EN ISO 80601-2-69-2014 医用电气设备 第2-69部分 基本安全和制氧设备的基本性能的特殊要求
医疗器械检测对应的标准

心电监护仪 YY1079-2008 医用电气设备 第2-49 部分:多参数 患者监护设备安全专用要求 YY0668-2008 医用电气设备 第2-27 部分:心电监 护设备安全专用要求
GB9706.25-2005 / IEC 60601-227:2005
眼科用刀通用技术条件 YY 0072-1992 角膜剪 YY/T 0176.10-1997 泪囊牵开器 YY 0073-1992 睑板腺囊肿镊 YY 0074-1992 虹膜剪 YY/T 0176.9-1997 眼睑拉钩 YY/T 0180-1994 睫毛镊 YY/T 0295.10-1997 晶体囊镊 YY/T 0295.11-1997 虹膜镊 YY/T 0295.7-1997 沙眼镊 YY/T 0295.9-1997
体视显微镜
光学系统
激光产品 光动力治疗机 多功能电子视频喉镜 窥喉镜传感视野仪 一次性摄像吸引管 医用冷光源 电子阴道镜 医用皮肤镜 图像分析和捕获系统
体视显微镜 第1部分 普及型体视显
GB/T 19864.1-2005
体视显微镜 第2部分 高性能体视湿
GB/T 19864.2-2005
光学系统 参数的测定
玻璃注射器 第1部分:全玻璃注射
玻璃注射器
YY1001.1-2004
玻璃注射器 第2部分:蓝芯全玻璃 注射器 YY1001.2-2004
腰椎穿刺针
腰椎穿刺针 YY/T 1148-2009
体温计 血压计 血压计
医用电子体温计
GB/T21416-2008 医用红外体温计 第1部分:耳腔式 GB/T21417.1-2008 SJ数字电子体温计 YZB/粤0054-2003 无创血压计规范 第1部分:通用要 EN1060-1:1996 无创血压计规范 第2部分:机械式 血压计的增补要求 EN1060-2:1996 无创血压计规范 第3部分:电子血 压测量系统的增补要求 EN1060-3:1997 手腕式电子血压计 YZB/粤0055-2002
医用电气设备 第2-71部分:功能性近红外光谱(NIRS)设备的基本安全和基本性能专用要求说明书

ICS11.040.55CCS C30中华人民共和国国家标准GB 9706.271—202×医用电气设备第2-71部分:功能性近红外光谱(NIRS)设备的基本安全和基本性能专用要求Medical electrical equipment –Part 2-71: Particular requirements for the basic safety and essential performance of functional near-infrared spectroscopy (NIRS)equipment(IEC 80601-2-71:2015,MOD)(征求意见稿)在提交反馈意见时,请将您知道的相关专利连同支持性文件一并附上。
××××- ××- ××发布××××- ××- ××实施国家市场监督管理总局发布国家标准化管理委员会目录前言 (II)引言 (IV)201.1 范围、目的和相关标准 (1)201.2 规范性引用文件 (2)201.3 术语和定义 (2)201.4 通用要求 (5)201.5 ME设备试验的通用要求 (5)201.6 ME设备和ME系统的分类 (5)201.7 ME设备标识、标记和文件 (5)201.8 ME设备对电击危险的防护 (5)201.9 ME设备和ME系统对机械危险的防护 (5)201.10 对不需要的或过量的辐射危险(源)的防护 (6)201.11 对超温和其他危险(源)的防护 (6)201.12 控制器和仪表的准确性和危险输出的防护 (6)201.13 ME设备危险情况和故障情况 (13)201.14 可编程医用电气系统(PEMS) (13)201.15 ME设备的结构 (13)201.16 ME系统 (13)201.17 ME设备和ME系统的电磁兼容性 (13)附录 (14)附录C (15)附录AA (资料性)专用指南和原理说明 (16)附录BB (规范性)应用功能性近红外光谱成像法估ME设备性能 (18)附录CC (资料性)引用基本原则 (26)前言本文件按照GB/T 1.1-2020《标准化工作导则第1部分:标准化文件的结构和起草规则》的规定起草。
6.ISO 80601-2-56{ed1.0}en文件

Reference numberISO 80601-2-56:2009(E)INTERNATIONAL STANDARD ISO80601-2-56First edition2009-10-01Medical electrical equipment —Part 2-56:Particular requirements for basic safetyand essential performance of clinicalthermometers for body temperaturemeasurementAppareils électromédicaux —Partie 2-56: Exigences particulières relatives à la sécurité fondamentaleet aux perfomances essentielles des thermomètres médicaux pour mesurer la température de corpsISO 80601-2-56:2009(E) PDF disclaimerThis PDF file may contain embedded typefaces. In accordance with Adobe's licensing policy, this file may be printed or viewed butshall not be edited unless the typefaces which are embedded are licensed to and installed on the computer performing the editing. In downloading this file, parties accept therein the responsibility of not infringing Adobe's licensing policy. The ISO Central Secretariat accepts no liability in this area.Adobe is a trademark of Adobe Systems Incorporated.Details of the software products used to create this PDF file can be found in the General Info relative to the file; the PDF-creation parameters were optimized for printing. Every care has been taken to ensure that the file is suitable for use by ISO member bodies. In the unlikely event that a problem relating to it is found, please inform the Central Secretariat at the address given below.COPYRIGHT PROTECTED DOCUMENT© ISO 2009All rights reserved. Unless otherwise specified, no part of this publication may be reproduced or utilized in any form or by any means, electronic or mechanical, including photocopying and microfilm, without permission in writing from either ISO at the address below or ISO's member body in the country of the requester.ISO copyright officeCase postale 56 • CH-1211 Geneva 20Tel. + 41 22 749 01 11Fax + 41 22 749 09 47E-mail copyright@Web Published in SwitzerlandISO 80601-2-56:2009(E)Contents PageForeword (v)Introduction (vi)201.1 * Scope, object and related standards (1)201.1. 