甲醛代替MS1100(1)
备考2023年中考科学一轮复习-溶液的酸碱性与pH值的关系(三)

备考2023年中考科学一轮复习-溶液的酸碱性与pH值的关系(三)溶液的酸碱性与pH值的关系专训单选题:1、(2016杨浦.中考模拟) 向稀盐酸中逐滴加入试剂X后,溶液的pH变化情况如图所示。
试剂X是()A . MgB . H2O C . NaOH D . CaCO32、(2017滨州.中考真卷) 下列图象能正确反映其对应操作中各量变化关系的是()A . 图①是给氯酸钾和二氧化锰的混合物加热B . 图②是向一定温度下的饱和氢氧化钙溶液中不断加入氧化钙固体C . 图③是向pH=1的酸溶液中不断加水D . 图④是向一定质量的稀硫酸中加入锌粒3、(2020四会.中考模拟) 某同学测得常见物质的pH如下表,其中能使紫色石蕊试物质牙膏肥皂水橘汁草木灰水食盐水pH 8.2 9.2 3.5 10.6 7.04、(2016潍坊.中考真卷) 如图对应关系正确的是()A . 一定量的MgSO溶液中加入NaOH溶液,沉淀质量与加4入NaOH溶液质量的关系 B . 相同质量的Mg、Fe分别与足量的同浓度稀硫酸反应,氢气的质量与反应时间的关系 C . 一定量的饱和石灰水中加入氧化钙,溶液中溶质质量与加入氧化钙质量的关系 D .向氢氧化钠溶液中不断加水,溶液的pH与加入水的质量的关系5、(2016十堰.中考真卷) 下列图象分别与选项中的实验过程相对应,其中正确的是()A . 向部分变质的氢氧化钠溶液中滴加稀盐酸B .向一定量稀盐酸中逐滴加入水 C . 常温下,等质量的锌和铁分别与足量同溶质质量分数的稀硫酸反应 D .电解水生成气体的体积6、(2016邵阳.中考真卷) 某化学兴趣小组利用稀盐酸和氢氧化钠溶液来探究中和反应的规律时,某溶液的pH随时间的变化如图所示,则a点所示溶液中溶质的是()A . HCl NaOHB . NaClC . NaCl NaOHD . NaCl HCl7、(2019永昌.中考模拟) 某化学课外活动小组开展下列实验活动:取刚降到地面的雨水水样,用pH计(测pH的仪器)每隔5分钟测一次pH,其数据如下表所示。
医疗器械公司 BSC-1100IIA2-X生物安全柜使用、清洁、维护和维修SOP

BSC-1100ⅡA2-X生物安全柜使用、清洁、维护和维修SOP1.目的:建立BSC-1100IIA2-X生物安全柜的使用、清洁、维护和维修SOP,以规范其操作。
2.范围:适用于设备操作者对BSC-1100IIA2-X生物安全柜的使用、清洁、维护和维修。
3.职责:3.1 检验员:按该SOP进行使用、清洁和维护。
3.2 设备机修员:按该SOP进行维护和维修。
3.3 质量部经理:监督检查本程序的执行情况。
4.内容:4.1适用范围II级A2级型安全柜具有前窗操作口的安全柜,操作者可以通过前窗操作口在安全柜内进行操作,对操作过程中的人员、产品及环境进行保护。
工作环境:(1)仅适用于室内;(2)环境温度:15~35℃;(3)相对温度:≤75%;(4)大气压力范围:70KPa~106KPa。
4.2技术参数4.3性能指标4.3.1生物安全性能人员安全性,用碘化钾(KI)法测试,前窗操作口的保护因子应小于1×105;产品保护,微生物菌落数≤5个;交叉污染保护,微生物菌落数≤2个。
4.3.2柜体防泄露安全柜加压到500Pa,保持30min后气压不低于450Pa。
4.3.3高效过滤器完整性可扫描检测过滤器在任何点的漏过率不超过0.01%。
4.3.4振动幅值频率10Hz和10KHz之间的振动净振幅不超过5um(rms)。
4.3.5照度平均不小于650lx,每个照度实测值不小于430lx。
4.3.6机械性能安全柜的设计与结构能抗外力引起的翻倒或变形、抗工作台面负载所引起的向下弯曲、抗工作负荷的倾翻。
工作台面中心加载23kg压力后,工作台面不产生永久变形。
4.3.7电器性能耐压:电压值5s内上升至1390V交流电压时,保持5s不击穿。
接地电阻≤0.1Ω。
4.4结构组成4.4.1结构组成图4.4.2前玻璃门驱动系统前玻璃门驱动系统由门电机、前玻璃门、牵引机构、限位开关等组成。
4.4.3空气过滤系统空气过滤系统是保证本设备性能最主要的系统。
食品中甲醛次硫酸氢钠(吊白块)测定结果分析

食品中甲醛次硫酸氢钠(吊白块)测定结果的分析摘要:本文讨论在食品中添加甲醛次硫酸氢钠常用的测定方法及测定结果的判定标准,根据测定结果与样品的本底值进行比较。
使检测结果合理准确。
关键词:甲醛食品甲醛次硫酸氢钠近年来,随着经济的发展,一些生产厂家利用吊白块又称雕白块(其化学名称:甲醛次硫酸氢钠;分子式为:nahs02·ch20·2h02;分子量:154.1l)有凝固蛋白的特性,在诸如面粉、腐竹、粉丝等食品中加入这一化学物质,以增加成品产出率,提高产量,改善食品的外观和口感。
但一次性食用甲醛次硫酸氢钠剂量仅l0g就会有生命危险,长期食用更是后患无穷。
为了维护消费者的利益,保障期食用更是后患无穷。
为了维护消费者的利益,保障人民群众的身体健康,严厉打击食品领域的违法行为,必须对这类产品进行严格抽查。
目前没有测定吊白块的国家标准方法,现大多采用盐酸苯脐法、乙酞丙酮法或ahmt[1]法测定吊白块中的甲醛含量来间接计算吊白块的含量,但由于有些样品只用甲醛处理或有甲醛本底,易产生误判。
本法利用测定so2和甲醛的含量联合判定吊白块是否存在,并且综合考虑了阴性样品的本底和so2/hcho的比值,使吊白块含量的判定更准确可靠。
1.1分光光度法原理对甲醛次硫酸氢钠进行定性与定量,主要依据甲醛次硫酸氢钠可逆反应:在酸性溶液中,甲醛次硫酸氢钠分解成甲醛与亚硫酸氢钠。
通过对水蒸汽蒸馏馏份中的nahso3和hcho测定来进行定性与定量检测,以确定检品中是否存在甲醛次硫酸氢钠,同时根据甲醛的定量结果,作为甲醛次硫酸氢钠存在量的量化依据。
1.2仪器与试剂紫外分光光度计uv102(扫描波长190~1100mm,岛津公司,日本)甲醛次硫酸氢钠:分析纯,天津。
甲醛:100mg/ml,国家标物中心提供,使用时稀释至所需浓度。
其它所用试剂均为国产分析纯,超纯水。
1.3样品处理准确称取约20g均匀样品于250ml的平底蒸馏烧瓶中,加入20g 硫酸钠和80ml去离子水(液体样品可只加65ml),装上冷凝装置,冷凝管下端插人装有1ml1%naoh吸收液的100ml容量瓶中,置于冰块浴中。
107胶水的制备实验报告

实验报告
班级13化工姓名日期2016 年 6 月17 日
实验()
六、思考题及讨论(Exercises and Discussion)
1、如何加速聚乙烯醇的溶解速度?
