晁辉公司型录-中文版20110914
10.1007_s00253-010-2443-4

BIOTECHNOLOGICAL PRODUCTS AND PROCESS ENGINEERINGEffects of biotic and abiotic elicitors on cell growth and tanshinone accumulation in Salvia miltiorrhiza cell culturesJiang-Lin Zhao &Li-Gang Zhou &Jian-Yong WuReceived:7September 2009/Revised:6January 2010/Accepted:6January 2010/Published online:2March 2010#Springer-Verlag 2010Abstract This study examined the effects of biotic and abiotic elicitors on the production of diterpenoid tanshi-nones in Salvia miltiorrhiza cell culture.Four classes of elicitors were tested,heavy metal ions (Co 2+,Ag +,Cd 2+),polysaccharides (yeast extract and chitosan),plant response-signaling compounds (salicylic acid and methyl jasmonate),and hyperosmotic stress (with sorbitol).Of these,Ag (silver nitrate),Cd (cadmium chloride),and polysaccharide from yeast extract (YE)were most effective to stimulate the tanshinone production,increasing the total tanshinone content of cell by more than ten-fold (2.3mg g -1versus 0.2mg g -1in control).The stimulating effect was concentration-dependent,most significant at 25μM of Ag and Cd and 100mg l -1(carbohydrate content)of YE.Of the three tanshinones detected,cryptotanshinone was stimulat-ed most dramatically by about 30-fold and tanshinones I and IIA by no more than 5-fold.Meanwhile,most of the elicitors suppressed cell growth,decreasing the biomass yield by about 50%(5.1–5.5g l -1versus 8.9g l -1in control).The elicitors also stimulated the phenylalanine ammonia lyase activity of cells and transient increases in the medium pH and conductivity.The results suggest that the elicitor-stimulated tanshinone accumulation was a stress response of the cells.Keywords Salvia miltiorrhiza .Cell culture .Tanshinones .Elicitors .Stress responseIntroductionSalvia miltiorrhiza Bunge (Lamiaceae),called Danshen in Chinese,is a well-known and important medicinal plant because its root is an effective herb for treatment of menstrual disorders and cardiovascular diseases and for the prevention of inflammation (Tang and Eisenbrand 1992).As its Chinese name refers,Danshen root is characterized by the abundance of red pigments which are mainly ascribed to numerous diterpene quinones generally known as tanshinones,e.g.,tanshinone I (T-I),tanshinone-IIA (T-IIA),and T-IIB,isotanshinone I and II,and cryptotanshinone (CT).Tanshinones constitute a major class of bioactive compounds in S .miltiorrhiza roots with proven therapeutic effects and pharmacological activities (Wang et al.2007).Danshen in combination with a few other Chinese herbs is an effective medicine widely used for the treatment of cardiovascular diseases and used as an emergency remedy for coronary artery disease and acute ischemic stroke.According to WHO statistics,cardiovas-cular diseases are and will continue to be the number one cause of death in the world (www.who.int/cardiovascular_diseases ).It is of significance to develop more efficient means for the production of Danshen and its active constituents.Although field cultivation is currently the major produc-tion means for Danshen and most other plant herbs,plant tissue cultures provide more well-controlled and sustainable systems for efficient production of desired bioactive compounds of the herb.Plant tissue cultures are the most useful and convenient experimental systems for examiningJ.-L.Zhao :L.-G.Zhou (*)Department of Plant Pathology,China Agricultural University,Beijing 100193,China email:lgzhou@J.-Y .Wu (*)Department of Applied Biology and Chemical Technology,The Hong Kong Polytechnic University,Hung Hom,Kowloon,Hong Kong email:bcjywu@.hkAppl Microbiol Biotechnol (2010)87:137–144DOI 10.1007/s00253-010-2443-4various factors on the biosynthesis of desired products and for exploring effective measures to enhance their produc-tion.The importance of Danshen for traditional and modern medicines has promoted the long-lasting research interest in the development of tiorrhiza tissue cultures for production of bioactive compounds for more than two decades.In an early study,Nakanishi et al.(1983)induced several cell lines from plant seedlings and screened out a cell line capable of producing significant amounts of CT and another diterpene,ferruginol.In later studies,the group performed a fuller evaluation and optimization of the medium for cell growth and CT production and,eventually,derived an effective production medium with a simpler composition(ten components)than the original Murashige and Skoog(MS) medium(about20components),achieving a high CT yield of 110mg l-1(Miyasaka et al.1987).Many recent studies have been focused on hairy root cultures of tiorrhiza transformed by Agrobacterium rhizogenes(Hu and Alfermann1993;Chen et al.2001)and by our group (Zhang et al.2004;Ge and Wu2005;Shi et al.2007).Most of the bioactive compounds in medicinal plants belong to secondary metabolites which are usually less abundant than primary metabolites in the plants.Since the accumulation of secondary metabolites in plants is a common response of plants to biotic and abiotic stresses, their accumulation can be stimulated by biotic and abiotic elicitors.Therefore,elicitation,treatment of plant tissue cultures with elicitors,is one of the most effective strategies for improving secondary metabolite production in plant tissue cultures(Chong et al.2005;Smetanska2008).The most common and effective elicitors used in previous studies include the components of microbial cells especially poly-and oligosaccharides(biotic)and heavy metal ions, hyperosmotic stress,and UV radiation(abiotic),and the signaling compounds in plant defense responses such as salicylic acid(SA)and methyl jasmonate(MJ;Zhou and Wu2006;Smetanska2008).Some of these elicitors,yeast extract(mainly the polysaccharide fraction),silver ion Ag+, and hyperosmotic stress(by an osmoticum)have also been applied and shown effective to enhance the production of tanshinones in tiorrhiza hairy root cultures(Chen et al.2001;Zhang et al.2004;Shi et al.2007).To the best of our knowledge,only a few studies have been documented on the effects of elicitors,YE,SA,and MJ,on the secondary metabolite production in Agro-bacterium tumefaciens transformed tiorrhiza cell cultures from one research group(Chen and Chen1999, 2000)but not any study in normal cell cultures.The present study focuses on the effects of common biotic and abiotic elicitors including polysaccharides,heavy metal ions, SA and MJ,and osmotic stress(with sorbitol)on the growth and accumulation of three major tanshinones T-I, T-IIA,and CT in suspension culture of normal tior-rhiza cells.In addition to the effects of various elicitors on the total tanshinone content of cells,the study will examine the effects on different tanshinone species and the potential relationship to plant stress response.Material and methodsCallus induction and cell suspension cultureYoung stem explants of tiorrhiza Bunge were collected from the botanical garden at the Institute of Medicinal Plant Development,Chinese Academy of Med-ical Sciences,Beijing,China,in May2005.The fresh explants were washed with tap water,surface-sterilized with 75%ethanol for1min,and then soaked in0.1%mercuric chloride for10min and rinsed thoroughly with sterilized water.The clean and sterilized explants were cut into∼0.5-cm segments and placed on solid MS medium(Murashige and Skoog1962)supplemented with sucrose(30g l-1),2,4-D(2mg l-1)and6-BA(2mg l-1)to induce callus formation. The callus culture of tiorrhiza was maintained on a solid,hormone-free MS medium with8g l-1agar and 30g l-1sucrose at25°C in the dark and subcultured every 4weeks.The culture was deposited in Lab Y1210at The Hong Kong Polytechnic University with a collection number of Danshen cell-1.All experiments in this study were performed in suspension culture of tiorrhiza cells in a liquid medium of the same composition as for the solid culture but excluding agar.The cell suspension culture was maintained in shake-flasks,i.e.,125-ml Erlenmeyer flasks on an orbital shaker operated at110–120rpm,at 25°C in the dark.Each of the flasks was filled with25ml medium and inoculated with0.3g fresh cells from18–21-day-old shake–flask culture.Elicitor preparation and administrationEight elicitors were tested,each at three concentrations,in the initial elicitation experiments(Table1).These are representative of the four major classes of elicitors for the induction of plant responses and the stimulation of secondary metabolite production in plant tissue cultures (Zhou and Wu2006;Smetanska2008).All elicitors except MJ were prepared as a concentrated stock solution in water and autoclaved at121°C for15min,and stored at4°C in a refrigerator prior to use.Yeast elicitor(YE)was the polysaccharide fraction of yeast extract(Y4250,Sigma, St.Louis,MO,USA)prepared by ethanol precipitation as described previously(Hahn and Albersheim1978;Ge and Wu2005).In brief,yeast extract was dissolved in distilled water(20g/100ml)and then mixed with400ml of ethanol and allowed to precipitate for4days at4°C in arefrigerator.The precipitate was redissolved in100ml of distilled water and subjected to another round of ethanol precipitation.The final gummy precipitate was dissolved in 50ml of distilled water and stored at4°C before use.The concentration of YE was represented by total carbohydrate content which was determined by the Anthrone test using sucrose as a reference.Chitosan solution was prepared by dissolving0.5g crab shell chitosan(C3646,Sigma)in1ml glacial acetic acid at55–60°C for15min,and then the final volume was adjusted to50ml with distilled water and the pH adjusted to5.8with NaOH(Prakash and Srivastava 2008).MJ(Cat.39,270-7,Sigma-Aldrich)was dissolved in 95%ethanol and sterilized by filtering through a microfilter (0.2µm).SA(10,591-0,Sigma-Aldrich),sorbitol(S3755, Sigma),and the salts of heavy metals including cobalt chloride(C8661,Sigma-Aldrich),silver nitrate(S7276, Sigma-Aldrich),and cadmium chloride(C5081,Sigma-Aldrich)were dissolved in distilled water to the desired concentrations and adjusted to pH5.8.Elicitor treatment was administered to the shake–flask culture of tiorrhiza cell on day18,which was about 2–3days before reaching the stationary phase.This time point is usually favorable for elicitation when the biomass concentration is high(compared with earlier days of growth),and the cell metabolism is still active(compared with that during or after stationary phase;Buitelaar et al. 1992;Cheng et al.2006).Each of the elicitor solutions was added into the culture medium with a micropipette at the desired concentration.After the elicitor addition,the shake–flask culture of cells was maintained for another7days and then harvested for analysis.All treatments were performed in triplicate,and the results were averaged.After the initial experiments on the eight elicitors,the three most effective ones,Ag(25µM),Cd(25µM),and YE(100mg l-1)were applied in the following experiments on the time courses of elicitor-treated cell growth and tanshinone accumulation in the tiorrhiza cell culture.Measurement of cell weight,sucrose concentration, medium pH,and conductivityThe cells were separated from the liquid medium by filtration.The cell mass on the filter paper was rinsed thoroughly with water and filtered again,and blotted dry by paper towels and then dried at50°C in an oven to attain the dry weight.Sucrose concentration in the liquid medium was determined by the Anthrone test using sucrose as a reference(Ebell1969),and the medium pH and conduc-tivity were measured with the respective electrodes on an Orion720A+pH meter(Thermo Fisher Scientific,Inc., Beverly,MA,USA)and a CD-4303conductivity meter (Lutron,Taiwan),respectively.Measurement of PAL activityPhenylalanine ammonia lyase(PAL)was extracted from fresh tiorrhiza cells with borate buffer(pH8.8).The cells were ground in the buffer(0.15g ml-1)for2min with a pestle and mortar on ice,and then centrifuged at10,000rpm and4°C for20min to obtain a solid-free extract.The PAL activity was determined based on the conversion of L-phenylalanine to cinnamic acid as described by Wu and Lin(2002).Analysis of tanshinone contentsThe cell mass from culture was dried and ground into powder and extracted with methanol/dichloromethane(4:1, v/v,10mg ml-1)under sonication for60min.After removal of the solid,the liquid extract was evaporated to dryness and redissolved in methanol/dichloromethane(9:1,v/v). Tanshinone content was determined by high performance liquid chromatography(HPLC)on a HP1100system using C18column,acetonitrile/water(55:45,v/v)as the mobile phase,and UV detection at275nm as described previously (Shi et al.2007).Three tanshinone species CT,T-I,and T-IIA were detected and quantified with authentic standards obtained from the Institute for Identification of Pharmaceu-tical and Biological Products(Beijing,China).Total tanshinone content is the total content of the three tanshinones in the cells.Tanshinone content in the culture medium was negligible and not determined.ResultsCell growth and tanshinone accumulation in tiorrhiza cell cultureThe time course of tiorrhiza cell growth exhibited a lag phase or slow growth period in the first3–6days, a rapid,linear growth period between day9–18,and aTable1Elicitors and concentrations tested in the initial experiments Elicitors Unit ConcentrationC1C2C3Cobalt chloride(Co)µM 5.02550 Silver nitrate(Ag)µM 5.02550 Cadmium chloride(Cd)µM 5.02550 Salicylic acid(SA)µM1050100 Methyl jasmonate(MJ)µM1050100 Yeast elicitor(YE)mg l-150100200 Chitosan(CH)mg l-150100200 Sorbitol(SO)g l-152550stationary or declining phase in the later days,reaching the maximum biomass concentration (8.1g l -1)around day 21.The total tanshinone content of cells remained at a very low level from days 1–12and then increased steadily from days 12–27to a maximum of 0.16mg g -1.A significant portion (65%)of the tanshinone accumulation or content increase occurred during the stationary phase from days 21–27(Fig.1a ),which is characteristic of secondary metabolite production in a batch culture process.The time course of sugar (sucrose)concentration (Fig.1b )was nearly sym-metrical to that of cell growth,indicating a direct correlation of the cell growth (or biomass production)to sugar consumption.As the major carbon source,sugar was required for the S .miltiorrhiza cell growth,and when it was depleted (around day 21),the cell growth stopped,and the biomass concentration began to drop.As seen from Fig.1b ,the medium pH showed a notable drop in the first 3days (due to consumption of NH 4+and release of protons)and a gradual increase after day 6(due to consumption of nitrate NO 3-)(Morard et al.1998).Effects of various elicitors on cell growth and tanshinone productionFigure 2shows the effects of elicitor treatments on the cell growth and tanshinone accumulation in S .miltiorrhiza cell cultures,which were dependent both on the elicitor species and elicitor dose.As seen from Fig.2a ,most of the elicitor treatments except Co 2+and sorbitol at lower concentrations suppressed the cell growth to a lower