伏立诺他 (2)
五种他汀药哪种最好副作用小

五种他汀药哪种最好副作用小
他汀药是一类常用的降脂药物,可以有效降低胆固醇和血脂水平,预防心血管疾病的发生。
然而,不同的他汀药在副作用方面可能会有一些差异。
下面是五种常见的他汀药,它们的副作用情况如下:
1. 氟伐他汀(Fluvastatin):副作用较轻微,包括头疼、恶心、胃肠道不适等。
2. 氟伐他汀(Rosuvastatin):与其他他汀药物相比,副作用
相对较少,但仍可能引起肌肉痛、肝功能异常等。
3. 氟伐他汀(Atorvastatin):可能引起肌肉痛、乏力、头痛等不适症状;偶尔会导致肌肉炎症和肝脏损伤。
4. 氟伐他汀(Pravastatin):副作用较轻,常见的不适症状包
括腹泻、头晕、乏力等。
5. 氟伐他汀(Simvastatin):可能引起肌肉痛、乏力、腹泻等,严重时可导致肌肉炎症和肝功能异常。
综上所述,不同的他汀药在副作用方面可能会有一些差异,但一般来说,副作用较轻的他汀药物包括氟伐他汀和氟伐他汀。
然而,每个人的体质和药物耐受性是不同的,因此在选择使用他汀药物时,应根据个体情况和医生的建议进行权衡。
反流性食管炎---伏诺拉生(新型抑酸药)

反流性⾷管炎---伏诺拉⽣(新型抑酸药)反流性⾷管炎患者⽆法严格餐前服药怎么办?全新抑酸机制药物了解⼀下~1反流性⾷管炎国内⾼患病率,⽬前尚存未满⾜需求RE的中国患病率⾼达6.4%,是进展为Barrett⾷管的主要独⽴危险因素2018年⼀项全球流⾏病学研究[1]显⽰:胃⾷管反流病(GERD)症状的全球总患病率为14.8%,2011年发⽣于我国上海的⼀项基于⼈⼝的调查研究显⽰反流性⾷管炎(RE)患病率为6.4%[2]。
⽬前已有证据表明RE是进展为巴雷特⾷管(Barrett)的重要独⽴危险因素[3],⽽Barrett⾷管患者较⼀般⼈群罹患⾷管腺癌的风险⾼出10-55倍[4]。
RE患者黏膜愈合率及症状控制并不满意RE的治疗⽬标为黏膜愈合、症状缓解。
质⼦泵抑制剂(PPI)是⽬前治疗RE的⼀线药物,但RE患者(尤其是LA- C/D级患者)采⽤PPI初始治疗后黏膜未愈合⽐例仍⾼达20%-30%。
RE患者在接受平均约1.1年的PPI维持治疗后,仍有近40%的患者症状未得到缓解[5]。
2PPI治疗遭遇瓶颈,患者依从性差是重要原因之⼀尽管指南对RE治疗进⾏规范,但实际临床效果仍不令⼈满意,抑酸不充分是原因之⼀,⽽依从性差⼜是导致抑酸不充分的重要因素。
患者⽤药依从性差的原因是什么?如下图,据研究显⽰,GERD患者⽤药依从性差的原因主要有以下:图1:GERD患者⽤药依从性差的原因[8]为何患者易忘记服药?1.服药疗程久:指南推荐RE治疗需服⽤PPI⾄8周,服药时间长,患者难以坚持,导致依从性降低。
2.服药次数多:指南推荐单次⽤药效果不佳时,可增加⾄两次,据2018年中华医学会消化年会参会医⽣调研数据显⽰[6],32%~57%的消化医⽣会为RE患者处⽅PPI标准剂量BID,若患者夜间烧⼼、反酸等症状严重,可能会增⾄三次,⽽服药次数越多,患者的依从性越低。
3.需要餐前半⼩时服⽤:由于PPI需酸激活,餐前半⼩时服⽤才能发挥最优效果,但是研究显⽰54.6%的患者⽆法严格在餐前服⽤[7],但是现代⼈⼯作⽣活节奏加快,难以做到精准时间服药。
FDA-伏立康唑说明书
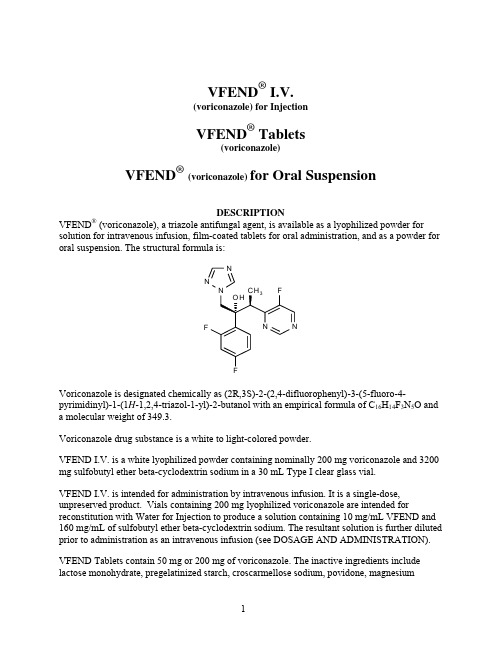
VFEND® I.V.(voriconazole) for InjectionVFEND® Tablets(voriconazole)VFEND®(voriconazole) for Oral SuspensionDESCRIPTIONVFEND® (voriconazole), a triazole antifungal agent, is available as a lyophilized powder for solution for intravenous infusion, film-coated tablets for oral administration, and as a powder for oral suspension. The structural formula is:Voriconazole is designated chemically as (2R,3S)-2-(2,4-difluorophenyl)-3-(5-fluoro-4-pyrimidinyl)-1-(1H-1,2,4-triazol-1-yl)-2-butanol with an empirical formula of C16H14F3N5O and a molecular weight of 349.3.Voriconazole drug substance is a white to light-colored powder.VFEND I.V. is a white lyophilized powder containing nominally 200 mg voriconazole and 3200 mg sulfobutyl ether beta-cyclodextrin sodium in a 30 mL Type I clear glass vial.VFEND I.V. is intended for administration by intravenous infusion. It is a single-dose, unpreserved product. Vials containing 200 mg lyophilized voriconazole are intended for reconstitution with Water for Injection to produce a solution containing 10 mg/mL VFEND and 160 mg/mL of sulfobutyl ether beta-cyclodextrin sodium. The resultant solution is further diluted prior to administration as an intravenous infusion (see DOSAGE AND ADMINISTRATION). VFEND Tablets contain 50 mg or 200 mg of voriconazole. The inactive ingredients include lactose monohydrate, pregelatinized starch, croscarmellose sodium, povidone, magnesiumstearate and a coating containing hypromellose, titanium dioxide, lactose monohydrate and triacetin.VFEND for Oral Suspension is a white to off-white powder providing a white to off-white orange-flavored suspension when reconstituted. Bottles containing 45 g powder for oral suspension are intended for reconstitution with water to produce a suspension containing 40mg/mL voriconazole. The inactive ingredients include colloidal silicon dioxide, titanium dioxide, xanthan gum, sodium citrate dihydrate, sodium benzoate, anhydrous citric acid, natural orange flavor, and sucrose.CLINICAL PHARMACOLOGYPharmacokineticsGeneral Pharmacokinetic CharacteristicsThe pharmacokinetics of voriconazole have been characterized in healthy subjects, special populations and patients.The pharmacokinetics of voriconazole are non-linear due to saturation of its metabolism. The interindividual variability of voriconazole pharmacokinetics is high. Greater than proportional increase in exposure is observed with increasing dose. It is estimated that, on average, increasing the oral dose in healthy subjects from 200 mg Q12h to 300 mg Q12h leads to a 2.5-fold increase in exposure (AUCτ), while increasing the intravenous dose from 3 mg/kg Q12h to 4 mg/kg Q12h produces a 2.3-fold increase in exposure (Table 1).Table 1Population Pharmacokinetic Parameters of Voriconazole in Subjects200 mg Oral Q12h 300 mg Oral Q12h 3 mg/kg IV Q12h 4 mg/kg IV Q12hAUCτ* (μg•h/mL) (CV%) 19.86(94%)50.32(74%)21.81(100%)50.40(83%)*Mean AUCτ are predicted values from population pharmacokinetic analysis of data from 236 subjectsDuring oral administration of 200 mg or 300 mg twice daily for 14 days in patients at risk of aspergillosis (mainly patients with malignant neoplasms of lymphatic or hematopoietic tissue), the observed pharmacokinetic characteristics were similar to those observed in healthy subjects (Table 2).Table 2Pharmacokinetic Parameters of Voriconazole in Patients at Risk for Aspergillosis200 mg Oral Q12h(n=9) 300 mg Oral Q12h(n=9)AUCτ* (μg•h/mL ) (CV%) 20.31(69%)36.51(45%)C max* (μg/mL) (CV%) 3.00(51%)4.66(35%)*Geometric mean values on Day 14 of multiple dosing in 2 cohorts of patientsSparse plasma sampling for pharmacokinetics was conducted in the therapeutic studies in patients aged 12-18 years. In 11 adolescent patients who