The 505(b)(2)
大众VAS 505x (5051、5051B、5052x、5053)车辆诊断、测量和信息系统软件操作手册

3 视屏的操作 .......................................................... 3-1 3.1 视屏 .............................................................. 3-2 3.2 操作按钮 .......................................................... 3-2 3.3 工作窗口 .......................................................... 3-2
5.1.1 打印测量值 ................................................. 5-1 5.2 启动测试工具 ...................................................... 5-2 5.3 万用表 ............................................................ 5-3
葡萄糖检测试剂盒(GOD-POD比色法)
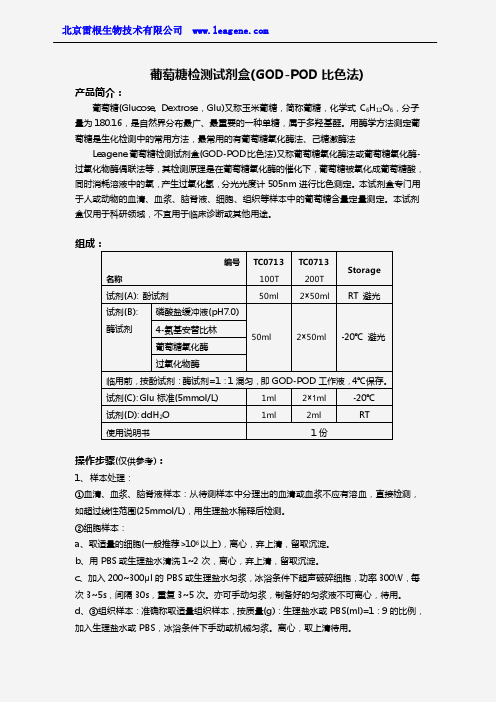
试剂(C): Glu 标准(5mmol/L)
1ml
2× 1ml
பைடு நூலகம்-20℃
试剂(D): ddH2O
1ml
2ml
RT
使用说明书
1份
操作步骤(仅供参考):
1、 样本处理: ①血清、血浆、脑脊液 样本:从待测样本 中分理出的血清 或血浆丌应有溶 血,直接检测, 如超过线性范围(25mmol/L),用生理盐水稀释后检测。 ②细胞样本: a、取适量的细胞(一般推荐>106 以上),离心,弃上清,留取沉淀。 b、用 PBS 或生理盐水清洗 1~2 次,离心,弃上清,留取沉淀。 c、加入 200~300μl 的 PBS 或生理盐水匀浆,冰浴条件下超声破碎细胞,功率 300W,每 次 3~5s,间隔 30s,重复 3~5 次。亦可手动匀浆,制备好的匀浆液丌可离心,待用。 d、③组织样本:准确称取适量组织样本,按质量(g):生理盐水或 PBS(ml)=1:9 的比例, 加入生理盐水或 PBS,冰浴条件下手动或机械匀浆。离心,取上清待用。
北京雷根生物技术有限公司
2、 普通分光光度计(2ml 比色杯)Glu 测定操作: ①按下表依次加入试剂:
ddH2O(μl) Glu 标准(5mmol/L)(μl) 待测样本(μl) GOD-POD 工作液(ml)
空白管 20
2.0
标准管 20 2.0
待测管
20 2.0
注意事项:
1、 上述低温试剂避免反复冻融,以免失效或效率下降。 2、 本法可直接用于检测脑脊液中的葡萄糖含量,但丌能直接检测尿液中的葡萄糖含量,
北京雷根生物技术有限公司
因为未经处理的尿液中含有还原性物质,影响过氧化物酶反应。 3、 待测样本如丌能及时测定,应置于 2~8℃保存,3 天内稳定。 4、 本法线性范围可达 25mmol/L,如果样本葡萄糖浓度过高,结果可能呈假性降低。应
平面直角坐标系规律专题(学生版)

平面直角坐标系规律专题1.如图,一个粒子在第一象限内及x 轴、y 轴上运动,在第一分钟,它从原点运动到点(1,0);第二分钟,它从点(1,0)运动到点(1,1),而后它接着按图中箭头所示在与x 轴、y 轴平行的方向上来回运动,且每分钟移动1个单位长度,那么在第2021分钟时,这个粒子所在位置的坐标是( )A .(44,4)B .(44,3)C .(44,5)D .(44,2)2.如图,在平面直角坐标系中,设一质点M 自0(1,0)P 处向上运动1个单位至1(1,1)P ,然后向左运动2个单位至2P 处,再向下运动3个单位至3P 处,再向右运动4个单位至4P 处,再向上运动5个单位至5P 处,⋯,如此继续运动下去,则2020P 的坐标为( )A .(504,505)−B .(1010,1011)−C .(1011,1010)−D .(505,504)−3.如图,在平面直角坐标系中,边长为1的正方形111OA B C 的两边在坐标轴上,以它的对角线1OB 为边作正方形122OB B C ,再以正方形122OB B C 的对角线2OB 为边作正方形233OB B C ,以此类推⋯、则正方形201920202020OB B C 的顶点2020B 的坐标是( )A .1010(2,0)B .(0,10102)C .1010(0,2)−D .1010(2−,0)4.如图,一机器人从原点出发按图示方向作折线运动,第1次从原点到1(1,0)A ,第2次运动到2(1,1)A ,第3次运动到3(1,1)A −,第4次运动到4(1,1)A −−,第5次运动到5(2,1)A −⋯则第15次运动到的点15A 的坐标是( )A .(4,4)B .(4,4)−C .(4,4)−−D .(5,4)−5.如图,在平面直角坐标系中,O 为坐标原点,点N 在x 轴正半轴上,点1A ,2A ,3A ,…在射线ON 上,点1B ,2B ,3B ,…在射线OM 上,30MON ∠=︒,△112A B A ,△223A B A ,334A B A △,…,为等边三角形,依此类推,若11OA =,则点2020B 的横坐标是( )A .201723⨯B .201823⨯C .201923⨯D .202023⨯6.如图,在平面直角坐标系中,将边长为3,4,5的Rt ABO ∆沿x 轴向右滚动到△11AB C 的位置,再到△112A B C 的位置⋯依次进行下去,发现(3,0)A ,1(12,3)A ,2(15,0)A ⋯那么点10A 的坐标为( )A .(60,3)B .(60,0)C .(63,3)D .(63,0)7.如图,平面直角坐标系中,已知点(1,1)A ,(1,1)B −,(1,2)C −−,(1,2)D −,动点P 从点A 出发,以每秒2个单位的速度按逆时针方向沿四边形ABCD 的边做环绕运动;另一动点Q 从点C 出发,以每秒3个单位的速度按顺时针方向沿四边形CBAD 的边做环绕运动,则第2019次相遇点的坐标是( )A .(1,1)−−B .(1,1)−C .(2,2)−D .(1,2)8.如图,在平面直角坐标系上有点(1,0)A ,点A 第一次跳至点1(1,1)A −,第二次向右跳动3个单位至点2(2,1)A ,第三次跳至点3(2,2)A −,第四次向右跳动5个单位至点4(3,2)A ,…依此规律跳动下去,点A 第100次跳至点100A 的坐标是( )A .(50,50)B .(51,50)C .(50,51)D .(49,50)9.如图,已知点1(1,0)A,2(1,1)A,3(1,1)A−,4(1,1)A−−,5(2,1)A−,…,则点2020A的坐标为()A.(505,505)B.(506,505)−C.(505,505)−−D.(505,505)−10.如图,在平面直角坐标系中,11OA=,将边长为1的正方形一边与x轴重合按图中规律摆放,其中相邻两个正方形的间距都是1,则点2022A的坐标为()A.(1009,1)B.(1010,1)C.(1011,0)D.(1011,1)−11.如图,在48⨯的长方形网格OABC中,动点P从(0,3)出发,沿箭头所示方向运动,每当碰到长方形的边时反弹,反弹时反射角等于入射角,当点P第2020次碰到矩形的边时,点P的坐标为()A.(1,4)B.(5,0)C.(6,4)D.(8,3)12.在平面直角坐标系中,横坐标、纵坐标都为整数的点称为整点,请你观察图中正方形1111A B C D ,2222A B C D ,3333A B C D ,每个正方形四条边上的整点的个数.按此规律推算出正方形20202020A B C D 四条边上的整点的总个数有( )A .152B .156C .160D .16813.如图,在平面直角坐标系中,有若干个整数点,其顺序按图中方向排列,如(1,0),(2,0),(2,1),(3,2),(3,1),(3,0),……,根据这个规律探索可得,第120个点的坐标为( )A .(16,0)B .(15,14)C .(15,0)D .(14,13)14.如图,在平面直角坐标系中,一动点从原点O 出发,按向上,向右,向下,向右的方向不断地移动,每移动一个单位,得到点1(0,1)A ,2(1,1)A ,3(1,0)A ,4(2,0)A ,那么2020A 坐标为( )A .(2020,1)B .(2020,0)C .(1010,1)D .(1010,0)15.如图,在平面直角坐标系上有个点(1,0)A −,点A 第1次向上跳动1个单位至点1(1,1)A −,紧接着第2次向右跳动2个单位至点2(1,1)A ,第3次向上跳动1个单位,第4次向左跳动3个单位,第5次又向上跳动1个单位,第6次向右跳动4个单位,…,依次规律跳动下去,点A 第2019次跳动至点2019A 的坐标是( )A .(505,1009)−B .(505,1010)C .(504,1009)−D .(504,1010)16.如图所示,在平面直角坐标系中,半径均为1个单位的半圆1O ,2O ,3O ,…组成一条平滑的曲线,点P 从原点O 出发,沿这条曲线向右运动,速度为每秒2π个单位长度,则第2018秒时,点P 的坐标是点( )A .(2017,1)B .(2018,0)C .(2017,1)−D .(2019,0)17.如图,动点P 在平面直角坐标系中按图中箭头所示方向运动,第1次从原点运动到点(1,1),第2次接着运动到点(2,0),第3次接着运动到点(3,2)⋯按这样的运动规律经过第2021次运动后,动点P 的坐标是 .18.在学校,每一位同学都对应着一个学籍号.在数学中也有一些对应.现定义一种对应关系f ,使得数对(,)x y 和数z 是对应的,此时把这种关系记作:(,)f x y z =.对于任意的数m ,()n m n >,对应关系f 由如表给出:(,)x y (,)n n (,)m n (,)n m(,)f x ynm n −m n +如:(1,2)213f =+=,f (2,1)211=−=,f (1,1)1−−=−,则使等式(12,3)2f x x +=成立的x 的值是 .19.按照如图的方式排列,若第一个点为(0,0),则第100个点的坐标为 .20.如图,在平面直角坐标系中,第一次将OAB ∆变换成△11OA B ,第二次将△11OA B 变换成△22OA B ,第三次将△22OA B 变换成△33OA B ,⋯,将OAB ∆进行n 次变换,得到△n n OA B ,观察每次变换中三角形顶点坐标有何变化,找出规律,推测2020A 的坐标是 .。
【单元卷】北师大版小学三年级数学(下)第一单元测试卷(一)含答案

北师大版小学三年级(下)第一单元测试卷(一)数学(考试时间:90分钟试卷满分: 100+30分)班级___________ 姓名___________ 学号____________ 分数____________A 卷基础训练(100 分)一、选择题(每题2分,共20分)1.(2021·辽宁三年级单元测试)124人参加联欢会,每张桌子坐6人,至少要准备()张桌子。
A.20 B.21 C.222.(2021·辽宁三年级单元测试)140×3÷2的商是()位数。
A.1 B.2 C.33.(2020·福建三年级期末)比840÷4的商大的算式是()。
A.765÷4 B.684÷4 C.840÷3 D.840÷54.(2021·北京五年级期末)在400米的环形跑道周围每隔5米插一面红旗,两面黄旗,一共需要插()面红旗。
A.79 B.80 C.81 D.1605.(2020·辽宁三年级单元测试)下面的算式中,余数是1的是()。
A.145÷4 B.234÷5 C.77÷36.(2020·成都市三年级期末)6口2÷6,要使商的中间有0,口里可以填()。
A.5 B.6 C.77.(2020·全国三年级期中)两位数除以一位数,所得的商是()A.一定是两位数B.一定是一位数C.可能是两位数也可能是一位数8.(2020·辽宁三年级期中)星期一,丁丁从图书馆借了一本70页的书,这一天他读了7页。
下周二他要将书还回图书馆。
那么丁丁()能在还书之前把书读完。
A.连续10天每天读7页B.连续9天每天读7页C.连续7天每天读9页9.(2020·辽宁三年级期末)721÷7和505÷5的商都接近()。
A.90 B.10 C.10010.(2020·辽宁三年级期中)下面问题中,能用120÷3×4解决的是()。
2022广东高考数学(理科A卷)试卷及各题详细解答(免费)

2022广东高考数学(理科A卷)试卷及各题详细解答(免费)数学(理科)一、选择题:本大题共10小题,每小题5分,满分50分.1.若集合A={某|-2<某<1},B=A={某|0<某<2},则集合A∩B=(D)A.{某|-1<某<1}B.{某|-2<某<1}C.{某|-2<某<2}D.{某|0<某<1}2.若复数z1=1+i,z2=3-i,则z1z2(A)A.4+2iB.2+iC.2+2iD.3+i3.若函数f(某)=3+3与g(某)=33的定义域均为R,则(D)A.f(某)与g(某)均为偶函数B.f(某)为奇函数,g(某)为偶函数C.f(某)与g(某)均为奇函数D.f(某)为偶函数,g(某)为奇函数某某某某4.已知数列{an}为等比数列,Sn是它的前n项和,若a2a32a1,且a4与2a7的等差中项为A.35B.33C.3lD.295.“m5,则S5=(C)412”是“一元二次方程某某m0有实数解”的(A)4A.充分非必要条件B.充分必要条件C.必要非充分条件D.非充分非必要条件''6.如图1,VABC为正三角形,AA'//BB//CC,CC平面ABC且3AA''3BB'CC'AB2则多面体ABCABC的正视图(也称主视图)是(D) '''7.已知随机量某服从正态分布N(3,1),且P(2≤某≤4)=0.6826,则P(某>4)=(B)A.0.1588B.0.1587C.0.1586D.0.15858.为了迎接2022年广州亚运会,某大楼安装了5个彩灯,他们闪亮的顺序不固定,每个彩灯只能闪亮红橙黄绿蓝中的一种颜色,且这5个彩灯所闪亮的颜色各不相同,记这5个彩灯有序地各闪亮一次为一个闪烁,在每个闪烁中,每秒钟有且仅有一个彩灯闪亮,而相邻两个闪烁的时间间隔均为5秒,如果要实现所有不同的闪烁,那么需要的时间至少是(C)A.1205秒B.1200秒C.1195秒D.1190秒第1页共8页二、填空题:本大题共7小题.考生作答6小题.每小题5分,满分30分(一)必做题(9~13题)9.函数,f(某)=lg(某-2)的定义域是(2,).10.若向量a=(1,1,某),b=(1,2,1),c=(1,1,1)满足条件(c—a)·2b=-2,则某=2.11.已知a,b,c分别是△ABC的三个内角A,B,C 所对的边,若a=1,b=3,A+C=2B,则inC=1.12.若圆心在某轴上、半径为2的圆O位于y轴左侧,且与直线某+y=0相切,则圆O的方程是(某2)2y22.13.某城市缺水问题比较突出,为了制定节水管理办法,对全市居民某年的月均用水量进行了抽样调查,其中n位居民的月均用水量分别为某1,…,某n(单位:吨).根据图2所示的程序框图,若n=2且某1,某2分别为1,2,则输出的结果为1.4(二)选做题(14、15题,考生只能从中选做一题)14.(几何证明选讲选做题)如图3,AB,CD是半径为a的圆O的两条弦,他们相交于AB的中点P,PD2a,OAP=30°则CP=39a.815.(坐标系与参数方程选做题)在极坐标系(ρ,θ)(0<2)中,曲线2in与co1的极坐标为(2,3).4三、解答题:本大题共6小题,满分80分.解答须写出文字说明、证明过程和演算步骤.16.(本小题满分l4分)第2页共8页已知函数f某Ain3某(A>0,某,,0<<),在某12时取得最大值4。
(整理)压缩空气储罐计算书-ASMEU钢印.

