细胞衰老 β-半乳糖苷酶-1
细胞15.细胞衰老与细胞程序性死亡知识考点

细胞15.细胞衰老与细胞程序性死亡知识考点●cell senescence 细胞衰老●定义:除了生殖干细胞,绝大多数正常细胞在经历有限次数的分裂后会进入“衰老”状态,不在具有增殖能力,细胞的形态结构和代谢活动也发生显著改变●Hayflick limit, Hayflick界限定义:原代培养细胞在进入增殖停滞状态前的有限倍增次数●特征●形态结构:大而扁平,细胞质膜的流动性降低,细胞骨架结构改变,细胞黏附性增强,细胞内溶酶体体积增大,内部含有大量未分解的脂质成分●分子特征●细胞不可逆地停止分裂,即使添加生长因子也无济于事●若干细胞周期的负调节因子表达上调或活性增强●衰老相关的β-半乳糖苷酶活化衰老细胞中pH 6.0条件下即表现出活性将细胞固定后,用pH 6.0的β-半乳糖苷酶底物溶液进行染色,能明显区分年轻和年老的培养细胞●衰老细胞端粒长度明显减少●出现衰老相关异染色质集中现象●产生一系列衰老特征性分泌物●分子机制●replicative senescence, RS 复制衰老●end replication problem 末端复制问题DNA聚合酶不能从头合成子链,复制母链3’端时,子链5’端与之配对的RNA引物被切除后会产生末端缺失,导致子链5’末端随着细胞分裂次数的增加而逐渐缩短●端粒的缩短●telomerase 端粒酶定义:以自身含有的RNA为模板,反转录出端粒DNA,从而避免端粒的缩短●p53信号通路引发细胞衰老●端粒的缩短导致细胞内DNA修复体系p53的活化,p53继而诱导p21的表达,p21使得CDK失去活性,从而阻止Rb蛋白的磷酸化,Rb不能与E2F分离,E2F处于持续失活状态,不能正常起始G_1/S检查点若干关键因子的转录,细胞周期停滞导致细胞衰老●另一条通路中,氧化损伤等因素可以通过诱导p16的表达导致细胞衰老●stress-induced premature senescence, SIPS 胁迫诱导的早熟性衰老定义:一些刺激因素(超量的过氧化物、原癌基因的非正常活化、非端粒的DNA损伤)能够缩短细胞的复制寿命,使细胞提前进入衰老状态●细胞衰老与个体衰老的关系●个体衰老在分子及细胞水平的标志●基因和蛋白质水平●基因组DNA损伤明显积累●染色体端粒长度明显缩短●表观遗传修饰(DNA及组蛋白某些位点甲基化或乙酰化修饰发生改变、染色质高级结构变化)引起基因表达异常●协助蛋白质折叠的体系以及降解非正常折叠蛋白质的体系发生障碍●细胞水平●失去增殖能力和功能减退的细胞累积●细胞组织器官生存微环境恶化●干细胞减弱甚至丧失组织更新能力●细胞通信的失调●线粒体功能障碍●细胞衰老与个体衰老的间接关联●组织干细胞衰老导致细胞再生受阻,器官机能下降,进而影响全身各系统之间的协调配合为什么组织干细胞会衰老?除了生殖干细胞外,多数单能和多能干细胞如表皮、骨髓和神经干细胞具有一定活性的端粒酶,但活性不足以完全弥补细胞复制过程中端粒的缩短,它们的增殖能力随着个体生命进程而下降。
细胞衰老与相关基因的关系

细胞衰老与相关基因的关系摘要】衰老是细胞的重要生命现象之一,主要受遗传与环境两个反面的影响,对细胞衰老相关基因的研究,可了解细胞衰老的分子机制,可揭示细胞衰老相关基因间相互作用及在衰老过程中的调节、损伤、应激、修复等内在联系,为老年病,细胞癌变、器官移植等提供了新的研究途径。
【关键词】细胞损伤促衰老因子自由基近年来,国内外对细胞衰老相关基因的研究非常活跃。
研究多以线虫、酵母、果蝇、小鼠为模型。
目前已发现有数十种促衰老因子(DAF)与之有关,改变某些基因的活性可使寿命延长或促进衰老发生,本文综述了衰老相关基因的分布、定位、分子生物学表达调控及临床应用。
1969年Haffman报道了一种存在于人类红细胞基质提取物夜相中的物质,它能控制抗体包被的绵羊红细胞的补体介导的溶血;Nichoson-weller[1]等通过丁醇提取,采用连续色谱法,从豚鼠和人类红细胞基质中纯化一种固有的膜糖蛋白,在纯化过程中监测到它能加速C3转化酶的衰老,从而命名为DAF。
1、DAF的分布与定位DAF广泛分布于外周血细胞[2],包括红细胞、粒细胞、TB淋巴细胞、单核细胞、骨髓单核细胞和红细胞系统的祖细胞上。
在动物模型证实可存在于心脏的脉管系统,肾脏、肝脏的各种器官中,表达在正常人的结肠、直肠粘膜及膀胱、子宫、胸膜等上皮细胞的表面,但自然杀伤细胞(NK)上没有DAF,不同细胞中DA F个数也不相同。
衰老基因可分布于多条染色体,如Newbold[3]将3号染色体上的衰老基因定位于3p2111~21113,可抑制端粒酶活性,Uejima[4]等将2号染色体上的衰老基因定位于2q37,不影响端粒酶活性,这也表明衰老存在多种调控途径。
2、细胞衰老的机理细胞衰老的研究有多种学说,20世纪60年代中期英国学者Harman首先提出的自由基学说是具有代表性的衰老学说之一。
目前影响力较大的是氧化-损伤学说[5],即代谢产生的氧化产物导致分子损伤,由于氧化产物不断积累,最终细胞衰老和死亡,自由基的种类繁多,其中以活性氧簇自由基(ROS)最为重要。
衰老相关β-半乳糖苷酶染色

衰老相关β-半乳糖苷酶染色细胞衰老β-半乳糖苷酶染色试剂盒产品编号产品名称规格BL133A细胞衰老β-半乳糖苷酶染色试剂盒100 T产品简介:细胞衰老时,SA-β-Gal (senescence-associated β-galactosidase)活性水平会上调,此时本试剂盒可以对衰老的细胞或组织进行染色检测。
正常细胞经过有限次数的分裂后停止分裂,出现不可逆的生长停滞,此时细胞仍然是存活的,但是细胞形态和生理代谢活性发生明显变化,通常表现为细胞体积变大,与衰老相关的β-半乳糖苷酶为活化状态。
β-半乳糖苷酶是细胞溶酶体内的水解酶,通常在pH 4.0时表现活性,但在衰老细胞内该酶在pH 6.0条件下表现活性。
本试剂盒即是基于此现象及原理,以X-Gal为底物,衰老细胞特异性β-半乳糖苷酶催化该底物生成蓝色产物,表现为细胞胞质有蓝色沉积物,在光学显微镜下即可很容易观察到变成蓝色的表达β-半乳糖苷酶的细胞或组织。
