盐酸地尔硫卓合成图解
盐酸地尔硫卓合成研究进展

盐酸地尔硫卓合成研究进展戴会彬;王国超;何高楠;罗小冬【摘要】介绍了重要的心血管药物盐酸地尔硫卓的特性、用途,叙述了其化学拆分、不对称合成和生物酶水解拆分的制备方法。
中间体(2S,3S)-dl-trans-(4-甲氧基苯基)-2,3-环氧丙酸甲酯(MPGM)通过化学拆分虽然有效,但会有很多的原料被浪费;而通过脂肪酶催化拆分得到的对映体过量值>99.9%,减少了反应步骤,但已有的拆分用固定化脂肪酶的化学性质很不稳定,拆分产物的利用率也还不确定。
因此应研究出一条收率高、成本低、反应条件温和、操作简单、绿色、符合工业化大规模生产的工艺路线。
【期刊名称】《化工生产与技术》【年(卷),期】2016(023)002【总页数】5页(P31-35)【关键词】盐酸地尔硫卓;手性中间体;化学拆分;不对称合成【作者】戴会彬;王国超;何高楠;罗小冬【作者单位】浙江永太科技股份有限公司,浙江临海 317016;浙江永太科技股份有限公司,浙江临海 317016;浙江永太科技股份有限公司,浙江临海 317016;浙江永太科技股份有限公司,浙江临海 317016【正文语种】中文【中图分类】TQ463+.7心血管药物地尔硫卓(Diltiazem)是苯并硫氮卓类钙离子通道阻滞剂,钙拮抗剂对扩张冠状血管有非常明显的作用,临床中多用于心律失常、心绞痛、高血压等心血管疾病的治疗。
盐酸地尔硫卓(1)的化学名为顺-(2S,3S)-(+)-5-[(2-二甲氨基)乙基]-2-(4-甲氧基苯基)-3-乙酰氧基-2,3-二氢-1,5-苯并硫氮杂卓-4(5氢)-酮,化学结构为[1-2]:盐酸地尔硫卓存在2个手性中心,其中只有(2S,3S)-异构体具有药理活性。
盐酸地尔硫卓的合成目前主要有化学拆分、不对称合成及运用生物酶拆分技术合成等方法,本文对这些方法及其研究进展进行了介绍和评述。
目前地尔硫卓的合成仍然以拆分法应用的最多,很多文献都报道了它的化学合成路线及相对应的拆分方法,目前已经用于大生产的3条路线合成路线,都是以茴香醛为原料,经多步反应得到最终的产物(Me为甲基)[3]。
盐酸地尔硫卓合成图解
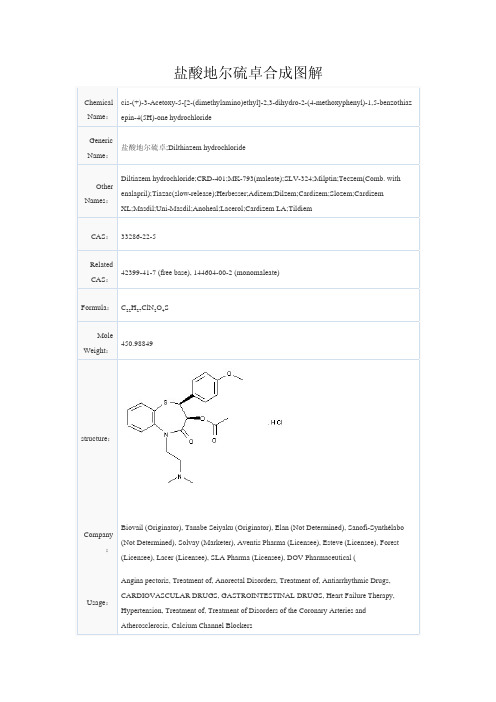
盐酸地尔硫卓合成图解 Chemical Name :cis-(+)-3-Acetoxy-5-[2-(dimethylamino)ethyl]-2,3-dihydro-2-(4-methoxyphenyl)-1,5-benzothiaz epin-4(5H)-one hydrochlorideGenericName :盐酸地尔硫卓;Dilthiazem hydrochloride Other Names :Diltiazem hydrochloride;CRD-401;MK-793(maleate);SLV-324;Milptin;Teczem(Comb. with enalapril);Tiazac(slow-release);Herbesser;Adizem;Dilzem;Cardizem;Slozem;Cardizem XL;Masdil;Uni-Masdil;Anoheal;Lacerol;Cardizem LA;Tildiem CAS : 33286-22-5RelatedCAS : 42399-41-7 (free base), 144604-00-2 (monomaleate)Formula : C 22H 27ClN 2O 4SMoleWeight : 450.98849structure :Company :Biovail (Originator), Tanabe Seiyaku (Originator), Elan (Not Determined), Sanofi-Synthélabo (Not Determined), Solvay (Marketer), Aventis Pharma (Licensee), Esteve (Licensee), Forest (Licensee), Lacer (Licensee), SLA Pharma (Licensee), DOV Pharmaceutical (Usage : Angina pectoris, Treatment of, Anorectal Disorders, Treatment of, Antiarrhythmic Drugs, CARDIOVASCULAR DRUGS, GASTROINTESTINAL DRUGS, Heart Failure Therapy,Hypertension, Treatment of, Treatment of Disorders of the Coronary Arteries andAtherosclerosis, Calcium Channel BlockersRoute 1Electrophilic bromination of ethyl p-methoxycinnamate (I) by means of N-bromosuccinimide in moist acetone gave rise to the racemic erythro bromohydrin (II), which was esterified with butyric anhydride to produce the racemic bromoester (III). Kinetic resolution of (III) employing Candida cylindracea lipase caused the enantioselective hydrolysis of the (S,S)-enantiomer, yielding the chiral bromohydrin (V). Cyclization of (V) in the presence of NaOMe furnished epoxide (VI). The target thiazepinone (VIII) was then obtained by condensation between the glycidic ester (VI) and 2-aminobenzenethiol (VII)Intermediates: Seri al No.1S,5R,13R,16S,17R)-4-cyano-16-ethyl-10-methoxy-12-oxa-4-azapentacyclo[9.6.1.0(1,13).0(5,17).0(7,18)]octadeca-7(18),8,10-trien-14-one(A)[(2R,3S,5R)-5-(6-hydroxy-9H-purin-9-yl)-3-[(phenoxycarbothioyl)oxy]tetrahydro-2-furanyl]methyl acetate (VII)Reference 1:McCague, R.; Wang, S.; Taylor, S.J.C. (Celltech Group plc); Chiral arylpropionates and their use. WO 9413828 .Route 2A different method for the preparation of glycidic ester (VI) consists in the asymmetric epoxidation of ethyl 4-methoxycinnamate (I) with oxone(R) in the presence of chiral macrocyclic ketones, such as the binaphthyl ketone (IX)Intermediates: Serial No.Reference 1:Seki, M.; Furutani, T.; Imashiro, R.; Kuroda, T.; Yamanaka, T.; Harada, N.; Arawaka, H.; Kusama, M.; Hashiyama, T.; A novel synthesis of a key intermediate for diltiazem. Tetrahedron Lett 2001, 42, 46, 8201. Reference 2:Ozaki, Y.; Arakawa, H.; Harada, N.; Hashiyama, T.; Kuroda, T.; Seki, M.; Kusama, M. (Tanabe Seiyaku Co., Ltd.); Process for preparing optically active phenyloxirane cpds.. WO 9856762 .Route 3In a further process, the racemic trans glycidic ester (XII), prepared by Darzens condensation between anisaldehyde (X) and methyl chloroacetate (XI), was resolved by enantioselective enzymatic hydrolysis, using several different enzymes and reaction conditions to produce the undesired (2S,3R) acid (XIII), while leaving intact the required (2R,3S)-glycidic ester (XIV) (4-7). Opening of the chiral epoxide (XIV) with2-aminobenzenethiol (VII) proceeded with retention of the configuration, producing methyl(2S,3S)-2-hydroxy-3-(2-aminophenylsulfanyl)-3-(4-methoxyphenyl)propionate (XV) (8). Alternatively, the (S,S)-amino ester (XV) was obtained by resolution with tartaric acid of the racemic three-adduct resulting from epoxide (XII) and 2-aminobenzenethiol (VII) (9). Cyclization of amino ester (XV) in refluxing xylene in the presence of p-toluenesulfonic acid afforded the target lactam (VIII) (9). The cyclization of (XV) to lactam (VIII) was also accomplished by means of trichloroacetic acid or under basic conditionsIntermediates: Serial No.(3R,4R)-4-(difluoromethyl)-1-(4-methoxyphenyl)-3-[(triisopropylsilyl)oxy]-2-azetidinone (XI) 1-Hydrazinecarboxamide (XII) [(2R,3S,5R)-5-(6-hydroxy-9H-purin-9-yl)-3-[(phenoxycarbothioyl)oxy]tetrahydro-2-furanyl]methyl acetate(VII) methyl 2-phenoxyacetate (X) Reference 1:Bhushan, L.B.; Jayachandran, B.E.; Bhushan, L.V.; Thottappillil, R. (Council of Scientific and Industrial Research); Process for preparing diltiazem. US 5869697 .Route 4A new enantioselective method for the preparation of glycidic ester (XIV) has been disclosed. Methyl trichloroacetate (XVI) was converted to the dichloroketene silyl acetal (XVII) by treatment with zinc powder and chlorotrimethylsilane. Asymmetric aldol condensation of (XVII) with anisaldehyde (X) in the presence of the chiral oxazaborolidine catalyst (XVIII) at -78 C produced methyl(S)-2,2-dichloro-3-hydroxy-3-(4-methoxyphenyl)propionate (XIX). Reductive mono-dechlorination of (XIX) furnished chlorohydrin (XX), which was then cyclized to glycidic ester (XIV) in the presence of NaOMeIntermediates: Serial No. methyl 2-phenoxyacetate (X) Reference 1:Imashiro, R.; Kuroda, T.; Asymmetric synthesis of methyl (2R,3S)-3-(4-methoxyphenyl) glycidate, a