在低温水中溶胀,然后再升温加速溶胀溶解,并采用机械搅拌使其分散。
2、两次调节PH的目的分别是什么?
第一次调节是因为反应在酸环境下才能进行,第二次是因为该反应是可逆反应,调回中性防止产量下降。
3、甲醛过量和不足对实验结果分别有什么样的影响?
甲醛过量:因为多链之间有范德华作用力,产物易形成凝胶;
甲醛过少:粘接性不够,不稳定且低温时易失去粘性。
4、本实验中影响107胶粘剂粘合性能的因素有哪些?
甲醛的量、ph、和温度的控制。
Keggin型多元杂多化合物催化氧化苯甲醇制苯甲醛

2010年第29卷第1期CHEMICAL INDUSTRY AND ENGINEERING PROGRESS ·71·化工进展Keggin型多元杂多化合物催化氧化苯甲醇制苯甲醛李贵贤,余 华,滕志君,卢有胜,汤 茜(兰州理工大学石油化工学院,甘肃兰州 730050)摘 要:合成了一系列具有Keggin结构的P-V-Mo-W四元杂多化合物(Cpyr)3+x PV x Mo y W12-x-y O40·n H2O [其中,Cpyr=(C16H32C5H4N)+,x=0、1、2和3],采用傅里叶变换红外光谱和X射线衍射等方法表征了杂多化合物的结构;以磷钒钼钨杂多化合物为相转移催化剂,用m(H2O2)=30%双氧水氧化苯甲醇制备苯甲醛。
考察了催化剂的种类、溶剂的类型和反应条件(催化剂用量、反应温度、反应时间、H2O2用量)对苯甲醇氧化反应的影响。
在优化条件下,即苯甲醇0.1 mol、催化剂(Cpyr)5PV2Mo5W5O40 0.04 mmol、m(H2O2)=30%H2O2 0.1 mol、在100 ℃反应8 h、溶剂水10 mL,苯甲醇的转化率达88.51%、苯甲醛的选择性达96.06%。
关键词:苯甲醇;苯甲醛;磷钒钼钨杂多化合物;相转移;氧化中图分类号:TQ 426.91文献标识码:A文章编号:1000–6613(2010)01–0071–05Oxidation of benzyl alcohol to benzaldehyde over Keggin typemulticomponent heteropoly compoundsLI Guixian,YU Hua,TENG Zhijun,LU Yousheng,TANG Qian(College of Petrochemical Technology,Lanzhou University of Technology,Lanzhou 730050,Gansu,China) Abstract:Multicomponent heteropoly compounds (Cpyr)3+x PV x Mo y W12-x-y O40·n H2O with Keggin structure were synthesized and proved by FTIR and X-ray spectroscopies.With 30% aqueous hydrogen peroxide as oxidant,benzyl alcohol was efficiently oxidized to benzaldehyde over reaction-controlled phase-transfer catalysts (Cpyr)3+x PV x Mo y W12-x-y O40. Effects of the type of catalyst,the amount of catalyst and H2O2,the kind of solvent,reaction temperature and time were investigated.Under the optimal conditions,i.e.,with 0.1 mol of benzyl alcohol,0.04 mmol of (Cpyr)5PV2Mo5W5O40 catalyst and 0.1mol of 30% H2O2,reaction temperature 100 ℃,10 mL water as the solvent,88.51% conversionof benzyl alcohol and 96.06% selectivity for benzaldehyde were achieved within 8h.Key words:benzyl alcohol;benzaldehyde;(Cpyr)5PV2Mo5W5O40;phase-transfer;oxidation苯甲醛,又称苦杏仁油,是制造染料用的中间体,也是制造医药品、香料、调味品、涂料等精细化学产品的重要原料。
隔热保温涂料(新)配方
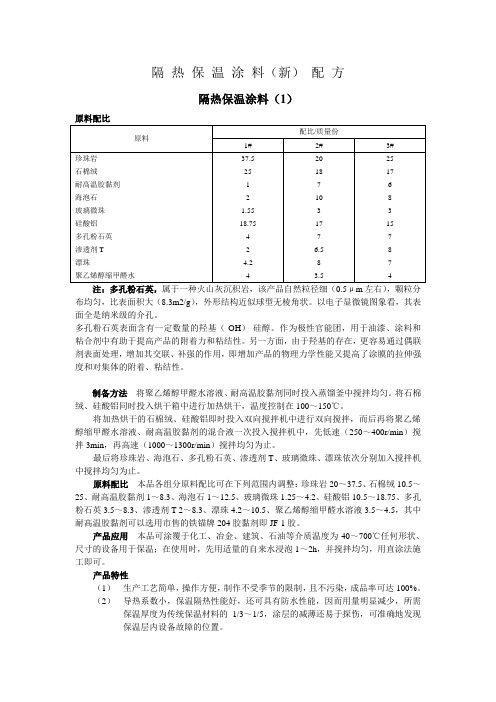
隔热保温涂料(新)配方隔热保温涂料(1)注:多孔粉石英,属于一种火山灰沉积岩,该产品自然粒径细(0.5μm左右),颗粒分布均匀,比表面积大(8.3m2/g),外形结构近似球型无棱角状。
以电子显微镜图象看,其表面全是纳米级的介孔。
多孔粉石英表面含有一定数量的羟基(-OH)-硅醇。
作为极性官能团,用于油漆、涂料和粘合剂中有助于提高产品的附着力和粘结性。
另一方面,由于羟基的存在,更容易通过偶联剂表面处理,增加其交联、补强的作用,即增加产品的物理力学性能又提高了涂膜的拉伸强度和对集体的附着、粘结性。
制备方法将聚乙烯醇甲醛水溶液、耐高温胶黏剂同时投入蒸馏釜中搅拌均匀。
将石棉绒、硅酸铝同时投入烘干箱中进行加热烘干,温度控制在100~150℃。
将加热烘干的石棉绒、硅酸铝即时投入双向搅拌机中进行双向搅拌,而后再将聚乙烯醇缩甲醛水溶液、耐高温胶黏剂的混合液一次投入搅拌机中,先低速(250~400r/min)搅拌3min,再高速(1000~1300r/min)搅拌均匀为止。
最后将珍珠岩、海泡石、多孔粉石英、渗透剂T、玻璃微珠、漂珠依次分别加入搅拌机中搅拌均匀为止。
原料配比本品各组分原料配比可在下列范围内调整:珍珠岩20~37.5、石棉绒10.5~25、耐高温胶黏剂1~8.3、海泡石1~12.5、玻璃微珠1.25~4.2、硅酸铝10.5~18.75、多孔粉石英3.5~8.3、渗透剂T 2~8.3、漂珠4.2~10.5、聚乙烯醇缩甲醛水溶液3.5~4.5,其中耐高温胶黏剂可以选用市售的铁锚牌204胶黏剂即JF-1胶。
产品应用本品可涂覆于化工、冶金、建筑、石油等介质温度为-40~700℃任何形状、尺寸的设备用于保温;在使用时,先用适量的自来水浸泡1~2h,并搅拌均匀,用直涂法施工即可。
产品特性(1)生产工艺简单,操作方便,制作不受季节的限制,且不污染,成品率可达100%。
(2)导热系数小,保温隔热性能好,还可具有防水性能,因而用量明显减少,所需保温厚度为传统保温材料的1/3~1/5,涂层的减薄还易于探伤,可准确地发现保温层内设备故障的位置。
投标产品的技术说明【模板】
2400*1100*760
3
2200*1100*760
4
1800*900*760
5
2800*1100*760
基材:台面,优质“和龙”牌E1级环保中密度纤维板,其他部位选用优质“根河”E1级环保刨花板,甲醛释放量≤5mg/100g;面材:台面,双面贴美国进口大西洋牌一级普通树种木皮,木皮厚度不小于0.6mm,纹理自然,颜色一致,美观大方。封边:四周显露部位使用与木皮材质一致的实木封边,木材含水率8-11%;走线孔内缘及隐蔽部位全部做封边处理;五金件:采用优质诚信林肯,带锁;油漆:优质“华润”高级隐孔亚光环保油漆;胶粘剂:采用马来西亚“WP101”环保热压贴面胶环保胶粘剂;班台构成:主台、副台、活动柜;结构性能描述:台面带薄抽,带真皮书写板,带走线功能;三屉活动推拒下设前置导向轮,内置文件挂架;副台可移动,插入台面下三分之一,中间设键盘屉,屉下保证中间净空高≥580,中间净空宽≥520,结构设计便于办公。柜门和抽屉带锁,三节无声滑轨,杜邦尼龙纤维合成脚轮。
22
900*400*1900
双门更衣柜
23
1200*600*2200
基材:著名“红宝石”品牌E1级优质环保中纤板;著名品牌E1级“根河”环保刨花板,甲醛释放量≤5mg/100g;面层:一级美国进口大西洋品牌胡桃木皮厚度不小于0.6mm,木皮宽度≥200mm,纹理自然,颜色一致,美观大方。封边:四周显露部位使用与木皮材质一致的实木封边;隐蔽部位全部做封边处理,木材含水率8-11%;五金件:采用著名德国“海福乐”品牌,带锁;油漆:著名德国“易涂宝”品牌高级隐孔亚光环保油漆;胶粘剂:采用著名马来西亚“WP101”环保热压贴面胶;结构性能描述:两门,带锁,配挂衣杆,衣帽钩;内置4层板,层板厚不小于25mm,可调整,木边采用使用与木皮材质一致的实木封边,背板厚不小于12mm,可保证柜体的稳定性,不易变形。
以苯甲醛为催化剂的盐酸中铝腐蚀的热力学和动力学研究(IJEM-V9-N6-5)
I.J. Engineering and Manufacturing, 2019, 6, 53-64Published Online November 2019 in MECS ()DOI: 10.5815/ijem.2019.06.05Available online at /ijemThermodyamic and Kietic Study on the Corrosion of Aluminium in Hydrochloric Acid using Benzaldehyde as Corrosion Inhibitor*Musa Husaini, Muhammad Bashir Ibrahima Department of Pure and Industrial Chemistry ,Faculty of Physical Sciences, Bayero University, P.M.B. 3011BUK, Kano. NigeriaReceived: 07 September 2019; Accepted: 15 October 2019; Published: 08 November 2019AbstractThe inhibition of the corrosion of aluminium by benzaldehyde in 1.4 M HCl was investigated using weight loss method and characterized by FT-IR analysis. The results showed that the corrosion rate of aluminium in 1.4 M HCl decreases with increase in concentration of the inhibitor. The inhibition efficiency increases progressively as the concentration of the inhibitor increases. Effects of temperature on the inhibition efficiency of the inhibitor showed that inhibition efficiency decreases with