欧盟对草莓中的农残限量要求
农药含量合格标准范围

农药含量合格标准范围农药是农业生产中常用的一种化学物质,可以有效地控制害虫和病害,提高农作物的产量和质量。
然而,过量使用农药会对环境和人体健康造成严重的危害。
因此,为了保障农产品的安全和消费者的健康,各国都制定了严格的农药残留标准。
在中国,农药残留标准由国家卫生健康委员会和农业农村部联合发布。
根据《食品安全国家标准食品中农药最大残留限量》(GB2763-2019)的规定,农产品中农药残留的最大限量是指在食品中允许存在的农药残留量的最高值,以毫克/千克(mg/kg)为单位。
不同的农产品和农药种类有不同的最大残留限量,具体标准如下:1. 蔬菜类:最大残留限量一般为0.01-5mg/kg,其中叶菜类和根茎类的最大残留限量较低,为0.01-0.5mg/kg;果实类和瓜果类的最大残留限量较高,为0.1-5mg/kg。
2. 水果类:最大残留限量一般为0.01-5mg/kg,其中柑橘类和浆果类的最大残留限量较低,为0.01-1mg/kg;热带水果类的最大残留限量较高,为0.1-5mg/kg。
3. 谷物类:最大残留限量一般为0.01-0.5mg/kg,其中稻谷和小麦的最大残留限量较低,为0.01-0.1mg/kg;玉米和大豆的最大残留限量较高,为0.05-0.5mg/kg。
4. 畜禽肉类:最大残留限量一般为0.01-0.1mg/kg,其中禽肉的最大残留限量较低,为0.01-0.05mg/kg;畜肉的最大残留限量较高,为0.05-0.1mg/kg。
需要注意的是,这些最大残留限量只是一个参考值,实际上,农产品中的农药残留量受到多种因素的影响,如农药的使用方法、剂量、作物品种、生长环境等。
因此,为了确保农产品的安全,消费者在购买农产品时应选择正规渠道,尽量选择有机农产品或者经过认证的绿色食品。
总之,农药残留是一个严重的食品安全问题,各国都在积极采取措施加强农药残留的监管和管理。
消费者也应该提高食品安全意识,选择安全、健康的农产品,共同维护食品安全。
欧盟农药残留限量标准

欧盟农药残留限量标准
欧盟农药残留限量标准是指欧盟对农产品中农药残留的限量标准,旨在保护消
费者免受农药残留的危害,保障食品安全。
农药残留是指农药在农产品中残留的量,其合理控制对人体健康和环境保护至关重要。
欧盟对农药残留的限量标准严格监管,对农产品进行抽样检测,确保其符合相关标准。
欧盟对农药残留的限量标准主要包括两个方面,一是对农产品中农药残留的具
体限量标准,二是对农药残留监测和检测方法的规定。
在欧盟法规中,针对不同类型的农产品,规定了相应的农药残留限量标准,如水果、蔬菜、谷物、肉类等。
同时,欧盟还规定了农药残留的监测和检测方法,确保对农产品进行全面、准确的检测。
欧盟农药残留限量标准的制定和执行,对欧盟成员国的农产品生产和贸易有着
重要影响。
符合欧盟农药残留限量标准的农产品可以进入欧盟市场,而不符合标准的农产品将受到限制。
因此,欧盟成员国的农产品生产企业和贸易商都必须严格遵守欧盟的农药残留限量标准,确保其产品符合相关要求。
在实际生产中,农产品生产企业需要加强对农药使用的管理,严格按照农药的
使用说明进行施药,避免超量使用或违规使用农药。
同时,农产品生产企业还需加强对农药残留的监测和检测,确保产品的农药残留量符合欧盟的标准要求。
只有通过严格管理和监测,才能确保农产品的质量和安全,满足欧盟的进口要求。
总之,欧盟农药残留限量标准对农产品生产和贸易有着重要影响,农产品生产
企业必须严格遵守相关标准,加强对农药使用和残留的管理和监测,确保产品的质量和安全。
只有如此,才能保障农产品进入欧盟市场,促进农产品贸易的顺利进行。
(EU)No 1881-2006 欧盟食品污染物限量标准(中文版)
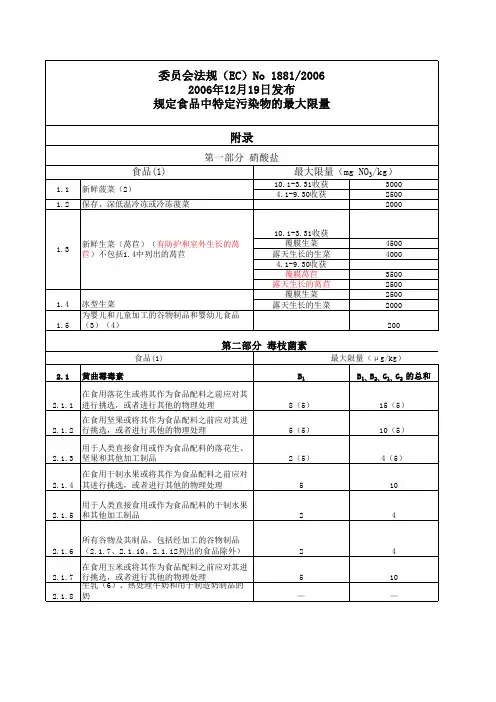
6.1.5 6.1.6 双壳贝类(26) 6.1.7 专供婴幼儿及儿童食用的经加工的谷物制品 婴幼儿奶粉和较大婴幼儿奶粉、包括婴幼儿牛 6.1.8 奶和较大婴幼儿奶(8、29) 6.1.9 专供婴幼儿食用的以医疗为目的的食疗食品
委员会法规(EC)No 1881/2006 2006年12月19日发布 规定食品中特定污染物的最大限量 附录
第一部分 硝酸盐 食品(1)
1.1 1.2 新鲜菠菜(2) 保存、深低温冷冻或冷冻菠菜 10.1-3.31收获 覆膜生菜 露天生长的生菜 4.1-9.30收获 覆膜莴苣 露天生长的莴苣 覆膜生菜 露天生长的生菜
3.3.1
0.5
3.3.2
1 200 100 50
3.4
3.4.1 3.4.2 3.4.3
锡
非饮料类罐装食品 罐装饮料,包括水果汁及蔬菜汁 专供婴幼儿食用的罐装婴儿食品及经加工的谷物食 品,不包括干燥粉末状制品(3、29) 罐装婴幼儿奶粉及较大婴幼儿罐装奶粉(包括也婴 幼儿牛奶及较大婴幼儿牛奶),不包括干燥精粉制 品(8、29) 专供婴幼儿的特殊医疗用罐装食品不包括干燥精粉 制品(8、29)
谷物、豆类蔬菜及豆类 蔬菜,不包括芸薹属蔬菜、叶类蔬菜、新鲜草本植 物及真菌(27).马铃薯的限量按照去皮马铃薯计 3.1.10 算 3.1.11 芸薹蔬菜、叶类蔬菜及养殖真菌 3.1.12 水果,不包括草莓及小水果(27) 3.1.13 草莓及小水果(27) 3.1.14 脂肪和油,不包括乳脂 3.1.15 水果汁、浓缩果汁及水果花蜜(14) 葡萄酒(包括汽酒、不包括烈酒)、苹果酒、梨酒 3.1.16 及果酒(11) 3.1.17 加香葡萄酒、加香葡萄酒饮料及加香鸡尾酒(13)
2(12) 2(12)
0.5 0.5
欧盟最新农残标准

欧盟最新农残标准————————————————————————————————作者:————————————————————————————————日期:欧盟最新农残标准茶叶农药英文名称农药中文名称MRL(mg/kg)检测限(mg/kg)指令备注ethylan 乙滴涕0.1 0.1 00 24 EC 实施日期:01/01/2001 Ethylene dibromide 二溴乙烷0.1 0.1 93 58 EEC2,4,5-T 2,4,5-涕0.05 0.05 93 58 EECAcephate 乙酰甲胺磷(欧杀松) 0.1 0.1 93 58 EECAldicarb 涕灭威(得灭克) 0.05 0.05 95 38 ECAldrin 艾氏剂见狄氏剂Amitraz 双甲脒0.1 0.1 95 38 ECAmitrole(Aminotriazole) 杀草强0.1 0.1 93 58 EECAramite 杀螨特0.1 0.1 00 24 EC 实施日期:01/01/2001 Atrazine 莠去津(草脱净) 0.1 0.1 93 58 EECAzoxystrobin 安灭达0.1 0.1 99 48 EC 2001/04/01执行Barban 燕麦灵0.1 0.1 00 24 EC 实施日期:01/01/2001 Benalaxyl 灭菌安(本达乐) 0.1 0.1 94 30 ECBenfuracarb 丙硫克百威(免扶克) 0.1 0.1 94 30 ECBenomyl 苯菌灵(免赖得) 见多菌灵Bifenthrin 联苯菊酯、氟氯菊酯 5 98 82 ECBinapacryl 双苯唑菌醇0.1 0.1 93 58 EECBromophos-ethyl 乙基溴硫磷0.1 0.1 93 58 EECBromopropylate 溴螨醇0.1 0.1 95 61 ECCamphechlor(Toxaphene) 毒杀芬0.1 0.1 93 58 EECCaptafol 敌菌丹(四氯丹) 0.1 0.1 93 58 EECCarbendazim 多菌灵(贝芬替) 0.1 0.1 93 58 EECCarbofuran 克百威(加保扶) 0.2 0.2 94 30 ECCarbosulfan 丁呋丹(丁基加保扶) 0.1 0.1 94 30 ECCartap 杀螟丹、培丹、巴丹、派丹0.1 0.1 00 24 EC 实施日期:01/01/2001Chlorbenside 氯杀螨0.1 0.1 00 24 EC 实施日期:01/01/2001 Chlorbufam 氯草灵0.1 0.1 00 24 EC 实施日期:01/01/2001 Chlordane 氯丹0.02 0.02 93 58 EECChlorfenson 杀螨酯0.1 0.1 00 24 EC 实施日期:01/01/2001 Chlormequat 矮壮素0.1 0.1 96 32 ECChlorobenzilate 乙酯杀螨醇0.1 0.1 00 24 EC 实施日期:01/01/2001 Chlorothalonil 百菌清(四氯异苯腈) 0.1 0.1 93 58 EECChloroxuron 枯草隆0.1 0.1 00 24 EC 实施日期:01/01/2001 Chlorpyrifos 毒死蜱0.1 0.1 93 58 EECChlorpyrifos-methyl 甲基毒死蜱0.1 0.1 93 58 EECCyfluthrin 氟氯氰菊酯0.1 0.1 00 42 EC 实施日期:01/07/2001Cypermethrin 氯氰菊酯(赛灭宁、三氟氯氰菊酯) 0.50.05 98 82 EC Daminozide 比久 0.1 0.1 94 30 EC DDT 滴滴涕0.2 0.05 93 58 EEC Deltamethrin 溴氰菊酯(第灭宁) 5 0.05 93 58 EECDi-allate 燕麦敌(燕麦威) 0.1 0.1 00 24 EC 实施日期:01/01/2001 Diazinon 敌草净、二嗪磷(大力松) 0.050.05 96 32 EC Dichlorprop 2,4滴丙酯 0.1 0.1 93 58 EEC Dichlorprop-P 精2,5滴丙酯见2,4滴丙酯 Dichlorvos 敌敌畏(二氯松) 0.1 0.1 98 82 EC Dicofol 三氯杀螨醇 20 98 82 EC Dieldrin 狄氏剂(包括艾氏剂) 0.02 93 57 EEC 单独或与艾氏剂合用,以狄氏剂表示Dimethoate 乐果(大灭松) 0.2 93 58 EEC Dinoseb 地乐酚 0.1 0.1 93 58 EEC Dioxathion 二恶硫磷 0.1 0.1 93 58 EECDiphenylamine 对二苯胺 0.05 0.05 00 57 EC 2001/04/01执行 Disulfoton 乙拌磷(二硫松) 0.05 0.05 96 32 EC Endosulfan 硫丹(安杀番) 30 93 58 EEC Endrin 异狄氏剂 0.01 0.01 93 58 EECEsfenvalerate 高氰戊菊酯 0.05 0.05 00 42 EC 2001/07/01执行 Ethephon 乙烯利 0.1 0.1 94 30 EC Ethion 乙硫磷(爱杀松) 2 93 58 EEC Fenarimol 氯苯嘧啶醇(芬瑞莫) 0.05 0.05 94 30 EC Fenbutatin oxide 苯丁锡 0.1 0.1 96 32 EC Fenchlorphos 皮绳硫磷 0.1 0.1 93 58 EEC Fenitrothion 杀螟硫磷(扑灭松) 0.5 98 82 EC Fentin acetate 三苯锡醋酸盐 见三苯锡化合物 Fentin compounds 三苯基锡化合物 0.1 0.1 96 32 ECFentin hydroxide羟基三苯锅见三苯锡化合物 Fenvalerate & Esfenvalerate(Sum of RR & SS isomers)氰戊菊酯和其异构体(RR 和SS 同分异构体总和)0.050.050042EC 实施日期:01/07/2001Fenvalerate & Esfenvalerate(Sum of RS & SR isomers)氰戊菊酯和其异构体(RS 和SR 同分异构体总和)0.05 0.05 00 42 EC 实施日期:01/07/2001Flucythrinate氟氰戊菊酯、扩赛宁、氟氰菊酯、甲氟菊酯、中西氟氰菊酯0.1 0.19561ECFluroxypyr 氟草烟 0.1 0.1 01 57 EC 2002/03/01执行 Furathiocarb 呋线威 0.1 0.1 94 30 EC Glyphosate 草甘膦(嘉磷塞) 0.1 0.1 93 58 EEC HCH 六六六 0.2 93 58 EEC Heptachlor七氯0.02 0.02 93 58 EEC Hexachlorobenzene 六氯苯,六氯代苯 0.01 0.01 93 58 EEC Imazalil 烯菌灵 0.1 0.1 93 58 EEC Iprodione异菌脲(侬普同)0.10.19358EECKresoxim-methyl 亚胺菌0.1 0.1 00 58 EC 实施日期:01/04/2001 Lambda-Cyhalothrin 氯氟氰菊酯 1 0.05 94 30 ECMalathion 马拉硫磷(马拉松) 0.5 98 82 ECMaleic hydrazide 抑芽丹(抑芽素) 1 1 93 58 EECMancozeb 代森锰锌(锰锌丁)浦) 见代森锰Maneb 代森锰(锰乃浦) 0.1 0.1 93 58 EECMecarbam 灭蚜磷0.05 0.05 96 32 EC 实施日期:01/07/2001 Metalaxyl 甲霜灵0.1 0.1 94 32 