葡萄糖酸钠溶解度曲线
葡萄糖酸钠是什么?
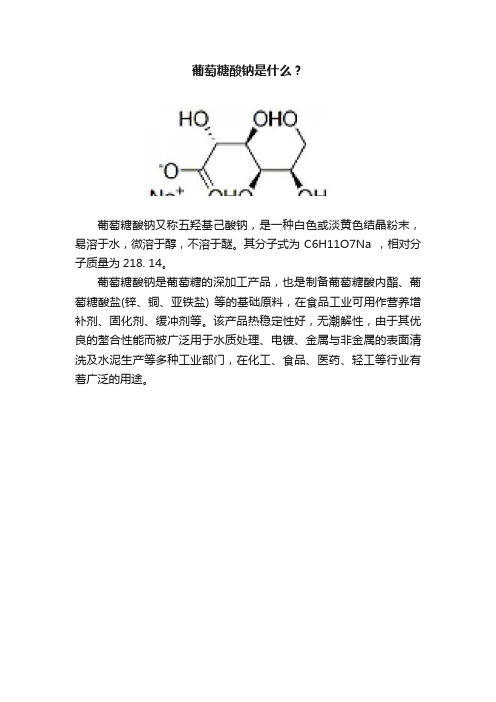
葡萄糖酸钠是什么?
葡萄糖酸钠又称五羟基己酸钠,是一种白色或淡黄色结晶粉末,易溶于水,微溶于醇,不溶于醚。
其分子式为C6H11O7Na ,相对分子质量为218. 14。
葡萄糖酸钠是葡萄糖的深加工产品,也是制备葡萄糖酸内酯、葡萄糖酸盐(锌、铜、亚铁盐) 等的基础原料,在食品工业可用作营养增补剂、固化剂、缓冲剂等。
该产品热稳定性好,无潮解性,由于其优良的螯合性能而被广泛用于水质处理、电镀、金属与非金属的表面清洗及水泥生产等多种工业部门,在化工、食品、医药、轻工等行业有着广泛的用途。
葡萄糖酸钠化学式 -回复

葡萄糖酸钠化学式-回复葡萄糖酸钠是一种常见的无机化合物,化学式为C6H11O7Na。
它是一种白色结晶固体,可溶于水。
葡萄糖酸钠在生命科学、医药和食品工业中具有广泛的应用。
在本文中,我们将一步一步地探讨葡萄糖酸钠的化学式,以及它的性质、制备和应用。
葡萄糖酸钠的化学式是C6H11O7Na,其中C表示碳元素,H表示氢元素,O表示氧元素,Na表示钠元素。
化学式告诉我们了葡萄糖酸钠分子的组成和结构,有助于我们理解这种化合物的性质和反应。
首先,让我们看一下葡萄糖酸钠的性质。
葡萄糖酸钠是一种无色结晶固体,在水中溶解度很高。
它是一种酸性盐,可以和酸反应生成相应的葡萄糖酸盐。
葡萄糖酸钠在水溶液中呈酸性,pH值较低。
它也是一种还原剂,可以与氧化剂发生反应,被氧化为较低的氧化态。
接下来,我们将探讨葡萄糖酸钠的制备方法。
葡萄糖酸钠的制备有几种常用的方法。
一种常见的方法是将葡萄糖与氧化钠或氢氧化钠反应,生成葡萄糖酸钠和水。
这个反应方程式可以表示为:C6H12O6 + NaOH -> C6H11O7Na + H2O另一种方法是将葡萄糖与碳酸钠反应,生成葡萄糖酸钠和二氧化碳。
这个反应方程式可以表示为:C6H12O6 + Na2CO3 -> C6H11O7Na + CO2 + H2O葡萄糖酸钠在多个领域都有广泛的应用。
在医药领域,葡萄糖酸钠常被用作药物的缓冲剂,用于稳定和调节药物的pH值。
它也可以用作静脉注射液的添加剂,帮助药物在体内更好地吸收和分解。
在食品工业中,葡萄糖酸钠被广泛用作食品的添加剂。
它可以作为酸味剂、调味剂和抗氧化剂使用。
葡萄糖酸钠可以延长食品的保鲜期,抑制食品中的微生物生长,提高食品的品质和口感。
除了医药和食品工业,葡萄糖酸钠还被广泛应用于生命科学研究中。
它可以用作细胞培养的缓冲剂,帮助细胞维持合适的环境。
葡萄糖酸钠还可以用于DNA和RNA的提取和保存,以及分子生物学实验中的一些反应。
总结起来,葡萄糖酸钠是一种常见的无机化合物,化学式为C6H11O7Na。
葡萄糖酸钠生产新工艺

++_.+
翎甸
瓦坩H
铭
A阪
青
^眦仃
立,
团迟
▲●■ I
工艺
糖姗 钠纛 生.‰ n秘
的设备工艺,提高了产品收率及产品质量。
就别..鲡
【摘要】本文简要介绍了葡萄糖酸钠产品在国内的生产现状及市场情况,重点探讨了发酵法生产 葡萄糖酸钠的生产工艺,在黑曲霉的培养诱变方面做了重点研究实现了高浓度、低营养盐、高温下的发
针对这些问题,我公司相关工艺、设备人员 根据在葡萄糖酸钠生产上积累经验以及对葡萄糖 酸钠产品性质的了解、查阅相关资料;依据自身 的经验及专业知识,提出了将多效降膜式蒸发器 与强截外循环式缩晶器裰结合,做成一个连续蒸 发结晶系统的构想; 在与降膜式蒸发器生产厂家的沟通中发现还 有很多问题,因为在国内葡萄糖酸钠行业还没有 厂家使用这种连续蒸发结晶系统的先例,很多数 据需要进行摸索设计。针瓣这些情况,我公司依 据连续蒸发结晶系统在柠檬酸上使用的经验及教 训,并让实验员在实验室对蔼萄糖酸钠发酵液在 蒸发方面的黏度、沸点升商、溶解度曲线等相荚 性质进行了摸索,掌握了第一手的资料,向设备 生产厂家提凄了合理豹设计数据。嚣翁这套三效 连续蒸发结晶系统已经连续运行了半年多了,与 当初的设计一致,没有出现任何阕题。 采用三效连续蒸发结晶系统后,对葡萄糖酸
・244・
2007年全国发酵行业高峰论坛
但是国内在蓣萄糖酸镳生产方匿还存在苓少闻 题,主要是生产工艺老化能耗较高,产品滤过质 爨、颜色等方面无法达到食品级的要求,有不少 园矫客户赠买匡内的镶萄糖酸钠后需要在进行精 制提取。 三、罴麴霉发酵生产葡萄糕酸钠戆生产工艺
改进
提赢了初糖浓度。 我公司技术人员对原来使用的黑翻霉菌种进 行培养、筛选诱变,成功解决了微生物在高浓度 金属离子下难以生长的技术难题,并将发酵培养 基中的营养盐浓度大幅度的降低。最终新菌种提 高了发酵酌初糖浓度,由原来的30%提高到瑗在 的34%,提高了四个百分点,缩短了发酵周期,
1107 葡萄糖酸钠

1107 葡萄糖酸钠Sodium Gluconate葡萄糖酸钠在建筑工业领域加入混凝土中具有很好的减水作用和阻滞缓凝作用。
减水作用即减少水在混凝土中的用量,混凝土中的水比例减少后,能增加混凝土的可塑性,促进了水泥水化;能增强混凝土的牢度、强度。
它的阻滞缓凝的作用即是推迟混凝土的最初凝固时间延长十几倍,即是将混凝土的可塑时间从几小时延长到几天而不影响其牢度,这就解决了现代混凝土工程因在一个地方配制,然后用车运到工地使用,因时间差而出现裂纹等一系列质量问题。
从而为重要工程建设获得成功提供了技术保障。
配制一定量的葡萄糖溶液加入于四口烧瓶中,称取适量催化剂加入到此烧瓶中,恒温。
