阿托伐他汀合成
阿托伐他汀合成图解
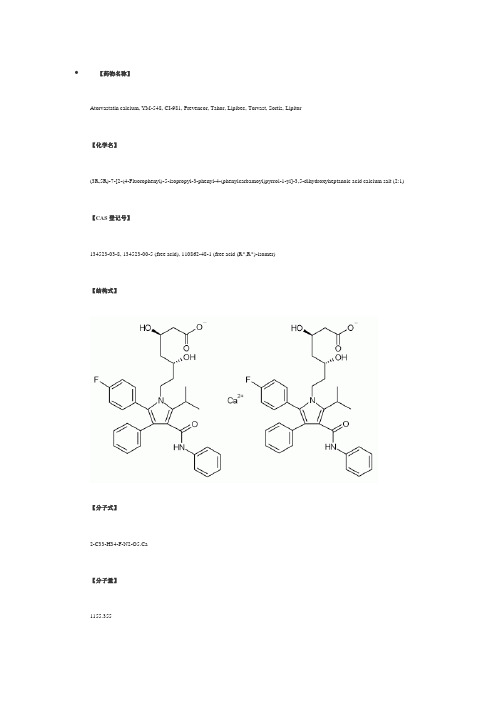
【药物名称】Atorvastatin calcium, YM-548, CI-981, Prevencor, Tahor, Lipibec, Torvast, Sortis, Lipitor【化学名】(3R,5R)-7-[2-(4-Fluorophenyl)-5-isopropyl-3-phenyl-4-(phenylcarbamoyl)pyrrol-1-yl]-3,5-dihydroxyheptanoic acid calcium salt (2:1) 【CAS登记号】134523-03-8, 134523-00-5 (free acid), 110862-48-1 (free acid (R*,R*)-isomer)【结构式】【分子式】2-C33-H34-F-N2-O5.Ca【分子量】1155.355【原研厂家】Jouveinal (Originator), Pfizer (Originator), Almirall Prodesfarma (Licensee), Syncro (Licensee), Yamanouchi (Licensee), Stanford University (Codevelopment)【作用类别】Alzheimer's Dementia, Treatment of , CARDIOVASCULAR DRUGS, Cognition Disorders, Treatment of, Immunologic Neuromuscular Disorders, Treatment of, Lipoprotein Disorders, Treatment of , METABOLIC DRUGS, Multiple Sclerosis, Agents for, NEUROLOGIC DRUGS, Treatment of Disorders of the Coronary Arteries and Atherosclerosis, HMG-CoA Reductase Inhibitors, TNFSF6 Expression Inhibitors【研发状态】Launched-1997【合成情况】NB2〖来源〗Drugs Fut〖合成路线〗〖标题〗Atorvastatin Calcium〖合成方法〗1) The condensation of 2-(1,3-dixolan-2-yl)ethylamine (I) with ethyl 2-bromo-2-(4-fluorophenyl)acetate (II) by means of triethylamine in acetonitrile gives ethyl 2-[2-(1,3-dioxolan-2-yl)ethylamino]-2-(4-fluorophenyl)acetate (III), which is acylated with isobutyryl chloride (IV) and triethylamine in dichloromethane yielding the corresponding amide (V). Saponification of the ester (V) with NaOH in methanol/water affords the free acid (VI), which is cyclized with N,3-diphenylpropynamide (VII) [obtained in the reaction of 3-phenylpropynoic acid (VIII) with aniline (IX) by means of dicyclohexylcarbodiimide (DCC)] by heating at 90 C in acetic anhydride giving1-[2-(1,3-dioxolan-2-yl)ethyl]-5-(4-fluorophenyl)-2-isopropyl-N,4-diphenylpyrrole-3-carboxamide (X). The hydrolysis of the dioxolane group of (X) with HCl yields the corresponding aldehyde (XI), which is condensed with methyl acetoacetate (XII) by means of NaH in THF affording 7-[2-(4-fluorophenyl)-5-isopropyl-3-phenyl-4-(N-phenylcarbamoyl)pyrrol-1-yl]-5-hydroxy-3-oxoheptanoic acid methyl ester (XIII). The reduction of the carbonyl group of (XIII) with tributylborane and NaBH4 in THF gives the (3R*,5R*)-dihydroxy ester (XIV), which is saponified with NaOH in water yielding the corresponding free acid (XV). The lactonization of (XV) by heating in refluxing toluene affords the (R*,R*)-lactone (XVI), which is submitted to optical resolution by reaction with (R)-1-phenylethylamine (XVII) followed by fractional crystallization thus obtaining the amide (XVII) as the pure (R,R,R)-enantiomer. The hydrolysis of the amide (XVIII) with NaOH, followed by heating in refluxing toluene gives the (R,R)-lactone (XIX), which is finally treated first with NaOH in methanol/water, and then with CaCl2 or calcium acetate.〖作者〗Graul, A.; Casta馿r, J.〖参考〗Graul, A.; Casta馿r, J.; Atorvastatin Calcium. Drugs Fut 1997, 22, 9, 956〖出处〗Drugs Fut1997,22,(9):956〖备注〗Synthesis Atorvastatin calcium has been obtained by several different ways: 1) The condensation of 2-(1,3-dixolan-2-yl)ethylamine (I) with ethyl 2-bromo-2-(4-fluorophenyl)acetate (II) by means of triethylamine in acetonitrile gives ethyl2-[2-(1,3-dioxolan-2-yl)ethylamino]-2-(4-fluorophenyl)acetate (III), which is acylated with isobutyryl chloride (IV) and triethylamine in dichloromethane yielding the corresponding amide (V). Saponification of the ester (V) with NaOH in methanol/water affords the free acid (VI), which is cyclized with N,3-diphenylpropynamide (VII) [obtained in the reaction of 3-phenylpropynoic acid (VIII) with aniline (IX) by means of dicyclohexylcarbodiimide (DCC)] by heating at 90 癈in acetic anhydride giving1-[2-(1,3-dioxolan-2-yl)ethyl]-5-(4-fluorophenyl)-2-isopropyl-N,4 -diphenylpyrrole-3-carboxamide (X). The hydrolysis of the dioxolane group of (X) with HCl yields the corresponding aldehyde (XI), which is condensed with methyl acetoacetate (XII) by means of NaH in THF affording 7-[2-(4-fluorophenyl)-5-isopropyl-3-phenyl-4-(N-phenylcarbamoyl)pyrrol -1-yl]-5-hydroxy-3-oxoheptanoic acid methyl ester (XIII). The reduction of the carbonyl group of (XIII) with tributylborane and NaBH4 in THF gives the (3R*,5R*)-dihydroxy ester (XIV), which is saponified with NaOH in water yielding the corresponding free acid (XV). The lactonization of (XV) by heating in refluxing toluene affords the (R*,R*)-lactone (XVI) (1, 2), which is submitted to optical resolution by reaction with (R)-1-phenylethylamine (XVII) followed by fractional crystallization thus obtaining the amide (XVII) as the pure (R,R,R)-enantiomer. The hydrolysis of the amide (XVIII) with NaOH, followed by heating in refluxing toluene gives the (R,R)-lactone (XIX) (2, 3), which is finally treated first with NaOH inmethanol/water, and then with CaCl2 or calcium acetate (3, 4). 