富马酸替诺福韦酯--------印度药典
富马酸替诺福韦二吡呋酯片说明书
核准日期:年月日修改日期:年月日富马酸替诺福韦二吡呋酯片说明书请仔细阅读说明书并在医师指导下使用【药品名称】通用名称:富马酸替诺福韦二吡呋酯片英文名称:Tenofovir Disoproxil Fumarate Tablets汉语拼音:FumasuanTinuofuwei’erbifuzhi Pian【成份】本品主要成份为富马酸替诺福韦二吡呋酯。
化学名称:9-[(R)-2[[双[[(异丙氧基羰基)氧基]甲氧基]氧膦基]甲氧基]-丙基]腺嘌呤富马酸盐(1:1)。
化学结构式:分子式:C19H30N5O10P·C4H4O4分子量:635.52【性状】本品为薄膜衣片,除去包衣后显白色。
【适应症】HIV-1感染富马酸替诺福韦二吡呋酯适用于与其他抗反转录病毒药物联用,治疗成人HIV-1感染。
使用富马酸替诺福韦二吡呋酯开始治疗HIV-1感染时,应考虑以下几点:富马酸替诺福韦二吡呋酯不应与含有替诺福韦的固定剂量复方制剂联用,包括:●依非韦伦/恩曲他滨/富马酸替诺福韦二吡呋酯、●利匹韦林/恩曲他滨/富马酸替诺福韦二吡呋酯、●恩曲他滨/丙酚替诺福韦、●艾维雷韦/考比司他/恩曲他滨/丙酚替诺福韦、●恩曲他滨/利匹韦林/丙酚替诺福韦、●艾维雷韦/考比司他/恩曲他滨/富马酸替诺福韦二吡呋酯、●恩曲他滨替诺福韦、●丙酚替诺福韦慢性乙型肝炎富马酸替诺福韦二吡呋酯适用于治疗慢性乙肝成人和≥12岁的儿童患者。
在开始使用富马酸替诺福韦二吡呋酯治疗HBV感染时,应考虑到以下要点:•成人患者中该适应症的确立基于从初次接受核苷治疗的受试者和既往接受过治疗且证实拉米夫定耐药的受试者中获得的安全性和疗效数据。
受试者为肝功能代偿的HBeAg阳性和HBeAg阴性慢性乙肝成人受试者。
•富马酸替诺福韦二吡呋酯在数量有限的患有失代偿期肝病的慢性乙肝受试者中进行过评价。
•临床试验中基线时存在阿德福韦相关突变的受试者数量太少,因此尚无法对疗效下结论。
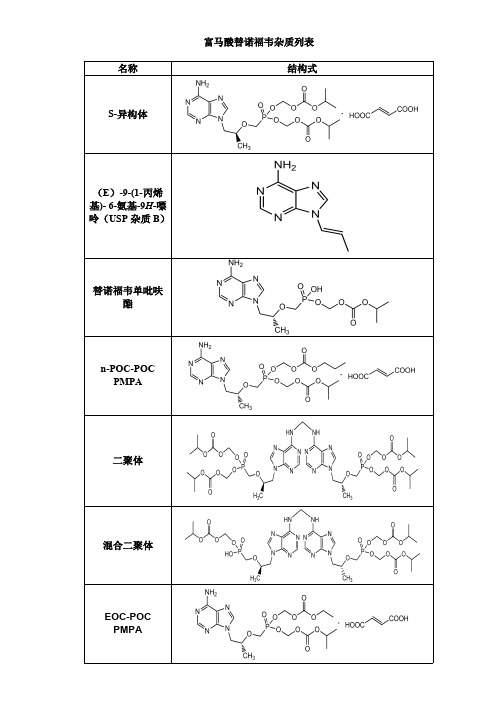
富马酸替诺福韦杂质列表
深圳菲斯是专业进口标准品代理商,主要品牌有 TRC、TLC、Molcan、EP、BP、 USP、Sinco 等品牌标准品。 1.替卡格雷杂质 2.利伐沙班杂质 3.阿考替胺杂质 4.普拉克索杂质 5.阿哌沙班杂 质 6.氨氯地平杂质 7.硼替佐米杂质 8.比索洛尔杂质 9.阿比特龙杂质 10.非布司 他杂质 11.安立生坦杂质 12.依折麦布杂质 13.厄洛替尼杂质 14.索拉非尼杂质 15. 维格列汀杂质 16.阿伐那非杂质 17.托法替尼杂质 18.米格列奈杂质 19.沃替西汀 杂质 20 尼贝地平杂质 21.艾帕列净杂质 22.阿普斯特杂质 23.门冬氨酸缩合物 24. 依托考昔杂质 25.达格列净杂质 26.尼达尼布杂质 27.托匹司他杂质 28.坎格列净 杂质 29.帕泊昔利布杂质 30.依鲁替尼杂质 31.盐酸氨溴索杂质 32.达比加群酯杂 质等库存 库存新产品,当天发货!