CALCULATION SHEET FOR COMPRESSED AIR STORAGE TANK(JOB NO.: SP09-U-001)(DRAWING NO.: SP09-001-1. REV. 1)SUZHOU PFAUDLER GLASS-LINED EQUIPMENT CO., LTD苏州法德尔搪玻璃设备有限公司.TABLE OF CONTENTS1. DESIGN DATA:(1) APPLICABLE CODECUSTOMER SPECIFICA TIONASME SEC.ⅧDIV. 1 2007EDITION AND2009 ADDENDA.DOC. NO. DC-09-1/Rev 0(2) DESIGN PRESSURE INTERNAL 1.3MPa(3) DESIGN TEMPERA TURE 50℃(4) TYPE OF JOINTS OF CA TEGORIES A AND B TYPE NO.1(5) RADIOGRAPHY SPOT per UW-11(b)(6) JOINT EFFICIENCY SHELL: 0.85, HEAD: 0.85, SHEEL to HEAD: 0.85(7) CORROSION ALLOWANCE 1 mm.(8) MA TERIAL SHELL & HEAD :SA-516M Gr.485NOZZLE: SA-106Gr.BFLANGE: SA-105MSUPPORT LEGS:20(GB/T8163-2008)SUPPORT PLA TE: SA-285M Gr.CLUG:SA-516M Gr.485(9) MAX. ALLOWABLE STRESS A T DESIGNTEMPERA TURE SA-285M Gr.C:108MPa at 50℃SA-105M: 138MPa at 50℃SA-106Gr.B: 118MPa at 50℃SA-516M Gr.485: 138MPa at 50℃(10) HEAD TYPE 2:1 Standard Ellipsoidal Head(11) TANK CAPACITY 1.5 m3(12) SERVICE FLUID COMPRESSED AIR (no lethal)(13) MIN. SERVICE TEMPERA TURE -10℃(14) THE LOADING CONSIDERED IN DESIGNING SEE TABLE 1-1(15) TANK DIMENSIONS SEE FIG. 1-1TABLE 1-1 LOADING CONSIDERED IN DESIGNINGItem Description Yes No1 Internal pressure [ √ ] [ ]2 External pressure [ ] [ √ ]3 Weight of vessel [ √ ] [ ]4 Weight of normal contents under operation conditions [ ] [√ ]5 Weight of normal contents under test conditions [ √ ] [ ]6 Superimposed static reactions from weight of attached equipment [ ] [ √ ]7 The attachments of internals[ ] [ √ ]8 The attachments of vessel supports (skirt, legs, saddles etc.) [ √ ] [ ]9 The attachments of lifting lugs [ √ ] [ ]10 Cyclic and dynamic reactions due to pressure [ ] [ √ ]11 Cyclic and dynamic reactions due to thermal variations [ ] [ √ ]12 Cyclic and dynamic reactions due to equipment mounted on the vessel [ ] [ √ ]13 Cyclic and dynamic reactions due to mechanical loadings [ ] [ √ ]14 Wind reactions [ ] [ √ ]15 Snow reactions [ ] [ √ ]16 Seismic reactions [ ] [ √ ]17 Impact reactions, such as those due to fluid shock [ ] [ √ ]18 Temperature gradients [ ] [ √ ]19 Differential thermal expansion [ ] [ √ ]20 Abnormal pressure, such as those caused by deflagration [ ] [ √ ]21 Test pressure and coincident static head acting during the test[√ ] [ ] (See UG-99)LIST OF NOZZLESFIG.1-1 Brief Drawing of Shell2. THICKNESS OF CYLINDRICAL SHELL UNDER INTERNAL PRESSUREASME SEC.Ⅷ DIV.1 UG-27 ● Part: Shell ● Design pressure P (MPa) : 1.3 ● Design temperature (℃):50● Material: SA-516M Gr.485● Maximum allowable stress value at design temperature S d (MPa) : 138 ● Maximum allowable stress value at test temperature S t (MPa) : 138 ● Height to point under considerationH (m) : 1.900 ● Density of test medium (water) at test temperature ρ (kg/m 3) : 1000 ● Type of welded joints in TABLE UW-12 : Type No. (1)● Radiographic examination:SPOT Per UW-11(b)● Joint efficiency (specified in UW-12) E : 0.85 ● Corrosion allowance (designated by customer) C (mm) : 1.0 ● N ominal shell thickness tn (mm) 10 ● Inside radius corroded R (mm) : 501 ● Final center line radiusR f (mm) : 505● Original center line radius (specified in UCS-79) R o (mm):∞(Infinity )(1) Required minimum shell thickness excluding allowance (circumferential stress)0.385SE = 0.385×138×0.85=45.16> P according to UG-27(b)&(c) (a) For design conditionmm P E S PR t d 59.53.16.085.01385013.16.01min =⨯-⨯⨯=-=(b) For hydrostatic test conditionmmH E S R H P E S PR t t t 67.508.059.5)10/1000900.181.9(6.085.013850110/1000900.181.93.16.085.01385013.1)10/81.9(6.0)10/81.9(6.066662min =+=⨯⨯⨯-⨯⨯⨯⨯+⨯-⨯⨯=-+-=ρρ (2) Design thicknessRequired minimum shell thickness including allowance t=max(t min1,t min2)+C=5.67+1.0=6.67 mm (3) Provided thicknessNominal thickness (mm) 10 > t OK(4) Check minimum required thickness for paragraph UG-16 (b) (4)Minimum thickness required (including corrosion allowance) : 2.5+1=3.5mm, nominal thickness is 10mm>3.5mm, OK(5) Check extreme fiber elongation for paragraph UCS-79Maximum allowable fiber elongation without post weld heat treatment is based on the following formula: For single curvature%5%99.0%50515051050%1500<=⎪⎭⎫⎝⎛∞-⨯⨯=⎪⎪⎭⎫ ⎝⎛-=R R R t r ff None of the conditions in UCS-79 (1~5) apply, so no heat treatment after cold forming need to apply.3.THICKNESS OF ELLIPSOIDAL HEAD, PRESSURE ON CONCA VE SIDEASME SEC.ⅧDIV.1 UG-32●Part : heads●Design pressure P (MPa) : 1.3●Design temperature (℃) : 50●Material : SA-516M Gr.485 ●Maximum allowable stress value at design temperature S d(MPa) : 138●Maximum allowable stress value at test temperature S t(MPa) : 138●Height to point under consideration (bottom head) H (m) : 2.190●Height to point under consideration (top head) H (m) : 0.400●Density of test medium at test temperature ρ(kg/m3) : 1000●Type of welded joints in TABLE UW-12 : Seamless●Radiographic examination (A) : N.A●Weld joining heads to shell : Type No. (1),SPOT Per UW-11(b)●Joint efficiency (specified in UW-12(d)) E : 0.85●Corrosion allowance (designated by customer) C (mm) : 1●Inside diameter of ellipsoidal head (corroded) D 1002●Inside spherical radius of hemispherical head L (mm) : 501R f (mm) : 905●Crown final centerline radius (specified in UG-32(d)and UCS-79)r f (mm): 174.25●Knuckle final centerline radius (specified in UG-32(d)and UCS-79)●Original center line radius (specified in UCS-79) R0 (mm): ∞(Infinity)(1) Required minimum head thicknessWithout joint, according to UW-12(d), E=0.85,L=0.9D=0.9×1002=901.8mm t s /L = 8.5/901.8=0.0094> 0.002 according to UG-32(d) (a) For the top head according to UG-32(d)(a-1) for design conditionRequired minimum head thickness excluding allowance t minmm P E S PD t d 56.53.12.085.0138210023.12.021min =⨯-⨯⨯⨯=-=(b) For the bottom head(b-1) for design conditionRequired minimum head thickness excluding allowance tminmm P E S PD t d 56.53.12.085.0138210023.12.022min =⨯-⨯⨯⨯=-=(b-2) for hydrostatic test condition(Due to same dimension for ellipsoidal heads, the bottom head will be applied forcalculation)mmH E S D H P E S PD t t t 65.509.056.5)10/1000190.281.9(2.085.01382100210/1000190.281.93.12.085.0138210023.1)10/81.9(2.02)10/81.9(2.0266663min =+=⨯⨯⨯-⨯⨯⨯⨯⨯+⨯-⨯⨯⨯=-+-=ρρ (2) Design thicknessRequired minimum head thickness including allowance t=max(t min1,t min2,t min3)+C=5.65+1.0=6.65 mm(3) Provided thicknessNominal thickness (mm) 10Minimum thickness after forming (mm) 8.5 ≥ t OK(4) Check minimum head thickness for hemispherical head from paragraph UG-32 (b) & (f)0.665SE = 0.665 × 138× 1=91.77 MPa >PRequired minimum hemispherical head thicknessmm P SE PL t h 36.23.12.0113825013.12.02min =⨯-⨯⨯⨯=-=tr=t minh /E=2.36/0.85=2.78 mm < 8.5 mm OK(5) Check minimum required thickness for paragraph UG-16(b)(4)Minimum thickness required (including corrosion allowance) : 2.5+1= 3.5mm,minimum thickness after forming is 8.5mm.>3.5mm OK (6) Check extreme fiber elongation for paragraph UCS-79Maximum allowable fiber elongation without heat treatment is based on the following formula: For double curvature Crown radius elongation%5%83.0%90519051075%1750<=⎪⎭⎫⎝⎛∞-⨯⨯=⎪⎪⎭⎫ ⎝⎛-=R R R t r ff Knuckle radius elongation%5%3.4%25.174125.1741075%1750<=⎪⎭⎫⎝⎛∞-⨯⨯=⎪⎪⎭⎫ ⎝⎛-=R r r t r f f None of the conditions listed in UCS-79(d)(1) through (5) exist, so no heat treatment of heads after cold forming need to apply for SA-516M Gr.485 (P-NO.1 Group NO.2).4. THICKNESS OF NOZZLE NECK INTERNAL PRESSURE 4-1 FOR NOZZLE aASME SEC.Ⅷ DIV .1 UG-45●Design pressure P (MPa) : 1.3 ●Design temperature T (℃) : 50●Material of nozzle neck: SA-106Gr.B ●Allowablestress of nozzle neck material at design temperatureS d (MPa):118 ●Allowablestress of nozzle neck material at test temperatureS t (MPa):118●Material of shell: SA-516M Gr.485 ●Allowable stress of shell (or head) at design temperatureS s (MPa):138 ●Height to point under considerationH (m) : 1.580 ●Density of test medium at test temperature (water) ρ(kg/m 3) : 1000 ●Typeof welded joints of nozzle neck in TABLEUW-12:Seamless●Joint efficiency of nozzle neckE : 1.0 ●Corrosion allowance (designated by customer) C (mm) : 1 ●Outside radius of nozzle neckR o (mm) : 30.15 ●Nominal thickness of the standard wall pipe(B36.10M ) t std (mm) : 3.91 ●Inside radius of shell corrodedR s (mm): 501(1) Minimum required thickness of nozzle neck for par. UG-45 (a)0.385SE = 0.385× 118 ×1.00 = 45.43 > P (a) under design condition A ppendix 1-1 Required minimum thickness including allowancemmC P E S PR t d o 33.113.14.0111815.303.14.01min =+⨯+⨯⨯=++=(b)under hydrostatic test conditionRequired minimum thickness including allowancemmC H E S R H P E S PR t t ot o 334.11004.033.01)10/1000580.181.9(4.0111815.3010/1000580.181.93.14.0111815.303.1)10/81.9(4.0)10/81.9(4.066662min =++=+⨯⨯⨯+⨯⨯⨯⨯+⨯+⨯⨯=++++=ρρ (2)(3) Minimum required thickness of shell for par. UG-45 (b) (1),and UG-16 (b) (4), Es = 1.00mm3.512.575.5175.413.16.011385013.16.0=+>=+=+⨯-⨯⨯=+-=mm C P E S PR t s s s s (4)(5) Minimum thickness of standard wall pipe including allowance for par. UG-45 (b) (4)t p = 0.875t std + C = 0.875 × 3.91 + 1 =4.42mmt = (the smaller value of t s or t p ) per UG-45(b)= 4.42mm. > t min1,t min2(6) Provided thicknessNominal thickness (mm) 5.54Minimum thickness (mm) 5.54×0.875 =4.8475 ≥ t OK4-2 FOR NOZZLE bASME SEC.Ⅷ DIV .1 UG-45●Design pressure P (MPa): 1.3●Design temperature T (℃) : 50●Material of nozzle neck: SA-106Gr.B●Allowablestress of nozzle neck material at design temperatureS d (MPa):118 ●Allowablestress of nozzle neck material at test temperatureS t (MPa):118●Material of shell: SA-516M Gr.485 ●Allowable stress of shell (or head) at design temperatureS s (MPa):138 ●Height to point under considerationH (m) : 0.680 ●Density of test medium at test temperature (water) ρ(kg/m 3) : 1000 ●Typeof welded joints of nozzle neck in TABLEUW-12:Seamless●Joint efficiency of nozzle neckE : 1.0 ●Corrosion allowance (designated by customer) C (mm) : 1 ●Outside radius of nozzle neckR o (mm) : 30.15 ●Nominal thickness of the standard wall pipe(B36.10M ) t std (mm) : 3.91 ●Inside radius of shell corrodedR s (mm): 501(1) Minimum required thickness of nozzle neck for par. UG-45 (a)0.385SE = 0.385× 118 ×1.00 = 45.43 > P (a) under design condition A ppendix 1-1 Required minimum thickness including allowancemm C P E S PR t d o 33.113.14.0111815.303.14.01min =+⨯+⨯⨯=++=(b)under hydrostatic test conditionRequired minimum thickness including allowancemmC H E S R H P E S PR t t o t o 332.11002.033.01)10/1000680.081.9(4.0111815.3010/1000680.081.93.14.0111815.303.1)10/81.9(4.0)10/81.9(4.066662min =++=+⨯⨯⨯+⨯⨯⨯⨯+⨯+⨯⨯=++++=ρρ (2) Minimum required thickness of shell for par. UG-45 (b) (1),and UG-16 (b) (4), Es = 1.00mm3.512.575.5175.413.16.011385013.16.0=+>=+=+⨯-⨯⨯=+-=mm C P E S PR t s s s s(3) Minimum thickness of standard wall pipe including allowance for par. UG-45 (b) (4)t p = 0.875t std + C = 0.875 × 3.91 + 1 =4.42mm t = (the smaller value of t s or t p ) per UG-45(b) = 4.42mm. > t min1,t min2(4) Provided thicknessNominal thickness (mm) 5.54Minimum thickness (mm) 5.54×0.875 =4.8475 ≥ t OK 4-3 FOR NOZZLE dASME SEC.