苯磺酸氨氯地平片英文说明书
络活喜(苯磺酸氨氯地平片) (1)

络活喜(苯磺酸氨氯地平片)【药品名称】商品名称:络活喜通用名称:苯磺酸氨氯地平片英文名称:Amlodipine Besylate T ablets【成份】本品主要成分为苯磺酸氨氯地平,化学名称为:3-乙基-5-甲基-2-(2-氨乙氧甲基)-4-(2-氯苯基)-1,4-二氢-6-甲基-3,5-吡啶二羧酸酯苯磺酸盐。
【适应症】(1)高血压(单独或与其他药物合并使用)。
(2)心绞痛:尤其自发性心绞痛(单独或与其他药物合并使用)。
【用法用量】通常口服起始剂量为5mg,每日一次,最大不超过10mg,每日一次。
瘦小者、体质虚弱者、老年患者或肝功能受损者从2.5mg,每日一次开始用药;合用其它抗高血压药者也从此剂量开始用药。
用药剂量根据个体需要进行调整,调整期应不少于7-14天,以便医生充分评估患者对该剂量的反应。
但在临床有保障的前提下,可以加快调整速度。
治疗心绞痛的推荐剂量是5-10mg,老年患者或肝功能受损者需减量。
【不良反应】本品在10mg/日的剂量范围内有良好的耐受性,大多数不良反应是轻中度的。
本品因不良反应而停药的仅为1.5%,与安慰剂没有明显差别(约1%)。
最常见的不良反应是头痛和水肿。
发生率>1%的剂量相关性不良反应如下:水肿、头晕、潮红和心悸。
与剂量关系不明确,但发生率超过1.0%的不良反应如下:头痛、疲倦、恶心、腹痛和嗜睡。
以上不良反应中,水肿、潮红、心悸和嗜睡在女性中的发生率超过男性。
以下不良事件发生率? 1%但> 0.1%,与药物的因果关系不明确:一般:过敏反应,虚弱,背痛,潮热,不适,疼痛,僵硬,体重增加;心血管:心律失常(包括心动过速、心动过缓或房颤),胸痛,低血压,外周缺血,昏厥,体位性头晕,体位性低血压和脉管炎;中枢和外周神经系统:感觉减退,外周神经病,感觉异常,震颤,眩晕;胃肠道:厌食症,便秘,消化不良,吞咽困难,腹泻,胃胀气,胰腺炎,呕吐,牙龈增生;骨骼肌系统:关节痛,关节炎,肌肉痛性痉挛,肌痛;精神:性功能障碍,失眠,紧张,抑郁,梦魇,焦虑,人格解体;皮肤及附属物:血管性水肿,红斑,搔痒,皮疹,斑丘疹;特殊感觉:视觉异常,结膜炎,复视,眼痛,耳鸣;泌尿系统:尿频,排尿障碍,夜尿;自主神经系统:口干,盗汗;代谢和营养:高血糖,口渴;造血系统:白细胞减少症,紫癜,血小板减少症。
络活喜(Norvasc)苯磺酸氨氯地平片说明书

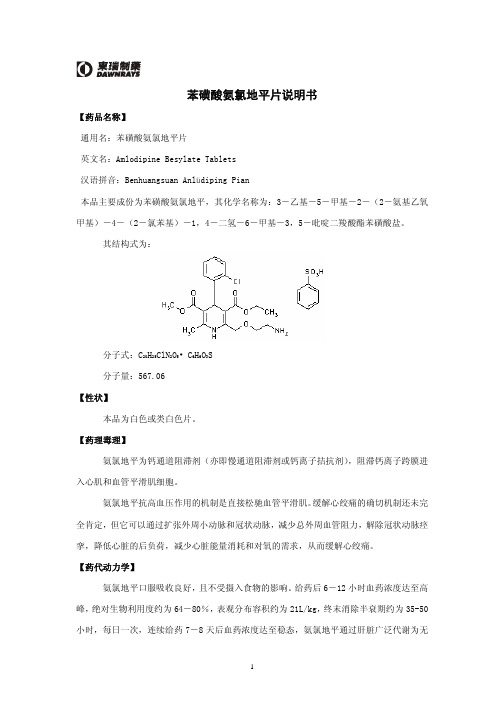
1核准日期:2007年03月09日修改日期:2009年06月26日;2009年12月09日;2010年03月25日;2010年06月07日;2011年05月23日;2012年04月09日;2014年01月03日;2015年12月01日;2016年05月11日;2016年07月12日;2017年02月14日;2018年05月28日;2019年08月19日;2020年05月12日;2020年06月15日;2020年07月01日;2020年12月30日;2022年06月14日;2023年06月23日苯磺酸氨氯地平片说明书请仔细阅读说明书并在医师或药师指导下使用【药品名称】通用名称: 苯磺酸氨氯地平片商品名称: 络活喜(Norvasc )英文名称: Amlodipine Besylate Tablets汉语拼音: Benhuangsuan Anlüdiping Pian【成份】本品主要成份为苯磺酸氨氯地平,其化学名称为:3-乙基-5-甲基-2-(2-氨基乙氧甲基)-4-(2-氯苯基)-1,4-二氢-6-甲基-3, 5-吡啶二羧酸酯苯磺酸盐。
化学结构式:分子式:C 20H 25N 2O 5Cl C 6H 6O 3S分子量:567.1本品辅料包括:微晶纤维素,无水磷酸氢钙,乙醇酸淀粉钠,硬脂酸镁。
【性状】本品为白色片。
【适应症】1.高血压本品适用于高血压的治疗。
本品可单独应用或与其他抗高血压药物联合应用。
高血压的控制是心血管风险综合管理的一部分,综合管理措施可能需要包括:血脂控制、糖尿病管理、抗血栓治疗、戒烟、体育锻炼和限制钠盐摄入。
收缩压或舒张压的升高均增高心血管风险。
在更高的基础血压水平上,每毫米汞柱血压的升高所带来的绝对风险增加会更高。
降低血压获得风险降低的相对程度在有不同心血管绝对风险的人群中是相似的。
严重高血压患者,略微降低血压就能带来较大的临床获益。
对成人高血压患者,通常而言,降低血压可降低心血管事件的风险,主要是卒中、以及心肌梗死的风险。
苯磺酸氨氯地平说明书
遮光,密封,阴凉处保存。 【包装】
铝塑板包装。7 片/板×1/盒;7 片/板×2/盒;7 片/板×4/盒。 【有效期】
暂定二年。 【批准文号】
2.5mg 国药准字 H20031087 5mg 国药准字 H20020390
4
10mg 国药准字 H20031088 【】 通用名:苯磺酸氨氯地平片 英文名:Amlodipine Besylate Tablets 汉语拼音:Benhuangsuan Anlüdiping Pian 本品主要成份为苯磺酸氨氯地平,其化学名称为:3-乙基-5-甲基-2-(2-氨基乙氧
甲基)-4-(2-氯苯基)-1,4-二氢-6-甲基-3,5-吡啶二羧酸酯苯磺酸盐。 其结构式为:
企业名称:苏州东瑞制药有限公司 地 址:江苏省苏州吴中经济开发区天灵路 22 号 邮政编码:215128 电话号码:0512-65626868 传真号码:0512-65628688 网 址:
5
2
Ⅲ-Ⅳ级)进行的长期、安慰剂对照研究(PRAISE-2)中,虽然心衰加重的发生率与安慰剂 相比无明显差异,但与氨氯地平有关的肺水肿报道有增加。 4.肝功能受损患者的使用:与其他所有钙拮抗剂相同,本品的半衰期在肝功能受损时延长。 但尚未确定相应的推荐剂量,因此,使用本品应谨慎。 5.肾功能衰竭患者的使用:氨氯地平的血药浓度改变与肾功能损害程度无相关性。因此, 可以采用正常剂量。本品不能被透析清除。 【孕妇及哺乳期妇女用药】 对孕妇用药缺乏相应的研究资料,故本品只在非常必要时方可用于孕妇。 尚不知本品能否通过乳汁分泌,服药的哺乳期妇女应中止哺乳。 【儿童用药】 尚无本品用于儿童的资料。 【老年患者用药】 本品血药浓度的达峰时间在老年和年轻患者中是相似的,老年患者药-时曲线下面积(AUC) 增加和消除半衰期的延长使消除率有下降趋势。有报导在接受相似剂量的氨氯地平时,老年 患者具有与年轻患者相同的良好耐受性。因此,老年患者可用正常剂量。但开始宜用较小剂 量,再渐增量为妥。 【药物相互作用】 本品与下列药物的合用是安全的:噻嗪类利尿剂、α-肾上腺素能受体阻滞剂、β-肾上腺素 能受体阻滞剂、血管紧张素转换酶抑制剂、长效硝酸酯类药物、舌下含服硝酸甘油、非甾体 类抗炎药、抗生素和口服降糖药。 用人血浆进行的体外研究数据显示本品不影响地高辛、苯妥英钠、华法林或吲哚美辛的血浆 蛋白结合率。 其他药物对氨氯地平的作用: 西咪替丁:与西咪替丁合用不改变氨氯地平的药代动力学。 柚子汁:20 名健康志愿者同时服用 240ml 柚子汁和 10mg 氨氯地平,未见对氨氯地平的药代 动力学有明显影响。 铝/镁(抗酸剂):同时服用铝/镁抗酸剂和单剂量氨氯地平,未见对氨氯地平的药代动力学
络活喜(苯磺酸氨氯地平片)说明书

络活喜(苯磺酸氨氯地平片)说明书【络活喜药品名称】通用名:苯磺酸氨氯地平片商品名:络活喜英文名:Amlodipine Besylate Tablets汉语拼音:Benhuangsuan Anlüdiping Pian络活喜化学名称为:3-乙基-5-甲基-2-(2-氨乙氧甲基)-4-(2-氯苯基)-1,4-二氢-6-甲基-3,5-吡啶二羧酸酯苯磺酸盐。
分子式:C20H25N2O5Cl·C6H6O3S分子量:567.1【络活喜性状】络活喜为白色片。
【络活喜药理毒理】药理作用苯磺酸氨氯地平是二氢吡啶类钙拮抗剂(钙离子拮抗剂或慢通道阻滞剂)。
心肌和平滑肌的收缩依赖于细胞外钙离子通过特异性离子通道进入细胞。
络活喜选择性抑制钙离子跨膜进入平滑肌细胞和心肌细胞,对平滑肌的作用大于心肌。
其与钙通道的相互作用决定于它和受体位点结合和解离的渐进性速率,因此药理作用逐渐产生。
络活喜是外周动脉扩张剂,直接作用于血管平滑肌,降低外周血管阻力,从而降低血压。
治疗剂量下,体外实验可观察到负性肌力作用,但在整体动物实验中未见。
络活喜不影响血浆钙浓度。
15项随机双盲、安慰剂对照的临床试验证实了络活喜的抗高血压作用。
轻中度高血压患者每日服药一次,可以24小时降低卧位和立位血压,长期使用不引起心率或血浆儿茶酚胺显著改变。
降压效果平稳。
降压效果和剂量相关,降压幅度与治疗前血压相关,中度高血压者(舒张压105-114mmHg)的疗效比轻度高血压者(舒张压90-104mmHg)高,血压正常者服药后没有明显作用。
络活喜降低舒张压的作用在老年人和年轻人中相似,降低收缩压的作用对老年人强。
络活喜缓解心绞痛的准确机制尚不明确,但可能在运动时,络活喜通过降低外周阻力(后负荷)减少心脏做功和心率血压乘积,减少心肌氧需,治疗劳力型心绞痛;通过抑制钙离子、肾上腺素、5-羟色胺和血栓素A2引起的冠状动脉和小动脉收缩,恢复缺血区血供治疗自发性心绞痛。
压氏达(苯磺酸氨氯地平片)

压氏达(苯磺酸氨氯地平片)【药品名称】商品名称:压氏达通用名称:苯磺酸氨氯地平片英文名称:Amlodipine Besylate Tablets【成份】本品主要成分为苯磺酸氨氯地平,其化学名称为:3-乙基-5-甲基-2-(2-氨乙氧甲基)-4-(2-氯苯基)-1,4-二氢-6-甲基-3,5-吡啶二羧酸酯苯磺酸盐。
【分子式】C20H25N2O5Cl·C6H6O3S【分子量】567.1【适应症】1.高直压。
本品适用于高血压的治疗。
可单独应用或与其他抗高血压药物联合应用。
2.冠心病(CAD)、慢性稳定型心绞痛。
本品适用于慢性稳定性心绞痛的对症治疗,可...【用法用量】成人:通常本品治疗高血压的起始剂量为5mg,每日一次,最大剂量为10mg,每日一次。
身材小、虚弱、老年或伴肝功能不全患者,起始剂量为2.5mg,每日一次,此剂量也可为本品联合其它抗高血压药物治疗的剂量.剂量调整应根据患者个体反应进行。
一般的剂量调整应在7-14天后开始进行。
如临床需要,在对患者进行严密监测的情况下,可更快地进行剂量调整。
治疗慢性稳定性或血管痉挛性心绞痛的推荐剂量是5-10mg,每日-次,老年及肝功能不全的患者建议使用较低剂量治疗,大多数患者的有效剂量为10mg每日一次。
【不良反应】本品在10mg/日的剂量范围内有良好的耐受性,大多数不良反应是轻中度的。
本品因不良反应而停药的仅为1.5%,与安慰剂没有明显差别(约1%)。
最常见的不良反应是头痛和水肿。
发生率>1%的剂量相关性不良反应如下:水肿、头晕、潮红和心悸。
与剂量关系不明确,但发生率超过1.0%的不良反应如下:头痛、疲倦、恶心、腹痛和嗜睡。
以上不良反应中,水肿、潮红、心悸和嗜睡在女性中的发生率超过男性。
以下不良事件发生率≤1%但>0.1%,与药物的因果关系不明确:一般:过敏反应,虚弱,背痛,潮热,不适,疼痛,僵硬,体重增加。
【禁忌】对二氢吡啶类钙拮抗剂过敏者。
苯磺酸氨氯地平片英文说明书

HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed touse amlodipine besylate tablets safely and effectively. See full prescribing information for amlodipine besylate tablets. Amlodipine Besylate Tablets Initial U.S. Approval: 1987INDICATIONS AND USAGEAmlodipine besylate tablets are a calcium channel blocker and may be used alone or in combination with other antihypertensive and antianginal agents for the treatment of:•Hypertension ( ) 1.1•Coronary Artery Disease ( ) 1.2•Chronic Stable Angina•Vasospastic Angina (Prinzmetal's or Variant Angina)•Angiographically Documented Coronary Artery Disease in patients without heart failure or an ejection fraction < 40%DOSAGE AND ADMINISTRATION•Adult recommended starting dose: 5 mg once daily with maximum dose 10 mg once daily. ( ) 2.1•Small, fragile, or elderly patients, or patients with hepatic insufficiency may be started on 2.5 mg once daily. ( ) 2.1•Pediatric starting dose: 2.5 mg to 5 mg once daily. ( ) 2.2: Doses in excess of 5 mg daily have not been studied in pediatric patients. ( ) Important Limitation2.2DOSAGE FORMS AND STRENGTHS• 2.5 mg, 5 mg, and 10 mg Tablets ( ) 3CONTRAINDICATIONS•Known sensitivity to amlodipine ( ) 4WARNINGS AND PRECAUTIONS •Symptomatic hypotension is possible, particularly in patients with severe aortic stenosis. However, because of the gradual onset of action, acute hypotension is unlikely. ( ) 5.1•Worsening angina and acute myocardial infarction can develop after starting or increasing the dose of amlodipine, particularly in patients with severe obstructive coronary artery disease. ( ) 5.2•Titrate slowly when administering calcium channel blockers to patients with severe hepatic impairment. ( ) 5.4ADVERSE REACTIONSMost common adverse reactions are headache and edema which occurred in a dose related manner. Other adverse experiences not dose related but reported with an incidence >1% are headache, fatigue, nausea, abdominal pain, and somnolence. ( ) 6To report SUSPECTED ADVERSE REACTIONS, contact Qualitest Pharmaceuticals at 1-800-444-4011 or FDA at 1-800-FDA-1088 or /medwatch.To report SUSPECTED ADVERSE REACTIONS, contact at or FDA at 1-800-FDA-1088 or /medwatchUSE IN SPECIFIC POPULATIONS •Pregnancy: Use only if the potential benefit justifies the potential risk. ( ) 8.1•Nursing: Discontinue when administering amlodipine. ( ) 8.3•Pediatric: Effect on patients less than 6 years old is not known. ( ) 8.4•Geriatric: Start dosing at the low end of the dose range, due to thegreater frequency of decreased hepatic, renal, or cardiac function and of concomitant disease or other drug therapy. ( ) 8.5Revised: 05/2010FULL PRESCRIBING INFORMATION: CONTENTS *1 INDICATIONS AND USAGE2 DOSAGE AND ADMINISTRATION3 DOSAGE FORMS AND STRENGTHS4 CONTRAINDICATIONS5 WARNINGS AND PRECAUTIONS6 ADVERSE REACTIONS7 DRUG INTERACTIONS7.10 Drug/Laboratory Test Interactions8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy8.3 Nursing Mothers8.4 Pediatric Use8.5 Geriatric Use10 OVERDOSAGE11 DESCRIPTION12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action12.2 Pharmacodynamics12.3 Pharmacokinetics and Metabolism12.4 Pediatric Patients13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility14 CLINICAL STUDIESAMLODIPINE 5MG TABLET* Sections or subsections omitted from the full prescribing information are not listedFULL PRESCRIBING INFORMATION1 INDICATIONS AND USAGE2 DOSAGE AND ADMINISTRATION3 DOSAGE FORMS AND STRENGTHS2.5, 5, and 10 mg Tablets4 CONTRAINDICATIONSAmlodipine is contraindicated in patients with known sensitivity to amlodipine.5 WARNINGS AND PRECAUTIONS6 ADVERSE REACTIONS7 DRUG INTERACTIONS7.10 Drug/Laboratory Test InteractionsNone known.8 USE IN SPECIFIC POPULATIONS8.1 PregnancyPregnancy Category C There are no adequate and well-controlled studies in pregnant women. Amlodipine should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.No evidence of teratogenicity or other embryo/fetal toxicity was found when pregnant rats and rabbits were treated orally with amlodipine maleate at doses up to 10 mg amlodipine/kg/day (respectively, 8 times and 23 times the maximum recommended human dose of 10 mg on a mg/m basis) during their respective periods of major organogenesis. However, litter size was significantly decreased (by about 50%) and the number of intrauterine deaths was significantly increased (about 5-fold) in rats receiving amlodipine maleate at a dose equivalent to 10 mg amlodipine/kg/day for 14 days before mating and throughout mating and gestation. Amlodipine maleate has been shown to prolong both the gestation period and the duration of labor in rats at this dose. 1121Based on patient weight of 50 kg.8.3 Nursing MothersIt is not known whether amlodipine is excreted in human milk. In the absence of this information, it is recommended that nursing be discontinued while amlodipine is administered.8.4 Pediatric UseEffect of amlodipine on blood pressure in patients less than 6 years of age is not known.8.5 Geriatric UseClinical studies of amlodipine did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy. Elderly patients have decreased clearance of amlodipine with a resulting increase of AUC of approximately 40–60%, and a lower initial dose may be required . [see ] Dosage and Administration (2.1)10 OVERDOSAGEOverdosage might be expected to cause excessive peripheral vasodilation with marked hypotension and possibly a reflex tachycardia. In humans, experience with intentional overdosage of amlodipine is limited.Single oral doses of amlodipine maleate equivalent to 40 mg amlodipine/kg and 100 mg amlodipine/kg in mice and rats, respectively, caused deaths. Single oral amlodipine maleate doses equivalent to 4 or more mg amlodipine/kg or higher in dogs (11 or more times the maximum recommended human dose on a mg/m basis) caused a marked peripheral vasodilation and hypotension. 