注射用雷替曲塞说明书
肿瘤科常用化疗药物输注注意事项
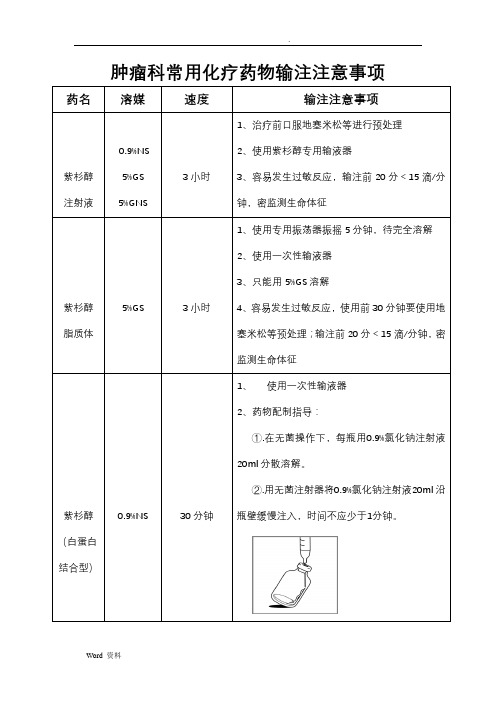
肿瘤科常用化疗药物输注注意事项药名溶媒速度输注注意事项
紫杉醇注射液0.9%NS
5%GS
5%GNS
3小时
1、治疗前口服地塞米松等进行预处理
2、使用紫杉醇专用输液器
3、容易发生过敏反应,输注前20分<15滴/分
钟,密监测生命体征
紫杉醇脂质体5%GS 3小时
1、使用专用振荡器振摇5分钟,待完全溶解
2、使用一次性输液器
3、只能用5%GS溶解
4、容易发生过敏反应,使用前30分钟要使用地
塞米松等预处理;输注前20分<15滴/分钟,密
监测生命体征
紫杉醇(白蛋白结合型)0.9%NS 30分钟
1、使用一次性输液器
2、药物配制指导:
①.在无菌操作下,每瓶用0.9%氯化钠注射液
20ml分散溶解。
②.用无菌注射器将0.9%氯化钠注射液20ml沿
瓶壁缓慢注入,时间不应少于1分钟。
药物说明书drins-雷替曲塞-正大

目前尚无确切有效解毒剂。一旦超量使用可考虑使用亚叶酸治疗。根据经验,每6小时静脉注射25mg/m2亚叶酸,越晚使用亚叶酸效果越差。超量用药预期不良反应容易扩大。应仔细监测有关胃肠道和血液学的毒性征兆并有针对性采取措施治疗。
【药理作用】
雷替曲塞为抗代谢类叶酸类似物,特异性地抑制胸苷酸合酶(TS)。与5-FU或氨甲喋呤相比,霍替曲塞是直接的和特异性的TS抑制剂。TS是胸腺嘧啶脱氧核苷三磷酸盐(TTP)合成过程的关键酶,而TTP又是DNA合成的必须核苷酸。抑制TS可导致DNA断裂和细胞凋亡。雷替曲塞经还原叶酸载体摄入细胞被叶酰聚谷氨酸合成酶转化成聚谷氨酸盐形式贮存细胞中,发挥更强TS抑制作用。雷替曲塞聚谷氨酸盐通过增强TS抑制能力、延长抑制时间而提高其抗肿瘤活性。但其在正常组织中的贮留可能会使毒性增加。
【适应症】
在患者无法接受联合化疗时,本品可单药用于治疗不适合5-Fu/亚叶酸钙的晚期结直肠癌患者。
【规格】
2mg。
【用法用量】
成人:推荐剂量为3mg/m2,用50-250ml 0.9%氯化钠注射液或5%葡萄糖注射液溶解稀释后静脉输注,给药时间15分钟,如果未出现毒性,可考虑按上述治疗每3周重复给药1次。本药应避免与其它药物混合输注。增加剂量会致使危及生命或致死性毒性反应的发生率升高,所以不推荐剂量大于3mg/m2。每次用药治疗前需检查全血细胞计数(包括白细胞分类计数和血小板计数)和肝、肾功能。治疗前应该白细胞计数>4.0×108/L、中性粒细胞计数>2.0×109/L和血小板计数>1.0×1011/L。出现毒性反应时,下一周期用药需延迟至不良反应消退;尤其是胃肠道毒性(腹泻或粘膜炎)及血液学毒性(中性粒细胞减少或血小板减少)需完全恢复才可进行后续治疗,出现胃肠道毒性者应至少每周检查一次全血细胞计数以监测血液学毒性。根据前一治疗周期观察到的最严重的胃肠道及血液学毒性等级,如果此类毒性已完全缓解,推荐按前一周期最严重的胃肠道、血液学毒性(以下毒性均按WHO标准分级)进行剂量调整:剂量减少25%:血液学毒性(中性粒细胞减少或者血小板减少)3级或胃肠道毒性2级(腹泻或粘膜炎);剂量减少50%:血液学毒性(中性粒细胞减少或者血小板减少)4级或胃肠道毒性3级(腹泻或粘膜炎)。一旦减量,后续治疗的剂量也须按减量后给药。