富马酸替诺福韦杂质列表 名称 结构式
S-异构体
(E)-9-(1-丙烯 基)- 6-氨基-9H-嘌 呤(USP 杂质 B)
替诺福韦单吡呋 酯
n-POC-POC PMPA
二聚体
混合二聚体
EOC-POC PMPA
DEC-POC PMPA
Des-Methyl TDF (去甲基替诺福 韦二吡呋酯)
MOC-POC PMPA
富马酸替诺福韦酯杂质 标准品 对照品

富马酸替诺福韦酯是一种化学物质,核苷酸逆转录酶抑制剂,是替诺福韦(PMPA,2)的前药,临床主要用于治疗人类免疫缺陷病毒(HIV)感染。
富马酸替诺福韦酯标准品列表名称结构式替诺福韦单异丙氧碳酸甲基酯Tenofovir Disoproxil RelatedCompound ECAS:211364-69-1替诺福韦酯异丙氧碳酸甲基甲基酯CAS: 1246812-16-7替诺福韦异丙氧碳酸甲基甲氧碳酸甲基酯Mono-POC Methyl TenofovirCAS: 1246812-43-0Tenofovir Impurity Q替诺福韦异丙氧碳酸甲基异丙基酯Tenofovir Disoproxil RelatedCompound GCAS: 1422284-15-8替诺福韦-N-异丙氧羰基异丙氧碳酸甲基酯Tenofovir Impurity ECAS:1244022-56-7替诺福韦酯杂质FTenofovir Impurity F(S)-替诺福韦二异丙氧碳酸甲基酯替诺福韦异丙氧碳酸甲基丙基碳酸甲基酯CAS: 1217542-13-6替诺福韦二聚物单酯CAS:1093279-77-6替诺福韦二聚物Tenofovir Disoproxil DimerCAS:1093279-76-59-丙烯腺嘌呤Tenofovir Disoproxil RelatedCompound BCAS:4121-40-8替诺福韦酯杂质RTenofovir Impurity R替诺福韦异丙氧碳酸甲基乙基碳酸甲基酯替诺福韦二乙酯Tenofovir impurity V替诺福韦单乙酯Tenofovir DisoproxilCarbamateDiethylaminocarboxymethylPOC Tenofovir Fumarate6N-Hydroxymethyl TenofovirDisoproxil1244022-53-4Isopropyl Tenofovir Diisopropyl Tenofovir Fumarate 160616-04-6 (free base)(S)-Tenofovir147127-19-3替诺福韦酯杂质R6N-Bromomethyl TenofovirDisoproxilTenofovir Disoproxil Fumarate Impurity(N6-CH2OH-POC PMPA) Tenofovir Related Compound 2 379270-35-6Tenofovir Related Compound 352364-31-5Tenofovir Related Compound 4851456-00-3Tenofovir Related Compound 51234081-04-9 Tenofovir Related Compound 6 383365-04-6Tenofovir Related Compound 7 79487-89-1Tenofovir monophosphate 206646-04-0Tenofovir Diphosphate166403-66-3Tenofovir Trimer Impurity 1Tenofovir Trimer Impurity 2。
富马酸替诺福韦酯杂质

富马酸替诺福韦酯杂质富马酸替诺福韦酯(Tenofovir disoproxil fumarate,CAS:202138-50-9)中文名:富马酸替诺福韦酯杂质A英文名:Tenofovir disoproxil fumarate impurity A规格:10mg-100mg纯度:>95%用途:实验室分析及新药研究中文名:富马酸替诺福韦酯杂质B英文名:Tenofovir disoproxil fumarate impurity B规格:10mg-100mg纯度:>95%用途:实验室分析及新药研究中文名:富马酸替诺福韦酯杂质C英文名:Tenofovir disoproxil fumarate impurity C规格:10mg-100mg纯度:>95%用途:实验室分析及新药研究中文名:富马酸替诺福韦酯杂质D英文名:Tenofovir disoproxil fumarate impurity D规格:10mg-100mg纯度:>95%用途:实验室分析及新药研究中文名称:富马酸替诺福韦酯中文别名:(R)-9-(2-磷酸甲氧基丙基)腺嘌呤二(异丙氧羰基氧甲基)酯富马酸盐;富马酸泰诺福韦酯;富马酸替诺福韦二吡呋酯英文名称:Tenofovir disoproxil fumarate英文别名:9-((R)-2-((Bis(((isopropoxycarbonyl)oxy)methoxy)phosphinyl)methoxy)propyl)adenine fumarate; bis({[(1-methylethoxy)carbonyl]oxy}methyl){[(1R)-2-(6-amino-9H-purin-9-yl)-1-methylethoxy]methyl}phosphonate(2E)-but-2-enedioate CAS号:202138-50-9该产品信息由广州优瓦仪器有限公司整理上传,相关杂质还有新利司他杂质、沃替西汀杂质、巴多昔芬杂质、醋酸巴多昔芬杂质、非马沙坦杂质、芬戈莫德杂质、枸橼酸托法替尼杂质、瑞替加滨杂质等,品牌有CATO、USP、Reagecon、EP、BP、TRC、TLC020-********。
揭秘:乙肝新药韦立得taf,为什么印度那么便宜,后背真实原因!

揭秘:⼄肝新药韦⽴得taf,为什么印度那么便宜,后背真实原因!韦⽴得这个词,⼤家都不陌⽣,是慢性⼄肝抗病毒的⼀款新药。
2018年12⽉8⽇,韦⽴得中国上市会在北京举⾏,下图为韦⽴得中国上市会现场媒体答问环节:重庆医科⼤学第⼆附属医院院长任红教授说:“虽然⽬前还⽆法彻底清除病毒,随着抗病毒药物在中国的升级换代,韦⽴得对于⼄肝患者来说可以有极低耐药率和更好安全性保障的⽤药选择,以降低肝硬化和肝癌发⽣的风险。
”“韦⽴得'靶向'肝脏,能有效抗病毒,肾脏和⾻骼安全实验室参数相较富马酸替诺福韦酯(TDF)有所提⾼,为⼄肝患者提供了新的希望。
”韦⽴得在中国上市后,很多⼈纷纷表⽰可望⽽不可及,到底是因为什么呢?⾸先从效果上来看:它确实是改变了传统⼄肝⽤药的副作⽤,从药的剂量上来看,⽤TDF⼗分之⼀的剂量就可以达到相同的效果,同样在三期的研究数据表明,经过1632名患者的⼄肝病毒检测,服⽤过韦⽴得的患者⽐服⽤韦瑞德的患者在⾻骼和肾脏实验室参数⽅⾯有所改善。
此外,在接受为期96周治疗期间,没有⼀例患者发⽣替诺福韦耐药的,尤其是年纪⼤的,本⾝⾻骼流失较快的,⾻质疏松的,可以考虑;⾃TAF上市以来这半年内,多位患者⾃曝达到了临床转阴的效果,唯⼀的缺点就是价格太贵,以⽬前的价格来说,可真是⼀笔不⼩的开⽀,但是对于家庭条件不那么好的患者来说,确实可望⽽不可及。
⼀条路⾛不通可以换⼀条路,据⼩编了解到,印版的价是国内三分之⼀,有⼀些家庭困难的⼄肝患者通过合众美康获得印版的,印度原⼚授权的taf瞬时间成为了⼄肝患者的挚爱!⼄肝要治,但是我们也是要考虑⾃⼰的家庭实际情况的,选择对的合适的抗病毒。
为啥印度的价格只要380就可以?为什么会相差这么多呢?⾸先,印度的法制化进程和国际化历程,没有脱离本国的发展阶段和需求,⽽是尽可能地去寻找适应⾃⾝现状的⽅式。
作为世界第⼆⼤⼈⼝国家,印度居民贫富分化悬殊,⼀直是世界上贫困⼈⼝最多的国家之⼀。
替诺福韦(韦瑞德)美国FDA批准用于治疗慢性乙肝

导读:替诺福韦(韦瑞德)于2008年被美国FDA批准用于治疗慢性乙型肝炎,目前仅在欧美国家应用。
而在亚太地区,由于临床试验资料原因及药物本身价格问题,尚未投入临床应用。
替诺福韦(韦瑞德)是一种新型核苷酸类逆转录酶抑制剂。
可有效对抗多种病毒,用于治疗病毒感染性疾病。
替诺福韦早已开始在美国和欧洲投入临床使用。
最新的美国肝病年会和欧洲肝病年会都认为,替诺福韦的抗病毒作用强、耐药性低,已经成为全球口服抗病毒药物中的佼佼者。
该药的化学名称为“富马酸替诺福韦二吡呋酯”,英文名称为“Viread”,属于新型的核苷酸类逆转录酶抑制剂。
替诺福韦(韦瑞德)能通过干扰人体内乙肝病毒DNA聚合酶的功能,抑制乙肝病毒的复制,从而降低人血清及肝组织内的病毒载量。
此药除了可治疗乙肝外,还可与其他抗逆转录病毒药物相配合,治疗成人艾滋病病毒的感染。
●替诺福韦Viread对初次进行抗乙肝病毒治疗患者效果的研究替诺福韦Viread已被证明对于以下情况的成人效果良好:·感染慢性乙肝的人·刚开始使用Viread进行治疗的人·肝脏功能正常的人两项研究(102号及103号研究)显示Viread能够帮助降低成人体内的乙肝病毒(HBV)数量。
特别需提醒的是,这些研究集中于那些患有慢性乙肝但肝脏仍有必要肝脏功能(代偿性肝病)的患者。
此外,大部分参加研究的患者此前从未使用过任何抗乙肝病毒的治疗药物(即初次抗病毒治疗)。
两项研究根据患者是否有乙肝E-抗原而进行分组。
和那些没有此抗原的人相比,有E-抗原的人对药物的反应可能不同。
●益处在102号及103号研究中,共有641名患者接受了评估。
这641人中有426名慢性乙肝患者接受了替诺福韦Viread的治疗,其他215名在第一年里接受了另一种药物的治疗。
此后,所有患者都接受Viread治疗。
共有389名患者完成了三年的Viread使用期。
其中的235人是E-抗原呈阴性的患者,154人是E-抗原呈阳性的患者。
富马酸泰诺福韦酯片说明书(英文)

HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use VIREAD safely and effectively. See full prescribing informationfor VIREAD.VIREAD® (tenofovir disoproxil fumarate) tabletsInitial U.S. Approval: 2001WARNINGS: LACTIC ACIDOSIS/SEVERE HEPATOMEGALY WITH STEATOSIS and POST TREATMENT EXACERBATION OFHEPATITISSee full prescribing information for complete boxed warning. • Lactic acidosis and severe hepatomegaly with steatosis, including fatal cases, have been reported with the use ofnucleoside analogs, including VIREAD. (5.1)• Severe acute exacerbations of hepatitis have been reported in HBV-infected patients who have discontinued anti-hepatitis B therapy, including VIREAD. Hepatic functionshould be monitored closely in these patients. Ifappropriate, resumption of anti-hepatitis B therapy may bewarranted. (5.2)---------------------------RECENT MAJOR CHANGES--------------------------- Indications and Usage (1.2) 10/2010 Dosage and Administration (2.1, 2.2, 2.3) 10/2010 Warnings and PrecautionsNew Onset or Worsening Renal Impairment (5.3) 10/2009Decreases in Bone Mineral Density (5.6) 03/2010----------------------------INDICATIONS AND USAGE--------------------------- VIREAD is a nucleotide analog HIV-1 reverse transcriptase inhibitor and an HBV reverse transcriptase inhibitor.VIREAD is indicated in combination with other antiretroviral agents for the treatment of HIV-1 infection in adults and pediatric patients 12 years of age and older. (1)VIREAD is indicated for the treatment of chronic hepatitis B in adults. (1)---------------------------DOSAGE AND ADMINISTRATION-------------------- • Recommended dose for the treatment of HIV-1 or chronic hepatitis B in adults: 300 mg once daily taken orally withoutregard to food. (2.1)• Recommended dose for the treatment of HIV-1 in pediatric patients (≥12 years of age and ≥35 kg): 300 mg once daily taken orally without regard to food. (2.2)• Dose recommended in renal impairment in adults:Creatinine clearance 30-49 mL/min: 300 mg every 48 hours.