盐酸林可霉素检验标准
注射用盐酸克林霉素质量标准

注射用盐酸克林霉素质量标准注射用盐酸克林霉素质量标准:全面评估与深度探讨1. 前言注射用盐酸克林霉素作为一种重要的抗生素药物,其质量标准尤为重要。
本文将对注射用盐酸克林霉素的质量标准进行全面评估,并深入探讨其相关主题。
2. 了解盐酸克林霉素盐酸克林霉素是一种广谱抗生素,对革兰氏阳性菌和某些革兰氏阴性菌均具有抑菌作用。
其在临床上被广泛应用,因此药品质量标准尤为关键。
3. 注射用盐酸克林霉素的质量标准(1)产品外观:注射用盐酸克林霉素应呈无色透明液体,无悬浮物、沉淀和异物。
(2)溶解度:注射用盐酸克林霉素在水中的溶解度应符合国家药典规定。
(3)含量测定:药品中盐酸克林霉素的含量应符合国家药典规定的标准。
(4)微生物限度:注射用盐酸克林霉素中细菌、真菌等微生物的限度应符合国家药典规定。
……(依次逐条深入评估)4. 盐酸克林霉素质量标准的重要性盐酸克林霉素作为临床上常用的抗生素药物,其质量标准关乎患者的用药效果和安全性。
高质量的盐酸克林霉素产品至关重要。
5. 个人观点和理解在我看来,注射用盐酸克林霉素的质量标准不仅仅是一项法定要求,更是对患者生命健康的责任。
只有严格执行质量标准,才能保证药品的有效性和安全性。
6. 结语通过本文对注射用盐酸克林霉素质量标准的全面评估与深度探讨,相信读者能够对这一主题有更深入的理解。
我们也应该意识到,药品质量是医疗安全的基石,应该倍加重视。
(以上内容仅供参考,具体文章内容还需根据实际情况具体撰写。
)盐酸克林霉素作为一种重要的抗生素药物,在临床上被广泛应用于治疗感染性疾病。
其质量标准尤为重要,直接关系到患者的用药效果和安全性。
本文将从不同角度对注射用盐酸克林霉素的质量标准进行全面评估,并深入探讨相关主题,旨在引起人们对药品质量的重视,并提高对药品质量标准的认识。
我们需要了解盐酸克林霉素的相关知识。
盐酸克林霉素是一种广谱抗生素,具有对革兰氏阳性菌和某些革兰氏阴性菌的抑菌作用。
其广泛的抗菌活性使得它成为治疗临床感染性疾病的重要药物之一。
019盐酸林可霉素检验记录
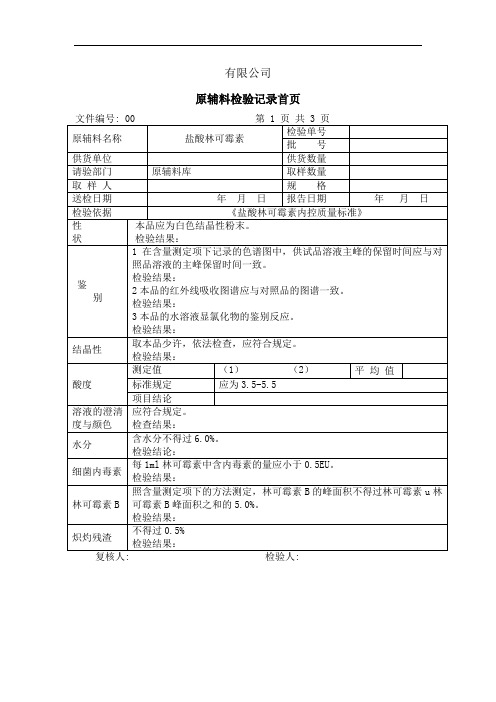
原辅料检验记录首页
文件编号: 00第 1 页 共 3 页
原辅料名称
盐酸林可霉素
检验单号
批 号
供货单位
供货数量
请验部门
原辅料库
取样数量
取 样 人
规 格
送检日期
年 月 日
报告日期
年 月 日
检验依据
《盐酸林可霉素内控质量标准》
性
状
本品应为白色结晶性粉末。
检验结果:
鉴
别
1在含量测定项下记录的色谱图中,供试品溶液主峰的保留时间应与对照品溶液的主峰保留时间一致。
对照品制备:另取林可霉素对照品,同上法制得 。
样品编号
内 容
1
2
样品取量Vs(g)
样品峰面积As
对照品取量Wc(g)
对照品峰面积Ac
计算公式:
As×Wc×N N(对照品含量)=
X = ×100%
Ac×Vs×S S(样品标示量)=
按无水物计,样品含量X(%)
含 量 平 均 值(%)
A-B
相对偏差 ×100%主要Fra bibliotek分析仪器
名 称
型 号
精度
柱温
测定用波长
编 号
分 析 天 平
BT25S
0.00001
____
_______
MHT-007
高效液相色谱仪
UV200II
----
30℃
254nm
MHT-008
色谱柱
C-18
流动相:0.05mol/L硼砂溶液-甲醇(4:6)
样品制备:取本品约50mg,精密称定,置25ml量瓶中,加流动相溶解并稀释至刻度,摇匀,精密量取10ul,注入液相色谱仪 。
