AOAC 2001.03 测定特定食品中的总膳食纤维 包含抗性麦芽糊精 酶重量法和液相色谱法
食品中碳水化合物检测项目(科标检测)
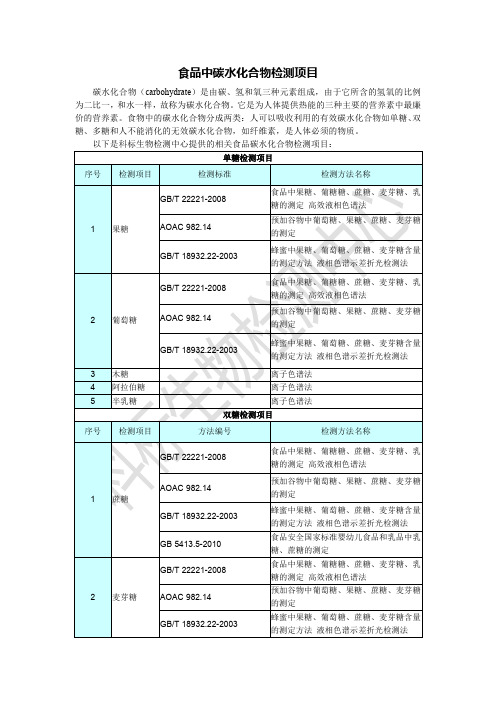
GB/T 22221-2008 3 乳糖 GB 5413.5-2010
食品中果糖、葡糖糖、蔗糖、麦芽糖、乳 糖的测定 高效液相色谱法 食品安全国家标准婴幼儿食品和乳品中乳 糖、蔗糖的测定
低聚糖检测项目 序号 检测项目 蔗果三糖 1 低聚果糖 蔗果四糖 蔗果五糖 异麦芽糖 2 低聚异麦芽 异麦芽三糖 糖 潘糖 异麦芽四糖 3 4 5 大豆低聚糖 棉籽糖 水苏糖 离子色谱法 离子色谱法 离子色谱法 离子色谱法 离子色谱法 离子色谱法 离子色谱法 离子色谱法 离子色谱法 选定食品产品中反式低聚半乳糖的测定 ——离子色谱法 品中的聚葡萄糖——离子色谱法 检测方法名称
食品中碳水化合物检测项目
碳水化合物(carbohydrate)是由碳、氢和氧三种元素组成,由于它所含的氢氧的比例 为二比一,和水一样,故称为碳水化合物。它是为人体提供热能的三种主要的营养素中最廉 价的营养素。食物中的碳水化合物分成两类:人可以吸收利用的有效碳水化合物如单糖、 双 糖、多糖和人不能消化的无效碳水化合物,如纤维素,是人体必须的物质。 以下是科标生物检测中心提供的相关食品碳水化合物检测项目:
低聚半乳糖 AOAC 2001.02 聚葡萄糖 AOAC 2000.11
膳食纤维检测项目 序号 1 2 3 4 5 检测项目 膳食纤维 总膳食纤维 不溶性膳食纤维 AOAC 991.43 可溶性膳食纤维 膳食纤维 GB/T 22224-2008 方法编号 GB/T 5009.88-2008 检测方法名称 食品中膳食纤维的测定 食品中总膳食纤维、不溶性膳食纤维、可 溶性膳食纤维的测定 酶重量法 MES TRIS 缓冲 食物中的膳食纤维的测定 酶重量法和酶 重量法-液相色谱测定 含有抗性麦芽糊精(简称:RMD)的食物中 6 总膳食纤维 AOAC2001.03 的膳食纤维总量 酶-重量法和液相色谱法 测定 7 8 不溶性膳食纤维 GB 5413.6-2010 不溶性膳食纤维 GB/T 9822-2008 食品安全国家标准婴幼儿食品和乳品中不 溶性膳食纤维的测定 谷物不溶性膳食纤维测定法
aoac标准

aoac标准AOAC标准。
AOAC国际是一个全球性的食品分析标准组织,成立于1884年,总部位于美国马里兰州。
AOAC标准是全球公认的食品分析标准,被广泛应用于食品安全检测、食品质量控制和食品生产过程中。
AOAC标准的制定严格遵循科学、客观、公正、透明的原则,确保标准的可靠性和准确性。
AOAC标准的制定过程经历了多个环节,包括提案、评审、公示、修订等多个步骤。
在提案阶段,任何相关单位和个人都可以向AOAC提出新标准的制定建议,AOAC将对提案进行评估,确定是否具备制定新标准的必要性和可行性。
经过评审通过的提案将进入公示阶段,公示期内,任何单位和个人都可以对提案进行意见反馈。
之后,AOAC将根据公示期内收集的意见对提案进行修订,最终确定新标准的制定方案。
AOAC标准涵盖了食品中的营养成分分析、微生物检测、农药残留检测、重金属检测、食品添加剂检测等多个领域。
在营养成分分析方面,AOAC标准规定了食品中脂肪、蛋白质、碳水化合物、维生素等营养成分的检测方法和标准数值,确保食品标签上的营养成分信息准确无误。
在微生物检测方面,AOAC标准规定了食品中常见的致病菌、霉菌、酵母菌等微生物的检测方法和限量标准,保障食品的微生物安全。
在农药残留检测方面,AOAC标准规定了食品中农药残留的检测方法和安全标准,防止因农药残留导致的食品安全问题。
AOAC标准的制定和应用,对于保障食品安全、促进食品质量提升、维护消费者权益具有重要意义。
通过严格的标准制定和检测方法认证,可以有效防范食品中的各类安全隐患,保障公众健康。
同时,AOAC标准也为食品生产企业提供了科学、规范的质量管理体系,有助于提升企业的竞争力和信誉度。
此外,AOAC标准的国际认可性和权威性,也为全球食品贸易提供了统一的标准基础,促进了国际贸易的顺畅进行。
总之,AOAC标准作为全球食品分析领域的权威标准,对于食品安全、食品质量和国际贸易具有重要意义。
未来,AOAC将继续致力于完善现有标准、制定新的标准,为全球食品行业的可持续发展贡献力量。
【精品】食物中膳食纤维的测定

膳食纤维的测定方法酶-重量法1.原理:样品分别用α-淀粉酶、蛋白酶、葡萄糖苷酶进行酶解消化以去除蛋白质和可消化的淀粉。
总膳食纤维(TDF)是先酶解,然后用乙醇沉淀,再将沉淀物过滤,将TDF残渣用乙醇和丙酮冲洗,干燥称重。
不溶性和可溶性膳食纤维(IDF和SDF)是酶解后将IDF过滤,过滤后的残渣用热水冲洗,经干燥后称重。
SDF是将上述滤出液用4倍量的95%乙醇沉淀,然后再过滤,干燥,称重。
TDF、IDF 和SDF量通过蛋白质、灰分含量进行校正。
2.适用范围AOAC991.43本方法适用于各类植物性食物和保健食品。
3.仪器3.1烧杯:400或600ml高脚型。
3.2过滤用坩埚:玻料滤板,美国试验和材料学会(ASTM)40-60μm,Pyrex60ml(CorningNo.36060buchner,或同等的)。
如下处理:(1)在灰化炉525℃灰化过夜。
炉温降至130℃以下取出坩埚。
(2)用真空装置移出硅藻土和灰质。
(3)室温下用2%清洗溶液浸泡1小时。
(4)用水和去离子水冲洗坩埚;然后用15ml丙酮冲洗然后风干。
(5)在干燥的坩埚中加0.5g硅藻土,在130℃烘干恒重。
(6)在干燥器中冷却1小时,记录坩埚加硅藻土重量,精确至0.1mg。
3.3真空装置:(1)真空泵或抽气机作为控制装置。
(2)1L的厚壁抽滤瓶。
(3)与抽滤瓶相配套的橡皮圈。
3.4振荡水浴箱:(1)自动控温使温度能保持在98±2℃。
(2)恒温控制在60℃。
3.5天平:分析级,精确至±0.1mg。
3.6马福炉:温度控制在525±5℃。
3.7干燥箱:温度控制在105和130±3℃。
3.8干燥器:用二氧化硅或同等的干燥剂。
干燥剂两周一次在130℃烘干过夜。
3.9PH计:注意温控,用pH4.0、7.0和10.0缓冲液标化。
3.10移液管及套头:容量100μl和5ml。
3.11分配器或量筒:(1)15±0.5ml,供分配78%的乙醇,95%的乙醇以及丙酮。
酶重量法-液相色谱法测定食品中总膳食纤维

酶重量法-液相色谱法测定食品中总膳食纤维余枭然;余慧剑【期刊名称】《河南科技》【年(卷),期】2021(40)28【摘要】本研究利用酶重量法-液相色谱法,测定含水溶性膳食纤维食品的总膳食纤维含量。
采用酶重量法测定试样中不溶性膳食纤维(Insoluble Dietary Fiber,IDF)和高分子质量可溶性膳食纤维(Soluble Dietary Fiber,SDF)的含量,采用液相色谱法测定试样的低分子质量抗性麦芽糊精(Resistant Malto Dextrin,RMD)含量。
液相色谱法的方法检出限为0.142%,定量限为0.474%,线性方程为y=1.207x+0.030,相关系数R^(2)为0.999,加标回收率为96.6%~99.7%,相对标准偏差(Relative Standard Deviation,RSD)为1.53%~1.69%(次数n=6)。
精密度试验以酶重量法-液相色谱法测试样品6份,平均含量为65.78%,RSD为0.52%。
实验结果表明,该方法灵敏度、准确率高,适用于添加抗性淀粉、含低分子质量的抗性糊精等水溶性膳食纤维的食品中总膳食纤维的测定。
【总页数】4页(P127-130)【作者】余枭然;余慧剑【作者单位】上海工程技术大学;劲研(上海)生物科技有限公司【正文语种】中文【中图分类】R151.3【相关文献】1.应用酶重量法测定全麦粉的总膳食纤维2.酶——重量法测定食品中的膳食纤维3.应用酶-重量法测定食物中的总膳食纤维4.关于测定粮食中总纤维素的中性净化法和酶重量法的改良5.食品中总的、不溶性及可溶性膳食纤维的酶-重量测定法因版权原因,仅展示原文概要,查看原文内容请购买。
aoac标准

aoac标准
AOAC标准。
AOAC国际是一个专门从事食品分析的非营利性科学组织,成立于1884年,
总部位于美国马里兰州。
AOAC标准是该组织发布的一系列用于食品和环境样品
分析的标准方法,被广泛应用于全球范围内的食品安全监测、质量控制和法规遵从性检验中。
本文将对AOAC标准进行介绍和分析。
首先,AOAC标准的制定是经过严格的科学验证和实践检验的。
这些标准方法
的制定过程需要经过多个实验室的验证和比对,确保其准确性、可重复性和可靠性。
因此,AOAC标准被公认为是行业内的权威标准,被广泛应用于食品和环境样品
的分析检测中。
其次,AOAC标准的内容涵盖了多个方面的分析方法,包括但不限于样品的制备、分离、测定和确认等步骤。
这些方法涵盖了食品中的营养成分、添加剂、农药残留、重金属、微生物污染等多个方面,可以满足不同类型样品的分析需求。
另外,AOAC标准的应用范围非常广泛,不仅可以应用于传统食品,还可以应
用于新型食品、保健食品、农产品和环境样品等多个领域。
这些标准方法的准确性和可靠性为食品行业的质量控制、风险评估和法规遵从性检验提供了有力的支持。
总的来说,AOAC标准是食品分析领域的权威标准,其制定经过严格的科学验
证和实践检验,内容涵盖了多个方面的分析方法,应用范围广泛。
因此,我们可以充分信赖AOAC标准,将其应用于食品和环境样品的分析检测中,以保障食品安
全和环境质量。
食品中膳食纤维的测定

1.1.1.1.1.3食品安全国家标准食品中膳食纤维的测定(征求意见稿)发布实施中华人民共和国卫生部发布前言本标准代替《食品中膳食纤维的测定》。
本标准与相比,主要变化如下:——修改了方法适用范围;——增加了膳食纤维、总膳食纤维、不溶性膳食纤维、可溶性膳食纤维的术语和定义;——修改了试剂顺序和文字格式;——修改了总膳食纤维计算公式;——添加了当食品中含有低分子质量可溶性膳食纤维时总膳食纤维计算方法的注释;——将酶重量法作为第一法,中性洗涤剂法作为第二法。
食品安全国家标准食品中膳食纤维的测定1 范围本标准规定了食品中膳食纤维的测定方法。
本标准酶重量法适用于植物类食品及其制品中总的、可溶性和不溶性膳食纤维的测定;中性洗涤剂法适用于谷物原料中不溶性膳食纤维的测定。
本标准第一法为仲裁法。
2 术语和定义下列术语和定义适用于本标准。
2.1 膳食纤维指植物中天然存在的、提取或合成的、聚合度 的碳水化合物聚合物,不能被人体小肠消化吸收、对人体有健康意义,包括纤维素、半纤维素、木质素、果胶、菊粉及其他一些膳食纤维单体成分等。
2.2 可溶性膳食纤维指能溶于水的膳食纤维部分。
2.3 不溶性膳食纤维指不能溶于水的膳食纤维部分,包括木质素、纤维素、部分半纤维素等。
2.4 总膳食纤维可溶性膳食纤维与不溶性膳食纤维之和。
第一法总的、可溶性和不溶性膳食纤维的测定(酶重量法)3 原理干燥试样经热稳定α淀粉酶、蛋白酶和葡萄糖苷酶酶解消化去除蛋白质和淀粉后,酶解液经乙醇沉淀、过滤,残渣用乙醇和丙酮洗涤,干燥后称重,即为总膳食纤维残渣。
另取同样经酶解的酶解液直接过滤,用热水洗涤残渣,干燥后称重,即得不溶性膳食纤维残渣;滤液用倍体积的乙醇沉淀、过滤、干燥后称重,得可溶性膳食纤维残渣。
扣除残渣中相应的蛋白质、灰分和空白即可计算出试样中总的、不溶性和可溶性膳食纤维的含量。
采用酶重量法测定的总膳食纤维包括不溶性膳食纤维和能被乙醇沉淀的高分子质量可溶性膳食纤维,如纤维素、半纤维素、果胶、其它非淀粉多糖及木质素等;不包括低分子质量的可溶性膳食纤维,如抗性麦芽糊精、果寡糖、低聚半乳糖、多聚葡萄糖等,及部分被加热破坏的抗性淀粉。
AOAC 2001.03 测定特定食品中的总膳食纤维 包含抗性麦芽糊精 酶重量法和液相色谱法