(2.3)Creatinine clearance 10-29 mL/min: 300 mg every 72 to 96 hours.(2.3)Hemodialysis: 300 mg every 7 days or after approximately 12hours of dialysis. (2.3)-----------------------DOSAGE FORMS AND STRENGTHS-------------------- Tablets: 300 mg. (3)--------------------------------CONTRAINDICATIONS------------------------------ None. (4)--------------------------WARNINGS AND PRECAUTIONS--------------------- • New onset or worsening renal impairment: Can include acute renal failure and Fanconi syndrome. Assess creatinine clearance (CrCl) before initiating treatment with VIREAD. Monitor CrCl and serum phosphorus in patients at risk. Avoid administeringVIREAD with concurrent or recent use of nephrotoxic drugs. (5.3) • Coadministration with Other Products: Do not use with other tenofovir-containing products (e.g., ATRIPLA and TRUVADA). Do not administer in combination with HEPSERA. (5.4) • HIV testing: HIV antibody testing should be offered to all HBV-infected patients before initiating therapy with VIREAD. VIREAD should only be used as part of an appropriate antiretroviralcombination regimen in HIV-infected patients with or without HBV coinfection. (5.5)• Decreases in bone mineral density (BMD): Observed in HIV-infected patients. Consider assessment of BMD in patients with a history of pathologic fracture or other risk factors for osteoporosis or bone loss. (5.6)• Redistribution/accumulation of body fat: Observed in HIV-infected patients receiving antiretroviral combination therapy. (5.7)• Immune reconstitution syndrome: Observed in HIV-infected patients. May necessitate further evaluation and treatment. (5.8)• Triple nucleoside-only regimens: Early virologic failure has been reported in HIV-infected patients. Monitor carefully and considertreatment modification. (5.9)-----------------------------ADVERSE REACTIONS--------------------------------In HIV-infected subjects: Most common adverse reactions (incidence ≥10%, Grades 2 - 4) are rash, diarrhea, headache, pain, depression, asthenia, and nausea. (6)In HBV-infected subjects with compensated liver disease: most common adverse reaction (all grades) was nausea (9%). (6)In HBV-infected subjects with decompensated liver disease: most common adverse reactions (incidence ≥10%, all grades) were abdominal pain, nausea, insomnia, pruritus, vomiting, dizziness, and pyrexia. (6)To report SUSPECTED ADVERSE REACTIONS, contact Gilead Sciences, Inc. at 1-800-GILEAD-5 or FDA at 1-800-FDA-1088 or /medwatch------------------------------DRUG INTERACTIONS--------------------------------• Didanosine:Coadministration increases didanosineconcentrations. Use with caution and monitor for evidence ofdidanosine toxicity (e.g., pancreatitis, neuropathy). Considerdose reductions or discontinuations of didanosine if warranted.(7.1)• Atazanavir:Coadministration decreases atazanavirconcentrations and increases tenofovir concentrations. Useatazanavir with VIREAD only with additional ritonavir; monitor for evidence of tenofovir toxicity. (7.2)• Lopinavir/ritonavir:Coadministration increases tenofovir concentrations. Monitor for evidence of tenofovir toxicity. (7.3)----------------------------USE IN SPECIFIC POPULATIONS-------------------• Pregnancy: There is a registry available. Enroll patients by calling 1-800-258-4263.• Nursing mothers: Women infected with HIV should be instructed not to breast feed. (8.3)• Safety and efficacy not established in patients less than 12 years of age. (8.4)See 17 for PATIENT COUNSELING INFORMATION and FDA-Approved Patient LabelingRevised: October 2010 FULL PRESCRIBING INFORMATION: CONTENTS*WARNINGS: LACTIC ACIDOSIS/SEVERE HEPATOMEGALY WITH STEATOSIS and POST TREATMENT EXACERBATION OF HEPATITIS1 INDICATIONS AND USAGE1.1 HIV-1 Infection1.2 Chronic Hepatitis B2 DOSAGE AND ADMINISTRATION2.1 Recommended Dose in Adults2.2 Recommended Dose in Pediatric Patients (≥12 Years ofAge and ≥35 kg)2.3 Dose Adjustment for Renal Impairment in Adults3 DOSAGE FORMS AND STRENGTHS4 CONTRAINDICATIONS5 WARNINGS AND PRECAUTIONS5.1 Lactic Acidosis/Severe Hepatomegaly with Steatosis5.2 Exacerbation of Hepatitis after Discontinuation ofTreatment5.3 New Onset or Worsening Renal Impairment5.4 Coadministration with Other Products5.5 Patients Coinfected with HIV-1 and HBV5.6 Decreases in Bone Mineral Density5.7 Fat Redistribution5.8 Immune Reconstitution Syndrome5.9 Early Virologic Failure6 ADVERSE REACTIONS6.1 Adverse Reactions from Clinical Trials Experience6.2 Postmarketing Experience7 DRUG INTERACTIONS7.1 Didanosine7.2 Atazanavir7.3 Lopinavir/Ritonavir7.4 Drugs Affecting Renal Function8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy8.3 Nursing