电化学基础 单元检测
陕西省西安中学2017-2018年高二第二学期《电化学单元检测》试题 无答案-精选文档

电化学基础单元检测题一、选择题(每小题2分,共50分,每小题都只有一个选项符合题意)1.关于右图所示的原电池,下列说法正确的是A .电子从铜电极通过检流计流向锌电极B .盐桥中的阳离子向硫酸铜溶液中迁移C .锌电极发生还原反应,铜电极发生氧化反应D .铜电极上发生的电极反应是↑=+-+222H e H2.观察下列几个装置示意图,有关叙述正确的是A .装置①中阳极上析出红色固体(两电极均为石墨电极)B .装置②的待镀铁制品应与电源正极相连C .装置③闭合电键后,外电路电子由a 极流向b 极D .装置④的离子交换膜允许阳离子、阴离子、水分子自由通过(提示:图中离子交换膜为阳离子交换膜)3.如图所示,a 、b 、c 均为石墨电极,d 为碳钢电极,通电进行电解。
假设在电解过程中产生的气体全部逸出,下列说法正确的是A .甲、乙两烧杯中溶液的pH 均保持不变B .甲烧杯中a 的电极反应式为:4OH -―4e -=O 2↑+2H 2OC .当电解一段时间后,将甲、乙两溶液混合,一定会产生蓝色沉淀D .当b 极增重3.2g 时,d 极产生的气体为2.24L (标况)4.下列反应中,不能设计成原电池的是A .2KMnO 4+16HCl=2KCl+2MnCl 2+ 5Cl 2+8H 2OB .Ba (OH )2·8H 2O+2NH 4Cl=BaCl 2+2NH 3↑+10H 2OC . 2CO+O 2=2CO 2D .2FeCl 2+Cl 2=2FeCl 35.将AsO -34+2I -+2H+ AsO -33+I 2+H 2O 设计成右图所示的电化学装置,其中C 1、C 2均为碳棒。
甲、乙两组同学分别进行下述操作:甲组:向B烧杯中逐滴加入浓盐酸乙组:向B烧杯中逐滴滴加入40%NaOH溶液下列描述中,正确的是A.甲组操作过程中,C2做负极B.乙组操作过程中,C1上发生的电极反应为:2I--2e-=I2C.两次操作过程中,微安表(G)指针的偏转方向相反D.甲组操作时该装置为原电池,乙组操作时该装置为电解池6.某同学按右图所示的装置进行电解实验。
电化学基础阶段质量检测答案

(满分100分时间90分钟)一、选择题(本题包括16小题,每小题3分,共48分)1.解析:干电池将化学能转化为电能;电解是将电能转化为化学能;水力发电是将势能转化为电能;太阳能热水器是将太阳能转化为热能。
答案:D2.解析:铜锌原电池工作时,电子由负极(锌)沿外电路流向正极(铜),B错误。
答案:B3.解析:此原电池放电时,反应消耗硫酸,使溶液的酸性降低。
答案:B4.解析:A、B中形成原电池都是铁作负极,加速铁的腐蚀;D中铸铁管作阳极,加速腐蚀;C中锌比铁活泼,铁作正极,受到保护。
答案:C5.解析:铅蓄电池放电时铅电极失去电子发生氧化反应,A错误;电解饱和食盐水,阳极上得到氯气,阴极上得到氢气,B错误;电镀时电镀液采用镀层金属的可溶性盐溶液,C正确;食盐水为中性溶液,所以应发生吸氧腐蚀,D错误。
答案:C6.解析:由图知,烧杯b中的Zn棒失去电子,发生氧化反应,电子转移到Fe棒上,烧杯a中通入的氧气在Fe棒表面得电子生成氢氧根离子,使a中溶液的pH升高。
所以正确的为A、B。
答案:AB7.解析:在氢氧燃料电池的负极上反应的是氢气;粗铜精炼时,纯铜与电源的负极相连,钢铁腐蚀的负极反应是Fe-2e-===Fe2+。
答案:A8.解析:水电解时阴极为2H++2e-===H2↑,C项错误;燃料电池放电时负极为H2+2OH--2e-===2H2O,故D项错误;A项中H2O可以循环利用,错误;B项正确。
答案:B9.解析:根据原电池工作原理,电子由负极(Cu)沿导线传递给正极(Ag),电解质溶液中阴离子沿盐桥从乙流向甲;正极(Ag):2Ag++2e-===2Ag,负极(Cu):Cu-2e-===Cu2+,总反应:Cu+2Ag+===Cu2++2Ag,与将Cu片浸入AgNO3溶液中发生的化学反应相同。
故选D。
答案:D10.解析:电解时阳极不能选用活泼的金属材料,故A错;电解饱和食盐水时阴极产物为还原产物H2,故B错;电解时阳极产物为氧化产物氯气,故C正确;电解饱和食盐水除生成氢气和氯气外,在阴极还有NaOH产生,所以电解实验结束后,搅拌溶液,溶液中有NaOH,显碱性,故D错。
化学单元测考试试题-电化学基础-A卷-附答案.doc

单元训练金卷■高三■化学卷(A )第十二单元电化学基础注意事项:1. 答题前,先将自己的姓名、准考证号填写在试题卷和答题卡上,并将准考证号条 形码粘贴在答题卡上的指定位罝。
2. 选择题的作答:每小题选出答案后,用2B 铅笔把答题卡上对应题0的答案标号涂 黑,写在试题卷、草稿纸和答题卡上的非答题区域均无效。
3. 非选择题的作答:用签字笔直接答在答题卡上对应的答题区域内。
写在试题卷、 草稿纸和答题卡上的非答题区域均无效。
4. 考试结束后,请将本试题卷和答题卡一并上交。
可能用到的相对原子质量:HJ C:12 N:14 0:16 Al:27 Cu:64 Zn:65一、选择题(每小题3分,共48分)1. 课堂学习中,同学们利用铝条、锌片、铜片、导线、电流计、橙汁、烧杯等用品探究原租 池的组成。
下列结论错误的是A. 原电池是将化学能转化成电能的装置B. 原电池巾电极、电解质溶液和导线等组成C. 图屮a 极为铝条、b 极为锌片时,导线屮会产生电流D. 图中a 极为锌片、b 极为铜片时,电子由铜片通过导线流向锌片2. 如图,在盛有稀H 2SO 4的烧杯中放入用导线连接的电极X 、Y,外电路中电子流向如图所 示,关于该装置的下列说法正确的是稀 H 2SO 4 fOlT — ---- ------------- --- —) 电极a%,电极b■ ■ ■ —*y 橙汁 -A.外电路的电流方向为X->外电路一>YB.若两电极分别为铁和碳棒,则X为碳棒,Y为铁C.X极上发生的是还原反应,Y极上发生的是氧化反应D.若两电极都是金属,则它们的活动性强弱为X〉Y3.下列有关电化学装賈完全正确的是4.烧杯A中盛放0.1 mol/L的H2SO4溶液,烧杯B中盛放0.1 mol/L的CuCl2溶液(两种溶液均足量),组成的装置如图所示。
下列说法不正确的是■ MB .•• •» •• •» •稀硫酸氯化铜溶液A BA.A为原电池,B为电解池B.A为电解池,B力原电池C.当A烧杯屮产生0.1 mol气体时,B烧杯中产生气体的物质的呈也为0.1 molD.经过一段时间,B烧杯中溶液溶质的浓度减小5.锌空气燃料电池可用作电动车动力电源,电池的电解质溶液为KOH溶液,反应为2Zn +O2+4OH_+2H2O===2Zn(OH)f。
强烈推荐电化学测试题(含答案)

电化学单元测试题可能用到的相对原子质量 H 1 C 12 N 14 O 16 Cu 64 Zn 65 第Ⅰ卷(选择题 共48分)一、选择题(本题包括16小题,每题3分,共48分。
每小题只有一个选项符合题意。
) 1、实验室中欲快速制取氢气,最好的方法应该是A .纯锌与稀硫酸 B .纯锌与浓硫酸 C .粗锌与稀硫酸D .粗锌与稀硝酸 2、金属防护的方法不正确的是A .对健身器材涂油漆以防止生锈B .将炊具制成铁铜合金而不用纯铁制品C .用牺牲锌块的方法来保护船身D .自行车的钢圈上镀上一层铬防锈 3、关于如右图所示装置的叙述,正确的是A .铜是阳极,铜片上有气泡产生B .铜片质量逐渐减少C .电流从锌片经导线流向铜片D .氢离子在铜片表面被还原4、下列关于原电池的叙述中,正确的是A .原电池中,正极就是阳极,负极就是阴极B .电子从负极经电解质溶液流向正极C .原电池工作时,溶液中的阳离子向负极移动D .形成原电池时,在负极上发生氧化反应5、下列关于原电池、电解池、电镀的说法中正确的是A .原电池中负极金属材料一定失电子被氧化B .用惰性电极电解Na 2SO 4溶液本质是电解水C .在铁片表面镀锌,将锌作阳极,铁做阴极D .在电镀池中电解质溶液浓度和溶液pH 都不变6、化学用语是学习化学的重要工具,下列用来表示物质变化的化学用语中,正确..的是 A .电解饱和食盐水时,阳极的电极反应式为:2Cl - -2e -=Cl 2 ↑B .氢氧燃料电池的负极反应式:O 2 + 2H 2O + 4e - =4OH -C .粗铜精炼时,与电源正极相连的是纯铜,电极反应式为:Cu-2e - = Cu 2+D .钢铁发生电化学腐蚀的正极反应式:Fe-2e - = Fe 2+7、对于金属冶炼的工业方法。
下列有关说法中正确的是A .可用电解饱和的MgCl 2溶液的方法获得金属镁B .工业上常用电解CuSO 4溶液的方法冶炼金属铜C .电解熔融Al 2O 3方法冶炼金属铝时,同时要加入冰晶石作熔剂D .工业上常采用活泼金属还原法冶炼金属银8、若某电能与化学能的转化装置(电解池或原电池)中发生反应的总反应离子方程式是:Cu+2H +=Cu 2++H 2↑,则关于该装置的有关说法正确的是A .该装置可能是原电池,也可能是电解池B .该装置只能是原电池,且电解质溶液为硝酸C .该装置只能是电解池,电解质溶液不可能是盐酸D .该装置只能是电解池,且金属铜为该电解池的阳极9、用铂电极电解CuSO 4溶液,当阴极端发现有气体产生时,继续再通电一会,则加入下面C K 1 ° ° ° ° K 2NaCl )Li 接,浸入硫酸铜溶液中,发现A 棒从加是(向(序为(填写字母)。
人教版高中化学选修4第4章 电化学基础 测试题
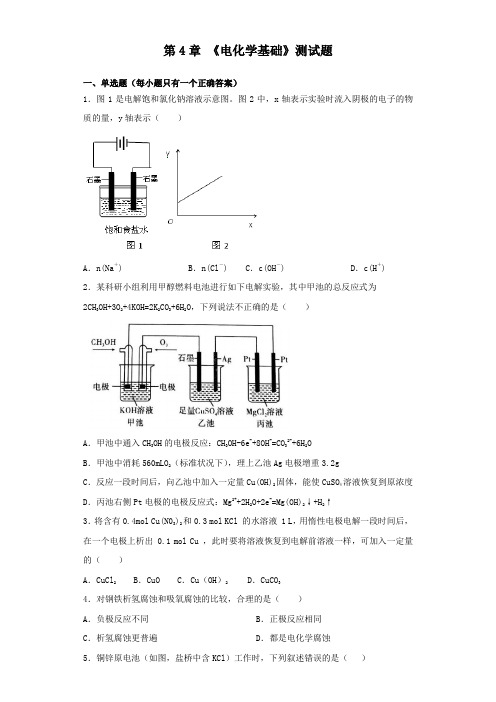
第4章《电化学基础》测试题一、单选题(每小题只有一个正确答案)1.图1是电解饱和氯化钠溶液示意图。
