Performance characterization of Ti substrate lead dioxide electrode with different solid
材料参数英文

材料参数英文Material ParameterMaterials are fundamental components in various industries, from construction to electronics, and their properties play a crucial role in determining the performance and functionality of the products they are used in. Understanding the parameters that define a material's characteristics is essential for engineers, scientists, and manufacturers to make informed decisions during the design, development, and production stages. In this essay, we will explore the key material parameters and their significance in the world of materials science and engineering.One of the primary material parameters is the chemical composition, which refers to the specific elements and their relative proportions that make up a material. The chemical composition of a material can have a significant impact on its physical, mechanical, and thermal properties. For example, the addition of certain alloying elements to steel can enhance its strength, corrosion resistance, or hardenability. Likewise, the composition of ceramics, polymers, and composites can be tailored to meet specific requirements, such as high-temperature resistance, electrical insulation, or lightweight properties.Another critical parameter is the microstructure, which describes the internal structure of a material at the microscopic level. The microstructure is influenced by the material's composition, as well as the manufacturing processes it undergoes, such as heat treatment, mechanical processing, or solidification. The arrangement and distribution of the material's constituent phases, grains, and defects can have a profound impact on its mechanical properties, corrosion behavior, and even its electrical and magnetic characteristics.The physical properties of a material, such as density, melting point, thermal conductivity, and coefficient of thermal expansion, are also important parameters that need to be considered. These properties can affect the material's performance in various applications, such as heat transfer systems, thermal insulation, or structural integrity under temperature variations.Mechanical properties, including strength, toughness, hardness, and fatigue resistance, are crucial parameters that determine a material's ability to withstand various stresses and deformations. These properties are often the primary drivers in the selection of materials for structural, aerospace, and automotive applications, where the material's ability to bear loads and resist failure is of utmost importance.Electrical and magnetic properties are also significant material parameters, particularly in the electronics and telecommunications industries. Parameters such as electrical conductivity, dielectric constant, and permeability can influence the performance of electronic components, circuits, and devices. The ability to control and manipulate these properties is crucial for the development of advanced electronic and electromagnetic technologies.In addition to the physical, mechanical, and electrical/magnetic properties, materials can also exhibit unique surface and interfacial characteristics, such as wettability, adhesion, and corrosion resistance. These parameters are particularly important in applications where the material's interaction with its environment, or with other materials, plays a critical role, such as in coatings, thin films, and biomedical implants.The characterization and measurement of material parameters are essential for understanding the behavior and performance of materials. A wide range of analytical techniques, including microscopy, spectroscopy, thermal analysis, and mechanical testing, are employed to quantify and evaluate the various material parameters. These techniques provide valuable data that can be used to optimize material selection, improve manufacturing processes, and develop new materials with enhanced properties.In the modern era of advanced materials and rapid technological progress, the understanding and control of material parameters have become increasingly crucial. Materials scientists and engineers are constantly pushing the boundaries of what is possible, developing new materials with tailored properties to meet the ever-evolving demands of industry and society. From lightweight and high-strength composites for aerospace applications to smart materials with programmable responses, the ability to precisely engineer and manipulate material parameters is the key to unlocking the full potential of materials and driving innovation across a wide range of fields.In conclusion, material parameters are the fundamental building blocks that define the characteristics and behavior of materials. By understanding and controlling these parameters, we can design, develop, and optimize materials to meet the demanding requirements of modern applications, ultimately paving the way for technological advancements and a more sustainable future.。
糠醛渣基木质素

第62卷 第1期吉林大学学报(理学版)V o l .62 N o .12024年1月J o u r n a l o f J i l i nU n i v e r s i t y (S c i e n c eE d i t i o n )J a n 2024d o i :10.13413/j .c n k i .jd x b l x b .2023064糠醛渣基木质素/纤维素复合材料的表征及氧还原电催化性能曲 霞1,2,任素霞1,李 政1,2,冯宇伟1,2,杨延涛1,雷廷宙1(1.常州大学城乡矿山研究院,江苏常州213164;2.常州大学石油化工学院,江苏常州213164)摘要:为解决糠醛渣堆放导致的环境污染问题,并拓展废弃物资源的高值化利用途径,通过超微研磨和高压均质等预处理过程制备糠醛渣基木质素/纤维素复合材料.采用扫描电子显微镜(S E M )和原子力显微镜(A F M )表征样品形貌,用范式洗涤法和元素分析对复合材料的组分以及元素含量进行分析,通过F o u r i e r 变换红外光谱(F T I R )和紫外光谱(U V )对复合材料的表面官能团进行分析,采用非等温条件下的多重升温速率热重法(T G )进行热动力学分析.结果表明,糠醛渣基木质素/纤维素复合材料中C 的质量分数较高(55.95%),可作为一种理想碳源,且该材料含有丰富的木质素(53.18%)和纤维素(39.48%),可利用价值较高,整体表观活化能较低(30k J /m o l ),其在0.1m o l /L 的K O H 碱性电解质中半波电位(E 1/2=0.83V )达到商业P t /C (E 1/2=0.86V )的96.5%,因此以糠醛渣基木质素/纤维素复合材料为生物质前驱体制备的碳材料可作为理想的燃料电池氧还原催化剂.关键词:糠醛渣;生物质;木质素/纤维素;动力学分析;氧还原活性中图分类号:O 469 文献标志码:A 文章编号:1671-5489(2024)01-0156-09C h a r a c t e r i z a t i o no f F u r f u r a lR e s i d u eB a s e dL i gn i n /C e l l u l o s e C o m p o s i t e a n dO x y g e nR e d u c t i o nE l e c t r o c a t a l yt i cP e r f o r m a n c e Q U X i a 1,2,R E NS u x i a 1,L I Z h e n g 1,2,F E N G Y u w e i 1,2,Y A N G Y a n t a o 1,L E IT i n gz h o u 1(1.U r b a na n dR u r a lM i n i n g R e s e a r c h I n s t i t u t e ,C h a n g z h o uU n i v e r s i t y ,C h a n g z h o u 213164,J i a n gs uP r o v i n c e ,C h i n a ;2.S c h o o l o f P e t r o c h e m i c a lE n g i n e e r i n g ,C h a n g z h o uU n i v e r s i t y ,C h a n g z h o u 213164,J i a n gs uP r o v i n c e ,C h i n a )收稿日期:2023-02-23.第一作者简介:曲 霞(1998 ),女,汉族,硕士研究生,从事生物质功能材料的研究,E -m a i l :2621903540@q q.c o m.通信作者简介:杨延涛(1980 ),男,汉族,博士,副研究员,从事生物质资源化利用的研究,E -m a i l :y y t @c c z u .e d u .c n ;雷廷宙(1963 ),男,汉族,博士,研究员,从事生物质能源技术开发利用的研究,E -m a i l :l e i t i n gz h o u @163.c o m.基金项目:国家重点研发计划项目(批准号:2021Y F C 2101604).A b s t r a c t :I no r d e r t o s o l v e t h e e n v i r o n m e n t a l p o l l u t i o n c a u s e db y t h e s t a c k i n g of f u r f u r a l r e s i d u e a n d e x p a n dt h e h igh -v a l u e u t i l i z a t i o n o f w a s t er e s o u r c e s ,t h ef u r f u r a lr e s i d u e b a s e dl i g n i n /c e l l u l o s e c o m p o s i t ew a s p r e p a r e dt h r o u g ht h e p r e t r e a t m e n t p r o c e s s e ss u c ha su l t r a -f i n e g r i n d i n g a n dh i g h -p r e s s u r eh o m o g e n i z a t i o n .T h es a m p l e m o r p h o l o g y w a sc h a r a c t e r i z e d b y u s i n g s c a n n i n g e l e c t r o n m i c r o s c o p y (S E M )a n da t o m i c f o r c e m i c r o s c o p y (A F M ),t h ec o m po s i t i o na n de l e m e n t a l c o n t e n to f t h ec o m p o s i t e w e r ea n a l y z e d b y u s i n g n o r m a lf o r m w a s h i n g m e t h o da n de l e m e n t a la n a l ys i s ,t h e s u r f a c ef u n c t i o n a l g r o u p s o ft h e c o m p o s i t e w e r e a n a l y z e d b y u s i n g Fo u r i e rt r a n s f o r m i n f r a r e d s p e c t r o s c o p y (F T I R )a n d u l t r a v i o l e ts p e c t r o s c o p y (U V ),a n d t h et h e r m o k i n e t i c a n a l ys i s w a s p e r f o r m e db y u s i n g m u l t i p l eh e a t i n g r a t e t h e r m o g r a v i m e t r y (T G )u n d e rn o n -i s o t h e r m a l c o n d i t i o n s .T h e r e s u l t s s h o wt h a t t h e m a s s f r a c t i o no fCi nf u r f u r a l r e s i d u eb a s e dl i g n i n /c e l l u l o s ec o m po s i t e i s h i g h (55.95%),w h i c hc a nb eu s e d a s a n i d e a l c a r b o ns o u r c e .T h em a t e r i a l c o n t a i n s a b u n d a n t l i gn i n (53.18%)a n dc e l l u l o s e (39.48%),a n dh a sh i g hu t i l i z a t i o nv a l u e .T h eo v e r a l l a p pa r e n ta c t i v a t i o n e n e r g y i s l o w (30k J /m o l ),a n d t h eh a l f -w a v e p o t e n t i a l (E 1/2=0.83V )i n0.1m o l /L K O Ha l k a l i n e e l e c t r o l y t e r e a c h e s 96.5%o f c o mm e r c i a l P t /C (E 1/2=0.86V ).T h e r e f o r e ,c a r b o nm a t e r i a l s p r e p a r e d b y u s i n g f u r f u r a l r e s i d u eb a s e d l i g n i n /c e l l u l o s e c o m po s i t e s a s b i o m a s s p r e c u r s o r s c a nb eu s e d a s i d e a l o x y g e n r e d u c t i o n c a t a l ys t s f o r f u e l c e l l s .K e yw o r d s :f u r f u r a l r e s i d u e ;b i o m a s s ;l i g n i n /c e l l u l o s e ;k i n e t i c a n a l y s i s ;o x y g e n r e d u c i n g a c t i v i t y 糠醛是一种多用途的工业化学品,是一种可再生㊁不可或缺的平台化合物,用于有机合成㊁溶剂㊁炼油和制药.