诊断试剂China Diagnostic Reagent Industry Report, 2010
体外诊断试剂基础

7
基本性能-线性范围
在给定测量范围内,给出的测量结果与样本中实际存在的被测量物的 值成比例的能力。线性是描述一个测量系统的测量示值或测量结果相 关于样本的赋值符合直线的属性。 数值区间 线性范围涵盖的数值区间越宽,那么对临床样本的覆盖也就越广。 两种检测同一待测物质的诊断试剂,试剂A线性范围为0至100,试 剂B线性范围为10至90;那试剂A会更受欢迎,因为照顾到了那些0 至10和90至100的样本,不用单独再想办法重新检测,节省了时间 和精力。
标检测的试剂。
第三类
➢ 与致病性病原体抗原、抗体以及核 酸等检测相关的试剂;
➢ 与血型、组织配型相关的试剂; ➢ 与人类基因检测相关的试剂; ➢ 与遗传性疾病相关的试剂; ➢ 与麻醉药品、精神药品、医疗用毒
性药品检测相关的试剂; ➢ 与治疗药物作用靶点检测相关的试
剂; ➢ 与肿瘤标志物检测相关的试剂; ➢ 与变态反应(过敏原)相关的试剂。
5
体外诊断试剂基本分析性能
基本指标有试剂空白、分析灵敏度、精密度、准确度、线性 范围、可报告范围、分析干扰、稳定性等。 性能的评价应当按照相应的评价方案进行,且应当把仪器、 试剂、校准品、检测程序等作为一个整体的检测系统进行评 价。
6
基本性能-试剂空白&灵敏度
试剂空白 当待测物质含量为理论零值时检测到的数值。 一般做法是以生理盐水为样本进行测定,理论上这个数值应该为零。 但实际情况并非如此,即使加的样品是水,也会显示出一定的反应度, 恒定的空白反应度不影响试剂性能。 但是如果空白反应度波动大就可能导至临床结果不准,通常是由于试 剂的反应体系不稳定导至的,如防腐体系、缓冲体系、酶促反应体系 等不稳定都会导至空白反应度的变化。
体外诊断(IVD)行业必不可少的抗体抗原原料供应商

体外诊断(IVD)行业必不可少的抗体抗原原料供应商——优宁维公司作为目前国内最专业、最全面的抗体供应商,上海优宁维公司为IVD(体外诊断试剂)生产商提供高质量单克隆抗体和抗原的最重要供应商之一,Meridian Life Science(Biodesign)、Fitzgerald、Medix Biochemica、HyTest、Progen、Calbioreagents、USbio、BiosPacific 和Exbio等已成为最大IVD生产商的首选合作伙伴。
美国Meridian Life Science是全球生产抗原(天然抗原和重组抗原), 抗体(单克隆和多克隆),人抗小鼠抗体阻滞剂以及用于体外诊断和生物制药关键试剂的领军企业,提供用于分子诊断学的各种试剂,以及用于研发的各种外包生产服务包括cGMP规范的病毒疫苗的临床一期和二期实验。
旗下Biodesign主要的工业及研究用免疫学产品供应商,公司符合ISO 9001,FDA,GMP等多项认证,获得USDA许可,其产品超过4500种,在全球有40多个分销商,为生命科学研究,药物制造,生物技术及诊断产品开发及生产等多个领域提供抗体、抗原、免疫检测全套免疫学产品。
美国Fitzgerald 公司是一家国际性知名抗体研发生产企业。
Fitzgerald公司为全球试剂诊断公司与研究机构提供专业的抗体、纯化抗原以及工业诊断用大包装免疫原料。
公司产品多达9000种,能够满足您对于最前沿科学研究的需求。
主要产品涉及:1. 免疫比浊法用特种蛋白抗体— ApoAI, ApoB, IgG, IgA, IgM, C3, C4, TRF, CRP, Lp(a), PA, Albumin, β2-Microglobulin, etc;2. 化学发光免疫测定法(CLIA)、酶联免疫测定法(EIA)、放射免疫测定法(RIA)、荧光免疫法(FIA)测定用各类高纯单克隆、多克隆抗体,纯化抗原;3. 癌症系列抗原和抗体,如CA50, CEA, CA, AFP, CA 125, CA15-3, CA19-9, CA72-4, etc;4. 配对抗体和抗原,如生育类、传染病类、TORCH系列、心脏标记物、神经科学类、病毒类、性腺,甲状腺系列等。
迈克沃伊和法默的中国体外诊断分销指南说明书

McEvoy and Farmer's Complete Guide to IVD Distribution in Chinahttps:///r/W875106C98AEN.htmlDate: May 2009Pages: 240Price: US$ 3,995.00 (Single User License)ID: W875106C98AENAbstractsWho is Who in Clinical Diagnostics in China, produced jointly with the firm McEvoy & Farmer, is the product of on-the-ground primary research on Chinese labs conducted in fall and winter 2008. Included in this report:Market Size Estimate by Major Category (Chemistry, Critical Care Chemistry, Urine Chemistry, Hematology, Flow Cytometry, Coagulation, Immunochemistry, Molecular Testing, Other IVD)Country Industry OverviewChinese Hospital StatisticsProfiles of 42 International Diagnostic Companies with operations in ChinaProfiles of 130 Domestic Diagnostic CompaniesProfiles of 24 Local Distributors, indicating the companies they distribute forThe Chinese market represents a significant opportunity for IVD diagnostic companies. But actionable information about this emerging market is often difficult to get. Only with an exhaustive, on-the-ground research team can