WHO-富马酸替诺福韦Tenofovir disoproxil fumarate质量标准
尿β2微球蛋白与HIV感染者长期服用富马酸替诺福韦酯致肾脏损伤的相关性分析

四川医学2022年3月第43卷(第3期)Sichuan Medical Journal,2022,Vol.43,No.3・213・•基金论文•doi:10.16252/ki.issnl004-0501-2022.03.001论著尿p2微球蛋白与HIV感染者长期服用富马酸替诺福韦酯致肾脏损伤的相关性分析袁媛打何盛华,刘欢霞1,吕春容蔦何青莲S姚远I,程静I,何沅鸿打尹科I(1.成都市公共卫生临床医疗中心,四川成都610066;2,成都市第二人民医院,四川成都610021)【摘要】目的探讨尿B2微球■蛋自(尿阻-MG)与长期服用富马酸替诺福韦酯(TDF)致丈滋病病毒感染者(HIV感染者)肾脏濒伤的相关性。
方法选择在成琳市公共卫生临床医疗中心门诊治疗的HIV感染者建立前瞻性研究队列,初始并持续TOF+3TC+EFV(TLE)方案抗病毒洛疗(ART)3=2年,随访观察共30个月,每3个月随访1次。
收集患者基线及随访期间估算肾小球滤过率(eGFR)、尿B2-MG等资料。
TDF相关肾脏损伤定义为eGFR下降超过基线的25%或者eGFR<90m]/(min• 1.73m2),计算肾损伤的发生率。
分析尿P2-MG与TDF相关肾脏损伤的关系。
结果纳入ART中位3.19年的HIV感染者100例,随访期间惠者eGFR中位值波动在114.9-120.3ml/(min• 1.73m?),较基线125.2ml/ (min- 1.73m2)均有轻度下降(P<0.05)o肾损伤发生率从2%(2/100)累积增加至26%(26/100)…尿32-MG呈波动上升,研究后期(第18-30个月)尿B2-MG水平及异常率均高于前期(第0~15个月)。
尿32-MG水平与肾濒伤累积发生率呈正相关(r=0.668,P<0.05)o eGFR下降程度超过40ml/(min• 1.73m2)时,尿p2-MG中位值最高(0.8mg/L)(P<0.05)…结论长期服用含TDF的抗病毒方案可致HIV感染者eGFR下降及肾脏濒伤发生率增加。
(优质)富马酸丙酚替诺福韦片(韦立得)(TAF)-详细说明书及重点

富马酸丙酚替诺福韦片(韦立得)(TAF)【主要成份】丙-2-基N-[(S)-({[(2R)-1-(6-氨基-9H-嘌呤-9-基)丙-2-基]-氧化}甲基)(苯氧基)磷酰基]-l-丙氨酸酯,(2E)-丁-2-烯二酸(2:1)【成份】化学名:丙-2-基N-[(S)-({[(2R)-1-(6-氨基-9H-嘌呤-9-基)丙-2-基]-氧化}甲基)(苯氧基)磷酰基]-l-丙氨酸酯,(2E)-丁-2-烯二酸(2:1)分子量:C21H29O5N6P·1/2(C4H4O4)【性状】本品为黄色、圆形的薄膜衣片。
除去包衣后,呈白色或类白色。
片剂直径8 mm,一面凹刻有“GSI”,另一面凹刻有“25”。
【适应症/功能主治】富马酸丙酚替诺福韦片适于治疗成人和青少年(年龄12 岁及以上,体重至少为35 kg)慢性乙型肝炎(参见【药理毒理】)。
【规格型号】25mg*30片【用法用量】应当由具备慢性乙型肝炎管理经验的医生开始治疗。
成人和青少年(年龄为12 岁及以上且体重至少为35 kg):每日一次,一次一片。
口服。
需随食物服用。
漏服剂量如果漏服一剂富马酸丙酚替诺福韦片且已超过通常服药时间不足18 小时,则患者应尽快服用一剂,并恢复正常给药时间。
如果已超过通常服药时间18 小时以上,则患者不应服用漏服药物,仅应恢复正常给药时间。
如果患者在服用富马酸丙酚替诺福韦片后1 小时内呕吐,则该患者应再服用一片。
如果患者在服用富马酸丙酚替诺福韦片后超过1 小时呕吐,则该患者无需再服用一片。
特殊人群老年人无需针对年龄为65 岁及以上的患者进行富马酸丙酚替诺福韦片剂量调整(参见【药理毒理】)。
肾功能损害对于肌酐清除率(CrCl) 估计值≥15 mL/min 的成人或青少年(年龄至少为12岁,并且体重至少为35 kg)或CrCl < 15 mL/min且正在接受血液透析的患者,无需调整富马酸丙酚替诺福韦片剂量。
在进行血液透析当天,应在血液透析治疗完成后给予富马酸替诺福韦片(参见【药理毒理】)。
替诺福韦 (Tenofovir) 抗病毒药物

替诺福韦 (Tenofovir) 抗病毒药物替诺福韦 (Tenofovir) 抗病毒药物代号:替诺福韦 (Tenofovir)英文名:Tenofovir药物类别:抗病毒药物药物特征:壮大免疫系统,用于治疗艾滋病毒感染和乙肝病毒感染药物用途:阻止病毒复制,减轻病情,控制病毒传播副作用:饮食限制,肝肾功能注意,皮肤过敏反应替诺福韦 (Tenofovir) 是一种广泛应用于抗病毒治疗的药物,具有壮大免疫系统的作用。
本文将详细介绍替诺福韦的药物特征、用途以及副作用,并提供一些使用注意事项。
1. 药物特征替诺福韦是一种抗病毒药物,以英文名Tenofovir命名。
它具有广谱的抗病毒活性,特别用于治疗艾滋病毒感染和乙肝病毒感染。
替诺福韦可通过抑制病毒的反转录酶活性,阻止病毒的复制过程,从而减轻病情和控制病毒的传播。
2. 药物用途替诺福韦被广泛用于治疗艾滋病毒感染和乙肝病毒感染。
艾滋病毒感染是一种严重威胁人类健康的疾病,而乙肝病毒感染同样会给患者带来危害。
替诺福韦的使用可以帮助延缓病情发展,降低病毒复制速度,提高患者免疫系统的抵抗力。
对于正在接受治疗的患者来说,替诺福韦可以有效减少传染性,降低传播风险。
3. 副作用在使用替诺福韦时,需要注意一些副作用。
首先,饮食方面需要有所限制,尤其是高脂肪食物和饮料,因为它们会加重药物在肝脏和肾脏中的代谢负担。
其次,由于替诺福韦的代谢主要发生在肝脏和肾脏中,所以需要定期监测肝肾功能,以确保药物的安全使用。
最后,部分患者可能会对替诺福韦产生皮肤过敏反应,如瘙痒、红肿等,如果出现这些症状,应立即停药并就医。
4. 使用注意事项在使用替诺福韦之前,患者应该告知医生自己的过敏史以及正在服用的其他药物,以免产生药物相互作用。
同时,根据医生的指导,按照规定剂量和使用时间进行服药。
