Gen Chem Review Sample Problems-BCH110A(2)
九天国际教育GCSE真题June 2017 QP - Paper 1C Edexcel Chemistry IGCSE

. . . . . . . . . . . ..................... . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . ............................................................................................................................................................ .. .. .. .. .. .. .. .. .. ..
. . . . . . . . . . . ..................... . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . ............................................................................................................................................................ .. .. .. .. .. .. .. .. .. ..
. . . . . . . . . . . ..................... . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . ............................................................................................................................................................ .. .. .. .. .. .. .. .. .. ..
分析化学B卷英文

扬州大学试题纸
( 2006 - 2007 学年第 一 学期) 化学化工 学院化学 06 年级课程 Analytical Chemistry (考试) B 卷 题目 得分 一 二 三 四 五 六 七 八 总分
姓名 学院 系 班( 2 points per question, 30%) 1. What is the standard deviation value for the data set listed below: 1.1668, 1.1559, 1.1833, 1.1622, 1.1713, 1.1699, 1.1655; A. 0.0085 B. 0.0079 C. 0.0274 D. 0.0188 E. none of these 2. What is the gravimetric factor for the determination of the w/w% Mg when precipitated as Mg2P2O3? At wts: Mg = 24.305 g, P = 30.974 g O = 15.999 g A.0.30658 B. 3.26178 C. 0.15329 D. 6.52355 E. none of these 3. If the true value of a measurement does not fall within the confidence limits this tells us: ( ) A) nothing B) that our measurement is not precise C) that our measurement is neither accurate nor precise D) that our measurement is not accurate E) that our true value is wrong 4. A liter of CuCl2- water solution which contains 1.0 mg of CuCl2 has a concentration of CuCl2. A. 1.0 ppb B. 1.0 ppm C. 1.0 ppt D. none of these 5. of a absorbing complex is always dependent upon the_____ A. the stability of the complex B. the thickness of the container C. the wavelength of the electromagnetic radiation D. the concentration of the comples 6. The best way to remove impurities resulting from the mechanical entrapment and occlusion is to__________ the precipitate. ( ) A. peptidize B. reprecipitate C. digest D. wash E. none of these
CHEM107(Fall-2006)FinalExam(1004pts)

CHEM 107 (Fall-2006)Final Exam (100 + 4 pts)Name: ------------------------------------------------------------------------, Clid # ------------------------------LAST NAME, First(Circle the alphabet segment of your LAST NAME): A, B C-G H-N O-S T-Z Please answer the following questions:Part I: Multiple Choices (44 pts: 22 @ 2 pts each + 4 pts bonus). Circle the ONE best answer:1. Which of the following species have bent (angular) molecular shape?I) CO2II) OF2III) NO2+IV) NO2-a) only II b) only IVc) II and IV d) I and III2.Which of the following ions is expected to be paramagnetic?Zn2+b) S2-a)Ti3+Cu+d)c)3.The ground state electron configuration of tantalum, Ta (Z = 73) isa)[Xe] 5d3 6s2b) [Xe] 4f14 5d3 6s2c) [Xe] 4f14 6d3 6s2 d) [Xe] 4f14 5d54. The oxidation numbers of phosphorus, P in PCl3 and P2O74- are:a) -3 and +10, respectively b) -3 and +7, respectivelyc) -3 and +5, respectively