biomass concentra-tion than that of the untreated control culture,and the growth suppression was more severe at a high elicitor dose.On the other hand,most of the elicitor treatments except Co 2+,sorbitol,SA,and MJ at lower concentrations increased the total tanshinone content of cell to a higher level than in the control (Fig.2b ).Overall the results indicated that the enhancement of tanshinone accumulation by an elicitor treatment concurred with a notable suppres-sion of cell growth or biomass production.Nevertheless,some of the elicitors had a much stronger stimulating effect on the tanshinone accumulation than the suppressing effect on the cell growth.In particular,Ag and Cd both at 25μM,and YE at 100mg l -1increased the total tanshinone content to 2.30mg g -1,about 11.5-fold versus that of the control (0.20mg g -1),but decreased the biomass production by no more than 50%(5.1–5.5g l -1versus 8.9g l -1).Another three elicitors,SA,MJ (both at 50μM),and sorbitol (50g l -1)increased the total tanshinone content by 2–3-fold but decreased the biomass by 30–45%compared with the control.The stimulating effect of chitosan on tanshinone accumulation (about 6-fold)was stronger than SA,MJ,and sorbitol but much weaker than Ag,Cd,and YE,while its suppressing effect on the cell growth was as severe as Ag,Cd,and YE.In summary,the results indicate that Ag,Cd,YE are the most favorable elicitors for the tanshinone production in S .miltiorrhiza cell culture and were used in the following experiments.Figure 3shows the time courses of cell growth and tanshinone production after treatment with the three most effective elicitors Ag (25μM),Cd (25μM),and YE (100mg l -1)and the control culture.All three elicitor treatments caused a steady decline of biomass concentration from initially 8.5g l -1to 5.3g l -1on day 6while biomass in00.040.080.120.160.20246810TT content (mg g -1)C e l l b i o m a s s (g d w l -1)dw TTa4.85.1 5.45.76.001020304036912151821242730p HS u c r o s e (g l -1)Culture time (d)bSucrosepHFig.1Time courses of biomass and total tanshinone content (a ),residue sugar (sucrose)and medium pH (b )in S .miltiorrhiza cell cultures (error bars for standard deviations,n =3)246810C e l l b i o m a s s (g l -1)0.00.51.01.52.02.5Control AgCdSAMJYECH SOT T c o n t e n t (m g g -1)Elicitor treatmentCo Fig.2Effects of various elicitors on biomass growth (a )andtanshinone production (b )in S .miltiorrhiza cell cultures (elicitors added to cultures on day 18at three concentrations C1,C2,and C3as shown in Table 1,and cultures harvested on day 25;error bars for standard deviations,n =3)the control culture was increased during this period (Fig.3a ).In the meantime,the tanshinone content of cells in the three elicitor-treated cultures increased sharply and most rapidly by Ag (from 0.14to 1.98mg g -1),while that of control increased slightly (from 0.14to 0.21mg g -1;Fig.3b ).The volumetric total tanshinone yields (the products of total tanshinone content and cell dry weight)were 1.9mg l -1in the control,and 9.2mg l -1,10.7mg l -1and 11.7mg l -1in cultures treated with 100mg l -1YE,25μM Cd,and Ag,respectively (on day 6).Another test was performed on the effects of two and three elicitors in combinations in the S .miltiorrhiza cell culture.As shown in Fig.4,the tanshinone content was increased about 20%with either two elicitors and about 40%with all three elicitors in combination compared with that with a single elicitor.The results suggest an additive or synergistic effect of these elicitors on the tanshinone accumulation in the cells.However,the combined use of two or three elicitors also suppressed the cell growth (biomass)more severely than with a single elicitor.Effects of elicitor treatments on different tanshinone species Of the three tanshinone species detected,CT was stimulated most significantly by all elicitors without exception;T-IIA was stimulated by most elicitors,and T-I was stimulated significantly only by chitosan but slightly stimulated or suppressed by other elicitors (Table 2).The highest CT content was about 2mg g -1(1,854–2,011μg g -1)in cellcultures treated with 25μM Ag and Cd,and 100mg l -1YE,about 31–34fold of the control level (60μg g -1),the highest T-I content 0.27mg g -1with 100mg l -1chitosan (3.4-fold of the control 80μg g -1)and the highest T-IIA content 0.37mg g -1with 25μM Cd (6-fold of the control 60μg g -1).As seen from the HPLC chromatograms (Fig.5),the cultures treated with the three different elicitors exhibited a similar profile with virtually identical major peaks.The experimental results do not suggest any specificity of particular tanshinone species to the type of elicitors,YE and chitosan as biotic polysaccharides,Cd and Ag as abiotic heavy metals,or SA and MJ as plant stress signaling pared with that of control,the HPLC profiles of elicitor-treated cultures also had three new unknown peaks appearing before the CT peak,between 10.0–11.5min and a high peak at 11.1min,which0.00.51.01.52.02.5123456T T c o n t e n t (m g g -1)Time after treatment (d)b4681012C e l l b i o m a s s (g l -1)Control Ag 25Cd 25YE 100aFig.3Time courses of biomass (a )and total tanshinone content (b )in S .miltiorrhiza cell cultures after treatment with Ag (25µM),Cd (25µM),and YE (100mg l -1;error bars for standard deviations,n =3)24681012345Cell dry weight (g l -1)T T c o n t e n t (m g g -1)Elicitor treatmentTTdwFig.4Effects of single and combined elicitors on S .miltiorrhiza cell growth and tanshinone accumulation (elicitors added to cell cultures on day 18at the same concentrations as in Fig.3,and cultures harvested on day 25;error bars for standard deviations,n =3)Table 2Effects of various elicitors on the accumulation of three tanshinones in S .miltiorrhiza cells Treatment aContent,μg/g (fold of content control)CTT-IT-IIA Control 59.9(1)81.6(1)57.6(1)Co-50263.7(4.4)67.5(0.83)55.5(0.96)Ag-251,817.5(30)71.0(0.87)225.8(3.9)Cd-251,854.0(31)80.3(0.98)369.0(6.4)SA-100390.0(6.5)78.5(0.96)72.8(1.3)MJ-100299.8(5.0)109.5(1.3)82.6(1.4)YE-1002,011.4(34)90.3(1.1)190.3(3.3)CH-100597.2(10)276.0(3.4)98.8(1.7)SO-50584.6(9.8)56.9(0.70)83.0(1.4)CT cryptotanshinone,T-I tanshinone I,T-IIA tanshinone-IIAaNumber after each elicitor symbol represents the elicitor concentra-tion as shown in Table 1may be ascribed to tanshinone relatives of higher polarity than CT induced by the elicitors.PAL activity,pH,and conductivity changes induced by elicitorsFigure 6shows the changes of intracellular PAL activity and medium pH and conductivity in the S .miltiorrhiza cell culture after the treatment by Ag (25μM),Cd (25μM),and YE (100mg l -1).The PAL activity of cells was stimulated by all three elicitors to the similar level,from 1.4-to 1.9-fold of the control level over 6days (Fig.6a ).PAL is a key enzyme at the entrance step in the phenylpropanoid pathway in plants,and its activity increase stimulated by the elicitors is suggestive of an enhanced secondary metabolism in the plant cells (Taiz and Zeiger 2006).The pH and conductivity of culture medium were also increased (to higher levels than those of the control)by all three elicitors but more significantly by YE (Fig.6b,c ).Most significant increases (differences from the control level)in the medium pH and conductivity were shown in the very early period from day 0–1.The increase in medium conductivity in the early period was most probably attributed to the release of potassium K +ion from the cells or K +efflux across the cell membrane (Zhang et al.2004).Transient medium pH increase (alkalinization)and K +efflux across the cell membrane are early and important events in the elicitation of plant responses and phytoalexin production (Ebel and Mithöer 1994;Roos et al.1998).The conductivity decline in the later period after day 1of Ag +and Cd 2+-treated cultures and the control cultures can be attributed to the consumption of inorganic and mineral nutrients in the culture medium (Kinooka et al.1991).Overall,the results here provide further evidence forthe01234R e l a t i v e P A L a c t i v i t yControl Ag CdYEa5.05.45.86.26.6M e d i u m p H b2.03.04.05.06.00246M e d i u m c o n d u c t i v i t y (m S )Time after treatment (d)cFig.6Time courses of PAL activity (a ),medium pH (b ),and conductivity (c )of S .miltiorrhiza cell cultures after elicitor treatments in comparison with the control (error bars for standard deviation,n =3)elicitor activities of Ag,Cd,and YE in stimulating the stress responses and secondary metabolism of the S. miltiorrhiza cells.DiscussionThe effects of various elicitors on tanshinone accumulation found here in the normal tiorrhiza cell cultures are in general agreement with those found in transformed cell and hairy root cultures of tiorrhiza.In transformed cell cultures(Chen and Chen1999),the CT accumulation was also stimulated significantly by YE but not by SA or MJ alone,and YE also inhibited the cell growth.The tanshinone(mainly CT)production in hairy root cultures was also enhanced significantly(3–4fold)by Ag(Zhang et al.2004)and YE(Shi et al.2007).In all these culture systems,CT was the major tanshinone species stimulated by various elicitor treatments.CT has been identified as a phytoalexin in tiorrhiza plant which plays a defense role against pathogen invasion of the plant(Chen and Chen 2000).In this connection,the stimulated CT accumulation by the elicitors may be a defense or stress response of the cells.CT was also the major diterpenoid produced by a normal tiorrhiza cell line which was initially grown in the MS medium and then transferred to a production medium containing only about half of the nutrient compo-nents of the MS medium(Miyasaka et al.1987).It is very possible that the improvement of CT yield in this production medium was also attributed,at least partially, to the stress imposed by the nutrient deficiency which suppressed growth but stimulated secondary metabolite accumulation.MJ or its relative jasmonic acid has been shown effective for stimulating a variety of secondary metab-olites in plant tissue cultures such as hypericin in Hypericum perforatum L.(St.John’s Wort)cell cultures (Walker et al.2002),paclitaxol(diterpenoid)and related taxanes in various Taxus spp.and ginsenosides in Panax spp.(Zhong and Yue2005),and bilobalide and ginkgo-lides in Ginkgo biloba cell cultures(Kang et al.2006). However,MJ showed only a moderate or insignificant stimulating effect on tanshinone accumulation in normal and transformed tiorrhiza cell cultures.The discrep-ancy suggests that the effects of various elicitors on secondary metabolite production in plant tissue cultures are dependent on the specific secondary metabolites.This argument is also supported by the much stronger stimu-lation of CT than T-I and T-IIA by most elicitors found in our tiorrhiza cell cultures.In addition,the hairy roots appeared more tolerant to the elicitor stress,and the growth was less inhibited by the elicitors or even enhanced in some cases,e.g.,by YE(Chen et al.2001)and sorbitol(Shi et al.2007).Moreover,sorbitol as an osmotic agent significantly stimulated the tanshinone accumulation(3–4folds)in tiorrhiza hairy root cultures,but not so significantly in the cell cultures.This shows that the elicitor activities for the same metabolites can vary with the tissue culture systems.In conclusion,the polysaccharide fraction of yeast extract and two heavy metal ions Ag+and Cd2+were potent elicitors for stimulating the tanshinone production in tiorrhiza cell culture.The stimulated tanshinone production by most elicitors was associated with notable growth suppression.CT was more responsive to the elicitors and enhanced more dramatically than another two tanshinones,T-I and IIA.The results from this study in the tiorrhiza cell cultures and from previous studies in hairy root cultures suggest that the cell and hairy root cultures may be effective systems for CT production, provided with the elicitors.As most of the elicitor chemicals are commercially available or can be readily prepared in the laboratory and easily administered to the cell and root cultures,they are suitable for practical applications in the laboratory or large-scale production. Acknowledgements This work was supported by grants from The Hong Kong Polytechnic University(G-U502and1-BB80)and the China Hi-Tech Research and Development Program(2006AA10A209).ReferencesBuitelaar RM,Cesário MT,Tramper J(1992)Elicitation of thiophene production by hairy roots of Tagetes patula.Enzyme Microb Technol14:2–7Chen H,Chen F(1999)Effects of methyl jasmonate and salicylic acid on cell growth and cryptotanshinone formation in Ti transformed Salvia miltiorrhiza cell suspension cultures.Biotechnol Lett 21:803–807Chen H,Chen F(2000)Effect of yeast elicitor on the secondary metabolism of Ti-transformed Salvia miltiorrhiza cell suspension cultures.Plant Cell Rep19:710–717Chen H,Chen F,Chiu FCK,Lo CMY(2001)The effect of yeast elicitor on the growth and secondary metabolism of hairy root cultures of Salvia miltiorrhiza.Enzyme Microb Technol28:100–105Cheng XY,Zhou HY,Cui X,Ni W,Liu CZ(2006)Improvement of phenylethanoid glycosides biosynthesis in Cistanche deserticola cell suspension cultures by chitosan elicitor.J Biotechnol 121:253–260Chong TM,Abdullah MA,Lai QM,Nor’Aini FM,Lajis NH(2005) Effective elicitation factors in Morinda elliptica cell suspension culture.Process Biochem40:3397–3405Ebel J,Mithöer A(1994)Early events in the elicitation of plant defence.Planta206:335–348Ebell LF(1969)Variation in total soluble sugars of conifer tissues with method of analysis.Phytochemistry8:227–233Ge XC,Wu JY(2005)Tanshinone production and isoprenoid pathways in Salvia miltiorrhiza hairy roots induced by Ag+and yeast elicitor.Plant Sci168:487–491。
会计实训

说明:
1实收资本增加-----2012年9月3日,在上海证券交易所发行A股 2营业总收入、营业利润增长较快------打开欧洲市场 3净利润增长巨大---维他麦在当地以及欧洲市场优惠的税收政策
光明上 市公司 股票交 易走向
2012年9 月之后 明显上 升
资产负债率
2012年一季度盈利能力
2010年1月12日光明食品集团提出以15亿澳元收购CSR公司旗下的糖业
和可再生能源业务。由于光明食品突然降低报价,使得潜在竞争对手新 加坡丰益国际趁机而入,以0.7亿澳元的差额成功赢得并购。 失败 2010年7月收购新西兰牛奶加工商之一Synlait ,吸取收购澳洲的高调教 训,开始低调收购新西兰。 成功 2010年9月,光明食品集团与联合饼干进入排他性谈判,收购价格约为 31.6亿美元(约合20亿英镑)。双方由于并购价格不能达成共识而导致 谈判破裂。 失败 2010年3月5日,光明食品集团与GNC签署谅解备忘录,双方建立了战 略合作关系,通过合资企业健安喜(中国)共同进军中国保健品市场。 然而,2011年1月下旬,光明食品集团却宣布退出了收购GNC的谈判。 由于光明食品未能在对方规定的时间内完成交易 ,一方面,光明食品集 团需要支付高额的并购对价和巨大的融资成本;另一方面,并购之后还 可能面临资产管理失败的巨大风险,在权衡后选择退出并购也许是明智 之举。 失败 2010年末 并购法国优诺 而竞标结果通用磨坊以16亿欧元获得优诺51% 的股权。 失败
Weetabix是一种营养早餐食品的品牌,中文“维他麦”, 公司位于英国乡 村的中心地带,自1932年起生产优质的谷物早餐产品。拥有一系列著名 的谷物早餐品牌,包括维他麦Weetabix、欧宝Alpen、Oatibix、乐迪 Ready Brek以及维多滋等。 维他麦食品公司共有员工2500余人,位于凯特灵区的工厂每年生产30亿块 维他麦饼干,即每天生产800万块饼干,每小时生产6000块饼干。该公 司的主打品牌维他麦是当今英国家喻户晓的早餐产品,占整个谷物早餐 市场份额的8%,每年的销售额超过1亿7千8百万美元。维他麦食品公司 的产品现在已经销往全世界80多个国家。
01-2013_-_Aawoot_Srikhaow_-_PreparationofCu2OH3NO3ZnOanovelcatalystformethylor[retrieved-2016-11-15]
![01-2013_-_Aawoot_Srikhaow_-_PreparationofCu2OH3NO3ZnOanovelcatalystformethylor[retrieved-2016-11-15]](https://img.taocdn.com/s3/m/da277a3dcfc789eb172dc83a.png)
