北京市朝阳区2014届高三下学期第一次综合练习( 化学试题
2014届北京市朝阳区高三年纪第一次综合练习

2014届北京市朝阳区高三年纪第一次综合练习英语试卷 2014.3第二部分知识运用(共两节,45分)第一节单项填空21. In China, you _____ vote when you are 18.A. mustB. mightC. canD. would22. I’ve been away for only three years, _____I can hardly recognize my hometown.A. soB. orC. forD. yet23. This article is well written because special attention ______ to the choice of words.A. has paidB. has been paidC. has paidD. had been paid24. They always give the vacant seat to ____ comes first.A. anyoneB. whomC. whomeverD. whoever25. In some countries, friends kiss____ both checks when they say goodbye.A. onB. inC. atD. of26. It is hard to imagine ____ makes a top-level athlete.A. whichB. howC. thatD. what27. The movie Gravity won seven awards at the Oscar night, ____was beyond t he director’s wildest dream.A. whichB. thatC. whereD. what28. ---Did he get the job finally?---Though he ____ well before the gob interview, he failed to answer some important questions.A. Was preparingB. would prepareC. had preparedD. has prepared29. When ____ about one of the biggest concerns today, many citizens mentioned the sharp rise inhousing price.A. askingB. to askC. askedD. having asked30. _____time, the teacher had us do half of the exercises in class and complete the other half forour homework.A. To saveB. SaveC. SavingD. Having saved31. Remember me to your parents when you ____them.A. are callingB. callC. will callD. have called32. ---What’s that noise?---There’s a truck stopping outside. It’s someone ____ something.A. deliverB. to deliverC. deliveringD. delivered33. Quietness seems impossible _____ everyone takes their seats and begins to write.A. untilB. afterC. forD. once34. ---How long have you been here, Susan?---Only about five minutes. Alisa ____ here with me.A. walksB. walkedC. has walkedD. had walked35. I didn’t know your mobile phone number; otherwise I ____you the moment I got to Canada.A. would ringB. would have rungC. had rungD. rang第二节完型填空Wallet BackThis is a story about a learning experience that had a big effect on the way that I live my life. The ____36___in the story did not give me tests or even grade me on my work. I was taught by one of the most effective ___37___ of teaching, one that only people with lots of love can do.“My wallet! Where is it?” were my first words___38___ I found my wallet was missing. I ____39___ my memory for a few good seconds, then realized that I had left my precious wallet in the library’s public ___40___!Because the library was now __41__, I had to wait until the next morning to look for it. When I got there the next day, all I found was a clean restroom. This was the first time I could remember ever ___42___ to see a clean restroom. As I walked out, I looked at myself in the mirror and shook my head at the ___43____ fool in front of me.I politely ___44____ the librarian at the front desk and asked her if a wallet had been found in the restroom yesterday. “No.” That was that. I walked off with a sense of ____45____.I __46___ what I would do if I had found a wallet containing sixty dollars, a phone card and many other irreplaceable ___47____things. Finally, I ___48___ accepted the fact that my wallet was gone.A week later, I received a ___49____ in the mail. it was my wallet And most ___50____, nothing was missing!But there was a little yellow sheet of paper folded up in one of the wallet pockets that had not been there before. I slowly unfolded the paper, which ___51____something like this:When we continue to help around, we will live in a larger and more rewarding world.This person didn’t even leave a return address. So I couldn’t ___52___whoever it was. From that day on, I __53__ myself that I will follow this ___54___ and help others and make them as ___55__ as I was when I opened that parcel!36. A. leader B. writer C. teacher D. scholar37. A. aids B. systems C. materials D. methods38. A. once B. when C. before D. since39. A. searched B. improved C. developed D. recorded40. A. canteen B. lounge C. restroom D. showroom41. A. closed B. deserted C. crowded D. occupied42. A. hoping B. hating C. refusing D. preferring43. A. grateful B. curious C. forgetful D. nervous44. A. approached B. interviewed C. identified D. reminded45. A. safety B. forgiveness C. satisfaction D. disappointment46. A. described B. wondered C. discovered D. expressed47. A. personal B. strange C. surprising D. reasonable48. A. gladly B. naturally C. willingly D. painfully49. A. letter B. note C. package D. suitcase50. A. sincerely B. amazingly C. honestly D. obviously51. A. read B. printed C. explained D. wrote52. A. owe B. thank C. admire D. inspire53. A. supported B. suggested C. improved D. promised54. A. plan B. course C. regulation D. example55. A. shocked B. cautions C. delighted D. optimistic第三部分:阅读理解(共两节,40分)第一部分AJohnny the ExplorerJohnny was three when he ran away from home for the first time. Somebody left the garden gate open. Johnny wandered out, crossed some fields, and two hours later, and arrived in the next village. He was just able