45.4.13AOAC Official Method2001.03Total Dietary Fiber in FoodsContaining Resistant MaltodextrinEnzymatic-Gravimetric Methodand Liquid Chromatography DeterminationFirst Action2001[This method is applicable to resistant maltodextrin(RMD)and to foods containing RMD listed in Table2001.03at 1.4%RMD.] A.PrincipleThis method determines total dietary fiber(TDF)value of pro-cessed foods containing insoluble dietary fiber(IDF)and high mo-lecular weight soluble dietary fiber(HMWSDF),which are precipitated in ethanol and low molecular weight resistant maltodextrin(LMWRMD),which is soluble in ethanol.This method defines dietary fiber(DF)as consisting of nondigestible car-bohydrates having a degree of polymerization with3sugar moieties (DP3)or higher after enzymatic hydrolysis.All the starches con-tained in food are converted to glucose after this enzymatic hydroly-sis.This method to determine TDF content in processed foods containing RMD is a combination of985.29(see45.4.07)for DF and a LC method for LMWRMD.A food is first analyzed for the to-tal quantity of IDF and HMWSDF,precipitated in ethanol,accord-ing to985.29(see45.4.07).Then an LC determination is conducted on the desalted filtrate to obtain the quantity of LMWRMD not pre-cipitated in the78%alcohol preparation.These2values[(IDF+ HMWSDF)+LMWRMD]are summed to obtain the TDF value in the food.B.Apparatus(a)Balance.—Analytical,weighing to0.1mg.(b)Beakers.—Tall-form,500mL.(c)Water baths.—To maintain a temperature of95–100C and 60C with ability to shake the containers.(d)Filtering crucibles.—Coarse,ASTM,40–60m pore size, Pyrex,50mL.(e)Glass or plastic columns.—To hold ion exchange resins, 75cm15mm id;a shorter(40–75cm15mm id)column can also be used.(f)Liquid chromatograph(LC).—With oven to maintain a col-umn temperature of80C and a20L injection loop.Column oper-ating conditions are:Temperature,80C;mobile phase,distilled water,C(d);flow rate,0.5mL/min.(g)Guard column(or precolumn).—TSK®guard column PWXL,6.0mm id4cm(Tosoh Corp.,distributed by TosoHaas, Montgomeryville,PA,USA;)or equivalent. (h)LC columns.—Two LC columns connected in series, TSK-GEL®G2500PWXL,7.8mm id30cm(Tosoh Corp.),or equivalent.(i)Detector.—Refractive index(RI);maintained at40C. (j)Data integrator or computer.—For peak area measurement. (k)Filters for disposable syringe.—0.2m membrane,13mm. (l)Filters for water.—0.2m,47mm.(m)Filter apparatus.—To hold47mm,0.2m filter,(l);to filter larger volumes of water,C(d).(n)Glass rods.—With fire-polished ends,ca20cm long. (o)Syringes.—10mL,plastic disposable.(p)Pasteur pipet.(q)Volumetric pipet.—10mL.(r)Volumetric flasks.—10,50,250,and1000mL.(s)Top loading balance.—4000g capacity.(t)Tubing.—PVC,2.79mm id(for ion exchange columns). (u)Glass LC syringe.—50L.(v)Teflon scraping rod.—Use in place of glass stirring rod to scrape precipitate from tall-form beaker.(w)Rotary evaporator.—R-3000VW“Student”(Büchi,Swit-zerland;)or equivalent.C.Reagents(a)Ethanol.—95%.Technical grade,used at60C.(b)Ethanol.—78%.Place207mL water in1L volumetric flask and dilute to volume with95%ethanol,(a).(c)Acetone.—Reagent grade.(d)Distilled water.(e)Sodium phosphate dibasic.(f)Sodium phosphate monobasic.(g)Phosphate buffer.—0.08M,pH6.0.Dissolve1.400g Na2HPO4(or1.753g dihydrate)and9.68g NaH2PO4H2O(or10.94g dihydrate)in ca700mL water,(d).Dilute to1L with water,(d),and verify pH with pH meter.(h)Heat stable a-amylase solution(Termamyl).—No.120L(ac-tivity:12units/mg protein;Novo Laboratories,Inc.,59Danbury Rd, Wilton,CT06897,USA),or equivalent(should not contain glycerol).(i)LC retention time standard.—Standard source of the distribu-tion of oligosaccharides(DP$3)in the LMWRMD fraction of RMD, corn syrup solids(DE25;Matsutani Chemical Industry Co.,Ltd.,ItamiTable2001.03Interlaboratory results for the determination of total dietary fiber in selected foods containing resistant maltodextrin by enzymatic-gravimetric method and liquid chromatographyFood x,%bs a(b)s r RSD r,%s R RSD R,% Resistant maltodextrin95.368(0) 1.63 1.71 2.37 2.48Hard candy37.997(1)0.58 1.530.68 1.79 Chicken and vegetable soup25.418(0)0.74 2.89 1.18 4.65 Grapefruit juice 1.388(0)0.02 1.330.04 3.20 White bread9.608(0)0.33 3.410.64 6.66 Strawberry Jell-O9.918(0)0.60 6.100.939.39a(b)a=Number of laboratories retained after eliminating outliers;b=number of laboratories removed as outliers.City,Hyogo,Japan;),analyzed by LC(Fig-ure2001.03A)as in D.(j)Protease.—No.P-3910or P-5380(activity:7–15units/mg pro-tein;Sigma Chemical Co.,St.Louis,MO,USA),or equivalent(should not contain glycerol).Prepare protease stock solution just before use by adding100mg protease enzyme to a10mL volumetric flask and bring-ing to volume with water,(d),(amount is sufficient for9test portions in duplicate).(k)Amyloglucosidase.—No.A-9913(activity:400units/mg pro-tein;Sigma Chemical Co.),or equivalent(should not contain glycerol). (l)Celite.—No.C-8656(Sigma Chemical Co.)or No.C-211,acid washed(Fisher Scientific Co.,Fair Lawn,NJ,USA),or equivalent. (m)Mixed-bed ion exchange resins for each test por-tion.—(1)m-1.—25g Amberlite IRA-67(OH-type;Organo Corp., Tokyo,Japan,/organo_corp.htm),or equivalent.