盐酸大观霉素盐酸林可霉素可溶性粉质量标准

盐酸大观霉素盐酸林可霉素可溶性粉Y ansuan Daguanmeisu Y ansuan Linkemeisu Kerongxingfen Spectinomycin Hydrochloride and Lincomycin Hydrochloride Soluble Powder 本品含大观霉素(C14H24N2O7)、林可霉素(C18H34N2O6S)均应为标示量的90.0%~110.0%。
【性状】本品为白色或类白色粉末。
【鉴别】取本品加水溶解并稀释成每1ml中约含大观霉素10mg与林可霉素5mg的溶液,作为供试品溶液;另取大观霉素标准品与林可霉素对照品,分别加水溶解并稀释成每1ml中约含大观霉素10mg、林可霉素5mg的溶液,作为标准品溶液与对照品溶液。
吸取上述三种溶液各5μl,照盐酸大观霉素可溶性粉鉴别项试验,供试品溶液所显主斑点的位置和颜色应与大观霉素标准品溶液、林可霉素对照品溶液的主斑点相同。
【检查】水分取本品,照水分测定法(附录0832第一法A)测定,含水分不得过15.0%。
林可霉素B照林可霉素含量测定项下的方法测定,林可霉素B的峰面积不得过林可霉素与林可霉素B峰面积和的5.0%。
其他应符合可溶性粉剂项下有关的各项规定(附录0113)。
【含量测定】大观霉素精密称取本品适量,照盐酸大观霉素项下的方法测定,即得。
林可霉素精密称取本品适量,加流动相溶解并定量稀释制成每1ml中约含林可霉素2mg的溶液,照盐酸林可霉素项下的方法测定,即得。
【作用与用途】抗生素类药。
用于革兰氏阴性细菌、革兰氏阳性细菌及支原体感染。
【用法与用量】以大观霉素计。
混饮:每1L水,5~7日龄雏鸡0.2~0.32g,连用3~5日。
【注意事项】仅用于5~7日龄雏鸡。
【规格】(1)5g;大观霉素2g(200万单位)与林可霉素1g(按C18H34N2O6S 计算)(2)50g;大观霉素20g(2000万单位)与林可霉素10g(按C18H34N2O6S 计算)(3)100g;大观霉素10g(1000万单位)与林可霉素5g(按C18H34N2O6S 计算)(4)100g;大观霉素40g(4000万单位)与林可霉素20g(按C18H34N2O6S 计算)【贮藏】密闭,在干燥处保存。
盐酸林可霉素检验标准

盐酸林可霉素检验标准【通用名称】盐酸林可霉素【其他名称】盐酸林可霉素拼音名:Yansuan Linkemeisu 英文名:Lincomycin Hydrochloride 书页号:2000年版二部-629 C18H34N2O6S.HCl.H2O 461.02 本品为6-(1- 甲基-反-4- 丙基-L-2- 吡咯烷甲酰氨基)-1-硫代-6,8-二脱氧-D- 赤式-a-D-半乳辛吡喃糖苷盐酸盐一水合物。
按无水物计算,含林可霉素(C18H34N2O6S) 不得少于82.5%。
【性状】本品为白色结晶性粉末;有微臭或特殊臭;味苦。
本品在水或甲醇中易溶,在乙醇中略溶。
【鉴别】(1) 本品的红外光吸收图谱应与对照的图谱(光谱集833图)一致。
(2) 取本品与林可霉素对照品,分别加流动相制成每1ml 中约含2mg 的溶液,照含量测定项下的高效液相色谱条件进行试验,供试品主峰的保留时间应与对照品主峰的保留时间一致。
(3) 本品的水溶液显氯化物的鉴别反应(附录Ⅲ)。
【检查】结晶性取本品少许,依法检查(附录ⅨD),应符合规定。