本试剂盒可以用于培养细胞的衰老检测,也可以用于组织切片的衰老检测。
本试剂盒仅染色衰老细胞,对衰老前的细胞(presenescent cells)、静止期细胞(quiescent cells)、永生细胞(immortal cells)或肿瘤细胞等不会染色。
产品组成:组分名称规格BL133A-1β-半乳糖苷酶染色固定液100 mLBL133A-2β-半乳糖苷酶染色液A100 mLBL133A-3β-半乳糖苷酶染色液B 1.2 mLBL133A-4DMF(二甲基甲酰胺) 5 mlBL133A-5X-Gal(粉末)100 mg使用方法:见说明书注意事项:1、X-Gal粉末配制成溶液后,分装成小份-20℃保存,3个月内有效,X-Gal溶液需室温完全解冻混匀后再使用。
2、β-半乳糖苷酶染色液A和B需提前恢复至室温后再使用,配制好的染色工作液需充分混匀无沉淀方可使用。
3、衰老细胞β-半乳糖苷酶染色反应依赖于特定的pH条件,不能在CO2培养箱中进行孵育显色,否则会影响染色工作液的pH,导致染色失败。
β-半乳糖苷酶染色(组织染色)

β-半乳糖苷酶染色(组织)检测原理:一般认为绝大多数正常细胞仅有有限的分裂能力,在不能分裂后就进入衰老(senescence)状态。
此时细胞仍然是存活的,但细胞的基因和蛋白的表达谱发生了很大改变。
衰老的细胞不能在一些常规的刺激下再诱导细胞分裂,并且衰老细胞的细胞周期分布也比较特殊,不同于一些损伤诱导的细胞休眠,也不同于细胞生长接触抑制的情况。
衰老细胞通常体积变大,并表达在pH6.0时有高酶活性的β-半乳糖苷酶(β-galactosidase)。
细胞衰老也被认为是生物体抑制肿瘤的一种方式,同时也是生物体老化(aging)的一种潜在原因。
由此以X-Gal为底物,在衰老特异性的β-半乳糖苷酶催化下会生成深蓝色产物。
从而在光学显微镜下很容易观察到变成蓝色的表达β-半乳糖苷酶的细胞。
操作步骤:(1)将石蜡组织切片置于65°C恒温箱,烤片1h左右。
(2)脱蜡:二甲苯I 10min→二甲苯II 10min →梯度酒精(由高到低)各5min。
(3)1×PBS 洗涤3次,每次 5min。
注:对于冷冻切片直接按照以下步骤进行。
(4)加入适当体积的β-半乳糖苷酶染色固定液,以充分盖住组织为宜,室温固定不少于 15min。
(5)1×PBS 洗涤3次,每次 5min。
(6)加入适当量的染色工作液。
(7)37℃孵育过夜,最好把整个切片浸泡在染色工作液中。
注:37℃孵育不能在二氧化碳培养箱中进行。
(8)1×PBS 洗涤3次,每次 5min。
(9)脱水:梯度酒精(由低到高)各5min → 二甲苯I 10min→ 二甲苯 II 10min。
(10)加上封片液封片后4℃可以保存较长时间。
(11)普通光学显微镜下观察。
光学显微镜下很容易观察到变成蓝色的表达β-半乳糖苷酶的细胞。
注:所用试剂按照β-半乳糖苷酶染色试剂盒说明配制。
β-半乳糖苷酶染色试剂盒市面上有很多种,但检测方法几近相同,具体还是要参照相应的试剂说明书来操作。
β-半乳糖苷酶(β-Galactosidase, β-GAL)试剂盒说明书
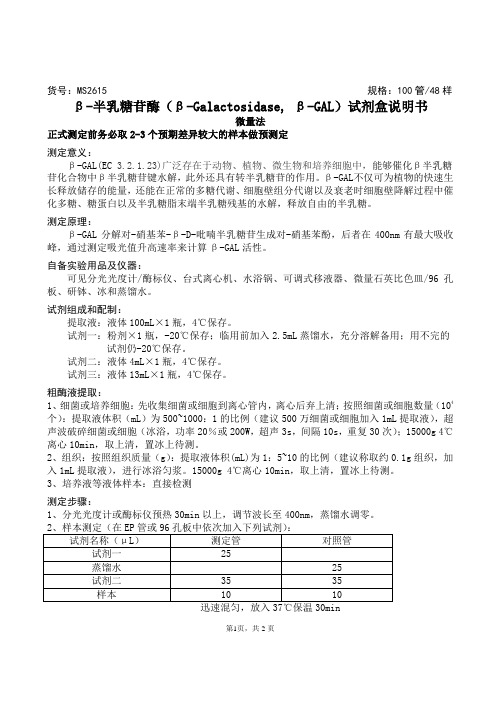
货号:MS2615 规格:100管/48样β-半乳糖苷酶(β-Galactosidase, β-GAL)试剂盒说明书微量法正式测定前务必取2-3个预期差异较大的样本做预测定测定意义:β-GAL(EC 3.2.1.23)广泛存在于动物、植物、微生物和培养细胞中,能够催化β半乳糖苷化合物中β半乳糖苷键水解,此外还具有转半乳糖苷的作用。
β-GAL不仅可为植物的快速生长释放储存的能量,还能在正常的多糖代谢、细胞壁组分代谢以及衰老时细胞壁降解过程中催化多糖、糖蛋白以及半乳糖脂末端半乳糖残基的水解,释放自由的半乳糖。
测定原理:β-GAL分解对-硝基苯-β-D-吡喃半乳糖苷生成对-硝基苯酚,后者在400nm有最大吸收峰,通过测定吸光值升高速率来计算β-GAL活性。
自备实验用品及仪器:可见分光光度计/酶标仪、台式离心机、水浴锅、可调式移液器、微量石英比色皿/96孔板、研钵、冰和蒸馏水。
试剂组成和配制:提取液:液体100mL×1瓶,4℃保存。
试剂一:粉剂×1瓶,-20℃保存;临用前加入2.5mL蒸馏水,充分溶解备用;用不完的试剂仍-20℃保存。
试剂二:液体4mL×1瓶,4℃保存。
试剂三:液体13mL×1瓶,4℃保存。
粗酶液提取:1、细菌或培养细胞:先收集细菌或细胞到离心管内,离心后弃上清;按照细菌或细胞数量(104个):提取液体积(mL)为500~1000:1的比例(建议500万细菌或细胞加入1mL提取液),超声波破碎细菌或细胞(冰浴,功率20%或200W,超声3s,间隔10s,重复30次);15000g 4℃离心10min,取上清,置冰上待测。
2、组织:按照组织质量(g):提取液体积(mL)为1:5~10的比例(建议称取约0.1g组织,加入1mL提取液),进行冰浴匀浆。
15000g 4℃离心10min,取上清,置冰上待测。
3、培养液等液体样本:直接检测测定步骤:1、分光光度计或酶标仪预热30min以上,调节波长至400nm,蒸馏水调零。
衰老

1998年,Wright(Science,1998)
将人的端粒酶反转录酶亚基(hTRT) 基因通过转染,引入正常的人二倍
体细胞,发现表达端粒酶的转染细
胞,其端粒长度明显增加,分裂旺 盛,作为细胞衰老指标的β-半乳糖
苷酶活性则明显降低,与对照细胞
形成极鲜明的反差。 此外,表达端粒酶的细胞寿命比正 常细胞至少长20代,且其核型正常。
体细胞突变理论(wear and tear theory):
衰老是构成机体的生物大分子损伤累积,尤其是DNA分子的损 伤,导致其功能缺陷。
Determined genetically
Interacting epigenetically
Metabolic capacity
Stress response
3.