ECMethamidophos 甲胺磷(达马松) 0.1 0.1 93 58 EECMethidathion 甲噻硫磷(灭大松) 0.1 0.1 95 61 ECMethomyl 灭多威(纳乃得) 0.1 0.1 95 38 ECMethoxychlor 甲氧滴滴涕0.1 0.1 00 24 EC 实施日期:01/01/2001 Methyl bromide 溴甲烷0.05 0.05 93 58 EECMetiram 代森联(免得烂) 见代森锰Monocrotophos 久效磷、亚素灵、纽瓦克0.1 0.1 98 82 ECOmethoate 氧乐果(欧灭松、氧化乐果)0.1 93 58 EECParaquat 百草枯0.1 0.1 93 58 EEC Permethrin 氯菊酯 2 0.1 96 82 EC Phorate 甲拌磷(福瑞松) 0.1 0.1 96 32 EC Phosmet 亚胺硫磷、益灭松0.1 0.1 98 82 ECPhoxim 辛硫磷、巴赛松、肟硫磷、倍腈松0.1 0.1 98 82 ECPirimiphos-methyl 甲基嘧啶磷(亚特松、甲基嘧啶硫磷)0.05 0.05 95 38 ECProcymidone 腐霉利(扑灭宁) 0.1 0.1 93 58 EECProfenofos 溴丙磷、布飞松、溴氯磷0.1 0.1 95 61 ECPropargite 克螨特、丙炔螨特 5 98 82 ECPropiconazole 丙环唑0.1 0.1 94 30 ECPropineb 甲基代森锌(甲基锌乃浦) 见代森锰Propoxur 残杀威(安丹) 0.1 0.1 96 32 ECPropyzamide 拿草特0.05 0.05 96 32 ECQuinalphos 喹硫磷0.1 0.1 00 42 EC 实施日期:01/07/2001 Tecnazene 四氯硝基苯0.1 0.1 00 82 EC 2003/01/01执行TEPP 特普0.02 0.02 93 58 EECThiabendazole 噻菌灵(腐绝) 0.1 0.1 95 38 ECThiodicarb 杀虫威(硫敌克) 见灭多威Thiophanate-methyl 甲基硫菌灵(甲基多保净) 见多菌灵Triazophos 三唑磷0.05 0.05 00 42 EC 2001/07/01执行Triforine 嗪胺灵0.1 0.1 96 32 ECVinclozolin 乙烯菌核利(免克宁) 0.1 0.1 93 58 EECZineb 代森锌(锌乃浦) 见代森锰Buprofezin 噻嗪酮0.02 0.02 德国Diflubenzuron 除草尿0.05 0.02 德国Fenpropathrin 甲氰菊酯0.02 德国Prothiofos 丙硫磷 1 0.01 德国Phosalone 伏杀硫磷0.1 0.05 德国欧盟农药最高残留限量标准茶叶。
欧盟食品中微生物限量标准

L 322/12 EN Official Journal of the European UnionCOMMISSION REGULATION (EC) No 1441/2007of 5 December 20077.12.2007amending Regulation (EC) No 2073/2005 on microbiological criteria for foodstuffs(Text with EEA relevance)THE COMMISSION OF THE EUROPEAN COMMUNITIES,Having regard to the Treaty establishing the European Community,Having regard to Regulation (EC) No 852/2004 of the European Parliament and of the Council of 29 April 2004 on the hygieneof foodstuffs (1), and in particular Article 4(4) thereof,Whereas:(1) Commission Regulation (EC) No 2073/2005 of 15November 2005 on microbiological criteria for food-stuffs (2) lays down microbiological criteria for certainmicro-organisms and the implementing rules to becomplied with by food business operators when im-plementing the general and specific hygiene measuresreferred to in Article 4 of Regulation (EC) No852/2004. Regulation (EC) No 2073/2005 alsoprovides that food business operators are to ensurethat foodstuffs comply with the relevant microbiological criteria set out in Annex I to that Regulation.(2) Chapters 1 and 2 of Annex I to Regulation (EC) No2073/2005 set out food safety criteria and processhygiene criteria regarding dried infant formulae anddried dietary foods for special medical purposesintended for infants below six months of age (driedinfant formulae and dried dietary foods). Part 2.2 ofChapter 2 of that Annex provides that where driedinfant formulae and dried dietary foods are tested andEnterobacteriaceae are detected in any of the sampleunits, the batch is to be tested for Enterobacter sakazakiiand Salmonella.(3) On 24 January 2007, the Scientific Panel on BiologicalHazards (BIOHAZ Panel) of the European Food SafetyAuthority (EFSA) issued an opinion with regard toEnterobacteriaceae as indicators of Salmonella andEnterobacter sakazakii. It concluded that it is not possible(1) OJ L 139, 30.4.2004, p. 1, as corrected by OJ L 226, 25.6.2004,p. 3.(2) OJ L 338, 22.12.2005, p. 1.to establish a correlation between Enterobacteriaceae andSalmonella, and no universal correlation between Entero-bacteriaceae and Enterobacter sakazakii exists. At individualplant level, a correlation between Enterobacteriaceae andEnterobacter sakazakii may however be established.(4) Therefore the requirement laid down in Regulation(EC) No 2073/2005 as regards the testing of driedinfant formulae and dried dietary foods for Salmonellaand Enterobacter sakazakii where Enterobacteriaceae aredetected in any of the sample units should no longerapply. Part 2.2 of Chapter 2 of Annex I to that Regu-lation should therefore be amended accordingly.(5) In line with the opinion on the microbiological risks ininfant formulae and follow-on formulae issued by theBIOHAZ Panel of EFSA on 9 September 2004, micro-biological criteria on Salmonella and Enterobacteriaceaeshould be laid down for dried follow-on formulae.(6) The BIOHAZ Panel of EFSA issued an opinion on Bacilluscereus and other Bacillus spp. in foodstuffs on 26 and 27January 2005. It concluded that one of the major controlmeasures is to control temperature and to establish asystem based on hazard analysis and critical controlpoint principles. Dehydrated foods, in which thepresence of spores of pathogenic Bacillus spp. isfrequent, might permit the growth of Bacillus cereusonce rehydrated in warm water. Some dehydratedfoods, including dried infant formulae and dried dietaryfoods, are consumed by potentially fragile consumers. In line with the EFSA opinion, the numbers of Bacillus cereusspores in dried infant formulae and dried dietary foodsshould be as low as possible during processing and aprocess hygiene criterion should be laid down inaddition to good practices designed to reduce delaybetween preparation and consumption.(7) Chapter 1 of Annex I to Regulation (EC) No 2073/2005provides for the analytical reference method for staphy-lococcal enterotoxins in certain cheeses, milk powder andwhey powder. That method has been revised by theCommunity reference laboratory for coagulase positivestaphylococci. The reference to that analytical referencemethod should therefore be amended. Chapter 1 ofAnnex I to that Regulation should therefore beamended accordingly.7.12.2007 EN Official Journal of the European Union L 322/13(8) Chapter 3 of Annex I to Regulation (EC) No 2073/2005sets out sampling rules for carcasses of cattle, pig, sheep,goats and horses for Salmonella analyses. Pursuant tothose rules the sampling area is to cover a minimumof 100 cm2per site selected. However, neither thenumber of sampling sites nor the minimum total areaof sampling is specified. In order to improve theimplementation of these rules in the Community, it isappropriate to further specify in Regulation (EC) No2073/2005 that the areas most likely to be contaminatedshould be selected for sampling and that the totalsampling area should be increased. Chapter 3 ofAnnex I to that Regulation should therefore beamended accordingly.