a company truly understand the chinese market. Now, a resource is available that can make on-the-ground research available to all companies at a fraction of the cost.Published jointly with trusted Asian IVD market experts, this Kalorama report is acomplete survey of the IVD market in China today. Market size for major categories of the IVD market, products on the market, important Chinese market trends and intense company profiles are part of this exhaustive study.This country of 1.3 billion people is now America’s sixth largest export market, and China’s economy, while showing some effects of the world recession, has been less impacted than other nations and is showing growth, although not as rapid as in recent years. A number of recent events and trends in the Chinese healthcare environment are making China an increasingly attractive market opportunity for in vitro diagnostics companies. The increasing numbers of private laboratories and expanded reference laboratories are expanding the market for tests of all kinds.Although there are a number of challenges for diagnostic manufacturers to understand and overcome, the market for clinical diagnostics in China (both reagents and instruments) remains one of the most promising emerging markets in the world.ContentsCOUNTRY SUMMARYMarket TotalsWhat is New in ChinaThe Healthcare System in ChinaMedical InsuranceA Brief Guide to the BureaucracyPrivate and Reference LaboratoriesPrivate Medical PracticeProduct RegistrationReimbursementTendersReagent RentalQuality ControlVacuum Tube ConversionSecond hand InstrumentsImported and Domestic Sales INTERNATIONAL MANUFACTURER PROFILESAbbott LaboratoriesABXAcon Biotech/InvernessAdaltisAffymetrixAgilent TechnologiesApplied BiosystemsArkrayAudit DiagnosticsBeckman CoulterBDBioneer TradeBio Rad LaboratoriesbioMérieuxDiaSorinDiaSys Diagnostic SystemsEppendorfEuroimmunFujirebio/CanAg DiagnosticsHitachi High Technologies CorporationHospitexHumanInverness MedicalJei Daniel (JD) BiotechJokohMedicine Devices Company (MDC) Melet Schloesing LaboratoriesMP BiomedicalsOrtho ClinicalDiagnosticsPerkinElmerPromegaQiagenRadiometerRandoxR BiopharmRocheSiemens Healthcare Diagnostics StagoSysmex CorporationThermoFisherVirionSerionYD DiagnosticsDOMESTIC MANUFACTURER PROFILES3V BioengineeringAccuBio TechAddcare Bio TechAi De Diagnostic (IND)AmpllyAntai DiagnosticsAudicom Medical InstrumentAutobio DiagnosticsAVE Science & TechnologyB & E Scientific InstrumentBasoDiagnosticsBeijing Genomics Institute (BGI Healthcare)Biocell InstituteBiocreateBiocup Biotech CompanyBioer TechnologyBiosino BiotechnologyBiote CompanyBioway BiotechnologyBlue Cross Bio MedicalBowlinman Sunshine Science & TechnologyCaihong Analytical InstrumentCaltech GroupCapitalBio CorporationCaretium Medical InstrumentsChang Chun Brother BiotechChangdao