key intermediate of diltiazem, via Mukaiyama aldol reaction. Tetrahedron Lett 2001, 42, 7, 1313.Route 5Addition of phenylmagnesium bromide to cyclohexene oxide (XXI) in the presence of CuCl gavetrans-2-phenylcyclohexanol (XXII), which was further esterified with chloroacetyl chloride to afford2-phenylcyclohexyl chloroacetate (XXIII). Enantioselective hydrolysis of the racemic ester (XXIII) by means of Pseudomonas fluorescens lipase provided pure (1R,2S)-2-phenylcyclohexanol (XXIV), which was again esterified with chloroacetyl chloride, yielding the chiral ester (XXVI). Darzens condensation of chloro ester (XXVI) with anisaldehyde (X) led to the chiral glycidic ester (XXVII). Epoxide ring opening in (XXVII) with 2-aminothiophenol (VII) furnished amino ester (XXVIII). Intermediate thiazepinone (VIII) was then obtained by cyclization of (XXVIII) using p-toluenesulfonic acid in refluxing xylene. Alternatively, amino ester (XXVIII) was first hydrolyzed to amino acid (XXIX), which was subsequently cyclized with p-toluenesulfonic acid as above (11). The final cyclization of amino acid (XXIX) to the intermediate thiazepinone (VIII) has also been carried out in the presence of trichloroacetic acidIntermediates: Serial No. 4-(dimethylamino)-N-(2,6-dimethylphenyl)-4-piperidinecarboxamide (XXIV) (2R,3R,4S,5S,6R)-3,5-bis(acetoxy)-6-[(acetoxy)methyl]-2-bromo-2-cyanotetrahydro-2H-pyran-4-yl acetate(XXI) [(2R,3S,5R)-5-(6-hydroxy-9H-purin-9-yl)-3-[(phenoxycarbothioyl)oxy]tetrahydro-2-furanyl]methyl acetate(VII) methyl 2-phenoxyacetate (X)4-{4-[4-(benzyloxy)phenyl]-1-piperazinyl}aniline;4-{4-[4-(benzyloxy)phenyl]-1-piperazinyl}phenylamine (+/--XXII)Reference 1:Plaum, M.J.M.; Boesten, W.H.J.; Process for the preparation of a benzothiazepine. EP 0796853; JP 1998007667; US 5859241 .Route 6A different strategy to reach the amino ester precursor (XXIX) was developed starting from the chiral diol (XXX), readily accessible by asymmetric dihydroxylation of cinnamate (I). Reaction of diol (XXX) with SOCl2 produced the cyclic sulfite (XXXI) (12,14). Optionally, diol (XXX) was condensed with phosgene to produce the cyclic carbonate (XXXII) (12). Opening of either sulfite (XXXI) or carbonate (XXXII) with2-aminothiophenol (VIII) proceeded with retention of the configuration, leading to the desired intermediate aminoacid (XXIX)Intermediates: Serial No.[(2R,3S,5R)-5-(6-hydroxy-9H-purin-9-yl)-3-[(phenoxycarbothioyl)oxy]tetrahydro-2-furanyl]methyl acetate(VIII)Reference 1:Lohray, B.B.; Jayachandran, B.; Bhushan, V.; Nandanan, E.; Ravindranathan, T.; Anchimeric assisted unprecedented SN(i)-type cleavage of cyclic sulfite: Application in the synthesis of the calcium channel blocker diltiazem. J Org Chem 1995, 60, 18, 5983.Reference 2:Hulshof, L.A.; Kuilman, T.; Process for preparing 1,5-benzothiazepin derivs.. EP 0450705 .Route 7The racemic thiazepinone (XXXIII) has been converted to the pure enantiomer (VIII) through a different strategy. Oxidation of hydroxy lactam (XXXIII) by means of DMSO and Ac2O produced the enol ester (XXXV). Basic hydrolysis of (XXXI) gave rise to the keto lactam (XXXV). This was then subjected to asymmetric reduction utilizing a reducing reagent generated in situ from NaBH4 and (S)-tert-leucine to afford the intermediate thiazepinone (VIII)Intermediates: Serial No. (1R,2R)-3-(acetoxy)-1-(4-methoxyphenyl)-2-methylpropyl acetateReference 1:Yamada, S.; Mori, Y.; Morimatsu, K.; Ishizu, Y.; Ozaki, Y.; Yoshioka, R.; Nakatani, T.; Seko, H.; Asymmetric reduction of a 1,5-benzothiazepine derivative with sodium borohydride-(S)-alpha-amino acids: An efficient synthesis of a key intermediate of diltiazem. J Org Chem 1996, 61, 24, 8586.Route 8The precursor cis-cinnamate (XXXIX) can be obtained by several synthetic routes. Bromination of ethyl trans-4-methoxycinnamate (I) afforded the dibromo ester (XXXVI), which underwent dehydrohalogenation and hydrolysis to the arylpropiolic acid (XXXVII) upon treatment with ethanolic KOH. Acid (XXXVII) was converted to the corresponding isopropyl ester (XXXVIII) by DCC-mediated coupling with isopropanol. Semihydrogenation of (XXXVIII) in the presence of Lindlar catalyst led to the required cis cinnamate (XXXIX). Alternatively, anisaldehyde (X) was converted to the gem-dibromostyrene (XL) by condensation with CBr4 in the presence of PPh3. Reaction of (XL) with BuLi, followed by addition of isopropyl chloroformate to the resultant lithium acetylide, furnished the arylpropiolate (XXXVIII). In another method to obtain the cis-cinnamate (XXXIX), trans-4-methoxycinnamic acid (XLI) was converted to the isopropyl ester (XLII), which was then photochemically isomerized to the desired cis-cinnamate (XXXIX)Intermediates: Serial No. methyl 2-phenoxyacetate (X) 2-[(2R)-4-benzoyl-2-(3,4-difluorophenyl)morpholinyl]ethyl benzoate (XLI) Reference 1:Jacobsen, E.N.; Deng, L.; Furukawa, Y.; Martinez, L.E.; Enantioselective catalytic epoxidation of cinnamate esters. Tetrahedron 1994, 50, 15, 4323.Route 9The key cis glycidate ester (XLIII) was prepared by (salen)Mn(III)-catalyzed asymmetric epoxidation of the cis cinnamate (XXXIX). Epoxide opening in (XLIII) with 2-nitrothiophenol (XLIV), with inversion of the configuration, led to the nitro ester adduct (XLV). The nitro group of (XLV) was then reduced to the aniline derivative (XLVI) by means of FeSO4. Subsequent isopropyl ester group saponification in (XLVI) furnished amino acid (XXIX). This was then cyclized to the target thiazepinone (VIII) in refluxing xyleneIntermediates: Serial No.Reference 1:Jacobsen, E.N.; Deng, L.; Furukawa, Y.; Martinez, L.E.; Enantioselective catalytic epoxidation of cinnamate esters. Tetrahedron 1994, 50, 15, 4323.Route 10In a variation of the preceding methods, the chiral glycidic amide (XLVII) was used as the synthetic precursor.Amide (XLVII) was either prepared by treatment of the chiral glycidic ester (VI) with ammonia, or by enzymatic resolution of (XII), followed by amidation. Iron-catalyzed addition of 