1 Scope (1)201.1. 2 Object (2)201.1. 3 Collateral standards (2)201.1. 4 Particular standards (2)references (3)201.2 Normativeand definitions (3)201.3 Terminologyrequirements (6)201.4 General201.4. 2 R ISK MANAGEMENT PROCESS for ME EQUIPMENT or ME SYSTEMS (6)201.4. 2.101 Additional requirements for RISK MANAGEMENT PROCESS for ME EQUIPMENT orME SYSTEMS (7)201.4. 3 E SSENTIAL PERFORMANCE (7)201.4. 3.101 * Additional requirements for ESSENTIAL PERFORMANCE (7)201.4. 101 Environmental conditions of use (7)201.5 General requirements for testing of ME EQUIPMENT (7)of201.6 ClassificationME EQUIPMENT and ME SYSTEMS (7)201.7 M E EQUIPMENT identification, marking and documents (7)201.7. 2.1 Minimum requirements for marking on ME EQUIPMENT and interchangeable parts (8)201.7. 2.1.101 Additional requirements for minimum requirements for marking on ME EQUIPMENT and interchangeable parts, marking of the packaging (8)201.7. 2.2 Marking on the outside of ME EQUIPMENT or ME EQUIPMENT parts (8)201.7. 2.101 Additional requirements for marking on the outside of ME EQUIPMENT orME EQUIPMENT parts (8)201.7. 4.3 Unit of measure (8)201.7. 4.3.101 Additional requirements for unit of measure (8)201.7. 9 A CCOMPANYING DOCUMENT (9)201.7. 9.1 Additional general requirements (9)201.7. 9.2 Additional requirements for instructions for use (9)201.7. 9.2.14.101 Additional requirements for ACCESSORIES, supplementary equipment,used material (9)201.7. 9.2.101 Instructions for use (9)201.7. 9.101 Additional requirements for ACCOMPANYING DOCUMENT (10)201.8 Protection against electrical HAZARDS from ME EQUIPMENT (10)mechanicalagainst201.9 ProtectionHAZARDS of ME EQUIPMENT and ME SYSTEMS (10)201.10 Protection against unwanted and excessive radiation HAZARD s (10)201.11 Protection against excessive temperatures and other HAZARDS (10)and instruments and protection against hazardous outputs (10)controlsof201.12 Accuracy201.12. 1 Accuracy of controls and instruments (10)201.12. 1.101 Additional requirements for accuracy of controls and instruments (10)201.12. 2 U SABILITY (11)201.12. 2.101 * Additional requirements for USABILITY (11)201.13 H AZARDOUS SITUATIONS and fault conditions (11)201.14 P ROGRAMMABLE ELECTRICAL MEDICAL SYSTEMS (PEMS) (11)ISO 80601-2-56:2009(E)201.15 Construction of ME EQUIPMENT ................................................................................................11 201.16 M E SYSTEMS ...............................................................................................................................11 201.17 * Electromagnetic compatibility of ME EQUIPMENT and ME SYSTEMS . (11)201.101 Laboratory performance requirements (11)201.101. 1 * General test requirements (11)201.101. 2 * Laboratory accuracy (12)201.101. 3 * Time response for a continuous clinical thermometer ........................................................13 201.102* Clinical accuracy validation.................................................................................................13 201.102. 1Method......................................................................................................................................13 201.102. 2* Human subject population requirements...........................................................................14 201.102. 3* Clinical bias calculation.......................................................................................................15 201.102. 4* Limits of agreement calculation..........................................................................................15 201.102. 5* Clinical repeatability calculation.........................................................................................16 201.103* Probes, probe cable extenders and probe covers............................................................16 201.103. 