2) The condensation of the already described aldehyde (XI) with(S)-(+)-2-acetoxy-1,1,2-triphenylethanol (XX) by means of lithium diisopropylamide (LDA) in THF gives5-[2-(4-fluorophenyl)-5-isopropyl-3-phenyl-4-(N-phenylcarbamoyl)pyrrol -1-yl]-3(R)-hydroxypentanoic acid2-hydroxy-1(S),2,2-triphenylethyl ester (XXI), which is trans-esterified with sodium methoxide in methanol/THF yielding the expected methyl ester (XXII). The condensation of (XXII) with tert-butyl acetate (XXIII) by means of LDA in THF affords(R)-7-[2-(4-fluorophenyl)-5-isopropyl-3-phenyl-4-(N-phenylcarbamoyl) pyrrol-1-yl]-5-hydroxy-3-oxoheptanoic acid tert-butyl ester (XXIV), which is reduced with triethylborane and NaBH4 in THF, hydrolyzed with NaOH, lactonized by heating in refluxing toluene and finally submitted to fractional crystallization in order to separate the two diastereomers of the obtained lactone, (R,R) and (R,S) (2, 3). The(R,R)-diastereomer (XIX), already obtained, is finally treated with NaOH and then with CaCl2 (2-4). 3) The condensation of4-cyano-3(R)-hydroxybutyric acid ethyl ester (XXV) with N,N-diphenylacetamide (R1 = R2 = Ph in XXVI) by means of LDA in THF gives 6-cyano-5(R)-hydroxy-3-oxo-N,N-diphenylhexanamide (XXVII), which is reduced with diethylmethoxyborane and NaBH4 in THF yielding 6-cyano-3(R),5(R)-dihydroxy-N,N-diphenylhexanamide (XXVIII). The protection of the two OH groups of (XXVIII) with acetone dimethylketal (XXIX) and methanesulfonic acid affords the 1,3-dioxane (XXX), which by reduction of its CN group by hydrogenation with H2 over RaNi in methanol/liquid ammonia gives (4R,6R)-2-[6-(2-aminoethyl)-2,2-dimethyl-1,3-dioxan-4-yl]-N,N -diphenylacetamide (XXXI). The cyclization of (XXXI) with 4-(4-fluorophenyl)-2-isobutyryl-4-oxo-N-phenylbutyramide (XXXII) (its synthesis is in section 6, Scheme 4) in refluxing toluene yields the protected dihydroxyheptanamide (XXXIII), which is deprotected with HCl in methanol to afford (3R,5R)-7-[2-(4-fluorophenyl)-5-isopropyl-3-phenyl-4-(N -phenylcarbamoyl)pyrrol-1-yl]-3,5-dihydroxy-N,N-diphenylheptanamide (XXXIV). Finally, this compound is hydrolyzed with NaOH and treated with calcium acetate in water (5). Scheme 18007203a. 4) The preceding reaction pathway can be repeated using other substituents for R1 and R2 in acetamide (XXVI) such as R1 = R2 = CH2Ph; R1 = R2 = Et; R1 = Bu, R2 = Me; R1 = t-Bu, R2 = CH2Ph; R1,R2 = -(CH2)5- (5). 5) The hydrolysis of methyl (Et or Bu)3(R)-(tert-butyldimethylsilyloxy)-4-cyanobutyrate (XXV) with NaOH gives the corresponding free acid (XXXVI), which is condensed with malonic acid mono-tert-butyl ester magnesium salt (XXXVII) by means of carbonyldiimidazole (CDI) yielding tert-butyl5(R)-(tert-butyldimethylsilyloxy)-6-cyano-3-oxohexanoate (XXXVIII). The desilylation of (XXXVIII) with tetrabutylammonium fluoride in acetic acid affords the expected hydroxylated ketoester (XXXIX), which is reduced with diethylmethoxyborane and NaBH4 in methanol giving tert-butyl 6-cyano-3(R),5(R)-dihydroxyhexanoate (XL). The protection of the two OH groups of (XL) with acetone dimethylketal(XXIX) and methanesulfonic acid affords the 1,3-dioxane (XLI) (6), which by reduction of its CN group by hydrogenation with H2 overPd/C gives intermediate (4R,6R)-2-[6-(2-aminoethyl)-2,2-dimethyl-1,3-dioxan-4-yl]acetic acid tert-butyl ester (XLII). The cyclization of (XLII) with 4-(4-fluorophenyl-2-isobutyryl-4-oxo-N-phenylbutyramide (XXXII) in refluxing toluene yields the protected dihydroxyheptanoate (XLIII), which is deprotected with HCl in methanol and finally hydrolyzed with NaOH and treated with calcium acetate in water (7). 8) The synthesis of the 4-(4-fluorophenyl)-2-isobutyryl-4-oxo-N-phenylbutyramide (XXXII) is carried out as follows: The condensation of 4-methyl-3-oxo-N-phenylpentanamide (XLIV) with benzaldehyde (XLV) gives2-benzylidene-4-methyl-3-oxo-N-phenylpentanamide (XLVI), which is then condensed with 4-fluorobenzaldehyde (XLVII) by means of triethylamine in hot ethanol (7). 6) The (4R,6R)-2-[6-(cyanomethyl)-2,2-dimethyl-1,3-dioxan-4-yl]acetic acid tert-butyl ester (XLI) can also be obtained by reaction of (4R,6R)-2-[6-(2-hydroxyethyl)-2,2-dimethyl-1,3-dioxan-4-yl]acetic acid tert-butyl ester (XLVIII) with tosyl chloride to give the corresponding tosylate (XVIX), which is then treated with NaCN (6). 7) The tert-butyl6-cyano-5(R)-hydroxy-3-oxohexanoate (XXXIX) can also be obtained by condensation of methyl 4-cyano-3(R)-hydroxybutyrate (L) with tert-butyl acetate (XXIII) by means of LDA in THF (6). 