2-aminothiophenol (VII) to the glycidamide (XLVII) in refluxing chlorobenzene yielded the desired threo adduct (XLVIII) as the major isomer. Cyclization of amino amide (XLVIII) under acidic conditions furnished thiazepinone (VIII)Intermediates: Serial No.1-Hydrazinecarboxamide (XII) [(2R,3S,5R)-5-(6-hydroxy-9H-purin-9-yl)-3-[(phenoxycarbothioyl)oxy]tetrahydro-2-furanyl]methyl acetate(VII)Reference 1:Yamada, S.; Tsujioka, I.; Shibatini, T.; Yoshioka, R.; Efficient alterative synthetic route to diltiazem via (2R,3S)-3-(4-methoxyphenyl)glycidamide. Chem Pharm Bull 1999, 47, 2, 146.Route 11Aldol condensation of anisaldehyde (X) with the lithium enolate of the N-acyl oxazolidinone (XLIX) gave adduct (L). Dehydration of alcohol (L) was accomplished by formation of the corresponding mesylate (LI),which underwent elimination in the presence of DBU, to produce a 4:1 mixture of Z and E olefins. After chromatographic isolation of the major Z isomer (LII), diastereoselective Michael addition using a 1:2 mixture of 2-aminothiophenol (VII) and the corresponding lithium thiophenoxide furnished (LIII) as the major diastereoisomer. Intramolecular cyclization of the amino imide (LIII) to the benzothiazepinone (LIV) was accomplished in the presence of trimethylaluminium in refluxing CH2Cl2. The methoxymethyl group of (LIV) was then removed by treatment with TiCl4, leading to the key precursor the thiazepinone (VIII)Intermediates: Serial No.[(2R,3S,5R)-5-(6-hydroxy-9H-purin-9-yl)-3-[(phenoxycarbothioyl)oxy]tetrahydro-2-furanyl]methyl acetate(VII) methyl 2-phenoxyacetate (X)Reference 1:Miyata, O.; Shinada, T.; Ninomiya, I.; Naito, T.; Asymmetric construction of two contiguous stereocenters by diastereoface differentiating addition reaction of thiols to chiral imides: Formal synthesis of (+)-diltiazem. Tetrahedron 1997, 53, 7, 2421.Reference 2:Miyata, O.; Shinada, T.; Ninomiya, I.; Naito, T.; Asymmetric induction at two contiguous stereogenic centers by diastereoface differentiating nucleophilic addition reaction. Tetrahedron Lett 1991, 32, 29, 3519.Route 12Alkylation of the lactam N of thiazepinone (I) with 2-(dimethylamino)ethyl chloride (II) in the presence ofK2CO3 or under phase-transfer conditions gave thiazeinone (III). Subsequent esterification of (III) with acetic anhydride furnished the title compound.Alternatively, diltiazem has been prepared by acetylation of thiazeinone (I) with Ac2O to yield the lactam acetate ester (IV), which is then alkylated with chloride (II) under phase-transfer conditionsIntermediates: Serial No. ethyl (Z)-3-(2-methoxy-4-pyridinyl)-2-propenoate (II) Reference 1:Coffen, D.L.; Madan, P.B.; Schwartz, A. (F. Hoffmann-La Roche AG); Process for the preparation of optically pure aminophenylthio- and aminonaphthalenylthio-propanoic acids. EP 0343474 .Reference 2:Honma, T.; Igarashi, K. (Shionogi & Co. Ltd.); Process for production of diltiazem hydrochloride. US 4552695 .Reference 3:Murthy, K.; Weeratunga, G.; Burchat, A. (ACIC (Canada) Inc.); Method for the manufacture of benzothiazepine derivs.. WO 9638429 .Reference 4:Piselli, F.L.; Boschi, P.; Navoni, C.; A process for the preparation of diltiazem. EP 0594101 .Reference 5:Bhushan, L.B.; Jayachandran, B.E.; Bhushan, L.V.; Thottappillil, R. (Council of Scientific and Industrial Research); Process for preparing diltiazem. US 5869697 .Reference 6:Hytonen, M. (Orion Corporation); Process for the manufacture of diltiazem. EP 0728751; JP 1996253464; US 5644054 .Reference 7:Hulshof, L.A.; Kuilman, T.; Process for preparing 1,5-benzothiazepin derivs.. EP 0450705 .Reference 8:Manghisi, E.; Cascio, G. (Lusofarmaco); Process for the optical resolution of thedl-alpha-hydroxy-3-(4-methoxyphenyl)-3-(2-aminophenylthio)propionic acid. US 4533748 .Reference 9:Simonovitch, H.; Hoffmann, T.; Sassoon, S. (Abic, Ltd.); A process for the preparation of benzothiazepin derivs.. EP 0158303 .Reference 10:Hytonen, M. (Orion Corporation); Method for the preparation of pharmaceutically active benzothiazepine derivs.. EP 0702009 .Reference 11:Manghisi, E.; Perego, B.; A process for the preparation of diltiazem. WO 9210485 .Route 13Similarly, thiazepinone (I) was transesterified with isopropenyl acetate (V) to afford acetate ester (IV). Subsequent alkylation of the lactam N of (IV) with 2-(dimethylamino)ethyl mesylate (VI) in the presence of K2CO3 furnished diltiazem.Similarly, thiazepinone (I) was transesterified with isopropenyl acetate (V) to afford acetate ester (IV). Subsequent alkylation of the lactam N of (IV) with 2-(dimethylamino)ethyl mesylate (VI) in the presence of K2CO3 furnished diltiazem.Intermediates: Serial No. 4,5-diethyl-2,4-dihydro-3H-1,2,4-triazol-3-one (V) Reference 1:Harsanyi, K.; Gizur, T.; Demeter, A.; Aracs, Z.; Felmeri, J.; Berki, K.; Trischeler, F.; Vincze, Z. (Gedeon Richter Ltd.); Process for the preparation of benzothiazepin-4(5H)-one derivs.. ES 2001146; JP 1987108872 .Route 14Several related procedures utilize racemic intermediates that are resolved in more advanced synthetic steps. The racemic trans-glycidic ester (IX) was prepared by Darzens condensation between anisaldehyde (VII) and methyl chloroacetate (VIII). Opening of the epoxide group of (IX) with 2-aminothiophenol (X) in hot chlorobenzene in the presence of FeCl3 gave rise to the racemic threo adduct (XI) which, without isolation, was cyclized to the cis-lactam (XII) by addition of methanesulfonic acid and then heating to reflux. Alkylation of the lactam N of (XII) with 2-(dimethylamino)ethyl chloride (II) led to the racemic precursor (XIII). Resolution of (XIII) to provide the (S,S)-isomer (VII) was then accomplished by preferential crystallization of supersaturated solutions of several sulfonate salts of (XIII) upon seeding with the desired enantiomer. Racemic diltiazem (XIV), obtained by acetylation of (XIII), has been resolved via formation of the corresponding diastereoisomeric salts with (S)-naproxenIntermediates: Serial No.