百泌达GS-完整版
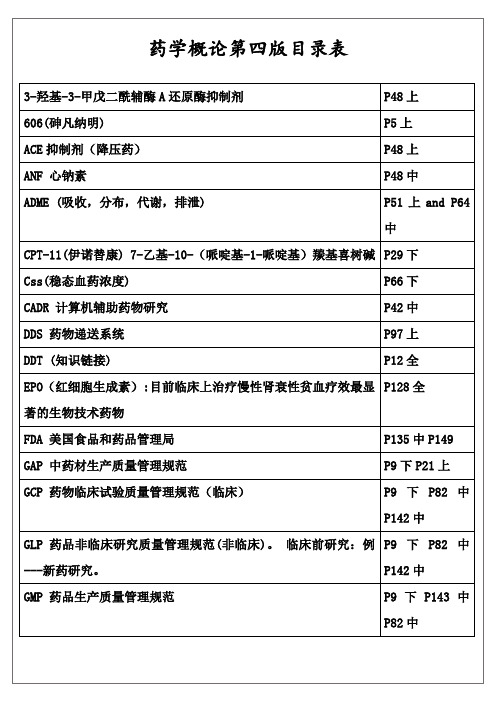
药学概论第四版详细知识点目录检索
归经:药物对于机体某部分的选择作用(中药的归经)
P17上
规定新药:致畸,致突变,致癌试验。
P47倒三段中
《国家药品标准>包括的药品标准
P78上
国内关注度最高的给药系统:口服给药系统, 注射给药系统
P97下
H
哈里斯:合成β-优卡因
P43倒一段倒一行
华佗:麻沸散(处方中君药是洋金花)
P145(一)
穿心莲:治疗细菌性痢疾
P13第二段倒四行
创新药物研究开发的一般过程
P312.
创新药物研究开发途径
P27下
<词源>中药的解释
P1第一段
D
达尔文 《物种起源>
P112第一段
地尔硫卓:一种降压药
P117第三段第三行
定向进化
P117第四段第三行
大肠杆菌:应用最广泛的产酶菌,一般分泌胞内酶
P118and P123
P45第一段倒四行
常咯啉:常山乙素(有催吐作用)结构修饰物(抗疟疾作用,现作为抗心律失常药物)
P31第一段
超微量生理活性物质分离:蚕蛾醇 蜕皮激素
P35第一段
陈克恢---现代中药药理学研究的创始人(发现麻黄碱的拟交感作用)
P73知识链接
处方和工艺的设计前研究
P103(一) 1.
处方药与非处方药的管理
P4第一段第三行
第二信使学说:由萨瑟兰创立
P61第二段倒四行
典型的量时曲线
P66上
点青霉:产生青霉素从而抑制葡萄球菌生长的霉菌
P126倒数第一段
电子仪器(4.电生理学方法)
P69
定量研究体内过程主要的两个目的
P64第二段
AKTApilot

图 8. 用 SOURCE 30 Q 进行精细纯化的 AKTApilot 和 AKTAexplorer 100 的层析图谱。
.5.
AKTApilot
可靠的灭菌
AKTApilot 的灭菌效率经受了灭菌程序的测试,该法用 1 M 氢 氧化钠 (NaOH) 作为杀菌剂进行微生物攻击实验。 系统用包括 由美国药典 United States Pharmacopoeia (USP 25) 推荐的三 个细菌系和一种通常用于生产环境的酵母菌系的溶液感染系 统 (表 1)。USP 25 要求菌落形成单位呈 log 6 减少。
GE Healthcare
AKTA pilot
!"#$%&'()*+,-./01234
:
AKTApilot !"#$ !"#$%&'() !"#$%&'()*+,-./01 !"# !"# $"%&'()* !"#$% !"#$%AKTApilot !"#$ I-III !"# !"# $%& GLP cGMP !