出现4级胃肠道毒性(腹泻或粘膜炎),或3级胃肠道毒性伴4级血液学毒性时必须中止治疗;同时迅速给予标准支持治疗,包括静脉补水和造血功能支持。临床前研究提示可以使用亚叶酸治疗,按照临床经验需每6小时静脉注射25mg/m2亚叶酸直至症状缓解。对于此类患者建议停用本药。肾功能不全:血清肌酐异常者,每次用药治疗前应监测肌酐清除率。对于因年龄或体重下降等因素使血清肌酐可能与肌酐清除率相关性不好而血清肌酐正常的患者,应按相同程序操作。如果肌酐清除率<65ml/min,作如下剂量调整:肝功能不全:对于轻到中度的肝功能损害患者不需调整剂量,但是因为部分药物经粪便排出(见药代动力学),且这些患者预后较差,故应慎用本药。本药未在重度肝功能损害患者中进行研究,因此对于显性黄疸或肝功能失代偿的患者不推荐使用。
注射剂药物输注时间(滴速)要求汇总(2023)

注射剂药物输注时间(滴速)要求汇总(2023)注射剂药物说明书提到有滴速要求的,临床实际操作中建议根据输液器说明中的点滴系数进行换算。
静脉输液中ImI有多少滴?输液皮条厂家不同滴速不同,约在15-20滴/毫升左右,精密输液器为15滴/毫升,普通输液器20滴/毫升,压力套装为30滴/毫升。
加入一些药物,如头袍类、红霉素等药物后,药液变浓稠,液滴张力随之变大,液珠变小,这样的液体滴注时可为22滴/毫升。
一般输液器包装上标明:规格0.7mm的针头,滴蒸储水20滴/毫升。
药品说明书对输注时间(滴速)有要求的药物:一、抗菌药物1左氧氟沙星(扬子江药业)每250m1不得少于2小时,500m1不得少于3小时2莫西沙星(拜耳先灵医药)静脉给药0.4g的滴注时间应为90分钟3万古霉素(V IANEXSA)以至少IOOm1溶媒进行稀释溶解后,静脉滴注时间应60分钟以上4亚胺培南西司他丁(杭州默沙东)剂量W500mg,给药时,静脉滴注时间应不少于20〜30分钟;剂量>500mg,给药时,应不少于40〜60分钟。
如病人滴注时出现恶心状态,可减慢滴注速度5阿奇霉素(辉瑞制药)配置成浓度为2mg∕m1,25On11的溶液滴注时间应不少于1小时;配置成浓度为1mg∕m1,50Om1的溶液时滴注时间应不少于3小时6两性霉素B(华北制药)浓度不超过IOmg∕100m1,避光缓慢静滴,每次滴注时间6小时以上7伏立康哇(PharmaciaUpjohn)浓度不应高于5mg∕m1,滴注速度最快不超过每小时3mg∕kg,每瓶滴注时间须1至2小时8注射用更昔洛韦(科益药业)不少于1小时9注射用醋酸卡泊芬净(MerckSharpDohme)静脉缓慢输注约1小时以上10注射用替加环素(惠氏制药)成人:静脉滴注时间约30〜60分钟;儿童:静脉滴注时间至少60分钟二、抗肿瘤药物1吉西他滨(江苏豪森)30分钟2注射用奥沙利钻(赛诺菲)2〜6小时3白消安注射液(浙江大冢制药)2小时4盐酸多柔比星脂质体注射液(石药集团)起始给药速率应不大于1mg∕min o如果无滴注反应,以后的滴注可在60分钟完成。
注射用雷替曲塞产品介绍

。
雷替曲塞药理
做为新一代水溶性胸苷酸合酶(TS)抑制剂, 该药通过细胞膜外还原型叶酸盐载体系统 将本品主动摄入细胞内,而后迅速代谢为 多谷氨酸类化合物抑制胸苷酸合酶活性, 并能在细胞内潴留,长时间发挥作用。它 对结肠直肠癌细胞系抑制作用强于5-氟尿嘧 啶.