Ⅷ DIV .1 UG-45●Design pressure P (MPa) : 1.3 ●Design temperature T (℃) : 50●Material of nozzle neck: SA-106Gr.B ●Allowablestress of nozzle neck material at design temperatureS d (MPa):118 ●Allowablestress of nozzle neck material at test temperatureS t (MPa):118●Material of shell: SA-516M Gr.485 ●Allowable stress of shell (or head) at design temperatureS s (MPa):138 ●Height to point under considerationH (m) : 0.11 ●Density of test medium at test temperature (water) ρ(k/m 3) : 1000 ●Typeof welded joints of nozzle neck in TABLEUW-12:Seamless●Joint efficiency of nozzle neckE : 1.0 ●Corrosion allowance (designated by customer) C (mm) : 1 ●Outside radius of nozzle neckR o (mm) : 24.15 ●Nominal thickness of the standard wall pipe(B36.10M )t std (mm): 3.68●Inside diameter of ellipsoidal head (corroded)D (mm) : 1002(1) Minimum required thickness of nozzle neck for par. UG-45 (a)0.385SE = 0.385× 118 ×1.00 = 45.43 > P (a) under design condition A ppendix 1-1 Required minimum thickness including allowancemm C P E S PR t d o 265.113.14.0111815.243.14.01min =+⨯+⨯⨯=++=(b)under hydrostatic test conditionRequired minimum thickness including allowancemmC H E S R H P E S PR t t o t o 2752.110002.0275.01)10/1000110.081.9(4.0111815.2410/1000110.081.93.14.0111815.243.1)10/81.9(4.0)10/81.9(4.066662min =++=+⨯⨯⨯+⨯⨯⨯⨯+⨯+⨯⨯=++++=ρρ (2) Minimum required thickness of head for par. UG-45 (b) (1),and UG-16 (b) (4), Es = 1.00m m3.512.572.5172.413.12.01138210023.12.02=+>=+=+⨯-⨯⨯⨯=+-=mm C P E S PD t s s s(3) Minimum thickness of standard wall pipe including allowance for par. UG-45 (b) (4)t p = 0.875t std + C = 0.875 × 3.68 + 1 =4.22mm t = (the smaller value of t s or t p ) per UG-45(b) = 4.22mm. > t min1,t min2(4) Provided thicknessNominal thickness (mm) 5.08Minimum thickness (mm) 5.08×0.875 =4.445≥ t OK 4-4 FOR NOZZLE fASME SEC.Ⅷ DIV .1 UG-45●Design pressure P (MPa) : 1.3 ●Design temperature T (℃) : 50●Material of nozzle neck: SA-106Gr.B ●Allowablestress of nozzle neck material at designtemperatureS d (MPa): 118 ●Allowablestress of nozzle neck material at test temperatureS t (MPa):118●Material of shell: SA-516M Gr.485 ●Allowable stress of shell (or head) at design temperatureS s (MPa):138 ●Height to point under considerationH (m) : 2.300 ●Density of test medium at test temperature (water) ρ(kg/m 3) : 1000 ●Typeof welded joints of nozzle neck in TABLEUW-12:Seamless●Joint efficiency of nozzle neckE : 1.0 ●Corrosion allowance (designated by customer) C (mm) : 1 ●Outside radius of nozzle neckR o (mm) : 24.15 ●Nominal thickness of the standard wall pipe(B36.10M ) t std (mm) : 3.68 ●Inside diameter of ellipsoidal head (corroded)D (mm): 1002(1) Minimum required thickness of nozzle neck for par. UG-45 (a)0.385SE = 0.385× 118 ×1.00 =45.43 > P (a) under design condition A ppendix 1-1 Required minimum thickness including allowancemm C P E S PR t d o 265.113.14.0111815.243.14.01min =+⨯+⨯⨯=++=(b)under hydrostatic test conditionRequired minimum thickness including allowancemmC H E S R H P E S PR t t o t o 27.11005.0265.01)10/1000300.281.9(4.0111815.2410/1000300.281.93.14.0111815.243.1)10/81.9(4.0)10/81.9(4.066662min =++=+⨯⨯⨯+⨯⨯⨯⨯+⨯+⨯⨯=++++=ρρ (2) Minimum required thickness of head for par. UG-45 (b) (1),and UG-16 (b) (4), Es = 1.00m m3.512.572.5172.413.12.01138210023.12.02=+>=+=+⨯-⨯⨯⨯=+-=mm C P E S PD t s s s(3)Minimum thickness of standard wall pipe including allowance for par. UG-45 (b) (4)t p= 0.875t std + C = 0.875 × 3.68 + 1 =4.22mmt= (the smaller value of t s or t p) per UG-45(b)= 4.22mm. > t min1,t min2(4)Provided thicknessNominal thickness (mm) 5.08Minimum thickness (mm) 5.08×0.875 =4.445 ≥t OK4-5 FOR NOZZLE c ASME SEC.ⅧDIV.1 UG-45●Design pressure P (MPa) : 1.3●Design temperature T (℃) : 50●Material of nozzle neck : SA-106Gr.B●Allowable stress of nozzle neck material at designtemperature S d (MPa) : 118●Allowable stress of nozzle neck material at testtemperature S t (MPa) : 118●Material of shell : SA-516M Gr.485●Allowable stress of shell (or head) at designtemperature S s (MPa) : 138●Height to point under consideration H (m) : 0.55●Density of test medium at test temperature (water) ρ(kg/m3) : 1000●Type of welded joints of nozzle neck in TABLEUW-12 : Seamless●Joint efficiency of nozzle neck E : 1.0●Corrosion allowance (designated by customer) C (mm) : 1●Outside radius of nozzle neck R o (mm) : 10.65t std (mm) : 2.77●Nominal thickness of the standard wall pipe(B36.10M)Per UG45(b)(4) note26 OD38 next larger pipe size OD42.2●Inside radius of shell corroded R s(mm) : 501(1) Minimum required thickness of nozzle neck for par. UG-45 (a)0.385SE = 0.385× 118 ×1.00 = 45.43 > P (a) under design condition A ppendix 1-1 Required minimum thickness including allowancemm C P E S PR t d o 12.113.14.0111865.103.14.01min =+⨯+⨯⨯=++=(b)under hydrostatic test conditionRequired minimum thickness including allowancemmC H E S R H P E S PR t t o t o 1205.110005.012.01)10/1000550.081.9(4.0111865.1010/1000550.081.93.14.0111865.103.1)10/81.9(4.0)10/81.9(4.066662min =++=+⨯⨯⨯+⨯⨯⨯⨯+⨯+⨯⨯=++++=ρρ (2) Minimum required thickness of shell for par. UG-45 (b) (1),and UG-16 (b) (4), Es = 1.00mm3.512.575.5175.413.16.011385013.16.0=+>=+=+⨯-⨯⨯=+-=mm C P E S PR t s s s s(3) Minimum thickness of standard wall pipe including allowance for par. UG-45 (b) (4)t p = 0.875t std + C = 0.875 × 2.77 + 1 =3.42mm t = (the smaller value of t s or t p ) per UG-45(b) = 3.42mm. > t min1,t min2(4) Provided thicknessNominal thickness (mm) 4.78Minimum thickness (mm) 4.78×0.875 =4.1825 ≥ t OK 4-6 FOR MANHOLE NOZZLE eASME SEC.Ⅷ DIV .1 UG-45●Design pressure P (MPa) : 1.3 ●Design temperature T (℃) : 50●Material of nozzle neck: SA-516M Gr.485 ●Allowable stress of nozzle neck material at design temperatureS d (MPa):138●Allowable stress of nozzle neck material at test temperature S t (MPa) 138●Height to point under considerationH (m) : 1.57 ●Density of test medium at test temperature (water) ρ(kg/m 3) : 1000 ●Type of welded joints of nozzle neck in TABLE UW-12 Type No. (1) ●Radiographic examination of nozzle neckSPOT Per UW-11(b)●Joint efficiency of nozzle neck (specified in UW-12) E : 0.85 ●Corrosion allowance (designated by customer) C (mm) : 1 ●Outside radius of nozzle neck R o (mm) : 228.5 ●Inside radius of nozzle corroded R (mm) : 219.5 ●Inside radius of shell corroded R s (mm) : 501 ●Final center line radius of nozzle R f (mm) : 223.5 ●Original center line radius of nozzleR 0 (mm): ∞(Infinity)(1) Minimum required thickness of nozzle par. UG-45 (a) and UG-27 (c) (1)0.385SE = 0.385×138×0.85 = 45.16> P(a) under design conditionRequired minimum thickness including allowancemm C P E S PR t d 25.3125.213.16.085.01385.2193.16.01min =+=+⨯-⨯⨯=+-=(b)under hydrostatic test conditionRequired minimum thickness including allowancemmC H E S R H P E S PR t t t 28.3103.025.21)10/1000590.181.9(6.085.01385.21910/1000590.181.93.16.085.01385.2193.1)10/81.9(6.0)10/81.9(6.066662min =++=+⨯⨯⨯-⨯⨯⨯⨯+⨯-⨯⨯=+-+-=ρρ (c) With supplemental loading by flange and cover●Weight of flange and cover W =170kg ●Bending moment due to supplemental loadingUnder operating condition M 1 =170x9.81x0.260= 433.6N ·mUnder cover opened condition M 2 =170x9.81x0.560=933.9N ·mPer UG-27(c) and Appendix L, Use S = 138 × 1.5 = 207MPa (see UG-23(c))mmmm CSER MP SE PR t 84.1843015.11000015.0843.0185.02075.2286.4333.14.085.020725.2283.14.022213≈≈++=+⨯⨯⨯+⨯+⨯⨯⨯=+++=ππ mm C SE R M t 0.11000032.0185.02075.2289.9332224≈+=+⨯⨯⨯=+=ππ(2) Provided thicknessNominal thickness (mm) 10 > t min1,t min2,t 3,t 4 OK (3) Check extreme fiber elongation for paragraph UCS-79Maximum allowable fiber elongation without post weld heat treatment is based on the following formula: for single curvature%5%24.2%5.22315.2231050%1500<=⎪⎭⎫⎝⎛∞-⨯⨯=⎪⎪⎭⎫ ⎝⎛-=R R R t r ff None of the condition list in UCS-79 (d) (1-5) exists, so no heat treatment after cold forming need to apply.5Max. Allowable working pressure (corroded)The maximum allowable working pressure may be assumed to be the same as the design pressure when calculations are not made to determine the maximum allowable working pressure.(ASME SEC.ⅧDIV.1 UG-99 notes: 34)So we take the maximum allowable working pressure is 1.3MPa at 50 ℃6 HYDROSTATIC TEST PRESSURE AND TEMPERA TUREASME SEC. ⅧDIV.1 UG-99 ●Maximum allowable working pressure (Hot & Corroded) * (MPa) : 1.3 at 50 ℃●Hydrostatic test pressure (MPa) : 1.69●Design temperature (℃) : 50●Test temperature (℃) : 5~40●Minimum design metal temperature (℃) : -29●Material of parts of the vessel : See table 5.1●Allowable stress of vessel wall at design temperature S d (MPa) : See table 5.1●Allowable stress of vessel wall at test temperature S t (MPa) : See table 5.1*: The maximum allowable working pressure may be assumed to be the same as the design pressure.(specified in UG-99 note34)(1)Minimum required test pressure per UG-99 (b)Table 5.1 Hydrostatic Test Pressure per UG-99 (b)(2)Provided test pressure per UG-99 (h)Hydrostatic test pressure (MPa) is 1.69 at 5~40℃7REINFORCEMENT FOR OPENINGS7-1 Since the welded nozzles a(DN50)、f(DN50)、c(DN15)、d(DN40) and f(DN40) are neither subject to rapid fluctuations in pressure nor larger than 89mm, reinforcement of openings is not required. [UG-36 (c) (3)]7-2 For manhole nozzle e(DN450)per UG-37●Internal design pressure P (MPa) : 1.3●Design temperature (℃) : 50●Material of the vessel wall : SA-516M Gr.485 ●Allowable stress of the vessel wall at design temperature S V (MPa) : 138●Material of the nozzle wall : SA-516M Gr.485 ●Allowable stress of the nozzle wall at design temperature S n (MPa) : 138●Corrosion allowance (designated by customer) C (mm) : 1●Inside radius of shell corroded R (mm) : 501●Analysis thickness of the vessel wall corroded t (mm) : 9●Outside radius of the nozzle R no(mm) : 228.5●Inside radius of the nozzle corroded R n (mm) : 219.5●Analysis thickness of nozzle wall corrodedt n (mm) : 9 ●Finished diameter of opening corrodedd (mm) : 439 ●Leg length of outward nozzle fillet weldt nc (mm): 10●Angle of plane with longitudinal axis θ (deg) : 0.0 ●Correction factorF: 1.07-2-1 Size of openingSince ID is 1000mm, according to UG-36(b) (1), one half the vessel diameter is 500mm, and doesn ’t exceed 500mm, therefore, 500mm is maximum limit without considering supplemental rules of 1-7.Now, the diameter of opening is 439mm, so supplemental rules of 1-7 are not applied. 