Effects of morphology and composition on
氧化钇对TC4_钛合金表面包埋渗硼的影响

第52卷第8期表面技术2023年8月SURFACE TECHNOLOGY·451·氧化钇对TC4钛合金表面包埋渗硼的影响韩宾龙,田晓东,卢鹏军,祁贤(长安大学 材料科学与工程学院 交通铺面材料教育部工程研究中心,西安 710064)摘要:目的加快TC4钛合金表面固体渗硼时渗层的生长,研究渗剂中添加Y2O3对渗硼的影响。
方法采用固体粉末包埋渗法对TC4基材进行1 050 ℃/8 h渗硼,包括渗剂中不添加Y2O3以及渗剂中分别添加质量分数为1%、3%、5%、7%Y2O3的试验研究。
通过扫描电子显微镜、能谱仪、波谱仪和X射线衍射仪分析渗硼样品的截面形貌、元素含量和表面物相,并测量渗硼样品的表面硬度和摩擦系数。
结果在渗剂中加入1%~7%的Y2O3,渗硼层结构与未添加氧化钇渗剂形成的相同,由致密连续的TiB2层和TiB 晶须扩散层组成。
Y2O3促进渗层生长的作用与其添加量密切相关。
渗剂中加入1%~3%的Y2O3有促进渗硼层生长的作用,且加入3%的Y2O3时,催渗效果最佳,可使渗硼层厚度增加40.24%,但加入5%~7%的Y2O3时反而会抑制渗硼层的生长。
能谱分析表明,Y原子能够扩散到渗硼层内,且渗硼层中存在原子数分数为0.01%~0.34%的微量Y元素,其随渗剂中Y2O3含量的增加而增加。
热力学分析发现,Y2O3参与渗剂反应形成活性Y原子而渗入基体。
向渗硼试剂中加入3%的Y2O3,样品的表面硬度较未添加Y2O3时提高35.54%,摩擦系数较未添加Y2O3时降低28.57%。
结论向渗硼试剂中加入适量氧化钇,是获得TC4合金表面高渗速、高硬度和低摩擦系数渗硼层的一种有效方法。
关键词:渗硼;包埋渗;氧化钇;催渗中图分类号:TG156.8+7文献标识码:A 文章编号:1001-3660(2023)08-0451-07DOI:10.16490/ki.issn.1001-3660.2023.08.041Effects of Y2O3 on Pack Boronizing of TC4 Titanium AlloyHAN Bin-long, TIAN Xiao-dong, LU Peng-jun, QI Xian(Engineering Research Center of Transportation Materials of Ministry of Education,School of Materials Science and Engineering, Chang'an University, Xi'an 710064, China)ABSTRACT: In order to accelerate the growth of the boronizing layer on TC4 titanium alloy prepared through pack cementation process, the effects of adding Y2O3 into the pack mixture were investigated. The TC4 matrix was boronized at1 050 ℃for 8 h with the pack mixtures composed of NaF, B and Al2O3 powders with Y2O3 free and Y2O3 addition of 1, 3, 5收稿日期:2022-08-04;修订日期:2023-02-08Received:2022-08-04;Revised:2023-02-08基金项目:大学生创新创业训练项目(X202210710586);长安大学中央高校基本科研业务费专项资金(300102311403)Fund:The Innovation and Entrepreneurship Training Program for College Students (X202210710586); Fundamental Research Funds for the Central Universities, CHD (300102311403)作者简介:韩宾龙(1997—),男,硕士研究生,主要研究方向为材料表面改性。
磁性铝基MOF的表征和对水体中氟化物吸附性能研究

Vol.34,No.5May,2021第 34 卷 第 5 期2021 年 5 月环 境 科 学 研 究Research of Environmental Sciences磁性铝基mof 的表征和对水体中氟化物吸附性能研究段平洲・2,贾晓波・2,后希康・2,夏 瑞1>2**,郭 勇3,张新飞・2收稿日期:2020-06-10 修订日期:2020-09-28作者简介:段平洲(1990-),男,河北保定人,助理研究员,博士,主要从事水环境化学研究,****************.*责任作者,夏瑞(1982-),男,湖北武汉人,副研究员,博士,主要从事流域水环境模拟研究,xiarui@ 基金项目:“美丽中国”生态文明建设科技工程专项资助项目(No.XDA23040500);国家水体污染控制与治理科技重大专项(No.2018ZX07601-003)Supported by Strategic Priority Research Program of the Chinese Academy of Sciences (No. XDA23040500) ; National Major Science and TechnologyProjects for Water Pollution Control and Treatment, China (No.2018ZX07601-003)1. 中国环境科学研究院,环境基准与风险评估国家重点实验室,北京1000122. 中国环境科学研究院水生态环境研究所,北京1000123. 宿州市环境保护监测站,安徽宿州234000摘要:我国多地的地表水受到成土母质或背景值的影响,氟离子浓度均超过1. 0 mg/L ,即高于GB 3838—2002(地表水环境质量标准》皿类标准限值.为了实现地表水的快速降氟和吸附材料的便捷回收,通过水热法制备了磁性Al-MOF@Fe 3O 4吸附材料,使 用扫描电极(SEM)、X 射线衍射仪(XRD)、X 射线光电子能谱仪(XPS)和孔隙度分析仪(BET)对材料的形貌和化学组成进行了表征.结果表明:①Al-MOF@Fe 3O 4具有不规则的晶体形状和直径更小的介孔结构,能够提供更高的比表面积吸附氟离子.②吸 附试验结果表明’Al-MOF/Fe j O "的吸附量达到了 75. 2 mg/g ,吸附过程更加符合拟二阶动力学模型,证明了化学吸附是该除氟过 程的主要机理.③增加吸附剂投加和降低氟离子初始浓度,有助于提高除氟效率,但却难以得到较高的吸附量,同时碱性条件不利于氟离子的吸附,阴离子对除氟性能的影响程度表现为CO 32->HCO 3->SO 42->PO 43->Cl -.④对吸附机理的研究表明,氟离子是 通过取代Al —0H 实现稳定和快速地脱离水体,使用NaOH 溶液淋洗可以实现吸附剂的高效再生.⑤5次循环吸附试验后,复合 材料依然保留了 71. 4 mg/g 的吸附能力和良好的磁性.在实际地表水中进行除氟试验,该吸附剂可以将氟化物浓度从1. 17 mg/L降至0.2 mg/L 以下.研究显示,Al-MOF@Fe j O 4纳米材料可以作为地表水除氟材料,实现对低浓度氟离子的高效去除.关键词:Al-MOF@Fe 3O 4;氟化物;吸附动力学;吸附机理;地表水修复中图分类号:X21文章编号:1001-6929(2021)05-1139-09文献标志码:A DOI : 10. 13198/j. issn. 1001-6929. 2020. 10. 25Characterization and Adsorption Properties of Magnetic AI-MOF Composite for FluorideDUAN Pingzhou 1,2, JIA Xiaobo 1,2, HOU Xikang 1,2, XIA Rui 1,2* , GUO Yong 3, ZHANG Xinfei 1,21. State Key Laboratory of Environment Criteria and Risk Assessment, Chinese Research Academy of Environmental Sciences, Beijing 100012, China2. Research Institute of Water Ecology and Environment , Chinese Research Academy of Environmental Sciences , Beijing 100012, China3.Suzhou Environmental Protection Monitoring Station, Suzhou 234000, ChinaAbstract : Surface water in many regions of China is affected by the soil or water background value , and the concentration of fluoride exceeds 1. 0 mg/L , which is higher than the Class 皿 water specified in the Enr/ronmental Quality Standard for Sur/ace Water ( GB 3838-2002). Inorder to reduce fluoride in surface water and recover adsorption materials, magnetic Al-MOF @ Fe 3 O 4 was prepared by hydrothermal method. The morphology and composition of the adsorbents were characterized by scanning electron microscope ( SEM) , X-ray diffraction ( XRD) , X-ray photoelectron spectroscopy ( XPS) and porosity analyzer ( BET) . The results showed that Al-MOF@ Fe 3 O 4 had irregularshape, rough surface and smaller mesoporous structure, which can provide a large specific surface area to absorb fluoride. The adsorption experiment indicated that the adsorption capacity of Al-M0F@ Fe 3O 4 was 75. 2 mg/g, which was in accordance with the pseudo second-order kinetic model, indicating that chemical adsorption was the main mechanism of the defluorination process. In addition, higheradsorbent dosage and lower initial concentration enhanced the efficiency of defluorination, but decreased the adsorption capacity. Alkaline conditions were not conducive to the adsorption of fluoride. The effects of co-existing anions on defluorination performance was in the order of CO 32- >HCO 3- >S0"2- >PO 43->Cl -. The study showed that fluoride could be separated from the water rapidly by replacing the surface1140环境科学研究第34卷hydroxyl groups of the adsorbent,and the adsorbent could be efficiently regenerated with NaOH solution.After five cycles of adsorption experiments,the composite still retained71.4mg/g adsorption capacity and good magnetic properties.Finally,defluorination experiments using real surface water showed that the adsorbent effectively reduced fluoride concentration from1.17mg/L to less than0.2mg/L.In conclusion,Al-M0F@Fe3O4nanocomposite can be used as adsorbent to effectively remove fluoride in surface water.Keywords:Al-M0F@Fe3O4;fluoride;adsorption kinetics;adsorption mechanism;surface water remediation受自然本底值、地壳运动或人为活动的影响,地表水中都有一定浓度的氟离子存在.虽然氟离子是人体必需的微量元素,然而一旦超过了特定限值,会导致严重的骨骼问题,损害幼儿肝和肾的正常功能等人体健康风险.我国将饮用水中氟化物浓度限值设定为1.0mg/L,低于世界卫生组织(WHO)规定的1.5mg/L.我国皖北[1]、宁夏回族自治区[2]、京津冀[3]及苏北[4]等地区,由于土壤中氟浓度较高,或受到长期采矿、挖沙的影响,造成一些地区天然地表水中氟化物浓度背景值高于1.0mg/L,多处国控和省控断面水质难以达到GB3838—2002(地表水环境质量标准》皿类标准,对国家和地方水污染管理和治理造成了较大的挑战,也是当前水环境修复面临的主要难题.迄今为止,许多相关领域专家和学者针对去除和回收水体中氟化物的技术开展了广泛的研究.其中,最受关注的除氟方法有混凝沉淀法[5]、电凝聚法⑷、反渗透法[7]、分子筛[8]等.在这些方法中,吸附技术以其操作简单、效率高、成本低、环境友好等优点被认为是最具竞争力的方法之一•总结现有研究结果表明,活性炭、活性氧化铝⑼、稀土金属氧化物(Ce O3和ZrO等)[l0]、羟基磷灰石[ll]等材料在除氟工艺中应用较多.由于氟离子活泼性强,碳材料对其的物理吸附难以达到30%以上.羟基磷灰石可以通过Ca—OH 与F-发生取代反应,但并不适合在自然水体中应用[l2].含铝吸附剂由于可以和Al3+形成较多的且带正电的Al—OH,被证明可以高效吸附F-.HE等[l3]制备了Al修饰的羟基磷灰石(Al-HAP),在200mg/L 的氟离子溶液中实现了93.84mg/g的吸附量.近年来,由金属节点(簇)和桥接有机连接体组成的金属有机骨架(MOF)材料,得到了广泛的研究和重视.它们具有比表面积大、孔隙率高、活性位点丰富等优势[l4].鉴于这些特性,MOFs已逐步开始应用于氟化物的吸附去除.Al-MOF被证明具有良好的水稳定性,晶体结构类似于MIL-53,具有无限连接的Al—OH—Al,以及方形孔道的Al(OH)(O2C—CH—CO2)结构,这样可以更加充分利用Al—OH与F-进行交换[l5-l6].Karmakar等[l7]研究表明,0.75g/L的Al-MOF 可以实现30mg/L的氟化物完全去除.然而,在大多数情况下,所制备的MOFs基材料很难从水溶液中分离出来,阻碍了其实际应用.因此,磁性纳米粒子在复合材料中的掺杂引起了人们的极大关注.磁性氧化铁纳米颗粒具有成本低、制备容易、稳定性高等优点,是一种很好的选择[l8].该研究针对当前水生态环境修复对于氟化物本底值超标的主要难点问题,制备了磁性的Al-MOF@Fe3O4材料,使用扫描电极(SEM)、X射线衍射仪(XRD)、X射线光电子能谱仪(XPS)和孔隙度分析仪(BET)等对该材料的表面形貌和元素组成进行了表征,分析其对水体中氟化物的吸附能力,并考察了吸附剂投加量、初始浓度和共存阴离子等参数的影响,科学辨析了其吸附动力学和机理,旨在探索提出一种针对高浓度氟化物背景值地区的水生态环境修复材料技术.1材料与方法1.1试验材料和仪器试验材料:上海麦克林生化科技有限公司生产的NaF、Al2(SO4)3・18H2O、NaOH和氨水(25%);国药集团试剂有限公司提供的丁二酸、FeC^dH。
益生菌和中草药在改善蛋品质方面的研究进展