雷替曲塞药理
雷替曲塞IC50(50%抑制浓度)长期给药为 1.3~3.9nmol/L,短期给药为80nmol/L,而 5-氟尿嘧啶与甲酰四氢叶酸合用长期给药 IC50为330~5800nmol/L,短期给药为 150000nmol/L。
注射用雷替曲塞产品介绍
南京正大天晴 广州办事处
通用名:注射用雷替曲塞 英文名:Raltitrexed Injection(Tumudex)
O
HN H N N CH3 H3 C N
O OH
S
O
O
OH
被列入《国家级化学医药新产品开发指南》
〖功用主治〗:
用于晚期结直肠肠癌患者。
雷替曲塞药理
高选择性的胸腺嘧啶合成酶(TS)抑制剂 其代谢物为多聚谷氨酸类化合物,比母药发挥更 强的酶抑制作用
结论
综上所述:雷替曲赛作为近几十年来第一 个新的一线细胞毒治疗药,可成为5-FU为 主的化疗方案的较好替代药。他的问世可 为医生和患者提供更多选择。为患者带来 方便,治疗效益显著。
注意事项
注意事项:本能只做单独给药,避免与其 它药物混合使用。本品用0.9%生理盐水或 5%葡萄糖水溶液稀释后应避光保存,在24 小时内使用。轻度和中毒肝损伤患者使用 时无需调整本品剂量,但本品部分经粪便 排泄,严重肝损伤患者使用时应注意。孕 妇及哺辱期妇女禁用本品。
雷替曲塞

雷替曲塞雷替曲塞,Raltitrexed英文化学名:S)-2-[(1-{5-[Methyl-(2-methyl-4-oxo-3,4-dihydro-quinazolin-6-ylm ethyl)-amino]-thiophen-2-yl}-methanoyl)-amino]-pentanedioic acid 中文化学名:N-[5-[N-甲基-N-(2-甲基-4-氧代-3,4-二氢喹唑啉-6-基甲基)氨基]-2-噻吩甲酰基]-L-谷氨酸分子式: C21H22N4O6S分子量:458.49质量标准:企标成分:雷替曲塞、甘露醇、氢氧化钠和磷酸氢二钠。
性状:白色或类白色疏松块状物或粉末规格2mg/支【药物名称】雷替曲塞粉针 Raltitrexed Injection【分子式成分】N-[5-[N-甲基-N-(2-甲基-4-氧代-3,4-二氢喹唑啉-6-基甲基)氨基]-2-噻吩甲酰基]-L-谷氨酸【制剂规格】本品为白色冻干粉末,每瓶含雷替曲塞2 mg。
【药理毒理】药理学研究表明,雷替曲塞为新一代水溶性胸苷酸合酶抑制剂,该药通过细胞膜外还原型叶酸盐载体系统将本品主动摄入细胞内,而后迅速代谢为多谷氨酸类化合物抑制胸苷酸合酶的活性,并能在细胞内潴留,长时间发挥作用。
它对结肠直肠癌细胞系的抑制作用强于5-氟尿嘧啶,雷替曲塞的IC50长期给药为1.3~3.9 nmol/L,短期给药为80 nmol/L,而5-氟尿嘧啶与甲酰四氢叶酸合用长期给药IC50为330~5800 nmol/L,短期给药为150000 nmol/L。
体外研究观察到雷替曲塞与5-氟尿嘧啶联合用药有协同作用,这种作用依赖于给药方案和剂量。
对176例晚期结肠直肠癌患者进行的II期临床试验,给予雷替曲塞3 mg/㎡,每3周1次,有25.6%产生综合疗效,从治疗到病情进展平均时间为4.2周,存活期平均为11.2月。
1300多例晚期结肠直肠癌患者的3项III期临床研究结果表明,雷替曲塞治疗组(每3周1次,每次3 mg/㎡)与5-氟尿嘧啶加甲酰四氢叶酸治疗组(5-氟尿嘧啶425mg/㎡加甲酰四氢叶酸20 mg/㎡或5-氟尿嘧啶400 mg/㎡加甲酰四氢叶酸200 mg/㎡,每天1次,连续5天,每4~5周重复1次),所产生的客观有效率相似,分别为14.3%~19.3%和15.2%~18.1%,中位缓解时间分别为3.1~4.8和3.6~5.3个月,中位生存期分别为9.7~10.9和10.2~12.7个月。
雷替曲塞临床研究

雷替曲塞临床研究进展雷替曲塞(ZD1694,'Tomudex')是一种新型的,直接的和特异的TS抑制剂,由Zeneca 医药和肿瘤研究中心(UK)共同研发,1991年进行临床试验。
1992年开始进行II期临床试验,推荐雷替曲塞 3.0 mg/m2,每3周一次,15分钟静脉滴注。
一、雷替曲塞作用机制雷替曲塞为抗代谢类叶酸类似物,是胸苷酸合成酶(TS)特异性选择性抑制剂。
TS是胸腺嘧啶脱氧核苷三磷酸盐(TTP)合成过程的关键酶,而TTP又是DNA合成的必需核苷酸。
抑制TS可导致DNA断裂和细胞凋亡。
肿瘤细胞DNA从头合成途径的关键酶是TS,因此抑制TS的活性常常作为抗肿瘤治疗的一个靶点。
雷替曲塞经还原叶酸载体摄入细胞被叶酰聚谷氨酸合成酶转化成聚谷氨酸盐形式贮存细胞中,聚谷氨酸盐通过较强地抑制TS活性、延长抑制时间而提高其抗肿瘤活性。
5-FU也是一种TS抑制剂,它在体内代谢成很多活性物质,包括氟尿嘧啶脱氧核苷单磷酸盐,结合在酶的嘧啶结合区域,抑制TS的活性。
其他的5-FU代谢产物有很多的效应,比如非特异性地干扰RNA和DNA的合成。
5-FU是进展期结直肠癌标准治疗方案的基本药物之一,但是治疗肿瘤的有效率往往不尽人意。
此外,用药的选择也不明确,例如联合生物调节剂特别是亚叶酸钙类(LV)等。
5-FU/LV联合用药可应用在很多肿瘤领域,但是目前联合奥沙利铂或伊立替康是治疗进展期结直肠癌的标准治疗方案。
但是一些5-FU为基础的治疗方案由于毒性持续存在等问题,而迫切需要5-FU的替代物单药或联合化疗。
标准5-FU/LV方案是一线治疗进展期结直肠癌的基础方案,而雷替曲塞是替代药物。
4个大型的III期临床研究结果显示,雷替曲塞的中位总生存期在9.7-10.7个月,而5-FU/LV在10.0-12.7个月,没有统计学差异;总有效率两种方案相似。
四个临床试验中有两个雷替曲塞治疗的PFS明显短于5-FU/LV,但是总生存期没有差异。
雷替曲塞用于腹腔镜结直肠癌根治术中腹腔灌注化疗的安全性分析

雷替曲塞用于腹腔镜结直肠癌根治术中腹腔灌注化疗的安全性分析1. 引言1.1 背景介绍雷替曲塞是一种新型的抑制肿瘤血管生成的药物,在肿瘤治疗中展现出了较好的疗效。
在腹腔镜结直肠癌根治术中,结合雷替曲塞进行腹腔灌注化疗可以有效杀灭残留的肿瘤细胞,进而降低复发率,提高患者的生存率。
腹腔灌注化疗的安全性一直是一个备受关注的问题。
本研究旨在探讨雷替曲塞在腹腔镜结直肠癌根治术中的应用,分析腹腔灌注化疗的作用机制,评估雷替曲塞在腹腔灌注化疗中的安全性,为临床治疗提供更为科学的依据。
愿通过本研究的开展,为结直肠癌的治疗和预后带来新的突破。
1.2 研究目的参考文献的引用格式等。
本研究的目的是探讨雷替曲塞用于腹腔镜结直肠癌根治术中腹腔灌注化疗的安全性。
具体包括评估雷替曲塞在结直肠癌手术中腹腔灌注化疗的安全性和有效性,分析雷替曲塞对手术患者的不良反应及并发症,为临床医生提供更安全有效的治疗方案,降低手术并发症的发生率,提高手术成功率。
本研究旨在为进一步明确雷替曲塞在结直肠癌根治术中的作用机制提供实验依据,为临床实践提供科学依据,为将来的研究提供参考。
通过本研究的开展,希望为临床医生提供更加全面的治疗方案,提高患者的生存率和生活质量,对结直肠癌的治疗提供新的思路和方法。
1.3 研究意义本研究的意义在于深入探讨雷替曲塞在腹腔镜结直肠癌根治术中腹腔灌注化疗的安全性,为临床医生提供更多治疗选择和决策依据。
通过对雷替曲塞在腹腔镜手术中的应用、腹腔灌注化疗的作用机制以及安全性分析等方面的研究,可以更好地评估该治疗方法的疗效和安全性,为患者的治疗提供更加科学、有效的方案。
本研究也有助于推动相关领域的学术研究和临床实践,为进一步完善结直肠癌治疗方案和提高患者生存率贡献力量。
通过本研究的开展,可以为临床医生提供更多选择和决策依据,促进结直肠癌治疗的进步,为患者提供更好的治疗效果和生存质量。
2. 正文2.1 雷替曲塞在腹腔镜结直肠癌根治术中的应用雷替曲塞是一种新型的抗肿瘤药物,已在腹腔镜结直肠癌根治术中得到广泛应用。
雷替曲塞二线治疗晚期结直肠癌临床护理体会

当使 用 思 密达 及 漱 口液 等 对 症 药 物 , 密 切 观 察 腹 泻 的 次 数 及性质 , 有无发热及感染 , 必 要 时 使 用 抗 生 素 。 适 当 使 用 降 温食品 , 减低 口腔 温 度 , 可 减 少 黏 膜 炎 的发 作 或 症 状 。注 意
~
3 0 mi n , 与 氟尿 嘧 啶 持 续 静 点 相 比 , 用 药时 间短 , 刺 激 血
管轻 , 静脉炎发生率低 、 程度轻 , 患者的耐受性 好 , 可 减 少 药
评价 1 次, 直 到疾 病进展 或 毒性不 能耐 受 , 最多 4 ~ 6个 周 期 。按 实 体 瘤 的 疗 效 评 价 标 准 : 完全 缓解 ( C R) 、 部 分 缓 解
止 吐药 物 ( 包 括 静 脉 及 口服 药 物 ) 可 明 显 减 轻 相 关 症 状 。 对
1 . 1 临床资料 : 本组 3 O例 , 男性 1 8例 , 女性 1 2 例, 年龄 4 7
~
8 6 岁, 平均 7 3 . 6 岁, 结肠癌 1 1 例, 直肠癌 1 9例 。 初 治 患
者 9 例, 复治患者 2 1 例 。均 有 明 确 的 病 理 学 诊 断 及 影 像 学
表 现 和 可 观 察 及 测 量 病 灶 。3 O例 患 者 均 接 受 过 F o l { o x或
呕 吐剧 烈 、 难 以进 食 者 应 注 意 补 液 及 电解 质 , 注 意 生 命 体 征
的变 化 , 防 止 脱 水 情 况 出现 。② 腹 泻 及 口腔 黏 膜 炎 : 本 组 发 病率分别 为 2 3 和 4 7 , 绝大多数 均为轻度 , 可 以耐 受 , 适
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