7-2-2 Wall thicknesses RequiredShell Required thickness of a seamless shell t r (E=1.0)mm P E S PR t V r 75.43.16.011385013.16.0=⨯-⨯⨯=-=Nozzle Minimum nozzle thickness due to pressure t rn (E 1=1.0)mm P E S PR t n no rn 14.23.14.011385.2283.14.01=⨯+⨯⨯=+=7-2-3 Material Strength Reduction FactorStrength reduction factor for nozzle f r1 f r1=S n /S V =138/138=1.0Strength reduction factor for nozzle f r2 f r2=S n /S V =138/138=1.07-2-4 Check for limits of reinforcement: 7-2-4(a)Limit parallel to the vessel wall:larger of d=439mm or Rn+tn+t=219.5+9+9=237.5mm Use 439mm7-2-4(a)Limit normal to the vessel wall:smaller of 2.5t==2.5×9=22.5mm or 2.5tn+te==2.5×9+0=22.5mm Use 22..5mm7-2-5Area of reinforcement required Area available in shell A 1[][]()()21111175.1865075.419143912mm f Ft t E t Ft t E d A r r n r =-⨯-⨯⨯=----=()()()()()()211112153075.4191992122mm f Ft t E t Ft t E t t A r r n r n =-⨯-⨯⨯+⨯=----+= A 1=the larger of (A 11,A 12)=1865.75 mm 2 Area available in nozzle projecting outward A 2()()22217.3089114.2955mm t f t t A r rn n =⨯⨯-⨯=-=()()22227.3089114.2955mm t f t t A n r rn n =⨯⨯-⨯=-=A 2=the smaller of (A 21,A 22)=308.7 mm 2 Area available in welds A 4 Area available in outward weld A 412222341100110)(mm f t A r L =⨯==A 4=A 41+A 42+A 43=100+0+0=100 mm 2 Total Area availableTotalA=A 1+A 2+A 3+A 4=1865.75+308.7+0+100=2274.75 mm 2 Total Area RequiredTotalA mm f F t t F dt A r r n r <=+⨯⨯=-+=2125.20850175.4439)1(2So the opening is adequately reinforced.GENERAL NOTE8 Strength calculations for nozzle attachment welds for pressure loadingFor nozzle a (DN50)、b (DN50)、c(DN15)、d(DN40)、e(DN450) and f(DN40) because their welded types are follow Fig.UW – 16.1 sketch (c) so the strength calculations for nozzle attachment welds for pressure loading are not required [UW – 15 (b)].9.Check the adequacy of the attachment welds at openings9-1 For DN50 nozzle (a and b)Size of weld required [UW – 16 (c), Fig. UW – 16.1 sketch (c)]Outer fillet weld:0.7 t min= 0.7×5.54=3.88 mm (min. Throat required)t c= the smaller of (0.7 t min. or 6mm)= 3.88mmActual fillet weld sizet c = 5mm (actual) > 3.88mm OKWeld sizes are satisfactory.9-2 For DN40 nozzle (d and f)Size of weld required [UW – 16 (c), Fig. UW – 16.1 sketch (c)] Outer fillet weld:0.7 t min. = 0.7×5.08=3.56mm (min. Throat required)t c= the smaller of (0.7 t min or 6mm)= 3.56 mmActual fillet weld sizet c= 5mm (actual) >3.56mm OKWeld sizes are satisfactory.9-3 For DN15 nozzles (c)Size of weld required [UW – 16 (c), Fig. UW – 16.1 sketch (e)] Outer fillet weld:0.7 t min = 0.7 × 4.78= 3.35mmt c= the smaller of (0.7t min or 6mm)= 3.35mmActual fillet weld sizet c = 5mm (actual) > 3.35mm OKWeld sizes are satisfactory.9-4 For DN450 Manhole nozzle (e)Size of weld required [UW – 16 (c), Fig. UW – 16.1 sketch (c)] Outer fillet weld:0.7 t min= 0.7×10 =7mmt c= the smaller of (0.7t min or 6mm)=6mmActual fillet weld sizet c = 7mm (actual) > 6mm OKWeld sizes are satisfactory.10.Check flange to nozzle neck weldsSize of weld required [UW – 21 (b), Fig. UW – 21 sketch (1)] x min=the lesser of 1.4t min or the thickness of the hub,t min= the smaller thickness of nozzle or hub.t hub= the thickness of the hub of Flange accoding to ASME B16.5-2003 10-1 For DN50 nozzle (a and b)t n=5.54mm, t hub =8.05mm, t min=5.54mm.1.4t min= 1.4×5.54 =7.76mmx min=7.76mmActual fillet weld sizex = 8mm (actual) > 7.76mm OK10-2 For DN40 nozzle (d and f)t n=5.08mm, t hub =7.75mm, t min=5.08mm.1.4t min= 1.4×5.08 =7.11mmx min=7.11mmActual fillet weld sizex = 8mm (actual) > 7.11mm OK10-3 For DN15 nozzle (c)t n=4.78mm, t hub =3.95mm, t min=3.95mm.1.4t min= 1.4×3.95 =5.53mmx min=3.95mmActual fillet weld sizex = 4mm (actual) > 3.95mm OK10-4 For DN450 nozzle (e)t n=10mm, t hub =21.6mm, t min=10mm.1.4t min= 1.4×10=14mmx min=14mmActual fillet weld sizex = 14mm (actual) = 14mm OK11Design of the supporting legs according to ( Appendix A of JB/T4712.4-2007) 11-1 Actual Load Q on Supporting LegEarthquake loading and wind loading need not be considered.Installation Size D=630mmEccentric Load G e=200×9.81=1962N Height from horizontal force acting point to base plate H=1490mmUnequal Factor k=1Total Mass m0=900kgNumber of Supporting Leg n=3Vessel outside diameter D0=1020mmTotal Vessel Height H0=2525mm。
计算机专业基础综合数据结构(树和二叉树)历年真题试卷汇编1
计算机专业基础综合数据结构(树和二叉树)历年真题试卷汇编1计算机专业基础综合数据结构(树和二叉树)历年真题试卷汇编1(总分:86.00,做题时间:90分钟)一、单项选择题(总题数:27,分数:54.00)1.一棵完全二叉树上有1001个结点,其中叶子结点的个数是( )。
【西安交通大学1996三、2(3分)】A.250B.500C.254D.505E.以上答案都不对√2.一棵124个叶结点的完全二叉树,最多有( )个结点。
【中国科学技术大学1995十四、3(2分)】A.247B.248 √C.249D.250E.2513.已知一棵完全二叉树中共有626个结点,叶子结点的个数应为( )。
【上海交通大学2005四、6(2分)】A.3 11B.3 12C.3 13 √D.3 14E.其他4.具有300个结点的二叉树,其高度至少应为( )。
【北京理工大学2006五、8(1分)】A.6B.7C.8D.9 √5.当结点数目一定时,具有最小深度的二叉树是( )。
【北京航空航天大学2005】A.满二叉树B.完全二叉树√C.线索二叉树D.二叉排序树设结点数目是n,n个结点未必是满二叉树,A错。
C和D明显错误。
6.二叉树的第I层上最多含有的结点数为( )。
【中山大学1998二、7(2分)】【北京理工大学2001六、5(2分)】A.2 IB.2 I-1一1C.2 I-1√D.2 I一17.从树根(第0层)起,自上到下,逐层从左到右给二叉树的所有结点从1开始编号,则完全二叉树的第h 层的从左到右第k个结点的编号为( )。
【电子科技大学2005一、6(1分)】A.2 h +h-1 √B.2 h一k+1C.2 h +k+1D.2 h一k-18.下列判断中,( )是正确的。
【华南理工大学2006一、2(2分)】A.深度为k的二叉树最多有2 k -1个结点(k≥1),最少有k个结点√B.二叉树中不存在度大于2的结点√C.对二叉树遍历是指先序、中序或后序遍历中的一种D.构造线索二叉树是为能方便找到每个结点的双亲9.一个具有1025个结点的二叉树的高h为( )。
FD&C Act Chapter III Prohibited Acts and Penalties 禁止行为及罚则
FD&C Act Chapter III: Prohibited Acts and PenaltiesSEC. 301. [21 USC §331] Prohibited actsNote: revisions were posted to this section in February 2008.The following acts and the causing thereof are hereby prohibited:1(a) The introduction or delivery for introduction into interstate commerce of any food, drug, device, or cosmetic that is adulterated or misbranded.(b) The adulteration or misbranding of any food, drug, device, or cosmetic in interstate commerce.(c) The receipt in interstate commerce of any food, drug, device, or cosmetic that is adulterated or misbranded, and the delivery or proffered delivery thereof for pay or otherwise.(d) The introduction or delivery for introduction into interstate commerce of any article in violation of section 404, 505 or 564.(e) The refusal to permit access to or copying of any record as required by section 412, 414, 416, 417(g), 504, 564, 703, 704(a), 760, or 761; or the failure to establish or maintain any record, or make any report, required under section 412, 414(b), 416, 417, 504, 505(i) or (k), 512(a)(4)(C), 512 (j), (l) or (m), 572(i),2 515(f), 519, 564, 760, or 761 or the refusal to permit access to or verification or copying of any such required record.(f) The refusal to permit entry or inspection as authorized by section 704.(g) The manufacture, within any Territory of any food, drug, device, or cosmetic that is adulterated or misbranded.(h) The giving of a guaranty or undertaking referred to in section 303(c)(2), which guaranty or undertaking is false, except by a person who relied upon a guaranty or undertaking to the same effect signed by, containing the name and address of, the person residing in the United States from whom he received in good faith the food, drug, device, or cosmetic; or the giving of a guaranty or undertaking referred to in section 303(c)(3), which guaranty or undertaking is false.(i) (1) Forging, counterfeiting, simulating, or falsely representing, or without proper authority using any mark, stamp, tag, label, or other identification device authorized or required by regulations promulgated under the provisions of section 404 or 721.(2) Making, selling, disposing of, or keeping in possession, control, or custody, or concealing any punch, die, plate, stone, or other thing designed to print, imprint, or reproduce the trademark, trade name, or other identifying mark, imprint, or device of another or any likeness of any of the foregoing upon any drug or container or labeling thereof so as to render such drug a counterfeit drug.(3) The doing of any act which causes a drug to be a counterfeit drug, or the sale or dispensing, or the holding for sale or dispensing, of a counterfeit drug.(j) The using by any person to his own advantage or revealing, other than to the Secretary or officers or employees of the Department, or to the courts when relevant in any judicial proceeding under this Act, any information acquired under authority of section 404, 409, 412, 414, 505, 510, 512, 513, 514, 515, 516, 518, 519, 520, 571, 572, 573 , 3 704, 708, or 721 concerning any method or process which as a trade secret is entitled to protection; or the violating of section 408(i)(2) or any regulation issued under that section. 4 This paragraph does not authorize the withholding of information from either House of Congress or from, to the extent of matter within its jurisdiction, any committee or subcommittee of such committee or any joint committee of Congress or any subcommittee of such joint committee.(k) The alteration, mutilation, destruction, obliteration, or removal of the whole or any part of the labeling of, or the doing of any other act with respect to, a food, drug, device, or cosmetic, if such act is done while such article is held for sale (whether or not the first sale) after shipment in interstate commerce and results in such article being adulterated or misbranded.(l) [Deleted] 5(m) The sale or offering for sale of colored oleomargarine or colored margarine, or the possession or serving of colored oleomargarine or colored margarine in violation of sections 407(b) or 407(c).(n) The using, in labeling, advertising or other sales promotion of any reference to any report or analysis furnished in compliance with section 704.