(2)m-2.—25g Amberlite200CT(HG)H(H-type;Organo Corp.),or equivalent,are mixed and packed in column for analysis of each test portion.The converted resin should satisfy the following specifications:(a)Total ion exchange capacity:1.74meq/mL (min);(b)Effective ion exchange capacity(R-H exchange capac-ity):1.6meq/mL(min);(c)pH:4–7.Before mixing and packing the 2resins into a column,wash each resin with H2O to obtain a pH value of7–8.8for m-1and4–7for m-2.If Amberlite200CT(HG)H cannot be obtained,Amberlite200(Na-type;Sigma Chemical Co.) or Amberlite200CT(Organo Corp.)can be used by converting it to “H-type”by the following procedure:Fill column(100cm40mm id),B(e),with600mL(500g) Amberlite200“Na-type”resin and determine approximate resin volume.Wash resin with2volumes of water,(d),at the rate of 60mL/min.Pass2volumes of10%HCl(1+3,w/w)through the resin at the rate of60mL/min.Remove HCl with3volumes of water, (d),passed through the resin at the rate of60mL/min.Add3–6vol-umes of additional water,(d),at the rate of120mL/min.The columnis adequately washed of HCl when a pH value of4–7is obtained.(It takes2–3h to charge and rinse these resins.)(n)Sodium hydroxide.—0.275M;reagent grade.Dissolve11.0g NaOH in ca700mL water,(d),in a1L volumetric flask.Dilute to volume with water,(d).(o)Hydrochloric acid.—0.325M;reagent grade.Dilute stock so-lution of known titer,e.g.,325mL1M HCl,to1L with water. (p)Glycerol(LC standard).—10mg/mL.For stock solution: weigh10g glycerol>99.5%purity into a small beaker.Quantita-tively transfer to1L volumetric flask with repeated washes with wa-ter,(d),and dilute to volume.It is important to measure and record the exact weight of the glycerol,weighing as close to10g as possi-ble.Take purity and weight of glycerol into consideration when cal-culating concentration of final glycerol LC standard.(q)Glycerol(for dextrose–glycerol standard).—100mg/mL.Weigh 10g high purity glycerol into a small beaker,transfer to a100mL volu-metric flask with water,(d),and dilute to volume.(r)Ammonium sulfate.—Reagent grade;standard to test mi-cro-Kjeldahl procedure.(s)Dextrose.—LC grade,high purity>99.5%.(t)Silver nitrate solution.—0.1M.Dissolve1.70g AgNO3in ca 70mL water,(d),in a100mL volmetric flask,and dilute to volume with water,(d).D.Determination(a)Enzymatic hydrolysis and filtration.—Weigh1.0g test por-tion(crushed,sieved to10mesh,fat extracted if>10%fat,and dried) into a500mL previously weighed tall-form beaker,B(b).Prepare induplicate with2blank digestion determinations.Disperse in50mL 0.08M phosphate buffer,C(g),and sonicated to ensure complete hydration.Add100L heat stable-amylase,C(h),and cover beaker with aluminum foil.Place beaker in shaker water bath and hold at95C for30min with shaking.Cool to room temperature,and adjust the pH of the solution to pH7.50.1with0.275M NaOH, C(n).Add0.5mL protease solution,C(j),and digest solution for 30min at60C.Cool solution to room temperature(25C),and ad-just pH to4.50.2with0.325M HCl,C(o).Add0.3mL amyloglucosidase,C(k),and digest at60C for30min.Upon com-pletion of the3enzyme digestion sequence,add4volumes of95% ethanol,C(a),by weight,previously heated e the top loading balance to weigh beaker with digestion mixture when add-ing ethanol(obtain tare weight of beaker before adding test portion). Assay the2blank digestions(i.e.,2beakers and2crucibles)in an identical manner.Let solutions stand overnight to form a precipitate.Filter by suc-tion,using a water aspirator or vacuum pump,through1.0g Celite layered on a Pyrex glass crucible filter that previously has been dried to constant weight.Wash the500mL tall-form beaker and the resi-due3times with20mL78%ethanol,C(b),2times with10mL95% ethanol,C(a),and2times with10mL acetone,C(c). Quantitatively transfer filtrate and washings to a1L round bottom flask.Dry residue in an air oven at105C overnight and record weight.This residue weight,minus the protein,ash,and blank resi-due weights represents the weight of the dietary fiber(IDF+ HMWSDF)recovered by the AOAC method.(b)Filtrate recovery,desalting,and LC analysis.—Evaporate with a rotary evaporator to near dryness.Dissolve the residue with a minimum amount of water,C(d),and transfer quantitatively to a 50mL volumetric flask.Add10mL of10mg/mL glycerol LC stan-dard and dilute to volume with water,C(d).Transfer contents of the 50mL volumetric flask to a column(75cm15mm id)containing 25g each,thoroughly mixed,of Amberlite IRA-67(m-1)and Amberlite200CT(HG)H(m-2)prepared just before use.Wash ex-tract through the column with250mL water,C(d),at the rate of 0.8mL/min.Collect250mL eluant from the ion exchange column and quanti-tatively transfer into a500mL round bottom flask.Evaporate to near dryness and quantitatively transfer to a10mL volumetric flask and dilute to volume with water,C(d).Transfer the contents of the 10mL volumetric flask to a10mL disposable syringe,B(o),and fil-ter through a0.2m filter,B(k).Use a50L LC glass syringe,B(u), to fill the20L injection loop on the LC,B(f).(c)Determining the response factor for dextrose;dextrose is equivalent to RMD in LC response.