如果在使用替诺福韦期间出现任何不适症状,应及时告知医生,并密切配合医生进行检测和调整治疗方案。
总结:替诺福韦是一种用于抗病毒治疗的药物,可应用于艾滋病毒感染和乙肝病毒感染的治疗中。
富马酸丙酚替诺福韦治疗慢性乙型肝炎的研究现状

富马酸丙酚替诺福韦治疗慢性乙型肝炎的研究现状王素娜,连建奇,贾战生,张 颖(空军军医大学唐都医院感染病科,西安710038)摘要:富马酸丙酚替诺福韦(TAF)是一种新型的核苷酸逆转录酶抑制剂,用于治疗HIV感染和慢性HBV感染。
与富马酸替诺福韦二吡呋酯(TDF)相比,TAF具有更好的血浆稳定性和更强的肝脏靶向性,并且极大的降低了肾功能损伤、骨密度降低等副作用。
概述了TAF的药理学特点、代谢途径、药物相互作用、耐药性、肾脏安全性及其在慢性HBV感染者中的最新临床研究进展。
关键词:乙型肝炎,慢性;替诺福韦;富马酸丙酚替诺福韦;富马酸替诺福韦二吡呋酯;治疗结果中图分类号:R512.62 文献标志码:A 文章编号:10001-5256(2019)08-1828-06ResearchadvancesintheclinicaleffectoftenofoviralafenamideintreatmentofchronichepatitisBWANGSuna,LIANJianqi,JIAZhansheng,etal.(DepartmentofInfectiousDiseases,TangduHospitalofAirForceMedicalUniversity,Xi’an710038,China)Abstract:Tenofoviralafenamide(TAF)isanovelnucleosidereversetranscriptaseinhibitorusedinthetreatmentofhumanimmunodeficiencyvirus(HIV)infectionandchronichepatitisBvirus(HBV)infection.Comparedwithtenofovirdisoproxilfumarate,TAFhasbetterplasmastabilityandstrongerliver-targetingabilityandcansignificantlyreducetheadverseeventsofrenalinjuryandreducedbonemineraldensity.Thisarticlesummarizestheresearchadvancesinthepharmacologicalcharacteristics,metabolicpathways,druginteractions,drugresistance,andrenalsafetyofTAFanditsroleinpatientswithchronicHBVinfection.Keywords:hepatitisB,chronic;tenofovir;tenofoviralafenamide;tenofovirdisoproxilfumarate;treatmentoutcomedoi:10.3969/j.issn.1001-5256.2019.08.040收稿日期:2019-04-04;修回日期:2019-05-23。
恩替卡韦和富马酸替诺福韦二吡呋酯治疗慢性乙型肝炎及乙型肝炎肝

恩替卡韦和富马酸替诺福韦二吡呋酯治疗慢性乙型肝炎及乙型肝炎肝硬化抗病毒效果目的比較恩替卡韦(ETV)、富马酸替诺福韦二吡呋酯(TDF)治疗慢性乙型肝炎及乙型肝炎肝硬化患者的抗病毒效果。
方法收集2016年1月~2017年1月在吉林省一汽总医院接受ETV或TDF抗病毒治疗的80例初治慢性乙型肝炎或肝硬化患者,其中接受TDF治疗者38例(TDF组),接受ETV治疗者42例(ETV组)。
监测患者治疗前及治疗后第24、36、48周的实验室指标:肝肾功能、血钙、血磷、肌酸激酶、HBV DNA水平、肝炎标志物,以及药物的不良反应。
结果治疗第24周时,ETV组患者血清乙型肝炎表面抗原(HBsAg)明显低于TDF组(P 0.05);治疗第24、36、48周时,两组丙氨酸氨基转移酶(ALT)比较差异无统计学意义(P > 0.05)。
治疗第24、36周时,ETV组HBV DNA水平明显低于TDF组(P 0.05)。
TDF组有2例患者出现血磷轻度下降,骨密度示轻度骨质疏松。
结论在第24周及36周时ETV抑制HBV DNA的能力明显优于TDF,第48周时两组比较无显著差异。
[Abstract] Objective To compare the antiviral effect of Entecavir (ETV)and Tenofovir Disoproxil Fumarate (TDF)in initial treatment patients with chronic hepatitis B and liver cirrhosis. Methods A total of 80 initial treatment patients with chronic hepatitis B and liver cirrhosis who received antiviral therapy with TDF or ETV and were regularly followed up in the FAW General Hospital,from January 2016 to January 2017 were enrolled,and 38 patients were treated with TDF (TDF group)and 42 patients were treated with ETV (ETV group). Laboratory markers were measured before and at 24th,36th,and 48th week of treatment,including liver and renal function parameters,serum calcium,serum phosphate,creatine