(3R,4R)-4-(difluoromethyl)-1-(4-methoxyphenyl)-3-[(triisopropylsilyl)oxy]-2-azetidinone (VIII) 1-Hydrazinecarboxamide (IX) ethyl (Z)-3-(2-methoxy-4-pyridinyl)-2-propenoate (II) [(2R,3S,5R)-5-(6-hydroxy-9H-purin-9-yl)-3-[(phenoxycarbothioyl)oxy]tetrahydro-2-furanyl]methyl acetate(X) methyl 2-phenoxyacetate (VII)Reference 1:Gizur, T.; Harsanyi, K.; Fogassy, E.; Studies of the resolution of racemates in the synthesis of diltiazem. J Prakt Chem Chem-Ztg 1994, 336, 7, 628.Reference 2:Yamada, S.; Yoshioka, R.; Shibatani, T.; Optical resolution of a 1,5-benzothiazepine derivative,a synthetic intermediate of diltiazem, by preferential crystallization and diastereomeric salt formation. Chem Pharm Bull 1997, 45, 12, 1922.Route 15. 4-Hydroxycinnamic acid (XV) was acetylated with Ac2O in pyridine, and the resultant 4-acetoxycinnamic acid (XVI) was converted to the cinnamyl alcohol (XVIII) via conversion to the mixed anhydride (XVII) and subsequent reduction with NaBH4. Sharpless asymmetric epoxidation of (XVIII) furnished the chiral epoxide alcohol (XIX). After oxidation of alcohol (XIX) to the glycidic acid (XX) by means of RuO2/NaIO4, treatment with dimethyl sulfate and Et3N gave rise to the corresponding methyl ester (XXI). Epoxide opening in (XXI) with HCl and pyridine hydrochloride produced chlorohydrin (XXII) as a mixture of the desired(2S,3R)-isomer and minor amounts of the corresponding 3-chloro epimer. Condensation of (XXII) with2-nitrothiophenol (XXIII) provided, after recrystallization from EtOH, the pure (S,S)-thioether adduct (XXIV). The hydroxyl group of (XXIV) was then protected as the methoxymethyl ether (XXV) by means of methylal in the presence of P2O5Intermediates: Serial No. 2-Fluoro-5-(trifluoromethyl)phenol (XV) Reference 1:Honma, T.; Igarashi, K. (Shionogi & Co. Ltd.); Process for production of diltiazem hydrochloride. US 4552695 .Route 16Selective hydrolysis of the acetate ester of (XXV) using benzylamine in THF led to phenol (XXVI), which was further methylated by means of diazomethane, yielding methyl ether (XXVII). Reduction of the nitrogroup (XXVII) to the corresponding aniline (XXVIII) was then performed employing ferrous sulfate and ammonium hydroxide. Subsequent saponification of the methyl ester group of (XXVIII) gave amino acid (XXIX). Cyclization of (XXIX) with ethyl chloroformate and triethylamine furnished lactam (XXX). The lactam N of (XXX) was then alkylated by 2-(dimethylamino)ethyl chloride (II) to produce (XXXI). Finally, deprotection of the methoxymethyl group of (XXXI) and concomitant O-acetylation was accomplished by treatment with acetyl chloride and TiCl4Intermediates: Serial No. ethyl (Z)-3-(2-methoxy-4-pyridinyl)-2-propenoate (II) Reference 1:Honma, T.; Igarashi, K. (Shionogi & Co. Ltd.); Process for production of diltiazem hydrochloride. US 4552695 .Route 17The racemic precursor threo-hydroxy nitro ester (III), prepared by addition of 2-nitrothiophenol (II) to the racemic trans-glycidate (I), has been optically resolved by enantioselective lipase-catalyzed esterification of the (R,R)-isomer, producing the (R,R)-acetate (IV), while leaving unaltered the target intermediate, the (S,S)-hydroxy nitroester enantiomer (V). The racemic hydroxy nitro ester (III) has also been resolved through formation of the diastereoisomeric salts with L-lysineIntermediates: Serial No. ethyl (Z)-3-(2-methoxy-4-pyridinyl)-2-propenoate (I) Reference 1:Kanerva, L.T.; Sundholm, O.; Lipase catalysis in the resolution of racemic intermediates of diltiazem synthesis in organic solvents. J Chem Soc - Perkins Trans I 1993, 13, 1385.Reference 2:Senuma, M.; Shibazaki, M.; Nishimoto, S.; Shibata,K.; Okamura, K.; Date, T.; The practical resolution of (2RS,3RS)-2-hydroxy-3-(4-methoxyphenyl)-3-(2-nitrophenylthio)propionic acid, a key intermediate for diltiazem, with L-lysine. Chem Pharm Bull 1989, 37, 12, 3204.。
心内科常用泵入药物

心内科常用泵入药物微量泵能根据医嘱要求将少量药液微量、精确、持续、均匀地泵入患者体内,使药物在体内能保持有效血药浓度以抢救危重病人。
心血管疾病常用微量泵药物根据药理作用分为以下七类:一、降压药物,降压药物使用过程中均需要监测患者血压水平,警惕低血压的发生。
1.硝普钠(50mg/支)配制方法:硝普钠注射剂50mg+5%GS(NS)50mL 微量泵入1.5ml/h(据血压调整剂量),注意避光。
用量:初始泵入速度为0.2~0.5μg/kg/min,根据血压控制情况以0.5~1mg/次递增,极量为10μg/kg/min。
总量为按体重3.5mg/kg。
监护事项:硝普钠对光敏感,溶液稳定性差,滴注溶液应新鲜配置并迅速将输液泵避光,同时使用避光输液器;硝普钠的毒性来自于其代谢产物氰化物,使用过程中需警惕氰化物中毒。
若出现氰化物中毒等情况,可考虑应用硫代硫酸钠解毒,用灭菌注射用水溶解成5%溶液后应用,肌肉或静脉注射一次0.5~1g。
2.盐酸乌拉地尔(5ml:25mg)配制方法:乌拉地尔注射液100mg+NS30mL 静脉泵入6mg/h(据血压调整剂量)。
用量:初始泵入速度为6~10mg/h,根据目标血压调整微量泵流速,每次调整2~4mg,每15~30min调整一次。
3.盐酸尼卡地平配制方法:使用原液20mg(20mL)直接泵入;使用NS或5%GS稀释成浓度为0.01%~0.02%的溶液,即1mL 输液中含有盐酸尼卡地平0.1~0.2mg。
用量:高血压急症时,初始剂量2mg/h,最大剂量20mg/h,或以0.5~6μg/kg/min给药,根据血压控制情况调节滴注速度。
监护事项:本品为钙拮抗剂,通过抑制钙离子内流而发挥血管扩张作用,可能会引起反射性心动过速;同时由于其强扩血管作用,颅内高压及可疑活动性颅内出血的患者应予以禁用。
二、降压降心率药物,此类药物兼有降血压和减慢心率的作用。
1. 艾司洛尔配制方法:艾司洛尔注射液为水针剂,可以使用原液2000mg/20mL泵入。
盐酸地尔硫唑片工艺-概述说明以及解释

盐酸地尔硫唑片工艺-概述说明以及解释1.引言1.1 概述概述部分的内容可以如下所示:引言部分旨在介绍本文的主题——盐酸地尔硫唑片工艺。
盐酸地尔硫唑片是一种常见的药物,具有广泛的应用领域,特别是在治疗感染性疾病方面。
本文将对盐酸地尔硫唑片的制备工艺进行详细介绍,以帮助读者了解该药物的生产过程以及相关的工艺参数。
在本文中,我们将首先介绍盐酸地尔硫唑片工艺流程和工艺参数。
工艺流程部分将逐步介绍制备盐酸地尔硫唑片的各个步骤,包括原料的选择与准备、合成反应的条件与控制、结晶与干燥等关键工艺环节。
而工艺参数部分将详细阐述影响制备质量和产量的关键参数,例如反应温度、反应时间、溶剂选择等。
在正文的最后部分,我们将对整个工艺进行总结,回顾工艺的主要步骤和参数,并评估工艺的优点和不足之处。
同时,我们也将展望未来,对盐酸地尔硫唑片工艺的改进和优化进行一些设想。
通过本文的阅读,读者将能够全面了解盐酸地尔硫唑片的制备工艺,并且能够根据工艺流程和参数进行工艺的控制和优化,以提高产品质量和产量。
同时,本文也为进一步研究和改进盐酸地尔硫唑片工艺提供了一定的参考和指导。
1.2 文章结构文章结构部分是对整篇文章的框架进行介绍和概括。
在这部分,我们可以简要说明文章的各个章节和各个章节的内容。
文章结构部分的内容可以如下所示:文章结构如下:第一部分:引言这一部分首先概述了盐酸地尔硫唑片的工艺,并说明了整篇文章的目标和意义。
第二部分:正文2.1 工艺流程本部分详细描述了盐酸地尔硫唑片的工艺流程。
包括原料准备、药物制备、制剂工艺等多个步骤,并对每个步骤的关键操作进行了阐述。
2.2 工艺参数这一部分介绍了盐酸地尔硫唑片制备过程中的一些重要参数,如温度、时间、pH值等。
同时,还对这些参数的选择和控制进行了讨论和分析。
第三部分:结论3.1 总结本部分对整篇文章进行总结,对盐酸地尔硫唑片工艺的关键点进行概括,并指出了工艺的优点和不足之处。
3.2 展望最后一部分展望了盐酸地尔硫唑片工艺的未来发展,提出了一些可以改进和优化的方向,并对未来研究方向进行了展望。
gepirone hydrochloride结构式

gepirone hydrochloride结构式1. 介绍gepirone hydrochloride是一种药物,其结构式为C16H20N2O2·HCl。
它是一种对抗焦虑和抑郁症的药物,常用于治疗各种焦虑症和抑郁症。
其化学结构和药理作用使得它成为一种独特的药物,对于患有焦虑和抑郁症的患者有着重要的治疗作用。
2. 结构式解释gepirone hydrochloride的化学结构如下:图片:C16H20N2O2·HCl的结构式图3. 结构分析gepirone hydrochloride是一种含氯有机化合物,分子结构中包含环丙基酮和吩嗪环,并且与盐酸形成盐的结构。
由结构式可以看出,它含有16个碳原子,20个氢原子,2个氧原子,2个氮原子和一个氯离子。
这种结构使得gepirone hydrochloride具有特定的药理作用和生物活性。