to give his name and address.By the time he was seven, Johnny used to vanish from home two to three times a year. Sometimes he covered quite long distances on foot. On other occasions he got on a bus or even a train, and simply sat there until someone asked for his ticket. Generally the police brought him home. "Why do you do it?" they used to ask. "You aren't unhappy at home, are you? .... ““Of course not," Johnny replied.” Then why?" "I just like seeing places," Johnny told them.Johnny continued to "see places" although everyone tried to stop him. His parents used to watch him closely, and so did his teachers; but sooner or later Johnny managed to slip away. As he grew older, his favorite trick was to hide on a long distance truck. Sometimes he used to travel hundreds of miles before anyone discovered him.It is hardly surprising that eventually Johnny managed to get on board a plane. He was twelve at the time. It was a cargo plane and, a few hours later, Johnny found himself in Cairo. How did he get on board? No one knows! According to Johnny himself, it was easy: he just went into the airport, walked along some corridors and got on board the nearest plane.In spite of all this, Johnny did well at school. He enjoyed maths and languages and, perhaps not surprisingly, he was especially good at geography. "What do you want to be when you grow up?" his teachers asked him. Johnny did not take long to answer that question. "An explorer!" he answered. "But it's difficult to become an explorer in this modem age." they tried to tell him, But it was no use: Johnny knew what he wanted!Just before he left school, Johnny saw a notice in one of the daily papers. An expedition was about to go to Brazil to travel up the Amazon River. There were vacancies for three young people "willing to work hard and with a sense of adventure". Johnny applied, and, two months later, he was on his way to Brazil.56. Johnny frequently left home because_________.A. he preferred to stay homeB. he enjoyed seeing new placesC. he couldn’t do well at schoolD. he didn’t get along well with his parents57. People around Johnny_______.A. tried to stop him from slipping awayB. kept following him to get him backC. booked tickets for him if necessaryD. were closer about how he traveled abroad58. From the passage, we can learn that _______.A. Johnny worked for a daily paperB. Johnny lacked a sense of adventureC. Johnny went exploring along the Amazon RiverD. Johnny went to Brazil months after he finished school.BEditor Henry Slocum,I read your May 10th article in the local newspaper Honesdale Times about electronic books, or e-books, with great interest. You made several good points about the disadvantage of e-books. You may have overlooked, however, some of the ways in which they are superior to traditional books. Yes, e-books are expensive, but they are also convenient. In addition, duo to their environment-friendly nature, e-books have the potential to change our planets for the better.E-books, for anyone who is unfamiliar with the term, are about the same size and shape as regular books. They have a large screen in the middle, however, this screen shows the reader a page of the text that has been downloaded from a computer. Once the reader has finished reading the page on the e-book screen, he or she scrolls down to see a new page. The process continues until the entire book has been read.As you pointed out, Mr. Slocum, it’s great to lie on a warm, sandy beach with a book. You can do that just as easily with an e-book as you can with a traditional paper book. In fact, because e-books are so light, you can carry themselves. Say, for example, that you like to read on the bus. Which would you rather carry with you---a heavy 800-page novel, or an e-book that weighs only a few ounces?Another important advantage is offered by e-books as well. They are more environment-friendly than traditional books. At present, thousands of trees cut down each year to meet the publishing industry’s demand for paper. Books that don’t sell are eventually returned to the publisher and destroyed, This terrible waste could be avoided if everyone used e-books, which require no paper.Sincerely,David Eng59. What does Editor Henry Slocum think about e-books?A. InterestingB. ExpensiveC. ConvenientD. Environment-friendly60. What is mainly talked about in Paragraph 2?A. What an e-book isB. Why e-books are popularC. How e-books have developedD. Who are the readers of e-books61. In David’s letter, he thinks that_______A. E-books will come down in priceB. E-books come in various sizes and shapesC. E-books do little harm to the environmentD. E-books are no better than traditional books62. The main purpose of the letter is to ________A. explain how to read e-booksB. honor the person who invented e-booksC. scold publishers for wasting so much paperD. provide evidence that e-books are a good ideaCLosing weight comes with a lot of health benefits---including make your brain sharper.Yes, it turns out that overweight may damage