received a mean voriconazole maintenance dose of 4 mg/kg IV, the median of the calculated mean plasma concentrations was 1.60 μg/mL (inter-quartile range 0.28 to 2.73 μg/mL).In 17 adolescent patients for whom mean plasma concentrations were calculated following a mean oral maintenance dose of 200 mg Q12h, the median of the calculated mean plasma concentrations was 1.16 μg/mL (inter-quartile range 0.85 to 2.14 μg/mL).When the recommended intravenous or oral loading dose regimens are administered to healthy subjects, peak plasma concentrations close to steady state are achieved within the first 24 hours of dosing. Without the loading dose, accumulation occurs during twice-daily multiple dosing with steady-state peak plasma voriconazole concentrations being achieved by day 6 in the majority of subjects (Table 3).Table 3Pharmacokinetic Parameters of Voriconazole from Loading Dose and Maintenance Dose Regimens(Individual Studies in Subjects)400 mg Q12h on Day 1, 200 mg Q12h on Days 2 to 10(n=17)6 mg/kg IV** Q12h on Day 1, 3 mg/kg IV Q12h on Days 2 to 10(n=9)Day 1, 1st dose Day 10 Day 1, 1st dose Day 10AUCτ* (μg•h/mL) (CV%) 9.31(38%)11.13(103%)13.22(22%)13.25(58%)C max (μg/mL) (CV%) 2.30(19%)2.08(62%)4.70(22%)3.06(31%)*AUCτ values are calculated over dosing interval of 12 hoursPharmacokinetic parameters for loading and maintenance doses summarized for same cohort of subjects**IV infusion over 60 minutesSteady state trough plasma concentrations with voriconazole are achieved after approximately 5 days of oral or intravenous dosing without a loading dose regimen. However, when an intravenous loading dose regimen is used, steady state trough plasma concentrations are achieved within 1 day.AbsorptionThe pharmacokinetic properties of voriconazole are similar following administration by the intravenous and oral routes. Based on a population pharmacokinetic analysis of pooled data in healthy subjects (N=207), the oral bioavailability of voriconazole is estimated to be 96% (CV 13%). Bioequivalence was established between the 200 mg tablet and the 40 mg/mL oral suspension when administered as a 400 mg Q12h loading dose followed by a 200 mg Q12h maintenance dose.Maximum plasma concentrations (C max) are achieved 1-2 hours after dosing. When multiple doses of voriconazole are administered with high-fat meals, the mean C max and AUCτ are reduced by 34% and 24%, respectively when administered as a tablet and by 58% and 37% respectively when administered as the oral suspension (see DOSAGE AND ADMINISTRATION).In healthy subjects, the absorption of voriconazole is not affected by coadministration of oral ranitidine, cimetidine, or omeprazole, drugs that are known to increase gastric pH.DistributionThe volume of distribution at steady state for voriconazole is estimated to be 4.6 L/kg, suggesting extensive distribution into tissues. Plasma protein binding is estimated to be 58% and was shown to be independent of plasma concentrations achieved following single and multiple oral doses of 200 mg or 300 mg (approximate range: 0.9-15 μg/mL). Varying degrees of hepatic and renal insufficiency do not affect the protein binding of voriconazole.MetabolismIn vitro studies showed that voriconazole is metabolized by the human hepatic cytochrome P450 enzymes, CYP2C19, CYP2C9 and CYP3A4 (see CLINICAL PHARMACOLOGY - Drug Interactions).In vivo studies indicated that CYP2C19 is significantly involved in the metabolism of voriconazole. This enzyme exhibits genetic polymorphism. For example, 15-20% of Asian populations may be expected to be poor metabolizers. For Caucasians and Blacks, the prevalence of poor metabolizers is 3-5%. Studies conducted in Caucasian and Japanese healthy subjects have shown that poor metabolizers have, on average, 4-fold higher voriconazole exposure (AUCτ) than their homozygous extensive metabolizer counterparts. Subjects who are heterozygous extensive metabolizers have, on average, 2-fold higher voriconazole exposure than their homozygous extensive metabolizer counterparts.The major metabolite of voriconazole is the N-oxide, which accounts for 72% of the circulating radiolabelled metabolites in plasma. Since this metabolite has minimal antifungal activity, it does not contribute to the overall efficacy of voriconazole.ExcretionVoriconazole is eliminated via hepatic metabolism with less than 2% of the dose excreted unchanged in the urine. After administration of a single radiolabelled dose of either oral or IV voriconazole, preceded by multiple oral or IV dosing, approximately 80% to 83% of theradioactivity is recovered in the urine. The majority (>94%) of the total radioactivity is excreted in the first 96 hours after both oral and intravenous dosing.As a result of non-linear pharmacokinetics, the terminal half-life of voriconazole is dose dependent and therefore not useful in predicting the accumulation or elimination of voriconazole.Pharmacokinetic-Pharmacodynamic RelationshipsClinical Efficacy and SafetyIn 10 clinical trials, the median values for the average and maximum voriconazole plasma concentrations in individual patients across these studies (N=1121) was 2.51 μg/mL (inter-quartile range 1.21 to 4.44 