Mothers8.4 Pediatric Use8.5 Geriatric Use8.6 Patients with Impaired Renal Function10 OVERDOSAGE11 DESCRIPTION12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action12.3 Pharmacokinetics12.4 Microbiology13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility13.2 Animal Toxicology and/or Pharmacology14 CLINICAL STUDIES14.1 Clinical Efficacy in Patients with HIV-1 Infection14.2 Clinical Efficacy in Patients with Chronic Hepatitis B16 HOW SUPPLIED/STORAGE AND HANDLING17 PATIENT COUNSELING INFORMATION AND FDAAPPROVED PATIENT LABELING* Sections or subsections omitted from the full prescribing information are not listedFULL PRESCRIBING INFORMATIONWARNINGS: LACTIC ACIDOSIS/SEVERE HEPATOMEGALY WITHSTEATOSIS and POST TREATMENT EXACERBATION OF HEPATITISLactic acidosis and severe hepatomegaly with steatosis, including fatalcases, have been reported with the use of nucleoside analogs, including VIREAD, in combination with other antiretrovirals [See Warnings and Precautions (5.1)].Severe acute exacerbations of hepatitis have been reported in HBV-infected patients who have discontinued anti-hepatitis B therapy, including VIREAD. Hepatic function should be monitored closely with both clinical andlaboratory follow-up for at least several months in patients who discontinueanti-hepatitis B therapy, including VIREAD. If appropriate, resumption ofanti-hepatitis B therapy may be warranted [See Warnings and Precautions(5.2)].1 INDICATIONS AND USAGEInfection1.1 HIV-1VIREAD® is indicated in combination with other antiretroviral agents for the treatment of HIV-1 infection in adults and pediatric patients 12 years of age and older.The following points should be considered when initiating therapy with VIREAD for the treatment of HIV-1 infection:• VIREAD should not be used in combination with TRUVADA® or ATRIPLA® [See Warnings and Precautions (5.4)].1.2 Chronic Hepatitis BVIREAD is indicated for the treatment of chronic hepatitis B in adults.The following points should be considered when initiating therapy with VIREAD for the treatment of HBV infection:• This indication is based primarily on data from treatment of subjects who were nucleoside-treatment-naïve and a smaller number of subjects who had previously received lamivudine or adefovir dipivoxil. Subjects were adults with HBeAgpositive and HBeAg-negative chronic hepatitis B with compensated liver disease [See Clinical Studies (14.2)].• VIREAD was evaluated in a limited number of subjects with chronic hepatitis B and decompensated liver disease. [See Adverse Reactions (6.1), Clinical Studies(14.2)]• The numbers of subjects in clinical trials who had lamivudine- or adefovirassociated substitutions at baseline were too small to reach conclusions ofefficacy [See Microbiology (12.4), Clinical Studies (14.2)].2 DOSAGE AND ADMINISTRATION2.1 Recommended Dose in AdultsFor the treatment of HIV-1 or chronic hepatitis B: The dose is one 300 mg VIREAD tablet once daily taken orally, without regard to food.In the treatment of chronic hepatitis B, the optimal duration of treatment is unknown.2.2 Recommended Dose in Pediatric Patients (≥12 Years of Age and ≥35 kg) For the treatment of HIV-1 in pediatric patients 12 years of age and older with body weight ≥35 kg (≥77 lb): The dose is one 300 mg VIREAD tablet once daily taken orally, without regard to food.2.3 Dose Adjustment for Renal Impairment in AdultsSignificantly increased drug exposures occurred when VIREAD was administered to subjects with moderate to severe renal impairment [See Clinical Pharmacology (12.3)]. Therefore, the dosing interval of VIREAD should be adjusted in patients with baseline creatinine clearance <50 mL/min using the recommendations in Table 1. These dosing interval recommendations are based on modeling of single-dose pharmacokinetic data in non-HIV and non-HBV infected subjects with varying degrees of renal impairment, including end-stage renal disease requiring hemodialysis. The safety and effectiveness of these dosing interval adjustment recommendations have not been clinically evaluated in patients with moderate or severe renal impairment, therefore clinical response to treatment and renal function should be closely monitored in these patients [See Warnings and Precautions (5.3)].No dose adjustment is necessary for patients with mild renal impairment (creatinine clearance 50–80 mL/min). Routine monitoring of calculated creatinine clearance and serum phosphorus should be performed in patients with mild renal impairment [See Warnings and Precautions (5.3)].Table 1 Dosage Adjustment for Patients with Altered Creatinine ClearanceCreatinine Clearance(mL/min)aHemodialysis Patients≥50 30–49 10–29Recommended 300 mg Dosing Interval Every 24hoursEvery 48hoursEvery 72 to96 hoursEvery 7 days or after a total ofapproximately 12 hours ofdialysis ba. Calculated using ideal (lean) body weight.b. Generally once weekly assuming three hemodialysis sessions a week of approximately 4 hours duration.VIREAD should be administered following completion of dialysis.The pharmacokinetics of tenofovir have not been evaluated in non-hemodialysis patients with creatinine clearance <10 mL/min; therefore, no dosing recommendation is available for these patients.No data are available to make dose recommendations in pediatric patients 12 years of age and older with renal impairment.3 DOSAGE FORMS AND STRENGTHSVIREAD is available as tablets. Each tablet contains 300 