通常糠醛由玉米芯㊁甘蔗渣㊁稻壳和其他农业废物中戊糖(半纤维素)脱水制备[1],大多数糠醛的转化率只有50%~60%[2-3],由于糠醛生产的主要催化剂为硫酸,因此糠醛渣呈高酸性以及富含盐类物质的特征[4],大量堆积易污染土壤和空气,导致严重的环境问题[5].在糠醛生产过程中,除生物质中的半纤维素大部分转化为糠醛外,木质素和纤维素大部分被保留,导致糠醛渣富含木质素和纤维素,具有较高的再利用价值.在生产糠醛过程中,生物质原料中的半纤维素发生水解,导致糠醛渣形成丰富的孔结构,具有相对较高的比表面积,成为制备生物质碳材料的良好前驱体.Y i n 等[6]以糠醛渣为原料,研究了通过回收热解气体自活化制备具有可控比表面积和中孔比的生物炭样品,活化后生物炭的比表面积为567m 2/g ,比孔体积为0.380c m 3/g;Z h o u 等[7]将经磷酸处理的糠醛渣进行了快速热解,制备了介孔率超高(93.90%)㊁孔径分布窄㊁比表面积大(1769.40m 2/g)的P 掺杂糠醛渣基碳材料,其对亚甲基蓝具有良好的吸附性能,平衡吸附容量为486m g /g,去除率为97.2%;C h e n 等[8]用碱性过氧化氢在不同温度和时间下提取糠醛渣中的木质素,结果表明,木质素的产率随反应时间和温度的增加而增大,在80ħ反应3h ,木质素的产率最大为41.40%,提取的木质素平均相对分子质量约为糠醛残渣磨碎木质素的1/4,表明糠醛残渣木质素在处理过程中发生严重降解;L i 等[9]用碱煮技术从工业糠醛渣中提取木质素,研究了不同碱处理条件对提取木质素的影响,结果表明,提取的木质素含有丰富愈创木酰㊁丁香酰和对羟基苯基结构单元,并且富含羟基;L i u 等[10]以漂白玉米芯残渣(C C R )为前驱体,采用4种不同方法(硫酸水解㊁甲酸水解㊁2,2,6,6-四甲基哌啶氧化物介导氧化和纸浆精制)制备纳米纤维素,并对纳米纤维素产品进行比较,结果表明,糠醛渣可作为制备纳米纤维素的原料.目前对糠醛渣提取纤维素和木质素或利用糠醛渣制备多孔碳的研究较多,但对糠醛渣的化学组成㊁表面化学性质及热动力学的研究文献报道较少.基于此,本文以糠醛渣为原料制备的糠醛渣基木质素/纤维素复合材料为研究对象,对其化学组成㊁表面化学性质㊁热动力学和电化学性能进行研究.1 实 验1.1 材料与仪器糠醛渣购自河南宏业控股集团有限公司;三聚氰胺(C 3H 6N 6)㊁硫脲(C H 4N 2S )和氢氧化钾(K O H )均为分析纯,购自上海麦克林生化科技有限公司;无水乙醇(C 2H 6O )为分析纯,购自江苏强盛功能化学股份有限公司;N a f i o n (质量分数为5%)分析纯,购自美国杜邦公司;氮气(N 2)和氧气(O 2)购自常州市华阳气体有限公司.MK Z A 10-15J 型超微研磨机(日本M a s u k o 公司);M -110P 型高压微射流均质机(美国M F I C公司);D H G -9030A 型鼓风干燥箱(上海一恒科学仪器有限公司);O T F -1200X 型真空管式炉(合肥科晶技术有限公司);C H I 760E 型电化学工作站(上海辰华设备有限公司).1.2 实验步骤1.2.1 糠醛渣基木质素/纤维素复合材料的制备称取200g 糠醛渣,加入200g 去离子水,搅拌均匀后抽滤并反复洗涤至中性,将糠醛渣分散成751 第1期 曲 霞,等:糠醛渣基木质素/纤维素复合材料的表征及氧还原电催化性能851吉林大学学报(理学版)第62卷质量分数为8%的悬浮液.用MK Z A10-15J型超微研磨机对悬浮液进行超微研磨,在研磨间隙加入约1000g去离子水用以稀释悬浮液,得到质量分数约为3%含木质素的纤维素粗产物悬浮液,向悬浮液中倒入适量去离子水并用M-110P型高压均质机对其进行高压均质,最终得到质量分数为1%的糠醛渣木质素/纤维素复合材料.糠醛渣基木质素/纤维素复合材料的制备流程如图1所示.图1糠醛渣基木质素/纤维素复合材料的制备流程F i g.1P r e p a r a t i o n p r o c e s s o f f u r f u r a l r e s i d u e b a s e d l i g n i n/c e l l u l o s e c o m p o s i t e s1.2.2糠醛渣基木质素/纤维素碳材料的制备将200m L糠醛渣基木质素/纤维素复合材料置于鼓风干燥箱(80ħ)中干燥24h,得到固体产物,将产物转移到石英舟中,在N2气氛下,以5ħ/m i n升温至特定温度,保持2h,自然冷却至室温.将碳化后的样品用1m o l/L H C l酸洗后水洗至中性,最终得到糠醛渣基木质素/纤维素碳材料(F R/C).1.2.3糠醛渣基木质素/纤维素氮硫共掺杂碳材料的制备将8g三聚氰胺和8g硫脲溶解在200m L糠醛渣基木质素/纤维素复合材料中,加热搅拌至均匀,将混合物置于鼓风干燥箱(80ħ)中干燥24h,得到固体产物,将产物转移到石英舟中,在N2气氛下,以5ħ/m i n升温至特定温度,保持2h,自然冷却至室温.将碳化后的样品用1m o l/L H C l酸洗后水洗至中性,最终得到糠醛渣基木质素/纤维素氮硫共掺杂碳材料(F R/C-N-S).1.3样品表征与分析利用场发射电子显微镜(S i g m a300型,德国Z e i s s公司)㊁元素分析仪(V a r i oE Lc u b e型,德国U N I C U B E公司)㊁F o u r i e r红外变换光谱仪(T e n s o r27型,德国B r u k e r公司)和紫外可见分光光度计(U V-2450型,日本S h i m a d z u公司)对糠醛渣基木质素/纤维素复合材料进行结构表征;使用S D T Q600型差热热重联用仪(美国T A仪器公司)对糠醛渣基木质素/纤维素复合材料进行热性能分析测试;参考G B/T20806 2006,D B37/T2969 2017和G B/T20805 2006对糠醛渣基木质素/纤维素复合材料中三大元素的质量分数进行测量;参考G B/T28731 2012对糠醛渣基木质素/纤维素复合材料进行工业分析和元素分析.采用C o a t s-R e d f e r n法和F l y n n-W a l l-O z a w a(F WO)法对糠醛渣基木质素/纤维素复合材料进行动力学分析,由于聚合物的热解反应可视为一级动力学反应,因此糠醛渣基木质素/纤维素复合材料的热解反应方程可表示为A(s)ңB(s)+C(g),(1)dαd t=k(1-α),(2)由于k =A e x p-E {}R T,(3)因此,式(2)可转化为d αd t=A e x p -E {}R T (1-α),(4)其中α=W 0-W W 0-W ɕ,W 0为初始样品质量(m g ),W 为t 时刻样品质量(m g),W ɕ为反应结束时的样品质量(m g),T 为反应温度(K ),A 为指前因子(s -1),E 为活化能(J /m o l ),R =8.314J /(m o l ㊃K )为气体常数[11-12].将升温速率常数β=d T d t(K /m i n )代入式(4)可得d αd T =Aβe x p -E {}R T (1-α).(5)用C o a t s -R e d f e r n 法对式(5)进行处理可得l n -l n (1-α)T éëêêùûúú2=l n A R βE 1-2R T æèçöø÷éëêêùûúúE -E R T.(6) 对多数的裂解反应[13],R T /E ≫1,1-2R T /E ʈ1,由于式(6)右端第一项几乎均为常数,因此式(6)可表示为l n -l n (1-α)T éëêêùûúú2=l n A R βE -E R T ,(7)由l n -l n (1-α)T éëêêùûúú2对T -1做图可得到一条直线,令Y =l n -l n (1-α)T éëêêùûúú2,α=-E R ,X =1T ,b =l n A R βE ,则有Y =αX +b ,由式(7)做图可直接得到该直线的斜率-E R 和截距l n A R βE [14],进而求出E 和A .当用F WO 法求E 时,不涉及反应机理函数,避免了相对误差,由于温度积分采用近似方法,因此引入了近似误差.对温度积分后可得G (a )=A E βR P (u )=A E βR e -u u 21u 0-2!u 1+3!u 2-4!u 3+æèçöø÷ ,(8)对式(8)两边取对数可得l n P (u )=-u +l n (u -2)-3l n u ,(9)若20≪u ≪60,则用T a yl o r 级数展开对数项并取一阶近似,可得l g P (u )=-2.315-0.4567E R T,(10)l g β与1/T 呈线性相关,通过曲线斜率可求出转化率对应的表观活化能[15].用辰华C H I 760E 型电化学工作站进行电化学测试,将2m g 糠醛渣基木质素/纤维素碳材料与乙醇(50μL )㊁去离子水(50μL )和N a f i o n (50μL )混合制备浆液,超声均匀后将12μL 的浆液滴到打磨抛光后的玻碳上,自然干燥.采用三电极体系(玻碳电极作为工作电极,铂片作为对电极,H g /H g 2C l 2作为参比电极)测试样品氧还原(O R R )性能,电解质溶液为0.1m o l /LK O H ,电位均已校正为可逆氢电极(R H E )的电位.2 结果与讨论2.1 木质素㊁纤维素和半纤维素的质量分数分析糠醛渣基木质素/纤维素复合材料的化学组成分析列于表1.由表1可见,木质素和纤维素的质量分数较高,木质素和纤维素的质量分数分别为53.18%和39.48%,远高于其他普通植物,因此用糠醛渣可提取木质素和纤维素.在制备糠醛过程中生物质中的半纤维素大部分被水解成戊糖,因此半纤维素的质量分数较低,仅占4.16%,由于糠醛渣中存在一定量的灰分等其他物质,不利于糠醛渣的高值951 第1期 曲 霞,等:糠醛渣基木质素/纤维素复合材料的表征及氧还原电催化性能化利用,因此可采取合适的方法去除.表1 糠醛渣基木质素/纤维素复合材料的化学组成分析T a b l e 1 C h e m i c a l c o mo s i t i o na n a l s i s o f f u r f u r a l r e s i d u e b a s e d l i n i n /c e l l u l o s e c o m o s i t e s 2.2 元素分析糠醛渣基木质素/纤维素复合材料与未处理糠醛渣的元素分析列于表2.由表2可见,糠醛渣基木质素/纤维素复合材料主要由C ,H ,O 三种元素组成,并含有微量的N 和S 元素.其中C 元素为主要成分,其质量分数为55.98%,糠醛渣基木质素/纤维素复合材料与未经处理糠醛渣元素中C 元素的质量分数相差较小,均可作为一种良好的可再生碳源加以利用.表2 糠醛渣基木质素/纤维素复合材料的元素分析T a b l e 2 E l e m e n t a l a n a l y s i s o f f u r f u r a l r e s i d u e b a s e d l i g n i n /c e l l u l o s e c o m po s i t e s %样品工业分析M a dA dV d a f元素分析C d a fH d a fN d a fS t ,dO d a f糠醛渣基木质素/纤维素复合材料5.993.1964.6255.985.110.500.1338.28糠醛渣4.4617.1576.1656.805.870.740.2036.392.3 微观结构分析糠醛渣及糠醛渣基木质素/纤维素复合材料的扫描电子显微镜(S E M )照片和原子力显微镜(A F M )照片分别如图2和图3所示.图2 糠醛渣(A )及糠醛渣基木质素/纤维素复合材料(B )的S E M 照片F i g .2 S E Mi m a g e s o f f u r f u r a l r e s i d u e (A )a n d f u r f u r a l r e s i d u e b a s e d l i g n i n /c e l l u l o s e c o m po s i t e s (B )图3 糠醛渣基木质素/纤维素复合材料的A F M 照片F i g .3 A F Mi m a ge of f u r f u r a l r e s i d u e b a s e d l ig n i n /c e l l u l o s e c o m po s i t e s 由图2(A )可见,糠醛渣表面较粗糙㊁致密,呈不规则的块状结构.由图2(B )可见,糠醛渣基木质素/纤维素复合材料呈明显的纤维状结构,其周围分散较多颗粒.由图3可见,糠醛渣基木质素/纤维素复合材料为粗细不同㊁长短不一的纤维素纤维,其周围分散大小不一的木质素颗粒.根据A F M 照片统计出糠醛渣基木质素/纤维素复合材料中木质素粒径和纤维素粒径分布,如图4所示.由图4(A )可见,木质素直径为20~300n m ,直径集中在(100ʃ15)n m 处;由图4(B )可见,纤维素长度为0~1200n m ,长度集中在(250ʃ150)n m 处;由图4(C )可见,纤维素直径为0~100n m ,直径集中在(35ʃ15)n m 处.2.4 红外光谱分析糠醛渣基木质素/纤维素复合材料的红外光谱如图5所示.由图5可见:糠醛渣宽频吸收带在3340~3460c m -1处,对应纤维素和木质素中061 吉林大学学报(理学版) 第62卷图4 糠醛渣基木质素/纤维素复合材料中木质素和纤维素的粒径分布F i g .4 P a r t i c l e s i z e d i s t r i b u t i o no f l i g n i na n d c e l l u l o s e i n f u r f u r a l r e s i d u e b a s e d l i g n i n /c e l l u l o s e c o m po s i t e s O H 的伸缩振动[16],表明糠醛渣中存在大量属于苯酚类和脂肪族结构的羟基;在2934c m -1附近出现的吸收峰主要是由于侧链的甲基和亚甲基中C H 拉伸振动[17]所致;在1703c m -1附近出现了木质素中非共轭酮和羧基中C O 伸缩振动的特征[16,18];在1621c m -1附近纤维素㊁半纤维素和木质素中出现了芳香环骨架C C 伸缩振动的特征;在1463c m -1附近出现了纤维素和木质素中甲基和亚甲基的C H 弯曲振动;在1325c m -1附近的特征峰为木质素中苯环骨架振动吸附所致[19];在1265,1064c m -1附近的强吸收峰可能为木质素中苯环甲氧基的C O 键伸缩振动导致[9].2.5 紫外光谱分析糠醛渣基木质素/纤维素复合材料的紫外光谱如图6所示.根据元素分析可知糠醛渣基木质素/纤维素复合材料中含有大量的木质素,木质素作为芳香族化合物含有丰富的苯环结构,可强烈吸收紫外光,因此通过紫外光谱可进一步了解复合材料的表面官能团.由图6可见,糠醛渣基木质素/纤维素复合材料在300n m 处出现一个明显的紫外光谱特征吸收峰,表明糠醛渣基木质素/纤维素复合材料的侧链结构中存在较多共轭烯键[20].图5 糠醛渣基木质素/纤维素复合材料的红外光谱F i g .5 I n f r a r e d s pe c t r u mof f u r f u r a l r e s i d u e b a s e d l ig n i n /c e l l u l o s e c o m po s i t es 图6 糠醛渣基木质素/纤维素复合材料的紫外光谱F i g .6 U Vs pe c t r u mof f u r f u r a l r e s i d u e b a s e d l ig n i n /c e l l u l o s e c o m po s i t e s 2.6 热解过程及热解动力学分析不同升温速率下糠醛渣基木质素/纤维素复合材料的热重(T G )曲线和热重微分(D T G )曲线如图7所示.由图7可见,不同升温速率下的T G 和D T G 曲线趋势大致相同,主要分为3个阶段[21].第一阶段发生在室温~190ħ,总质量损失为3.95%,主要是水分的去除.第二阶段发生在190~460ħ,总质量损失为47.60%,这是由于糠醛渣基木质素/纤维素复合材料中纤维素㊁半纤维素和木质素在该阶段产生分解所致.随着升温速率从5ħ/m i n 增加到30ħ/m i n ,D T G 曲线的峰值温度从341ħ增加到378ħ,这是由于生物质的热导率较低,糠醛渣作为一种生物质,若升温速率太快则受热时存在时间延迟,从而出现热滞后现象.第三阶段(>460ħ)对应炭化过程,随着加热速率从5ħ/m i n 增加到30ħ/m i n,固体残渣的质量占比变大,这是由于温度相同时,样品升温越慢,物料传热和传质过程越充分所致[22].为进一步验证第二阶段(190~460ħ)的活化能,用C o a t s -R e d f e r n 法对糠醛渣基木质素/纤维素161 第1期 曲 霞,等:糠醛渣基木质素/纤维素复合材料的表征及氧还原电催化性能复合材料进行动力学分析,结果列于表3.