4.
生命周期短(平均寿命仅20天) 。
体细胞少,易于追踪。
线虫(clock,clk-)突变体: clk- / clk- ) 生活过程的时间延长:发育、细胞周期、成体的节律性行为 生命期延长! Clk基因作用于染色体的沉默区? Puca 等(2001) 跟踪观察137 组年龄91~109 岁的长寿同胞 ( siblings) , 308 名长寿老人,发现他们的4 号染色体D4S1564
这些多肽均是呼吸链复
合物亚单位的组成部分。
缺乏组蛋白的保护,缺乏校正DNA损伤的修复酶。 氧化压力为环境因素的原始应力,首先作用于线粒体DNA, 导致线粒体DNA (mtDNA) 的突变。 正常人心脏,随年龄增加可发生358型mtDNA的缺失,野生型 mtDNA下降至总mtDNA的11%。 骨骼肌细胞中,老年个体比青年个体mtDNA重排发生的数目和 种类明显增高。
端粒DNA序列由特殊的富含GT区的简单串联重复
细胞衰老检查的实验技术及原理(一)

细胞衰老检查的实验技术及原理(一)关键词:细胞色素成纤维细胞酶磷酸试剂标准物质北京标准物质网衰老细胞的形态变化主要表现为形状变大、变平、胞核增大、核膜内陷、染色质固缩、胞内溶酶体变多等。
衰老细胞中细胞器数量尤其是线粒体数量减少,胞质内有色素堆积和空泡形成,最终导致细胞死亡。
总体来说衰老细胞的各种结构呈退行性变化。
根据其特征,目前常用于检测衰老的方法如下:一、β-半乳糖苷酶活性1995年,Dimiri等发现体外培养二倍体成纤维细胞在培养基pH值为6时,其β-半乳糖苷酶染色的阳性率随代龄增加而逐渐上调,他们把这种中性β-半乳糖苷酶定义为SA-β-gal,即衰老相关的β-半乳糖苷酶。
衰老细胞或组织产生的β-半乳糖苷酶可以催化底物X—Gal,生成深蓝色产物,从而在光学显微镜下很容易观察到(图5-5-1)。
在人体表皮角质层细胞中,也可以发现SA-β-gal 随年龄的增加而增加。
并且,SA-β-gal不依赖于DNA复制,可以区分衰老细胞与静止期的细胞。
SA-β-gal是一种体内体外都适用的检测衰老的生物标记物。
由于检测SA-β-gal的方法简单易行,其在检测衰老细胞方面有很广泛的应用,目前已经有2400多篇论文应用了这种方法。
二、端粒长度的检查端粒是位于真核细胞染色体末端顶部的核蛋白结构,由高度保守的TTAGGG 重复序列组成,其存在可以保护染色体末端,是维持染色体稳定的重要因素。
鉴于端粒的特殊结构,端粒的长度会随着每次细胞的分裂而缩短,因此,端粒长度是衰老的一个重要生物标志。
这里我们主要讨论端粒限制性片段(TRF)分析及荧光原位杂交(FISH)法。
端粒限制性片段分析:TRF分析也称作端粒的Southern印迹法,是应用针对端粒重复序列的探针来检测限制性酶切后所保留的端粒的方法。
限制性酶会将基因组DNA消化为短的片段,留下大量完好的端粒,即所谓的端粒限制性片段。
以凝胶电泳分离基因组片段,通过放射性探针(CCCTAA)。
衰老可导致间充质干细胞氧化损伤修复能力降低

f 2 0 1 2 1 0 0 9 o o 9 , M w J s )
: 0 协 1 2 - D 1 0 1 _ - 0 ] g
Agi ng r e du ces oxi da t i ve dam a ge r ep ai r abi l i t y of mes ench ym al s t em cel l s
中国组织工程研 宄
谚 仃 誊 7 0
2 01 3—0 3—0 5出版
Chi n e s e J o u r n a l o fT i s s ue En g i n e e r i ng Re s ea r c h Ma r c h 5 2 01 3 V o L 1 7 No. 1 O
摘 要 背景 :DN A损 伤及 损伤 后 的应答 异 常会 导致 基因 组不稳 定 ,基 因组 不稳 定是 肿瘤 形成 的重 要 因素之 一 。
通 讯作 者 :杨 劲 ,博 士 生 导 师 ,教授 ,解放 军第 三 军 医 大学基 础 部 细胞 生物 学教 研室 ,重庆 市
40 0 0 38
衰老可导致间充质干细胞氧化损伤修复能力降低术 ★
张 岳, 余 瑾 ,张 艺 ,石家 仲 ,黄亚 琴 ,杨 劲
解放 军第 三军 医 大学基础 医学部 细胞 生物 学教研 室,重 庆 市 4 0 0 0 3 8
文 章亮 点 :
1 实验 以培 养 2 O代 以前 , 衰 老相 关 p 一 半乳糖 苷酶 阳 性率 < 1 O %的间 充质 干细 胞作 为年轻 间充质 干细 胞 ; 培养 4 O代 以后 ,衰老 相关 D 一 半乳 糖 苷酶 阳性 率> 7 0 %的间剂 , 比较 培养衰 老干细胞 与年 轻干细胞 对氧化 损伤 的敏感程度 及 D N A损伤 的修复 能力 。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Genistein protects against UVB-induced senescence-like characteristics in human dermalfibroblast by p66Shc down-regulationYi Na Wang a,1,Wei Wu b,1,Hong Chao Chen a,Hong Fang a,*a Department of Dermatology,1st Affiliated Hospital,Zhejiang University School of Medicine,79#Qing Chun Road,Hangzhou310003,Chinab State Key Laboratory for Diagnosis and Treatment of Infectious Disease,First Affiliated Hospital,College of Medicine,Zhejiang University,Hangzhou310003,China1.IntroductionIt has been noticed that the appearance of facial wrinkling in theAsian population is delayed for about10years when compared tothe Caucasian population.The pattern and degree of facialwrinkling is different as well.There are many factors contributingto this difference,such as lifestyle,genetic background,andnutrition.The Asian diet is well known for being rich in soy or soy-containing products and the estrogen-like compounds in soyprotein,along with their antioxidant activities,are regarded aspotential weapons against the aging process.Isoflavones,a group of polyphenolic compounds found in andisolated from a number of plants,with soybeans and soy productslike tofu and textured vegetable protein being the primary foodsource,have attracted a great deal of interest,especially forpossible properties in the prevention and treatment of cancer andchronic disease including cardiovascular diseases and diabetesmellitus[1,2].Recent studies further suggested that isoflavonesmight act as a photoprotection and inhibit the initiation andpromotion of skin carcinomas[3].One of the main isoflavones is genistein.Genistein has beenreported to modulate molecular functions mainly by acting as atyrosine kinase inhibitor[4].Also genistein has anti-oxidation andanti-angiogenesis effects as well as estrogenic activities[5].Previousevidences have suggested that genistein down-regulates UVB-induced signal transduction cascades in carcinogenesis and confersphoto-protective effect in SKH-1murine skin and in humanreconstituted skin[6,7].Recent studies revealed that genisteinprevent UV-induced photoaging and photodamage in humanskin[8].However,although studies have been reported on theJournal of Dermatological Science58(2010)19–27A R T I C L E I N F OArticle history:Received4July2009Received in revised form9February2010Accepted10February2010Keywords:GenisteinPhotoagingUVBp66ShcFKHRL1A B S T R A C TBackground:Genistein,as an active compound of dietary antioxidants,has shown considerable promiseas an effective agent against aging process.However,the effect of genistein on skin photoaging and theassociated mechanism remain unclear.Objective:To delineate the effect of genistein on UVB-induced senescence in human dermalfibroblasts(HDFs)with emphasis on the mechanism of oxidative pathway regulated by p66Shc involved in the events.Methods:HDFs were induced to premature senescence by repetitive subcytotoxic doses of UVBirradiation.Cellular apoptosis and DNA cell cycle were analyzed usingflow cytometry.Intracellularlevels of superoxide dismutase(SOD)and malondialdehyde(MDA)were detected by ELISA.Mutationlevels of two large deletions of mitochondrial DNA,4977bp and3895bp deletion,were determined byquantitative PCR.Western blot was applied to detect the expression and activation of p66Shc(the66-kilodalton isoform of the growth factor adapter Shc)and FKHRL1(a forkhead protein that is intimatelylinked with intracellular oxidation).Results:Strong activity of senescence-associated beta-galactosidase(SA-b-gal),high percent of cellapoptosis as well as cell cycle arrest in G0/G1phase,and increased intracellular oxidative stress wereobserved in HDFs irradiated by UVB.Genistein exerted dramatically protective effects on HDFs in a dose-dependent manner.Elevated copy numbers of large deletions in mitochondrial DNA were also inhibitedby genistein.Down-regulation of total and phosphorylated p66Shc on Ser36,as well as FKHRL1and itsphosphorylation on Thr32,were observed after genistein treatment.Conclusion:The results indicate that genistein protects UVB-induced senescence-like characteristics inHDFs via maintenance of antioxidant enzyme activities and modulation of mitochondrial oxidativestress through down-regulation of a p66Shc-dependent signaling pathway,which may provide potentialprevention against skin aging and even photoaging.