PURAC - Agrochemicals 2011
培美曲塞二钠 质量标准

培美曲塞二钠质量标准培美曲塞二钠(Perampanel Sodium)是一种新型的抗癫痫药物,属于非竞争性α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid受体拮抗剂。
它通过抑制神经元的兴奋性和减少谷氨酸释放,对癫痫的发作起到治疗作用。
培美曲塞二钠是钠盐形式,与其自由酸形式具有相同的药效。
培美曲塞二钠的化学结构是C23H20N4NaO,其分子量为414.42。
其化学名称是7-(3-氰基苯基)-5-苯基[1,2,4]三嗪-6-蓝烯-3-甲酸钠盐。
它是一种白色或类白色晶体粉末,溶于水、甲醇和乙醇,化学性质稳定。
培美曲塞二钠在临床上主要用于治疗癫痫。
目前,培美曲塞二钠已被批准用于治疗部分性癫痫随机化,双盲安慰剂对照研究显示,对癫痫的治疗有效率显著高于安慰剂组。
它可用作单药治疗或辅助治疗,并可用于儿童和成人患者。
剂量的选择应根据患者的具体情况来确定,一般从起始剂量开始,然后逐渐增加,直到达到有效的维持剂量。
通常,起始剂量为2毫克/天,剂量可逐步增加至最大剂量为12毫克/天。
培美曲塞二钠对于癫痫的治疗可以通过多种机制发挥作用。
首先,培美曲塞二钠作为一种非竞争性α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid(AMPA)受体拮抗剂,它能够直接抑制AMPA 受体的活性,降低神经元的兴奋性。
其次,培美曲塞二钠能够抑制谷氨酸的释放,从而减少神经元间的兴奋性传递。
最后,培美曲塞二钠可以通过调节钙离子通道和钾离子通道的活性,影响神经元内外钙离子和钾离子的浓度,从而影响神经元的兴奋性。
培美曲塞二钠的治疗效果已在大量的临床试验中得到证实。
一项对于片剂配伍剂量的研究显示,培美曲塞二钠治疗癫痫的有效率达到了50%以上。
而另一项多中心、随机、双盲、安慰剂对照研究对治疗癫痫的患者进行了为期12周的评估,结果显示,培美曲塞二钠组的癫痫发作次数显著减少,有效率达到了70%以上。
国际化妆品原料标准中文名称目录

国际化妆品原料标准中文名称目录国食药监许…2010? 479号附件:国际化妆品原料标准中文名称目录(2010年版)前言为加强对化妆品原料的监督管理,进一步规范国际化妆品原料标准中文名称命名,在卫生部发布的《国际化妆品原料标准中文名称目录》(2007年版)的基础上,国家食品药品监督管理局组织对美国化妆品盥洗用品及香水协会2008年出版的《国际化妆品原料字典和手册(第十二版)》[International Cosmetic Ingredient Dictionary and Handbook Twelfth Edition(2008)](以下简称《字典》)中所收录的原料命名进行了翻译,完成了《国际化妆品原料标准中文名称目录》(2010年版)(以下简称《目录》)。
一、《目录》主要内容《目录》共收录15649个原料,按照国际化妆品原料命名(INCI 名称)的英文字头排序。
内容包括原料的INCI名称、中文名称、CAS 号等。
其中INCI名称与CAS号,对应《字典》中的相应内容。
二、INCI名称编译原则和有关问题的说明(一)编译原则主要参照INCI名称中文译名有关通则。
(二)有关问题的说明1.为保持编译工作的一致性和连续性,《目录》编译参照了INCI 名称中文译名有关通则,《目录》中着色剂的中文名称以《字典》中的INCI 名称为准进行编译,删除了原通则中第3条有关着色剂原料括符CI号的内容。
《字典》中着色剂原料INCI名称为CI号的,中文名称以CI号表示。
原料INCI名称既有日本着色剂索引号,又有CI号的,以CI号作为中文名称。
2.《目录》中的原料名称尽量做到与《字典》中的原料名称一一对应,《字典》中原料名称出现重复的,《目录》中作为名称相同原料仅收录一次。
3.《字典》中个别原料的有关表述无实际意义的,如:AND OTHER INGREDIENTS,《目录》中编译为“和其他成分”,无具体化妆品原料与之相对应,主要是考虑尊重原版《字典》。
改良山梨醇麦康凯琼脂或改良麦康凯肉汤配套试剂安全数据单说明书

化学品安全技术说明书第一部分化学品及企业标识产品中文名称:改良山梨醇麦康凯琼脂或改良麦康凯肉汤配套试剂产品英文名称:Additives for CT-Sorbitol MacConkey Agar orCT-MacConkey Broth产品编号:SR0240企业名称:广东环凯微生物科技有限公司地址:广东省广州市黄埔区广州开发区科学城神舟路788号邮编:510663公司网址电子邮件地址:*********************传真号码:************销售热线:************-8602技术热线:************-8877/8876推荐用途和限制用途:生化研究/分析第二部分危险性概述GSH危害性类别皮肤腐蚀/刺激(类别3), 呼吸过敏(类别1);皮肤过敏(类别1)。
GSH标签要素象形图:无警示词:警告危险信息:H316 造成轻微皮肤刺激;H317 可能造成皮肤过敏反应;H334 吸入可能导致过敏或哮喘病症状或呼吸困难。
防范说明事故响应:P333 + P313 如发生皮肤刺激或皮疹:求医/就诊;P342 + P311 如有呼吸系统病症:呼叫急救中心/医生。
废弃处置:P501将内装物/容器送到批准的废物处理厂处理。
物理和化学危险目前掌握信息,没有物理或化学的危险性。
健康危害H316 造成轻微皮肤刺激;H317 可能造成皮肤过敏反应;H334吸入可能导致过敏或哮喘病症状或呼吸困难。
环境危害无数据资料。
其它危害无。
第三部分成分/组成信息混合物化学品成分:参考使用说明。
有害物质成分:化学名称CAS No. 浓度亚碲酸钾7790-58-10.25%头孢克圬79350-37-10.0005%第四部分急救措施一般信息:无特殊的措施要求。
皮肤接触:立即用清水彻底清洗。
眼睛接触:立即提起眼睑,用大量流动清水冲洗。
如不适就医。
吸入:如不适就医。
食入:如不适就医。
就医信息:出示产品使用说明或者此SDS。
芳香族酰胺

➢ PPTA纤维横向强度(压缩强度和剪切强度)比纵向拉伸 强度低很多
➢ 耐疲劳性问题较突出。 为什么?
12
芳香族聚酰胺纤维
对位芳香族聚酰胺(PPTA)纤维
热性能 ✓ 玻璃化温度为345℃,分解温度为560℃,极限氧指数为28
~30%。 ✓ 耐热性能接近无机纤维,在高温下不熔融,并可以保持较高
的强度和较好的尺寸稳定性。 ✓ 有很强的自熄性和阻燃性
✓ 相邻的片状微晶之间主要由范 德华力结合在一起。
11
芳香族聚酰胺纤维
对位芳香族聚酰胺(PPTA)纤维
力学性能
✓ PPTA纤维其强度比一般有机纤维高3倍以上 ✓ 模量是尼龙的10倍,涤纶的9倍,钢丝的2~3倍 ✓ 比重只有钢丝的1/5左右,因此其比强度相当于钢丝的
6-7倍,作为结构材料可以替代钢铁及钛合金等金属材料
NMP-CaCl2
对苯二胺
对苯二甲酰氯
溶剂回收
缩聚 萃取、水洗
干燥聚合体 4
芳香族聚酰胺纤维
对位芳香族聚酰胺(PPTA)纤维
聚合物的制备:低温溶液缩聚法 ✓ 聚合体系必须严格保持无水,否则聚合将难以进行。 ✓ 在可增N-强甲体基系吡的咯溶烷剂酮化(作N用M,P)促溶进剂缩中聚加反入应了的L程iC度l、。CaCl2等盐, ✓ 对苯二胺和对苯二甲酰氯的反应很快,反应开始后几分钟就出
NH
NH CO
CO n
✓ 分子中的骨架原子通过强共价键结合,键能非常大。