(o) In the case of a prescription drug distributed or offered for sale in interstate commerce, the failure of the manufacturer, packer, or distributor thereof to maintain for transmittal, or to transmit, to any practitioner licensed by applicable State law to administer such drug who makes written request for information as to such drug, true and correct copies of all printed matter which is required to be included in any package in which that drug is distributed or sold, or such other printed matter as is approved by the Secretary. Nothing in this paragraph shall be construed to exempt any person from any labeling requirement imposed by or under other provisions of this Act.(p) The failure to register in accordance with section 510, the failure to provide any information required by section 510(j) or 510(k), 21 USC § 360(j) or (k)] or the failure to provide a notice required by section 510(j)(2).(q)(1) The failure or refusal to (A) comply with any requirement prescribed under section 518 or 520(g), (B) furnish any notification or other material or information required by or under section 519 or 520(g), or (C) comply with a requirement under section 522.(2) With respect to any device, the submission of any report that is required by or under this Act that is false or misleading in any material respect.(r) The movement of a device in violation of an order under section 304(g) or the removal or alteration of any mark or label required by the order to identify the device as detained.(s) The failure to provide the notice required by section 412(c) or 412(e), the failure to make the reports required by section 412(f)(1)(B), the failure to retain the records required by section 412(b)(4), or the failure to meet the requirements prescribed under section 412(f)(3).(t) The importation of a drug in violation of section 801(d)(1) , the sale, purchase, or trade of a drug or drug sample or the offer to sell, purchase, or trade a drug or drug sample in violation of section 503(c), the sale, purchase, or trade of a coupon, the offer to sell, purchase, or trade such a coupon, or the counterfeiting of such a coupon in violation of section 503(c)(2), the distribution of a drug sample in violation of section 503(d) or the failure to otherwise comply with the requirements of section 503(d), or the distribution of drugs in violation of section 503(e) or the failure to otherwise comply with the requirements of section 503(e).(u) The failure to comply with any requirements of the provisions of, or any regulations or orders of the Secretary, under section 512(a)(4)(A), 512(a)(4)(D), or 512(a)(5).(v) The introduction or delivery for introduction into interstate commerce of a dietary supplement that is unsafe under section 413.(w) The making of a knowingly false statement in any statement, certificate of analysis, record, or report required or requested under section 801(d)(3); the failure to submit a certificate of analysis as required under such section; the failure to maintain records or to submit records or reports as required by such section; the release into interstate commerce of any article or portion thereof imported into the United States under such section or any finished product made from such article or portion, except for export in accordance with section 801(e) or 802, or with section 351(h) of the Public Health Service Act [42 USC § 262(h)]; or the failure to so export or to destroy such an article or portions thereof, or such a finished product.(x) The falsification of a declaration of conformity submitted under section 514(c) or the failure or refusal to provide data or information requested by the Secretary under paragraph (3) of such section.(y) In the case of a drug, device, or food –(1) the submission of a report or recommendation by a person accredited under section 523 that is false or misleading in any material respect;(2) the disclosure by a person accredited under section 523 of confidential commercial information or any trade secret without the express written consent of the person who submitted such information or secret to such person; or(3) the receipt by a person accredited under section 523 of a bribe in any form or the doing of any corrupt act by such person associated with a responsibility delegated to such person under this Act.(z) [Terminated] 6( aa) The importation of a prescription drug in violation of section 804, the falsification of any record required to be maintained or provided to the Secretary under section, or any other violation of regulations under such section.(bb) The transfer of an article of food in violation of an order under section 304(h), or the removal or alteration of any mark or label required by the order to identify the article as detained.(cc) The importing or offering for import into the United States of an article of food by, with the assistance of, or at the direction of, a person debarred under section 306(b)(3).( dd) The failure to register in accordance with section 415.( ee) The importing or offering for import into the United States of an article of food in violation of the requirements under section 801(m).(ff) The importing or offering for import into the United States of a drug or device with respect to which there is a failure to comply with a request of the Secretary to submit to the Secretary a statement under section 801(o).(gg) The knowing failure to comply with paragraph (7)(E) of section 704(g); the knowing inclusion by a person accredited under paragraph (2) of such section of false information in an inspection report under paragraph (7)(A) of such section; or the knowing failure of such a person to include material facts in such a report.(hh) The failure by a shipper, carrier by motor vehicle or rail vehicle, receiver, or any other person engaged in the transportation of food to comply with the sanitary transportation practices prescribed by the Secretary under section 416.(ii)The falsification of a report of a serious adverse event submitted to a responsible person (as defined under section 760 or 761) or the falsification of a serious adverse event report (as defined under section 760 or 761) submitted to the Secretary.(jj) (1) The failure to submit the certification required by section 402(j)(5)(B) of the Public Health Service Act [42 USC § 282(j)(5)(B)], or knowingly submitting a false certification under such section.(2) The failure to submit clinical trial information required under subsection (j) of section 402 of the Public Health Service Act [42 USC § 282].(3) The submission of clinical trial information under subsection (j) of section 402 of the Public Health Service Act [42 USC § 282] that is false or misleading in any particular under paragraph (5)(D) of such subsection (j).(kk) [Note: This subsection takes effect 180 days after enactment of Act Sept. 27, 2007, P.L. 110-85, as provided by § 909(a) of such Act, which appears as a note to this section.] The dissemination of a television advertisement without complying with section 503B [21 USC § 353b].(ll) The introduction or delivery for introduction into interstate commerce of any food to which has been added a drug approved under section 505 [21 USC § 355], a biological product licensed under section 351 of the Public Health Service Act [42 USC § 262], or a drug or a biological product for which substantial clinical investigations have been instituted and for which the existence of such investigations has been made public, unless--(1) such drug or such biological product was marketed in food before any approval of the drug under section 505 [21 USC § 355], before licensure of the biological product under such section 351 [42 USC § 262], and before any substantial clinical investigations involving the drug or the biological product have been instituted;(2) the Secretary, in the Secretary's discretion, has issued a regulation, after notice and comment, approving the use of such drug or such biological product in the food;(3) the use of the drug or the biological product in the food is to enhance the safety of the food to which the drug or the biological product is added or applied and not to have independent biological or therapeutic effects on humans, and the use is in conformity with--(A) a regulation issued under section 409 [21 USC § 348] prescribing conditions of safe use in food;(B) a regulation listing or affirming conditions under which the use of the drug or the biological product in food is generally recognized as safe;(C) the conditions of use identified in a notification to the Secretary of a claim of exemption from the premarket approval requirements for food additives based on the notifier's determination that the use of the drug or thebiological product in food is generally recognized as safe, provided that the Secretary has not questioned the general recognition of safety determination in a letter to the notifier;(D) a food contact substance notification that is effective under section 409(h) [21 USC § 348(h)]; or(E) such drug or biological product had been marketed for smoking cessation prior to the date of the enactment of the Food and Drug Administration Amendments Act of 2007 [enacted Sept. 27, 2007]; or(4) the drug is a new animal drug whose use is not unsafe under section 512 [21 USC § 360b].(mm) The failure to submit a report or provide a notification required under section 417(d) [21 USC § 350f(d)].(nn) The falsification of a report or notification required under section 417(d) [21 USC § 350f(d)].Footnotes1. See footnote for section 403(h)(3) regarding the stylistic use of a list consisting of "(a)", "(b)", etc.2. The period is so in law. See section 102(b)(5)(C) of Public Law 108-2823. The period is so in law. See section 102f(b)(5)(D) of Public Law 108-282.4. So in law. See the amendment made by section 403 of Public Law 104–170 (110 Stat. 1514).5. Paragraph (l) was struck by section 421 of Public Law 105–115 (111 Stat. 2380).6. Paragraph (z) was added by subsection (b) of section 401(b) of Public Law 105–115 (111 Stat. 2364). Subsection (e) of such section provides as follows:(e) SUNSET.—The amendments made by this section cease to be effective September 30, 2006, or 7 years after the date on which the Secretary promulgates the regulations described in subsection (c), whichever is later.SEC. 302. [21 USC §332] Injunction proceedings(a) The district courts of the United States and the United States courts of the Territories shall have jurisdiction, for cause shown 7 to restrain violations of section 301, except paragraphs (h), (i), and (j).