(9) In the interests of clarity of Community legislation, it isappropriate to replace Annex I to Regulation (EC) No2073/2005 by the text set out in the Annex to thisRegulation. (10) The measures provided for in this Regulation are inaccordance with the opinion of the StandingCommittee on the Food Chain and Animal Health,HAS ADOPTED THIS REGULATION:Article 1Annex I to Regulation (EC) No 2073/2005 is replaced by the text in the Annex to this Regulation.Article 2This Regulation shall enter into force on the 20th day following its publication in the Official Journal of the European Union.This Regulation shall be binding in its entirety and directly applicable in all Member States. Done at Brussels, 5 December 2007.For the CommissionMarkos KYPRIANOUMember of the CommissionL 322/14 ENChapter 1.Chapter 2.Official Journal of the European UnionANNEX‘ANNEX IMicrobiological criteria for foodstuffsFood safety criteria . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 15Process hygiene criteria . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 207.12.20072.1 Meat and products thereof . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 202.2 Milk and dairy products . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 232.3 Egg products . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 262.4 Fishery products . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 272.5 Vegetables, fruits and products thereof . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 28 Chapter3. Rules for sampling and preparation of test samples . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 293.1 General rules for sampling and preparation of test samples . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 293.2 Bacteriological sampling in slaughterhouses and at premises producing minced meat and meatpreparations . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 297.12.2007ENOfficial Journal of the European UnionL 322/15C h a p t e r 1. F o o d s a f e t y c r i t e r i aeeeeeeeeL 322/16 EN Official Journal of the European Union 7.12.2007e e e e e e e e e e7.12.2007 EN Official Journal of the European Union L 322/17e e e e e e e eL 322/18ENOfficial Journal of the European Union7.12.2007e1 (2 ) F o r p o i n t s 1.1-1.25 m = M . (3 ) T h e m o s t r e c e n t e d i t i o n o f t h e s t a n d a r d s h a l l b e u s e d . (4 ) R e g u l a r t e s t i n g a g a i n s t t h e c r i t e r i o n i s n o t r e q u i r e d i n n o r m a l c i r c u m s t a n c e s f o r t h e f o l l o w i n g r e a d y -t o -e a t f o o d s : — t h o s e w h i c h h a v e r e c e i v e d h e a t t r e a t m e n t o r o t h e r p r o c e s s i n g e f f e c t i v e t o e l i m i n a t e L . m o n o c y t o g e n e s , w h e n r e c o n t a m i n a t i o n i s n o t p o s s i b l e a f t e r t h i s t r e a t m e n t (f o r e x a m p l e , p r o d u c t s h e a t t r e a t e d i n t h e i r f i n a l p a c k a g e ), — f r e s h , u n c u t a n d u n p r o c e s s e d v e g e t a b l e s a n d f r u i t s , e x c l u d i n g s p r o u t e d s e e d s , — b r e a d , b i s c u i t s a n d s i m i l a r p r o d u c t s , — b o t t l e d o r p a c k e d w a t e r s , s o f t d r i n k s , b e e r , c i d e r , w i n e , s p i r i t s a n d s i m i l a r p r o d u c t s , — s u g a r , h o n e y a n d c o n f e c t i o n e r y , i n c l u d i n g c o c o a a n d c h o c o l a t e p r o d u c t s , — l i v e b i v a l v e m o l l u s c s . (5 ) T h i s c r i t e r i o n s h a l l a p p l y i f t h e m a n u f a c t u r e r i s a b l e t o d e m o n s t r a t e , t o t h e s a t i s f a c t i o n o f t h e c o m p e t e n t a u t h o r i t y , t h a t t h e p r o d u c t w i l l n o t e x c e e d t h e l i m i t 100 c f u /g t h r o u g h o u t t h e s h e l f -l i f e . T h e o p e r a t o r m a y f i x i n t e r m e d i a t e l i m i t s d u r i n g t h e p r o c e s s t h a t m u s t b e l o w e n o u g h t o g u a r a n t e e t h a t t h e l i m i t o f 100 c f u /g i s n o t e x c e e d e d a t t h e e n d o f s h e l f -l i f e . (6 ) 1 m l o f i n o c u l u m i s p l a t e d o n a P e t r i d i s h o f 140 m m d i a m e t e r o r o n t h r e e P e t r i d i s h e s o f 90 m m d i a m e t e r . (7 ) T h i s c r i t e r i o n s h a l l a p p l y t o p r o d u c t s b e f o r e t h e y h a v e l e f t t h e i m m e d i a t e c o n t r o l o f t h e p r o d u c i n g f o o d b u s i n e s s o p e r a t o r , w h e n h e i s n o t a b l e t o d e m o n s t r a t e , t o t h e s a t i s f a c t i o n o f t h e c o m p e t e n t a u t h o r i t y , t h a t t h e p r o d u c t w i l l n o t e x c e e d t h e l i m i t o f 100 c f u /g t h r o u g h o u t t h e s h e l f -l i f e . (8 ) P r o d u c t s w i t h p H ≤ 4,4 o r a w ≤ 0,92, p r o d u c t s w i t h p H ≤ 5,0 a n d a w≤ 0,94, p r o d u c t s w i t h a s h e l f -l i f e o f l e s s t h a n f i v e d a y s s h a l l b e a u t o m a t i c a l l y c o n s i d e r e d t o b e l o n g t o t h i s c a t e g o r y . O t h e r c a t e g o r i e s o f p r o d u c t s c a n a l s o b e l o n g t o t h i s c a t e g o r y , s u b j e c t t o s c i e n t i f i c j u s t i f i c a t i o n . ( 9 ) T h i s c r i t e r i o n s h a l l a p p l y t o m e c h a n i c a l l y s e p a r a t e d m e a t (M S M ) p r o d u c e d w i t h