✓ 长链分子中的酰胺基被苯环间隔且与苯环形成共轭效应,内 旋转位能相当高,键的自由旋转受阻,酰胺基和苯环能在一 个平面内稳定共存,大分子呈平面刚性伸直链构象。
8
结构
芳香族聚酰胺纤维
对位芳香族聚酰胺(PPTA)纤维
USP美国药典,二甘醇、乙二醇及其他杂质

GlycerinC 3H 8O 3 92.101,2,3-Propanetriol.Glycerol [56-81-5].» Glycerin contains not less than 99.0 percent and not more than 101.0 percent of C 3H 8O 3, calculated on the anhydrous basis. The amount of any individual impurity, excluding diethylene glycol and ethylene glycol, if detected, meets the requirements under Other Impurities (NMT 0.1%) and the amount of total impurities, including diethylene glycol and ethylene glycol, is NMT 1.0%. Packaging and storage— Preserve in tight containers.USP Reference standards 11— USP Diethylene Glycol RS . USP Ethylene Glycol RS . USP Glycerin RS .Color— Its color, when viewed downward against a white surface in a 50-mL color-comparison tube, is not darker than the color of a standard made by diluting 0.40 mL of ferric chloride CS with water to 50 mL and similarly viewed in a color-comparison tube of approximately the same diameter and color as that containing the Glycerin.Identification— [NOTE—Compliance is determined by meeting the requirements for both Identification A and B .]A: Infrared Absorption 197F .B: Standard stock solution 1—Transfer 50 mg of USP Diethylene Glycol RS , accurately weighed, to a 100-mL volumetric flask, dilute with methanol to volume, and mix. Standard stock solution 2— Transfer 50 mg of USP Ethylene Glycol RS , accurately weighed, to a 100-mL volumetric flask, dilute with methanol to volume, and mix.Standard stock solution 3— Transfer 50 mg of USP Glycerin RS , accurately weighed, to a 100-mL volumetric flask, dilute with methanol to volume, and mix.Resolution solution— Transfer 5.0 mL each of Standard stock solution 1, Standard stock solution 2, and Standard stock solution 3, to a 100-mL volumetric flask, dilute withmethanol to volume, and mix.Test solution— Transfer about 5 g of Glycerin to a 100-mL volumetric flask, dilute with methanol to volume, and mix.Chromatographic system (see Chromatography 621)— The gas chromatograph is equipped with a flame-ionization detector, a 0.53-mm × 30-m fused-silica analytical column coated with 3.0-µm G43 stationary phase. The injection port temperature is maintained at 220 and the detector temperature is maintained at 250. The carrier gas is helium with a flow rate of about 4.5 mL per minute. The split flow ratio is about 10:1. The chromatograph is programmed as follows: Initially, the column temperature is equilibratedat 100 for 4 minutes, then increased at a rate of 50 per minute to 120, and is maintained at 120 for 10 minutes. It is then increased at a rate of 50 per minute to 220, and is maintained at 220 for 6 minutes. Chromatograph the Resolution solution, and record the peak responses and retention times as directed for Procedure: the relative retention times are about 0.3 for ethylene glycol, 0.8 for diethylene glycol, and 1.0 for glycerin; and the relative standard deviation for replicate injections for the diethylene glycol is not more than 10%.Procedure— Separately inject equal volumes (about 1 µL) of the Resolution solution and the Test solution into the chromatograph, and record the chromatograms. If a peak at the relative retention time for the diethylene glycol or ethylene glycol