2If massive overdose should occur, initiate active cardiac and respiratory monitoring. Frequent blood pressure measurements are essential. Should hypotension occur, provide cardiovascular support including elevation of the extremities and the judicious administration of fluids. If hypotension remains unresponsive to these conservative measures, consider administration of vasopressors(such as phenylephrine) with attention to circulating volume and urine output. As amlodipine is highly protein bound, hemodialysis is not likely to be of benefit.11 DESCRIPTIONAmlodinpine besylate is the besylate salt of amlodipine, a long-acting calcium channel blocker.Amlodipine besylate is chemically described as 3-Ethyl-5-methyl (±)-2-[(2-aminoethoxy)methyl]-4-(2-chlorophenyl)-1,4-dihydro-6-methyl-3,5-pyridinedicarboxylate, monobenzenesulphonate. Its molecular formula is C H ClN O •C H O S, and its structural formula is: 202525663Amlodipine besylate is a white crystalline powder with a molecular weight of 567.1. It is slightly soluble in water and sparingly soluble in ethanol. Amlodipine besylate tablets are formulated as white tablets equivalent to 2.5, 5, and 10 mg of amlodipine fororal administration. In addition to the active ingredient, amlodipine besylate, each tablet contains the following inactive ingredients: dibasic calcium phosphate anhydrous, magnesium stearate, microcrystalline cellulose, and sodium starch glycolate.12 CLINICAL PHARMACOLOGY12.1 Mechanism of ActionAmlodipine is a dihydropyridine calcium antagonist (calcium ion antagonist or slow-channel blocker) that inhibits the transmembrane influx of calcium ions into vascular smooth muscle and cardiac muscle. Experimental data suggest that amlodipine binds to both dihydropyridine and nondihydropyridine binding sites. The contractile processes of cardiac muscle and vascular smooth muscleare dependent upon the movement of extracellular calcium ions into these cells through specific ion channels. Amlodipine inhibits calcium ion influx across cell membranes selectively, with a greater effect on vascular smooth muscle cells than on cardiac muscle cells. Negative inotropic effects can be detected but such effects have not been seen in intact animals at therapeutic doses. Serum calcium concentration is not affected by amlodipine. Within the physiologic pH range, amlodipine is an ionized compound (pKa=8.6), and its kinetic interaction with the calcium channel receptor is characterized by a gradual rate of association and dissociation with the receptor binding site, resulting in a gradual onset of effect. in vitroAmlodipine is a peripheral arterial vasodilator that acts directly on vascular smooth muscle to cause a reduction in peripheral vascular resistance and reduction in blood pressure.The precise mechanisms by which amlodipine relieves angina have not been fully delineated, but are thought to include the following: 12.2 Pharmacodynamics12.3 Pharmacokinetics and MetabolismAfter oral administration of therapeutic doses of amlodipine, absorption produces peak plasma concentrations between 6 and 12 hours. Absolute bioavailability has been estimated to be between 64 and 90%. The bioavailability of amlodipine is not altered by the presence of food.Amlodipine is extensively (about 90%) converted to inactive metabolites via hepatic metabolism with 10% of the parent compound and 60% of the metabolites excreted in the urine. studies have shown that approximately 93% of the circulating drug is bound to plasma proteins in hypertensive patients. Elimination from the plasma is biphasic with a terminal elimination half-life of about 30–50 hours. Steady-state plasma levels of amlodipine are reached after 7 to 8 days of consecutive daily dosing. Ex vivoThe pharmacokinetics of amlodipine are not significantly influenced by renal impairment. Patients with renal failure may therefore receive the usual initial dose.Elderly patients and patients with hepatic insufficiency have decreased clearance of amlodipine with a resulting increase in AUC of approximately 40–60%, and a lower initial dose may be required. A similar increase in AUC was observed in patients with moderate to severe heart failure.12.4 Pediatric PatientsSixty-two hypertensive patients aged 6 to 17 years received doses of amlodipine between 1.25 mg and 20 mg. Weight-adjusted clearance and volume of distribution were similar to values in adults.13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of FertilityRats and mice treated with amlodipine maleate in the diet for up to two years, at