BiotechnologyChemClin Bio Tech / China Diagnostics Medical Chemtron BiotechChina Medical/Yuande Bio Medical EngineeringCondor Teco Medical TechnologyCornley Hi TechDa An GeneDecipher BioscienceDirui IndustrialDL Medical BiotechDoubleQ LabDragon MedicalElikan Biological TechnologyFenghua BioengineeringFengHui Medical Science & Technology First Sun ElectronicFosun DiagnosticsGenetel PharmaceuticalsGenius ElectronicsGoldsite DiagnosticsHai Tai BiologicalHaoyuan BiotechnologyHeal Force/Nison InstrumentHealthDigitHongcheng (HC) BiopharmaceuticalHongshi Medical TechnologyHope Industry and TradeHua Sin ScienceHua Tong Medical InstrumentHuaguan Biochip CompanyHuatai Biotechnology IndustrialHuayang Analysis InstrumentHybriBioInTec ProductsJian Ye Medical EquipmentinSangTe Medicine InstrumentKanghua Biotech CompanyKehua Bio EngineeringKinghawk TechnologyLabnovation TechnologiesLandwind International Medical Science LaoLa ElectronicLeadman Biochemistry TechnologyLengguang TechnologyLingyi Medical ScienceLivzon GroupLongx TechnologiesMaker Science TechnologyMaysun TechnologyMaxcom ElectronicMD Pacific TechnologyMeiyilin Electronics InstrumentMerit Choice BioengineeringMindray Medical ElectronicsModern Gold BiotechnologyNanfen Medical Biochemical InstrumentNeusoft Medical SystemsNew MoonNewScen Coast Bio PharmaceuticalOption Science & Technology Development Company Perlong GroupPG Biotech/QiagenPrecil InstrumentProcan ElectronicsRayto Life and Analytical SciencesRich Science IndustryRongsheng Biotech.Runbio Biotech .Sanco InstrumentSan Jose Medical Products..Sciarray BiotechSciendox Bio-Technology CompanySenlong Biotech..Share SunShensuo Medical DiagnosticsShenzhen New Industries Biomedical Engineering (SNIBE) Shining Sun TechnologySinnowa Medical Science & TechnologySTAC Medical Science & TechnologySteellex Scientific InstrumentStrong Biotechnologies...Success Technology DevelopmentSun BiotechSunostik Biomedical TechnologySym-Bio Lifescience.Tecom ScienceTechcompTellgen LifeTiangen Biotech...TianHai Medical Equipment (THME)Tianlong Science and TechnologyTigsun Biotinge Science & TechnologyTZD Technological.Urit Medical ElectronicWanCheng Bio-elect.Wantai BiologicalWasson An-Ze Bio-tech Company..WearmaxWeirikang Biological TechnologyW.H.P.M. Bioresearch & Technology/Hemosure Wondfo BiotechXun-Da Medical InstrumentYasen IndustrialYaxin Sheng WuZhong Tai BiotechZJ Bio-TechDISTRIBUTOR PROFILESAdvanced Clinical Laboratory Science (ACLS)Ailex Technology.....AusBio LaboratoriesBio-Asia DiagnosticsBiochem GroupBio-Star Technology DevelopmentChindex InternationalChinMax Medical SystemsDiamond BiotechnologyDong Hu Instrument/East LakeFu Li Tai (FLT) MeditecGene CompanyGiantech Medical Science & TechnologyGolden-Grand Medical Hongtex Bio-techLangkaNewtime TradingRainbow-Mega Scientific InstrumentScience International/Science