kinase,HBV DNA level,hepatitis markers. Adverse drug reactions were also observed. Results At 24th week of treatment,HBsAg of ETV group was lower than that of TDF group (P 0.05). At 24th,36th,48th week of treatment,the two groups had no statistical difference in ALT level (P > 0.05). At 24th,36th week of treatment,HBV DNA level of ETV group was lower than that of TDF group (P 0.05). In TDF group,2 cases occured serum inorganic phosphorus declined slightly,and bone mineral density was detected mild osteoporosi. Conclusion At 24th and 36th week of treatment,ETV restrains HBV DNA better than TDF,while at 48th week there is no differece between the two drugs.[Key words] Chronic hepatitis B;Hepatitis cirrhosis B;Hepatitis B surface antigens;Tenofovir Disoproxil Fumarate;Entecavir全球约有2.4亿慢性乙型肝炎病毒(HBV)感染者,每年约有65万人死于HBV感染所致的肝衰竭、肝硬化和肝细胞癌。
富马酸丙酚替诺福韦在慢乙肝患者中的应用

富马酸丙酚替诺福韦在慢乙肝患者中的应用刘雪;蒋雪梅【期刊名称】《临床医学进展》【年(卷),期】2024(14)2【摘要】乙型肝炎病毒(Hepatitis B virus, HBV)的慢性持续感染是慢性乙型肝炎(Chronic hepatitis B, CHB)、肝硬化和肝癌发生发展的重要原因,是世界范围内的一个主要公共卫生问题。
目前尚缺乏有效清除病毒的药物,用于一线治疗的药物为核苷(核苷酸)类似物(NUC)及干扰素(IFN)。
其中富马酸丙酚替诺福韦片(tenofovir alafenamide tablets,TAF,商品名:Vemlidy),是2018年年底在我国上市的慢乙肝治疗药物,其三期临床研究显示与富马酸替诺福韦二吡呋酯(tenofovir disoproxil fumarate, TDF)相比具有同等的抗病毒效应,更高的肾脏及骨骼安全性更好的肝脏靶向性等优势。
本文介绍了该药在真实世界中应用的疗效及安全性。
得出了该药无论是对于初治患者还是经治患者,与TDF具有相当的病毒抑制作用,且具有更好的ALT复常率、更好的骨肾安全性,并且对于低病毒血症的患者,换用TAF也能获得更好的完全病毒学应答率。
【总页数】10页(P3550-3559)【作者】刘雪;蒋雪梅【作者单位】山东大学医学融合与实践中心济南;山东省公共卫生临床中心济南【正文语种】中文【中图分类】R51【相关文献】1.富马酸丙酚替诺福韦与富马酸替诺福韦二吡呋酯治疗慢性乙型肝炎患者疗效与安全性比较2.富马酸丙酚替诺福韦与富马酸替诺福韦二吡呋酯治疗慢性乙型肝炎的临床疗效及安全性3.Hepatology International|富马酸丙酚替诺福韦片和富马酸替诺福韦二吡呋酯能够安全有效地阻断高病毒载量孕妇HBV的母婴垂直传播4.富马酸丙酚替诺福韦对阿德福韦酯经治肾功能损伤慢乙肝患者疗效及肾功能影响观察因版权原因,仅展示原文概要,查看原文内容请购买。
替诺福韦艾拉酚胺(TFA);Tenofoviralafenamide;GS7340 产品说明书
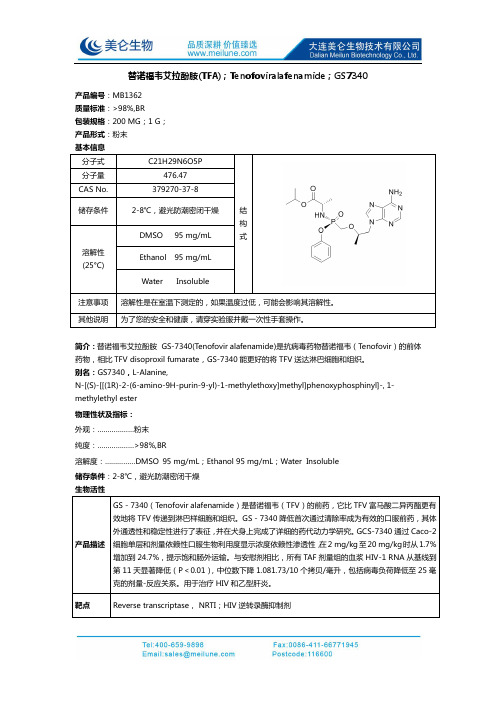
替诺福韦艾拉酚胺(TFA);Tenofoviralafenamide ;GS7340产品编号:MB1362 质量标准:>98%,BR 包装规格:200 MG ;1 G ; 产品形式:粉末结构式简介:替诺福韦艾拉酚胺 GS-7340(Tenofovir alafenamide)是抗病毒药物替诺福韦(Tenofovir )的前体药物,相比TFV disoproxil fumarate ,GS-7340能更好的将TFV 送达淋巴细胞和组织。
别名:GS7340,L-Alanine,N-[(S)-[[(1R)-2-(6-amino-9H-purin-9-yl)-1-methylethoxy]methyl]phenoxyphosphinyl]-, 1- methylethyl ester 物理性状及指标: 外观:………………粉末 纯度:………………>98%,BR溶解度:……………DMSO 95 mg/mL ;Ethanol 95 mg/mL ;Water Insoluble 储存条件:2-8℃,避光防潮密闭干燥为Reverse transcriptase。
●部分产品我司仅能提供部分信息,我司不保证所提供信息的权威性,以上数据仅供参考交流研究之用。
活性化合物操作注意事项1产品分装:您收到货物后最好不要自己进行分包,因为分包环境、包装材料等因素可能导致分包后的产品变质;如您有特殊包装要求,请在订购时候与我们客服代表阐明,当然价格会做适当调整。
对于开盖后,长期未使用的,请务必重新密封好,建议Parafilm封口膜,并按照相应储存条件使用。
如果放置时间过长,超过产品有效期,建议您重新购买,以免影响实验质量。
2储备液制备:大部分试剂的溶液形式稳定性较差,请优先采用现用现配的方式。
如需制备储存液,请选用合适溶剂,细胞培养类多选择DMSO,储备液制备完成后请于零下80摄氏度储存,一般可以稳定存在3-6个月以上。
孕期富马酸替诺福韦二吡呋酯抗病毒治疗对孕妇骨代谢及新生儿骨密度的影响