concentrations calculated to provide daily dosage levels of 0.5, 1.25, and 2.5 amlodipine mg/kg/day, showed no evidence of a carcinogenic effect of the drug. For the mouse, the highest dose was, on a mg/m basis, similar to the maximum recommended human dose of 10 mg amlodipine/day. For the rat, the highest dose was, on a mg/m basis, about twice the maximum recommended human dose. 2222Mutagenicity studies conducted with amlodipine maleate revealed no drug related effects at either the gene or chromosome level. There was no effect on the fertility of rats treated orally with amlodipine maleate (males for 64 days and females for 14 days prior to mating) at doses up to 10 mg amlodipine/kg/day (8 times the maximum recommended human dose of 10 mg/day on a mg/m basis). 222Based on patient weight of 50 kg14 CLINICAL STUDIESPATIENT INFORMATION AMLODIPINE BESYLATE TABLETSRead this information carefully before you start taking and each time you refill your prescription. There may be new information. This information does not replace talking with your doctor. If you have any questions about , ask your doctor. Your doctor will know if is right for you. amlodipineamlodipineamlodipineis a type of medicine known as a calcium channel blocker (CCB). It is used to treat high blood pressure (hypertension) and a type of chest pain called angina. It can be used by itself or with other medicines to treat these conditions. What is ? amlodipine AmlodipineHigh blood pressure comes from blood pushing too hard against your blood vessels. relaxes your blood vessels, which lets your blood flow more easily and helps lower your blood pressure. Drugs that lower blood pressure lower your risk of having a stroke or heart attack. High Blood Pressure (hypertension)AmlodipineAngina is a pain or discomfort that keeps coming back when part of your heart does not get enough blood. Angina feels like a pressing or squeezing pain, usually in your chest under the breastbone. Sometimes you can feel it in your shoulders, arms, neck, jaws, or back. can relieve this pain. AnginaAmlodipineDo not use if you are allergic to amlodipine (the active ingredient in ), or to the inactive ingredients. Your doctor or pharmacist can give you a list of these ingredients. Who should not use amlodipine?amlodipine besylate tablets amlodipine besylate tabletsTell your doctor about any prescription and non-prescription medicines you are taking, including natural or herbal remedies. Tell your doctor if you: What should I tell my doctor before taking ? amlodipine•ever had heart disease•ever had liver problems•are pregnant, or plan to become pregnant. Your doctor will decide if is the best treatment for you. amlodipine•are breastfeeding. Do not breastfeed while taking . You can stop breastfeeding or take a different medicine. amlodipineHow should I take ? amlodipine•Take once a day, with or without food. You can take with most drinks, including grapefruit juice. amlodipineamlodipine•It may be easier to take your dose if you do it at the same time every day, such as with breakfast or dinner, or at bedtime. Do not take more than one dose of at a time. amlodipine•If you miss a dose, take it as soon as you remember. Do not take if it has been more than 12 hours since you missed your last dose. Wait and take the next dose at your regular time. amlodipine•You can use nitroglycerin and together. If you take nitroglycerin for angina, don't stop taking it while you are taking Other medicines:amlodipineamlodipine.•While you are taking , do not stop taking your other prescription medicines, including any other blood pressure medicines, without talking to your doctor. amlodipine•If you took too much , call your doctor or Poison Control Center, or go to the nearest hospital emergency room right away. amlodipineWhat should I avoid while taking ? amlodipine•breastfeed. It is not known if will pass through your milk. Do notamlodipine•start any new prescription or non-prescription medicines or supplements, unless you check with your doctor first. Do notmay cause the following side effects. Most side effects are mild or moderate: What are the possible side effects of ? amlodipine Amlodipine•headache•swelling of your legs or ankles•tiredness, extreme sleepiness•stomach pain, nausea•dizziness•flushing (hot or warm feeling in your face)•arrhythmia (irregular heartbeat)•heart palpitations (very fast heartbeat)It is rare, but when you first start taking or increase your dose, you may have a heart attack or your angina may get worse. If that happens, call your doctor right away or go directly to a hospital emergency room. amlodipineTell your doctor if you are concerned about any side effects you experience. These are not all the possible side effects of . For a complete list, ask your doctor or pharmacist. amlodipineCall your doctor for medical advice about side effects. You may report side effects to the FDA at 1-800-FDA-1088 or by visiting /medwatch.Keep away from children. Store at 20° to 25°C (between 68° and 77°F) [see USP Controlled Room Temperature]. Keep out of the light. Do not store in the bathroom. Keep in a dry place. How do I store ? amlodipineamlodipineamlodipinebesylate tabletsamlodipineamlodipineSometimes, doctors will prescribe a medicine for a condition that is not written in the patient information leaflets. Only use the way your doctor told you to. Do not give to other people, even if they have the same symptoms you have. It may harm them. General advice about amlodipineamlodipineamlodipineYou can ask your pharmacist or doctor for information about . amlodipineManufactured for: Huntsville, AL 35811QUALITEST PHARMACEUTICALS8182524 Revised: 5/2010 R3AMLODIPINE 5MG TABLETRevised: 05/2010Distributed by: Unit Dose Services。