向溶液中通入空气,并不断滴加一定浓度的NaOH 溶液来维持一定的pH 值。
反应后的溶液经冷却、抽滤( 催化剂回收) ,滤液减压蒸馏浓缩、结晶,风干后得到葡萄糖酸钠晶体。
该法工艺简单,反应平稳,易于控制,反应条件温和,其葡萄糖转化率在95 %左右。
缺点是所用催化剂在循环使用一定次数后,催化效率下降,使葡萄糖转化率降低,反应时间延长甚至基本无催化活性,催化剂必须报废更新,相应提高了单位产品催化剂耗量,也使葡萄糖酸钠产品生产成本较高。
由此可见,催化剂性能的好坏是使用此法的关键。
催化氧化法还是目前国内葡萄糖酸钠生产的主要方法,其产量占到80 %以上。
别名:五羟基已酸钠分子式Molecular formula : C 6 H 11 O 7 Na结构式Structural formula :分子量Molecular weight :218.14性状Properties :白色结晶颗粒或粉末,极易溶于水,略溶于酒精,不溶于乙醚。
White crystalline granule or powder,easy to dissolve in the water 。
包装Packing :25 ㎏外套塑料编织袋,内用塑料薄膜袋,或按客户要求定制包装。
25kg in plastic film bag lined plastic woven bag,or following your demand 。
葡萄糖酸钠-USP

USP 36Official Monographs / Sodium5165ride ion, to a 1000-mL volumetric flask, add water to dis-solve, dilute with water to volume, and mix.Sodium Fluoride and Phosphoric Acid Procedure—Proceed as directed for Procedure in the Assay Gelunder Sodium Fluoride Oral Solution. Calculate the quantity,in mg, of fluoride ion in each mL of the Topical Solutiontaken by the formula:» Sodium Fluoride and Phosphoric Acid Gel con-tains not less than 90.0percent and not moreC/V than 110.0percent of the labeled amount offluoride ion, in an aqueous medium containing a in which C is the determined concentration of fluoride ion,in µg per mL, in the Assay preparation, and V is the volume,suitable viscosity-inducing agent.in mL, of Topical Solution taken.Packaging and storage—Preserve in tight, plastic con-TEST2—[NOTE—Use Purified Water (resistivity not less than tainers.18 megohm-cm) for the Mobile phase and all preparations.]Labeling—Label Gel in terms of the content of sodium Mobile phase—Dissolve about 300mg of anhydrous so-fluoride (NaF) and in terms of the content of fluoride ion.dium carbonate in 2000mL of water, add 2.0mL of 1NUSP Reference standards 〈11〉—sodium hydroxide, mix, and pass through a 0.45-µm nylonUSP Sodium Fluoride RSfilter.Identification—Standard stock preparation—Dissolve a suitable quantityof USP Sodium Fluoride RS in water to obtain a solution A: Place a quantity of Gel, equivalent to about 500mg of having a known concentration of about 0.11mg per mL.fluoride ion, in a platinum crucible in a well-ventilated hood,and add 15mL of sulfuric acid. Cover the crucible with a Standard preparation—Transfer 5.0mL of the Standardpiece of clear, polished glass, and heat on a steam bath for stock solution