Strobilurin类杀菌剂作用靶标研究进展
简析氨基甲酸酯类杀菌剂
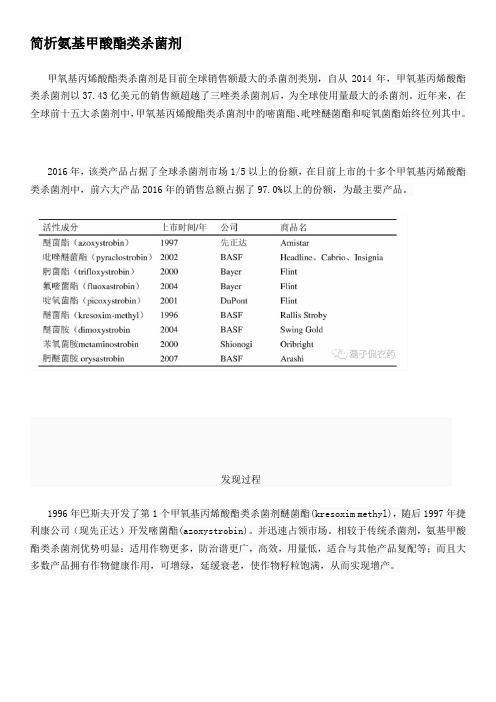
简析氨基甲酸酯类杀菌剂甲氧基丙烯酸酯类杀菌剂是目前全球销售额最大的杀菌剂类别,自从2014年,甲氧基丙烯酸酯类杀菌剂以37.43亿美元的销售额超越了三唑类杀菌剂后,为全球使用量最大的杀菌剂。
近年来,在全球前十五大杀菌剂中,甲氧基丙烯酸酯类杀菌剂中的嘧菌酯、吡唑醚菌酯和啶氧菌酯始终位列其中。
2016年,该类产品占据了全球杀菌剂市场1/5以上的份额,在目前上市的十多个甲氧基丙烯酸酯类杀菌剂中,前六大产品2016年的销售总额占据了97.0%以上的份额,为最主要产品。
发现过程1996年巴斯夫开发了第1个甲氧基丙烯酸酯类杀菌剂醚菌酯(kresoxim methyl),随后1997年捷利康公司(现先正达)开发嘧菌酯(azoxystrobin)。
并迅速占领市场。
相较于传统杀菌剂,氨基甲酸酯类杀菌剂优势明显:适用作物更多,防治谱更广,高效,用量低,适合与其他产品复配等;而且大多数产品拥有作物健康作用,可增绿,延缓衰老,使作物籽粒饱满,从而实现增产。
2000年,一些类似物进入市场,著名的药剂是诺华公司开发的肟菌酯(trifloxystrobin)。
但不久因诺华与捷利康合并成立先正达,基于反垄断的需要,肟菌酯被剥离给拜耳。
先正达公司授权杜邦公司开发啶氧菌酯和杀虫剂氯虫苯甲酰胺混剂。
2002年,巴斯夫公司开发吡唑醚菌酯(pyraclostrobin),代号BAS。
2004年巴斯夫公司又推出醚菌胺(dimoxystrobin),拜耳公司推出氟嘧菌酯(fluoxastrobin),均用于谷物。
2007年巴斯夫公司推出的肟醚菌胺(orysastrobin)。
住友化学2016年上市的mandestrobin是最近上市的甲氧基丙烯酸酯类杀菌剂。
作用机理甲氧基丙烯酸酯类杀菌剂是基于天然抗生素strobilurin A为先导化合物开发的新型杀菌剂,是能量生成抑制剂。
它的作用机理是通过与病原菌细胞线粒体中cytb和C1复合体Oo部位的结合而抑制线粒体的电子传递,从而阻断细胞色素与细胞色素之间的电子传递(阻断的氧化),进而抑制线粒体呼吸作用,使得线粒体无法并供给细胞正常代谢所需能量(ATP)。
Strobilurins-new fungicides for crop protection(strobilurin类杀菌剂综述)

Strobilurins––newfungicides forcrop protection Kresoxim-methyl1.IntroductionSince time immemorial,fungi have played an important part in the history of mankind,for example in the production of foodstuffs such as cheese,bread dough,and soy sauce or in alcoholic fermentation processes.In some Asian and Amer-ican cultures fungi have been used by cults as the source of hallucinogens.Also when it comes to delicacies,the fruiting bodies of fungi(to the layman the actual,that is,visible, manifestations of fungi)have been valued since olden times, none more so than the truffles prized by gourmets.However, the importance of fungi as medicines was traditionally rather low compared with that of plants.This situation remained basically unchanged until the groundbreaking discovery of penicillin by Alexander Fleming in1928and its development as the first highly active antibiotic obtained from a mold. Since then the importance of fungi as sources of antibiotics and other pharmacologically active natural products has become ever more evident.[1]2.The Natural Strobilurins and Oudemansins2.1.BackgroundStrobilurins were first identified within the framework of a program begun in late1976aimed at discovering new antibiotic agents from basidiomycetes.This class of higher fungi,which includes many familiar species such as fly agaric and edible boletus,was in those days spurned by natural product researchers.The often sluggish growth of mycelial cultures of basidiomycetes meant they were regarded as being more difficult to handle than the extremely active substance-rich streptomycetes,which are classified as bacteria.Never-theless,studies of the pigments and toxins of the fruiting bodies[2]promised an original and diverse secondary metab-olism in mycelial cultures too.In their work on the formation of polyacetylenes in basidiomycetes,Jones and Thaller had drawn parallels between the secondary metabolism of basi-diomycetes and that of higher plants,notably Apiaceae andStrobilurins:Evolution of a New Class of Active SubstancesHubert Sauter,*Wolfgang Steglich,and Timm AnkeDedicated to Professor Hans-Jürgen Quadbeck-Seeger on the occasion of his60thbirthday[*]Dr.H.SauterBASF Aktiengesellschaft HauptlaboratoriumForschung FungizideD-67056Ludwigshafen(Germany)Fax:( 49)621-60-21156E-mail:hubert.sauter@basf-ag.deProf.Dr.W.SteglichInstitut für Organische Chemie der UniversitätButenandt Strasse5±13/F,D-81377München(Germany)Prof.Dr.T.AnkeFachbereich Biologie,Lehrbereich BiotechnologiePaul-Ehrlich-Strasse,Gebäude23D-67663Kaiserslautern(Germany)REVIEWSREVIEWS H.Sauter et al.Asteraceae.[3]This followed from the pioneering work by the groups of Anchel,Kavanagh,and Hervey,[4]who in the1940s and1950s had screened hundreds of extracts of fruiting bodies,and in some cases of corresponding fungal mycelial cultures too,for the formation of antibiotics.A pivotal discovery to emerge from their studies was pleuromutilin,a new antibacterial antibiotic with an interesting site of action,[4b]which was launched some years later by Sandoz as the semisynthetic derivative tiamulin for use as a veterinary medicine.