d) +3 and +5, respectively5. If a gold ring has a mass of 5.50 g in temperature from 25.0 to 28.0 ºC, how much heat isabsorbed? (specific heat of gold is 0.129 J/g ºC)a) 2.1 J b) -2.1 J c) 0.24 J d) 130 J6 Which of the following statements is false?a)An electron transition from n = 1 to n = 3 absorbs energy.b)Light emitted by an n = 5 to n = 2 transition will have a shorter wavelength than that fromn = 4 to n = 2 transition.c)An atom of group 16 has two unpaired electrons.d)All of the above.7. Which of the following molecules is expected to be polar?SF6a) BeF2b)c) XeF4d) OF28. Calculate ∆H° for the following reaction using the given enthalpies of formation.2 NO (g) + 5 O2 (g) 2 NO2 (g)∆H f° NO (g) = 90.25 kJ/mol & ∆H f° NO2 (g) = 33.18 kJ/molb)+57.07 kJ b) -57.02 kJc) -114.14d) +114.14 kJ9. Which of the following is most likely to involve sp3d hybrid orbitals at the central atoms?I) ClF3II) BrF5III) XeF4 IV) SF4a) I and IV b) only IIc)only IV d) II and III10. Which of the following has the greatest ionization energy?a)Br b) Sn c) Se d) Cl11. Which name is incorrect?Ca3P2: calcium phosphide b) Na2S: sodium sulfidea)c)P2S5: diphosphorus pentasulfide d) Co2O3: cobalt(III) trioxide12. Aluminum and oxygen react according to the following equation:4 Al (s) + 3 O2 (g) 2 Al2O3 (s)What mass of Al2O3 (102.0 g/mol), in grams, can be made by reacting 2.3 g of Al with excess oxygen?a) 8.7 g b) 17.4 gc) 4.3 g d) no answer was give13. If 400.0 mL of 0.375 M NaOH is added to 280.0 mL of 0.600 M HNO3, will the mixture bea) neutral b) basicc) acidic d) amphoteric14. Calcium metal and bromine react to form calcium bromide, CaBr2. Which statement is correct?a) Ca atom loses two electrons and becomes Ar atom.b) Br atom gains an electron and become Kr atom.c) The Ca and Br atoms change into different, more stable atoms.d) CaBr2 is predominantly an ionic compound.15. The standard enthalpy change for the following reaction is -53.0 kJ.2 HI (g) H2 (g) + I2 (s)What is the standard enthalpy of formation of HI (g)?a) 53.0 kJ b) -105.9 kJc) 26.5 kJ d) -53.0 kJ16.An atom containing 26 protons and a mass number of 59, would be:Coa) Fe b)c) As d) Ni17. The element X occurs naturally to the extent of 20.0% 12X and 80.0% 13X. the atomic mass of Xis nearest12.2a) 12.8 b)c) 12.5 d) 13.018.Equal volumes of an unknown gas and nitrogen (N2) gas takes 5.37 min and 3.55 minutes,respectively to effuse through a small hole. Calculate the molar mass of the gas.a) 64.1 b)12.2128c)42.4 d)19. The greatest deviation from the Ideal Gas Law is expected at:a) high T, high P b) low T, high Pc) high T, low P d) low T, low P20. A 4.8 g sample of a compound of nitrogen and oxygen contains 3.02 g of oxygen. What is thesimplest formula?NO2b) N2O3a)c)d)NON2O21.Which of the following ions has the largest ionic radius?Se2-b) Cs+a)S2-d) Sr2+c)22. In the phase diagram shown here. Which statement applies? Phase Diagram of Substance Za)The path A C represents sublimation.b)Following the path A B C, thecompound would first liquefy and then vaporize.c)If the compound is in state A, continued reductionof the pressure (at constant T) will cause it to melt.d)None of these statements is correct.23. Consider the above phase diagram, what is the normal melting point of the pure