标准电极电势表(酸碱)
电极电势表
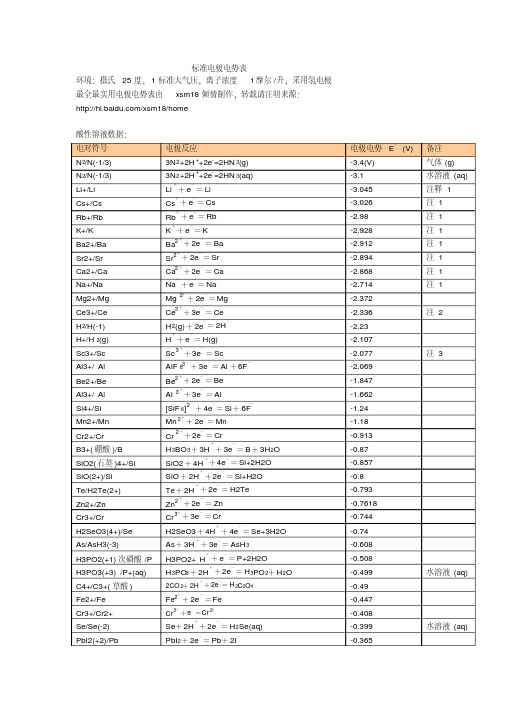
备注 气体 (g) 水溶液 (aq) 注释 1 注 1 注 1 注 1 注 1 注 1 注 1 注 1
+2+
+
+
-
+ 2e = Mg
- -
-
Ce3 + 3e = Ce H 2(g) + 2e = 2H H + e = H(g) Sc
3+
+ -
注 2
-2.23 -2.107 -2.077 注 3
- + - - - - + - + - -
-
0.2476 0.268 0.308 0.337 0.354 0.3572
HAsO 2 .H2O
SO42 + 8H + 6e = S+4H 2 O
2 Ag 2 CrO 4+ 2e = 2Ag + CrO 4
+ - -
0.447 0.449 0.4647 0.521 0.5355
2+
+ 2e = Cr
+ - + -
H 3BO 3+ 3H + 3e = B + 3H 2O SiO2 + 4H + 4e = Si+2H2O SiO + 2H + 2e = Si+H2O Te + 2H + 2e = H2Te Zn 2 + 2e = Zn Cr 3 + 3e = Cr H2SeO3 + 4H + 4e = Se+3H2O As + 3H + 3e = AsH 3 H3PO2+ H + e = P+2H2O H 3PO3 + 2H + 2e = H 3PO 2+ H 2O
3 Fe + 3e = Fe 2S Ag 2 S+ 2H + 2e = 2Ag +H
+ - + - - - + -
+
-
-0.12 -0.063 -0.0405 -0.037 -0.0366 0.00 气体 (g)
标准电极电势表
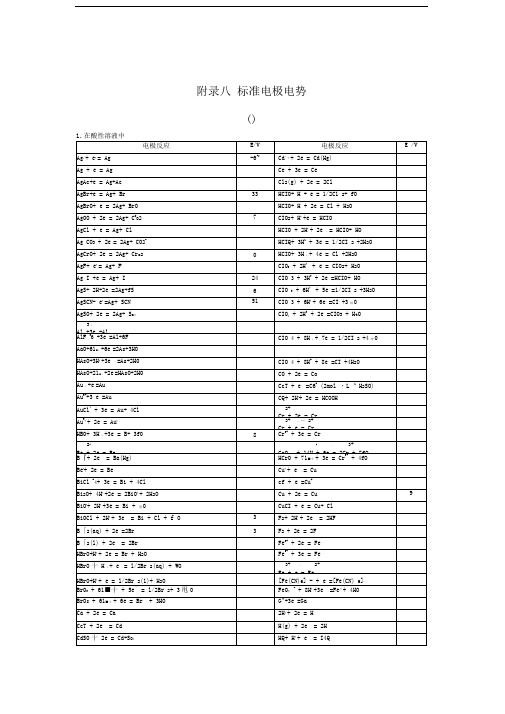
3十—
Al +3e =Al
AlF36+3e =Al+6F
CIO4+8H十+7e=1/2CI2+4冲0
AaO+61■十+6e—=2As+3HO
HAsO+3H++3e—=As+2H0
CIO4+8H++8e—=CI—+4H2O
HAsO+21■十+2e—=HAsO+2HO
CO+2e=Co
Au十+e—=Au
CcT+e一=C6+(2mol・L—1H2SO)
Au3++3 e—=Au
CQ+2H++2e—=HCOOH
AuCl4+3e=Au+ 4Cl
2+—
Cr+2e=Cr
Au3++2e—=Au+
3+—―2+
Cr+e=Cr
HBO+3H十+3e—=B+ 3f0
8
Cr3++3e—=Cr
HCIO+ H+2e=Cl+H2O
AgOO+2e=2Ag+C2o2
7
CIO2+H++e=HCIO
AgCl+e=Ag+Cl
HCIO+2H++2e一=HCIO+ HO
Ag CO3+2e「=2Ag+ CO2-
HCIQ+3H1+3e=1/2CI2+2H2O
标准电极电势表

S2O +2e-=2SO
Mn +e-=MO
Mn +4H++3e-=MnO2+2H2O
2H2SO3+H++2e-=H2SO4-+2H20
Mn +8H++5e-=Mn2++4H2O
H2SO3+4H++4e-=S+3H2O
MO3++3e-=MO
电极反应
E/V
Te4++4e-=Te
V3++e-=V2+
TeO2+4H++4e-=Te+2H2O
VO2++2H++e-=V3++H2O
Te +8H++7e-=Te+4H2O
VO +2H++e-=VO2++H2O
H6TeO6+2H++2e-=TeO2+4 H2O
V(OH) +2H++e-=VO2++3H2O
3
F2+2e-=2F-
Br2(l)+2e-=2Br-
Fe2++2e-=Fe
HBrO+H++2e-=Br-+H2O
Fe3++3e-=Fe
HBrO+H++e-=l/2Br2(aq)+H2O
Fe3++e-=Fe2+
HBrO+H++e-=l/2Br2(l)+H2O
[Fe(CN)6]3-+e-=[Fe(CN)6]4-
Sb2O5+6H++4e-=2SbO++3H2O
N2O4+2H++2e-=2HNO2
SbO++2H++3e-=Sb+H2O
N2O4+4H++4e-=2NO+2H2O
Sc3++3e-=Sc
2NO+2H++2e-=N2O+H2O
Se+2H++2e-=H2Se(aq)
HNO2+H++e-=NO+H2O
标准电极电势

标准电极电势表维基百科,自由的百科全书标准电极电势可以用来计算化学电池或原电池的电化学势或电极电势。
本表中所给出的电极电势以标准氢电极为参比电极,溶液中离子有效浓度为1mol/L,气体分压为100kPa,温度为298K,所有离子的数据都在水溶液中测得。
[1][2][3][4][5][6][7][8][9]单击每栏上方的符号可将数据按元素符号或标准电极电势值排序。
注:(s) – 固体;(l) – 液体;(g) – 气体;(aq) – 水溶液;(Hg) – 汞齐。
Ba+ + e− Ba(s)−4.38[10][1][3] Sr+ + e− Sr(s)−4.10[11][1][3] Ca+ + e− Ca(s)−3.8[11][1][3] Pr3+ + e− Pr2+−3.1[11] N HN 3(aq)−3.09[6]Li+ + e− Li(s)−3.0401[5]N2(g) + 4 H2O + 2 e− 2 NH2OH(aq) + 2 OH−−3.04[6]Cs+ + e− Cs(s)−3.026[5]Ca(OH)2(s) + 2 e− Ca(s) + 2 OH−−3.02[11] Rb+ + e− Rb(s)−2.98[4]K+ + e− K(s)−2.931[5]Mg+ + e− Mg(s)−2.93[10] Ba2+ + 2 e− Ba(s)−2.912[5]La(OH)3(s) + 3 e− La(s) + 3OH−−2.90[5]Fr+ + e− Fr(s)−2.9[11]Sr2+ + 2 e− Sr(s)−2.899[5]Sr(OH)2(s) + 2 e− Sr(s) + 2 OH−−2.88[11] Ca2+ + 2 e− Ca(s)−2.868[5]Eu2+ + 2 e− Eu(s)−2.812[5]Ra2+ + 2 e− Ra(s)−2.8[5]Yb2+ + 2 e− Yb(s)−2.76[11][1] Na+ + e− Na(s)−2.71[5][9] Sm2+ + 2 e− Sm(s)−2.68[11][1] No2+ + 2 e− No(s)−2.50[11] HfO(OH)2(s) + H2O + 4 e− Hf(s) + 4 OH−−2.50[11]Th(OH)4(s) + 4 e− Th(s) + 4 OH−−2.48[11] Md2+ + 2 e− Md(s)−2.40[11]La + 3 e La(s)−2.379[5] Y3+ + 3 e− Y(s)−2.372[5] Mg2+ + 2 e− Mg(s)−2.372[5] ZrO(OH)2(s) + H2O + 4 e− Zr(s) + 4OH−−2.36[5] Pr3+ + 3 e− Pr(s)−2.353[11] Ce3+ + 3 e− Ce(s)−2.336[11] Er3+ + 3 e− Er(s)−2.331[11] Ho3+ + 3 e− Ho(s)−2.33[11] Al(OH)4− + 3 e− Al(s) + 4 OH−−2.33Al(OH)3(s) + 3 e− Al(s) + 3OH−−2.31Tb3+ + 3 e− Tb(s)−2.28H2(g) + 2 e− 2 H−−2.25Ac3+ + 3 e− Ac(s)−2.20Be+ + e− Be(s)−2.12[10] Cf2+ + 2 e− Cf(s)−2.12[11] Am3+ + 3 e− Am(s)−2.048[11] Cf3+ + 3 e− Cf(s)−1.94[11] Am2+ + 2 e− Am(s)−1.9[11] Be2+ + 2 e− Be(s)−1.85Rf4+ + 4 e− Rf(s)−1.67[12] U3+ + 3 e− U(s)−1.66[7] Al3+ + 3 e− Al(s)−1.66[9] Ti2+ + 2 e− Ti(s)−1.63[9] Bk2+ + 2 e− Bk(s)−1.6[11] ZrO2(s) + 4 H+ + 4 e− Zr(s) + 2 H2O−1.553[5] Hf4+ + 4 e− Hf(s)−1.55[11] Zr4+ + 4 e− Zr(s)−1.45[5]Ti + 3 e Ti(s)−1.37[13] TiO(s) + 2 H+ + 2 e− Ti(s) + H2O−1.31Ti2O3(s) + 2 H+ + 2 e− 2 TiO(s) + H2O−1.23Zn(OH)42− + 2 e− Zn(s) + 4 OH−−1.199[14] Mn2+ + 2 e− Mn(s)−1.185[14] Fe(CN)64− + 6 H+ + 2 e− Fe(s) + 4HCN(aq)−1.16[15] V2+ + 2 e− V(s)−1.175[2] Te(s) + 2 e− Te2−−1.143[2] Nb3+ + 3 e− Nb(s)−1.099Sn(s) + 4 H+ + 4 e− SnH4(g)−1.07In(OH)3(s) + 3 e− In(s) + 3 OH−−0.99[11] SiO2(s) + 4 H+ + 4 e− Si(s) + 2 H2O−0.91B(OH)3(aq) + 3 H+ + 3 e− B(s) + 3 H2O−0.89Fe(OH)2(s) + 2 e− Fe(s) + 2 OH−−0.89[15] Fe2O3(s) + 3 H2O + 2 e− 2Fe(OH)2(s) + 2 OH−−0.86[15] TiO2+ + 2 H+ + 4 e− Ti(s) + H2O−0.862 H2O + 2 e− H2(g) + 2 OH−−0.8277[5] Bi(s) + 3 H+ + 3 e− BiH3−0.8[14] Zn2+ + 2 e− Zn(Hg)−0.7628[5] Zn2+ + 2 e− Zn(s)−0.7618[5] Ta2O5(s) + 10 H+ + 10 e− 2 Ta(s) + 5 H2O−0.75Cr3+ + 3 e− Cr(s)−0.74[Au(CN)2]− + e− Au(s) + 2 CN−−0.60Ta3+ + 3 e− Ta(s)−0.6PbO(s) + H2O + 2 e− Pb(s) + 2 OH−−0.582 TiO2(s) + 2 H+ + 2 e− Ti2O3(s) + H2O−0.56Ga3+ + 3 e− Ga(s)−0.53U4+ + e− U3+−0.52[7] H3PO2(aq) + H+ + e− P(白磷[16]) + 2 H2O−0.508[5] H3PO3(aq) + 2 H+ + 2 e− H3PO2(aq) + H2O−0.499[5] H3PO3(aq) + 3 H+ + 3 e− P(红磷)[16] + 3H2O−0.454[5] Fe2+ + 2 e− Fe(s)−0.44[9] 2 CO2(g) + 2 H+ + 2 e− HOOCCOOH(aq)−0.43Cr3+ + e− Cr2+−0.42Cd2+ + 2 e− Cd(s)−0.40[9] SeO32− + 4e− + 3H2O ⇌ Se + 6OH−−0.37[17] GeO2(s) + 2 H+ + 2 e− GeO(s) + H2O−0.37Cu2O(s) + H2O + 2 e− 2 Cu(s) + 2 OH−−0.360[5] PbSO4(s) + 2 e− Pb(s) + SO42−−0.3588[5] PbSO4(s) + 2 e− Pb(Hg) + SO42−−0.3505[5] Eu3+ + e− Eu2+−0.35[7] In3+ + 3 e− In(s)−0.34[2] Tl+ + e− Tl(s)−0.34[2] Ge(s) + 4 H+ + 4 e− GeH4(g)−0.29Co2+ + 2 e− Co(s)−0.28[5] H3PO4(aq) + 2 H+ + 2 e− H3PO3(aq) + H2O−0.276[5] V3+ + e− V2+−0.26[9] Ni2+ + 2 e− Ni(s)−0.25As(s) + 3 H+ + 3 e− AsH3(g)−0.23[2] AgI(s) + e− Ag(s) + I−−0.15224[14] MoO2(s) + 4 H+ + 4 e− Mo(s) + 2 H2O−0.15Si(s) + 4 H+ + 4 e− SiH4(g)−0.14Sn2+ + 2 e− Sn(s)−0.13O2(g) + H+ + e− HO2•(aq)−0.13Pb2+ + 2 e− Pb(s)−0.13[9] WO2(s) + 4 H+ + 4 e− W(s) + 2 H2O−0.12P(红磷) + 3 H+ + 3 e− PH3(g)−0.111[5] CO2(g) + 2 H+ + 2 e− HCOOH(aq)−0.11Se(s) + 2 H+ + 2 e− H2Se(g)−0.11CO2(g) + 2 H+ + 2 e− CO(g) + H2O−0.11SnO(s) + 2 H+ + 2 e− Sn(s) + H2O−0.10SnO2(s) + 2 H+ + 2 e− SnO(s) + H2O−0.09WO3(aq) + 6 H+ + 6 e− W(s) + 3 H2O−0.09[2] P(白磷) + 3 H+ + 3 e− PH3(g)−0.063[5] Fe3+ + 3 e− Fe(s)−0.04[15] HCOOH(aq) + 2 H+ + 2 e− HCHO(aq) + H2O−0.032 H+ + 2 e− H2(g) 0.00≡ 0 AgBr(s) + e− Ag(s) + Br−+0.07133[14] S4O62− + 2 e− 2 S2O32−+0.08Fe3O4(s) + 8 H+ + 8 e− 3 Fe(s) + 4 H2O+0.085[8] N2(g) + 2 H2O + 6H+ + 6 e− 2 NH4OH(aq)+0.092HgO(s) + H2O + 2 e− Hg(l) + 2 OH−+0.0977Cu(NH3)42+ + e− Cu(NH3)2+ + 2 NH3+0.10[2] Ru(NH3)63+ + e− Ru(NH3)62++0.10[7] N2H4(aq) + 4 H2O + 2 e− 2 NH4+ + 4 OH−+0.11[6] H2MoO4(aq) + 6 H+ + 6 e− Mo(s) + 4 H2O+0.11Ge4+ + 4 e− Ge(s)+0.12C(s) + 4 H+ + 4 e− CH4(g)+0.13[2] HCHO(aq) + 2 H+ + 2 e− CH3OH(aq)+0.13S(s) + 2 H+ + 2 e− H2S(g)+0.14Sn4+ + 2 e− Sn2++0.15Cu2+ + e− Cu++0.159[2] HSO4− + 3 H+ + 2 e− SO2(aq) + 2 H2O+0.16UO22+ + e− UO2++0.163[7] SO42− + 4 H+ + 2 e− SO2(aq) + 2 H2O+0.17TiO2+ + 2 H+ + e− Ti3+ + H2O+0.19Bi3+ + 2e− Bi++0.2SbO+ + 2 H+ + 3 e− Sb(s) + H2O+0.20AgCl(s) + e− Ag(s) + Cl−+0.22233[14] H3AsO3(aq) + 3 H+ + 3 e− As(s) + 3 H2O+0.24GeO(s) + 2 H+ + 2 e− Ge(s) + H2O+0.26UO2+ + 4 H+ + e− U4+ + 2 H2O+0.273[7] At2 + e− 2 At-+0.3[11] Re3+ + 3 e− Re(s)+0.300Bi3+ + 3 e− Bi(s)+0.32VO2+ + 2 H+ + e− V3+ + H2O+0.34Cu2+ + 2 e− Cu(s)+0.340[2] [Fe(CN)6]3− + e− [Fe(CN)6]4−+0.36Tc2+ + 2 e− Tc(s)+0.40[11] O2(g) + 2 H2O + 4 e− 4 OH−(aq)+0.40[9] H2MoO4 + 6 H+ + 3 e− Mo3+ + 2 H2O+0.43Ru2+ + 2 e− Ru(s)+0.455[11] Bi+ + e− Bi(s)+0.50CH3OH(aq) + 2 H+ + 2 e− CH4(g) + H2O+0.50SO2(aq) + 4 H+ + 4 e− S(s) + 2 H2O+0.50Cu+ + e− Cu(s)+0.520[2] CO(g) + 2 H+ + 2 e− C(s) + H2O+0.52I3− + 2 e− 3 I−+0.53[9] I2(s) + 2 e− 2 I−+0.54[9] [AuI4]− + 3 e− Au(s) + 4 I−+0.56H3AsO4(aq) + 2 H+ + 2 e− H3AsO3(aq) + H2O+0.56[AuI2]− + e− Au(s) + 2 I−+0.58MnO4− + 2 H2O + 3 e− MnO2(s) + 4 OH−+0.59Rh+ + e− Rh(s)+0.600[11] S2O32 − + 6 H+ + 4 e− 2 S(s) + 3 H2O+0.60Fc+ + e− Fc(s)+0.641[18]Ag + −+0.643[11]H2MoO4(aq) + 2 H+ + 2 e− MoO2(s) + 2 H2O+0.65+ 2 H+ + 2 e−H2O2(aq)+0.70Tl3+ + 3 e− Tl(s)+0.72PtCl62− + 2 e− PtCl42− + 2 Cl−+0.726[7] H2SeO3(aq) + 4 H+ + 4 e− Se(s) + 3 H2O+0.74Rh3+ + 3 e− Rh(s)+0.758[11] PtCl42− + 2 e− Pt(s) + 4 Cl−+0.758[7] Fe3+ + e− Fe2++0.77Ag+ + e− Ag(s)+0.7996[5] Hg22+ + 2 e− 2 Hg(l)+0.80NO3−(aq) + 2 H+ + e− NO2(g) + H2O+0.80FeO42− + 5 H2O + 6 e− Fe2O3(s) + 10 OH−+0.81[15] H2(g) + 2 OH− 2 H2O + 2 e−+0.828[19] [AuBr4]− + 3 e− Au(s) + 4 Br−+0.85Hg2+ + 2 e− Hg(l)+0.85MnO4− + H+ + e− HMnO4−+0.902 Hg2+ + 2 e− Hg22++0.91[2] Pd2+ + 2 e− Pd(s)+0.915[7] [AuCl4]− + 3 e− Au(s) + 4 Cl−+0.93MnO2(s) + 4 H+ + e− Mn3+ + 2 H2O+0.95[AuBr2]− + e− Au(s) + 2 Br−+0.96 [HXeO6]3− + 2 H2O + 2 e− + [HXeO4]− + 4 OH−+0.99[20] HNO2 + H+ + e- = NO(g) + H2O+0.996H6TeO6(aq) + 2 H+ + 2 e− TeO2(s) + 4 H2O+1.02[21] Br2(l) + 2 e− 2 Br−+1.07Br2(aq) + 2 e− 2 Br−+1.09[9] NO2(g) + H+ + e- = HNO2+1.093IO3− + 5 H+ + 4 e− HIO(aq) + 2 H2O+1.13[AuCl2]− + e− Au(s) + 2 Cl−+1.15HSeO4− + 3 H+ + 2 e− H2SeO3(aq) + H2O+1.15Ir3+ + 3 e− Ir(s)+1.156[11] Ag2O(s) + 2 H+ + 2 e− 2 Ag(s) + H2O+1.17ClO3− + 2 H+ + e− ClO2(g) + H2O+1.18 [HXeO6]3− + 5 H2O + 8 e− Xe(g) + 11 OH−+1.18[20] Pt2+ + 2 e− Pt(s)+1.188[7] ClO2(g) + H+ + e− HClO2(aq)+1.192 IO3− + 12 H+ + 10 e− I2(s) + 6 H2O+1.20ClO4− + 2 H+ + 2 e− ClO3− + H2O+1.20O2(g) + 4 H+ + 4 e− 2 H2O+1.229[9] MnO2(s) + 4 H+ + 2 e− Mn2+ + 2H2O+1.23 [HXeO4]− + 3 H2O + 6 e− Xe(g) + 7 OH−+1.24[20]Tl3+ + 2 e− Tl++1.25Cr2O72 − + 14 H+ + 6 e− 2 Cr3+ + 7 H2O+1.33Cl2(g) + 2 e− 2 Cl−+1.36[9] CoO2(s) + 4 H+ + e− Co3+ + 2 H2O+1.422 NH3OH+ + H+ + 2 e− N2H5+ + 2 H2O+1.42[6] 2 HIO(aq) + 2 H+ + 2 e− I2(s) + 2 H2O+1.44Ce4+ + e− Ce3++1.44BrO3− + 5 H+ + 4 e− HBrO(aq) + 2 H2O+1.45β-PbO2(s) + 4 H+ + 2 e− Pb2+ + 2 H2O+1.460[2]α-PbO2(s) + 4 H+ + 2 e− Pb2+ + 2 H2O+1.468[2] 2 BrO3− + 12 H+ + 10 e− Br2(l) + 6 H2O+1.482ClO3− + 12 H+ + 10 e− Cl2(g) + 6 H2O+1.49HO2 + H+ + e− H2O2+1.495[11] MnO4− + 8 H+ + 5 e− Mn2+ + 4 H2O+1.51HO2• + H+ + e− H2O2(aq)+1.51Au3+ + 3 e− Au(s)+1.52NiO2(s) + 4 H+ + 2 e− Ni2+ + 2 OH−+1.592 HClO(aq) + 2 H+ + 2 e− Cl2(g) + 2 H2O+1.63Ag2O3(s) + 6 H+ + 4 e− 2 Ag+ + 3 H2O+1.67HClO2(aq) + 2 H+ + 2 e− HClO(aq) + H2O+1.67Pb4+ + 2 e− Pb2++1.69[2] MnO4− + 4 H+ + 3 e− MnO2(s) + 2 H2O+1.70AgO(s) + 2 H+ + e− Ag+ + H2O+1.77 H2O2(aq) + 2 H+ + 2 e− 2 H2O+1.776Co3+ + e− Co2++1.82Au+ + e− Au(s)+1.83[2] BrO4− + 2 H+ + 2 e− BrO3− + H2O+1.85半反应E° (V)[注 1]来源Ag2+ + e− Ag++1.98[2]S2O82− + 2 e− 2 SO42−+2.07O3(g) + 2 H+ + 2 e− O2(g) + H2O+2.075[7]HMnO4− + 3 H+ + 2 e− MnO2(s) + 2 H2O+2.09XeO3(aq) + 6 H+ + 6 e− Xe(g) + 3 H2O+2.12[20]H4XeO6(aq) + 8 H+ + 8 e− Xe(g) + 6 H2O+2.18[20]FeO42− + 3 e− + 8 H+ Fe3+ + 4 H2O+2.20[22]XeF2(aq) + 2 H+ + 2 e− Xe(g) + 2HF(aq)+2.32[20]H4XeO6(aq) + 2 H+ + 2 e− XeO3(aq) + H2O+2.42[20]F2(g) + 2 e− 2 F−+2.87[2][9]F2(g) + 2 H+ + 2 e− 2 HF(aq)+3.05[2]Tb4+ + e− Tb3++3.05[11]1.^ Clicking on this column to re-sort by potential didn’t work in the Safari webbrowser in v. 4.0.3 or earlier (but works in v. 4.0.5). In this case just reload the page to restore the original order.参考资料1.^ 1.01.11.21.31.41.5 Milazzo, G., Caroli, S., and Sharma, V. K. (1978). Tables ofStandard Electrode Potentials (Wiley, Chichester).2.^ 2.002.012.022.032.042.052.062.072.082.092.102.112.122.132.142.152.162.172.182.19 Bard, A. J., Parsons, R., and Jordan, J. (1985). Standard Potentials in Aqueous Solutions (Marcel Dekker, New York).3.^ 3.03.13.23.3 Bratsch, S. G. (1989). Journal of Physical Chemistry Reference DataVol. 18, pp. 1–21.4.^ 4.04.1 Vanýsek, Petr (2006). "Electrochemical Series," in Handbook of Chemistry and Physics: 87th Edition (/) (Chemical RubberCompany).^ 5.005.015.025.035.045.055.065.075.085.095.105.115.125.135.145.155.165.175.185.195.205.215.22 5.5.235.245.255.265.275.285.295.30 Vanýsek, Petr (2007). “Electrochemical Series”(/articles/08_08_88.pdf) , in Handbook of Chemistryand Physics: 88th Edition (/) (Chemical RubberCompany).6.^ 6.06.16.26.36.4 Greenwood, N. N.; Earnshaw, A.. Chemistry of the Elements. 2ndEdition. Oxford:Butterworth-Heinemann. 1997. ISBN0-7506-3365-4.^ 7.00 7.01 7.02 7.03 7.04 7.05 7.06 7.07 7.08 7.09 7.10 7.11 Bard, A.J., Faulkner, L.R.(2001). Electrochemical Methods. Fundamentals and Applications , 2nd edition (John Wiley and Sons Inc).7.^ 8.0 8.1 Marcel Pourbaix (1966). Atlas of Electrochemical Equilibria in Aqueous Solutions (NACE International, Houston, Texas; Cebelcor, Brussels).8.^ 9.00 9.01 9.02 9.03 9.04 9.05 9.06 9.07 9.08 9.09 9.10 9.11 9.12 9.13 9.14 Peter Atkins (1997). Physical Chemistry , 6th edition (W.H. Freeman and Company, New York).9.^ 10.0 10.1 10.2 Ca Sr Ba 一价[11]与两价间的标准电极电势正好有规律关系,因此可以估计近似值10.^ 11.00 11.01 11.02 11.03 11.04 11.05 11.06 11.07 11.08 11.09 11.10 11.11 11.12 11.13 11.14 11.15 11.16 11.17 11.18 11.19 11.20 11.21 11.22 11.23 11.24 11.25 11.26 11.27 11.28 11.29 11.30 11.31 Standard Redox Potential Table (/time-to-wake-up/docs/electrochemical_redox_potential)11.^ Ti Zr Hf 的标准电极电势变化较规律,因此可估计Rf 的标准电极电势12.^ Gordon Aylward & Tristan Findlay (2008). "SI Chemical Data", 6th edition (John Wiley & Sons, Australia), ISBN 9780470816387.13.^ 14.0 14.1 14.2 14.3 14.4 14.5 14.6 Vanýsek, Petr (2007). “Electrochemical Series”, in Handbook of Chemistry and Physics: 88th Edition (Chemical Rubber Company).14.^ 15.0 15.1 15.2 15.3 15.4 WebElements Periodic Table of the Elements | Iron | compounds information (/iron/compounds.html)15.^ 16.0 16.1 由−0.454和(2×−0.499 + −0.508) ÷ 3 = −0.502推算出。
(完整版)标准电极电势表(非常全)
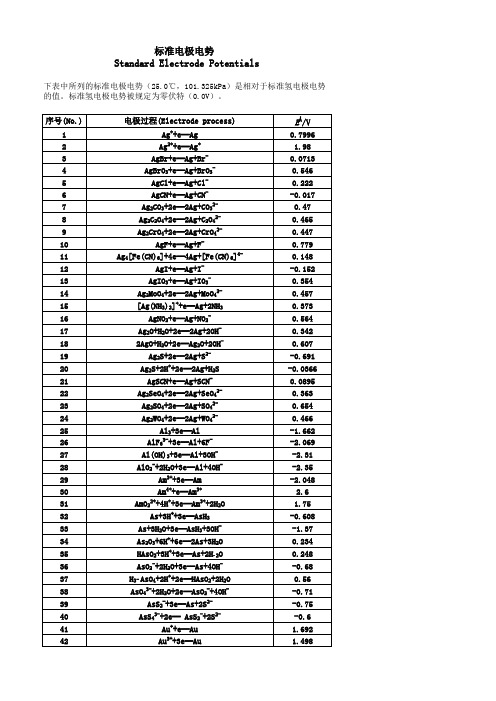
标准电极电势Standard Electrode Potentials下表中所列的标准电极电势(25.0℃,101.325kPa)是相对于标准氢电极电势的值。
标准氢电极电势被规定为零伏特(0.0V)。