酸度取本品,加水制成每1ml 中含0.1g的溶液,依法测定(附录ⅥH),pH值应为 3.0~5.5。
溶液的澄清度与颜色取本品5 份,各2g,分别加水5ml使溶解,溶液应澄清;如显浑浊,与1 号浊度标准液(附录ⅨB)比较,均不得更浓;如显色,与黄色或黄绿色1号标准比色液(附录ⅨA)比较,均不得更深。
水分取本品,照水分测定法(附录ⅧM第一法A)测定,含水分不得过6.0%。
炽灼残渣不得过0.5%(附录ⅧN)。
异常毒性取本品,加氯化钠注射液制成每1ml 中含5mg的溶液,依法检查(附录ⅪC),按静脉注射法给药,应符合规定(供注射用)。
细菌内毒素取本品,依法检查(附录ⅪE),每1mg林可霉素中含内毒素的量应小于0.5EU (供注射用)。
降压物质取本品,依法检查(附录ⅪG),剂量按猫体重每1kg 注射5mg,应符合规定(供注射用)。
盐酸林可霉素检验记录

结果:
检验人:复核人:
检查
(4)水分
仪器名称与型号:ZSD-2型自动水分滴定仪仪器编号:0310-04-103
测定温度:
费休氏试液的标定:
F……………为每1ml费休氏试液相当于水的重量(mg)
W…………为称取重蒸馏水的重量(mg)
A……………为滴定所消耗费休氏试液的容积(ml)
B……………为空白所消耗费休氏试液的容积(ml)
W1=mg W2=mg W3=mg
A1=ml A2=ml A3=ml
B=ml
计算得:F1=F2=F3=
F平均值为相对平均偏差=
供试品测定:供试品中水分含量X=
W…………为称取供试品的重量(mg)
计算:含量X
…………为称取对照品的重量(g) …………为对照品峰面积
…………为称取供试品的重量(g) …………为供试品峰面积 ……………为对照品含量
MR=
Mx1=Mx2=
AR1=AR2=
AX1=AX2=
X1=X2=
检验人:复核人:
含量测定
平均值:
含量(按干品计)为
结果:
检验人:复核人:
(2)测定:取本品约50mg,精密称定,置25ml量瓶中,用适量流动相溶解并稀释至刻度,摇匀,精密量取10μl注入液相色谱仪,记录色谱图;另取盐酸林可霉素对照品适量,同法测定。按外标法以峰面积计算供试品中C18H34N2O6S的含量。
(3)含量
对照品来源:对照品批号:
对照品规格:对照品含量:
对照品溶液配制:精密称取盐酸林可霉素对照品,置于25ml量瓶中,用适量流动相溶解并稀释至刻度。
盐酸林可霉素含量分析

含量测定:
流动相——加13.5ml磷酸到1000ml水中,用氨水调节pH到6.0.此溶液-乙腈-甲醇(780:150:150)。
(色谱适用性参照色谱分析621)
标准溶液制备——精确称取一定量的USP标准品溶于流动相,使其浓度为1.2mg/ml,如有必要用超声波溶解。
分析准备——精密称量约12mg盐酸林可霉素至10ml流动相,用振荡器震荡5min使其溶解,如有必要采用超声波溶解。
色谱系统——检测波长210nm,色谱柱4.6mm*25cm,5-μm,L7填料。
柱温保持在46℃,流速约为1ml/min,主峰拖尾因子不大于1.3,理论塔板数不低于4000.连续进样的系统重复性的相对标准偏差不大于2.0%。
精确量取一定体积的供试液,相当于林可霉素600mg到50ml容量瓶中,用流动相稀释至刻度,混匀。
量取两毫升至25ml容量瓶,用流动相稀释至刻度并混匀。
按如下公式计算每毫升供试品中林可霉素的含量,单位mg/ml
0.625(CP / V)(rU / rS)
C是对照品的浓度——mg/mL
P是USP盐酸林可霉素标准品的效率——ug/mg
V是供试液的体积——mL.