substance Z?a)+18 ºC b) -22 ºCc) - 2 ºC d) +4 ºC24. Using the same diagram, if the average atmospheric pressure at the top of certain mount, which isabout 19,000 feet above the sea level is 0.526 atm, What is the approximate boiling temperature of the liquid?a) 20 ºC b) 140 ºCc) 5 ºCd) can not be determined from this graphPart II. Bonding and Chemical Equations (26 pts):A) Bonding & molecular structure (12 pts):1. Draw the Lewis structure for the SO 2 molecule, draw the resonance structures, if there is any then predict:a) the molecular shape b) hybridization of the central atom c) number σ and π bonds d) bond angleB) Chemical Equations (16 pts):2. (8 pts ) Balance the following redox equation in acidic solution: UO 22+ (aq) + Te (s) U 4+ (aq) + TeO 42- (aq),Then identify the oxidizing agent and how many electron(s) are transferred per one mole of the oxidizing agent.2. (6 pts ) Write the balanced chemical equation and the net ionic equation for the reaction betweenaqueous solutions of HNO 3 (aq) and CaCO 3 (s) and then identify the spectator ion(s)Part III. Calculations (30 pts: 5 @ 6 pts each) Show all work for full credit. Please express allanswers with the proper units and correct number of significant figures.1. Given the following equations:2 H 2 (g) + O 2(g) 2 H 2O (l)∆H rxn ° = -571.6 kJ N 2 (g) + O 2 (g) 2 NO (g) ∆H rxn ° = +180.5 kJN 2 (g) + 3 H 2 (g) 2 NH 3 (g)∆H rxn ° = -92.2 kJDetermine the enthalpy change, ∆H rxn ° for the following reaction:2 NO (g) + 5 H 2 (g) 2 NH3 (g) + 2 H 2O (l) ∆H rxn ° = ??2. If a 17.0 g of impure nickel metal reacts with excess carbon monoxide, CO forming 6.25 L ofNi(CO)4 gas (MM = 170.75 g/mol) under standard temperature and pressure conditions. What is the percent by mass of nickel in the impure nickel metal sample?Ni(s) + 4 CO (aq) Ni(CO)4(g)3. Oxyacetylene torches used for welding reach temperatures near 2000 °C. The reaction involved in the combustion of acetylene is2 C 2H 2 (g) + 5 O 2 (g) 4 CO 2 (g) + 2 H 2O (g)Starting with 175 g of both acetylene, C 2H 2 and oxygen: a) What is the limiting reactant?b) If 68.5 L of carbon dioxide (d = 1.85 g/L) is produced, what is the percent yield of the reaction atthe same conditions of temperature and pressure?4. The reaction of a copper penny with nitric acid results in the formation of a red-brown gaseouscompound containing nitrogen and oxygen. A sample of the gas at a pressure of 727 mmHg and a temperature of 18 ºC weighs 0.289 g in a flask with a volume of 157.0 mL. Calculate the molar mass of the gas, and suggest a reasonable chemical formula for the compound (NO, NO2, N2O, N2O5, N2O4).5. Vitamin C, the compound that believes to prevent the common cold, has the composition 40.92% C,4.58% H and 54.50% O. Determine the simplest formula of the compound. In another experiment,the molar mass of the vitamin was found to be about 180 g/mol. What is the molecular formula of vitamin C?Final Exam – Fall 2006You will have 140 minutes to complete this exam. The exam has 7 pages plus the Periodic Table and Reference page.When you are told to do so, tear off the Periodic Table cover sheet and use as required during the exam.11H1.01Periodic Table of the