is present in the Test solution, the peak must be identifed and quantified as directed in the test for Diethylene glycol and ethylene glycol impurities.Specific gravity 841: not less than 1.249.Residue on ignition 281—Heat 50 g in an open, shallow 100-mL porcelain dish until it ignites, and allow it to burn without further application of heat in a place free from drafts. Cool, moisten the residue with 0.5 mL of sulfuric acid, and ignite to constant weight: the weight of the residue does not exceed 5 mg (0.01%).Water, Method I 921: not more than 5.0%.Chloride 221—A 7.0-g portion shows no more chloride than corresponds to 0.10 mL of 0.020 N hydrochloric acid (0.001%).Sulfate 221—A 10-g portion shows no more sulfate than corresponds to 0.20 mL of 0.020 N sulfuric acid (about 0.002%).Heavy metals 231—Mix 4.0 g with 2 mL of 0.1 N hydrochloric acid, and dilute with water to 25 mL: the limit is 5 µg per g.Limit of chlorinated compounds— Accurately weigh 5 g of Glycerin into a dry, round-bottom, 100-mL flask, add 15 mL of morpholine, and connect the flask by a ground joint to a reflux condenser. Reflux gently for 3 hours. Rinse the condenser with 10 mL of water,receiving the washing in the flask, and cautiously acidify with nitric acid. Transfer the solution to a suitable comparison tube, add 0.50 mL of silver nitrate TS, dilute with water to 50.0 mL, and mix: the turbidity is not greater than that of a blank to which 0.20 mL of 0.020 N hydrochloric acid has been added, the refluxing being omitted (0.003% of Cl).Fatty acids and esters— Mix 50 g of Glycerin with 50 mL of freshly boiled water and 5 mL of 0.5 N sodium hydroxide VS, boil the mixture for 5 minutes, cool, add phenolphthalein TS, and titrate the excess alkali with 0.5 N hydrochloric acid VS. Performa blank determination (see Residual Titrations under Titrimetry 541): not more than 1 mL of 0.5 N sodium hydroxide is consumed.Diethylene glycol and ethylene glycol impurities—Internal standard solution— Transfer 100 mg of 2,2,2-trichloroethanol (internal standard), accurately weighed, to a 100-mL volumetric flask, dilute with methanol to volume, and mix.Standard stock solution 1— Transfer 50 mg of USP Diethylene Glycol RS, accurately weighed, to a 100-mL volumetric flask, dilute with methanol to volume, and mix.Standard stock solution 2— Transfer 50 mg of USP Ethylene Glycol RS, accurately weighed, to a 100-mL volumetric flask, dilute with methanol to volume, and mix.Standard stock solution 3— Transfer 50 mg of USP Glycerin RS, accurately weighed, to a 100-mL volumetric flask, dilute with methanol to volume, and mix.Standard solution— Transfer 5.0 mL each of Standard stock solution 1, Standard stock solution 2, and the Internal standard solution to a 100-mL volumetric flask, and dilute with methanol to volume, and mix.Resolution solution— Transfer 500 mg of USP Glycerin RS to a 10-mL volumetric flask, add 0.5 mL each of Standard stock solution 1 and Standard stock solution 2, dilute with methanol to volume, and mix.Test solution— Transfer about 5 g of Glycerin to a 100-mL volumetric flask, add 5.0 mL of Internal standard solution, dilute with methanol to volume, and mix.Chromatographic system (see Chromatography 621)— The gas chromatograph is