图7 糠醛渣基木质素/纤维素复合材料在不同升温速率下的T G (A )和D T G (B )曲线F i g .7 T G (A )a n dD T G (B )c u r v e s o f f u r f u r a l r e s i d u e b a s e d l i g n i n /c e l l u l o s e c o m p o s i t e s a t d i f f e r e n t h e a t i n g ra t e s 表3 用C o a t s -R e d f e r n 法对糠醛渣基木质素/纤维素复合材料进行热解反应动力学分析T a b l e3 P y r o l y s i s r e a c t i o nk i n e t i c s a n a l y s i s o f f u r f u r a l r e s i d u e b a s e d l i g n i n /c e l l u l o s e c o m p o s i t e s b y u s i n g Co a t s -R e d f e r nm e t h o d β/(ħ㊃m i n -1)θ/ħnE /(k J ㊃m o l-1)A /m i n-1R 25205~465132.21903.160.9510224~484134.121935.850.9415210~490131.541933.450.9420220~500129.811782.450.9425220~500134.185049.110.9530220~500133.525132.430.94由表3可见,实验条件下升温速率对表观活化能的影响较小,但热解开始和结束的温度随升温图8 糠醛渣基木质素/纤维素复合材料在不同升温速率下l n β和1000/T 的关系F i g .8 R e l a t i o n s h i p be t w e e n l n βa n d1000/T of f u r f u r a l r e s i d u eb a s e dl ig n i n /c e l l u l o s ec o m p o s i t e sa td i f f e r e n th e a ti n g ra t e s 速率的增加而增加,主要失质量区间随升温速率的增加向高温区移动.整体表观活化能较低(30k J /m o l ),与文献[23]的结果一致,表明糠醛渣基木质素/纤维素复合材料的热解反应较易进行.糠醛渣基木质素/纤维素复合材料在不同升温速率下l n β和1000/T 的关系如图8所示.由图8可见,线性拟合较好.用F WO 法对糠醛渣基木质素/纤维素复合材料进行热解反应动力学分析,结果列于表4.由表4可见,通过F WO 法计算出的活化能为22.30~40.17k J /m o l ,其整体上随转化率的升高而增大,最大值为40.17k J /m o l .采用两种方法拟合的曲线线性相关系数R 2均较大(>0.93),因此糠醛渣基木质素/纤维素复合材料的主要热解阶段可视为一级动力学反应,表明单段一级动力学模型可靠.表4 用F W O 法对糠醛渣基木质素/纤维素复合材料进行热解反应动力学分析T a b l e 4 P y r o l y s i s r e a c t i o nk i n e t i c s a n a l y s i s o f f u r f u r a l r e s i d u e b a s e d l i g n i n /c e l l u l o s e c o m p o s i t e s b y u s i n g FW O m e t h o d2.7 糠醛渣基木质素/纤维素氮硫共掺杂碳材料电催化氧还原性能纯糠醛渣基木质素/纤维素复合碳材料(F R /C )与糠醛渣基木质素/纤维素氮硫共掺杂多孔碳材料(F R /C -N -S )的循环伏安(C V )曲线和1600r /m i n 下的线性扫描伏安(L S V )曲线如图9所示.由261 吉林大学学报(理学版) 第62卷图9(A )可见,碳材料在O 2饱和的0.1m o l /LK O H 电解液中出现明显还原峰,表明两种碳材料均具有电催化氧还原(O R R )活性.由图9(B )可见,F R /C -N -S 的起始电位(E o n s e t )为0.93V ,半波电位(E 1/2)为0.83V ,极限电流密度为1.75m A /c m 2,与商业P t /C (E o n s e t =1.04V 和E 1/2=0.86V )相近,远高于F R /C 的氧还原催化活性(起始电位为0.74V ,半波电位为0.6V ,极限电流密度为1.14m A /c m 2),可见掺杂杂原子增强了糠醛渣基木质素/纤维素复合碳材料的电催化氧还原性能,表明以糠醛渣基木质素/纤维素复合材料为生物质前驱制备的碳材料可作为理想的燃料电池氧还原催化剂.图9 碳材料的C V 曲线(A )和1600r /m i n 下的L S V 曲线(B )F i g.9 C Vc u r v e s (A )o f c a r b o nm a t e r i a l a n dL S Vc u r v e s (B )a t 1600r /m i n 综上,本文通过超微研磨和高压均质预处理过程制备了糠醛渣基木质素/纤维素复合材料,并研究了其化学组成㊁表面化学性质及热动力学性质等.结果表明:糠醛渣基木质素/纤维素复合材料含有丰富的纤维素和木质素,木质素和纤维素的质量分数分别为53.18%和39.48%;糠醛渣基木质素/纤维素复合材料主要由C ,H ,O 三种元素组成,并含有少量的N 和S 元素;糠醛渣基木质素/纤维素复合材料中含有O H ,CO ,C C 和C H 等官能团;升温速率对糠醛渣基木质素/纤维素复合材料的热解特性影响较大,其表观活化能较低,相关系数R 2>0.93;以糠醛渣基木质素/纤维素复合材料为生物质前驱体制备的碳材料起始电位为0.93V ,半波电位为0.83V ,极限电流密度为1.75m A /c m 2,氧还原催化活性较高.因此,以糠醛渣基木质素/纤维素复合材料为生物质前驱体制备的碳材料在电催化氧还原性能方面具有较好的应用前景.参考文献[1] R A C HAMO N T R E EP ,D O U Z O U T ,C H E E N K A C HO R N K ,e t a l .F u r f u r a l :AS u s t a i n a b l eP l a t f o r m C h e m i c a l a n dF u e l [J ].A p p l i e dS c i e n c e a n dE n g i n e e r i n g P r o gr e s s ,2020,13(1):3-10.[2] B I SX ,L I U W Y ,WA N GC H ,e t a l .A V e r s a t i l eA p p r o a c h t o t h eS y n t h e s i s o f B i o m a s sD e r i v e d f r o m F u r f u r a l R e s i d u e s a s aP o t e n t i a lA d s o r b e n t [J ].J o u r n a l o fE n v i r o n m e n t a l C h e m i c a l E n g i n e e r i n g,2018,6(4):5049-5052.[3] MA O L Y ,Z HA N G L ,G A O N B ,e ta l .F e C l 3a n d A c e t i c A c i d C o -c a t a l y z e d H y d r o l y s i so fC o r n c o bf o r I m p r o v i n g F u r f u r a lP r o d u c t i o na n d L i g n i n R e m o v a lf r o m R e s i d u e [J ].B i o r e s o u r c e T e c h n o l o g y,2012,123:324-331.[4] WA N G Q ,L I U Y Y ,L I U S N ,e ta l .C o m p r e h e n s i v eT h e r m o c h e m i c a lU t i l i z a t i o no fB i o m a s sR e s i d u e sf r o m F u r f u r a l P l a n t s a n dE L W T e c h n o l o g y [J ].F u e l ,2019,252:116-124.[5] A O W Y ,F U J ,MA O X ,e ta l .C h a r a c t e r i z a t i o na n d A n a l y s i so fA c t i v a t e dC a r b o n sP r e pa r e df r o m F u r f u r a l R e s i d u e sb y M ic r o w a v e -A s s i s t e dP y r o l y s i sa nd A c t i v a t i o n [J ].F ue lP r o c e s s i n g T e c h n o l o g y ,2021,213:106640-1-106640-13.[6] Y I N YL ,G A O Y ,L IA M.S e l f -a c t i v a t i o no fB i o c h a r f r o m F u r f u r a lR e s i d u e sb y R e c y c l e dP y r o l y s i sG a s [J ].W a s t eM a n a ge m e n t ,2018,77:312-321.[7] Z HO U X ,L I U X H ,Q IFL ,e t a l .Ef f i c i e n tP r e p a r a t i o no fP -D o p e dC a r b o nw i t hU l t r a -h igh M e s o p o r o u sR a ti o f r o m F u r f u r a lR e s i d u ef o rD y e R e m o v a l [J ].S e p a r a t i o na n d P u r i f i c a t i o n T e c h n o l o g y,2022,292:120954-1-120954-9.361 第1期 曲 霞,等:糠醛渣基木质素/纤维素复合材料的表征及氧还原电催化性能461吉林大学学报(理学版)第62卷[8] C H E NCZ,L IM F,WU Y Y,e t a l.S t r u c t u r a lC h a r a c t e r i z a t i o no fL i g n i nE x t r a c t e dw i t h A l k a l i n e H y d r o g e nP e r o x i d e f r o m F u r f u r a lR e s i d u e[J].C e l l u l o s eC h e m i s t r y a n dT e c h n o l o g y,2015,49(2):153-163.[9] L IR,WA N G X H,L I N Q X,e t a l.S t r u c t u r a l F e a t u r e s o fL i g n i nF r a c t i o n a t e d f r o mI n d u s t r i a l F u r f u r a lR e s i d u eU s i n g A l k a l i n eC o o k i n g T e c h n o l o g y a n d I t sA n t i o x i d a n t P e r f o r m a n c e[J].F r o n t i e r s i nE n e r g y R e s e a r c h,2020,8: 83-1-83-10.[10] L I U C,L IB,D U H S,e t a l.P r o p e r t i e so fN a n o c e l l u l o s e I s o l a t e df r o m C o r n c o bR e s i d u eU s i n g S u l f u r i cA c i d,F o r m i cA c i d,O x i d a t i v e a n d M e c h a n i c a lM e t h o d s[J].C a r b o h y d r a t eP o l y m e r s,2016,151:716-724.[11]肖瑞瑞,杨伟,陈雪莉,等.三种常见生物质热解动力学特性的研究[J].化学世界,2012,53(11):663-694.(X I A O R R,Y A N G W,C H E N X L,e ta l.R e s e a r c ho n P y r o l y s i s K i n e t i c sC h a r a c t e r i s t i c so fT h r e e T y p e sB i o m a s s[J].C h e m i s t r y W o r l d,2012,53(11):663-694.)[12]袁聪聪,王宇栋,张丁川,等.木屑颗粒热解过程动力学计算及热解气体分析[J].过程工程学报,2017,17(5):1102-1108.(Y U A NCC,WA N G Y D,Z HA N G D C,e t a l.P y r o l y s i sD y n a m i c sC a l c u l a t i o na n dP y r o l y s i sG a sA n a l y s i s o fW o o dP a r t i c l e s[J].T h eC h i n e s e J o u r n a l o fP r o c e s sE n g i n e e r i n g,2017,17(5):1102-1108.)[13]杨兴卫,杨茂立,安海,等.玉米芯炭质燃料的理化性能及热解过程分析[J].过程工程学报,2018,18(4):851-857.(Y A N G X W,Y A N G M L,A N H,e t a l.A n a l y s i s o f P h y s i c o c h e m i c a l P r o p e r t i e s a n dP y r o l y s i sP r o c e s s o fC o r n c o bC a r b o nF u e l[J].T h eC h i n e s e J o u r n a l o fP r o c e s sE n g i n e e r i n g,2018,18(4):851-857.) [14]王承志,李法社,张帅,等.生物质燃油热重特性分析[J].昆明理工大学学报(自然科学版),2016,41(2):15-19.(WA N GCZ,L IFS,Z HA N GS,e t a l.A n a l y s i so fT h e r m o g r a v i m e t r i cC h a r a c t e r i s t i c so fB i o m a s sF u e l [J].J o u r n a l o fK u n m i n g U n i v e r s i t y o f S c i e n c e a n dT e c h n o l o g y(N a t u r a l S c i e n c e),2016,41(2):15-19.) [15]蒋荣亮,魏刚,徐洪耀.N H-P O S S基耐高温环氧树脂的制备及动力学分析[J].当代化工,2019,48(9):1959-1963.(J I A N G R L,W E I G,X U H Y.P r e p a r a t i o na n d K i n e t i c s A n a l y s i so f N H-P O S S B a s e d H i g h T e m p e r a t u r eR e s i s t a n tE p o x y R e s i n[J].C o n t e m p o r a r y C h e m i c a l I n d u s t r y,2019,48(9):1959-1963.) [16] C H E N X Y,L IH P,L I U W Y,e t a l.E f f e c t i v eR e m o v a l o f M e t h y lO r a n g ea n dR h o d a m i n eBf r o m A q u e o u sS o l u t i o nU s i n g F u r f u r a l I n d u s t r i a l P r o c e s s i n g W a s t e:F u r f u r a l R e s i d u e a s a nE c o-F r i e n d l y B i o s o r b e n t[J].C o l l o i d sa n dS u r f a c e sA:P h y s i c o c h e m i c a l a n dE n g i n e e r i n g A s p e c t s,2019,583(C):123976-1-123976-9.[17] L I U Y,S O N G Y M,R A NC M,e t a l.C h a r a c t e r i z a t i o n a n dA n a l y s i s o f C o n d e n s a t e s a n dN o n-c o n d e n s a b l eG a s e sf r o m F u r f u r a lR e s i d u ev i aF a s tP y r o l y s i s i naB u b b l i ng F l u i d i z e dB e d R e a c t o r[J].W a s t e M a n a g e m e n t,2021,125:77-86.[18] WA N G Y,X UZY,S O N GX,e t a l.T h eP r e p a r a t i o n o f L o w-C o s tA d s o r b e n t f o rH e a v y M e t a l B a s e d o nF u r f u r a lR e s i d u e[J].M a t e r i a l s a n d M a n u f a c t u r i n g P r o c e s s e s,2017,32(1):87-92.[19]张晓君,赵明珠,赵志海,等.稻草制浆黑液中木质素/二氧化硅复合材料的制备[J].吉林大学学报(理学版),2015,53(2):340-343.