to a 500-mL volumetric flask, dilute with water1hour. Remove the glass cover, rinse it in water, and dry: to volume, and pass through a 0.45-µm nylon or PTFE filter.the glass surface exposed to vapors from the crucible is This solution contains about 1.1µg of sodium fluoride peretched.mL.B: It responds to the tests for Phosphate 〈191〉.Assay preparation—Transfer 10.0mL of the Topical Solu-tion to a suitable volumetric flask, dilute with water quan-Rotational Rheometer Methods 〈912〉—Place a quantity titatively, and stepwise if necessary, to obtain a solution hav-of Gel in a suitable plastic container, insert the stopper se-ing a known concentration of about 1.1µg of sodium curely, and allow to stand until the gel is free from air bub-fluoride per mL, based on the label claim, and pass through bles. Place it in a water bath maintained at a temperature of a 0.45-µm nylon or PTFE filter.25±0.5° until it adjusts to the temperature of the waterbath (30minutes or longer). Do not stir the gel while it is in Chromatographic system (see Chromatography 〈621〉)—Thethe bath. Remove the sample from the bath, stir the gel liquid chromatograph is equipped with a conductivity de-gently for 5seconds, and without delay, using a rotational tector with a suitable suppression unit and a 4.6-mm ×viscometer, determine the viscosity using the appropriate 25-cm column and a 4.6-mm × 50-mm guard column, bothspindle to obtain a scale reading between 10% and 90% of containing packing L46 (see Chromatographic Reagentsfull scale at a speed of 60 rpm or of 30 rpm. Calculate the under Reagents, Indicators, and Solutions). The flow rate isviscosity, in centipoises, by multiplying the scale reading by about 1.0mL per minute. [NOTE—It is recommended to usethe constant for the spindle and speed used: the viscosity is polymethylpentene HPLC vials.] Chromatograph the Stan-between 7000 and 20,000 centipoises.dard preparation, and record the peak responses as directedfor Procedure: the elution order is fluoride, followed by the pH 〈791〉—Place about 40mL in a plastic beaker, and deter-chloride peak; the resolution, R, between fluoride and chlo-mine the pH using a suitable electrode system: the pH is ride is not less than 1.5; the column efficiency is not less between 3.0 and 4.0.than 1500 theoretical plates; the tailing factor