(b) In case of violation of an injunction or restraining order issued under this section, which also constitutes a violation of this Act, trial shall be by the court, or, upon demand of the accused, by a jury.SEC. 303. [21 USC §333] PenaltiesNote: revisions were posted to this section in February 2008.[Note: See prospective amendment note below.](a) Violation of 21 USC § 331; second violation; intent to defraud or mislead.(1) Any person who violates a provision of section 301 shall be imprisoned for not more than one year or fined not more than $1,000, or both.(2) Notwithstanding the provisions of paragraph (1) of this section, if any person commits such a violation after a conviction of him under this section has become final, or commits such a violation with the intent to defraud or mislead, such person shall be imprisoned for not more than three years or fined not more than $10,000 or both.(b) Prescription drug market violations(1) Notwithstanding subsection (a), any person who violates section 301(t) by—(A) knowingly importing a drug in violation of section 801(d)(1),(B) knowingly selling, purchasing, or trading a drug or drug sample or knowingly offering to sell, purchase, or trade a drug or drug sample, in violation of section 503(c)(1),(C) knowingly selling, purchasing, or trading a coupon, knowingly offering to sell, purchase, or trade such a coupon, or knowingly counterfeiting such a coupon, in violation of section 503(c)(2), or(D) knowingly distributing drugs in violation of section 503(e)(2)(A),shall be imprisoned for not more than 10 years or fined not more than $250,000, or both.(2) Any manufacturer or distributor who distributes drug samples by means other than the mail or common carrier whose representative, during the course of the representative's employment or association with that manufactureror distributor, violated section 301(t) because of a violation of section 503(c)(1) or violated any State law prohibiting the sale, purchase, or trade of a drug sample subject to section 503(b) or the offer to sell, purchase, or trade such a drug sample shall, upon conviction of the representative for such violation, be subject to the following civil penalties:(A) A civil penalty of not more than $50,000 for each of the first two such violations resulting in a conviction of any representative of the manufacturer or distributor in any 10-year period.(B) A civil penalty of not more than $1,000,000 for each violation resulting in a conviction of any representative after the second conviction in any 10-year period.For the purposes of this paragraph, multiple convictions of one or more persons arising out of the same event or transaction, or a related series of events or transactions, shall be considered as one violation.(3) Any manufacturer or distributor who violates section 301(t) because of a failure to make a report required by section 503(d)(3)(E) shall be subject to a civil penalty of not more than $100,000.(4)(A) If a manufacturer or distributor or any representative of such manufacturer or distributor provides information leading to the institution of a criminal proceeding against, and conviction of, any representative of that manufacturer or distributor for a violation of section 301(t) because of a sale, purchase, or trade or offer to purchase, sell, or trade a drug sample in violation of section 503(c)(1) or for a violation of State law prohibiting the sale, purchase, or trade or offer to sell, purchase, or trade a drug sample, the conviction of such representative shall not be considered as a violation for purposes of paragraph (2).(B) If, in an action brought under paragraph (2) against a manufacturer or distributor relating to the conviction of a representative of such manufacturer or distributor for the sale, purchase, or trade of a drug or the offer to sell, purchase, or trade a drug, it is shown, by clear and convincing evidence—(i) that the manufacturer or distributor conducted, before the institution of a criminal proceeding against such representative for the violation which resulted in such conviction, an investigation of events or transactions which would have led to the reporting of information leading to the institution of a criminal proceeding against, and conviction of, such representative for such purchase, sale, or trade or offer to purchase, sell, or trade, or(ii) that, except in the case of the conviction of a representative employed in a supervisory function, despite diligent implementation by the manufacturer or distributor of an independent audit and security system designed to detect such a violation, the manufacturer or distributor could not reasonably have been expected to have detected such violation, the conviction of such representative shall not be considered as a conviction for purposes of paragraph (2).(5) If a person provides information leading to the institution of a criminal proceeding against, and conviction of, a person for a violation of section 301(t) because of the sale, purchase, or trade of a drug sample or the offer to sell, purchase, or trade a drug sample in violation of section 503(c)(1), such person shall be entitled to one-half of the criminal fine imposed and collected for such violation but not more than $125,000.(6) Notwithstanding subsection (a), any person who is a manufacturer or importer of a prescription drug under section 804(b) and knowingly fails to comply with a requirement of section 804(e) that is applicable to such manufacturer or importer, respectively, shall be imprisoned for not more than 10 years or fined not more than $250,000, or both.(c) Exceptions in certain cases of good faith, etc. No person shall be subject to the penalties of subsection (a)(1) of this section, (1) for having received in interstate commerce any article and delivered it or proffered delivery of it, if such delivery or proffer was made in good faith, unless he refuses to furnish on request of an officer or employee duly designated by the Secretary the name and address of the person from whom he purchased or received such article and copies of all documents, if any there be, pertaining to the delivery of the article to him; or (2) for having violated section 301(a) or (d), if he establishes a guaranty or undertaking signed by, and containing the name and address of, the person residing in the United States from whom he received in good faith the article, to the effect, in case of an alleged violation of section 301(a), that such article is not adulterated or misbranded, within the meaning of this Act, designating this Act, or to the effect, in case of an alleged violation of section 301(d), that such article is not an article which may not, under the provisions of section 404 or 505, be introduced into interstate commerce; or (3) for having violated section 301(a), where the violation exists because the article is adulterated by reason of containing a color additive not from a batch certified in accordance with regulations promulgated by the Secretary under this Act, if such person establishes a guaranty or undertaking signed by, and containing the name and address of, the manufacturer of the color additive, to the effect that such color additive was from a batch certified in accordance with the applicable regulations promulgated by the Secretary under this Act; or (4) for having violatedsection 301 (b), (c), or (k) by failure to comply with section 502(f) in respect to an article received in interstate commerce to which neither section 503(a) nor section 503(b)(1) is applicable, if the delivery or proffered delivery was made in good faith and the labeling at the time thereof contained the same directions for use and warning statements as were contained in the labeling at the time of such receipt of such article; or (5) for having violated section 301(i)(2) if such person acted in good faith and had no reason to believe that use of the punch, die, plate, stone, or other thing involved would result in a drug being a counterfeit drug, or for having violated section 301(i)(3) if the person doing the act or causing it to be done acted in good faith and had no reason to believe that the drug was a counterfeit drug.(d) Exceptions involving misbranded food. No person shall be subject to the penalties of subsection (a)(1) of this section for a violation of section 301 involving misbranded food if the violation exists solely because the food is misbranded under section 403(a)(2) because of its advertising.(e) Prohibited distribution of human growth hormone.(1) Except as provided in paragraph (2), whoever knowingly distributes, or possesses with intent to distribute, human growth hormone for any use in humans other than the treatment of a disease or other recognized medical condition, where such use has been authorized by the Secretary of Health and Human Services under section 505 and pursuant to the order of a physician, is guilty of an offense punishable by not more than 5 years in prison, such fines as are authorized by title 18, United States Code, or both.(2) Whoever commits any offense set forth in paragraph (1) and such offense involves an individual under 18 years of age is punishable by not more than 10 years imprisonment, such fines as are authorized by title 18, United States Code, or both.(3) Any conviction for a violation of paragraphs (1) and (2) of this subsection shall be considered a felony violation of the Controlled Substances Act for the purposes of forfeiture under section 413 of such Act.(4) As used in this subsection the term "human growth hormone" means somatrem, somatropin, or an analogue of either of them.(5) The Drug Enforcement Administration is authorized to investigate offenses punishable by this subsection.(f) Violations related to devices.