《中国肝脏病杂志(电子版)》2021年 第13卷 第2期
·论著· 35
group, 73 cases) and unantiviral therapy group (control group, 150 cases) according to whether accepted antiviral treatment during pregnancy or not. The bone metabolism indexes including serum calcium, phosphorus and alkaline phosphatase of patients before delivery and the bone mineral density (Z value) of newborns were analyzed by independent-samples t test. Pregnant women in observation group were divided into antiviral therapy from 28 weeks of pregnancy group (56 cases) and antiviral therapy for the whole pregnancy course group (17 cases). The bone metabolism indexes of patients before delivery and the bone mineral density of newborns were analyzed by independent-samples t test. Results There were no significant difference between patients in observation group and control group in the levels of serum calcium [(2.29 ± 0.09) mmol/L vs (2.26 ± 0.11) mmol/L; t = 1.697, P = 0.091] and phosphorus [(1.21 ± 0.14) mmol/L vs (1.20 ± 0.12) mmol/L; t = 0.810, P = 0.419]. The alkaline phosphatase level of patients in observation group was significantly higher than that of control group [(159.75 ± 41.53) U/L vs (125.35 ± 33.59) U/L; t = 6.609, P < 0.001]. There was no significant differences in Z value of newborns between the two groups [(0.70 ± 0.45) g/cm3 vs (0.75 ± 0.55) g/cm3; t = -0.654, P = 0.514]. Compared with those in antiviral therapy from 28 weeks of pregnancy group, the blood calcium [(2.27 ± 0.09) mmol/L vs (2.32 ± 0.09) mmol/L; t = 1.976, P = 0.052], phosphorus [(1.21 ± 0.14) mmol/L vs (1.25 ± 0.16) mmol/L; t = 0.828, P = 0.410] and neonatal bone mineral density [(0.66 ± 0.45) g/cm3 vs (0.81 ± 0.47) g/cm3; t = 0.917, P = 0.362] of pregnant women in antiviral therapy for the whole pregnancy course group decreased slightly, while alkaline phosphatase increased slightly[(161.59 ± 42.05) U/L vs (153.77 ± 40.75) U/L; t = -0.688, P = 0.494], however, the differences were not statistically significant. Conclusions The serum alkaline phosphatase of pregnant women with HBV infection can be increastherapy during pregnancy, which has no significant effect on neonatal bone mineral density. Key words: Pregnancy complicated with hepatitis B virus infection; Tenofovir disoproxil fumarate; Bone metabolism; Alkaline phosphatase