t h e t e c h n i q u e s r e f e r r e d t o i n p a r a g r a p h 3 o f C h a p t e r I I I o f S e c t i o n V o f A n n e x I I I t o R e g u l a t i o n (E C ) N o 853/2004 o f t h e E u r o p e a n P a r l i a m e n t a n d o f t h e C o u n c i l . ( 10 ) E x c l u d i n g p r o d u c t s w h e n t h e m a n u f a c t u r e r c a n d e m o n s t r a t e t o t h e s a t i s f a c t i o n o f t h e c o m p e t e n t a u t h o r i t i e s t h a t , d u e t o t h e r i p e n i n g t i m e a n d a wo f t h e p r o d u c t w h e r e a p p r o p r i a t e , t h e r e i s n o s a l m o n e l l a r i s k . ( 11 ) O n l y i c e c r e a m s c o n t a i n i n g m i l k i n g r e d i e n t s . ( 12 ) P r e l i m i n a r y t e s t i n g o f t h e b a t c h o f s e e d s b e f o r e s t a r t i n g t h e s p r o u t i n g p r o c e s s o r t h e s a m p l i n g m u s t b e c a r r i e d o u t a t t h e s t a g e w h e r e t h e h i g h e s t p r o b a b i l i t y o f f i n d i n g S a l m o n e l l a i s e x p e c t e d . ( 13 ) R e f e r e n c e : C o m m u n i t y r e f e r e n c e l a b o r a t o r y f o r c o a g u l a s e p o s i t i v e s t a p h y l o c o c c i . E u r o p e a n s c r e e n i n g m e t h o d f o r t h e d e t e c t i o n o f s t a p h y l o c o c c a l e n t e r o t o x i n s i n m i l k a n d m i l k p r o d u c t s . ( 14 ) P a r a l l e l t e s t i n g f o r E n t e r o b a c t e r i a c e a e a n d E . s a k a z a k i i s h a l l b e c o n d u c t e d , u n l e s s a c o r r e l a t i o n b e t w e e n t h e s e m i c r o -o r g a n i s m s h a s b e e n e s t a b l i s h e d a t a n i n d i v i d u a l p l a n t l e v e l . I f E n t e r o b a c t e r i a c e a e a r e d e t e c t e d i n a n y o f t h e p r o d u c t s a m p l e s t e s t e d i n s u c h a p l a n t , t h e b a t c h m u s t b e t e s t e d f o r E . s a k a z a k i i . I t s h a l l b e t h e r e s p o n s i b i l i t y o f t h e m a n u f a c t u r e r t o d e m o n s t r a t e t o t h e s a t i s f a c t i o n o f t h e c o m p e t e n t a u t h o r i t y w h e t h e r s u c h a c o r r e l a t i o n e x i s t s b e t w e e n E n t e r o b a c t e r i a c e a e a n d E . s a k a z a k i i . ( 15 ) E . c o l i i s u s e d h e r e a s a n i n d i c a t o r o f f a e c a l c o n t a m i n a t i o n . ( 16 ) A p o o l e d s a m p l e c o m p r i s i n g a m i n i m u m o f 10 i n d i v i d u a l a n i m a l s . ( 17 ) P a r t i c u l a r l y f i s h s p e c i e s o f t h e f a m i l i e s : S c o m b r i d a e , C l u p e i d a e , E n g r a u l i d a e , C o r y f e n i d a e , P o m a t o m i d a e , S c o m b r e s o s i d a e . ( 18 ) S i n g l e s a m p l e s m a y b e t a k e n a t r e t a i l l e v e l . I n s u c h a c a s e t h e p r e s u m p t i o n l a i d d o w n i n A r t i c l e 14(6) o f R e g u l a t i o n (E C ) N o 178/2002, a c c o r d i n g t o w h i c h t h e w h o l e b a t c h i s t o b e d e e m e d u n s a f e , s h a l l n o t a p p l y . ( 19 ) R e f e r e n c e s : 1 . M a l l e P ., V a l l e M ., B o u q u e l e t S . A s s a y o f b i o g e n i c a m i n e s i n v o l v e d i n f i s h d e c o m p o s i t i o n . J . A O A C I n t e r n a t . 1996, 79, 43-49. 2. D u f l o s G ., D e r v i n C . , M a l l e P ., B o u q u e l e t S . R e l e v a n c e o f m a t r i x e f f e c t i n d e t e r m i n a t i o n o f b i o g e n i c a m i n e s i n p l a i c e ( P l e u r o n e c t e s p l a t e s s a ) a n d w h i t i n g ( M e r l a n g u s m e r l a n g u s . J . A O A C I n t e r n a t . 1999, 82, 1097-1101.7.12.2007ENOfficial Journal of the European UnionL 322/19I n t e r p r e t a t i o n o f t h e t e s t r e s u l t sT h e l i m i t s g i v e n r e f e r t o e a c h s a m p l e u n i t t e s t e d , e x c l u d i n g l i v e b i v a l v e m o l l u s c s a n d l i v e e c h i n o d e r m s , t u n i c a t e s a n d g a s t r o p o d s i n r e l a t i o n t o t e s t i n g E . c o l i , w h e r e t h e l i m i t r e f e r s t o a p o o l e d s a m p l e .T h e t e s t r e s u l t s d e m o n s t r a t e t h e m i c r o b i o l o g i c a l q u a l i t y o f t h e b a t c h t e s t e d ( 1 ).L . m o n o c y t o g e n e s i n r e a d y -t o -e a t f o o d s i n t e n d e d f o r i n f a n t s a n d f o r s p e c i a l m e d i c a l p u r p o s e s :— s a t i s f a c t o r y , i f a l l t h e v a l u e s o b s e r v e d i n d i c a t e t h e a b s e n c e o f t h e b a c t e r i u m ,— u n s a t i s f a c t o r y , i f t h e p r e s e n c e o f t h e b a c t e r i u m i s d e t e c t e d i n a n y o f t h e s a m p l e u n i t s .L . m o n o c y t o g e n e s i n r e a d y -t o -e a t f o o d s a b l e t o s u p p o r t t h e g r o w t h o f L . m o n o c y t o g e n e s b e f o r e t h e f o o d h a s l e f t t h e i m m e d i a t e c o n t r o l o f t h e p r o d u c i n g f o o d b u s i n e s s o p e r a t o r w h e n h e i s n o t a b l e t o d e m o n s t r a t et h a t t h e p r o d u c t w i l l n o t e x c e e d t h e l i m i t o f 100 c f u /g t h r o u g h o u t t h e s h e l f -l i f e :— s a t i s f a c t o r y , i f a l l t h e v a l u e s o b s e r v e d i n d i c a t e t h e a b s e n c e o f t h e b a c t e r i u m ,— u n s a t i s f a c t o r y , i f t h e p r e s e n c e o f t h e b a c t e r i u m i s d e t e c t e d i n a n y o f t h e s a m p l e u n i t s .L . m o n o c y t o g e n e s i n o t h e r r e a d y -t o -e a t f o o d s a n d E . c o l i i n l i v e b i v a l v e m o l l u s c s :— s a t i s f a c t o r y , i f a l l t h e v a l u e s o b s e r v e d a r e ≤ t h e l i m i t ,— u n s a t i s f a c t o r y , i f a n y o f t h e v a l u e s a r e > t h e l i m i t .S a l m o n e l l a i n d i f f e r e n t f o o d c a