序号(No.)电极过程(Electrode process)EÅ/V 1Ag++e═Ag0.79962Ag2++e═Ag+ 1.983AgBr+e═Ag+Br-0.07134AgBrO3+e═Ag+BrO3-0.5465AgCl+e═Ag+Cl-0.2226AgCN+e═Ag+CN--0.0177Ag2CO3+2e═2Ag+CO32-0.478Ag2C2O4+2e═2Ag+C2O42-0.4659Ag2CrO4+2e═2Ag+CrO42-0.44710AgF+e═Ag+F-0.77911Ag4[Fe(CN)6]+4e═4Ag+[Fe(CN)6]4-0.14812AgI+e═Ag+I--0.152 13AgIO3+e═Ag+IO3-0.35414Ag2MoO4+2e═2Ag+MoO42-0.45715[Ag(NH3)2]++e═Ag+2NH30.37316AgNO2+e═Ag+NO2-0.56417Ag2O+H2O+2e═2Ag+2OH-0.342182AgO+H2O+2e═Ag2O+2OH-0.60719Ag2S+2e═2Ag+S2--0.691 20Ag2S+2H++2e═2Ag+H2S-0.0366 21AgSCN+e═Ag+SCN-0.0895 22Ag2SeO4+2e═2Ag+SeO42-0.36323Ag2SO4+2e═2Ag+SO42-0.65424Ag2WO4+2e═2Ag+WO42-0.46625Al3+3e═Al-1.662 26AlF63-+3e═Al+6F--2.069 27Al(OH)3+3e═Al+3OH--2.3128AlO2-+2H2O+3e═Al+4OH--2.3529Am3++3e═Am-2.048 30Am4++e═Am3+ 2.631AmO22++4H++3e═Am3++2H2O 1.7532As+3H++3e═AsH3-0.608 33As+3H2O+3e═AsH3+3OH--1.3734As2O3+6H++6e═2As+3H2O0.23435HAsO2+3H++3e═As+2H2O0.24836AsO2-+2H2O+3e═As+4OH--0.6837H3AsO4+2H++2e═HAsO2+2H2O0.5638AsO43-+2H2O+2e═AsO2-+4OH--0.7139AsS2-+3e═As+2S2--0.7540AsS43-+2e═ AsS2-+2S2--0.641Au++e═Au 1.69242Au3++3e═Au 1.49843Au3++2e═Au+ 1.401 44AuBr2-+e═Au+2Br-0.959 45AuBr4-+3e═Au+4Br-0.854 46AuCl2-+e═Au+2Cl- 1.15 47AuCl4-+3e═Au+4Cl- 1.002 48AuI+e═Au+I-0.5 49Au(SCN)4-+3e═Au+4SCN-0.66 50Au(OH)3+3H++3e═Au+3H2O 1.45 51BF4-+3e═B+4F--1.04 52H2BO3-+H2O+3e═B+4OH--1.79 53B(OH)3+7H++8e═BH4-+3H2O-0.0481 54Ba2++2e═Ba-2.912 55Ba(OH)2+2e═Ba+2OH--2.99 56Be2++2e═Be-1.847 57Be2O32-+3H2O+4e═2Be+6OH--2.63 58Bi++e═Bi0.5 59Bi3++3e═Bi0.308 60BiCl4-+3e═Bi+4Cl-0.16 61BiOCl+2H++3e═Bi+Cl-+H2O0.16 62Bi2O3+3H2O+6e═2Bi+6OH--0.46 63Bi2O4+4H++2e═2BiO++2H2O 1.593 64Bi2O4+H2O+2e═ Bi2O3+2OH-0.56 65Br2(水溶液,aq)+2e═2Br- 1.087 66Br2(液体)+2e═2Br- 1.066 67BrO-+H2O+2e═Br-+2OH0.761 68BrO3-+6H++6e═Br-+3H2O 1.423 69BrO3-+3H2O+6e═Br-+6OH-0.61 702BrO3-+12H++10e═Br2+6H2O 1.482 71HBrO+H++2e═Br-+H2O 1.331 722HBrO+2H++2e═Br2(水溶液,aq)+2H2O 1.574 73CH3OH+2H++2e═CH4+H2O0.59 74HCHO+2H++2e═CH3OH0.19 75CH3COOH+2H++2e═CH3CHO+H2O-0.12 76(CN)2+2H++2e═2HCN0.373 77(CNS)2+2e═2CNS-0.77 78CO2+2H++2e═CO+H2O-0.12 79CO2+2H++2e═HCOOH-0.199 80Ca2++2e═Ca-2.868 81Ca(OH)2+2e═Ca+2OH--3.02 82Cd2++2e═Cd-0.403 83Cd2++2e═Cd(Hg)-0.352 84Cd(CN)42-+2e═Cd+4CN--1.09 85CdO+H2O+2e═Cd+2OH--0.783 86CdS+2e═Cd+S2--1.17 87CdSO4+2e═Cd+SO42--0.246 88Ce3++3e═Ce-2.336 89Ce3++3e═Ce(Hg)-1.437 90CeO2+4H++e═Ce3++2H2O 1.4 91Cl2(气体)+2e═2Cl- 1.35892ClO-+H2O+2e═Cl-+2OH-0.89 93HClO+H++2e═Cl-+H2O 1.482 942HClO+2H++2e═Cl2+2H2O 1.611 95ClO2-+2H2O+4e═Cl-+4OH-0.76 962ClO3-+12H++10e═Cl2+6H2O 1.47 97ClO3-+6H++6e═Cl-+3H2O 1.451 98ClO3-+3H2O+6e═Cl-+6OH-0.62 99ClO4-+8H++8e═Cl-+4H2O 1.38 1002ClO4-+16H++14e═Cl2+8H2O 1.39 101Cm3++3e═Cm-2.04 102Co2++2e═Co-0.28 103[Co(NH3)6]3++e═[Co(NH3)6]2+0.108 104[Co(NH3)6]2++2e═Co+6NH3-0.43 105Co(OH)2+2e═Co+2OH--0.73 106Co(OH)3+e═Co(OH)2+OH-0.17 107Cr2++2e═Cr-0.913 108Cr3++e═Cr2+-0.407 109Cr3++3e═Cr-0.744 110[Cr(CN)6]3-+e═[Cr(CN)6]4--1.28 111Cr(OH)3+3e═Cr+3OH--1.48 112Cr2O72-+14H++6e═2Cr3++7H2O 1.232 113CrO2-+2H2O+3e═Cr+4OH--1.2 114HCrO4-+7H++3e═Cr3++4H2O 1.35 115CrO42-+4H2O+3e═Cr(OH)3+5OH--0.13 116Cs++e═Cs-2.92 117Cu++e═Cu0.521 118Cu2++2e═Cu0.342 119Cu2++2e═Cu(Hg)0.345 120Cu2++Br-+e═CuBr0.66 121Cu2++Cl-+e═CuCl0.57 122Cu2++I-+e═CuI0.86 123Cu2++2CN-+e═[Cu(CN)2]- 1.103 124CuBr2-+e═Cu+2Br-0.05 125CuCl2-+e═Cu+2Cl-0.19 126CuI2-+e═Cu+2I-0 127Cu2O+H2O+2e═2Cu+2OH--0.36 128Cu(OH)2+2e═Cu+2OH--0.222 1292Cu(OH)2+2e═Cu2O+2OH-+H2O-0.08 130CuS+2e═Cu+S2--0.7 131CuSCN+e═Cu+SCN--0.27 132Dy2++2e═Dy-2.2 133Dy3++3e═Dy-2.295 134Er2++2e═Er-2 135Er3++3e═Er-2.331 136Es2++2e═Es-2.23 137Es3++3e═Es-1.91 138Eu2++2e═Eu-2.812 139Eu3++3e═Eu-1.991 140F2+2H++2e═2HF 3.053190IO3-+2H2O+4e═IO-+4OH-0.15 191IO3-+3H2O+6e═I-+6OH-0.26 1922IO3-+6H2O+10e═I2+12OH-0.21 193H5IO6+H++2e═IO3-+3H2O 1.601 194In++e═In-0.14 195In3++3e═In-0.338 196In(OH)3+3e═In+3OH--0.99 197Ir3++3e═Ir 1.156 198IrBr62-+e═ IrBr63-0.99 199IrCl62-+e═IrCl63-0.867 200K++e═K-2.931 201La3++3e═La-2.379 202La(OH)3+3e═La+3OH--2.9 203Li++e═Li-3.04 204Lr3++3e═Lr-1.96 205Lu3++3e═Lu-2.28 206Md2++2e═Md-2.4 207Md3++3e═Md-1.65 208Mg2++2e═Mg-2.372 209Mg(OH)2+2e═Mg+2OH--2.69 210Mn2++2e═Mn-1.185 211Mn3++3e═Mn 1.542 212MnO2+4H++2e═Mn2++2H2O 1.224 213MnO4-+4H++3e═MnO2+2H2O 1.679 214MnO4-+8H++5e═Mn2++4H2O 1.507 215MnO4-+2H2O+3e═MnO2+4OH-0.595 216Mn(OH)2+2e═Mn+2OH--1.56 217Mo3++3e═Mo-0.2 218MoO42-+4H2O+6e═Mo+8OH--1.05 219N2+2H2O+6H++6e═2NH4OH0.092 2202NH3OH++H++2e═N2H5++2H2O 1.42 2212NO+H2O+2e═N2O+2OH-0.76 2222HNO2+4H++4e═N2O+3H2O 1.297 223NO3-+3H++2e═HNO2+H2O0.934 224NO3-+H2O+2e═NO2-+2OH-0.01 2252NO3-+2H2O+2e═N2O4+4OH--0.85 226Na++e═Na-2.713 227Nb3++3e═Nb-1.099 228NbO2+4H++4e═Nb+2H2O-0.69 229Nb2O5+10H++10e═2Nb+5H2O-0.644 230Nd2++2e═Nd-2.1 231Nd3++3e═Nd-2.323 232Ni2++2e═Ni-0.257 233NiCO3+2e═Ni+CO32--0.45 234Ni(OH)2+2e═Ni+2OH--0.72 235NiO2+4H++2e═Ni2++2H2O 1.678 236No2++2e═No-2.5 237No3++3e═No-1.2 238Np3++3e═Np-1.856239NpO2+H2O+H++e═Np(OH)3-0.962 240O2+4H++4e═2H2O 1.229 241O2+2H2O+4e═4OH-0.401 242O3+H2O+2e═O2+2OH- 1.24 243Os2++2e═Os0.85 244OsCl63-+e═Os2++6Cl-0.4 245OsO2+2H2O+4e═Os+4OH--0.15 246OsO4+8H++8e═Os+4H2O0.838 247OsO4+4H++4e═OsO2+2H2O 1.02 248P+3H2O+3e═PH3(g)+3OH--0.87 249H2PO2-+e═P+2OH--1.82 250H3PO3+2H++2e═H3PO2+H2O-0.499 251H3PO3+3H++3e═P+3H2O-0.454 252H3PO4+2H++2e═H3PO3+H2O--0.276 253PO43-+2H2O+2e═HPO32-+3OH--1.05 254Pa3++3e═Pa-1.34 255Pa4++4e═Pa-1.49 256Pb2++2e═Pb-0.126 257Pb2++2e═Pb(Hg)-0.121 258PbBr2+2e═Pb+2Br--0.284 259PbCl2+2e═Pb+2Cl--0.268 260PbCO3+2e═Pb+CO32--0.506 261PbF2+2e═Pb+2F--0.344 262PbI2+2e═Pb+2I--0.365 263PbO+H2O+2e═Pb+2OH--0.58 264PbO+4H++2e═Pb+H2O0.25 265PbO2+4H++2e═Pb2+2H2O 1.455 266HPbO2-+H2O+2e═Pb+3OH--0.537 267PbO2+SO42-+4H++2e═PbSO4+2H2O 1.691 268PbSO4+2e═Pb+SO42--0.359 269Pd2++2e═Pd0.915 270PdBr42-+2e═Pd+4Br-0.6 271PdO2+H2O+2e═PdO+2OH-0.73 272Pd(OH)2+2e═Pd+2OH-0.07 273Pm2++2e═Pm-2.2 274Pm3++3e═Pm-2.3 275Po4++4e═Po0.76 276Pr2++2e═Pr-2 277Pr3++3e═Pr-2.353 278Pt2++2e═Pt 1.18 279[PtCl6]2-+2e═[PtCl4]2-+2Cl-0.68 280Pt(OH)2+2e═Pt+2OH-0.14 281PtO2+4H++4e═Pt+2H2O1 282PtS+2e═Pt+S2--0.83 283Pu3++3e═Pu-2.031 284Pu5++e═Pu4+ 1.099 285Ra2++2e═Ra-2.8 286Rb++e═Rb-2.98 287Re3++3e═Re0.3337Th4++4e═Th-1.899 338Ti2++2e═Ti-1.63 339Ti3++3e═Ti-1.37 340TiO2+4H++2e═Ti2++2H2O-0.502 341TiO2++2H++e═Ti3++H2O0.1 342Tl++e═Tl-0.336 343Tl3++3e═Tl0.741 344Tl3++Cl-+2e═TlCl 1.36 345TlBr+e═Tl+Br--0.658 346TlCl+e═Tl+Cl--0.557 347TlI+e═Tl+I--0.752 348Tl2O3+3H2O+4e═2Tl++6OH-0.02 349TlOH+e═Tl+OH--0.34 350Tl2SO4+2e═2Tl+SO42--0.436 351Tm2++2e═Tm-2.4 352Tm3++3e═Tm-2.319 353U3++3e═U-1.798 354UO2+4H++4e═U+2H2O-1.4 355UO2++4H++e═U4++2H2O0.612 356UO22++4H++6e═U+2H2O-1.444 357V2++2e═V-1.175 358VO2++2H++e═V3++H2O0.337 359VO2++2H++e═VO2++H2O0.991 360VO2++4H++2e═V3++2H2O0.668 361V2O5+10H++10e═2V+5H2O-0.242 362W3++3e═W0.1 363WO3+6H++6e═W+3H2O-0.09 364W2O5+2H++2e═2WO2+H2O-0.031 365Y3++3e═Y-2.372 366Yb2++2e═Yb-2.76 367Yb3++3e═Yb-2.19 368Zn2++2e═Zn-0.7618 369Zn2++2e═Zn(Hg)-0.7628 370Zn(OH)2+2e═Zn+2OH--1.249 371ZnS+2e═Zn+S2--1.4 372ZnSO4+2e═Zn(Hg)+SO42--0.799。
标准电极电势表(全)