for the fluo-Assay—[NOTE—Store all solutions, except Buffer solution, in ride peak is not more than 2.0; and the relative standard plastic containers.]deviation for six replicate injections is not more than 2.0%Buffer solution and Standard preparations—Prepare as di-for the fluoride peak. [NOTE—Chloride is an impurity that is rected in the Assay under Sodium Fluoride Oral Solution. present in USP Sodium Fluoride RS.]Assay preparation—Transfer a quantity of Gel, equivalent Procedure—Separately inject equal volumes (about 20µL)to about 20mg of fluoride ion, accurately weighed, to aof the Standard preparation and the Assay preparation into1000-mL volumetric flask, add water to dissolve, dilute with the chromatograph, record the chromatograms, and meas-water to volume, and mix.ure the responses for the fluoride peaks. Calculate the per-Procedure—Proceed as directed for Procedure in the Assay centage, P (w/v), of sodium fluoride in the portion of Topi-under Sodium Fluoride Oral Solution. The quantity, in mg, of cal Solution taken by the formula:fluoride ion in the portion of Gel taken is equivalent to C,the determined concentration of fluoride ion, in µg per mL, 100×0.001×C S(D/V)(r U/r S)in the Assay preparation.in which 0.001 is the conversion factor from mg to g; C S isthe concentration, in mg per mL, of sodium fluoride in theStandard preparation;D is the dilution factor for the Assaypreparation; V is the volume, in mL, of Topical Solution Sodium Gluconatetaken to prepare the Assay preparation; and r U and r S are thefluoride peak responses obtained from the Assay preparationand the Standard preparation, respectively.Calculate the percentage (w/v) of fluoride ion in the por-tion of Topical Solution taken by the formulaP×19.00/41.99C6H11NaO7218.14D-Gluconic acid, monosodium salt;in which P is as defined above; 19.00 is the atomic weightMonosodium D-gluconate [527-07-1].of fluorine; and 41.99 is the molecular weight of sodiumfluoride.5166Sodium / Official Monographs USP 36DEFINITION accurately timed, and cool rapidly to room tempera-Sodium Gluconate contains NLT 98.0% and NMT 102.0%ture. Add 25mL of 0.6 N acetic acid, 10.0mL of 0.1 N of C6H11NaO7.iodine VS, and 10mL of 3N hydrochloric acid, and ti-trate with 0.1 N sodium thiosulfate VS, adding 3mL of IDENTIFICATION starch TS as the endpoint is