t e g o r i e s :— s a t i s f a c t o r y , i f a l l t h e v a l u e s o b s e r v e d i n d i c a t e t h e a b s e n c e o f t h e b a c t e r i u m ,— u n s a t i s f a c t o r y , i f t h e p r e s e n c e o f t h e b a c t e r i u m i s d e t e c t e d i n a n y o f t h e s a m p l e u n i t s .S t a p h y l o c o c c a l e n t e r o t o x i n s i n d a i r y p r o d u c t s :— s a t i s f a c t o r y , i f i n a l l t h e s a m p l e u n i t s t h e e n t e r o t o x i n s a r e n o t d e t e c t e d ,— u n s a t i s f a c t o r y , i f t h e e n t e r o t o x i n s a r e d e t e c t e d i n a n y o f t h e s a m p l e u n i t s .E n t e r o b a c t e r s a k a z a k i i i n d r i e d i n f a n t f o r m u l a e a n d d r i e d d i e t a r y f o o d s f o r s p e c i a l m e d i c a l p u r p o s e s i n t e n d e d f o r i n f a n t s b e l o w 6 m o n t h s o f a g e :— s a t i s f a c t o r y , i f a l l t h e v a l u e s o b s e r v e d i n d i c a t e t h e a b s e n c e o f t h e b a c t e r i u m ,— u n s a t i s f a c t o r y , i f t h e p r e s e n c e o f t h e b a c t e r i u m i s d e t e c t e d i n a n y o f t h e s a m p l e u n i t s .H i s t a m i n e i n f i s h e r y p r o d u c t s f r o m f i s h s p e c i e s a s s o c i a t e d w i t h a h i g h a m o u n t o f h i s t i d i n e :— s a t i s f a c t o r y , i f t h e f o l l o w i n g r e q u i r e m e n t s a r e f u l f i l l e d :1. t h e m e a n v a l u e o b s e r v e d i s ≤ m2. a m a x i m u m o f c /n v a l u e s o b s e r v e d a r e b e t w e e n m a n d M3. n o v a l u e s o b s e r v e d e x c e e d t h e l i m i t o f M ,— u n s a t i s f a c t o r y , i f t h e m e a n v a l u e o b s e r v e d e x c e e d s m o r m o r e t h a n c /n v a l u e s a r e b e t w e e n m a n d M o r o n e o r m o r e o f t h e v a l u e s o b s e r v e d a r e > M .___________( 1 ) T h e t e s t r e s u l t s m a y b e u s e d a l s o f o r d e m o n s t r a t i n g t h e e f f e c t i v e n e s s o f t h e h a z a r d a n a l y s i s a n d c r i t i c a l c o n t r o l p o i n t p r i n c i p l e s o r g o o d h y g i e n e p r o c e d u r e o f t h e p r o c e s s .L 322/20ENOfficial Journal of the European Union7.12.2007C h a p t e r 2. P r o c e s s h y g i e n e c r i t e r i a2.1 M e a t a n d p r o d u c t s t h e r e o fr f r f r f r f r s f r f f f r f f f7.12.2007ENOfficial Journal of the European UnionL 322/21f f f f f ( 2 ) F o r p o i n t s 2.1.3-2.1.5 m = M . ( 3 ) T h e m o s t r e c e n t e d i t i o n o f t h e s t a n d a r d s h a l l b e u s e d . ( 4 ) T h e l i m i t s (m a n d M ) s h a l l a p p l y o n l y t o s a m p l e s t a k e n b y t h e d e s t r u c t i v e m e t h o d . T h e d a i l y m e a n l og sh a l l b e c a l c u l a t e d b y fi r s t t a k i n g a l o g v a l u e o f e a c h i n d i v i d u a l t e s t r e s u l t a n d t h e n c a l c u l a t i n g t h e m e a n o f t h e s e l o g v a l u e s . ( 5 ) T h e 50 s a m p l e s s h a l l b e d e r i v e d f r o m 10 c o n s e c u t i v e s a m p l i n g s e s s i o n s i n a c c o r d a n c e w i t h t h e s a m p l i n g r u l e s a n d f r e q u e n c i e s l a i d d o w n i n t h i s R e g u l a t i o n . ( 6 ) T h e n u m b e r o f s a m p l e s w h e r e t h e p r e s e n c e o f s a l m o n e l l a i s d e t e c t e d . T h e c v a l u e i s s u bj e c t t o r e v i e w i n o r d e r t o t ak e i n t o a c c o u n t t h e p r o g r e s s m a d e i n r e d u c i n g t h e s alm on e l l a p r e v a l e n c e . M e m b e r S t a t e so r r e g i o n s h a v i n g l o w s a l m o n e l l ap r e v a l e n c e m a y u s e l o w e r c v a l u e s e v e n b e f o r e t h e r e v i e w . ( 7 ) T h i s c r i t e r i o n s h a l l n o t a p p l y t o m i n c e d m e a t p r o d u c e d a t r e t a i l l e v e l w h e n t h e s h e l f -l i f e o f t h e p r o d u c t i s l e s s t h e n 24 h o u r s . ( 8 ) E . c o l i i s u s e d h e r e a s a n i n d i c a t o r o f f a e c a l c o n t a m i n a t i o n . ( 9 ) T h e s e c r i t e r i a a p p l y t o m e c h a n i c a l l y s e p a r a t e d m e a t (M S M ) p r o d u c e d w i t h t h e t e c h n iq u e sr e f e r r e d t o i n p a r a g r a p h 3 o f C h a p t e r I I I o f S e c t i o n V o f A n n e x I I I t o R e g u l a t i o n (E C ) N o 853/2004 o f t h e E u r o p e a n P a r l i a m e n t a n d o f t h e C o u n c i l .L 322/22ENOfficial Journal of the European Union7.12.2007I n t e r p r e t a t i o n o f t h e t e s t r e s u l t sT h e l i m i t s g i v e n r e f e r t o e a c h s a m p l e u n i t t e s t e d , e x c l u d i n g t e s t i n g o f c a r c a s e s w h e r e t h e l i m i t s r e f e r t o p o o l e d s a m p l e s .T h e t e s t r e s u l t s d e m o n s t r a t e t h e m i c r o b i o l o g i c a l q u a l i t y o f t h e p r o c e s s t e s t e d .E n t e r o b a c t e r i a c e a e a n d a e r o b i c c o l o n y c o u n t i n c a r c a s e s o f c a t t l e , s h e e p , g o a t s , h o r s e s a n d p i g s :— s a t i s f a c t o r y , i f t h e d a i l y m e a n l o g i s ≤ m ,— a c c e p t a b l e , i f t h e d a i l y m e a n l o g i s b e t w e e n m a n d M ,— u n s a t i s f a c t o r y , i f t h e d a i l y m e a n l o g i s > M. S a l m o n e l l a i n c a r c a s e s :— s a t i s f a c t o r y , i f t h e p r e s e n c e o f S a l m o n e l l a i s d e t e c t e d i n a m a x i m u m o f c /n s a m p l e s ,— u n s a t i s f a c t o r y , i f t h e p r e s e n c e o f S a l m o n e l l a i s d e t e c t e d i n m o r e t h a n c /n s a m p l e s .A f t e r e a c h s a m p l i n g s e s s i o n , t h e r e s u l t s o f t h e l a s t t e n s a m p l i n g s e s s i o n s s h a l l b e a s s e s s e d i n o r d