—Each chromatogram must be evaluated or standardized for the RI response of RMD.This is ac-complished using glycerol standard,C(q).The peak areas,represent-ing concentration,obtained by LC analysis of equal amounts of RMD and dextrose are equivalent.Glycerol is used as the internal standard but its peak area compared to the peak area for an equal amount of dextrose or RMD is not equivalent.A glycerol standard curve is there-fore prepared to obtain a“response factor”to calculate the exact amount of RMD in a chromatogram of each test portion.Prepare3solutions in individual100mL volumetric flasks con-taining the same amount of glycerol and3levels of dextrose.It is im-portant to know and use the reported content(i.e.,>99.5%purity)of both glycerol and dextrose standards as reported by suppliers.Accu-rately weigh0.5,1.0,and2.0g dextrose into3separate100mL volu-metric flasks,respectively.To each flask add10mL of the 100mg/mL glycerol standard,C(q).Dilute each flask to volume with water,C(d).These3flasks represent the standard solutions to calculate the“response factor”for dextrose that is used to determine the amount of RMD as displayed in LC chromatograms.Use a50L LC syringe,B(u),to fill the20L injection loop for each standard glycerol–dextrose solution.Obtain the values for the peak areas of dextrose and glycerol from the3chromatograms.The reciprocal of the slope obtained by comparing the ratio of peak area of dextrose/peak area of glycerol(y-axis)to the ratio of the weight of dextrose/weight of glycerol(x-axis)is the“response factor.”The av-erage“response factor”among laboratories is0.82,varying slightly in each laboratory.Response factor=1/(PA-dex/PA-gly)(Wt-gly/Wt-dex) where PA-dex=peak area dextrose;PA-gly=peak area glycerol; Wt-dex=weight of dextrose in standard;Wt-gly=weight of glyc-erol in standard.A flow diagram for a combined enzymatic-gravimetric method and LC determination is shown in Figure2001.03B.E.CalculationAll values used in calculations are in mg,except for percent(%) values.Assay each test portion in duplicate,resulting in2test portion weight values,test portion weight and test portion weight(prime);2cruciblesfor eachblankandtestportion,blankandblank(prime);and test portion and test portion(prime).(a)Calculate average%(IDF+HMWSDF)as fol-lows.—(1)Blank ash(Ab)=(ash+Celite+blank crucible)–(Celite +blank crucible).(2)Blank residue weight(BRW)=((BR+BR)/2)–(Pb+Ab)where Pb=blank protein,determined by micro-Kjeldahl procedure;BR=weight of first blank crucible with residue;BR=weight of second blank cruci-ble with residue;Ab=weight of blank ash from step(a)(1). (3)Test portion residue weight(SR)=(residue+Celite+test portion crucible)–(Celite+test portion crucible).Duplicate test portion residue weight(SR)=(residue+Celite+test portion cru-cible)–(Celite+test portion crucible).(4)Test portion ash weight(As)=(ash+Celite+crucible)–(Celite+crucible).(5)Final test portion residue weight(FSR)=SR–Ps–As–BRW =FSR where Ps=protein,determined by micro-Kjeldahl procedure; SR=final test portion residue weight from step(a)(3);As=test por-tion ash weight from step(a)(4);BRW=blank residue weight from step(a)(2).Repeat this calculation for FSR using SR–Ps–As–BRW(using values from duplicate test portion weights).(6)Percent final test portion residue weight(%FSR)=(FSR/ SW)100=%FSR where FSR=final test portion residue weight from step(a)(5);SW=test portion weight.Repeat this calculation for%FSR using FSR and SW.(7)%(IDF+HMWSDF)=average%FSR=(%FSR+%FSR)/2where %FSR=percent final test portion residue weight;%FSR=percent final duplicate test portion residue weight.(b)C a l c u l a t e a v e r a g e%L M W R M D a s f o l-lows.—(1)LMWRMD=(peak area of LMWRMD/peak area of glycerol)(glycerol standard,mg response factor).(2)%LMWRMD=(LMWRMD/SW)100where LMWRMD =weight of LMWRMD from step(b)(1);SW=test portion weight. Repeat calculations for%LMWRMD using LMWRMD and SW.(3)%ALMWRMD=average%LMWRMD=(%LMWRMD+ %LMWRMD)/2where%LMWRMD=percent LMWRMD for test portion from step(b)(2);%LMWRMD=percent LMWRMD for duplicate test portion from step(b)(2).(c)Calculate average%total dietary fiber(TDF)as fol-lows.—Percent(%)TDF=%(IDF+HMWSDF)+%ALMWRMD where%(IDF+HMWSDF)=average percent IDF+HMWSDF from step(a)(7);%ALMWRMD=average percent LMWRMD from step(b)(3).F.Resistant MaltodextrinThe commercially available U.S.GRAS status RMD is a source of dietary fiber.Resistant maltodextrin is certified as an approved di-etary fiber ingredient for the Program for Foods for Specific Health Use(FOSHU)in Japan.Dietary fiber supplements prepared simply by packaging RMD(or agglomerated RMD)in sachet forms and la-beled as RMD have been on the market.Fibersol®-2,RMD,is manu-factured and was supplied by Matsutani Chemical Industry Co.,Ltd. (Itami City,Hyogo,Japan).The moisture content of the product is 2.7%and DE is10.5.The RMD is produced by the pyrolysis and subsequent enzyme treatment of corn starch.It is an aggregate of glucose polymers with the MW distribution of180(DP-1)to >10000(DP-62)daltons,but the average MW is2000daltons.It