益生菌和中草药在改善蛋品质方面的研究进展宋翔;郭杨丽;程福亮;徐海燕;谷巍【摘要】随着生活水和消费者消费水平的提高,人们对于饮食的需求不再局限于量上的满足,而更多关注于质的提高.鸡蛋是日常饮食中重要的蛋白质来源,如何安全绿色的提高蛋品质是重中之重,因而蛋品质的研究是当前比较热门的研究,中草药和益生菌作为绿色饲料添加剂,在防病、治病的基础上,还具有提高蛋品质的作用.选重点汇总和探讨了这两种添加剂在改善蛋壳厚度,蛋壳颜色,蛋黄颜色,蛋黄比率,蛋白粘稠度,降低鸡蛋胆固醇这些指标中的重要作用,为蛋品质研究提供新的思路.【期刊名称】《畜禽业》【年(卷),期】2018(029)010【总页数】3页(P16-17,20)【关键词】蛋品质;益生菌;中草药【作者】宋翔;郭杨丽;程福亮;徐海燕;谷巍【作者单位】山东宝来利来生物工程股份有限公司,山东泰安271000;山东宝来利来生物工程股份有限公司,山东泰安271000;山东宝来利来生物工程股份有限公司,山东泰安271000;山东宝来利来生物工程股份有限公司,山东泰安271000;山东宝来利来生物工程股份有限公司,山东泰安271000【正文语种】中文【中图分类】S816.70 引言随着社会经济的快速发展,人们的物质文化生活发生了很大的改变,由过去的追求经济、实惠逐步转变为为现在要求食品安全,绿色消费。
鸡蛋营养价值很高,是我们日常生活中重要的动物蛋白,人们对蛋品质的要求也越来越高。
在日常生活中,消费者往往通过感官来评价鸡蛋的质量。
蛋品质指标包括蛋壳厚度,蛋壳颜色,蛋黄颜色,蛋黄比率,哈氏单位,蛋白粘稠度,胆固醇等,这些指标对蛋产品的销量和价格有重要影响。
益生菌是一类对宿主有益的活性微生物,具有无毒、无耐药性,无残留等诸多优点,具有改善宿主微生态平衡,抗菌促生长作用,是抗生素的理想替代品。
目前市场占有率较高的主要是乳酸菌类和枯草芽孢杆菌类产品。
中草药添加剂具有资源广、成本低,毒副作用小或无,动物体不产生抗药性等优点。
新型钴基高温合金成分设计的研究进展

㊀第43卷㊀第3期2024年3月中国材料进展MATERIALS CHINAVol.43㊀No.3Mar.2024收稿日期:2021-07-29㊀㊀修回日期:2021-11-25基金项目:国家自然科学基金钢铁联合研究基金重点项目(U1960204);国家自然科学基金面上项目(51871042,52171107);中央高校基本科研业务费专项资金项目(N2023026)第一作者:张旭明,男,1998年生,硕士研究生通讯作者:高秋志,男,1981年生,副教授,硕士生导师,Email:neuqgao@马庆爽,女,1989年生,讲师,硕士生导师,Email:maqsneuq@DOI :10.7502/j.issn.1674-3962.202107062新型钴基高温合金成分设计的研究进展张旭明1,2,马庆爽1,2,张海莲3,毕长波4,张会杰1,2,李会军5,高秋志1,2(1.东北大学秦皇岛分校资源与材料学院,河北秦皇岛066004)(2.东北大学轧制技术及连轧自动化国家重点实验室,辽宁沈阳110819)(3.秦皇岛市道天高科技有限公司,河北秦皇岛066000)(4.东北大学秦皇岛分校控制工程学院,河北,秦皇岛066004)(5.天津大学材料科学与工程学院,天津300354)摘㊀要:传统钴基高温合金的强化机制为固溶强化和碳化物强化,弱于有序γᶄ相沉淀强化的镍基高温合金的强化效果,日本学者发现了有序γᶄ相强化的Co-Al-W 系新型钴基高温合金,其强化效果明显优于传统钴基高温合金㊂由于新型钴基高温合金具有较传统镍基高温合金更高的承温能力以及更加优异的高温抗蠕变性能和抗氧化性能,因此被认为是最具潜力的航空发动机热端材料之一,近年来得到迅速发展㊂基于国内外学者对新型钴基高温合金的研究成果,系统总结多种合金元素(如Ta,Ti,W 和Nb 等)对新型钴基高温合金组织和性能的影响㊂在组织方面,总结合金元素对合金相变温度㊁γᶄ相的体积分数及形态㊁γᶄ相的尺寸㊁γ/γᶄ两相晶格错配度和有害相的影响;在性能方面,总结合金元素对合金抗氧化性能㊁力学性能及抗蠕变性能的影响,以期为新型钴基高温合金的成分设计提供参考㊂最后对新型钴基高温合金成分的高效率设计进行展望㊂关键词:钴基高温合金;成分设计;γᶄ相;组织性能;蠕变中图分类号:TG146.1+6㊀㊀文献标识码:A㊀㊀文章编号:1674-3962(2024)03-0230-08引用格式:张旭明,马庆爽,张海莲,等.新型钴基高温合金成分设计的研究进展[J].中国材料进展,2024,43(3):230-237.ZHANG X M,MA Q S,ZHANG H L,et al .Research Progress on Composition Design of Novel Cobalt Based Superalloy[J].MaterialsChina,2024,43(3):230-237.Research Progress on Composition Design ofNovel Cobalt Based SuperalloyZHANG Xuming 1,2,MA Qingshuang 1,2,ZHANG Hailian 3,BI Changbo 4,ZHANG Huijie 1,2,LI Huijun 5,GAO Qiuzhi 1,2(1.School of Resources and Materials,Northeastern University at Qinhuangdao,Qinhuangdao 066004,China)(2.State Key Laboratory of Rolling and Automation,Northeastern University,Shenyang 110819,China)(3.Qinhuangdao Daotian High Technology Co.,Ltd.,Qinhuangdao 066000,China)(4.School of Control Engineering,Northeastern University at Qinhuangdao,Qinhuangdao 066004,China)(5.School of Materials Science and Engineering,Tianjin University,Tianjin 300354,China)Abstract :The strengthening mechanism of traditionalcobalt-based superalloys is solid solution strengthening and carbide strengthening whereas,both solid solution strength-ening and carbide strengthening are weaker than that of nickel-based superalloys with ordered γᶄprecipitation.Jap-anese scholars discovered a novel type of Co-Al-W superal-loys with ordered γᶄphase strengthening,and its strengthe-ning effect is significantly better than that of traditional co-balt-based pared with traditional nickel-based superalloys,the novel cobalt-based superalloys have higher temperature capability,more excellent high tempera-ture creep resistance and oxidation resistance,therefore,the novel cobalt-based superalloys are considered to be the㊀第3期张旭明等:新型钴基高温合金成分设计的研究进展most potential aeroengines hot side materials and have developed rapidly in recent years.In this review,based on the re-search results of the novel cobalt-based superalloys by scholars at home and abroad,the effects of various alloying elements (such as Ta,Ti,W,Nb and so on)on the structure and properties of novel cobalt-based superalloys were systematically summarized.In terms of microstructure,the effects of alloying elements on transformation temperature,volume fraction and morphology ofγᶄphase,the size ofγᶄphase,the lattice misfit ofγ/γᶄtwo phase and the harmful phase were summarized. Meanwhile,in terms of properties,the effects of alloying elements on oxidation resistance,mechanical property and creep resistance of the alloy were also discussed,it is expected to provide reference for the composition design of novel cobalt-based superalloys.Finally,the high efficiency design of novel cobalt-based superalloys are prospected.Key words:Co-based superalloy;composition design;γᶄphase;microstructure and properties;creep1㊀前㊀言高温合金是指能够在600ħ以上的高温环境下正常工作,承受较为复杂的机械应力,具有稳定性的同时又高合金化的金属材料[1]㊂常见的高温合金有铁基㊁镍基和钴基3种,高温合金具有组织稳定㊁强度高㊁抗氧化性好以及抗蠕变性能优良等特点,目前广泛应用于能源动力㊁航空航天等领域[2-4]㊂随着对高温合金性能要求越来越高,提高高温合金的承温能力尤为重要[5]㊂航空发动机和燃气轮机中应用最成功的是镍基高温合金,由于熔点的限制导致其承温能力的提升极为有限,因此开发承温能力更高的新型高温合金是未来该领域的重点研究方向[6]㊂沉淀强化型钴基高温合金即新型钴基高温合金,相比镍基高温合金具有更加优异的抗蠕变性能㊁抗腐蚀性能㊁耐磨性以及更高的熔点[7],开发潜力大,应用前景广阔[8]㊂实验证明,诸多合金化元素(如: Al,Ta,Ni等)能够提高钴基高温合金强化相的稳定性㊂目前关于合金元素对钴基高温合金组织和性能影响的研究相对独立,部分常见合金元素对钴基高温合金组织和性能的影响还尚未形成统一认识㊂本文系统总结了Ni, Ti,Mo和Cr等常见合金化元素对新型钴基高温合金组织性能的影响,以期为新型钴基高温合金的进一步成分设计和组织调控提供参考,并对该合金成分的设计进行了展望㊂2㊀新型钴基高温合金概述2006年,Sato等[9]开发了具有L12结构γᶄ-Co3(Al, W)强化相的新型Co-Al-W系高温合金,该合金的固㊁液相线温度比镍基单晶高温合金高100~150ħ[10-12]㊂相比常规镍基高温合金,新型Co-Al-W系高温合金具有更强的各向弹性异性[13],相关研究也表明Co-Al-W基新型高温合金的机械性能较为优异[14-17];但是γ/γᶄ两相区过窄[9,18]㊁γᶄ相的高温稳定性低[19-21]以及合金密度大等特点限制了该合金在航天工业中的应用㊂因此在提高新型钴基高温合金相稳定性的同时如何降低其质量密度是当前研究的重要问题[22]㊂钴基高温合金中常见相的晶体学参数如表1所示[5,23]㊂新型钴基高温合金的组织主要由γ-Co基体相和γᶄ-Co3X(X=Al,Ti和Ta等)两相组成㊂其中,γ-Co是面心立方(fcc)的相,高温下fcc结构的Co较为稳定㊂经热处理后的γᶄ相主要呈立方结构,但是由于晶格错配度的改变也可能呈球状[24]㊂一方面,固溶元素含量越高,固溶强化的效果也越显著,Mo和Ni等合金化元素可以提高γᶄ相的溶解温度[9,10,15,25-27];但另一方面,过量的合金化元素会导致有害二次相如β-CoAl㊁χ-Co3W和μ-Co7W6等在基体中析出,降低合金的组织稳定性㊂表1㊀钴基高温合金中常见相的晶体学参数[5,23] Table1㊀Crystallographic parameters of common phases in cobalt based superalloy[5,23]Phase Structure symbol ExampleεA3CoγA1CoγᶄL12Co3(Al,W)μD85Co7W6βB2CoAlηD024Ni3TiχD019Co3W3㊀合金化元素对新型钴基高温合金物理性能及组织的影响3.1㊀合金化元素对新型钴基高温合金相变温度及密度的影响㊀㊀高温合金相变温度的高低决定了合金承温能力的大小㊂合金相变温度越高,承温能力自然也就越高㊂Lass[28]利用CALPHAD热力学数据库探究了Ni元素对新型钴基高温合金的影响机理,结果表明,由于Ni元素倾向分布在γᶄ相中从而提高了γᶄ相的溶解温度,同时也扩大了Co-Al-W-Ni系新型钴基高温合金高温下稳定的γ/γᶄ两相区㊂Chen等[22]测量了分别添加多种合金化元素后的Co-5Al-14V-2X四元合金相变温度,如图1所示,Ti,Nb 和Ta等合金化元素可显著提高γᶄ相溶解温度,而Cr元132中国材料进展第43卷素增加了γᶄ相中Cr 原子与近邻原子的结合能,导致γᶄ相的生成能增加,使γᶄ相的溶解温度降低[29]㊂图1㊀Co-5Al-14V-2X 四元合金的γᶄ相溶解温度㊁固相线温度和液相线温度[22]Fig.1㊀γᶄsolvus,solidus and liquidus temperatures of the Co-5Al-14V-2X quaternary alloys [22]Jin 等[30]利用第一性原理计算了Co 3(Al,M )(M =Ti,V,Cr,Zr,Nb,Mo,Hf,Ta 和W)化合物的稳定性和力学性能,研究发现,大多数化合物都具有比较好的稳定性,Al 是稳定L12结构的重要元素㊂各种成分的钴基合金以及Mar-M-247镍基合金的相变温度如图2所示[15,22,31-34]㊂诸多新型钴基高温合金的相变温度高于传统镍基高温合金,尤其是含有难熔合金化元素的新型钴基高温合金,如Co-9Al-9W㊁Co-5Al-14V 等㊂这是因为Ti,Nb,Ta 和W等难熔合金化元素的加入在新型钴基高图2㊀基于文献整理的各种钴基合金的γᶄ相溶解温度㊁固相线温度和液相线温度[15,22,31-34]Fig.2㊀γᶄsolvus,solidus and liquidus temperatures of various Co-based alloys based on literature reviews [15,22,31-34]温合金中形成了高熔点的化合物,同时作为强γᶄ相形成元素,提高了γᶄ相的体积分数,从而实现了强化效果[26]㊂通常认为,高的γᶄ相溶解温度是提高高温合金服役温度的基础㊂低密度同样是高温结构材料不断追求的目标之一㊂图3为各种钴基高温合金的密度[22,33,35-39]㊂难熔元素的加入导致新型钴基高温合金密度大幅上升,其中Co-9Al-9.8W 高温合金密度最高,可达9.82g㊃cm -3,这是其较高的含W 量导致的㊂实验证明,其他合金化元素(Mo,Cr,V 和Ti 等)代替W 元素后,合金密度大幅下降,甚至可与传统镍基高温合金媲美㊂图3㊀基于文献整理的各种钴基高温合金的密度[22,33,35-39]Fig.3㊀Density of various Co-based superalloys based on literaturereviews [22,33,35-39]3.2㊀合金化元素对新型钴基高温合金中γᶄ相体积分数的影响㊀㊀合金中γᶄ相的体积分数主要由合金化元素向γᶄ相的分配决定,较高的γᶄ相体积分数使合金具有更优异的力学性能[40]㊂Chen 等[22]和Makineni 等[41]对不同Ni 含量的新型钴基高温合金中的γᶄ相体积分数进行了统计,发现γᶄ相的体积分数随着Ni 元素含量的增加大幅提升㊂Cr 元素含量增加会降低γᶄ相的体积分数,Cr 在合金中倾向于分布在γ相基体中[42],同时大量Cr 元素会导致合金中有害第二相的析出,从而消耗大量其他合金化元素,使γᶄ相体积分数降低㊂Ta,Ti 和Nb 