mg of tenofovir disoproxil fumarate, which is equivalent to 245 mg of tenofovir disoproxil. The tablets are almond-shaped, light blue, film-coated, and debossed with “GILEAD” and “4331” on one side and with “300” on the other side.4 CONTRAINDICATIONSNone.5 WARNINGS AND PRECAUTIONS5.1 Lactic Acidosis/Severe Hepatomegaly with SteatosisLactic acidosis and severe hepatomegaly with steatosis, including fatal cases, have been reported with the use of nucleoside analogs, including VIREAD, in combination with other antiretrovirals. A majority of these cases have been in women. Obesity and prolonged nucleoside exposure may be risk factors. Particular caution should be exercised when administering nucleoside analogs to any patient with known risk factors for liver disease; however, cases have also been reported in patients with no known risk factors. Treatment with VIREAD should be suspended in any patient who develops clinical or laboratory findings suggestive of lactic acidosis or pronounced hepatotoxicity (which may include hepatomegaly and steatosis even in the absence of marked transaminase elevations).5.2 Exacerbation of Hepatitis after Discontinuation of Treatment Discontinuation of anti-HBV therapy, including VIREAD, may be associated with severe acute exacerbations of hepatitis. Patients infected with HBV who discontinue VIREAD should be closely monitored with both clinical and laboratory follow-up for at least several months after stopping treatment. If appropriate, resumption of anti-hepatitis B therapy may be warranted.5.3 New Onset or Worsening Renal ImpairmentTenofovir is principally eliminated by the kidney. Renal impairment, including cases of acute renal failure and Fanconi syndrome (renal tubular injury with severe hypophosphatemia), has been reported with the use of VIREAD [See Adverse Reactions (6.2)].It is recommended that creatinine clearance be calculated in all patients prior to initiating therapy and as clinically appropriate during therapy with VIREAD. Routine monitoring of calculated creatinine clearance and serum phosphorus should be performed in patients at risk for renal impairment, including patients who have previously experienced renal events while receiving HEPSERA®.Dosing interval adjustment of VIREAD and close monitoring of renal function are recommended in all patients with creatinine clearance <50 mL/min [See Dosage and Administration (2.3)]. No safety or efficacy data are available in patients with renal impairment who received VIREAD using these dosing guidelines, so the potential benefit of VIREAD therapy should be assessed against the potential risk of renal toxicity.VIREAD should be avoided with concurrent or recent use of a nephrotoxic agent.5.4 Coadministration with Other ProductsVIREAD should not be used in combination with the fixed-dose combination products TRUVADA or ATRIPLA since tenofovir disoproxil fumarate is a component of these products.VIREAD should not be administered in combination with HEPSERA (adefovir dipivoxil) [See Drug Interactions (7.4)].Coinfected with HIV-1 and HBV5.5 PatientsDue to the risk of development of HIV-1 resistance, VIREAD should only be used in HIV-1 and HBV coinfected patients as part of an appropriate antiretroviral combination regimen.HIV-1 antibody testing should be offered to all HBV-infected patients before initiating therapy with VIREAD. It is also recommended that all patients with HIV-1 be tested for the presence of chronic hepatitis B before initiating treatment with VIREAD.5.6 Decreases in Bone Mineral DensityAssessment of bone mineral density (BMD) should be considered for adults and pediatric patients 12 years of age and older who have a history of pathologic bone fracture or other risk factors for osteoporosis or bone loss. Although the effect of supplementation with calcium and vitamin D was not studied, such supplementation may be beneficial for all patients. If bone abnormalities are suspected then appropriate consultation should be obtained.In HIV-1 infected adult subjects treated with VIREAD in Study 903 through 144 weeks, decreases from baseline in BMD were seen at the lumbar spine and hip in both arms of the study. At Week 144, there was a significantly greater mean percentage decrease from baseline in BMD at the lumbar spine in subjects receiving VIREAD + lamivudine + efavirenz (-2.2% ± 3.9) compared with subjects receiving stavudine + lamivudine + efavirenz (-1.0% ± 4.6). Changes in BMD at the hip were similar between the two treatment groups (-2.8% ± 3.5 in the VIREAD group vs. -2.4% ± 4.5 in the stavudine group). In both groups, the majority of the reduction in BMD occurred in the first 24–48 weeks of the study and this reduction was sustained through Week 144. Twenty-eightpercent of VIREAD-treated subjects vs. 21% of the stavudine-treated subjects lost at least 5% of BMD at the spine or 7% of BMD at the hip. Clinically relevant fractures (excluding fingers and toes) were reported in 4 subjects in the VIREAD group and 6 subjects in the stavudine group. In addition, there were significant increases in biochemical markers of bone metabolism (serum bone-specific alkaline phosphatase, serum osteocalcin, serum C-telopeptide, and urinary N-telopeptide) in the VIREAD group relative to the stavudine group, suggesting increased bone turnover. Serum parathyroid hormone levels and 1,25 Vitamin D levels were also higher in the VIREAD group. Except for bone