图2中,x轴表示实验时流入阴极的电子的物质的量,y轴表示()A.n(Na+) B.n(Cl-) C.c(OH-) D.c(H+) 2.某科研小组利用甲醇燃料电池进行如下电解实验,其中甲池的总反应式为2CH3OH+3O2+4KOH=2K2CO3+6H2O,下列说法不正确的是()A.甲池中通入CH3OH的电极反应:CH3OH-6e-+8OH-=CO32-+6H2OB.甲池中消耗560mLO2(标准状况下),理上乙池Ag电极增重3.2gC.反应一段时间后,向乙池中加入一定量Cu(OH)2固体,能使CuSO4溶液恢复到原浓度D.丙池右侧Pt电极的电极反应式:Mg2++2H2O+2e-=Mg(OH)2↓+H2↑3.将含有0.4mol Cu(N03)2和0.3 mol KCl 的水溶液 1 L,用惰性电极电解一段时间后,在一个电极上析出 0.1 mol Cu ,此时要将溶液恢复到电解前溶液一样,可加入一定量的()A.CuCl2 B.CuO C.Cu(OH)2 D.CuCO34.对钢铁析氢腐蚀和吸氧腐蚀的比较,合理的是()A.负极反应不同B.正极反应相同C.析氢腐蚀更普遍D.都是电化学腐蚀5.铜锌原电池(如图,盐桥中含KCl)工作时,下列叙述错误的是()A.正极反应为:Cu2++2e–=Cu B.电池反应为:Zn+Cu2+=Zn2+ +CuC.在外电路中,电子从负极流向正极 D.盐桥中的K+移向ZnSO4溶液6.下列有关电化学的说法正确的是()A.锌锰干电池工作一段时间后碳棒变细B.在海轮外壳上镶入锌块可减缓船体的腐蚀,是采用了牺牲阳极的阴极保护法C.铜锌原电池工作时,电子沿外电路从铜电极流向锌电极D.电解MgCl2饱和溶液,可制得金属镁7.某同学用如图所示的电化学装置电解硫酸铜溶液,有一个电极为Al,其它三个电极均为Cu,则下列说法正确的是()A.电子方向:电极Ⅳ→→电极ⅠB.电极Ⅰ发生还原反应C.电极Ⅱ逐渐溶解D.电极Ⅲ的电极反应:Cu-2e-═Cu2+ 8.下列事实不能用电化学原理解释的是( )A.铝片不用特殊方法保护B.轮船水线下的船体上装一定数量的锌块C.纯锌与稀硫酸反应时,滴入少量CuSO4溶液后速率增大D.镀锌铁比较耐用9.在盛有稀H2SO4的烧杯中放入用导线连接锌片和铜片,下列叙述正确的是()A.正极附近的SO42―离子浓度逐渐增大 B.电子通过导线由铜片流向锌片C.正极有O2逸出 D.铜片上有H2逸出10.某原电池装置如图所示,电池总反应为2Ag+Cl2=2AgCl。
2021届高三化学——电化学基础单元检测(有答案和详细解析)

2021届高三化学——电化学基础单元检测(有答案和详细解析)考生注意:
1.本试卷共4页。
2.答卷前,考生务必用蓝、黑色字迹的钢笔或圆珠笔将自己的姓名、班级、学号填写在相应位置上。
3.本次考试时间90分钟,满分100分。
4.请在密封线内作答,保持试卷清洁完整。
一、选择题(本题共12小题,每小题2分,共24分。
每小题只有一个选项符合题意。
)
1.某同学做了如下实验:
装置
现象电流表指针未发生偏转电流表指针发生偏转
下列说法中正确的是()
A.加热铁片Ⅰ所在烧杯,电流表指针会发生偏转
B.用KSCN溶液检验铁片Ⅲ、Ⅳ附近溶液,可判断电池的正、负极
C.铁片Ⅰ、Ⅲ的腐蚀速率相等
D.“电流表指针未发生偏转”,说明铁片Ⅰ、铁片Ⅱ均未被腐蚀
乙醇燃料电池中采用磺酸类质子溶剂,在200 ℃左右时供电,电池总反应式为C2H5OH+3O2===2CO2+3H2O,电池示意图如图所示,下列说法中正确的是()
A.电池工作时,质子向电池的负极迁移
B.电池工作时,电流由b极沿导线流向a极
C.a极上发生的电极反应是C2H5OH+3H2O+12e-===2CO2+12H+
D.b极上发生的电极反应是2H2O+O2+4e-===4OH-
3. 化学家正在研究尿素动力燃料电池,尿液也能发电。
用这种电池直接去除城市废水中的尿素,既能产生净化的水,又能发电,尿素燃料电池结构如图所示,下列有关描述正确的是()。
人教选修四第四章《电化学基础》单元检测题(含答案)
《电化学基础》单元检测题一、单选题1.下列关于如图所示原电池装置的叙述中,正确的是A.铜片是负极 B.铜片质量逐渐减少C.电流从锌片经导线流向铜片 D.氢离子在铜片表面被还原2.下列说法正确的是 ( )A.在原电池中,电子由正极流向负极B.在电解池中,物质在阴极发生氧化反应C.在原电池中,物质在负极发生氧化反应D.在电解池中,与电源正极相连的电极是阴极3.有两只串联的电解池(Pt为电极),甲池盛有足量的CuSO4溶液,乙池盛有足量的某硝酸盐的稀溶液。
电解时当甲池电极析出6.4gCu时,乙池电极析出21.6g金属,则乙池的溶质可能是A.NaNO3B.Cu(NO3)2C.Al(NO3)3D.AgNO34.如图所示电化学装置,X可能为“锌棒”或“碳棒”,下列叙述错误的是A.X为锌棒,仅闭合K1,Fe电极上发生还原反应B.X为锌棒,仅闭合K1,产生微量电流方向:Fe→XC.X为碳棒,仅闭合K2,该电化学保护法称为“牺牲阳极阴极保护法”D.若X为碳棒,仅闭合K1,铁电极的极反应为:Fe -2e-→ Fe2+5.燃料电池的优点是化学能直接转化为电能,而不经过热能这一中间环节,能量利用率高。
氢氧燃料电池可同时供应电和水蒸气,所需燃料为H2,电解质为熔融K2CO3。
已知该电池的正极反应为O2+2CO2+4e-2CO32-。
下列叙述正确的是( )A.放电时CO32-向正极移动B.随着反应的进行,CO32-在不断消耗C.负极反应为H2+CO32--2e-H2O+CO2D.当该电池产生的水蒸气折算成标准状况下的体积为22.4 L时,转移电子4 mol6.如图所示的装置,两烧杯中均为相应的水溶液,通电一段时间后,测得甲池中某电极质量增加2.16g,乙池中某电极上析出0.64g某金属,下列说法正确的是A.甲池是b极上析出金属银,乙池是c极上析出某金属B.甲池是a极上析出金属银,乙池是d极上析出某金属C.某盐溶液可能是CuSO4溶液D.某盐溶液可能是Mg(NO3)2溶液7.下列说法正确的是( )A.实验时酸或碱溅到眼中,应立即用水冲洗,并不断眨眼,不能用手搓揉眼睛B.检验硫酸亚铁铵溶液中Fe2+的方法是:先滴加新制氨水后滴加KSCN溶液C.证明钢铁吸收氧腐蚀的方法是:在镀锌铁皮上滴1~3滴含酚酞的饱和食盐水,静置1~2min,观察现象D.因为氧化铁是一种碱性氧化物,所以常用作红色油漆和涂料8.下列冶炼金属的原理不正确的是()A.电解饱和食盐水制备金属钠B.加热分解Ag2O制备金属银C.Fe2O3与CO高温下反应制备金属铁D.Cu2S与O2高温下反应制备金属铜9.下列各组的电极材料和电解液,不能组成原电池的是A.铜片、铜片、稀硫酸 B.铜片、石墨棒、硝酸银溶液C.锌片、铜片、稀硫酸 D.铜片、银片、FeCl3溶液10.下图中的电化学装置以甲醇(CH3OH)为主要原料合成碳酸二甲酯[(CH3O)2CO],相关说法错误的是A.B是直流电源的负极B.碳酸二甲酯中碳均为+4价C.阳极附近溶液pH降低D.每当有2molH+通过离子交换膜,消耗氧气的体积在标准状况下为11.2L 二、填空题11.某原电池的装置如图所示,看到b极上有红色金属析出,回答下列问题:①若a、b是两种活动性不同的金属,则活动性a____b(填>、<或=);②电路中的电子从____经导线流向_____(填a或b);③溶液中的SO42-向________极移动(填a或b);④若两电极分别是Al和C,则负极的电极反应式为_________________。
《电化学基础》测试卷.doc
《电化学基础》测试卷The electrochemical base test volume(1) basic practiceA,multiple choiceThe following processes need to be electrified to do so: ionization;(2) the electrolysis; (3) plating; (4) electrophoresis; The electrochemical corrosion;A,(1) (2) (3); B, (2) (3) (4); C, (2) (4) (5); D, all;At room temperature, the PH of 0. 1 molar of acid is:A, 1; B, > 1; C, < 1; D, can,t be sure;3, 1 liter contains NaOH under normal temperature of saturated salt water, the PH = 10, with platinum electrodes during electrolysis, when the cathode has a 11. 2 liter gases (standard conditions), the PH of the solution is close to (set after electrolytic solution volume is still 1 liter):A., 0; B, 12; C, 13; D, 14;The basic structure of the newly developed bromine-zinc battery in foreign countries is to use carbon rods as the poles, and the electrolyte is the zinc bromide solution. There are four electrode reactions:N - 2e = Zn = Zn + 2e 二ZnThe anode reaction and discharge when charged are:A, (4) (1); B, (2) (3); C, (3) (1); D, (2) (4);The colbay reaction is 2RC00K + 2H2OR -r + H2 + 2K0H, and the following statement is true (C)The products ofB. the products of hydrogen are produced in the cathode.C. the products of carbon are produced in the anode.D. The products of carbon are produced in the cathode.hydrogen are produced in the anode.Use 0.01 mo/litre H2S04 titrate 0. 