contains1–4and1–6glucosidic bonds,which originate from starch and1–2and1–3glucosidic bonds that are created by transglucosidation during pyrolysis.Internal utilization of RMD by in vitro and in vivo tests show that <10%is digested and absorbed in the small intestine.Approximately 50%of the products are fermented in the large intestine and ca40%of the products are excreted into the feces.In order to distinguish this substance from conventional maltodextrin(digestible),the term“re-sistant”is added and used to describe this compound.The sugar,oligosaccharide,and polysaccharide composition of the LMWRMD fraction of the RMD has been determined before and after hydrolytic enzyme treatments and is shown in Fig-ure2001.03A.The distribution of these oligosaccharides is not sig-nificantly changed when RMD is treated with hydrolytic enzymes. To assess the oligosaccharide moieties and their distribution in the LMWRMD of RMD,corn syrup solids were used as a standard source of these oligosaccharides(Figure2001.03A).The nondigestible portions of RMD consists of DP units of3(DP-3)and above(Figure2001.03A).These nondigestible oligosaccharides and polysaccharides constitute>90%of RMD.Approximately60% of RMD consist of polymers having>10DP.References:J.AOAC Int.83,1013(2000);(future issue).。
膳食纤维含量实验报告(3篇)
第1篇一、实验目的本次实验旨在测定不同食物中膳食纤维的含量,了解膳食纤维在食物中的分布情况,以及其对人体健康的重要性。
通过实验,我们可以掌握膳食纤维的测定方法,并对富含膳食纤维的食物进行评估。
二、实验材料1. 食物样品:大米、小麦、玉米、燕麦、豆类、蔬菜、水果等。
2. 试剂与仪器:无水乙醇、丙酮、热稳定α-淀粉酶、蛋白酶、葡萄糖苷酶、电子天平、离心机、烘箱、烧杯、漏斗、滤纸等。
三、实验方法1. 样品处理:将各种食物样品分别研磨成粉末,过筛,以去除杂质。
2. 酶解:取一定量的样品粉末,加入适量的热稳定α-淀粉酶、蛋白酶和葡萄糖苷酶,在适宜的温度和pH条件下进行酶解反应。
3. 沉淀与抽滤:酶解后的溶液加入无水乙醇和丙酮,充分混合,静置沉淀,抽滤,得到膳食纤维残渣。
4. 洗涤与干燥:将残渣用无水乙醇和丙酮洗涤,干燥称量,得到总膳食纤维(TDF)含量。
5. 可溶性膳食纤维(SDF)测定:将酶解后的溶液直接抽滤,用热水洗涤残渣,干燥称量,得到不溶性膳食纤维(IDF)含量;滤液用无水乙醇沉淀,抽滤,干燥称量,得到SDF含量。
四、实验结果1. 大米:TDF含量为2.2%,SDF含量为0.6%。
2. 小麦:TDF含量为2.5%,SDF含量为0.8%。
3. 玉米:TDF含量为2.8%,SDF含量为0.9%。
4. 燕麦:TDF含量为5.3%,SDF含量为1.2%。
5. 豆类:TDF含量为6.5%,SDF含量为1.8%。
6. 蔬菜:TDF含量为3.2%,SDF含量为0.9%。
7. 水果:TDF含量为2.7%,SDF含量为0.8%。
五、实验讨论1. 从实验结果可以看出,不同食物中膳食纤维的含量差异较大。
豆类、蔬菜和燕麦的膳食纤维含量较高,适合作为高纤维食物的来源。
2. 燕麦的膳食纤维含量最高,其TDF含量是大米的2倍多,小麦的2倍。
这说明燕麦是一种非常优秀的膳食纤维来源。
3. 豆类、蔬菜和水果中的膳食纤维含量较高,可以促进肠道蠕动,增加粪便体积,有助于缓解便秘症状。
膳食纤维 标准方法
膳食纤维标准方法
膳食纤维是指人体无法消化吸收的碳水化合物类物质。
膳食纤维对人体健康具有重要的作用,包括促进消化系统健康、调节血糖和胆固醇水平、预防便秘以及控制体重等。
为了准确测量食物中的膳食纤维含量,需要进行标准方法的测定。
目前,国际通用的膳食纤维含量测定方法有两种:AOAC (Association of Official Analytical Chemists)方法和ISO (International Organization for Standardization)方法。
1. AOAC方法:AOAC方法是美国官方方法,也是国际上最常用的方法。
根据AOAC 991.43或AOAC 985.29方法,首先将食物样品经过一系列处理,如酶解、水解等,获得可溶性和不可溶性纤维。
然后,借助酶解、滴定、重量等技术手段,可以得到总纤维、不可溶性纤维和可溶性纤维的含量。
2. ISO方法:ISO方法是由国际标准化组织制定的方法,与AOAC方法相似。
ISO 13904和ISO 15954方法是常用的ISO 方法。
这些方法主要利用酶解、水解、甲弹法等技术,将膳食纤维分为不可溶性纤维和可溶性纤维,并使用滴定、重量等手段进行测定。
无论使用AOAC方法还是ISO方法,都需要进行样品的预处理、酶解、滴定等步骤,以获得准确的膳食纤维含量。
这些方法在实验室条件下进行,需要仪器设备和专业操作人员进行操作。
需要注意的是,虽然AOAC和ISO方法都是国际通用的标准方法,但在具体的实验操作过程中,可能会存在一些差异,因此在测定过程中应当依据相应的方法详细操作,并遵循实验室的操作规程。
aoac标准
AOAC国际(Association of Official Analytical Chemists International)是一个专注于食品和药物分析的国际性组织,成立于1884年。
该组织发布的方法标准被广泛用于检测和分析食品、药物、化妆品等样品,为确保产品的质量和安全提供了可靠的分析方法。
AOAC标准是一系列详细描述分析方法的指南,确保在全球范围内实验室得到相同结果的一致性。
### AOAC标准的特点:1. **权威性:** AOAC标准是由一群专业的化学分析师和科学家共同制定的,确保了方法的科学性和可靠性。
2. **广泛适用性:** AOAC标准适用于各种类型的食品、饮料、药物和化妆品等产品的检测和分析。
3. **标准化:** AOAC标准是国际上广泛接受的标准,有助于提高实验室间和国际间的数据可比性。
4. **定期更新:** 鉴于科技的不断进步,AOAC标准定期进行修订和更新,以确保其方法与最新科学技术保持同步。
5. **多领域应用:** AOAC标准不仅仅关注于一种分析方法,而是覆盖了多个领域,包括化学、微生物学、生物技术等。
### AOAC标准的制定过程:AOAC标准的制定是一个系统且透明的过程,确保标准的可靠性和有效性。
主要步骤包括:1. **提案:** 任何AOAC成员都可以提出新的标准或修改现有标准的提案。
提案需要包括详细的实验步骤、数据分析和相关文献等。
2. **审查:** 提案将被提交给专门的委员会进行评审。
委员会由专业领域的专家组成,他们会仔细审查提案的科学性和可行性。
3. **公开评论:** 审查通过后,提案将被公开发表,接受来自公众和科学界的评论。
这有助于确保标准的广泛接受和适用性。
4. **修订:** 根据公开评论的反馈,委员会可能需要对提案进行修改。
这确保了标准的科学性和实用性。
5. **最终批准:** 一旦经过多轮的审查和修订,标准最终会被提交给AOAC的理事会,获得最终批准后,成为AOAC标准。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
45.4.13AOAC Official Method2001.03Total Dietary Fiber in FoodsContaining Resistant MaltodextrinEnzymatic-Gravimetric Methodand Liquid Chromatography DeterminationFirst Action2001[This method is applicable to resistant maltodextrin(RMD)and to foods containing RMD listed in Table2001.03at 1.4%RMD.] A.PrincipleThis method determines total dietary fiber(TDF)value of pro-cessed foods containing insoluble dietary fiber(IDF)and high mo-lecular weight soluble dietary fiber(HMWSDF),which are precipitated in ethanol and low molecular weight resistant maltodextrin(LMWRMD),which is soluble in ethanol.This method defines dietary fiber(DF)as consisting of nondigestible car-bohydrates having a degree of polymerization with3sugar moieties (DP3)or higher after enzymatic hydrolysis.All the starches con-tained in food are converted to glucose after this enzymatic hydroly-sis.This method to determine TDF content in processed foods containing RMD is a combination of985.29(see45.4.07)for DF and a LC method for LMWRMD.A food is first analyzed for the to-tal quantity of IDF and HMWSDF,precipitated in ethanol,accord-ing to985.29(see45.4.07).Then an LC determination is conducted on the desalted filtrate to obtain the quantity of LMWRMD not pre-cipitated in the78%alcohol preparation.These2values[(IDF+ HMWSDF)+LMWRMD]are summed to obtain the TDF value in the food.B.Apparatus(a)Balance.