1General.....................................................................................................................................16 201.103. 2Labeling....................................................................................................................................17 202 Medical electrical equipment — Part 1-2: General requirements for basic safetyand essential performance — Collateral standard: Electromagneticcompatibility — Requirements and tests (17)202.6.2.1.10 Compliance criteria.................................................................................................................17 AnnexesAnnex C (informative) Guide to marking and labelling requirements for ME EQUIPMENT andME SYSTEMS (19)Annex D (informative) Symbols on Marking (22)Annex AA (informative) Particular Guidance and rationale..........................................................................24 Annex BB (informative) R EFERENCE TEMPERATURE SOURCE .. (37)Annex CC (informative) Environmental aspects............................................................................................39 Annex DD (informative) Reference to the essential principles of safety and performance ofmedical devices in accordance with ISO/TR 16142 (40)Bibliography (42)Alphabetized index of defined terms used in this particular standard (45)ISO 80601-2-56:2009(E)ForewordISO (the International Organization for Standardization) is a worldwide federation of national standards bodies (ISO member bodies). The work of preparing International Standards is normally carried out through ISO technical committees. Each member body interested in a subject for which a technical committee has been established has the right to be represented on that committee. International organizations, governmental and non-governmental, in liaison with ISO, also take part in the work. ISO collaborates closely with the International Electrotechnical Commission (IEC) on all matters of electrotechnical standardization. International Standards are drafted in accordance with the rules given in the ISO/IEC Directives, Part 2.The main task of technical committees is to prepare International Standards. Draft International Standards adopted by the technical committees are circulated to the member bodies for voting. Publication as an International Standard requires approval by at least 75 % of the member bodies casting a vote.Attention is drawn to the possibility that some of the elements of this document may be the subject of patent rights. ISO shall not be held responsible for identifying any or all such patent rights.ISO 80601-2-56 was prepared by Technical Committee ISO/TC 121, Anaesthetic and respiratory equipment, Subcommittee SC 3, Lung ventilators and related equipment, in cooperation with Subcommittee 62D, Electrical equipment, of Technical Committee IEC/TC 62: Electrical equipment in medical practice.ISO 80601 consists of the following parts, under the general title Medical electrical equipment:⎯Part 2-12: Particular requirements for basic safety and essential performance of critical care ventilators⎯Part 2-13: Particular requirements for basic safety and essential performance of an anaesthetic workstation⎯Part 2-55: Particular requirements for the basic safety and essential performance of respiratory gas monitors⎯Part 2-56: Particular requirements for basic safety and essential performance of clinical thermometers for body temperature measurement⎯Part 2-61: Particular requirements for the basic safety and essential performance of pulse oximeter equipment for medical useIEC 80601-2-30: Particular requirements for basic safety and essential performance of automated non-invasive sphygmomanometers, IEC 80601-2-35: Particular requirements for basic safety and essential performance of blankets, pads and mattresses intended for heating in medical use, IEC 80601-2-58: Particular requirements for basic safety and essential performance of lens removal devices and vitrectomy devices for ophthalmic surgery, IEC 80601-2-59: Particular requirements for basic safety and essential performance of screening thermographs for human febrile temperature screening and IEC 80601-2-60: Particular requirements for basic safety and essential performance of dental equipment are published by IEC.ISO 80601-2-56:2009(E)IntroductionIn this International Standard, the following print types are used.