(Z HA N GXJ,Z HA O MZ,Z HA OZH,e t a l.P r e p a r a t i o n o f L i g n i n/S i l i c aH y b r i d f r o mB l a c kL i q u o r o fR i c eS t r a wP u l p i n g[J].J o u r n a l o f J i l i nU n i v e r s i t y(S c i e n c eE d i t i o n),2015,53(2):340-343.)[20] X I O N GFQ,HA N Y M,WA N GS Q,e t a l.P r e p a r a t i o na n dF o r m a t i o n M e c h a n i s m o fS i z e-C o n t r o l l e dL i g n i nN a n o s p h e r e sb y S e l f-a s s e m b l y[J].I n d u s t r i a l C r o p s a n dP r o d u c t s,2017,100:146-152.[21]郭平,王观竹,许梦,等.不同热解温度下生物质废弃物制备的生物质炭组成及结构特征[J].吉林大学学报(理学版),2014,52(4):855-860.(G U O P,WA N G G Z,X U M,e t a l.S t r u c t u r e a n d C o m p o s i t i o nC h a r a c t e r i s t i c s o f B i o c h a r sD e r i v e d f r o mB i o m a s sW a s t e s a tD i f f e r e n t P y r o l y s i sT e m p e r a t u r e s[J].J o u r n a l o f J i l i nU n i v e r s i t y(S c i e n c eE d i t i o n),2014,52(4):855-860.)[22]徐期勇,章佳文,刘虎,等.市政污泥与木屑共热解特性及动力学分析[J].可再生能源,2021,39(9):1150-1156.(X U Q Y,Z HA N G J W,L I U H,e ta l.C o-p y r o l y s i s C h a r a c t e r i s t i c sa n d K i n e t i c s A n a l y s i so f M u n i c i p a l S l u d g e a n d W o o dC h i p s[J].R e n e w a b l eE n e r g y R e s o u r c e s,2021,39(9):1150-1156.) [23]杜海清.木质类生物质催化热解动力学研究[D].哈尔滨:黑龙江大学,2008.(D U H Q.S t u d y o nC a t a l y t i cP y r o l y s i sK i n e t i c s o fW o o d y B i o m a s s[D].H a r b i n:H e i l o n g j i a n g U n i v e r s i t y,2008.)(责任编辑:王健)。
Materials Characterization

Materials Characterization Materials characterization is a crucial aspect of scientific research and development. It involves the study of the properties and behavior of different materials, and plays a significant role in various fields such as materials science, engineering, and manufacturing. By understanding the characteristics of materials, scientists and engineers can make informed decisions about their suitability for specific applications, design new materials with desired properties, and ensure the quality and reliability of products. One perspectiveon materials characterization is from the viewpoint of a materials scientist. For them, the process of characterization begins with the selection of appropriate techniques and instruments to analyze the material of interest. This could involve using techniques such as microscopy, spectroscopy, or diffraction to examine the structure, composition, and physical properties of the material. The scientist may also need to perform various tests, such as mechanical, thermal, or electrical tests, to assess the material's performance under different conditions. This comprehensive understanding of the material's properties is crucial for designing and optimizing materials for specific applications. From an engineer's perspective, materials characterization is essential for ensuring the reliability and performance of products. Engineers need to know how materials will behaveunder different operating conditions, such as temperature, pressure, or stress. By characterizing materials, engineers can make informed decisions about material selection, design components with appropriate dimensions and properties, andpredict the lifespan of products. For example, in the aerospace industry,materials characterization is critical for designing lightweight yet strong materials for aircraft structures, as well as understanding how these materialswill perform in extreme conditions. Another perspective on materials characterization comes from the manufacturing industry. Manufacturers rely on materials characterization to ensure the quality and consistency of their products. By characterizing raw materials and finished products, manufacturers can identify any variations or defects that may affect product performance or safety. For instance, in the pharmaceutical industry, materials characterization is used to analyze the composition and purity of drug substances and ensure that they meetregulatory standards. By doing so, manufacturers can guarantee the effectiveness and safety of their products. From a consumer's perspective, materials characterization may not be directly visible or apparent, but it greatly impacts the quality and performance of the products they use. For example, imagine buying a smartphone that claims to have a scratch-resistant screen. This claim is only possible because materials scientists and engineers have characterized the mechanical properties of the screen material and optimized it to resist scratches. Without materials characterization, consumers would not have access to products with the same level of performance and reliability. In conclusion, materials characterization is a vital aspect of scientific research, engineering, and manufacturing. It provides valuable insights into the properties and behavior of materials, enabling scientists, engineers, and manufacturers to make informed decisions about material selection, design, and quality control. From the perspective of a materials scientist, engineer, manufacturer, or consumer, materials characterization plays a crucial role in ensuring the performance, reliability, and quality of products.。
发动机开发试验中英文名词对照

发动机开发试验中英文名词对照1 热力学开发试验 Thermodynamics Test1.1 性能试验 Performance Test1.1.1 全负荷试验 Full Load Test1.1.2 部分负荷试验 Part Load Test1.1.3 排放试验 Emission Test1.1.4 油耗开发试验 Fuel Consumption Test1.2 冷却系统性能试验 Cooling Functional Test对整个发动机冷却系统的功能及特性(如,冷却液及机油的温度、压力和流量等)进行检验,试验在不同的冷却液及机油温度下进行,发动机需装配上面向批产的散热器、加热器及机油冷却器等。
V erification of the function and characteristic of the complete engine cooling system (e.g. coolant and oil temperatures / pressure / flow). Measurement with different coolant temperatures in the complete map shall be carried out production intend radiator, heater, oil cooler shall be installed on the engine.1.2.1 关键零件温度的测量(缸盖、活塞、气门、缸孔)Measurement of critical component temperatures (Cyl. Head, Pistons, Valves, Cyl. Bore) 1.2.2 节温器功能检查(静态/动态的控制特征)Check of thermostat function (Stat.& Dym. Control characteristics)1.2.3 热平衡分析Heat Balance analysis1.2.4 水泵气穴特性的确定Determination of water pump cavitation characteristing1.2.5 冷却系统的压力建立Cooling system pressure build-up1.2.6 开锅后的影响Check of after boiling effects1.3 润滑系统性能试验 Lubrication Function System对整个发动机润滑系统的功能及特性(如冷却液及机油温度、压力和流量等)进行检验,试验在不同的冷却液及机油温度下进行。
高稳定性钛系聚酯催化剂TiOC@SiO2的制备及应用

化工进展Chemical Industry and Engineering Progress2024 年第 43 卷第 3 期高稳定性钛系聚酯催化剂TiOC@SiO 2的制备及应用刘斌,王勇军,吕汪洋,陈文兴(浙江理工大学纺织纤维材料与加工技术国家地方联合工程实验室,浙江 杭州 310018)摘要:钛系聚酯催化剂因催化活性高、环境友好等优点,是传统锑系聚酯催化剂的理想替代品。
为了制备出耐水解性好、分散性好、催化性能稳定的钛系聚酯催化剂,采用反相微乳液法,制备得到核壳结构催化剂TiOC@SiO 2。
在钛有机化合物的表面包覆一层硅氧烷,以此稳定钛有机化合物的催化活性。
利用多种现代表征方法对TiOC@SiO 2的形貌、结构和性能进行了表征分析,并探究其在合成聚对苯二甲酸乙二醇酯(PET )中的催化性能。
研究结果表明,TiOC@SiO 2催化剂为粒径约200nm 的核壳球形结构,无Ti —O —Si 键,钛含量为6.95%。
TiOC@SiO 2催化剂在90℃下水浴2h 后,其结构和催化活性保持不变,复合结构显著提高了钛有机化合物的耐水解性和分散性。
在聚酯合成实验中,仅添加5μg/g TiOC@SiO 2,在270℃下缩聚反应92min ,即可制备出特性黏度为0.677dL/g 、端羧基含量为14.4mol/t 、b 值为2.16的PET 。
关键词:催化剂;聚合;纳米粒子;聚酯;催化性能中图分类号:TS15;TQ426 文献标志码:A 文章编号:1000-6613(2024)03-1395-08Preparation and application of high stability titanium polyester catalystTiOC@SiO 2LIU Bin ,WANG Yongjun ,LYU Wangyang ,CHEN Wenxing(National Engineering Laboratory for Textile Fiber Materials & Processing Technology, Zhejiang Sci-Tech University,Hangzhou 310018, Zhejiang, China)Abstract: Titanium-based polyester catalysts are ideal substitutes for traditional antimony-based catalysts due to their high catalytic activity and environmental friendliness. In order to prepare titanium polyester catalyst with hydrolysis resistance, good dispersibility and stable catalytic performance, TiOC@SiO 2 catalyst was prepared by reverse microemulsion method. A layer of siloxane was coated on the surface of titanium containing organic compound to stabilize the catalytic activity. The morphology, structure and properties of TiOC@SiO 2 were characterized by various modern characterization methods, and its catalytic performance in the synthesis of polyethylene terephthalate (PET) was evaluated. The results showed that the TiOC@SiO 2 catalyst had a core-shell spherical structure with a particle size of about 200nm, but no Ti —O —Si bond, and a Ti content of 6.95%. The structure and catalytic activity of TiOC@SiO 2 catalyst remained unchanged at 90℃ for 2h. The composite structure significantly improved the hydrolysis resistance of the titanium organic compounds and dispersibility. In the polyethylene terephthalate synthesis experiment, with only 5μg/g TiOC@SiO 2 added and polycondensation at 270℃ for研究开发DOI :10.16085/j.issn.1000-6613.2023-0349收稿日期:2023-03-07;修改稿日期:2023-06-01。