μg/mL) and 3.79 μg/mL (inter-quartile range 2.06 to 6.31 μg/mL), respectively. A pharmacokinetic-pharmacodynamic analysis of patient data from 6 of these 10 clinical trials (N=280) could not detect a positive association between mean, maximum or minimum plasma voriconazole concentration and efficacy. However, PK/PD analyses of the data from all 10 clinical trials identified positive associations between plasma voriconazole concentrations and rate of both liver function test abnormalities and visual disturbances (see ADVERSE REACTIONS).ElectrocardiogramA placebo-controlled, randomized, crossover study to evaluate the effect on the QT interval of healthy male and female subjects was conducted with three single oral doses of voriconazole and ketoconazole. Serial ECGs and plasma samples were obtained at specified intervals over a 24-hour post dose observation period. The placebo-adjusted mean maximum increases in QTc from baseline after 800, 1200 and 1600 mg of voriconazole and after ketoconazole 800 mg were all <10 msec. Females exhibited a greater increase in QTc than males, although all mean changes were <10 msec. Age was not found to affect the magnitude of increase in QTc. No subject in any group had an increase in QTc of ≥60 msec from baseline. No subject experienced an interval exceeding the potentially clinically relevant threshold of 500 msec. However, the QT effect of voriconazole combined with drugs known to prolong the QT interval is unknown (see CONTRAINDICATIONS, PRECAUTIONS-Drug Interactions).Pharmacokinetics in Special PopulationsGenderIn a multiple oral dose study, the mean C max and AUCτfor healthy young females were 83% and 113% higher, respectively, than in healthy young males (18-45 years), after tablet dosing. In the same study, no significant differences in the mean C max and AUCτ were observed between healthy elderly males and healthy elderly females (>65 years). In a similar study, after dosing with the oral suspension, the mean AUC for healthy young females was 45% higher than in healthy young males whereas the mean C max was comparable between genders. The steady state trough voriconazole concentrations (C min) seen in females were 100% and 91% higher than in males receiving the tablet and the oral suspension, respectively.In the clinical program, no dosage adjustment was made on the basis of gender. The safety profile and plasma concentrations observed in male and female subjects were similar. Therefore, no dosage adjustment based on gender is necessary.GeriatricIn an oral multiple dose study the mean C max and AUCτin healthy elderly males (≥ 65 years) were 61% and 86% higher, respectively, than in young males (18-45 years). No significant differences in the mean C max and AUCτ were observed between healthy elderly females ( ≥ 65 years) and healthy young females (18-45 years).In the clinical program, no dosage adjustment was made on the basis of age. An analysis of pharmacokinetic data obtained from 552 patients from 10 voriconazole clinical trials showed that the median voriconazole plasma concentrations in the elderly patients (>65 years) were approximately 80% to 90% higher than those in the younger patients (≤65 years) after either IV or oral administration. However, the safety profile of voriconazole in young and elderly subjects was similar and, therefore, no dosage adjustment is necessary for the elderly.PediatricA population pharmacokinetic analysis was conducted on pooled data from 35 immunocompromised pediatric patients aged 2 to <12 years old who were included in two pharmacokinetic studies of intravenous voriconazole (single dose and multiple dose). Twenty-four of these patients received multiple intravenous maintenance doses of 3 mg/kg and 4 mg/kg.A comparison of the pediatric and adult population pharmacokinetic data revealed that the predicted average steady state plasma concentrations were similar at the maintenance dose of 4 mg/kg every 12 hours in children and 3 mg/kg every 12 hours in adults (medians of 1.19 μg/mL and 1.16 μg/mL in children and adults, respectively) (see PRECAUTIONS, Pediatric Use). Hepatic InsufficiencyAfter a single oral dose (200 mg) of voriconazole in 8 patients with mild (Child-Pugh Class A) and 4 patients with moderate (Child-Pugh Class B) hepatic insufficiency, the mean systemic exposure (AUC) was 3.2-fold higher than in age and weight matched controls with normal hepatic function. There was no difference in mean peak plasma concentrations (C max) between the groups. When only the patients with mild (Child-Pugh Class A) hepatic insufficiency were compared to controls, there was still a 2.3-fold increase in the mean AUC in the group with hepatic insufficiency compared to controls.In an oral multiple dose study, AUCτwas similar in 6 subjects with moderate hepatic impairment (Child-Pugh Class B) given a lower maintenance dose of 100 mg twice daily compared to 6 subjects with normal hepatic function given the standard 200 mg twice daily maintenance dose. The mean peak plasma concentrations (C max) were 20% lower in the hepatically impaired group. It is recommended that the standard loading dose regimens be used but that the maintenance dose