8) The synthesis of the 4-(4-fluorophenyl)-2-isobutyryl-4-oxo-N-phenylbutyramide (XXXII) is carried out as follows: The condensation of 4-methyl-3-oxo-N-phenylpentanamide (XLIV) with benzaldehyde (XLV) gives2-benzylidene-4-methyl-3-oxo-N-phenylpentanamide (XLVI), which is then condensed with 4-fluorobenzaldehyde (XLVII) by means of triethylamine in hot ethanol (7). 9) The cyclization of (XXXII) with intermediate (XLII) (preceding synthesis) in refluxing toluene yields the protected dehydroxyheptanoate (XLIII), which is deprotected with HCl in methanol and finally hydrolyzed with NaOH and treated with calcium acetate in water. References 1. Roth, B.D. (Warner-Lambert Co.). Trans-6-[2-(3- or 4-carboxamido-substd.pyrrol-1-yl)alkyl]-4-hydroxypyran-2-one inhibitors of cholesterol synthesis. EP 247633, US 4681893. 2. Roth, B.D., Blankley, C.J., Chucholowski, A.W., Ferguson, E., Hoefle, M.L., Ortwine, D.F., Newton, R.S., Sekerke, C.S., Sliskovic, D.R., Stratton, C.D., Wilson, M.W. Inhibitors of cholesterol biosynthesis. 3. Tetrahydro-4-hydroxy-6-[2-(1H-pyrrol-1-yl)ethyl]-2H-pyran-2-one inhibitors of HMG-CoA reductase. 2. Effects of introducing substituents at positions three and four of the pyrrole nucleus. J Med Chem 1991, 34: 357-66. 3. Roth, B.D. (Warner-Lambert Co.). (R-(R*R*)-2-(4-Fluorophenyl)-beta,delta-dihydroxy-5-(1-methylethyl-3-phenyl-4-[(phenylamino)-carbonyl]-1H-pyrrole-1-heptanoic acid, its lactone form and salts thereof. EP 409281, JP 91058967, US 5273995.4. Milb, N., Muhammad, N.A., Weiss, J., Nesbitt, R.U. (Warner-Lambert Co.). Stable oral CI-981 formulation and process for preparing same. EP 680320, JP 96505640, WO 9416693.5. Butler, D.E., Le, T.V., Nanninga, T.N. (Warner-Lambert Co.). Process fortrans-6-[2-(substd.-pyrrol-1-yl)alkyl]pyran-2-one inhibitors of cholesterol synthesis. US 5298627. 6. Brower, P.L., Butler, D.E., Deering, C.F., Le, T.V., Millar, A., Nanninga, T.N., Roth, B.D. The synthesis of (4R-cis)-1,1-dimethylethyl6-cyanomethyl-2,2-dimethyl-1,3-dioxane-4-acetate, a key intermediate for the preparation of CI-981, a highly potent, tissue selective inhibitor of HMG-CoA reductase. Tetrahedron Lett 1992, 33: 2279-82. 7. Baumann, K.L., Butler, D.E., Deering, C.F., Mennen, K.E., Millar, A., Nanninga, T.N., Palmer, C.W., Roth, B.D. The convergent synthesis of CI-981, an optically active, highly potent, tissue selective inhibitor of HMG-CoA reductase. Tetrahedron Lett 1992, 33: 2283-4. 8. McKenzie, A.T. (Warner-Lambert Co.). Form III crystalline(R-(R*,R*))-2-(4-fluorophenyl)-beta,delta-dihydroxy-5-(1-methyl-ethyl) -3-phenyl-4-((phenylamino)carbonyl)-1H-pyrrole-1-heptanoic acid hemi calcium salt (atorvastatin). WO 9703958. 9. Lin, M., Schweiss, D. (Warner-Lambert Co.). Novel process for the production of amorphous [R-(R*,R*)]-2-(4-fluorophenyl)-beta,delta-dihydroxy-5-(1-methylethyl)-3-phenyl-4-[(phenylamino)carbonyl]-1H-pyrrole-1-heptanoic acid calcium salt (2:1). WO 9703960. 10. Briggs, C.A., Jennings, R.A., Wade, R.A., Harasawa, K., Ichikawa, S., Minohara, K., Nakagawa, S. (Warner-Lambert Co.). Crystalline[R-(R*,R*)]-2-(4-fluorophenyl)-beta,delta-dihydroxy-5-(1-methylethyl) -3-phenyl-4-[(phenylamino)carbonyl]-1H-pyrrole-1-heptanoic acid hemi calcium salt (atorvastatin). WO 9703959.〖来源〗J Med Chem〖合成路线〗〖标题〗Inhibitors of cholesterol biosynthesis. 3. Tetrahydro-4-hydroxy-6-[2-(1H-pyrrol-1-yl)ethyl]-2H-pyran-2-one inhibitors of HMG-CoA reductase. 2. Effects of introducing substituents at positions three and four of the pyrrole nucleus〖合成方法〗1) The condensation of 2-(1,3-dixolan-2-yl)ethylamine (I) with ethyl 2-bromo-2-(4-fluorophenyl)acetate (II) by means of triethylamine in acetonitrile gives ethyl 2-[2-(1,3-dioxolan-2-yl)ethylamino]-2-(4-fluorophenyl)acetate (III), which is acylated with isobutyryl chloride (IV) and triethylamine in dichloromethane yielding the corresponding amide (V). Saponification of the ester (V) with NaOH in methanol/water affords the free acid (VI), which is cyclized with N,3-diphenylpropynamide (VII) [obtained in the reaction of 3-phenylpropynoic acid (VIII) with aniline (IX) by means of dicyclohexylcarbodiimide (DCC)] by heating at 90 C in acetic anhydride giving1-[2-(1,3-dioxolan-2-yl)ethyl]-5-(4-fluorophenyl)-2-isopropyl-N,4-diphenylpyrrole-3-carboxamide (X). The hydrolysis of the dioxolane group of (X) with HCl yields the corresponding aldehyde (XI), which is condensed with methyl acetoacetate (XII) by means of NaH in THF affording 7-[2-(4-fluorophenyl)-5-isopropyl-3-phenyl-4-(N-phenylcarbamoyl)pyrrol-1-yl]-5-hydroxy-3-oxoheptanoic acid methyl ester (XIII). The reduction of the carbonyl group of (XIII) with tributylborane and NaBH4 in THF gives the (3R*,5R*)-dihydroxy ester (XIV), which is saponified with NaOH in water yielding the corresponding free acid (XV). The lactonization of (XV) by heating in refluxing toluene affords the (R*,R*)-lactone (XVI), which is submitted to optical resolution by reaction with (R)-1-phenylethylamine (XVII) followed by fractional crystallization thus obtaining the amide (XVII) as the pure (R,R,R)-enantiomer. The hydrolysis of the amide (XVIII) with NaOH, followed by heating in refluxing toluene gives the (R,R)-lactone (XIX), which is finally treated first with NaOH in methanol/water, and then with CaCl2 or calcium acetate.