4. 药理作用gepirone hydrochloride主要通过影响大脑中的神经递质来发挥药理作用。
它与5-羟色胺1A受体结合,从而调节神经递质的释放和重摄取,进而改善患者的情绪和心理状态。
这种作用使得gepironehydrochloride成为一种重要的治疗焦虑和抑郁症的药物,对于改善患者的心理健康起着重要的作用。
5. 临床应用gepirone hydrochloride广泛应用于各种焦虑和抑郁症的治疗中,特别是对于那些对传统抗抑郁药物有耐受性或者需要减轻焦虑症状的患者。
它可以单独使用或者与其他药物联合使用,根据患者的具体情况进行治疗。
临床研究表明,gepirone hydrochloride在改善患者的抑郁和焦虑症状上有显著的疗效,成为一种重要的治疗药物。
6. 临床研究大量的临床研究表明,gepirone hydrochloride在治疗焦虑和抑郁症方面具有显著疗效,尤其是对于那些对其他药物有耐受性或者存在严重不良反应的患者。
临床试验数据显示,gepirone hydrochloride可以显著改善患者的心理状态,提高生活质量,减轻焦虑和抑郁症状。
盐酸地尔硫卓注射液说明书

盐酸地尔硫卓注射液盐酸地尔硫卓注射液使用说明书•【药品名称】通用名称:盐酸地尔硫卓注射液汉语拼音:Yansuan Di'erliuzhuo Zhusheye•【成份】本品主要成份为:盐酸地尔硫卓•【适应症】可用于控制心房颤动的心室率。
•【规格】(1)10mg(2)50mg•【用法用量】静脉注射:成人用量,初次为10mg,临用前用氯化钠注射液或葡萄糖注射液溶解、稀释成1%浓度,在3分钟内缓慢注射,或按体重0.15~0.25mg/kg计算剂量,15分钟后可重复,也可按体重每分钟5~15μg/kg静脉滴注。
•【不良反应】1.国外治疗心绞痛时以安慰剂对照试验,结果表明,本品不良反应并不比安慰剂多。
2.临床治疗心绞痛病人观察到最常见的不良反应和发生率为:浮肿(2.4%)、头痛(2.1%)、恶心(1.9%)、眩晕(1.5%)、皮疹(1.3%)、无力(1.2%).3.不常有的(小于1%)有以下情况:(1)心血管系统:心绞痛、心律失常、房室传导阻滞(Ⅰ、Ⅱ、Ⅲ度),心动过缓、束支传导阻滞,充血性心力衰竭、心电图异常、低血压、心悸、晕厥、心动过速、室性期前收缩。
①本品延长房室交界不应期,除病窦综合征外并不明显延长窦房结恢复时间,罕见情况下此作用可异常减慢心率(特别在病窦综合征患者)或致Ⅱ或Ⅲ度房室传导阻滞。
本品与β阻滞剂或洋地黄同用可导致对心脏传导的协同作用。
②虽本品有负性肌力作用,但在心室功能正常的人血流动力学研究无心脏指数降低或对收缩性(dp/dt)持续负性作用。
在心室功能受损的患者单用本品或与β阻滞剂同用的经验有限,因而这些患者应用本品须谨慎。
③低血压者用本品治疗偶可致症状性低血压。
(2)神经系统:多梦、遗忘、抑郁、步态异常、幻觉、失眠、神经质、感觉异常、性格改变、嗜睡、震颤。
(3)消化系统:厌食、便秘、腹泻、味觉障碍、消化不良、口渴、呕吐、体重增加。
应用本品时急性肝损害为罕见情况,有碱性磷酸酶、乳酸脱氢酶、门冬氨酸氨基转移酶、丙氨酸氨基转移酶明显增高和其它伴有急性肝损害现象,停药可以恢复。
盐酸地尔硫卓片

禁忌
1.病态窦房结综合症未安装起搏器者。 2. II或III度房室传导阻滞未安装起搏器者。 3.收缩压低于12kPa(90mmHg)、心率低于50次/分者。 4.对本品过敏者。 5.充血性心力衰竭患者。
注意事项
1.本品可延长房室结不应期,除病态窦房结综合征外不明显延长窦房结恢复时间。罕见情况下此作用可异常 减慢心率(特别在病态窦房结综合征患者)或致II或III度房室传导阻滞。
适应症
1、心绞痛; 2、轻、中度高血压。
规格
30mg
用法用量
口服,每次1-2片,每日3-4次,餐前或睡前服药,每1-2天增加一次剂量,如需增加剂量,每日剂量不超过 360mg,但需在医生指导下服用。
不良反应
1.可能出现浮肿、头痛、恶心、眩晕、皮疹、无力。
2.极少出现以下情况(发生率1.0%以下):
1.对心绞痛的改善作用:
①本品可以有效扩张心外膜和心内膜下的冠状动脉及侧支血管,增加冠脉及侧支血管的血流量,从而增加缺 血心肌和血流,改善心肌缺血,缓解心绞痛。
②抑制冠脉血管的痉挛,对由冠状动脉痉挛所致的积压型心绞痛具有明显的临床疗效。
③扩张末梢血管,减轻心脏后负荷,减慢心率,降低心脏做功,对因心脏耗氧增加所致的心绞痛具有明显改 善作用。
房室传导阻滞、心动过缓、束支传导阻滞、充血性心衰、心电图异常、低血压、心悸、晕厥、心动过速、室 性早搏、多梦、遗忘、抑郁、步态异常、幻觉、失眠、神经质、嗜睡、震颤、厌食、便秘、腹泻、味觉障碍、消 化不良、口渴、呕吐、肝功能轻度异常、瘀点、光敏感、瘙痒、荨麻疹、多形红斑、剥脱性皮炎、弱视、肌酸磷 酸肌酶升高、呼吸困难、鼻出血、高血糖、高尿酸血症、阳痿、肌痉挛、多尿、耳鸣、骨关节痛、脱发、锥体外 系综合征、齿龈增生、溶血性贫血、出血时间延长、白细胞减少、紫癜、视膜病变、血小板减少。
盐酸地尔硫卓片说明书范文

盐酸地尔硫卓片说明书范文盐酸地尔硫是一种西药,患者服用前一定要先看盐酸地尔硫说明书,明确其主治功能是否和自己的病症相符合才能服用。
下面来看盐酸地尔硫说明书和其他相关内容。
1、盐酸地尔硫的成分本品主要成份盐酸地尔硫卓其化学名称为:顺-(+)-5-[(2-二甲氨基)乙基]-2-(4-甲氧基苯基)-3-乙酰氧基-2,3-二氢-1,5-苯并硫氮杂卓-4(5H)-酮盐酸盐。
2、盐酸地尔硫的功能主治冠状动脉痉挛引起的心绞痛和劳力型心绞痛。
高血压。
3、盐酸地尔硫的用法用量口服,起始剂量60~120mg/次,每日2次(一次2~4片,一日2次),平均剂量范围为240~360mg/天。
3.1、室上性心动过速:取10mg盐酸地尔硫卓溶于生理盐水中,约3分钟缓慢静脉滴注,并可根据年龄和症状适当增减;3.2、高血压急症:通常以5-15μg/kg/min,静脉滴注。
当血压降至目标值以后,可以根据血压变化适当调节滴速;3.3、手术时异常高血压的急救处理:成人1次约1分钟内缓慢静脉滴注盐酸地尔硫10mg,并可根据病人年龄和症状适当增减。
通常以5-15μg/kg/min,当血压降至目标值以后,边监测血压边调节点滴速度;3.4、不稳定心绞痛:1-5μg/kg/min,从小剂量开始静脉滴注,而后根据病情酌量增减,最大剂量不超过5μg/kg/min。
盐酸地尔硫的功效1、盐酸地尔硫的功效与作用2、盐酸地尔硫治什么病室上性心动过速;手术时异常高血压的急救处置;高血压急症;不稳定心绞痛。
服用盐酸地尔硫的注意事项1、盐酸地尔硫有副作用吗1.1、本品可延长房室结不应期,除病态窦房结综合症外不明显延长窦房结恢复时间。
罕见情况下此作用可异常减慢心率(特别在病态窦房结综合症患者)或致II或III度房室传导阻滞。
本品与β受体阻滞剂或洋地黄合用可导致对心脏传导减缓的协同作用。
有报导一例变异性心绞痛患者口服本品60mg致心脏停搏2-5秒。
1.2、本品有负性肌力作用,在心室功能受损的患者单用或与β受体阻滞剂合用的经验有限,因而这些患者应用本品须谨慎。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
盐酸地尔硫卓合成图解 Chemical Name :cis-(+)-3-Acetoxy-5-[2-(dimethylamino)ethyl]-2,3-dihydro-2-(4-methoxyphenyl)-1,5-benzothiaz epin-4(5H)-one hydrochlorideGenericName :盐酸地尔硫卓;Dilthiazem hydrochloride Other Names :Diltiazem hydrochloride;CRD-401;MK-793(maleate);SLV-324;Milptin;Teczem(Comb. with enalapril);Tiazac(slow-release);Herbesser;Adizem;Dilzem;Cardizem;Slozem;Cardizem XL;Masdil;Uni-Masdil;Anoheal;Lacerol;Cardizem LA;Tildiem CAS : 33286-22-5RelatedCAS : 42399-41-7 (free base), 144604-00-2 (monomaleate)Formula : C 22H 27ClN 2O 4SMoleWeight : 450.98849structure :Company :Biovail (Originator), Tanabe Seiyaku (Originator), Elan (Not Determined), Sanofi-Synthélabo (Not Determined), Solvay (Marketer), Aventis Pharma (Licensee), Esteve (Licensee), Forest (Licensee), Lacer (Licensee), SLA Pharma (Licensee), DOV Pharmaceutical (Usage : Angina pectoris, Treatment of, Anorectal Disorders, Treatment of, Antiarrhythmic Drugs, CARDIOVASCULAR DRUGS, GASTROINTESTINAL DRUGS, Heart Failure Therapy,Hypertension, Treatment of, Treatment of Disorders of the Coronary Arteries andAtherosclerosis, Calcium Channel BlockersRoute 1Electrophilic bromination of ethyl p-methoxycinnamate (I) by means of N-bromosuccinimide in moist acetone gave rise to the racemic erythro bromohydrin (II), which was esterified with butyric anhydride to produce the racemic bromoester (III). Kinetic resolution of (III) employing Candida cylindracea lipase caused the enantioselective hydrolysis of the (S,S)-enantiomer, yielding the chiral bromohydrin (V). Cyclization of (V) in the presence of NaOMe furnished epoxide (VI). The target thiazepinone (VIII) was then obtained by condensation between the glycidic ester (VI) and 2-aminobenzenethiol (VII)Intermediates: Seri al No.1S,5R,13R,16S,17R)-4-cyano-16-ethyl-10-methoxy-12-oxa-4-azapentacyclo[9.6.1.0(1,13).0(5,17).0(7,18)]octadeca-7(18),8,10-trien-14-one(A)[(2R,3S,5R)-5-(6-hydroxy-9H-purin-9-yl)-3-[(phenoxycarbothioyl)oxy]tetrahydro-2-furanyl]methyl acetate (VII)Reference 1:McCague, R.; Wang, S.; Taylor, S.J.C. (Celltech Group plc); Chiral arylpropionates and their use. WO 9413828 .Route 2A different method for the preparation of glycidic ester (VI) consists in the asymmetric epoxidation of ethyl 4-methoxycinnamate (I) with oxone(R) in the presence of chiral macrocyclic ketones, such as the binaphthyl ketone (IX)Intermediates: Serial No.Reference 1:Seki, M.; Furutani, T.; Imashiro, R.; Kuroda, T.; Yamanaka, T.; Harada, N.; Arawaka, H.; Kusama, M.; Hashiyama, T.; A novel synthesis of a key intermediate for diltiazem. Tetrahedron Lett 2001, 42, 46, 8201. Reference 2:Ozaki, Y.; Arakawa, H.; Harada, N.; Hashiyama, T.; Kuroda, T.; Seki, M.; Kusama, M. (Tanabe Seiyaku Co., Ltd.); Process for preparing optically active phenyloxirane cpds.. WO 9856762 .Route 3In a further process, the racemic trans glycidic ester (XII), prepared by Darzens condensation