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Monographs: Pharmaceutical substances: Tenofoviri disoproxili fumaras - Tenofovir disoproxil fumarateC19H30N5O10P,C4H4O4Relative molecular mass. 635.5Chemical name. 1,1'-bis(1-methylethyl)1,1'-[({[(1R)-2-(6-amino-9H-purin-9-yl)-1-methylethoxy]methyl}phosphonoyldioxy)dimet hyl] dicarbonate (ester) hydrogen (2E)-but-2-enedioate (salt); CAS Reg.No. 202138-50-9.Description. White to almost-white, crystalline powder.Solubility. Slightly soluble in water, soluble in methanol, very slightly soluble in dichloromethane.Category. Antiretroviral (Nucleotide Reverse Transcriptase Inhibitor).Storage. Tenofovir disoproxil fumarate should be kept in a tightly closed container, protected from light, and stored at a temperature between 2 and 8°C.Additional information. Tenofovir disoproxil fumarate may exhibit polymorphism.RequirementsDefinition. Tenofovir disoproxil fumarate contains not less than 98.5 percent and not more than 101.0 percent of tenofovir disoproxil fumarate (C19H30N5O10P,C4H4O4), calculated with reference to the anhydrous substance.Manufacture. The production method is validated to ensure that the substance, if tested, would comply with:- a limit of not more than 5 ppm for the mutagenic impurity9-(prop-1-enyl)-9H-purin-6-amine (impurity K), which may be a synthesis related substance, using a suitable method, and- a limit of not more than 1.0% for the tenofovir disoproxil (S)-enantiomer (impurity G), using a suitable chiral chromatographic method.Identity tests• Either tests A, B and C or test D may be applied.A. Carry out test A.1 or, where UV detection is not available, test A.2.A.1 Carry out the test as described under 1.14.1 Thin-layer chromatography, using silica gel R6 as the coating substance and a mixture of 67 volumes of dichloromethane R, 20 volumes of acetonitrile R, 10 volumes of methanol R and 3 volumes of ammonia (~260 g/l) TS as the mobile phase. Apply separately to the plate 5 μl of each of 2 solutions in methanol containing (A) 10 mg of the test substance per ml and (B) 10 mg of tenofovir disoproxil fumarate RS per ml. After removing the plate from the chromatographic chamber, allow it to dry exhaustively in air or in a current of air. Examine the chromatogram in ultraviolet light (254 nm).The principal spot obtained with solution A corresponds in position, appearance, and intensity with that obtained with solution B.A.2 Carry out the test as described under 1.14.1 Thin-layer chromatography, using the conditions described above under test A.1 but using silica gel R5 as the coating substance. Stain the plate with iodine vapour and examine the chromatogram in daylight.The principal spot obtained with solution A corresponds in position, appearance, and intensity with that obtained with solution B.B. Carry out test B.1 or, where UV detection is not available, test B.2.B.1 Carry out the test as described under 1.14.1 Thin-layer