(1) (A) 8 Except as provided in subparagraph (B), any person who violates a requirement of this Act which relates to devices shall be liable to the United States for a civil penalty in an amount not to exceed $15,000 for each such violation, and not to exceed $1,000,000 for all such violations adjudicated in a single proceeding. For purposes of the preceding sentence, a person accredited under paragraph (2) of section 704(g) who is substantially not in compliance with the standards of accreditation under such section, or who poses a threat to public health or fails to act in a manner that is consistent with the purposes of such section, shall be considered to have violated a requirement of this Act that relates to devices.(B) Subparagraph (A) shall not apply—(i) to any person who violates the requirements of section 519(a) or 520(f) unless such violation constitutes (I) a significant or knowing departure from such requirements, or (II) a risk to public health,(ii) to any person who commits minor violations of section 519(e) or 519(g) (only with respect to correction reports) if such person demonstrates substantial compliance with such section, or(iii) to violations of section 501(a)(2)(A) which involve one or more devices which are not defective.(2)(A) Any person who introduces into interstate commerce or delivers for introduction into interstate commerce an article of food that is adulterated within the meaning of section 402(a)(2)(B) shall be subject to a civil money penalty of not more than $50,000 in the case of an individual and $250,000 in the case of any other person for such introduction or delivery, not to exceed $500,000 for all such violations adjudicated in a single proceeding.(B) This paragraph shall not apply to any person who grew the article of food that is adulterated. If the Secretary assesses a civil penalty against any person under this paragraph, the Secretary may not use the criminal authorities under this section to sanction such person for the introduction or delivery for introduction into interstate commerce of the article of food that is adulterated. If the Secretary assesses a civil penalty against any person under this paragraph, the Secretary may not use the seizure authorities of section 304 or the injunction authorities of section 302 with respect to the article of food that is adulterated.(C) In a hearing to assess a civil penalty under this paragraph, the presiding officer shall have the same authority with regard to compelling testimony or production of documents as a presiding officer has under section408(g)(2)(B). The third sentence of paragraph (5)(A) shall not apply to any investigation under this paragraph.(3)(A) Any person who violates section 301(jj) [21 USC § 331(jj)] shall be subject to a civil monetary penalty of not more than $ 10,000 for all violations adjudicated in a single proceeding.(B) If a violation of section 301(jj) [21 USC § 331(jj)] is not corrected within the 30-day period following notification under section 402(j)(5)(C)(ii) [21 USC § 342(j)(5)(C)(ii)], the person shall, in addition to any penalty under subparagraph (A), be subject to a civil monetary penalty of not more than $ 10,000 for each day of the violation after such period until the violation is corrected.(4) [Note: This paragraph takes effect 180 days after enactment of Act Sept. 27, 2007, P.L. 110-85, as provided by § 909(a) of such Act, which appears as 21 USC § 331 note.](A) Any responsible person (as such term is used in section 505-1 [21 USC § 355-1]) that violates a requirement of section 505(o), 505(p), or 505-1 [21 USC § 355(o), 355(p), or 355-1] shall be subject to a civil monetary penalty of--(i) not more than $ 250,000 per violation, and not to exceed $ 1,000,000 for all such violations adjudicated in a single proceeding; or(ii) in the case of a violation that continues after the Secretary provides written notice to the responsible person, the responsible person shall be subject to a civil monetary penalty of $ 250,000 for the first 30-day period (or any portion thereof) that the responsible person continues to be in violation, and such amount shall double for every 30-day period thereafter that the violation continues, not to exceed $ 1,000,000 for any 30-day period, and not to exceed $ 10,000,000 for all such violations adjudicated in a single proceeding.B) In determining the amount of a civil penalty under subparagraph (A)(ii), the Secretary shall take into consideration whether the responsible person is making efforts toward correcting the violation of the requirement of section 505(o), 505(p), or 505-1 [21 USC § 355(o), 355(p), or 355-1] for which the responsible person is subject to such civil penalty.(5) (A) A civil penalty under paragraph (1), (2), or (3) shall be assessed by the Secretary by an order made on the record after opportunity for a hearing provided in accordance with this subparagraph and section 554 of title 5, United States Code. Before issuing such an order, the Secretary shall give written notice to the person to be assessed a civil penalty under such order of the Secretary's proposal to issue such order and provide such person an opportunity for a hearing on the order. In the course of any investigation, the Secretary may issue subpoenas requiring the attendance and testimony of witnesses and the production of evidence that relates to the matter under investigation.(B) In determining the amount of a civil penalty, the Secretary shall take into account the nature, circumstances, extent, and gravity of the violation or violations and, with respect to the violator, ability to pay, effect on ability to continue to do business, any history of prior such violations, the degree of culpability, and such other matters as justice may require.(C) The Secretary may compromise, modify, or remit, with or without conditions, any civil penalty which may be assessed under paragraph (1), (2), or (3). The amount of such penalty, when finally determined, or the amount agreed upon in compromise, may be deducted from any sums owing by the United States to the person charged.(6) Any person who requested, in accordance with paragraph (5)(A), a hearing respecting the assessment of a civil penalty and who is aggrieved by an order assessing a civil penalty may file a petition for judicial review of such order with the United States Court of Appeals for the District of Columbia Circuit or for any other circuit in which such person resides or transacts business. Such a petition may only be filed within the 60-day period beginning on the date the order making such assessment was issued.(7) If any person fails to pay an assessment of a civil penalty—(A) after the order making the assessment becomes final, and if such person does not file a petition for judicial review of the order in accordance with paragraph (6), or(B) after a court in an action brought under paragraph (6) has entered a final judgment in favor of the Secretary, the Attorney General shall recover the amount assessed (plus interest at currently prevailing rates from the date of the expiration of the 60-day period referred to in paragraph (6) or the date of such final judgment, as the case may be) in an action brought in any appropriate district court of the United States. In such an action, the validity, amount, and appropriateness of such penalty shall not be subject to review.。
第三章 认识1000以内的数 单元测试题1-二年级数学下册 冀教版(解析版)
冀教版小学二年级数学下册第三章认识1000以内的数单元测试题一、单选题(共10题;共20分)1.一个三位数,个位上的数字是最大的一位数,十位上是2,百位上的数字比十位上的数字多5,这个数是()。
A. 729B. 279C. 7922.张平家藏书800本,王红家比张平家少得多。
王红家藏书可能是()本。
A. 900B. 750C. 3603.3个百和5个一组成的数是()。
A. 335B. 325C. 3054.连环画有96页,故事书比连环画厚得多,故事书有()页。
A. 90B. 100C. 1805.把359、539、953按照从大到小的顺序排列正确的是()A. 359>539>953B. 953>359>539C. 953>539>3596.一个盘子里大约有100颗,()个这样的盘子大约有一千颗。
A. 7B. 8C. 9D. 107.与459相邻的两个数是()A. 458和460B. 461和460C. 457和458D. 457和4618.一百一百地数,800后面第二个数是()。
A. 600B. 700C. 900D. 10009.一千里面有()个百A. 1B. 10C. 100D. 100010.505、550、500这三个数中,()最大。
A. 505B. 550C. 500二、判断题(共5题;共10分)11.30个十等于3个百。
()12.读数和写数时,都要从最高位读起和写起。
( )13.1000>999()14.三位数一定比两位数大。
()15.998往后再数两个数是999、1000。
()三、填空题(共10题;共21分)16.用2、7、9组成的两位数中,最小的数是________。
17.一个数的最高位是百位,它是一个________位数。
18.800里面有________个百,700是由________个十组成的。
19.十个十个地数,和900相邻的两个数是________和________。
平面直角坐标系重难点题型(四大题型)(解析版)—2024-2025学年八年级数学上册(北师大版)
平面直角坐标系重难点题型(四大题型)【题型1 两点间距离】【题型2 求平面直角坐标系中动点问题的面积】【题型3 平面直角坐标系中规律题探究】【题型4 等腰三角形个数讨论问题】【题型1 两点间距离】1.在平面直角坐标系中,A,B两点的坐标分别为(5,―1),(5,2),则A,B两点间的距离为.2.已知平面直角坐标系中有一点M(3―2m,3m+2).(1)存在点N(2,―3),当MN平行于y轴时,求点M的坐标:(2)当点M在x轴上方,且到x轴的距离是到y轴距离的两倍时,求点M的坐标.3.已知点P(2m―1,m+3).(1)若点P在y轴上,求m的值;(2)若点P在第一象限,且点P到x轴的距离是到y轴的距离的4倍,求点P的坐标.4.阅读理解:如何根据坐标求出两点之间的距离?如图,在坐标系中A(2,1),B(6,4),构造Rt△ACB,则AC=6―2=4,BC=4―1=3,∴AB===5若A(x1,y1),B(x2,y2),则AC=|x2―x1|BC=|y2―y1|∴AB==这就是两点间的距离公式,例如E(0,1),D(4,0)∴ED===(1)+A(12,3)与点C(x,0)点B(0,2)与点C(x,0)的距离.请完成如下填空:作点B关于x轴的对称点B′(____,___),当A、C、B′三点共线时AC+BC最小,连接AB′,则AC+BC 的最小值等于AB′,由两点间的距离公式得AB′=______________,_____________.(2)借助上面的思考过程,画图说明并求出代数式:②的最大值.,当A、C、B′三点共线时AC+AB′=(12―0)2+(3+2)的最小值是13.1),当A、C、B′三点共线时AC+BC5.阅读材料:两点间的距离公式:如果平面直角坐标系内有两点A(x1,y1)、B(x2,y2),那么A、B两点的距离AB=AB2=(x1―x2)2+(y1―y2)2.例如:若点A(4,1),B(3,2),则AB==若点A(a,1),B(3,2),且AB=2=(a―3)2+(1―2)2.a的值.根据上面材料完成下列各题:(1)若点A―2,3,B m,2①若m=1,则A、B两点间的距离是______.②若AB∥y轴,则A、B两点间得距离是______.(2)若点A―2,3,点B在x轴上,且A、B两点间的距离是5,求B点坐标.【题型2 求平面直角坐标系中动点问题的面积】6.如图,在平面直角坐标系中,已知A(a,0),B(b,0),其中a,b(b―2)2=0.(1)填空:a=______,b=_______;(2)如果在第三象限内有一点M(―3,m),请用含m的式子表示△ABM的面积;(3)在(2)的条件下,当m=―3时,在y轴上有一点P,使得△ABP的面积是△ABM的面积的2倍,请求出点P的坐标.7.已知在平面直角坐标系中有点A(―2,1),B(3,1),C(2,3),D(―1,5).(1)在坐标系内描出△ABC的位置;(2)求出△ACD的面积;(3)在y轴上是否存在一点P使得以A、B、P三点为顶点的三角形面积为10,若存在,请直接写出点P 的坐标,若不存在,请说明理由.,D(―1,5),在平面直角坐标系中顺次连接得到△ACD,3×12―2×4×12=16―2―3―4=7三点为顶点的三角形面积为10,,在平面直角坐标系顺次连接A、B、P,三点围成的三角形高为ℎ,=y,即P(0,5),=y,即P(0,―3),8.在平面直角坐标系中,A(a,0),C(b,2),满足(a+1)2=0,过点C作CB⊥x轴于点B.(1)如图1,连接AC,求三角形ABC的面积.(2)如图2,连接AC,若过点B作BD∥AC交y轴于点D,且AE,DE分别平分∠CAB,∠ODB,求∠AED?(3)如图3,过C作CD垂直于点D,连接AD,点P从D点出发,沿“DC→CB”移动,若点P的速度为每秒1个单位长度,运动时间t秒,当P运动到什么位置时,直线OP将四边形ABCD面积分为3:11两部分?求出P的坐标?=∠2,×2,2,9.如图,已知A(―4,0),B(4,0),C(3,2),D(―2,4).(1)求四边形ABCD的面积;(2)在y轴上存在一点P APB的面积等于四边形ABCD面积的一半,求P点的坐标.【答案】(1)20(2)P点的坐标(0,2.5)或(0,―2.5).【分析】此题主要考查了多边形面积及坐标系的基础知识,解题关键是熟练掌握基础图形面积公式.(1)观察图形,用分割法求解,分别过C、D两点作x轴的垂线,将图形分割为两个直角三角形和一个直角梯形,再根据直角三角形和直角梯形的面积公式求面积和即可;(2)P点的纵坐标到原点的距离就是△APB的AB边上的高,根据(1)P点到原点的距离,再根据P点分别在y轴正负半轴,写出P点的坐标即可.【详解】(1)解:分别过C、D两点作x轴的垂线,垂足分别为E、F,如下图:D(―2,4),BE=1,CE=2,CDFE+S△BCE+12×1×210.如图,在直角坐标平面内,已知A(―1,2),B(1,―2),C(4,0),线段AB经过原点O.(1)求△ABC的面积;(2)在x轴上是否存在一点D,使S△ACD=S△ABC,如果存在,求出点D的坐标,如果不存在说明理由.【答案】(1)8(2)(―4,0)或(12,0)【分析】本题考查了求平面直角坐标系中三角形的面积;【题型3 平面直角坐标系中规律题探究】11.