cognitive functions such as memory and attention. There have been few studies of overweight and cognitive functioning, possibly because it is generally believed that it is not a primary risk cause for poor cognitive performance. Losing weight, therefore, may help improve these mental functions, according to a new research led by John Gunstad, assistant professor of psychology at Kent State University.Growing evidence suggests that being fat is linked to cognitive deficits(缺陷). So Gunstad and his team guessed that losing weight might improve mental function. For their study, they measured memory and attention in a group of 150 overweight participants, some of whom had some king of operation for weight loss and some did not. All of the volunteers completed mental skills tests to access their abilities if memory and attention at the beginning of the study, and again 12 weeks later. To begin with, about 24%of the patients showed damaged learning and 23%showed signs of poor memory when tested. At the end of the study, those who had lost weight after operation improved their scores into the average or above average for cognitive functions. Scores for the volunteer who didn’t lose weight dropped even further.The study helped, Gunstad to find out whether losing weight had any effect on mental function. Now that he’s been the positive effect that weight loss can have one memory and attention, he says he will next study those who choose to lose weight by the traditional way—eating healthier and getting more active. He expects that losing weight in this way will have a similarly positive effect on the brain. “If we can improve the condition with operation, then we can probably produce the same change with behavioral weight loss as well.” He says.63. There is less research on overweight and cognitive functions because researchers________.A. believe overweight only affects our bodyB. have focused on ways to sharpen people’s mindC. do not consider overweight a main cause for low cognitive abilityD. are clear about the relation between weight and mental functions64. The result of Gunstad’s study shows that_______A. losing weight has little effect on people’s memoryB. Losing weight can improve people’s mental functionsC. overweight people are likely to have psychology problemD. overweight people’s abilities of concentration differ greatly65. What is Gunstad planning to prove next in his research?A. Slim people are smarter than overweight peopleB. Healthy diet is better than exercise in losing weightC. Traditional ways of losing weight are better than operationD. Overweight people will get smarter by taking more exercise66. Which of the following is the best title for the text?A. Body Weight and HealthB. Losing Weight by OperationC. Ways to Improve Mental FunctionsD. Losing Weight to Sharpen Your MindDSeeing in StereoHave you wondered why you have two eyes and why they are set close together on the front of your face? The reasons are simple and important to the way you see the rest of your world.Your eyes are like two small cameras. A camera catches an image of an object and records it on film. Similarly, when you look at something, each eye takes in what it sees and sends this image to the back of the eyeball. Because your eyes are set close together, they view the world from about the same height but from slightly different angles. Working as a team, they eyes send the images to your brain, which puts them together into a single, centered image.Seeing in stereo means seeing with two eyes working together, which is called stereoscopic sight. This allows you to view the world in three dimensions, or 3-D. Seeing depth helps you to judge the distance between you and the objects you see and to adjust to the changing angle at which you see something as you move closer to or farther away from it. If images are coming from only one eye, however, only two of these dimensions—height and width---can be perceived.A world seen with one eye is thus two-dimensional, as in a photograph.Now consider why your two eyes are on the front of your face. Think of other animals with the same arrangement. They are all animal that hunt, like lions and wolves. Their eyes face directly in front of them. This provided a field of sight about 180 degree wide, which is called binocular sight. On the other hand, animals that are hunted have eyes on the sides of the head, which provides nearly 360-degree field of sight. In order to stay alive, they need to see things coming from the sides and from behind. However, without stereoscopic sight, they have difficulty determining how far a danger is.With sight both stereoscopic and binocular, human share with animal hunters the ability to see from side to side and to accurately determine the distance. If you think it would be great to have another type of sight, perhaps with hundreds of tiny eyes like many insects do, think again! Each tiny insect eye sees only a tiny part. Besides, what if you needed glasses? Be glad for the eyesight that you