specific alkaline phosphatase, these changes resulted in values that remained within the normal range.In a clinical study of HIV-1 infected pediatric subjects 12 years of age and older (Study 321), bone effects were similar to adult subjects. Under normal circumstances BMD increases rapidly in this age group. In this study, the mean rate of bone gain was less in the VIREAD-treated group compared to the placebo group. Six VIREAD treated subjects and one placebo treated subject had significant (>4%) lumbar spine BMD loss in 48 weeks. Among 28 subjects receiving 96 weeks of VIREAD, Z-scores declined by -0.341 for lumbar spine and -0.458 for total body. Skeletal growth (height) appeared to be unaffected. Markers of bone turnover in VIREAD-treated pediatric subjects 12 years of age and older suggest increased bone turnover, consistent with the effects observed in adults.The effects of VIREAD-associated changes in BMD and biochemical markers on long-term bone health and future fracture risk are unknown.Cases of osteomalacia (associated with proximal renal tubulopathy and which may contribute to fractures) have been reported in association with the use of VIREAD [See Adverse Reactions (6.2)].The bone effects of VIREAD have not been studied in patients with chronic HBV infection.Redistribution5.7 FatIn HIV-infected patients redistribution/accumulation of body fat including central obesity, dorsocervical fat enlargement (buffalo hump), peripheral wasting, facial wasting, breast enlargement, and "cushingoid appearance" have been observed in patients receiving combination antiretroviral therapy. The mechanism and long-term consequences of these events are currently unknown. A causal relationship has not been established. 5.8 Immune Reconstitution SyndromeImmune reconstitution syndrome has been reported in HIV-infected patients treated with combination antiretroviral therapy, including VIREAD. During the initial phase of combination antiretroviral treatment, patients whose immune system responds may develop an inflammatory response to indolent or residual opportunistic infections [such as Mycobacterium avium infection, cytomegalovirus, Pneumocystis jirovecii pneumonia (PCP), or tuberculosis], which may necessitate further evaluation and treatment.5.9 Early Virologic FailureClinical studies in HIV-infected subjects have demonstrated that certain regimens that only contain three nucleoside reverse transcriptase inhibitors (NRTI) are generally less effective than triple drug regimens containing two NRTIs in combination with either a non-nucleoside reverse transcriptase inhibitor or a HIV-1 protease inhibitor. In particular, early virological failure and high rates of resistance substitutions have been reported. Triple nucleoside regimens should therefore be used with caution. Patients on a therapy utilizing a triple nucleoside-only regimen should be carefully monitored and considered for treatment modification.6 ADVERSEREACTIONSThe following adverse reactions are discussed in other sections of the labeling:• Lactic Acidosis/Severe Hepatomegaly with Steatosis [See Boxed Warning, Warnings and Precautions (5.1)].• Severe Acute Exacerbation of Hepatitis [See Boxed Warning, Warnings and Precautions (5.2)].• New Onset or Worsening Renal Impairment [See Warnings and Precautions (5.3)]. • Decreases in Bone Mineral Density [See Warnings and Precautions (5.6)].• Immune Reconstitution Syndrome [See Warnings and Precautions (5.8)].6.1 Adverse Reactions from Clinical Trials ExperienceBecause clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.Clinical Trials in Adult Patients with HIV-1 InfectionMore than 12,000 subjects have been treated with VIREAD alone or in combination with other antiretroviral medicinal products for periods of 28 days to 215 weeks in clinical trials and expanded access studies. A total of 1,544 subjects have received VIREAD 300 mg once daily in clinical trials; over 11,000 subjects have received VIREAD in expanded access studies.The most common adverse reactions (incidence ≥10%, Grades 2–4) identified from any of the 3 large controlled clinical trials include rash, diarrhea, headache, pain, depression, asthenia, and nausea.Treatment-Naïve PatientsStudy 903 - Treatment-Emergent Adverse Reactions: The most common adverse reactions seen in a double-blind comparative controlled study in which 600 treatment-naïve subjects received VIREAD (N=299) or stavudine (N=301) in combination with lamivudine and efavirenz for 144 weeks (Study 903) were mild to moderate gastrointestinal events and dizziness.Mild adverse reactions (Grade 1) were common with a similar incidence in both arms, and included dizziness, diarrhea, and nausea. Selected treatment-emergent moderate to severe adverse reactions are summarized in Table 2.Table 2Selected Treatment-Emergent Adverse Reactions a (Grades 2–4) Reported in≥5% in Any Treatment Group in Study 903 (0–144 Weeks)VIREAD + 3TC + EFVd4T + 3TC + EFV N=299 N=301 Body as a WholeHeadache Pain Fever Abdominal pain Back painAsthenia 14%13% 8% 7% 9% 6% 17% 12% 7% 12% 8% 7% Digestive SystemDiarrhea Nausea Dyspepsia Vomiting 11% 8% 4% 5% 13% 9% 5% 9% Metabolic Disorders Lipodystrophy b1% 8%Musculoskeletal Arthralgia Myalgia 5% 3% 7% 5% Nervous SystemDepression InsomniaDizziness Peripheral neuropathy cAnxiety 11%5% 3% 1% 6% 10% 8% 6% 5%6% Respiratory Pneumonia 5% 5% Skin and Appendages Rash event d18% 12%a. Frequencies of