Elements2He4.0023Li6.944Be9.015B10.816C12.017N14.018O16.009F19.0010Ne20.18311Na22.9912Mg24.3013Al26.9814Si28.0815P30.9716S32.0617Cl35.4518Ar39.95419K39.120Ca40.0821Sc44.9622Ti47.8823V50.9424Cr52.0025Mn54.9426Fe55.8527Co58.9328Ni58.6929Cu63.5530Zn65.3831Ga69.7232Ge72.5933As74.9234Se78.9635Br79.9036Kr83.80537Rb85.4738Sr87.6239Y88.9140Zr91.2241Nb92.9142Mo95.9443Tc(98)44Ru101.145Rh102.946Pd106.447Ag107.948Cd112.449In114.850Sn118.751Sb121.852Te127.653I126.954Xe131.1655Cs132.956Ba137.357La138.972Hf178.573Ta181.074W183.875Re186.276Os190.277Ir192.278Pt195.179Au197.080Hg200.681Tl204.482Pb207.283Bi209.084Po(209)85At(210)86Rn(222)787Fr(223)88Ra226.089Ac227.0104Rf(261)105Db(262)106Sg(263)107Bh(262)108Hs(265)109Mt(266)110Uun(269)111Uuu(272)112Uub(277)58Ce140.159Pr140.960Nd144.261Pm(145)62Sm150.463Eu152.064Gd157.265Tb158.966Dy162.567Ho164.968Er167.369Tm168.970Yb173.071Lu175.090Th232.091Pa231.092U238.093Np237.094Pu(244)95Am(243)96Cm(247)97Bk(247)98Cf(251)99Es(252)100Fm(257)101Md(258)102No(259)103Lr(260)。
(NEW)华彤文《普通化学原理》(第4版)配套题库【名校考研真题+课后习题+章节题库+模拟试题】

,因
为 > > ,所以溶解度大小AgCl>AgBr>AgI,Ag2CrO4
的溶度积与溶解度的关系为
> =1.34×10
-5,故溶解度大小为Ag2CrO4>AgCl>AgBr>AgI。
7.难溶盐Th(IO3)4的溶解度S0(mol·dm-3)是其溶度积Ksp的函 数,它们的关系式为( )。[中国科学院-中国科学技术大学2004 研]
第15章 元素化学 第16章 化学与社会发展 第三部分 章节题库 第1章 绪 论 第2章 气 体 第3章 相变·液态 第4章 溶液 第5章 化学热力学 第6章 化学平衡 第7章 化学反应速率 第8章 酸碱平衡 第9章 沉淀溶解平衡 第10章 氧化还原·电化学 第11章 原子结构 第12章 化学键与分子结构 第13章 晶体与晶体结构 第14章 配位化合物
第15章 元素化学 第16章 化学与社会发展 第二部分 课后习题 第1章 绪 论 第2章 气 体 第3章 相变·液态 第4章 溶 液 第5章 化学热力学 第6章 化学平衡 第7章 化学反应速率 第8章 酸碱平衡 第9章 沉淀溶解平衡 第10章 氧化还原·电化学 第11章 原子结构 第12章 化学键与分子结构 第13章 晶体与晶体结构 第14章 配位化合物
目 录
第一部分 名校考研真题 第1章 绪 论 第2章 气 体 第3章 相变·液态 第4章 溶 液 第5章 化学热力学 第6章 化学平衡 第7章 化学反应速率 第8章 酸碱平衡 第9章 沉淀溶解平衡 第10章 氧化还原·电化学 第11章 原子结构 第12章 化学键与分子结构 第13章 晶体与晶体结构 第14章 配位化合物
解得Pb2+的浓度为 [Pb2+]=8.4×10-5mol·L-1
2.计算0.0500mol·dm-3 H2CO3溶液中的c(H+)、c(HCO3 -)、c(CO32-)各为多少?[已知H2CO3 =4.30×10-7, = 5.61×10-11][北京科技大学2013、2014研]
【清华】分0梅豪2010011810
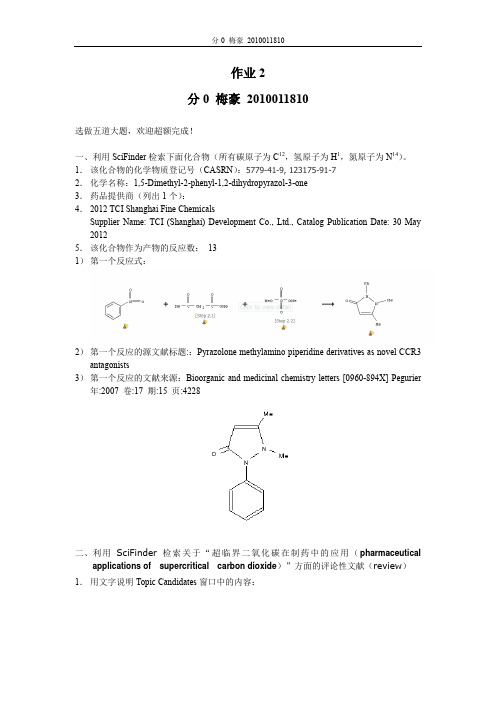
作业2分0 梅豪2010011810选做五道大题,欢迎超额完成!一、利用SciFinder检索下面化合物(所有碳原子为C12,氢原子为H1,氮原子为N14)。
1.该化合物的化学物质登记号(CASRN):5779-41-9, 123175-91-72.化学名称:1,5-Dimethyl-2-phenyl-1,2-dihydropyrazol-3-one3.药品提供商(列出1个):4.2012 TCI Shanghai Fine ChemicalsSupplier Name: TCI (Shanghai) Development Co., Ltd., Catalog Publication Date: 30 May 20125.该化合物作为产物的反应数:131)第一个反应式:2)第一个反应的源文献标题::Pyrazolone methylamino piperidine derivatives as novel CCR3 antagonists3)第一个反应的文献来源:Bioorganic and medicinal chemistry letters [0960-894X] Pegurier 年:2007 卷:17 期:15 页:4228二、利用SciFinder检索关于“超临界二氧化碳在制药中的应用(pharmaceuticalapplications of supercritical carbon dioxide)”方面的评论性文献(review)1.用文字说明Topic Candidates窗口中的内容:内容为各种不同形式包含所要检索的内容的文献进行分类以后有哪些。
他们有不同程度的符合性。
例如第一个就是完全符合之后就是包含不同的关键词的文献数。
2.同时具有pharmaceutical applications和supercritical carbon dioxide两个概念的文献记录数:833.对2中的记录进行限定检索,原文语种为中文的记录数:84.在3得到的结果中,第1条记录的标题:Application of supercritical CO2 extraction of traditional Chinese medicine5.在3得到的结果中,第1条记录能否在线看到全文:不能三、用中文介绍BIOSIS Previews数据库,包括:1)收录文献学科领域和文献出版类型;2)检索字段;3)记录字段;4)与Web of Science相比,BP的特色所在。
有机化学习题第四章 二烯烃和共轭体系习题
(考虑 C+稳定性!)
(十一) 下列两组化合物分别与 1,3-丁二烯[(1)组]或顺丁烯二酸酐[(2)组]进行 Diels-Alder 反应,试将其按反应活性由大到小排列成序。
CH3
(1)
(A) (B)
CN
(C)
CH2Cl
CH2=C CH=CH2
(2) (A)
CH3
(B)
CH2=CH CH=CH2
(C)
CHO
+
CH2Cl
CH2Cl KMnO4
H+ , ∆
HOOCCH2CHCH2CH2COOH CH2Cl (B)
(A)
CH3 hν CH3
CH3 CH3
(10)
CH3 CH3
CH3 CH3 CH3 H H CH3
(9)
CH3 ∆ H CH3 H
(四)
给出下列化合物或离子的极限结构式,并指出哪个贡献最大?