ß2010Japanese Society for Investigative Dermatology.Published by Elsevier Ireland Ltd.All rightsreserved.*Corresponding author.Tel.:+8657187236340;fax:+8657187236385.E-mail addresses:hongfangzy@,tango654321@(H.Fang).1These authors contributed equally to this work.Contents lists available at ScienceDirectJournal of Dermatological Sciencej o u r n a l h o m e p a g e:w ww.e l s e v i e r.c o m/j d s0923-1811/$36.00ß2010Japanese Society for Investigative Dermatology.Published by Elsevier Ireland Ltd.All rights reserved.doi:10.1016/j.jdermsci.2010.02.002photo-protective effect of genestein on skin tissue,little has been reported about its effects on aging process of skin cells.Here we report the supplementation of genistein,an active isoflavone,in inhibiting UVB-induced cellular senescence-like characteristics of human dermalfibroblasts(HDFs)and the possible mechanisms involved in the activity.2.Materials and methods2.1.Main reagentsPrimary human dermalfibroblast(HDFs)was obtained from Biotek Biotechnology Co.(Beijing,China).Genistein was obtained from Sigma Chemical Co.(St.Louis,MO,USA).The annexin V-FITC apoptosis detection kit was from Beckman Coulter Inc.(Beckman Coulter,Fullerton,CA,USA).Primary antibodies and phosphory-lated antibodies to shcA,FKHRL1and secondary antibodies were purchased from Upstate Biotechnology,Inc.(USA).Cytochemical staining kit of SA-b-gal and MTT assay kit was obtained from Beyotime Biotechnology(Haimen,China)and ELISA kit for detection of intracellular SOD and MDA was from Nanjing Jiancheng Biology Science Company(Nanjing,China).2.2.Cell culture,genistein treatment and UVB irradiationHDFs were cultured in dulbecco’s modified eagle’s medium (DMEM,Invitrogen,UK)supplemented with10%heat-inactivated fetal bovine serum(FBS,Invitrogen,UK),penicillin(100U/ml),and streptomycin(100mg/l)at378C in a humidified atmosphere containing5%CO2.Genistein was dissolved in dimethyl sulfoxide (DMSO)and used for the treatment of cells.50–60%confluent cells were treated with different concentrations of genistein,whereas DMSO treated cells served as control.After24h of genistein treatment,cells were subcultured at half confluence(1Â104cells/ cm2)in DMEM+1%FBS.They were washed once with phosphate-buffered saline(PBS)and exposed to UVB radiation with the lids removed,in a thin layer of PBS using one Philips TL20W/01lamps (Philips,Netherlands)emitting UVB peaking at311nm,which were placed30cm above the petri dishes.The emitted radiation was checked using a UVR radiometer with a UVB sensor(Bioblock Scientific,Belgium).After irradiation,PBS was replaced by DMEM+1%FBS.The radiation stress was performed three times a day and the accumulative dose for UVB exposure was250mJ/ cm2.Control cells were kept in the same culture conditions without UVB exposure.2.3.Cell viability assay(MTT dye assay)Proliferation of cells was determined by the3-[4,5-dimethylthylthiazol-2-yl]-2,5-diphenyltetrazolium bromide(MTT) method.Briefly,approximately10,000HDFs were plated in each well of96-well plates.After overnight incubation,the cells were treated with genistein(0–160m g/ml)for12h,24h,36h and48h.At the various times following genistein treatment,the medium was removed and MTT(20m l of5mg/ml)was added to each well and incubated at378C for4h.The plates were spun,and the purple colored precipitates of formazan were dissolved in150m l of DMSO. Absorbance was measured at490nm using an ELISA plate reader.The reduction in viability of in genistein-treated HDFs was expressed as a percentage compared to non-genistein(DMSO)treated cells and control cells were considered to be100%viable.2.4.Cytochemical stainingSenescence-associated beta-galactosidase(SA-b-gal)activity was determined at24h later after UVB irradiation as described by Dimri et al.[9].Briefly,the cells were washed with PBS andfixed in 0.2%glutaraldehyde solutions for5min.After dilution with PBS, the cells were washed again with PBS and stained with X-gal solution for6–24h at378C.The population of SA-b-gal-positive cells was determined by counting400cells per dish and photographs were taken using a phase-contrast microscope at 100Âand400Âmagnification(Olympus,Japan).The proportions of cells positive for the SA-b-gal activity are given as percentage of the total number of cells counted in each dish.The results are expressed as mean of triplicatesÆSD.2.5.Flow cytometryA quantitative assessment of apoptosis and DNA cell cycle was made24h later after UVB exposure using the annexin V-FITC apoptosis detection kit as described by the manufacturer.Briefly, for apoptosis analysis,(0.5–1)Â106cells were resuspended at a concentration of1Â106cells/ml in ice-cold PBS for three times and suspended in100m l of binding buffer solution(0.1M Hepes/ NaOH pH7.4,1.4M NaCl,25mM CaCl2).Cells were then treated with5m l of annexin V-FITC and5m l of propidium iodide(PI)and placed in the dark at room temperature for15min.Fluorescence-activated cell sorting(FACS)analysis was done on a cytometer (Beckman Coulter,Fullerton,CA,USA).For DNA cell cycle analysis, about1Â106cells werefixed with ice-cold70%ethanol over night at48C.The cells were subsequently resuspended in PBS for three times and added with2ml of Coulter DNA-Prep reagent at room temperature for30min.Then cells were stained with PI(50m g/ml in PBS)for30min and data was acquired on a Beckman Coulter XL (Beckman Coulter,Fullerton,CA,USA).2.6.ELISA assayIntracellular activity of SOD and level of MDA were also detected by ELISA kit under the instruction of manufacturer.The intracellular activity of SOD and level of MDA were calculated directly from the rate of absorbance of the sample versus the average rate of the blank