01 mo/liter NaOH solution, neutralize and add water to 100m 1. If there is an error in the end, the ratio of 1 drop of H2S04 to 1 drop of H2S04 (1 drop to 0. 05 ml), and the ratio of C (H +) is:A: ten b. fifty c. fiveIn a beaker of saturated sodium carbonate, insert inert electrodes, keep the temperature constant, and after a certain amount of time:The PH of the solution will increaseB.The ratio of sodium ions to carbonate ions will be smallerC.The concentration of the solution gradually increases, and a certain amount of crystal is precipitated outThe concentration of the solution stays the same8, room temperature, the pH = 1 hydrochloride average into 2 portions, 1 add right amount water, the other one to join with the mo1ar concentration of hydrochloric acid after the same amount of NaOH solution, pH is increased by 1, belong to the water and the volume of NaOH solution is as follows:A.10 c. 11 d. 129, there are five bottles of solution are respectively (1) 10 ml of 0. 60 mo/NaOH aqueous solution (2) 20 ml mo / 0. 50 litres of sulfuric acid aqueous solution (3) 30 ml of 0. 40 the 40 ml/1 HC1 solution (4) the 0. 30 / d) aqueous solution (5) 50 ml mo / 0. 20 litres of sucrose solution. The order of the total number of ions and molecules in each of these bottles is:A, B, >, >, >, >, >C, >, >, >, >, >, >To connect Al and Cu slices to a wire, a group of HN03 is inserted into a dilute NaOH solution, which forms the original cel 1. In the two original batteries, the two are:A, Al, Al pills; B, Cu, Al pills; C, Al, Cu pills; D, Cu and Cu pills;11, the mass fraction of 0. 052 (5. 2%) of the NaOlI solution 1 liter (density of 1.06 g/ml) with a platinum electrode electrolysis, when the mass fraction of the NaOH solution changed 0. 010 stops electrolysis(1. 0%), should comply with the relationships of the solution is: The mass fraction of NaOHThe mass of the anode (grams)・ The mass of the cathode deposition.19152B0. 062 (6. 2%)152 a.0. 062 (6. 2%)0. 042 (4. 2%)1.29.4D0. 042 (4. 2%)9.41.212, a team of extracurricular activities, will cut a piece of galvanized iron in the conical flask, and drops into a small amount of salt water to soak, addend phenolphthalein try drops again, according to the figure device experiment, observation, a few minutes later the following phenomenon is impossible:A,B, the air bubble in the ducts;B,B, a water column formed in the trachea;C,metal shears red; A BZinc is corroded;13, a new type of fuel cell, it is porous nickel plate as the electrode in KOH solution, then access to polar ethane and oxygen respectively, the overall reaction is: 2 c2h6 o2 + 7 + 8 KOH k2co3 + 10 二4 h2o; The correct inference about the batteryis:A, the negative reaction is: 14H20 + 702 + 28e -.B: after a period of discharge, the PH around the negative pole rises. C, 1 molc2h6, and 14 moles of electrons transferred on the circuit.D,the concentration of KOH in the process of discharge is essentially the same;14,contains two kinds of solute in a solution of NaCl and - H2S04, the amount of substance ratio of 3:1, the mixed solution with graphite electrode electrolysis, according to the electrode product, can be clearly divided into three stages・ The following statements are incorrect:A, the cathode starts from the beginning and only comes out of H2; B, the anode comes out of the C12, and then it comes out of 02.The final stage of C and electrolysis is electrolytic water; D, the PH of the solution goes up, up to 7.The anode is made of iron, and copper is an electrolyte of the sufficient amount of NaOH solution, and after a period of time, two moles of Fe (OH) 3 solid are obtained, and the water is consumed in this room:A, , 3 mol. B, 4 mol. C, 5 mol; D, 6 mol;16, 100 ml, 2 mo/liter of NaOH solution, 100 ml, 2 mo/1 - H2S04 solution and mix a certain amount of ammonia, the solution obtained make phenolphthalein solution show pale red, the relationship between ion concentration in the solution is correct:A, C (S042 —)二C (Na +) > C (NH4 +) >・B, C (Na +) > C (S042 -)> C (NH4 +)・C, C (NH4 +), >, C (S042 —)二 C (Na +), >, C (OH C(H +)・C (H +) + C (NH4 +) + C (Na +)二C (OH -)+ 2C (S042 -);The PH value of the acid and acetic acid are diluted to the original n times m, and the PH of the two solutions is still the same, and the relationship between n and m isA, m = n B, m > n C, m < n D, m? nThe volume of hydrochloric acid with the same volume, the same pH, NaOH, and NH3 H20 is VI, V2, and V3, and the three relationships are:A, VI, >, V2 bl, V3, B, V3, V2, > VI, C, V3, >, V2, V2, VI, V2, VI, V2, VI, V2, VI, V2, all the way to V2Second, fills up the topicHydrogen peroxide (H202) and water are extremely weak electrolytes, but H202 is more acidic than H20.