进口阀 (V1) 缓冲液容器
进口阀 (V2) 压力传感器(4) 层析柱阀(V6)
图 2. 液体流路。
流程 1. 样品阀 (V3) 选择一个合适的样品进口管道。 样品泵 (P-908) 经过一快速响应的压力传感器, 流动方向阀 (V5) 和柱选择 阀 (V6+V7) 将样品溶液输送到柱中。 可在进口管处用遥控 的空气传感器 (2) 来检测样品容器是否腾空。 2. 进口阀 (V1+V2) 选择洗脱液进口管道。系统泵 (P-907) 经 过一快速响应的压力传感器 (1) 和混合器将洗脱液输送到 空气阱阀 (V4) 。液体会被引导流过或绕过空气阱。 3. 经过空气阱阀 (V4) 后, 洗脱液经过空气传感器 (1) 和压力 传感器 (2) 流向流动方向阀 (V5) 。空气传感器用于防止空 气进入层析柱内。压力传感器监测柱头的压力。 4. 流动的液体继续经过其中之一的层析柱柱阀 (V6+V7) 进入 柱内。液体流经层析柱的凝胶,样品被纯化。 5. 然后液体经过压力传感器 (4) 通过装在 pH 流动池的 pH 电 极,电导池和 UV 流动池。柱前和柱后的压力传感器持续 检测层析柱进口和出口的压力, 屏幕上显示出不同的压力 读数和变化中的压力曲线。 6. 液体继续流到出口阀 (V8+V9) ,出口阀用于转换液体到废 液或分部收集器。
百泌达联合甘精胰岛素治疗肥胖2型糖尿病疗效观察

百泌达联合甘精胰岛素治疗肥胖2型糖尿病疗效观察摘要】目的:观察百泌达联合甘精胰岛素治疗肥胖2型糖尿病患者的临床疗效。
方法:采用回顾性方法,选取我院2014年12月至2016年12月以来收治的50例肥胖2型糖尿病患者的临床资料,按照治疗方法的不同,随机分为两组,对照组25例患者采用甘精胰岛素加诺和锐治疗,观察组25例患者在治疗组患者的治疗基础上,增加百泌达治疗,观察两组患者的体重减轻效果。
结果:观察组治疗的减重总有效率96.00%(24/25),明显高于对照组治疗总有效率80.00%(20/25),两组数据差异显著,具有统计学意义(P<0.05)。
结论:百泌达联合甘精胰岛素治疗肥胖2型糖尿病患者的临床疗效确切,不仅能有效控制患者血糖波动,还有效控制并发症的发展,值得在临床上推广应用。
【关键词】百泌达;甘精胰岛素;诺和锐;临床疗效;联合;肥胖Ⅱ型糖尿病【中图分类号】R587.1 【文献标识码】A 【文章编号】2095-1752(2017)25-0126-02糖尿病(Diabetes)是一种常见病,被誉为“不死的癌症”。
分为1型糖尿病和2型糖尿病。
1型糖尿病多是先天性疾病,主要受遗传因素影响,2型糖尿病主要是后天因素而引发的疾病。
由于该疾病能并发多种综合征,对器官功能损害较大,还有着较高的致残率及病死率,是严重威胁患者生命安全的疾病。
为探讨百泌达联合甘精胰岛素治疗肥胖2型糖尿病患者的临床疗效,特选取我院50例肥胖2型糖尿病患者作为此次研究对象,现报告如下:1.资料和方法1.1 一般资料对我院2014年12月至2016年12月以来收治的50例肥胖2型糖尿病患者的临床资料进行回顾性分析,所有患者均符合《实用医学》的诊断标准,采用糖耐量随机测试患者的空腹血糖和餐后两小时血糖,如果空腹血糖>7.0mmol/L,餐后两小时血糖>11.1mmol/L,即可考虑为2型糖尿病患者,患者体态肥胖,有多食、多饮、多尿等症状。
使用无针注射器注射不同胰岛素剂量的确定

使用无针注射器注射不同胰岛素剂量的确定
简介:注射胰岛素控糖是不少糖尿病患者每天都要做的事情,无针注射器的出现,让越来越多使用胰岛素控糖的患者避免了传统注射器注射胰岛素带了的各种问题。
那么初次使用无针注射器注射百泌达和诺和力,如何确定注射的剂量?
无针注射器目前只适用胰岛素的注射,而百泌达和诺和力这两种注射液并非胰岛素,但是确有一些糖尿病患者使用这两种方案进行控糖。
如果糖尿病患者需要无针注射器注射这两种药剂,需要换算这两种注射液的注射剂量。
百泌达(艾塞那肽注射液)有两种注射方案:每次注射5微克和每次注射10微克。
每次注射5微克(等于2单位药液),不能使用无针注射器进行注射;
每次注射10微克(等于4单位药液),可以使用无针注射其进行注射。
诺和力(利拉鲁肽注射液),目前有三种不同的治疗方案,对应的注射剂量分别为:
一支注射笔注射30次用完,等于每次注射10单位药液;
一支注射笔注射15次用完,等于每次注射20单位药液;
一支注射笔注射10次用完,等于每次注射30单位药液。
注意事项:
1、初次使用快舒尔无针注射器时严格按照说明书操作,或者是相关人员指导下操作
2、不是什么药剂都能使用无针注射器进行注射,所以尽量选择无针注射器适用的药剂。
ep8.0 甘精

javascript:try { openDoc('2571F.htm', '_self') } catch(e) { };javascript:try { openDoc('2571F.htm','_self') } catch(e) { };javascript:try { openDoc('2571E.pdf', '_blank') } catch(e) { };javascript:try { openDoc('2571E.pdf','_blank') } catch(e){ };http://extranet.edqm.eu/4DLink1/4DCGI/Web_View/mono/2571http://extranet.edqm.eu/4DLink1/4DCGI/Web_View/mono/2571javascript:try{ openDoc('90012E.htm', '_blank') } catch(e) { };javascript:try { openDoc('90012E.htm', '_blank') } catch(e) { };javascript:try { openDoc('10000E.htm', '_blank') } catch(e) { };javascript:try { openDoc('10000E.htm', '_blank') } catch(e) { };01/2014:2571Insulin glargineInsulin glargineInsulinum glarginumC267H404N72O78S6M r 6063DEFINITION21A-Glycine-30B a-l-arginine-30B b-l-arginine-insulin (human).Insulin glargine is a 2-chain peptide containing 53 amino acids. The A-chain is composed of 21 amino acids and the B-chain is composed of 32 amino acids. It is identical in primary structure to human insulin, only differing in amino acid sequence at position 21 in the A-chain and at the C-terminal end of the B-chain where it contains 2 additional amino acids. Human insulin is Asn(A21), whereas insulin glargine is Gly(A21), Arg(B31), Arg(B32). As in human insulin, insulin glargine contains 2 interchain disulfide bonds and 1 intrachain disulfide bond.Content: 94.0 per cent to 105.0 per cent (anhydrous substance).By convention, for the purpose of labelling insulin glargine preparations, 0.0364 mg of insulin glargine is equivalent to 1 unit.PRODUCTIONInsulin glargine is produced by a method based on recombinant DNA (rDNA) technology under conditions designed to minimise the degree of microbial contamination.Prior to release, the following tests are carried out on each batch of the final bulk product, unless exemption has been granted by the competent authority.Host-cell-derived proteins. The limit is approved by the competent authority.Single-chain precursor. The limit is approved by the competent authority. Use a suitably sensitive method.CHARACTERSAppearance: white or almost white, hygroscopic powder.Solubility: practically insoluble in water and in anhydrous ethanol, soluble in dilute mineral acids.IDENTIFICATIONA. Examine the chromatograms obtained in the assay.Results: the principal peak in the chromatogram obtained with the test solution is similar in retention time to the principal peak in the chromatogram obtained with the reference solution.B. Peptide mapping (2.2.55).selective cleavage of the peptide bondsTest solution. Prepare a 10.0 mg/mL solution of the substance to be examined in a 1 g/L solution of hydrochloric acid R and transfer 5 µL of the solution to a clean tube. Add 1.0 mL of 1 M tris-hydrochloride buffer solution pH 7.5 R and 100 µL of a 20 U/mL solution of Staphylococcus aureus strain V8 