be halved in patients with mild to moderate hepatic cirrhosis (Child-Pugh Class A and B) receiving voriconazole. No pharmacokinetic data are available for patients with severe hepatic cirrhosis (Child-Pugh Class C) (see DOSAGE AND ADMINISTRATION).Renal InsufficiencyIn a single oral dose (200 mg) study in 24 subjects with normal renal function and mild to severe renal impairment, systemic exposure (AUC) and peak plasma concentration (C max) of voriconazole were not significantly affected by renal impairment. Therefore, no adjustment is necessary for oral dosing in patients with mild to severe renal impairment.In a multiple dose study of IV voriconazole (6 mg/kg IV loading dose x 2, then 3 mg/kg IV x 5.5 days) in 7 patients with moderate renal dysfunction (creatinine clearance 30-50 mL/min), the systemic exposure (AUC) and peak plasma concentrations (C max) were not significantly different from those in 6 subjects with normal renal function.However, in patients with moderate renal dysfunction (creatinine clearance 30-50 mL/min), accumulation of the intravenous vehicle, SBECD, occurs. The mean systemic exposure (AUC) and peak plasma concentrations (C max) of SBECD were increased 4-fold and almost 50%, respectively, in the moderately impaired group compared to the normal control group. Intravenous voriconazole should be avoided in patients with moderate or severe renal impairment (creatinine clearance <50 mL/min), unless an assessment of the benefit/risk to the patient justifies the use of intravenous voriconazole (see DOSAGE AND ADMINISTRATION - Dosage Adjustment).A pharmacokinetic study in subjects with renal failure undergoing hemodialysis showed that voriconazole is dialyzed with clearance of 121 mL/min. The intravenous vehicle, SBECD, is hemodialyzed with clearance of 55 mL/min. A 4-hour hemodialysis session does not remove a sufficient amount of voriconazole to warrant dose adjustment.Drug InteractionsEffects of Other Drugs on VoriconazoleVoriconazole is metabolized by the human hepatic cytochrome P450 enzymes CYP2C19,CYP2C9, and CYP3A4. Results of in vitro metabolism studies indicate that the affinity of voriconazole is highest for CYP2C19, followed by CYP2C9, and is appreciably lower forCYP3A4. Inhibitors or inducers of these three enzymes may increase or decrease voriconazole systemic exposure (plasma concentrations), respectively.The systemic exposure to voriconazole is significantly reduced or is expected to be reduced by the concomitant administration of the following agents and their use is contraindicated: Rifampin (potent CYP450 inducer): Rifampin (600 mg once daily) decreased the steady state C max and AUCτ of voriconazole (200 mg Q12h x 7 days) by an average of 93% and 96%, respectively, in healthy subjects. Doubling the dose of voriconazole to 400 mg Q12h does not restore adequate exposure to voriconazole during coadministration with rifampin. Coadministration of voriconazole and rifampin is contraindicated (see CONTRAINDICATIONS, PRECAUTIONS - Drug Interactions).Ritonavir (potent CYP450 inducer; CYP3A4 inhibitor and substrate): The effect of the coadministration of voriconazole and ritonavir (400 mg and 100 mg) was investigated in twoseparate studies. High-dose ritonavir (400 mg Q12h for 9 days) decreased the steady state C max and AUCτ of oral voriconazole (400 mg Q12h for 1 day, then 200 mg Q12h for 8 days) by an average of 66% and 82%, respectively, in healthy subjects. Low-dose ritonavir (100 mg Q12h for 9 days) decreased the steady state C max and AUCτ of oral voriconazole (400 mg Q12h for 1 day, then 200 mg Q12h for 8 days) by an average of 24% and 39%, respectively, in healthy subjects. Although repeat oral administration of voriconazole did not have a significant effect on steady state C max and AUCτ of high-dose ritonavir in healthy subjects, steady state C max and AUCτ of low-dose ritonavir decreased slightly by 24% and 14% respectively, when administered concomitantly with oral voriconazole in healthy subjects. Coadministration of voriconazole and high-dose ritonavir (400 mg Q12h) is contraindicated.Coadministration of voriconazole and low-dose ritonavir (100 mg Q12h) should be avoided, unless an assessment of the benefit/risk to the patient justifies the use of voriconazole. (see CONTRAINDICATIONS, PRECAUTIONS - Drug Interactions).St. John’s Wort(CYP450 inducer; P-gp inducer): In an independent published study in healthy volunteers who were given multiple oral doses of St. John’s Wort (300 mg LI 160 extract three times daily for 15 days) followed by a single 400 mg oral dose of voriconazole, a 59% decrease in mean voriconazole AUC0-∞was observed. In contrast, coadministration of single oral doses of St. John’s Wort and voriconazole had no appreciable effect on voriconazole AUC0-∞. Because long-term use of St. John’s Wort could lead to reduced voriconazole exposure, concomitant use of voriconazole with St. John’s Wort is contraindicated (see CONTRAINDICATIONS, PRECAUTIONS – Drug Interactions).Carbamazepine and long-acting barbiturates (potent CYP450 inducers): Although not studied in vitro or in vivo, carbamazepine and long-acting barbiturates (e.g., phenobarbital, mephobarbital) are