LaboratoriesSunlionSuns-GroupTruth EnterpriseUnited Science InternationalVastec Medical..Zhi Cheng Biotech.Appendix: China’s Hospitals by Province.I would like to orderProduct name:McEvoy and Farmer's Complete Guide to IVD Distribution in ChinaProduct link:https:///r/W875106C98AEN.htmlPrice:US$ 3,995.00 (Single User License / Electronic Delivery)If you want to order Corporate License or Hard Copy, please, contact our CustomerService:*************************PaymentTo pay by Credit Card (Visa, MasterCard, American Express, PayPal), please, clickbutton on product page https:///r/W875106C98AEN.htmlTo pay by Wire Transfer, please, fill in your contact details in the form below:First name:Last name:Email:Company:Address:City:Zip code:Country:Tel:Fax:Your message:**All fields are requiredCustumer signature _______________________________________Please, note that by ordering from you are agreeing to our Terms& Conditions at https:///docs/terms.htmlTo place an order via fax simply print this form, fill in the information belowand fax the completed form to +44 20 7900 3970。
临床诊断试剂市场分析报告

临床诊断试剂市场分析报告1.引言1.1 概述概述:临床诊断试剂市场是医疗行业中一个备受关注的领域,随着人们对健康的日益重视,临床诊断试剂市场也在不断发展壮大。
临床诊断试剂是指用于临床检测和诊断的试剂和仪器,包括但不限于血液、尿液、唾液等生物样本的分析和检测。
这些试剂和仪器在医疗诊断和治疗中起着至关重要的作用,对于提高临床诊断的准确性和效率具有重要意义。
本报告旨在对临床诊断试剂市场进行深入分析,了解其市场规模、发展趋势、市场竞争格局和市场前景展望,以帮助相关企业和投资者更好地把握市场动态,制定更有效的战略规划。
1.2 文章结构文章结构部分的内容:本报告分为三个主要部分,包括引言、正文和结论。
在引言部分,我们将首先介绍临床诊断试剂市场的概况,包括其发展历程和现状,以及市场所面临的挑战和机遇。
接着,我们将阐明本报告的结构和目的,以及对市场的展望和总结。
在正文部分,我们将对临床诊断试剂市场进行深入分析,包括市场概况、主要市场参与者分析以及市场发展趋势展望。
我们将详细介绍市场的规模、市场份额、竞争格局和趋势变化,为读者提供全面的市场情况分析。
在结论部分,我们将对市场前景展望进行深入阐述,并对市场所面临的挑战和机遇进行分析。
最后,我们将对本报告进行总结,并提出一些建议,以便读者更好地理解市场情况,做出相应的决策。
通过以上结构的安排,我们将为读者提供一份全面、深入的临床诊断试剂市场分析报告,以帮助他们更好地了解市场情况,并制定有效的战略。
1.3 目的本报告的目的是对临床诊断试剂市场进行深入分析,了解市场的概况、主要参与者及其竞争态势,以及市场发展的趋势展望。
通过对市场的全面了解,可以为行业内企业制定发展战略和市场营销策略提供参考,同时也为投资者提供市场投资决策的依据。
同时,我们也将在报告中分析市场面临的挑战和机遇,展望未来市场的发展前景,为相关企业和投资者提供决策支持。
1.4 总结综上所述,通过对临床诊断试剂市场的概况、主要市场参与者分析以及市场发展趋势展望的深入分析,我们可以看到该市场的潜力巨大。
体外诊断试剂注册 中英文对照
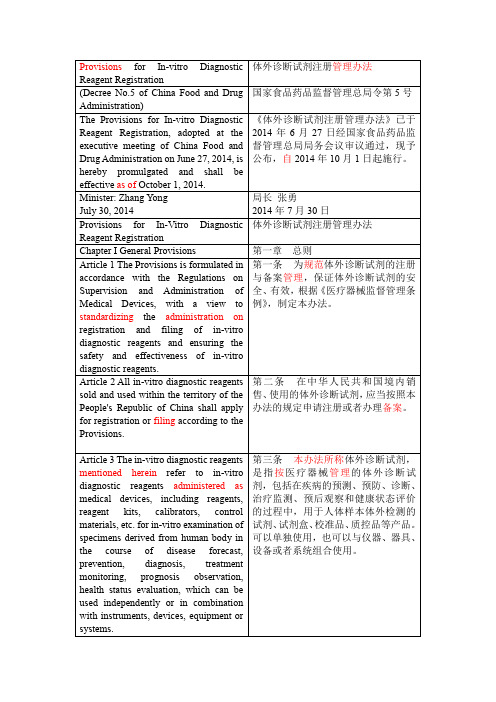
体外诊断试剂注册管理办法
(Decree No.5 of China Food and Drug Administration)
国家食品药品监督管理总局令第5号
The Provisions for In-vitro Diagnostic Reagent Registration, adopted at the executive meeting of China Food and Drug Administration on June 27, 2014, is hereby promulgated and shall be effectiveas ofOctober 1, 2014.
境内第一类体外诊断试剂备案,备案人向设区的市级食品药品监督管理部门提交备案资料。
Class II in-vitro diagnostic reagents shall be reviewed by the food and drug regulatory department of the provinces, autonomous regions and municipalities directly under the central government, and themedical device registration certificateshall be issued after approval.
香港、澳门、台湾地区体外诊断试剂的注册、备案,参照进口体外诊断试剂办理。
Article 7 Where aregistration applicantor filing entityof an in-vitro diagnostic reagent brings the products to the marketin his own name,he shallproduct.