1电对方程式E /VLi(I)-(0) Li++e-=Li -3.0401 Cs(I)-(0) Cs++e-=Cs -3.026 Rb(I)-(0) Rb++e-=Rb -2.98 K(I)-(0) K++e-=K -2.931 Ba(II)-(0) Ba2++2e-=Ba -2.912 Sr(II)-(0) Sr2++2e-=Sr -2.89 Ca(II)-(0) Ca2++2e-=Ca -2.868 Na(I)-(0) Na++e-=Na -2.71 La(III)-(0) La3++3e-=La -2.379 Mg(II)-(0) Mg2++2e-=Mg -2.372 Ce(III)-(0) Ce3++3e-=Ce -2.336 H(0)-(-I) H2(g)+2e-=2H--2.23Al(III)-(0) AlF63-+3e-=Al+6F--2.069 Th(IV)-(0) Th4++4e-=Th -1.899 Be(II)-(0) Be2++2e-=Be -1.847 U(III)-(0) U3++3e-=U -1.798Hf(IV)-(0) HfO2++2H++4e-=Hf+H2O -1.724 Al(III)-(0) Al3++3e-=Al -1.662 Ti(II)-(0) Ti2++2e-=Ti -1.630Zr(IV)-(0) ZrO2+4H++4e-=Zr+2H2O -1.553Si(IV)-(0) [SiF6]2-+4e-=Si+6F--1.24 Mn(II)-(0) Mn2++2e-=Mn -1.185 Cr(II)-(0) Cr2++2e-=Cr -0.913 Ti(III)-(II) Ti3++e-=Ti2+-0.9B(III)-(0) H3BO3+3H++3e-=B+3H2O -0.8698*Ti(IV)-(0) TiO2+4H++4e-=Ti+2H2O -0.86Te(0)-(-II) Te+2H++2e-=H2Te -0.793 Zn(II)-(0) Zn2++2e-=Zn -0.7618Ta(V)-(0) Ta2O5+10H++10e-=2Ta+5H2O -0.750Cr(III)-(0) Cr3++3e-=Cr -0.744Nb(V)-(0) Nb2O5+l0H++10e-=2Nb+5H2O -0.644As(0)-(-III) As+3H++3e-=AsH3-0.608 U(IV)-(III) U4++e-=U3+-0.607 Ga(III)-(0) Ga3++3e-=Ga -0.549P(I)-(0) H3PO2+H++e-=P+2H2O -0.508P(III)-(I) H3PO3+2H++2e-=H3PO2+H2O -0.499*C(IV)-(III) 2CO2+2H++2e-=H2C2O4-0.49Fe(II)-(0) Fe2++2e-=Fe -0.447Cr(III)-(II) Cr3++e-=Cr2+-0.407 Cd(II)-(0) Cd2++2e-=Cd -0.4030 Se(0)-(-II) Se+2H++2e-=H2Se(aq) -0.399Pb(II)-(0) PbI2+2e-=Pb+2I--0.365 Eu(III)-(II) Eu3++e-=Eu2+-0.36Pb(II)-(0) PbSO4+2e-=Pb+SO42--0.3588In(III)-(0) In3++3e-=In -0.3382 Tl(I)-(0) Tl++e-=Tl -0.336 Co(II)-(0) Co2++2e-=Co -0.28P(V)-(III) H3PO4+2H++2e-=H3PO3+H2O -0.276Pb(II)-(0) PbCl2+2e-=Pb+2Cl--0.2675 Ni (II)-(0) Ni2++2e-=Ni -0.257 V(III)-(II) V3++e-=V2+-0.255Ge(IV)-(0) H2GeO3+4H++4e-=Ge+3H2O -0.182Ag(I)-(0) AgI+e-=Ag+I--0.15224 Sn(II)-(0) Sn2++2e-=Sn -0.1375 Pb(II)-(0) Pb2++2e-=Pb -0.1262*C(IV)-(II) CO2(g)+2H++2e-=CO+H2O -0.12P(0)-(-III) P(white)+3H++3e-=PH3(g) -0.063Hg(I)-(0) Hg2I2+2e-=2Hg+2I--0.0405Fe(III)-(0) Fe3++3e-=Fe -0.037 H(I)-(0) 2H++2e-=H20.0000 Ag(I)-(0) AgBr+e-=Ag+Br-0.07133S(II.V)-(II) S4O62-+2e-=2S2O32-0.08*Ti(IV)-(III) TiO2++2H++e-=Ti3++H2O 0.1S(0)-(-II) S+2H++2e-=H2S(aq) 0.142 Sn(IV)-(II) Sn4++2e-=Sn2+0.151Sb(III)-(0) Sb2O3+6H++6e-=2Sb+3H2O 0.152Cu(II)-(I) Cu2++e-=Cu+0.153 Bi(III)-(0) BiOCl+2H++3e-=Bi+Cl-+H2O 0.1583S(VI)-(IV) SO42-+4H++2e-=H2SO3+H2O 0.172Sb(III)-(0) SbO++2H++3e-=Sb+H2O 0.212Ag(I)-(0) AgCl+e-=Ag+Cl-0.22233As(III)-(0) HAsO2+3H++3e-=As+2H2O 0.248Hg(I)-(0) Hg2Cl2+2e-=2Hg+2Cl-(饱和KCl) 0.26808Bi(III)-(0) BiO++2H++3e-=Bi+H2O 0.320U(VI)-(IV) UO22++4H++2e-=U4++2H2O 0.327C(IV)-(III) 2HCNO+2H++2e-=(CN)2+2H2O 0.330V(IV)-(III) VO2++2H++e-=V3++H2O 0.337 Cu(II)-(0) Cu2++2e-=Cu 0.3419Re(VII)-(0) ReO4-+8H++7e-=Re+4H2O 0.368Ag(I)-(0) Ag2CrO4+2e-=2Ag+CrO42-0.4470S(IV)-(0) H2SO3+4H++4e-=S+3H2O 0.449Cu(I)-(0) Cu++e-=Cu 0.521I(0)-(-I) I2+2e-=2I-0.5355I(0)-(-I) I3-+2e-=3I-0.536As(V)-(III) H3AsO4+2H++2e-=HAsO2+2H2O 0.560Sb(V)-(III) Sb2O5+6H++4e-=2SbO++3H2O 0.581Te(IV)-(0) TeO2+4H++4e-=Te+2H2O 0.593U(V)-(IV) UO2++4H++e-=U4++2H2O 0.612**Hg(II)-(I) 2HgCl2+2e-=Hg2Cl2+2Cl-0.63Pt(IV)-(II) [PtCl6]2-+2e-=[PtCl4]2-+2Cl-0.68O(0)-(-I) O2+2H++2e-=H2O20.695Pt(II)-(0) [PtCl4]2-+2e-=Pt+4Cl-0.755*Se(IV)-(0) H2SeO3+4H++4e-=Se+3H2O 0.74Fe(III)-(II) Fe3++e-=Fe2+0.771 Hg(I)-(0) Hg22++2e-=2Hg 0.7973 Ag(I)-(0) Ag++e-=Ag 0.7996Os(VIII)-(0) OsO4+8H++8e-=Os+4H2O 0.8N(V)-(IV) 2NO3-+4H++2e-=N2O4+2H2O 0.803Hg(II)-(0) Hg2++2e-=Hg 0.851Si(IV)-(0) (quartz)SiO2+4H++4e-=Si+2H2O 0.857Cu(II)-(I) Cu2++I-+e-=CuI 0.86N(III)-(I) 2HNO2+4H++4e-=H2N2O2+2H2O 0.86Hg(II)-(I) 2Hg2++2e-=Hg22+0.920N(V)-(III) NO3-+3H++2e-=HNO2+H2O 0.934Pd(II)-(0) Pd2++2e-=Pd 0.951N(V)-(II) NO3-+4H++3e-=NO+2H2O 0.957N(III)-(II) HNO2+H++e-=NO+H2O 0.983I(I)-(-I) HIO+H++2e-=I-+H2O 0.987V(V)-(IV) VO2++2H++e-=VO2++H2O 0.991V(V)-(IV) V(OH)4++2H++e-=VO2++3H2O 1.00Au(III)-(0) [AuCl4]-+3e-=Au+4Cl- 1.002Te(VI)-(IV) H6TeO6+2H++2e-=TeO2+4H2O 1.02N(IV)-(II) N2O4+4H++4e-=2NO+2H2O 1.035N(IV)-(III) N2O4+2H++2e-=2HNO21.065I(V)-(-I) IO3-+6H++6e-=I-+3H2O 1.085Br(0)-(-I) Br2(aq)+2e-=2Br- 1.0873Se(VI)-(IV) SeO42-+4H++2e-=H2SeO3+H2O 1.151Cl(V)-(IV) ClO3-+2H++e-=ClO2+H2O 1.152Pt(II)-(0) Pt2++2e-=Pt 1.18Cl(VII)-(V) ClO4-+2H++2e-=ClO3-+H2O 1.189I(V)-(0) 2IO3-+12H++10e-=I2+6H2O 1.195Cl(V)-(III) ClO3-+3H++2e-=HClO2+H2O 1.214Mn(IV)-(II) MnO2+4H++2e-=Mn2++2H2O 1.224O(0)-(-II) O2+4H++4e-=2H2O 1.229Tl(III)-(I) T13++2e-=Tl+ 1.252Cl(IV)-(III) ClO2+H++e-=HClO21.277N(III)-(I) 2HNO2+4H++4e-=N2O+3H2O 1.297**Cr(VI)-(III) Cr2O72-+14H++6e-=2Cr3++7H2O 1.33Br(I)-(-I) HBrO+H++2e-=Br-+H2O 1.331Cr(VI)-(III) HCrO4-+7H++3e-=Cr3++4H2O 1.350Cl(0)-(-I) Cl2(g)+2e-=2Cl- 1.35827Cl(VII)-(-I) ClO4-+8H++8e-=Cl-+4H2O 1.389Cl(VII)-(0) ClO4-+8H++7e-=1/2Cl2+4H2O 1.39Au(III)-(I) Au3++2e-=Au+ 1.401Br(V)-(-I) BrO3-+6H++6e-=Br-+3H2O 1.423I(I)-(0) 2HIO+2H++2e-=I2+2H2O 1.439Cl(V)-(-I) ClO3-+6H++6e-=Cl-+3H2O 1.451Pb(IV)-(II) PbO2+4H++2e-=Pb2++2H2O 1.455Cl(V)-(0) ClO3-+6H++5e-=1/2Cl2+3H2O 1.47Cl(I)-(-I) HClO+H++2e-=Cl-+H2O 1.482Br(V)-(0) BrO3-+6H++5e-=l/2Br2+3H2O 1.482Au(III)-(0) Au3++3e-=Au 1.498Mn(VII)-(II) MnO4-+8H++5e-=Mn2++4H2O 1.507Mn(III)-(II) Mn3++e-=Mn2+ 1.5415Cl(III)-(-I) HClO2+3H++4e-=Cl-+2H2O 1.570Br(I)-(0) HBrO+H++e-=l/2Br2(aq)+H2O 1.574N(II)-(I) 2NO+2H++2e-=N2O+H2O 1.591I(VII)-(V) H5IO6+H++2e-=IO3-+3H2O 1.601Cl(I)-(0) HClO+H++e-=1/2Cl2+H2O 1.611Cl(III)-(I) HClO2+2H++2e-=HClO+H2O 1.645Ni(IV)-(II) NiO2+4H++2e-=Ni2++2H2O 1.678Mn(VII)-(IV) MnO4-+4H++3e-=MnO2+2H2O 1.679Pb(IV)-(II) PbO2+SO42-+4H++2e-=PbSO4+2H2O 1.6913Au(I)-(0) Au++e-=Au 1.692 Ce(IV)-(III) Ce4++e-=Ce3+ 1.72N(I)-(0) N2O+2H++2e-=N2+H2O 1.766O(-I)-(-II) H2O2+2H++2e-=2H2O 1.776Co(III)-(II) Co3++e-=Co2+(2mol·L-1 H2SO4) 1.83Ag(II)-(I) Ag2++e-=Ag+ 1.980S(VII)-(VI) S2O82-+2e-=2SO42- 2.010O(0)-(-II) O3+2H++2e-=O2+H2O 2.076O(II)-(-II) F2O+2H++4e-=H2O+2F- 2.153Fe(VI)-(III) FeO42-+8H++3e-=Fe3++4H2O 2.20O(0)-(-II) O(g)+2H++2e-=H2O 2.421F(0)-(-I) F2+2e-=2F- 2.866F2+2H++2e-=2HF 3.0532电对方程式E /VCa(II)-(0) Ca(OH)2+2e-=Ca+2OH--3.02Ba(II)-(0) Ba(OH)2+2e-=Ba+2OH--2.99La(III)-(0) La(OH)3+3e-=La+3OH--2.90Sr(II)-(0) Sr(OH)2·8H2O+2e-=Sr+2OH-+8H2O -2.88Mg(II)-(0) Mg(OH)2+2e-=Mg+2OH--2.690Be(II)-(0) Be2O32-+3H2O+4e-=2Be+6OH--2.63Hf(IV)-(0) HfO(OH)2+H2O+4e-=Hf+4OH--2.50Zr(IV)-(0) H2ZrO3+H2O+4e-=Zr+4OH--2.36Al(III)-(0) H AlO-+H O+3e-=Al+OH--2.33P(I)-(0) H2PO2-+e-=P+2OH--1.82B(III)-(0) H2BO3-+H2O+3e-=B+4OH--1.79P(III)-(0) HPO32-+2H2O+3e-=P+5OH--1.71Si(IV)-(0) SiO32-+3H2O+4e-=Si+6OH--1.697P(III)-(I) HPO32-+2H2O+2e-=H2PO2-+3OH--1.65Mn(II)-(0) Mn(OH)2+2e-=Mn+2OH--1.56Cr(III)-(0) Cr(OH)3+3e-=Cr+3OH--1.48*Zn(II)-(0) [Zn(CN)4]2-+2e-=Zn+4CN--1.26Zn(II)-(0) Zn(OH)2+2e-=Zn+2OH--1.249Ga(III)-(0) H2GaO3-+H2O+2e-=Ga+4OH--1.219Zn(II)-(0) ZnO22-+2H2O+2e-=Zn+4OH--1.215Cr(III)-(0) CrO2-+2H2O+3e-=Cr+4OH--1.2Te(0)-(-I) Te+2e-=Te2--1.143P(V)-(III) PO43-+2H2O+2e-=HPO32-+3OH--1.05*Zn(II)-(0) [Zn(NH3)4]2++2e-=Zn+4NH3-1.04*W(VI)-(0) WO42-+4H2O+6e-=W+8OH--1.01*Ge(IV)-(0) HGeO3-+2H2O+4e-=Ge+5OH--1.0Sn(IV)-(II) [Sn(OH)6]2-+2e-=HSnO2-+H2O+3OH--0.93S(VI)-(IV) SO42-+H2O+2e-=SO32-+2OH--0.93Se(0)-(-II) Se+2e-=Se2--0.924Sn(II)-(0) HSnO2-+H2O+2e-=Sn+3OH--0.909P(0)-(-III) P+3H2O+3e-=PH3(g)+3OH--0.87N(V)-(IV) 2NO3-+2H2O+2e-=N2O4+4OH--0.85H(I)-(0) 