等作为强γᶄ相形成元素,在合金中分布于γᶄ相之中,其含量增加可增加γᶄ相的体积分数;而Mo 元素在γ/γᶄ两相之间接近平均分232㊀第3期张旭明等:新型钴基高温合金成分设计的研究进展配,对合金中γᶄ相体积分数的影响较小[22,23,43-45]㊂Wang等[46]通过第一性原理计算发现Ru,Rh,Pd,Ir 和Pt 元素倾向于占据Co 3Ta 中的Co 位,而Re 元素倾向于占据Co 3Ta 中Ta 的位置,从而提高γᶄ的相体积分数㊂应该明确的是,较大的γᶄ相体积分数可增大位错运动的阻力,从而使得合金的瞬时拉伸强度和持久强度提高㊂3.3㊀合金化元素对新型钴基高温合金中γ/γᶄ相晶格错配度的影响㊀㊀新型钴基高温合金中γᶄ相的形态由界面自由能和错配应变能两方面因素共同决定㊂界面自由能与错配应变能之和越小,γᶄ相的形态越稳定㊂一般来说,界面自由能与错配应变能分别与界面面积和γ/γᶄ相的晶格错配度有关,晶格错配度绝对值越大,错配应变能越大[47]㊂新型钴基高温合金中晶格错配度一般为正值,当晶格错配度较小时,γᶄ相的形态由界面自由能主导,体积相同时球体的表面积最小,故γᶄ相倾向于呈球状;当晶格错配度较大时,γᶄ相的形态由错配应变能主导,由于金属弹性一般呈各向异性,故γᶄ相倾向于呈立方状㊂晶格错配度δ可定义为[41]:δ=2(a γᶄ-a γ)a γᶄ+a γ(1)其中,a γᶄ和a γ分别为γᶄ相和γ相的晶格常数㊂Ni 元素使γᶄ相的晶格常数变小,导致晶格错配度减小,促使γᶄ相球化㊂在含W 钴基高温合金中添加Cr 元素,由于Cr 原子占据W 原子的位置,导致合金晶格错配度减小而使γᶄ相趋于球状[48,49]㊂Gao 等[50]研究了不同成分钴基高温合金时效后的晶格错配度(图4),发现Cr 元素的加入降低了合金的晶格错配度㊂Ti 是钴基高温合金中γᶄ相形成元素之一,会增大γ/γᶄ两相的晶格错配度进而使合金中γᶄ相倾向于呈立方状㊂Ta 原子掺杂会引起更大的晶格畸变,所以Ta 元素对晶格错配度增加的贡献要大于Ti 元素[51]㊂Hf 也可以增大合金中γ/γᶄ相的错配度,因此同样有利于改善合金强度[52]㊂一般来说,合金化元素的原子半径与Co 原子半径相差越大,引起的图4㊀利用XRD 测量的γ/γᶄ两相之间的晶格错配度[50]Fig.4㊀Lattice misfit between the γ-and γᶄ-phases measured by high-energy synchrotron X-ray diffraction [50]晶格畸变越大,越会导致合金晶格错配度的提高,从而使γᶄ相越倾向于呈立方状㊂Zenk 等[49]发现提高γ/γᶄ两相界面处的晶格畸变,能够有效阻碍合金变形过程中位错的运动,提高合金力学性能㊂凡是能够增大γᶄ相晶格常数的合金元素(如Nb,Ti 和Ta 等),都能增加γᶄ相周围的共格应变,起到强化作用㊂但错配度太大会降低高温下γᶄ相的稳定性,容易聚集长大从而松弛弹性应力[52]㊂晶格错配度越小的γᶄ相则具有更高的高温稳定性,因而此类合金的抗蠕变性能也更加优异[53]㊂3.4㊀合金化元素对新型钴基高温合金中γᶄ相尺寸的影响㊀㊀影响γᶄ相尺寸和长大的因素主要有合金元素的扩散㊁晶格错配度㊁弹性模量等,γᶄ相的尺寸大小对合金的性能也具有至关重要的影响,一般来说γᶄ相的尺寸越小,分布越弥散,合金的性能越好[54]㊂不同含量的合金组织如图5所示,Chen 等[22]研究统计了不同Ni 质量分数(10,20,30)的合金组织中γᶄ相的平均尺寸分别为(324ʃ74),(425ʃ150)和(496ʃ153)nm,发现随着Ni 含量的增加γᶄ相出现了明显的粗化现象㊂图5㊀Co-x Ni-8Al-12V 合金在900ħ固溶退火处理72h 后的SEM 照片[22]:(a)x =10,(b)x =20,(c)x =30Fig.5㊀Field emission scanning electron microscope images of Co-x Ni-8Al-12V quaternary alloys annealed at 900ħfor 72h after solu-tion annealing treatment [22]:(a)x =10,(b)x =20,(c)x =30332中国材料进展第43卷㊀㊀Gao 等[50]对γᶄ相的尺寸统计结果显示,γᶄ相的平均尺寸随Ti 元素含量的增加而增加㊂Ti 原子在合金中的扩散速率比Al 原子更快,降低了两相之间的界面能导致γᶄ相生长的驱动力增大㊂Cr 和Mo 元素都能促进合金中γᶄ相的粗化,且Mo 元素的影响更大㊂Pandey 等[47]认为Lifshitz-Slyozov-Wagner(LSW)模型仅适用于含Ti 量较低的高温合金㊂一般来说,γᶄ相的长大分为2个过程,在时效时间较短即时效初期,γᶄ相依靠原子的扩散进行生长;在时效时间较长即时效后期,γᶄ相主要依靠互相合并进行长大[44,55]㊂3.5㊀合金化元素对新型钴基高温合金中μ相和η相的影响㊀㊀μ相是一种主要由2种不同大小的金属原子构成的拓扑密排相,其结构为D85结构㊂作为一种硬脆相,μ相可能会成为裂纹的形核位置和拓展通道[38],μ相析出的同时会消耗大量的合金元素,减弱合金固溶强化及沉淀强化作用㊂有害相一般在晶界析出,但当Cr 元素的含量足够高时,有害相也会在晶粒内部析出,从而强烈降低合金力学性能㊂图6为不同新型钴基高温合金的显微组织照片㊂可以发现,Cr 元素含量的增加导致W 元素在γ相和γᶄ相中的溶解度降低,促进μ相的沉淀析出[32,36,44]㊂同时有文献表明,Ni 元素能够提高合金的组织稳定性,有效减少μ-Co 7W 6有害相的析出,提高合金的力学性能[56]㊂η相是一种具有D024结构的有害相,与μ相类似,倾向于在晶界析出减弱强化作用,会对合金性能产生不良影响[23]㊂郭建亭[57]认为,Al /Ti 原子数比值是合金中能否形成η相的决定性因素,同时Al +Ti 含量和Al /Ti 原子数比值也是影响合金中γᶄ相体积分数和γᶄ/γ两相晶格错配度的关键因素,一般地,Al +Ti 含量越高γᶄ相体积分数越高,γᶄ/γ两相晶格错配度也越高;Al /Ti 原子数比值越高,γᶄ相体积分数越高,γᶄ/γ两相晶格错配度越低㊂因此要严格控制合金Al +Ti 含量和Al /Ti 原子比,避免η相的析出对合金组织稳定性和力学性能产生不良影响,同时保证钴基合金具有较高的γᶄ相体积分数和较宽的加工窗口㊂图6㊀不同Cr 含量合金固溶处理后的SEM 照片:(a)9Cr-A 合金[36],(b)12Cr 合金[44],(c)8Cr 合金[32],(d)12Cr 合金[44]Fig.6㊀SEM images of alloys with different Cr contents after solution treatment:(a)9Cr-A alloys [36],(b)12Cr alloys [44],(c)8Cralloys [32],(d)12Cr alloys [44]4㊀合金化元素对合金性能的影响4.1㊀合金化元素对钴基高温合金抗氧化性、抗热腐蚀性的影响㊀㊀抗氧化性和抗热腐蚀性也是衡量合金高温性能好坏的一项重要指标[58,59]㊂在新型钴基高温合金中,Al 除稳定γᶄ相外,还能在合金表面形成致密的Al 2O 3氧化薄膜来提高合金的抗氧化性[60]㊂但Ti 的存在会引入空位,降低Al 2O 3的热力学稳定性,从而降低合金的抗氧化性㊂Chung 等[32]证实Cr 降低了合金的氧化层厚度,随着Cr 浓度的增加,更薄的氧化层足以形成耐氧化的表面(图7)㊂同时有实验证明较高的Cr 含量有助于形成结构致密的Cr 2O 3和Al 2O 3,阻止O 进一步扩散到基体中[23]㊂Cr 元素与Al 元素可以协同作用加速Al 2O 3的形成,即降低形成Al 2O 3层所需的临界Al 浓度[36,61]㊂合金表面致密的Al 2O 3和Cr 2O 3氧化层阻断O 向基体的扩散,提432㊀第3期张旭明等:新型钴基高温合金成分设计的研究进展图7㊀不同合金的氧化层截面组织照片[32]:(a)L24-0Cr 合金,(b)L24-12Cr 合金Fig.7㊀Micrographs of oxide layer structure of different alloys[32]:(a)L24-0Cr,(b)L24-12Cr alloys高合金的抗氧化性㊂Chen 等[42]发现6Cr 钴基高温合金并没有优异的抗氧化性,因为合金中γᶄ相的体积分数减小导致γ相基体优先氧化,适当高的γᶄ相体积分数也能提高合金抗氧化性㊂Ni 元素能够促进Cr 2O 3的生长及延缓合金的结节性氧化,提高合金的抗氧化性能[62]㊂此外,Ta 的添加也被证实能在一定程度上提高合金的抗热腐蚀性能[52]㊂4.2㊀合金化元素对新型钴基高温合金力学性能及抗蠕变性能的影响㊀㊀作为结构构件的物质基础,结构材料的性能直接影响到构件能否满足使用要求,因此结构材料的设计往往对其力学性能提出要求㊂图8为Makineni 等[41]测试的Co-10Al-5Mo-2Nb 和Co-30Ni-10Al-5Mo-2Nb Co 基高温合金的拉伸性能,2种合金依靠高γᶄ相含量,室温下强度达到了800MPa,超过了诸多含W 钴基高温合金㊂W 能够引起明显的晶格膨胀,阻止位错运动,同时提高γᶄ相的体积分数,提高合金强度㊂Mo元素在钴基高温合金中易图8㊀不同Co 基高温合金在不同条件下的拉伸应力-应变曲线[41]:(a)室温下Co-10Al-5Mo-2Nb,(b)室温下Co-30Ni-10Al-5Mo-2Nb,(c)870ħ时Co-30Ni-10Al-5Mo-2NbFig.8㊀Tensile stress-strain curves of different Co-based alloys at dif-ferent conditions [41]:(a)Co-10Al-5Mo-2Nb at room temper-ature,(b )Co-30Ni-10Al-5Mo-2Nb at room temperature,(c)Co-30Ni-10Al-5Mo-2Nb at 870ħ与C 形成大量的MoC 碳化物,细小弥散的碳化物也可以改善合金的力学性能,同时也在一定程度上达到细晶强化的效果㊂Ti 会增大γᶄ相的粗化速率,对合金力学性能产生不利影响,但Bocchini 等[63]证明Ti 提高了合金的高温强度,这说明γᶄ相体积分数增大对合金的强度提升效果超过了组织粗化带来的负面影响㊂在Co-Al-W 基合金中,少量的B 元素能够促进富W 硼化物在晶界的析出,起到晶界强化的作用,有利于提高合金的力学性能[64]㊂高温合金需要在高温环境下长时间服役,因此要求它具有优异的抗蠕变性能㊂蠕变是指在恒应力或载荷下所发生的缓慢而连续的塑性变形,关于蠕变的研究对高温合金具有非常重要的意义㊂可通过探究合金化元素对新型钴基高温合金抗蠕变性能的影响及其机理进而对它进行针对性的设计㊂Cr 元素含量的增加显著增大了蠕变最小稳态应变速率[65],Povstugar 等[66]认为当合金中加入Cr 元素以后会生成有害的二次相并改变合金的堆垛层错能,恶化合金的抗蠕变性能,而Ni 能够部分抵消Cr 对合金抗蠕变性能的恶化[44]㊂W 和Nb 元素均能够强烈降低γ相基体的堆垛层错能,有效改善高温合金的抗蠕变性能㊂得益于晶界强化的作用,含B 合金拥有较其他合金更优异的抗蠕变性能㊂在Co-Al-W 基合金中加入Ta 元素能够明显提高合金的蠕变寿命,但与其他元素如Si 和Mo 等同时存在时会析出大量金属间化合物,降低合金抗蠕变性能[67]㊂在合金蠕变的过程中,经常出现γᶄ相的定向粗化,通常称之为筏化[66,68-70]㊂钴基高温合金一般表现出正晶格错配,在压缩状态下γᶄ相会在所施加压应力的垂直方向与拉应力的平行方向发生筏化[71]㊂如图9所示,0Cr 和4Cr 合金中的γᶄ相出现了筏化现象㊂8Cr 合金没有发生筏化是因为大量Cr 原子占据W 原子的晶格后降低了晶格错配度,导致γᶄ相缺乏各向异性的应力场,进而使筏化的驱动力减小[44]㊂5㊀结㊀语高温合金不仅是航空发动机的重要材料,也是能源㊁化工领域高温耐蚀部件的重要材料㊂新型钴基高温合金具有比镍基高温合金更高的γᶄ相溶解温度和熔点,但γᶄ相的高温稳定性还有待提高㊂本文主要针对不同合金化元素对新型钴基高温合金组织性能的影响做了总结梳理㊂Ni 能够有效提高合金性能,但过量的Ni 导致γᶄ相形态改变,新型钴基高温合金中的Ni 含量应保持在30%(原子数分数,下同)以下;Ti,Ta 和Nb 等强γᶄ相形成元素能够大幅提高γᶄ相的体积分数,过量将导致γᶄ相的加速粗化和密度增加,常见钴镍基高温合金中Ti,Ta 和Nb532中国材料进展第43卷图9㊀不同Co基合金蠕变后的SEM照片[44]:(a,b)0Cr,(c,d) 4Cr,(e,f)8CrFig.9㊀Post-creep SEM images of different Co-based alloys[44]:(a,b) 0Cr,(c,d)4Cr,(e,f)8Cr含量为2%~4%;Cr在提高合金的抗氧化性[72]的同时可促进有害相的析出,降低合金力学性能,新型钴基高温合金中Cr含量一般控制在4%~6%以下㊂新型钴基高温合金具有多项优于传统钴基高温合金的性能,是最具潜力的高温合金之一㊂但与发展相对成熟的镍基高温合金相比,新型钴基高温合金的发展和应用仍然具有很大的挑战,如合金的制造工艺以及零件的加工和热处理工艺尚不成熟等㊂目前我国合金成分设计数据库仍然不够健全,但随着计算材料学㊁材料基因工程等领域的发展,CALPHAD㊁第一性原理计算㊁机器学习等方法将在合金的高效设计中发挥更大的作用,将材料计算㊁计算机仿真模拟等多种设计思路与实验相结合有望实现新型钴基高温合金的高通量设计㊂参考文献㊀References[1]㊀杜金辉,吕旭东,董建新,等.金属学报[J],2019,55(9):1115-1132.DU J H,LV X D,DONG J X,et al.Acta Metallurgica Sinica[J], 2019,55(9):1115-1132.[2]㊀LIU Z,GAO Q,ZHANG H,et al.Materials Science&Engineering:A[J],2019,755:106-115.[3]㊀程远,赵新宝,岳全召,等.稀有金属材料与工程[J],2023,52(7):2599-2611.CHENG Y,ZHAO X B,YUE Q Z,et al.Rare Metal Materials and Engineering[J],2023,52(7):2599-2611.[4]㊀JIANG J,LIU Z,GAO Q,et al.Materials Science&Engineering:A[J],2020,797:140219.[5]㊀刘健.元素对γᶄ沉淀强化型钴基高温合金组织及力学性能的影响[D].合肥:中国科学技术大学,2019.LIU J.Effects of Alloying Elements on the Microstructure and Mechan-ical Behavior ofγᶄ-Strengthed Co-Base Superalloys[D].Hefei:Uni-versity of Science and Technology of China,2019.[6]㊀刘兴军,陈悦超,卢勇,等.金属学报[J],2020,56(1):1-20.LIU X J,CHEN Y C,LU Y,et al.Acta Metallurgica Sinica[J], 2020,56(1):1-20.[7]㊀KLEIN L,SHEN Y,KILLIAN M S,et al.Corrosion Science[J],2011,53(9):2713-2720.[8]㊀JINSHAN H,MIN Z,LONGFEI L,et al.Materials Letters[J],2020,262:127042.[9]㊀SATO J,OMORI T,OIKAWA K,et al.Science[J],2006,312(5770):90-91.[10]SUZUKI A.Acta Materialia[J],2008,56(6):1288-1297.[11]WALTER C,HALLSTEDT B,WARNKEN N.Materials Science andEngineering:A[J],2005,397(1/2):385-390.[12]PARK H,LI C,JAKUS A E,et al.Scripta Materialia[J],2020,188:146-150.[13]SUZUKI A,INUI H,POLLOCK T M.Annual Review of MaterialsResearch[J],2015,45(1):345-368.[14]BAUER A,NEUMEIER S,PYCZAK F,et al.Superalloys[J],2012,2012:695-703.[15]AKANE S,GARRET C D,TRESA M P.Scripta Materialia[J],2006,56(5):385-388.[16]LU S,ANTONOV S,LI L,et al.Metallurgical and Materials Transac-tions A[J],2018,49(9):4079-4089.