〖作者〗Roth, B.D.; Blankley, C.J.; Chucholowski, A.W.; Ferguson, E.; Hoefle, M.L.; Ortwine, D.F.; Newton, R.S.; Sekerke, C.S.; Sliskovic, D.R.; Stratton, C.D.; Wilson, M.W.〖参考〗Roth, B.D.; Blankley, C.J.; Chucholowski, A.W.; Ferguson, E.; Hoefle, M.L.; Ortwine, D.F.; Newton, R.S.; Sekerke, C.S.; Sliskovic, D.R.; Stratton, C.D.; Wilson, M.W.; Inhibitors of cholesterol biosynthesis. 3. Tetrahydro-4-hydroxy-6-[2-(1H-pyrrol-1-yl)eth IĶ 셈睋서睋쓰睎ℐ ul> li>a href=쓜睎 Ʈ 0 ๙ιᇐ ꀀÑ밸-00AA004CLSID\{0E59F1D5-1FBE-11D0-8FF2-00A0D10038BC}e Ǔ〖出处〗J Med Chem1991,34,(1):357-66〖备注〗〖来源〗Drugs Fut〖合成路线〗〖标题〗Atorvastatin Calcium〖合成方法〗2) The condensation of the previously described aldehyde (XI) with (S)-(+)-2-acetoxy-1,1,2-triphenylethanol (XX) by means of lithium diisopropylamide (LDA) in THF gives5-[2-(4-fluorophenyl)-5-isopropyl-3-phenyl-4-(N-phenylcarbamoyl)pyrrol-1-yl]-3(R)-hydroxypentanoic acid2-hydroxy-1(S),2,2-triphenylethyl ester (XXI), which is trans-esterified with sodium methoxide in methanol/THF yielding the expected methyl ester (XXII). The condensation of (XXII) with tert-butyl acetate (XXIII) by means of LDA in THF affords(R)-7-[2-(4-fluorophenyl)-5-isopropyl-3-phenyl-4-(N-phenylcarbamoyl) pyrrol-1-yl]-5-hydroxy-3-oxoheptanoic acid tert-butyl ester (XXIV), which is reduced with triethylborane and NaBH4 in THF, hydrolyzed with NaOH, lactonized by heating in refluxing toluene and finally submitted to fractional crystallization in order to separate the two diastereomers of the obtained lactone, (R,R) and (R,S). The(R,R)-diastereomer (XIX), already obtained, is finally treated with NaOH and then with CaCl2.〖作者〗Graul, A.; Casta馿r, J.〖参考〗Graul, A.; Casta馿r, J.; Atorvastatin Calcium. Drugs Fut 1997, 22, 9, 956〖出处〗Drugs Fut1997,22,(9):956〖备注〗Synthesis Atorvastatin calcium has been obtained by several different ways: 1) The condensation of 2-(1,3-dixolan-2-yl)ethylamine (I) with ethyl 2-bromo-2-(4-fluorophenyl)acetate (II) by means of triethylamine in acetonitrile gives ethyl2-[2-(1,3-dioxolan-2-yl)ethylamino]-2-(4-fluorophenyl)acetate (III), which is acylated with isobutyryl chloride (IV) and triethylamine in dichloromethane yielding the corresponding amide (V). Saponification of the ester (V) with NaOH in methanol/water affords the free acid (VI), which is cyclized with N,3-diphenylpropynamide (VII) [obtained in the reaction of 3-phenylpropynoic acid (VIII) with aniline (IX) by means of dicyclohexylcarbodiimide (DCC)] by heating at 90 癈in acetic anhydride giving1-[2-(1,3-dioxolan-2-yl)ethyl]-5-(4-fluorophenyl)-2-isopropyl-N,4 -diphenylpyrrole-3-carboxamide (X). The hydrolysis of the dioxolanegroup of (X) with HCl yields the corresponding aldehyde (XI), which is condensed with methyl acetoacetate (XII) by means of NaH in THF affording 7-[2-(4-fluorophenyl)-5-isopropyl-3-phenyl-4-(N-phenylcarbamoyl)pyrrol -1-yl]-5-hydroxy-3-oxoheptanoic acid methyl ester (XIII). The reduction of the carbonyl group of (XIII) with tributylborane and NaBH4 in THF gives the (3R*,5R*)-dihydroxy ester (XIV), which is saponified with NaOH in water yielding the corresponding free acid (XV). The lactonization of (XV) by heating in refluxing toluene affords the (R*,R*)-lactone (XVI) (1, 2), which is submitted to optical resolution by reaction with (R)-1-phenylethylamine (XVII) followed by fractional crystallization thus obtaining the amide (XVII) as the pure (R,R,R)-enantiomer. The hydrolysis of the amide (XVIII) with NaOH, followed by heating in refluxing toluene gives the (R,R)-lactone (XIX) (2, 3), which is finally treated first with NaOH inmethanol/water, and then with CaCl2 or calcium acetate (3, 4). 2) The condensation of the already described aldehyde (XI) with(S)-(+)-2-acetoxy-1,1,2-triphenylethanol (XX) by means of lithium diisopropylamide (LDA) in THF gives5-[2-(4-fluorophenyl)-5-isopropyl-3-phenyl-4-(N-phenylcarbamoyl)pyrrol -1-yl]-3(R)-hydroxypentanoic acid2-hydroxy-1(S),2,2-triphenylethyl ester (XXI), which is trans-esterified with sodium methoxide in methanol/THF yielding the expected methyl ester (XXII). The condensation of (XXII) with tert-butyl acetate (XXIII) by means of LDA in THF affords(R)-7-[2-(4-fluorophenyl)-5-isopropyl-3-phenyl-4-(N-phenylcarbamoyl) pyrrol-1-yl]-5-hydroxy-3-oxoheptanoic acid tert-butyl ester (XXIV), which is reduced with triethylborane and NaBH4 in THF, hydrolyzed with NaOH, lactonized by heating in refluxing toluene and finally submitted to fractional crystallization in order to separate the two diastereomers of the obtained lactone, (R,R) and (R,S) (2, 3). The(R,R)-diastereomer (XIX), already obtained, is finally treated with NaOH and then with CaCl2 (2-4). 