equipped with a flame-ionization detector, a 0.53-mm × 30-m fused-silica analytical column coated with 3.0-µm G43 stationary phase. The injection port temperature is maintained at 220 and the detector temperature is maintained at 250. The carrier gas is helium, flowing at a rate of about 4.5 mL per minute. The split flow ratio is about 10:1. The chromatograph is programmed as follows. Initially, the column temperature is equilibratedat 100 for 4 minutes, then increased at a rate of 50 per minute to 120, and is maintained at 120 for 10 minutes then increased at a rate of 50 per minute to 220, andis maintained at 220for 6 minutes. Chromatograph the Standard solution, and record thepeak response ratio and retention times as directed for Procedure: the relative retention times are about 0.3 for ethylene glycol, 0.8 for diethylene glycol, and 1.0 for glycerin; the relative standard deviation for replicate injections for the diethylene glycol is not more than 10%; and the limit of quantitation of diethylene glycol and ethylene glycol is not more than 0.025%.Procedure— Separately inject equal volumes (about 1 µL) of the Standard solution and the Test solution into the chromatograph, record the chromatograms, and measure the responses for the ethylene glycol and diethylene glycol peaks. Calculate the percentage of diethylene glycol and ethylene glycol in the portion of Glycerin taken by the formula:100(CS / CU)(RU/ RS)in which CSis the concentration, in mg per mL, of diethylene glycol (or ethylene glycol) inthe Standard solution; CUis the concentration, in mg per mL, of Glycerin in the Testsolution; and RU and RSare the peak response ratios for diethylene glycol (or ethyleneglycol) to the internal standard peak obtained from the Test solution and the Standard solution: NMT the limit of quantitation for each, is found (0.025%).Assay—Sodium periodate solution— Dissolve 60 g of sodium metaperiodate in sufficient water containing 120 mL of 0.1 N sulfuric acid to make 1000 mL. Do not heat to dissolve the periodate. If the solution is not clear, pass through a sintered-glass filter. Store the solution in a glass-stoppered, light-resistant container. Test the suitability of this solution as follows. Pipet 10 mL into a 250-mL volumetric flask, dilute with water to volume, and mix. To about 550 mg of Glycerin dissolved in 50 mL of water add 50 mL of the diluted periodate solution with a pipet. For a blank, pipet 50 mL of the solution into a flask containing 50 mL of water. Allow the solutions to stand for 30 minutes, then to each add 5 mL of hydrochloric acid and 10 mL of potassium iodide TS, and rotate to mix. Allow to stand for 5 minutes, add 100 mL of water, and titrate with 0.1 N sodium thiosulfate, shaking continuously and adding 3 mL of starch TS as the endpoint is approached. The ratio of the volume of 0.1 N sodium thiosulfate required for the glycerin–periodate mixture to that required for the blank should be between 0.750 and 0.765.Procedure— Transfer about 400 mg of Glycerin, accurately weighed, to a 600-mL beaker, dilute with 50 mL of water, add bromothymol blue TS, and acidify with 0.2 N sulfuric acid to a definite green or greenish yellow color. Neutralize with 0.05 N sodium hydroxide to a definite