between anisaldehyde (X) and methyl chloroacetate (XI), was resolved by enantioselective enzymatic hydrolysis, using several different enzymes and reaction conditions to produce the undesired (2S,3R) acid (XIII), while leaving intact the required (2R,3S)-glycidic ester (XIV) (4-7). Opening of the chiral epoxide (XIV) with2-aminobenzenethiol (VII) proceeded with retention of the configuration, producing methyl(2S,3S)-2-hydroxy-3-(2-aminophenylsulfanyl)-3-(4-methoxyphenyl)propionate (XV) (8). Alternatively, the (S,S)-amino ester (XV) was obtained by resolution with tartaric acid of the racemic three-adduct resulting from epoxide (XII) and 2-aminobenzenethiol (VII) (9). Cyclization of amino ester (XV) in refluxing xylene in the presence of p-toluenesulfonic acid afforded the target lactam (VIII) (9). The cyclization of (XV) to lactam (VIII) was also accomplished by means of trichloroacetic acid or under basic conditionsIntermediates: Serial No.(3R,4R)-4-(difluoromethyl)-1-(4-methoxyphenyl)-3-[(triisopropylsilyl)oxy]-2-azetidinone (XI) 1-Hydrazinecarboxamide (XII) [(2R,3S,5R)-5-(6-hydroxy-9H-purin-9-yl)-3-[(phenoxycarbothioyl)oxy]tetrahydro-2-furanyl]methyl acetate(VII) methyl 2-phenoxyacetate (X) Reference 1:Bhushan, L.B.; Jayachandran, B.E.; Bhushan, L.V.; Thottappillil, R. (Council of Scientific and Industrial Research); Process for preparing diltiazem. US 5869697 .Route 4A new enantioselective method for the preparation of glycidic ester (XIV) has been disclosed. Methyl trichloroacetate (XVI) was converted to the dichloroketene silyl acetal (XVII) by treatment with zinc powder and chlorotrimethylsilane. Asymmetric aldol condensation of (XVII) with anisaldehyde (X) in the presence of the chiral oxazaborolidine catalyst (XVIII) at -78 C produced methyl(S)-2,2-dichloro-3-hydroxy-3-(4-methoxyphenyl)propionate (XIX). Reductive mono-dechlorination of (XIX) furnished chlorohydrin (XX), which was then cyclized to glycidic ester (XIV) in the presence of NaOMeIntermediates: Serial No. methyl 2-phenoxyacetate (X) Reference 1:Imashiro, R.; Kuroda, T.; Asymmetric synthesis of methyl (2R,3S)-3-(4-methoxyphenyl) glycidate, a key intermediate of diltiazem, via Mukaiyama aldol reaction. Tetrahedron Lett 2001, 42, 7, 1313.Route 5Addition of phenylmagnesium bromide to cyclohexene oxide (XXI) in the presence of CuCl gavetrans-2-phenylcyclohexanol (XXII), which was further esterified with chloroacetyl chloride to afford2-phenylcyclohexyl chloroacetate (XXIII). Enantioselective hydrolysis of the racemic ester (XXIII) by means of Pseudomonas fluorescens lipase provided pure (1R,2S)-2-phenylcyclohexanol (XXIV), which was again esterified with chloroacetyl chloride, yielding the chiral ester (XXVI). Darzens condensation of chloro ester (XXVI) with anisaldehyde (X) led to the chiral glycidic ester (XXVII). Epoxide ring opening in (XXVII) with 2-aminothiophenol (VII) furnished amino ester (XXVIII). Intermediate thiazepinone (VIII) was then obtained by cyclization of (XXVIII) using p-toluenesulfonic acid in refluxing xylene. Alternatively, amino ester (XXVIII) was first hydrolyzed to amino acid (XXIX), which was subsequently cyclized with p-toluenesulfonic acid as above (11). The final cyclization of amino acid (XXIX) to the intermediate thiazepinone (VIII) has also been carried out in the presence of trichloroacetic acidIntermediates: Serial No. 4-(dimethylamino)-N-(2,6-dimethylphenyl)-4-piperidinecarboxamide (XXIV) (2R,3R,4S,5S,6R)-3,5-bis(acetoxy)-6-[(acetoxy)methyl]-2-bromo-2-cyanotetrahydro-2H-pyran-4-yl acetate(XXI) [(2R,3S,5R)-5-(6-hydroxy-9H-purin-9-yl)-3-[(phenoxycarbothioyl)oxy]tetrahydro-2-furanyl]methyl acetate(VII) methyl 2-phenoxyacetate (X)4-{4-[4-(benzyloxy)phenyl]-1-piperazinyl}aniline;4-{4-[4-(benzyloxy)phenyl]-1-piperazinyl}phenylamine (+/--XXII)Reference 1:Plaum, M.J.M.; Boesten, W.H.J.; Process for the preparation of a benzothiazepine. EP 0796853; JP 1998007667; US 5859241 .Route 6A different strategy to reach the amino ester precursor (XXIX) was developed starting from the chiral diol (XXX), readily accessible by asymmetric dihydroxylation of cinnamate (I). Reaction of diol (XXX) with SOCl2 produced the cyclic sulfite (XXXI) (12,14). Optionally, diol (XXX) was condensed with phosgene to produce the cyclic carbonate (XXXII) (12). Opening of either sulfite (XXXI) or carbonate (XXXII) with2-aminothiophenol (VIII) proceeded with retention of the configuration, leading to the desired intermediate aminoacid (XXIX)Intermediates: Serial No.[(2R,3S,5R)-5-(6-hydroxy-9H-purin-9-yl)-3-[(phenoxycarbothioyl)oxy]tetrahydro-2-furanyl]methyl acetate(VIII)Reference 1:Lohray, B.B.; Jayachandran, B.; Bhushan, V.; Nandanan, E.; Ravindranathan, T.; Anchimeric assisted unprecedented SN(i)-type cleavage of cyclic sulfite: Application in the synthesis of the calcium channel blocker diltiazem. J Org Chem 1995, 60, 18, 5983.Reference 2:Hulshof, L.A.; Kuilman, T.; Process for preparing 1,5-benzothiazepin derivs.. EP 0450705 .Route 7The racemic thiazepinone (XXXIII) has been converted to the pure enantiomer (VIII) through a different strategy. Oxidation of hydroxy lactam (XXXIII) by means of DMSO and Ac2O produced the enol ester (XXXV). Basic hydrolysis of (XXXI) gave rise to the keto lactam (XXXV). This was then subjected to asymmetric reduction utilizing a reducing reagent generated in situ from NaBH4 and (S)-tert-leucine to afford the intermediate thiazepinone (VIII)Intermediates: Serial No. (1R,2R)-3-(acetoxy)-1-(4-methoxyphenyl)-2-methylpropyl acetateReference 1:Yamada, S.; Mori, Y.; Morimatsu, K.; Ishizu, Y.; Ozaki, Y.; Yoshioka, R.; Nakatani, T.; Seko, H.; Asymmetric reduction of a 1,5-benzothiazepine derivative with sodium borohydride-(S)-alpha-amino acids: An efficient synthesis of a key intermediate of diltiazem. J Org Chem 1996, 61, 24, 8586.Route 8The precursor cis-cinnamate (XXXIX) can be obtained by several synthetic routes. Bromination of ethyl trans-4-methoxycinnamate (I) afforded the dibromo ester (XXXVI), which underwent dehydrohalogenation and hydrolysis to the arylpropiolic acid (XXXVII) upon treatment with ethanolic KOH. Acid (XXXVII) was converted to the corresponding isopropyl ester (XXXVIII) by DCC-mediated coupling with isopropanol. Semihydrogenation of (XXXVIII) in the presence of Lindlar catalyst led to the required cis cinnamate (XXXIX). Alternatively, anisaldehyde (X) was converted to the gem-dibromostyrene (XL) by condensation with CBr4 in the presence of PPh3. Reaction of (XL) with BuLi, followed by addition of isopropyl chloroformate to the resultant lithium acetylide, furnished the arylpropiolate (XXXVIII). In another method to obtain the cis-cinnamate (XXXIX), trans-4-methoxycinnamic acid (XLI) was converted to the isopropyl ester (XLII), which was then photochemically isomerized to the desired cis-cinnamate (XXXIX)Intermediates: Serial No. methyl 2-phenoxyacetate (X) 2-[(2R)-4-benzoyl-2-(3,4-difluorophenyl)morpholinyl]ethyl benzoate (XLI) Reference 1:Jacobsen, E.N.; Deng, L.; Furukawa, Y.; Martinez, L.E.; Enantioselective catalytic epoxidation of cinnamate esters. Tetrahedron 1994, 50, 15, 4323.Route 9The key cis glycidate ester (XLIII) was prepared by (salen)Mn(III)-catalyzed asymmetric epoxidation of the cis cinnamate (XXXIX). Epoxide opening in (XLIII) with 2-nitrothiophenol (XLIV), with inversion of the configuration, led to the nitro ester adduct (XLV). The nitro group of (XLV) was then reduced to the aniline derivative (XLVI) by means of FeSO4. Subsequent isopropyl ester group saponification in (XLVI) furnished amino acid (XXIX). This was then cyclized to the target thiazepinone (VIII) in refluxing xyleneIntermediates: Serial No.Reference 1:Jacobsen, E.N.; Deng, L.; Furukawa, Y.; Martinez, L.E.; Enantioselective catalytic epoxidation of cinnamate esters. Tetrahedron 1994, 50, 15, 4323.Route 10In a variation of the preceding methods, the chiral glycidic amide (XLVII) was used as the synthetic precursor.Amide (XLVII) was either prepared by treatment of the chiral glycidic ester (VI) with ammonia, or by enzymatic resolution of (XII), followed by amidation. Iron-catalyzed addition of 2-aminothiophenol (VII) to the glycidamide (XLVII) in refluxing chlorobenzene yielded the desired threo adduct (XLVIII) as the major isomer. Cyclization of amino amide (XLVIII) under acidic conditions furnished thiazepinone (VIII)Intermediates: Serial No.1-Hydrazinecarboxamide (XII) [(2R,3S,5R)-5-(6-hydroxy-9H-purin-9-yl)-3-[(phenoxycarbothioyl)oxy]tetrahydro-2-furanyl]methyl acetate(VII)Reference 1:Yamada, S.; Tsujioka, I.; Shibatini, T.; Yoshioka, R.; Efficient alterative synthetic route to diltiazem via (2R,3S)-3-(4-methoxyphenyl)glycidamide. Chem Pharm Bull 1999, 47, 2, 146.Route 11Aldol condensation of anisaldehyde (X) with the lithium enolate of the N-acyl oxazolidinone (XLIX) gave adduct (L). Dehydration of alcohol (L) was accomplished by formation of the corresponding mesylate (LI),which underwent elimination in the presence of DBU, to produce a 4:1 mixture of Z and E olefins. After chromatographic isolation of the major Z isomer (LII), diastereoselective Michael addition using a 1:2 mixture of 2-aminothiophenol (VII) and the corresponding lithium thiophenoxide furnished (LIII) as the major diastereoisomer. Intramolecular cyclization of the amino imide (LIII) to the benzothiazepinone (LIV) was accomplished in the presence of trimethylaluminium in refluxing CH2Cl2. The methoxymethyl group of (LIV) was then removed by treatment with TiCl4, leading to the key precursor the thiazepinone (VIII)Intermediates: Serial No.[(2R,3S,5R)-5-(6-hydroxy-9H-purin-9-yl)-3-[(phenoxycarbothioyl)oxy]tetrahydro-2-furanyl]methyl acetate(VII) methyl 2-phenoxyacetate (X)Reference 1:Miyata, O.; Shinada, T.; Ninomiya, I.; Naito, T.; Asymmetric construction of two contiguous stereocenters by diastereoface differentiating addition reaction of thiols to chiral imides: Formal synthesis of (+)-diltiazem. Tetrahedron 1997, 53, 7, 2421.Reference 2:Miyata, O.; Shinada, T.; Ninomiya, I.; Naito, T.; Asymmetric induction at two contiguous stereogenic centers by diastereoface differentiating nucleophilic addition reaction. Tetrahedron Lett 1991, 32, 29, 3519.Route 12Alkylation of the lactam N of thiazepinone (I) with 2-(dimethylamino)ethyl chloride (II) in the presence ofK2CO3 or under phase-transfer conditions gave thiazeinone (III). Subsequent esterification of (III) with acetic anhydride furnished the title compound.Alternatively, diltiazem has been prepared by acetylation of thiazeinone (I) with Ac2O to yield the lactam acetate ester (IV), which is then alkylated with chloride (II) under phase-transfer conditionsIntermediates: Serial No. ethyl (Z)-3-(2-methoxy-4-pyridinyl)-2-propenoate (II) Reference 1:Coffen, D.L.; Madan, P.B.; Schwartz, A. (F. Hoffmann-La Roche AG); Process for the preparation of optically pure aminophenylthio- and aminonaphthalenylthio-propanoic acids. EP 0343474 .Reference 2:Honma, T.; Igarashi, K. (Shionogi & Co. Ltd.); Process for production of diltiazem hydrochloride. US 4552695 .Reference 3:Murthy, K.; Weeratunga, G.; Burchat, A. (ACIC (Canada) Inc.); Method for the manufacture of benzothiazepine derivs.. WO 9638429 .Reference 4:Piselli, F.L.; Boschi, P.; Navoni, C.; A process for the preparation of diltiazem. EP 0594101 .Reference 5:Bhushan, L.B.; Jayachandran, B.E.; Bhushan, L.V.; Thottappillil, R. (Council of Scientific and Industrial Research); Process for preparing diltiazem. US 5869697 .Reference 6:Hytonen, M. (Orion Corporation); Process for the manufacture of diltiazem. EP 0728751; JP 1996253464; US 5644054 .Reference 7:Hulshof, L.A.; Kuilman, T.; Process for preparing 1,5-benzothiazepin derivs.. EP 0450705 .Reference 8:Manghisi, E.; Cascio, G. (Lusofarmaco); Process for the optical resolution of thedl-alpha-hydroxy-3-(4-methoxyphenyl)-3-(2-aminophenylthio)propionic acid. US 4533748 .Reference 9:Simonovitch, H.; Hoffmann, T.; Sassoon, S. (Abic, Ltd.); A process for the