体外诊断试剂相关英文专业术语解释

体外诊断试剂相关英文专业术语解释英文回答:In Vitro Diagnostic (IVD): A medical device intendedfor the in vitro examination of specimens derived from the human body, alone or in combination with reagents or other devices. IVDs are used to provide information for the diagnosis, monitoring, or treatment of disease, or for assessing health status.Analytical Sensitivity: The lowest concentration of an analyte that can be detected with a reasonable degree of certainty.Analytical Specificity: The ability of an IVD to distinguish between the target analyte and other substances that may be present in the specimen.Assay: A procedure performed on a specimen to detect or measure an analyte.Biochemical Marker: A substance that is present in the body in specific amounts or concentrations and can be used to indicate the presence or absence of a particular disease or condition.Calibration: The process of adjusting an IVD to ensure that it provides accurate results.Clinical Sensitivity: The ability of an IVD tocorrectly identify individuals who have a particular disease or condition.Clinical Specificity: The ability of an IVD tocorrectly identify individuals who do not have a particular disease or condition.Control: A sample or material that is used to monitor the performance of an IVD.Cutoff Value: The concentration of an analyte that is used to distinguish between positive and negative results.Diagnostic Sensitivity: See Clinical Sensitivity.Diagnostic Specificity: See Clinical Specificity.External Quality Assessment Scheme (EQAS): A programthat provides laboratories with proficiency testing samples to assess the accuracy and reliability of their IVD testing.False Negative: A negative test result when the individual actually has the disease or condition.False Positive: A positive test result when the individual does not actually have the disease or condition.Immunoassay: An IVD that uses antibodies to detect or measure an analyte.Internal Quality Control (IQC): A system of procedures that a laboratory uses to monitor the performance of its IVDs on a regular basis.Limit of Blank (LoB): The highest concentration of an analyte that can be detected in the absence of the target analyte.Limit of Detection (LoD): See Analytical Sensitivity.Limit of Quantification (LoQ): The lowest concentration of an analyte that can be accurately measured.Molecular Diagnostic: An IVD that uses nucleic acid technology to detect or measure an analyte.Negative Predictive Value: The probability that an individual who tests negative for a disease or condition does not actually have the disease or condition.Positive Predictive Value: The probability that an individual who tests positive for a disease or condition does actually have the disease or condition.Quality Control: The system of procedures that a laboratory uses to ensure the accuracy and reliability ofits IVD testing.Reagent: A substance that is used in an IVD to produce a visible or measurable reaction.Reference Interval: The range of values that are considered normal for a particular analyte in a healthy population.Sensitivity: See Analytical Sensitivity or Clinical Sensitivity.Specimen: A sample of biological material that is collected for testing.Specificity: See Analytical Specificity or Clinical Specificity.Validation: The process of demonstrating that an IVD meets its intended performance specifications.