control using the provided ratio table, repeated at least three times for each sample.2.7.Quantitative real-time PCRQuantitative real-time PCR was performed on an ABI Prism 7700instrument(Applied Biosystems,Foster City,CA,USA)after extraction of genomic DNA from HDFs by total DNA extraction kit (Takara Bio,Otsu,Japan)according to the manufacturer’s instruc-tions.Mutation levels of two large deletions of mitochondrial DNA (mtDNA),4977bp deletion and3895bp deletion,were deter-mined by quantitative PCR by using specific primers:4977bp deletion(8469–13,446nt in mitochondrial DNA),50-ACTACGGT-CAATGCTCTG-30(sense primer)and50-GGAGGTTGAAGTGAGAGGT ATG-30(antisense primer),315bp;3895bp deletion(548–4443nt in mitochondrial DNA)50-GCTTCTGGCCACAGCACTTA-30(sense primer)and50-TAGCGCTGTGAT GAGTGTGC-30(antisense primer), 323bp.The presence of mitochondrial DNA was assessed by PCR amplification of a83bp conservative region of the mitochondrial DNA as an internal control by using the following primers:50-GATTTGGGTACCACCCAAGTATTG-30(sense primer)and50-AATATTCA TGGTGGCTGGCAGTA-30(antisense primer)[10].A BioEasy SYBR Green I PCR Kit(Bioer Technology,Hangzhou city, China)was used in this study.The PCR amplification cycles consisted of an initial denaturation at958C for3min;30cycles of denaturation at958C for30s,annealing at608C for30s,and extension at728C for30s;and afinal extension for10min at 728C.The dissociation curve for each amplification was analyzed to confirm that there were no non-specific PCR products.TheY.N.Wang et al./Journal of Dermatological Science58(2010)19–27 20comparative cycle threshold(Ct)method(2DÀCt)was established for the relative quantification of large deletions in mtDNA.2.8.Western blot analysisCells were harvested at24h following UVB treatment as described above,washed and lysed with lysis buffer.Protein concentration in the resulting lysate was determined using the Lowry assay[11].Appropriate amounts of protein(about40m g) were resolved by electrophoresis in12%Tris–glycine polyacryl-amide gels and transferred to nitrocellulose membranes.Mem-branes were blocked by5%bovine albumin serum in TBST(20mM Tris–HCl pH7.4,150mM NaCl,0.05%Tween20)and then incubated overnight with the appropriate primary antibody at 1:1000or1:200dilution(total or phosphorylated antibodies). They were next washed and incubated with the corresponding horseradish peroxidase conjugated secondary antibody at1:1000 dilution in TBST.Bound secondary antibody was detected using an enhanced chemiluminescence(ECL)system(Pierce Biotechnology Inc.,Rockford,IL,USA).Membranes were exposed to light-sensitive film.As a control,the corresponding b-actin levels were determined in the same cell lysates using the antibody for b-actin(Santa Cruz Biotechnology,Santa Cruz,CA,USA).2.9.Statistical analysisAll values are expressed as meansÆparisons between treatment groups were made by performing t-tests on data derived from triplicates.P values of<0.05were considered statistically significant.The results are representative of at least three indepen-dent experiments with reproducible results.3.Results3.1.Inhibitory effect of genistein and UVB irradiation on HDFs cell viabilityThe cytotoxic effect of genistein on HDFs cells was determined with varying concentrations of genistein and times(12–48h)by MTT assay.As shown in Fig.1A,no inhibitory effect of genisterin on HDFs cell viability was observed when concentration of genistein was lower than80m g/ml.The lowest concentration of genistein that exhibited an inhibitory effect on cell viability was120m g/ml for36h.Based on these observations,we selected a maximum dose of80m g/ml and a time period of24h treatment for further mechanistic studies.We also examined the effect of UVB exposure on HDFs cell viability.There was a decrease in the viability of HDFs with increasing doses of UVB radiation(50–350mJ/cm2)(Fig.1B), and UVB at doses of300and350mJ/cm2significantly inhibited the cell viability.Therefore the subcytotoxic dose of UVB used throughout this study was250mJ/cm2.3.2.UVB-induced SA-b-gal activity is suppressed by genisteinAt present,the most commonly used method to detect senescent cells is a modified b-gal assay[9].Detectable b-gal at pH6was found to increase during replicative senescence of fibroblast cultures in vitro and in vivo and was absent in immortal cell cultures.This was termed senescence-associated beta-galactosidase or SA-b-gal.Our study revealed strong activity of SA-b-gal in UVB-irradiated HDFs,which,as one of the biomarkers of senescence,indicated that cells were induced to a senescence-like state by UVB exposure.The effect of genistein on SA-b-gal activity was also observed and genistein was found to effectively suppress the expression of SA-b-gal in a dose-dependent manner (Fig.2).3.3.UVB-induced apoptosis and cell cycle arrest is inhibited by genisteinWe next determined the effect of genistein on UVB-induced apoptosis by PI staining and the annexin V method usingflow cytometry.As shown in Fig.3A,UVB irradiation resulted in induction of apoptosis on HDFs,while genistein had an anti-apoptotic effect in a dose-dependant manner.It was observed that treatment of HDFs with40and80m g/ml of genistein for24h decreased the number of early apoptotic cells(LR)significantly when compared to18.1%in vehicle control(0m g/ml)group.The number of late apoptotic cells(UR)decreased from15.9%in0m g/ ml control group to3.8%(80m g/ml of genistein-treated cells).The total percent of apoptotic cells(UR+LR)decreased from34%in 0m g/ml control HDFs to8.5%with80m g/ml of genistein treatment for24h.Slow proliferation serves as a biochemical event during aging process,and it has been demonstrated that cells can be arrested in some certain phase of cell cycle[12].For these reasons, we next quantified the extent of cell cycle arrest byflow-cytometric analysis of genistein-treated cells labeled with PI and annexin V.As shown by PI staining and the annexin V method,weFig.1.Genistein shows little inhibitory effect on HDFs cell viability,while UVB irradiation induces decrease of HDFs cell viability in a dose-dependent manner.