Applied Catalysis B:Environmental 130–131 (2013) 84–92Contents lists available at SciVerse ScienceDirectApplied Catalysis B:Environmentalj o u r n a l h o m e p a g e :w w w.e l s e v i e r.c o m /l o c a t e /a p c a tbPreparation of Cu 2(OH)3NO 3/ZnO,a novel catalyst for methyl orange oxidation under ambient conditionsAssadawoot Srikhaow a ,b ,Siwaporn Meejoo Smith b ,c ,∗aMaterials Science and Engineering Graduate Program,Faculty of Science,Mahidol University,Rama VI Road,Rajathevi,Bangkok 10400,Thailand bCenter of Excellence for Innovation in Chemistry,Faculty of Science,Mahidol University,Rama VI Road,Rajathevi,Bangkok 10400,Thailand cDepartment of Chemistry,Faculty of Science,Mahidol University,Rama VI Road,Rajathevi,Bangkok 10400,Thailanda r t i c l ei n f oArticle history:Received 4May 2012Received in revised form 1September 2012Accepted 16October 2012Available online 29 October 2012Keywords:Copper hydroxide nitrate Zinc oxideLayered hydroxyl salts Catalytic wet oxidation Wastewater treatmenta b s t r a c tThis work reports a novel process to synthesize copper hydroxyl nitrate/zinc oxide composites (Cu 2(OH)3NO 3/ZnO),and their application as a highly effective and reusable catalyst for wet oxidation of methyl orange (MO)under ambient conditions.No additional air or oxygen flow is ing a metal oxide assisted method,the Cu 2(OH)3NO 3/ZnO composites were hydrothermally obtained by varying the Cu:Zn mole ratio (2:1,4:1,and 6:1)and the structural,chemical and surface properties of the composites were investigated.Decolorization of 500ppm MO can be effectively catalyzed by the Cu 2(OH)3NO 3/ZnO composite (Cu:Zn =4:1)with the color,chemical oxygen demand (COD)and total organic carbon (TOC)removal efficiencies being greater than 99%,98%and 94%,respectively,after 20min treatments using the catalyst loading of 3g L −1.Results from systematic catalytic activity tests strongly suggested that MO was oxidized by oxygen dissolved in the dye solution,and that the degradation pathway of MO possibly occurred through radical and H 2O 2generation.The application of highly efficient Cu 2(OH)3NO 3/ZnO cat-alysts in wastewater remediation may be attractive alternative to existing oxidation catalyst systems as they are low-cost,simple to prepare,feasible to operate under ambient conditions.© 2012 Elsevier B.V. All rights reserved.1.IntroductionWater pollution is one of the main environmental concerns impacting the world ecology.Therefore,water protection plans and wastewater treatments should receive a great attention as they impact the world’s economic growth,global food production and industrial development.It is widely accepted that agricultural runoff and industrial wastewater may contain hazardous chemicals [1,2]and hence discharge of the polluted water without treatment can cause major damage to the quality of natural water reservoirs.Textile industries,in particular,intensively use chemicals (such as dyes and transfer reagents)and massive amounts of water in dyeing processes.Thus,significant amounts of dye contaminated effluent might be produced and effective treatment processes must be put in place to clean the water before releasing into waterways.One of the main research areas in wastewater treatment is developing a novel technology to effectively remove residual dyes and organic pollut-ants from wastewater [3–5].Conventional methods used to remove∗Corresponding author at:Department of Chemistry,Faculty of Science,Mahidol University,Rama VI Road,Rajathevi,Bangkok 10400,Thailand.Tel.:+66222015164;fax:+6623547151.E-mail addresses:siwaporn.smi@mahidol.ac.th ,siwaporn.meejoo@ (S.M.Smith).pollutants from wastewater are adsorption,biological treatment and chemical oxidation [3,6].Although adsorption is the simplest method,but less effective as pollutants were only transferred to the sorbent surface,and additional treatment of the contaminated solid is required [7].Biological treatments are considered as promis-ing alternatives.However,it may be rather difficult to biologically degrade some conjugated aromatic compounds due to their struc-tural stability [8].In addition,microorganisms may not be survived under extreme conditions (extreme pH or highly toxic)inhibiting effective biological treatments of wastewater [9,10].Advanced oxidation processes (AOPs)have been described as chemical methods operated to induce the oxidative degradation of organic compounds by radical species [11,12].Examples of AOPs include ozonation [13],Fenton process [14],photocatalysis [15],sonolysis [16]and other chemical oxidation processes induced by oxidizers such as air,O 2,O 3,and H 2O 2[11,17,18].To practically operate in industrial sites,the oxidation process should remove organic pollutants effectively under mild conditions,and with low cost.Photocatalytic oxidation and sonolysis are of interest as they can operate under ambient conditions with no requirement of additional oxidizer such as ozone and hydrogen peroxide [16,18].However,industrial integration is difficult,as these require special maintenance/operation of high power light sources,or special-ized sonication equipments.In recent years,a limited number of works have reported oxidation of organic compounds via catalytic0926-3373/$–see front matter © 2012 Elsevier B.V. All rights reserved./10.1016/j.apcatb.2012.10.018A.Srikhaow,S.M.Smith/Applied Catalysis B:Environmental130–131 (2013) 84–9285wet oxidation(CWO)at near ambient conditions,in which the organic substances undergo aerial oxidation over metal catalysts such as Fe2O3–CeO2–TiO2/␥-Al2O3[19],MoO3/Ce[20],polyoxo-molybdate nanotubes[21],and ZnO/MoO3mixed oxide nanotubes [22].Although no harmful chemical reagent is required,air or oxy-gen is necessary to activate these oxidation processes.Apart from the intrinsic properties of the catalyst,factors such as catalyst loading,concentration of the organic substrate,airflow rate(and, perhaps,its purity)can play significant roles in the efficiency and rate of the processes[5,12,23].A few copper hydroxyl salts were reported as promising cat-alysts in azo dyes removal via catalytic wet peroxide oxidation (CWPO)in which H2O2is required as oxidizer[24,25].In2010, Zhan and Chen reported the degradation of azo dyes over copper hydroxyphosphate,Cu2(OH)PO4,under near-neutral pH conditions [24],whereas copper hydroxide nitrate,Cu2(OH)3NO3is an effec-tive CWPO catalyst for oxidative degradation of azo dyes in a wide pH range[25].Previously reported,Cu2(OH)3NO3can be synthe-sized by several routes,such as precipitation of Cu(NO3)2in a basic aqueous solution[26],urea hydrolysis of Cu(NO3)2[27],chemical reaction between CuO and aqueous Cu(NO3)2solution[28],cation exchange of Mg(II)for Cu(II)in aqueous Cu(NO3)2solution and evaporation of aqueous Cu(NO3)2solution[29].To gain a significant advantage over the previously reported cop-per hydroxyl salt catalysts,an ideal catalyst should enable organic substrates to be oxidized under ambient conditions,with no requirement of additional continuously supplied oxidizing agent. In this work,Cu2(OH)3NO3(a copper hydroxyl salt)was incor-porated with microcrystalline ZnO by a one-step hydrothermally metal oxide assisted method.This study also reports the application of this material(denoted as Cu2(OH)3NO3/ZnO)as a highly effec-tive and reusable catalyst for wet oxidation of methyl orange(MO) under ambient conditions without addition of oxidizer.Details on systematic investigations of the structural and catalytic activity of this material and possible degradation pathway of MO are dis-cussed.2.Experimental2.1.MaterialsAll chemicals used in this work were commercially supplied as analytical grade reagents,and used without further purification. De-ionized water was used throughout the experiments.Copper nitrate trihydrate and copper sulphate pentahydrate were com-mercially obtained from Univar,while ZnO powdered material was obtain from Merck.Tert-butanol(Merck)and NaOH(Rankem)were employed as hydroxyl radical scavenger reagents.2.2.Preparation method offlower-like ZnOA d-glucose assisted precipitation method[30]was applied to obtainflower-like ZnO materials by using NaOH and Zn(NO3)2 (Qrëc)as starting reagents,a mixture of H2O:acetone:ethyl acetate (3:3:2by volume)as co-solvent,and d-glucose(Univar)as ion sta-bilizer.Obtained white precipitate was subsequently calcined at 400◦C for3h in an air atmosphere,giving aflower-like ZnO sample with S BET=11.7m2/g.2.3.Preparation of the Cu2(OH)3NO3/ZnO samplesA series of suspensions containing theflower-like ZnO(or com-mercial ZnO)powder in Cu(NO3)2aqueous solutions were obtained by varying the Cu:Zn molar ratios(2:1,4:1and6:1).Next,these sus-pensions were sonicated for30min in an ultrasonic bath,followed by a hydrothermal treatment at100◦C in a50mL Teflon-lined stain-less steel autoclave for30min.Subsequently,the reaction mixtures were left to stand at room temperature,allowing the suspensions to cool down.Afterfiltering and washing with de-ionized water, the precipitates were freeze dried,and kept in a dry condition at room temperature.2.4.Sample characterizationStructural properties of as-prepared samples were stud-ied on a Bruker(AXS model D8advance)powder X-ray diffractometer equipped with Cu K␣radiation, =1.5419˚A,2Ârange=5–50◦,step=0.050◦,scan step=1s/step.FT-IR spectra were obtained on a Perkin Elmer(Spectrum GX FT-IR System) fourier-transform infrared spectrometer.The microstructure of catalyst samples was examined on a JEOL(JSM-6400)scan-ning electron microscope.Furthermore,energy dispersive X-ray (EDX)microanalyses were carried out to identify the chemi-cal composition of catalyst samples.The surface properties were studied by using Brunauer–Emmett–Teller method(BET Model Quantachrome/Autosorb-1,Thermo Finnigan/Sorp-tomatic1990). The concentration of elements in the samples was also determined on an X-rayfluorescence spectrometer(Bruker S4EXPLORER) equipped operating in a He working mode(X-ray tube win-dow=75m;excitation=4kW)using a loose powder preparation method and a34mm sample cup.2.5.Catalytic degradation experimentsAll catalytic reactions were carried out using the same catalyst loading;3g L−1of aqueous methyl orange(MO,500ppm)under stirring.The color removal efficiency of MO was monitored as a function of time by measuring absorbance of the dye solution after catalytic treatment at given time intervals.In order to terminate the reaction at specific reaction times,the catalyst was immediatelyfil-tered off using a Buchner funnel equipped with a water aspirator pump.UV–vis absorption spectra of the dyefiltrates were recorded on a Perkin Elmer(Lamda800)UV–vis spectrophotometer.Subse-quently,the concentration of MO in thefiltrates was quantified using the absorbance at464nm(corresponding to unreacted MO) and a curvefitting method using the Beer Lambert law.The color removal efficiency(Á,%)of methyl orange was calculated by using this equation:Á(%)=C0−C tC0×100(1)where C0is the initial concentration of MO and C t is the concentra-tion of MO after‘t’min.Total organic carbon(TOC)content in the dye solutions was determined by an in-house method(SGS laboratory service):LBEN-09149based on United States Environmental Protection Agency, 2004,EPT method9060A.Moreover,chemical oxygen demand (COD)was measured by a standard closed reflux/titration method [31].The TOC removal efficiency is defined as:TOC removal efficiency(%)=TOC0−TOC tTOC0×100(2)where TOC0is initial TOC of the solution and TOC t is TOC of solution after‘t’min reaction time.Similarly,COD0and COD t values were used instead of TOC0and TOC t values in Eq.(2)respectively,to calculate COD removal efficiencies.The data from triplicate mea-surements were analyzed to obtain average values and standard deviation(SD).The significance of difference of data was evaluated using the Student’s T-test and one-way ANOVA[32]at a significance level of0.05.86 A.Srikhaow,S.M.Smith/Applied Catalysis B:Environmental130–131 (2013) 84–92To investigate the possible mechanism of decolorization of MO, the experiments were conducted under air atmosphere,vacuum in a closed system and in the presence of radical scavengers i.e.tert-butanol[33,34]and NaOH[35],into the solution at25◦C.Detailed processes are described in the supplementary data.The stability of catalyst,Cu2(OH)3NO3/ZnO(Cu:Zn=4:1)was studied by monitoring the generation of metal ions in the dye solution during catalytic wet oxidation.After20-min reaction,the concentration of Cu and Zn ions in the dyefiltrates was then deter-mined by using a graphite furnace atomic absorption spectrometer (GFAAS,Perkin Elmer AAnalyst100).A hollow cathode zinc lamp (Perkin Elmer)operated with10-mA current was employed,with argonflow throughout the heating program,except during the atomization step.2.6.Characterization of the degradation productsLiquid chromatography with ion trap mass analyzer(LC–MS, Agilent technology,Agilent1100equipped with Esquire3000plus) was employed to detect the degradation products upon the oxi-dation of methyl orange.The LC–MS system was equipped with C18column and30%of acetonitrile and70%of0.01M ammonium acetate(pH6.8)were used as a mobile phase.Theflow rate used was0.6mL min−1.The mass spectrometer was equipped with an electrospray ionization(ESI)source operating at negative polar-ity.This LC/MS system could detect mass ranged between100and 400m/z.3.Results and discussion3.1.Characterization of the catalystsPowder X-ray diffractrograms of ZnO powder and the syn-thesized Cu2(OH)3NO3/ZnO samples with varying Cu:Zn mole ratios are shown in Fig.1a.The diffraction peak at∼13◦corre-sponds to the basal distance(6.96˚A)typically reported for the Cu2(OH)3NO3layered materials[26,27,29,36].It was observed that the Cu2(OH)3NO3/ZnO derived from the Cu:Zn molar ratio=2:1 contains two crystalline phases,monoclinic Cu2(OH)3NO3(JCPDF card no.74-1749)and hexagonal ZnO(JCPDF card no.36145).How-ever,with the increased Cu:Zn molar ratios to4:1and6:1,the structural characteristics of Cu2(OH)3NO3becomes more evident, whereas the diffraction peaks corresponding to the ZnO phase(at 34.4◦and47.4◦)become weaker in intensity.This is possibly due to a full coverage of Cu2(OH)3NO3layers on the ZnO particles.The sharp and well-defined peaks reflected high degree of crystallinity for all synthesized samples.No diffraction peaks corresponding to neither Zn(OH)2,CuO nor Cu(OH)2phases were observed.It should be pointed out that,to our knowledge,this metal oxide assisted route to synthesize copper hydroxide nitrate has never been reported.The conversion of copper nitrate to copper hydroxyl nitrate possibly resulted from the availability of hydroxyl groups on the ZnO solid base.In a previous study,the basic strength of a ZnO sample was reported as7.2<H<9.3in an Hammett indicator scale, indicating a fairly high strength comparing with those of ZrO2,TiO2, CaO and SrO[37].From Fig.1b,IR measurements also confirm the formation of copper hydroxide nitrate in the system studied.In a good agree-ment with previously reported works[26–29]the IR peaks at876, 785and676cm−1can be assigned to hydrogen bonding frequencies related to Cu O H.The peaks at1048( 1),810( 2),1340,1348and 1429cm−1( 3)can be attributed to the vibration modes of NO3−ions[27].The symmetric and asymmetric stretching modes of NO3−at1429and1340cm−1suggested the presence of NO3−between copper hydroxide layers.The IR band at1048cm−1corresponds to the N O stretching vibration of a monodentrate O NO groups[38],Fig. 1.