3) The condensation of4-cyano-3(R)-hydroxybutyric acid ethyl ester (XXV) with N,N-diphenylacetamide (R1 = R2 = Ph in XXVI) by means of LDA in THF gives 6-cyano-5(R)-hydroxy-3-oxo-N,N-diphenylhexanamide (XXVII), which is reduced with diethylmethoxyborane and NaBH4 in THF yielding 6-cyano-3(R),5(R)-dihydroxy-N,N-diphenylhexanamide (XXVIII). The protection of the two OH groups of (XXVIII) with acetone dimethylketal (XXIX) and methanesulfonic acid affords the 1,3-dioxane (XXX), which by reduction of its CN group by hydrogenation with H2 over RaNi in methanol/liquid ammonia gives (4R,6R)-2-[6-(2-aminoethyl)-2,2-dimethyl-1,3-dioxan-4-yl]-N,N -diphenylacetamide (XXXI). The cyclization of (XXXI) with 4-(4-fluorophenyl)-2-isobutyryl-4-oxo-N-phenylbutyramide (XXXII) (its synthesis is in section 6, Scheme 4) in refluxing toluene yields the protected dihydroxyheptanamide (XXXIII), which is deprotected with HCl in methanol to afford(3R,5R)-7-[2-(4-fluorophenyl)-5-isopropyl-3-phenyl-4-(N -phenylcarbamoyl)pyrrol-1-yl]-3,5-dihydroxy-N,N-diphenylheptanamide (XXXIV). Finally, this compound is hydrolyzed with NaOH and treated with calcium acetate in water (5). Scheme 18007203a. 4) The preceding reaction pathway can be repeated using other substituents for R1 and R2 in acetamide (XXVI) such as R1 = R2 = CH2Ph; R1 = R2 = Et; R1 = Bu, R2 = Me; R1 = t-Bu, R2 = CH2Ph; R1,R2 = -(CH2)5- (5). 5) The hydrolysis of methyl (Et or Bu)3(R)-(tert-butyldimethylsilyloxy)-4-cyanobutyrate (XXV) with NaOH gives the corresponding free acid (XXXVI), which is condensed with malonic acid mono-tert-butyl ester magnesium salt (XXXVII) by means of carbonyldiimidazole (CDI) yielding tert-butyl5(R)-(tert-butyldimethylsilyloxy)-6-cyano-3-oxohexanoate (XXXVIII). The desilylation of (XXXVIII) with tetrabutylammonium fluoride in acetic acid affords the expected hydroxylated ketoester (XXXIX), which is reduced with diethylmethoxyborane and NaBH4 in methanol giving tert-butyl 6-cyano-3(R),5(R)-dihydroxyhexanoate (XL). The protection of the two OH groups of (XL) with acetone dimethylketal (XXIX) and methanesulfonic acid affords the 1,3-dioxane (XLI) (6), which by reduction of its CN group by hydrogenation with H2 overPd/C gives intermediate (4R,6R)-2-[6-(2-aminoethyl)-2,2-dimethyl-1,3-dioxan-4-yl]acetic acid tert-butyl ester (XLII). The cyclization of (XLII) with 4-(4-fluorophenyl-2-isobutyryl-4-oxo-N-phenylbutyramide (XXXII) in refluxing toluene yields the protected dihydroxyheptanoate (XLIII), which is deprotected with HCl in methanol and finally hydrolyzed with NaOH and treated with calcium acetate in water (7). 8) The synthesis of the 4-(4-fluorophenyl)-2-isobutyryl-4-oxo-N-phenylbutyramide (XXXII) is carried out as follows: The condensation of 4-methyl-3-oxo-N-phenylpentanamide (XLIV) with benzaldehyde (XLV) gives2-benzylidene-4-methyl-3-oxo-N-phenylpentanamide (XLVI), which is then condensed with 4-fluorobenzaldehyde (XLVII) by means of triethylamine in hot ethanol (7). 6) The (4R,6R)-2-[6-(cyanomethyl)-2,2-dimethyl-1,3-dioxan-4-yl]acetic acid tert-butyl ester (XLI) can also be obtained by reaction of (4R,6R)-2-[6-(2-hydroxyethyl)-2,2-dimethyl-1,3-dioxan-4-yl]acetic acid tert-butyl ester (XLVIII) with tosyl chloride to give the corresponding tosylate (XVIX), which is then treated with NaCN (6). 7) The tert-butyl6-cyano-5(R)-hydroxy-3-oxohexanoate (XXXIX) can also be obtained by condensation of methyl 4-cyano-3(R)-hydroxybutyrate (L) with tert-butyl acetate (XXIII) by means of LDA in THF (6). 8) The synthesis of the 4-(4-fluorophenyl)-2-isobutyryl-4-oxo-N-phenylbutyramide (XXXII) is carried out as follows: The condensation of 4-methyl-3-oxo-N-phenylpentanamide (XLIV) with benzaldehyde (XLV) gives2-benzylidene-4-methyl-3-oxo-N-phenylpentanamide (XLVI), which is then condensed with 4-fluorobenzaldehyde (XLVII) by means oftriethylamine in hot ethanol (7). 9) The cyclization of (XXXII) with intermediate (XLII) (preceding synthesis) in refluxing toluene yields the protected dehydroxyheptanoate (XLIII), which is deprotected with HCl in methanol and finally hydrolyzed with NaOH and treated with calcium acetate in water. References 1. Roth, B.D. (Warner-Lambert Co.). Trans-6-[2-(3- or 4-carboxamido-substd.pyrrol-1-yl)alkyl]-4-hydroxypyran-2-one inhibitors of cholesterol synthesis. EP 247633, US 4681893. 