TomudexSummary of Product Characteristics Updated 04-Dec-2017 | Hospira UK Ltd1. Name of the medicinal product'Tomudex'2. Qualitative and quantitative composition'Tomudex' contains 2 mg raltitrexed in each vial.3. Pharmaceutical formPowder for solution for infusion.4. Clinical particulars4.1 Therapeutic indicationsThe palliative treatment of advanced colorectal cancer where 5-Fluorouracil and folinic acid based regimens are either not tolerated or inappropriate.4.2 Posology and method of administrationFor instructions on reconstitution and dilution of the product before administration, see section 6.6 Instructions for use/handling.Adults: - The dose of 'Tomudex' is calculated on the basis of the body surface area. The recommended dose is 3 mg/m2 given intravenously, as a single short, intravenous infusion in 50 to 250 ml of either 0.9% sodium chloride solution or 5% dextrose (glucose) solution. It is recommended that the infusion is given over a 15 minute period. Other drugs should not be mixed with 'Tomudex' in the same infusion container. In the absence of toxicity, treatment may be repeated every 3 weeks.Dose escalation above 3 mg/m2 is not recommended, since higher doses have been associated with an increased incidence of life-threatening or fatal toxicity.Prior to the initiation of treatment and before each subsequent treatment a full blood count (including a differential count and platelets), liver transaminases, serum bilirubin and serum creatinine measurements should be performed.The total white cell count should be greater than 4,000/mm3, the neutrophil count greater than 2,000/mm3 and the platelet count greater than 100,000/mm3 prior to treatment. In the event of toxicity the next scheduled dose should be withheld until signs of toxic effects regress. In particular, signs of gastrointestinal toxicity (diarrhoea or mucositis) and haematological toxicity (neutropenia or thrombocytopenia) should have completely resolved before subsequent treatment is allowed. Patients who develop signs of gastrointestinal toxicity should have their full blood counts monitored at least weekly for signs of haematological toxicity.Based on the worst grade of gastrointestinal and haematological toxicity observed on the previous treatment and provided that such toxicity has completely resolved, the following dose reductions are recommended for subsequent treatment:● 25% dose reduction: in patients with WHO grade 3 haematological toxicity (neutropenia or thrombocytopenia) or WHOgrade 2 gastrointestinal toxicity (diarrhoea or mucositis).● 50% dose reduction: in patients with WHO grade 4 haematological toxicity (neutropenia or thrombocytopenia) or WHOgrade 3 gastrointestinal toxicity (diarrhoea or mucositis).Once a dose reduction has been made, all subsequent doses should be given at the reduced dose.Treatment should be discontinued in the event of any WHO grade 4 gastrointestinal toxicity (diarrhoea or mucositis) or in the event of a WHO grade 3 gastrointestinal toxicity associated with WHO grade 4 haematological toxicity. Patients with such toxicity should be managed promptly with standard supportive care measures including i.v. hydration and bone marrow support. In addition, preclinical data suggest that consideration should be given to the administration ofleucovorin (folinic acid). From clinical experience with other antifolates, leucovorin may be given at a dose of 25 mg/m2i.v. every 6 hours until the resolution of symptoms. Further use of 'Tomudex' in such patients is not recommended.It is essential that the dose reduction scheme should be adhered to since the potential for life threatening and fatal toxicity increases if the dose is not reduced or treatment not stopped as appropriate.Elderly: - Dosage and administration as for adults. However, 'Tomudex' should be used with caution in elderly patients (see section 4.4 Special warnings and precautions for use).Children: - 'Tomudex' is not recommended for use in children as safety and efficacy have not been established in this group of patients.Renal impairment:- For patients with abnormal serum creatinine, before the first or any subsequent treatment, a creatinine clearance should be performed or calculated.For patients with a normal serum creatinine when the serum creatinine may not correlate well with the creatinine clearance due to factors such as age or weight loss, the same procedure should be followed. If creatinine clearance is≤65 ml/min, the following dose modifications are recommended:Dose modification in the presence of renal impairmentCreatinine Clearance Dose as % of 3.0 mg/m2Dosing Interval> 65 ml/min Full dose3-weekly55 to 65 ml/min75%4-weekly25 to 54 ml/min50%4-weekly< 25 ml/min No therapy Not applicableSee Contraindications for use in patients with severe renal impairmentHepatic Impairment:- No dosage adjustment is recommended for patients with mild to moderate hepatic impairment. However, given that a proportion of the drug is excreted via the faecal route, (see section 5.2 Pharmacokinetic Properties) and that these patients usually form a poor prognosis group, patients with mild to moderate hepatic impairment need to be treated with caution (see section 4.4 Special warnings and special precautions