⎯Requirements and definitions: roman type.⎯Test specifications: italic type.⎯Informative material appearing outside of tables, such as notes, examples and references: in smaller type. Normative text of tables is also in a smaller type.⎯T ERMS DEFINED IN C LAUSE 3 OF THE GENERAL STANDARD, IN THIS PARTICULAR STANDARD OR AS NOTED: SMALL CAPITALS.In referring to the structure of this International Standard, the term⎯“clause” means one of the 20 numbered divisions within the table of contents, inclusive of all subdivisions(e.g. Clause 7 includes subclauses 7.1, 7.2, etc.);⎯“subclause” means a numbered subdivision of a clause (e.g. 7.1, 7.2 and 7.2.1 are all subclauses of Clause 7).References to clauses within this International Standard are preceded by the term “Clause” followed by the clause number. References to subclauses within this International Standard are by number only.In this International Standard, the conjunctive “or” is used as an “inclusive or” so a statement is true if any combination of the conditions is true.The verbal forms used in this International Standard conform to usage described in Annex H of the ISO/IEC Directives, Part 2. For the purposes of this International Standard, the auxiliary verb:⎯“shall” means that compliance with a requirement or a test is mandatory for compliance with this standard;⎯“should” means that compliance with a requirement or a test is recommended but is not mandatory for compliance with this standard;⎯“may” is used to describe a permissible way to achieve compliance with a requirement or test.An asterisk (*) as the first character of a title or at the beginning of a paragraph or table title indicates that there is guidance or rationale related to that item in Annex AA.This international standard deals with electrical CLINICAL THERMOMETERS, either already available or that will come available in the future.The purpose of a CLINICAL THERMOMETER is to assess the true temperature of a REFERENCE BODY SITE. The temperature of the PATIENT'S body is an important vital sign in assessing overall health, typically in combination with blood pressure and pulse rate. Determining whether a PATIENT is afebrile or febrile is an important purpose of a CLINICAL THERMOMETER, since being febrile suggests that the PATIENT is ill.ISO 80601-2-56:2009(E) There are different temperatures at each REFERENCE BODY SITE according to the balance between the production, transfer, and loss of heat.[38]C LINICAL ACCURACY of a CLINICAL THERMOMETER is VERIFIED by comparing its OUTPUT TEMPERATURE with that of a REFERENCE THERMOMETER, which has a specified uncertainty for measuring true temperature. For an equilibrium CLINICAL THERMOMETER, the CLINICAL ACCURACY can be sufficiently determined under laboratory conditions that create an equilibrium state between the two thermometers.For a CLINICAL THERMOMETER that operates in the ADJUSTED MODE, laboratory VERIFICATION alone is not sufficient because the adjustment algorithm for deriving the OUTPUT TEMPERATURE includes the characteristics of the PATIENT and the environment.