likely to significantly decrease plasma voriconazole concentrations. Coadministration of voriconazole with carbamazepine or long-acting barbiturates is contraindicated (see CONTRAINDICATIONS, PRECAUTIONS - Drug Interactions).Minor or no significant pharmacokinetic interactions that do not require dosage adjustment:Cimetidine (non-specific CYP450 inhibitor and increases gastric pH): Cimetidine (400 mgQ12h x 8 days) increased voriconazole steady state C max and AUCτ by an average of 18% (90% CI: 6%, 32%) and 23% (90% CI: 13%, 33%), respectively, following oral doses of 200 mgQ12h x 7 days to healthy subjects.Ranitidine (increases gastric pH): Ranitidine (150 mg Q12h) had no significant effect on voriconazole C max and AUCτfollowing oral doses of 200 mg Q12h x 7 days to healthy subjects.Macrolide antibiotics: Coadministration of erythromycin (CYP3A4 inhibitor;1g Q12h for 7 days) or azithromycin (500 mg qd for 3 days) with voriconazole 200 mg Q12h for 14 days had no significant effect on voriconazole steady state C max and AUCτ in healthy subjects. The effects of voriconazole on the pharmacokinetics of either erythromycin or azithromycin are not known.Effects of Voriconazole on Other DrugsIn vitro studies with human hepatic microsomes show that voriconazole inhibits the metabolic activity of the cytochrome P450 enzymes CYP2C19, CYP2C9, and CYP3A4. In these studies, the inhibition potency of voriconazole for CYP3A4 metabolic activity was significantly less than that of two other azoles, ketoconazole and itraconazole. In vitro studies also show that the major metabolite of voriconazole, voriconazole N-oxide, inhibits the metabolic activity of CYP2C9 and CYP3A4 to a greater extent than that of CYP2C19. Therefore, there is potential for voriconazole and its major metabolite to increase the systemic exposure (plasma concentrations) of other drugs metabolized by these CYP450 enzymes.The systemic exposure of the following drugs is significantly increased or is expected to be significantly increased by coadministration of voriconazole and their use is contraindicated: Sirolimus (CYP3A4 substrate): Repeat dose administration of oral voriconazole (400 mg Q12h for 1 day, then 200 mg Q12h for 8 days) increased the C max and AUC of sirolimus (2 mg single dose) an average of 7-fold (90% CI: 5.7, 7.5) and 11-fold (90% CI: 9.9, 12.6), respectively, in healthy male subjects. Coadministration of voriconazole and sirolimus is contraindicated (see CONTRAINDICATIONS, PRECAUTIONS - Drug Interactions).Terfenadine, astemizole, cisapride, pimozide and quinidine (CYP3A4 substrates): Although not studied in vitro or in vivo, concomitant administration of voriconazole with terfenadine, astemizole, cisapride, pimozide or quinidine may result in inhibition of the metabolism of these drugs. Increased plasma concentrations of these drugs can lead to QT prolongation and rare occurrences of torsade de pointes. Coadministration of voriconazole and terfenadine, astemizole, cisapride, pimozide and quinidine is contraindicated (see CONTRAINDICATIONS, PRECAUTIONS - Drug Interactions).Ergot alkaloids: Although not studied in vitro or in vivo, voriconazole may increase the plasma concentration of ergot alkaloids (ergotamine and dihydroergotamine) and lead to ergotism. Coadministration of voriconazole with ergot alkaloids is contraindicated (see CONTRAINDICATIONS, PRECAUTIONS - Drug Interactions).Coadministration of voriconazole with the following agents results in increased exposure or is expected to result in increased exposure to these drugs. Therefore, careful monitoring and/or dosage adjustment of these drugs is needed:Cyclosporine (CYP3A4 substrate): In stable renal transplant recipients receiving chronic cyclosporine therapy, concomitant administration of oral voriconazole (200 mg Q12h for 8 days) increased cyclosporine C max and AUCτ an average of 1.1 times (90% CI: 0.9, 1.41) and 1.7 times (90% CI: 1.5, 2.0), respectively, as compared to when cyclosporine was administered without voriconazole. When initiating therapy with voriconazole in patients already receiving cyclosporine, it is recommended that the cyclosporine dose be reduced to one-half of the original dose and followed with frequent monitoring of the cyclosporine blood levels. Increased cyclosporine levels have been associated with nephrotoxicity. When voriconazole is discontinued, cyclosporine levels should be frequently monitored and the dose increased as necessary (see PRECAUTIONS - Drug Interactions).Methadone (CYP3A4, CYP2C19, CYP2C9 substrate): Repeat dose administration of oral voriconazole (400mg Q12h for 1 day, then 200mg Q12h for 4 days) increased the C max and AUCτ of pharmacologically active R-methadone by 31% (90% CI: 22%, 40%) and 47% (90% CI: 38%, 57%), respectively, in subjects receiving a methadone maintenance dose (30-100 mg QD). The C max and AUC of (S)-methadone increased by 65% (90% CI: 53%, 79%) and 103% (90% CI: 85%, 124%), respectively. Increased plasma concentrations of methadone have been associated with toxicity including QT prolongation. Frequent monitoring for adverse events and toxicity related to methadone is recommended during