approached. Perform a •A. I DENTIFICATION T ESTS—G ENERAL, Sodium〈191〉blank determination, omitting the specimen, and note Sample solution:1 in 20the difference in volumes required (see Titrimetry 〈541〉).Acceptance criteria:Meets the requirements Each mL of the difference in volume of 0.1 N sodium •B. T HIN-L AYER C HROMATOGRAPHY thiosulfate consumed is equivalent to 2.7mg of reduc-Standard solution:10mg/mL of USP Potassium Gluco-ing substances (as dextrose).nate RS in water. [N OTE—Heat in a water bath at 60° if Acceptance criteria:NMT 0.5%necessary to dissolve.]Sample solution:10mg/mL of Sodium Gluconate in ADDITIONAL REQUIREMENTSwater. [N OTE—Heat in a water bath at 60° if necessary•P ACKAGING AND S TORAGE:Preserve in well-closedto dissolve.]containers.Chromatographic system•USP R EFERENCE S TANDARDS〈11〉(See Chromatography 〈621〉, Thin-Layer Chromatography.)USP Potassium Gluconate RSMode:TLCAdsorbent:0.25-mm layer of chromatographic silicagelApplication volume:5µLDeveloping solvent system:Alcohol, ethyl acetate,Sodium Hypochlorite Solutionammonium hydroxide, and water (50:10:10:30)Spray reagent:Dissolve 2.5g of ammonium molyb-NaClO74.44date in 50mL of 2N sulfuric acid in a 100-mL volu-Hypochlorous acid, sodium salt.metric flask, add 1.0g of ceric sulfate, swirl to dissolve,Sodium hypochlorite [7681-52-9].and dilute with 2N sulfuric acid to volume.» Sodium Hypochlorite Solution contains not less AnalysisSamples:Standard solution and Sample solution than 4.0percent and not more than 6.0percent, Develop in the Developing solvent system until the sol-by weight, of NaClO.vent front has moved three-fourths of the length of Caution—This Solution is not suitable for applica-the plate. Remove the plate from the chamber, and tion to wounds.dry at 110° for 20 min. Allow to cool and spray withthe Spray reagent. Heat the plate at 110° for 10 min.Packaging and storage—Preserve in tight, light-resistant Acceptance criteria:The principal spot from the Sample containers, at a temperature not exceeding 25°.solution corresponds in color, size, and R F value to thatIdentification—from the Standard solution.A: The Solution at first colors red litmus blue and then ASSAY bleaches it.