2H2O+2e-=H2+2OH--0.8277Cd(II)-(0) Cd(OH)2+2e-=Cd(Hg)+2OH--0.809Co(II)-(0) Co(OH)2+2e-=Co+2OH--0.73Ni(II)-(0) Ni(OH)2+2e-=Ni+2OH--0.72As(V)-(III) AsO43-+2H2O+2e-=AsO2-+4OH--0.71Ag(I)-(0) Ag2S+2e-=2Ag+S2--0.691As(III)-(0) AsO2-+2H2O+3e-=As+4OH--0.68Sb(III)-(0) SbO2-+2H2O+3e-=Sb+4OH--0.66*Re(VII)-(IV) ReO4-+2H2O+3e-=ReO2+4OH--0.59*Sb(V)-(III) SbO3-+H2O+2e-=SbO2-+2OH--0.59Re(VII)-(0) ReO4-+4H2O+7e-=Re+8OH--0.584*S(IV)-(II) 2SO32-+3H2O+4e-=S2O32-+6OH--0.58Te(IV)-(0) TeO32-+3H2O+4e-=Te+6OH--0.57Fe(III)-(II) Fe(OH)3+e-=Fe(OH)2+OH--0.56S(0)-(-II) S+2e-=S2--0.47627Bi(III)-(0) Bi2O3+3H2O+6e-=2Bi+6OH--0.46N(III)-(II) NO2-+H2O+e-=NO+2OH--0.46*Co(II)-C(0) [Co(NH3)6]2++2e-=Co+6NH3-0.422Se(IV)-(0) SeO32-+3H2O+4e-=Se+6OH--0.366Cu(I)-(0) Cu2O+H2O+2e-=2Cu+2OH--0.360Tl(I)-(0) Tl(OH)+e-=Tl+OH--0.34 *Ag(I)-(0) [Ag(CN)2]-+e-=Ag+2CN--0.31Cu(II)-(0) Cu(OH)2+2e-=Cu+2OH--0.222Cr(VI)-(III) CrO42-+4H2O+3e-=Cr(OH)3+5OH--0.13*Cu(I)-(0) [Cu(NH3)2]++e-=Cu+2NH3-0.12O(0)-(-I) O2+H2O+2e-=HO2-+OH--0.076Ag(I)-(0) AgCN+e-=Ag+CN--0.017N(V)-(III) NO3-+H2O+2e-=NO2-+2OH-0.01Se(VI)-(IV) SeO42-+H2O+2e-=SeO32-+2OH-0.05Pd(II)-(0) Pd(OH)2+2e-=Pd+2OH-0.07S(II,V)-(II) S4O62-+2e-=2S2O32-0.08Hg(II)-(0) HgO+H2O+2e-=Hg+2OH-0.0977Co(III)-(II) [Co(NH3)6]3++e-=[Co(NH3)6]2+0.108Pt(II)-(0) Pt(OH)2+2e-=Pt+2OH-0.14Co(III)-(II) Co(OH)3+e-=Co(OH)2+OH-0.17Pb(IV)-(II) PbO2+H2O+2e-=PbO+2OH-0.247I(V)-(-I) IO3-+3H2O+6e-=I-+6OH-0.26Cl(V)-(III) ClO3-+H2O+2e-=ClO2-+2OH-0.33Ag(I)-(0) Ag2O+H2O+2e-=2Ag+2OH-0.342Fe(III)-(II) [Fe(CN)6]3-+e-=[Fe(CN)6]4-0.358Cl(VII)-(V) ClO4-+H2O+2e-=ClO3-+2OH-0.36*Ag(I)-(0) [Ag(NH3)2]++e-=Ag+2NH30.373O(0)-(-II) O2+2H2O+4e-=4OH-0.401I(I)-(-I) IO-+H2O+2e-=I-+2OH-0.485*Ni(IV)-(II) NiO2+2H2O+2e-=Ni(OH)2+2OH-0.490Mn(VII)-(VI) MnO4-+e-=MnO42-0.558Mn(VII)-(IV) MnO4-+2H2O+3e-=MnO2+4OH-0.595Mn(VI)-(IV) MnO42-+2H2O+2e-=MnO2+4OH-0.60Ag(II)-(I) 2AgO+H2O+2e-=Ag2O+2OH-0.607。
标准电极电势表 (碱)
电对方程式E /VCa(II)-(0) Ca(OH)2+2e-=Ca+2OH--3.02 Ba(II)-(0) Ba(OH)2+2e-=Ba+2OH--2.99 La(III)-(0) La(OH)3+3e-=La+3OH--2.90 Sr(II)-(0) Sr(OH)2·8H2O+2e-=Sr+2OH-+8H2O -2.88 Mg(II)-(0) Mg(OH)2+2e-=Mg+2OH--2.690 Be(II)-(0) Be2O32-+3H2O+4e-=2Be+6OH--2.63 Hf(IV)-(0) HfO(OH)2+H2O+4e-=Hf+4OH--2.50 Zr(IV)-(0) H2ZrO3+H2O+4e-=Zr+4OH--2.36 Al(III)-(0) H2AlO3-+H2O+3e-=Al+OH--2.33 P(I)-(0) H2PO2-+e-=P+2OH--1.82 B(III)-(0) H2BO3-+H2O+3e-=B+4OH--1.79 P(III)-(0) HPO32-+2H2O+3e-=P+5OH--1.71 Si(IV)-(0) SiO32-+3H2O+4e-=Si+6OH--1.697 P(III)-(I) HPO32-+2H2O+2e-=H2PO2-+3OH--1.65 Mn(II)-(0) Mn(OH)2+2e-=Mn+2OH--1.56 Cr(III)-(0) Cr(OH)3+3e-=Cr+3OH--1.48 *Zn(II)-(0) [Zn(CN)4]2-+2e-=Zn+4CN--1.26 Zn(II)-(0) Zn(OH)2+2e-=Zn+2OH--1.249 Ga(III)-(0) H2GaO3-+H2O+2e-=Ga+4OH--1.219 Zn(II)-(0) ZnO22-+2H2O+2e-=Zn+4OH--1.215 Cr(III)-(0) CrO2-+2H2O+3e-=Cr+4OH--1.2 Te(0)-(-I) Te+2e-=Te2--1.143 P(V)-(III) PO43-+2H2O+2e-=HPO32-+3OH--1.05*Zn(II)-(0) [Zn(NH3)4]2++2e-=Zn+4NH3-1.04*W(VI)-(0) WO42-+4H2O+6e-=W+8OH--1.01*Ge(IV)-(0) HGeO3-+2H2O+4e-=Ge+5OH--1.0Sn(IV)-(II) [Sn(OH)6]2-+2e-=HSnO2-+H2O+3OH--0.93S(VI)-(IV) SO42-+H2O+2e-=SO32-+2OH--0.93Se(0)-(-II) Se+2e-=Se2--0.924 Sn(II)-(0) HSnO2-+H2O+2e-=Sn+3OH--0.909P(0)-(-III) P+3H2O+3e-=PH3(g)+3OH--0.87N(V)-(IV) 2NO3-+2H2O+2e-=N2O4+4OH--0.85H(I)-(0) 2H2O+2e-=H2+2OH--0.8277 Cd(II)-(0) Cd(OH)2+2e-=Cd(Hg)+2OH--0.809 Co(II)-(0) Co(OH)2+2e-=Co+2OH--0.73Ni(II)-(0) Ni(OH)2+2e-=Ni+2OH--0.72As(V)-(III) AsO43-+2H2O+2e-=AsO2-+4OH--0.71Ag(I)-(0) Ag2S+2e-=2Ag+S2--0.691 As(III)-(0) AsO2-+2H2O+3e-=As+4OH--0.68Sb(III)-(0) SbO2-+2H2O+3e-=Sb+4OH--0.66*Re(VII)-(IV) ReO4-+2H2O+3e-=ReO2+4OH--0.59*Sb(V)-(III) SbO3-+H2O+2e-=SbO2-+2OH--0.59Re(VII)-(0) ReO4-+4H2O+7e-=Re+8OH--0.584 *S(IV)-(II) 2SO32-+3H2O+4e-=S2O32-+6OH--0.58Te(IV)-(0) TeO32-+3H2O+4e-=Te+6OH--0.57Fe(III)-(II) Fe(OH)3+e-=Fe(OH)2+OH--0.56S(0)-(-II) S+2e-=S2--0.47627 Bi(III)-(0) Bi2O3+3H2O+6e-=2Bi+6OH--0.46N(III)-(II) NO2-+H2O+e-=NO+2OH--0.46 *Co(II)-C(0) [Co(NH3)6]2++2e-=Co+6NH3-0.422 Se(IV)-(0) SeO32-+3H2O+4e-=Se+6OH--0.366 Cu(I)-(0) Cu2O+H2O+2e-=2Cu+2OH--0.360 Tl(I)-(0) Tl(OH)+e-=Tl+OH--0.34 *Ag(I)-(0) [Ag(CN)2]-+e-=Ag+2CN--0.31 Cu(II)-(0) Cu(OH)2+2e-=Cu+2OH--0.222 Cr(VI)-(III) CrO42-+4H2O+3e-=Cr(OH)3+5OH--0.13 *Cu(I)-(0) [Cu(NH3)2]++e-=Cu+2NH3-0.12 O(0)-(-I) O2+H2O+2e-=HO2-+OH--0.076 Ag(I)-(0) AgCN+e-=Ag+CN--0.017 N(V)-(III) NO3-+H2O+2e-=NO2-+2OH-0.01 Se(VI)-(IV) SeO42-+H2O+2e-=SeO32-+2OH-0.05 Pd(II)-(0) Pd(OH)2+2e-=Pd+2OH-0.07S(II,V)-(II) S4O62-+2e-=2S2O32-0.08 Hg(II)-(0) HgO+H2O+2e-=Hg+2OH-0.0977 Co(III)-(II) [Co(NH3)6]3++e-=[Co(NH3)6]2+0.108 Pt(II)-(0) Pt(OH)2+2e-=Pt+2OH-0.14 Co(III)-(II) Co(OH)3+e-=Co(OH)2+OH-0.17 Pb(IV)-(II) PbO2+H2O+2e-=PbO+2OH-0.247 I(V)-(-I) IO3-+3H2O+6e-=I-+6OH-0.26Cl(V)-(III) ClO3-+H2O+2e-=ClO2-+2OH-0.33 Ag(I)-(0) Ag2O+H2O+2e-=2Ag+2OH-0.342 Fe(III)-(II) [Fe(CN)6]3-+e-=[Fe(CN)6]4-0.358 Cl(VII)-(V) ClO4-+H2O+2e-=ClO3-+2OH-0.36*Ag(I)-(0) [Ag(NH3)2]++e-=Ag+2NH30.373 O(0)-(-II) O2+2H2O+4e-=4OH-0.401 I(I)-(-I) IO-+H2O+2e-=I-+2OH-0.485 *Ni(IV)-(II) NiO2+2H2O+2e-=Ni(OH)2+2OH-0.490 Mn(VII)-(VI) MnO4-+e-=MnO42-0.558 Mn(VII)-(IV) MnO4-+2H2O+3e-=MnO2+4OH-0.595 Mn(VI)-(IV) MnO42-+2H2O+2e-=MnO2+4OH-0.60 Ag(II)-(I) 2AgO+H2O+2e-=Ag2O+2OH-0.607 Br(V)-(-I) BrO3-+3H2O+6e-=Br-+6OH-0.61 Cl(V)-(-I) ClO3-+3H2O+6e-=Cl-+6OH-0.62 Cl(III)-(I) ClO2-+H2O+2e-=ClO-+2OH-0.66 I(VII)-(V) H3IO62-+2e-=IO3-+3OH-0.7 Cl(III)-(-I) ClO2-+2H2O+4e-=Cl-+4OH-0.76 Br(I)-(-I) BrO-+H2O+2e-=Br-+2OH-0.761 Cl(I)-(-I) ClO-+H2O+2e-=Cl-+2OH-0.841 *Cl(IV)-(III) ClO2(g)+e-=ClO2-0.95。
标准电极电势表(全)
电对
方程式
EN
Li(l)—(0)
Li++e-=Li
—
Cs(l)-(0)
CS+e=Cs
—
Rb(l)—(0)
Rb++e-=Rb
—
K(l)-(0)
K+e-=K
—
Ba(ll)-(0)
BsT+2e-=Ba
—
Sr(ll)-(0)
Si2++2e-=Sr
—
Ca(ll-(0)
Cc2+2e=Ca
TeQ+4H++4e—=Te+2H2O
U(V)—(IV)
UQ++4H++e—=U4++2H2O
**Hg(II)—(I)
2HgC2+2e—=Hg?Cb+2C「
Pt(IV)—(II)
[PtC6]2-+2e—=[PtC4]2-+2C「
O(0)—(—I)
O2+2H+2e=H2O2
Pt(II)—(0)
[PtCl4]2-+2e—=Pt+4C「
—
ln (lll)-(0)
In3++3e-=In
—
Tl(l)-(0)
Tl++e-=Tl
—
Co(ll)- (0)
Cc?+2e=Co
—
P(V)-(lll)
H3PO4+2H++2e-=H3PO3+H2O
—
Pb(ll)-(0)
PbC2+2e-=Pb+2C-
最全的标准电极电势(无表格版)