chromatography, using silica gel R6 as the coating substance and a mixture of 50 volumes of heptane R, 30 volumes of glacial acetic acid R and 20 volumes of dichloromethane R as the mobile phase. Apply separately to the plate 5 μl of each of the following 2 solutions in ethanol R. For solution (A) use 10 mg of the test substance per ml and for solution (B) use 2 mg of fumaric acid R per ml. Develop the plate in an unsaturated tank over a path of 10 cm. After removing the plate from the chromatographic chamber, allow it to dry exhaustively in air or in a current of air. Examine the chromatogram in ultraviolet light (254 nm).One of the principal spots obtained with solution A corresponds in position, appearance, and intensity with that obtained with solution B.B.2 Carry out the test as described under 1.14.1 Thin-layer chromatography, using the conditions described above under test B.1 but using silica gel R5 as the coating substance. Spray lightly with a 16 g/l solution of potassium permanganate R and examine the chromatogram in daylight.The principal spot obtained with solution A corresponds in position, appearance, and intensity with that obtained with solution B.C. The absorption spectrum (1.6) of a 25 µg/ml solution, when observed between 220 nmand 320 nm, exhibits a maximum at about 261 nm; the specific absorbance () is 230 to 250.D. Carry out the examination as described under 1.7 Spectrophotometry in the infrared region. The infrared absorption spectrum is concordant with the spectrum obtained from tenofovir disoproxil fumarate RS or with the reference spectrum of tenofovir disoproxil fumarate. If the spectra thus obtained are not concordant, repeat the test using the residues obtained by separately dissolving the test substance and tenofovir disoproxil fumarate RS in a small amount of methanol R and evaporating to dryness. The infrared absorption spectrum is concordant with the spectrum obtained from tenofovir disoproxil fumarate RS.Specific optical rotation (1.4). Use a 10.0 mg/ml solution in hydrochloric acid (0.1 mol/l)VSand calculate with reference to the anhydrous substance; = -20° to -26°.Water. Determine as described under 2.8 Determination of water by the Karl Fischer method, Method A. Use about 1.0 g of the substance; the water content is not more than 10 mg/g.Heavy metals. Use 1.0 g in 30 ml of methanol R for the preparation of the test solution as described under 2.2.3 Limit test for heavy metals, Procedure 2; determine the heavy metals content accordi ng to Method A; not more than 20 μg/g.Sulfated ash (2.3). Not more than 1.0 mg/g.Related substances. Carry out