—Analytical,weighing to0.1mg.(b)Beakers.—Tall-form,500mL.(c)Water baths.—To maintain a temperature of95–100C and 60C with ability to shake the containers.(d)Filtering crucibles.—Coarse,ASTM,40–60m pore size, Pyrex,50mL.(e)Glass or plastic columns.—To hold ion exchange resins, 75cm15mm id;a shorter(40–75cm15mm id)column can also be used.(f)Liquid chromatograph(LC).—With oven to maintain a col-umn temperature of80C and a20L injection loop.Column oper-ating conditions are:Temperature,80C;mobile phase,distilled water,C(d);flow rate,0.5mL/min.(g)Guard column(or precolumn).—TSK®guard column PWXL,6.0mm id4cm(Tosoh Corp.,distributed by TosoHaas, Montgomeryville,PA,USA;)or equivalent. (h)LC columns.—Two LC columns connected in series, TSK-GEL®G2500PWXL,7.8mm id30cm(Tosoh Corp.),or equivalent.(i)Detector.—Refractive index(RI);maintained at40C. (j)Data integrator or computer.—For peak area measurement. (k)Filters for disposable syringe.—0.2m membrane,13mm. (l)Filters for water.—0.2m,47mm.(m)Filter apparatus.—To hold47mm,0.2m filter,(l);to filter larger volumes of water,C(d).(n)Glass rods.—With fire-polished ends,ca20cm long. (o)Syringes.—10mL,plastic disposable.(p)Pasteur pipet.(q)Volumetric pipet.—10mL.(r)Volumetric flasks.—10,50,250,and1000mL.(s)Top loading balance.—4000g capacity.(t)Tubing.—PVC,2.79mm id(for ion exchange columns). (u)Glass LC syringe.—50L.(v)Teflon scraping rod.—Use in place of glass stirring rod to scrape precipitate from tall-form beaker.(w)Rotary evaporator.—R-3000VW“Student”(Büchi,Swit-zerland;)or equivalent.C.Reagents(a)Ethanol.—95%.Technical grade,used at60C.(b)Ethanol.—78%.Place207mL water in1L volumetric flask and dilute to volume with95%ethanol,(a).(c)Acetone.—Reagent grade.(d)Distilled water.(e)Sodium phosphate dibasic.(f)Sodium phosphate monobasic.(g)Phosphate buffer.—0.08M,pH6.0.Dissolve1.400g Na2HPO4(or1.753g dihydrate)and9.68g NaH2PO4H2O(or10.94g dihydrate)in ca700mL water,(d).Dilute to1L with water,(d),and verify pH with pH meter.(h)Heat stable a-amylase solution(Termamyl).—No.120L(ac-tivity:12units/mg protein;Novo Laboratories,Inc.,59Danbury Rd, Wilton,CT06897,USA),or equivalent(should not contain glycerol).(i)LC retention time standard.—Standard source of the distribu-tion of oligosaccharides(DP$3)in the LMWRMD fraction of RMD, corn syrup solids(DE25;Matsutani Chemical Industry Co.,Ltd.,ItamiTable2001.03Interlaboratory results for the determination of total dietary fiber in selected foods containing resistant maltodextrin by enzymatic-gravimetric method and liquid chromatographyFood x,%bs a(b)s r RSD r,%s R RSD R,% Resistant maltodextrin95.368(0) 1.63 1.71 2.37 2.48Hard candy37.997(1)0.58 1.530.68 1.79 Chicken and vegetable soup25.418(0)0.74 2.89 1.18 4.65 Grapefruit juice 1.388(0)0.02 1.330.04 3.20 White bread9.608(0)0.33 3.410.64 6.66 Strawberry Jell-O9.918(0)0.60 6.100.939.39a(b)a=Number of laboratories retained after eliminating outliers;b=number of laboratories removed as outliers.City,Hyogo,Japan;),analyzed by LC(Fig-ure2001.03A)as in D.(j)Protease.—No.P-3910or P-5380(activity:7–15units/mg pro-tein;Sigma Chemical Co.,St.Louis,MO,USA),or equivalent(should not contain glycerol).Prepare protease stock solution just before use by adding100mg protease enzyme to a10mL volumetric flask and bring-ing to volume with water,(d),(amount is sufficient for9test portions in duplicate).(k)Amyloglucosidase.—No.A-9913(activity:400units/mg pro-tein;Sigma Chemical Co.),or equivalent(should not contain glycerol). (l)Celite.—No.C-8656(Sigma Chemical Co.)or No.C-211,acid washed(Fisher Scientific Co.,Fair Lawn,NJ,USA),or equivalent. (m)Mixed-bed ion exchange resins for each test por-tion.—(1)m-1.—25g Amberlite IRA-67(OH-type;Organo Corp., Tokyo,Japan,/organo_corp.htm),or equivalent.(2)m-2.—25g Amberlite200CT(HG)H(H-type;Organo Corp.),or equivalent,are mixed and packed in column for analysis of each test portion.The converted resin should satisfy the following specifications:(a)Total ion exchange capacity:1.74meq/mL (min);(b)Effective ion exchange capacity(R-H exchange capac-ity):1.6meq/mL(min);(c)pH:4–7.Before mixing and packing the 2resins into a column,wash each resin with H2O to obtain a pH value of7–8.8for m-1and4–7for m-2.If Amberlite200CT(HG)H cannot be obtained,Amberlite200(Na-type;Sigma Chemical Co.) or Amberlite200CT(Organo Corp.)can be used by converting it to “H-type”by the following procedure:Fill column(100cm40mm id),B(e),with600mL(500g) Amberlite200“Na-type”resin and determine approximate resin volume.Wash resin with2volumes of water,(d),at the rate of 60mL/min.Pass2volumes of10%HCl(1+3,w/w)through the resin at the rate of60mL/min.Remove HCl with3volumes of water, (d),passed through the resin at the rate of60mL/min.Add3–6vol-umes of additional water,(d),at the rate of120mL/min.The columnis adequately washed of HCl when a pH value of4–7is obtained.(It takes2–3h to charge and rinse these resins.)(n)Sodium hydroxide.—0.275M;reagent grade.Dissolve11.0g NaOH in ca700mL water,(d),in a1L volumetric flask.Dilute to volume with water,(d).