coadministration. Dose reduction of methadone may be needed (see PRECAUTIONS - Drug Interactions).Tacrolimus (CYP3A4 substrate): Repeat oral dose administration of voriconazole (400 mgQ12h x 1 day, then 200 mg Q12h x 6 days) increased tacrolimus (0.1 mg/kg single dose) C max and AUCτ in healthy subjects by an average of 2-fold (90% CI: 1.9, 2.5) and 3-fold (90% CI: 2.7, 3.8), respectively. When initiating therapy with voriconazole in patients already receiving tacrolimus, it is recommended that the tacrolimus dose be reduced to one-third of the original dose and followed with frequent monitoring of the tacrolimus blood levels. Increased tacrolimus levels have been associated with nephrotoxicity. When voriconazole is discontinued, tacrolimus levels should be carefully monitored and the dose increased as necessary (see PRECAUTIONS - Drug Interactions).Warfarin (CYP2C9 substrate): Coadministration of voriconazole (300 mg Q12h x 12 days) with warfarin (30 mg single dose) significantly increased maximum prothrombin time by approximately 2 times that of placebo in healthy subjects. Close monitoring of prothrombin time or other suitable anticoagulation tests is recommended if warfarin and voriconazole are coadministered and the warfarin dose adjusted accordingly (see PRECAUTIONS - Drug Interactions).Oral Coumarin Anticoagulants (CYP2C9, CYP3A4 substrates): Although not studied in vitro or in vivo, voriconazole may increase the plasma concentrations of coumarin anticoagulants and therefore may cause an increase in prothrombin time. If patients receiving coumarin preparations are treated simultaneously with voriconazole, the prothrombin time or other suitable anti-coagulation tests should be monitored at close intervals and the dosage of anticoagulants adjusted accordingly (see PRECAUTIONS - Drug Interactions).Statins (CYP3A4 substrates): Although not studied clinically, voriconazole has been shown to inhibit lovastatin metabolism in vitro (human liver microsomes). Therefore, voriconazole is likely to increase the plasma concentrations of statins that are metabolized by CYP3A4. It is recommended that dose adjustment of the statin be considered during coadministration. Increased statin concentrations in plasma have been associated with rhabdomyolysis (see PRECAUTIONS - Drug Interactions).Benzodiazepines (CYP3A4 substrates): Although not studied clinically, voriconazole has been shown to inhibit midazolam metabolism in vitro (human liver microsomes). Therefore, voriconazole is likely to increase the plasma concentrations of benzodiazepines that are metabolized by CYP3A4 (e.g., midazolam, triazolam, and alprazolam) and lead to a prolongedsedative effect. It is recommended that dose adjustment of the benzodiazepine be considered during coadministration (see PRECAUTIONS - Drug Interactions).Calcium Channel Blockers (CYP3A4 substrates): Although not studied clinically, voriconazole has been shown to inhibit felodipine metabolism in vitro (human liver microsomes). Therefore, voriconazole may increase the plasma concentrations of calcium channel blockers that are metabolized by CYP3A4. Frequent monitoring for adverse events and toxicity related to calcium channel blockers is recommended during coadministration. Dose adjustment of the calcium channel blocker may be needed (see PRECAUTIONS - Drug Interactions).Sulfonylureas (CYP2C9 substrates): Although not studied in vitro or in vivo, voriconazole may increase plasma concentrations of sulfonylureas (e.g., tolbutamide, glipizide, and glyburide) and therefore cause hypoglycemia. Frequent monitoring of blood glucose and appropriate adjustment (i.e., reduction) of the sulfonylurea dosage is recommended during coadministration (see PRECAUTIONS - Drug Interactions).Vinca Alkaloids (CYP3A4 substrates): Although not studied in vitro or in vivo, voriconazole may increase the plasma concentrations of the vinca alkaloids (e.g., vincristine and vinblastine) and lead to neurotoxicity. Therefore, it is recommended that dose adjustment of the vinca alkaloid be considered.No significant pharmacokinetic interactions were observed when voriconazole was coadministered with the following agents. Therefore, no dosage adjustment for these agents is recommended:Prednisolone (CYP3A4 substrate): Voriconazole (200 mg Q12h x 30 days) increased C max and AUC of prednisolone (60 mg single dose) by an average of 11% and 34%, respectively, in healthy subjects.Digoxin (P-glycoprotein mediated transport): Voriconazole (200 mg Q12h x 12 days) had no significant effect on steady state C max and AUCτ of digoxin (0.25 mg once daily for 10 days) in healthy subjects.Mycophenolic acid (UDP-glucuronyl transferase substrate): Voriconazole (200 mg Q12h x 5 days) had no significant effect on the C max and AUCτof mycophenolic acid and its major metabolite, mycophenolic acid glucuronide after administration of a 1 g single oral dose of mycophenolate mofetil.Two-Way InteractionsConcomitant use of the following agents with voriconazole is contraindicated:Rifabutin (potent CYP450 inducer): Rifabutin (300 mg once daily) decreased the C max and AUCτof voriconazole at 200 mg twice daily by an average of 67% (90% CI: 58%, 73%) and 79% (90% CI: 71%, 84%), respectively, in healthy subjects. During coadministration with rifabutin (300 mg once daily), the steady state C max and AUCτof voriconazole following an increased dose of 400 mg twice daily were on average approximately 2 times higher, compared。