the test as described under 1.14.4 High-performance liquid chromatography, using a stainless steel column (25 cm x 4.6 mm) packed withbase-deactivated particles of silica gel the surface of which has been modified with chemically bonded oct adecylsilyl groups (5 μm).The mobile phases for the gradient elution consist of a mixture of Mobile phase A and Mobile phase B, using the following conditions:Mobile phase A: 2 volumes of acetonitrile R, 20 volumes of phosphate buffer pH 6.0 and 78 volumes of water R.Mobile phase B: 65 volumes of acetonitrile R, 20 volumes of phosphate buffer pH 6.0 and 15 volumes of water R.Prepare the phosphate buffer pH 6.0 by dissolving 3.50 g of potassium dihydrogen phosphate R and 1.70 g of tetrabutyl ammonium hydrogen sulfate R in 800 ml of water R, adjust the pH to 6.0 by adding sodium hydroxide (1 mol/l) VS and dilute to 1000 ml with water R.After preparation, keep the solutions at about 6°C, or use an injector with cooling.Prepare the following solutions using water R as diluent. For solution (1) use 1.0 mg of the test substance per ml. For solution (2) dilute a suitable volume of solution (1) to obtain a concentration of 5 µg of tenofovir disoproxil fumarate per ml. For solution (3) use 0.2 mg of fumaric acid R per ml.For the system suitability test: prepare solution (4) by heating solution (1) carefully in a boiling water-bath for 20 minutes.Operate with a flow rate of 1.0 ml per minute. As a detector use an ultraviolet spectrophotometer set at a wavelength of 260 nm.Maintain the column temperature at 30 °C.Inject 20 μl of solution (4). The test is not valid unless the resolution between the principal peak (retention time about 40 minutes) and the peak due to the tenofovir monoester (with a relative retention of about 0.5) is not less than 25.Inject alternat ively 20 μl each of solutions (1) and (2) and (3). In the chromatogram obtained with solution (1), the following peak is eluted at the following relative retention, with reference to tenofovir (retention time about 40 minutes): fumarate about 0.15.In the chromatogram obtained with solution (1), the area of any peak due to the tenofovir monosoproxil (impurity A) is not greater than twice the area of the principal peak obtained with solution (2) (1.0%); the area of any other impurity peak is not greater than the area of the principal peak obtained with solution (2) (0.5%) and the areas of not more than two such peaks are greater than 0.4 times the area of the principal peak obtained with solution (2) (0.2%). The sum of the areas of all peaks, other than the principal peak, is not greater