for use).'Tomudex' has not been studied in patients with severe hepatic impairment, clinical jaundice or decompensated liver disease and its use in such patients is not recommended.4.3 Contraindications'Tomudex' should not be used in pregnant women, in women who may become pregnant during treatment or women who are breast feeding. Pregnancy should be excluded before treatment with 'Tomudex' is commenced. (see section 4.6 Pregnancy and lactation).'Tomudex' is contraindicated in patients with severe renal impairment (creatinine clearance < 25ml/min).Administration of leucovorin (folinic acid), folic acid or vitamin preparations containing these agents with 'Tomudex' is contraindicated (see section 4.5 Interaction with other medicinal products and other forms of interaction).4.4 Special warnings and precautions for use'Tomudex' must only given by or under the supervision of a physician who is experienced in cancer chemotherapy, and in the management of chemotherapy-related toxicity. Patients undergoing therapy should be subject to appropriate supervision so that signs of possible toxic effects or adverse reactions (particularly diarrhoea) may be detected and treated promptly (see section 4.2 Posology and method of administration).In common with other cytotoxic agents of this type, caution is necessary in patients with depressed bone marrow function, poor general condition, or prior radiotherapy.Patients whose disease progressed on previous treatment for advanced disease with 5-Fluorouracil based regimens may also be resistant to the effects of 'Tomudex'.Elderly patients are more vulnerable to the toxic effects of 'Tomudex'. Since renal function tends to decline with age and the plasma clearance of raltitrexed is reduced with renal function impairment, there is a potential for accumulation of raltitrexed in elderly patients. Extreme care should be taken to ensure adequate monitoring of adverse reactions especially signs of gastrointestinal toxicity (diarrhoea or mucositis) and myelosuppression (neutropenia, thrombocytopenia, infection) and dose should be reduced and /or delayed as appropriate. A proportion of the 'Tomudex' is excreted via the faecal route, (see section 5.2 Pharmacokinetic properties) therefore, patients with mild to moderate hepatic impairment should be treated with caution.Treatment with 'Tomudex' in patients with severe hepatic impairment is not recommended.It is recommended that pregnancy should be avoided during treatment and for at least 6 months after cessation of treatment if either partner is receiving 'Tomudex' (see also section 4.6 Pregnancy and lactation).There is no clinical experience with extravasation. However, perivascular tolerance studies in animals did not reveal any significant irritant reaction.'Tomudex' is a cytotoxic agent and should be handled according to normal procedures adopted for such agents (see section 6.6 Instructions for use/handling).4.5 Interaction with other medicinal products and other forms of interactionNo specific clinical drug - drug interaction studies have been conducted in man.Leucovorin (folinic acid), folic acid or vitamin preparations containing these agents must not be given immediately prior to or during administration of 'Tomudex', since they may interfere with its action.Clinical trials evaluating the use of Tomudex in combination with other antitumour therapies are currently ongoing.'Tomudex' is 93% protein bound and while it has the potential to interact with similarly highly protein bound drugs, no displacement interaction with warfarin has been observed in vitro. Data suggest that active tubular secretion may contribute to the renal excretion of raltitrexed, indicating a potential interaction with other actively secreted drugs such as non-steroidal antiinflammatory drugs (NSAIDS). However, a review of the clinical trial safety database did not reveal evidence of clinically significant interaction in patients treated with 'Tomudex' who also received concomitant NSAIDS, warfarin and other commonly prescribed drugs.4.6 Pregnancy and lactationPregnancyPregnancy should be avoided if either partner is receiving 'Tomudex'. It is also recommended that conception should be avoided for at least 6 months after cessation of treatment.'Tomudex' should not be used during pregnancy or in women who may become pregnant during treatment (see section 5.3 Preclinical safety data). Pregnancy should be excluded before treatment with 'Tomudex' is started.Breastfeeding'Tomudex' should not be given to women who are breast feeding.FertilityFertility studies in the rat indicate that 'Tomudex' can cause impairment of male fertility. Fertility returned to normal three months after dosing ceased. 