苯磺酸氨氯地平片说明书
苯磺酸氨氯地平片说明书核准日期: -11-29苯磺酸氨氯地平片说明书请仔细阅读说明书并在医师指导下使用【药品名称】通用名称:苯磺酸氨氯地平片英文名称:Amlodipine Besylate Tablets汉语拼音:Benhuangsuan Anlüdiping Pian【成份】本品主要成份为苯磺酸氨氯地平,其化学名称为:3-乙基-5-甲基-2-(2-氨基乙氧甲基)-4-(2-氯苯基)-1,4-二氢-6-甲基-3,5-吡啶二羧酸酯苯磺酸盐。
化学结构式:C6H6O3S分子式:C20H25N2O5Cl·C6H6O3S分子量:567.1【性状】本品为浅绿色片。
【适应症】1、高血压本品适用于高血压的治疗。
可单独应用或与其它抗高血压药物联合应用。
2、冠心病(CAD)慢性稳定性心绞痛本品适用于慢性稳定性心绞痛的对症治疗。
可单独应用或与其它抗心绞痛药物联合应用。
血管痉挛性心绞痛(Prinzmetal’s或变异型心绞痛)本品适用于确诊或可疑的血管痉挛性心绞痛的治疗。
可单独应用也可与其它抗心绞痛药物联合应用。
经血管造影证实的冠心病经血管造影证实为冠心病,但射血分数≧40%且无心衰的患者,本品可减少因心绞痛住院的风险以及降低冠状动脉重建术的风险。
【规格】5mg。
【用法用量】成人:一般本品治疗高血压的起始剂量为5mg,每日一次,最大剂量为10mg,每日一次。
身材小、虚弱、老年或伴肝功能不全患者,起始剂量为 2.5mg,每日一次;此剂量也可为本品联合其它抗高血压药物治疗的剂量。
剂量调整应根据患者个体反应进行,一般的剂量调整应在7-14天后开始进行。
如临床需要,在对患者进行严密监测的情况下,可更快地进行剂量调整。
治疗慢性稳定性或血管痉挛性心绞痛的推荐剂量是5-10mg,每日一次,老年及肝功能不全的患者建议使用较低剂量治疗,大多数患者的有效剂量为10mg,每日一次。
治疗冠心病的推荐剂量是5-10mg,每日一次。
苯磺酸氨氯地平片说明书
核准日期:2010-11-29苯磺酸氨氯地平片说明书请仔细阅读说明书并在医师指导下使用【药品名称】通用名称:苯磺酸氨氯地平片英文名称:Amlodipine Besylate Tablets汉语拼音:Benhuangsuan Anlüdiping Pian【成份】本品主要成份为苯磺酸氨氯地平,其化学名称为:3-乙基-5-甲基-2-(2-氨基乙氧甲基)-4-(2-氯苯基)-1,4-二氢-6-甲基-3,5-吡啶二羧酸酯苯磺酸盐。
化学结构式:C6H6O3S分子式:C20H25N2O5Cl·C6H6O3S分子量:567、1【性状】本品为浅绿色片。
【适应症】1、高血压本品适用于高血压得治疗。
可单独应用或与其她抗高血压药物联合应用。
2、冠心病(CAD)慢性稳定性心绞痛本品适用于慢性稳定性心绞痛得对症治疗。
可单独应用或与其她抗心绞痛药物联合应用。
血管痉挛性心绞痛(Prinzmetal’s或变异型心绞痛)本品适用于确诊或可疑得血管痉挛性心绞痛得治疗。
可单独应用也可与其她抗心绞痛药物联合应用。
经血管造影证实得冠心病经血管造影证实为冠心病,但射血分数≧40%且无心衰得患者,本品可减少因心绞痛住院得风险以及降低冠状动脉重建术得风险。
【规格】5mg。
【用法用量】成人:通常本品治疗高血压得起始剂量为5mg,每日一次,最大剂量为10mg,每日一次。
身材小、虚弱、老年或伴肝功能不全患者,起始剂量为2、5mg,每日一次;此剂量也可为本品联合其它抗高血压药物治疗得剂量。
剂量调整应根据患者个体反应进行,一般得剂量调整应在7-14天后开始进行。
如临床需要,在对患者进行严密监测得情况下,可更快地进行剂量调整。
治疗慢性稳定性或血管痉挛性心绞痛得推荐剂量就是5-10mg,每日一次,老年及肝功能不全得患者建议使用较低剂量治疗,大多数患者得有效剂量为10mg,每日一次。
治疗冠心病得推荐剂量就是5-10mg,每日一次。
在临床研究中,大多数患者需要10mg/日得剂量。
苯磺酸氨氯地平片说明书
核准日期:2010-11-29苯磺酸氨氯地平片说明书请仔细阅读说明书并在医师指导下使用【药品名称】通用名称:苯磺酸氨氯地平片英文名称:Amlodipine Besylate T ablets汉语拼音:Benhuangsuan Anlüdiping Pian【成份】本品主要成份为苯磺酸氨氯地平,其化学名称为:3-乙基-5-甲基-2-(2-氨基乙氧甲基)-4-(2-氯苯基)-1,4-二氢-6-甲基-3,5-吡啶二羧酸酯苯磺酸盐。
化学结构式:C6H6O3S分子式:C20H25N2O5Cl·C6H6O3S分子量:567.1【性状】本品为浅绿色片。
【适应症】1、高血压本品适用于高血压的治疗。
可单独应用或与其他抗高血压药物联合应用。
2、冠心病(CAD)慢性稳定性心绞痛本品适用于慢性稳定性心绞痛的对症治疗。
可单独应用或与其他抗心绞痛药物联合应用。
血管痉挛性心绞痛(Prinzmetal’s或变异型心绞痛)本品适用于确诊或可疑的血管痉挛性心绞痛的治疗。
可单独应用也可与其他抗心绞痛药物联合应用。
经血管造影证实的冠心病经血管造影证实为冠心病,但射血分数≧40%且无心衰的患者,本品可减少因心绞痛住院的风险以及降低冠状动脉重建术的风险。
【规格】5mg。
【用法用量】成人:通常本品治疗高血压的起始剂量为5mg,每日一次,最大剂量为10mg,每日一次。
身材小、虚弱、老年或伴肝功能不全患者,起始剂量为2.5mg,每日一次;此剂量也可为本品联合其它抗高血压药物治疗的剂量。
剂量调整应根据患者个体反应进行,一般的剂量调整应在7-14天后开始进行。
如临床需要,在对患者进行严密监测的情况下,可更快地进行剂量调整。
治疗慢性稳定性或血管痉挛性心绞痛的推荐剂量是5-10mg,每日一次,老年及肝功能不全的患者建议使用较低剂量治疗,大多数患者的有效剂量为10mg,每日一次。
治疗冠心病的推荐剂量是5-10mg,每日一次。
在临床研究中,大多数患者需要10mg/日的剂量。
苯磺酸氨氯地平片说明书
苯磺酸氨氯地平片说明书药品名称:通用名称:苯磺酸氨氯地平片英文名称:Amlodipine Besylate Tablets商品名称:络活喜成份:本品主要成份为苯磺酸氨氯地平适应症:高血压本品适用于高血压的治疗。
可单独应用或与其他抗高血压药物联合应用。
冠心病(CAD)慢性稳定性心绞痛本品适用于慢性稳定性心绞痛的对症治疗。
可单独应用或与其他抗心绞痛药物联合应用。
血管痉挛性心绞痛(P...用法用量:成人通常本品治疗高血压的起始剂量为5mg,每日一次,最大剂量为10mg,每日一次。
身材小、虚弱、老年、或伴肝功能不全患者,起始剂量为 2.5mg,每日一次;此剂量也可为本品联合其它抗高血压药物治疗的剂量... 不良反应:临床试验中的不良事件由于临床试验进行的条件存在很大差异,一种药物在临床试验中观察到的不良反应发生率无法与另一种药物在临床试验中不良反应发生率进行直接比较,可能也不能反映临床实践中观察到的发生率。
Word文档 1本品的...禁忌:对氨氯地平过敏的病人禁用本品。
注意事项:低血压症状性低血压可能发生,特别是在严重的主动脉狭窄患者中。
因本品的扩血管作用是逐渐产生的,服用本品后发生急性低血压的情况罕有报道。
心绞痛加重或心肌梗死极少数患者特别是伴有严重冠状动脉阻塞性疾病的患者...药物相互作用:体外数据体外研究数据显示:络活喜不影响地高辛、苯妥英钠、华法林或吲哚美辛与血浆蛋白的结合。
西咪替丁与西咪替丁合用不改变氨氯地平的药代动力学。
葡萄柚汁20名健康志愿者同时服用240ml葡萄柚汁和单剂量1...临床试验:对于高血压的作用成人患者苯磺酸氨氯地平的降压疗效在15个双盲、随机、安慰剂对照的研究中得到了证实,其中苯磺酸氨氯地平组800人,安慰剂组538人。
安慰剂校正后,接受每天一次本品给药治疗的轻中度高血压患...毒理研究:致癌作用,致畸作用,生殖毒性:大鼠和小鼠经食物给予氨氯地平0.5、1.25和2.5mg/kg/日,连续2年,未见致癌作用。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed touse amlodipine besylate tablets safely and effectively. See full prescribing information for amlodipine besylate tablets. Amlodipine Besylate Tablets Initial U.S. Approval: 1987INDICATIONS AND USAGEAmlodipine besylate tablets are a calcium channel blocker and may be used alone or in combination with other antihypertensive and antianginal agents for the treatment of:•Hypertension ( ) 1.1•Coronary Artery Disease ( ) 1.2•Chronic Stable Angina•Vasospastic Angina (Prinzmetal's or Variant Angina)•Angiographically Documented Coronary Artery Disease in patients without heart failure or an ejection fraction < 40%DOSAGE AND ADMINISTRATION•Adult recommended starting dose: 5 mg once daily with maximum dose 10 mg once daily. ( ) 2.1•Small, fragile, or elderly patients, or patients with hepatic insufficiency may be started on 2.5 mg once daily. ( ) 2.1•Pediatric starting dose: 2.5 mg to 5 mg once daily. ( ) 2.2: Doses in excess