[17]SHI L,YU J J,CUI C Y,et al.Materials Science and Engineering:A[J],2015,620:36-43.[18]BOCCHINI P J,LASS E A,MOON K W,et al.Scripta Materialia[J],2013,68(8):563-566.[19]KOBAYASHI S,TSUKAMOTO Y,TAKASUGI T,et al.Intermetallics[J],2009,17(12):1085-1089.[20]LASS E A,WILLIAMS M E,CAMPBELL C E,et al.Journal ofPhase Equilibria and Diffusion[J],2014,35(6):711-723. [21]LASS E A,GRIST R D,WILLIAMS M E.Journal of Phase Equilib-ria and Diffusion[J],2016,37(4):387-401.[22]CHEN Y,WANG C,RUAN J,et al.Acta Materialia[J],2019,170:62-74.[23]LLEWELYN S C H,CHRISTOFIDOU K A,ARAULLO-PETERS V J,et al.Acta Materialia[J],2017,131:296-304.[24]BANTOUNAS I,GWALANI B,ALAM T,et al.Scripta Materialia[J],2019,163:44-50.[25]BAUER A,NEUMEIER S,PYCZAK F,et al.Scripta Materialia[J],2010,63(12):1197-1200.[26]OOSHIMA M,TANAKA K,OKAMOTO N,et al.Journal of Alloys&Compounds[J],2010,508(1):71-78.632㊀第3期张旭明等:新型钴基高温合金成分设计的研究进展[27]POLLOCK T M,DIBBERN J,TSUNEKANE M,et al.JOM[J],2010,62(1):58-63.[28]LASS E A.Metallurgical and Materials Transactions A[J],2017,48(5):2443-2459.[29]CHEN M,WANG C Y.Journal of Applied Physics[J],2010,107(9):093705[30]JIN M,MIAO N,ZHAO W,et putational Materials Science[J],2018,148:27-37.[31]RUAN J,XU W,YANG T,et al.Acta Materialia[J],2020,186:425-433.[32]CHUNG D W,TOININ J P,LASS E A,et al.Journal of Alloys andCompounds[J],2020,832:154790.[33]ZHANG Y,FU H,ZHOU X,et al.Intermetallics[J],2019,112:106543.[34]ZHANG Y,FU H,ZHOU X,et al.Materials Science and Engineer-ing:A[J],2018,737:265-273.[35]MAKINENI S K,NITHIN B,CHATTOPADHYAY K.Scripta Materia-lia[J],2015,98:36-39.[36]LI W,LI L,ANTONOV S,et al.Journal of Alloys and Compounds[J],2020,826:154182.[37]QU S,LI Y,HE M,et al.Materials Science and Engineering:A[J],2019,761:138034.[38]LIU J,YU J J,YANG Y H,et al.Materials Science and Engineering:A[J],2019,745:404-410.[39]PHILIPPE T,VOORHEES P W.Acta Materialia[J],2013,61(11):4237-4244.[40]REYES T F L,DUNAND D C.Journal of Materials Research andTechnology[J],2021,11:2305-2313.[41]MAKINENI S K,NITHIN B,CHATTOPADHYAY K.Acta Materialia[J],2015,85:85-94.[42]CHEN Y,XUE F,WANG C,et al.Corrosion Science[J],2019,161:108179.[43]XU W W,SHANG S L,WANG C P,et al.Materials&Design[J],2018,142:139-148.[44]NG D S,CHUNG D W,TOININ J P,et al.Materials Science and En-gineering A[J],2020,778:139108.[45]PYCZAK F,BAUER A,GOKEN M,et al.Journal of Alloys andCompounds[J],2015,632:110-115.[46]WANG C,LI K,HAN J,et al.Journal of Alloys and Compounds[J],2019,808:151068.[47]PANDEY P,RAJ A,BALER N,et al.Materialia[J],2021,16:101072.[48]OMORI T,OIKAWA K,SATO J,et al.Intermetallics[J],2013,32:274-283.[49]ZENK C H,NEUMEIER S,STONE H J,et al.Intermetallics[J],2014,55:28-39.[50]GAO Q,JIANG Y,LIU Z,et al.Materials Science and Engineering:A[J],2020,779:139139.[51]YAN H Y,COAKLEY J,VORONTSOV V A,et al.Materials Scienceand Engineering:A[J],2014,613:201-208.[52]郭建亭.高温合金材料学[M].北京:科学出版社,2010:152.GUO J T.Materials Science and Engineering for Superalloys[M].Bei-jing:Science Press,2010:152.[53]MANIAR G N,BRIDGE J E.Metallurgical Transactions[J],1971,2(1):95-102.[54]CHEN J,GUO M,YANG M,et putational Materials Science[J],2021,191:110358.[55]SAUZA D J,DUNAND D C,SEIDMAN D N.Acta Materialia[J],2019,174:427-438.[56]周鹏杰,宋德航,吴海斌,等.航空材料学报[J],2019,39(6):73-80.ZHOU P J,SONG D H,WU H B,et al.Journal of Aeronautical Ma-terials[J],2019,39(6):73-80.[57]郭建亭.金属学报[J],2010,46(5):513-527.GUO J T.Acta Mentallurgica Sinica[J],2010,46(5):513-527.[58]GAO Q,LU B,MA Q,et al.Intermetallics[J],2021,138:107312.[59]GAO Q,SHANG H,MA Q,et al.Materials and Corrosion[J],2022,73(4):513-525.[60]YU H,UKAI S,HAYASHI S,et al.Corrosion Science[J],2017,118:49-59.[61]GAO Q,LIU Z,LI H,et al.Journal of Materials Science&Technolo-gy[J],2021,68:91-102.[62]GAO B,WANG L,LIU Y,et al.Corrosion Science[J],2019,157:109-115.[63]BOCCHINI P J,SUDBRACK C K,NOEBE R D,et al.Materials Sci-ence and Engineering A[J],2017,705:122-132. [64]马启慧,王清,董闯.材料导报[J],2020,34(3):03157-03164.MA Q H,WANG Q,DONG C.Materials Reports[J],2020,34(3): 03157-03164.[65]MURAKUMO T,KOBAYASHI T,KOIZUMI Y,et al.Acta Materialia[J],2004,52(12):3737-3744.[66]POVSTUGAR I,ZENK C H,LI R,et al.Materials Science and Tech-nology[J],2016,32(3):220-225.[67]BAUER A,NEUMEIER S,PYCZAK F,et al.Materials Science andEngineering:A[J],2012,550:333-341.[68]COAKLEY J,LASS E A,MA D,et al.Scripta Materialia[J],2017,134:110-114.[69]LI Y,PYCZAK F,PAUL J,et al.Materials Science and Engineering:A[J],2018,719:43-48.[70]XUE F,ZENK C H,FREUND L P,et al.Scripta Materialia[J],2018,142:129-132.[71]CHUNG D W,NG D S,DUNAND D C.Materialia[J],2020,12:100678.[72]高杉,邹俭鹏.稀有金属材料与工程.[J],2022,51(3):814-820.GAO S,ZOU J P.Rare Metal Materials and Engineering[J],2022, 51(3):814-820.(编辑㊀费蒙飞)732。
morph定义语言学英文

morph定义语言学英文Introduction to Morphology in LinguisticsMorphology is a branch of linguistics that focuses on the study of words, particularly their internal structure and how they are formed. It plays a crucial role in understanding the ways in which words are created and how they convey meaning. This article provides an introduction to the key concepts and principles of morphology in the field of language science.1. Definition:Morphology can be defined as the study of the internal structure and formation of words in language. It examines the smallest meaningful units in a language, known as morphemes, and analyzes their combination to form complex words. A morpheme can be a root, a prefix, a suffix, or aninflectional ending. By studying morphemes, linguists gain insights into the mechanisms that allow language users to create and comprehend an infinite number of words.2. Morphological Processes:In morphology, various processes are involved in the formation of words. Two important processes are derivation and inflection. Derivation refers to the creation of new words by adding affixes to a base or root word. For example, the noun "friend" can be derived into the adjective "friendly" by adding the suffix "-ly." Inflection, on the other hand, involves the modification of a word's form to indicate grammatical features such as tense, number, or case. For instance, the verb "sing" changes its form to "sang" in the past tense.3. Morphological Structure:Morphological structure can be classified into two main types: analytic and synthetic. Analytic languages, like Mandarin Chinese, rely heavily on word order and context rather than inflectional processes to convey meaning. In contrast,synthetic languages, such as Latin or Russian, use inflected forms to express grammatical relationships within a sentence. These inflections often denote features like tense, gender, number, and case.4. Morphophonology:Morphophonology is an important aspect of morphology that focuses on the interaction between morphological processesand phonological rules. It examines how morphemes may change their form based on the phonological properties of the surrounding sounds. For instance, the plural suffix "-s" in English can have different pronunciations based on the final sound of the preceding word.5. Cross-Linguistic Variation:Morphological processes can vary greatly across different languages. Some languages have highly productive and complex morphological systems, while others have less intricate systems with fewer affixes. The study of cross-linguistic variation in morphology allows linguists to gain a deeper understanding of the different ways in which languages represent meaning and structure words.Conclusion:Morphology is a fundamental component of language study, asit provides insights into how words are formed and how they convey meaning. By investigating the internal structure andformation of words, linguists can unravel the intricate connections between morphology, syntax, and semantics. Moreover, the study of morphology highlights the rich diversity of languages around the world, showcasing the various ways in which humans communicate and express themselves through words.。