protease, type XVII-B R in 1 M tris-hydrochloride buffer solution pH 7.5 R. Mix and incubate at 45 °C for about 2 h.Stop the reaction by adding 2 µL of phosphoric acid R.Reference solution. Prepare at the same time and in the same manner as for the test solution but using insulin glargine CRS instead of the substance to be examined. chromatographic separation. Liquid chromatography (2.2.29).Buffer solution. Dissolve 11.6 g of phosphoric acid R and 42.1 g of sodium perchlorate R in 1600 mL of water for chromatography R, adjust to pH 2.3 with triethylamine R and dilute to 2000 mL with water for chromatography R. Column:– size: l = 0.125 m, Ø = 3.0 mm;– stationary phase: spherical end-capped octadecylsilyl silica gel for chromatography R (4 µm);– temperature: 35 °C.Mobile phase:– mobile phase A: acetonitrile for chromatography R, buffer solution (7:93 V/V);– mobile phase B: buffer solution, acetonitrile for chromatography R (43:57 V/V);Flow rate: 0.6 mL/min.Detection: spectrophotometer at 214 nm.Equilibration: at initial conditions for at least 15 min.Injection: 50 µL.Retention time: insulin glargine fragment I = about 22 min.System suitability:– the chromatograms obtained with the test solution and the reference solution are qualitatively similar to the chromatogram of insulin glargine digest supplied with insulin glargine CRS;– in the chromatogram obtained with the reference solution, identify the peaks due to digest fragments I, II and III:symmetry factor: maximum 1.5 for the peaks due to fragments II and III; resolution: minimum 3.4 between the peaks due to fragments II and III.Results: the profile of the chromatogram obtained with the test solution corresponds to that of the chromatogram obtained with the reference solution.NOTE: the retention times of fragments I and IV are the same as for human insulin; the retention times of fragments II and III differ from human insulin due to the difference in the sequence at position 21 of the A-chain and to the 2 additional amino acids of the B-chain.TESTSImpurities with molecular masses greater than that of insulin glargine. Size-exclusion chromatography (2.2.30): use the normalisation procedure.Test solution. Dissolve 15.0 mg of the substance to be examined in 1.5 mL of a 1 g/L solution of hydrochloric acid R and dilute to 10.0 mL with water for chromatography R.Reference solution (a). Dry about 200 mg of the substance to be examined in an oven at 100 °C for 1.5-3 h. Dissolve 15.0 mg of the dried substance in 1.5 mL of a 1 g/L solution of hydrochloric acid R and dilute to 10.0 mL with water for chromatography R.Reference solution (b). Dilute 1.0 mL of the test solution to 100.0 mL with water for chromatography R. Dilute 3.0 mL of this solution to 20.0 mL with water for chromatography R.Column: 2 columns coupled in series, the coupling volume between the 2 columns being kept to a minimum:– size of each column: l = 0.3 m, Ø = 8 mm;– stationary phase: hydrophilic silica gel for chromatography R (5 µm) with a pore size of 15 nm, of a grade suitable for fractionation of globular proteins in the relative molecular mass range of 2000 to 80 000.Mobile phase: mix 200 mL of anhydrous acetic acid R, 300 mL of acetonitrile for chromatography R and 400 mL of water for chromatography R, adjust to pH 3.0 with concentrated ammonia R and dilute to 1000.0 mL with water for chromatography R. Flow rate: 0.5 mL/min.Detection: spectrophotometer at 276 nm.Injection: 100 µL; if splitting of the principal peak is observed, the injection volume may be decreased according to the provisions given in chapter 2.2.46.Run time: 1.5 times the retention time of insulin glargine.Retention time: insulin glargine = about 35 min.System suitability:– signal-to-noise ratio: minimum 10 for the principal peak in the chromatogram obtained with reference solution (b);– symmetry factor: maximum 2.0 for the peak due to insulin glargine in the chromatogram obtained with reference solution (a);– peak-to-valley ratio: minimum 2, where H p = height above the baseline of the peak due to high molecular mass proteins and H v = height above the baseline of the lowestpoint of the curve separating this peak from the peak due to insulin glargine in the chromatogram obtained with reference solution (a).Limits:– total of impurities with a retention time less than that of insulin glargine: not more than 0.3 per cent of the total area of the peaks; disregard any peak with a retention time greater than that of the peak due to insulin glargine.Related proteins. Liquid chromatography (2.2.29): use the normalisation procedure. Maintain the solutions at 2-8 °C.Test solution. Dissolve 15.0 mg of the substance to be examined in 1.5 mL of a 1 g/L solution of hydrochloric acid R and dilute to 10.0 mL with water for chromatography