rU和rS分别是从供试品和标准品分别获得的主峰面积。
盐酸林可霉素EP7.3质量标准

3688Lidocaine / Official Monographs USP 35volume, in mL, of the Assay preparation; W is the weight, inmg, of the Cream taken to prepare the Assay preparation; L is Lincomycin Hydrochloridethe individual label claim, in percent, for either lidocaine orprilocaine; and 220.31 and 256.77 are the molecular weights ofprilocaine and prilocaine hydrochloride, respectively (these areused only for calculating the percentage of prilocaine in theCream).LimeC18H34N2O6S·HCl·H2O461.02CaO56.08D-erythro-α-D-galacto-Octopyranoside, methyl 6,8-dideoxy-6-Calcium oxide [1305-78-8].[[(1-methyl-4-propyl-2-pyrrolidinyl)carbonyl]amino]-1-thio-,monohydrochloride, monohydrate, (2S-trans)-.» Lime, when freshly ignited to constant weight,Methyl 6,8-dideoxy-6-(1-methyl-trans-4-propyl-L-2-pyrrolidine-contains not less than 95.0 percent of CaO.carboxamido)-1-thio-D-erythro-α-D-galacto-octopyranosidemonohydrochloride monohydrate [7179-49-9]. Packaging and storage—Preserve in tight containers.Anhydrous 443.01 [859-18-7].Identification—» Lincomycin Hydrochloride has a potency equiv-A: Moisten it with water: heat is generated, and a whitealent to not less than 790 µg of lincomycin powder is obtained (calcium hydroxide or slaked lime). Mix thepowder with 3 or 4 times its weight of water: a smooth magma(C18H34N2O6S) per mg.of lime forms that is alkaline to litmus.Packaging and storage—Preserve in tight containers.B: Slake 1g with 20 mL of water, and add 6N acetic acidLabeling—Where it is intended for use in preparing injectable until the lime is dissolved: the resulting solution responds to thedosage forms, the label states that it is sterile or must be sub-test for Calcium 〈191〉.jected to further processing during the preparation of injectable Loss on ignition 〈733〉—Ignite a portion to constant weight indosage forms.a tared platinum crucible at 1100±50°: it loses not more than10.0% of its P Reference standards 〈11〉—Insoluble substances—Slake 5.0 g, then mix with 100 mL of USP Endotoxin RSwater, followed by hydrochloric acid, dropwise, with agitation,USP Lincomycin Hydrochloride RSuntil solution takes place: the resulting solution after boiling Identification, Infrared Absorption 〈197M〉.and cooling is acid, and when filtered through a tared crucible,Specific rotation 〈781S〉: between +135° and +150°. washed with water until free of chlorides, and dried at 105° for1 hour yields not more than 50 mg of insoluble substances Test solution: 20 mg per mL, in water.(1.0%).Crystallinity 〈695〉: meets the requirements. Carbonate—Slake 1g, mix with 50 mL of water, and decant pH 〈791〉: between 3.0 and 5.5, in a solution (1 in 10).the greater portion of the milky liquid: the addition of an ex-Water, Method I 〈921〉: between 3.0% and 6.0%.cess of 3N hydrochloric acid to the residue does not causeLimit of lincomycin B—Use the chromatogram obtained more than a slight effervescence.from the Assay preparation in the Assay: the area of the linco-Magnesium and alkali salts—Dissolve 500 mg in 30 mL of mycin B peak is not greater than 5.0% of the sum of the areas water and 15 mL of 3N hydrochloric acid. Neutralize the solu-of the lincomycin B peak and the lincomycin peak.tion with 6N ammonium hydroxide, heat to boiling, and addOther requirements—Where the label