神奇的纳米盒作文英语

神奇的纳米盒作文英语Title: The Marvel of Nanoboxes。
Nanotechnology has revolutionized the way we perceive and interact with the world around us. Among its myriad applications, one of the most fascinating is the concept of nanoboxes. These microscopic structures, often no larger than a few nanometers, possess a myriad of potential applications that span across various fields, from medicine to electronics. In this discourse, we shall delve into the marvel of nanoboxes, exploring their structure, functionality, and the diverse array of applications they offer.Firstly, let us understand what exactly nanoboxes are. Essentially, nanoboxes are nano-sized containers or cages composed of various materials, such as metals, polymers, or even biological molecules like proteins. Their structure resembles a tiny box with an empty interior space capable of encapsulating guest molecules. The remarkable aspect ofnanoboxes lies in their ability to precisely control the size, shape, and composition at the nanoscale, allowing for tailored properties and functionalities.The functionality of nanoboxes stems from their unique structural characteristics. Due to their miniature size, nanoboxes exhibit high surface area-to-volume ratios, making them ideal candidates for applications requiring efficient surface interactions. Moreover, their hollow interior provides an isolated environment conducive to encapsulating guest molecules, protecting them from external factors such as degradation or chemical reactions.One of the most promising applications of nanoboxes is in drug delivery systems. The ability to encapsulate drugs within nanoboxes offers several advantages, including targeted delivery, controlled release, and enhanced therapeutic efficacy. By functionalizing the surface of nanoboxes with targeting ligands, such as antibodies or peptides, they can selectively bind to specific cells or tissues, thereby minimizing off-target effects and reducing systemic toxicity.Furthermore, nanoboxes hold immense potential in the field of catalysis. The confined environment within the nanobox cavity can significantly alter the reactivity and selectivity of encapsulated catalysts, leading to improved catalytic performance. Additionally, the ability to precisely tune the composition and morphology of nanoboxes allows for the design of custom catalysts tailored for specific reactions, thus opening new avenues for sustainable chemical synthesis.In the realm of electronics, nanoboxes offer intriguing possibilities for nanoscale device fabrication. By incorporating nanoboxes into electronic components, such as transistors or sensors, researchers can exploit their unique properties to enhance device performance. For instance, nanoboxes functionalized with semiconducting materials could serve as building blocks for novel nanoelectronic devices with improved efficiency and miniaturization.Moreover, nanoboxes have garnered significant attentionin the field of photonics and plasmonics. Their ability to confine and manipulate light at the nanoscale enables the development of next-generation photonic devices with unprecedented functionality. By engineering the optical properties of nanoboxes through precise control of size, shape, and material composition, researchers can create nanophotonic structures with applications ranging from sensing and imaging to data communication.Beyond these applications, nanoboxes hold promise in various other fields, including environmental remediation, energy storage, and biomolecular sensing. Their versatility and tunability make them valuable tools for addressing complex challenges across diverse domains.In conclusion, the marvel of nanoboxes lies not only in their miniature size but also in their immense potential to revolutionize numerous fields of science and technology. From drug delivery and catalysis to electronics and photonics, nanoboxes offer a plethora of opportunities for innovation and advancement. As researchers continue to unravel the mysteries of nanoscale phenomena, the futureholds boundless possibilities for harnessing the power of nanoboxes to address some of the most pressing challenges facing humanity.。
Chapter-Morphology--形态学现代语言学

Chapter 3 Morphology 形态学1.Definition 定义Morphology is a branch of grammar which studies the internal structure of words and the rules by which words are formed.形态学是语法学的一个分支,它研究的是单词的内在结构及单词的构成规则。
The aim of morphology is to find out these rules.形态学的任务就是要找出这些规则(单词构成的规则)。
Morphology is divided into two sub-branches: inflectional morphology and lexical or derivational morphology. The former studies the inflections and the latter the study of word-formation.形态学可以划分两个分支:屈折形态学和词汇形态学(也叫派生形态学)。
前者研究的是单词的屈折变化,后者研究的是构词法。
2.Morpheme 词素Morpheme: the smallest meaningful unit of language 词素:语言中最小的意义单位Just as a phoneme is the basic unit in the study of phonology, so is a morpheme the basic unit in the study of morphology.正如音位是音系学研究中的基本单位一样,词素是形态学研究中的基本单位。
Monomorphemic words 单词素单词Types of morphemes 词素的类型Free morphemes 自由词素The morphemes that are independent units of meaning and can be used freely all by themselves are called free morphemes. Such as help, table,room, mate, quick, able.这些词素是独立的、可以自由使用的意义单位,所以它们就被称作自由词素。
自考英语语言学Chapter 3 Morphology
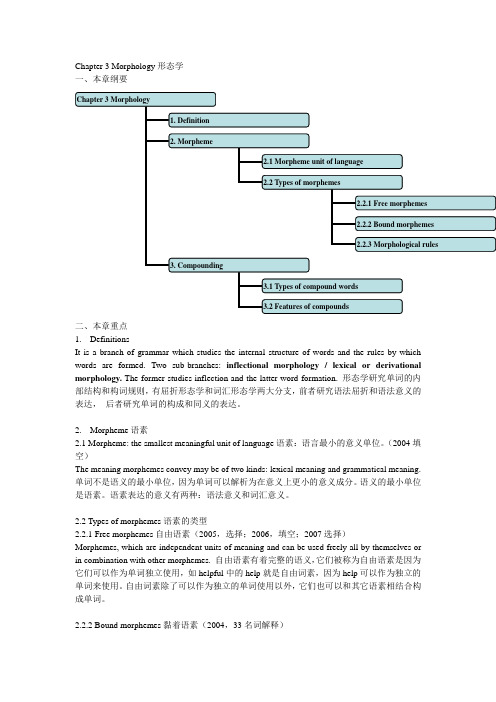
Chapter 3 Morphology形态学一、本章纲要二、本章重点1.DefinitionsIt is a branch of grammar which studies the internal structure of words and the rules by which words are formed. Two sub-branches: inflectional morphology / lexical or derivational morphology. The former studies inflection and the latter word-formation. 形态学研究单词的内部结构和构词规则,有屈折形态学和词汇形态学两大分支,前者研究语法屈折和语法意义的表达,后者研究单词的构成和同义的表达。
2.Morpheme语素2.1 Morpheme: the smallest meaningful unit of language语素:语言最小的意义单位。
(2004填空)The meaning morphemes convey may be of two kinds: lexical meaning and grammatical meaning. 单词不是语义的最小单位,因为单词可以解析为在意义上更小的意义成分。
语义的最小单位是语素。
语素表达的意义有两种:语法意义和词汇意义。