--标准--标准电极电势表--1在酸性溶液中(298K)电对方程式 E /VLi(l) —(0) Li + + e— = Li —3.0401Cs(l) —(0) Cs + + e — = Cs —3.026 Rb(l) —(0) Rb + + e — = Rb —2.98 K(l) —(0) K + + e— = K —2.931 Ba(ll) —(0) Ba2+ + 2e — = Ba —2.912 Sr(ll) —(0) Sr2+ + 2e — = Sr —2.89 Ca(ll) —(0) Ca2+ + 2e — = Ca —2.868 Na(l) —(0) Na + + e — = Na —2.71 La(lll) —(0) La3 + + 3e — = La —2.379 Mg(ll) —(0) Mg 2+ + 2e — = Mg —2.372 Ce(lll) —(0) Ce3 + + 3e — = Ce —2.336 H(0) —(—l) H2(g) + 2e — = 2H ——2.23 Al(lll) —(0) AIF63 — + 3e — = Al + 6F ——2.069 Th(IV) —(0) Th4+ + 4e — = Th —1.899 Be(ll) —(0) Be2+ + 2e — = Be —1.847 U(lll) —(0) U3+ + 3e — = U —1.798 Hf(IV) —(0) HfO 2+ + 2H ++ 4e — = Hf + H 20 —1.724 Al(lll) —(0) Al3+ + 3e —= Al —1.662 Ti(ll) —(0) Ti2+ + 2e — = Ti —1.630Zr(IV) -(0) ZrO 2+ 4H + + 4e 一= Zr + 2H 2O -1.553 Si(IV) -(0) [SiF6]2- + 4e - = Si + 6F --1.24 Mn(II) -(0) Mn 2 + + 2e 一= Mn -1.185 Cr(II) -(0) Cr2+ + 2e -= Cr -0.913 Ti(III) -(II) Ti3+ + e -= Ti2+-0.9B(III) -(0) H 3BO 3+ 3H + + 3e 一= B + 3H 2O-0.8698*Ti(IV) -(0) TiO 2 + 4H + + 4e 一= Ti + 2H 2O -0.86 Te(0) -(-II)Te + 2H + + 2e - = H2Te -0.793 Zn(II) -(0) Zn2 + + 2e —= Zn -0.7618 Ta(V) -(0) Ta2O5 + 10H + + 10e 一= 2Ta + 5H 2O -0.750 Cr(III) -(0) Cr3 + + 3e —= Cr -0.744 Nb(V) -(0) Nb 2O5+ 10H + + 10e —= 2Nb + 5H 2O -0.644 As(0) -(-III) As + 3H + + 3e —= AsH 3 -0.608 U(IV) -(III) U4+ + e-= U3+-0.607 Ga(III) -(0) Ga3 + + 3e 一= Ga -0.549 P(I) -(0) H3PO2 + H + + e —= P + 2H 2O -0.508 P(III) -(I) H3PO3 + 2H + + 2e 一= H3PO2 + H2O -0.499 *C(IV) -(III) 2CO2 + 2H + + 2e H2C2O4 -0.49 Fe(II) -(0) Fe2 ++ 2e —= Fe -0.447 Cr(III) -(II) Cr3 + + e- = Cr2 +-0.407 Cd(II) -(0) Cd2 + + 2e - = Cd -0.4030 Se(0) -(-II)Se + 2H + + 2e 一= H2Se(aq) -0.399Pb(II) -(0) Pbl 2+ 2e —= Pb + 2I —-0.365 Eu(III) -(II) Eu3+ + e- = Eu2+-0.36Pb(II) -(0) PbSO 4+ 2e - = Pb + SO42--0.3588 In(III) -(0) ln3+ + 3e —= In -0.3382 Tl(I) -(0) Tl ++ e —= Tl -0.336 Co(II) -(0) Co2 + + 2e - = Co -0.28P(V) -(III) H3PO4 + 2H + + 2e 一= H3PO3 + H2O -0.276 Pb(II) -(0) PbCI 2 + 2e —= Pb + 2CI —-0.2675 Ni (II) -(0) Ni2 + + 2e —= Ni -0.257V(III) -(II) V3 + + e- = V2+-0.255 Ge(IV) -(0) H 2GeO 3 + 4H + + 4e 一= Ge + 3H 2 O -0.182 Ag(I) -(0) Agl + e _= Ag + I —-0.15224 Sn(II) -(0) Sn2++2e-=Sn -0.1375 Pb(II) -(0) Pb2++2e-=Pb -0.1262**C(IV) -(II) CO2(g)+2H++2e-=CO+H2O -0.12P(0) -( -III) P(white) +3H++3e-=PH3(g) -0.063 Hg(I) -(0) Hg2I2+2e-=2Hg +2I--0.0405 Fe(III) -(0) Fe3+ + 3e -= Fe -0.037H(I) -(0) 2H + + 2e -= H2 0.0000 Ag(I) -(0) AgBr + e-= Ag + Br -0.07133 S(II.V) -(II) S4O62-+2e-=2S2O32-0.08*Ti(IV) -(III) TiO2++2H++e-=Ti3++H2O 0.1S(0) -( -II) S+ 2H + + 2e —= H2S(aq) 0.142 Sn(IV) -(II) Sn4+ + 2e —= Sn2+0.151 Sb(III) -(0) Sb2O3 + 6H + + 6e 一= 2Sb + 3H 20 0.152 Cu(II) -(I) Cu2+ + e- = Cu +0.153 Bi(III) -(0) BiOCI + 2H ++ 3e 一= Bi + Cl 一+ H20 0.1583 S(VI) -(IV) S04_+ 4H + + 2e —= H2SO3 + H20 0.172 Sb(III) -(0) SbO + + 2H ++ 3e 一= Sb + H20 0.212 Ag(I) -(0) AgCI + e = Ag + Cl 0.22233 As(III) -(0) HAsO 2 + 3H + + 3e 一= As + 2H 20 0.248 Hg(I) -(0) Hg 2CI2+ 2e-= 2Hg + 2CI -(饱和KCI) 0.26808 Bi(III) -(0) BiO ++ 2H ++ 3e -= Bi + H 2O 0.320U(VI) -(IV) UO 22++ 4H ++ 2e -= U4++ 2H 2O 0.327C(IV) -(III) 2HCNO + 2H ++ 2e -= (CN) 2+ 2H 2O 0.330V(IV) -(III) VO 2++ 2H ++ e-= V3++ H 2O 0.337 Cu(II) -(0) Cu 2++ 2e -= Cu 0.3419 Re(VII) -(0) ReO 4-+ 8H ++7e -= Re+ 4H 2O 0.368 Ag(I) -(0) Ag 2CrO 4+ 2e -= 2Ag + CrO 42-0.4470 S(IV) -(0) H 2SO 3+ 4H + + 4e -= S+ 3H 2O 0.449 Cu(I) -(0) Cu + + e -= Cu 0.521I(0) -(-I) I2+ 2e -= 2I-0.5355 I(0) -(-I) I3 -+ 2e -= 3I -0.536 As(V) -(III) H 3AsO 4+ 2H ++ 2e -= HAsO 2+ 2H 2O 0.560Sb(V)(III) Sb2O5 + 6H + + 4e 一= 2SbO 十+ 3H 20 0.581 -Te(IV) -(0) TeO 2+ 4H + + 4e 一= Te + 2H 20 0.593 U(V) -(IV) UO2 ++ 4H + + e —= U4+ + 2H 2O 0.612**** Hg(II) -(I) 2HgCI 2 + 2e 一= Hg 2CI2 + 2CI —0.63 Pt(IV) -(II) [PtCI 6]2- + 2e —= [PtCI 4]2- + 2CI —0.68O(0) - (-I) O2+ 2H + + 2e —= H2O2 0.695 Pt(II) -(0) [PtCI 4]2- + 2e - = Pt + 4CI -0.755 *Se(IV) -(0) H 2SeO 3 + 4H ++ 4e 一= Se + 3H 2O 0.74 Fe(III) -(II) Fe3++ e —= Fe2+0.771 Hg(I) -(0) Hg 22+ + 2e 一= 2Hg 0.7973 Ag(I) -(0) Ag + + e -= Ag 0.7996 Os(VIII) -(0) OsO 4 + 8H + + 8e 一= Os + 4H 2O 0.8N(V) -(IV) 2NO 3- + 4H + + 2e - = N2O4 + 2H 2O 0.803 Hg(II) -(0) Hg 2+ + 2e _ = Hg 0.851Si(IV)(0) (quartz)SiO 2 + 4H + + 4e 一= Si + 2H 2O 0.857 -Cu(II)(I) Cu2 ++厂 + e-= CuI 0.86 -N(III) -(I) 2HNO 2 + 4H + + 4e 一= H2N2O2 + 2H 2O 0.86 Hg(II) -(I) 2Hg 2 + + 2e - = Hg 22+0.920 N(V) -(III) NO 3- + 3H + + 2e HNO 2+ H2O 0.934Pd(II)(0) Pd2 + + 2e —= Pd 0.951 -N(V) -(II) NO 3一+ 4H ++ 3e 一= NO + 2H2O 0.957 N(III) -(II) HNO 2+ H + + e —= NO + H2O 0.983I(I) -(-I) HIO + H + + 2e —=厂+ H2O 0.987 V(V) -(IV) VO 2 + + 2H + + e - = VO2+ + H2O 0.991 V(V) -(IV) V(OH) 4 + + 2H + + e —= VO2十+ 3H 2O 1.00 Au(III) -(0) [AuCI 4] 一+ 3e —= Au + 4Cl — 1.002 Te(VI) -(IV) H6TeO 6 + 2H ++ 2e —= TeO 2 + 4H 2O 1.02N(IV) -(II) N2O4 + 4H + + 4e —= 2NO + 2H 2O 1.035 N(IV) -(III) N2O4 + 2H + + 2e 一= 2HNO 2 1.065 I(V) -( -I) IO 3- + 6H + + 6e —=厂+ 3H 2O 1.085 Br(0) -(-I) Br2(aq) + 2e —= 2Br — 1.0873 Se(VI) -(IV) SeO42- + 4H + + 2e 一= H2SeO3 + H 2O 1.151 Cl(V) -(IV) CIO 3一+ 2H + + e —= ClO2 + H2O 1.152 Pt(II) -(0) Pt2+ + 2e —= Pt 1.18 Cl(VII) -(V) CIO 4- + 2H + + 2e - = CIO 3-+ H2O 1.189 I(V) -(0) 2IO 3- + 12H + + 10e —= I2+ 6H 2O 1.195 Cl(V) -(III) ClO 3- + 3H + + 2e —= HClO 2+ H2O 1.214 Mn(IV) -(II) MnO 2 + 4H + + 2e 一= Mn 2++ 2H 2O 1.224 O(0) -(-II) O2+ 4H + + 4e —= 2H 2O 1.229 Tl(III) -(I) T13+ + 2e - = Tl + 1.252 Cl(IV) -(III) ClO 2 + H + + e -= HClO 2 1.277 N(III) -(I) 2HNO 2 + 4H + + 4e 一= N2O + 3H 2O 1.297**Cr2O7_ + 14H + + 6e 一= 2Cr3+ + 7H 2O 1.33 **Cr(VI) -(III)Br(I) -(-I) HBrO + H + + 2e 一= BL+ H2O 1.331Cr(VI) -(III) HCrO 4- + 7H + + 3e -=Cr3 ++ 4H2O 1.350 Cl(0) -( -I) Cl2(g) + 2e - = 2CI - 1.35827Cl(VII) -(-I)ClO 4-+ 8H+ + 8e -=CIT 4H 2O 1.389Cl(VII) -(0)ClO 4-+ 8H+ + 7e -=1/2CI 2+ 4H 2O 1.39Au(III) -(I)Au 3 ++ 2e —= Au + 1.401Br(V) -(-I) BrO 3 -+ 6H + + 6e -=BL + 3H 2O 1.423 I(I) -(0) 2HIO + 2H+ + 2e —= I2+ 2H 2O 1.439Cl(V) -(-I) ClO 3-+ 6H + + 6e -=CI- + 3H 2O 1.451 Pb(IV) -(II) PbO 2 + 4H + + 2e —Pb 2++ 2H 2O 1.455Cl(V) -(0) ClO 3-+ 6H + + 5e -=1/2CI 2+ 3H 2O 1.47 Cl(I) -( -I) HClO + H + + 2e —=CI -+ H 2O 1.482 Br(V) -(0) BrO 3 -+ 6H + + 5e -=I/2Br 2 + 3H 2O 1.482 Au(III) -(0) Au3 ++ 3e —= Au 1.498 Mn(VII) -(II) MnO 4 -+ 8H ++ 5e -=Mn 2+ + 4H 2O 1.507 Mn(III) -(II) Mn 3+ + e —= Mn 2+ 1.5415 Cl(III) -( -I) HClO 2+ 3H ++ 4e -=CU 2H 2O 1.570 Br(I) -(0) HBrO + H + + e —= I/2Br 2(aq) + H2O 1.574 N(II) -(I) 2NO + 2H ++ 2e —N2O+H2O 1.591 I(VII) -(V) H 5IO 6 + H + + 2e —= IO 3-+ 3H 2O 1.601 Cl(I) -(0) HClO + H + + e —= 1/2CI 2 + H2O 1.611 Cl(III) -(I) HClO 2+ 2H ++ 2e -=HCIO + H2O 1.645 Ni(IV) -(II) NiO 2+ 4H + + 2e 一=Ni2++2H2O 1.678Mn (VII) —(IV) MnO 4 — + 4H + + 3e — = MnO 2 + 2H 20 1.679 Pb(IV) —(II) PbO 2 + SO42— + 4H + + 2e — = PbSO 4+2H 2O 1.6913 Au(I) —(0) Au + + e — = Au 1.692 Ce(IV) —(III) Ce4+ + e — = Ce3+ 1.72N(I) —(0) N2O + 2H + + 2e — = N2+ H2O 1.766O( —I) —( —H2O2 + 2H + + 2e —= 2H 2O 1.776 II)Co(III) —(II) Co3 + + e — = Co2 +(2mol •—1 H 2SO 4) 1.83 Ag(II) —(I) Ag 2 + + e— = Ag + 1.980 S(VII) —(VI) S2O82 — + 2e — = 2SO42— 2.010 0(0) —(—II) O3+ 2H + + 2e — = O2+ H2O 2.076 O(II) —(—II) F2O + 2H + + 4e — = H2O + 2F — 2.153 Fe(VI) —(III) FeO42 — + 8H + + 3e —= Fe3+ + 4H 2O 2.200(0) —(—II) O(g) + 2H + + 2e — = H2O 2.421 F(0) —( —I) F2 + 2e — = 2F — 2.866F2 + 2H + + 2e — = 2HF 3.053 2在碱性溶液中(298K)电对方程式 E /V Ca(II) —(0) Ca(OH) 2+ 2e — = Ca + 2OH ——3.02 Ba(II) —(0) Ba(OH) 2+ 2e — = Ba + 2OH ——2.99 La(III) —(0) La(OH) 3+ 3e —= La + 3OH ——2.90 Sr(II) —(0) Sr(OH) 2 • 8HO + 2e — = Sr + 2OH — + 8H 2O — 2.88Mg(II) —(0) Mg(OH) 2 + 2e — = Mg + 2OH ——2.690Be(II) -(0) Be 2O32_+ 3H 20 + 4e —= 2Be + 60H ——2.63Hf(IV) -(0)HfO(OH) 1 2+ H 20 + 4e —= Hf + 40H ——2.50Zr(IV) -(0) H 2ZrO 3 + H20 + 4e — = Zr + 40H ——2.36Al(III) -(0) H 2AlO 3 —+ H20 + 3e — = Al + 0H ——2.33P(I) -(0) H 2PO 2-+ e — = P+ 20H ——1.82B(III) -(0) H 2BO 3-+ H20 + 3e — = B + 40H ——1.79P(III) -(0)HPO 32-+ 2H 20 + 3e —= P+ 50H ——1.71 Si(IV) -(0) SiO 32-+ 3H 2O + 4e- = Si + 60H -—1.697 P(III) -(I) HPO 32-+ 2H 20 + 2e —= H2PO2— + 30H ——1.65 Mn(II) -(0) Mn(OH) 2 + 2e — = Mn + 20H ——1.56 Cr(III) -(0) Cr(OH) 3 + 3e —= Cr + 30H ——1.48 *Zn(II) -(0) [Zn(CN) 4]2-+ 2e — = Zn + 4CN ——1.26 Zn(II) -(0) Zn(OH) 2+2e—=Zn+20H ——1.249 Ga(III) -(0) H 2GaO 3—+H20+2e—=Ga+40H ——1.219 Zn(II) -(0) ZnO 22-+2H 20+2e—=Zn +40H ——1.215 Cr(III) -(0) CrO 2一+ 2H 20 + 3e 一= Cr + 40H ——1.2Te(0) -(-I)Te +2e—= Te2——1.143P(V)-(III) PO43- + 2H 20 + 2e - = HPO 32- + 30H -—1.05*Zn(II) -(0) [Zn(NH 3)4]2++2e—=Zn+4NH 3 —1.04*W(VI) -(0) WO42-+4H20+6e—=W+80H ——1.01**Ge(IV) -(0) HGeO 3 —+2H20+4e—=Ge+50H ——1.0Sn(IV) -(II) [Sn(OH) 6]2—+2e—=HSn0 2—+H20+30H—0.93S(VI) -(IV) S04—+ H20 + 2e-= SO32- + 2OH --0.93Se(0) -(-II) Se + 2e - = Se —-0.924 Sn(II) -(0) HSnO 2一+ H2O + 2e 一= Sn + 3OH —-0.909P(0) -( -III) P+ 3H 2O + 3e —= PH 3(g) + 3OH —-0.87N(V) -(IV) 2NO 3 - + 2H2O + 2e 一= N2O4+ 4OH —-0.85H(I) -(0) 2H2O + 2e〜H2 + 2OH —-0.8277 Cd(II) -(0) Cd(OH) 2+ 2e —= Cd(Hg) + 2OH —-0.809 Co(II) -(0) Co(OH) 2+ 2e - = Co + 2OH --0.73Ni(II) -(0) Ni(OH) 2+ 2e 一= Ni + 2OH —-0.72As(V) -(III) AsO 43- + 2H 2O + 2e 一= AsO 2- + 4OH —-0.71Ag(I) -(0) Ag 2S+ 2e 一= 2Ag + S2—-0.691 As(III) -(0) AsO 2 一+ 2H 20 + 3e 一= As + 4OH —-0.68Sb(III) -(0) SbO 2- + 2H 2O + 3e - = Sb + 4OH --0.66*Re(VII) -(IV) ReO4一+ 2H 2O+ 3e 一= ReO 2 + 4OH —-0.59**Sb(V) -(III) SbO 3-+ H2O + 2e 一= SbO 2- + 2OH —-0.59Re(VII) -(0) ReO 4一+ 4H 2O+ 7e 一= Re + 8OH —-0.584**S(IV) -(II) 2SO 32- + 3H 2O + 4e - = S2O32+ 6OH --0.58Te(IV) -(0) TeO 32- + 3H 2O + 4e - = Te + 6OH --0.57Fe(III) -(II) Fe(OH) 3+ e 一= Fe(OH) 2+ OH —-0.56S(0) -( -II) S+ 2e - = S2--0.47627 Bi(III) -(0) Bi2O3 + 3H 2O + 6e 一= 2Bi + 6OH —-0.46N(III) -(II) NO 2 一+ H2O + e —= NO + 2OH —-0.46*Co(II) -C(0) [Co(NH3)6]2++ 2e —= Co + 6NH 3 -0.422 Se(IV) -(0) SeO 32- + 3H 2 O + 4e -= Se + 6OH --0.366 Cu(I) -(0) Cu2O + H2O + 2e -= 2Cu + 2OH —-0.360 Tl(I) -(0) Tl(OH) + e-= Tl + OH —-0.34 *Ag(I) -(0) [Ag(CN) 2]- + e-= Ag + 2CN —-0.31 Cu(II) -(0) Cu(OH) 2+ 2e- = Cu + 2OH —-0.222 Cr(VI) -(III) CrO 42 - + 4H 2O + 3e- = Cr(OH) 3 + 5OH —-0.13 *Cu(I) -(0) [Cu(NH 3)2] + + e- = Cu + 2NH 3 -0.12 O(0) -(-I) O2+ H2O + 2e- = HO 2- + OH —-0.076 Ag(I) -(0) AgCN + e - = Ag + CN —-0.017 N(V) -(III) NO 3 - + H2O + 2e - = NO 2 - + 2OH —0.01 Se(VI) -(IV) SeO42- + H2O + 2e -= SeO32- + 2OH -0.05 Pd(II) -(0) Pd(OH) 2+ 2e - = Pd + 2OH —0.07S(II,V) -(II) S4O62-+ 2e - = 2S2O32-0.08 Hg(II) -(0) HgO + H2O + 2e- = Hg + 2OH —0.0977 Co(III) -(II) [Co(NH 3)6]3++ e- = [Co(NH 3)6]2+0.108 Pt(II) -(0) Pt(OH) 2+ 2e -= Pt + 2OH —0.14 Co(III) -(II) Co(OH) 3+ e -= Co(OH) 2 + OH -0.17 Pb(IV) -(II) PbO 2 + H2O + 2e -= PbO + 2OH -0.247 I(V) -( -I) IO3- + 3H 2O + 6e- = l- + 6OH —0.26 Cl(V) -(III) CIO 3- + H2O + 2e - = ClO 2—+ 2OH —0.33Ag(I) -(0) Ag 2O + H2O + 2e - = 2Ag + 2OH —0.342Fe(III) -(II) [Fe(CN) 6]3一+ e一= [Fe(CN) 6]4—0.358 Cl(VII) -(V) CIO 4一+ H 20 + 2e —= ClO 3—+ 2OH —0.36*Ag(I) -(0) [Ag(NH 3)2] + + e一= Ag + 2NH 3 0.373 O(0) -(-II) 02+ 2H 20 + 4e —= 40H —0.401 I(I) -(-I) I0 一+ H20 + 2e —=厂 + 20H —0.485 *Ni(IV) -(II) NiO 2+ 2H 20 + 2e 一= Ni(0H) 2 + 20H —0.490 Mn(VII) -(VI) Mn0 4- + e —= Mn0 42-0.558 Mn(VII) -(IV) Mn0 4- + 2H 20 + 3e 一= Mn0 2 + 40H —0.595 Mn(VI) -(IV) Mn0 42一+ 2H 20 + 2e 一= Mn0 2+ 40H —0.60 Ag(II) -(I) 2Ag0 + H20 + 2e一= Ag 20 + 20H —0.607 Br(V) -(-I) Br0 3一+ 3H 20 + 6e 一= BL + 60H —0.61 Cl(V) -(-I) CI03—+ 3H 20 + 6e 一= Cl_+ 60H —0.62 Cl(III) -(I) Cl02-+H20+2e-=Cl0-+20H -0.66 I(VII) -(V) H3I062-+2e-=I03-+30H-0.7 Cl(III) -( -I) CI02-+2H20+4e-=CI-+40H-0.76 Br(I) -(-I) Br0-+H20+2e-=Br-+20H-0.761 Cl(I) -( -I) CI0-+H20+2e-=CI-+20H -0.841 *Cl(IV) -(III) CI0 2(g) +e-= CI0 2-0.95O(0) -(-II) 03+H20+2e-=02+20H - 1.24 R.Lide, Handbook of Chemistry and Physics, 8 -25 -8 -30, 78th. edition, 1997 -1998 Dean Ed,Lange ' s Handbook of Chemistry, 13th. edition, 1985 参考书.。
标准电极电势表(酸+碱)
0.761
68
BrO 3-+6H ++6e═ Br -+3H 2O
1.423
69
BrO 3-+3H 2O+6e═ Br -+6OH -
0.61
70
2BrO 3-+12H ++10e═Br 2+6H 2O
1.482
71
HBrO+H ++2e═Br -+H 2O
1.331
72
2HBrO+2H ++2e═Br 2(水溶液 ,aq)+2H 2O
0.465
9
Ag2CrO
4+2e═ 2Ag+CrO
24
0.447
10
AgF+e═ Ag+F -
6]+4e═ 4Ag+[Fe(CN) 6]4-
0.148
12
AgI+e ═ Ag+I -
-0.152
13
AgIO
3+e═ Ag+IO
3
0.354
14
Ag2MoO
4+2e═ 2Ag+MoO
2ClO 3-+12H ++10e═Cl 2+6H 2O
1.47
97
ClO 3-+6H ++6e═ Cl -+3H 2O
1.451
98
ClO 3-+3H 2O+6e═ Cl -+6OH -
0.62
99
ClO 4-+8H ++8e═ Cl -+4H 2O
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
标准电极电势表 --酸性溶液中(298K)>>> 碱性溶液中(298K)>>>1电对方程式E /VLi(I)-(0)Li++e-=Li-3.0401 Cs(I)-(0)Cs++e-=Cs-3.026 Rb(I)-(0)Rb++e-=Rb-2.98 K(I)-(0)K++e-=K-2.931 Ba(II)-(0)Ba2++2e-=Ba-2.912 Sr(II)-(0)Sr2++2e-=Sr-2.89 Ca(II)-(0)Ca2++2e-=Ca-2.868 Na(I)-(0)Na++e-=Na-2.71 La(III)-(0)La3++3e-=La-2.379 Mg(II)-(0)Mg2++2e-=Mg-2.372 Ce(III)-(0)Ce3++3e-=Ce-2.336 H(0)-(-I)H2(g)+2e-=2H--2.23 Al(III)-(0)AlF63-+3e-=Al+6F--2.069 Th(IV)-(0)Th4++4e-=Th-1.899 Be(II)-(0)Be2++2e-=Be-1.847 U(III)-(0)U3++3e-=U-1.798 Hf(IV)-(0)HfO2++2H++4e-=Hf+H2O-1.724 Al(III)-(0)Al3++3e-=Al-1.662 Ti(II)-(0)Ti2++2e-=Ti-1.630 Zr(IV)-(0)ZrO2+4H++4e-=Zr+2H2O-1.553 Si(IV)-(0)[SiF6]2-+4e-=Si+6F--1.24 Mn(II)-(0)Mn2++2e-=Mn-1.185 Cr(II)-(0)Cr2++2e-=Cr-0.913 Ti(III)-(II)Ti3++e-=Ti2+-0.9B(III)-(0)H3BO3+3H++3e-=B+3H2O-0.8698 *Ti(IV)-(0)TiO2+4H++4e-=Ti+2H2O-0.86 Te(0)-(-II)Te+2H++2e-=H2Te-0.793 Zn(II)-(0)Zn2++2e-=Zn-0.7618 Ta(V)-(0)Ta2O5+10H++10e-=2Ta+5H2O-0.750 Cr(III)-(0)Cr3++3e-=Cr-0.744 Nb(V)-(0)Nb2O5+l0H++10e-=2Nb+5H2O-0.644 As(0)-(-III)As+3H++3e-=AsH3-0.608 U(IV)-(III)U4++e-=U3+-0.607 Ga(III)-(0)Ga3++3e-=Ga-0.549 P(I)-(0)H3PO2+H++e-=P+2H2O-0.508 P(III)-(I)H3PO3+2H++2e-=H3PO2+H2O-0.499 *C(IV)-(III)2CO2+2H++2e-=H2C2O4-0.49 Fe(II)-(0)Fe2++2e-=Fe-0.447Cr(III)-(II)Cr3++e-=Cr2+-0.407 Cd(II)-(0)Cd2++2e-=Cd-0.4030 Se(0)-(-II)Se+2H++2e-=H2Se(aq)-0.399 Pb(II)-(0)PbI2+2e-=Pb+2I--0.365 Eu(III)-(II)Eu3++e-=Eu2+-0.36 Pb(II)-(0)PbSO4+2e-=Pb+SO42--0.3588 In(III)-(0)In3++3e-=In-0.3382 Tl(I)-(0)Tl++e-=Tl-0.336 Co(II)-(0)Co2++2e-=Co-0.28P(V)-(III)H3PO4+2H++2e-=H3PO3+H2O-0.276 Pb(II)-(0)PbCl2+2e-=Pb+2Cl--0.2675 Ni (II)-(0)Ni2++2e-=Ni-0.257 V(III)-(II)V3++e-=V2+-0.255 Ge(IV)-(0)H2GeO3+4H++4e-=Ge+3H2O-0.182 Ag(I)-(0)AgI+e-=Ag+I--0.15224 Sn(II)-(0)Sn2++2e-=Sn-0.1375 Pb(II)-(0)Pb2++2e-=Pb-0.1262 *C(IV)-(II)CO2(g)+2H++2e-=CO+H2O-0.12P(0)-(-III)P(white)+3H++3e-=PH3(g)-0.063 Hg(I)-(0)Hg2I2+2e-=2Hg+2I--0.0405 Fe(III)-(0)Fe3++3e-=Fe-0.037 H(I)-(0)2H++2e-=H20.0000 Ag(I)-(0)AgBr+e-=Ag+Br-0.07133 S(II.V)-(II)S4O62-+2e-=2S2O32-0.08*Ti(IV)-(III)TiO2++2H++e-=Ti3++H2O0.1S(0)-(-II)S+2H++2e-=H2S(aq)0.142Sn(IV)-(II)Sn4++2e-=Sn2+0.151Sb(III)-(0)Sb2O3+6H++6e-=2Sb+3H2O0.152Cu(II)-(I)Cu2++e-=Cu+0.153Bi(III)-(0)BiOCl+2H++3e-=Bi+Cl-+H2O0.1583S(VI)-(IV)SO42-+4H++2e-=H2SO3+H2O0.172Sb(III)-(0)SbO++2H++3e-=Sb+H2O0.212Ag(I)-(0)AgCl+e-=Ag+Cl-0.22233 