启动速度比硬盘快10倍。
十、广泛的应用范围和广阔的开发前景
检测项目广泛应用于甲状腺、性激素、骨代谢、心肌梗塞、肿瘤标志物、传染病抗原抗体等的定量测定。
ECL分析原理中采用三联吡啶钌作为标记物,其活化衍生物是三联吡啶钌+N羟基琥珀酸胺酯(NHS)。
该衍生物性能稳定,且分子量很小,产生的空间位阻小,与免疫球蛋白的分子比超过20仍不会影响抗体的可溶性和免疫活性,因此它可与抗体、半抗原、激素、核酸等各种生物分子结合形成稳定的标记物,使检测的菜单大大丰富,为其检测菜单的开发前景提供了广阔空间。
罗氏收购宝灵曼后,设有专职的研发部门为电化学发光分析仪开发配套试剂,所以每年都会有5-10个新项目问世,为用户的项目拓展奠定了基础。