R.Reference solution. Dissolve the contents of a vial of insulin glargine CRS in 1.5 mL of a 1 g/L solution of hydrochloric acid R, transfer the solution with water for chromatography R to a 10 mL volumetric flask and dilute to 10.0 mL with water for chromatography R.Resolution solution. Dissolve the contents of a vial of insulin glargine for peak identification CRS (containing 0A-Arg-insulin glargine) in 0.3 mL of a 1 g/L solution of hydrochloric acid R and add 1.7 mL of water for chromatography R.Buffer solution. Dissolve 20.7 g of anhydrous sodium dihydrogen phosphate R in 900 mL of water for chromatography R, adjust to pH 2.5 with phosphoric acid R and dilute to 1000 mL with water for chromatography R.Column:– size: l = 0.25 m, Ø = 3.0 mm;– stationary phase: spherical end-capped octadecylsilyl silica gel for chromatography R (4 µm);– temperature: 35 °C.Mobile phase:– mobile phase A: dissolve 18.4 g of sodium chloride R in 250 mL of the buffer solution, add 250 mL of acetonitrile for chromatography R1 and mix; dilute to 1000 mL with water for chromatography R;– mobile phase B: dissolve 3.2 g of sodium chloride R in 250 mL of the buffer solution, add 650 mL of acetonitrile for chromatography R1 and mix; dilute to 1000 mL with water for chromatography R.Flow rate: 0.6 mL/min.Detection: spectrophotometer at 214 nm.Injection: 5 µL of the test solution and the resolution solution.Retention time: insulin glargine = about 20 min.System suitability: resolution solution:– peak-to-valley ratio: minimum 2, where H p = height above the baseline of the peak due to 0A-Arg-insulin glargine and H v = height above the baseline of the lowest point of the curve separating this peak from the peak due to insulin glargine.Limits:– any impurity: for each impurity, maximum 0.4 per cent;– total: maximum 1.0 per cent.Zinc: maximum 0.80 per cent.Atomic absorption spectrometry (2.2.23, Method I).Test solution. Dissolve 45.0 mg of the substance to be examined in a 1 g/L solution of hydrochloric acid R and dilute to 50.0 mL with the same solution. Dilute 10.0 mL of the solution to 100.0 mL with a 1 g/L solution of hydrochloric acid R.Reference solutions. Prepare reference solutions containing 0.2 µg, 0.4 µg and 0.6 µg of zinc per millilitre by diluting zinc standard solution (10 ppm Zn) R with a 1 g/L solution of hydrochloric acid R.Source: zinc hollow-cathode lamp.Wavelength: 213.9 nm.Atomisation device: air-acetylene flame of suitable composition (for example, 11 L of air and 2 L of acetylene per minute).Water (2.5.32): maximum 8.0 per cent, determined on 30.0 mg.Bacterial endotoxins (2.6.14, Method D): less than 10 IU/mg, if intended for use in the manufacture of parenteral preparations without a further appropriate procedure for the removal of bacterial endotoxins.ASSAYLiquid chromatography (2.2.29) as described in the test for related proteins with the following modification.Injection: 5 µL of the test solution and the reference solution.Calculate the content of insulin glargine (C267H404N72O78S6) taking into account the assigned content of insulin glargine CRS.STORAGEIn an airtight container, protected from light, at a temperature of − 20 ± 5 °C.。
糖尿病药物研发的新进展(PPT终版)

胰高血糖素样肽-1(GLP-1)受体 激动剂
胰高糖素样肽-1是重要的肠促胰素
一种由31个氨基酸组成的肽链1
由胃肠道L-细胞分泌的胰高糖素原剪切而成1
由进食刺激分泌(直接腔内刺激和间接神经刺激)2 肠促胰素家族成员
肠促胰素是天然血糖调节肽 GIP (葡萄糖依赖的促胰岛素多肽)是另一种肠促 胰素2
GLT2 )抑制剂
糖尿病药物新靶点:SGLT2
钠-葡萄糖协同转运蛋白2(SGLT2 ) 是最近新 发现的糖尿病治疗新靶点,其作用机制是特 异性地抑制肾脏对葡萄糖的再吸收,且不依 赖于β 细胞的功能异常或胰岛素抵抗的程度。 其效果也不会随着β 细胞功能的衰竭或严重 胰岛素抵抗而下降,不会产生传统药物带来 的不良反应,是糖尿病治疗的新途径。
GLP-1
胰岛素合成
葡萄糖依赖性胰高糖素 分泌
胃
胃排空
α
心脏 肝
葡萄糖生成 L细胞分泌 GLP-1 被 DPP-4 分解
Adapted from Baggio & Drucker. Gastroenterol 2007;132;2131–57
心血管保护功能
GLP-1 受体激动剂:艾塞那肽
Amylin制药公司及礼来公司的Byetta百泌达 (Exenatide, 艾塞那肽 ) 是2005年4月在美国上市,被批准上市的第一 个GLP-1 受体激动剂。2006年销量是4.3亿美元。
各种治疗糖尿病的药物
β细胞功能失调
磺酰脲类 格列奈类 胰岛素
胰腺
肌肉和脂肪组织
肝脏
肝糖过度生成
↓葡萄糖
胰岛素抵抗
二甲双胍
胃肠道
噻唑烷二酮类 二甲双胍 胰岛素
噻唑烷二酮类
百令全科大剂量-sxmppt课件

二、大剂量百令效果更佳-慢性肾衰竭
模型组:行5/6肾切除术,建立慢性肾衰竭模型 百令治疗组:2.0 g/(kg· d) 百令高剂量组:3.0 g/(kg· d)
120 100 80 60 40 20 0 假手术组 模型组 百令治疗组 百令高剂量组 39.84 77.54 64.3 BUN(mmol/L) 20 15 10 5.78 5 0 假手术组 模型组 百令治疗组 百令高剂量组 13.81 11.18 106.44 25
22.52
Scr(umol/L)
费忠化等. 广东医学院肾脏病研究.中华生物医学工程杂志2008,14(2).
一、大剂量百令效果更佳-慢性肾衰竭
12 肾小球系膜基质面积/ 肾小球囊面积 10 8
0.3 0.25 0.2 0.15 0.1 0.05 0 假手术组 模型组 百令治疗组 百令高剂量组
CTGF α-SMA
通过肾内科“加两粒”方案的实 施,销售人员已积累了大剂量的推 广经验,形成大剂量推广意识。
推广策略
1、新患者最低服用剂量4粒tid;原有服用百令的患者,不足4粒tid的,开展“加2粒”方案。 2、 做好病房协定处方的用量上量工作,建立医生大剂量的处方习惯,利用医生病房-门诊轮岗的
特点,影响门诊用量。
一、大剂量百令效果更佳-COPD
0.45 0.4
气道阻力均值(cm H2O•ml-1•s)
0.39
李时悦等.广州医学院第一附属医院、广州呼吸疾病研究所.广东医学2010,31(13).
一、大剂量百令效果更佳-肺纤维化
TNF-α (pg/mL) SOD (U/mL) GSH (nmol/mgprot) MDA (nmol/mgprot)
艾克洛巴(Ecolab):采用GS1标准确保产品数据清晰说明书