As(III)-(0)HAsO2+3H++3e-=As+2H2O0.248Hg(I)-(0)Hg2Cl2+2e-=2Hg+2Cl-(饱和KCl)0.26808 Bi(III)-(0)BiO++2H++3e-=Bi+H2O0.320U(VI)-(IV)UO22++4H++2e-=U4++2H2O0.327C(IV)-(III)2HCNO+2H++2e-=(CN)2+2H2O0.330V(IV)-(III)VO2++2H++e-=V3++H2O0.337Cu(II)-(0)Cu2++2e-=Cu0.3419 Re(VII)-(0)ReO4-+8H++7e-=Re+4H2O0.368Ag(I)-(0)Ag2CrO4+2e-=2Ag+CrO42-0.4470S(IV)-(0)H2SO3+4H++4e-=S+3H2O0.449Cu(I)-(0)Cu++e-=Cu0.521I(0)-(-I)I2+2e-=2I-0.5355 I(0)-(-I)I3-+2e-=3I-0.536 As(V)-(III)H3AsO4+2H++2e-=HAsO2+2H2O0.560 Sb(V)-(III)Sb2O5+6H++4e-=2SbO++3H2O0.581 Te(IV)-(0)TeO2+4H++4e-=Te+2H2O0.593 U(V)-(IV)UO2++4H++e-=U4++2H2O0.612 **Hg(II)-(I)2HgCl2+2e-=Hg2Cl2+2Cl-0.63 Pt(IV)-(II)[PtCl6]2-+2e-=[PtCl4]2-+2Cl-0.68 O(0)-(-I)O2+2H++2e-=H2O20.695 Pt(II)-(0)[PtCl4]2-+2e-=Pt+4Cl-0.755 *Se(IV)-(0)H2SeO3+4H++4e-=Se+3H2O0.74 Fe(III)-(II)Fe3++e-=Fe2+0.771 Hg(I)-(0)Hg22++2e-=2Hg0.7973 Ag(I)-(0)Ag++e-=Ag0.7996 Os(VIII)-(0)OsO4+8H++8e-=Os+4H2O0.8N(V)-(IV) 2NO3-+4H++2e-=N2O4+2H2O 0.803 Hg(II)-(0)Hg2++2e-=Hg0.851 Si(IV)-(0)(quartz)SiO2+4H++4e-=Si+2H2O0.857 Cu(II)-(I)Cu2++I-+e-=CuI0.86 N(III)-(I)2HNO2+4H++4e-=H2N2O2+2H2O0.86 Hg(II)-(I)2Hg2++2e-=Hg22+0.920 N(V)-(III)NO3-+3H++2e-=HNO2+H2O0.934 Pd(II)-(0)Pd2++2e-=Pd0.951 N(V)-(II)NO3-+4H++3e-=NO+2H2O0.957 N(III)-(II)HNO2+H++e-=NO+H2O0.983 I(I)-(-I)HIO+H++2e-=I-+H2O0.987 V(V)-(IV)VO2++2H++e-=VO2++H2O0.991 V(V)-(IV)V(OH)4++2H++e-=VO2++3H2O 1.00 Au(III)-(0) [AuCl4]-+3e-=Au+4Cl- 1.002 Te(VI)-(IV)H6TeO6+2H++2e-=TeO2+4H2O 1.02 N(IV)-(II)N2O4+4H++4e-=2NO+2H2O 1.035 N(IV)-(III)N2O4+2H++2e-=2HNO2 1.065 I(V)-(-I)IO3-+6H++6e-=I-+3H2O 1.085 Br(0)-(-I)Br2(aq)+2e-=2Br- 1.0873 Se(VI)-(IV)SeO42-+4H++2e-=H2SeO3+H2O 1.151 Cl(V)-(IV)ClO3-+2H++e-=ClO2+H2O 1.152 Pt(II)-(0)Pt2++2e-=Pt 1.18 Cl(VII)-(V)ClO4-+2H++2e-=ClO3-+H2O 1.189 I(V)-(0)2IO3-+12H++10e-=I2+6H2O 1.195 Cl(V)-(III)ClO3-+3H++2e-=HClO2+H2O 1.214 Mn(IV)-(II)MnO2+4H++2e-=Mn2++2H2O 1.224 O(0)-(-II)O2+4H++4e-=2H2O 1.229 Tl(III)-(I)T13++2e-=Tl+ 1.252 Cl(IV)-(III)ClO2+H++e-=HClO2 1.277N(III)-(I)2HNO2+4H++4e-=N2O+3H2O 1.297 **Cr(VI)-(III)Cr2O72-+14H++6e-=2Cr3++7H2O 1.33Br(I)-(-I)HBrO+H++2e-=Br-+H2O 1.331 Cr(VI)-(III)HCrO4-+7H++3e-=Cr3++4H2O 1.350 Cl(0)-(-I)Cl2(g)+2e-=2Cl- 1.35827 Cl(VII)-(-I)ClO4-+8H++8e-=Cl-+4H2O 1.389 Cl(VII)-(0)ClO4-+8H++7e-=1/2Cl2+4H2O 1.39 Au(III)-(I)Au3++2e-=Au+ 1.401 Br(V)-(-I)BrO3-+6H++6e-=Br-+3H2O 1.423 I(I)-(0)2HIO+2H++2e-=I2+2H2O 1.439 Cl(V)-(-I)ClO3-+6H++6e-=Cl-+3H2O 1.451 Pb(IV)-(II)PbO2+4H++2e-=Pb2++2H2O 1.455 Cl(V)-(0)ClO3-+6H++5e-=1/2Cl2+3H2O 1.47Cl(I)-(-I)HClO+H++2e-=Cl-+H2O 1.482 Br(V)-(0)BrO3-+6H++5e-=l/2Br2+3H2O 1.482 Au(III)-(0)Au3++3e-=Au 1.498 Mn(VII)-(II)MnO4-+8H++5e-=Mn2++4H2O 1.507 Mn(III)-(II)Mn3++e-=Mn2+ 1.5415 Cl(III)-(-I)HClO2+3H++4e-=Cl-+2H2O 1.570 Br(I)-(0)HBrO+H++e-=l/2Br2(aq)+H2O 1.574 N(II)-(I)2NO+2H++2e-=N2O+H2O 1.591 I(VII)-(V)H5IO6+H++2e-=IO3-+3H2O 1.601 Cl(I)-(0)HClO+H++e-=1/2Cl2+H2O 1.611 Cl(III)-(I)HClO2+2H++2e-=HClO+H2O 1.645 Ni(IV)-(II)NiO2+4H++2e-=Ni2++2H2O 1.678 Mn(VII)-(IV)MnO4-+4H++3e-=MnO2+2H2O 1.679 Pb(IV)-(II)PbO2+SO42-+4H++2e-=PbSO4+2H2O 1.6913 Au(I)-(0)Au++e-=Au 1.692 Ce(IV)-(III)Ce4++e-=Ce3+ 1.72N(I)-(0)N2O+2H++2e-=N2+H2O 1.766 O(-I)-(-II)H2O2+2H++2e-=2H2O 1.776 Co(III)-(II)Co3++e-=Co2+(2mol·L-1 H2SO4) 1.83 Ag(II)-(I)Ag2++e-=Ag+ 1.980 S(VII)-(VI) S2O82-+2e-=2SO42- 2.010 O(0)-(-II)O3+2H++2e-=O2+H2O 2.076 O(II)-(-II)F2O+2H++4e-=H2O+2F- 2.153 Fe(VI)-(III)FeO42-+8H++3e-=Fe3++4H2O 2.20O(0)-(-II)O(g)+2H++2e-=H2O 2.421 F(0)-(-I)F2+2e-=2F- 2.866F2+2H++2e-=2HF 3.0532电对方程式E /V Ca(II)-(0)Ca(OH)2+2e-=Ca+2OH--3.02Ba(II)-(0)Ba(OH)2+2e-=Ba+2OH--2.99 La(III)-(0)La(OH)3+3e-=La+3OH--2.90 Sr(II)-(0)Sr(OH)2·8H2O+2e-=Sr+2OH-+8H2O-2.88 Mg(II)-(0)Mg(OH)2+2e-=Mg+2OH--2.690 Be(II)-(0)Be2O32-+3H2O+4e-=2Be+6OH--2.63 Hf(IV)-(0)HfO(OH)2+H2O+4e-=Hf+4OH--2.50 Zr(IV)-(0)H2ZrO3+H2O+4e-=Zr+4OH--2.36 Al(III)-(0)H2AlO3-+H2O+3e-=Al+OH--2.33 P(I)-(0)H2PO2-+e-=P+2OH--1.82 B(III)-(0)H2BO3-+H2O+3e-=B+4OH--1.79 P(III)-(0)HPO32-+2H2O+3e-=P+5OH--1.71 Si(IV)-(0)SiO32-+3H2O+4e-=Si+6OH--1.697 P(III)-(I)HPO32-+2H2O+2e-=H2PO2-+3OH--1.65 Mn(II)-(0)Mn(OH)2+2e-=Mn+2OH--1.56 Cr(III)-(0)Cr(OH)3+3e-=Cr+3OH--1.48 *Zn(II)-(0)[Zn(CN)4]2-+2e-=Zn+4CN--1.26 Zn(II)-(0)Zn(OH)2+2e-=Zn+2OH--1.249 Ga(III)-(0)H2GaO3-+H2O+2e-=Ga+4OH--1.219 Zn(II)-(0)ZnO22-+2H2O+2e-=Zn+4OH--1.215 Cr(III)-(0)CrO2-+2H2O+3e-=Cr+4OH--1.2 Te(0)-(-I)Te+2e-=Te2--1.143 P(V)-(III)PO43-+2H2O+2e-=HPO32-+3OH--1.05 *Zn(II)-(0)[Zn(NH3)4]2++2e-=Zn+4NH3-1.04 *W(VI)-(0)WO42-+4H2O+6e-=W+8OH--1.01 *Ge(IV)-(0)HGeO3-+2H2O+4e-=Ge+5OH--1.0 Sn(IV)-(II)[Sn(OH)6]2-+2e-=HSnO2-+H2O+3OH--0.93 S(VI)-(IV)SO42-+H2O+2e-=SO32-+2OH--0.93 Se(0)-(-II)Se+2e-=Se2--0.924 Sn(II)-(0)HSnO2-+H2O+2e-=Sn+3OH--0.909 P(0)-(-III)P+3H2O+3e-=PH3(g)+3OH--0.87 N(V)-(IV)2NO3-+2H2O+2e-=N2O4+4OH--0.85 H(I)-(0)2H2O+2e-=H2+2OH--0.8277 Cd(II)-(0)Cd(OH)2+2e-=Cd(Hg)+2OH--0.809 Co(II)-(0)Co(OH)2+2e-=Co+2OH--0.73 Ni(II)-(0)Ni(OH)2+2e-=Ni+2OH--0.72 As(V)-(III)AsO43-+2H2O+2e-=AsO2-+4OH--0.71 Ag(I)-(0)Ag2S+2e-=2Ag+S2--0.691 As(III)-(0)AsO2-+2H2O+3e-=As+4OH--0.68 Sb(III)-(0)SbO2-+2H2O+3e-=Sb+4OH--0.66 *Re(VII)-(IV)ReO4-+2H2O+3e-=ReO2+4OH--0.59 *Sb(V)-(III)SbO3-+H2O+2e-=SbO2-+2OH--0.59 Re(VII)-(0)ReO4-+4H2O+7e-=Re+8OH--0.584 *S(IV)-(II)2SO32-+3H2O+4e-=S2O32-+6OH--0.58 Te(IV)-(0)TeO32-+3H2O+4e-=Te+6OH--0.57Fe(III)-(II)Fe(OH)3+e-=Fe(OH)2+OH--0.56S(0)-(-II)S+2e-=S2--0.47627 Bi(III)-(0)Bi2O3+3H2O+6e-=2Bi+6OH--0.46N(III)-(II)NO2-+H2O+e-=NO+2OH--0.46*Co(II)-C(0)[Co(NH3)6]2++2e-=Co+6NH3-0.422 Se(IV)-(0)SeO32-+3H2O+4e-=Se+6OH--0.366 Cu(I)-(0)Cu2O+H2O+2e-=2Cu+2OH--0.360 Tl(I)-(0)Tl(OH)+e-=Tl+OH--0.34*Ag(I)-(0)[Ag(CN)2]-+e-=Ag+2CN--0.31 Cu(II)-(0)Cu(OH)2+2e-=Cu+2OH--0.222 Cr(VI)-(III)CrO42-+4H2O+3e-=Cr(OH)3+5OH--0.13*Cu(I)-(0)[Cu(NH3)2]++e-=Cu+2NH3-0.12O(0)-(-I)O2+H2O+2e-=HO2-+OH--0.076 Ag(I)-(0)AgCN+e-=Ag+CN--0.017 N(V)-(III)NO3-+H2O+2e-=NO2-+2OH-0.01Se(VI)-(IV)SeO42-+H2O+2e-=SeO32-+2OH-0.05Pd(II)-(0)Pd(OH)2+2e-=Pd+2OH-0.07S(II,V)-(II)S4O62-+2e-=2S2O32-0.08Hg(II)-(0)HgO+H2O+2e-=Hg+2OH-0.0977 Co(III)-(II)[Co(NH3)6]3++e-=[Co(NH3)6]2+0.108Pt(II)-(0)Pt(OH)2+2e-=Pt+2OH-0.14Co(III)-(II)Co(OH)3+e-=Co(OH)2+OH-0.17Pb(IV)-(II)PbO2+H2O+2e-=PbO+2OH-0.247I(V)-(-I)IO3-+3H2O+6e-=I-+6OH-0.26Cl(V)-(III)ClO3-+H2O+2e-=ClO2-+2OH-0.33Ag(I)-(0)Ag2O+H2O+2e-=2Ag+2OH-0.342Fe(III)-(II)[Fe(CN)6]3-+e-=[Fe(CN)6]4-0.358Cl(VII)-(V)ClO4-+H2O+2e-=ClO3-+2OH-0.36*Ag(I)-(0)[Ag(NH3)2]++e-=Ag+2NH30.373O(0)-(-II)O2+2H2O+4e-=4OH-0.401I(I)-(-I)IO-+H2O+2e-=I-+2OH-0.485*Ni(IV)-(II)NiO2+2H2O+2e-=Ni(OH)2+2OH-0.490Mn(VII)-(VI)MnO4-+e-=MnO42-0.558Mn(VII)-(IV)MnO4-+2H2O+3e-=MnO2+4OH-0.595Mn(VI)-(IV)MnO42-+2H2O+2e-=MnO2+4OH-0.60Ag(II)-(I)2AgO+H2O+2e-=Ag2O+2OH-0.607Br(V)-(-I)BrO3-+3H2O+6e-=Br-+6OH-0.61Cl(V)-(-I)ClO3-+3H2O+6e-=Cl-+6OH-0.62Cl(III)-(I)ClO2-+H2O+2e-=ClO-+2OH-0.66I(VII)-(V)H3IO62-+2e-=IO3-+3OH-0.7Cl(III)-(-I)ClO2-+2H2O+4e-=Cl-+4OH-0.76Br(I)-(-I)BrO-+H2O+2e-=Br-+2OH-0.761Cl(I)-(-I)ClO-+H2O+2e-=Cl-+2OH-0.841*Cl(IV)-(III)ClO2(g)+e-=ClO2-0.95。