increase in temperature. The value of activation energy (Ea) was found to be 20.55 Kjmol-1 for aluminium corrosion in 1.4 M HCl which was increased to 34.49 Kjmol-1 in the presence of 0.1 M inhibitor concentration. The calculated values for enthalpy of activation (ΔHa) were all positive indicating the endothermic nature of the aluminium dissolution process. The obtained values of Gibbs free energy (ΔGads) was in the range of -17.94 to -18.27 kJ mol-1. Kinetics of the reaction in the presence of the inhibitor revealed that it follows a first order reaction. The value of rate constant (k) was reduced from uninhibited acid to the inhibited acid solution, while the half-life values in the presence of the inhibitor were higher compared to the value in uninhibited acid solution suggesting that inhibition efficiency increases with increase in the concentration of the inhibitor.Index Terms: Aluminum, benzaldehyde, FT-IR analysis, kinetic parameters, thermodynamic parameters and weight loss.© 2019 Published by MECS Publisher. Selection and/or peer review under responsibility of the Research Association of Mode rn Education and Computer Science* Corresponding author.E-mail address: musahusaini36@54 Thermodyamic and Kietic Study on The Corrosion of Aluminium in Hydrochloric Acid UsingBenzaldehyde as Corrosion Inhibitor1.IntroductionAluminium is the most widely used non-ferrous metal in all the fields of domestic and industrial applications like construction, transportation, packaging, a wide range of household items, electrical transmission and outer shell of consumer electronics etc. Aluminium is remarkable for its low density and ability to resist corrosion due to the passivation phenomenon [1]. The protective coating of aluminium destroyed when exposed to acid or alkaline environment and corrosion of aluminium occurs, yielding Al+3 ion in acid solution and AlO2 in the alkaline solution [2]. Acid pickling is usually used for aluminium surface treatment. Aluminium can react with hydrochloric acid to produce hydrogen gas. The chloride ions of the HCl cause a substantial loss of the metal by corrosion and Cl- ions of the acid create extensive localized attack over the surface of aluminium. Hence to avoid such kind of attack on the base metal during pickling and cleaning aluminium surfaces with acidic solutions, the use of inhibitors become necessary. The use of inhibitors is among the most practical methods for protection against corrosion in acidic media. Various organic compounds are reported as good corrosion inhibitors for aluminium in hydrochloric acid media [3, 4].A great number of research have being devoted to the subject of corrosion inhibitors in both the laboratory and fields. Corrosion inhibition is of great practical importance, as it’s extensively employed in shortening wastage of engineering materials and reducing corrosion control costs. Inhibitor applications is quite varied usually playing an important role to minimize localized corrosions and unexpected sudden failures in water treatment facility, oil extraction and processing industries, ferrous metal cleaners, heavy industrial manufacturing, water treatment chemicals, water-containing hydraulic fluids, automatic transmission fluids, automotive component manufacture, cutting fluids, engine coolants etc. Most of the well-known acid inhibitors are organic compounds containing nitrogen, oxygen, phosphorus, sulfur and π bonds, as well as aromatic rings in their structure which are the major adsorption centers [5]. Generally, a strong interaction causes higher inhibition efficiency, the inhibition effect increases in the sequence O < N < S [6, 7]. Compounds with π-bonds also exhibit good inhibitive properties due to interaction of π orbital with metal surface. The objectives of the present research is to study the kinetic parameters, thermodynamic parameters, adsorption isotherm and the inhibition effect of benzaldehyde as inhibitor on the corrosion of aluminium in hydrochloric acid at different inhibitor concentration and temperature. The weight loss method was used in this study as its quantitative and possibly most accurate method in monitoring and measuring corrosion of metallic structures.1.1 Significance of the ResearchThe investigation of the corrosion inhibition of benzaldehyde on aluminium provided a useful information on surface coverage, inhibition efficiency, thermodynamic and kinetic data, and the type of adsorption of benzaldehyde inhibitor on the surface of aluminium. The use of benzaldehyde inhibitor will reduce the rates of usage of inorganic inhibitors which are toxic to environment. This submission is in advancement of contribution toward the sustained world-wide in looking for friendly, non-toxic and commercially available