(a)Powder XRD patterns of Cu2(OH)3NO3/ZnO samples at varying the Cu:Zn molar ratios in comparison to that of ZnO and(b)Infrared spectra of Cu2(OH)3NO3/ZnO(Cu:Zn=4:1)and ZnO powder.whereas the band at1637cm−1can be ascribed to a HOH bending mode.The peaks at3543cm−1and3433cm−1indicated more than one type of hydroxyl groups in the structure[27,29].Note that the characteristic peak of ZnO at430wavenumber[39]was not clearly observed in the Cu2(OH)3NO3/ZnO(Cu:Zn=4:1)sample,possibly due to signal overlapping and the full coverage of Cu2(OH)3NO3 layers formed on the ZnO particles as previously discussed.Noticed from Fig.2a,the microcrystalline ZnO substrate resem-bles to a bunch of doubleflowers.Although the Cu2(OH)3NO3/ZnO composites did not retain theflower-like microstructure of their substrate(Fig.2b–d)a resemblance of aggregates offlake-like plates(∼400nm in thickness)morphology can be still observed. X-rayfluorescence(XRF)was employed to perform elemental anal-ysis in the composite samples,and the results were included in Table1.The XRF results suggest that the content of Cu was found to increase in the composites with increased Cu:Zn molar ratios.This finding was consistent with the results from EDX microanalyses, Fig.3,revealing the presence of Cu and Zn on the composite surface, and that the content of Cu was found to be higher in the compositesA.Srikhaow,S.M.Smith/Applied Catalysis B:Environmental130–131 (2013) 84–9287Fig.2.SEM images of theflower-like ZnO(a)and(b–d)Cu2(OH)3NO3/ZnO at the Cu:Zn molar ratios of2:1,4:1and6:1,respectively.Table1Elemental concentration in the Cu2(OH)3NO3/ZnO samples at various Cu:Zn molar ratios obtained by XRF analysis.Cu:Zn Concentration(wt%)Depth ofpenetration(m) Cu Zn2:147.97±0.04815.09±0.0140.13–0.164:149.86±0.04811.30±0.0120.14–0.176:160.09±0.054 1.59±0.0040.13–0.16 derived from the higher Cu:Zn.However,as shown in Table2,the Cu:Zn ratios vary in different areas of the sample surface,suggest-ing that the Cu-containing compound does not homogeneously incorporated with the ZnO particles.3.2.Catalytic activity of Cu2(OH)3NO3/ZnOThe performance in decolorization of500ppm aqueous methyl orange(MO)solution over the Cu2(OH)3NO3/ZnO composites at varying Cu:Zn ratios were examined over a period of time as shown in Fig.4a.Notably,the color removal efficiencies reached99% within1.5min of the treatments by all composites at25◦C under atmospheric pressure(Fig.4a).It was noticed from Fig.4a and b,that the Cu2(OH)3NO3/ZnO composites prepared with relatively high Cu:Zn molar ratios oxidized MO slightly faster than the sam-ples having lower Cu content,implying that the amount of copper may play a crucial part to the reaction kinetics of MO degradation. Determined usingfirst-order kinetic model,it was found that the higher rate constants for the catalytic wet oxidation of MO were obtained from the Cu2(OH)3NO3/ZnO with the higher Cu:Zn molar ratios(6:1,4.4min−1;4:1,3.9min−1;2:1,3.6min−1).Thefirst order kinetic plot in Fig.4b was focused in the range of shorter reaction time,because after1.5min the color removal efficiencies reached 99%.Statistical analyses suggested that the kinetic constants for the MO decoloriaztion by each catalyst(2:1,4:1and6:1)are signifi-cantly different due to the Cu:Zn molar ratios at the level of p<0.05. Fig.4c shows a characteristic absorption band at464nm corre-sponding to a conjugated azo bond structure in the MO molecule [40].In this work,the absorption band at464nm become weaker in intensity after treatment with Cu2(OH)3NO3/ZnO composites. Therefore,in consistent with the result in Fig.4b,the UV–vis spec-tra of MO after1-min treatments over the composites at varying ratios indicate that the composites with higher Cu:Zn molar ratios lead to the higher color removal rates.Chemical oxygen demand(COD)and total organic carbon(TOC) values are generally determined to examine the water quality. According to the results(Fig.5)COD removal efficiencies over theTable2EDX analysis in different areas of the Cu2(OH)3NO3/ZnO samples at various Cu:Zn molar ratios.Cu:Zn%ElementArea#1Area#2Area#3Cu Zn O Cu Zn O Cu Zn O2:125.7414.8059.4639.85 5.5254.6323.5226.3050.18 4:137.78 5.1057.1145.08 1.9053.0154.09 2.8243.08 6:153.70 3.4042.9048.12 1.4850.4035.73 1.3262.9588 A.Srikhaow,S.M.Smith /Applied Catalysis B:Environmental 130–131 (2013) 84–92Fig.3.EDX analysis of the Cu 2(OH)3NO 3/ZnO samples at various Cu:Zn molar ratios (a)2:1,(b)4:1and (c)6:1,respectively.catalyst are about 88%and 98%after treatment for 5and 20min,respectively.In addition,the results in Fig.5also suggest organic carbon mineralization and CO 2evolution from the oxidation of MO after 5-and 20-min of the treatments by Cu 2(OH)3NO 3/ZnO (Cu:Zn =4:1)resulting in 84%,and 94%TOC removal efficiencies,respectively.Thus,at this catalyst loading condition,the effective decolorization of MO occurred through the fragmentation of the dye into some other colorless compounds,as well as,the mineral-ization of MO.3.3.Possible mechanism and degradation pathwayThe BET surface area of catalysts prepared by the Cu:Zn molar ratios of 2:1,4:1,6:1are 10.81,8.85,and 5.76m 2/g,respectively.From Fig.4a,the Cu 2(OH)3NO 3/ZnO with lower specific surface area gave the higher color removal efficiency,suggesting that the effective color removal was not due to adsorption of dye onto the solid surface.It was found that the higher performance catalysts have lower surface areas,and thus the MO degradation rates are not proportional to the BET surface area of the catalyst.This maybe because copper hydroxyl nitrate deposited on the surface and filled in the pores of the ZnO,resulting in the materials with lower surface areas.It should be also pointed out that the lower surface area materials also have reduced pore volumes,as the pore volumes of catalysts prepared by the Cu:ZnmolarFig.4.(a)Color removal efficiency upon time using Cu 2(OH)3NO 3/ZnO catalysts as a function of Cu:Zn molar ratio and surface area (m 2/g),(b)kinetic of methyl orange oxidation catalyzed by Cu 2(OH)3NO 3/ZnO as a function of Cu dosage and (c)UV–vis absorption spectrum of fresh MO (50ppm)and those of oxidized MO after 1-min treatment by the catalysts with various Cu:Zn molar ratiosunder ambient conditions.Initial concentration of MO =500ppm;catalyst loading =3g L −1.ratios of 2:1,4:1,6:1are 0.08,0.07,and 0.02cc/g,respectively.In addition,approximately the same color removal efficiency (∼99%)was also observed when a dispersion of Cu 2(OH)3NO 3/ZnO (Cu:Zn =4:1)in aqueous MO solution was kept in the dark under similar experimental conditions mentioned above.Thus,light had no influence to the catalytic activity of composite.As a result,one could suggest a catalytic wet oxidation (CWO)process,in which the dye undergoes aerial oxidation over the composites.Note that,commercially supplied ZnO powder can also be replaced the flower-like ZnO to produce the Cu 2(OH)3NO 3/ZnO composites,A.Srikhaow,S.M.Smith/Applied Catalysis B:Environmental130–131 (2013) 84–9289Fig. 5.Color(Á),COD and TOC removal efficiencies upon treatment of MO aqueous solution by the Cu2(OH)3NO3/ZnO(Cu:Zn=4:1).Initial concentration of MO=500ppm;catalyst loading=3g L−1.having almost the same catalytic activity.In an attempt to under-stand the nature of MO degradation over the Cu2(OH)3NO3/ZnO composites,additional MO degradation reactions were conducted in various experimental conditions and reported in Fig.6.It is well known that CWO catalysts require oxygen to degrade organic compounds.Accordingly,if catalytic wet oxidation(CWO) was the major process responsible for MO degradation,the oxi-dation rate of MO should be directly proportional to the oxygen concentration.One of the experiments was conducted under vac-uum(setup Fig.S2)here the MO solution was thoroughly degassed prior to being used.As reported in Fig.6,the color removal effi-ciency of MO under vacuum was about7.72%,which is much lower than under ambient conditions(∼100%).The proposed mechanism of CWO reactions outlined by Ma et al.[44]may be applied to describe the CWO reactions occurring here.RH+Cu2+→R•+Cu++H+(3) Cu++O2→Cu2++O2−(4) 2O2−+2H2O→2OH−+H2O2+O2(5) H2O2+Cu2+→HO•+OH−+Cu2+(6)HO•+MO→degradationproducts(7)parative results of the color removal efficiency after5min treatment of MO with Cu2(OH)3NO3/ZnO(Cu:Zn=4:1),with varying experimentalconditions.Fig.7.Powder XRD patterns of Cu2(OH)3NO3/ZnO samples(Cu:Zn=4:1)before and after reaction comparing with that of MO.From the proposed model,Cu(II)in the catalyst undergoes reduction reaction forming Cu(I)which further reacts with oxygen dissolved in an aqueous solution.Subsequently,H2O2is generated as intermediates through the reaction of O2−and water molecules. Following this model,it is possible that hydroxyl radical is cre-ated when Cu2(OH)3NO3decomposed H2O2intermediates.Finally, MO molecule was attacked by hydroxyl radicals.According to the proposed CWO reaction mechanism,the presence of radical scavenging reagents such as NaOH and tert-butanol,should signif-icantly inhibit the oxidation of MO.The result in Fig.6represents that adding NaOH and tert-butanol gave low color removal effi-ciencies of2.40%and5.95%,respectively after5-min treatments using Cu2(OH)3NO3/ZnO(Cu:Zn=4:1).These results strongly sup-port that the decomposition of MO occurred through a radical pathway.Previously discussed from the result in Fig.4c,the decrease in intensity of the absorption band corresponding to MO indi-cated the cleavage of the azo group,and hence decolorization of the dye solution.No spectral shift corresponding to possible complexation between dye molecules and metal cations[41–43] was observed in our system.Besides this,Fig.7shows that the Cu2(OH)3NO3/ZnO catalyst undergoes no significant structural change after20min reaction.Apart from the typical features cor-responding to the Cu2(OH)3NO3crystalline phase,extra diffraction peaks were observed at8.9◦and17.1◦which correspond to crystal-lized MO on the catalyst surface.The observed slight shift in peak positions is possibly due to microstrains on the sample occurring during the drying process.According to XRD results,there is no evidence of any new crystalline phase in the used catalyst,ruling90 A.Srikhaow,S.M.Smith /Applied Catalysis B:Environmental 130–131 (2013) 84–92Fig.8.Possible MO degradation pathway producing molecular fragments as detected by LC/MS.out the possibility of complexation between the Cu 2(OH)3NO 3/ZnO composite and MO.Consequently,all results discussed above sup-port the degradation of MO over Cu 2(OH)3NO 3/ZnO composites via a catalytic wet oxidation process.Nevertheless,in contrast to conventional CWO catalysts,Cu 2(OH)3NO 3/ZnO is highly active with no requirement of air or oxygen flow or any additional oxidant.In an attempt to determine the nature of MO degradation prod-ucts,LC/MS analysis of decolorized MO solutions revealed the presence of three chemical species after a 5min reaction period.At level of 99%decolorization,the chemical species identified were unreacted MO (M w =304)and two product species with m /z =290and 208,corresponding to MO fragments (Fig.S3).The reaction steps in wet oxidation of MO catalyzed by Cu 2(OH)3NO 3/ZnO observed by LC/MS are given in Fig.8.3.4.Catalyst reusabilityThis part focuses on possibility of recovery,recyclization,and regeneration of the catalyst.By simple centrifugation and decanta-tion,it was found that the Cu 2(OH)3NO 3/ZnO (Cu:Zn =4:1)catalyst can be reused for three consecutive runs,without any further treat-ment,maintaining the color removal efficiencies of 99%,and the COD and TOC removal efficiencies of greater than 90%after 20-min reaction as shown in Fig.9a.As previously discussed,adsorption of MO on the catalyst surface occurred.However,based on the high color removal efficiencies for three cycle utilization,the presence of adsorbed MO on the surface of used catalyst did not affect the color removal efficiencies.Nevertheless,when the catalyst was fur-ther reused without treatment in the 4th run,it was found that the color removal efficiency dropped from 99%to about 37%as reported in Fig.9b.Therefore,the used catalyst after the 3rd run requires suitable regeneration prior to further use.Possible regen-eration methods include refluxing method,calcination under a suitable atmosphere,rinsing by appropriate solvent or some combi-nations of processes [12,19,45,46].Due to the low thermalstabilityFig.9.(a)Color (Á),COD and TOC removal efficiency for suspension of the Cu 2(OH)3NO 3/ZnO (Cu:Zn =4:1)in MO aqueous solution during consecutive runs and (b)comparative results of the color removal efficiencies (Á)of MO by the Cu 2(OH)3NO 3/ZnO (Cu:Zn =4:1)in the 1st and 4th cycles without regeneration,and that of the 4th cycle obtained by employing the regenerated catalyst after the 3rd run via mild acid wash.Initial concentration of MO =500ppm;catalyst loading =3g L −1.of copper hydroxide nitrate and the solubility of metal oxide in acid,the spent Cu 2(OH)3NO 3/ZnO was regenerated by washing with weak acid to remove the unreacted MO and,possibly,degra-dation products adsorbed on the catalyst surface without causing serious damages to the catalyst.It was found that,after filtration,washing the spent catalyst (from the 3rd run)with 5mM HCl(aq)for 20min,and rinsing with water followed by drying at 100◦C,the regenerated catalyst can be employed in the 4th cycle giving the color removal efficiency of 95%as shown in Fig.9b.This color removal efficiency is lower than those obtained from the first three cycles,probably due to some loss of active copper species during acid washing.Table 3reports that,after 20-min MO degradation,the Cu 2(OH)3NO 3/ZnO (Cu:Zn =4:1)catalyst slightly dissolved in the dye solution giving the concentration of Cu and Zn ions of 4.3and 0.5ppm in the first cycle.In the subsequent cycles,the solubil-ity of catalyst was found lower.According to the high color,COD,and TOC removal efficiencies (>90%)for three cycle utilization,this trace amount of meal leaching did not affect the removal efficien-cies,implying that the Cu 2(OH)3NO 3/ZnO catalyst was stable for three consecutive cycles with no requirement of catalyst regen-eration (Fig.9a).However,due to the results of metal leaching,there may be questioning of the MO degradation via homogeneous。
看穿你的报表

通过现金流追踪企业财务舞弊线索。
20 0 8年 4月 ,随 着 上市 公司 2 0 0 7年 年 报 的全 面发
布,郑朝 晖署名 “ 夏草” ,在其 “ 财务侦探”博客接连发
表《 深市 中小板十大涉嫌偷漏税过会公司》 沪市 2 0 、《 07
最不容易掺假 的资产。可我在分析中发现,1I 0 账户也是 不安全 的。尤其是在经济下滑时期,货币资金最容易被
挪用。一些企业资金链其实已经 出现 紧张征兆,但账面
9 2
同 S L& P RU1E T E OP TN I 月 读 Y O TS
上仍有巨额的现金, 或者_直以来有巨额现金躲在账面上。
月 读
S TY & O P ORTUN I ES LE P Tl
对照审视自己 见人摔 跤而抚掌大笑 ,只是看客的心理 ;
的脚下,才是智者所为 。
●
看穿你的报表
◎ 子 非 /文
20 0 8年 8 月,一本针对上市公司财务造假 的新书面 世,其作者郑朝晖也再次成为公众视 线的焦点。 郑 朝晖,笔 名飞 草、申草、夏草 ,从 2 0 年开始 01
品质! ”国
▲
哲 倒
一
识破产业链 阴谋 j n
_
懿
l 凰 g
.