blue endpoint, free from green color. Prepare a blank containing 50 mL of water, and neutralize in the same manner. Pipet 50 mL of the Sodium periodate solution into each beaker, mix by swirling gently, cover with a watch glass, and allow to stand for 30minutes at room temperature (not exceeding 35) in the dark or in subdued light. Add 10 mL of a mixture of equal volumes of ethylene glycol and water, and allow to stand for 20 minutes. Dilute each solution with water to about 300 mL, and titrate with 0.1 N sodiumhydroxide VS to a pH of 8.1 ± 0.1 for the specimen under assay and 6.5 ± 0.1 for the blank, using a pH meter. Each mL of 0.1 N sodium hydroxide, after correction for the blank, is equivalent to 9.210 mg of C 3H 8O 3.Auxiliary Information— Please check for your question in the FAQs before contacting USP.USP32–NF27 Page 2513Pharmacopeial Forum : Volume No. 28(4) Page 1245Chromatographic Column— GLYCERINChromatographic columns text is not derived from, and not part of, USP 32 or NF 27. Topic/Question Contact Expert Committee Monograph Kevin T. Moore, Ph.D.Scientist1-301-816-8369(EM105) Excipient Monographs 1Reference StandardsLili Wang, Technical ServicesScientist1-301-816-8129RSTech@。
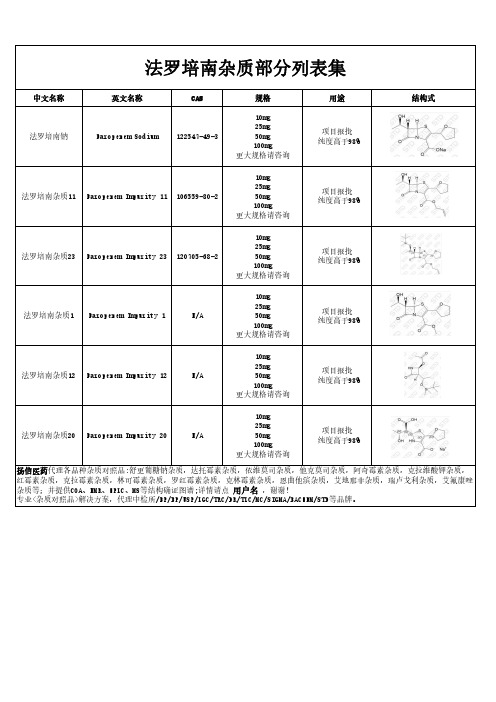
法罗培南杂质部分汇总
Байду номын сангаас
中文名称 法罗培南钠
英文名称 Faropenem Sodium
CAS 122547-49-3
规格
10mg 25mg 50mg 100mg 更大规格请咨询
用途
项目报批 纯度高于98%
结构式
法罗培南杂质11 Faropenem Impurity 11 106559-80-2
10mg 25mg 50mg 100mg 更大规格请咨询
项目报批 纯度高于98%
法罗培南杂质23 Faropenem Impurity 23 120705-68-2
10mg 25mg 50mg 100mg 更大规格请咨询
项目报批 纯度高于98%
法罗培南杂质1 Faropenem Impurity 1
N/A
10mg 25mg 50mg 100mg 更大规格请咨询
项目报批 纯度高于98%
法罗培南杂质12 Faropenem Impurity 12
N/A
10mg 25mg 50mg 100mg 更大规格请咨询
项目报批 纯度高于98%
法罗培南杂质20 Faropenem Impurity 20
N/A
10mg 25mg 50mg 100mg 更大规格请咨询
项目报批 纯度高于98%
扬信医药代理各品种杂质对照品:舒更葡糖钠杂质,达托霉素杂质,依维莫司杂质,他克莫司杂质,阿奇霉素杂质,克拉维酸钾杂质, 红霉素杂质,克拉霉素杂质,林可霉素杂质,罗红霉素杂质,克林霉素杂质,恩曲他滨杂质,艾地那非杂质,瑞卢戈利杂质,艾氟康唑 杂质等;并提供COA、NMR、HPLC、MS等结构确证图谱;详情请点 用户名 ,谢谢! 专业<杂质对照品>解决方案,代理中检所/EP/BP/USP/LGC/TRC/DR/TLC/MC/SIGMA/BACHEM/STD等品牌。
作为DHODH抑制剂的5-环丙基-2-{[2-(2,6-二氟苯基)嘧啶-5-基]胺基}苯甲酸
专利名称:作为DHODH抑制剂的5-环丙基-2-{[2-(2,6-二氟苯基)嘧啶-5-基]胺基}苯甲酸钠盐
专利类型:发明专利
发明人:N·嘉西亚古赛里兹,F·卡雷拉卡雷拉,M·朱莉娅简,L·德贝裘恩,X·塞拉马西亚
申请号:CN201080011541.4
申请日:20100311
公开号:CN102348687A
公开日:
20120208
专利内容由知识产权出版社提供
摘要:本发明涉及5-环丙基-2-{[2-(2,6-二氟苯基)嘧啶-5-基]胺基}苯甲酸钠盐及其药学上可接受的溶剂合物。
申请人:奥米罗有限公司
地址:西班牙巴塞罗那
国籍:ES
代理机构:北京同达信恒知识产权代理有限公司
代理人:黄志华
更多信息请下载全文后查看。
洋甘菊提取液MSDS英文版
1. IDENTIFICATION OF THE SUBSTANCE/TREPARATION AND THE COMPANY/UNDERTAKING3.HAZARDS IDENTIFICATION4. FIRST AID MEASURESMATERIAL SAFETY DATA SHEETProduct name:Supplier:Tel:EMERGENCY OVERVIEW: May cause skin irritation and/or dermatitisPrinciple routes of exposure: Inhalation: Ingestion: Skin contact: Eye contact:SkinMay cause irritation of respiratory tract May be harmful if swallowed May cause allergic skin reaction Avoid contact with eyesStatements of hazard MAY CAUSE ALLERGIC SKIN REACTION.Statements of Spill of Leak Label Eliminate all ignition sources. Absorb and/or contain spill with inert materials (e.g., sand, vermiculite). Then place in appropriate container. For large spills, use water spray to disperse vapors, flush spill area. Prevent runoff from entering waterways or sewers.General advice:POSITION/INFORMATION ON