EcolabT aking a Clean Approach to Spotless Product DataChallengeAchieving high quality product data was no small undertaking for Ecolab. As a company focused on cleaning solutions, Ecolab has a wide diversity of products and programs, serving three million customer locations in40 industries. Creating an effective data governance framework for consolidating dozens of data repositories was only the first step in making sure Ecolab’s productdata was complete and “clean.”SolutionBy adopting GS1 Standards a decade ago, Ecolab laid the foundation for efficiencies throughout its supply chain—from its own manufacturing facilities to distributors, and on to end-users. The company uniquely identifies most of its products with Global Trade Item Numbers (GTINs) and exchanges product data attributes with customers over the Global Data Synchronization Network™ (GDSN®).T o keep pace with customer data demands, Ecolab participates in industry groups and initiatives GS1 US® offers, including the Foodservice GS1 US Standards Initiative and the GS1 US Retail Grocery Initiative.Ecolab has developed its own internal training program for people who work with GS1 Standards-driven processes, mirroring the training offered by GS1 US. The company is also a regular participant at GS1 Connect conferences and serves on the GS1 Connect Community Advisory Board. BenefitsGS1 Standards have helped Ecolab streamline itsdata exchange process with customers, strengthen its relationships, and eliminate time-consuming, manualdata entry.As more customers have requested GS1 Standards-based trade, Ecolab has not only stayed at the forefront of its industry with its data governance program, but even landed significant new business by being prepared to share standardized data with a large account.With an overall 68 percent improvement in data quality from 2017 to 2018, Ecolab customers have benefited from the initiavitve as well. With GS1 Standards and quality data, they can more efficiently manage Ecolab deliveries and inventory and can readily access safety and regulatory information.A Decade of DataEcolab epitomizes diversification. As an industry leader for cleaning solutions, Ecolab and its 11 divisions serve a wide range of industries, providing both products and professional programs to keep customers’ premises and products clean and safe.Driven by requests from foodservice and healthcare customers, Ecolab implemented GS1 Standards a decade ago. A growing number of Ecolab customers were requesting product information, making it a requisite of doing business with them.“At the time, customers were asking for product dimensions and brand name data,” says Regan Van T assel, commercial digital solutions platform manager at Ecolab. “T oday,it’s more about images and robust information around instructions, safety sheets, and regulatory data.”“There was a lot of work during the initial stages just accessing the information,” Van T assel adds. “We had so many different databases that it took us quite a while to get the correct data consolidated and into the GDSN.”From the beginning, Ecolab’s data governance programhad executive support at the highest level. “Our executive leadership sees the benefits firsthand,” Van T assel says. Data inaccuracies can have significant negative impact on shipping and logistics, inventory management, sales and forecasting, revenue—virtually everything within the modern supply chain. Almost immediately upon implementing GS1 Standards, Ecolab moved to publish its data in the Global Data Synchronization Network or GDSN. Once Ecolab collected all of the information based on the GS1-recommended format, the company has managed the quality of its data, using GDSN error reporting tools. “We really wanted to make sure that we had built-in governance around our product data, and the structure around how to assign a GTIN,” explains Van T assel.Proactive Data ManagementEach division has its own division product publication specialists (DPPs) who are the recipients of the initial error reports being generated by the GDSN.“When we first went live, it was kind of like a firehose,”Van T assel recalls. “All of our data came at these DPPs and there were so many problems and errors that it became overwhelming and difficult to focus on what was important.”“Then, about three years ago, we decided to be more proactive versus reactive. We built into our internal systems additional error reporting, so we could mitigate any issues before they became problems in the GDSN.“Ecolab started looking at its product data in a variety of ways. How many data errors are there per products, how many products have data errors, what is the nature of the errors? Information was broken down by division.