即 咸 半 /义
一 _ 露 _ _ ● I % 捌
我 希望我们 的新 一代年轻人——我们未来 的领袖们都
‘ l
}
●
能具备逆 向思维的能力。对于很 多专家学者提出的 “ 中国是 个制造业大国”这个命题 ,我们应该多一些辨证的思考。 任何行业的产业链 ,除了加工制造,还有 6 大环节 : 产 品设计、原料采购、物流运输、订单处理、批发经营、终端
企业的简单介绍

企业的简单介绍
一、公司背景
本公司成立于2010年,是一家专注于提供高品质产品和服务的企业,总部位于国内一线城市。
公司拥有一批高素质的管理人才和技术人才,以及一支专业的销售和客服团队。
公司秉承“以质量求生存、以信誉求发展”的经营理念,不断追求卓越,为客户提供更好的产品和服务。
二、主营业务
公司的主营业务包括XXX和XXX两个领域,其中XXX业务主要是XXX,XXX,XXX等;而XXX业务主要是XXX,XXX,XXX等。
公司的业务范围广泛,能够满足客户不同需求的需求。
三、公司愿景
本公司的愿景是成为全球领先的XXXX企业,在不断创新和发展的道路上,为客户提供更优质的产品和服务。
四、公司文化
本公司的企业文化是以创新为核心,以服务客户为宗旨,以诚信为基础。
公司注重员工的职业发展和素质提升,为员工提供丰富的培训和发展机会。
同时,公司也积极参与公益事业,回馈社会,做出自己的贡献。
五、公司荣誉
多年来,公司凭借卓越的产品和服务,赢得了广大客户的信赖和支持,取得了一系列荣誉和奖项。
例如:XXX奖,XXX奖,XXX奖等。
这些荣誉是对公司一直以来不断追求卓越的肯定,也是公司未来发展的动力。
六、发展规划
为了适应市场变化和满足客户需求,本公司将不断进行技术创新和升级,提高产品的质量和性能;同时,公司还将加强与国内外优秀企业的合作,开拓新的市场和业务领域。
未来,本公司将继续以客户需求为导向,不断优化自身业务和服务体系,为客户提供更优质的产品和服务。
企业类型对照表
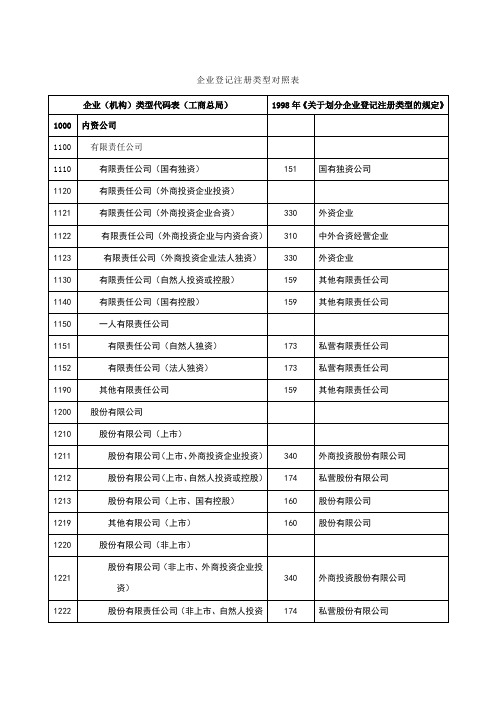
企业登记注册类型对照表
说明:1.凡在工商局登记为“股份制”、“股份制企业”、“股份制企业(非法人)”
的单位,如是按照《中华人民共和国公司登记管理条例》注册为股份制企业,
并以募集方式筹集资本的,对应160“股份有限公司”;否则,对应151“国
有独资公司”或159“其他有限责任公司”。
2.“联营”企业按照实际联营情况,对应141“国有联营企业”、142“集体联
营企业”、143“国有与集体联营企业”、149“其他联营企业”。
3.“有限责任公司(台港澳与外国投资者合资)”按照台港澳与外国投资者的
出资比例,对应230“港、澳、台商独资经营企业”或330“外资企业”。
如果出资比例各为50%,则按照协议,以拥有企业实际控制权(协议控股)
作为判断依据。
4.“股份有限公司(台港澳与外国投资者合资、未上市、上市)”按照台港澳
与外国投资者的股份比例,对应240“港、澳、台商投资股份有限公司”或
340“外商投资股份有限公司”。
如果双方股份各为50%,则按照协议,以拥
有公司实际控制权(协议控股)作为判断依据。
5.“集团”随核心企业判断注册登记类型。
公司简介的翻译(中英文版)
公司简介的翻译企业的历史history发展现状和成就公司信息corporate information行政管理状况executives and management前景,企业文化和价值观value, ethics, culture, vision and philosophy公司治理corporate governance产品服务范围products and services企业领导人致辞president’s message功能:一,提供公司信息二,宣传公司,引起注意,呼吁合作语言特点:一份企业简介,除概要性地介绍企业的情况外,对其产品也做了简略的宣传。
从文体上看,企业简介是说明书的一种,属于外贸应用文文体。
从语篇类型上看,它属于信息股东类语篇,起着宣传介绍的功能。
预期译文功能是在译语语境中,使译文读者对该公司及所生产的产品留下深刻印象,最终达到促其购买产品的目的。
所以,企业简介不同于一般的说明书,具有自己的语言特点:1.选词富有鼓动性原文:拥有雄厚的技术力量。
译文:Boasting tremendous technological strength原文:该厂最近又开发出珍珠牙膏系列产品,收到消费者的青睐。
译文:The pearl king, the latest achievement of NPC, is very well—received by customers at home and abroad2、有一定的程式化用语1) “主要经营”. . . . . . 可译成: engage in , handle a large range of business including . . .2) “奉行/坚持⋯⋯原则; 以⋯⋯宗旨”可译成: hold/abide by the principles of . . . , adhere tothe aims of . . . , follow the te . . . , based by the motto of the pany , with the enterprise spiritof . . .3) “经⋯⋯批准”可译成: approved , appointed , permitted.4) “集⋯⋯于一体”可译成: feature , integrate , bine.5) 企业的性质: “外资企业”可译成: foreign - funded enterprise ,“合资企业”可译成:joint venture ,“合作企业”可译成: cooperativeenterprise ,“独资企业”可译成: wholly foreign- owned enterprise.●获得奖项: “获得金奖”可译成: be awarded the gold prize. “通过ISO9002 质量认证”可译成: pass/gain/obtain/be granted the Certificate of ISO9002 International Quality System.●“最受欢迎产品奖”可译成: be awarded most wele goods.●“荣誉企业”可译成: honorable enterprise.●“优质企业”可译成: qualified enterprise.●“一级企业”可译成: class A enterprise.3、经常使用标语口号式的文字例1 : “质量第一, 信誉第一, 服务至上, 平等互利”。
关键词索引
2009年15卷第17期关键词索引自然科学数学Bayes分析 09170075 Cerami条件 09170074 Hardy位势 09170074 S-粗集 09170076 α,β-迁移 09170076 存在性 09170074 多阶段试验 09170075 可靠性增长 09170075 临界指数 09170074 实时调度 09170076 双调和方程 09170074 双向IS-粗集 09170076 序化建模 09170075信息科学与系统科学B92协议 09170077 GM(1,1)模型 09170079 波动性 09170078 沉降变形 09170079 多式联运 09170080 反比参与率 09170078 复杂网络 09170078 恒生指数 09170078 互信息 09170077 交易量 09170078 介数指标 09170078 快速货物运输 09170080 量子密码术 09170077 时间窗 09170080 蚁群算法 09170080 优化 09170079 预测 09170079 最佳窃听角度 09170077力学“拟静力”方法 09170129 1∶10模型结构 09170124 Ⅲ型界面裂纹 09170110 Bautin系统 09170093 Biot动力方程 09170129 Davenport谱 09170123 Fourier级数 09170112 Levy型索穹顶 09170096 LLS模型 09170134 LMI不等式 09170139 Lyapunov方程 09170112 Lyapunov量 09170093 Maple符号程序 09170093 Navier-Stokes方程 09170127 Noether守恒量 09170087Shepard形函数 09170115Simulink软件 09170152Timoshenko梁 09170091UDF 09170128Y型分支管 09170146板结构 09170108贝塞尔函数 09170103倍化分岔 09170095被动减振器 09170123本构关系 09170102本征频率 09170121崩落法 09170118边界层理论 09170147边界元法 09170144波动方程 09170122博弈 09170109薄板 09170138薄层法 09170137参数共振 09170136参数激励 09170135层状地基 09170137颤振 09170126长期项 09170133超细长弹性杆 09170090驰振 09170128尺寸效应 09170100冲击 09170120冲击动力学 09170143初始条件 09170121初始预应力分布 09170096穿透裂纹 09170151纯滚动 09170084粗集 09170104大尺度采空区 09170117大展弦比模型机翼 09170127代表性体积元 09170098弹簧 09170081弹性振动 09170127道路工程 09170102低周反复加载试验 09170099地震反应 09170125跌落冲击 09170105叠加原理 09170111定解问题 09170122动力失稳 09170117动水作用力模型 09170125动态断裂韧度 09170100动态塑性响应 09170120动网格 09170128冻土 09170106短肢剪力墙 09170101断裂动力学 09170110多刚体系统 09170150多孔饱和地基 09170129多目标 09170109多体系统动力学 09170094多重孔隙介质 09170111反分析 09170113反复荷载 09170116反向动力学 09170150仿真 09170145放矿 09170118非局部弹性 09170091非均匀矿岩散体 09170118非齐次项 09170122非线性 09170097非线性分析 09170124非线性粘弹性 0917010209170105分叉 09170138分岔 0917008909170131分离流 09170127分数阶导数Maxwell模型 09170102风荷载取值 09170123风特性 09170142附连水质量 09170144复杂时滞动力网络 09170132改性沥青 09170102概率神经网络 09170104干摩擦 09170095杆 09170096刚柔耦合系统 09170092钢板-混凝土组合梁 09170097钢筋混凝土桥墩 09170099钢丝绳隔振器 09170130港口、海岸及近海工程 09170125高强度螺栓 09170152高压水射流 09170147各向异性 09170111拱坝 09170109共谋合作模型 09170109共振传感器 09170135广义Maxwell模型 09170102广义质量 09170150果实 09170105哈密顿函数 09170087海底悬跨管道 09170125海量数据 09170148含水量 09170106耗能能力 09170114弧长 09170086滑移 09170116滑移模型 09170097混沌 0917009509170131混沌FHN神经元振子 09170132基因调控网 09170140基于性能抗震设计 09170099激变 09170138极限环分岔 09170093几何概念 09170090几何相似试样 09170100几何优化 09170148加载率 09170100尖叫 09170126剪切滑移滞回模型 09170114简捷计算法 09170096建模方法 09170092建筑结构 09170101渐进法 09170136节点 09170096结构影响系数 09170114结构优化 09170109解析解 091701030917011009170123解析试函数法 09170101界面参数 09170113金属-FGM-陶瓷复合板 09170149金属薄膜 09170113近似解 09170133精确模型 09170090精细积分 09170112径向刚度 09170103静电驱动 09170135聚类 09170140开普勒定律 09170085抗侧刚度 09170098抗震安全 09170124考虑自重 09170096颗粒运动 09170146空间算子代数 09170150孔道孔径 09170143控制规则 09170134快慢变系统 09170133拉格朗日变量 09170121拉格朗日函数 09170087拉格朗日力学逆问题 09170087离散变量 09170108离散元法 09170118离心率 09170085理想弹塑性滞回模型 09170114力矩 09170088力敏传感器 09170141力学模型 09170105梁 09170120两自由度模型 09170128临界坡度 09170119灵敏度分析 09170094流体 09170091流体力学 09170143鲁棒脉冲同步 09170132路堑边坡 09170119煤层切槽 09170147密肋复合墙体 09170098面积速度 09170085 摩擦 09170126 内波 09170145 能量公式 09170082 拟动力试验 09170124 黏弹性 09170136 扭转刚度 09170107 欧拉方程组 09170103 耦合 09170106 耦合变形影响 09170092 爬行步态 09170134 频散 09170091 平均值 09170121 苹果 09170105 坡面流 09170119 奇异摄动 09170133 气固两相流 09170146 潜体 09170145 浅埋煤层 09170117 侵彻 09170143 缺口 09170151 容积法 09170137 柔性梁 09170092 三维弹性理论 09170103 三维应力强度因子 09170151 设计流程 09170096 射流 09170143 神经网络 0917008909170130 失速 09170127 时滞 09170089 输电线 09170133 数据融合 09170104 数学建模 09170152 数值分析 09170125 数值模拟 0917011809170146 数值研究 09170106 栓钉 09170097 双盘转子-轴承系统 09170131 撕裂试验 09170113 四边形网格 09170148 速率 09170082 塑性铰模型 09170099 随机风振 09170123 随机振子 09170140 损伤 09170105 损伤识别 09170104 损伤指标 09170099 索 09170096 索支撑系统 09170107 台风 09170142 泰勒多项式基 09170115 碳纳米管 09170091 体胀系数 09170141 条形基础 09170137 同步 09170140 土壤侵蚀 09170119 湍流尾迹 09170145 椭圆轨道 09170083 拓扑优化 09170148 网格畸变 09170101微机电系统 09170135微粒群算法 09170108韦帕 09170142位错分布函数 09170110位移法 09170103温度 09170106稳定性 09170136稳态热应力 09170149涡激振动 09170128无量纲数 09170120无限长线荷载 09170137无限域流场 09170144无源化 09170139细观力学有限元 09170098现场实测 09170142线弹性力学 09170111线性时变系统 09170112橡胶筒 09170103星形支架 09170107形状函数 09170121形状记忆合金 09170138型钢混凝土柱 09170116虚拟原点 09170143虚位移 09170090悬点 09170086悬链线 09170107悬索桥 09170142压膜阻尼 09170135延性 09170114演示实验 09170088遥感 09170145叶片 09170131移动荷载 09170129应力 09170106应力腐蚀 09170152优化 09170107优化设计 0917009409170108有限元 0917010109170126有限元法 091701080917014909170151有限元分析 09170097有效弹性模量 09170098有效应力 09170111有心运动 09170085预反射 09170134预应力混凝土安全壳 09170124圆弧摆 09170086圆锥曲线轨道 09170085运动轨迹 09170084运动认知 09170134运动时间 09170083运动学 09170090杂交建模 09170130噪声 09170126增广Lagrange乘子法 