2. Roth, B.D., Blankley, C.J., Chucholowski, A.W., Ferguson, E., Hoefle, M.L., Ortwine, D.F., Newton, R.S., Sekerke, C.S., Sliskovic, D.R., Stratton, C.D., Wilson, M.W. Inhibitors of cholesterol biosynthesis. 3. Tetrahydro-4-hydroxy-6-[2-(1H-pyrrol-1-yl)ethyl]-2H-pyran-2-one inhibitors of HMG-CoA reductase. 2. Effects of introducing substituents at positions three and four of the pyrrole nucleus. J Med Chem 1991, 34: 357-66. 3. Roth, B.D. (Warner-Lambert Co.). (R-(R*R*)-2-(4-Fluorophenyl)-beta,delta-dihydroxy-5-(1-methylethyl-3-phenyl-4-[(phenylamino)-carbonyl]-1H-pyrrole-1-heptanoic acid, its lactone form and salts thereof. EP 409281, JP 91058967, US 5273995.4. Milb, N., Muhammad, N.A., Weiss, J., Nesbitt, R.U. (Warner-Lambert Co.). Stable oral CI-981 formulation and process for preparing same. EP 680320, JP 96505640, WO 9416693.5. Butler, D.E., Le, T.V., Nanninga, T.N. (Warner-Lambert Co.). Process fortrans-6-[2-(substd.-pyrrol-1-yl)alkyl]pyran-2-one inhibitors of cholesterol synthesis. US 5298627. 6. Brower, P.L., Butler, D.E., Deering, C.F., Le, T.V., Millar, A., Nanninga, T.N., Roth, B.D. The synthesis of (4R-cis)-1,1-dimethylethyl6-cyanomethyl-2,2-dimethyl-1,3-dioxane-4-acetate, a key intermediate for the preparation of CI-981, a highly potent, tissue selective inhibitor of HMG-CoA reductase. Tetrahedron Lett 1992, 33: 2279-82. 7. Baumann, K.L., Butler, D.E., Deering, C.F., Mennen, K.E., Millar, A., Nanninga, T.N., Palmer, C.W., Roth, B.D. The convergent synthesis of CI-981, an optically active, highly potent, tissue selective inhibitor of HMG-CoA reductase. Tetrahedron Lett 1992, 33: 2283-4. 8. McKenzie, A.T. (Warner-Lambert Co.). Form III crystalline(R-(R*,R*))-2-(4-fluorophenyl)-beta,delta-dihydroxy-5-(1-methyl-ethyl) -3-phenyl-4-((phenylamino)carbonyl)-1H-pyrrole-1-heptanoic acid hemi calcium salt (atorvastatin). WO 9703958. 9. Lin, M., Schweiss, D. (Warner-Lambert Co.). Novel process for the production of amorphous [R-(R*,R*)]-2-(4-fluorophenyl)-beta,delta-dihydroxy-5-(1-methylethyl)-3-phenyl-4-[(phenylamino)carbonyl]-1H-pyrrole-1-heptanoic acid calcium salt (2:1). WO 9703960. 10. Briggs, C.A., Jennings, R.A., Wade, R.A., Harasawa, K., Ichikawa, S., Minohara, K., Nakagawa, S. (Warner-Lambert Co.). Crystalline[R-(R*,R*)]-2-(4-fluorophenyl)-beta,delta-dihydroxy-5-(1-methylethyl) -3-phenyl-4-[(phenylamino)carbonyl]-1H-pyrrole-1-heptanoic acid hemi calcium salt (atorvastatin). WO 9703959.〖来源〗J Med Chem〖合成路线〗〖标题〗Inhibitors of cholesterol biosynthesis. 3. Tetrahydro-4-hydroxy-6-[2-(1H-pyrrol-1-yl)ethyl]-2H-pyran-2-one inhibitors of HMG-CoA reductase. 2. Effects of introducing substituents at positions three and four of the pyrrole nucleus〖合成方法〗2) The condensation of the previously described aldehyde (XI) with (S)-(+)-2-acetoxy-1,1,2-triphenylethanol (XX) by means of lithium diisopropylamide (LDA) in THF gives5-[2-(4-fluorophenyl)-5-isopropyl-3-phenyl-4-(N-phenylcarbamoyl)pyrrol-1-yl]-3(R)-hydroxypentanoic acid2-hydroxy-1(S),2,2-triphenylethyl ester (XXI), which is trans-esterified with sodium methoxide in methanol/THF yielding the expected methyl ester (XXII). The condensation of (XXII) with tert-butyl acetate (XXIII) by means of LDA in THF affords(R)-7-[2-(4-fluorophenyl)-5-isopropyl-3-phenyl-4-(N-phenylcarbamoyl) pyrrol-1-yl]-5-hydroxy-3-oxoheptanoic acid tert-butyl ester (XXIV), which is reduced with triethylborane and NaBH4 in THF, hydrolyzed with NaOH, lactonized by heating in refluxing toluene and finally submitted to fractional crystallization in order to separate the two diastereomers of the obtained lactone, (R,R) and (R,S). The(R,R)-diastereomer (XIX), already obtained, is finally treated with NaOH and then with CaCl2.〖作者〗Roth, B.D.; Blankley, C.J.; Chucholowski, A.W.; Ferguson, E.; Hoefle, M.L.; Ortwine, D.F.; Newton, R.S.; Sekerke, C.S.; Sliskovic, D.R.; Stratton, C.D.; Wilson, M.W.〖参考〗Roth, B.D.; Blankley, C.J.; Chucholowski, A.W.; Ferguson, E.; Hoefle, M.L.; Ortwine, D.F.; Newton, R.S.; Sekerke, C.S.; Sliskovic, D.R.; Stratton, C.D.; Wilson, M.W.; Inhibitors of cholesterol biosynthesis. 3. Tetrahydro-4-hydroxy-6-[2-(1H-pyrrol-1-yl)eth IĶ 셈睋서睋쓰睎쓜睎 Ʈ 0 ๙ιᇐ ꀀÑ밸-00AA004CLSID\{0E59F1D5-1FBE-11D0-8FF2-00A0D10038BC}e Ǔ ℐ ul> li>a href=〖出处〗。
调血脂药阿托伐他汀钙的合成探究

调血脂药阿托伐他汀钙的合成探究摘要】综合国内外关于阿托伐他汀钙的报道和文献,通过大量的实验研究,对阿托伐他汀钙的合成技术进行优化,探讨适合工业化生产阿托伐他汀钙的新工艺技术,在进行阿托伐他汀钙的合成过程中,考察水解反应时间、水解反应温度及溶剂对阿托伐他汀钙收率的影响,得出阿托伐他汀钙合成技术的优缺点,为大规模、工业化生产阿托伐他汀钙提供依据。
【关键词】阿托伐他汀钙;调血脂药;合成【中图分类号】R97 【文献标识码】A 【文章编号】1007-8231(2015)11-0218-02阿托伐他汀钙是临床上常见的用于治疗高胆固醇血症等胆固醇升高患者,为临床安全有效的降脂药物。
其是还原酶的选择性及竞争性抑制剂,主要通过抑制肝脏内HMG-CoA还原酶及胆固醇的合成从而起到降低脂蛋白及胆固醇水平的作用[1]。
该药是美国Warner-Lambert公司的Paker-Dvais药厂和Pfizer公司共同研制出来的,是第三代他汀类的调脂药,在临床上被广泛的运用[2]。
本文详细论述了阿托伐他汀钙的合成方法。