[3] Therefore the CLINICAL ACCURACY of a CLINICAL THERMOMETER that operates in the ADJUSTED MODE has to be VALIDATED clinically, using statistical methods of comparing its OUTPUT TEMPERATURE with that of a REFERENCE CLINICAL THERMOMETER which has a specified CLINICAL ACCURACY in representing a particular REFERENCE BODY SITE temperature.For a CLINICAL THERMOMETER that operates in the ADJUSTED MODE, the LABORATORY ACCURACY is VERIFIED in a DIRECT MODE and the CLINICAL ACCURACY is VALIDATED in the ADJUSTED MODE(OPERATING MODE) with a sufficiently large group of human subjects.The intention of this International Standard is to specify the requirements and the test procedures for the VERIFICATION of the LABORATORY ACCURACY for all types of electrical CLINICAL THERMOMETERS as well as for the VALIDATION of the CLINICAL ACCURACY of a CLINICAL THERMOMETER that operates in the ADJUSTED MODE.INTERNATIONAL STANDARD ISO 80601-2-56:2009(E)Medical electrical equipment —Part 2-56:Particular requirements for basic safety and essential performance of clinical thermometers for body temperature measurement201.1 * Scope, object and related standardsIEC 60601-1:2005, Clause 1 applies, except as follows:201.1.1 ScopeReplacement:This International Standard applies to the BASIC SAFETY and ESSENTIAL PERFORMANCE of a CLINICAL THERMOMETER in combination with its ACCESSORIES, hereafter referred to as ME EQUIPMENT. This standard specifies the general and technical requirements for electrical CLINICAL THERMOMETERS. This standard applies to all electrical CLINICAL THERMOMETERS that are used for measuring the body temperature of PATIENTS.C LINICAL THERMOMETERS can be equipped with interfaces to accommodate secondary indicators, printing equipment, and other auxiliary equipment to create ME SYSTEMS. This standard does not apply to auxiliary equipment.M E EQUIPMENT that measures a temperature not as a primary purpose, but as a secondary function, is outside the scope of this standard.EXAMPLE 1 Swan-Ganz thermodilution determination of cardiac output is not in the scope of this standard. EXAMPLE 2 A Foley catheter that includes a temperature PROBE is in the scope of this standard.EXAMPLE 3 P ATIENT heating ME EQUIPMENT that includes a skin temperature measurement such as infant incubators, heating blankets, heating pads and heating mattresses are not in the scope of this standard, unless they indicate a temperature of a REFERENCE BODY SITE in which they are in the scope of this standard.Requirements for ME EQUIPMENT intended to be used for non-invasive human febrile temperature screening of groups of individuals under indoor environmental conditions are given in IEC 80601-2-59:2008 and such ME EQUIPMENT is not covered by this standard.If a clause or subclause is specifically intended to be applicable to ME EQUIPMENT only, or to ME SYSTEMS only, the title and content of that clause or subclause will say so. If that is not the case, the clause or subclause applies both to ME EQUIPMENT and to ME SYSTEMS, as relevant.H AZARDS inherent in the intended physiological function of ME EQUIPMENT or ME SYSTEMS within the scope of this standard are not covered by specific requirements in this standard except in IEC 60601-1:2005, 7.2.13 and 8.4.1.NOTE Additional information can be found in IEC 60601-1:2005, 4.2.ISO 80601-2-56:2009(E)201.1.2 ObjectReplacement:The object of this particular standard is to establish particular BASIC SAFETY and ESSENTIAL PERFORMANCE requirements for a CLINICAL THERMOMETER, as defined in 201.3.206, and its ACCESSORIES.NOTE A CCESSORIES are included because the combination of the CLINICAL THERMOMETER and the ACCESSORIES needs to be safe. A CCESSORIES can have a significant impact on the BASIC SAFETY and ESSENTIAL PERFORMANCE of a CLINICAL THERMOMETER.standards201.1.3 CollateralAddition:This particular standard refers to those applicable collateral standards that are listed in IEC 60601-1:2005, Clause 2, as well as Clause 2 of this particular standard.IEC 60601-1-3 does not apply.standards201.1.4 ParticularReplacement:In the IEC 60601 series, particular standards may modify, replace or delete requirements contained in the general standard as appropriate for the