(1)if you view H202 as a di-acid, write the ionization equation in the water:(2)because of the weak acidity of H202, it can form positive salt with strong base, and also form acid salt under certain conditions・ Write the chemical equation of the salt in the form of H202 and Ba (OH) 2:(3)hydroelectricity is dissociated from the generation of H30 + and OH 一called water・Like water, hydrogen peroxide has a very weak since I ionization, it accidentally ionization equation is as follows:;The industrial wastewater containing cr2o72-acid industrial waste water is used in the following way: the appropriate amount of NaCl is added to the industrial waste water, and the mixing is uniform・Using Fe to electrolyte the electrode, over a period of time there are Cr (OH) 3 and Fe (OH) 3 deposition; The waste water reaches the discharge standard. Try to answer:(1)electrode reaction in electrolysis: anode cathode(2)write an ion reaction equation that changes Cr2072 to Cr3 + •■(3)how does the precipitation of the Cr (OH) 3, Fe (OH) 3 deposit occur in the electrolysis process?It is known that the ionizing degree of ammonia is equivalent to the degree of ionization of acetic acid, which is equal to the same temperature, and the solution of ammonium chloride is acidic・ Now, in a small amount of Mg (OH) 2 suspension, add the right amount of saturated ammonium chloride solution, and the solid is completely dissolved. A classmate,s explanation is:It's Mg (OH) 2.(2) NH4 + + H20NH3? H20 + H +; H + OH 二H20;Because NH4 + hydrolysis is acidic, H + and OH - react to form water, causing the reaction to balance right and dissolve・The interpretation of classmate b is:It's Mg (OH) 2. NH4 plus OH minus NH3? H20.Because NH4 cl is ionized by an NH4 plus the OH, which is ionized by Mg (OH) 2, which produces a weak electrolyte NH3? H20, which causes the equilibrium between the reaction, right, and Mg (OH) 2 is dissolved ・(1) c student can,t sure which students reasonable explanation, then choose one of the following reagents, to prove that a, b two classmates explain only a right, he chooses reagent is: (fill in the number)A,NH4N03; B, CH3C00NH4; C, Na2C03; D, NH3? H20.(2)please explain why the classmate made the choice・(3) in the suspension of Mg (OH) 2, the selected reagent is put into the suspension of Mg (OH) 2. As a result of this, the interpretation of the classmate is more reasonable (the or 〃b〃);Completing the NH4C1 saturation solution makes the ion equation for Mg (OH) 2 dissolved:.22. From H +, Na +, Cu2 +, Ba2 +, Cl 一,S042 一ion, choose appropriate ion of an electrolyte, electrolyte solution according to the following requirements to electrolysis (are inert electrode):(1)the electrolyte is reduced in electrolyte and the amount of water is constant・(2)the quality of the electrolyte is kept constant while electrolyte, the amount of water is reduced, and the electrolyte used is:(3)the quality of electrolytes and water changes when electrolysis, and the electrolyte used is;At room temperature, 0. 01 molCH3C00Na and 0. 004 mollICl dissolve in water and make 0. 5 L mixed solution. Judge:There is a particle in the solution;The amount of matter in the solution that has two particles must be equal to 0.01 moles, and they're sum;In this case, it's n (CH3COO 一) + n (OH -) -n (H +)二mol.Three, calculation problem24.Under a certain temperature to a certain volume density of 1. 15 g/ml of sodium chloride solution with inert electrodes electrolytic, set exactly the electrolysis of sodium chloride and no other reaction occurs, the oxygen mass fraction of 80% in the solution, try to calculate:(1)the ratio of the amount of solute and solvent in an electrolyte solution.