(o)Hydrochloric acid.—0.325M;reagent grade.Dilute stock so-lution of known titer,e.g.,325mL1M HCl,to1L with water. (p)Glycerol(LC standard).—10mg/mL.For stock solution: weigh10g glycerol>99.5%purity into a small beaker.Quantita-tively transfer to1L volumetric flask with repeated washes with wa-ter,(d),and dilute to volume.It is important to measure and record the exact weight of the glycerol,weighing as close to10g as possi-ble.Take purity and weight of glycerol into consideration when cal-culating concentration of final glycerol LC standard.(q)Glycerol(for dextrose–glycerol standard).—100mg/mL.Weigh 10g high purity glycerol into a small beaker,transfer to a100mL volu-metric flask with water,(d),and dilute to volume.(r)Ammonium sulfate.—Reagent grade;standard to test mi-cro-Kjeldahl procedure.(s)Dextrose.—LC grade,high purity>99.5%.(t)Silver nitrate solution.—0.1M.Dissolve1.70g AgNO3in ca 70mL water,(d),in a100mL volmetric flask,and dilute to volume with water,(d).D.Determination(a)Enzymatic hydrolysis and filtration.—Weigh1.0g test por-tion(crushed,sieved to10mesh,fat extracted if>10%fat,and dried) into a500mL previously weighed tall-form beaker,B(b).Prepare induplicate with2blank digestion determinations.Disperse in50mL 0.08M phosphate buffer,C(g),and sonicated to ensure complete hydration.Add100L heat stable-amylase,C(h),and cover beaker with aluminum foil.Place beaker in shaker water bath and hold at95C for30min with shaking.Cool to room temperature,and adjust the pH of the solution to pH7.50.1with0.275M NaOH, C(n).Add0.5mL protease solution,C(j),and digest solution for 30min at60C.Cool solution to room temperature(25C),and ad-just pH to4.50.2with0.325M HCl,C(o).Add0.3mL amyloglucosidase,C(k),and digest at60C for30min.Upon com-pletion of the3enzyme digestion sequence,add4volumes of95% ethanol,C(a),by weight,previously heated e the top loading balance to weigh beaker with digestion mixture when add-ing ethanol(obtain tare weight of beaker before adding test portion). Assay the2blank digestions(i.e.,2beakers and2crucibles)in an identical manner.Let solutions stand overnight to form a precipitate.Filter by suc-tion,using a water aspirator or vacuum pump,through1.0g Celite layered on a Pyrex glass crucible filter that previously has been dried to constant weight.Wash the500mL tall-form beaker and the resi-due3times with20mL78%ethanol,C(b),2times with10mL95% ethanol,C(a),and2times with10mL acetone,C(c). Quantitatively transfer filtrate and washings to a1L round bottom flask.Dry residue in an air oven at105C overnight and record weight.This residue weight,minus the protein,ash,and blank resi-due weights represents the weight of the dietary fiber(IDF+ HMWSDF)recovered by the AOAC method.(b)Filtrate recovery,desalting,and LC analysis.—Evaporate with a rotary evaporator to near dryness.Dissolve the residue with a minimum amount of water,C(d),and transfer quantitatively to a 50mL volumetric flask.Add10mL of10mg/mL glycerol LC stan-dard and dilute to volume with water,C(d).Transfer contents of the 50mL volumetric flask to a column(75cm15mm id)containing 25g each,thoroughly mixed,of Amberlite IRA-67(m-1)and Amberlite200CT(HG)H(m-2)prepared just before use.Wash ex-tract through the column with250mL water,C(d),at the rate of 0.8mL/min.Collect250mL eluant from the ion exchange column and quanti-tatively transfer into a500mL round bottom flask.Evaporate to near dryness and quantitatively transfer to a10mL volumetric flask and dilute to volume with water,C(d).Transfer the contents of the 10mL volumetric flask to a10mL disposable syringe,B(o),and fil-ter through a0.2m filter,B(k).Use a50L LC glass syringe,B(u), to fill the20L injection loop on the LC,B(f).(c)Determining the response factor for dextrose;dextrose is equivalent to RMD in LC response.—Each chromatogram must be evaluated or standardized for the RI response of RMD.This is ac-complished using glycerol standard,C(q).The peak areas,represent-ing concentration,obtained by LC analysis of equal amounts of RMD and dextrose are equivalent.Glycerol is used as the internal standard but its peak area compared to the peak area for an equal amount of dextrose or RMD is not equivalent.A glycerol standard curve is there-fore prepared to obtain a“response factor”to calculate the exact amount of RMD in a chromatogram of each test portion.Prepare3solutions in individual100mL volumetric flasks con-taining the same amount of glycerol and3levels of dextrose.It is im-portant to know and use the reported content(i.e.,>99.5%purity)of both glycerol and dextrose standards as reported by suppliers.Accu-rately weigh0.5,1.0,and2.0g dextrose into3separate100mL volu-metric flasks,respectively.To each flask add10mL of the 100mg/mL glycerol standard,C(q).Dilute each flask to volume with water,C(d).These3flasks represent the standard solutions to calculate the“response factor”for dextrose that is used to determine the amount of RMD as displayed in LC chromatograms.Use a50L LC syringe,B(u),to fill the20L injection loop for each standard glycerol–dextrose solution.Obtain