伏诺拉生在幽门螺杆菌根除治疗中作用及影响因素的研究进展

·综述·伏诺拉生在幽门螺杆菌根除治疗中作用及影响 因素的研究进展吕一鸣 秦向荣 于 静 王晓勇【摘要】 伏诺拉生是一种新型钾离子竞争性酸阻滞剂,与PPI 相比,其抑酸效果更好,2015年日本批准其用于幽门螺杆菌(Hp )根除治疗。
研究显示,伏诺拉生方案根除Hp 的疗效与PPI 方案相当或Hp 根除率更高,且具有良好的机体耐受性。
该文就伏诺拉生方案在Hp 根除治疗中的临床疗效及影响因素,以及其对肠道菌群和13C 呼气试验的影响等方面的研究进展作一综述,为选择Hp 根除治疗方案提供参考。
【关键词】 伏诺拉生;幽门螺杆菌;根除治疗;影响因素;肠道菌群DOI: 10. 3969/j. issn. 1673-534X. 2023. 03. 002基金项目:江苏省博士后科研资助项目(2020Z182)作者单位:213003 南京医科大学附属常州第二人民医院消化内科通信作者:王晓勇,幽门螺杆菌(Hp )与多种消化系统疾病(萎缩性胃炎、消化性溃疡和胃癌等)和非消化系统疾病(血小板减少性紫癜、缺铁性贫血、糖尿病和冠状动脉疾病等)有关,1994年被WHO 列为Ⅰ类致癌物[1],根除Hp 能够有效预防相关疾病的发生。
目前中国以含铋剂的四联疗法为根除Hp 的标准治疗方案,该方案包括铋剂、1种PPI 和2种抗生素。
然而近年来该方案的Hp 根除率下降,除克拉霉素、甲硝唑及左氧氟沙星耐药率升高和患者依从性不高外,胃酸抑制不充分也是原因之一。
PPI 只能抑制活化态的质子泵,且半衰期短,起效慢,通常需要3~5 d 才能达到最佳疗效;夜间抑酸不充分,容易引起阿莫西林和克拉霉素分解,且受到细胞色素P450酶CYP2C19的基因型影响,抑酸效果的个体差异较大[2]。
伏诺拉生是一种新型钾离子竞争性酸阻滞剂,可抑制活化态和静息态的质子泵,且半衰期长,起效迅速,首剂即可达到最佳抑酸效果,24 h 内可持续抑制胃酸分泌,其维持胃内pH >4的时间占比高于PPI [3]。
伏立康唑注射液的输注注意事项

利福平
苯巴比妥
联用时增加给药剂量
依非韦伦(400mgqd)
苯妥英
利福布汀
卡马西平
奈韦拉平
联用时密切监测
安全性和有效性
糖皮质激素、西咪替丁
奥美拉唑、艾司奥美拉唑、泮托拉唑、雷贝拉唑、兰索拉唑
口服避孕药
利托那韦(100mgq12h)
茚地那韦、阿扎那韦、沙奎那韦、利匹韦林、依曲韦林
银杏叶
推荐伏立康唑注射液单独输注,禁止与其他药物配伍。
不推荐在同一Y型管输注
两性霉素B普通制剂;
头孢吡肟、环孢素、丹曲林、白消安、地西泮;
柔红霉素脂质体、多柔比星普通制剂、伊达比星;
米托蒽醌、莫西沙星、硝普钠、泮托拉唑、苯妥英钠;
硫喷妥钠、替加环素。
不推荐联合使用
依非韦伦(400mgqd)
利托那韦(400mgq12h)
红霉素、阿奇霉素、克拉霉素
伏立康唑注射液的输注注意事项
不良反应
常见恶心、呕吐、腹痛或腹泻等;
肝功能异常;
视觉损害、肝功、肾功;
连续用药超过28d,需要监测视觉功能(包括视敏度、视力范围、色觉);
每年进行一次全身皮肤检查;
应于用药第3日及之后的4-6周每周监测血药谷浓度。
指南推荐
伏诺拉生作用机理

伏诺拉生作用机理
伏诺拉生(Vonoprazan)是一种新型的抑酸药物,其作用机理是通过阻断H+/K+-ATP酶的K+通道,竞争性阻滞K+与该酶的结合,长时间停留于胃壁细胞,从而快速抑制胃酸的分泌。
与传统的质子泵抑制剂(PPI)相比,伏诺拉生具有更快速、更强效的抑酸作用,并且其作用时间更持久。
此外,伏诺拉生还可以通过抑制胃酸分泌,改善胃黏膜的微环境,促进胃黏膜的修复和愈合。
在治疗反流性食管炎等胃食管反流性疾病方面,伏诺拉生展现出了良好的疗效和安全性。
需要注意的是,伏诺拉生并非适用于所有人群,禁忌症包括对该药物任何一种组成的成分过敏的人群、正在接受阿扎那韦或利匹韦林治疗的患者以及哺乳期妇女等。
在使用伏诺拉生前,应仔细阅读药品说明书并遵从医生的建议。
伏立康唑化学结构式
伏立康唑化学结构式伏立康唑是一种常用的抗真菌药物,其化学结构式如下所示:伏立康唑的化学名为2-(2,4-dichlorophenyl)-1,1-dimethyl-3-(1H-1,2,4-triazol-1-yl)butan-2-ol,属于酮类抗真菌药物。
伏立康唑的结构中含有多个功能团,每个功能团都对其抗真菌活性起到重要作用。
首先,该药物的苯环上有两个氯原子,这两个氯原子增加了伏立康唑与真菌细胞壁中的酵母菌脂质的相互作用,从而抑制了真菌细胞壁的合成。
其次,伏立康唑分子中的三唑环与真菌的细胞色素P450酶结合,抑制了该酶的活性,进而阻断了真菌细胞的麦角固醇生物合成途径,最终导致真菌细胞的死亡。
伏立康唑广泛用于治疗多种真菌感染疾病,包括念珠菌感染、皮肤黏膜念珠菌病、肺念珠菌病、内脏念珠菌病等。
此外,伏立康唑还可用于治疗由皮肤癣菌引起的癣症、头癣和甲癣等皮肤病。
伏立康唑的抗真菌活性广谱,且对多种真菌具有较高的选择性,因此被广泛应用于医疗领域。
伏立康唑的药代动力学特性也是其临床应用的重要考虑因素之一。
该药物口服后能迅速吸收,达到最高血药浓度的时间大约在1-2小时之间。
伏立康唑的生物利用度高达90%以上,与血浆蛋白结合率也较高。
该药物经过肝脏代谢,主要通过CYP3A4酶代谢为多种代谢物,其中活性代谢物伏立康唑醇的抗真菌活性较高。
伏立康唑主要通过肾脏排泄,半衰期约为2-4小时。
然而,伏立康唑也存在一些不良反应和药物相互作用的风险。
常见的不良反应包括恶心、呕吐、腹泻、头痛、皮疹等。
此外,伏立康唑还有一定的肝毒性,使用时需注意监测肝功能。
伏立康唑与其他药物的相互作用也需要注意,特别是与抗逆转录病毒药物、抗凝血药物、口服避孕药等药物的联用可能会导致药物浓度的改变,增加不良反应的风险。
伏立康唑是一种重要的抗真菌药物,其化学结构中的多个功能团对其抗真菌活性起到关键作用。
该药物广泛应用于治疗多种真菌感染疾病,但在使用时需注意其不良反应和药物相互作用的风险。
富马酸伏诺拉生片及其临床应用
富马酸伏诺拉生片及其临床应用富马酸伏诺拉生片是一种常用的药物,广泛应用于临床治疗中。
本文将介绍富马酸伏诺拉生片的基本信息、药理作用以及临床应用情况。
一、富马酸伏诺拉生片的基本信息富马酸伏诺拉生片是一种含有富马酸伏诺拉的药物制剂,常见的剂型有片剂、胶囊剂等。
富马酸伏诺拉是一种非处方药,具有抗炎、镇痛和退热的作用。
该药物主要通过口服给药途径进行使用。
二、富马酸伏诺拉生片的药理作用富马酸伏诺拉生片具有多种药理作用。
首先,它可抑制前列腺素的合成,从而减少炎症反应和组织损伤。
其次,富马酸伏诺拉生片可调控疼痛传导途径,减轻疼痛感。
此外,它还能够降低身体内的体温,具有退热作用。
三、富马酸伏诺拉生片的临床应用1. 抗炎镇痛富马酸伏诺拉生片常用于治疗各种炎症性疾病引起的疼痛,如风湿性关节炎、骨关节炎等。
它可以缓解炎症引起的疼痛和肿胀,改善患者的生活质量。
2. 退热富马酸伏诺拉生片可用于退热治疗,对于感染性疾病引起的发热症状,它能够迅速降低体温,减轻患者的不适感。
3. 急性扁桃体炎富马酸伏诺拉生片也可以用于治疗急性扁桃体炎。
该疾病常伴有咽喉疼痛、发热等症状,富马酸伏诺拉生片能够缓解这些症状,促进患者康复。
4. 骨折、创伤后疼痛富马酸伏诺拉生片对于骨折、创伤等引起的疼痛有一定的治疗效果。
通过镇痛作用,它可以减轻患者的疼痛感,提高患者的生活质量。
4. 其他临床应用富马酸伏诺拉生片还可以用于其他一些疾病的治疗,如牙痛、月经痛等。
但在使用过程中,应注意严格按照医生的建议使用,避免超量使用和长期使用。
综上所述,富马酸伏诺拉生片是一种非常常用的药物,用于治疗各种炎症性疾病引起的疼痛和不适感。
它具有抗炎、镇痛和退热的作用,能够改善患者的生活质量。
在使用过程中,我们应注意按照医生的建议使用,以期取得更好的治疗效果。
参考文献:1. Schjerning Olsen AM, Fosbøl EL, Lindhardsen J, et al. Duration of treatment with nonsteroidal anti-inflammatory drugs and impact on risk of death and recurrent myocardial infarction in patients with prior myocardial infarction. A nationwide cohort study. Circulation. 2011;123(20):2226-2235.2. Ray WA, Stein CM, Daugherty JR, et al. COX-2 selective non-steroidal anti-inflammatory drugs and risk of serious coronary heart disease. Lancet. 2002;359(9307):118-123.。
沃诺拉赞(伏诺拉生),一种钾离子竞争性酸阻滞剂
沃诺拉赞伏诺拉生(Vonoprazan)又名沃诺拉赞,是武田制药原研的一款钾离子竞争性酸阻滞剂(P-CAB),是一种可逆性质子泵抑制剂。
富马酸沃诺拉赞,商品名为Takecab®,由武田制药(Takeda)和大冢制药(Otsuka)联合研发,于2014 年12 月在日本获PMDA 批准上市。
富马酸伏诺拉生已被中国《胃食管反流病专家共识(2020)》《胃食管反流病多学科诊疗共识(2022)》日本《胃食管反流病循证临床实践指南(2021)》等国内外权威指南和专家共识推荐。
01作用机制及临床应用传统PPIs 抑制剂主要通过抑制H+/K+-ATP 酶的活性来阻断由任何刺激引起的胃酸分泌。
但PPI 并不总是能够提供足够的疗效,而且抑制胃酸分泌效果常因人而异。
而钾竞争性酸阻滞剂(P-CABs)是一类新型质子泵抑制剂, 具有速效、强劲、持久的胃酸分泌抑制作用;同时,在胃壁细胞胃酸分泌的最后一步中,通过抑制K+对H+,K+—ATP 酶(质子泵)的结合作用,对胃酸分泌也具有提前终止作用。
沃诺拉赞为全新一代抑酸剂,较临床应用最广的质子泵抑制剂,具有起效更快(首剂全效)、抑酸效果更持久、夜间抑酸效果更佳、黏膜愈合率更高等优势。
用于治疗酸相关疾病,包括幽门螺杆菌感染、胃食管返流、消化性溃疡、十二指肠溃疡、胃溃疡以及食管炎。
02市场销售数据情况富马酸伏诺拉生片目前在我国还处于前期扩张模式,据药融云统计,2021年该药院内销售额大涨,一举破亿。
2022年又同比增长了173%,院内销售额达到4.29亿,上升趋势明显,前景可观。
由于国产仿制药获批较晚,目前该P-CAB抑酸药市场由武田制药一家独享。
本品种2019年全球销售额为6.9亿美元,同比增长25%。
2020年,伏诺拉生全球销售额达848亿日元(约7.94亿美元),同比增长16.7%。
2021年财年销售额为1023.97亿日元(折合约9.33亿美元)。
富马酸伏诺拉生片2022年仅在日本销售额约7.2亿美元,达到“重磅炸弹”级水平毋容置疑。
伏诺拉生的临床应用及不良反应
伏诺拉生的临床应用及不良反应
叶磊;傅燕;杨瑶
【期刊名称】《胃肠病学和肝病学杂志》
【年(卷),期】2023(32)2
【摘要】富马酸伏诺拉生(Vonoprazan, TAK-438)是临床应用中引入的第二种P-CABs药物,于2020年12月28日纳入我国医保,作为第二代P-CABs药物,伏诺拉生具有独特的吡咯衍类结构,其碱性pKa为9.06的特性,促使其能在酸性环境(如胃壁细胞的细胞内小管)中高水平积累,显示出了更强的抑酸作用。