INGREDIENTSInhalation:Skin contact:Ingestion:Eye contact:Protection of first – aiders:Medical conditions aggravated by exposure: In the case of accident or if you fell unwell, seek medical advice immediately (show the label where possible).Move to fresh air, call a physician immediately.Rinse immediately with plenty of water and seek medical adviceDo not induce vomiting without medical advice.In the case of contact with eyes, rinse immediately with plenty of water and seek medical advice.No information availableNone knownSuitable extinguishing media:Specific hazards:Special protective equipment for firefighters:Flash point:Autoignition temperature:NFPA rating Use dry chemical, CO2, water spray or “alcohol” foam Burning produces irritant fumes.As in any fire, wear self-contained breathing apparatus pressure-demand, MSHA/NIOSH (approved or equivalent) and full protective gearNot determinedNot determinedNFPA Health: 1 NFPA Flammability: 1 NFPA Reactivity: 0Personal precautions: Environmental precautions: Methods for cleaning up: Use personal protective equipment.Prevent product from entering drains.Sweep up and shovel into suitable containers for disposalStorage:7. HANDLING AND STORAGE5.FIRE-FIGHTING MEASURES6. ACCIDENTAL RELEASE MEASURESRoom temperature Handling:Safe handling advice: Incompatible products:Use only in area provided with appropriate exhaust ventilation.Wear personal protective equipment.Oxidising and spontaneously flammable productsEngineering measures: Respiratory protection: Skin and body protection:Eye protection: Hand protection: Hygiene measures:Ensure adequate ventilation.Breathing apparatus only if aerosol or dust is formed. Usual safety precautions while handling the product will provide adequate protection against this potential effect. Safety glasses with side-shieldsPVC or other plastic material glovesHandle in accordance with good industrial hygiene and safety practice.Melting point/range: Boiling point/range: Density: Vapor pressure: Evaporation rate: Vapor density: Solubility (in water): Flash point:Autoignition temperature:No Data available at this time. No Data available at this time. No data available No data available No data available No data available No data available Not determined Not determinedStability: Stable under recommended storage conditions. Polymerization: None under normal processing.Hazardous decomposition products: Thermal decomposition can lead to release of irritating gases and vapours such as carbon oxides.Materials to avoid: Strong oxidising agents.10. STABILITY AND REACTIVITY9. PHYSICAL AND CHEMICAL PROPERTIES8. EXPOSURE CONTROLS/PERSONAL PROTECTION11. TOXICOLOGICAL INFORMATIONConditions to avoid: Exposure to air or moisture over prolonged periods.Product information Acute toxicityChronic toxicity:Local effects: Chronic exposure may cause nausea and vomiting, higher exposure causes unconsciousness.Symptoms of overexposure may be headache, dizziness, tiredness, nausea and vomiting.Specific effects:May include moderate to severe erythema (redness) and moderate edema (raised skin), nausea, vomiting,headache.Primary irritation: Carcingenic effects: Mutagenic effects: Reproductive toxicity:No data is available on the product itself. No data is available on