(A)Cell viability was not affected by genistein when concentration was lower than80m g/ml.Reduced cell viability of HDFs was observed with genistein treatment(120–160m g/ml) at36and48h(compared with0m g/ml control group,#p<0.05at36h,*p<0.05at48h).Viability of cells was determined by the MTT assay as described in materials and methods.(B)Dose-dependent effect of UVB irradiation on HDFs cell viability.Reduced cell viability was observed with UVB irradiation(50–350mJ/cm2),and UVB at doses of 300and350mJ/cm2significantly inhibited the cell viability(compared with0mJ/cm2group,*p<0.05).The data are presented as meansÆSD(n=8)and all experiments were done in triplicate.Y.N.Wang et al./Journal of Dermatological Science58(2010)19–2721found UVB irradiation caused cell cycle arrest,with most HDFs being arrested on G0/G1phase,and it might play a key role in aging process by initiating cell aging (Fig.3B).Fig.3B also shows that HDFs in G0/G1phase was diminished in quantity upon different doses of genistein (20m g/ml,40m g/ml,and 80m g/ml),indicating a dose-dependent inhibition in cell cycle arrest by genistein.Taken together,flow cytometry analysis indicated that treatment of HDFs cells with genistein resulted in a dose-dependent inhibitory effect of UVB-induced apoptosis and cell cycle arrest.3.4.Genistein has regulatory effect on decreased activity of intracellular SOD and increased level of MDA induced by UVB SOD,as a primary defense,could be a crucial enzyme in eliminating oxygen free radicals and reducing the oxidative stress,while MDA,a pro-oxidative product,was regarded as a marker for free radicals-induced lipid peroxidation [13,14].Therefore,de-creased activity of SOD and increased level of MDA were often used as an indication of oxidative damage.In this experiment,wealsoFig.2.UVB irradiation induces SA-b -gal expression in HDFs and protective effect of genistein on it.The expression of SA-b -gal was detected using cytochemical staining method as described in materials and methods,and cell in blue was considered as positive for SA-b -gal staining.Genistein treatment for 24h inhibits the activity of SA-b -gal in a dose-dependent manner in HDFs,and a significant inhibitory effect on SA-b -gal activity was observed at doses of 20,40and 80m g/ml (compared with 0m g/ml control group,*p <0.05).The data are presented as means ÆSD (n =8)and all experiments were done in triplicate.ND,not detectable.A representative staining image is shown from three independent experiments with identical results.Y.N.Wang et al./Journal of Dermatological Science 58(2010)19–2722examined the effect of genistein treatment on the intracellular activity of SOD and level of MDA in HDFs irradiated by UVB.As shown in Fig.4,even in the absence of UVB irradiation,genistein treatment induced the activity of SOD and inhibited the intracellular level of MDA in HDFs.Repeated exposure to subcytotoxic doses of UVB resulted in a significant decrease in intracellular activity of SOD and a concomitant increase in intracellular level of MDA,strongly suggesting an increased oxidative stress response of HDFs to UVB.However,genistein treatment of HDFs was found to have regulatory effects on the expression of intracellular SOD and MDA.Our data clearly show that genistein treatment caused an increase in intracellular SOD activity,and a significant decrease in MDA level at the highest dose (80m g/ml).Thus there was an overall shift in the ratio of anti-oxidative and pro-oxidative products following genistein treat-ment.3.5.UVB-induced mtDNA mutations are down-regulated by genistein Mitochondrial processes –especially those involving free radical production,damage and propagation –are deeply implicated in the advance of aging [15].Previous work has provided support for the significance of mtDNA mutation during aging process.There is evidence to suggest that deletions in mtDNA accumulate with age,and the most common and also the most often-assayed mtDNA deletion mutation is 4,977bp deletion (also known as common deletion)[16,17].Furthermore,recent investigation revealed a possible link between another kind of large deletion,3895bp mtDNA deletion and UVR exposure [10].Therefore,we next determined the relative copy number of 4977bp deletion and 3895bp deletion in mtDNA using real-time quantitative PCR.Products from real-time PCR were confirmed by DNA gel electrophoresis (Fig.5A).As shown in Fig.5B,compara-Fig.3.Genistein treatment dose-dependently inhibits UVB-induced apoptosis and cell cycle arrest in HDFs.(A)Flow cytometry of genistein-treated HDFs using a double-staining method with FITC-conjugated annexin V and PI.The LR quadrant indicates the percentage of early apoptotic cells (annexin V-stained cells)and the UR quadrant the percentage of late apoptotic cells (annexin V +PI-stained cells).(B)Genistein treatment positively regulates cell cycle activity in HDFs.Cell cycle was analyzed by flow cytomety using DNA content analysis kit.A representative figure is shown from three independent experiments with identical results.Y.N.Wang et al./Journal of Dermatological Science 58(2010)19–2723Fig.4.Genistein treatment increases intracellular SOD activity and decreases MDA intracellular level in HDFs.Cells were treated with different concentrations of genistein for 24h as indicated.Intracellular levels of SOD as well as MDA in HDFs were analyzed by ELISA method as detailed in Section 2.Reduced SOD activity and increased MDA level were observed in HDFs with UVB exposure,while genistein treatment at concentrations of 20,40and 80m g/ml significantly up-regulates the intracellular activity of SOD (compared with 0m g/ml control group,*p <0.05).Genistein treatment at concentration of 80m g/ml was observed to down-regulate intracellular level of MDA (compared with 0m g/ml control group,#p <0.05).The data are presented as means ÆSD (n =8)and all experiments were done intriplicate.Fig.5.Quantitative polymerase chain reaction analysis revealed that genistein treatment decreases the relative copy number of common deletion (4977bp deletion)and 3895bp deletion of mtDNA in UVB-exposed HDFs.