除此之外,起效快、抑酸效果持续时间长、结合可逆等方面优势较传统质子泵抑制剂(proton pump inhibitors, PPIs)药物展示了更广阔的应用前景。
目前伏诺拉生已被证实可以用于
临床治疗胃食管反流病、消化性溃疡、H.pylori的根除以及ESD术后延迟出血等
疾病。
本文拟对伏诺拉生的临床应用以及不良反应进行综述。
【总页数】3页(P218-220)
【作者】叶磊;傅燕;杨瑶
【作者单位】昆明医科大学第二附属医院消化内科
【正文语种】中文
【中图分类】R57
【相关文献】
1.伏诺拉生与雷贝拉唑治疗反流性食管炎的临床疗效比较
2.伏诺拉生联合阿莫西林、克拉霉素三联七日疗法根除幽门螺杆菌的临床研究
3.基于AHP-TOPSIS法和数据
挖掘综合评价新型抑酸药伏诺拉生临床应用的合理性4.富马酸伏诺拉生片联合兰索拉唑治疗胃食管反流性咽喉病的临床效果5.新型抑酸药物伏诺拉生临床应用的研究进展
因版权原因,仅展示原文概要,查看原文内容请购买。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
伏立诺他 Vorinostat (Zolinza) 一、背景资料 皮肤T细胞淋巴瘤( Cutaneous T cell lymphoma, CTCL),为一种先由皮肤产生病变后,再扩散至淋巴结或其它器官的淋巴瘤。在各种不同型态的淋巴瘤中,皮肤T细胞淋巴瘤所占的比例并不高,仅约占 非何杰氏巴瘤(non-Hodgkin T-cell lymphomas)2~3%左右。但是,根据美国医学会皮肤医学档案期刊( Archives of Dermatology) 一篇研究报告指出,这种之前十分罕见的疾病,目前在美国愈来愈普遍,病例有逐渐增加的趋势。 皮肤T细胞淋巴瘤最早是在1806年,由法国皮肤病学者Alibert诊断发现,患者身上皮肤会长出许多与霉菌感染无关,但外观却极似蕈状的肿块。这种疾病发生率每十年就全面性地增加,数据显示,从1973~2002年,这三十年间,在美国诊断出皮肤T细胞淋巴瘤共有四千七百八十三例,大约每百万人有六点四人罹患这种疾病。目前皮肤T细胞淋巴瘤的致病原因尚不清楚,可能和环境中的化学物质,如“杀虫剂”的慢性长期接触有关;而流行病学显示,本疾病大多发生在40岁至60岁的成年人,男性与女性的比例约为2比1,且随着年龄的增长,罹患率也相对提高。皮肤T细胞淋巴瘤的主要表现型有两种,一种为红皮症(Sezary syndrome),患者全身皮肤发红,非常痒且对冷敏感,并有淋巴瘤细胞进入外围血液中;另一种为蕈样肉芽肿(Mycosis fungoides),疾病晚期患者的皮肤会呈现突起如蘑菇样的肿块,这些肿块极易并发细菌感染而引起败血症,而随着疾病的变化,愈晚期的患者愈容易合并其它内脏器官(如肺脏、肝脏、脾脏)的侵犯。 在治疗上,依疾病的不同进程而有所差异,通常初期病灶只局限在皮肤上,所以使用外用药膏或光治疗(phototherapy)即可有效控制病情;若已侵犯到淋巴结或其它内脏器官,以生物制剂(如bexarotene)合并放射或化学治疗,则可收到较佳的反应率,但对于较难缠的复发性皮肤T细胞淋巴瘤,上述的治疗模式似乎无法得到明显的疗效。 然而皮肤T细胞淋巴瘤属于低恶性度、疾病变化较缓慢的淋巴瘤,所以,若以强力的化学治疗,并无法延长患者的存活期,只会增加毒性且引起不适。因此,针对此疾病,科学家们致力研究的目标在于减轻患者的症状,降低治疗引起的副作用,甚至是延长患者的存活期。根据研究,许多肿瘤或癌症的患者体内的组织蛋白乙酰化调节酶(histone-acetylation regulatory enzymes)会改变,这个发现提供了一个新的治疗方向,经由不断的努力,vorinostat (Zolinza)终于诞生了,美国食品药物管理局(FDA)在2006年10月6日核准了vorinostat (Zolinza)上市,用于治疗已经使用其它药物,但疾病仍持续恶化或复发的皮肤T细胞淋巴瘤(Cutaneous T cell lymphoma, CTCL)。 当研究人员在很多巴瘤患者的病标本无意中发现了一些抑癌基因的基因促进区 (promoter region)常被接上甲基根(methylation),其中组织蛋白(histone)的甲基化显得相当重要,因为会影响染色体的组成紧密与否,进而影响基因的表现(当形成染色体时,由于排列紧密即无法进行转译的工作),故甲基化之后会吸引组织蛋白去乙酰酶(HDAC; histone deacetylase)可使得细胞的组织蛋白(histone)去乙酰化(deacetylation),这个过程之后将会吸引很多基因的抑制分子(repressor),同时 DNA 结构也会因为 histone deacetylation 而变成复制延长,导致染色体紧缩和抑制转录(transcription)的效果。因此这些抑癌基因的功能被阻断,造成肿瘤的生成。Vorinostat (Zolinza)在分类上属于组织蛋白去乙酰酶抑制剂(histone deacetylase,HDAC inhibitor),其作用原理即组织蛋白去乙酰酶抑制剂则会与组织蛋白去乙酰酶结合,使其失去作用进而促使基因表现。它可藉由抑制组织蛋白去乙酰酶(HDAC1, HDAC2, HDAC3, HDAC6),促使乙酰化组织蛋白增加,造成癌细胞的转录、转译过程异常,而使得癌细胞的复制、生长停止,甚至是死亡,但目前确切的抗肿瘤机转仍未完全明了。 Vorinostat (Zolinza)的药理分类属于组织蛋白去乙酰酶抑制剂(histone deacetylase「HDAC」inhibitor),怀孕分级为D级,蛋白质结合率为71%,平均半衰期约为二小时,服药后约四小时可达最高药物血中浓度,由肝脏代谢,而主要由尿中(52%)排出,临床上建议剂量为口服每次400毫克,每天一次。因为食物(尤其是高脂肪含量)会提高vorinostat (Zolinza)33%的吸收,所以最好饭中服用,而为了预防脱水现象,除了限水患者外,每天应补充适当水份(约每天两公升)。由于Vorinostat (Zolinza)本身为胶囊剂型(规格:每颗粒为100毫克),厂商建议服药时不可打开胶囊或咬碎,避免皮肤或黏膜组织直接接触药品粉末。假如患者无法耐受治疗副作用时,可调整剂量至每次300毫克,每天一次或每次300毫克,每天一次,一星期服用五天。 Vorinostat (Zolinza)较常见的非血液相关的副作用(如表三)为腹泻(52%)、疲倦(52%)、恶心(41%)、味觉障碍(28%)、厌食(24%)及体重减轻(21%);而血液相关的副作用则为血小板减少(26%)及贫血(14%)。另外,有某些检验数据也会因服用vorinostat (Zolinza)而出现异常,如胆固醇、三酸甘油脂、血糖都会有升高的现象,也有可能会有静脉栓塞或心律不整的情形,所以服用vorinostat (Zolinza)初期(前二个月),必须两星期检测一次相关的血液检查以确保用药安全。 二、Vorinostat (Zolinza)相关的临床试验 Vorinostat (Zolinza)属于新一类作用机转的治疗用药,在2006年的美国血液医学会(American Society of Hematology)年会中,发表针对vorinostat (Zolinza)用于治疗复发性皮肤T细胞淋巴瘤的第二期(phase II)研究试验结果。此临床试验在北美洲(包括美国及加拿大)18家医疗中心进行,总共纳入74位患者(基本数据, 如表一),这些患者都曾以平均三种以上的治疗方式治疗无效,本试验患者平均年龄为61岁,其中13位属于早期患者(IB~IIA),另外61位则属于较后期(IIB或更严重),而这61位较后期的患者中,有30位有Sezary syndrome。他们以vorinostat (Zolinza) 400毫克,每天一次进行治疗,若无法忍受这种剂量,则将药量降低至每天300毫克,或每天300毫克且一星期只服用五天,用药后的疗效反应依Severity Weighted Assessment Tool (SWAT)的标准,根据身体皮肤的侵犯面积程度及呈现的外观(片状、斑状或肿瘤)来评估。在经过平均约两个月(55天)的治疗后,试验结果(如表二)共有22位(30%)的患者,依SWAT的评估标准,有>50%的皮肤症状减少率,属于有效反应,这22位有治疗反应的患者中,包含4位(31%)的早期患者及18位(30%)的较后期患者,这些患者的反应期中位数(Median duration of response)为168天,而疾病恶化时间中位数(Median time to progression)为202天(依SWAT的评估标准,有>50%的皮肤症状增加率)。 而另一项发表于血液期刊(Blood)的第二期(phase II)研究试验,则是针对vorinostat (Zolinza)不同的用量、用法,进行疗效的探讨。在另一项第二期的研究试验,纳入33位患者,其中5位期别在IIB以下,而另外28位期别则在IIB以上,将这些患者分成三个不同组别,第一组剂量为每次400毫克,每天一次;第二组剂量为每次先以300毫克,每天两次,一星期服用三天,四周之后再增加到每次300毫克,每天两次,一星期服用五天;第三组剂量则是每次300毫克,每天两次,服用14天后,休息7天,再以每次200毫克,每天两次来治疗。本试验患者的平均年龄为67岁(26-82),性别(男性占18位, 55%),种族(白人占25位, 76%),其中11位(33%)有Sezary syndrome,平均治疗时间为八星期,试验结果显示共有8位(24.2%)患者有治疗反应率,包括1位疾病期别IIB以下,及7位疾病期别IIB以上的患者。整体反应率在三组分别为30.8%, 9.1%, 33.3%。而在以不同剂量治疗后,证实300毫克,每天两次的治疗方式,不仅毒性增加,疗效也不会明显优于400毫克,每天一次的治疗方式。在有治疗反应率的8位患者中,出现反应天数的中位数(The Median time to response)为83.5天(25 - 153),反应期中位数(Median duration of response)为106天(66–136),疾病恶化时间中位数(Median time to progression)为211.5天(94–255)。 此外,由于皮肤T细胞淋巴瘤也有可能以放射疗法治疗,所以也有针对vorinostat (Zolinza)合并放射疗法的研究,令人振奋的是,虽然机转尚不十分明确,但vorinostat (Zolinza)似乎可以提高肿瘤细胞对放射线的敏感度,而使放射疗法效果更明显。所以,目前为止,vorinostat (Zolinza)相关的临床研究都证实,对于皮肤T细胞淋巴瘤的治疗,vorinostat (Zolinza)确实占有一席之地。 三、申报情况 截止2016年4月14日,以以伏立诺他为关键词,共搜到26个受理号。(利用咸大数据库)
受理号 标准药品名 药品 类型 申请 类型 承办 日期 标准企业名称 办理状态 状态开始 时间 CDE技术 审评结论
CXHL0700530 伏立诺他 化药 新药 2008-01-23 江苏正大天晴药业股份有限公司 制证完毕-已发批件江苏省 ED185066042CS 2009-12-15 批准临床
CXHL0700531 伏立诺他胶囊 化药 新药 2008-01-23 江苏正大天晴药业股份有限公司 制证完毕-已发批件江苏省 ED185066042CS 2009-12-15 批准临床 CXHL0900092 伏立诺他 化药 新药 2009-03-27 吉林一心制药股份有限公司 制证完毕-已发批件吉林省 EF798405752CS 2010-07-05 批准临床 CXHL0900093 伏立诺他胶囊 化药 新药 2009-03-27 吉林一心制药股份有限公司 制证完毕-已发批件吉林省 EF798405752CS 2010-07-05 批准临床 CXHL0900060 伏立诺他 化药 新药 2009-04-14 杭州容立医药科技有限公司 制证完毕-已发批件浙江省 EF798405386CS 2010-07-05 批准临床