'Tomudex' caused embryolethality and foetal abnormalities in pregnant rats.4.7 Effects on ability to drive and use machines'Tomudex' may cause malaise or asthenia following infusion and the ability to drive/use machinery could be impaired whilst such symptoms continue.4.8 Undesirable effectsAs with other cytotoxic drugs, 'Tomudex' may be associated with certain adverse drug reactions. These mainly include reversible effects on the haemopoietic system, liver enzymes and gastrointestinal tract. Table 1 presents the possible adverse drug reactions occurring with 'Tomudex' treatment.In this section undesirable effects are defined as follows: Very common (≥1/10); common (≥1/100 to <1/10); uncommon (≥1/1,000 to ≤1/100); rare (≥1/10,000 to ≤1/1,000); very rare (≤1/10,000), not known (cannot be estimated from the available data).Table 1: Adverse drug reactions in patients treated with Tomudex for advanced colorectal carcinoma divided by System Organ Class and frequencySystem Organ Class Frequency Adverse drug reactionInfections & infestations Common CellulitisSepsisFlu-like syndrome Blood and lymphatic disorders Very Common Leucopenia (neutropenia in particular) a, bAnaemia aCommon Thrombocytopenia a, b Metabolism and Nutrition Disorders Very Common AnorexiaCommon DehydrationNervous system disorders Common HeadacheHypertonia (usually muscular cramps)Taste perversion Eye disorders Common ConjunctivitisGastrointestinal disorders Very Common Nausea cDiarrhoea d,eVomiting c,eConstipationAbdominal PainCommon StomatitisDyspepsiaMouth ulcerationFrequency unknown Gastrointestinal Bleeding f,gHepato-biliary disorder Common HyperbilirubinemiaSkin & subcutaneous tissue disorders Very Common RashCommon AlopeciaPruritusSweatingUncommon DesquamationCommon ArthralgiaMusculoskeletal, Connective tissue & bonedisordersVery Common Asthenia hGeneral disorders and administration siteconditionsFever hMucositisCommon Peripheral oedemaPainMalaiseInvestigations Very Common AST increased iALT increased iCommon Weight lossAlkaline phosphatase increaseda Leucopenia (neutropenia in particular), anaemia and thrombocytopenia, alone or in combination, are usually mild to moderate and occur in the first or second week after treatment and recover by the third week.b Severe (WHO grade 3 and 4) leucopenia (neutropenia in particular) and thrombocytopenia of WHO grade 4 can occur and may be life-threatening or fatal especially if associated with signs of gastrointestinal toxicity.c Nausea and Vomiting are usually mild (WHO grade 1 and 2), occur usually in the first week following the administration of 'Tomudex', and are responsive to antiemetics.d Diarrhoea is usually mild or moderate (WHO grade 1 and 2) and can occur at any time following the administration of 'Tomudex'. However, severe diarrhoea (WHO grade 3 and 4) can occur, and may be associated with concurrent haematological suppression especially leucopenia (neutropenia in particular). Subsequent treatment may need to be discontinued or dose reduced according to the grade of toxicity (see Section 4.2 Posology and method of administration).e Diarrhoea and vomiting may be severe and if untreated may proceed to dehydration, hypovolaemia and renalimpairmentf from spontaneous reportingg Gastrointestinal bleeding may be associated with mucositis and/or thrombocytopenia.h Asthenia and fever were usually mild to moderate following the first week of administration of 'Tomudex' and reversible.Severe asthenia can occur and may be associated with malaise and a flu-like syndrome.i Increases in AST and ALT have usually been asymptomatic and self-limiting when not associated with progression ofthe underlying malignancy.Reporting of suspected adverse reactionsReporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions viaYellow Card SchemeWebsite: /yellowcard or search for MHRA Yellow Card in the Google Play or Apple App Store4.9 OverdoseThere is no clinically proven antidote available. In the case of inadvertent or accidental administration of an overdose, preclinical data suggest that consideration should be given to the administration of leucovorin. From clinical experience with other antifolates leucovorin may be given at a dose of 25mg/m2 i.v. every 6 hours. As the time interval between 'Tomudex' administration and leucovorin rescue increases, its effectiveness in counteracting toxicity may diminish.The expected manifestations of overdose are likely to be an exaggerated form of the adverse drug reactions anticipated with the administration of the drug. Patients should, therefore, be carefully monitored for signs of gastrointestinal and haematological toxicity. Symptomatic