中文回答:体外诊断试剂 (IVD),在体外检查人体标本的医疗器械,单独使用或与试剂或其他设备配合使用。
丙型肝炎国产诊断试剂与第三代进口试剂的临床比较研究
中华医学检验杂志科技期刊CHINESE JOURNAL OF MEDICAL LABORATORYSCIENCES1998年 第6期 No.6 November论著丙型肝炎国产诊断试剂与第三代进口试剂的临床比较研究祁自柏 周诚 谷金莲 扬振 张庶民凌世淦 张贺秋 王国华 宋晓国 陈琨 李河民 【摘要】 目的 考察丙型肝炎(丙肝)国产改进酶标诊断试剂与国外第三代主流试剂的灵敏度与特异性。
方法 用改进后的国产新伟凯公司丙肝试剂(XWK)和两种国外第三代主流试剂(A3,O3)检测了2 882份供血员血样和556份各种肝炎病人血样。
结果 3种试剂(XWK,A3,O3)分别检出380,378和371份阳性样品,经免疫印迹条法(RIBA)和聚合酶链反应(PCR)方法确证,3种试剂的假阳性数分别为7,6和1份,假阴性数分别为1,2和4份。
结果说明,改进后的国产丙肝试剂,其检出灵敏度与目前国际上最新一代试剂接近,但其特异性略逊于国外两种第三代主流试剂。
结论 分析RIBA和PCR所测得的3种试剂漏检样品的结果可以看出,目前国际上的第三代丙肝酶标试剂对丙肝核心抗体的检出灵敏度较低,这一点与我们以前的研究结果相同。
而改进后的国产丙肝试剂既赶上了国外第三代试剂对丙肝NS3抗体的检出力度,同时又避免了国外试剂所暴露出的对丙肝核心抗体检出力弱的缺点。
【关键词】 肝炎,丙型 试剂盒,诊断 聚合酶链反应Evaluating Chinese anti-HCV EIA kit and foreign third-generation anti-HCV EIA kit Qi Zibai, Zhou Cheng, GuJinlian, et al. National Institute for the Control of Pharmaceutical and Biological Products, Beijing 100050 【Abstract】 Objective Toeevaluate the sensitivity and specificity of the improved Chinese anti-HCV EIA kitand foreign third-generation anti-HCV EIA kit. Methods 2 882 Chinese blood donors and 556 Chinese hepatitispatient sera were tested with the improved Chinese anti-HCV EIA kit (XWK) and two foreign third-generation anti-HCV EIA kits (A3,O3).Results The positive samples detected by XWK, A3 and O3 were 380, 378 and 371respectively. According to the confirmed results by RIBA and PCR, the false positive samples given by XWK, A3 andO3 were 7, 6 and 1. And the false negative samples were 1, 2 and 4 respectively. The results showed that the sensitivityof the improved Chinese anti-HCV EIA kit was similar to that of the third-generation kit, but the specificity was poorthan that of the late.Conclusion The conclusion also can be drawn that the capability of A3 and O3 in detecting anti-HCV core antibody is poor. 【Key words】 Hepatitis C Reagent kits, diagnostic Polymerase chain reaction 丙型肝炎(丙肝)病毒(HCV)于1989年被发现以来[1],国外的丙肝酶标诊断试剂(抗HCV EIA)已发展到第三代。
体外诊断试剂分析报告
体外诊断试剂(IVD)分析报告及建议体外诊断试剂是指按医疗器械管理的体外诊断试剂,包括可单独使用或与仪器、器具、设备或系统组合使用,在疾病的预防、诊断、治疗监测、预后观察、健康状态评价以及遗传性疾病的预测过程中,用于对人体样本(各种体液、细胞、组织样本等)进行体外检测的试剂、试剂盒、校准品(物)、质控品(物)等。
国家法定用于血源筛查的体外诊断试剂、采用放射性核素标记的体外诊断试剂不属于《体外诊断试剂注册管理办法(试行)》的管理范围。
一、体外诊断试剂(IVD)市场分析中国体外诊断(IVD)产业兴起于20世纪80年代初期,经历近30年的发展,从无到有,从弱到强,现在整个产业已经具备了规模化发展条件。
中国诊断产品用户主要包括19800 多家医院、39000多个乡镇卫生院和300多家血站,以及日新月异的体检中心和正在兴起的临床检验独立实验室。
总体来看,中国诊断行业仍处在成长初期目前,我国诊断试剂市场基本可以分成7个部分,其中免疫学诊断试剂市场规模最大,市场份额29.61%,其次是:生化诊断试剂,血糖自我监测试剂血液诊断试剂,尿液诊断试剂,生物学诊断试剂3.97%,分子及其他诊断试剂12.01%。
分子诊断试剂是20世纪80年代后期发展起来的,无论技术还是市场,都是目前所有诊断试剂产品中发展最快的。
免疫诊断试剂的市场规模已逐渐超过临床化学试剂,但由于二者的检验对象有所区别,生化诊断试剂还将保持较大份额。