电致变色材料综述
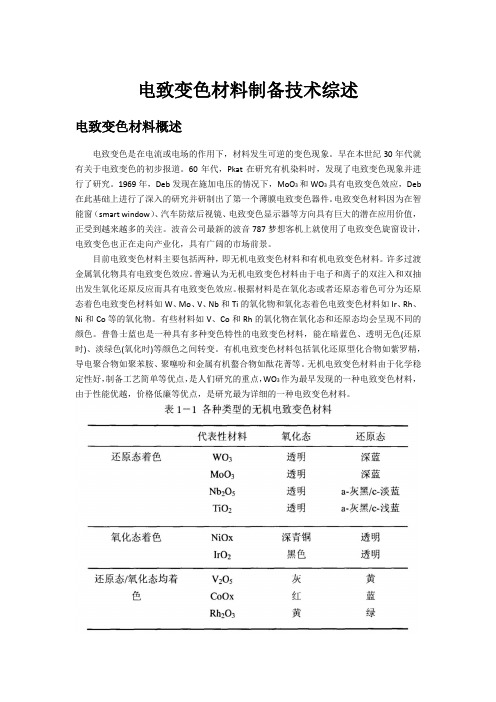
电致变色材料制备技术综述电致变色材料概述电致变色是在电流或电场的作用下,材料发生可逆的变色现象。
早在本世纪30年代就有关于电致变色的初步报道。
60年代,Pkat在研究有机染料时,发现了电致变色现象并进行了研究。
1969年,Deb发现在施加电压的情况下,MoO3和WO3具有电致变色效应,Deb 在此基础上进行了深入的研究并研制出了第一个薄膜电致变色器件。
电致变色材料因为在智能窗(smart window)、汽车防炫后视镜、电致变色显示器等方向具有巨大的潜在应用价值,正受到越来越多的关注。
波音公司最新的波音787梦想客机上就使用了电致变色旋窗设计,电致变色也正在走向产业化,具有广阔的市场前景。
目前电致变色材料主要包括两种,即无机电致变色材料和有机电致变色材料。
许多过渡金属氧化物具有电致变色效应。
普遍认为无机电致变色材料由于电子和离子的双注入和双抽出发生氧化还原反应而具有电致变色效应。
根据材料是在氧化态或者还原态着色可分为还原态着色电致变色材料如W、Mo、V、Nb和Ti的氧化物和氧化态着色电致变色材料如Ir、Rh、Ni和Co等的氧化物。
有些材料如V、Co和Rh的氧化物在氧化态和还原态均会呈现不同的颜色。
普鲁士蓝也是一种具有多种变色特性的电致变色材料,能在暗蓝色、透明无色(还原时)、淡绿色(氧化时)等颜色之间转变。
有机电致变色材料包括氧化还原型化合物如紫罗精,导电聚合物如聚苯胺、聚噻吩和金属有机螯合物如酞花菁等。
无机电致变色材料由于化学稳定性好,制备工艺简单等优点,是人们研究的重点,WO3作为最早发现的一种电致变色材料,由于性能优越,价格低廉等优点,是研究最为详细的一种电致变色材料。
目前对电致变色材料性能的研究主要集中在四点:1.颜色和对比度的提高,包括变色对比度的提高和变色光谱的展宽,例如将铌氧化物和ITO纳米晶复合,使材料同时具备对可见光和近红外光电致变色的效应。
2.变色效率,电致变色薄膜的吸光度的变化值与所注入的电荷直接相关,变色效率即电致变色薄膜的吸光度的变化值与单位面积所注入的电荷的比值。
歧化和异构化的反应数据

△,G:=△,职一TA,s:
(3)
1n∥=一簪
(4)
则3.0 MPa时气相反应的平衡转化率可由各物 质平衡组成),i表示,采用公式(5)计算(设此体系为 真实气体的理想混合物),结果见表1。
n(yj叻)巧=(5)以叶∥
(5)
式(5)中逸度系数妒,利用普遍化的Vifial系数法求取。
Table 1
表1歧化反应的△,醒。△,s:,△,G:。∥以及平衡转化率数值
唯一成功用于工业装置的歧化反应催化剂。该技术 丁烯的单程转化率可以在60%以上,丙烯的选择性 在90%以上Mj。由于w基催化剂具有抗毒性能好 的优点隋】,采用该类催化剂进行歧化制丙烯的研究 备受关注,到目前为止,催化性能最好的W基催化 剂载体依然是由氧化硅材料制备的载体。虽然 OCT技术已工业化十余套,但是以WO,/SiP:为催 化剂,专门针对c。烯烃歧化生产丙烯这一目标,进 行相关催化剂的详细研究以及相关工艺条件的考 察,这方面的报道并不多见。
1. 任务书 Assignment.docx

第一部分:毕业设计(论文)任务书(Part I:Senior Project Assignment)2015年(Year)1月(Month)10日(Day)1.Background and ObjectivesWith the development of highway in our country,more and more asphalt was used.Besides,less petroleum was existed.Therefore,the renewable energy were searched. In a variety of renewable energy sources,renewable biomass has huge reserves,widely distributed in features.Biomass resources are:crop straw,cereal grass shell,bark branches, bamboo bagasse,waste food oil,all kinds of livestock feces.Among them,crop stalks Leftover stalks left after harvest seeds containing high fiber content of crop residues, including cereals,beans,potatoes,oil type,ing the biomass to produce bio-oil and put the oil into application is very important.One of the thermochemical processes used to produce bio-oil is fast pyrolysis.Fast pyrolysis is the rapid decomposition of organic matter(biomass)in the absence of oxygen to produce solids such as,char, pyrolysis liquid or oil(bio-oils),and gas.In order to use the bio-oil to produce the suitable bio-binder.The performance of bio-binder need to be studied.Besides,some additives may be need to put into the bio-binder.So the performance of modified bio-binder also need to be studied.2.Design Specification(Research Scopes)The study should be based on the wide range of research and application of bio-asphalt at home and abroad to further study the content of bio-oil,the preparation temperature,mixing time,test temperature,the aging and other factors on the properties of the bio-oil modified asphalt.Preparation,penetration,and softening point are the most important test tools.With the growing global energy crisis and environmental degradation,finding renewable resources and protecting the natural environment are the major problems to be solved.In the field of road engineering,petroleum asphalt is a major road building material, according to statistics,China's annual consumption of asphalt is about20million tons. Because of non-renewable petroleum resources,the price of petroleum asphalt began to soar in recent years.Meanwhile,the process of preparation and use of asphalt is also accompanied by significant carbon emissions.Our country is a large agricultural country with a large population,a huge amount of plant stalks and waste oil residues in everyday life put a huge pressure on the ecosystem.A reasonable treatment and utilization of energy to alleviate tensions would promote sustainable social and economic development and the improvement of the ecological environment has important significance.In the process of biodiesel and gasoline production,because the required quality of bio-diesel automotive engines and gasoline are higher,in the preparation process,mainly by distillation or cracking of the heavy fraction is the residual biomass,heavy oil.The efficient use of biomass cannot only reduce the heavy road construction costs but also improve the performance of asphalt pavement,which is the significance of this thesis lies.3.Main TasksThrough this senior project,the following tasks should be fulfilled:Task1:The important reason of the application of bio-binder should be understood.Task2:The high performance of bio-binder and modified bio-binder should be studied.Task3:The low performance of bio-binder modified bio-binder should be studied.Task4:The aging properties of bio-binder should be explored.4.Main Deliverables1.Senior project proposal.2.Bi-week memo.3.Graduation thesis.5.Time TableThe senior project advisor should help students plan ahead through a list of time slots as shown in table below.It should be noted however,the time table shown below is the basic or overall requirements of senior project committee.Advisors should have their6.Main References[1]Y.Xu,Z.You,Performance Evaluation of Asphalt Binder Modified by Bio-oil Generated from Waste Wood Resources[J].International Journal of Pavement Research and Technology,Jul.2013.6(4):431-439[2]Bridgewater,A.V.,“An Introduction to Fast Pyrolysis of Biomass for Fuels and Chemicals”in Fast Pyrolysis of Biomass:A Handbook,edited by Bridgewater,A.et.al. CPL Scientific Publishing Services Limited,Newbury,1999,pp.1-13.[3]Raouf,M.A.,and Williams,R.C.(2009)Determination of Pre-Treatment Procedure Required for Developing Bio-Binders from Bio-Oils.Proceedings of the2009Mid-Continent Transportation Research Symposium[J].Ames,Iowa State University, Iowa,USA[4]胡兴涛,生物质快速热解制生物质油的实验研究[D],山东:山东科技大学,2010[5]Julian Mills-Beale,and Z.You,Aging Influence on Rheology Properties of Petroleum-based Asphalt Modified with Biobinder[J],Journal of Materials in Civil Engineering,accepted manuscript,October11,2012.doi:10.1061[6]Fini,E.H.,Kalberer,E.W.,Shahbazi,A.,Basti,M.,You,Z.,Ozer,H.,and Aurangzeb, Q.(2011).Chemical characterization of biobinder from swine manure:Sustainable modifier for asphalt binder[J].Journal of Materials in Civil Engineering,23(11),1506-1513.[7]郑典模,屈海宁,孙云.地沟油催化裂解制备生物燃油[J],南昌大学学报(工学版),2010,32(3),242-245[8]万益琴,王应宽,林向阳.微波裂解海藻快速制取生物燃油的试验[J],农业工程学报,2010,26(1):295-300[9]Ellie H.Fini,Synthesis and Characterization of Bio-modified Rubber(BMR)Asphalt:A Sustainable Waste Management Solution for Scrap Tire and Swine Manure[J],Journalof Environmental Engineering,accepted manuscript,July13,2013.doi:10.1061[10]Onochie,A.,Fini,E.,Yang,X.,Mills-Beale,J.,and You,Z.(2013)Rheological Characterization of Nano-particle based Bio-modified Binder[J].Transportation Research Board,TRB2013CD-ROM,Washington D.C.,Paper ID:13-4895.[11]Hill,B.,Oldham,D.,Behnia,B.,Fini,E.H.,Buttlar,W.G.,and Reis,H.(2013)Low Temperature Performance Characterization of Bio-Modified Asphalt Mixtures Containing Reclaimed Asphalt Pavement[J].Transportation Research Board,TRB2013CD-ROM, Washington D.C.,Paper ID:13-3773.[12]Tang,S.,and Williams,R.C.(2009)Antioxidant Effect of Bio-Oil Additive ESP on Asphalt Binder[J].Proceedings of the2009Mid-Continent Transportation Research Symposium,Ames,Iowa State University,Iowa,USA[13]Raouf,M.A.,and Williams,R.C.Temperature and Shear Susceptibility of aNon-petroleum Binder as a Pavement Material[J].Transportation Research Board,2010 TRB CD-ROM,Washington D.C.,Paper ID:10-0812.[14]Fini,E.H.,Yang,S.-H.,and Xiu,S.(2010).Characterization and Application of Manure-Based Bio-binder in Asphalt Industry[J].Transportation Research Board,TRB 2010CD-ROM,Washington D.C.Paper ID:10-2871[15]Fini,E.H.,Al-Qadi,I.L.,You,Z.,Zada,B.,and Mills-Beale,J.(2012).Partial replacement of asphalt binder with bio-binder:Characterisation and modification[J]. International Journal of Pavement Engineering,13(6),515-522.[16]SEIDEL,J.C.,and HADDOCK,J.E.(2012)Soy Fatty Acids as Sustainable Modifier for Asphalt Binders[J].Workshop of"Alternative Binders for Sustainable Asphalt Pavements",E-C165,Transportation Research Board,Washington D.C.pp:15-22[17]WEN,H.,BHUSAL,S.,and WEN,B.(2012)Laboratory Evaluation of Waste Cooking Oil Based Bioasphalt as Sustainable Binder for Hot-Mix Asphalt[J].Workshop of "Alternative Binders for Sustainable Asphalt Pavements",E-C165,Transportation Research Board,Washington D.C.pp:49-60.