09170094粘结退化 09170116粘结应力 09170116粘聚力模型 09170113赵氏响应数 09170120振动隔离 09170130整星隔振 09170139正实控制 09170139制动 09170126质量 09170121质量分布 09170081质心 09170081滞后非线性 09170130中心直裂纹平台巴西圆盘 09170100重力场 09170081周期 091700820917008409170086周期系数 09170112周期运动 0917008909170095轴向变速运动梁 09170136属性约简 09170104转动动能 09170088锥体上滚 09170088自然单元法 09170115自然相邻结点插值 09170115自相似函数 09170110综合试验 09170117阻抗函数 09170137阻尼谐振子 09170087最小二乘法 09170115最小势能原理 09170081物理学BEC 09170002Clebsch-Gordan系数 09170158C语言编程 09170165Dirac符号法 09170163H波导 09170166n维 09170160Wigner-Seitz原胞 09170192X射线组合折射透镜 09170178爱因斯坦 09170001保长同胚 09170192本征函数 09170161本征矢的完备性 09170156变密度法 09170179波束跟踪理论 09170167波速 09170175玻尔能级公式 09170157玻尔兹曼常量 09170171不变量本征算符 09170159不定方程 09170165布朗运动 09170171布里奇曼 09170154布里渊区 09170192参量 09170193测量电路 09170183掺杂镍 09170189成像特性 09170176成像质量 09170180电磁场 09170003电磁感应 09170184电磁理论 09170172电抗性质线 09170182电桥的输出电压灵敏度 09170186电容式传感器 09170183对称性 09170158二阶常系数线性微分方程 09170187二维耦合量子谐振子 09170159二维无限深势阱 09170165反对易c数 09170155反演对称性 09170192范德瓦尔斯方程 09170169非辐射介质波导 09170166非平衡电桥 09170186非球面人工晶体 09170180非线性代数方程组 09170162非线性误差 09170186费米法 09170170费米算符 09170155分子平均场理论 09170169干涉 09170173高频交流电磁铁 09170184高压实验技术 09170154高压物理学 09170154各向异性晶体 09170172固有频率 09170153光量子假说 09170001光学性能 0917017809170180光栅 09170175黑体辐射 0917016009170191混响 09170167极大值 09170174极限环 09170164简并度 09170165简谐振子 09170002简正波 09170167渐变周期弯曲光栅 09170176交流发电机 09170187角动量 09170158阶跃光纤 09170177截止条件 09170177介电函数 09170188介观耦合电路 09170185精确解 09170162净热 09170170静态假设 09170170矩形波导 09170166矩阵 09170191聚焦 09170178卡诺循环 09170170开口谐振环 09170166颗粒物质 09170193克劳修斯方法 09170170空间反射镜 09170179库仑散射 09170190勒让德变换 09170168粒子数算符的本征矢 09170156 量纲分析 09170191 量子化 09170185 量子力学表象 09170163 量子态 09170165 量子态密度 09170160 灵敏度 09170174 隆格-楞兹矢量 09170157 卢瑟福散射公式 09170190 马吕斯定律 09170174 麦克斯韦方程 09170003 脉动直流电 09170187 能量本征值 09170159 钕铁硼永磁铁 09170184 耦合 09170153 偏心距 09170181 偏心球形微粒 09170181 平行平板波导 09170166 气垫导轨 09170184 轻量化 09170179 球差 09170180 热力学 09170168 热力学势能 09170170 萨尔茨堡会议 09170001 三点式正弦振荡器 09170182 三角级数 09170162 射线模型 09170177 受迫振动 09170153 双折射 09170172 同伦延拓法 09170164 投影算符的积分 09170156 拓扑优化 09170179 外推法 09170153 微分析 09170178 涡电流 09170184 吸收系数 09170188 显微摄像系统 09170171 线偏振 09170172 相对论粒子 09170160 相干态 09170155 相积分 09170157 相位平衡 09170182 消光系数 09170188 谐波平衡 09170164 修正算法 09170164 旋转式 09170002 雅可比行列式 09170168 衍射 09170173 衍射特性 09170176 杨氏模量 09170153 氧化钨钼薄膜 09170189 液体表面波 09170175 液体密度 09170183 一维表示 09170161 异向介质 09170166 有效介电常量 09170181 宇称算符 09170163 圆锥曲线 09170190 真实气体 09170169 正三角形 09170161 正则变换 09170185阻抗特性 09170189最大变化率 09170174作用半径 09170169坐标伸缩 09170003坐标算符的本征矢 09170156化学“分子胶囊” 091702441-烯丙基-3-甲基咪唑盐酸盐 091704661,3-二苯基异苯并呋喃 0917033810-乙基吖啶酮-2-磺酰氯 09170342091703531H核磁共振 091704082-(对羟苯基)丙酸 091703372-亚烷基-5-羟甲基四氢呋喃 091703103-苯甲基-2,5-吗啉二酮 091704334,4′-联吡啶-2,2′,6,6′-四羧酸 091702244,4′-偶氮二(4-氰基戊酸) 091704564′-对二甲氨基苯基-2,2′∶6′,2′′-三联吡啶 091702205-羟基-6,7,8,3′,4′-五甲氧基黄酮 091703695-羟色胺 091703635-羟吲哚乙酸 091703636-羟基-1-甲基-1,2,3,4-四氢-β-咔啉 09170363ABA三嵌段聚合物 09170457Au-Pd催化剂 09170397B3LYP 09170421BaFe12O19 09170219BAFP 09170373Brønsted 酸 09170329C3H2 09170418Ca2Zn4Ti16O38∶Pr3+ 09170204Cd(OH)2 09170301Cd1−x Zn x S 09170411Ce-Si共掺杂 09170402CeO2-TiO2复合物 09170383CeO2改性 09170397CHEMSPEC 09170221CMC 09170330Co/Fe催化剂 09170395Corrole 09170408Cr(Ⅵ) 09170294CT DNA结合 09170332Cu(Ⅱ)配合物 09170199Cu(II)化合物 09170426Cu2+ 09170295Cu6Sn5合金 09170401CuBr 09170206CuCl2 09170406CuMn/γ-Al2O3催化剂 09170398CuSO4 09170406DL-氨基酸 09170279DNA 0917025409170299DNA切割 09170243FCC废催化剂 09170201Fe-AlPO4-5 09170400Freundlich方程 09170266Gemini型咪唑基离子液体 09170464Herschel-Bulkley模型 09170381HMS 09170288HOMO-LUMO 09170441HPLC/ESI-Q-TOF MS 09170340K2La2Ti3O10 09170210K562 09170333Keggin结构 09170283KF-蒙脱土 09170325Kolbe-Schmitt反应 09170006L-半胱氨酸 09170296L-异亮氨酸 09170279L-组氨酸 09170377La1- x Sr x YO3- α 09170213La2O3颗粒 09170416LaYO3 09170213Lewis酸 09170330LiCo1/3Ni1/3Mn1/3O2 09170403MAG3衍生物 09170477MDA 09170261MDMA 09170261Mg掺杂 09170197MnCr2O4纳米线 09170198MnO2 09170256MTT 09170333N-Boc保护谷氨酸 09170323n-C5H11I 09170405N-异丙基丙烯酰胺-N-乙烯基吡咯烷酮共聚物 09170425NaY分子筛 0917020109170247NiO 09170208NO x 09170399O(3P) 09170418OH自由基 09170427Pb(II) 09170218Pb2+ 09170295PbS 09170259Pd-Ce/γ-Al2O3 09170394Pd催化剂 09170223pH敏感性 09170471PS/SiO2纳米复合微球 09170473pUC19 DNA 09170306QuEChERS方法 09170347Rh(I)-Cr催化剂 09170372RNA干扰 09170472Ru-Fe/C催化剂 09170257Ru/C催化剂 09170257Salen Mn(III) 09170222salen 09170335Salen型配合物 09170200SBA-15 09170262Schrock钼催化剂 09170228SiO2∶Sm 09170250siRNA载体 09170472Ta2O5 09170233Taguchi技术 09170247TiO2 09170402TiO2包覆 09170403Tutton盐 09170232UF6 09170221Williamson成醚反应 09170267XRD精修 09170205Xylopyridine A 09170332X射线单晶 09170420X射线光电子能谱 09170469Yb3+掺杂 0917024109170223ZnO 09170236α-噻吩甲酰三氟丙酮-哌啶合铈 09170229α-氧化铁 09170252α,β-不饱和酮 09170325β-FeOOH 09170252β-二酮环金属铂配合物 09170249β-环糊精 0917031509170388β-榄香烯 09170230β-酮酸酯 09170310阿卡波糖 09170362阿维菌素类药物残留 09170351癌胚抗原 09170312氨 09170399氨基酸 09170320氨解 09170449胺 09170334钯 09170334钯催化剂 09170396白度值 09170377白藜芦醇 09170303白藜芦醇白蛋白纳米粒 09170303白铜B10 09170424半导体 09170236包结物 0917031509170388保水性 09170276杯芳冠醚 09170320杯芳烃 0917032009170323本构方程 09170428本征动力学 09170395苯-N,N-二甲基甲酰胺 0917038409170260苯并咪唑 09170271苯酚 0917026609170392苯基化 09170258苯基三甲氧基硅烷 09170258苯甲醇 09170288苯甲醛 09170288苯乙胺-N,N-双甲基膦酸 09170215 苯乙胺-N,N-双甲基膦酸锆 0917021509170215 苯乙双胍 09170362 苯乙烯基硼酸 09170268 比色氟离子识别 09170370 吡唑 09170272 铋 09170297 表观摩尔体积 09170384 表面活性剂 09170414 表面自由能 09170277 冰相 09170392 丙二腈 09170325 丙交酯 09170433 丙烯腈 09170466 丙烯酸丁酯 09170284 铂 0917038909170410 卟啉 09170304 不对称 09170335 不对称环氧化 09170222 不匹配层状化合物 09170196 不匹配结构 09170196 不锈钢箔 09170198 草酸二甲酯 09170396 层间距 09170215 层状化合物 09170215 层状双金属氢氧化物 0917000809170456 查耳酮 09170268 茶叶 09170351 超额摩尔体积 09170384 超分子 09170194 超高效液相色谱 09170360 超高效液相色谱-串联质谱法 09170350 超高效液相色谱-质谱联用法 09170351 超临界二氧化碳 09170425 超声波散射 09170440 超声波在线跟踪 09170440 超声化学 09170236 超疏水 09170387 超支化聚氨酯 09170286 超支化聚合物 09170453 沉淀聚合 09170451 陈皮 09170369 橙皮苷 09170369 弛豫速率 09170444 除草活性 09170313 储氧材料 0917023709170407 磁共振成像造影剂 09170444 磁化反转 09170234 磁性 0917025309170426 雌二醇 09170353 雌三醇 09170353 次级代谢产物 09170274 从头测序 09170346从头计算 09170203粗化 09170439醋酸纤维丝束 09170285醋酸乙烯酯 09170284催化 09170223091702390917028909170326催化层 09170416催化剂 0917023709170394催化燃烧 091700070917039409170407催化性能 09170389大豆 09170355大环 09170318大气氧化反应 09170427大叶藤黄 09170371代谢产物 09170332丹磺酰氯 09170353单壁碳纳米管 091703310917037609170389单分散大孔亲水交联聚甲基丙烯酸环氧丙酯微球 09170358单分散树脂 09170279单线态氧 09170338胆固醇传感器 09170341胆固醇吸附 09170470胆固醇氧化酶 09170341胆固醇酯酶 09170341蛋白质 09170375蛋白质纯化 09170358蛋白质组 09170374氮掺杂 09170217氮氧化物 09170239氘代试剂 09170348导电 09170430导电性 09170273等规聚丙烯/炭黑复合材料 09170442等离子体 09170390等温结晶 09170442低黏度 09170448低温 09170399低温选择性催化还原 09170239第一性原理 09170210缔合流体 09170422电沉积 091702060917041009170412电催化 091702650917038209170423电化学超级电容器 09170415电化学辅助沉积 09170474电化学合成 09170310电化学检测 0917036309170365电化学聚合 09170441电化学容量 09170415电化学阻抗谱 091702420917039309170401电解质 09170276电容性能 09170208电致变色器件 09170383电致发光 09170445电致磷光 09170211电子光谱 0917024909170409凋亡素基因 09170312迭代搜索 09170374丁吡吗啉 09170315动力学方程 09170395动态共价键化学 09170318独居石CePO4 09170195端氨基聚(醚-氨酯-酰胺) 09170444端基 09170434端羟基聚酯低聚物 09170438对氨基苯磺酸 09170209对氨基苯乙酸 09170203对映体分离 09170279对映体选择性合成 09170328多巴胺 09170296多吡啶配体 09170254多壁碳纳米管 091702960917030709170419多残留 09170348多齿配体 09170306多角星 09170236多金属氧酸盐 091702620917027309170283多晶格晶体 09170196多孔硅 09170251多孔铜集流体 09170401多孔氧化铝模板 09170234多孔支架 09170474多树枝 09170259多肽 09170251多肽分离 09170357多肽合成 09170477多羰基 09170475朵蕾烷二萜 09170321二醇 0917031909170328二次活化 09170415二氮杂萘联苯 09170211二碘化钐 09170326二芳醚合成 09170336二甲醚 0917037809170398二甲双胍 09170362二阶非线性光学性质 