preparation of benzothiazepin derivs.. EP 0158303 .Reference 10:Hytonen, M. (Orion Corporation); Method for the preparation of pharmaceutically active benzothiazepine derivs.. EP 0702009 .Reference 11:Manghisi, E.; Perego, B.; A process for the preparation of diltiazem. WO 9210485 .Route 13Similarly, thiazepinone (I) was transesterified with isopropenyl acetate (V) to afford acetate ester (IV). Subsequent alkylation of the lactam N of (IV) with 2-(dimethylamino)ethyl mesylate (VI) in the presence of K2CO3 furnished diltiazem.Similarly, thiazepinone (I) was transesterified with isopropenyl acetate (V) to afford acetate ester (IV). Subsequent alkylation of the lactam N of (IV) with 2-(dimethylamino)ethyl mesylate (VI) in the presence of K2CO3 furnished diltiazem.Intermediates: Serial No. 4,5-diethyl-2,4-dihydro-3H-1,2,4-triazol-3-one (V) Reference 1:Harsanyi, K.; Gizur, T.; Demeter, A.; Aracs, Z.; Felmeri, J.; Berki, K.; Trischeler, F.; Vincze, Z. (Gedeon Richter Ltd.); Process for the preparation of benzothiazepin-4(5H)-one derivs.. ES 2001146; JP 1987108872 .Route 14Several related procedures utilize racemic intermediates that are resolved in more advanced synthetic steps. The racemic trans-glycidic ester (IX) was prepared by Darzens condensation between anisaldehyde (VII) and methyl chloroacetate (VIII). Opening of the epoxide group of (IX) with 2-aminothiophenol (X) in hot chlorobenzene in the presence of FeCl3 gave rise to the racemic threo adduct (XI) which, without isolation, was cyclized to the cis-lactam (XII) by addition of methanesulfonic acid and then heating to reflux. Alkylation of the lactam N of (XII) with 2-(dimethylamino)ethyl chloride (II) led to the racemic precursor (XIII). Resolution of (XIII) to provide the (S,S)-isomer (VII) was then accomplished by preferential crystallization of supersaturated solutions of several sulfonate salts of (XIII) upon seeding with the desired enantiomer. Racemic diltiazem (XIV), obtained by acetylation of (XIII), has been resolved via formation of the corresponding diastereoisomeric salts with (S)-naproxenIntermediates: Serial No.(3R,4R)-4-(difluoromethyl)-1-(4-methoxyphenyl)-3-[(triisopropylsilyl)oxy]-2-azetidinone (VIII) 1-Hydrazinecarboxamide (IX) ethyl (Z)-3-(2-methoxy-4-pyridinyl)-2-propenoate (II) [(2R,3S,5R)-5-(6-hydroxy-9H-purin-9-yl)-3-[(phenoxycarbothioyl)oxy]tetrahydro-2-furanyl]methyl acetate(X) methyl 2-phenoxyacetate (VII)Reference 1:Gizur, T.; Harsanyi, K.; Fogassy, E.; Studies of the resolution of racemates in the synthesis of diltiazem. J Prakt Chem Chem-Ztg 1994, 336, 7, 628.Reference 2:Yamada, S.; Yoshioka, R.; Shibatani, T.; Optical resolution of a 1,5-benzothiazepine derivative,a synthetic intermediate of diltiazem, by preferential crystallization and diastereomeric salt formation. Chem Pharm Bull 1997, 45, 12, 1922.Route 15. 4-Hydroxycinnamic acid (XV) was acetylated with Ac2O in pyridine, and the resultant 4-acetoxycinnamic acid (XVI) was converted to the cinnamyl alcohol (XVIII) via conversion to the mixed anhydride (XVII) and subsequent reduction with NaBH4. Sharpless asymmetric epoxidation of (XVIII) furnished the chiral epoxide alcohol (XIX). After oxidation of alcohol (XIX) to the glycidic acid (XX) by means of RuO2/NaIO4, treatment with dimethyl sulfate and Et3N gave rise to the corresponding methyl ester (XXI). Epoxide opening in (XXI) with HCl and pyridine hydrochloride produced chlorohydrin (XXII) as a mixture of the desired(2S,3R)-isomer and minor amounts of the corresponding 3-chloro epimer. Condensation of (XXII) with2-nitrothiophenol (XXIII) provided, after recrystallization from EtOH, the pure (S,S)-thioether adduct (XXIV). The hydroxyl group of (XXIV) was then protected as the methoxymethyl ether (XXV) by means of methylal in the presence of P2O5Intermediates: Serial No. 2-Fluoro-5-(trifluoromethyl)phenol (XV) Reference 1:Honma, T.; Igarashi, K. (Shionogi & Co. Ltd.); Process for production of diltiazem hydrochloride. US 4552695 .Route 16Selective hydrolysis of the acetate ester of (XXV) using benzylamine in THF led to phenol (XXVI), which was further methylated by means of diazomethane, yielding methyl ether (XXVII). Reduction of the nitrogroup (XXVII) to the corresponding aniline (XXVIII) was then performed employing ferrous sulfate and ammonium hydroxide. Subsequent saponification of the methyl ester group of (XXVIII) gave amino acid (XXIX). Cyclization of (XXIX) with ethyl chloroformate and triethylamine furnished lactam (XXX). The lactam N of (XXX) was then alkylated by 2-(dimethylamino)ethyl chloride (II) to produce (XXXI). Finally, deprotection of the methoxymethyl group of (XXXI) and concomitant O-acetylation was accomplished by treatment with acetyl chloride and TiCl4Intermediates: Serial No. ethyl (Z)-3-(2-methoxy-4-pyridinyl)-2-propenoate (II) Reference 1:Honma, T.; Igarashi, K. (Shionogi & Co. Ltd.); Process for production of diltiazem hydrochloride. US 4552695 .Route 17The racemic precursor threo-hydroxy nitro ester (III), prepared by addition of 2-nitrothiophenol (II) to the racemic trans-glycidate (I), has been optically resolved by enantioselective lipase-catalyzed esterification of the (R,R)-isomer, producing the (R,R)-acetate (IV), while leaving unaltered the target intermediate, the (S,S)-hydroxy nitroester enantiomer (V). The racemic hydroxy nitro ester (III) has also been resolved through formation of the diastereoisomeric salts with L-lysineIntermediates: Serial No. ethyl (Z)-3-(2-methoxy-4-pyridinyl)-2-propenoate (I) Reference 1:Kanerva, L.T.; Sundholm, O.; Lipase catalysis in the resolution of racemic intermediates of diltiazem synthesis in organic solvents. J Chem Soc - Perkins Trans I 1993, 13, 1385.Reference 2:Senuma, M.; Shibazaki, M.; Nishimoto, S.; Shibata,K.; Okamura, K.; Date, T.; The practical resolution of (2RS,3RS)-2-hydroxy-3-(4-methoxyphenyl)-3-(2-nitrophenylthio)propionic acid, a key intermediate for diltiazem, with L-lysine. Chem Pharm Bull 1989, 37, 12, 3204.。