(A)Two large deletions of mtDNA were detected in HDFs after UVB irradiation using real-time PCR method and the products were electrophoresised in 2%agarose gel.(B)Genistein treatment in concentrations of 40and 80m g/ml significantly down-regulates the relative copy number of common deletion (compared with 0m g/ml control group,#p <0.05).Also genestein was found to down-regulate the relative copy number of 3895bp deletion of mtDNA at 80m g/ml (compared with 0m g/ml control group,*p <0.05).The data are presented as means ÆSD (n =8)and all experiments were done in triplicate.ND,not detectable.Y.N.Wang et al./Journal of Dermatological Science 58(2010)19–2724tively higher levels of 4977bp deletion and 3895bp deletion were found in UVB-induced HDFs,while 24h-treatment of genistein at dose of 80m g/ml led to remarkable down-regulations in the relative copy number of these two large deletions of mtDNA.3.6.p66Shc and FKHRL1is involved in anti-aging effect of genistein The 66-kilodalton isoform of the growth factor adapter Shc (p66Shc)has been shown to intimately associate with cellular stress,mitochondrial proapoptotic activity and cellular senes-cence,and it appears to regulate the transcription of antioxidant proteins that scavenge superoxide and hydrogen peroxide.Studies have also linked p66Shc in a signaling pathway that includes FKHRL1,another mammalian forkhead protein that tightly regulates the transcription of antioxidant genes such as MnSOD and catalase [18,19].Therefore,to further examine whether p66Shc and FKHRL1was involved in UVB-induced senescence of HDFs and mechanism underlying anti-aging effect of genistein,we analyzed the expression and activation pattern of p66Shc as well asFKHRL1protein by western blot.As shown in Fig.6,p66Shc and FKHRL1were significantly up-regulated by UVB irradiation,and genistein treatment of HDFs resulted in a dose-dependent decrease in their expression.The phosphorylation of p66Shc and FKHRL1were also down-regulated by different concentrations of genistein,which indicated genistein,as one of antioxidants,counteracts the UVB-induced oxidative stress at least in part by regulating the expression and activation of p66Shc and FKHRL1protein.4.DiscussionIn contrast to other tissues,the skin is subject to both chronological aging as well as environmental insult in the form of UVR,which triggers increased intracellular levels of reactive oxygen species (ROS)and other markers of oxidative stress (e.g.,8-oxo-guanosine,isoprostane,nitrotyrosine),and finally contributes to the aging process of the skin (photoaging).Although UVB is mostly absorbed in the epidermis and predominantly affects epidermal cells,about 10–30%of UVB can penetrate theepidermis,Fig.6.Genistein treatment reduces the expression of p66Shc and FKHRL1in UVB-exposed HDFs.Cells were treated with different concentrations of genistein for 24h as indicated.The expression of proteins in treated cells was analyzed by Western blotting as detailed in Section 2.(A)Representative blots are shown from three independent experiments with identical results.(B)Protein expression levels of p66Shc and FKHRL1were normalized to that of b -actin and are presented as the fold change compared to 0m g/ml control group.UVB irradiation induced increased expression of p66Shc and FKHRL1,while genistein treatment down-regulates the relative expressions of p66Shc and FKHRL1dose-dependently (compared with 0m g/ml control group,#p <0.05,*p <0.05).(C)Relative expression levels of phospho-Ser36-p66Shc/p66Shc and phospho-Thr32-FKHRL1/FKHRL1are presented as the fold change compared to 0m g/ml control group.Increased expression of phosphorylated p66Shc and FKHRL1were detected in HDFs after UVB irradiation.Genistein treatment at concentrations of 10,20,40and 80m g/ml significantly down-regulates the expressions of phosphorylated p66Shc (compared with 0m g/ml control group,*p <0.05)and phosphorylated FKHRL1(compared with 0m g/ml control group,#p <0.05).Y.N.Wang et al./Journal of Dermatological Science 58(2010)19–2725reach the upper dermis,and do harm tofibroblasts and extracellular matrix as well[20].Chronic exposure to high intensity UVB causes cumulative damage over time,and it is regarded as one of the most important environmental hazards affecting human skin.Recent studies have observed that UVB has far more potent carcinogenic potential and can also generate ROS, and the molecular mechanisms which are responsible for UVB-induced gene expression differ from those involved in UVA-induced gene regulation[21,22].However,the exact mechanisms of UVB on skin aging and related signaling pathways are still a matter of debate.In2005,stress-induced premature senescence (SIPS)of human diploidfibroblasts(HDFs)was induced by a series of subcytotoxic exposures to UVB[23],which helps to explore the mechanisms of UVB-induced senescence,and it has been proved to be an unique and attractive in vitro model for photoaging study [24,25].In our study,based on this in vitro model system,we evaluated the protective effect of genistein against photoaging in HDFs and its mechanism of action.Consistent with previous studies[23–25], we found that repetitive subcytotoxic doses of UVB irradiation successfully induces the expression of SA-b-gal,causes cell apoptosis and cell cycle arrest in HDFs,while genistein protects cells against senescence-like state in a dose-dependant manner. The result clearly demonstrated the anti-aging effect of genistein in premature HDFs induced by UVB exposure.Genistein is well known for its diverse biological actions including protective effects against cellular aging[26].Accumulating evidence from cell culture and laboratory animal experiments also indicates that genistein has the potential to prevent or delay the photoaging process[27].However,we are interested in not only the anti-aging effect in HDFs of genistein but also