09170249二硫键稳定性单链抗体 09170312二噻吩-苯并硒二唑 09170287二维结构 09170225二氧化钛 091702170917037909170387二氧化碳 0917000609170469二氧化碳乳化液 09170005二乙氧基丙烯酰胺甲氧基硫代磷酸酯 09170293二正丁基锡 09170203发光共聚物 09170287钒(Ⅳ/Ⅴ)电对 09170227钒氧配合物 09170413钒液流电池 09170227反渗透膜 09170385反相高效液相色谱 09170353反相气相色谱法 09170212反相微乳液09170472反应机理 091702280917041809170427反应诱导相分离 09170440芳胺 09170409芳基三氟甲磺酸酯 09170334芳酰胺 09170318芳酰基氨基硫脲 09170271芳香醛 09170280仿生合成 09170377纺丝液 09170248放射性药物 09170477非晶/纳米晶Ni-Mo合金 09170412非牛顿指数 09170248分光光度法 09170266分离 0917023009170332分离纯化 09170305分散固相萃取 09170355分子动力学模拟 0917038809170404分子烙印 09170263分子量 0917042909170435分子模拟 0917037309170385分子印迹聚合物 09170344分子印迹聚合有机凝胶 09170470酚酞型聚醚砜 09170431呋喃妥因 09170367呋喃唑酮 09170367伏格列波糖 09170362氟嘧菌酯 09170360浮动催化法 09170380腐蚀 09170424腐蚀防护 09170461负极 09170401负载型固体酸 09170289复分解反应 09170228复合材料 09170207复合粒子 09170458复合物 09170389 复合氧化物 09170007 傅立叶红外光谱法 09170373 富集 09170352 钙掺杂 09170238 钙钛矿 0917000709170197 钙钛石复合氧化物 09170260 干燥控制化学添加剂 09170378 甘缬二肽 09170254 肝癌 09170311 高分子辅助自组装 09170445 高固含量 09170448 高岭土 09170247 高取向 09170206 高速逆流色谱 09170369 高效液相色谱-串联质谱 09170348 高效液相色谱-离子阱质谱 0917034209170363 高效液相色谱法 0917036109170362091703640917036709170368 高压液相色谱-电喷雾质谱联用 09170311 高折射率 0917044609170447 锆 09170009 锆溶胶 09170270 格子Boltzmann方法 09170439 铬酸根 09170275 功能单体 09170263 功能高分子材料 09170010 功能化修饰 09170331 功能聚合物微球 09170451 汞 09170297 共沉淀法 09170240 共混 09170464 共混膜 09170431 共价结合 09170308 共聚 09170469 共聚物组成分析 09170433 共缩聚 09170475 共振拉曼光谱 09170400 共振增强多光子电离 09170405 狗枣猕猴桃 09170302 构象分析 09170417 构效关系 09170314 谷胱甘肽 09170285 谷胱甘肽过氧化物酶 09170308 钴 09170226 钴卟啉负载碳黑催化剂 09170391 固定化 09170006 固沙剂 09170284 固相萃取 091703490917035609170361 固相反应法 09170242 胍 09170326关环复分解反应 09170321光催化 0917021709170379光催化活性 09170210光电化学 09170424光交联 09170449光谱 09170229光散射 09170011光学活性聚合物 09170460光学透明性 09170446光致发光 0917025009170278光转化 09170392规范不变原子轨道 09170408硅胶 09170344硅铝比 09170247硅橡胶 09170443贵金属 09170007铪 09170009海藻酸钠 09170414含氟丙烯酸酯 09170277合成 09170271091702800917028209170286合成与性能 09170475核-壳微球 09170473核磁共振 0917001109170343核磁共振系数 09170406核酸酶 09170254横截面积 09170386红色长余辉 09170204红树林 09170274红外定量 09170433红外拟合 09170205互穿网络 09170471化学共沉淀 09170245化学机械抛光 09170202化学交换 09170343化学气相沉积 09170411化学位移 09170406化学形态 09170221化学振荡 09170294化妆品 09170367环糊精 09170012环三聚磷腈 09170306环氧 09170324环氧化物 09170328环氧树脂固化 09170440缓释 09170291黄瓜子叶生根 09170313黄芪 09170365黄酮 09170302磺胺类药物 09170348磺丁基-β-环糊精 09170368磺化酚酞型聚醚砜 09170431磺化聚醚醚酮 09170431磺化羧甲基壳聚糖 09170455磺化修饰 09170352磺基异硫氰酸苯酯 09170346回收利用 09170201混合电容器 09170208活性成分 09170365活性炭 09170415活性自由基聚合 09170013机理 091702590917037209170421机械性能 09170471鸡肉 09170361积炭 09170390基因传递 09170014基因传输 09170454基因治疗 09170472基质辅助激光解吸电离飞行时间串联质谱 0917034609170352基质效应 09170349激发态 09170231激光解吸/电离质谱 09170251极限氧指数 09170293计时电流 09170226季铵盐 0917027509170463甲醇部分氧化 09170397甲醇电氧化 09170416甲醇氧化 09170410甲基丙烯酸缩水甘油酯 09170451甲烷 0917000709170394甲烷重整 09170390甲酰胺 09170217剪切粘度 09170414碱熔活化 09170201键合特性 09170229姜黄素 09170338浆状催化剂 09170378交流阻抗 09170226胶束 09170454胶束状聚集体 09170452胶原 09170455接枝聚合 09170275结构表征 09170267结构确证 09170343结构水分子 09170404结构与性能 09170464结构与性质的关系 09170008结合能力 09170263结晶 09170434结晶度 09170247解离 09170421介孔氧化锆 09170238金纳米颗粒 09170251金属参与 09170322近红外光谱 09170345晶化动力学 09170412晶面控制 09170256晶体结构 09170194091701990917020009170203091702090917022409170229091702320917023709170243091702440917025309170282091702830917030009170413静电纺丝 09170387静电吸附 09170450静电相互作用 09170275桔皮素 09170369拒食剂 09170324聚(N-甲基甲基丙烯酰亚胺)/聚偏氟乙烯 09170436聚[乙烯-alt-R-N-(1-苯乙基)马来酰胺酸] 09170459聚氨酯微乳液 09170448聚苯胺 0917024609170383聚苯胺材料 09170235聚苯乙烯 09170295091704370917045609170458聚吡咯 09170423聚吡唑硼酸盐 09170413聚丙烯 091704290917043709170464聚丙烯酰胺/氧化锌纳米线薄膜 09170445聚对二氧环己酮 0917043509170465聚二甲基二烯丙基氯化铵 09170264聚芳醚砜醚酮酮 09170434聚芳醚酮 09170475聚合 09170423聚合物-表面活性剂作用 0917041409170301聚琥珀酸丁二酯 09170468聚己内酯 09170454聚轮烷 09170012聚碳酸1,2-丙二酯 09170468聚碳酸酯 09170006聚酰胺 09170207聚酰胺酰亚胺 09170447聚酰亚胺 09170446聚乙二醇 09170251091703750917045209170465聚乙烯吡咯烷酮 09170473聚乙烯亚胺 0917027509170454 绝对构型 09170319 均三唑 09170272 均三唑并噻二唑 09170272 咔唑乙酸 09170282 抗癌活性 09170282 抗菌 09170463 抗菌活性 09170272 抗氧化活性 09170371 抗肿瘤活性 09170274 壳核结构 09170004 壳聚糖 091702550917029109170341091703440917038209170474 可纺性 09170270 可见光催化 09170402 可见光激发 09170204 可降解 09170462 可聚合凝胶剂 09170470 可控合成 09170301 可逆加成-断裂链转移 09170013 可生物降解高分子 09170014 可再生资源 09170462 克球酚 09170349 刻蚀 09170458 空气“自呼吸” 09170281 空心球 09170233 控制步骤 09170227 控制释放 09170425 口占 吨酮 09170371 库尔提斯重排 09170337 喹诺酮类药物 0917034809170361 喹喔啉甲醛 09170298 扩散系数 09170385 拉曼光谱 09170241 镧掺杂 09170219 镧系配合物 09170300 酪胺 09170337 累托石 09170467 离子交换整体柱 09170359 离子速度成像 09170405 离子液 09170222 离子液凝胶 09170010 离子液体 09170010091702060917026809170423 离子液体单体 09170466 理论计算 09170199 理论研究 09170426 理论研究进展 09170008 锂 09170273 锂离子电池 0917040109170403 立方结构的 09170259 立体选择性 09170321 联炔 09170319两亲聚合物 09170457两亲性 09170465量子点 09170308量子化学 09170413劣化 09170393邻苯二胺 09170280邻二取代苯 09170441淋巴癌病人 09170340淋巴结转移 09170311磷钼钒杂多酸盐 09170265磷酸根离子 09170220磷酸化 09170255磷钨酸 09170276膦配体 09170334流变-导电行为同步测试 09170442流变性 0917024809170381硫代乙酸 09170421硫酸铟 09170194六铝酸盐 09170007绿色合成 09170310氯丙基三乙氧基硅烷 09170267罗丹明B 09170402螺缩酮 09170324麻黄碱 09170263迈克尔加成反应 09170325鳗鱼 09170348毛细管电泳 09170365毛细管整体柱 09170357咪唑 09170330咪唑配体 09170199猕猴桃科 09170302密度 09170384密度泛函和从头算方法 09170231密度泛函理论 091702490917037209170400091704080917040909170418密度梯度理论 09170422嘧螨酯 09170360模板 09170235模拟水 09170424膜材料 09170385膜电极集合体 09170281木质素 09170290内标法 09170348内生真菌 09170274纳米MCM-41分子筛 09170258纳米SiO2 09170450纳米棒 09170278纳米发光材料 09170004纳米复合材料 09170246091704300917045609170467纳米晶体 09170214纳米空心球 09170458纳米粒子 09170465纳米凝胶 09170472纳米器件原型 09170445纳米碳酸钙 09170458纳米铁微粒 09170292纳米微球 09170291纳米纤维 09170387纳米线 0917019509170411纳米阵列 09170234纳升级液相色谱 09170357奶粉 09170350耐烧蚀性能 09170443囊泡结构 09170457能量转移 09170287拟薄水铝石 09170378拟除虫菊酯 09170354拟除虫菊酯农药 09170356黏土 09170437尿 09170353尿液 09170340镍的配合物 09170420镍基催化剂 09170390镍铜锌铁氧体 09170245柠檬酸 09170219凝胶渗透色谱 09170360牛奶 0917035009170366牛血红蛋白 09170344牛血清白蛋白 0917021809170303牛血清蛋白 09170291农药残留 091703540917035509170360农药多残留 09170347浓缩果蔬汁 09170347偶氮-醌腙互变异构 09170316偶氮苯液晶聚合物 09170457偶氮基杯[4]芳烃 09170316偶氮聚合物 09170476偶极环加成 09170298偶联反应 09170268哌啶衍生物 09170333哌嗪 09170272配合物 091700090917022909170339配位体辅助模板 09170238膨润土 09170289膨胀型阻燃剂 09170293皮肤再生材料 09170455芘探针 09170449葡萄糖 09170294谱学表征 09170305齐聚物 09170320气-液-固生长机制 09170198气/液界面 09170388气氛 09170198气敏性 09170235气体传感器 09170276气体浓差电池 09170213气相色谱-电子轰击离子源质谱 09170354气相色谱-负化学离子源质谱法 09170355气相色谱-质谱 0917036609170261汽液成核 09170422铅 09170339前线轨道 09170409嵌段共聚物 09170452091704600917046509170476嵌段聚合物 09170439强化置换甲烷 09170005强阳离子交换固相萃取 09170350强阳离子交换色谱 09170352羟丙基 09170291羟基磷灰石 091702050917020709170474切割 0917029909170306亲水扩链 09170448亲水取代基 09170222禽类产品 09170349氢氟相互作用 09170329氢化反应 09170006氢化物发生-原子荧光 09170297氢甲酰化 09170372氢键 0917031809170426氢气 09170210氢钨青铜 09170410氢氧化镍 0917019609170216氰乙基 09170409取代苯并咪唑 09170280取代反应 09170334取代基效应 09170231去对称化 09170321全氟烷基碘 09170327犬尿氨酸 09170364犬尿喹啉酸 09170364炔基化 09170335缺陷氧基磷灰石 09170242群蛀虫内酯 09170321燃料电池 09170391燃烧热 09170292热处理 09170391热和水热稳定性 09170262热力学 09170428热力学分析 09170245热熔胶 09170286热稳定性 0917019509170446热性能 0917026909170447热致相分离 09170429人免疫球蛋白 09170373人源单链抗体 09170308 溶剂浸取 09170262 溶剂热 09170233 溶剂热法 09170216 溶剂热合成 09170194 溶剂诱导结晶 09170434 溶胶-凝胶-水热法 0917040209170250 溶胶-凝胶法 091702480917044309170207 溶解性 0917026909170447 溶液混合 09170207 熔融共混 09170468 熔体黏度 09170437 肉桂酸酯 09170449 乳酸-3-苯甲基-2,5-吗啉二酮共聚物 09170433 乳液 09170285 乳液聚合 09170284 弱阳离子交换填料 09170358 噻二唑 09170446 噻吩 09170446 三氟甲基 09170329 三核锰配合物 09170253 三聚氰胺 09170366 三聚氰酸 09170366 三联吡啶铜配合物 09170243 三氯乙烯 09170379 三嗪 09170304 三嗪卟啉 09170304 三元共聚物 