(2)the concentration of the substance of the original sodium chloride solution・25.To 8 grams of divalent metal oxide solid dilute sulphuric acid is added to make it completely dissolved, known to the consumption of sulfuric acid volume is 100 ml, insert the platinum electrode in the solution of electrolysis, electricity after a certain period of time, collected on an electrode 224 ml (standard conditions) of oxygen, in another 1. 28 grams of the metal electrode・(1)the name of the metal oxide is determined by calculation.(2)the concentration of the substance in the solution of sulfuric acid (the volume of solution) is calculated by 100 ml.(2) ability testingA,multiple choiceAs people's quality of life improves, the problem that the battery must be focused on is raised to the agenda, which is the primary reasone the metal material of the battery caseB.prevent the contamination of soil and water by heavy metal ions such as mercury, cadmium and leadC.not to corrode other substances from the electrolyte that leaks inthe batteryRecycle the graphite electrodesThe following statement is trueZinc reacts with dilute sulphuric acid to make hydrogen,Adding a small amount of copper sulfate can accelerate the reaction. When the coating is broken, the white iron (galvanized iron) is more corrosive than the tin plate・ When plating, it is necessary to place the plated in the cathode of the electrolyte・When smelting aluminum, the aluminum oxide is added to the liquid crystal and it becomes the molten body and then the surface of the steel surface is easily corroded to produce Fe203 nh20A. in b. by c. with d. inThe right image is an electrolytic CuC12 solution, where c and d are graphite electrodes・ The following are the right onesA. a is negative, b is positive B・a is anode and b is cathodeIn the process of electrolysis, d electrode mass increases d. In the process of electrolysis, the concentration of chloride ions remains constantElectrolysis with inert electrodes・The following statement is correctA. electrolytic dilute sulphuric acid solution, which is essentiallyelectrolytic water, so the solution p H remains unchangedThe solution of sodium hydroxide is to consume OH -,so the pH of the solution decreasesC. electrolysis of sodium sulphate, which is a ratio of 1 to 2 in the cathode and anodeD.Electrolytic chlorinated copper solution, the ratio of the amount of matter in the cathode and anode is 1:1The correct description of the device in the right picture is correct A: this is an original batteryThis is an electrolytic NaOH solutionC. P is positive, and the electrode reaction is 2H + + 2e -二H2 arrowThe d. f. e is negative, and its electrodes react to it: 40H 一二2H20 + 02What happens when you get the reaction Cu + 2H20 二Cu (OH) 2 and the H2 arrowA.the copper is the anode of the original battery, the carbon is the anode of the original battery, and the sodium chloride is the electrolyte solutionB.copper zinc alloy is chemically corroded in damp aire copper to make Yin, electropositive, and electrolyte sodium sulphate solutione copper to make Yin, electropositive, electrolysis of copper sulfate solution 7. Hydrogen fuel cell is a kind of high performance battery, total reaction of 112 + 02 二2 1120, electrolyte solution of KOH solution, in the following concerning the describe of the battery is not correctA.H 2 is negative, 02 is positiveB. The PH of electrolyte solution is increasing at workThe negative reaction: 2h2-4e - + 40H - 二4H20When iron rods are connected with graphite rods, they are immersed in 0. 01 mol/L of salt solution, which may occurAn oh~b in the vicinity of an iron bar is corrodedRelease the C12 D. On the graphite rodUse the inert electrode electrolyte to electrolyte the Na2C03 solution, and if the temperature remains constant, it will be over a period of timeThe ratio of the pH of the solution to the c (Na +) and c (C032 -)is greaterC. The concentration of the solution is large, and the crystal is precipitated out・ The concentration of the solution is constant10. With a platinum electrode electrochemical a certain concentration of aqueous solution of the following materia1. After the electrolysis, add right amount of water to the rest of the electrolyte, can make the solution and electrolyticbefore is the sameA. gnoc3b・ cA, B, and C are three kinds of metals, in which case A and B are immersed in A dilute H2S04. (2) the amount of electrolytic material concentration in the same A and C of the mixed solution of salt, on the cathode precipitation C first, (using inert electrode), determineB, B, B, B, B, B, B, B, B, B, B, B, B, B, B, B, B, B, B, B, B, B, B, B, B, C, B, B, B, B, B, B, B, C, B, B, B, B, C, B, B, C, their reducing strength for sequenceB, B, B 12. With graphite electrode, electrolytic silver nitrate solution in the anode collected 0. 