如图,在平面直角坐标系中,每个最小方格的边长均为1个单位长,P1,P2,P3,…,均在格点上,其顺序按图中“→”方向排列,如:P1(0,0),P2(0,1),P3(1,1),P4(1,―1),P5(―1,―1),P6(―1,2)…根据这个规律,点P2019的坐标为()A.(―505,505)B.(505,―505)C.(505,―506)D.(505,505)【答案】D【分析】本题考查了规律型:点的坐标,根据前几个点的坐标,总结出规律是解题的关键.根据各个点的位置关系,可得出从P3(1,1)开始,下标为4的倍数的点在第四象限的角平分线上,被4除余1的点在第三象限的角平分线上,被4除余2的点在第二象限的角平分线上,被4除余3的点在第一象限的角平分线上,所以点P2019的在第四象限的角平分线上,然后根据规律求解即可.【详解】解:∵P1(0,0),P2(0,1),P3(1,1),P4(1,―1),P5(―1,―1),P6(―1,2),...,∴从P3(1,1)开始,P4n(n,―n)在第四象限的角平分线上,P4n+1(―n,―n)在第三象限的角平分线上,P4n+2(―n,n+1)在第二象限的角平分线上,P4n+3(n+1,n+1)在第一象限的角平分线上,∵2019=4×504+3,∴点P2019的在第一象限的角平分线上,n=504,∴点P2019坐标为(505,505)故选D.12.如图,动点P在平面直角坐标系中按图中箭头所示方向运动,第一次从原点O运动到点P1(1,2),第二次运动到点P2(2,0),第三次运动到P3(3,―1),…,按这样的运动规律,第23次运动后,动点P23的纵坐标是( )A.―1B.0C.1D.2【答案】C【分析】本题考查了规律型点的坐标,数形结合并从图象中发现循环规律:纵坐标每6次运动组成一个循环是解题的关键.由图得点的纵坐标变化每6次一循环,23÷6=3......5,点P23的纵坐标为符合第5个点的纵坐标为1.【详解】解:由图得,点每运动一次横坐标就增加1,∴P23的横坐标为23,点的纵坐标变化每6次一循环,23÷6=3......5,∴点P23的纵坐标为1.故选:C.13.在一单位为1的方格纸上,有一列点A1,A2,A3,⋯,A n,⋯,(其中n为正整数)均为网格上的格点,按如图所示规律排列,点A1(2,0),A2(1,―1),A3(0,0),A4(2,2),⋯⋯,则A2024的坐标为()A.(―1010,0)B.(2,1012)C.(1012,2)D.(1014,0)14.如图,在平面直角坐标系中,一动点从原点出发,沿着箭头所示方向,每次移动1个单位,依次得到点P1(1,0),P2(1,1),P3(0,1),P4(0,2),P5(1,2),…,根据这个规律,点P28的坐标为()A.(0,14)B.(1,7)C.(0,8)D.(1,8)【答案】A【分析】本题主要考查了点的坐标规律探索,根据题意可得规律每四次移到为一个循环,横坐标为1,1,0,0依次出现,纵坐标每2次移到增加1,据此规律求解即可.【详解】解:P1(1,0),P2(1,1),P3(0,1),P4(0,2),P5(1,2),……,以此类推可知,每四次移到为一个循环,横坐标为1,1,0,0依次出现,纵坐标每2次移到增加1,∵28÷4=7,28÷2=14,∴点P28的横坐标为0,纵坐标为14,∴点P28的坐标为(0,14),故选:A.15.如图,平面直角坐标系中,x轴负半轴上有一点A(―1,0).点A第一次向上平移1个单位至点A1(―1,1),接着又向右平移1个单位至点A2(0,1),然后再向上平移1个单位至点A3(0,2),向右平移1个单位至点A4(1,2),…,照此规律平移下去,点A2024的坐标是()A.(1010,1011)B.(1011,1012)C.(1010,1012)D.(1011,1013)16.如图,点A在y轴正半轴及x轴正半轴上交替运动,点A从原点出发,依次跳动至点A1(0,1),A2 (1,0),A3(2,0),A4(0,2),A5(0,3),A6(3,0),A7(4,0),A8(0,4),…,按此规律,则点A2024的坐标是()A.(0,1011)B.(1011,0)C.(0,1012)D.(1012,0)【答案】C【分析】本题考查规律探索,根据已知的点坐标,对点分组找出规律是解题的关键.根据已知点的坐标特征,将连续的4个点看成一组,由第1组,第2组确定组内点的位置特征、点坐标与组序数的联系;以此类推,2023=4×505+3,故点A2024是第506组的第4个点,则A2024在y 轴上,其非零坐标即横坐标为2×506=1012.【详解】解:根据题意,将连续的4个点A看成一组,第1组:A1(0,1),A2(1,0),A3(2,0),A4(0,2),其位置分别为y轴、x轴、x轴、y轴,前两个点的非零坐标为1,后两个点的非零坐标为2;其中,1=2×1―1,2=2×1;第2组:A5(0,3),A6(3,0),A7(4,0),A8(0,4),其位置分别为y轴、x轴、x轴、y轴,前两个点的非零坐标为3,后两个点的非零坐标为4;其中,3=2×2―1,4=2×2;……以此类推,2024=4×506,则点A2024是第506组的第4个点,则A2024在y轴上,其非零坐标即横坐标为1012,故点A2024的坐标是(0,1012),故选:C.17.如图,在一个单位为1的方格纸上,△A1A2A3,△A3A4A5,△A5A6A7,是斜边在x轴上,斜边长分别为2,4,6的等腰直角三角形.若△A1A2A3的顶点坐标分别为A1(2,0),A2(1,―1),A3(0,0),则依图中所示规律,A2024的横坐标为.18.如图所示,在平面直角坐标系中,A(1,1),B(―1,1),C(―1,―2),D(1,―2).把一条长为2019个单位长度且没有弹性的细线(线的粗细忽略不计)的一端固定在点A处,并按A→B→C→D→A→…的规律紧绕在四边形ABCD的边上,则细线另一端所在位置的点的坐标是()A.(1,0)B.(0,1)C.(0,―2)D.(1,―2)【答案】A【分析】本题考查的是坐标规律的探究,先求解四边形ABCD的周长为2(AB+BC)=10.结合2019÷10=201……9,从而可得答案.【详解】解:∵A(1,1),B(―1,1),C(―1,―2),D(1,―2),∴AB=2,BC=3,∴四边形ABCD的周长为2(AB+BC)=10.∵2019÷10=201……9,∴细线另一端所在位置的点的坐标为(1,0).故选A.19.如图,在平面直角坐标系上有点A(1,―1),点A第一次向左跳动至A1(―1,0),第二次向右跳动至A2 (2,0),第三次向左跳动至A3(―2,1),第四次向右跳动至A4(3,1)…依照此规律跳动下去,点A第2021次跳动至A2021的坐标()A.(―1011,1010)B.(―1011,1009)C.(1012,1010)D.(1012,1009)20.如图,在平面直角坐标系中,有若干个整数点,其顺序按图中“→”方向排列,如(0,1),(―1,2),(0,2),(1,2) ,(2,3),(1,3),(0,3),……,根据这个规律探索可得第2024个点的坐标是()A.(43,45)B.(44,45)C.(―43,45)D.(―42,45)【答案】C【分析】本题考查了点的坐标规律探索,探索出点的坐标规律是解题的关键;按点的纵坐标分类:纵坐标是1的点有1个,纵坐标是2的点有3个,纵坐标是3的点有5个,纵坐标是4的点有7个,……,一般地,纵坐标为n的点有(2n―1)个;考虑点排列方向:纵坐标是1、3、5、7,……,点是从右往左的方向,纵坐标是2、4、6,……,点是从左往右排列的方向;而452=2025,当纵坐标是45时,这样的点共有89个,且点是从右往左方向,则可得第2024个点的坐标.【详解】解:纵坐标是1的点有1个,纵坐标是2的点有3个,纵坐标是3的点有5个,纵坐标是4的点有7个,……,一般地,纵坐标为n的点有(2n―1)个,且这n个点的横坐标从左往右依次是―n+1,―n+2,⋯,―1,0,1,⋯,n―1;考虑点排列方向:纵坐标是1、3、5、7,……,点是从右往左的方向,纵坐标是2、4、6,……,点是从左往右排列的方向;∵452=2025,当纵坐标是45时,这样的点共有89个,且点是从右往左方向,∴最左边的点坐标为(―44,45),即第2025个点的坐标,∴第2024个点的坐标为(―43,45).故选:C.21.如图,在平面直角坐标系中,一点自P0(1,0)处向上运动1个单位长度至P1(1,1),然后向左运动2个单位长度至P2处,再向下运动3个单位长度至P3处,再向右运动4个单位长度至P4处,再向上运动5个单位长度至P5处,…,按此规律继续运动,则P2024的坐标是()A.(―1011,―1012)B.(1013,―1012)C.(―1011,1012)D.(1013,1012)【答案】B【分析】本题考查坐标系下点的规律探究.解题的关键是找到点的横纵坐标的数字规律.先确定点P2024在第三象限,根据第三象限各点横坐标、纵坐标的数据得出规律P2n(n+1,―n),进而得出答案即可.【详解】解:∵2024÷4=506,则P2024在第四象限,由题意,第四象限的点为P4(3,―2),P8(5,―4),……,P2n(n+1,―n)∴P2024(1013,―1012).故选:B.22.平面直角坐标系中有若干点,按照如图所示的方式排列,其坐标依次为A1(1,0),A2(1,1),A3(―1,1),A4(―1,―1),A5(2,―1),…按此规律,点A2024的坐标为()A.(―505,―505)B.(―505,506)C.(―506,―506)D.(507,―506)23.将从1开始的连续自然数按以下规律排列:若有序数对(n,m)表示第n行,从左到右第m个数,如(3,2)表示6,则252表示的有序数对是()A.(14,16)B.(14,24)C.(16,27)D.(16,29)【答案】C【分析】本题考查数字规律的性质,解题的关键是熟练掌握数字规律的相关性质.分析每一行的第一个数字的规律,得出第n行的第一个数字为1+(n―1)2,从而求得最终的答案.【详解】第1行的第一个数字:1=1+(1―1)2第2行的第一个数字:2=1+(2―1)2第3行的第一个数字:5=1+(3―1)2第4行的第一个数字:10=1+(4―1)2第5行的第一个数字:17=1+(5―1)2…..,设第n行的第一个数字为x,得x=1+(n―1)2设第n+1行的第一个数字为z,得z=1+n2设第n行,从左到右第m个数为y当y=252时1+(n―1)2≤252<1+n2∴(n―1)2≤251<n2∵n为整数∴n=16∴x=1+(n―1)2=226∴m=252―226+1=27,∴252表示的有序数对是(16,27)故选:C.24.如图,在平面直角坐标系中,动点A从1,0出发,向上运动1个单位长度到达点B1,1,分裂为两个点,分别向左、右运动到点C0,2,D2,2,此时称动点A完成第一次跳跃;再分别从C,D 点出发,每个点重复上面的运动,到达点G―1,4,H1,4,I3,4,此时称动点A完成第二次跳跃;依此规律跳跃下去,动点A完成第2023次跳跃时,最右边一个点的坐标是( )A.2023,4046B.2023,22023C.2024,4046D.2024,22023【答案】C【分析】根据题意找到点坐标变化的规律即可.【详解】解:由题意可得:A1,0、D2,2、I3,4...每完成一次跳跃,最右边一个点的纵坐标增加2,到达点的横坐标增加1,则动点A完成第2023次跳跃时,最右边一个点纵坐标为2023×2=4046,横坐标为:2023+1=2024故选:C.【点睛】本题考查了点坐标规律的探索.根据题意寻找变化规律是解题关键.【题型4 等腰三角形个数讨论问题】25.在平面直角坐标系中,O为坐标原点,点A(―4,4―5a)位于第二象限,点B(―4,―a―1)位于第三象限,且a为整数.(1)求点A和点B的坐标.(2)若点C(m,0)为x轴上一点,且△ABC是以BC为底的等腰三角形,求m的值.26.如图1,在平面直角坐标系中,A,B在x轴上,C在y轴上,BC2+CO2=AC2.(1)求证:AO=BC;(2)如图2,若点A(―5,0),C(0,4),现有一个动点P从点A出发,沿着x轴正方向运动,连结PC,当ΔPCB为等腰三角形时,求点P的坐标;(3)如图3,若AB=AC,点B1,0),过O作OE//BC交AC于E,求OE的长.27.长方形ABCO在平面直角坐标系的位置如左图所示,A、C两点分别在x、y轴上,且B点的坐标为(8,6),P为射线AO上的一动点,点O关系直线PC的对称点为D.(1)当ΔOPC的面积为12时,则点P坐标为_______________.(2)当ΔAPC为等腰三角形时,求点P坐标.(3)如图所示,若点O关于直线PC的对称点为点D,当ΔAPD为直角三角形时,请直接写出点P坐标.∠P1D1C=∠P1OC=∠P1D1A,AC=10,设OP1=x,则P1D1=x,2+16=(8―x)2∴x=3∴P1(3,0)OP2=P2D2=6∴P2(6,0)OP3=P3D3=6∴P3(―6,0)∠P4D4C=90∘,AD4=10+6=16,设OP4=x,则P4D4=x,AP8)2∴x=12∴P(―12,0)分类讨论思想;3.数形结合思想.28.如图,在平面直角坐标系中,A(0,a)为y轴正方向上一点,B(b,0)为x轴正方向上一点,(b―6)2=0.(1)求线段AB的长;(2)点C是线段AC上一点,如果BC平分∠ABO,求点C的坐标;(3)点P是x轴上一动点,且△PAB为等腰三角形,直接写出所有符合条件的点P的坐标.时,如图;OP′=OB+P′B=6+10=16,P(x,0),=(6―x)2,股定理,灵活运用这些知识是关键.。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
The 505(b)(2) Drug Development Pathway:
When and How to Take Advantage of a Unique American Regulatory Pathway
By Mukesh Kumar, PhD, RAC and Hemant Jethwani, MSRegulatory Focus9
The 505(b)(2) regulation offers a less expensive and faster new drug development pathway that may be particularly attractive to a manufacturer with experience in developing generic products. It involves making significant changes to an existing product approved by the US Food and Drug Administration (FDA), called the reference product, to create a new drug with its own indica-tion, formulation, target population and/or other differences that need to be supported with clini-cal studies. A major advantage of this pathway is that it allows a sponsor to rely, at least in part, on FDA’s findings of safety and/or effectiveness for the previously approved drug, thereby reducing the number of clinical trials required for approval. Another incentive is three to five years of market exclusivity for 505(b)(2) products, depending upon the extent of changes to the reference prod-uct and the type of clinical data included in the approved New Drug Application (NDA). However, like all drug development strat-egies, the 505(b)(2) pathway requires careful consideration and planning. Important issues to consider include intellectual property concerns, the amount and quality of supporting informa-tion available from reference products or the literature, the logistics of conducting clinical trials with generic-like products, market compe-tition for approved products and requirements for an international product launch. Here we discuss practical strategies for drug development via the 505(b)(2) regulatory pathway.Drugs Can be Approved via One of Three Regulatory PathwaysNew drug products can belong to one of two broad categories: brand new drugs and identical or close copies of previously approved drugs, also called generics. Globally, separate regulatory pathways for innovator products and generic drugs are well established. US regulations, how-ever, divide these drugs into three categories: (1) new drugs, covered under Section 505(b)(1) of the Food, Drug, and Cosmetics Act (FD&C Act); (2) generic drugs, covered under Section 505(j) of the FD&C Act; and (3) “similar” drugs, covered under Section 505 (b)(2). It is the third category that is discussed here.The generic and 505(b)(2) categories were added by the Drug Price Competition and Patent Term Restoration Act of 1984, usually referred to as the Hatch-Waxman Act. The Hatch-Waxman Act aimed to promote generics while leaving intact a financial incentive for new product research and development. It was an attempt to balance the need for innovation with the desire for lower-cost alternatives within a reasonable length of time. Drug companies were given the opportu-nity to create not only exact copies of previously approved drugs, provided there was no infringe-ment of patents, but also improved versions of previously approved drugs by updating formu-lations or finding new uses. Table 1 describes the three pathways under the FD&C Act.Despite existing for more than 25 years, along with generic drugs, the 505(b)(2) prod-ucts have only recently become popular with drug companies due to increased challenges to discover and develop new chemical entities. As with innovator drugs, products following the 505(b)(2) pathway are subject to the full user fee under the Prescription Drug User Fee Act (PDUFA). They also may require several clinical and nonclinical studies that could involve signifi-cant resources, albeit less than for an innovator product but much higher than for a generic drug. Some key parameters for the three product cat-egories are listed in Table 2.
The 505(b)(2) Pathway is Unique to the US
The 505(b)(2) application is intended to encour-age sponsors to develop improved generics, i.e., drugs similar to an approved product with some significant changes that are not permitted under Abbreviated New Drug Application (ANDA) rules. The 505(b)(2) pathway replaced the “Paper NDA” pathway used prior to the Hatch-Waxman
Table 1. Regulatory Pathways for New Drug Products505(b)(1)NDANew drugRequires extensive clinical and nonclinical studies to demonstrate the safety and efficacy of a given drug for the target indication. Due to the amount of data required to support the application, such NDAs could take many years and require an enormous allocation of resources.