•P ROCEDURE B: The addition of 3N hydrochloric acid to the Solution Sample:150mg causes an evolution of chlorine.Analysis:Transfer the Sample to a suitable conical flaskC: The solution obtained in Identification test B responds and dissolve in 75mL of glacial acetic acid, warming ifto the flame test for Sodium 〈191〉.necessary to dissolve. Cool, add quinaldine red TS, andAssay—Weigh accurately, in a glass-stoppered flask, about titrate with 0.1 N perchloric acid VS to a colorless3mL of Solution, and dilute it with 50mL of water. Add 2g endpoint. Each mL of 0.1 N perchloric acid is equiva-of potassium iodide and 10mL of 6N acetic acid, and ti-lent to 21.81mg of C6H11NaO7.trate the liberated iodine with 0.1 N sodium thiosulfate VS, Acceptance criteria:98.0%–102.0%adding 3mL of starch TS as the endpoint is approached. IMPURITIES Perform a blank determination, and make any necessary •C HLORIDE AND S ULFATE, Chloride〈221〉correction. Each mL of 0.1 N sodium thiosulfate is equiva-Sample:1.0g lent to 3.722mg of NaClO.Acceptance criteria:The Sample shows no more chlo-ride than corresponds to 1mL of 0.020 N hydrochloricacid (NMT 0.07%).•C HLORIDE AND S ULFATE, Sulfate〈221〉Sample:2.0g Sodium Hypochlorite Topical Solution Acceptance criteria:The Sample, dissolved in boilingwater, shows no more sulfate than corresponds to 1mL» Sodium Hypochlorite Topical Solution contains of 0.020 N sulfuric acid (NMT 0.05%).•L EAD〈251〉not less than 0.20g and not more than 0.32g of Test preparation:1.0g in 25mL of water Sodium Hypochlorite (NaClO) in 1000mL of Acceptance criteria:NMT 10ppm Topical Solution. Prepare Sodium Hypochlorite •H EAVY M ETALS, Method I〈231〉Topical Solution as follows (see Pharmaceutical Test preparation:Dissolve 1.0g in 10mL of water, addCompounding—Nonsterile Preparations 〈795〉): 6mL of 3N hydrochloric acid, and dilute with water to25mL.Acceptance criteria:NMT 20ppm•R EDUCING S UBSTANCES Sodium Hypochlorite Solution.... 5.0 mL Sample:1.0gAnalysis:Transfer the Sample to a 250-mL conical flask,Monobasic Sodium Phosphatedissolve in 10mL of water, and add 25mL of alkaline monohydrate................ 1.02 g cupric citrate TS. Cover the flask, boil gently for 5 min,。
葡萄糖酸钠纯度的检测方法

葡萄糖酸钠纯度的检测方法新乡市华旭化工助剂有限公司专业生产葡萄糖酸钠网址:葡萄糖酸钠的盐味接近食盐,阀值为食盐的五倍,无苦涩味,没有刺激性,可以取代食盐制备低钠食品满足有高血压等需要低钠饮食人群的需要;其次可以代替食盐用于改良特性,调整发酵,赋予食品保存性、脱水作用等功能;可以应用于水质处理、电镀、金属与非金属的表面清洗、水泥生产、制药工业等领域。