the product itself. No data is available on the product itself. No data is available on the product itself.Mobility:Bioaccumulation: Ecotoxicity effects: Aquatic toxicity:No data available No data available No data availableMay cause long-term adverse effects in the aquatic environment.12. ECOLOGICAL INFORMATION13. DISPOSAL CONSIDERATIONSWaste from residues/unused products:Contaminated packaging:Waste disposal must be in accordance with appropriate Federal, State and local regulations. This product, if unaltered by use, may be disposed of treatment at a permitted facility or as advised by your local hazardous waste regulatory authority. Residue from fires extinguished with this material may be hazardous.Do not re-use empty containers.UN/Id No:Not regulated14. TRANSPORT INFFORMATIONDOTProper shipping name: Not regulatedTGD(Canada)WHMIS hazard class: Non - controlledIMDG/IMOIMDG – Hazard Classifications Not ApplicableIMO – labels:15. REGULATORY INFOTMATION International Inventories16. OTHER INFORMATIONPrepared by: Health & SafetyDisclaimer: The information and recommendations contained herein are based upon tests believed to be reliable.However, XABC does not guarantee the accuracy or completeness NOR SHALL ANY OF THIS INFORMATION CONSTITUTE A WARRANTY, WHETHER EXPRESSED OR IMPLIED, AS TO THE SAFETY OF THE GOOD, THE MERCHANTABILITY OF THE GOODS, OR THE FITNESS OF THE FITNESS OF THE GOODS FOR A PARTICULAR PURPOSE. Adjustment to conform to actual conditions of usage maybe required. XABC assumes no responsibility for results obtained or for incidental or consequential damages, including lost profits arising from the use of these data. No warranty against infringement of any patent, copyright or trademark is made or implied.End of safety data sheet。
匹可硫酸钠资料汇总
大于 95% 现货
匹可硫酸钠杂质 7 杂质 J
N O
NaO3SO
OSO3Na
Chemical Formula: C18H13NNa2O9S2 Exact Mass: 496.98
Molecular Weight: 497.40
大于 95%
现货
匹可硫酸钠杂质 8 匹克硫酸钠 EP 杂 质B
大于 95% 现货
匹可硫酸钠杂质 23
HO O
N
Chemical Formula: C12H9NO2 Exact Mass: 199.06
Molecular Weight: 199.21
大于 95% 现货
广州隽沐生物科技代理各种杂质对照品:舒更葡糖钠杂质,达托霉素杂质,依维莫司杂质, 他克莫司杂质,阿奇霉素杂质,克拉维酸钾杂质,红霉素杂质,克拉霉素杂质,林可霉素 杂质,罗红霉素杂质,克林霉素杂质等;
OH
N Chemical Formula: C18H15NO2
Exact Mass: 277.11 Molecular Weight: 277.32
NaO3SO
大于 95% 现货
匹可硫酸钠杂质 19
OSO3Na
N
Chemical Formula: C18H13NNa2O8S2 Exact Mass: 480.99
NaO3SO
OSO3Na OH
N
Chemical Formula: C18H13NNa2O9S2 Exact Mass: 496.98
Molecular Weight: 497.40
大于 95%
四周
匹可硫酸钠杂质 6 杂质 I
N O
阿克苏诺贝尔分散剂介绍 (中文)
21% 21%
Surface Chemistry
Chemicals Pakistan
20%
4%
* Before incidentals
Surface Chemistry | Title
4
What Akzo Nobel Chemicals supply
Surface Chemistry 表面化 学
• Surfactants 表面活性剂 • Fatty amines 脂肪胺 • Polymers (Dispersants & HASE Thickeners) 聚合物
Akzonobel product for ink industry
Wetting and dispersant agent
Pigment dispersion steps
1. 2. 3. Wet out particle surface Break-up aggregate Surfactants Surfactants + Polymers Polymers
Pulp and Performance – 纸浆和性能
• Colloidal silica 胶体氧化硅. LevasilTM . BindzilTM
Functional Chemicals – Polymer additives 功能化学 品-聚合物添加剂
Initiators polymerization (radical ). 聚合反应的引发剂(自由基) PerkadoxTM and TrigonoxTM. Accelerators.加速剂 Co-based and Co-free. NouryactTM
• Aqueous Emulsion Polymerization水 性乳液聚合
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
OH
…………..