treatment and standard supportive care measures for the management of this toxicity should be applied.5. Pharmacological properties5.1 Pharmacodynamic propertiesRaltitrexed is a folate analogue belonging to the family of anti-metabolites and has potent inhibitory activity against the enzyme thymidylate synthase (TS). Compared to other antimetabolites such as 5-Fluorouracil or methotrexate, raltitrexed acts as a direct and specific TS inhibitor. TS is a key enzyme in the de novo synthesis of thymidine triphosphate (TTP), a nucleotide required exclusively for deoxyribonucleic acid (DNA) synthesis. Inhibition of TS leads to DNA fragmentation and cell death. Raltitrexed is transported into cells via a reduced folate carrier (RFC) and is then extensively polyglutamated by the enzyme folyl polyglutamate synthetase (FPGS) to polyglutamate forms that are retained in cells and are even more potent inhibitors of TS. Raltitrexed polyglutamation enhances TS inhibitory potency and increases the duration of TS inhibition in cells which may improve antitumour activity. Polyglutamation could also contribute to increased toxicity due to drug retention in normal tissues.In clinical trials, 'Tomudex' at the dose of 3mg/m2 i.v. every 3 weeks has demonstrated clinical antitumour activity with an acceptable toxicity profile in patients with advanced colorectal cancer.Four large clinical trials have been conducted with 'Tomudex' in advanced colorectal cancer. Of the three comparative trials, two showed no statistical difference between 'Tomudex' and the combination of 5-fluorouracil plus folinic acid for survival while one trial showed a statistically significant difference in favour of the combination of 5-fluorouracil plus folinic acid. 'Tomudex' as a single agent was as effective as the combination of 5-fluorouracil and folinic acid in terms of objective response rate in all trials.5.2 Pharmacokinetic propertiesFollowing intravenous administration at 3.0 mg/m2, the concentration-time profile in patients was triphasic: Peak concentrations, found at the end of the infusion, were followed by a rapid initial decline in concentration. This was followed by a slow elimination phase. The key pharmacokinetic parameters are presented below:Summary of mean pharmacokinetic parameters in patients administered 3.0 mg/m2 Raltitrexed byintravenous infusionC m ax (ng/ml)AUC o-∞(ng.h/ml)CL(ml/min)CL r(ml/min)V ss(l)t1/2β(h)t1/2γ(h)656185651.625.1548 1.79198 Key:C m ax: Peak plasma concentration.AUC: Area under plasma concentration-time curve.CL: Clearance.V ss: Volume of distribution at steady state. t ½γ: Terminal half life.CL r: Renal clearancet½β: Half life of the second (β) phase.The maximum concentrations of raltitrexed increased linearly with dose over the clinical dose range tested.During repeated administration at three week intervals, there was no clinically significant plasma accumulation of raltitrexed in patients with normal renal function.Apart from the expected intracellular polyglutamation, raltitrexed was not metabolised and was excreted unchanged, mainly in the urine, 40 - 50%. Raltitrexed was also excreted in the faeces with approximately 15% of the radioactive dose being eliminated over a 10 day period. In the [14C] - raltitrexed trial approximately half of the radiolabel was not recovered during the study period. This suggests that a proportion of the raltitrexed dose is retained within tissues, perhaps as raltitrexed polyglutamates, beyond the end of the measurement period (29 days). Trace levels of radiolabel were detected in red blood cells on Day 29.Raltitrexed pharmacokinetics are independent of age and gender. Pharmacokinetics have not been evaluated in children.Mild to moderate hepatic impairment led to a small reduction in plasma clearance of less than 25%.Mild to moderate renal impairment (creatinine clearance of 25 to 65 ml/min) led to a significant reduction (approximately 50%) in raltitrexed plasma clearance.5.3 Preclinical safety dataPerivascular tolerance in studies in animals did not reveal any significant irritant reaction.Acute toxicityThe approximate LD50 values for the mouse and rat are 875-1249 mg/kg and >500 mg/kg respectively. In the mouse, levels of 750 mg/kg and above caused death by general intoxication.Chronic toxicityIn one month continuous and six month intermittent dosing studies in the rat, toxicity was related entirely to the cytotoxic nature of the drug. Principal target organs were the gastrointestinal tract, bone marrow and the testes. In similar studies in the dog, cumulative dose levels similar to that used clinically, elicited only pharmacologically-related changes to proliferating