1. 试验材料1.1 试剂葡萄糖酸钠(分析纯),甲醇(色谱纯),磷酸(分析纯),超纯水,氢氧化钠,硫酸铜,冰醋酸,喹哪啶红,高氯酸1.2 仪器设备DIONEX高效液相色谱系统:P680 HPLC Pump,ASI-100 Automated Sample Injector,Thermostatted Column Compartment TCC-100,PDA-100 Photodiode Array Detector;反相色谱C18柱,Apollo C18(250mm*4.6mm);Milli-Q○R Millipore 超纯水器;KQ5200DE型数控超声波清洗器,DHG-9145A型鼓风干燥箱,UNICO UV-2102 PC型紫外可见分光光度计;TIM 840 TITRATION MANAGER(工作电极:PHG 311-9;参比电极:REF 921);WZZ-2A自动数显旋光仪。
2. 实验部分2.1 HPLC法[5]准确称取1.5040g于105℃下烘至恒重的葡萄糖酸钠,用超纯水溶解并定容至500mL。
分别取1,2,3,4,5,6,7,8,9mL葡萄糖酸钠溶液用超纯水稀释至15mL。
将其分别过0.45μm滤膜,再超声处理后即可进样,在HPLC仪器上分析,取其中6点做标准曲线。
高效液相色谱采用的流动相为甲醇︰水︰1%磷酸(2︰48︰50),流速为1.0mL/min,柱温为25℃,进样量为15μL,检测波长为210nm。
2.2 分光光度法[6]准确称取13.4779g于105℃下烘至恒重的葡萄糖酸钠,用蒸馏水定容至50mL。
葡萄糖酸钠MSDS

葡萄糖酸钠葡萄糖酸钠CAS号: 527-07-1英文名称:Sodium gluconate英文同义词:glonsen;pasexon100t;BVD Addicrete;Sunmorl N60S;DisparlightDV;Developer,partB;Natriumgluconat;gluconatodisodio;SODIUM GLUCONATE;SOIDUMD-GLUCONATE中文名称:葡萄糖酸钠中文同义词:葡糖酸钠;葡萄糖酸钠;葡糖酸钠盐;D-葡糖酸钠;五羟基己酸钠;五羟基已酸钠;D-葡糖糖酸钠;D-葡萄糖酸钠;D-葡糖酸单钠盐;D-葡萄糖酸钠,97%CBNumber:CB9229989分子式: C6H11NaO7分子量: 218.14MOLFile:527-07-1.mol葡萄糖酸钠化学性质熔点: 206 °C (dec.)(lit.)溶解度: H2O: 0.1 g/mL, clearMerck : 14,4456BRN : 3919651稳定性: Stable. Incompatible with strong oxidizing agents. CAS 数据库: 527-07-1(CAS DataBase Reference)EPA化学物质信息: D-Gluconic acid, monosodium salt(527-07-1)安全信息安全说明: 24/25WGK Germany : 1RTECS号: LZ5235000海关编码: 29181600葡萄糖酸钠 MSDSGluconic acid sodium salt葡萄糖酸钠性质、用途与生产工艺含量分析精确称取试样约750mg,移入一干燥的200ml锥形烧瓶中,加冰醋酸75ml,在加热板上加热溶解后冷却,加喹哪啶红试液(TS-203)数滴,用0.1mol/L高氯酸的冰醋酸溶液经10ml微量滴定管滴定至无色终点。
每ml0.1mol/L高氯酸相当于葡萄糖酸钠(C6H11NaO7)21.81mg。
葡萄糖酸钠分析方法

文件编号:SOP.QAD—2001葡萄糖酸钠的检测方法起草:日期:审核:日期:批准:日期:发布日期:实施日期:1.目的规范葡萄糖酸钠的检测操作,以达到准确的检测结果。
2.适用范围适用于葡萄糖酸钠的性状、确认实验、含量、溶状、pH、硫酸盐、氯化物、重金属、铅、砷、还原糖、干燥减量、晶体色度(b)、过滤实验、异物检查、溶液色度(10%w/v)、粒度分布和色调共计18个检测项目。
3.职责3.1分析人员在进行葡萄糖酸钠的相关检验时,应严格执行本规定。
3.2葡萄糖酸钠的检测负责人负责本规程的实施,监督与修订。
4.标准4.1性状:无色~黄色晶体粉末或颗粒,稍有特殊气味。
4.2确认实验:符合实验4.3含量:98.0-102.0%4.4溶状:无色,几乎澄清4.5 pH(1.0g/10ml):6.2-7.84.6硫酸盐:0.24% 以下4.7氯化物:0.071% 以下4.8重金属(以Pb计):10μg/g以下4.9铅:2μg/g以下4.10砷(以As2O3计):2.0μg/g以下4.11还原糖(以葡萄糖计):0.50% 以下4.12干燥减量:0.30% 以下4.13晶体色度(b):3.5以下4.14过滤实验:C-C以上4.15异物检查:C以上4.16溶液色度(10%w/v):15以下4.17粒度分布:符合实验4.18色调(420nm):0.02以下5.工作程序5.1性状称取10g样品,放在洁净的白纸上,目视观察同时用鼻子闻其气味,做出判断。
5.2确认实验5.2.1焰色反应呈现黄色5.2.2取被中和的葡萄糖酸钠溶液(1→20),加入焦锑酸钾试液,有白色结晶性沉淀生成(用玻璃棒磨擦试管内壁,加速沉淀形成)。
5.2.3取5ml葡萄糖酸钠溶液(1→10),加入0.7ml冰醋酸和1ml新配制的苯肼,水浴加热30分钟。
冷却后,用玻璃棒磨擦容器内壁,出现晶体,过滤收集晶体,溶于10ml沸水中,加少量活性炭脱色,过滤。
冷却后,用玻璃棒磨擦容器内壁,出现晶体,过滤收集并干燥沉淀的晶体。