(3) 氧化时可以生成β-羧基己二酸
COOH
根据以上事实,试推测该二聚体的构造式,并写出各步反应式。
解:该二聚体的构造式为: 各步反应式略。
(1) CH3 C
N
(2)
(CH3)2C=CH C(CH3)2
CH2 C CH3 O
(3)
CH2=CH CH2
CH3
(6)
C CH=CH2 O
(4)
(5)
解:
(1)
CH3 C
N
CH3 C
N
CH3 C
N
贡献最大(非电荷分离)
(2)
(CH3)2C=CH C(CH3)2
(CH3)2C
CH=C(CH3)2
(2)
1,3-戊二烯
General Chemistry Examination (2)
General Chemistry Examination (2)I. True or False1. Molecules in which the central atom is attracted to varying groups, and those in which the groups are symmetrically distributed, are polar. ( )2. In any system involving electrical charges, the charges tend to neutralize each other on a scale as small as possible. ( )3. Pure HCl is NOT an acid. ( )4. Sodium bicarbonate is the basic component of the major buffer system in human blood. ( )5. Elements that lie in same period have similar properties, have the same number of valence electrons and belong to the same chemical family. ( )Ⅱ. There are 30 Questions. Circle your answer clearly; otherwise no credit will be given. Circle only one answer. If you circle two or more, you will receive no credit. [TOP]1. How many moles of solid Ca(NO3)2should be added to 450 milliliters of 0.35 mol⋅L-1Al(NO3)3toNO to 1.7 mol⋅L-1?(Assume that the volume of the solution remains increase the concentration of the -3constant.) ( )A. 0.07 molB. 0.15 molC. 0.29 molD. 0.45 molE. 0.77 mol2. Which of the following elements has the lowest ionization energy? ( )A. FB. CoC. KD. BeE. O3. Which of the following molecules is a nonpolar molecule with polar double bonding? ( )A. SO2B. SiH4C. CO2D. Be2E. NO4. A well is sunk in a bed of rock containing fluorspar (CaF2). Given that K sp (CaF2) = 3.45×10-11 at 298 K, and assuming that the water in the well is saturated with CaF2, and that the fluorspar is the only source of Ca2+ and F- ions, then what is the molality of the Fluoride ion at 298 K? ( )A. 5.87⨯10-6B. 2.05⨯10-4C. 3.26⨯10-4D. 2.94⨯10-6E. 4.0 ⨯10-115. Considering the reaction 2A → 4B+C, if plotting log c A versus time, a straight-line plot is obtained, What is the order of this reaction? ( )A. half-order reactionB. second-order reactionC. zero-order reactionD. first-order reactionE. not enough information is providedⅢ. Fill in the following blanks with correct terms [TOP]1. A (1) reaction is one involving two (2) reactions, one of them an oxidation, the other a reduction. When a substance is oxidized it (3) ; when a substance is reduced it (4) .2. A 1.00% by mass NaCl(aq) solution has a freezing point of –0.593︒C, so the total molality of all solute species is (5) ; the percentage dissociation of NaCl in this solution is (6) .(Attention: The molality calculated from the freezing-point depression is the sum of the molalities of undissociated ion pairs, the Na + ions, and the Cl - ions.)3. The (7) definition of an acid and a base, states that an acid is anything that releases a (8) in water, while a base is anything that releases a (9) . The (10) definition eliminates the need for water in the definition by defining acid-base reactions in terms of a (11) transfer from an acid to base, regardless of solvent.A third definition, the (12) definition, treats an acid-base reaction in terms of the donation of an (13) frm a base to an acid.Ⅳ. Explain why? [TOP]1. We wish to separate magnesium ions and barium ions by selective precipitation. Which anion, fluoride or carbonate, would be the better choice for achieving this precipitation? Why?K sp K sp MgF 25.16⨯10-11 BaF 21.84⨯10-7 MgCO 3 6.82⨯10-6 BaCO 32.58⨯10-92. Solutions that are injected into the bloodstream (for blood transfusions and intravenous feeding) must be “isotonic” with the blood (that is, have the same osmotic pressure). If the injected solution i s too dilute, it is termed “hypotonic” and if too concentrated, “hypertonic”. Explain the effects of hypotonic and hypertonic solutions on the cell in the bloodstream.V. Calculation [TOP]1. Ethylamine reacts with water as follows:C 2H 5NH 2(aq) + H 2O(l )→+352NH H C (aq) + OH -(aq)The base-dissociation constant, K b , for the ethylamine ion is 4.5×10-4.