corrosion inhibitors.1.2 Literature ReviewSeveral report have been made to the organic compounds that contain nitrogen, sulfur and nitrogen as polar groups and conjugated double bonds in their structures to be good corrosion inhibitors for many metals and alloys in corrosive environment [8]. The inhibiting effect of these organic compounds is usually attributedto their interactions with the metallic surfaces through their adsorption. Polar functional groups serves as the reaction center that stabilitizes the adsorption process. Nevertheless the adsorption of an inhibitor on a metal surface depends on certain factors which include; the inhibitor’s chemical structure, nature and surface charge of the metal, the type of the electrolyte solution and the adsorption mode [9].The Inhibition of Aluminum corrosion in 3.5% NaCl solution using diisopropyl thiourea (DISOTU) has been reported by Karthikeyan et al [10] using weight loss, electrochemical polarization technique, impedance method and quantum mechanical measurement. It was found that the aluminium corrosion in the sea water medium was minimize effectively by the compound. Adsorption of the compound on metal was noticed to follow Langmuir adsorption isotherm. The inhibition efficiency (IE) increases with increase in inhibitor concentration. The adsorption of protective layer of inhibitor on aluminium surface was confirmed by using Quantum mechanical studies.The effect of formazan of benzaldehyde (FB) on the corrosion of mild steel in acidic media (1.0 M HCl and 2 M HCl) has been investigated by Anand [11] using weight loss measurements, electrochemical studies and surface analysis. These studies have also shown that formazan of benzaldehyde is a good inhibitor for mild steel in 1.0 M HCl and 2 M HCl acid solutions at room temperature in 2h. In 1.0 M HCl the inhibition efficiency was high when compared to 2 M HCl acid solutions. The surface analysis also confirms the corrosion inhibition of the mild steel by the inhibitor (FB).Aluminium and its alloys are vitally preferred materials of construction in many chemical and engineering fields due to their light weight corrosion resistant properties. Nitrogen-containing organic compounds, like amines and diamine derivatives offer good protection of metallic materials on the corrosion for many metals in acidic solutions. Arvnabh et al [12] analyzed the corrosion inhibition of aluminium alloys of grade 1060, 1100 and 3003 in trichloroacetic by using conductivity and potentiostatic polarization in different inhibitors of diamine such as ethyl amino ethylamine, di-methyl amino ethylamine, 1:3 di-amino propane, tetra methyl ethylene diamine. Diamines are act as mixed inhibitors as revealed by polarization curves in the case of alloy of 3003 grade.In the recent investigation effect of Triethylenetetramine [TETA] and 2-(2-aminoethylamino) ethanol [AEAE] as corrosion inhibitors for N80 steel in 15% HCl solution was studied by Yadav et al [13] using polarization, AC impedance (EIS) and weight loss measurements. Both inhibitors were found to be effective inhibitors. Inhibition efficiency was found to increase significantly with increasing inhibitor concentration. Mixed-type inhibitors were revealed from polarization studies. Charge transfer resistance increases and double layer capacitance decreases in presence of inhibitors as revealed by AC impedance studies. Inhibitors adsorption on the surface of N80 steel follows Langmuir adsorption isotherm.2.Material and Methods2.1 Specimen PreparationRectangular specimen of aluminium (99.5 %) obtained from Metal Focus Fabrication Technology Incubation Centre Kano State, Nigeria was used in this study [14]. The specimen of size 3 x 2 x 0.1 cm with a small hole near the upper edge was used for the determination of corrosion rate. The specimen was polished using different grade of emery paper, degreased in absolute ethanol, dried in acetone and stored in a moisture-free desiccators prior to use.2.2 Preparation of SolutionsDouble distilled water was used to prepare a stock solution of analytical grade hydrochloric acid (36.5%, 1.18g/ cm3). The required concentration of the acid solution was prepared by appropriate dilutions. The used inhibitor was benzaldehyde (95% 1.04 g/cm3), and concentrations of the inhibitor used for the study was 0.02, 0.04, 0.06, 0.08 and 0.1 M. Each of these concentrations was diluted in the prepared desired concentrations of acids for use as test solutions in weight loss.2.3 Weight loss MeasurementThe prepared weighted specimens were immersed in 100 ml beaker containing the acid solution in the absence and presence of various concentrations of the inhibitor (0.02, 0.04, 0.06, 0.08 & 0.1 M) at 308 K and 3hrs immersion time, after which they were retrieved, washed, dried, re-weighted and recorded. The experiment were performed in replicate. The effect of temperature was studied at a temperature range of 308, 313 and 318 K. The weight loss of aluminium was calculated in grams as the difference between the initial weight and the weight after the removal of the corrosion product. The weight loss (Δw) corrosion rate (C.R), inhibition efficiency (I.E) and degree of surface coverage (Ө) were calculated using the Equations 1, 2, 3 and 4 respectively.