states that Lincomycin ammonium oxalate TS to precipitate the calcium completely.Hydrochloride is sterile, it meets the requirements for Sterility Heat the mixture on a steam bath for 1 hour, cool, dilute withand Bacterial endotoxins under Lincomycin Injection. Where the water to 100 mL, mix, and filter. To 50 mL of the filtrate addlabel states that Lincomycin Hydrochloride must be subjected to 0.5 mL of sulfuric acid, evaporate to dryness, and ignite in afurther processing during the preparation of injectable dosage tared platinum crucible to constant weight. The weight of theforms, it meets the requirements for Bacterial endotoxins under residue does not exceed 9 mg.Lincomycin Injection.Assay—Ignite about 1g of Lime in a muffle furnace to con-Assay—stant weight, cool, weigh accurately, and dissolve in 20 mL ofMobile phase—Add 13.5 mL of phosphoric acid to 1000 mL 3N hydrochloric acid. Cool the solution, transfer to a 500-mLof water, and adjust with ammonium hydroxide to a pH of 6.0. volumetric flask with the aid of water, dilute with water to vol-Prepare a filtered and degassed mixture of this solution, aceto-ume, and mix. Transfer 50.0 mL to a suitable container, addnitrile, and methanol (780:150:150). Make adjustments if nec-100 mL of water, 15 mL of 1N sodium hydroxide, and 300 mgessary (see System Suitability under Chromatography 〈621〉).of hydroxy naphthol blue, and titrate with 0.05 M edetate diso-dium VS until the solution is deep blue in color. Each mL of Standard preparation—Dissolve an accurately weighed quan-0.05 M edetate disodium is equivalent to 2.804 mg of CaO.tity of USP Lincomycin Hydrochloride RS in Mobile phase to ob-tain a solution having a known concentration of about 1.2 mgper mL, using sonication if necessary to effect solution.Assay preparation—To about 12 mg of Lincomycin Hydro-chloride, accurately weighed, add 10.0 mL of Mobile phase.Shake by mechanical means for 5 minutes, and sonicate if nec-essary to effect solution.Chromatographic system (see Chromatography 〈621〉)—Theliquid chromatograph is equipped with a 210-nm detector anda 4.6-mm × 25-cm column that contains 5-µm packing L7 andis maintained at a temperature of 46°. The flow rate is about 1mL per minute. Chromatograph the Standard preparation, andrecord the responses as directed for Procedure: the tailing factorfor the main lincomycin peak is not more than 1.3; the columnUSP 35Official Monographs / Lincomycin 3689efficiency determined from the main lincomycin peak is not less bile phase , and shake by mechanical means for 5 minutes. Use than 4000 theoretical plates; and the relative standard deviation the solution thus obtained as the Assay preparation.for replicate injections is not more than 2.0%.Procedure—Proceed as directed for Procedure in the Assay Procedure—Separately inject equal volumes (about 20 µL) of under Lincomycin Hydrochloride. Calculate the quantity, in mg,the Standard preparation and the Assay preparation into the of lincomycin (C 18H 34N 2O 6S) in the portion of Capsule contents chromatograph, record the chromatograms, and measure the taken by the formula:areas for the major peaks. The relative retention times are about (CP /20)(r U /r S )0.5 for lincomycin B and 1.0 for lincomycin. Calculate thequantity, in µg, of lincomycin (C 18H 34N 2O 