(a) A student carefully measures out 65.987 mL of a 0.250mol ⋅L -1 solution of ethylamine. Calculate the OH - ion concentration.(b) Calculate the pOH of the solution.(c) Calculate the % ionization of the ethylamine in the solution in part (a).(d) What would be the pH of a solution made by adding 15.000g of ethylammonium bromide(C 2H 5NH 3Br) to 250.00 mL of a 0.100-mol solution of ethylamine?2. In the following primary cell: Pt|Fe 3+(0.1mol·L -1), Fe 2+(0.01mol·L -1)||Ag +(0.1mo·L -1)|Ag, it is known that φθ(Fe 3+/Fe 2+) = 0.771V , φθ(Ag +/Ag) = 0.7996V ,(a) Caculate the electromotive force of the cell at 25︒C;(b) Indicate the positive electrode and the negative electrode of cell formulation;(c) Write out the cell reaction and electrode reaction;(d) If add NaCl into electrode Ag +/Ag, make [Cl -]=1mol ⋅L -1, calculate φ(Ag +/Ag) at 25︒C.[ K sp (AgCl)=1.77×10-10]【英文综合测试题参考答案】 [TOP]I. 1.F 2.T 3.T 4.T 5.FII. 1.B 2.C 3.C 4.B 5.DIII.1. (1) Redox (2) half-cell (3) loses electrons (4) gains electrons2. (5) 0.319mol ⋅kg -1 (6) 86.6%3. (7) Arrhenius (8) proton (9) hydroxide ion (10) Brønsted-Lowry (11) proton (12) Lewis (13) electron pair Ⅵ1. fluoride, because the solubility difference is larger.2. Hypotonic solutions will cause a net flow of solvent into the cell to equalize the osmotic pressure. The cell will burst and die (hemolysis). Hypertonic solutions will cause a net flow of solvent out of the cells to equalize the osmotic pressure. The cells will shrink and die.V.1. (a) K b ·c b ≥20K w ,and c b /K b ≥500,011.0250.0105.4][OH 4b b =⨯⨯==--c K (mol ⋅L -1)(b) pOH = -lg0.011= 1.96 (c) ionization % =%4.4%100250.0011.0%100whole part =⨯=⨯(d) acid base a log p pH n n K +==(14.00+lg4.5×10-4)+lg 1mol 126.0g 15.000g0.100mol -⋅=10.58 2. (a) )Fe ()Ag ()Fe (lg V 05916.023θ+++-=c c c n E E V 0897.001.01.01.0lg 1V 05916.0)771.07996.0(-=⨯--= (b) Fe 3+/Fe 2+ is the positive electrode and Ag +/Ag is the negative electrode (c) The cell reaction: Fe 3++Ag→Fe 2++Ag +;Electrode reaction: Fe 3++e -→Fe 2+ and Ag - e -→Ag +; (d) 1)Ag (lg V 05916.0)/Ag Ag ()/Ag Ag (θ++++=c n ϕϕ V 223.01101.77lg 1V 05916.07996.0]Cl [(AgCl)lg 1V 05916.07996.010-sp =⨯+=+=-K。
谱图解析样题
NAME______________________Chem 203Organic SpectroscopyMidterm Examination, Part II (50 points total)Problem 1 of 3 (two out of three required, 25 points)Saturday, November 5, 2011, 9 am - ???SUBMIT TWO OF THE THREE PROBLEMS FOR GRADING AND DO NOT SUBMIT THE PROBLEM THAT YOU DO NOT WANT GRADED. IF THREE PROBLEMS ARESUBMITTED, ONLY THE FIRST TWO (PROBLEMS 1 AND 2) WILL BE GRADED Books, notes, calculators, rulers, and laptop computers are permitted as is wireless (or wired) internet access and appropriate software (e.g, PyMOL, Maestro/MacroModel, Excel, ChemDoodle, Chemdraw, ElComp, MolE, etc.). Communication with other students by e-mail, text, or in person is not permitted. Catalogs of molecular structures (e.g., the Aldrich catalog, the Merck Index, etc.) or databases of molecular structures (such as wireless access to SciFinder Scholar, the Sigma-Aldrich website, etc.) are NOT PERMITTED. INAPPROPRIATE COMMUNICATION OR USE OF SUCH ITEMS CONSTITUTES ACADEMIC DISHNONESTY, WILL RESULT IN A FAILING GRADE (F) IN THE CLASS, AND MAY RESULT IN EXPULSION FROM THE Ph.D. PROGRAM.If you wish to use a laptop computer, please