e r t o o b t a i n t h e n n u m b e r o f s a m p l e s .E . c o l i a n d a e r o b i c c o l o n y c o u n t i n m i n c e d m e a t , m e a t p r e p a r a t i o n s a n d m e c h a n i c a l l y s e p a r a t e d m e a t (M S M ):— s a t i s f a c t o r y , i f a l l t h e v a l u e s o b s e r v e d a r e ≤ m ,— a c c e p t a b l e , i f a m a x i m u m o f c /n v a l u e s a r e b e t w e e n m a n d M , a n d t h e r e s t o f t h e v a l u e s o b s e r v e d a r e ≤ m ,— u n s a t i s f a c t o r y , i f o n e o r m o r e o f t h e v a l u e s o b s e r v e d a r e > M o r m o r e t h a n c /n v a l u e s a r e b e t w e e n m a n d M .7.12.2007ENOfficial Journal of the European UnionL 322/232.2 M i l k a n d d a i r y p r o d u c t sf s f s d --L 322/24ENOfficial Journal of the European Union7.12.2007f l- 1 ( 2 ) F o r p o i n t s 2.2.7, 2.2.9 a n d 2.2.10 m = M . ( 3 ) T h e m o s t r e c e n t e d i t i o n o f t h e s t a n d a r d s h a l l b e u s e d . ( 4 ) T h e c r i t e r i o n s h a l l n o t a p p l y t o p r o d u c t s i n t e n d e d f o r f u r t h e r p r o c e s s i n g i n t h e f o o d i n d u s t r y . ( 5 ) E . c o l i i s u s e d h e r e a s a n i n d i c a t o r f o r t h e l e v e l o f h y g i e n e . ( 6 ) F o r c h e e s e s w h i c h a r e n o t a b l e t o s u p p o r t t h e g r o w t h o f E . c o l i , t h e E . c o l i c o u n t i s u s u a l l y t h e h i g h e s t a t t h e b e g i n n i n g o f t h e r i p e n i n g p e r i o d , a n d f o r c h e e s e s w h i c h a r e a b l e t o s u p p o r t t h e g r o w t h o f E . c o l i , i t i s n o r m a l l y a t t h e e n d o f t h e r i p e n i n g p e r i o d . ( 7 ) E x c l u d i n g c h e e s e s w h e r e t h e m a n u f a c t u r e r c a n d e m o n s t r a t e , t o t h e s a t i s f a c t i o n o f t h e c o m p e t e n t a u t h o r i t i e s , t h a t t h e p r o d u c t d o e s n o t p o s e a r i s k o f s t a p h y l o c o c c a l e n t e r o t o x i n s . ( 8 ) O n l y i c e c r e a m s c o n t a i n i n g m i l k i n g r e d i e n t s . ( 9 ) P a r a l l e l t e s t i n g f o r E n t e r o b a c t e r i a c e a e a n d E . s a k a z a k i i s h a l l b e c o n d u c t e d , u n l e s s a c o r r e l a t i o n b e t w e e n t h e s e m i c r o -o r g a n i s m s h a s b e e n e s t a b l i s h e d a t a n i n d i v i d u a l p l a n t l e v e l . I f E n t e r o b a c t e r i a c e a e a r e d e t e c t e d i n a n y o f t h e p r o d u c t s a m p l e s t e s t e d i n s u c h a p l a n t , t h e b a t c h h a s t o b e t e s t e d f o r E . s a k a z a k i i . I t s h a l l b e t h e r e s p o n s i b i l i t y o f t h e m a n u f a c t u r e r t o d e m o n s t r a t e t o t h e s a t i s f a c t i o n o f t h e c o m p e t e n t a u t h o r i t y w h e t h e r s u c h a c o r r e l a t i o n e x i s t s b e t w e e n E n t e r o b a c t e r i a c e a e a n d E . s a k a z a k i i . ( 10 ) 1 m l o f i n o c u l u m i s p l a t e d o n a P e t r i d i s h o f 140 m m d i a m e t e r o r o n t h r e e P e t r i d i s h e s o f 90 m m d i a m e t e r .7.12.2007ENOfficial Journal of the European UnionL 322/25I n t e r p r e t a t i o n o f t h e t e s t r e s u l t sT h e l i m i t s g i v e n r e f e r t o e a c h s a m p l e u n i t t e s t e d .T h e t e s t r e s u l t s d e m o n s t r a t e t h e m i c r o b i o l o g i c a l q u a l i t y o f t h e p r o c e s s t e s t e d .E n t e r o b a c t e r i a c e a e i n d r i e d i n f a n t f o r m u l a e , d r i e d d i e t a r y f o o d s f o r s p e c i a l m e d i c a l p u r p o s e s i n t e n d e d f o r i n f a n t s b e l o w s i x m o n t h s o f a g e a n d d r i e d f o l l o w -o n f o r m u l a e :— s a t i s f a c t o r y , i f a l l t h e v a l u e s o b s e r v e d i n d i c a t e t h e a b s e n c e o f t h e b a c t e r i u m ,— u n s a t i s f a c t o r y , i f t h e p r e s e n c e o f t h e b a c t e r i u m i s d e t e c t e d i n a n y o f t h e s a m p l e u n i t s .E . c o l i , E n t e r o b a c t e r i a c e a e (o t h e r f o o d c a t e g o r i e s ) a n d c o a g u l a s e -p o s i t i v e s t a p h y l o c o c c i :— s a t i s f a c t o r y , i f a l l t h e v a l u e s o b s e r v e d a r e ≤ m ,— a c c e p t a b l e , i f a m a x i m u m o f c /n v a l u e s a r e b e t w e e n m a n d M , a n d t h e r e s t o f t h e v a l u e s o b s e r v e d a r e ≤ m ,— u n s a t i s f a c t o r y , i f o n e o r m o r e o f t h e v a l u e s o b s e r v e d a r e > M o r m o r e t h a n c /n v a l u e s a r e b e t w e e n m a n d M .P r e s u m p t i v e B a c i l l u s c e r e u s i n d r i e d i n f a n t f o r m u l a e a n d d r i e d d i e t a r y f o o d s f o r s p e c i a l m e d i c a l p u r p o s e s i n t e n d e d f o r i n f a n t s b e l o w s i x m o n t h s o f a g e :— s a t i s f a c t o r y , i f a l l t h e v a l u e s o b s e r v e d a r e ≤ m ,— a c c e p t a b l e , i f a m a x i m u m o f c /n v a l u e s a r e b e t w e e n m a n d M , a n d t h e r e s t o f t h e v a l u e s o b s e r v e d a r e ≤ m ,— u n s a t i s f a c t o r y , i f o n e o r m o r e o f t h e v a l u e s o b s e r v e d a r e > M o r m o r e t h a n c /n v a l u e s a r e b e t w e e n m a n d M .。
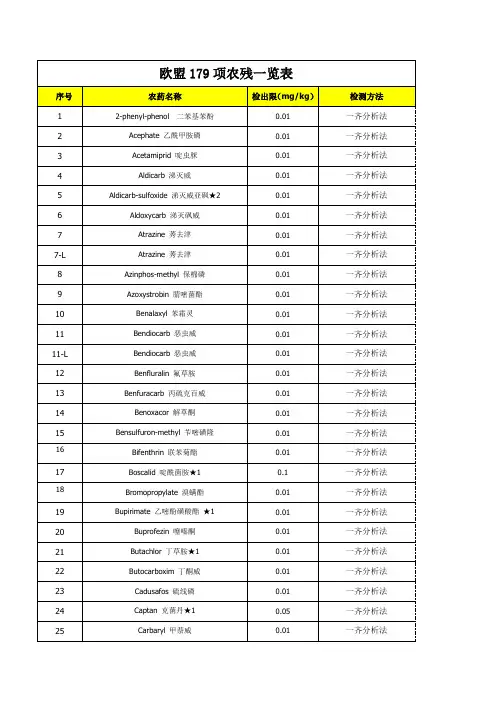
欧盟179项农残一览表
一齐分析法
141
Pyrimethanil嘧霉胺
0.01
一齐分析法
142
Quinalphos喹硫磷
0.01
一齐分析法
143
Quintozene五氯硝基苯★1
0.01
一齐分析法
144
Quizalofop-ethyl喹禾灵
0.01
一齐分析法
145
Rimsulfuron砜嘧磺隆
0.01
一齐分析法
146
一齐分析法
101
Methoxyfenozide甲氧虫酰肼★1
0.01
一齐分析法
102
Metolachlor异丙甲草胺
0.01
一齐分析法ຫໍສະໝຸດ 103Mevinphos速灭磷
0.01
一齐分析法
104
Monocrotophos久效磷
0.01
一齐分析法
105
Myclobutanil腈菌唑
0.01
一齐分析法
106
Napropamide敌草胺
0.01
一齐分析法
107
Nicosulfuron烟嘧磺隆
0.01
一齐分析法
108
Nitrothal-isopropyl酞菌酯
0.01