particular ME EQUIPMENT under consideration, and may add other BASIC SAFETY and ESSENTIAL PERFORMANCE requirements.A requirement of a particular standard takes priority over IEC 60601-1.For brevity, IEC 60601-1 is referred to in this particular standard as the general standard. Collateral standards are referred to by their document number.The numbering of sections, clauses and subclauses of this particular standard corresponds to that of the general standard with the prefix “201” (e.g. 201.1 in this standard addresses the content of Clause 1 of the general standard) or applicable collateral standard with the prefix “20x” where x is the final digit(s) of the collateral standard document number (e.g. 202.4 in this particular standard addresses the content of Clause 4 of the 60601-1-2 collateral standard, 203.4 in this particular standard addresses the content of Clause 4 of the 60601-1-3 collateral standard, etc.). The changes to the text of the general standard are specified by the use of the following words:“Replacement” means that the clause or subclause of the IEC 60601-1 or applicable collateral standard is replaced completely by the text of this particular standard.“Addition” means that the text of this particular standard is additional to the requirements of the IEC 60601-1 or applicable collateral standard.“Amendment” means that the clause or subclause of the IEC 60601-1 or applicable collateral standard is amended as indicated by the text of this particular standard.Subclauses or figures which are additional to those of the general standard are numbered starting from 201.101, additional annexes are lettered AA, BB, etc., and additional items aa), bb), etc.Subclauses or figures which are additional to those of a collateral standard are numbered starting from 20x, where “x” is the number of the collateral standard, e.g. 202 for IEC 60601-1-2, 203 for IEC 6060-1-3, etc.The term “this standard” is used to make reference to the IEC 60601-1, any applicable collateral standards and this particular standard taken together.ISO 80601-2-56:2009(E) Where there is no corresponding section, clause or subclause in this particular standard, the section, clause or subclause of the IEC 60601-1 or applicable collateral standard, although possibly not relevant, applies without modification; where it is intended that any part of the IEC 60601-1 or applicable collateral standard, although possibly relevant, is not to be applied, a statement to that effect is given in this particular standard.201.2 NormativereferencesNOTE Informative references are listed in the bibliography beginning on page 43.IEC 60601-1:2005, Clause 2 applies, except as follows:Replacement:IEC 60601-1-2:2007, Medical electrical equipment — Part 1-2: General requirements for basic safety and essential performance — Collateral Standard: Electromagnetic compatibility — Requirements and testsIEC 60601-1-6:2006, Medical electrical equipment — Part 1-6: General requirements for basic safety and essential performance — Collateral Standard: UsabilityIEC 60601-1-8:2006, Medical electrical equipment — Part 1-8: General requirements for basic safety and essential performance — Collateral Standard: General requirements, tests and guidance for alarm systems in medical electrical equipment and medical electrical systemsAddition:ISO 14155-1:2003, Clinical investigation of medical devices for human subjects — Part 1: General requirementsISO 14155-2:2003, Clinical investigation of medical devices for human subjects — Part 2: Clinical investigation plansISO 15223-1:2007, Medical devices — Symbols to be used with medical device labels, labelling and information to be supplied — Part 1: General requirementsAmendment 1:2008IEC 60601-1:2005, Medical electrical equipment — Part 1: General requirements for basic safety and essential performanceIEC 60601-1-9:2007, Medical electrical