Δw = (1)C.R = (2)Ө = (3)I.E = × 100 (4)Where wi and wf are the initial and final weight of Aluminium samples, w1 and w0 are the weight loss values in presence and absence of inhibitor, respectively. A is the total area of the aluminium specimen and t is the immersion time.2.4 Fourier Transform Infrared Spectroscopic AnalysisFT-IR analysis was carried out for the fresh inhibitor and that of the corrosion product obtained from the reaction of aluminium immersed in 1.4 M HCl solution for 3 hrs immersion time in the presence of 0.1 M benzaldehyde at 308 K using Agilent Technology, FTIR (Cary 630) Fourier Transform Infrared Spectrophotometer. 650 – 4000 cm-1 wave number was used to scan the sample during the analysis.3.Results and DiscussionThe data of all figures presented in this section are obtained from the result presented in Table 1.3.1 Effect of inhibitor on corrosion rate and inhibition efficiencyFigure 1 shows the plot of corrosion rate against inhibitor concentration for aluminium corrosion in 1.4 M HCl at different temperatures. The figure reveals that the rate of corrosion of aluminium in 1.4 M hydrochloric acid decreases with increase in inhibitor concentration at all the temperatures studied. The values of the corrosion rate of inhibited system was found to be lower compared to the uninhibited system due to the inhibitive effect of the inhibitor. Increase in inhibitor concentration causes the increase in the rate at which the inhibitor molecules adsorbed on the surface of the aluminium thereby forming a barrier for charge and mass transfer which results into a decrease in the interaction between the metal surface and the corrosive media and also, reduces the rate of corrosion.Table 1. Variation of Corrosion Parameters for Corrosion of Aluminium in 1.4 M HCl in the Absence and Presence of Different Concentrations of Inhibitor. InhibitorConc. (M) Corrosion rate (mgcm -2h -1) 308 K 313 K 318 K Surface Coverage (Ө) 308 K 313 K 318 K Inhibition efficiency (%) 308 K 313 K 318 K Blank0.020.040.060.080.10 19.527 21.290 25.138 13.366 16.566 19.761 12.450 15.483 18.577 11.888 14.633 17.916 11.266 13.483 17.072 10.733 12.527 16.405 - - - 0.3155 0.2451 0.2131 0.3624 0.2945 0.2602 0.3911 0.3332 0.2866 0.4230 0.3855 0.3202 0.4503 0.4292 0.3468 - - - 31.55 24.51 21.31 36.24 29.45 26.02 39.11 33.32 28.66 42.30 38.55 32.02 45.03 42.92 34.68Fig.1. Variation of Corrosion Rate with Inhibitor Concentration for Al Corrosion in 1.4 M HClFig.1 shows the variation of inhibition efficiency against the different concentration of the inhibitor at 308, 313 and 318 K. The figure reveal that the inhibition efficiency increases with increase in the concentration of the inhibitor. This is due to the formation of more protective barrier film of the inhibitor molecules on the metal surface, which further supporting the inhibitive action of the inhibitor against the corrosion of the metal. Similar result was reported by Karthikaiselvi and Subhashini [15].3.2 Effect of Temperature on Corrosion Rate and Inhibition EfficiencyThe corrosion rates of aluminium in the absence and presence of various concentrations of the inhibitor at different temperatures was shown in Fig.3. The result obtained showed that the rate of corrosion of aluminium58 Thermodyamic and Kietic Study on The Corrosion of Aluminium in Hydrochloric Acid UsingBenzaldehyde as Corrosion Inhibitorincreased with increase in temperature. This phenomenon is due to the fact that chemical reaction rates normally increases when the temperature increase. Increase in temperature leads to increase in the average kinetic energy possessed by the reacting molecules thereby making the molecules to overcome the energy barrier and react faster [16].Fig.2. Variation of Inhibition Efficiency with Inhibitor Concentration for Al Corrosion in 1.4M HClFig.3. Variation of Corrosion Rate with Temperature for Al Corrosion in 1.4 M HClVariation of inhibition efficiency with temperature at different concentration of the inhibitors is shown in Figure 4. The result obtained showed that the inhibition efficiency decreases with increase in temperature, this is due to the desorption of adsorbed inhibitor molecules from aluminium surface. The significant difference between values of inhibition