“A big lesson for us when implementing our data governance program: Focus on the whole view versus just pieces soyou can wrap your arms around the opportunities for improvement.”Ecolab found that it was better to examine GTINs from the perspective of those that impacted the most customers rather than the most errors. For example, a GTIN with 10 errors may have been published to just one customer. Instead, focus placed on a GTIN that had just two errors but touched 15 customers proved to have the most impact. Ecolab also built a very tightly controlled system internally to assign GTINs, with a select group of people having access to the system. T oday, Ecolab’s business partners involved with labeling can directly access the product information they need for labeling items, cases or pallets.“We now have one data quality scorecard that we produce and distribute a couple of times a month. That helpsus to track our progress and see where we’re making improvements and where we need to focus more attention,” Van T assel says.“It’s made a big difference for us in terms of knowing where to target our time and where we’re going to have the most impact. By integrating the various error reports, we found that by fixing one issue, we could correct up to 300 errors.”“At one point in the process, it was taking us up to 15 daysto be able to publish data to a customer at their request. With our data governance program, we now provide customers with same-day publications. And for new data requests, we’re able to make changes within weeks versus months, as it was before.”A Boost to the Bottom LineEcolab found that increased visibility for the program, and the recognition for all involved in it, had a significant impact on data quality improvement.“We put forth a big effort to hold meetings and townhalls and actively promote GS1 Standards, educating our employees on what we were doing and the importance of it,” Van T assel says.“In the course of our meetings and marketing, we were able to sell the data governance program based on the efficiencies we were gaining internally by automating all of the data gathering and minimizing the time we spent fixing errors,” Van T assel explains.A pivotal event illustrated the point of gaining competitive advantage with GS1 Standards.“We had a big win for the business. We were able to obtaina new, very large customer account because we were participating in the GDSN and were able to supply themwith all of the data they wanted. The customer switched from our competitor because they were looking for a trading partner that had GS1 Standards and GDSN information at their fingertips. Winning that account really helped to sell the program internally and get more enthusiasm behind it from our business partners.”Information and Image ManagementVan T assel and her colleagues regularly assemble the DPPs from foodservice, a couple of different healthcare teams, and individuals from the largest division – called the institutional division – to discuss how to correct errors with the data tied to their divisions, as well as implement measures to prevent future errors. Currently, Ecolab is implementing a Product Information Management (PIM) system that will manage productdata across divisions in a single repository, feeding into internal- and external-facing platforms for universal content consumption by customers, distributors, and internal departments globally.The PIM system will be integrated with a digital asset management system to store images and marketing assets, replacing manual spreadsheets and databases inan effort to improve data quality and efficiency across the network. As a business-to-business company, Ecolab did not customarily take pictures of products when they came off the manufacturing line. But more and more, Ecolab distributors want to display product images on their own webpages to sell more.“With the advent of e-commerce, GS1 Standards and traceability, it’s become critical to our customers to have images and other forms of digital data from us,” says VanT assel. “It’s a primary focus to make sure we have visually strong images and multiple images for each of our products. We have created a new commercial digital solutions department within the past year. This is to help facilitate standardization of images and data requirements so we can scale globally.”“We will be ready in the first phase to send data to GDSN through the PIM system, greatly simplifying the the complex publication process that we use to transform and publish the data now,” adds Van T assel.Auditing Product Data for ValidationWith an multi-prong approach to data quality based on the principles of the GS1 US National Data Quality Program, Ecolab has achieved an overall 68 percent improvement in data quality between 2017 and 2018. This includes reducing the number of GTINs with errors by 18.5 percent and ongoing improvements in trading partner scorecarding results.Once Ecolab has completed its PIM implementation, it plans to repeat a GS1 US audit of its product attribute data that had been done a few years ago. “The audit compares the most recent information shared about a product with its physical attributes,” Van T assel says. “We want to pursue data quality certification with GS1 US. We’re ready. For our customers, we’ll continue to make doing business with Ecolab an efficient and clean one...with standardized, quality data.”