equipment — Part 1-9: General requirements for basic safety and essential performance — Collateral Standard: Requirements for environmentally conscious designIEC 60601-1-10:2007, Medical electrical equipment — Part 1-10: General requirements for basic safety and essential performance — Collateral Standard: Requirements for the development of physiologic closed-loop controllers201.3 Terminology and definitionsFor the purposes of this document, the terms and definitions given in IEC 60601-1:2005, IEC 60601-1-6:2006, IEC 60601-1-8:2006, and the following definitions apply.NOTE An alphabetized index of defined terms is found beginning on page 45.Additions:201.3.201* ADJUSTED MODEOPERATING MODE where the OUTPUT TEMPERATURE is calculated by adjusting the signal from the input SENSOR。
各国医疗器械标准目录(中英文对照)
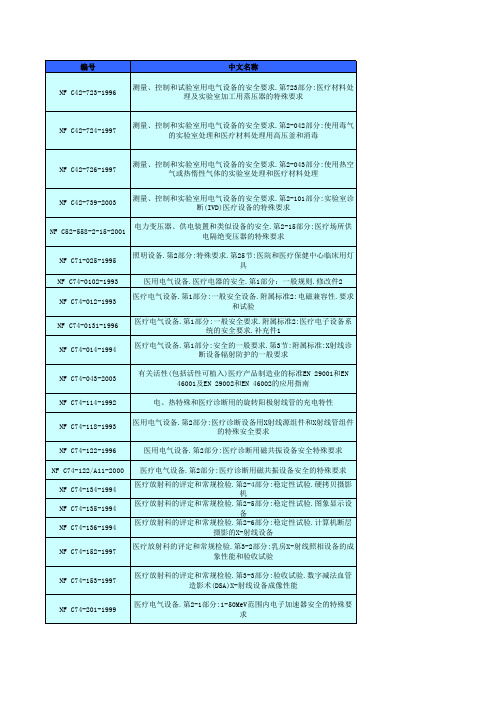
NF S90-410-1988
NF S90-411-1988
NF S90-414-1988
NF S90-420-1986
NF S90-426-1988
外科医疗器械.外科植入物.不锈钢粗糙面骨干板.尺寸和表面特性
NF S90-பைடு நூலகம்27-1990
外科医疗器械.脊椎用接骨器材.不锈钢制或钴基合金制脊椎板.A系 统.尺寸与几何形状 外科医疗器械.矫形外科设备.传动连接.第2部分:十字形和一字形旋 具 外科医疗器械.外科植入物.嵌入胯骨中的人造大腿部件.嵌合物顶端 部分和阳部分的规范 外科医疗器械.外科植入物.局部和全部髋关节修复术.第2部分: 由 金属和塑料材料制成的关节面 外科医疗器械.骨科关节假体.基本规范 外科医疗器械.膝关节全体和部分假肢.第1部分:分类、定义和尺寸 名称 外科医疗器械.髋关节全部和部分假体.第1部分:分类和,尺寸的标识 和要求 外科医疗器械.止血钳.尺寸和试验 外科医疗器械.钳、穿线管和持针器.尺寸和试验 外科医疗器械.一般用途.手术刀、解剖镊、重复使用的手术刀、导 管 医用外科设备.外科医疗器械.剪刀.尺寸和试验 外科医疗器械.外科植入物.心血管植入物、心脏瓣膜 外科医疗器械.矫形和假肢组件.肩轴和颈轴 外科医疗器械.矫形和假肢组件.带凸耳的凸圆头开槽全螺纹螺钉 外科医疗器械.矫形和假肢组件.带凸耳的平圆头开槽合螺纹螺钉
NF C52-558-2-15-2001
NF C71-025-1995 NF C74-0102-1993 NF C74-012-1993
NF C74-0131-1996
NF C74-014-1994
NF C74-043-2003
NF C74-114-1992
NF C74-118-1993
电磁兼容国内注册委托检验报告检验依据(重新整理)

医用电气设备注册检验依据
医用电气设备在进行电磁兼容的注册或委托检验时,检验依据除YY0505标准外,还需要考虑一些国家标准或行业标准。
因为除YY0505对电磁兼容提出要求外,还有一些国家标准和行业标准对电磁兼容性提出了不同于YY0505的电磁兼容性要求。
如GB9706.4-2009、GB9706.9-2008等,这些标准或是在限值上与YY0505的要求不同,或是在判据上提出具体要求,或是在试验布置上做出具体规定等。
只要产品预期功能涉及这些国家标准或行业标准,检验依据必须引用。
有些专标电磁兼容章节直接引用通标,有些涉及特殊要求,尤其注意电磁兼容特殊要求。
我们将这些标准中特殊要求列出来,方便查阅。
1、对电磁兼容有要求的国家标准和行业标准列表
IEC60601-2-26(GB9706.26)电磁兼容特殊要求
IEC60601-2-30(YY0667)高频手术设备试验布置
ESU试装置图
患者模拟器图12、IEC60601-2-34(YY0783)电磁兼容特殊要求
13、IEC60601-2-35(YY0834)电磁兼容特殊要求
14、IEC60601-2-37(GB9706.9)电磁兼容特殊要求
15、IEC60601-2-46(YY0570)电磁兼容特殊要求
16、IEC60601-2-47(YY0885)电磁兼容特殊要求
IEC60601-2-47(YY0885)电磁兼容试验布置
传导发射试验装置。
《医用电气设备第2-12部分治疗呼吸机的基本安全和基本

《医用电气设备第2-12部分:治疗呼吸机的基本安全和基本性能专用要求》编制说明1任务来源及背景1.1根据国家食品药品监督管理总局办公厅的食药监办械管﹝2017﹞94号文《食品药品监管总局办公厅关于印发2017年医疗器械行业标准制修订项目的通知》和财政部食品药品监管总局文件财社[2017]65号《财政部食品药品监管总局关于下达2017年公共卫生服务补助资金的通知》有关要求,由上海德尔格医疗器械有限公司、上海市医疗器械检测所和深圳迈瑞生物医疗电子股份有限公司共同负责起草《医用电气设备第2-12部分:治疗呼吸机的基本安全和基本性能专用要求》国家标准(项目编号:)。
1.2本标准按照GB/T 1.1-2009《标准化工作导则第1部分:标准的结构和编写》的规定进行编写。
2标准制定内容说明2.1标准“范围”的内容本专用标准适用于下列呼吸机及其附件(简称ME设备)的基本安全和基本性能:——预期用于依赖于机械通气的患者、且由专业操作者照料的;和——预期用于专业医疗场所的危急护理环境中,或预期用于专业医疗场所内的转运。
本专用标准也适用于制造商声称连接到呼吸系统或呼吸机的附件,这些附件的特性可能会影响呼吸机的基本安全和基本性能。
本专用标准不适用于不依赖机械通气的患者所使用的通气模式下运行的ME设备和ME系统。
本专用标准不适用于持续气道正压(CPAP)ME设备、睡眠呼吸暂停治疗ME设备、家庭护理环境呼吸机、通气支持ME设备、急救和转运用呼吸机、麻醉呼吸机、高频喷射呼吸机(HFJVs)和高频震荡呼吸机(HFOVs)。
本专用标准没有规定预期主要在专业护理场所中用于增加自主呼吸患者通气的ME设备的要求。
本专用标准没有规定预期用于由GB9706.29定义的麻醉应用的呼吸机或附件的要求。
本专用标准没有规定预期用于由YY0600.2定义的依赖呼吸机患者使用的家用呼吸机或附件的要求。
本专用标准没有规定预期用于由YY0600.3定义的急救和转运用呼吸机或附件的要求。
《医用电气设备第2-69部分氧气浓缩器的基本安全
《医用电气设备第2-69部分:氧气浓缩器的基本安全和基本性能专用要求》编制说明1 任务来源及背景1.1 根据全国麻醉和呼吸设备标准化技术委员会2017年标准制修订计划有关要求,由山东尚健医疗科技有限公司和上海市医疗器械检测所共同负责起草《医用电气设备第2-69部)。
分:氧气浓缩器的基本安全和基本性能专用要求》行业标准(项目编号:)1.2 本标准按照GB/T 1.1-2009《标准化工作导则第 1 部分:标准的结构和编写》的规定进行编写。
2 标准制定内容说明2.1 标准“范围”的内容GB 9706.1-20XX(IEC 60601-1:2005+修订1:2012)的1.1替换为:本专用标准规定了氧气浓缩器及其附件(以下简称ME设备)的基本安全和基本性能专用要求,氧气浓缩器预期用于提高输送给单个患者的气体氧浓度。
此类氧气浓缩器主要用于家庭保健环境中,包括多种环境中转移时可运行的、由单个患者操作的氧气浓缩器,环境包括任何私人交通、公共交通、商用飞机在内。
注1:此类氧气浓缩器也可用于专业保健机构。
本专用标准适用于转移时可运行的和转移时不可运行的氧气浓缩器。
本专用标准适用于集成于或与其他医疗器械、ME设备或ME系统一起使用的氧气浓缩器。
示例1:带有储氧装置[10]或湿化器[4]的氧气浓缩器。
示例2:与单独的流量计一起使用的氧气浓缩器。
示例3:在电力和麻醉气体后勤供应受限区域使用的麻醉系统[3]上使用的氧气浓缩器。
示例4:带有液氧罐或气瓶灌充系统的氧气浓缩器。
本专用标准也适用于那些预期连接到氧气浓缩器,并且其特性影响氧气浓缩器的基本安全和基本性能的附件。
本专用标准未规定用于医用气体管道系统的氧气浓缩器的要求,其要求见YY 1468。
如果某章或某条仅适用于ME设备,或仅适用于ME系统,则该章或该条的标题和内容将说明。