efficiency of the given inhibitor obtained at the studied temperature suggested that the mechanism of adsorption of inhibitor on aluminum surface is consistent with physical adsorption mechanism. For a physical adsorption mechanism, inhibition efficiency of the inhibitor increases with decrease in temperature which mean physical adsorption mechanism occurred on the aluminium surface. Similar result was reported by reported by Eddy et al [17] and Abdallah [18].3.3Adsorption behaviourTo understand the mechanism of corrosion inhibition process, the adsorption behaviour of the adsorbate on the aluminium surface must be known. The information on the interaction between the metal surface andBenzaldehyde as Corrosion Inhibitorthe inhibitor molecules can be provided by adsorption isotherm. The degree of surface coverage (Ө) for different concentrations of inhibitor was evaluated from weight loss measurements. The data was applied to various isotherms. Attempts were made to fit the experimental data into different isotherms. The result indicates that Langmuir adsorption isotherm model best described the adsorption characteristics of the inhibitor on the aluminium surface. Langmuir adsorption isotherm is the ideal adsorption isotherm for physical and chemical adsorption on a smooth surface, and it is valid for monolayer adsorption onto a surface containing a finite number of identical sites. According to this isotherm, the surface coverage (Ө) is related to the inhibitor concentration Cinh by the equation given below;Fig.4. Variation of Inhibition Efficiency with Temperature for Al Corrosion in 1.4 M HClinh C 1= C inhads K θ+ (5)Where K ads is the adsorption equilibrium constant, C inh is the inhibitor concentration in the solution, and Ө is the surface coverage. The plot of C inh /Ө against C inh gave a straight line with slope equal to unity and R 2 close to 1 indicating that the adsorption of the inhibitor on the surface of aluminium is consistent with Langmuir adsorption isotherm. The correlation coefficient (R 2) and adsorption equilibrium constant (K ads ) are presented in Table 3.3.4 Kinetic Study3.4.1 Rate Constant (k) and Half Life (t 1/2)Kinetic analysis of the data is considered necessary since corrosion reaction is a heterogeneous process that composed of anodic and cathodic reactions with the same or different rate. In this current study, the initial weight of aluminum specimen at given time, t is designated as W i , the weight loss is W L and the weight change at time t is (W i - W L ), while k 1 is the first order rate constant.ln (Wi - W L ) = -k 1t + lnW L (6) The plots of ln (Wi - W L ) against time (hr) at 308 K showed a linear variation which confirms a first order reaction kinetics with respect to the corrosion of aluminium in 1.4 M HCl solutions in the presence of the inhibitor. The first order reaction rate constants (k 1) calculated from the slope of the plot are presented in Table 2. The result shows that the values of rate constant (k 1) for the corrosion of aluminium was found to be higher in the case of uninhibited acid solution than inhibited acid solution. This confirmed the inhibition ofBenzaldehyde as Corrosion Inhibitoraluminium corrosion in acids solution by the presence of inhibitors.The half-life (t1/2) was calculated from equation 7t1/2 = 0.693 / k(7) From Table 2, the values of the half-lives (t1/2) increased from uninhibited solution to inhibited solution. The increase in half-lives (t1/2) in the presence of the inhibitor compared to the uninhibited solution support the earlier results that corrosion rate decreases in the presence of the inhibitor compared to the uninhibited solution. It can also be seen that as the concentration of the inhibitor increases,the half-life also increases which results into a decrease in the corrosion rate suggesting that more protection of the aluminium by the inhibitor has been established.3.4.2 Activation Energy (Ea)The rate of most chemical reactions increases with temperature following Arrhenius equation [17]. In the case of the electrochemical reactions, temperature favors the kinetics of corrosion reactions and more specifically, the anodic dissolution of the metal. The activation energy of the corrosion process was calculated using the Arrhenius law equationln(C.R) =EB alnRT(8)Where B is a constant which depends on the metal type, R is the universal gas constant, and T is the absolute temperature. The plot of ln (C.R) Vs reciprocal of absolute temperature (1/T) gave a straight line with slope = −Ea/R, from which the activation energy values for the corrosion process was calculated. The calculated activation energy values were tabulated in Table 2. The activation energy value in uninhibited hydrochloric acid solution is low