1.阿托伐他汀钙的合成1.1 线性合成方法仪器:选用核磁共振波谱仪、元素分析仪器、高效液相色谱仪,红外分光光度仪。
1.1.1中间体合成在圆底烧瓶中加入(4R,6R)一6氨乙基一2,2一二甲基一1,3一二氧己环一4一乙酸叔丁酯,氟一d一[2一甲基一1一氧丙基]一7一氧代一N,13一二苯基苯丁混合,加入甲苯200ml及对甲苯磺酸,对其进行加热,回流,采用分流器将反应生成的水分去除,6小时后,进行降温,随后进行减压蒸发掉甲苯,加入MTBE100ml分散,将固体过滤。
1.1.2阿托伐他汀钙的合成方法在圆底烧瓶中加入上述中间体合成的产物,并加入40毫升甲醇、200ml氢氧化钠的水溶液,加热,待水解后溶液变清,进行降温,采用叔丁基甲基醚80毫升重复洗涤;洗涤完成后,将水溶液重新加热,搅拌均匀,加入水溶液醋酸钙搅拌2小时后,冷却至20摄氏度,过滤,将产物水洗,进行减压干燥,直至白色固体出现。
阿托伐他汀侧链结构式

阿托伐他汀侧链结构式
阿托伐他汀(Atorvastatin)是一种降脂药物,属于他汀类药物,用于治疗高胆固醇血症和预防心血管疾病。
其侧链结构是该分子中与羟基苯甲酸酯基团相连的部分,对于药物的吸收、分布、代谢和排泄具有重要作用。
阿托伐他汀的侧链结构包括一个2-[4-氟苯基]-β,δ-二羟基戊酸酯基团。
这个侧链通过酯键与阿托伐他汀分子的主体部分相连。
具体的化学结构如下:
OH
│
HOOC-CH=CH-CH2-CH2-O-CO-R
│
OCH2CH2NHCO
│
R'-COOH
其中,R代表阿托伐他汀分子的主体部分,包含一个内酯环和一个噻唑烷酮环;R'则是指与酯基相连的烃基,在阿托伐他汀中,R'为一个氢原子。
请注意,这个侧链结构式是一个简化的表示,实际的分子结构可能更为复杂,并且需要考虑立体化学因素。
在药物设计和合成中,侧链的精确结构对药物的活性、稳定性和生物利用度都至关重要。
阿托伐他汀钙的合成工艺

摘 要 :对阿托伐他汀钙合成工 艺进行分析 。通过 大量 实验 ,全 面优化 阿托伐他 汀钙的合成路线 ,选择与工业化生产需求 相符合 的新 工艺,构建 阿托伐他 汀钙 的生 产工艺。结果表 明 :利用元素分析、核磁共振氢谱 、红外光谱等分析手段 ,分析 阿托 伐他 汀钙 的结构表征 ,该合 成工艺具有有机 溶剂残 留量低、产品纯度 高、产率 高、操作 简单 、原料 易得 、反应条件温和 等基本 特征 ,与 药用标准相符合 ,适合 用于工业化生产 中。
明显 降 低 ,使 社 会 效 益 、 经 济 效 益 明 显 提 高 。本 文 对 阿 托 伐 收率为 70.00% ;水解反应 时 间为 i.2h时,收率为 81.00% ;水
他 汀 钙 的 合 成 工 艺进 行全 面 分 析 ,现 总 结 如 下 。
解 反 应 时 间 为 1.5h时 ,收 率 为 85.00%;水 解 反 应 时 间 为 1.8h时 ,
为 美 国 辉 瑞 公 司 ,可 用 于 高 胆 固 醇 血 症 的 临 床 治 疗 以及 预 防 行 2h搅 拌 , 并 给 予 抽 滤 ,在 70℃ 温 度 下 将 滤 饼 放 置 在 真 空 状
中 【1]。 阿 托 伐 他 汀 钙 主 要 是 采 用 对 血 脂 异 常 患 者 和 载 脂 蛋 白 态 下 30h,测 量产 品质 量 。
阿 托 伐 他 汀 钙 在 最 后 一 步 合 成 时 , 在 改 变 水 解 反 应 时 间
最近几 年, 国内外开始重视 研究 阿托 伐他 汀钙药物 的制作 工 的 情 况 下 , 维 持 其 他 条 件 不 变 ,可 以得 知 以下 数 据 :水 解 反
艺 ,通 过对其 合成工 艺进行优 化 ,促进 用药成 本、生产 成本 应 时 间 为 0.5h时 ,收 率 为 45.00% ;水 解 反 应 时 间 为 1.0h时 ,
阿托伐他汀说明书

商品名:立普妥英文名:Lipitor通用名:阿托伐他汀钙外文名:Lipitor,AtorvastatinCalcium汉语拼音:liputuo性质:立普妥是白色、椭圆、薄膜衣的阿托伐他汀钙盐片,是一种合成的选择性、竞争性HMG-CoA还原酶抑制剂(他汀类),其分子式为(C33H34FN2O5)2Ca·3H2O,分子量为1209.42。
片剂的一面凹刻“PD155”,另一面有“10”的字样。
药理毒理:立普妥为HMG-CoA还原酶选择性抑制剂,通过抑制HMG-CoA还原酶和胆固醇在肝脏的生物合成而降低血浆胆固醇和脂蛋白水平,并能通过增加肝细胞表面低密度脂蛋白(LDL)受体数目而增加LDL的摄取和分解代谢。
立普妥也能减少LDL的生成和其颗粒数。
立普妥还能降低某些纯合子型家族性高胆固醇血症(FH)的低密度脂蛋白胆固醇(LDL-C)水平,而一类型的人群对其他类型的降脂药物治疗很少有应答。
立普妥能降低纯合子和杂合子家族性高胆固醇血症、非家族性高胆固醇血症以及混合性脂类代谢障碍患者的血浆总胆固醇(TC)、LDL-C和载脂蛋白B(ApoB),还能降低极低密度脂蛋白胆固醇(VLDL-C)和三酰甘油(TG)的水平,并能不同程度地提高血浆高密度脂蛋白胆固醇(HDL-C)和载脂蛋白A1(ApoA1)的水平。
药代动力学:吸收:口服后迅速吸收,1-2小时内达到最大血浆浓度,吸收程度随口服剂量的增加而成正比例地增加。
绝对生物利用度约为12%,抑制HMG-CoA还原酶的全身利用度约为30%。
无论是否与食物同时服用或在一天中无论何时服用,其降低血浆LDL-C的效果都相似。
分布:平均分布容积是381升,其中98%以上与血浆蛋白结合。
代谢:阿托伐他汀在体内被代谢成为邻羟基化和对羟基化代谢产物,以及各种β-氧化产物。
其对循环HMG-CoA还原酶抑制活性大约70%源于活性代谢产物。
消除:阿托伐他汀及其代谢产物通过肝脏和/或肝外途径代谢后主要经胆汁排除。
调血脂药阿托伐他汀钙的合成研究进展

随着 对 高 脂 血 症 的 不 断 研 究 和综 合 治 疗 经 验 的 积 累 , 疗 高 治
几 得 到 关 键 中 间 体 2一( 3一[ 4一氟 苯 基 ) 一异 丙 基 一 苯 基 一 一5 3一
+
F
脂血症的药物品种及其使用情况也在不断地发生变化…。 羟基 一 3一
3一甲基 戊 二 酰 辅 酶 A H ( MG— o 还 原 酶 抑 制 剂 , 称 他 汀 类 C A) 简 (a n) 物 , s t s药 ti 由于 作 用 机 制 新 颖 , 用 范 围 较为 是 目前 最 为 经典 和有 效 的 调血 脂 药 - 。 耐 被 3 ]
a i s u t e i o te r g 1 er snh s ) T e oh ro e i t pe ae ci l3 5一 i —d y r y h pa o c rg e tte f m te c t c r n h i ( n a y tei . h te n s o rp r hr , es i do e t i ai f m n,h o h d r u t n i s a h x n c d a r
线提 供 参 考 。 关键 词 : 血 脂 药 : 调 阿托 伐 他 汀钙 : 成 合
中图 分 类号 : 7 6 T 6 . 1 R92 . ; Q40 3 文 献标 识 码 : A 文章 编 号 :0 6— 9 1 2 1 )1— 0 4 3 1 0 4 3 (0 0 2 0 0 —0
药学专论
21 年第 l 卷第 2 期 00 9 1
调血 脂 药 阿托伐 他 汀 钙 的合成 研 究 进展
张宜凡 虞心红 ,
(.上 海 医药高等 专科 学校 药学系 , 海 1 上 2 11 ; 2 0 3 8 .华东理 工 大学 药学院 , 上海 20 3 ) 0 1 7
阿托伐他汀钙中间体合成研究

阿托伐他汀钙中间体合成研究作者:刘艳玲乔向勇来源:《卷宗》2016年第06期摘要:本文以2-[2-(4-氟苯基)-2-氧代-1-苯基乙基]-4-甲基-3-氧代-N-苯基戊酰胺和(4R,6R)-6-氨乙基-2,2-二甲基-1,3-二氧六环-4-乙酸特戊酯通过Paal–Knorr反应合成出了阿托伐他汀钙中间体缩合物,分析得出了合成阿托伐他汀钙缩合物的最佳反应时间为28h,且收率高达85%。
这对阿托伐他汀钙工业化生产具有重要意义。
关键词:阿托伐他汀钙;Paal–Knorr反应;中间体;合成Abstract:The Intermediate of Atorvastatin Calcium was synthesized from 2-(2-(4-fluorophenyl)-2-oxo-1-phenylethyl)-4-methyl-3-oxo-N-phenylpentanamide and 1-((4R,6R)-6-(2-aminoethyl)-2,2-dimethyl-1,3-dioxan-4-yl)-3-(neopentyloxy)propan-2-one by Paal-Knorr reaction with yield of about 85%. Also, the best reaction condition is made certain: if the resulting mixtureis heated under reflux for 28h. The present method is advantageous for the large-scale synthesis.Key words:Atorvastatin Calcium, Paal-Knorr reaction, Intermediate, Synthesis7-[2-(4-氟苯基)-3-苯基-4-(苯胺基甲酰基)-5-(2-丙基)吡咯-1-基]-3,5-二羟基庚酸钙(阿托伐他汀钙)可治疗其总胆固醇升高,低密度脂蛋白胆固醇升高,载脂蛋白B升高和甘油三酯升高。
阿托伐他汀

主治:
阿托伐他汀的主要用途是治疗血脂异常 和预防心血管疾病。 • 血脂异常:高胆固醇血症、混合性高血 脂和高甘油三酯血症等 • 心血管疾病:预防二级冠状动脉心脏病 和多种危险因素,如心肌梗塞,中风,不 稳定心绞痛和血运重建。 •
禁忌:
•
1、活动性肝病:胆汁淤积,肝性脑 病,肝炎,黄疸 2、AST或ALT水平 3、原因不明的海拔 4、怀孕 5、母乳喂养
阿托伐他汀(Atorvastatin, INN、Lipitor/Pfizer)是治疗高 胆固醇血症和冠心病的常见药 物。由辉瑞公司装造,销售药 品名为胆固清或立普妥(Lipitor)。
它的结构式与立体结构式
药物作用机理 :•阿托伐他汀是HMG-CoA还原酶的竞争性抑制剂。 然而,不同于大多数人来说,它是一个完全人工 合成的化合物。 HMG-CoA还原酶催化还原3 - 羟 基-3 - 甲基 - 辅酶A(HMG-COA)甲羟戊酸,这 是在肝脏胆固醇合成的限速步骤。抑制酶降低从 头合成胆固醇,提高肝细胞表达的低密度脂蛋白 受体(LDL受体)。增加肝细胞低密度脂蛋白的吸 收,降低血液中的低密度脂蛋白胆固醇量。像其 他他汀类药物,阿托伐他汀也降低血甘油三酯水 平,并略有增加高密度脂蛋白胆固醇水平。在临 床试验中,阿托伐他汀阻止胆固醇的吸收。