tissue. Target organs in the dog were therefore similar to the rat.Mutagenicity'Tomudex' was not mutagenic in the Ames test or in supplementary tests using E. coli or Chinese hamster ovary cells.'Tomudex' caused increased levels of chromosome damage in an in vitro assay of human lymphocytes. This effect was ameliorated by the addition of thymidine, thus confirming it to be due to the anti-metabolic nature of the drug. An in vivo micronucleus study in the rat indicated that at cytotoxic dose levels, 'Tomudex' is capable of causing chromosome damage in the bone marrow.Reproductive toxicologyFertility studies in the rat indicate that 'Tomudex' can cause impairment of male fertility. Fertility returned to normal three months after dosing ceased. 'Tomudex' caused embryolethality and foetal abnormalities in pregnant rats.CarcinogenicityThe carcinogenic potential of 'Tomudex' has not been evaluated.6. Pharmaceutical particulars6.1 List of excipientsMannitol Ph Eur, USPDibasic sodium phosphate (heptahydrate USP or dodecahydrate Ph Eur)Sodium hydroxide Ph Eur, USNF6.2 IncompatibilitiesThere is no information on incompatibilities at present and therefore 'Tomudex' should not be mixed with any other drug.6.3 Shelf lifeThe expiry life of 'Tomudex' is 36 months when stored below 25°C, protected from light.Once reconstituted, 'Tomudex' is chemically stable for 24 hours at 25°C exposed to ambient light. For storagerecommendation, see Instructions for Use/Handling.6.4 Special precautions for storageAddressHorizon, Honey Lane, Hurley, Maidenhead, SL6 6RJ, UK WWWMedical Information Direct LineUnopened vial - Do not store above 25°C. Keep container in the outer carton.Reconstituted vial - Refrigerate at 2-8°C.6.5 Nature and contents of container'Tomudex' is packed in 5ml clear neutral type I glass vials, with a bromobutyl rubber closure and an aluminium crimp seal with a plastic flip-off cover.The vials are packed in individual cartons to protect the product from light.6.6 Special precautions for disposal and other handlingEach vial, containing 2mg of raltitrexed, should be reconstituted with 4ml of sterile water for injections to produce a 0.5 mg/ml solution.The appropriate dose of solution is diluted in 50 - 250 ml of either 0.9% sodium chloride or 5% glucose (dextrose) injection and administered by a short intravenous infusion over a period of 15 minutes.There is no preservative or bacteriostatic agent present in 'Tomudex' or the materials specified for reconstitution or dilution. 'Tomudex' must therefore be reconstituted and diluted under aseptic conditions and it is recommended that solutions of 'Tomudex' should be used as soon as possible. Reconstituted 'Tomudex' solution may be stored refrigerated(2 - 8°C) for up to 24 hours.In accordance with established guidelines, when diluted in 0.9% sodium chloride or 5% glucose (dextrose) solution, it is recommended that administration of the admixed solution should commence as soon as possible after admixing. The admixed solution must be completely used or discarded within 24 hours of reconstitution of 'Tomudex' intravenous injection.Reconstituted and diluted solutions do not need to be protected from light.Do not store partially used vials or admixed solutions for future patient use.Any unused injection or reconstituted solution should be discarded in a suitable manner for cytotoxics.'Tomudex' should be reconstituted for injection by trained personnel in a designated area for the reconstitution of cytotoxic agents. Cytotoxic preparations such as 'Tomudex' should not be handled by pregnant women.Reconstitution should normally be carried out in a partial containment facility with extraction e.g. a laminar air flow cabinet, and work surfaces should be covered with disposable plastic-backed absorbent paper.Appropriate protective clothing, including normal surgical disposable gloves and goggles, should be worn. In case of contact with skin, immediately wash thoroughly with water. For splashes in the eyes irrigate with clean water, holding the eyelids apart, for at least 10 minutes. Seek medical attention.Any spillages should be cleared up using standard procedures.Waste material should be disposed of by incineration in a manner consistent with the handling of cytotoxic agents.7. Marketing authorisation holderHospira UK LimitedHorizon, Honey Lane, HurleyMaidenhead,SL6 6RJUnited Kingdom8. Marketing authorisation number(s)PL 04515/02259. Date of first authorisation/renewal of the authorisation25th June 200010. Date of revision of the text11/2017Ref: gxTM 3_1Company Contact DetailsHospira UK Ltd+44 (0)1304 616161 Fax+44 (0)800 098 8653Medical Information Fax+44 (0)1304 656221。