2.2 Types of morphemes语素的类型2.2.1 Free morphemes自由语素(2005,选择;2006,填空;2007选择)Morphemes, which are independent units of meaning and can be used freely all by themselves or in combination with other morphemes. 自由语素有着完整的语义,它们被称为自由语素是因为它们可以作为单词独立使用,如helpful中的help就是自由词素,因为help可以作为独立的单词来使用。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Applied Catalysis A:General 240(2003)263–271Effects of morphology and composition on catalytic performance of double metal cyanide complex catalystYi Jun Huang a ,Guo Rong Qi a ,∗,Lin Shen Chen baDepartment of Polymer Science and Engineering,Zhejiang University,Hangzhou 310027,PR Chinab Analysis and measurement center of Zhejiang University,Hangzhou 310028,PR China Received 19November 2001;received in revised form 8March 2002;accepted 1August 2002AbstractDouble metal cyanide (DMC)complexes based on Zn 3[Co(CN)6]2were prepared using different zinc salts and complexing agents and They were characterized by elemental analysis,IR and XRD.The catalytic properties of DMC complexes were determined in polymerization of propylene oxide.Great differences in morphology of DMC complexes was were correlated with preparation route as well as with the kind of zinc salts employed.The catalytic activity was strongly dependent on themorphology of DMC complexes and composition of catalysts.The study revealed that the amorphous catalyst made from ZnCl 2and t -BuOH had the highest activity (up to 26kg polymer/g catalyst).The DMC catalyst was non-stoichiometric.Polymerization of propylene oxide (PO)catalyzed by DMC proceeded in a controlled manner.The molecular weight of each polymer was controlled by the monomer/initiator ratio,the values of molecular weight distribution (MWD)were in the range of 1.23–1.48.The polymer has an atactic structure and a head-to-tail regiosequance.©2002Elsevier Science B.V .All rights reserved.Keywords:Double metal cyanide complex;Morphology;Catalytic activity;Propylene oxide;Polyether polyol1.IntroductionPolyether polyols are widely used as precursors in the manufacture of polyurethanes.These polymers are currently prepared by anionic polymerization of alkene-oxide (mainly propylene oxide),catalyzed by an alkaline base such as KOH.As a result of trans-fer reaction to the monomer,the molecular weight is limited to a relatively low value (equilvalent weight <2000)and the molecular weight distribution (MWD)is broadened.Moreover,the polyols obtained have a high unsaturation that results in a loss of hydroxylfunctionality [1].∗Corresponding author.Fax:+86-571-87951773.E-mail address:qiguorong@ (G.R.Qi).Aluminum porphyrins are excellent catalysts for polymerization of propylene oxide,affording the cor-responding polymers of controlled molecular weight with narrow MWD [2].However,the high cost of these catalysts prevents for an industrial application.Other coordination catalysts such as AlR 3–H 2O [3],ZnR 2–H 2O [4],Bu 2SnO–Bu 3PO 4condensate [5],alumoxane-propylene oxide complex [6]and rare earth metal compounds [7]have been used to prepare high molecular weight (Mn up to 105)polyoxypropy-lenes,which contain a fraction of atactic polymers in addition to isotactic polymers.Moreover,the molec-ular weight of polymers prepared by these catalysts is not predictable.Double metal cyanide complexes (DMC)are well-known catalysts for alkene-oxide polymerization.Recent improvements have made0926-860X/02/$–see front matter ©2002Elsevier Science B.V .All rights reserved.PII:S 0926-860X (02)00452-无定形的聚醚多元醇羟基部分264Y.J.Huang et al./Applied Catalysis A:General240(2003)263–271DMC catalysts much more attractive for commercialmanufacture of polyoxypropylene polyols[8–11].Thecatalysts are highly active,and give polyether poly-ols that have low unsaturation and narrow molecularweight distribution compared with similar polyolssynthesized using conventional KOH catalysis.DMC catalysts are usually prepared by react-ing aqueous solutions of water-soluble metal salts(M1X w)and metal cyanide salts to form a precipitateof DMC compound.A water-soluble organic com-plexing agent(L)is included in the preparation ofeach catalyst.The resulting catalyst has the generalformula:M1u[M2(CN)6]v·x M1X w·y L·z H2O.In fact,it is found from patent reports that the mostwidely used DMC catalysts which are of importancein industry applications are based on Zn3[Co(CN)6]2.However,DMC catalysts are very versatile,and evenif prepared with the same starting material,they mayshow great differences in catalytic activity.In ad-dition to patent reports,few open studies on DMCand DMC catalyzed polymerization of alkene-oxideare reported.The present work was mainly aimed atstudying the interrelation of differences in morphol-ogy and composition with catalytic activity.2.Experimental2.1.MaterialK3Co(CN)6(ACROS95%)was recrystallized be-fore use,ZnCl2,ZnSO4·7H2O,zinc acetate(Zn(Ac)2), dioxane,tetrahydrofuran(THF),dimethoxyethane (DME)are all analyticallg pure grade reagents. Tert-butyl alcohol(98%)was used without further pu-rification.Propylene oxides(>99.8%pure)were used as received.PPG-400was a polyoxypropylene diol (Mn≈400)made with KOH and was a commercial product.2.2.Catalyst preparationIn order to investigate the dependence of catalytic activity on preparation conditions,several different methods were adopted to prepare DMC catalysts. (a)A solution of3g K3Co(CN)6in40ml of H2O and8ml of complexing agent(solution1)was addeddropwise with stirring to a solution of0.08mol Zn2+in40ml water and15ml complexing agent (solution2).The resulting white suspension was pressurefiltered.DMC solids were isolated,and were resuspended with vigorous stirring in a solu-tion of complexing agent and H2O(v/v=3/1).The solids were again isolated by pressurefil-tering.This process(alternatefiltration and re-suspending)was repeated several times.Finally, the solids were resuspended in neat complexing agent,and isolated as described above.The result-ing DMC catalyst solids were dried under vacuum at50◦C for more than8h.(b)The preparation was similar to method(a),exceptthat volumes of initial complexing agent included in solutions1and2were4and8ml,respectively.(c)A solution of3g K3Co(CN)6in40ml of H2Owas added dropwise with stirring to a solution of0.08mol Zn2+in40ml water at30◦C.Then theDMC solids were isolated by pressurefiltration, and were resuspended in50ml H2O.This process was repeated several times.Finally,the solids were vacuum-dried.(d)DMC catalysts prepared with method(c)were re-suspended in neat complexing agent again,and specified amounts of ZnCl2(dissolved in a mix-ture of H2O and complexing agent)were added with stirring.The resulting suspensions were di-rectly dried under vacuum.(e)A solution of3g K3Co(CN)6in40ml of H2Owas added dropwise with stirring to a solution of0.08mol Zn2+in40ml water at30◦C.After theaddition,23ml of complexing agent was added to the suspension.Stirring continued for another1h, then the DMC solids were isolated.The subse-quent steps were similar to those in method(a).(f)The preparation was similar to method(e),ex-cept that after the addition of K3Co(CN)6,the resulting suspension was heated for12min at 100◦C.All these above-prepared catalysts are insoluble in monomers,polyether polyols and in all common or-ganic solvents.Thus these DMC catalysts seem to be heterogeneous.Interestingly,unlike normal heteroge-neous catalysts,most of the DMC catalysts in this study became soluble(the reaction mixture became clear)after polymerization started.