09170449 三元肽铜(Ⅱ)配合物 09170254 三唑啉酮 09170313 三唑醛 09170298 扫描Kelvin探针 09170393 扫描电子显微镜 09170293 色氨酸 09170364 杀菌率 09170264 杀菌性能 09170264 砷 09170297 渗透率 09170380 生长机理 09170301 生物标记 09170214 生物活性 0917030909170317 生物降解聚合物 09170438 生物降解形状记忆聚合物 09170438 生物质 09170462 十甲基五元瓜环 09170244 十六烷基三甲基溴化铵 09170388 十字结构 09170386 石墨纳米薄片 09170246 时温依赖性 09170436 手性 09170323 手性联萘酚 09170267 手性流动相添加剂 09170368 手性配体交换色谱固定相 09170279 手性元 09170328疏水性 09170463鼠脑 09170363数据库搜索 09170374双-噻唑啉酮衍生物 09170298双(N-氨基酞酰亚胺)硫醚 09170269双U形 09170209双功能螯合剂 09170477双功能催化剂 09170335双光子吸收 09170386双极膜 09170255双席夫碱 09170298水 09170268水果 09170360水滑石 09170212水基导电聚苯胺 09170461水结构 09170406水凝胶 09170471水热法 09170195水热合成 091702240917022509170252091702560917028309170300水性涂料 09170461水杨酸 09170295水蒸气重整 09170398四(4-羟基-3-乙氧基苯基)卟啉 09170339四重氢键 09170453松子仁 09170356酸处理 09170257酸化膨润土 09170381酸敏感 09170452酸效应 09170316隧道逾渗模型 09170430羧甲基壳聚糖 09170467羧甲基纤维素 09170255羧酸配体 09170225缩聚 09170462肽腈化合物 09170314钛 09170009钛酸盐 09170196炭黑 09170430碳-氟键活化 09170329碳糊修饰电极 09170265碳纳米管场效应管 09170376碳纳米管复合膜 09170380碳酸根取代 09170205碳酸酯 09170006碳纤维 09170466碳纸 09170227糖-凝集素识别 09170376糖-碳纳米管复合物 09170376糖基化 09170376糖原合成酶激酶-3β 09170404烫伤 09170455特征黏度 09170264藤黄属 09170371锑精矿 09170297体外降解 09170435天然产物 0917031909170324天然气水合物 09170005条状样品 09170435铁锰复合氧化物 09170399同轴静电纺丝 09170432铜催化交叉偶联反应 09170336酮康唑对映体 09170368透过率 09170241透明激光陶瓷 09170241透明陶瓷 09170240涂层 0917045309170474退火 0917023409170278脱脂棉 09170219外消旋化合物 09170209完全催化氧化 09170260完全液相法 09170378微波萃取 09170261微波辐射 091702710917027709170331微观结构 09170235微管 09170219微管蛋白 09170417微过氧化酶-11 09170382微孔膜 09170429微米棒 09170216微纳管 09170432微凝胶 09170011微球 09170467微乳法 0917020809170226微相分离 09170439温度敏感 09170463温控分子动力学 09170417稳定性 09170305无机-有机杂化 09170265无机培养基 09170264五氟碘乙烷 09170327五氟氯乙烷 09170327芴 09170287吸附 0917026609170295吸附热力学 09170212吸油值 09170377烯烃聚合 09170009硒 09170297稀土 09170469稀土负载 09170330稀土离子掺杂 09170004稀土磷酸盐 09170004稀土配合物 09170209稀土元素 09170214锡 09170297席夫碱缩合反应 09170200细胞红蛋白 09170305细乳液聚合 09170277虾 09170348纤维素 09170290酰胺 09170334显色剂 09170339线粒体膜电位 09170307相对量子产率 09170405相分离 09170436相转变 09170252硝苯地平 09170359硝酸根 09170392小波变换 09170345偕二氟高烯丙基胺 09170322锌配合物 09170220新蛋白质 09170346新型AHAS抑制剂 09170309星形聚合物 09170013形状记忆 09170438形状记忆聚合物 09170428性能 09170453修饰电极 09170296修饰核苷 09170340旋光聚电解质 09170459旋节线分离 09170440选择离子监测 09170355选择性催化还原 09170399血管化 09170455血红蛋白 09170341血红素氧合酶-1 09170307血浆 09170359血清 09170364血液 09170261循环伏安 09170391循环伏安法 09170232循环性能 09170403亚胺 09170318亚磺化脱氯 09170327亚甲蓝-四苯硼化钠荧光探针 09170375亚硝酸甲酯 09170396烟草 0917034509170354盐 09170256阳极氧化 09170223氧化钡 09170237氧化铝 09170396氧化膜 09170202氧化锌 09170278氧化钇粉体 09170240氧化应激 09170307氧还原 09170391氧还原电催化性能 09170257液化 09170290液相还原法 09170292液相色谱-串联质谱 09170347液相色谱-电喷雾串联质谱联用 09170349一步法 09170425一锅法合成 09170304一氧化碳 0917039609170410铱(III)配合物 09170211乙醇部分氧化重整 09170395 乙醇电催化 09170226 乙醇电催化氧化 09170419 乙二胺 09170419 乙二醇 09170290 乙二醇二甲基丙烯酸酯 09170451 乙烯 09170372 乙烯聚合 09170420 乙酰丙酸 09170462 乙酰基 09170302 乙酰羟酸合成酶 09170317 乙酰水杨酸 09170289 钇铝石榴石纤维 09170248 异丙氧基稀土 09170330 异构 09170421 异核Au基电荷转移配合物 09170231 抑制 09170333 抑制剂 09170314 抑制性 09170317 镱和铒的α-噻吩甲酰丙酮-哌啶配合物 09170299 阴极水管理 09170281 阴极微孔层 09170281 阴离子分散剂 09170381 阴离子识别 0917022009170370 银离子配位色谱 09170230 引发剂活性 09170450 吲哚二酮类化合物 09170309 饮料 09170360 饮食油烟 09170407 印迹 09170308 荧光 0917021409170332 荧光差异双向凝胶电泳 09170311 荧光传感器 09170323 荧光猝灭 0917021809170338 荧光猝灭法 0917037309170375 荧光检测 0917034209170364 荧光效率 09170386 荧光性能 09170004 荧光性质 0917022409170300 硬盘基片 09170202 优先插层 09170437 铀工艺 09170221 有机电解合成 09170233 有机化合物 09170006 有机金属化合物 09170006 有机磷 09170354 有机氯 09170354 有机氯农药 09170356 有机培养基 09170264 有机涂层 09170393 有机锡(Ⅳ)配合物 09170282 铕(III)配位聚合物 09170225 鱼精蛋白 09170358 玉米 09170355原核表达 09170312原酸酯 09170452原位红外光谱 09170379原位乳液聚合 09170450原位生成SiO2 09170443原子力显微镜 09170011原子转移自由基聚合 0917045709170460圆二色性 09170200杂多酸 09170235杂化材料 0917026209170461杂化纤维 09170270杂双位点受体 09170370载体 09170444在线分析 09170359在线红外 09170290在线凝胶渗透色谱-气相色谱/质谱 09170356在线衍生 09170364造孔剂 09170416增敏 09170375增韧 09170468增塑剂 09170468粘度系数 09170406折叠机制 09170417针状纳米碳酸钙 09170377真菌 09170332振荡反应 09170294整体式催化剂 0917023909170407整体型催化剂 09170007正辛烷 09170427支持向量机 09170345脂肪胺 09170342直接甲醇燃料电池 091702810917041609170431植物油脂 09170266制备 0917028909170448制氢 091703950917039709170398质量控制 09170374质谱 09170374质子导电性 09170213中成药 09170362中间态 09170417中空碳纤维 09170432中药 09170365中子散射 09170011重氮化-偶合反应 09170316重排反应 09170324周期振荡 09170294竹粉 09170290柱前衍生 0917034209170353转化 09170221准聚轮烷 09170012紫外-可见吸收光谱 09170218紫外光接枝 09170463紫外光滤波器 09170232紫外光谱 09170263紫外照射 09170218自旋玻璃态 09170197自由基 09170285自由基共聚 09170466自组装 09170476自组装膜 09170459腙 09170322腙类配体 09170336阻抗谱 09170213组氨酸标签肽 09170352组织蛋白酶K 09170314组织工程 09170014左旋咪唑 09170350作用 09170299地球科学CHAMP 09170502EGM96 09170488ETERNA潮汐分析方法 09170511GLONASS 09170016GNSS 09170016GPS 0917001609170490091704960917050209170506GPS气象学 09170498GPS数据 09170479GRACE 0917049709170502IERS 09170490IGS 09170499ITRF2005 09170505K-Ar年龄测试 09170528Kuhn-Tucker条件 09170510Lagrange插值 09170499Lemke算法 09170510L曲线法 09170494Mogi模型 09170494PB2002 09170493ScanSAR 09170015Tikhonov正则化 09170504V A V潮汐分析方法 09170511WGS84 09170505阿克苏 09170528白云岩 09170527板块边界 09170493板块边界模型 09170493板块构造 09170493板块运动 09170015北缘 09170524边界效应 09170508病态模型 09170504波幅 09170490剥蚀 09170517补给 09170512不等式约束 09170510不适定问题 09170494布格异常图 09170492参数试验 09170486测井曲线 09170532层序 09170517柴达木盆地 09170524产能 0917053109170533超限率分析 09170484潮汐摄动 09170507车载数据采集系统 09170482沉积特征 0917052509170526沉积相 09170527成都 09170520成烃特征 09170524城市活断层 09170486城市生活垃圾 09170513储集层 09170527储集层预测 09170519储量 09170518触发器 09170480大坝安全监测 09170500大坝变形 09170501大地构造 09170521大地水准面 09170483大地震 09170520大气降水 09170512大气阻力 09170497导航定位 09170016导航卫星 09170507低点位移 09170496低轨卫星 09170506低通滤波器 09170491地层 09170517地层吸收 09170487地电阻率 09170496地壳结构 09170483地倾斜观测 09170489地球参考框架 09170505地球重力场 09170497地热台网 09170485地应力 09170532地应力场 09170495地震 0917049509170517缔合勒让德函数 09170503电磁卫星 09170496电磁异常 09170496电力线检测 09170482电路结构 09170481迭代 09170511动力学演化 09170515动态预测 09170501断层端部 09170495断层活动性 09170486断陷盆地 09170534额济纳旗 09170529仿真实验 09170501非差 09170506。
课件:《拥抱阳光-公司介绍》
是我们对目标达成不到最后一刻绝不放弃的那么多一点坚持!
39
跟
着
阳
光
走
40
国之声、湖南卫视、新浪网联合发起,由我司独家冠名关爱
女性大型活动。
24
第二篇
阳光品牌:价值无限
洋河集团有限公司
纳爱斯集团有限公司 云南白药集团股份有限公司
阳光 新势力
淘宝网
苏宁电器 郎酒集团 格力电器股份有限公司 招商银行 雨润控股集团有限公司 阳光保险 (集团)股份有限公司
31
第二章
阳光公益:大爱无疆
阳光 新势力
阳 光 志 愿 者 协 会
在业内率先成立了全国性的“阳光保险 青年志愿者协会”组织,目前全系统内志愿
者人数超过20000人。
32
第二章
阳光公益:大爱无疆
阳光 新势力
捐 建 阳 光 博 爱 学 校
阳光保险自成立以来,一直勇于承担社会责任,致力于社会公益 事业。已陆续在湖南、贵州、四川、山东、福建、西藏、云南、广西 等地捐建了23所阳光保险博爱学校。
养老储蓄型
投资收益型
百万身价0元购的产品在阳光
十全十美的产品在阳光 20
第二章
阳光金融:全面覆盖
阳光产品体系
个人 寿险
车险与 家财险
居民大病 保险
阳光 新势力
企业财 产保险
信托 业务
团体 保险 基金与 各类理财
一个客户 一个账户 一个阳光
小额 信贷
21
第二篇
阳光品牌:价值无限
连续三年独家冠名《我要上春晚》 并开展一系列客户与员工共同上春晚的活动
阳光 大未来
“终极客户”战略
先进企业に学ぶ「顾客の声を生かす力」
苦情やクレームといった、いわゆるマイナス情報を、プラスの力として生かすためには、斬新な発想と仕組みの転換が不可欠だ。
アグレッシブに顧客の声を求め、確かな実績を生み出している2つの企業を徹底レポートする。
INDEX >>従来の広聴の枠を超え、「お客様相談センター」を経営の主幹組織に転換-株式会社ニチレイフーズのケース-■リコール問題を糧にクレーム対応の先進企業へ■寄せられたすべての「声」を集約し、リアルタイムで提供■顧客との「窓口」を担う部門こそ企業の核となるべき顧客の要望を商品改良で実現真摯な対応姿勢が企業価値を決める-株式会社伊藤園のケース-■お客様相談室が商品開発の提案を行う■過剰なサービスではなく真摯な対応を追求■時代とともに変化する常識への臨機応変な対応が必要■「苦情・クレーム」をサイト上で集めて活用 中小企業の商品開発を支援~苦情・クレーム博覧会■リコール問題を糧にクレーム対応の先進企業へクレームに対する企業の意識は近年大きく変わってきている。
回避すべきものではなく、顧客の率直な声と捉えるようになり、“クレームは宝の山”といったフレーズさえ浸透している。
そして、賞味期限や産地偽装問題が続出し、昨今特に消費者の声に敏感になっている食品業界において、クレーム対応策を積極的に推進しているのがニチレイフーズである。
冷凍食品の出荷額第1位で消費者との接点が多い同社は、お客様相談センターに機能を集約し、全社でその内容を徹底して共有することで、ニチレイフーズ全体の活動に顧客の声を生かす体制を築いている。
お客様相談センターが開設された約30年前は苦情対応が主な業務だったが、1992年から品質保証部に属して、安全性向上への取り組みを強化。
2001年には営業企画部に移り、顧客の声をマーケティングへ活用するようになった。
そして、03年からは商品部付となり、開発や改良改善にもかかわるなど進化を続けてきたが、大きな転機となったのは、02年に発生したホウレン草の残留農薬の問題だ。