the values for the peak areas of dextrose and glycerol from the3chromatograms.The reciprocal of the slope obtained by comparing the ratio of peak area of dextrose/peak area of glycerol(y-axis)to the ratio of the weight of dextrose/weight of glycerol(x-axis)is the“response factor.”The av-erage“response factor”among laboratories is0.82,varying slightly in each laboratory.Response factor=1/(PA-dex/PA-gly)(Wt-gly/Wt-dex) where PA-dex=peak area dextrose;PA-gly=peak area glycerol; Wt-dex=weight of dextrose in standard;Wt-gly=weight of glyc-erol in standard.A flow diagram for a combined enzymatic-gravimetric method and LC determination is shown in Figure2001.03B.E.CalculationAll values used in calculations are in mg,except for percent(%) values.Assay each test portion in duplicate,resulting in2test portion weight values,test portion weight and test portion weight(prime);2cruciblesfor eachblankandtestportion,blankandblank(prime);and test portion and test portion(prime).(a)Calculate average%(IDF+HMWSDF)as fol-lows.—(1)Blank ash(Ab)=(ash+Celite+blank crucible)–(Celite +blank crucible).(2)Blank residue weight(BRW)=((BR+BR)/2)–(Pb+Ab)where Pb=blank protein,determined by micro-Kjeldahl procedure;BR=weight of first blank crucible with residue;BR=weight of second blank cruci-ble with residue;Ab=weight of blank ash from step(a)(1). (3)Test portion residue weight(SR)=(residue+Celite+test portion crucible)–(Celite+test portion crucible).Duplicate test portion residue weight(SR)=(residue+Celite+test portion cru-cible)–(Celite+test portion crucible).(4)Test portion ash weight(As)=(ash+Celite+crucible)–(Celite+crucible).(5)Final test portion residue weight(FSR)=SR–Ps–As–BRW =FSR where Ps=protein,determined by micro-Kjeldahl procedure; SR=final test portion residue weight from step(a)(3);As=test por-tion ash weight from step(a)(4);BRW=blank residue weight from step(a)(2).Repeat this calculation for FSR using SR–Ps–As–BRW(using values from duplicate test portion weights).(6)Percent final test portion residue weight(%FSR)=(FSR/ SW)100=%FSR where FSR=final test portion residue weight from step(a)(5);SW=test portion weight.Repeat this calculation for%FSR using FSR and SW.(7)%(IDF+HMWSDF)=average%FSR=(%FSR+%FSR)/2where %FSR=percent final test portion residue weight;%FSR=percent final duplicate test portion residue weight.(b)C a l c u l a t e a v e r a g e%L M W R M D a s f o l-lows.—(1)LMWRMD=(peak area of LMWRMD/peak area of glycerol)(glycerol standard,mg response factor).(2)%LMWRMD=(LMWRMD/SW)100where LMWRMD =weight of LMWRMD from step(b)(1);SW=test portion weight. Repeat calculations for%LMWRMD using LMWRMD and SW.(3)%ALMWRMD=average%LMWRMD=(%LMWRMD+ %LMWRMD)/2where%LMWRMD=percent LMWRMD for test portion from step(b)(2);%LMWRMD=percent LMWRMD for duplicate test portion from step(b)(2).(c)Calculate average%total dietary fiber(TDF)as fol-lows.—Percent(%)TDF=%(IDF+HMWSDF)+%ALMWRMD where%(IDF+HMWSDF)=average percent IDF+HMWSDF from step(a)(7);%ALMWRMD=average percent LMWRMD from step(b)(3).F.Resistant MaltodextrinThe commercially available U.S.GRAS status RMD is a source of dietary fiber.Resistant maltodextrin is certified as an approved di-etary fiber ingredient for the Program for Foods for Specific Health Use(FOSHU)in Japan.Dietary fiber supplements prepared simply by packaging RMD(or agglomerated RMD)in sachet forms and la-beled as RMD have been on the market.Fibersol®-2,RMD,is manu-factured and was supplied by Matsutani Chemical Industry Co.,Ltd. (Itami City,Hyogo,Japan).The moisture content of the product is 2.7%and DE is10.5.The RMD is produced by the pyrolysis and subsequent enzyme treatment of corn starch.It is an aggregate of glucose polymers with the MW distribution of180(DP-1)to >10000(DP-62)daltons,but the average MW is2000daltons.It contains1–4and1–6glucosidic bonds,which originate from starch and1–2and1–3glucosidic bonds that are created by transglucosidation during pyrolysis.Internal utilization of RMD by in vitro and in vivo tests show that <10%is digested and absorbed in the small intestine.Approximately 50%of the products are fermented in the large intestine and ca40%of the products are excreted into the feces.In order to distinguish this substance from conventional maltodextrin(digestible),the term“re-sistant”is added and used to describe this compound.The sugar,oligosaccharide,and polysaccharide composition of the LMWRMD fraction of the RMD has been determined before and after hydrolytic enzyme treatments and is shown in Fig-ure2001.03A.The distribution of these oligosaccharides is not sig-nificantly changed when RMD is treated with hydrolytic enzymes. To assess the oligosaccharide moieties and their distribution in the LMWRMD of RMD,corn syrup solids were used as a standard source of these oligosaccharides(Figure2001.03A).The nondigestible portions of RMD consists of DP units of3(DP-3)and above(Figure2001.03A).These nondigestible oligosaccharides and polysaccharides constitute>90%of RMD.Approximately60% of RMD consist of polymers having>10DP.References:J.AOAC Int.83,1013(2000);(future issue).。