have.67. According to the passage, an eye is like a camera because both_______A. can record imagesB. can imagine objectsC. provide centered imagesD. work at the same height68. Stereoscopic sight is a result of having_________.A. a three-hundred-sixty-degree field of sightB. hundreds of eyes, all seeing parts of an imageC. two eyes close to one another that work togetherD. one eye on either side of the head, each seeing a different image69. The underlined word “perceived” in paragraph 3means________.A. setB. takenC. seenD. understood70. The author implies that “seeing in stereo”________.A. is similar to the way camera workB. is an ability humans are fortunate to haveC. would be better for a wider field of sightD. helps people to have better sight than animals第二节Puppy FoodA puppy (young dog) is a precious addition to any family. The excitement of bringing home a little furry friend will always live in our memories. Like all of us, though, your puppy must have adequate nutrition right from the start to make cer tain that it’ll have a long and healthy life.____71____You might ask yourself what is the best for your new pet with all of the different varieties on the market in all of their attractive packaging. Some dry food produced especially for puppies is the best for the development of their teeth. _____72_____Contrary to the belief of many first time puppy owners, it is not always the best idea to purchase food that is too high in calcium, protein, and vitamin levels. _____73_____In addition, a high intake of calcium is associated with bone disease in large-breed dogs. If you have a small-breed puppy, you can buy that kind of food.It is not a good idea to feed the food you eat to your puppy frequently, as your puppy may become selective about food. ___74____Some people think that dog biscuits and other treats are fine, but they should not be a main part of puppy’s diet.Young puppies should be fed three times a day. Each puppy is unique, however, so feeding them twice a day is acceptable. After ten to twelve weeks of age, feed your puppy twice per day. Allow your puppy to cat as much as he would like in fifteen minutes. ____75____If you let your puppy eat too much or too often by keeping food accessible at all time, he may become overweight and have health problem as an adult. Like humans, your puppy will not enjoy the food any longer if it is there at all times.A.Pick up the dish with the remaining foodB.Proper food id a basic necessity for your puppiesC.Remember to give your puppy the food he would like to haveD.It can also cause an upset stomach due to an unbalanced dietE. A further benefit is that it is less expensive than the canned foodF.One example is that extra helpings of nutrients do harm to digestive organsG.It’s good to provide the extra nutrients a puppy n eeds until he grows larger.第四部分:书面表达(共两节,35分)第一节你的英国朋友Tim打算暑假来北京学习汉语,来信向你咨询你校国际版的汉语课程。
北京市朝阳区高中示范校2014_2015学年度高二化学第一学期综合测试卷3选修4

北京市朝阳区高中示范校2014-2015学年度第一学期高二化学选修4综合测试卷 3(试卷满分为100分,考试时间为100分钟)第Ⅰ卷(选择题共50分)可能用到的相对原子质量:H:1 N:14 O:16 Na:23 S:32 Cl:35.5 Zn:65 Cu:64选择题(每小题只有一个选项符合题意,每小题2分,共50分)1.下列物质中属于强电解质的是A.NH3·H2O B.H2O C.CH3COOH D.醋酸铵2.有关化学反应的说法中,正确的是A.自发反应都是放热反应B.自发反应都是熵增大的反应C.能自发进行的吸热反应一定是熵增大的反应D.非自发反应在任何条件下都不能实现3.下列与化学反应能量变化相关的叙述正确的是A.生成物能量一定低于反应物总能量B.放热反应的反应速率总是大于吸热反应的反应速率C.应用盖斯定律,可计算某些难以直接测量的反应焓变D.同温同压下,H2(g) + Cl2(g) = 2 HCl(g)在光照和点燃条件下的ΔH不同4.“嫦娥奔月”是一个充满浪漫主义的中国神话故事。
2007年10月24日我国“嫦娥一号”探月卫星由长三甲火箭送入预定的轨道。
长三甲火箭第三级推进剂采用低温液氧/液氢。
已知在298K时,2g氢气与氧气完全反应生成液态水放热285.8kJ,则此反应的热化学方程式为A.2H2(g)+O2(g) === 2H2O(l) △H=-285.8KJ·mol-1B.2H2(g)+O2(g) === 2H2O(l) △H=+285.8KJ·mol-1C.H2(g)+O2(g) === H2O(l) △H=-285.8KJ·mol-1D.H2(g)+ O2(g) === H2O(g) △H=-285.8KJ·mol-15.工业上制备纯硅反应的热化学方程式如下:SiCl4(g)+2H2(g)=Si(s)+4HCl(g);ΔH = + Q kJ/m ol(Q>0)某温度、压强下,将一定量反应物通入密闭容器进行以上反应(此条件下为可逆反应),下列叙述正确的是A.反应过程中,若增大压强能提高SiCl4的转化率B.若反应开始时SiCl4为1 mol,则达平衡时,吸收热量为Q kJC.反应至4 min时,若HCl浓度为0.12 mol/L,则H2的反应速率为0.03 mol/(L·min) D.当反应吸收热量为0.025Q kJ时,生成的HCl通入100 mL 1 mol/L的NaOH溶液恰好反应6.下列电离方程式中书写正确的是A.NaHSO4 Na++H++SO42- B.NaHCO3 Na++H++CO32-C.HClO = H++ClO- D.H2S H++HS-;HS- H++S2-7.合成氨所需氢气可由煤和水反应制得,其中一步反应为CO(g)+H2O(g) 错误!未找到引用源。
北京市朝阳区示范性高中校2014—2015学年度第一学期高一化学期中复习练习卷 3 Word版含答案

北京市朝阳区示范性高中校2014—2015学年度第一学期高一化学期中复习练习卷 3可能用到的相对原子质量:H ~1 C~12 O~16Na~23Cl~35.5K~39 S~32 N~14 Mg~24 Zn~65第一卷选择题(每题2分,共50分)一、选择题(每小题只有一个正确答案)1.胶体区别于其他分散系的本质特征是()A.胶体的分散质能通过滤纸空隙,而浊液的分散质不能B.产生丁达尔现象C.分散质粒子直径在1nm~100nm之间D.胶体在一定条件下能稳定存在2.以下过滤操作中,不正确的是()A.蒸发时用玻璃棒不断搅动B.过滤时,玻璃棒与三层滤纸的一边接触C.分液时,先将上层液体倒出D.将浓硫酸慢慢注入盛有水的烧杯中进行稀释3. 关于用CCl4萃取碘水的说法中不正确的是()A.碘在CCl4中的溶解度大于在水中的溶解度B.萃取后水层颜色变浅C.萃取后CCl4溶液层为紫红色D.萃取后水层颜色变红且在下层4.过滤后的食盐水仍含有可溶性的CaCl2、MgCl2、Na2SO4 等杂质,通过如下几个实验步骤,可制得纯净的食盐水:①加入稍过量的Na2CO3溶液;②加入稍过量的NaOH溶液;③加入稍过量的BaCl2 溶液;④滴入稀盐酸至无气泡产生;⑤过滤,正确的操作顺序是()A.③②①⑤④ B.①②③⑤④C.②③①④⑤ D.③⑤②①④5.下列电离方程式错误的是()A.NaHCO3 = Na+ + H+ + CO32-B.NaHSO4 = Na ++ H+ + SO42-C.H2SO4 = 2H+ + SO42-D.KClO3 = K+ + ClO3-6.实验室有三瓶失去标签的试剂,分别是Na2CO3、NaCl、AgNO3,实验员选择了一种试剂就把它们区别开来了,这种试剂是( )A.盐酸B.氢氧化钠溶液C.氯化钡溶液D.硝酸7.下列各物质中含氢原子数最多的是()A.1mol NH4C1 B.1.5mol NH4NO3C.1.204×1024 CO(NH2)2D.1mol NH3·H2O8.在物质分类中,前者包括后者的是()A.氧化物、化合物B.