6S) in each mg of the in which the terms are as defined therein.Lincomycin Hydrochloride taken by the formula:10(CP /W )(r U /r S )in which C is the concentration, in mg per mL, of USP Linco-Lincomycin Injectionmycin Hydrochloride RS in the Standard preparation; P is the designated potency, in µg of lincomycin per mg, of USP Linco-mycin Hydrochloride RS; W is the weight, in mg, of the portion » Lincomycin Injection contains an amount of of Lincomycin Hydrochloride taken to prepare the Assay prepa-Lincomycin Hydrochloride in Water for Injection ration; and r U and r S are the lincomycin peak responses obtained equivalent to not less than 90.0 percent and not from the Assay preparation and the Standard preparation ,more than 120.0 percent of the labeled amount respectively.of lincomycin (C 18H 34N 2 O 6S). It contains benzyl alcohol as a preservative.Packaging and storage—Preserve in single-dose or in multi-Lincomycin Hydrochloride Capsulesple-dose containers, preferably of Type I glass.USP Reference standards 〈11〉—» Lincomycin Hydrochloride Capsules contain an USP Endotoxin RSamount of C 18H 34N 2O 6S ·HCl ·H 2O equivalent to USP Lincomycin Hydrochloride RSnot less than 90.0 percent and not more than Bacterial endotoxins 〈85〉—It contains not more than 0.5120.0 percent of the labeled amount of linco-USP Endotoxin Unit per mg of lincomycin.mycin (C 18H 34N 2O 6S).Sterility 〈71〉—It meets the requirements when tested as di-rected for Membrane Filtration under Test for Sterility of the Prod-Packaging and storage—Preserve in tight containers.uct to be Examined .pH 〈791〉: between 3.0 and 5.5.USP Reference standards 〈11〉—USP Lincomycin Hydrochloride RS Particulate matter 〈788〉: meets the requirements for small-volume injections.Dissolution 〈711〉—Other requirements—It meets the requirements under Injec-Medium: water; 500 mL.tions 〈1〉.Apparatus 1: 100 rpm.Assay—Time: 45 minutes.Mobile phase, Standard preparation, and Chromatographic sys-Procedure—Filter a portion of about 20 mL of the solution tem—Proceed as directed in the Assay under Lincomycin under test. Transfer about 5 mL of the eluant into a small test Hydrochloride .tube, and add 250 µL of 0.01 M sodium sulfate internal stan-Assay preparation—Transfer an accurately measured volume dard solution. Evaporate until dry using a vacuum centrifuge.of Injection, equivalent to about 600 mg of lincomycin, to a Add 10.0 µL of water to the precipitate and place on a vortex 50-mL volumetric flask, dilute with Mobile phase to volume, and mixer until all solid material is dissolved. Transfer this solution to mix. Transfer 2.0 mL of this solution to a 25-mL volumetric a capillary tube, place it in a Raman spectrometer, and obtain flask, dilute with Mobile phase to volume, and mix.the Raman spectrum using suitable instrumental conditions (see Spectrophotometry and Light-scattering 〈851〉). Integrate the Procedure—Proceed as directed for Procedure in the Assay Raman intensity, applying baseline corrections, between 660under Lincomycin Hydrochloride . Calculate the quantity, in mg,cm −1 and 720 cm −1. Divide this result by the integrated intensity of lincomycin (C 18H 34N 2O 6S) in each mL of the Injection taken between 966 cm −1 and 994 cm −1. Determine the amount of by the formula:C 18H 34N 2O 6S dissolved in