be willing to share briefly with others when needed.1. Analyze the spectra and solve the structure of the molecule for which data are provided.Identify any noteworthy heteroatoms present. Determine the molecular formula and unsaturation number. Identify functional groups that are present from the IR and other spectra. Identify key fragments from NMR. Assign the 1H NMR and 13C NMR resonances to the respective atoms in the molecules. Mass spectra are EIMS, unless otherwise indicated.ONLY WORK SHOWN ON THIS PAGE WILL BE GRADED.Exact Mass: 151.0633Noteworthy Heteroatoms:Molecular Formula:Unsaturation Number:Functional Groups (be as specific as possible):Fragments (from NMR):Structure (Make sure to properly indicate stereochemistry, if applicable):Structure with 1H NMR resonances lettered from the most downfield to the most upfield (a, b, c, d, etc.): (Note: Not all resonances can be assigned with certainty. If assignments are uncertain, indicate so by showing possible letters.)Structure with 13C NMR resonances numbered from the most downfield to the most upfield (1, 2, 3, 4, etc.): (Note: Not all resonances can be assigned with certainty. If assignments are uncertain, indicate so by showing possible numbers.)Molecular Model: Build an energy-minimized molecular model of the structure using PyMOL and the "clean" function. Save the .pse files as structure1.pse. E-mail the .pse file to me (jsnowick@).DU=/v, USER=nmr11t, NAME=nmr11t-X-sample2, EXPNO=1, PROCNO=1F1=15.020ppm, F2=-1.021ppm, MI=0.40cm, MAXI=10000.00cm, PC=1.000 # ADDRESS FREQUENCY INTENSITY[Hz] [PPM]1 23630.2 4613.554 9.2362 3.642 23642.0 4612.116 9.2333 3.513 25487.4 4386.486 8.7816 2.574 25501.2 4384.801 8.7782 2.645 25526.9 4381.649 8.7719 2.566 25540.8 4379.962 8.7685 2.447 27396.7 4153.044 8.3142 1.638 27412.4 4151.125 8.3104 2.469 27428.4 4149.166 8.3065 1.5810 27461.6 4145.112 8.2984 1.6511 27477.3 4143.184 8.2945 2.4512 27493.3 4141.236 8.2906 1.4613 31107.4 3699.345 7.4059 1.8714 31112.7 3698.701 7.4047 1.9215 31147.2 3694.485 7.3962 1.9216 31152.5 3693.841 7.3949 1.9617 31172.2 3691.427 7.3901 1.8518 31177.5 3690.774 7.3888 1.8519 31212.0 3686.565 7.3804 1.6720 31217.1 3685.932 7.3791 1.6621 31480.6 3653.725 7.3146 1.3822 43204.2 2220.323 4.4450 2.5823 43262.6 2213.188 4.4307 7.3624 43320.9 2206.053 4.4164 7.3025 43379.3 2198.916 4.4021 2.4526 55508.7 715.902 1.4332 7.9027 55567.0 708.773 1.4189 15.0028 55625.5 701.626 1.4046 7.3729 61363.9 0.013 0.0000 0.87。
福山机理题
3
NH2 p-Cl-C6H4CHO
1) PMe3 , Toluene , rt; p-Cl-C6H4 Ph2C=C=O
N3
cat. AcOH EtOH , reflux
2) Pd/C , Toluene, reflux
N N Ph
4
O H3C
CH3 CH3
1) Eu(fod)3 (10 mol%), toluene, 80 °C, 36 h, 84%
tert-BuLi;
i-PrO
;
O
i-PrO HO i-PrO
H
Li
; aq NH4Cl
CH3
O CH3
3
CO2Me
1) SnCl4, toluene, –78 °C;
OH H
NaHCO3, toluene, 80 °C
H Me
NO2 Me
2) H2, RaneyNi, MeOH 160 psi, rt
2) hν, Corex, 19 h, 79% 3) LiDBB, THF, –78 °C to 10 °C, 35 h, 57%
LiDBB; lithium di-tert-butylbiphenylide
HO CH3
H3C H3C
H CH3
5
N
1) MCPBA 2) (CF3CO)2O;
aq. Na2CO3
1 P N H NO2
1) COCl2, DMAP, DMF 25 °C, 1 hr
2) NH2CH2CH2NH2, DMAP CH2Cl2, 25 °C, 1 hr
3) hν (lamp equipped with pyrex filter) THF
O HN NH
哈佛大学有机化学卷3
C. Nucleophilic ring closures sub-classified according to hybridization state of electrophilic component: (tetrahedral = tet; trigonal = trig; digonal = dig) D. Nucleophilic ring closures further subclassified according to size of the fomed ring. For example:
predominant inversion
predominant retention
Stereochemistry frequently determined by electrophile structure
See A. Basu, Angew. Chem. Int. Ed. 2002, 41, 717-738 (PDF)
SO3CH3
(CH3)3N
+
SO3
–
X
What is the absolute requirement for the Nu–C–X bond angle?? 180° +/– ??