4 g of oxygen, and electrolytic acid generated 250 ml of a sodium hydroxide solution, is the concentration of a solution of sodium hydroxideA. 0. 20 moles of IT. 0. 15 moles of 1-1 c. 10 moles of 1-1. 0. 05 moles of 1~113.The solid-oxide fuel cell was developed by Westinghouse・ It is an electrolyte from zirconium oxide, a solid electrolyte that allows oxygen ions (02 -) to pass through during high temperatures・ The battery,s working principle is shown in the diagram, where the porous electrode a and b are not involved in the electrode reaction・ The following is trueA.there's A very strong battery in the reaction of 02B.there is an H2 participating in the positive electrode of the BThe corresponding electrode for c a is 02 + 2H20The total reaction equation of the battery is 2H2 + 02 二2H2014. With a platinum electrode electrolysis of NaCl and the mixture of CuS04 solution, when circuit through the 4 mol, electronic power, Yin and Yang both produce 1. 4 mol, gas, after electrolytic solution volume is 4 1, the final pH value of electrolyte nearestA. four b. two c. 13 d. 14There is a button microcell with an electrode of Ag20 and Zn. Electrolytic cell is KOH solution, so commonly known as silver-zinc battery, the battery electrode reaction to zinc + OH - 2 + 2 二e zinc (OH) 2, Ag20 + H20 + 2 二2 e ag + OH - the following claims, the right is for (1) zinc anode, Ag20 (2) for the positive discharge, pH near the anode rise (3) discharging, near the cathode solution pH value lower (4) anion in the solution to the positive direction, cation to negative directionA. in b. by c. in d. inWhen using graphite as an electrode for electrolysis of 3 mol/LNaCl dnd 0.4 molar 2 (S04) 3, the following curve is correct ()Second, fills up the topic17. Molten salt fuel cells with high power generation efficiency, therefore, available Li2CO3, Na2C03 and molten salt mixture of electrolyte, CO as the anode gas, a mixture of air and C02 gas for cathode to gas and work under 650 °C of the f uel cell system, complete the relevant battery reactive: reactive: battery anode reaction: 2 CO + 2 co32 — > 4 C02 + 4 e -・The cathode reaction:, total reaction: battery.Add the Na2S04 solution to the u-tube, add a few drops of litmus test liquid, insert the inert electrode, and turn on the power suppl y.(1)the observable phenomenon is observed after a period of time.(2)to cut off the power supply, remove the electrodes will be in the u-tube solution in a small beaker, stirring, observable phenomenon is that the reason for this is that・If (3) to switch to the device electrolysis with a few drops of phenolphthalein try drops Na2C03 saturated solution of anode was observed after a period of time, the cathode・ 19. A research study to explore the influencing factors of steel rust, design the following solutions: A, B, C, D four little flask respectively into dry chicken wire, soaked in salt water of fine wire, fine wire soaked in water, salt water and fine wire, and make the wire completely immersed in salt water, and then to assemble as shown in the four sets of devices, every once in A while measuring the height of the catheter in the water rise, result in the following table data (listed in the table for the catheter in the height of water rise/cm)・The rise of water at different timesTime/hour0. 51.01.52.02.53.0A bottle (dry wire)B (iron wire with salt water)0.41.23.45.67.69.8C (the wire with water in it)(the wire that is fully immersed in the salt water)If you are the group leader, please answer the following questions: (1)why the water in the catheter rises・(2)in these experiments,The speed of wire rust is in the order of large to smal1.