of 5 mg daily have not been studied in pediatric patients. ( ) Important Limitation2.2DOSAGE FORMS AND STRENGTHS• 2.5 mg, 5 mg, and 10 mg Tablets ( ) 3CONTRAINDICATIONS•Known sensitivity to amlodipine ( ) 4WARNINGS AND PRECAUTIONS •Symptomatic hypotension is possible, particularly in patients with severe aortic stenosis. However, because of the gradual onset of action, acute hypotension is unlikely. ( ) 5.1•Worsening angina and acute myocardial infarction can develop after starting or increasing the dose of amlodipine, particularly in patients with severe obstructive coronary artery disease. ( ) 5.2•Titrate slowly when administering calcium channel blockers to patients with severe hepatic impairment. ( ) 5.4ADVERSE REACTIONSMost common adverse reactions are headache and edema which occurred in a dose related manner. Other adverse experiences not dose related but reported with an incidence >1% are headache, fatigue, nausea, abdominal pain, and somnolence. ( ) 6To report SUSPECTED ADVERSE REACTIONS, contact Qualitest Pharmaceuticals at 1-800-444-4011 or FDA at 1-800-FDA-1088 or /medwatch.To report SUSPECTED ADVERSE REACTIONS, contact at or FDA at 1-800-FDA-1088 or /medwatchUSE IN SPECIFIC POPULATIONS •Pregnancy: Use only if the potential benefit justifies the potential risk. ( ) 8.1•Nursing: Discontinue when administering amlodipine. ( ) 8.3•Pediatric: Effect on patients less than 6 years old is not known. ( ) 8.4•Geriatric: Start dosing at the low end of the dose range, due to thegreater frequency of decreased hepatic, renal, or cardiac function and of concomitant disease or other drug therapy. ( ) 8.5Revised: 05/2010FULL PRESCRIBING INFORMATION: CONTENTS *1 INDICATIONS AND USAGE2 DOSAGE AND ADMINISTRATION3 DOSAGE FORMS AND STRENGTHS4 CONTRAINDICATIONS5 WARNINGS AND PRECAUTIONS6 ADVERSE REACTIONS7 DRUG INTERACTIONS7.10 Drug/Laboratory Test Interactions8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy8.3 Nursing Mothers8.4 Pediatric Use8.5 Geriatric Use10 OVERDOSAGE11 DESCRIPTION12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action12.2 Pharmacodynamics12.3 Pharmacokinetics and Metabolism12.4 Pediatric Patients13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility14 CLINICAL STUDIESAMLODIPINE 5MG TABLET* Sections or subsections omitted from the full prescribing information are not listedFULL PRESCRIBING INFORMATION1 INDICATIONS AND USAGE2 DOSAGE AND ADMINISTRATION3 DOSAGE FORMS AND STRENGTHS2.5, 5, and 10 mg Tablets4 CONTRAINDICATIONSAmlodipine is contraindicated in patients with known sensitivity to amlodipine.5 WARNINGS AND PRECAUTIONS6 ADVERSE REACTIONS7 DRUG INTERACTIONS7.10 Drug/Laboratory Test InteractionsNone known.8 USE IN SPECIFIC POPULATIONS8.1 PregnancyPregnancy Category C There are no adequate and well-controlled studies in pregnant women. Amlodipine should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.No evidence of teratogenicity or other embryo/fetal toxicity was found when pregnant rats and rabbits were treated orally with amlodipine maleate at doses up to 10 mg amlodipine/kg/day (respectively, 8 times and 23 times the maximum recommended human dose of 10 mg on a mg/m basis) during their respective periods of major organogenesis. However, litter size was significantly decreased (by about 50%) and the number of intrauterine deaths was significantly increased (about 5-fold) in rats receiving amlodipine maleate at a dose equivalent to 10 mg amlodipine/kg/day for 14 days before mating and throughout mating and gestation. Amlodipine maleate has been shown to prolong both the gestation period and the duration of labor in rats at this dose. 1121Based on patient weight of 50 kg.8.3 Nursing MothersIt is not known whether amlodipine is excreted in human milk. In the absence of this information, it is recommended that nursing be discontinued while amlodipine is administered.8.4 Pediatric UseEffect of amlodipine on blood pressure in patients less than 6 years of age is not known.8.5 Geriatric UseClinical studies of amlodipine did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy. Elderly patients have decreased clearance of amlodipine with a resulting increase of AUC of approximately 40–60%, and a lower initial dose may be required . [see ] Dosage and Administration (2.1)10 OVERDOSAGEOverdosage might be expected to cause excessive peripheral vasodilation with marked hypotension and possibly a reflex tachycardia. In humans, experience with intentional overdosage of amlodipine is limited.Single oral doses of amlodipine maleate equivalent to 40 mg amlodipine/kg and 100 mg amlodipine/kg in mice and rats, respectively, caused deaths. Single oral amlodipine maleate doses equivalent to 4 or more mg amlodipine/kg or higher in dogs (11 or more times the maximum recommended human dose on a mg/m basis) caused a marked peripheral vasodilation and hypotension. 