the underlying molecular mechanism.Previous studies have been observed that genistein might protect against the cellular aging process by modulating oxidative stress and subsequent events[28,29].It has been well established that cellular aging is accompanied by increased oxidative stress,DNA damage(including mtDNA damage)and altered expression of aging-related genes.Oxidative stress,as the major event in the pathogenesis of the aging process,in turn, increases the occurrence of cell damages,especially by apoptosis, which contributes to cellular aging[30].Our data showed that genistein treatment resulted in significant inhibition of elevated levels of MDA as well as copy number of large deletion mutations (including4977bp deletion and3895bp deletion)in mtDNA,and increased the intracellular activity of SOD in UVB-irradiated HDFs. The4977bp‘‘common’’deletion and3895bp deletion in mtDNA have been proposed to be valuable as biomarkers of photodamage or photoaging in skin,and to reflect the dysfunctions of mitochondria[31–34],which are both generators and targets of radical damage in aging.Our results supported the hypothesis that genistein protects skinfibroblasts against senescence by inducing antioxidant enzymes and preventing intracellular oxidative stress that originates in the mitochondria.Still,it is unclear what events are involved in genistein’s effects of protecting both intracellular oxidative stress and mitochondrial damages.In this respect,it is of interest that p66Shc adaptor protein has been involved in mitochondrial signal transduction pathways that regulate cellular responses to oxidative stress.It has been demonstrated that p66Shc plays a critical role in linking mitochondrial functions,oxidative stress and cellular aging. p66Shc translates oxidative damage into cell death by acting as reactive oxygen species producer within mitochondria[35–37], and causes alterations of mitochondrial responses and three-dimensional structure,thus inducing apoptosis[38,39].Mice lacking expression of p66Shc are less susceptible to oxidative stress and have an extended life span[40].Phosphorylation of p66Shc at serine36in the N-terminal CH2domain is critical for the cell aging response elicited by oxidative damage,such as UV and H2O2treatment[19,41,42].Thesefindings prompted us to investigate whether p66Shc was involved in the anti-aging effect of genistein in HDFs.We found that expression and phosphoryla-tion of p66Shc on Ser36in cultured HDFs increased significantly upon UVB irradiation,while a decrease in total and phosphorylated p66Shc was observed in HDFs after treatment with genistein. These data support the concept that p66Shc is correlated with oxidative stress and plays a pivotal role in the cell aging process, and it participates in genistein’s protective effect against senes-cence-like characteristics at least as an important signaling molecule.Therefore,genistein might block the oxidative and aging pathway by a mechanism regulated by p66Shc.Additionally,p66Shc protein seems to interfere with oxidative stress-induced aging by regulating forkhead transcription factors [43].Forkhead family members are well known for their role in regulating stress responses[44–46].They can be activated by a wide range of stress stimuli,in response to which they can regulate a broad variety of cellular mechanisms(e.g.,DNA repair, scavenging of ROS,cell cycle progression,apoptosis,or metabo-lism)[45–48].Recent studies revealed that deletion of p66shc largely blocks phosphorylation of FKHRL1,a homologue of the forkhead family members,indicating the functional interaction with FKHRL1of p66shc[49].To determine whether FKHRL1 expression is regulated by p66Shc in human HDFs during genistein’s anti-aging process,total and phosphorylated FKHRL1 on T1(Thr32)were assessed by western blot in this study.Our data show UVB irradiation of HDFs induced up-regulation of not only p66Shc but also FKHRL1as well as its phosphorylation.Similar with the expression of p66Shc protein,a down-regulation of total and phosphorylated FKHRL1expression was also observed with the treatment of genistein.These data suggested the possibility that redox-dependent forkhead activation was regulated by intracellular oxidative stress in a p66Shc-dependent pattern, and this interplay may modulate the anti-aging effect of genistein on HDFs.Overall,this study presents evidence to explore the protective effect of genistein against senescence-like characteristics on HDFs and the mechanism underlying it.The above data indicate that genistein reverses the senescence process in HDFs by anti-oxidative action,which is mediated through down-regulation of p66Shc protein that involves forkhead protein suppression. Therefore,targeting of this pathway may benefit skin-aging (including photoaging and photodamage)treatment.Given that oxidative stress has been implicated in a lot of human aging processes besides skin aging,this role for p66Shc as signaling agents may have important therapeutic implications. AcknowledgmentsThis work is supported by Zhejiang Health Department Foundation of China(No.2006B865)and Skin Grant Program (2007)of L’Oreal-Chinese Medical Association(CMA). References[1]Bartlett HE,Eperjesi F.Nutritional supplementation for type2diabetes:asystematic review.Ophthalmic Physiol Opt2008;28:503–23.[2]Montenegro MF,Pessa LR,Tanus-Santos JE.Isoflavone genistein inhibits theangiotensin-converting enzyme and alters the vascular responses to angio-tensin I and bradykinin.Eur J Pharmacol2009;607:173–7.[3]Widyarini S,Husband AJ,Reeve VE.Protective effect of the isoflavonoid equolagainst hairless mouse skin carcinogenesis induced by UV radiation alone or with a chemical cocarcinogen.Photochem Photobiol2005;81:32–7.[4]Dixon RA,Ferreira D.Genistein.Phytochemistry2002;60:205–11.[5]Su SJ,Yeh TM,Chuang WJ,Ho CL,Chang KL,Liu HS,et al.The novel targets foranti-angiogenesis of genistein on human cancer cells.Biochem Pharmacol 2005;69:307–18.Y.N.Wang et al./Journal of Dermatological Science58(2010)19–27 26。