(3)the factors that affect the rust of steel are mainly.There are two different points of view about the "pH change in the electrolytic CuC12 solution":The idea is that the "theorist" thinks that the pH of the solution after electrolytic CuC12 is elevated・Point 2 it is, "experimental" after repeated several times, precise experimental determination, prove that the electrolytic CuC12 solution pH change as shown in the curve relationship with・ Please answer the following questions:(1)of CuC12 before electrolytic solution pH value at A point location is (with ion equation)・(2)the theoretical basis of the theory is that it isIt is.(3)^experimental^ experimental conclusion is that they are "precise experiment" is described through the accurate determination of the pH of the solution.(4)what do you think? Your point of view of the reason is that (briefly) through chemistry.A battery is a device that can charge and discharge repeatedly, with a battery that occurs when charging and dischargingThe reaction is: Ni02 + Fe + 2H20 Fe (OH) 2 + Ni (OH) 2Use this battery for electrolytic M (N03) x solution:(1)the anode (inert) of the electrolytic pool shall be connected (the serial number)・A.NIf the battery works for a period of time, it consumes 0. 36 grams of water・(2)the calculation of the relative atomic mass of M (N03) x when the M (N03) x solution increases to mg(using m and x).(3)this battery electrolysis contains 0. 01 moles of CuS04 and 0.01 moles of NaCl, and the anode produces gasL (standard condition), the solution of electrolysis after electrolysis is released to IL, the pH of the solution is.Three, calculation problem22.To 8 g of divalent metal oxide solid adding rare - 1I2S04, make its just completely dissolved, known by sulfuric acid volume is 100ml, insert the platinum electrode in electrolysis in the solution obtained, electricity after a certain period of time, collected on an electrode 224 ml (standard conditions) of oxygen, in another electrode on the metal precipitation 1. 28 g.(1)the name of the metal oxidation is determined by calculation.(2)the concentration of the substance in the solution of sulfuric acid (the volume of solution) is calculated by lOOmL・The basic chemistry foundation of chapter four(1)basic practiceone23456co DDB9 001415161718CBa.CDCCDBC(1)H02 - H++ 022 -(2)H202 + Ba (OH) 2 = Ba 02 + 2 H20.2H202 + Ba (OH) 2 = Ba (H02) 2+2 H20;(3) 2 H202 H02 - + H302 +(1)the anode・ Cathode: 2H + + 2e -二H2 arrow(2)Cr2072 - + 6Fe2 + + 14H + 二2Cr3 + + + 7H20(3)as the reaction progresses, the H + concentration decreases, the oh-concentration increases, and eventually the Cr (OH) 3, Fe (OH) 3 precipitates・21,(1) B; (2) the CH3C00NH4 solution appears neutral, whichcan prove which classmate is correct・⑶ b;22,(1) CuC12, HC1; (2) H2S04, Na2S04 (3) NaCl, BaC12, CuS0423,7 (1) ; (2) CH3COOH + CH3COO 二0. 01 moles; 0. 006 mol, (3);24,(1) 1:10 (2) 4.48 molar(1)the concentration of copper oxide (2) sulphuric acid is 0. 2 molar ・[please note: 224 ml (standard condition) oxygen is less than 8 grams with 1.28 grams・This shows that MS04 is not fully electrolytic!(2)ability testing oneC 広 DCO O02 + 2C02 + 4e - 二2C032-2C0 + 02 二2C02(1)the area is red near the anode and blue near the cathode・ The solution is also purple, and by electron conservation, the amount of H + and OH minus of the Yin and Yang is exactly the same, while the Na2S04 solution is not hydrolyzed and is neutra1.(2)the red is getting lighter; The red gradually deepens・(1)when iron rusts, it reacts with oxygen in the air, depleting oxygen and reducing the gas pressure in a small beaker.(2) B > C > A = D・(3)contact with oxygen; The presence of water; There is an electrolyte (or a salt) that is present, and at the same time the iron rusts the fastest (or from the condition of the battery that makes up the original battery)・(1)Cu2 + hydrolysis, the solution is acidic, Cu2 + + 2H20 Cu (OH) 2 + 2H +・(2)when electrolysis, the Cu2 + is separated by the cathode, and because the concentration of Cu2 + decreases, the hydrolysis equilibrium moves to the left, so the H + concentration decreases and the pH increases・(3)the pH of the solution is lower, pH.(4)the "experimentalisls" are correct because, while electrolysis, the anode is dialyse and C12 is dissolved in water to form hydrochloric acid・(1) A (2) 50mx (3) 0. 16& 222.(1)the metal is M, n (02)二0.01 mol, and the amount of matter measured by M is 0. 02 moles, according to the mo 1 ar mass of M, and the metal oxide is Cu0・(2)in accordance with the Cu ~ - H2S04,N of H2S04 is equal to 0. 02 moles・ one。