一齐分析法
109
Omethoate氧乐果
0.01
一齐分析法
110
Oxadixyl恶霜灵
0.05
一齐分析法
111
Oxadiazon恶草酮
0.1
一齐分析法
112
Oxydemeton-methyl砜吸磷
0.01
一齐分析法
34
Chlorpyrifos毒死蜱
欧盟最新农残标准
欧盟最新农残标准茶叶农药英文名称农药中文名称MRL(mg/kg)检测限(mg/kg)指令备注ethylan 乙滴涕0.1 0.1 00 24 EC 实施日期:01/01/2001 Ethylene dibromide 二溴乙烷0.1 0.1 93 58 EEC2,4,5-T 2,4,5-涕0.05 0.05 93 58 EECAcephate 乙酰甲胺磷(欧杀松) 0.1 0.1 93 58 EECAldicarb 涕灭威(得灭克) 0.05 0.05 95 38 ECAldrin 艾氏剂见狄氏剂Amitraz 双甲脒0.1 0.1 95 38 ECAmitrole(Aminotriazole) 杀草强0.1 0.1 93 58 EECAramite 杀螨特0.1 0.1 00 24 EC 实施日期:01/01/2001 Atrazine 莠去津(草脱净) 0.1 0.1 93 58 EECAzoxystrobin 安灭达0.1 0.1 99 48 EC 2001/04/01执行Barban 燕麦灵0.1 0.1 00 24 EC 实施日期:01/01/2001 Benalaxyl 灭菌安(本达乐) 0.1 0.1 94 30 ECBenfuracarb 丙硫克百威(免扶克) 0.1 0.1 94 30 ECBenomyl 苯菌灵(免赖得) 见多菌灵Bifenthrin 联苯菊酯、氟氯菊酯 5 98 82 ECBinapacryl 双苯唑菌醇0.1 0.1 93 58 EECBromophos-ethyl 乙基溴硫磷0.1 0.1 93 58 EECBromopropylate 溴螨醇0.1 0.1 95 61 ECCamphechlor(Toxaphene) 毒杀芬0.1 0.1 93 58 EECCaptafol 敌菌丹(四氯丹) 0.1 0.1 93 58 EECCarbendazim 多菌灵(贝芬替) 0.1 0.1 93 58 EECCarbofuran 克百威(加保扶) 0.2 0.2 94 30 ECCarbosulfan 丁呋丹(丁基加保扶) 0.1 0.1 94 30 ECCartap 杀螟丹、培丹、巴丹、派丹0.1 0.1 00 24 EC 实施日期:01/01/2001Chlorbenside 氯杀螨0.1 0.1 00 24 EC 实施日期:01/01/2001 Chlorbufam 氯草灵0.1 0.1 00 24 EC 实施日期:01/01/2001 Chlordane 氯丹0.02 0.02 93 58 EECChlorfenson 杀螨酯0.1 0.1 00 24 EC 实施日期:01/01/2001 Chlormequat 矮壮素0.1 0.1 96 32 ECChlorobenzilate 乙酯杀螨醇0.1 0.1 00 24 EC 实施日期:01/01/2001 Chlorothalonil 百菌清(四氯异苯腈) 0.1 0.1 93 58 EECChloroxuron 枯草隆0.1 0.1 00 24 EC 实施日期:01/01/2001 Chlorpyrifos 毒死蜱0.1 0.1 93 58 EECChlorpyrifos-methyl 甲基毒死蜱0.1 0.1 93 58 EECCyfluthrin 氟氯氰菊酯0.1 0.1 00 42 EC 实施日期:01/07/2001Cypermethrin 氯氰菊酯(赛灭宁、三氟氯氰菊酯)0.5 0.05 98 82 ECDaminozide 比久 0.1 0.1 94 30 EC DDT 滴滴涕0.2 0.05 93 58 EEC Deltamethrin 溴氰菊酯(第灭宁) 5 0.05 93 58 EECDi-allate 燕麦敌(燕麦威) 0.1 0.1 00 24 EC 实施日期:01/01/2001 Diazinon 敌草净、二嗪磷(大力松) 0.050.05 96 32 EC Dichlorprop 2,4滴丙酯 0.1 0.1 93 58 EEC Dichlorprop-P 精2,5滴丙酯见2,4滴丙酯 Dichlorvos 敌敌畏(二氯松) 0.1 0.1 98 82 EC Dicofol 三氯杀螨醇 20 98 82 EC Dieldrin 狄氏剂(包括艾氏剂) 0.02 93 57 EEC 单独或与艾氏剂合用,以狄氏剂表示Dimethoate 乐果(大灭松) 0.2 93 58 EEC Dinoseb 地乐酚 0.1 0.1 93 58 EEC Dioxathion 二恶硫磷 0.1 0.1 93 58 EECDiphenylamine 对二苯胺 0.05 0.05 00 57 EC 2001/04/01执行 Disulfoton 乙拌磷(二硫松) 0.05 0.05 96 32 EC Endosulfan 硫丹(安杀番) 30 93 58 EEC Endrin 异狄氏剂 0.01 0.01 93 58 EECEsfenvalerate 高氰戊菊酯 0.05 0.05 00 42 EC 2001/07/01执行 Ethephon 乙烯利 0.1 0.1 94 30 EC Ethion 乙硫磷(爱杀松) 2 93 58 EEC Fenarimol 氯苯嘧啶醇(芬瑞莫) 0.05 0.05 94 30 EC Fenbutatin oxide 苯丁锡 0.1 0.1 96 32 EC Fenchlorphos 皮绳硫磷 0.1 0.1 93 58 EEC Fenitrothion 杀螟硫磷(扑灭松) 0.5 98 82 EC Fentin acetate 三苯锡醋酸盐 见三苯锡化合物 Fentin compounds 三苯基锡化合物 0.1 0.1 96 32 ECFentin hydroxide羟基三苯锅见三苯锡化合物 Fenvalerate & Esfenvalerate(Sum of RR & SS isomers)氰戊菊酯和其异构体(RR 和SS 同分异构体总和)0.050.050042EC 实施日期:01/07/2001Fenvalerate & Esfenvalerate(Sum of RS & SR isomers)氰戊菊酯和其异构体(RS 和SR 同分异构体总和)0.05 0.05 00 42 EC 实施日期:01/07/2001Flucythrinate氟氰戊菊酯、扩赛宁、氟氰菊酯、甲氟菊酯、中西氟氰菊酯0.1 0.19561ECFluroxypyr 氟草烟 0.1 0.1 01 57 EC 2002/03/01执行 Furathiocarb 呋线威 0.1 0.1 94 30 EC Glyphosate 草甘膦(嘉磷塞) 0.1 0.1 93 58 EEC HCH 六六六 0.2 93 58 EEC Heptachlor七氯0.02 0.02 93 58 EEC Hexachlorobenzene 六氯苯,六氯代苯 0.01 0.01 93 58 EEC Imazalil 烯菌灵 0.1 0.1 93 58 EEC Iprodione 异菌脲(侬普同) 0.1 0.1 93 58 EECKresoxim-methyl 亚胺菌 0.1 0.1 00 58 EC 实施日期:01/04/2001 Lambda-Cyhalothrin 氯氟氰菊酯 1 0.05 94 30 EC Malathion马拉硫磷(马拉松)0.59882ECMaleic hydrazide 抑芽丹(抑芽素) 1 1 93 58 EECMancozeb 代森锰锌(锰锌丁)浦) 见代森锰Maneb 代森锰(锰乃浦) 0.1 0.1 93 58 EECMecarbam 灭蚜磷0.05 0.05 96 32 EC 实施日期:01/07/2001 Metalaxyl 甲霜灵0.1 0.1 94 32 ECMethamidophos 甲胺磷(达马松) 0.1 0.1 93 58 EECMethidathion 甲噻硫磷(灭大松) 0.1 0.1 95 61 ECMethomyl 灭多威(纳乃得) 0.1 0.1 95 38 ECMethoxychlor 甲氧滴滴涕0.1 0.1 00 24 EC 实施日期:01/01/2001 Methyl bromide 溴甲烷0.05 0.05 93 58 EECMetiram 代森联(免得烂) 见代森锰Monocrotophos 久效磷、亚素灵、纽瓦克0.1 0.1 98 82 ECOmethoate 氧乐果(欧灭松、氧化乐果)0.1 93 58 EECParaquat 百草枯0.1 0.1 93 58 EEC Permethrin 氯菊酯 2 0.1 96 82 EC Phorate 甲拌磷(福瑞松) 0.1 0.1 96 32 EC Phosmet 亚胺硫磷、益灭松0.1 0.1 98 82 ECPhoxim 辛硫磷、巴赛松、肟硫磷、倍腈松0.1 0.1 98 82 ECPirimiphos-methyl 甲基嘧啶磷(亚特松、甲基嘧啶硫磷)0.05 0.05 95 38 ECProcymidone 腐霉利(扑灭宁) 0.1 0.1 93 58 EECProfenofos 溴丙磷、布飞松、溴氯磷0.1 0.1 95 61 ECPropargite 克螨特、丙炔螨特 5 98 82 ECPropiconazole 丙环唑0.1 0.1 94 30 ECPropineb 甲基代森锌(甲基锌乃浦) 见代森锰Propoxur 残杀威(安丹) 0.1 0.1 96 32 ECPropyzamide 拿草特0.05 0.05 96 32 ECQuinalphos 喹硫磷0.1 0.1 00 42 EC 实施日期:01/07/2001 Tecnazene 四氯硝基苯0.1 0.1 00 82 EC 2003/01/01执行TEPP 特普0.02 0.02 93 58 EECThiabendazole 噻菌灵(腐绝) 0.1 0.1 95 38 ECThiodicarb 杀虫威(硫敌克) 见灭多威Thiophanate-methyl 甲基硫菌灵(甲基多保净) 见多菌灵Triazophos 三唑磷0.05 0.05 00 42 EC 2001/07/01执行Triforine 嗪胺灵0.1 0.1 96 32 ECVinclozolin 乙烯菌核利(免克宁) 0.1 0.1 93 58 EECZineb 代森锌(锌乃浦) 见代森锰Buprofezin 噻嗪酮0.02 0.02 德国Diflubenzuron 除草尿0.05 0.02 德国Fenpropathrin 甲氰菊酯0.02 德国Prothiofos 丙硫磷 1 0.01 德国Phosalone 伏杀硫磷0.1 0.05 德国欧盟农药最高残留限量标准茶叶。
欧盟农药
w ww .t bt -s ps .g ov .c n欧盟食品中农药残留限量标准欧盟农药残留限量标准由欧盟健康与消费者保护总司负责制定。
为解决各成员国食品及饲料中农药残留限量标准不一致的问题,欧盟于2005年5月颁布了关于“植物和动物源性食品和饲料中农药最大残留标准以及修订理事会91/414/EEC 指令的第396/2005号议会与委员会法规”,要求欧盟各成员必须实施统一的农药最高残留限量标准,对于无具体限量标准且不属于豁免物质的农药残留则实施0.01mg/kg 的一律标准。
该法规共有7个附录,包括:食品与饲料分类表(附录1)、正式限量标准(附录2,包括关于谷物中农药残留限量的86/362/EEC 指令、关于动物源性食品中农药残留限量的86/363/EEC 指令、关于植物源性食品中农药残留限量的90/642/EEC 指令等三个指令制定的协调标准)、临时限量标准(附录3包括欧盟水平尚未协调的农药残留限量标准以及86/362/EEC 、86/363/EEC 、90/642/EEC 等三个指令未涉及的食品中农药残留限量标准)、豁免物质清单(附录4)、受分析方法限制产品的特定一律标准(附录5)、某些加工产品的稀释因子(附录6)、成员国可制定高于欧盟协调标准的特定农药/产品清单(附录七,主要是在成员国内部作为熏蒸剂使用的农药)。
目前,欧盟已完成附录I 、II 、III 、IV 、VII 等的制定,法规也于2008年9月1日生效。
附录I 、II 、III 、IV 的数据可在数据库中查询。
附录I 和VII 具体内容分别见附件1和附件2 :附件1:食品与饲料分类表wwwwwwwwwwwwwwwwwwwwwwwwwwwwwwwww附件2:表VII被当作熏蒸剂在收获后的产品上使用且产品不直接被消费者食用时,成员国可以制定本国标准的农药及其适用产品清单ww.sps-tbt.www。
欧盟对草莓中的农残限量要求
0,02*
0,02*
0,02* 0,05*