Me N Me
H
R
H
O S O O
exclusively intermolecular
C
H
Eschenmoser: Tether Nu and X
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
BCH 110A General Chemistry Review Sample Problems (Dr. Ziegler) p. 1 BCH 110A – Dr. Ziegler General Chemistry Review: Chemical Equilibrium, Ionization of H2O, pH, pKa
PRACTICE/SAMPLE PROBLEMS
Work these without looking at the answers, which are on the next page. NOTE ESPECIALLY PROBLEM 2B, how to go from the ratio of base/acid (Henderson-Hasselbalch calculation) to the fraction of the total (acid + base) that's in the form of the acid (or what fraction is in the form of the base). In fact, anytime you calculate a RATIO and get a number, STATE the (understood) "1" in the denominator. If ratio is 0.4, state it as 0.4 / 1. Also, start by writing chemical equations to describe acid dissociation reactions, with CHARGE BALANCE.
1. For a solution whose pH is 6.0, what is [H+]? If [H+] is 5 x 10–7 M, what is the pH? (Use a simple scientific calculator if necessary to do log problems.)
2. A. For a weak acid such as the R group carboxyl group of Glu or Asp in a protein, if the pKa of that specific residue's carboxyl group is exactly 4.0, in an environment in which the pH = 5.0, what would be the ratio of base to acid ( [COO–]/[COOH] )?
B. For that same carboxyl group, what fraction, or what percentage, of the total (population of all the molecules in solution) is present in the form of the ACID (COOH) at pH 5.0? What fraction of the total is present in the form of the BASE (COO–) at pH 5?
3. Consider the state of ionization of the side chain (R group, an imidazole group) of a specific His residue, residue #20 (counting from the amino terminus) in the amino acid sequence of a certain protein. If about 1% of the molecules of that protein in solution have the imidazole group of residue #20 in the uncharged (neutral) form at pH 4.5., calculate the pKa of that specific His residue in that protein.
(You need to know the ionization properties of the functional group of His to answer this question -- look at the ionization equilibrium for His in Fig. 3-12b, p. 81, of Lehninger Principles of Biochemistry, 5th ed. All that concerns you is the ionization of the side chain (R group), the imidazole group, which for this amino acid is the middle pKa. The pKa of the imidazole group of the free amino acid histidine is 6.0, but the exact pKa values of amino acid functional groups in proteins vary somewhat, depending on the specific environment around that residue in the 3-dimensional structure of that protein. You're being asked to calculate this specific His residue's pKa.) BCH 110A General Chemistry Review Sample Problems (Dr. Ziegler) p. 2
SOLUTIONS TO PRACTICE/SAMPLE PROBLEMS 1. pH = – log[H+] (Remember, the log is the exponent.) If pH = 6.0, [H+] =10–pH = 10–6.0 M (Use a calculator for this.) If [H+] = 5 x 10–7 M, pH = – log(5 x 10–7) = 6.3
2. acid dissocation reaction for a carboxyl group: (R)-COOH (R)-COO– + H+ A. Use the Henderson-Hasselbalch Equation whenever you see a problem involving the relationship of pH, pKa, and base/acid ratio (or fraction of the total group that's in form of the base, or of the acid).
pH = pKa + log([base]/[acid]) log([base]/[acid]) = pH – pKa = 5.0-4.0 = 1.0 ([base]/[acid]) = 101 = 10/1 (There's 10 times as much of the carboxyl group in the form of the base as in the form of the acid when the pH is 1 unit above the pKa.)
B. At pH 5.0, the COO–/COOH ratio is 10/1. We can do this problem most simply by using proportions; the total of the carboxyl group is COOH + COO– = 1 + 10 = 11. The fraction of the total in the form of the acid (COOH) is (COOH) / total = (COOH) / (COOH + COO–) = 1/11 = 0.091 9.1% of the molecules in solution have that carboxyl group in its acid form.
COO– / total = COO– / (COOH + COO–) = 10/11 = 0.909 Alternatively, if you've already calculated that acid/total = 0.091, then base/total = 1 – (acid/total) = 1 – (0.091) = 0.909. Either way, 90.9% of the molecules in solution have that carboxyl group in its base form.
3. We'll abbreviate the imidazole group in question as Im, so neutral form is Im, the conjugate base, and charged form is Im+, the conjugate acid. Im+ <--> Im + H+ Use the Henderson-Hasselbalch Equation again. "About 1% neutral" means that base (Im) / total = about 0.01 or 1/100. Thus base/acid ratio would be base/(total – base) = 1 / (100 – 1) = 1/99.
pH = pKa + log([base]/[acid]) pKa = pH – log([base]/[acid]) = 4.5 – log (1/99) = 4.5 – (– 2.0) = 4.5 + 2.0 = 6.5
The pKa of this specific His residue in this protein must be about 6.5. (Note that in this protein, the pKa of this imidazole group is slightly different from the pKa of the R group of the free amino acid histidine.)