化合物、电解质C.溶液、胶体D.溶液、分散系9.设N A 表示阿伏加德罗常数,下列叙述中不正确的是( ) A. 常温常压下,11.2L 氧气所含的原子数为N AB .1.8g 的NH 4+离子中含有的电子数为N AC .常温常压下,48gO 3含有的氧原子数为3N AD .2.4g 金属镁变为镁离子时失去的电子数为0.2N A10.与50 mL 0.1 mol· L -1 Na 2CO 3溶液中Na +的物质的量浓度相同的溶液( ) A. 50mL 0.2mol·L -1 的 NaCl 溶液 B. 100mL 0.1mol·L -1的 NaCl 溶液 C. 25mL 0.2mol·L -1 的 Na 2SO 4 溶液 D. 10mL 0.5mol·L -1 的 Na 2CO 3 溶液11.将5mol/L 的Mg (NO 3)2溶液a mL 稀释至b mL ,稀释后溶液中NO 3-的物质的量浓度为( ) A .b a 5mol/L B .b a 10mol/L C .a b 5mol/L D .ba mol/L12.已知1.505×1023个X 气体分子的质量为8g ,则X 气体的摩尔质量是( ) A .16g B .32g C .64g /mol D .32g /mol13.下列物质能导电的是( )A .硫酸铜晶体B .CO 2C .熔融状态下的氯化钾D .无水乙醇14.将饱和FeCl 3溶液分别滴入下列液体中,能形成胶体的是( ) A. 冷水 B. 沸水 C. NaOH 溶液 D. NaCl 溶液15.下列关于电解质的判断中,正确的观点是( ) A .在熔融状态下能导电的物质 B .在水溶液中能导电的物质C .在水溶液中或熔融状态下能导电的化合物D .在熔融和溶液的状态下都能导电的化合物16.下列反应的离子方程式书写正确的是( ) A.氯化铜溶液与铁粉反应:Cu 2++Fe=Fe 2++Cu B.稀 H 2SO 4与铁粉反应:2Fe+6H +=2Fe 3++3H 2↑C.氢氧化钡溶液与稀 H 2SO 4 反应:Ba 2++SO 42-=BaSO 4↓D.碳酸钙与盐酸反应:CO 32-+2H +=H 2O+CO 2↑17.能用H ++OH -=H 2O 来表示的化学反应是( )A. 氢氧化镁和稀盐酸反应B. Ba(OH)2溶液滴入稀硫酸中C. 澄清石灰水和稀硝酸反应D. 二氧化碳通入澄清石灰水中18.有四位同学分别对四种溶液中所含的离子进行检测,所得结果如下,其中所得结果错误的是( ) A .K +、Na +、Cl -、NO 3- B .OH -、CO 32-、Cl -、K + C .Ba 2+、Na +、OH -、NO 3- D .Cu 2+、NO 3-、OH -、Cl -19.将标准状况下,将VL A 气体(摩尔质量为Mg/mol )溶于0.1L 水中,所得溶液密度为ρg/cm 3,则此溶液的物质的量浓度(mol/L )为( )A .)2240(+MV V ρ B .)2240(1000+MV V ρC .ρ)1.0(4.22+V MVD .100VρM (MV+2240)20.某位同学配制一定物质的量浓度的NaOH 溶液时,造成所配溶液浓度偏高的原因是( ) A .所用NaOH 已经潮解 B .向容量瓶中加水未到刻度线 C .有少量NaOH 溶液残留在烧杯里D .用带游码的托盘天平称2.4gNaOH 时误用了“左码右物”方法21.浓度为0.50 mol ·L -1的某金属阳离子M n+的溶液10.00 mL ,恰好与0.40 mol ·L -1的NaOH 溶液12.50 mL 完全反应,生成沉淀,则n 等于( ) A.1 B.2 C.3 D.422.欲配制100ml 1.0 mol/L Na 2SO 4溶液,正确的方法是( ) ① 将14.2 g Na 2SO 4 溶于100ml 水中② 将32.2g Na 2SO 4·10H 2O 溶于少量水中,再用水稀释至100 ml ③ 将20 ml 5.0 mol/L Na 2SO 4溶液用水稀释至100 ml A .①② B .②③ C .①③ D .①②③23.在无土栽培中,需配制一定量含50 mol NH 4Cl; 6 mol KCl 和24 mol K 2SO 4的营养液。
【2014朝阳一模】北京市朝阳区2014届高三第一次综合练习 数学理试题 Word版含答案
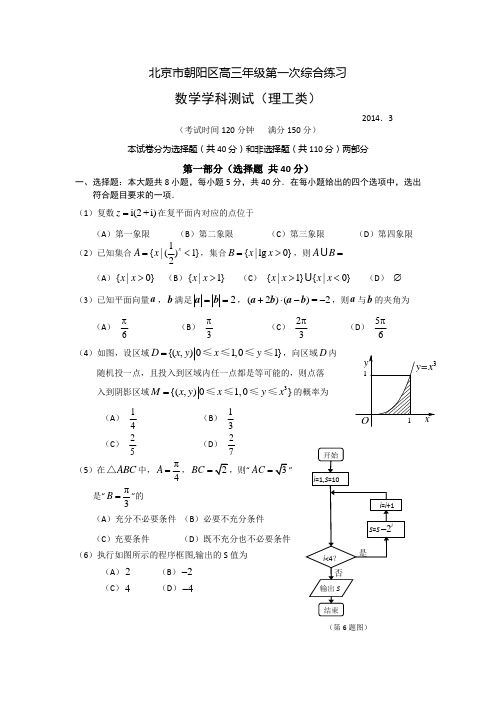
(第6题图)北京市朝阳区高三年级第一次综合练习数学学科测试(理工类)2014.3(考试时间120分钟 满分150分)本试卷分为选择题(共40分)和非选择题(共110分)两部分第一部分(选择题 共40分)一、选择题:本大题共8小题,每小题5分,共40分.在每小题给出的四个选项中,选出符合题目要求的一项. (1)复数i(2+i)z =在复平面内对应的点位于(A )第一象限 (B )第二象限 (C )第三象限 (D )第四象限 (2)已知集合1{|(1}2xA x =<,集合{|lg 0}B x x =>,则AB =(A ){|0}x x > (B ){|1}x x > (C ) {|1}{|0}x x x x >< (D ) ∅ (3)已知平面向量a ,b 满足2==a b ,(2)()=2⋅--a +b a b ,则a 与b 的夹角为(A )6π (B ) 3π(C )32π (D ) 65π(4)如图,设区域{(,)01,01}D x y x y =≤≤≤≤,向区域D 内随机投一点,且投入到区域内任一点都是等可能的,则点落 入到阴影区域3{(,)01,0}M x y x y x =≤≤≤≤的概率为(A )14(B )13(C ) 25 (D ) 27(5)在ABC △中,π4A =,BC =“AC ”是“π3B =”的(A )充分不必要条件 (B )必要不充分条件 (C )充要条件 (D )既不充分也不必要条件 (6)执行如图所示的程序框图,输出的S 值为(A )2 (B )2- (C )4 (D )4-(7)已知函数2sin ()1xf x x =+.下列命题: ①函数()f x 的图象关于原点对称; ②函数()f x 是周期函数; ③当2x π=时,函数()f x 取最大值;④函数()f x 的图象与函数1y x=的图象没有公共点,其中正确命题的序号是(A ) ①③ (B )②③ (C ) ①④ (D )②④(8)直线y x m =+与圆2216xy +=交于不同的两点M ,N ,且3MN O M O N ≥+,其中O 是坐标原点,则实数m 的取值范围是 (A )(2,22⎡-⎣ (B )(22,4⎡--⎣(C) [2,2]- (D ) [-第二部分(非选择题 共110分)二、填空题:本大题共6小题,每小题5分,共30分.把答案填在答题卡上. (9)在各项均为正数的等比数列{}n a 中,12a =,2312a a +=,则该数列的前4项和为 .(10)在极坐标系中,A 为曲线2cos ρθ=上的点,B 为曲线cos 4ρθ=上的点,则线段AB 长度的最小值是 .(11)某三棱锥的三视图如图所示,则这个三棱锥的体积为 ;表面积为 .(12)双曲线2221(0)y x b b-=>的一个焦点到其渐近线的距离是2,则b = ;此双曲线的离心率为 .(13)有标号分别为1,2,3的红色卡片3张,标号分别为1,2,3的蓝色卡片3张,现将全部的6张卡片放在2行3列的格内 (如图).若颜色相同的卡片在同一行,则不同的放法种数为 .(用数字作答)正视图俯视图(14)如图,在四棱锥S ABCD -中,SB ⊥底面ABCD .底面ABCD 为梯形,AB AD ⊥,AB ∥CD ,1,3AB AD ==,2CD =.若点E 是线段AD 上的动点,则满足90SEC ∠=︒的点E 的个数是 .三、解答题:本大题共6小题,共80分.解答应写出文字说明,演算步骤或证明过程. (15)(本小题满分13分)已知函数22()2sin()cos sin cos f x x x x x =π-⋅+-,x ∈R . (Ⅰ)求()2f π的值及函数()f x 的最小正周期; (Ⅱ)求函数()f x 在[]0,π上的单调减区间.(16)(本小题满分13分)某单位从一所学校招收某类特殊人才.对20位已经选拔入围的学生进行运动协调能力和逻辑思维能力的测试,其测试结果如下表:例如,只知道从这20位参加测试的学生中随机抽取一位,抽到运动协调能力或逻辑思维能力优秀的学生的概率为25. (I )求a ,b 的值;(II )从参加测试的20位学生中任意抽取2位,求其中至少有一位运动协调能力或逻辑思维能力优秀的学生的概率;(III )从参加测试的20位学生中任意抽取2位,设运动协调能力或逻辑思维能力优秀的学生人数为ξ,求随机变量ξ的分布列及其数学期望E ξ.BCDESA(17)(本小题满分14分)如图,四棱锥P ABCD -的底面为正方形,侧面PAD ⊥底面ABCD .PAD △为等腰直角三角形,且PA AD ⊥. E ,F 分别为底边AB 和侧棱PC 的中点.(Ⅰ)求证:EF ∥平面PAD ; (Ⅱ)求证:EF ⊥平面PCD ; (Ⅲ)求二面角E PD C --的余弦值.(18)(本小题满分13分)已知函数21()ln 2f x ax x =-,a ∈R . (Ⅰ)求函数()f x 的单调区间;(Ⅱ)若函数()f x 在区间[1,e]的最小值为1,求a 的值.(19)(本小题满分14分)已知椭圆2222:1(0)x y C a b a b +=>>经过点(Ⅰ)求椭圆C 的方程;(Ⅱ)直线(1)(0)y k x k =-≠与椭圆C 交于,A B 两点,点M 是椭圆C 的右顶点.直线AM 与直线BM 分别与y 轴交于点,P Q ,试问以线段PQ 为直径的圆是否过x 轴上的定点?若是,求出定点坐标;若不是,说明理由.(20)(本小题满分13分)从1,2,3,,n 中这n 个数中取m (,m n *∈N ,3m n ≤≤)个数组成递增等差数列,所有可能的递增等差数列的个数记为(,)f n m .(Ⅰ)当5,3n m ==时,写出所有可能的递增等差数列及(5,3)f 的值; (Ⅱ)求(100,10)f ;(Ⅲ)求证:()(1)(,)2(1)n m n f n m m -+>-.A E BCDPF北京市朝阳区高三年级第一次综合练习数学答案(理工类) 2014.3三、解答题15. (本小题满分13分) 解: ()f x =sin 2cos 2x x -)4x π=-.(Ⅰ)())12242f πππ=⋅-==.显然,函数()f x 的最小正周期为π. …………… 8分 (Ⅱ)令ππ3π2π22π242k x k +-+≤≤得 37ππππ88k x k ++≤≤,k ∈Z .又因为[]0,πx ∈,所以3π7π,88x ⎡⎤∈⎢⎥⎣⎦. 函数()f x 在[]0,π上的单调减区间为3π7π,88⎡⎤⎢⎥⎣⎦. …………… 13分 16. (本小题满分13分)解:(I )设事件A :从20位学生中随机抽取一位,抽到运动协调能力或逻辑思维能力优秀的学生.由题意可知,运动协调能力或逻辑思维能力优秀的学生共有(6)a +人. 则62()205a P A +==. 解得 2a =.所以4b =. …………… 4分(II )设事件B :从20人中任意抽取2人,至少有一位运动协调能力或逻辑思维能力优秀的学生.由题意可知,至少有一项能力测试优秀的学生共有8人.则21222062()1()195C P B P B C =-=-=. …………… 7分(III )ξ的可能取值为0,1,2.20位学生中运动协调能力或逻辑思维能力优秀的学生人数为8人.所以21222033(0)95C P C ξ===,1112822048(1)95C C P C ξ===,2822014(2)95C P C ξ===.