四氢呋喃二甲氧基乙烷Y.J.Huang et al./Applied Catalysis A:General 240(2003)263–2712652.3.Polymerization experimentPolymerizations with one-step and semi-continuous addition of monomer were carried out as described previously [12].A polyoxypropylene diol was used as an initiator.A clear liquid product was ob-tained.Herein the catalytic activity is defined as the weight of finished polymer per weight of Zn 3[Co(CN)6]2.2.4.MeasurementWide-angle X-ray diffraction (W AXD)patterns of the DMC catalyst were obtained by means of a Ru-gaku D/MAX-B X-ray diffractometer using Cu K ␣ra-diation.The scan rate was 3◦/min and the scan range is 30–70◦.The elemental analysis of catalysts was performed by using a Hitachi 180–50atomic absorp-tion spectrophotometer and a ThermoQuest EA 1110instrument.13C NMR spectra of the polymer were obtained on a Avance DMX 500spectrometer with CDCl 3as a solvent.Infra-red spectra of catalysts were measured with a VECTOR-22IR spectrometer.Num-ber average molecular weight of the polymers was de-termined by using a KNUER vapor phase osmometer (THF as solvent).Molecular weight distributions were measured using a Waters 150-C instrument,operated at 25◦C,with a set at 104,103and 500Åcolumns.Table 1Effect of preparation method of DMC catalyst on catalytic activity a Catalyst Method CompositionK +(mg/kg)Activity (kg/g)1a Zn 3[Co(CN)6]2·1.0ZnCl 2·2.5t -BuOH ·4H 2O 30514.12a Zn 3[Co(CN)6]2·0.85ZnCl 2·2.2t -BuOH ·5H 2O 27914.63a Zn 3[Co(CN)6]2·1.2ZnCl 2·2.5t -BuOH ·5H 2O 29613.84b Zn 3[Co(CN)6]2·0.94ZnCl 2·2.5t -BuOH ·5H 2O 21713.95b c Zn 3[Co(CN)6]2·8H 2O850.126c c Zn 3[Co(CN)6]2·1.2ZnCl 2·6H 2O 980.417d c Zn 3[Co(CN)6]2·1.3t -BuOH ·5H 2O950.268d Zn 3[Co(CN)6]2·0.22ZnCl 2·1.6t -BuOH ·4H 2O 90 6.59d Zn 3[Co(CN)6]2·0.65ZnCl 2·2.5t -BuOH ·6H 2O 987.210d Zn 3[Co(CN)6]2·1.6ZnCl 2·4.1t -BuOH ·3H 2O 125 6.811e Zn 3[Co(CN)6]2·0.86ZnCl 2·3.8t -BuOH ·3H 2O 1808.212fZn 3[Co(CN)6]2·1.1ZnCl 2·3.8t -BuOH ·4H 2O95–aOne-step polymerization:catalyst level =55mg/kg (concentration in a mixture of monomer and initiator on a solid Zn 3[Co(CN)6]2weight basis),PO/PPG-400(w /w )=40/1,temperature =100±3◦C,reaction time =6h.b Catalyst level =3500mg/kg.c Catalyst 6was prepared by treating catalyst 5with ZnCl 2,catalyst level =2500mg/kg.d Catalyst 7was prepared by treating catalyst 5with BuOH,catalyst level =2500mg/kg.Polystyrene standards with low polydispersities were used to generate a calibration curve.The unsaturation of each polymer was determined according to ASTM D4671–D87.3.Results and discussion3.1.The non-stoichiometric nature of the DMC catalystUsing K 3Co(CN)6,ZnCl 2and t -BuOH as starting materials,we prepared various catalysts by different methods.Table 1shows their compositions detected according to elemental analysis and the correspond-ing polymer yields in polymerization of PO.From Table 1,it is seen that catalysts 1–3,which were pre-pared by the same method had different composition formulas (existence of t -BuOH and its coordination to Zn 2+had also been confirmed by IR spectra (omit-ted)).The reason for such differences in composi-tion may be unavoidable variations during operation or post-treatment.Fortunately,these differences had no effect on activity.Catalysts 8–10,in which dif-ferent amounts ZnCl 2were added deliberately,also had similar catalytic activities.There was no ZnCl 2retained in the catalysts prepared with method (c),though excess ZnCl 2was used in the preparation,and266Y.J.Huang et al./Applied Catalysis A:General 240(2003)263–271the catalysts in which ZnCl 2and t -BuOH were ab-sent showed rather low activity.Probably retention of certain amounts of ZnCl 2in these DMC catalysts is associated with use of t -BuOH during pared with catalyst 4,introducing ZnCl 2(cata-lyst 6)and t -BuOH (catalyst 7)raised the activity of Zn 3[Co(CN)6]2by factors of about 3.4and 2.1,re-spectively.Surprisingly,a combination of these two agents (catalyst 8–10)resulted in a great increase in catalytic activity of Zn 3[Co(CN)6]2(up to 50times).This result indicatesthat ZnCl 2and t -BuOH (complex-ing agent)were both required as important promoter for high activity of DMC catalyst,and that the consid-erable variations of the content of ZnCl 2and t -BuOH had no significant effect on catalytic activity.Thus the DMC catalyst is of non-stoichiometric nature.3.2.Effect of morphology of DMC on catalytic activityAs shown in Table 1,DMC catalysts prepared by different methods exhibited great differences in cat-alytic activity.The catalytic activity of these catalysts decreased in order of preparation method:a ∼b >e >d >f.Fig.1.XRD patterns of DMC catalysts prepared with different methods.Fig.1shows XRD patterns of catalysts prepared by different methods.From Fig.1,the crystalline phase of all samples was Zn 3[Co(CN)6]2since there were no peaks related to ZnCl 2.Catalyst 1was low crys-talline or substantially amorphous,lacking sharp lines in the XRD pattern,whereas catalyst 11exhibited a crystalline pattern,which ascribed to a cubic lattice structure (d value:5.12,3.62and 2.56Å).Indeed,cat-alysts prepared by methods (c)and (d)also exhibited a cubic lattice structure.Catalyst 10had a rhombo-hedral lattice structure (d value:9.03,6.54,5.42and 4.51Å).In contrast to catalyst 1,catalyst 4exhibited a crystalline pattern.Unfortunately,this crystal struc-ture modification of Zn 3[Co(CN)6]2can not be identi-fied using the standard JCPDS.These results indicate that the morphology of each catalyst was largely de-pendent on preparation conditions.On the other hand,it can be concluded that the morphology had a signif-icant effect on catalytic activity.3.3.Effect of complexing agent on catalytic activity Table 2shows the catalytic activities of DMC cat-alysts prepared by methods (a)and (b)using vari-ous complexing agents.The complexing agent clearlyY.J.Huang et al./Applied Catalysis A:General 240(2003)263–271267Table 2Effect of complexing agent on catalytic properties of DMC catalyst a Catalyst Preparation method Complexing agent Catalyst level (mg/kg)Reaction time (h)Conversion (%)Activity (kg/g)1a t -BuOH 5056813.613a Dioxane 505469.214a DME 100543 4.315a THF1505––16a t -BuOH/dioxane b 5056212.517a t -BuOH/DME b 5055811.618e t -BuOH 100565 6.519e DME5055310.620e t -BuOH/DME 502551120et -BuOH/DME5055711.2a Other polymerization conditions as described in Table 1.bMixed ratio =1/1(v/v).played an important role.Catalytic activities of cat-alysts prepared by method (a)decreased as follows:t -BuOH >dioxane >DME >THF.However,when prepared with method (e),DME was a better candi-date than t -BuOH.Interestingly,if a mixture of DME and t -BuOH was used as complexing agent,the cata-lyst prepared with method (e)could provide the high-est rate of polymerization,though the polymer yield was not the highest.These results indicate the exis-tence of an optimum combination of preparation and complexing agent regarding catalytic activity.3.4.Effect of water-soluble metal salts (M 1X w )on catalytic activityAs mentioned in the introduction of this paper,on the one hand M 1X w was used as cation source inTable 3Effect of M 1X w on catalytic activity of DMC catalyst a Catalyst M 1X w Catalyst level (mg/g)Time (h)Conversion (%)Activity (kg/g catalyst)1ZnCl 25557814.121ZnSO 44005––22+HCl b 3005––23+ZnCl 2c 3005––24Zn(Ac)22005––25+HCl b 5556511.826+ZnCl 2c 5556010.627CoCl 22006––28NiCl 22006––29NiCl 22006––a Other polymerization conditions as described in Table 1.bCatalysts 21and 24were prepared by treating catalysts 20and 23with 1.0ml HCl (37%)+10ml t -BuOH,respectively.c Catalysts 22and 26was prepared by treating catalysts 20and 24with 0.4g ZnCl 2+0.6ml H 2O +10ml t -BuOH,respectively.formation of precipitate,while on the other hand it was retained or intentionally added to the finished catalyst as a promoter.Table 3shows the catalytic activities of DMC catalysts prepared by method (a)using various M 1X w .Table 2shows that the catalyst prepared with ZnCl 2had the highest activity,whereas those prepared with ZnSO 4(catalyst 21)and Zn(Ac)2(catalyst 24)had low activity.Interestingly,after being treated with HCl or ZnCl 2,catalysts prepared with Zn(Ac)2exhibited considerable higher activity.The reason may be that Zn(Ac)2retained in the catalyst was changed to ZnCl 2.However,either treated with HCl or with ZnCl 2,no improvement of activity of the