[18]Yang,S.-H.,Suciptan,T.,and Chang,Y.-H.(2013)Investigation of Rheological Behavior of Japanese Cedar Based Bio-Binder As Partial Replacement For Bituminous Binder[J].Transportation Research Board,TRB2013CD-ROM,Washington D.C.,Paper ID:13-3801.[19]Peralta,J.,Raouf,M.A.,Tang,S.,and Williams,R.C.(2012).Bio-Renewable Asphalt Modifiers and Asphalt Substitutes[J].Sustainable Bioenergy and Bioproducts, Green Energy and Technology,Springer,89-115.[20]Mohammad,L.N.,Elseifi,M.A.,Cooper,S.B.,Challa,H.,and Naidoo,P.(2013) Laboratory Evaluation of Asphalt Mixtures Containing Bio-Binder Technologies[J]. Transportation Research Board,TRB2013CD-ROM,Washington D.C.,Paper ID:13-3784.[21]Hajj,E.Y.,Souliman,M.I.,Alavi,M.Z.,and Salazar,L.G.(2013)Influence of Hydrogreen Bio-asphalt on Viscoelastic Properties of Reclaimed AsphaltMixtures[J],Transportation Research Board,TRB2013CD-ROM,Washington D.C.,Paper ID:13-2253.[22]Williams,R.C.,Satrio,J.,Rover,M.,Brown,R.C.,and Teng,S.(2009)Utilization of Fractionated Bio Oil in Asphalt[J].Transportation Research Board,TRB2009CD-ROM,Washington D.C.Paper ID:09-3187[23]Abdel,M.,Metwally,R.M.,and Williams,R.C.(2010).Development ofNon-Petroleum Based Binders for Use in Flexible Pavements[J].TR-594,Iowa State University,Ames,IA.[24]Hill,D.R.(2012).Bioasphalt and Biochar from Pyrolysis of Urban Yard Waste[D], Master of Science,Case Western Reserve University.[25]AUDO,E.C.,QUEFFELEC,B.B.,LEGRAND,J.,and L.PINE,O.(2012) Alternative Binder from Microalgae Algoroute Project[J].Workshop of“Alternative Binders for Sustainable Asphalt Pavements”,E-C165,Transportation Research Board, Washington D.C.pp:7-14.。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Performance characterization of Ti substrate lead dioxide electrode with different solid solution interlayersHaishen Kong •Haiyan Lu •Wenli Zhang •Haibo Lin •Weimin HuangReceived:25March 2012/Accepted:23May 2012/Published online:16June 2012ÓSpringer Science+Business Media,LLC 2012Abstract In this study,Ti substrate was coated by three different mixed oxides (SnO 2–Sb 2O 5,RuO 2–TiO 2,IrO 2–Ta 2O 5),then PbO 2was electrodeposited on them to prepare PbO 2electrode.The microstructure of the solid solution interlayers and PbO 2coatings was characterized by scan-ning electronic microscopy and X-ray diffraction.The results indicated that the above oxides interlayer exited in the form of solid solution and the interlayer covered the Ti substrate largely.Accelerated life test proved that the presence of solid solution interlayer can increase the sta-bility of PbO 2electrodes in electrolysis.Cyclic voltam-mogram indicated that the PbO 2electrode with the solid solution interlayer have more active surface area in sulfuric acid solution.Linear scanning voltammograms showed that the over potentials of the oxygen evolution are decreased with the addition of the solid solution interlayer.Electro-chemical impedance spectroscopy showed that the pres-ence of interlayer can increase the electrochemical activity of oxygen evolution reaction and electrical conductivity.IntroductionTi substrate lead dioxide is a promising anode widely used in practice owing to its high excellent conductivity and chemical inertness [1,2].The PbO 2electrode shows high catalytic activity for contaminants abatement and organic mineralization [3,4].It is also employed as O 2evolution electrode in some practical applications,such as the elec-trowinning of metals [5].Nevertheless,the main problem of the Ti/PbO 2electrode is its poor stability.The known failure mechanisms of Ti/PbO 2electrode include flaking off of the fragile PbO 2coating to the electrolyte and generating TiO 2insulating layer between the substrate and coating [1,6].A lot of efforts have been made to improve the stability of the Ti/PbO 2electrode.The most useful method is introducing a transition layer between Ti substrate and PbO 2coating [7,8].Comn-inellis and Plattner [9]have shown that the presence of Au or Pt thin interlayer can effectively prevent the growth of TiO 2insulating layer between the substrate and the PbO 2coating.These attempts have shown that the successful interlayer should have high conductivity,excellent chemical stability,and corrosion resistance in electrolysis.And the interlayer should combine closely with the PbO 2layer to decrease the interface resistance and improve adhesive strength.The dimensionally stable anodes (DSA)invented by Beer in the late 1960s have become the most important electrodes in electrochemical engineering.These electrodes consist of Ti substrate and oxides coating,such as Ti/SnO 2–Sb 2O 5[10–12],Ti/RuO 2–TiO 2[13,14],and Ti/IrO 2–Ta 2O 5[15,16]electrodes,etc.All the three mixed oxides exist in the form of solid solution,they can cover the Ti substrate completely and combine with the substrate closely.The solid solution electrodes show several advantages of good electrical conductivity,excellent chemical stability andH.Kong ÁH.Lu (&)ÁW.Zhang ÁH.Lin (&)ÁW.Huang College of Chemistry,Jilin University,Changchun 130012,People’s Republic of China e-mail:luhy@ H.Line-mail:lhb910@H.LinState Key Laboratory of Theoretical and Computational Chemistry,Jilin University,Changchun 130012,People’s Republic of ChinaJ Mater Sci (2012)47:6709–6715DOI 10.1007/s10853-012-6613-xcorrosion resistance in electrolysis.Otherwise,the rough surface of the DSA provides more sites for deposition of PbO2crystallite,the adhesive strength between the inter-layer and the PbO2coating may be improved.In the study,we applied three different mixed oxides (SnO2–Sb2O5,RuO2–TiO2,and IrO2–Ta2O5)as interlayer between the Ti substrate and the PbO2coating.The objec-tives of this study are increasing the stability of Ti/PbO2 electrode and searching for the function of different solid solution interlayers on Ti/PbO2electrode.The electro-chemical behaviors of the PbO2electrode with the solid interlayer were characterized by electrochemical and phys-ical pared with the Ti/PbO2electrode without interlayer,Ti/solid solution interlayer/PbO2anode showed more superior in stability,electric conductivity,and elec-trochemical activity.And the Ti/IrO2–Ta2O5/PbO2electrode has the best stability and electrochemical activity of oxygen evolution.ExperimentalPreparation of interlayer onto Ti substratePrior to coating,the Ti substrate(geometric size:2.09 1.090.1cm3)underwent sandblasting,30min of etching in boiling10%hydrochloric acid and5min of ultrasonic cleaning in ultra pure water(18.25M X cm).Three kinds of thin interlayers(SnO2–Sb2O5,RuO2–TiO2,and IrO2–Ta2O5)were prepared by thermal decomposition tech-nique.The applied mole ratio of SnCl4Á5H2O to SbCl3, RuCl3Áx H2O to Ti(C4H9O)4and H2IrCl6Á6H2O to TaCl5is 9:1,1:5,and7:6in this paper,respectively[12,13,17,18]. The total mass of oxides loading are about1.5,4,and 0.8mg cm-2,respectively.Ti/solid solution interlayer/PbO2electrode preparation PbO2was deposited onto Ti/interlayer(geometric area: 1.091.0cm2)under the current density of20mA cm-2at 65°C for2h.The electrolyte was composed of0.5M Pb(NO3)2,0.04M NaF and0.1M HNO3.The resultant electrodes were called Ti/SnO2–Sb2O5/PbO2,Ti/RuO2–TiO2/PbO2,and Ti/IrO2–Ta2O5/PbO2electrode.PbO2 coating weights are about0.163,0.154,and0.150g, respectively.Ti/PbO2electrode without interlayer made in the same method was used as a reference,the coating weight is about0.140g.Microstructure characterizationThe morphology and particle size of solid solution inter-layer and PbO2coating were studied by JSM-6700F SEM (Japan Electron Co.,Japan).The crystalline phase was identified by XRD using a Ragaku diffractometer(Japan) with CuK a-radiation operating at40kV and200mA. Composition analysis was carried out by XPS(Thermo Scientific ESCALAB250,USA)with monochromatized radiations(Al,K a).Electrochemical measurementElectrochemical measurements were performed by a conventional three electrode glass cell and an electro-chemical workstation(PARSTAT2273,Princeton Applied Research,USA).Ti/PbO2,Ti/SnO2–Sb2O5/PbO2, Ti/RuO2–TiO2/PbO2,and Ti/IrO2–Ta2O5/PbO2electrodes were used as working electrode(geometric area: 1.091.0cm2),Ti/RuO2–TiO2–SnO2net(geometric area: 3.093.0cm2)was used as the counter-electrode because it was stable in the media and saturated calomel electrode (SCE)was used as the reference electrode in a separate