高中化学选修四《电化学基础》单元检测(整理含答案)
高中化学选修四《电化学基础》单元检测时间:90分钟,总分:100分一、选择题(本题包括17个小题,每小题3分,共51分)1.化学用语是学习化学的重要工具,下列用来表示物质变化的化学用语中,正确的是()A.惰性电极电解饱和食盐水时,阳极的电极反应式为:2Cl--2e-===Cl2↑B.氢氧燃料电池的负极反应式为:O2+2H2O+4e-===4OH-C.粗铜精炼时与电源正极相连的是纯铜电极反应式为:Cu-2e-===Cu2+D.钢铁发生电化学腐蚀的正极反应式为:Fe-2e-===Fe2+2.下列叙述中,正确的是()①电解池是将化学能转变成电能的装置②原电池是将电能转变成化学能的装置③金属和石墨导电均为物理变化,电解质溶液导电是化学变化④不能自发进行的氧化还原反应,通过电解的原理有可能实现⑤电镀过程相当于金属的“迁移”,可视为物理变化A.①②③④B.③④C.③④⑤ D.④3.下列各装置中铜电极上能产生气泡的是()4.下列说法不正确的是()A.充电电池充电时,发生电解反应;放电时,发生原电池反应B.电镀时,应将镀层金属与电源正极相连C.电解饱和NaCl溶液时,阳极上放出黄绿色气体的同时还产生大量的氢氧化钠D.利用电化学原理保护金属主要有两种方法,分别是牺牲阳极的阴极保护法和外加电流的阴极保护法5.如图所示,铜片、锌片和石墨棒用导线连接后插入番茄里,电流表中有电流通过,则下列说法正确的是()A.锌片是负极B.两个铜片上都发生氧化反应C.石墨是阴极D.两个番茄都形成原电池6.在理论上不能用于设计成原电池的化学反应是()A. 4Fe(OH)2(s)+2H2O(l)+O2(g)===4Fe(OH)3(s)ΔH<0B.CH3CH2OH(l)+3O2(g)===2CO2 (g)+3H2O(l)ΔH<0C.Al(OH)3(s)+NaOH(aq)===NaAlO2(aq)+2H2O(l)ΔH<0D.H2(g)+Cl2(g)===2HCl(g)ΔH<07.下列事实与电化学腐蚀无关的是()A.钢铁制品生锈后用盐酸处理B.黄铜(Cu、Zn合金)制的铜锣不易产生铜绿C.铜、铝电线一般不连接起来作导线D.生铁比熟铁(几乎是纯铁)容易生锈8.下列过程需要通电才能进行的是()①电离②电解③电镀④电泳⑤电化学腐蚀A.①②③B.②④⑤C.②③④D.全部9.对外加电流的金属保护中,下列叙述正确的是()A,被保护的金属与电源的正极相连B.被保护的金属与电源的负极相连C.在被保护的金属表面上发生氧化反应D.被保护的金属为阴极,其表面上不发生氧化反应,而发生还原反应10.我国某大城市今年夏季多次降下酸雨。
电化学基础专题测试
电化学基础专题测试一、选择题1、综合下图判断,下列正确的说法是A. 装置I 和装置II 中负极反应均是B. 装置I 和装置II 中正极反应均是C. 装置I 和装置II 中盐桥中的阳离子均向右侧烧杯移动D. 放电过程中,装置I 左侧烧杯和装置II 右侧烧杯中溶液的pH 均增大2、如图所示,将铁棒和石墨棒插入饱和食盐水中。
下列说法正确的是( )A .甲中铁被保护不会腐蚀B .甲中正极反应式为4OH --4e - =2H 2O +O 2↑C .乙中铁电极上发生氧化反应D .乙中石墨电极附近滴几滴碘化钾淀粉溶液变蓝色3、右图所示装置中,已知电子由b 极沿导线流向锌。
下列判断正确的是( )A 、该装置中Cu 极为阴极B 、一段时间后锌片质量减少C 、b 极反应的电极反应式为:H 2-2e -+20H -=2H 2OD 、当铜极的质量变化为32g 时,a 极上消耗的O2的体积为5.6L4、用铅蓄电池电解甲、乙电解池中的溶液。
己知铅蓄电池的总反应为:Pb+PbO 2+2H 2SO 4放电充电2PbSO 4十2H 2O 电解一段时间后向c 极和d 极附近分别滴加酚酞试剂,c 极附近溶液变红,下列说法正确的是( )A .d 极为阴极B .放电时铅蓄电池负极的电极反应式为:PbO 2+4H +十SO 42-+4e -=PbSO 4+2H 2OC .若利用甲池精炼铜,b 极应为粗铜D .若四个电极材料均为石墨,当析出6.4 gCu 时,两池中共产生标准状况下的气体3.36L5、电渗析法是一种利用离子交换膜进行海水淡化的方法,其原理如图所示。
已知海水中含Na +、Cl -、Ca 2+、Mg 2+、SO 42-等离子,电极为惰性电极。
下列叙述中正确的是 ( )A .A 膜是阳离子交换膜B .通电后,海水中阴离子往b 电极处运动C .通电后,a 电极的电极反应式为 4OH --4e -=O 2↑ +2H 2OD .通电后,b 电极上产生无色气体,溶液中出现少量白色沉淀6、氯碱工业常利用阳离子交换膜电解食盐水,下列说法不正确的是 ( )A .随着电解的进行,c (NaCl)降低,需不断补充饱和食盐水B .电解过程中采用增大阳极区溶液pH 的方法,可以减少Cl 2在水中的溶解量C .阳离子交换膜的作用是阻止OH -移向阳极,以使氢氧化钠在阴极区富集D .阳极表面用钛氧化物涂层处理,目的是降低电解产物Cl 2对电极的腐蚀7、锂钒氧化物凝胶电池能量密度高,成本低,便于大量推广。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
《第四章电化学基础》单元检测
一、选择题(本题包括18小题,每小题3分,共54分;每小题只有一个选项符合题意)
1、将镁条和铝条平行插入一定浓度的氢氧化钠溶液中,用导线连接形成原电池。
下列有关该装置的叙述正确的是( )
A.因镁比铝活泼,故镁是原电池的负极,铝为正极
B.铝条表面虽有氧化膜,但可不必处理
C.该电池的内、外电路中,电流均由电子定向移动形成
D.铝是电池的负极,工作时溶液中会立即有白色沉淀生成
答案 B
2关于下图所示的原电池,下列说法正确的是( )
A.电子从锌电极通过电流表流向铜电极
B.盐桥中的阴离子向硫酸铜溶液中迁移
C.铜电极发生还原反应,其电极反应是2H++2e-===H2↑
D.取出盐桥后,电流表仍会偏转,铜电极在反应前后质量不变
答案 A
3下列事实与电化学腐蚀无关的是( )
A.光亮的自行车钢圈不易生锈
B.黄铜(Cu、Zn合金)制的铜锣不易生锈
C.铜、铝电线一般不连接起来作导线
D.生铁比熟铁(几乎是纯铁)容易生锈
答案 A
4、如图装置中,小试管内为红墨水,具支试管内盛有pH=4的雨水和生铁片。
观察到:开始导管内液面下降,一段时间后导管内液面回升,略高于小试管液面。
以下有关解释合理的是( )
A.生铁片中的碳是原电池的负极,发生还原反应
B.雨水酸性较强,生铁片仅发生析氢腐蚀
C.墨水回升时,碳极反应式为O2+2H2O+4e-===4OH-
D.具支试管中溶液pH逐渐减小
答案 C
5下列关于如图所示的实验装置的判断中错误的是( )
A.若X为碳棒,开关K置于A处可减缓铁的腐蚀
B.若X为锌棒,开关K置于A或B处都可减缓铁的腐蚀
C.若X为锌棒,开关K置于B处时,为牺牲阳极的阴极保护法
D.若X为碳棒,开关K置于B处时,铁电极上发生的反应为2H++2e-===H2↑
答案 D
6如图是工业电解饱和食盐水的装置示意图,下列有关说法中不正确的是( )
A.装置中出口①处的物质是氯气,出口②处的物质是氢气
B.该离子交换膜只能让阳离子通过,不能让阴离子通过
C.装置中发生反应的离子方程式为Cl -+2H +=====通电Cl 2↑+H 2↑
D.该装置是将电能转化为化学能
答案 C
7、(2019·腾冲县期末)锂空气电池放电时的工作原理如图所示。
下列叙述正确的是(
)
A.放电时Li +由B 极向A 极移动
B.电池放电反应为4Li +O 2+2H 2O===4LiOH
C.B 电极反应式为O 2+4H ++4e -===2H 2O
D.电解液a 可以为氯化锂溶液
答案 B
8某电池以K2FeO4和锌为电极材料,氢氧化钾溶液为电解质溶液。
下列说法不正确的是( )
A.锌为电池的负极
B.正极反应式为2FeO2-4+10H++6e-===Fe2O3+5H2O
C.该电池放电过程中电解质溶液浓度增大
D.电池工作时OH-向负极迁移
答案 B
9金属镍有广泛的用途。
粗镍中含有少量铁、锌、铜、铂等杂质,可用电解法制备高纯度的镍,下列叙述正确的是(已知:氧化性Fe2+<Ni2+<Cu2+)( )
A.阳极发生还原反应,其电极反应式:Ni2++2e-===Ni
B.电解过程中,阳极质量的减少与阴极质量的增加相等
C.电解后,溶液中存在的金属阳离子只有Fe2+和Zn2+
D.电解后,电解槽底部的阳极泥中有铜和铂,没有锌、铁、镍
答案 D
10某原电池构造如图所示,下列叙述正确的是( )
A.在外电路中,电子由银电极流向铜电极
B.取出盐桥后,电流表的指针仍发生偏转
C.外电路中每通过0.1 mol电子,铜的质量理论上减小6.4 g
D.原电池的总反应式为Cu+2AgNO3===2Ag+Cu(NO3)2
答案 D
11关于下图所示①②两个装置的叙述,正确的是( )
A.装置名称:①是原电池,②是电解池
B.硫酸浓度变化:①增大,②减小
C.电极反应式:①中阳极:4OH--4e-===2H2O+O2↑,②中正极:Zn-2e-===Zn2+
D.离子移动方向:①中H+向阴极方向移动,②中H+向负极方向移动
答案 B
12.(2020·内江市高三质检)以SO2为原料,通过下列工艺可制备化工原料H2SO4和清洁能源H2。
下列说法中不正确的是( )
A.该生产工艺中Br2被循环利用
B.在电解过程中,电解槽阴极附近溶液的pH变大
C.原电池中负极发生的反应为SO2+2H2O-2e-===SO2-4+4H+
D.该工艺总反应的化学方程式表示为SO2+Br2+2H2O===2HBr+H2SO4。