adverse reactions are based on all treatment-emergent adverse events, regardless of relationshipto study drug.b. Lipodystrophy represents a variety of investigator-described adverse events not a protocol-defined syndrome.c. Peripheral neuropathy includes peripheral neuritis and neuropathy.d. Rash event includes rash, pruritus, maculopapular rash, urticaria, vesiculobullous rash, and pustular rash.Laboratory Abnormalities: With the exception of fasting cholesterol and fastingtriglyceride elevations that were more common in the stavudine group (40% and 9%)compared with VIREAD (19% and 1%) respectively, laboratory abnormalities observedin this study occurred with similar frequency in the VIREAD and stavudine treatmentarms. A summary of Grade 3 and 4 laboratory abnormalities is provided in Table 3.Table 3 Grade 3/4 Laboratory Abnormalities Reported in ≥1% of VIREAD-TreatedSubjects in Study 903 (0–144 Weeks)VIREAD + 3TC + EFV d4T + 3TC + EFVN=299 N=301Any ≥ Grade 3 Laboratory Abnormality 36% 42%Fasting Cholesterol (>240 mg/dL) 19% 40%Creatine Kinase (M: >990 U/L; F: >845 U/L) 12% 12%Serum Amylase (>175 U/L) 9% 8%AST (M: >180 U/L; F: >170 U/L) 5% 7%ALT (M: >215 U/L; F: >170 U/L) 4% 5%Hematuria (>100 RBC/HPF) 7% 7%Neutrophils (<750/mm3) 3%1% Fasting Triglycerides (>750 mg/dL) 1% 9%Study 934 - Treatment Emergent Adverse Reactions: In Study 934, 511 antiretroviralnaïve subjects received either VIREAD + EMTRIVA® administered in combination withefavirenz (N=257) or zidovudine/lamivudine administered in combination with efavirenz(N=254). Adverse reactions observed in this study were generally consistent with thoseseen in previous studies in treatment-experienced or treatment-naïve subjects(Table 4).Table 4Selected Treatment-Emergent Adverse Reactions a (Grades 2–4) Reported in≥5% in Any Treatment Group in Study 934 (0–144 Weeks) VIREAD b + FTC + EFV AZT/3TC + EFVN=257 N=254 Gastrointestinal Disorder Diarrhea Nausea Vomiting9% 9% 2% 5% 7% 5% General Disorders and Administration Site Condition Fatigue9% 8% Infections and Infestations SinusitisUpper respiratory tract infectionsNasopharyngitis 8% 8%5% 4% 5%3% Nervous System Disorders Headache Dizziness 6% 8% 5% 7%Psychiatric Disorders Depression Insomnia9% 5% 7% 7% Skin and Subcutaneous TissueDisorders Rash eventc7%9%a. Frequencies of adverse reactions are based on all treatment-emergent adverse events, regardless of relationshipto study drug.b. From Weeks 96 to 144 of the study, subjects received TRUVADA with efavirenz in place of VIREAD + EMTRIVAwith efavirenz. c. Rash event includes rash, exfoliative rash, rash generalized, rash macular, rash maculopapular, rash pruritic, andrash vesicular. Laboratory Abnormalities: Laboratory abnormalities observed in this study were generally consistent with those seen in previous studies (Table 5).Table 5 Significant Laboratory Abnormalities Reported in ≥1% of Subjects in AnyTreatment Group in Study 934 (0–144 Weeks)VIREAD a + FTC + EFV AZT/3TC + EFVN=257 N=254Any ≥ Grade 3 Laboratory Abnormality 30% 26%Fasting Cholesterol (>240 mg/dL) 22% 24%Creatine Kinase (M: >990 U/L; F: >845 U/L) 9% 7%Serum Amylase (>175 U/L) 8% 4%Alkaline Phosphatase (>550 U/L) 1% 0%AST (M: >180 U/L; F: >170 U/L) 3% 3%ALT (M: >215 U/L; F: >170 U/L) 2% 3%Hemoglobin (<8.0 mg/dL) 0% 4%Hyperglycemia (>250 mg/dL) 2% 1%Hematuria (>75 RBC/HPF) 3% 2%Glycosuria (≥3+) <1% 1%Neutrophils (<750/mm3) 3%5% Fasting Triglycerides (>750 mg/dL) 4% 2%a. From Weeks 96 to 144 of the study, subjects received TRUVADA with efavirenz in place of VIREAD + EMTRIVAwith efavirenz.Treatment-Experienced PatientsTreatment-Emergent Adverse Reactions: The adverse reactions seen in treatmentexperienced subjects were generally consistent with those seen in treatment naïvesubjects including mild to moderate gastrointestinal events, such as nausea, diarrhea,vomiting, and flatulence. Less than 1% of subjects discontinued participation in theclinical studies due to gastrointestinal adverse reactions (Study 907).A summary of moderate to severe, treatment-emergent adverse reactions that occurredduring the first 48 weeks of Study 907 is provided in Table 6.。
国产替诺福韦说明书

【商品名】倍信富马酸替诺福韦二吡呋酯片【通用名】富马酸替诺福韦二吡呋酯片【英文名】Teno fovirDisoproxilFumarate Tablets【汉语拼音】FuMaSua nTiNuoFuWeiErBiFuZhiPia n【主要成份】本品主要成分为富马酸替诺福韦二吡呋酯,其化学名称为9-[(R)-2-[[双[[(异丙氧基羰基)氧基]甲氧基]氧膦基卜丙基]腺嘌呤富马酸盐(1:1)。
【性状】本品为淡蓝色杏仁状薄膜衣片,除去包衣显白色。
【适应症】HIV-1感染富马酸替诺福韦二吡呋酯适用于与其它抗逆转录病毒药物联用,治疗成人HIV-1感染。
使用富马酸替诺福韦二吡呋酯开始治疗HIV-1感染时,应考虑一下几点:富马酸替诺福韦二吡呋酯不应与含有替诺福韦的固定剂量复方制剂联用,包括:•依非韦伦/恩曲他滨/富马酸替诺福韦二吡呋酯;•利匹韦林/恩曲他滨/富马酸替诺福韦二吡呋酯;•艾维雷韦/克比司特/恩曲他滨/富马酸替诺福韦二吡呋酯;•恩曲他滨替诺福韦。
【用法用量】HIV-1的治疗:剂量为每次300mg (—片),每日一次,口服,空腹或与食物同时服用。
成人肾功能损害患者使用剂量的调整在中至重度肾功能损害的受试者中给予富马酸替诺福韦二吡呋酯时,药物暴露显著增加(参见【药代动力学】)。
对基线肌酐清除率v 50mL/分钟的患者,应按照表1调整富马酸替诺福韦二吡呋酯的给药间期。
在此推荐的给药间期是根据在不同肾功能损害级别的非HIV和非HBV感染受试者,包括需要血液透析的晚间肾病的患者中单次给药的药代动力学数据模型得出。
在中度至重度肾功能损害的患者中,尚未对这些给药间期调整建议的安全性和疗效进行临床评价,因此在这些患者中应当密切监测对治疗的临床反应和肾功能(参见【注意事项】)。
对轻度肾功能损害(肌酐清除率50-80mL/分钟)的患者,无需调整剂量。
在这些患者中应定期监测计算出来的肌酐清除率和血清磷。
(参见【注意事项】)。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
NOTE - Prepare the solutions immediately before use. Test solution. Dissolve 100 mg of the substance under examination in 50 ml of methanol. Reference solution (a). A 0.2 per cent w/v solution of tenofovir disoproxil jitmarate RS in methanol. Reference solution (b). Dilute 1.0 ml ofreference solution (a) to 100.0 ml with methanol. Reference solution (c). Dissolve 10 mg ofthejUmaric acid in 50 ml of methanol.