comparison with an aqueous Standard solution having a known concentration of USP Lincomycin Hy-0.625(CP /V )(r U /r S )drochloride RS.in which V is the volume, in mL, of Injection taken, and the Tolerances—Not less than 75% (Q) of the labeled amount of other terms are as defined therein.C 18H 34N 2O 6S is dissolved in 45 minutes.Uniformity of dosage units 〈905〉: meet the requirements.Water, Method I 〈921〉: not more than 7.0%.Assay—Lincomycin Hydrochloride Soluble Mobile phase, Standard preparation, and Chromatographic sys-tem—Proceed as directed in the Assay under Lincomycin PowderHydrochloride.Assay preparation—Remove, as completely as possible, the » Lincomycin Hydrochloride Soluble Powder con-contents of not less than 10 Capsules, taking care to prevent tains an amount of Lincomycin Hydrochloride capsule shell fragments from being combined with the capsule equivalent to not less than 90.0 percent and not contents and to remove any shell fragments from the contents.more than 110.0 percent of the labeled amount Weigh and mix the combined contents, and transfer an accu-of lincomycin (C 18H 34N 2O 6S).rately weighed portion of the powder, equivalent to about 50mg of lincomycin, to a suitable container. Add 50.0 mL of Mo-。
盐酸林可霉素注射液(成品)检验操作规程

GMP管理文件一、目的:为规定盐酸林可霉素注射液生产过程中的质量控制和检验操作要求,特制定此操作规程。
二、适用范围:适用于盐酸林可霉素注射液成品的检验。
三、责任者:生产部经理、检验员、生产人员四、正文:质量标准:见盐酸林可霉素注射液(成品)内控质量标准操作内容:【性状】本品为无色的澄明液体。
【鉴别】(1)在含量测定项下记录的色谱图中,供试品溶液主峰的保留时间应与对照品溶液主峰的保留时间一致。
(2)本品显氯化物的鉴别反应。
【检查】 PH值取本品,加水制成每1ml中含0.1g的溶液,依法测定,PH值应为3.1~5.0。
颜色本品应无色;如显色,与黄色或绿色2号标准比色液比较,不得更深。
无菌取本品,转移至不少于500ml的0.9%无菌氯化钠溶液中,用薄膜过滤法处理后,依法检查,应符合规定。
细菌内毒素照盐酸林可霉素项下的方法检查,应符合规定。
林可霉素B 取本品适量,加流动相定量稀释成每1ml中含2mg的溶液,照盐酸林可霉素项下的方法检查,林可霉素B的峰面积不得过林可霉素与林可霉素B峰面积之和的5.0%。
其他应符合注射剂项下有关的各项规定。
【含量测定】照高效液相色谱法测定。
色谱条件与系统适用性试验用十八烷基硅烷键合硅胶为填充剂;以0.05mol/l硼砂溶液(用85%磷酸溶液调节PH值至5.0)-甲醇-乙腈(6.:36:4)为流动相;检测波长为214nm。
理论板数按林可霉素峰计算不低于1600。
测定法精密量取本品适量,用流动相稀释成每1ml中含林可霉素2mg的溶液,摇匀,精密量取10ul,注入液相色谱仪,记录色谱图;另取林可霉素对照品适量,同法测定,按外标法以峰面积计算供试品中C18H34N2O6S的含量。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
盐酸林可霉素检验标准
【通用名称】盐酸林可霉素
【其他名称】盐酸林可霉素拼音名:Yansuan Linkemeisu 英文名:Lincomycin Hydrochloride 书页号:2000年版二部-629 C18H34N2O6S.HCl.H2O 461.02 本品为6-(1- 甲基-反-4- 丙基-L-2- 吡咯烷甲酰氨基)-1-硫代-6,8-二脱氧-D- 赤式-a-D-半乳辛吡喃糖苷盐酸盐一水合物。
按无水物计算,含林可霉素(C18H34N2O6S) 不得少于82.5%。
【性状】本品为白色结晶性粉末;有微臭或特殊臭;味苦。
本品在水或甲醇中易溶,在乙醇中略溶。
【鉴别】(1) 本品的红外光吸收图谱应与对照的图谱(光谱集833图)一致。
(2) 取本品与林可霉素对照品,分别加流动相制成每1ml 中约含2mg 的溶液,照含量测定项下的高效液相色谱条件进行试验,供试品主峰的保留时间应与对照品主峰的保留时间一致。
(3) 本品的水溶液显氯化物的鉴别反应(附录Ⅲ)。
【检查】结晶性取本品少许,依法检查(附录ⅨD),应符合规定。
酸度取本品,加水制成每1ml 中含0.1g的溶液,依法测定(附录ⅥH),pH值应为 3.0~5.5。
溶液的澄清度与颜色取本品5 份,各2g,分别加水5ml使溶解,溶液应澄清;如显浑浊,与1 号浊度标准液(附录ⅨB)比较,均不得更浓;如显色,与黄色或黄绿色1号标准比色液(附录ⅨA)比较,均不得更深。
水分取本品,照水分测定法(附录ⅧM第一法A)测定,含水分不得过6.0%。
炽灼残渣不得过0.5%(附录ⅧN)。
异常毒性取本品,加氯化钠注射液制成每1ml 中含5mg的溶液,依法检查(附录ⅪC),按静脉注射法给药,应符合规定(供注射用)。
细菌内毒素取本品,依法检查(附录ⅪE),每1mg林可霉素中含内毒素的量应小于0.5EU (供注射用)。
降压物质取本品,依法检查(附录ⅪG),剂量按猫体重每1kg 注射5mg,应符合规定(供注射用)。
林可霉素B照含量测定项下的高效液相色谱法条件测定,林可霉素B的峰面积不得过林可霉素与林可霉素B峰面积之和的5.0%。
【含量测定】照高效液相色谱法(附录ⅤD)测定。
色谱条件与系统适用性试验用十八烷基硅烷键合硅胶为填充剂;0.05mol/L硼砂溶液(用85%磷酸溶液调节pH值至6.0 )-甲醇(4:6) 为流动相;检测波长为214nm 。
理论板数按林可霉素峰计算应不低于1500,林可霉素峰与林可霉素B峰的分离度应不小于2.6。
林可霉素B峰为林可霉素峰相对保留时间的0.5~0.7。
测定法取本品约20mg,精密称定,置25ml量瓶中,用适量流动相溶解并稀释至刻度,摇匀,取10μl 注入液相色谱仪,记录色谱图;另取盐酸林可霉素对照品适
量,同法测定。
按外标法以峰面积计算供试品中C18H34N2O6S 的含量。
【类别】抗生素类药。
【贮藏】密封保存。
【制剂】(1) 盐酸林可霉素片
(2) 盐酸林可霉素注射液
(3) 盐酸林可霉素胶囊。