后记:
阿托伐他汀于1985年由布鲁斯·罗斯-帕克-戴 维斯华纳-兰伯特公司首次合成(现为辉瑞公司 /Pfizer),是制药历史上销售最好的药物。它自 1996年被美国食品药品监督管理局批准以来,累 计销售额超过1250亿美元。并连续保持此销售冠 军纪录达十年。通用阿托伐他汀,由沃森制药公司 和兰伯西实验室制造,并于2011年11月30日开始 于美国上市。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
题目:阿托伐他汀中间体及其合成工艺研究姓名:髙晔学号:************指导老师:***时间:2014-06-14摘要:阿托伐他汀是一种羟甲戊二酰辅酶A还原酶抑制剂,通过抑制胆固醇生物合成的限速酶羟甲戊二酰辅酶A还原酶,从而起到抗高血脂症的作用。
大量临床研究表明,他汀类药物高效、安全,为调脂药物中的首选药物。
本文对近年来阿托伐他汀中间体,及阿托伐他汀合成工艺进行总结。
希望找到一种更好的合成阿托伐他汀的方法,应用于实践,帮助更多的患者。
关键词:阿托伐他汀中间体合成目录摘要 (I)第一章他汀类药物的研究背景及意义 (1)1.1他汀类药物简介 (1)1.2阿托伐他汀的简介 (2)第二章阿托伐他汀中间体的合成 (5)2.1 4-氟-α-(2-甲基-1-氧丙基)-γ-氧-N,β-二苯基苯丁酰胺的合成52.2异丁酰乙酸酯的合成 (5)2.3 4-甲基-3-氧-N-苯戊酰胺的合成 (6)2.3 4-甲基-3羰基-N-苯-2-(苯亚甲基)戊酰胺的合成 (6)2.4 4-氟-a-(2-甲基-卜氧丙基)-Y-氧-N,β-二苯基苯丁酰胺的合成6 第三章阿托伐他汀的合成路线 (7)3.1路线一:以2-(4-氟苯基)-2-溴-乙酸乙酯为起始原料 (7)3.2路线二;以异丁酰乙酰苯胺为起始原料 (8)3.3路线三 (8)3.4路线四:以阿托伐他汀醛为起始原料 (9)3.5路线五;以苯乙酸为起始原料 (10)3.6路线六:以L一缬氨酸为起始原料 (11)3.7路线七: (11)第四章总结 (13)参考文献 (14)第一章他汀类药物的研究背景及意义1.1他汀类药物简介心血管疾病(包括冠心病和动脉粥样硬化)是一类严重威胁人类健康的疾病。
近年来,随着人们生活水平的提高和工作节奏的加快,心血管疾病的发病率和死亡率都呈明显的上升态势。
研究表明,血浆胆固醇(CH)水平增高,特别是低密度脂蛋白胆固醇(LDL-CH)水平的增高,是早发性动脉粥样硬化和冠心病的重要因素。
抑制CH合成,特别是控制LDL-CH可有效防治动脉粥样硬化和冠心病。
胆固醇合成过程复杂,有近三十步酶促反应,可划分三个阶段:①由B-羟基-B-甲基戊二酰酶A(HMG-CoA)经羟甲基戊二酰酶A还原酶(HMG-CoAreductase)催化生成甲羟戊酸(mevflonicacid,MV A);②由甲羟戊酸经一系列反应生成鲨烯;③鲨烯进入微粒体环化为胆固醇。
理论上选择性抑制胆固醇合成中的某一部反应都可以达到控制胆固醇过高的目的。
然而,若抑制后面的步骤往往会导致甾醇中间体的积累而引起严重的副反应,这一发现使人们放弃了对抑制后续步骤的研究。
HMG-CoA还原酶作用于胆固醇合成的初始步骤且为重要的限速酶,寻找该酶的抑制剂用作调节高血脂症的有效药物成为近年来人们关注的热点。
他汀类药物,(3R,5S,6E)-3,5-二羟基-6-庚烯酸(酯或盐),就是一种HMG-CoA还原酶抑制剂,它可竞争性抑制胆固醇合成过程中的跟速酶HMG-CoA还原酶,而降低体内的内源性合成的胆固醇水平,选择性强,疗效确切,能显著降低LDL中胆固醇水平,并能提高HDL中胆固醇水平,使胆同醇形式从有害到无害。
这对冠心病的防治非常有益,是目前治疗高胆固醇血症中疗效良好的药物。
他汀类药物上市。
就表现出了强大的市场竞争力。
第一个上市的他汀类药物是美国默克公司(MerckCo.)研制开发的洛伐他汀(Lovastatin),于1987年在美国上市,该药上市后仅数年,销售额即突破10亿美元大关,90年代初期就已经成为世界十大畅销药物之一。
随后,疗效更好的辛伐他汀(Simvastatin)及普伐他汀钠(PavastatinSodium)也分别在1988年和1989年上市,获得了更大的成功。
90年代后,氟伐他汀(Fluvastatin),阿伐他汀(Atorvastatin)和赛伐他(Cerivastatin)又相继上市,90年代他汀类药物的年销售额以20%的年他汀类新药物的合成平均增长率增长。
1995年,他汀类药物的全球总售额为53亿美元,到1999年总销售额已超过了100亿美元。
在预测2005年全球最畅销的15种药物中,他汀类HMG-CoA还原酶抑制剂就占了三席,总金额超过200亿美元。
1.2阿托伐他汀的简介1.2.1阿托伐他汀的背景阿托伐他汀的化学名为( 3R, 5R ) -7-[ 2-( 4-氟苯基) -5-异丙基-3-苯基-4-(苯氨基甲酰基)吡咯-1-基] -3,5-二羟基庚酸钙盐,它是由美国Warner-Lambert公司和辉瑞( Pfizer)公司共同开发的他汀类血脂调节药, 1997年在英国率先上市。
由于可以大大降低总胆固醇和低密度脂蛋白的含量,具有高活性、低毒低副作用等优点。
活性优于在它之前的所有他汀类药物,且毒副作用小,因此一经上市就表现出不同凡响的上升势头, 2000年后一跃成为全球销售额最高的药物, 2004年全球销售收入高达120亿美元,成为全球首个销量额破百亿美元的药品,并连续七年全球销售总额过百亿。
其研究愈发的引起人们的关注,巨大的经济效益刺激着人们长期关注其合成方法的研究,有关其全合成的研究不断被报道。
1.2.2阿托伐他汀的物化性质分子式:C33H33CaFNO5分子量:582.6947熔点 176-178℃熔点:176-178℃结构式及立体结构式:结构式立体结构式1.2.3阿托伐他汀的作用机理是一种新型羟甲基戊二酶辅酶A(HMG—CoA)还原酶抑制剂,阻断HMG-CoA 还原成羟甲戊酸,它通过长时间持续竞争性抑制作用,降低血浆中胆固醇合成总量,从而抑制了VLDL的生成,降低甘油三酯水平;另外,阿托伐他汀可增加产生肝细胞表面LDL受体的mRNA表达,使LDL受体数量显著增加并使其活性增强,增加血浆LDL-胆固醇的清除,保持细胞内胆固醇内环境的稳定,同时也降低载脂蛋白的水平。
适用于杂合子家族性或非家族性高胆固醇血症和混合性高脂血症,也用于纯合子高胆固醇血症。
(1)抑制HMGu-CoA还原酶HMG—CoA还原酶是胆固醇合成酶系中的限速酶,通过对其的抑制作用,从而使胆固醇合成减少。
当前所使用的几种HMG-CoA丕原酶抑制剂都具有相同的作用机制。
然而,动物实验表明,这类药物并非完全一致胆固醇的合成,给药后仍有足够的胆固醇以完成生理功能,例如胆固醇的合成和细胞的生长,阿托伐他汀吸收后即有生物活性。
(2)增加低密度脂蛋白(LDL)受体阿托伐他汀通过对VLDL必需的。
阿托伐他汀通过使血浆胆固醇浓度降低,使VLDL合成分泌减少。
VLDL是携带和转用三酰甘油所必需的,VLDL-C又是LDL-C的前提,故阿托伐他汀可使三酰甘油、VLDL-C、LDL-C均降低。
(3)抗动脉粥样硬化作用阿托伐他汀通过降低血脂、减少脂质浸润和泡沫的形成,对延迟动脉粥样硬化病有利。
阿托伐他汀可防止动脉粥样硬化瓣破裂。
体外实验表明可抑制平滑肌增殖和转移。
1.2.4阿托伐他汀国内外研制情况1991年美国华纳-兰伯特公司发明了立普妥,于1997年首次上市,由辉瑞公司代理销售。
2001年辉瑞公司完成对华纳-兰伯特的收购,完全将立普妥归于帐下。
2000年,立普妥进入中国。
在国内,北京红惠制药有限公司与1999年9月获得了阿托伐他汀钙及片剂的新药证书和生产批件;2001年2月,广州南新制药有限公司、天然药物及仿生药物国家重点实验室、湖南迪诺制药有限公司也获得了阿托伐他汀钙及片剂的生产许可。
1.2.5国内外市场情况在国际上,阿托伐他汀品牌药全球销售一路飙升,2002年销售额创历史新高,也创下了重磅炸弹药全球销售的新记录,达到了79亿美元。
在国内,阿托伐他汀钙国内由红惠制药有限公司于1999年在辉瑞公司的利普妥获得行政保护前获得了生产批文。
2001年红惠公司开始向医院推广阿托伐他汀钙(商品名阿乐)。
2001年16大典型城市样本医院的购药金额为101.6万元,购药量为15.0万片,2002年购药金额增至771.4万元,增加了6.6倍,购药量为114.0万片,增加了6.6倍,可以说,无论是购药金额,还是购药量均呈快速递增态势。
2003年一季度购药金额为321.2万元,较2002年同期增长了260.5%;购药量为48.1万片,较2002年同期增长了265.0%。
根据ATPIII,由于高脂血症诊断标准的改变,预见会有更多的美国人因高胆固醇而接受治疗:接受膳食治疗者将会从5200万增至6500万,接受药物治疗者将会从1300万增至3600万。
在我国,心脑血管疾病发病率、死亡率近年来逐年上升,发病率高达8%,死亡率接近总死亡率的50%;平均每20分钟就有一人因心脑血管疾病而死亡。
近年来国内学者进行了一些调查研究,得出的结论是中国城镇居民的血脂水平要高于农民,成年人血TC随年龄而增高,50岁以前男性血TC高于女性,但50岁以后女性血TC增高的幅度超过男性;平均值亦比男性为高,LDL-C的变化趋势与TC相同。
TG亦随年龄而增高。
TC与TG均在60~80岁时达到最高水平,尔后便下降。
而HDL-C在男性各年龄组相对稳定而无明显差异,但较美国的同年龄人群为高,而中美两国中年女性的HDL-C水平无明显差别。
在地区分布上呈现北高南低,西高东低的特点。
由高脂血症发病的这些特点可以预见,随着我国人口老龄化进程的加速,城市化发展的加速以及生活方式的日渐西化,未来我国高脂血症的发病率和发病人数将会继续增长。
1.2.6知识产权情况阿托伐他汀具有欧洲专利,专利号EP0409281,2011年到期;美国专利,专利号US4681893。
在中国有行政保护,与1999年9月30日授权,授权号:B-US99093016,到期日:2004年7月21日。
1.2.7阿托伐他汀的研究意义阿托伐他汀(Astorvastatin)作为一种合适的一线降脂药物,它能有效降低冠心病的发生率和死亡率。
该药为最新合成药物,医学界公认比天然微生物发酵,半合成的他汀类药物好,患者使用时,起始剂量即有优异的调脂达标率。
2005年一季度销售数据山显示,增长率比上一年四季度有了较大增长,正在以大幅增加的速率逐渐吞噬着国内调血脂药物的市场。
目前阿托伐他汀为在保护期内的新药品种,但是在专利保护期终止后,必将出现群雄逐鹿的市场局面。
因此,对于我国研制开发阿托伐他汀降脂药具有巨大重要意义。
第二章阿托伐他汀中间体的合成2.1 4-氟-α-(2-甲基-1-氧丙基)-γ-氧-N,β-二苯基苯丁酰胺的合成4-氟a(2-甲基-1-氧丙基)-γ-氧-N,β-二苯基苯丁酰胺是合成阿托伐他汀所需要的重要中间体。
2.2异丁酰乙酸酯的合成文献报道了两种合成异丁酰乙酸乙酯的方法:一种是乙酰乙酸乙酯在乙醇镁的作用下,和异丁酰氯在0~5。