com¬pared to inhibited hydrochloric acid solution. The higher activa¬tion energy values in the presence of inhibitor molecules strongly support the results of corrosion rate studies and confirms the physisorption mechanism (inhibition efficiency decreases with an increase in solution temperature).Table 2. Kinetic Parameters for Aluminium Corrosion with and without various inhibitor concentrationsInhibitor Concentration(M) Activation Energy(kj mol-1)Rate Const. (k ×10-3) (hour-1) Half-life (hours)Blank 20.55 144.39 4.790.02 31.84 91.75 7.550.04 32.60 84.59 8.190.06 33.39 80.28 8.630.08 33.81 75.56 9.170.10 34.49 71.57 9.683.5 Thermodynamic StudyThe Thermodynamic parameters like enthalpy of activation (ΔHa) and entropy of activation (ΔSa) were calculated using the transition state equation. The linear form of transition state equation is given by the equation belowBenzaldehyde as Corrosion Inhibitora a RS H C R T Nh ln R RT ln ∆∆⎛⎫⎛⎫⎛⎫⎛⎫- ⎪ ⎪ ⎪ ⎪⎝⎭⎝⎭⎝⎭⎝=⎭+ (9)Where h is Plank’s constant and N is Avagadro’s number. A plot of ln (C.R /T) vs 1/T gave a straight line with slope = −ΔHa/T and intercept = ln(R/Nh) +ΔSa /R. The calculated values of enthalpy of activation (ΔHa) and entropy of activation (ΔSa) are tabulated in Table 3. The positive signs of the enthalpies reflect the endothermic nature of the aluminium dissolution process. Large negative values of entropies show that the activated complex in the rate determining step is an association rather than dissociation step meaning that a decrease in disordering takes place on going from reactants to the activated complex.Table 3. Enthalpy and Entropy change of the reaction process with various concentrations of the inhibitorInhibitor Concentration(M)ΔH (kJ mol -1) - ΔS (kJ mol -1k -1) blank17.95 219.63 0.0229.24 186.05 0.0430.00 184.19 0.0630.79 182.04 0.0831.21 181.21 0.10 31.89 179.483.5.1 Free Energy of Adsorption (ΔGads,)The free energy of adsorption (ΔGads) is related to adsorption equilibrium constant (Kads) by the equation given belowΔG ads = - RT ln (55.5 K ads ) (10) The negative values of free energy of adsorption (Gads) presented in Table 4 suggests that the inhibitor molecules are strongly adsorbed on the metal surface, the values also shows a spontaneous adsorption of the inhibitor molecules mainly characterized by the strong interactions with the metal surface. The values of ΔGads around -20 kJmol-1 are consistent with electrostatic interaction between charged molecules and a charged metal indicating physical adsorption (physisorption) mechanism, while those around -40 kJmol-1 involve charge sharing or transfer of electrons to form a co-ordinate type of bond otherwise known as chemical adsorption (chemisorption) mechanism. In the present study, the values of ΔGads are less than -20 kJ mol-1 which indicate that the adsorption of the inhibitor on the aluminium surface confirms a physical adsorption mechanism. Similar result was reported by Vimala et al [19].Table 4. Adsorption Parameters Deduced from Langmuir Adsorption Isotherm for Corrosion Inhibition of Aluminium.Temperature (K)R 2 K ads ΔG (kJ mol -1) 308313318 0.9943 0.9918 0.9986 19.90 18.69 18.08 -17.94 -18.07 -18.273.6 Infrared Spectroscopy Analysis ResultsBenzaldehyde as Corrosion InhibitorThe FTIR spectra of the inhibitor (benzaldehyde) and that of the corrosion product are presented in Figures 5 and 6. The analysis of the inhibitor presented in Figure 5 revealed the presence of carbonyl group C=O stretch, aromatic C-H stretch and aldehyde C-H stretch. The analysis of the corrosion product presented in Figure 6 shows the appearance of carbonyl group C=O stretch, aromatic C-H stretch and aldehyde C-H stretch and this confirmed that the adsorption of the inhibitor on the metal surface took place.Fig.5. FT-IR Spectra of BenzaldehydeFig.6. FT-IR Spectra of Aluminium in 1.4 M HCl with 0.1 M Benzaldehyde4.ConclusionFrom the result of this study it can be concluded that benzaldehyde served as good inhibitor in reducing the rate of hydrochloric acid attack on the surface of aluminium, by electrostatic interaction between the charged inhibitor molecules and the charged surface of aluminium. This make the adsorption process occurred through physical adsorption mechanism (physisorption). The calculated values of Gibbs free energy of adsorption, enthalpy and activation energy also support the physisorption mechanism of the process. The presence ofBenzaldehyde as Corrosion Inhibitorfunctional group of the inhibitor molecule in the FT-IR of the corrosion product confirmed that the adsorption of the inhibitor on the metal surface took place. The adsorption of different concentrations of the inhibitor on the surface of the aluminium in hydrochloric acid followed Langmuir adsorption isotherm.This work is only limited to the thermodyamic and kietic study on the corrosion of aluminium in hydrochloric acid using benzaldehyde as corrosion inhibitor using only weight loss method. It’s recommended that the feature work on the corrosion inhibition effect of benzaldehyde to include other methods of corrosion inhibition analysis such as gravimetric, thermometric, electrochemical impedance spectroscopy e.t.c. References[1] Abdel-Gaber, A. M., Abd-El-Nabey, B. A., Sidahmed, I. M. El-Zayady, A. M. and Saadawy, M. (2006). Kinetics and Thermodynamics of Aluminium Dissolution in 1.0 M Sulphuric Acid Containing Chloride Ions. Mater., Chem. Phys., 98: 291-297.