所以ξ的分布列为所以,0E ξ=⨯33951+⨯48952+⨯1495764955==. …………… 13分17. (本小题满分14分)(Ⅰ)证明:取PD 的中点G ,连接FG ,AG .因为F ,G 分别是PC ,PD 的中点, 所以FG 是△PCD 的中位线. 所以FG ∥CD ,且12FG CD =. 又因为E 是AB 的中点,且底面ABCD 为正方形,所以1122AE AB CD ==,且AE ∥CD . 所以AE ∥FG ,且AE FG =. 所以四边形AEFG 是平行四边形. 所以EF ∥AG .又EF ⊄平面PAD ,AG ⊂平面PAD ,AE BCDPFG所以EF平面PAD . ……………4分(Ⅱ)证明: 因为平面PAD ⊥平面ABCD ,PA AD ⊥,且平面PAD 平面ABCD AD =, 所以PA ⊥平面ABCD . 所以PA AB ⊥,PA AD ⊥.又因为ABCD 为正方形,所以AB AD ⊥, 所以,,AB AD AP 两两垂直.以点A 为原点,分别以, , AB AD AP 为, , x y z 轴, 建立空间直角坐标系(如图). 由题意易知AB AD AP ==, 设2AB AD AP ===,则(0,0,0)A ,(2,0,0)B ,(2,2,0)C ,(0,2,0)D ,(0,0,2)P ,(1,0,0)E ,(1,1,1)F .因为(0,11)EF =,,(022)PD =-,,,(200)CD =-,,, 且(0,11)(0,2,2)0EF PD ⋅=⋅-=,,(0,11)(2,00)0EF CD ⋅=⋅-=,,所以EF PD ⊥,EF CD ⊥.又因为PD ,CD 相交于D ,所以EF ⊥平面PCD . …………… 9分 (Ⅲ)易得(102)EP =-,,,(0,22)PD =-,.设平面EPD 的法向量为(, , )x y z =n ,则0,0.EP PD ⎧⋅=⎪⎨⋅=⎪⎩n n 所以 20,220. x z y z -+=⎧⎨-=⎩即2,. x z y z =⎧⎨=⎩令1z =,则(2,1,1)=n .由(Ⅱ)可知平面PCD 的法向量是(0,11)EF =,, 所以cos ,2EF EF EF⋅〈〉===⋅n n n .由图可知,二面角E PD C --的大小为锐角,所以二面角E PD C --的余弦值为3. ……………14分 18. (本小题满分13分)解:函数()f x 的定义域是(0,)+∞, 1()f x ax x '=-21ax x-=.(Ⅰ)(1)当0a =时,1()0f x x'=-<,故函数()f x 在(0,)+∞上单调递减. (2)当0a <时,()0f x '<恒成立,所以函数()f x 在(0,)+∞上单调递减.(3)当0a >时,令()0f x '=,又因为0x >,解得x =①当x ∈时,()0f x '<,所以函数()f x 在单调递减.②当)x ∈+∞时,()0f x '>,所以函数()f x 在)+∞单调递增. 综上所述,当0a ≤时,函数()f x 的单调减区间是(0,)+∞,当0a >时,函数()f x 的单调减区间是,单调增区间为)+∞.…7分 (Ⅱ)(1)当0a ≤时,由(Ⅰ)可知,()f x 在[1,e]上单调递减,所以()f x 的最小值为21(e)e 112f a =-=,解得240ea =>,舍去. (2)当0a >时,由(Ⅰ)可知,①1,即1a ≥时,函数()f x 在[1,e]上单调递增, 所以函数()f x 的最小值为1(1)12f a ==,解得2a =.②当1e <<,即211e a <<时,函数()f x 在上单调递减,在上单调递增,所以函数()f x 的最小值为11ln 122f a =+=,解得e a =,舍去.③e ,即210e a <≤时,函数()f x 在[1,e]上单调递减,所以函数()f x 的最小值为21(e)e 112f a =-=,得24ea =,舍去. 综上所述,2a =. ……………13分19. (本小题满分14分)解:(Ⅰ)由题意得22=21314c a a b ⎧⎪⎪⎨⎪+=⎪⎩,解得=2a ,1b =. 所以椭圆C 的方程是2214x y +=. …………… 4分 (Ⅱ)以线段PQ 为直径的圆过x 轴上的定点.由22(1)14y k x x y =-⎧⎪⎨+=⎪⎩得2222(14)8440k x k x k +-+-=. 设1122(,),(,)A x y B x y ,则有2122814k x x k +=+,21224414k x x k -=+.又因为点M 是椭圆C 的右顶点,所以点(2,0)M .由题意可知直线AM 的方程为11(2)2y y x x =--,故点112(0,)2y P x --. 直线BM 的方程为22(2)2y y x x =--,故点222(0,)2y Q x --. 若以线段PQ 为直径的圆过x 轴上的定点0(,0)N x ,则等价于0PN QN ⋅=恒成立.又因为1012(,)2y PN x x =-,2022(,)2y QN x x =-, 所以221212001212224022(2)(2)y y y y PN QN x x x x x x ⋅=+⋅=+=----恒成立. 又因为121212(2)(2)2()4x x x x x x --=-++2222448241414k k k k -=-+++ 22414k k=+, 212121212(1)(1)[()1]y y k x k x k x x x x =--=-++22222448(1)1414k k k k k -=-+++22314k k-=+, 所以222212000212212414304(2)(2)14k y y k x x x k x x k -++=+=-=--+.解得0x =.故以线段PQ 为直径的圆过x轴上的定点(. …………… 14分 20. (本小题满分13分) 解:(Ⅰ)符合要求的递增等差数列为1,2,3;2,3,4;3,4,5;1,3,5,共4个.所以(5,3)4f =. …………… 3分 (Ⅱ)设满足条件的一个等差数列首项为1a ,公差为d ,d *∈N .1019a a d =+,10110011199a a d --==≤,d 的可能取值为1,2,,11.对于给定的d ,11091009a a d d =--≤, 当1a 分别取1,2,3,,1009d -时,可得递增等差数列1009d -个(如:1d =时,191a ≤,当1a 分别取1,2,3,,91时,可得递增等差数列91个:1,2,3,,11;2,3,4,,12;;91,92,93,,100,其它同理).所以当d 取1,2,,11时,可得符合要求的等差数列的个数为:(100,10)100119(1211)1100966506f =⋅-⋅+++=-⋅=.…………… 8分(Ⅲ)设等差数列首项为1a ,公差为d ,1(1)m a a m d =+-,1111m a a n d m m --=--≤,记11n m --的整数部分是t ,则11111n n t m m ---<--≤,即111n m n t m m --<--≤. d 的可能取值为1,2,,t ,对于给定的d ,1(1)(1)m a a m d n m d =----≤,当1a 分别取1,2,3,,(1)n m d --时,可得递增等差数列(1)n m d --个.所以当d 取1,2,,t 时,得符合要求的等差数列的个数2(1)121(,)(1)222t t m n m f n m nt m t t +--+=--⋅=-+ 22121(21)()22(1)8(1)m n m n m t m m --+-+=--+-- 易证21112(1)1n m n m n m m m --+-<---≤. 又因为211||12(1)2(1)n m n m m m m m --++-=---,2113||2(1)12(1)n m n m m m m -+---=---, 所以21211||||12(1)2(1)1n m n m n m n m m m m --+-+-->-----. 所以(1)(,)(1)2t t f n m nt m +=--⋅ (1)()(1)11(1)122(1)n m n m n m n m n m m n m m m --+--+-->⋅--⋅=--. 即()(1)(,)2(1)n m n f n m m -+>-. …………… 13分。
【2014朝阳一模】北京市朝阳区2014届高三第一次综合练习 物理试题 Word版含答案.pdf

北京市朝阳区高三年级第次综合练习 理科综合 2014.3 本试卷共16页,共300分。
考试时长150分钟。
考生务必将答案答在答题卡上,在试卷上作答无效。
考试结束后,将本试卷和答题卡一并交回。
以下数据可供解题时参考: 可能用到的相对原子质量:H1 C12 O16 第一部分(选择题 共120分) 本部分共20小题,每小题6分,共120分。
在每小题列出的四个选项中,选出最符合题目要求的一项。
关于αβ、γ三种射线,下列说法正确的是 A.α射线是一种波长很短的电磁波B.γ射线是一种波长很短的电磁波 C.β射线的电离能力最强D.γ射线的电离能力最强 14.V C.电压的最大值为V D.电压瞬时值的表达式为(V) 16.如图所示,A、B两物块的质量分别为m和M,把它们靠在一起从光滑斜面的顶端由静止开始下滑。
已知斜面的倾角为θ,斜面始终保持静止。
则在此过程中物块B对物块A的压力为 A.MgsinθB.Mgcosθ C.0D.(M+m)gsinθ 17. A.t=0.3s时,质元Q的加速度达到正向最大 B.波的传播速度为20m/s C.波的传播方向沿x轴负方向 D.t=0.7s时,质元P的运动方向沿y轴负方向 18.如图所示,真空中有A、B两个等量异种点电荷,O、M、N是AB连线的垂线上的三个点,且AO>OB。
一个带负电的检验电荷仅在电场力的作用下,从M点运动到N点,其轨迹如图中实线所示。
若M、N两点的电势分别为φM和φN,检验电荷通过M、N两点的动能分别为EkM和EkN,则 A.φM=φN,EkM=EkNB.φM<φN,EkM<EkN C.φMEkND.φM>φN,EkM>EkN 19. A.换用最大阻值更大的滑动变阻器,将导线a的M端移到电流表“3”接线柱上 B.换用最大阻值更大的滑动变阻器,将导线b的N端移到电流表“0.6”接线柱上 C.换用最大阻值更小的滑动变阻器,将导线a的M端移到电流表“3”接线柱上 D.换用最大阻值更小的滑动变阻器,将导线b的N端移到电流表“0.6”接线柱上 20.给一定质量温度为0的水加热,在水的温度由0上升到4的结合力形成多分子结构作用势能 C.水分子的平均动能增大,吸收的热量一部分用于分子间的结合力做正功 D.水分子的平均动能增大,吸收的热量一部分用于克服分子间的结合力做功 第二部分(非选择题 共180分) 本部分共11小题,共180分。
北京市房山区2014届高三下学期一模化学试题解析(解析版)

北京市房山区2014届高三下学期一模理综化学试题解析(解析版)6.下面不属于居室污染物的是7.下列说法正确的是A.酿酒过程中,葡萄糖可通过水解反应生成酒精B.鸡蛋清溶液中加入饱和硫酸钠溶液,生成的沉淀物不能再溶解C.酸性高锰酸钾紫色溶液中加入植物油充分振荡后,溶液颜色会褪去D.维生素C ( )溶液中滴加KI淀粉溶液,立即变蓝色【答案】C【解析】试题分析:A.酿酒过程中,葡萄糖在酒曲酶的作用下通过氧化反应生成酒精。
错误。
B.鸡蛋清中含有的蛋白质分子达到胶体颗粒的大小,当向含有鸡蛋白的溶液中加入饱和硫酸钠溶液,鸡蛋白的溶解度降低,会生成的沉淀物。
由于物质的性质没有变化,所以加水还可以继续溶解。
错误。
C.酸性高锰酸钾紫色溶液有强氧化性,而植物油的烃基中含有不饱和的碳碳双键,容易被氧化,所以酸性高锰酸钾紫色溶液中加入植物油充分振荡后,溶液颜色会褪去。
正确。