Succinic Acid
O HO O OH
CONFIDENTIAL
© 4/19/2011
7
Product RASOLV – Lactate Esters
O
R
HO
S
O R1 O
HO
R1 O
Biocontent
(ASTM 6866)
R1 = H R1 = CH3 R1 = C2H5 R1 = C3H7 R1 = C4H9 R1=C8H17 R1=C12H25
CONFIDENTIAL
© 4/19/2011
11
CO2 Balance
1000
Build-up of CO2 emission for Purac Lactic acid
500
0
kg CO2 eq/ton
-500
-1000
-1500
-2000
Electricity
Total
Sugar
Sugar production
© 4/19/2011
3
Organization
PURAC
Chemicals & Pharma Food
Global leader of natural Lactic acid & Derivatives Global manufacturing footprint Over 70 years of experience
CONFIDENTIAL
© 4/19/2011
4
Application Areas
Green Chemicals
Home Care
Personal Care
Pharma
Bioplastics
Safe Solvents
Medical Care
Animal Nutrition
CONFIDENTIAL
Organization
Improve Environmental Performance - Higher biocontent - Better Safety profile
CONFIDENTIAL
© 4/19/2011
16
Functionalities
Good (Co)-solvent for Actives Relatively slow evaporation (Enhances contact time) Relatively high flash point (Safe) Adjuvancy Readily Biodegradable
PURAC Headquarters Gorinchem - Netherlands
Gorinchem, Netherlands Blair, Nebraska, USA
Campos, Rio, Brasil
PURAC plants PURAC sales offices Montmelo, Spain Map Tha Put, Thailand
Steam
CONFIDENTIAL
Aux Chemical
Sugar Transport
Agrochemicals
Improve Crop Production
Per Acre
Formulations
Active Ingredients
O MeO N R O Me Me Me OMe
Formulations: - (Co)Solvents - Adjuvents
© 4/19/2011
14
Typical Pesticide Formulation
Active Ingredient(s) Solvent(s) Surfactants Adjuvant Other components
5 - 50 % 20 - 60 % 1 - 10 % 15%
1 - 20 %
Concentrate, to be mixed with water before application Formulation can be solvent based, water based, powder or (micro-)emulsion
CONFIDENTIAL
© 4/19/2011
USA Lactate esters exempted from the requirement of a tolerance - EL and BL on FIFRA list 4A (40 CFR 180.950)) - NPL and EHL on FIFRA list 4B (40CFR 180.910 and 40CFR 180.930) EL and BL approved as food flavoring agents (FDA) Esters 100% VOC’s
Co-solvent
• • Enhance solubility properties of e.g (methylated) vegetable oils. Possible replacement for NMP, CH or isopherone
Bio-enhancement adjuvant
© 4/19/2011
1
ห้องสมุดไป่ตู้grochemical
Active Ingredients Solvents Adjuvents
CONFIDENTIAL
© 4/19/2011
2
Organization
csm
CSM Bakery Supplies CSM Bakery Supplies Europe Europe CSM Bakery Supplies CSM BakerySupplies North America North America Biochemical Division Biochemical Division PURAC
BL and esp. EHL can be used as oil phase in emulsions EC formulations with tebucanazole and PURASOLV EHL EC formulations with mixtures of actives of the azole family and PURASOLV EHL EC formulations with lactate esters as adjuvant EC formulations with PURASOLV BL and NPL
CONFIDENTIAL
© 4/19/2011
17
Applications
Solvent for emulsions (EC, EW and ME)
• • • • •
EC = Emulsion Concentrates EW = Emulsion in Water ME = Micro-emulsions
100% 75% 100% 50% 43% 27%
CONFIDENTIAL
© 4/19/2011
8
Production
Production Carbohydrates
Production Lactic Acid
Production Lactide
Polymerization
Carbohydrate
Active Ingredient Manufacturers
Formulators Tank Mixers
Farmers
Patented
Patented
Generic
Generic
All parties have IP or IP licenses among each other
CONFIDENTIAL
Lactic Acid Lactate Salts Lactate Esters
Lactide
PLA Resin
Methyl Lactate Ethyl Lactate N-Propyl Lactate Butyl Lactate Ethylhexyl Lactate
CONFIDENTIAL
© 4/19/2011
CONFIDENTIAL
© 4/19/2011
6
Product Line
O
PURAC® PURASAL ® PURAMEX ® PURACAL ® PURASOLV ® PURALACT ® Lactic Acid Lactate Salts
OH OH
O
Metal Lactates
OR
Calcium Lactate C1 – C8 Lactate Esters Lactides
15
Benefits PURAC/PURASOLV
Improve Crop production - Prevention of disease/weed etc.
Cost-effective formulations - Replacement of more expensive solvents - Reduce concentration of expensive Actives Ingredients (Adjuvancy)
Active Ingredients: - Fungicide - Herbicide - Insecticide
CONFIDENTIAL
© 4/19/2011
13
Agrochemicals
PURAC PURASOLV
- Chiral Synthesis
PURASOLV
- Solvents - Adjuvents
• PURASOLV BL as co-surfactant for micro-emulsions of hydrocarbons