[2] Stansbury, E. E. and Buchanan, R. A. (2000). Fundamentals of Electrochemical Corrosion, ASM International Materials Park, USA.[3] Li, X., Deng, S. and Fu, H. (2011). Inhibition by Tetradecylpyridinium Bromide of the Corrosion of Aluminium, Inhibition by Tetradecylpyridinium Bromide of the Corrosion of Aluminium in Hydrochloric Acid Solution. Corros. Sci., 53:1529-1536.[4] Musa, A. Y. Kadhum, A. A. H., Mohamad, A. B., Takriff, M. S. and Chee, E. P. (2012) Inhibition of Aluminium Corrosion by Phthalazinone Synergistic Effect of Halide Ion in 1.0 M HCl. Curr. Appl. Phys., 12, 325-330.[5] Lopez-Sesenes R, Gonzalez-Rodriguez, J. G., Casales, M., Martinez, L., and Sanchez-Ghenno, J. C. (2011). Corrosion Inhibition of Carbon Steel in 0.5M HCl by Monopropianate. Int. J. Electrochem. Sci., 6: 1772-1784.[6] Awad, H. S. and Gawad, S. A. (2005). Mechanism of inhibition of iron corrosion in hydrochloric acid by pyrimidine and series of its derivatives. Anti-Corros. Method Mater.,52: 328.[7] Chetouani, A., Aouniti, A., Hammouti, B., Benchat, N., Benhadda, T. and Kertit, S. (2003). Corrosion inhibitors for iron in hydrochloride acid solution by newly synthesized pyridazine derivatives. Corros. Sci., 45: 1675-1684.[8] Sherif, E.S.M. (2012). Electrochemical and Gravimetric Study on the Corrosion and Corrosion Inhibition of Pure Copper in Sodium Chloride Solutions by Two Azole Derivatives. International Journal of Electrochemical Science. 7: 1482-1859.[9] Fouda, A. S., Gadow, H. S. and Shalabi, K. (2015). Chemical and Electrochemical Investigations of Coffee Husk as Green Corrosion Inhibitor for Aluminum in Hydrochloric Acid Solutions. . International Journal of Innovative Research in Science. 23(1) 28-45.[10] Karthikeyan, S., Lakshmi, N.V. and Arivazhagan, N. (2013). The Corrosion Inhibition of Aluminium in 3.5% NaCl by Diisopropyl Thiourea. International Journal of Chemical Technology and Research. 5(4): 1959-1963.[11] Anand, B. (2013). Effect Formazan of Benzaldehyde as Corrosion Inhibitor on Preventing the Mild Steel Corrosion in Acidic Medium. Chemical Science Transactions. 2(4), 1126-1135.[12] Arvnabh, M., Godhani, D.R. And Sanghani, A. (2011). Diamines as corrosion inhibitors for aluminium alloy in organic acid. The Asian Journal of Experimental Chemistry. 6(1): 38-41.[13] Yadav, M., Kumar, S., Sharma, U. and Yadav, P.N. (2013). Substituted amines as corrosion inhibitors for。
氯化银提取
氯化银的还原精炼方法至少有七十多种,但可概括为两大类:液相化学还原和高温熔炼还原法。
前者如水合肼(水合联氨)、甲酸、甲醛、葡萄糖、抗坏血酸、双氧水、硼氢化钠等还原方法及铁粉、锌粉、铝等金属置换法。
后者如碳酸钠、硼砂、碳、氢气高温还原法。
下面介绍几种较常见的还原法:1、水合肼还原法水合肼是强还原剂,水合肼可以直接还原氯化银。
氨水将氯化银络合生成银氨离子进入溶液。
氨浸条件:液固比3~4:1,室温,搅拌条件下加入氨水,在PH=9左右的条件下浸出2~3小时。
过滤后,渣用氨水与碳酸铵的混合液洗涤。
经一次氨浸处理,银的浸出率可达97%~98%。
向氨浸液中加入水合肼,还原条件:温度50摄氏度,水合肼用量为理论量的2~3倍,人工或机械搅拌下缓慢加入水合肼,30分钟左右即可。
还原率大于99%2、甲醛还原法在碱性溶液中,氯化银可被甲醛还原为金属银。
在搅拌下,用氢氧化钠调节氯化银水悬料PH至11,缓慢加入甲醛。
甲醛还原氯化银为放热反应,反应速度快。
必须用水反应洗涤海绵银中过剩的甲醛,以免熔炼铸锭时甲醛挥发中毒。
海绵银熔炼铸锭纯度大于99%3、硼氢化钠还原法用氢氧化钠调节氯化银水悬料PH至12.5,在60~80摄氏度时加入3%硼氢化钠,所得海绵银纯度大于99.9%4、锌粉还原向氯化银悬浮料中加入浓硫酸,到硫酸浓度约5%,边搅拌边向氯化银水悬浮料中缓慢加入锌粉,至无白色氯化银为止。
待反应完成后,再用5%稀硫酸溶解过剩的锌粉,用水反复洗涤PH到7。
1KG锌粉可还原4KG海绵银,得到粗银纯度为99.5%,锌置换母液含银0.0001g/L。
5、铁还原将氯化银放入容器中,并把铁片埋于氯化银沉淀中,再加浓盐酸淹盖,然后加热至沸腾,同时搅拌,至白色氯化银沉淀全变成灰色的银粉为止。
取出残余铁片,再用稀盐酸溶解残余的铁,用水反复洗涤PH到7。
海绵银熔炼铸锭纯度大于99%6、碳酸钠熔炼还原将干的氯化银和碳酸钠混合,氯化银:碳酸钠=1:0.4。
臭氧消毒浓度和条件
臭氧消毒浓度和条件尽管在中国许多公司使用臭氧对洁净室进行消毒,许多业内人士对其消毒效果也将信将疑。
本文汇总了国内外不同法规/指南对臭氧消毒浓度及其条件的要求,供大家参考:消毒技术规X和GB 28232《臭氧发生器安全与卫生标准》空气消毒:臭氧对空气中的微生物有明显的杀灭作用,采用 20mg/m3 浓度的臭氧,作用 30min,对自然菌的杀灭率达到90% 以上。
表面消毒:用臭氧气体消毒,臭氧对物品表面上污染的微生物有杀灭作用,但作用缓慢,一般要求 60mg/m3 ,相对湿度≥70%,作用 60 min~120min 才能达到消毒效果。
验证指南消毒时关闭相应的新风进口和回风排放阀门,使整个被消毒的洁净区空气通过净化系统风管形成循环,臭氧发生器即开始工作。
如每日做空气灭菌,一般可开机1~1.5h;如每周以臭氧代替化学试剂熏蒸对物体表面、墙壁、地面及设备灭菌,一般可开机2~2.5h。
对空气中浮游菌,臭氧灭菌浓度为(2~4)×10^-6;对物体表面的沉降菌,为(10~15)×10^-6设计、应用臭氧灭菌60min 达到相对浓度后,继续保持一段时间(1~1.5h),即可达到对机器设备和建筑物体表面沉降菌杀灭的目的。
PDA TR 70 无菌生产设施的清洁消毒程序原理用气体处理小X围或大规模操作可选的另一种方式是使用臭氧。
臭氧是通过氧气加高电压制成。
该系统使用了高浓度的臭氧气体,集成一个气体发生器向待消毒区域内释放臭氧。
该系统的设计规X通常为臭氧浓度200ppm或更高(注释:臭氧1ppm≈2mg/m3, 200ppm≈400mg/m3),相对湿度80%或更高,处理时间取决于区域的大小,自身的生物负载和区域内的障碍物情况。
这个系统已经在多个产业环境内使用,并且现在正在被考虑作为GMP操作中可能的备选。
每当化学剂用于大规模气体处理或雾化处理洁净室时,必须考虑安全性。
如果未采用正确的防X措施来保证化学消毒剂被遏制在拟处理区域X围内,那么所讨论的所有消毒剂都能够导致人员的伤害或死亡。