D.维生素C 有还原性,没有氧化性,不能把KI氧化为I2,所以向维生素C溶液中滴加KI淀粉溶液,溶液不会变蓝色。
错误。
考点:考查酿酒、鸡蛋清、植物油、维生素C的性质的知识。
【结束】8.W 、X 、Y 、Z 四种短周期元素在元素周期表中的相对位置如图所示,其最外层电子数之和等于24,由此可知说法错误的是( )A .原子半径大小:W >XB .元素最高正价:W >ZC .简单阴离子的还原性:Y >ZD .气态氢化物的稳定性:X >Y【答案】B【解析】试题分析:A.W 、X 是同一周期的元素,原子序数越大,原子半径就越小。
因此原子半径:W >X 。
正确。
9.下列离子方程式的书写与结论均合理的是 10.含氮废水中的NH 4+在一定条件下可与O 2发生以下反应:① NH 4+(aq) + 3/2O 2(g) = NO 2-(aq) + 2H +(aq) + H 2O(l) ΔH = -273kL/mol② NO 2-(aq) + 1/2O 2(g) = NO 3-(aq) ΔH = -73kL/mol下列叙述不正确...的是A.升高温度,可使①②反应速率均加快B.室温下时0.1 mol/L HNO2(aq) pH>1,则NaNO2溶液显碱性C.NH4+(aq) + 2O2(g) = NO3 -(aq) + 2H+(aq) + H2O(l) ΔH = -346kJ/molD.1 mol NH4+在①反应中与1 mol NO2-在②反应中失电子数之比为1:3【答案】D【解析】试题分析:A.任何化学反应,升高温度,反应速率均加快。
北京市怀柔区2014届高三下学期适应性练习理综化学试题解析(解析版)
北京市怀柔区2014届高三下学期适应性练习理综化学试题解析(解析版)可能用到的相对原子质量H 1 N 14 O 16 C 126.化学与生活密切相关,下列说法正确的是A.淀粉、油脂和蛋白质都是高分子化合物B.煤的气化和液化均属于化学变化C.雾霾的发生与汽车尾气的直接排放无关D.合成纤维和光导纤维都是新型无机非金属材料7. 在实验室,称取一定量的粗盐经溶解、过滤、结晶等操作,可得到较纯净的食盐。
下列图示对应的操作不规范的是【答案】C【解析】试题分析:A、托盘天平称量固体时,符合左物右码的原则,指针指向分度盘的中间,正确;B、固体溶解时用玻璃棒搅拌加速溶解,正确;C、过滤时未用玻璃棒引流,错误;D、蒸发时用玻璃棒搅拌防止液体溅出,正确,答案选C。
考点:考查粗盐提纯的正确操作8.下列有关物质的性质、应用等的说法正确的是()A.SiO2不能溶于任何酸B.在同浓度、同体积的碳酸钠和碳酸氢钠溶液中,各加入两滴酚酞,碳酸氢钠溶液中红色较深C.浓硫酸能干燥SO2等气体,说明浓硫酸具有吸水性D.自来水厂可用明矾对水进行消毒杀菌9. 用N A表示阿伏加德罗常数的数值,下列说法正确的是A.46 g NO2和N2O4的混合物中含有的氮原子数为N AB.常温常压下,22.4L乙烯中含极性共价键数目为5N AC.1 L 0.1 mol/L的Fe2(SO4)3溶液中,Fe3+的数目为0.2 N AD.1 mol羟基(—OH)中含电子数为10 N A10.下列离子方程式正确的是A.Cl2通入水中:Cl2+H2O=2H+ +Cl-+ClO-B.双氧水中加入稀硫酸和KI溶液:H2O2+2H++2I-=I2+2H2OC.用铜作电极电解CuSO4溶液:2Cu2++2H2电解2↑+4H+D.钢铁发生吸氧腐蚀时,铁作负极被氧化:Fe - 3e- = Fe3+11. 下列陈述I、II正确并且有因果关系的是:12.美国圣路易斯大学研制新型的乙醇燃料电池,用质子(H+)溶剂,在200o C左右供电。
2014北京朝阳高三二模化学试卷与解析(易题库教研团队出品)
2014市某某区高三二模化学试题可能用到的相对原子质量:H 1 C 12 O 166. 在生活、生产中为增大反应速率而采取的措施合理的是A. 食物放在冰箱中B. 塑料制品中添加抑制剂C. 在糕点包装内放置小包除氧剂D. 燃煤发电时用煤粉代替煤块答案:D解析:食物放在冰箱中,温度低腐败变质的速率减慢;塑料制品中添加抑制剂是负催化剂减缓其老化速度;在糕点包装内放置小包除氧剂消除包装袋中的氧气防止器氧化;将煤粉代替煤块增大燃烧时候的接触面积速率加快。
D 正确。
7. 将装满气体X 的试管倒置于装有液体Y 的水槽中,下列说法合理的是答案:A解析:A 中,NH 3极易溶于水,溶液呈碱性,所以试管内充满红色溶液;正确。
NO 2+H 2O=2HNO 3+NO 溶液不能充满试管;SO2具有漂白性可使品红的水溶液褪色;C2H4可以使酸性KMnO 4溶液褪色。
B C D 错误。
8. 下列解释事实的方程式不准确...的是A .铝热法炼铁:Fe 2O 3 + 2Al ====Al 2O 3 + 2FeB .工业上用NH 3制备NO :4NH 3 + 5O 2 ==== 4NO + 6H 2OC .向受酸雨影响的湖泊中喷洒CaCO 3粉末:CO 32- + 2H +== H 2O + CO 2↑D. 过氧化钠用于呼吸面具中作为氧气的来源:2Na 2O 2 + 2CO 2 ==2Na 2CO 3 + O 2答案:C解析:CaCO 3难溶于水,在离子方程式中不能拆开,错误。
其他三个方程式比较简单,都正确。
9. 粗制的CuSO 4·5H 2O 晶体中含有Fe 2+。
提纯时,为了除去Fe 2+,常加入少量H 2O 2,然后再加入少量碱至溶液pH=4,可以达到除去铁离子而不损失硫酸铜的目的。
下列说法高温催化剂高温不正确...的是A. 溶解CuSO4·5H2O晶体时要加入少量稀H2SO4B. 加入H2O2,将Fe2+氧化,2Fe2+ + H2O2 + 2H+ == 2 Fe3+ + 2H2OC. 由于CuSO4的催化作用,会使部分H2O2分解而损失D. 调溶液pH=4的依据是Cu(OH)2比Fe(OH)3更难溶答案:D解析:为防止CuSO4在溶解时候水解要加少量稀H2SO4;加入H2O2目的是将Fe2+氧化为Fe3+,然后通过调节溶液P H值使其转化为比Cu(OH)2更难溶的Fe(OH)3,以除Fe2+。
北京市丰台区2014届高三下学期统一练习化学试题解析(解析版)
第 1 页 共 9 页 北京市丰台区2014届高三下学期统一练习化学试题解析(解析版) 可能用到的相对原子质量:H-1 C-12 N-14 O-16 S-32 Na-23 Cl—35.5 6.化学与生活密切相关,下列说法正确的是 A.食盐可作调味剂 B.CO2属于大气污染物 C.柠檬很酸,属于酸性食物 D.用聚氯乙烯塑料袋包装食品
7.下列解释事实的方程式表达不正确...的是 A.碳酸氢钠可作食品膨松剂:2NaHCO3 △ Na2CO3+ CO2↑+H2O B.铝制容器不能盛装碱液:2Al +2OH-+2H2O 2AlO2-+ 3H2↑
C.氯气可用于消毒:Cl2+H2O 2H++ Cl-+ClO- D.过氧化钠可用于呼吸面具:2Na2O2 + 2CO2 2 Na2CO3 + O2
8.图Ⅰ的目的是精炼铜,图Ⅱ的目的是保护钢闸门。下列说法不正确...的是 A.图Ⅰ中a为纯铜 B.图Ⅰ中SO42—向b极移动 C.图Ⅱ中如果a、b间连接电源,则a连接负极 D.图Ⅱ中如果a、b间用导线连接,则X可以是铜 第 2 页 共 9 页
9.下列说法正确的是 A.植物油的主要成分是高级脂肪酸 B.银氨溶液可用于检验淀粉是否完全水解 C.溴乙烷与氢氧化钠水溶液反应可制取乙烯
D.丙氨酸()缩聚产物的结构简式为
10.实验:① 向盛有1 mL 0.1 mol/L MgCl2溶液试管中加1 mL 0.2 mol/L NaOH溶液,得到浊液a,过滤得到滤液b和白色沉淀c。② 向沉淀c中滴加0.1mol/L FeCl3溶液,沉淀变为红褐色。下列分析不正确...的是 A.浊液a中存在沉淀溶解平衡:Mg (OH) 2(s)Mg2+(aq)+2OH-(aq) B.滤液b中不含有Mg2+ C.②中颜色变化说明Mg (OH)2转化为Fe(OH)3 D.实验可以证明Fe(OH)3比Mg (OH)2更难溶 【答案】B 【解析】 试题分析:①向盛有1 mL 0.1 mol/L MgCl2溶液试管中加1 mL 0.2 mol/L NaOH溶液,恰好发生反应:MgCl2+2NaOH=Mg(OH) 2↓+2NaCl。Mg(OH) 2在溶液中存在沉淀溶解平衡Mg (OH)
2016年北京市朝阳区高三年级第一次综合练习(一模)化学试题和答案
北京市朝阳区高三年级第一次综合练习理科综合化学试卷2016.4.16.中国传统文化对人类文明贡献巨大,古化文献中充分记载了古代化学研究成果.下列关于KNO3的古代文献,对其说明不合理的是7.N2 (g) 与H2 (g) 在铁催化剂表面经历如下过程生成NH3 (g) :下列说法正确的是A.Ⅰ中破坏的均为极性键B.Ⅳ中NH2 与H2 生成NH3C.Ⅱ、Ⅲ、Ⅳ均为放热过程D.8.下列检测方法不合理的是9.某厂用Na 除掉苯中的水分。
某次生产误将甲苯当做苯投进反应釜中,由于甲苯中含水量少,最后反应釜还残留大量的Na 。
下列处理方法更合理、更安全的是A.打开反应釜,将Na 暴露在空气中与氧气反应B.向反应釜通入Cl2 ,Na 在Cl2 中燃烧生成NaClC.向反应釜加大量H2 O,通过化学反应“除掉”金属钠D.向反应釜滴加C2 H5 OH,并设置放气管,排出氢气和热量10.《常用危险化学用品贮存通则》规定:“遇火、遇热、遇潮能引起燃烧、爆炸或发生化学反应,产生有毒气体的化学危险品不得在露天或在潮湿、积水的建筑物中贮存”。
下列解释事实的方程式中,不合理的是A.贮存液氮的钢瓶防止阳光直射:B.硝酸铵遇热爆炸:C.干燥的AlCl3遇水产生气体: D.火灾现场存有电石,禁用水灭火:11.下列“试剂”和“试管中的物质”不.能.完成“实验目的”的是12.某同学做如下实验:下列说法正确的是A.“电流计指针未发生偏转”,说明铁片Ⅰ、铁片Ⅱ均未被腐蚀B.用溶液检验铁片Ⅲ、Ⅳ附近溶液,可判断电池的正、负极C.铁片Ⅰ、Ⅲ所处的电解质溶液浓度相同,二者的腐蚀速率相等D.铁片Ⅳ的电极反应式为25.(17 分)有机物A 为缓释阿司匹林的主要成分。
用于内燃机润滑油的有机物Y 和用于制备水凝胶的聚合物P 的合成路线如下。
已知:⑴ D 的分子式为C7H6O3,D 中所含的官能团是。
⑵D→Y的化学方程式是。
⑶反应Ⅰ的另一种产物是M ,其相对分子质量是60,B 、M 均能与NaHCO3反应产生CO2。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
·1· 北京市朝阳区2014届高三3月第一次综合练习 化学部分 6. 当身处贴有下列标志的环境时,行为不正确...的是
7. 已知: 下列说法不正确...的是 A. ①和②变化过程中都会放出热量 B. 氯原子吸引电子的能力强于钠原子和氢原子 C. ①和②中的氯原子均得到1个电子达到8电子稳定结构 D. NaCl中含有离子键,HCl中含有共价键 8. 综合下图判断,下列叙述不正确...的是
A. Ⅰ、Ⅱ的反应原理均是Zn + Cu2+ = Zn2+ + Cu B. Ⅰ、Ⅱ中均有电子转移,均是把化学能转化为电能利用 C. 随着反应的进行,Ⅰ、Ⅱ中CuSO4溶液颜色均渐渐变浅 D. 取a中溶液,加足量Ba(NO3)2溶液,过滤后向滤液中加AgNO3溶液,有沉淀产生 ·2·
9. 下列解释事实的方程式不准确...的是 A. 氨水使湿润的红色石蕊试纸变蓝:NH3·H2ONH4+ + OH- B. 工业上用过量的NaOH溶液吸收SO2:SO2 + OH- == HSO3- C. 用烧碱溶液清洗铝表面的氧化膜:2OH- + Al2O3 = 2AlO2- + H2O D. 用石灰乳吸收泄漏的氯气:2Ca(OH)2 + 2Cl2== CaCl2 + Ca(ClO)2 + 2H2O 10.下列实验事实不能用...平衡移动原理解释的是