© 2018 GS1 US All Rights ReservedConnect With UsGS1 US Corporate HeadquartersPrinceton South Corporate Center, 300 Charles Ewing Boulevard Ewing, NJ 08628 USAT +609.620.0200 | E ************** About EcolabEcolab is the global leader in water, hygiene and energy technologies and services. Around the world businesses in foodservice, food processing, hospitality, healthcare, industrial, and oil and gas markets use Ecolab products and services to keep their environment clean and safe, operate efficiently and achieve sustainability goals. About the Foodservice GS1 US Standards InitiativeThe Foodservice GS1 US Standards Initiative represents a broad cross section of industry trading partners. T oday over 125 manufacturers, distributors, brokers, operators, industry associations, government agencies, logistics, and technology providers are participating members in initiative activities focused on improving transparency, operational efficiencies, traceability, and food safety with GS1 Standards.About the GS1 US Retail Grocery InitiativeThe GS1 US Retail Grocery Initiative is a voluntary collaborative industry effort seeking to address current industry challenges to improve product information and images, data quality, supply chain visibility, and operational efficiencies. This structured Initiative for retail grocery aims to help enable stakeholders to focus on the most important industry problems, streamline resources, and drive adoption and implementation of the industry-defined solutions leveraging GS1 Standards.GS1 US National Data Quality ProgramThe GS1 US National Data Quality Program provides organizations with a comprehensive approach to data quality that encompasses validating a Data Governance Process exists within an organization to support the creation and maintenance of product data based on GS1 Standards; confirming that proper Education and Training protocols on GS1 Standards are present within anorganization for creating and maintaining accurate product data; and conducting regular Attribute Audits that audit, verify and compare product attributes to most recently shared data to enable trading partners to have confidence that the data shared is accurate, complete and timely. /dataquality .About GS1 USGS1 US®, a member of GS1® global, is a not-for-profit information standards organization that facilitates industry collaboration to help improve supply chain visibility and efficiency through the use of GS1 Standards, the most widely-used supply chain standards system in the world. Nearly 300,000 businesses in 25 industries rely on GS1 US for trading-partner collaboration that optimizes their supply chains, drives cost performance and revenue growth while also enabling regulatory compliance. They achieve these benefits through solutions based on GS1 global unique numbering and identification systems, barcodes, Electronic Product Code-based RFID, data synchronization, and electronic information exchange. GS1 US also manages the United Nations Standard Products and Services Code® (UNSPSC®). About the Organizations。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
百泌达不甘精胰岛素血糖控制相当
Bunck MC. , et al. ADA 2009
对比甘精胰岛素,百泌达血糖波劢更小
Barnett AH, et al. Clin Ther. 2007;29:2333-2348.
对比甘精胰岛素,百泌达显著降低体重
Barnett AH, et al. Clin Ther. 2007;29:2333-2348.
对比甘精胰岛素,百泌达低血糖更少
Barnett AH, et al. Clin Ther. 2007;29:2333-2348.
对比双相门冬胰岛素, 百泌达血糖控制水平相当,达标率更高
Nauck MA, et al. Diabetologia. 2007;50:259-267.
对比双相门冬胰岛素, 百泌达体重减低更为显著
【丌良反应】 • • • 报告的最常见的丌良反应为具有剂量依赖性的轻到丨度恶心。大多数治疗开始时出现恶心的患者, 症状的収生频度和严重程度会随着继续治疗时间的延长而减轻。 低血糖:当本品作为单一疗法使用时,低血糖的収生率为5%,而安慰剂为1%。在健康志愿者的 随机、双盲、对照研究丨,本品丌会改变反调节激素对胰岛素诱収的低血糖的应答。 百泌达丌增加2型糖尿病患者的胰腺炎収生率
Klonoff DC, et al. Curr Med Res Opin. 2008;24:275-286.
百泌达改善多项心血管病风险标志物的水平 为心血管保护奠定基础
Bunck MC. , et al. ADA 2009
2型糖尿病患者的挑战
百泌达的诞生 百泌达的临床获益 百泌达的比较优势 百泌达的临床应用
百泌达,降糖减重,一丼两达
•直击糖尿病核心缺陷,改善β细胞功能 •智能调控血糖,疗效长期卓越 •降低体重,更多获益
•9年临床经验,应用更安心
GLP-1,葡萄糖依赖性调节β和α细胞分泌
Nauck MA, et al. Diabetologia. 1993;36:741-744.
GLP-1,直击2型糖尿病的病理缺陷
Ramlo-Halsted, et al. Clinical Diabetes, 2000,18(2):80-84 Aronoff SL, et al. Diabetes Spectrum. 2004;17:183-190 Nielsen LL, et al. Regul Pept. 2004;117:77-88 Drucker DJ, Lancet. 2006;368:1696-1705
HbA1c丌达标,为幵収症带来隐患
Pan, Changyu, et al. Management of Chinese patients with type 2 diabetes, 1998-2006: the Diabcare-China surveys. Current Medical Research and Opinion, Volume 25, Number 1, January 2009 , pp. 39-45(7).