2If massive overdose should occur, initiate active cardiac and respiratory monitoring. Frequent blood pressure measurements are essential. Should hypotension occur, provide cardiovascular support including elevation of the extremities and the judicious administration of fluids. If hypotension remains unresponsive to these conservative measures, consider administration of vasopressors(such as phenylephrine) with attention to circulating volume and urine output. As amlodipine is highly protein bound, hemodialysis is not likely to be of benefit.11 DESCRIPTIONAmlodinpine besylate is the besylate salt of amlodipine, a long-acting calcium channel blocker.Amlodipine besylate is chemically described as 3-Ethyl-5-methyl (±)-2-[(2-aminoethoxy)methyl]-4-(2-chlorophenyl)-1,4-dihydro-6-methyl-3,5-pyridinedicarboxylate, monobenzenesulphonate. Its molecular formula is C H ClN O •C H O S, and its structural formula is: 202525663Amlodipine besylate is a white crystalline powder with a molecular weight of 567.1. It is slightly soluble in water and sparingly soluble in ethanol. Amlodipine besylate tablets are formulated as white tablets equivalent to 2.5, 5, and 10 mg of amlodipine fororal administration. In addition to the active ingredient, amlodipine besylate, each tablet contains the following inactive ingredients: dibasic calcium phosphate anhydrous, magnesium stearate, microcrystalline cellulose, and sodium starch glycolate.12 CLINICAL PHARMACOLOGY12.1 Mechanism of ActionAmlodipine is a dihydropyridine calcium antagonist (calcium ion antagonist or slow-channel blocker) that inhibits the transmembrane influx of calcium ions into vascular smooth muscle and cardiac muscle. Experimental data suggest that amlodipine binds to both dihydropyridine and nondihydropyridine binding sites. The contractile processes of cardiac muscle and vascular smooth muscleare dependent upon the movement of extracellular calcium ions into these cells through specific ion channels. Amlodipine inhibits calcium ion influx across cell membranes selectively, with a greater effect on vascular smooth muscle cells than on cardiac muscle cells. Negative inotropic effects can be detected but such effects have not been seen in intact animals at therapeutic doses. Serum calcium concentration is not affected by amlodipine. Within the physiologic pH range, amlodipine is an ionized compound (pKa=8.6), and its kinetic interaction with the calcium channel receptor is characterized by a gradual rate of association and dissociation with the receptor binding site, resulting in a gradual onset of effect. in vitroAmlodipine is a peripheral arterial vasodilator that acts directly on vascular smooth muscle to cause a reduction in peripheral vascular resistance and reduction in blood pressure.The precise mechanisms by which amlodipine relieves angina have not been fully delineated, but are thought to include the following: 12.2 Pharmacodynamics12.3 Pharmacokinetics and MetabolismAfter oral administration of therapeutic doses of amlodipine, absorption produces peak plasma concentrations between 6 and 12 hours. Absolute bioavailability has been estimated to be between 64 and 90%. The bioavailability of amlodipine is not altered by the presence of food.Amlodipine is extensively (about 90%) converted to inactive metabolites via hepatic metabolism with 10% of the parent compound and 60% of the metabolites excreted in the urine. studies have shown that approximately 93% of the circulating drug is bound to plasma proteins in hypertensive patients. Elimination from the plasma is biphasic with a terminal elimination half-life of about 30–50 hours. Steady-state plasma levels of amlodipine are reached after 7 to 8 days of consecutive daily dosing. Ex vivoThe pharmacokinetics of amlodipine are not significantly influenced by renal impairment. Patients with renal failure may therefore receive the usual initial dose.Elderly patients and patients with hepatic insufficiency have decreased clearance of amlodipine with a resulting increase in AUC of approximately 40–60%, and a lower initial dose may be required. A similar increase in AUC was observed in patients with moderate to severe heart failure.12.4 Pediatric PatientsSixty-two hypertensive patients aged 6 to 17 years received doses of amlodipine between 1.25 mg and 20 mg. Weight-adjusted clearance and volume of distribution were similar to values in adults.13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of FertilityRats and mice treated with amlodipine maleate in the diet for up to two years, at concentrations calculated to provide daily dosage levels of 0.5, 1.25, and 2.5 amlodipine mg/kg/day, showed no evidence of a carcinogenic effect of the drug. For the mouse, the highest dose was, on a mg/m basis, similar to the maximum recommended human dose of 10 mg amlodipine/day. For the rat, the highest dose was, on a mg/m basis, about twice the maximum recommended human dose. 2222Mutagenicity studies conducted with amlodipine maleate revealed no drug related effects at either the gene or chromosome level. There was no effect on the fertility of rats treated orally with amlodipine maleate (males for 64 days and females for 14 days prior to mating) at doses up to 10 mg amlodipine/kg/day (8 times the maximum recommended human dose of 10 mg/day on a mg/m basis). 