compartment with a Luggin capillary.All the potentials were referred to SCE unless otherwise stated in this paper. Linear sweep voltammetry was performed to acquire their oxygen evolution potential in0.5M H2SO4solution.CV curves were recorded between0.8and1.95V in0.5M H2SO4solution.EIS were recorded at constant potential (1.95V vs.SCE)located in the oxygen evolution reaction (OER)domain.It was obtained in the frequency range of 100mHz–100kHz.The amplitude of the ac signal was 10mV.All electrochemical experiments were carried out at room temperature(25±2°C).All solutions were pre-pared with ultra pure water and all reagents used in the experiments were analytic grade.Accelerated life testThe accelerated life test was conducted under galvanostatic electrolysis at a current density of1A cm-2in1M H2SO4 solution with potentiostat–galvanostat(WYJ,Hangzhou Pingguo,China).The cell voltage between the working electrode and the counter-electrode(Ti net geometric area: 3.093.0cm2)was recorded.The cell voltage versus service time curve was recorded simultaneously.The ser-vice life of the electrodes was considered to be terminated when the cell voltage reaches up to10V.Results and discussionSurface morphologyFigure1a–c shows the SEM images of Ti/SnO2–Sb2O5, Ti/RuO2–TiO2,and Ti/IrO2–Ta2O5interlayers,respectively.It is observed that the surface of the solid solution prepared using the thermal decomposition method exhibits a ‘‘cracked-mud’’structure which is typical for DSA[10].The average size of the cracks of Ti/SnO2–Sb2O5,Ti/RuO2–TiO2,and Ti/IrO2–Ta2O5interlayers are about0.2,1,and 3l m,respectively.This structure can increase the contact area between the interlayer and the PbO2layer,which reduce the interface resistance and improve the adhesive strength.Figure2a–c and d shows the SEM images of Ti/PbO2, Ti/SnO2–Sb2O5/PbO2,Ti/RuO2–TiO2/PbO2and Ti/IrO2–Ta2O5/PbO2electrode surfaces,respectively.The SEM images show that the PbO2layer is compact,this structure can prevent free O2-from contacting the titanium substrate to generate an insulating TiO2layer[19].The average size of PbO2grain dimension is about3–4l m,and the average size of crack in IrO2–Ta2O5interlayer is also about3–4l m.Due to the existence of the crack,the PbO2could deposit in the crack of IrO2–Ta2O5interlayer to form an embedded structure with the IrO2–Ta2O5interlayer.The embedded structure can make the combination of surface coating and Ti substrate more compact andfirm[20,21].The IrO2–Ta2O5interlayer could increase the adhesive power between Ti substrate and PbO2coating with the embedded structure.The accelerate service life test demonstrated that the PbO2electrode with IrO2–Ta2O5interlayer has the longest service life for the studied four PbO2electrodes.The stability of Ti/IrO2–Ta2O5/PbO2electrode increase largely. Therefore,there could be the superior adhesive power over the other interlayer between Ti substrate and PbO2coating.XRD analysisTheoretically,whether or not a metal oxide mixture can form a solid solution is highly dependent on the ionic radius difference in the metal elements.The oxidation states of Sn,Sb,Ru,Ti,Ir,Ta,and Pb species are Sn(IV), Sb(V),Ru(IV),Ti(IV),Ir(IV),Ta(V),and Pb(IV),respec-tively.The ionic radii of Sn(IV),Sb(V),Ru(IV),Ti(IV), Ir(IV),Ta(V),and Pb(IV)are71,62,62,68,77,73,and 84nm,respectively(Table1).When the ratio of radii difference between two metal ions in oxides is less than 15%,the metal oxide mixture should be highly intermixed and exist in the form of solid solution[22].The different ratios of ionic radii among Sn4?and Sb5?,Ru4?and Ti4?, Ir4?,and Ta5?radii are13,9,and5%,respectively.These may explain why the oxide mixtures existed in the form of continuous solid solution.The differences ratio of ionic radii between Ti4?obtained for oxidation of Ti substrate and correlative ions in the interlayer also lie in the Hume-Rothery limit(15%),so combination of the substrate and interlayer is successful[22].The different ratios of ionic radii of Sn4?and Pb4?as well as Ir4?and Pb4?are15and Fig.1SEM images of the interlayer surface.a Ti/SnO2–Sb2O5,b Ti/RuO2–TiO2,c Ti/IrO2–Ta2O58.3%,respectively,which are also smaller than the limit.It can be inferred that SnO 2–PbO 2and IrO 2–PbO 2can form continuous solid solution.But TiO 2with PbO 2only form a limited solid solution due to the larger difference of their ion radii (19%)in Ti/RuO 2–TiO 2/PbO 2electrode.Figure 3a shows XRD patterns of different interlayers:Ti/SnO 2–Sb 2O 5,Ti/RuO 2–TiO 2,and Ti/IrO 2–Ta 2O 5.As shown in Fig.3a-1,the broad and symmetric peaks at 2h =26.6°(110),33.9°(101),52.0°(211)are attributed to diffraction peaks of SnO 2with tetragonal cassiterite structure.However,the peak positions are slightly different from the characteristic peaks of pure SnO 2crystal (PDF-21-1250)and no Sb 2O 5-rich peaks are detected.These facts reveal that various components in the Sb 2O 5–SnO 2have been highly intermixed.In other words,the SnO 2–Sb 2O 5mixture exists in the form of a solid solution.In addition,to the well-defined peaks of solid solution,the Ti metal peaks from the substrate are also observed.In Fig.3a-2,a series of broad and symmetric peaks were found at 2h =27.7°(110),36.0°(101),54.5°(211),56.9°(220),and 69.2°(301)corresponding to the rutile structure of TiO 2and no RuO2-rich peaks were detected.The facts also reveal that various components in the RuO 2–TiO 2interlayer have been highly intermixed in the form of a solid solution.In Fig.3a-3,a series of broad and symmetric peaks were found at 2h =27.7°(110),34.5°(101),and 53.3°(301)corresponding to the IrO 2–Ta 2O 5solid solution.The above results demonstrated that SnO 2–Sb 2O 5,RuO 2–TiO 2,and IrO 2–Ta 2O 5interlayers all exist in the form of solid solution.Figure 3b shows XRD patterns of Ti/PbO 2,Ti/SnO 2–Sb 2O 5/PbO 2,Ti/RuO 2–TiO 2/PbO 2,and Ti/IrO 2–Ta 2O 5/PbO 2electrodes.The diffractive peaks at 2h =25.4°,31.9°,49.0°,and 62.3°attribute to b -PbO 2structure (PDF-35-1222),they belong to (110),(011),(211),and (301)crystal face,respectively (Fig.3b-2).The results indicate that PbO 2electrode with and without solid solution inter-layer mostly contain b -PbO 2crystalline phases.The introduction of interlayer does not result in significant b -PbO 2phase changes.However,the corresponding diffractive peaks intensities are different among thefourFig.2SEM images of different PbO 2electrodes.a Ti/PbO 2surface,b Ti/SnO 2–Sb 2O 5/PbO 2surface,c Ti/RuO 2–TiO 2/PbO 2surface,d Ti/IrO 2–Ta 2O 5/PbO 2surfaceTable 1Radii of correlative ions in different solid solution Metal ions Ti 4?Sn 4?Sb 5?Ru 4?Ir 4?Ta 5?Pb 4?r (pm)68716262777384PbO 2electrodes.Moreover,the crystal orientations of the PbO 2with interlayer are richer than those without.The results suggest the better crystallinity of PbO 2at Ti/solid solution substrate.The solid solution interlayers affect the preferred orientation of PbO 2electrode.Accelerated life testFigure 4shows the accelerated life test of the Ti/PbO 2,Ti/SnO 2–Sb 2O 5/PbO 2,Ti/RuO 2–TiO 2/PbO 2,and Ti/IrO 2–Ta 2O 5/PbO 2electrode.The experiment is used to compare their electrochemical stabilities in electrolysis.When the cell voltage reaches up to 10V,the electrode service life is considered to be terminated.The service life of the Ti/PbO 2electrode is very short,which is about 11h.The presence of SnO 2–Sb 2O 5,RuO 2–TiO 2,and IrO 2–Ta 2O 5interlayers greatly prolong the service life of the PbO 2electrode.The service life of Ti/SnO 2–Sb 2O 5/PbO 2,Ti/RuO 2–TiO 2/PbO 2,and the Ti/IrO 2–Ta 2O 5/PbO 2elec-trode are about 58,207,and 672h,respectively.The above results show that the solid solution interlayers have greatlyincreased the service life of the PbO 2electrodes,especially IrO 2–Ta 2O 5interlayer.The rough surface of solid solution interlayers can increase the adhesive strength between Ti substrate and PbO 2coating.The presence of solid solution interlayers can inhibit inactivation of Ti substrate and avoid flaking of PbO 2layer.Thus,the stability of Ti/solid solution inter-layer/PbO 2electrodes is improved.Electrochemical activity of oxygen evolutionFigure 5shows the relationship of q*against the reciprocal of square root of scan rate.The charge q*was obtained by integration of the voltammetric curves over the whole potential range from 0.8to 1.95V.q*is considered as proportional to the active surface area of oxide electrodes [23].The results show