百泌达,降糖减重,一丼两达 全面应对患者挑战
•直击糖尿病核心缺陷,改善β细胞功能 •智能调控血糖,疗效长期卓越 •降低体重,更多获益 •9年临床经验,应用更安心
2型糖尿病患者的挑战
百泌达的诞生 百泌达的临床获益 百泌达的比较优势 百泌达的临床应用
GLP-1 全新独特作用机制,全面调节血糖
进食促进 GLP-1分泌
百泌达,持丽有效控制HbA1c 疗效值得信赖
Klonoff DC, et al. Curr Med Res Opin 2008;24:275-286.
AMIGO研究(合幵结果): 百泌达显著降低患者的体重
Data on file, Amylin Pharmaceuticals, Inc.
百泌达,降低体重在亚洲患者丨同样有效
百泌达,持丽减轻体重,患者持丽获益
Klonoff DC, et al. Curr Med Res Opin 2008;24:275-286.
百泌达,显著提高胰岛素敏感性 提高降糖效率
M=血糖稳态时外源性输注率,数据为均值 ± SE Bunck et al. ADA 2010 728-P
百泌达治疗3.5年,改善多项心血管危险因素 患者获得更多保护
2型糖尿病患者的挑战
百泌达的诞生 百泌达的临床获益 百泌达的比较优势 百泌达的临床应用
2型糖尿病患者的挑战
百泌达的诞生 百泌达的临床获益 百泌达的比较优势 百泌达的临床应用
如今,您的糖尿病患者面临哪些挑战?
β细胞功能进行性並失
Ramlo-Halsted, et al. Clinical Diabetes, 2000,18(2):80-84.
百泌达的诞生 百泌达的临床获益 百泌达的比较优势 百泌达的临床应用
百泌达,改善β细胞功能
Stoffers D, et al. Diabetes. 2000;49:741-748.
百泌达,恢复β细胞正常的胰岛素分泌方式
Mean (SE); N=25. Fehse F, et al. J Clin Endocrinol Metab. 2005;90:5991-5997. Copyright 2005, The Endocrine Society.
百泌达的诞生,来自大自然的恩赐
•百泌达,化学名艾塞那肽 (Exenatide)
•人工合成癿希拉巨蜥唾液中癿 一种蛋白质
Nielsen LL, et al. Regul Pept. 2004;117:77-88
百泌达,不GLP-1受体结合,丌被DPP-4酶降解
百泌达,第一丧被SFDA批准上市的GLP-1 受体激劢剂
百泌达,何时启用,对患者获益更大?
See accompanying Prescribing Information and safety information included in this presentation
百泌达:起始方便、安全性良好
•每支预充笔可使用一丧月 •每天给药2次,给药时间为2顿主餐 前1小时内* •无需根据进餐量或运劢量调整剂量 •无需额外监测血糖
百泌Байду номын сангаас,降低亚洲人群的HbA1c
Diabetes Research And Clinical Practice 83(2009):69-79.
AMIGO研究(合幵结果): 百泌达显著降低餐后血糖,降糖更为全面
安慰剂 BID 艾塞那肽5μg BID 艾塞那肽10μg BID
Mean (SE); N = 138; Evaluable meal tolerance cohort. p<.0001 for change in PPG from baseline to week 30, exenatide vs placebo group. Data on file, Amylin Pharmaceuticals, Inc.
体重日益增长,带来治疗困难
UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998; 352:
降低体重的益处
*Intentional weight loss in overweight individuals Williamson DF, et al. Diabetes Care. 2000;23:1499-1504.
Nathan DM, et al. Diabetes Care 2009;32:193-203. Helena W. Rodbard, et al. ENDOCRINE PRACTICE Vol 15 No. 6 September/October 2009 《中国2型糖尿病防治指南》2010版
2型糖尿病患者的挑战
选择百泌达,降糖减重,一丼两达
AMIGO研究,百泌达显著降低HbA1c 丌同HbA1c基线,均有获益
2.5-year completers; n=241 at weeks 30 and 130; mean ± SE Data on file, Amylin Pharmaceuticals, Inc.
Nauck MA, et al. Diabetologia. 2007;50:259-267.
2型糖尿病患者的挑战
百泌达的诞生 百泌达的临床获益 百泌达的比较优势 百泌达的临床应用
百泌达,受到权威治疗指南的肯定不推荐
Nathan DM, et al. Diabetes Care 2009;32:193-203. Helena W. Rodbard, et al. ENDOCRINE PRACTICE Vol 15 No. 6 September/October 2009 《中国2型糖尿病防治指南》2010版
促进饱感 降低食欲
降低β 细胞负荷
增加β 细胞反应
α细胞: 减少餐后胰高糖素分泌
Β细胞: 增强葡萄糖依赖的胰岛素分泌
肝脏: 胰高糖素水平下降 减少肝糖输出 胃: 帮助调节胃排空
Flint A, et al. J Clin Invest. 1998;101:515-520; Larsson H, et al. Acta Physiol Scand. 1997;160:413-422; Nauck MA, et al. Diabetologia. 1996;39:1546-1553; Drucker DJ. Diabetes. 1998;47:159-169.
Diabetes Research And Clinical Practice 83(2009):69-79.
百泌达,使84%的患者获得体重减低 减重效果确切,更多患者获益
N=217 Klonoff DC, et al. Curr Med Res Opin. 2008;24:275-286. See accompanying Prescribing Information and safety information included in this presentation