据《2013-2017年中国诊断试剂行业产销需求与投资预测分析报告》[4]分析,2007-2009年中国诊断试剂行业的市场规模年复合增长率达17.93%,呈现高速增长趋势。
2009年中国诊断试剂市场规模达到96.72亿元,人均年费用为7.3元,而日本2008年人均年使用量为227.5元,差距悬殊。
总体来看,中国诊断行业仍处在成长期初期。
诊断试剂行业是生物制药行业的重要组成部分,就国内的现状来说,中国的诊断试剂仍处于弱小成长期,才处于产品研发与生产的投入初期,成长期还没有真正到来。
一文读懂体外诊断试剂IVD
一文读懂体外诊断试剂IVD现如今去医院看病基本上都是医生未见,诊断先行,血检、尿检早已习以为常,而这些检查都是基于体外诊断试剂。
体外诊断试剂也以其精准性、便易性和高效性在整个医疗过程中占据越来越重要的位置,在现代医疗体系中不仅能大大降低医生的工作量,同时也极大的提高了诊断的准确性以及对疾病的预防性,因此,体外诊断又有着“医生的眼镜”之美誉。
今天的每日课堂栏目就一起来学习下体外诊断试剂IVD是什么。
IVD定义业内人士俗称其为IVD也就是英文In Vitro Diagnostic。
体外诊断试剂和器械在国外统一称为体外诊断医疗器械。
属于医疗器械的一部分。
在我国,体外诊断试剂是指:可单独使用或与仪器、器具、设备或系统组合使用,在疾病的预防、诊断、治疗监测、预后观察、健康状态评价以及遗传性疾病的预测过程中,用于对人体样本(各种体液、细胞、组织样本等)进行体外检测的试剂、试剂盒、校准品(物)、质控品(物)等。
内涵及市场分类体外诊断产品包括对人体样本(包括体液、细胞、组织样本等)进行收集、制备(定向处理)、检测的试剂仪器及分析系统。
通常体外诊断市场主要分为四大类:生化试剂、免疫诊断、分子诊断、即时检验(POCT)。
据《蓝皮书》数据:2014年,体外诊断细分领域占其总体市场比例前三位:占比接近四成的免疫诊断(38%)、占比接近两成的生化试剂(19%)和占比为15%的分子诊断。
目前市场份额占比最大的是免疫诊断。
其中生化试剂类产品主要以国产品牌为主;免疫诊断类产品以进口为主导,国产品牌占有一定份额;分子诊断类产品则是进口和国产品牌鱼龙混杂;而在POCT类产品方面,是以进口为主,国产尚处于起步阶段。
国内体外诊断产业现状中国医药工业信息中心发布的《蓝皮书》显示:2014年我国医疗器械市场总量达到2760亿元,依据市场占比来看,前三位的依次是:市场占比接近两成的医学影像设备(19%)、市场占比为16%的体外诊断产品和市场占比为13%的高值医用耗材及植入物。
体外诊断试剂审评要求
体外诊断试剂审评要求体外诊断试剂(In Vitro Diagnostic Reagents,IVD)是一种应用于体外临床实验室、医学检验科室和其他医疗机构中,用于诊断疾病、监测治疗效果和预防健康等的试剂。
为保证体外诊断试剂的质量和安全性,各国监管机构都制定了一系列的审评要求和规范。
在中国,国家药监局(NMPA)负责对体外诊断试剂进行审评。
技术要求是指试剂在临床应用中需要满足的性能指标和要求。
例如,试剂的准确性和精密度必须符合国家标准或国际标准,以保证试剂能够提供准确和可靠的结果;试剂的灵敏度和特异性必须足够高,能够检测到目标物质的低浓度和排除其他干扰物质的影响;试剂的线性范围必须足够宽,能够适应不同病情的检测需求;试剂在不同环境条件下的稳定性和一致性必须得到验证,能够保持长期使用的效果。
质量要求是指试剂在生产过程中需要满足的质量管理要求。
例如,试剂的原材料和辅料必须符合药品GMP的要求,并且必须经过严格的检验和验证;试剂的生产过程必须符合规定的程序和规范,保证每个批次的试剂能够达到相同的质量要求;试剂的包装和存储必须符合规定的条件,保证试剂在运输和使用过程中不受影响。
总之,体外诊断试剂的审评要求包括注册、技术、质量和安全等方面,旨在保证试剂的质量和安全性,提高临床应用的准确性和可靠性,保障患者的健康和安全。
这些要求对试剂生产企业来说是一项严峻的挑战,需要加强质量管理和安全管理,不断提高技术水平和生产能力,以满足监管机构和市场的要求。
同时,对监管机构来说,也需要做好审评的科学性和公正性,并加强对试剂的监督和管理,加强国际合作和信息共享,共同提高体外诊断试剂的质量和安全水平。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
• • • • • • • • • •
4.18 Beijing North Institute of Biological Technology 4.18.1 Profile 4.18.2 Operation 4.19 DiaSys Diagnostic Systems (Shanghai) 4.19.1 Profile 4.19.2 Operation 4.20 Other Enterprises 4.20.1 Autobio 4.20.2 Biobase 4.20.3 Boson Biotechnology
China Diagnostic Reagent Industry Report, 2010
In 2009, China’s diagnostic reagent market represented about 5% of the global market, and Chinese enterprises still concentrated on lowend biochemistry diagnostic reagent market. In China, there had been more than 70 biochemistry diagnostic reagent producers by the end of 2009, with sales over RMB 4 billion in total. At present, there are only about a dozen of immune diagnostic reagent producers with sales of RMB 1.5 billion in total, and there are no more than five nucleic acid diagnostic reagent producers with the market size of no more than RMB 500 million in all. By contrast, in foreign markets, the share of biochemistry diagnostic reagent has become very low, while immune diagnostic reagent and nucleic acid diagnostic reagent featuring higher detection sensitivity and specificity have become the mainstream.