A. B.
C. D.
11. 右图为实验室制取乙炔并验证其性质的装置图。下列说法不合理...的是 A. 逐滴加入饱和食盐水可控制生成乙炔的速率 B. KMnO4酸性溶液褪色,说明乙炔具有还原性 C. 用Br2的CCl4溶液验证乙炔的性质,不需要除杂 D. 将纯净的乙炔点燃,有浓烈的黑烟,说明乙炔不饱 和程度高 12. 在100℃时,将N2O4、NO2分别充入两个各为1 L的密闭容器中,反应过程中浓度变化如下:[2NO2(g) N2O4(g) ΔH<0]
下列说法正确的是
温度/℃ 20 100 FeCl3饱和溶液 棕黄色 红褐色
c(醋酸) / (mol·L-1) 0.1 0.01 pH 2.9 3.4
容器 物质 起始浓度/(mol·L-1) 平衡浓度/(mol·L-1) Ⅰ N2O4 0.100 0.040 NO2 0 0.120
Ⅱ N2O4 0 0.014 NO2 0.100 0.072 ·3·
A. 平衡时,Ⅰ、Ⅱ中反应物的转化率α(N2O4)<α(NO2) B. 平衡时,Ⅰ、Ⅱ中上述正反应的平衡常数K(Ⅰ) = 2K(Ⅱ) C. 平衡后,升高相同温度,以N2O4表示的反应速率ν(Ⅰ)<ν(Ⅱ) D. 平衡后,升高温度,Ⅰ、Ⅱ中气体颜色都将变深 ·4· 25. (16分)对羟基扁桃酸、香豆素–3–羧酸用于制备药物、香料,二者合成路线如下(部分产物及条件未列出):
(1)A的结构简式是__ ____。 (2)A生成 的反应类型是 ______。
(3)B中含氧官能团的名称是__ ____。 (4)对羟基扁桃酸的结构简式是____ __。 (5)乙醇→E的化学方程式是__ ____。 (6)有机物甲能发生银镜反应。下列说法正确的是______。 a. 甲中含有羟基 b. 芳香化合物中只有一种酯与甲互为同分异构体 c. F、X均不能发生加成反应 (7)F的结构简式是__ ____。 (8)X分子中存在两个六元环,X→W的化学方程式是___ ___。
CH2COOH Cl
催化剂 加热
催化剂 加热 W
(R、R′、R”为烃基) 溶液 △ ·5·
26. (12分) 综合利用CO2对环境保护及能开发意义重大。 (1)Li2O、Na2O、MgO均能吸收CO2。如果寻找吸收CO2的其他物质,下列建议合理的是______。 a . 可在碱性氧化物中寻找 b. 可在ⅠA、ⅡA族元素形成的氧化物中寻找 c. 可在具有强氧化性的物质中寻找 (2)Li2O吸收CO2后,产物用于合成Li4SiO4,Li4SiO4用于吸收、释放CO2。原理是:在500℃,CO2与Li4SiO4接触后生成Li2CO3;平衡后加热至700℃,反应逆向进行,放出CO2,Li4SiO4
再生,说明该原理的化学方程式是______。
(3)利用反应A可将释放的CO2转化为具有工业利用价值的产品。 反应A: 已知:
① 反应Ⅱ是_____反应(填“吸热”或“放热”),其原因是 。 ② 反应A的热化学方程式是_______。 (4)高温电解技术能高效实现(3)中反应A,工作原理示意图如下:
① 电极b发生 (填“氧化”或“还原”)反应。 ② CO2在电极a放电的反应式是______。 (5)CO与H2在高温下合成C5H12(汽油的一种成分)减少碳排放。已知燃烧1 mol C5H12(g)生成H2O(g)放出约3 540 kJ的热量。根据化学平衡原理,说明提高合成C5H12的产率可采取的措施是______。 ·6·
27. (14分) 某碳素钢锅炉内水垢的主要成分是碳酸钙、硫酸钙、氢氧化镁、铁锈、二氧化硅等。水垢会形成安全隐患,需及时清洗除去。清洗流程如下: Ⅰ.加入NaOH和Na2CO3混合液,加热,浸泡数小时; Ⅱ.放出洗涤废液,清水冲洗锅炉,加入稀盐酸和少量NaF溶液,浸泡; Ⅲ.向洗液中加入Na2SO3溶液; Ⅳ.清洗达标,用NaNO2溶液钝化锅炉。 (1)用NaOH溶解二氧化硅的化学方程式是_____。 (2)已知: 20℃时溶解度/g CaCO3 CaSO4 Mg(OH)2 MgCO3 1.4×10-3 2.55×10-2 9×10-4 1.1×10-2 根据数据,结合化学平衡原理解释清洗CaSO4的过程_____。 (3)在步骤Ⅱ中: ① 被除掉的水垢除铁锈外,还有 。 ② 清洗过程中,溶解的铁锈会加速锅炉腐蚀,用离子方程式解释其原因_____。 (4)步骤Ⅲ中,加入Na2SO3的目的是 。 (5)步骤Ⅳ中,钝化后的锅炉表面会覆盖一层致密的Fe2O3保护膜。 ① 完成并配平其反应的离子方程式: Fe + NO2- + H2O== N2↑+ + ②下面检测钝化效果的方法合理的是 。 a. 在炉面上滴加浓H2SO4,观察溶液出现棕黄色的时间 b. 在炉面上滴加酸性CuSO4溶液,观察蓝色消失的时间 c. 在炉面上滴加酸性K3[Fe(CN)6]溶液,观察出现蓝色沉淀的时间 d. 在炉面上滴加浓HNO3,观察出现红棕色气体的时间 ·7·
28. (16分) 某同学对铜与浓硫酸反应产生的黑色沉淀进行探究,实验步骤如下: Ⅰ. 将光亮铜丝插入浓硫酸,加热; Ⅱ. 待产生大量黑色沉淀和气体时,抽出铜丝,停止加热; Ⅲ. 冷却后,从反应后的混合物中分离出黑色沉淀,洗净、干燥备用。 (1)步骤Ⅱ产生的气体是______。 (2)步骤Ⅲ中,“从反应后的混合物中分离出黑色沉淀”的操作是______。 (3)该同学假设黑色沉淀是CuO。检验过程如下: 查阅文献:检验微量Cu2+的方法是:向试液中滴加K4[Fe(CN)6] 溶液,若产生红褐色沉淀,证明有Cu2+。 ① 将CuO放入稀硫酸中,一段时间后,未见明显现象,再滴加K4[Fe(CN)6] 溶液,产生红褐色沉淀。 ② 将黑色沉淀放入稀硫酸中,一段时间后,滴加K4[Fe(CN)6] 溶液,未见红褐色沉淀。 由该检验过程所得结论是 。 (4)再次假设,黑色沉淀是铜的硫化物。实验如下: 实验装置 现象
1. A试管中黑色沉淀逐渐溶解 2. A试管内上方出现红棕色气体 3. B试管中出现白色沉淀
① 现象2说明黑色沉淀具有______性。 ② 产生红棕色气体的化学方程式是______。 ③ 能确认黑色沉淀中含有S元素的现象是 ,相应的离子方程式是 。 ④ 为确认黑色沉淀是“铜的硫化物”,还需进行的实验是_____。 (5)以上实验说明,黑色沉淀中存在铜的硫化物。进一步实验后证明黑色沉淀是CuS与Cu2S的混合物。将黑色沉淀放入浓硫酸中加热一段时间后,沉淀溶解,其中CuS溶解的化学方程式是______。 ·8·
化学学科参考答案 2014.3 第一部分 (选择题 共42分) 本部分共7小题,每小题6分,共42分。在每小题列出的四个选项中,选出最符合题目要求的一项。 题号 6 7 8 9 10 11 12 答案 D C B B A C D 第二部分 (非选择题 共58分)
(答案合理均给分) 25. (16分) (1)CH3COOH (2)取代反应 (3)羟基 羧基
(4) (5)2CH3CH2OH + HOOC—CH2—COOH C2H5OOC—CH2—COOC2H5+2H2O
(6)ab
(7) (8) 26.(12分) (1)ab
(2)CO2 + Li4SiO4 Li2CO3 + Li2SiO3 (3)① 吸热 反应物总能量低于生成物总能量(或ΔH>0) ② CO2(g) + H2O(g) ==CO(g) + H2(g) +O2(g) ΔH = +524.8 kJ·mol-1 (4)① 氧化
O-COO-
O-COO-
OHCOOH500℃
700℃
HO CH—COOH OH △ 浓H2SO4
—OH —CH=C—COOC2H5 COOC2H5
O C=O CH COOC2H5 C + 3NaOH —ONa
—CH=C—COONa
COONa + C2H5OH + H2O