[5]All of this,plus the congenial research environ-ment offered by the Sonderforschungsbereich in Tübingen headed by ZähnerªChemical Biology of Microorganismsº, was the spur for one of us(T.A.)to commence work on basidiomycetes.Among the first antibiotics discovered were strobilurins A and B,obtained from fermentations of Strobi-lurus tenacellus(a fungus that grows on pinecones).[6]Up till then no natural products had been isolated from this fungus. Our first publication in1977[6]reported the physical and chemical data of strobilurins A and B as well as their powerful antibiotic activity against a range of fungal species.The high cytotoxic activity towards Ehrlich ascites tumor cells led Douros of the National Cancer Institute(USA)to ask us later the same year to provide samples of the strobilurins to screen for antitumor activity.The first117mg of strobilurin A were dispatched in1978.Soon afterwards the exciting news came that strobilurin A,although exhibiting only very weak anti-tumor activity,showed no acute toxicity in the tumor-bearing mice.The structure elucidation by the research group of one of us (W.S.)showed the strobilurins to have an unusually simple structure and apparently represent a new class of antifungal antibiotics whose abundance in nature was not confined to the genus Strobilurus.[7]Strobilurin A has also been isolated from Cyphellopsis cultures and from two Mycena species.This compound was originally thought to have a side chain with an all-trans9E configuration,but this was later(1984)revised to 9Z.[8]Strobilurin A was found to show great similarity to mucidin,an antifungal antibiotic published in1969[9]and patented in1970[10]without a proposed structure;they had the same empirical formula,the same UV spectrum,and equal activity.However,the optical activity reported for mucidin ( 338(c 10)at546nm)completely ruled out a common identity with the strobilurin A.Mucidin,which was isolated from Oudemansiella mucida(a fungus that grows on the trunks of beech trees),found use in Czechoslovakia as an antifungal ointment(MuciderminªSpofaº).To be more certain that strobilurin A and mucidin were different com-pounds,samples of O.mucida were collected as starter material for mycelial cultures,which were then investigated for the formation of antifungal antibiotics.To our surprise we did not detect mucidin in these cultures,but instead a novel natural product,the crystalline,optically active oudeman-sin A,and the oily strobilurin A.[11]Like strobilurins A and B, oudemansin A also exhibited powerful antifungal activity. The quest to establish which compound corresponded to ªmucidinºproved to be highly protracted.Czech patents and publications yielded contradictory information.One pat-ent,[12]citing previous patents of the same origin,[10]cor-rectly described the structure as(9Z)-strobilurin A.No reference was made in this work to the optical rotationH.Sauter W.Steglich T.AnkeREVIEWS Strobilurinspreviously reported for mucidin.[10]Then in1981with reference to the earlier patents,[10,12]among them also the patent stating the correct9Z configuration,Sedmera et al. confusingly reported the identity of mucidin as that of(9E)-strobilurin A.[13]The optical rotation reported in the Czech patent[10b]was referred to as aªprinting errorº.Final clarification was ultimately furnished in a1986paper,[14]in which a direct spectroscopic comparison established the structures of(9Z)-strobilurin A and mucidin to be one and the same.2.2.Abundance in FungiFungi that produce strobilurins and oudemansins are found all over the world in all climate zones.[17b]With one exception (Bolinea lutea,an ascomycete)all such species are basidio-mycetes.[15]Some of the structures(Scheme1)are extremely complex,as in the case of strobilurin E,which is characterized by high antifungal activity and very high cytostatic activity.[16] The structures of the9-methoxystrobilurins K and L,stro-bilurin D,and hydroxystrobilurin D have since been re-vised.