• • • • • • • • • • • • • • • • • • • • •
4.11 Kinghawk Pharmaceutical 4.11.1 Profile 4.11.2 Operation 4.12 ABON Biopharm (Hangzhou) 4.12.1 Profile 4.12.2 Operation 4.13 Shanghai Upper Bio-Tech Pharma 4.13.1 Profile 4.13.2 Operation 4.14 Beijing Leadman Biochemical 4.14.1 Profile 4.14.2 Operation 4.15 Blue Cross Bio-Medical (Beijing) 4.15.1 Profile 4.15.2 Operation 4.16 Inverness Medical (Shanghai) 4.16.1 Profile 4.16.2 Operation 4.17 Baso Diagnostics 4.17.1 Profile 4.17.2 Operation
The major diagnostic reagent producers in China include KHB and Da An Gene, all of which hold certain market shares by virtue of their own technology strength. Da An Gene mainly produces nucleic acid diagnostic reagent which can compete with the international advanced level in technology and quality. In 2009, Da An Gene’s nucleic acid diagnostic reagent business accounted for over 50% of the company’s operating income and about 60% of the domestic nucleic acid diagnostic reagent market. In addition, with the launch of China nucleic acid blood screening market, the nucleic acid blood screening business is expected to become a new profit point of Da An Gene. Operating Income and YoY Growth of Nucleic Acid Diagnostic Reagent Sector of Da An Gene, 2006-2009
• • • • • • •
2. Operating Environment in China 2.1 Global Market 2.1.1 Development of Biopharmaceutical Industry Worldwide 2.1.2 Development of Diagnostic Reagent Industry Worldwide 2.2 Chinese Market 2.2.1 Development of Biopharmaceutical Industry in China 2.2.2 Relevant Policies of China • • • • • • • • • 4. Domestic Major Producers 4.1 KHB 4.1.1 Profile 4.1.2 Operation 4.1.3 Operation of Diagnostic Reagent Business 4.2 Da An Gene 4.2.1 Profile 4.2.2 Operation 4.2.3 Operation of Diagnostic Reagent Business 4.3 Fosun Pharmaceutical (Group) 4.3.1 Profile 4.3.2 Operation
Selected Charts
• • • • • • • • • • • • • • • • • • • • Global Biopharmaceutical Market Scale and YoY Growth, 2000-2009 Global Biopharmaceutical Revenue by Region, 2008 Mergers and Acquisitions of Global Diagnostic Reagent Industry, Jan.2007Jan.2010 Sales and Proportion of In-Vitro Diagnostic Reagents of Major European Countries, 2008 China’s Biopharmaceutical Sales and YoY Growth, 2005-2009 Relevant Polices of Diagnostic Reagent Industry in China Major Diagnostic Reagent Producers in China Composition of In-Vitro Diagnostic Reagents in China, 2009 Import Value of Contrast Agent for X-Ray Inspection and Diagnostic Reagent for Patients, Jan.-Aug., 2010 Export Value of Contrast Agent for X-Ray Inspection and Diagnostic Reagent for Patients, Jan.-Aug., 2010 China’s Biochemical Reagent Market Scale and Growth Rate, 2007-2010 China’s Immune Reagent Market Scale and YOY Growth, 2007-2013 Operating Income and Net Profit of KHB, 2006-2010Q1 Operating Income of KHB by Product, 2007-2009 Sales Revenue of Diagnostic Reagent of KHB by Product, 2007-2009 Revenue from Blood Screening Business of KHB,2011 E Operating Income and Net Profit of Da An Gene, 2006-2010 Q1 Operating Income of Da An Gene by Product, 2007-2009 Operating Income and YOY Growth of Nucleic Acid Diagnostic Reagent of Da An Gene, 2006-2009 Revenue from Blood Screening Business of Da An Gene, 2011
• • •