that the introduction of solid solu-tion interlayer could enhance voltammetric charge quantity q*.The q *values increase in the order of Ti/PbO 2,Ti/SnO 2–Sb 2O 5/PbO 2,Ti/RuO 2–TiO 2/PbO 2,and Ti/IrO 2–Ta 2O 5/PbO 2.A possible explanation for the phenomenon is that there are richer crystalline orientations for the PbO 2electrodes with the solid solution interlayers than that without.Con-sequently,there is more activity surface area in Ti/solid solution interlayer/PbO 2electrode.Figure 6shows the liner scanning voltammograms of Ti/PbO 2,Ti/SnO 2–Sb 2O 5/PbO 2,Ti/RuO 2–TiO 2/PbO 2,and Ti/IrO 2–Ta 2O 5/PbO 2electrodes in 0.5M H 2SO 4solution.The oxygen evolution potential decreased as the following order of Ti/PbO 2,Ti/SnO 2–Sb 2O 5/PbO 2,Ti/RuO 2–TiO 2/PbO 2,and Ti/IrO 2–Ta 2O 5/PbO 2.The presence of solid solu-tion interlayer can increase the activity of oxygen evolution.For evaluating the oxygen evolution activity of different PbO 2electrode,EIS measurements were performed at constant potential (1.95V)located in the OER domain.Figure 7shows the Nyquist diagrams of different electrode in 0.5M H 2SO 4solution.The equivalent circuit which best fits the experimental EIS data is a R s (R ct Q dl )(R f Q f )com-bination (Fig.8).In the previous literature [19,24],itwasFig.3XRD patterns ofdifferent interlayers and PbO 2electrodes.a-1Ti/SnO 2–Sb 2O 5,a-2Ti/RuO 2–TiO 2,a-3Ti/IrO 2–Ta 2O 5interlayers,b-1Ti/PbO 2,b-2Ti/SnO 2–Sb 2O 5/PbO 2,b-3Ti/RuO 2–TiO 2/PbO 2,b-4Ti/IrO 2–Ta 2O 5/PbO 2electrodeFig.4Accelerated life test of different interlayers and PbO 2electrodes in 1M H 2SO 4under 1A cm -2.1Ti/PbO 2,2Ti/SnO 2–Sb 2O 5/PbO 2,3Ti/RuO 2–TiO 2/PbO 2,4Ti/IrO 2–Ta 2O 5/PbO 2electrodeoften used to simulate the impedance data for OER on metal oxide anodes.In this circuit,R s represents the uncompensated solution resistance,first capacitive loop (R f Q f )at high frequency region reflects electron transfer information through the oxide film,the R f and Q f stands for resistance through the oxide layer and the pseudo-capacitance of the oxide layer,respectively.The second capacitive loop (R ct Q dl )at low frequency region reflects the charge transfer information at the interface PbO 2/electro-lyte,R ct and Q dl indicate the charge transportation resis-tance at the interface and the double layer capacitance,respectively.In this experiment,R ct stands for the activity of oxygen evolution.The fitted electrical parameters are listed in Table 2.As shown in the table,the R f of the Ti/PbO 2,Ti/SnO 2–Sb 2O 5/PbO 2,Ti/RuO 2–TiO 2/PbO 2,and Ti/IrO 2–Ta 2O 5/PbO 2elec-trode are 0.0328,0.0172,0.0201,and 0.0187X cm 2,while the R ct are 2.021,1.749,1.512,and 1.15X cm 2,respectively.From above experimental data,it can be concluded that the presence of the solid solution interlayer can reduce the R f and R ct ,which indicates the conductivity and electrochemical activity of oxygen evolution for the Ti/solid solution inter-layer/PbO 2electrodes are higher than those for the Ti/PbO 2electrode.The reason of high conductivity could be explained as follows:first,the metal oxide formed by thermal decom-position is non-stoichiometric,there are more free electron and oxygen vacancies in the solid solution interlayer.The free electron and oxygen vacancies can enhance the elec-trical conductivity of the electrode.Second,the solid solution interlayer can prevent from forming an insulating TiO 2layer on Ti surface [25,26].Third,the solid solution interlayer can closely combine with the Ti substrate and the electrodeposited PbO 2coating,this structure can reduce interface resistance and increase the electrical conductivity of the whole Ti substrate PbO 2electrode.An explanation for high electrochemical activity of oxygen evolution is that there is more activity surface area on the Ti/solid solution interlayer/PbO 2electrodes.The mechanism for oxygen evolution on oxide electrode in acid solution might be proposed as follows [27]:Fig.5Relationship of voltammetric charge quantity (q *)versus the reciprocal of square root of scan rate in 0.5M H 2SO 4solution.a Ti/PbO 2,b Ti/SnO 2–Sb 2O 5/PbO 2,c Ti/RuO 2–TiO 2/PbO 2,and d Ti/IrO 2–Ta 2O 5/PbO 2electrodeFig.6Linear scanning voltammograms at different PbO 2electrode in 0.5M H 2SO 4:scan rate at 1mV s -1.1Ti/PbO 2,2Ti/SnO 2–Sb 2O 5/PbO 2,3Ti/RuO 2–TiO 2/PbO 2,4Ti/IrO 2–Ta 2O 5/PbO 2electrodeFig.7Nyqusit diagrams of different PbO 2electrodes in 0.5M H 2SO 4.a Ti/PbO 2electrode,b Ti/SnO 2–Sb 2O 5/PbO 2electrode,c Ti/RuO 2–TiO 2/PbO 2electrode,d Ti/IrO 2–Ta 2O 5/PbO 2electrode.E =1.95V (vs.SCE)Fig.8Equivalent circuit used in the analysis of the experimental EIS dataSþH2O¼SÁÁÁOHþHþþeÀð1ÞSÁÁÁOH¼SÁÁÁOþHþþeÀð2ÞSÁÁÁOHþSÁÁÁOH¼SÁÁÁOþSþH2Oð3ÞSÁÁÁOþSÁÁÁO¼2SþO2ð4ÞS is an active surface area on the oxide surface.SÁÁÁO and SÁÁÁOH denote the adsorption state.For the same PbO2 electrode,the number of S is contributive to the high electrochemical activity of oxygen evolution.When the S is large,there may be high electrochemical activity in the electrode surface.The results of q*is consistent with R ct. Therefore,Ti/solid solution interlayer/PbO2electrodes with low R ct have high oxygen evolution activity.Because the q* value of Ti/IrO2–Ta2O5/PbO2is the highest compared the other three electrodes,it has the highest electrochemical activity of oxygen evolution.Summary and conclusionsThe SnO2–Sb2O5,RuO2–TiO2,and IrO2–Ta2O5mixture oxides coated on Ti substrate exist in the form of a solid solution.The solid solution interlayers can completely cover the titanium substrate and improve the bond with the Ti substrate and PbO2coating.The PbO2electrodes with solid solution interlayers greatly increased the service life of the PbO2electrode.In particular,Ti/IrO2–Ta2O5/PbO2 electrode greatly prolongs the service life of the PbO2 electrode to672h,which is61times longer than that of the PbO2electrode without interlayer.The overpotential of the oxygen evolution decreases with the addition of the solid solution interlayer.The presence of interlayer can increase the electrochemical activity of oxygen evolution and electrical conductivity.The oxygen evolution activity increases in the order of Ti/PbO2,Ti/SnO2–Sb2O5/PbO2, Ti/RuO2–TiO2/PbO2,and Ti/IrO2–Ta2O5/PbO2electrodes.Acknowledgements The authors would like to acknowledge the support provided by the National Natural Science Foundation of China(No.20873051),key project in Jilin Province(No.20080306). References1.Feng J,Johnson DC(1991)J Electrochem Soc138:33282.Mohd J,Pletcher D(2006)Electrochim Acta52:7863.Velichenko AB,Girenko DV,Kovalyov SV(1998)J ElectroanalChem454:2034.Cong Y,Wu Z(2007)J Phys Chem C111:34425.Amadelli R,Maldotti A,Molinari A,Danilov FI,Velichenko AB(2002)J Electroanal Chem534:16.Matsumoto Y,Noguchi M,Matsunaga T(1999)J Phys Chem B103:71907.Ciriaco L,Anjo C,Correia J(2009)Electrochim Acta54:14648.Han W,Chen Y,Wang L(2011)Desalination276:82ninellis C,Plattner E(1982)J Appl Electrochem12:39910.Adams B,Tian M,Chen A(2009)Electrochim Acta54:149111.Chen X,Chen G,Yue PL(2001)J Phys Chem B105:462312.Wang YQ,Tong HY,Xu WL(2004)Chin J Appl Chem21:43713.Hine F,Yasuda M,Lida T(1984)Electrochim Acta29:144714.Zhitomirsky I(1999)J Mater Sci34:2441.doi:10.1023/A:100457091834715.Hu JM,Zhang JQ,Cao CN(2002)Corros Sci44:165516.Hu JM,Zhang JQ(2003)J Mater Sci38:705.doi:10.1023/A:1021840426997ninellis C,Vercesi GP(1991)J Appl Electrochem21:33518.Xu LK,Xin YL,Wang JT(2009)Electrochem Acta54:182019.Devilliers D,Mahe´E(2010)Electrochim Acta55:820720.Cui X,Zhao GH,Lei YZ(2009)Mater Chem Phys113:31421.Zhao GH,Cui X,Liu MC(2009)Environ Sci Technol43:148022.Chen X,Chen G(2005)Electrochim Acta50:415523.Trasatti S(1991)Electrochim Acta36:165924.Alves VA,Da Silva LA,Boodts JFC(1998)Electrochim Acta44:1525ssali TAF,Boodts JFC,Bulhoes LOS(2000)J Appl Electro-chem30:62526.Da Silva LM,De Faria LA,Boodts JFC(2002)J ElectroanalChem532:14127.Lodi G,Sivieri E,De Battisti A,Trasatti S(1978)J ApplElectrochem8:135Table2Impedance information for the four electrodesElectrode R s(X cm2)R ct(X cm2)Q dl(X-1cm-2s n)n R f(X cm2)Q f(X-1cm-2s n)n Chi-square(v2)a0.05845 2.0210.0226510.0328 1.07910-50.858.82910-3b0.05243 1.7490.0233210.0172 1.31 5.84910-3c0.05132 1.5120.030930.97820.02010.31621 2.79910-4d0.04578 1.150.024130.99990.01870.21311 3.21910-3a Ti/PbO2,b Ti/SnO2–Sb2O5/PbO2,c Ti/RuO2–TiO2/PbO2,d Ti/IrO2–Ta2O5/PbO2electrodeChi-square v2test,the smaller the better。