222Based on patient weight of 50 kg14 CLINICAL STUDIESPATIENT INFORMATION AMLODIPINE BESYLATE TABLETSRead this information carefully before you start taking and each time you refill your prescription. There may be new information. This information does not replace talking with your doctor. If you have any questions about , ask your doctor. Your doctor will know if is right for you. amlodipineamlodipineamlodipineis a type of medicine known as a calcium channel blocker (CCB). It is used to treat high blood pressure (hypertension) and a type of chest pain called angina. It can be used by itself or with other medicines to treat these conditions. What is ? amlodipine AmlodipineHigh blood pressure comes from blood pushing too hard against your blood vessels. relaxes your blood vessels, which lets your blood flow more easily and helps lower your blood pressure. Drugs that lower blood pressure lower your risk of having a stroke or heart attack. High Blood Pressure (hypertension)AmlodipineAngina is a pain or discomfort that keeps coming back when part of your heart does not get enough blood. Angina feels like a pressing or squeezing pain, usually in your chest under the breastbone. Sometimes you can feel it in your shoulders, arms, neck, jaws, or back. can relieve this pain. AnginaAmlodipineDo not use if you are allergic to amlodipine (the active ingredient in ), or to the inactive ingredients. Your doctor or pharmacist can give you a list of these ingredients. Who should not use amlodipine?amlodipine besylate tablets amlodipine besylate tabletsTell your doctor about any prescription and non-prescription medicines you are taking, including natural or herbal remedies. Tell your doctor if you: What should I tell my doctor before taking ? amlodipine•ever had heart disease•ever had liver problems•are pregnant, or plan to become pregnant. Your doctor will decide if is the best treatment for you. amlodipine•are breastfeeding. Do not breastfeed while taking . You can stop breastfeeding or take a different medicine. amlodipineHow should I take ? amlodipine•Take once a day, with or without food. You can take with most drinks, including grapefruit juice. amlodipineamlodipine•It may be easier to take your dose if you do it at the same time every day, such as with breakfast or dinner, or at bedtime. Do not take more than one dose of at a time. amlodipine•If you miss a dose, take it as soon as you remember. Do not take if it has been more than 12 hours since you missed your last dose. Wait and take the next dose at your regular time. amlodipine•You can use nitroglycerin and together. If you take nitroglycerin for angina, don't stop taking it while you are taking Other medicines:amlodipineamlodipine.•While you are taking , do not stop taking your other prescription medicines, including any other blood pressure medicines, without talking to your doctor. amlodipine•If you took too much , call your doctor or Poison Control Center, or go to the nearest hospital emergency room right away. amlodipineWhat should I avoid while taking ? amlodipine•breastfeed. It is not known if will pass through your milk. Do notamlodipine•start any new prescription or non-prescription medicines or supplements, unless you check with your doctor first. Do notmay cause the following side effects. Most side effects are mild or moderate: What are the possible side effects of ? amlodipine Amlodipine•headache•swelling of your legs or ankles•tiredness, extreme sleepiness•stomach pain, nausea•dizziness•flushing (hot or warm feeling in your face)•arrhythmia (irregular heartbeat)•heart palpitations (very fast heartbeat)It is rare, but when you first start taking or increase your dose, you may have a heart attack or your angina may get worse. If that happens, call your doctor right away or go directly to a hospital emergency room. amlodipineTell your doctor if you are concerned about any side effects you experience. These are not all the possible side effects of . For a complete list, ask your doctor or pharmacist. amlodipineCall your doctor for medical advice about side effects. You may report side effects to the FDA at 1-800-FDA-1088 or by visiting /medwatch.Keep away from children. Store at 20° to 25°C (between 68° and 77°F) [see USP Controlled Room Temperature]. Keep out of the light. Do not store in the bathroom. Keep in a dry place. How do I store ? amlodipineamlodipineamlodipinebesylate tabletsamlodipineamlodipineSometimes, doctors will prescribe a medicine for a condition that is not written in the patient information leaflets. Only use the way your doctor told you to. Do not give to other people, even if they have the same symptoms you have. It may harm them. General advice about amlodipineamlodipineamlodipineYou can ask your pharmacist or doctor for information about . amlodipineManufactured for: Huntsville, AL 35811QUALITEST PHARMACEUTICALS8182524 Revised: 5/2010 R3AMLODIPINE 5MG TABLETRevised: 05/2010Distributed by: Unit Dose Services。