Cypermethrin (cypermethrin including other mixtures of constituent isomers (sum of isomers)) (F) Cyproconazole (F) Cyprodinil (F) (R) Cyromazine Dalapon Daminozide (sum of daminozide and 1,1-dimethyl-hydrazine, expressed as daminozide) Dazomet (Methylisothiocyanate resulting from the use of dazomet and metam) DDT (sum of p,p´-DDT, o,p´-DDT, p-p´-DDE and p,p´-TDE (DDD) expressed as DDT) (F) Deltamethrin (cis-deltamethrin) (F) Desmedipham Diallate Diazinon (F) Dicamba Dichlobenil Dichlorprop: sum of dichlorprop (including dichlorprop-P) and its conjugates, expressed as dichlorprop Dichlorvos
1 0,01* 0,05* 0,01* 0,02 0,01* 0,01* 0,02* 0,1* 0,05* 0,02* 0,05
5 0,05* 0,05* 0,05* 0,2 0,5 0,05* 0,01* 0,01* 0,05* 0,01* 0,05*
0,5
0,02* 2
欧盟规定农药最大残留限量指标
0.01 氟草隆(Fluometuron)
0.01
三唑锡,三环锡(Azocyclotin, cyhexatin)
0.01 氟虫腈(Fipronil)
0.005
6
T/SNX 6-2020
三得芬(Tridemorph)
0.01 氟酰胺(Flutolanil)
0.01
三氟甲磺隆(Tritosulfuron)
1 甲基氟啶黄隆(Flupyrsulfuron-methyl) 0.01 甲基环丙烯(1-methylcyclopropene) 0.05 甲基立枯磷(Tolclofos-methyl)
0.01 0.01 0.5 0.02 0.01 0.01
0.01 甲基苯噻隆(Methabenzthiazuron)
0.02
乙丁烯氟灵(Ethalfluralin)
0.01 氯苯胺灵(Chlorpropham)
0.01
乙呋草黄(Ethofumesate)
氯草敏((Chloridazon (R) (sum of
0.03
0.1
chloridazon and chloridaz)
乙基多杀菌素(Spinetoram)
0.5 氯菊酯(Permethrin)
0.5 炔苯酰草胺(Propyzamide)
0.01
二氯喹啉酸(Quinclorac)
炔草酸及其异构体及其盐类((Clodinafop
0.01
0.02
and its S-isomers and their salts, exp)
二溴乙烷(EDB)
0.0பைடு நூலகம் 炔螨特(Propargite)
0.01
二甲四氯丙酸(Mecoprop)
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
0,02* 0,02* 0,05* 0,01*
10 0,05* 0,05*
0,05*
0,05* 0,02*
0,1*
0,01*
3 0,1 0,5 0,05* 0,01* 0,05* 0,01* 10 30 0,05* 0,01* 0,05* 0,05* 1 3 0,02* 0,01* 0,01* 0,1* 0,02* 3 0,01*
Pesticides Web Version - EU MRLs (File created on 12/12/2013 02:44)
截至到2013年12月12日
Pesticide residues and maximum residue levels (mg/kg)
(*) Indicates lower limit of analytical determination
2
0,02*
0,02*
0,02* 0,05*
Cypermethrin (cypermethrin including other mixtures of constituent isomers (sum of isomers)) (F) Cyproconazole (F) Cyprodinil (F) (R) Cyromazine Dalapon Daminozide (sum of daminozide and 1,1-dimethyl-hydrazine, expressed as daminozide) Dazomet (Methylisothiocyanate resulting from the use of dazomet and metam) DDT (sum of p,p´-DDT, o,p´-DDT, p-p´-DDE and p,p´-TDE (DDD) expressed as DDT) (F) Deltamethrin (cis-deltamethrin) (F) Desmedipham Diallate Diazinon (F) Dicamba Dichlobenil Dichlorprop: sum of dichlorprop (including dichlorprop-P) and its conjugates, expressed as dichlorprop Dichlorvos
1 0,01* 0,05* 0,01* 0,02 0,01* 0,01* 0,02* 0,1* 0,05* 0,02* 0,05
5 0,05* 0,05* 0,05* 0,2 0,5 0,05* 0,01* 0,01* 0,05* 0,01* 0,05*
0,5
0,02* 2
0,01* 0,5 0,02* 5 0,05* 0,01* 0,05*
Cyflufenamid: sum of cyflufenamid (Z-isomer) and its E-isomer Cyfluthrin (cyfluthrin including other mixtures of constituent isomers (sum of isomers)) (F) Cyhalofop-butyl (sum of cyhalofop butyl and its free acids) Cymoxanil
0152000: (b) Strawberries 1,1-dichloro-2,2-bis(4-ethylphenyl)ethane (F) 1,2-dibromoethane (ethylene dibromide) (F) 1,2-dichloroethane (ethylene dichloride) (F) 1,3-Dichloropropene 1-methylcyclopropene 1-Naphthylacetamide 1-Naphthylacetic acid 2,4,5-T (F) 2,4-D (sum of 2,4-D and its esters expressed as 2,4-D) 2,4-DB (sum of 2,4-DB, its salts, its esters and its conjugates, expressed as 2,4-DB) (R) 2-phenylphenol 8-hydroxyquinoline (sum of 8-hydroxyquinoline and its salts, expressed as 8hydroxyquinoline) Abamectin (sum of avermectin B1a, avermectinB1b and delta-8,9 isomer of avermectin B1a) (F) Acephate Acequinocyl Acetamiprid (R) Acetochlor Acibenzolar-S-nd acibenzolar acid (CGA 210007) expressed as acybenzolar-S-methyl) Aclonifen Acrinathrin (F) Alachlor
0,01* 0,01* 0,01* 0,05* 0,01* 0,05* 0,05* 0,05* 0,05* 0,05* 0,05*
0,01*
0,1
0,01* 0,01* 0,5 0,02
0,02*
0,05* 0,2 0,01* 0,02* 0,01* 0,01* 0,01* 0,01* 0,01*
0,05*
Aldicarb (sum of aldicarb, its sulfoxide and its sulfone, expressed as aldicarb)
Aldrin and Dieldrin (Aldrin and dieldrin combined expressed as dieldrin) (F) Ametoctradin (R) Amidosulfuron (R) Aminopyralid Amisulbrom Amitraz (amitraz including the metabolites containing the 2,4 -dimethylaniline moiety expressed as amitraz) Amitrole Anilazine Aramite (F) Asulam Atrazine (F) Azadirachtin
Clethodim (sum of Sethoxydim and Clethodim including degradation products calculated as Sethoxydim) Clodinafop and its S-isomers, expressed as clodinafop (F) Clofentezine (R) Clomazone Clopyralid Clothianidin Copper compounds (Copper) Cyanamide including salts expressed as cyanamide Cyazofamid Cyclanilide (F) Cycloxydim including degradation and reaction products which can be determined as 3(3-thianyl)glutaric acid S-dioxide (BH 517-TGSO2) and/or 3-hydroxy-3-(3thianyl)glutaric acid S-dioxide (BH 517-5-OH-TGSO2) or methyl esters thereof, calculated in total as cycloxydim
0,01 0,01* 0,01* 0,5 0,05*
1
Azimsulfuron Azinphos-ethyl (F) Azinphos-methyl (F) Azocyclotin and Cyhexatin (sum of azocyclotin and cyhexatin expressed as cyhexatin) Azoxystrobin Barban (F) Beflubutamid Benalaxyl including other mixtures of constituent isomers including benalaxyl-M (sum of isomers) Benfluralin (F) Benfuracarb Bentazone (sum of bentazone and the conjugates of 6-OH and 8-OH bentazone expressed as bentazone) (R) Benthiavalicarb (Benthiavalicarb-isopropyl (KIF-230 R-L) and its enantiomer (KIF-230 S-D) and diastereomers (KIF-230 R-L and KIF-230 S-D) Bifenazate Bifenox (F) Bifenthrin (F) Binapacryl (F) Biphenyl Bitertanol (F) Bixafen (R) Boscalid (F) (R) Bromide ion Bromophos-ethyl Bromopropylate (F) Bromoxynil (bromoxynil including its esters expressed as bromoxynil) (F) Bromuconazole (sum of diasteroisomers) (F) Bupirimate Buprofezin (F) Butralin Butylate Cadusafos Camphechlor (Toxaphene) (F) (R) Captafol (F) Captan (R) Carbaryl (F) Carbendazim and benomyl (sum of benomyl and carbendazim expressed as carbendazim) (R) Carbetamide Carbofuran (sum of carbofuran and 3-hydroxy-carbofuran expressed as carbofuran) Carbosulfan Carboxin Carfentrazone-ethyl (determined as carfentrazone and expressed as carfentrazoneethyl)