than 5 times the area of the principal peak obtained with solution (2) (2.5%). Disregard any peak corresponding to the peak obtained in the chromatogram with solution (3) and any peak with an area less than 0.1 times the area of the principal peak in the chromatogram obtained with solution (2) (0.05%).AssayDissolve 0.40 g, accurately weighed, in 30 ml of glacial acetic acid R1 and titrate with perchloric acid (0.1 mol/l) VS, determine the end point potentiometrically as described under 2.6 Non-aqueous titration Method A. Each ml of perchloric acid (0.1 mol/l) VS is equivalent to 63.55 mg of tenofovir disoproxil fumarate (C19H30N5O10P,C4H4O4).ImpuritiesA. (1-methylethyl)(8R)-9-(6-amino-9H-purin-9-yl)-5-hydroxy-8-methyl-5-oxo-2,4,7-trioxa-5-λ5-phosphanon anoate (tenofovir monosoproxil),B. (1-methylethyl)(5RS,8R)-9-(6-amino-9H-purin-9-yl)-5-methoxy-8-methyl-5-oxo-2,4,7-trioxa-5-λ5-phosph anonanoate,B. methyl (1-methylethyl)(5RS)-5-{[(1R)-2-(6-amino-9H-purin-9-yl)-1-methylethoxy]methyl}-5-oxo-2,4,6,8-tetrao xa-5-λ5-phosphanonanedioate,D. (1-methylethyl)(5RS,8R)-9-(6-amino-9H-purin-9-yl)-8-methyl-5-(1-methylethoxy)-5-oxo-2,4,7-trioxa-5-λ5-phosphanonanoate,E. (1-methylethyl)(8R)-5-hydroxy-8-methyl-9-(6-{[(1-methylethoxy)carbonyl]amino}-9H-purin-9-yl)-5-oxo-2,4,7-trioxa-5-λ5-phosphanonanoate,F. bis(1-methylethyl)9,9'-[methylenebis(imino-9H-purine-6,9-diyl)]bis[(8R)-5-hydroxy-8-methyl-5-oxo-2,4,7-tri oxa-5-λ5-phosphanonanoate] (tenofovir monosoproxil dimer),G. bis(1-methylethyl)5-{[(1S)-2-(6-amino-9H-purin-9-yl)-1-methylethoxy]methyl}-5-oxo-2,4,6,8-tetraoxa-5-λ5 -phosphanonanedioate (tenofovir disoproxil (S)-enantiomer) [see under Manufacture],H. 1-methylethyl propyl(5RS)-5-{[(1R)-2-(6-amino-9H-purin-9-yl)-1-methylethoxy]methyl}-5-oxo-2,4,6,8-tetrao xa-5-λ5-phosphanonanedioate,I. bis(1-methylethyl)5-{[(1R)-2-(6-{[({9-[(2R)-5-hydroxy-2,11-dimethyl-5,9-dioxo-3,6,8,10-tetraoxa-5-λ5-pho sphadodecyl]-9H-purin-6-yl}amino)methyl]amino}-9H-purin-9-yl)-1-methylethoxy]methyl }-5-oxo-2,4,6,8-tetraoxa-5-λ5-phosphanonanedioate (tenofovir di- and monosoproxil heterodimer),J. tetrakis(1-methylethyl)5,5'-(methylenebis{imino-9H-purine-6,9-diyl[(2R)-propane-1,2-diyl]oxymethylene})bis[5-oxo-2,4,6,8-tetraoxa-5-λ5-phosphanonanedioate] (tenofovir disoproxil dimer),K. 9-(prop-1-enyl)-9H-purin-6-amine, [see under Manufacture],L. (1-methylethyl)(5RS)-5-{[(1R)-2-(6-amino-9H-purin-9-yl)-1-methylethoxy]methyl}-10-methyl-5,9-dioxo-2,4,6,8-tetraoxa-10-aza-5-λ5-phosphaundecanoate,M. ethyl 1-methylethyl(5RS)-5-{[(1R)-2-(6-amino-9H-purin-9-yl)-1-methylethoxy]methyl}-5-oxo-2,4,6,8-tetrao xa-5-λ5-phosphanonanedioate.。