catalyst prepared with ZnSO 4was observed.We therefore investigated the morphology of the above samples.Fig.2shows XRD patterns of catalysts268Y.J.Huang et al./Applied Catalysis A:General 240(2003)263–271Fig.2.XRD patterns of DMC catalysts prepared using different M 1X w .prepared by method (a)using Zn(Ac)2and ZnSO 4as M 1X w .The morphology of these catalysts differed ob-viously from that prepared using ZnCl 2.The catalyst prepared with ZnSO 4exhibited a rhombohedral lat-tice structure (9.01,6.54,5.41and 4.50Å).This crys-tal structure and the corresponding low activity were consistent with that of the catalyst mentioned above.The catalyst prepared with Zn(Ac)2also exhibited a highly crystalline pattern which can not also be identi-fied using the standard JCPDS.Other kinds of transi-tion metal salts,such as CoCl 2,NiCl 2and CdCl 2had been used instead of ZnCl 2to prepare catalysts.How-ever,such catalysts exhibited a rather low activity.The above results indicate thatZnCl 2was by far the most suitable candidate as cation source and as an impor-tant promoter in DMC catalyst,and the morphology of DMC catalysts was largely dependent on the kind of M 1X w employed.3.5.Characteristic features of DMC-catalyzed polymerization of propylene-oxideFig.3shows a plot of propylene oxide consump-tion versus reaction time.The polymerization reac-tion exhibited a very short induction period (severalminutes).This is in contrast to the long induction pe-riod (several hours)of the typical DMC catalyst as reported by some patents [9,10].It can be seen that the reaction rate decreased gradually with incremental monomer addition and finally,further consumption of the monomer was limited,which means the presenceFig.3.Polymerization of propylene oxide with different amounts of catalyst (40g PPG-400as initiator,at 120±2◦C,with semi-continuous addition of monomer):()0.038g;(᭹)0.024g;(᭡)0.012g.Y.J.Huang et al./Applied Catalysis A:General240(2003)263–271269 Table4Polymerization of PO catalyzed with DMC catalyst aRun P/I(w/w)b M n(VPO)M w/M n M n(calculated)c Unsaturation(meq/g)1 4.31985 1.2321200.0082 5.52329 1.2526000.01137.73326 1.2834800.012 4176522 1.4872000.01257.53515 2.8134000.009616.26258 3.4768800.011a PPG-400(40g)as initiator,run1–4,polymerization with semi-continuous addition of monomer,at120±3◦C,catalyst 1=0.05g;run5and6polymerization with one-step addition of monomer:at100±3◦C,catalyst concentration=55mg/kg.b P/I:weight ratio offinished polymer to initiator.c M n(calculated)=(P/I)×M n(initial)=(P/I)×400.of a termination process.Both polymer yield and re-action rate decreased with clearly decrease of catalyst amounts.Table4shows the experimental results of poly-merization of propylene oxide.All polymers had a low level of unsaturation without respect to molecular weight,such levels demonstrated the relative absence of side reactions such as transfer reaction to monomer. Interestingly,polymers prepared by semi-continuous addition of monomer exhibited a narrower MWD than that prepared with prepared by one-step addition of monomer.Furthermore,molecular weight of polymers formed was consistent with the calculation based on monomer to initiator ratio(M/I),not with that based on the monomer to catalyst ratio(M/C),which in-dicates that every molecule of the initiator partici-pated in initiation and propagation steps.Considering the fact that the moles of initiator exceeded greatly the moles of catalyst,a rapid exchange must exist be-tween dormant species and active species.From the practical viewpoint,this control behavior of molec-ular weight is highly desirable.In our forthcoming paper[12],partial living polymerization characteris-tics of DMC system will be described in -pared with DMC catalyst based on Zn3[Fe(CN)6]2 [13],DMC catalyst prepared in this work(based on Zn3[Co(CN)6]2)provides high activity.In the case of polymerization with semi-continuous addition of monomer,catalytic activity up to26kg polymer/g cat-alyst was reached.This catalytic activity is approxi-mately100times that of aluminum porphyrins[2].In addition,the GPC traces of all polymers synthesized in this work exhibited a single and symmetrical peak, which is evidence that only one type of active centre existed in this system.It is well known that polyether oligomer produced with ZnCl2showed very low de-gree of polymerization.As mentioned in the previ-ous section,neat Zn3[Co(CN)6]2alone was also active for ROP of propylene oxide and polymers of medium molecular weight could be generated.Thus it can be concluded that the active site was on Zn3[Co(CN)6]2. However,the precise structure of the active species can’t be determined in situ because the catalyst level was very low.Fig.4shows the13C NMR spectrum of product prepared by DMC catalyst.The methyl,methylene, and methine carbon resonances can be easily assigned: a-CH(74.6–75.5ppm),b-CH2(72.6–73.5ppm),c-CH3 (16.9–17.2ppm).The simplicity of signal c indi-cates that the polymer chain consists exclusively of head-to-tail sequence,as typically observed for an-ionic polymerization of propylene oxide.Two signals of methylene carbon observed at72.0and73.1ppm are ascribed to meso(m)and racemic(r)diads re-spectively,and three signals of methine observed at 75.2,75.0and74.8ppm are ascribed to isotactic(i), heterotactic(h)and syndiotactic(s)triads,respec-tively.The similar intensity of there three signals(a) indicates that the polymer has a random distribution of the configurational sequences[14].In fact,the mi-crostructure of the product was similar to that of the one synthesized by base-catalyzed polymerization, and polyether polyols with this kind of microstruc-ture are very suitable candidates as precursors in the manufacture of polyurethanes.In conclusion,the preparation route and condition such as temperature,the kind and the concentration of starting material have significant effect on the mor-phology of DMC complexes.The results of the present work demonstrate that catalytic activity values were strongly dependent on composition as well as mor-phology of DMC catalyst and that the DMC catalyst is non-stoichiometric.The choices of suitable prepa-ration condition and starting materials are very im-portant to obtain DMC catalysts with high activity.So far,the substantially amorphous DMC catalyst made from ZnCl2and t-BuOH have the highest activity.The DMC catalysts are very active for polymerization of propylene oxide;high-quality polyether polyols thatY.J.Huang et al./Applied Catalysis A:General240(2003)263–271271have very low unsaturation,and narrow molecu-lar weight distributions can be prepared under mild conditions.References[1]C.P.Smith,J.W.Reisch,J.M.O’Connor,J.Elastoplast.24(1992)305.[2]M.Akatsuka,T.Aida,S.Inoue,Macromolecules27(1994)2820.[3]R.O.Colough,K.Wilknson,J.Polym.Sci.C4(1963)311.[4]N.Oquni,S.Watanabe,M.Maki,Macromolecules6(1973)195.[5]K.Miura,T.Kitayama,K.Hatada,T.Nakata,Polym.J.25(1993)655.[6]B.Wu,J.Harlan,R.W.Lenz,A.R.Barron,Macromolecules30(1997)316.[7]Y.S.Zheng,L.Q.Ying,Z.Q.Shen,Polymer41(2000)1641.[8]Le-Khac.Bi.EP0755716(1997).[9]K.G.McDaniel,M.J.Perry,J.E.Hayes,WO9914258(1999).[10]J.Hofmann,P.Gupta,H.Pielartzic,EP0892002(1999).[11]R.M.Wehmeyer,WO0104177(2001).[12]Y.J.Huang,G.R.Qi,Y.H.Wang,J.Polym.Sci.Part A:Polym.Chem.40(2002)1142.[13]Y.J.Huang,G.R.Qi,J.W.Qian,L.X.Feng,Acta PolymericaSinica2(2001)210.[14]F.C.Schilling,A.E.Tonelli,Macromolecules19(1986)1337.。