TELMISARTAN TABLETS
IP 2010
Solvent mixture. 80 volumes of buffer solution prepared by diluting 5.0 ml of triethylamine to 2000ml with water and 20 volumes of methanol. Test solution. Weigh and powder 20 tablets. Disperse a quantity ofpowder containing about 100 mg ofTelmisartan in 100.0 ml ofsolvent mixture, sonicate for 45 minutes and filter. Reference solution. A 0.0005 per cent w/v solution of telmisartan RS in the solvent mixture.
2188
IP 2010
TENOFOVIR DISOPROXIL FUMARATE
Identification
A. Detennine by infrared absorption spectrophotometry (2.4.6). Compare the spectrum with that obtained with tenofovir disoproxil jitmarate. RS or with the reference spectrum of tenofovir disoproxil fumarate.
Chromatographic system - a stainless steel column 15 cm x 4.6 mm, packed with octadecylsilane bonded to porous silica (5 /lm) (such as Inertsil ODS-3), - mobile phase: a mixture of60 volumes ofbuffer solution prepared by dissolving 2.72 g ofpotassium dihydrogen phosphate in 1000 ml of water; add 2 ml of triethylamine and adjust the pH to 2.4 with orthophosphoric acid and 40 volumes of acetonitrile, flow rate. 1 ml per minute, - spectrophotometer set at 298 nIn, - injection volume. 20 Ill. Inject the reference solution. The test is not valid unless the theoretical plates is not less than 3000, the tailing factor is not more than 2.0 and the relative standard deviation for replicate injections is not more than 2.0 percent. Inject the test solution and the reference solution. Calculate the content of C33H30N40z in the tablets.
,
HXGaaH
IБайду номын сангаас
HaaG
H
Mol. Wt. 635.5 Tenofovir Disoproxil Fumarate salt ofbis(isopropyloxycarbonyloxymethyl ester of (R)-9-(2-phosphonomethoxypropyl)adenine with fumaric acid. Tenofovir Disoproxil Fumarate contain not less than 97.0 per cent and not more than 102. 0 per cent ofCI9H30N501OP,C4~04, c!ll~lll_at~~ on tl1eanb:ydr()us basis. Category. Antiviral. Dose. 300 mg daily. Description. A white to off- white crystalline powder.
Tests
Related substances. Detennine by liquid chromatography (2.4.14).
Inject the test solution, reference solution (b) and reference solution (c). In the chromatogram obtained with the test solution, the area of any secondary peak is not more than the area of the peak in the chromatogram obtained with the reference solution (b) (1.0 per cent) and the sum of all the secondary peaks is not more than 2.5 times the' area of the peak in the chromatogram obtained with the reference solution (b) (2.5 per cent). Ignore any peak corresponding to the peak obtained in the chromatogram in the reference solution (c) and any peak having area less than 0.02 times the area of the principal peak in the chromatogram obtained with reference solution (b) (0.02 per cent). Fumaric Acid. 17.5 per cent to 19.0 percent. Detennine by liquid chromatography (2.4.14).
Chromatographic system a stainless steel column l5cm x 4.6 mm, packed with octadecylsilane bonded to porous silica (5 /lm) (suc4 as InertsilODS-3), mobile phase: A. dissolve 0.5 g of potassium dihydrogen phosphate in 1000 ml of water; add 2 ml of triethylamine, adjusted to pH 3.2 with orthophosphoric acid, B. acetonitrile, a linear gradient programme using the conditions given below, flow rate. 1.8 ml per minute, spectrophotometer set at 298 nIn, injection volume. 20 Ill. Time (min.) Mobile phase A (per cent v/v) 78 80 70 Mobile phase B (per cent v/v) 22 20 30 40 40
B. In the Assay, the principal peak in the chromatogram obtained with the test solution corresponds to the peak in the chromatogram obtained with the reference solution.
o
6 7 15
60 60
40 20 78
25
26 35 40
Tenofovir Disoproxil Fumarate
60
80 22
Inject the reference solution. The test is not valid unless the theoretical plates is not less than 3000, tailing factor is not more than 2.0 and relative standard deviation for replicate injections is not more than 5.0 per cent. Inject the test solution and the reference solution. In the chromatogram obtained with the test solution the area of any secondary peak is not more than the area ofthe principal peak in the chromatogram obtained with the reference solution (0.5 per cent), the sum ofthe area of all the secondary peaks is not more than 4 times the area of the principal peak in the chromatogram obtained with reference solution (2.0 per cent). Ignore any peak with an area less than 0.1 times the area ofthe -principal peakinthe chromatogram obtained with the reference solution (0.05 per cent). Other tests. Comply with the tests stated under Tablets. Assay. Determine by liquid chromatography (2.4.14).