否则,该章或该条同时适用于相关的ME设备和ME系统。
除了通用标准的7.2.13和8.4.1, 本标准中规定的要求并未覆盖本标准范围内的ME设备或ME系统的生理功能内在风险。
医用电气设备 第2-21部分:婴儿辐射保暖台的基本安全和基本性能专用要求
ICS11.040.55C 39 YY 中华人民共和国医药行业标准YY 0455—20XX代替YY0455-2011医用电气设备第2-21部分:婴儿辐射保暖台的基本安全和基本性能专用要求Medical electrical equipment- Part 2-21: Particular requirements for the basic safety and essential performance of infant radiant warmers(IEC 60601-2-21:2009/A1:2016,MOD)(征求意见稿)2018.6.25XXXX-XX-XX发布XXXX-XX-XX实施目次前言 (II)引言 (III)201.1范围,目的和相关标准 (1)201.2规范性引用文件 (2)201.3术语和定义 (2)201.4通用要求 (4)201.5ME设备试验的通用要求 (5)201.6ME设备和ME系统的分类 (5)201.7ME设备识别、标记和文件 (5)201.8ME设备对电击危险(源)的防护 (7)201.9ME设备和ME系统对机械危险的防护 (7)201.10对不需要的或过量的辐射危险(源)的防护 (8)201.11对超温和其他危险(源)的防护 (8)201.12控制器和仪表的准确性和危险输出的防护 (9)201.13ME设备危险情况和故障状态 (12)201.14可编程医用电气系统(PEMS) (12)201.15ME设备的结构 (12)201.16ME系统 (13)201.17ME设备和ME系统的电磁兼容性 (13)202电磁兼容性-要求和测试 (13)附录AA (资料性附录)专用指南和原理说明 (15)参考文献 (21)索引 (23)图201.101–试验装置布局 (3)图201.102–试验装置 (4)图AA.1-本标准主要要求的图解 (16)表201.101–增加的基本性能要求 (5)前言医用电气设备安全要求系列标准主要由两大部分组成:---第1部分:基本安全和基本性能的通用要求;---第2部分:基本安全和基本性能的专用要求。
国家标准 医用电气设备 第1-1部分:安全通用要求
GB ICS 11.040 C 30 中华人民共和国国家标准 GB 9706.15—2008/IEC 60601-1-1:2000 代替GB 9706.15—1999 医用电气设备第1-1部分:安全通用要求并列标准:医用电气系统安全要求 Medical electrical equipment——Part 1:General requirements for safety——1. Collateral standard:Safety requirements for medicaI electrical systems (IEC 60601-1-1:2000,IDT) 2008-12-15发布 2010—02—01实施 中华人民共和国国家质量监督检验检疫总局发布 中国国家标准化管理委员会发布 目次 前言 (Ⅲ) 第一 篇概述…………………………………………………………………………………………………1 1 适用范围和目的…………………………………………………………………………………………1 2 术语和定义………………………………………………………………………………………………1 3 通用要求…………………………………………………………………………………………………1 6 识别、标记和文件…………………………………………………………………………………………2 第二 篇环境条件……………………………………………………………………………………………3 10 环境条件…………………………………………………………………………………………………3 第三 篇对电击危险防护……………………………………………………………………………………3 16 外壳和防护罩……………………………………………………………………………………………3 17 隔离………………………………………………………………………………………………………3 19 连续漏电流和患者辅助电流……………………………………………………………………………3 第四篇 对机械危险防护……………………………………………………………………………………4 22 运动部件…………………………………………………………………………………………………4 第五篇 对不需要的或过量的辐射危险的防护……………………………………………………………4 第六篇 对易燃麻醉混合气点燃危险的防护………………………………………………………………4 第七篇 对超温和其他安全方面危险的防护………………………………………………………………4 44 溢流、液体泼洒、泄漏、受潮、进液、清洗、消毒、灭菌和相容性………………………………………4 49 供电电源的中断…………………………………………………………………………………………4 第八篇 工作数据的准确性和危险输出的防止……………………………………………………………5 第九篇 不正常的运行和故障状态;环境试验……………………………………………………………5 52 不正常的运行和故障状态………………………………………………………………………………5 第十篇 结构要求……………………………………………………………………………………………5 56 元器件和组件……………………………………………………………………………………………5 57 网电源部分、元器件和布线……………………………………………………………………………5 58 保护接地——端子和连接………………………………………………………………………………6 59 结构和布线………………………………………………………………………………………………6 图201 患者环境举例………………………………………………………………………………………6 附录AAA(资料性附录) 总导则和编制说明……………………………………………………………7 附录BBB(资料性附录) 医用电气设备与非医用电气设备组合的举例………………………………10 附录CCC(规范性附录) 规范性引用文件……………………………………………………………12 附录DDD(资料性附录) 参考文献……………………………………………………………………13 附录EEE(规范性附录) 对可移式多孔插座的要求…………………………………………………14 附录FFF(资料性附录) 可移式多孔插座应用举例……………………………………………………15 前言 GB 9706的本部分的全部技术内容为强制性。
医用机器人电磁兼容检测研究
中国医疗设备 2019年第34卷 09期 V OL.34 No.0930专 论FEATURES医用机器人电磁兼容检测研究王权,李澍,张超,孟祥峰,任海萍中国食品药品检定研究院 医疗器械检定所 光机电室,北京 102629引言近年来,作为一个国家科技发展和高端制造业水平的重要标志,机器人特别是医用机器人产业发展受到了世界各国的高度关注。
医用机器人在微创手术、骨科手术、远程医疗、智能康复等领域展现了巨大的潜力[1]。
与传统医疗器械相比,应用机器人技术的医疗器械是一种融合了医学、电子、机械、光学、人机接口、模式识别等多领域的综合性医疗器械,应用范围广泛,产品之间差异明显,研发、测试、验证、确认等环节都不同于传统医疗器械[2],并且在实际使用中可能存在较大的使用风险,尤其是电磁兼容方面的风险[3]。
在此条件下,如何进行产品上市前质量控制与评价,尤其是对于医用机器人这种涵盖多用途、多设备组合的创新医疗器械的电磁兼容[4]评价提出了新的挑战。
目前,为了促进行业的健康和有序发展,国内外都在积极研究和制定医用机器人的行业标准。
在国际方面国际标准化组织(International Organization for Standardization ,ISO )、国际电工委员会(International Electrotechnical Commission ,IEC )是机器人标准化工作的两大主要组织。
ISO 是最早进行机器人标准化研究的国际标准化组织。
目前由ISO/TC299承担机器人标准化工作,工作范围除工业机器人外也包含医用机器人领域,工作重点在于安全与性能测试标准。
ISO/TC299 /JWG5、ISO/TC299 /JWG35、ISO/TC299 /JWG36正在进行包括应用机器人技术的医疗设备安全自治程度指南、手术机器人和康复机器人的基本安全要求和性能的特殊要求的制定。
IEC 标准化工作主要由IEC/TC59、IEC/TC61、IEC/TC62、IEC/TC116技术委员会承担,制定的标准主要涉及家用服务机器人的安全和性能、工业机器人的功能安全和医疗机器人等方面。