[17]The antibacterial activity of9-methoxystrobilurin L published by the company Xenova has,like the proposed structure,since been found to be erroneous.[18]2.3.Function of the Natural Products for the Producers In the search for new active substances,in the group of one of us(T.A.)basidiomycetes are normally grown on complex, highly nutrient rich media to ensure good mycelial growth and high production of secondary metabolites.However,because these conditions in no way correspond to those of their natural substrates,such as soil,leaves,or(most commonly)wood, certain strobilurin producers were also grown on their natural, presterilized substrates and then investigated for the forma-tion of antibiotics.Formation of both oudemansin A and strobilurins was observed in a Pterula species,a tropical Favolaschia species,and in Mycena tintinnabulum.[19]In Mycena tintinnabulum strobilurin D was detected both in the fruiting bodies(caps)and in the mycelially colonized substrate(oak wood)in nature.The concentrations deter-mined in the mycelially colonized oak wood were sufficient to inhibit the growth of other fungal species.This finding and the worldwide distribution of strobilurins and oudemansins leads us to conclude that the production of these compounds affords an advantage in terms of survival over other parasitic fungal species or fungi colonizing the same substrate.But how do the fungi that produce strobilurins or oude-mansins protect themselves against their own antifungal toxins,which are often produced in considerable quantities? We investigated this matter in collaboration with the group ofScheme1.The natural strobilurins and oudemansins[17b,66]and myxothiazole A.[21a]The sterocenters whose configurations are unknown are marked with an asterisk.REVIEWSH.Sauter et al.Brand and von Jagow,and found that in Strobilurus tenacellus the site of action in the cytochrome b protein (see Section 2.4)is modified such that the binding of strobilurins and oude-mansins becomes much more difficult.This is essentially due to the substitution of a small amino acid (alanine or threonine)in position 127by a larger isoleucine residue.This resistance-inducing exchange occurs in a region that is highly preserved in the species sensitive to strobilurin-type toxins.In S.tenacellus an additional small amino acid in position 254is replaced by glutamine,with the asparagine in position 261being replaced by aspartic acid.All these amino acid residues are located in the binding envelope of the reduced coen-zyme Q.[20]2.4.Mode of ActionStudies on the mode of action of strobilurins and oude-mansins revealed very early on that these compounds inhibit the respiration of fungi.Investigations with Ehrlich ascites carcinoma cell cultures showed that the site of action lay exclusively in the respiratory chain (Figure 1).OthereffectsFigure 1.The site of action of strobilurins and oudemansins in the mitochondrial respiratory chain.such as the complete inhibition of protein,RNA,and DNA synthesis were attributed by one of us (T.A.)to the resulting intracellular deficiency in ATP .The oxygen uptake and ATP synthesis of rat liver mitochondria were inhibited with both a -ketoglutarate and succinate as substrates;hence the site of action had to be in either complex III or IV of the respiratory chain.[11]A fortunate turn of events led us to find in von Jagow a collaboration partner of the utmost competence,who succeeded in localizing fairly exactly the site of action of the strobilurins and oudemansins in the mitochondrial bc 1com-plex.In our first joint publication we reported that strobilur-ins A and B,oudemansin A,and myxothiazole,a compound isolated by the groups of Reichenbach and Höfle at the Gesellschaft für Biotechnologische Forschung (Society for Biotechnological Research)in Braunschweig,[21a]all bind at the same site of action.[21b]A common structural element of these compounds that is clearly essential to their mode of action is an (E )-b -methoxyacrylate subunit.Strobilurins,oudemansins,and myxothiazole bind reversibly to the ubihy-droquinone oxidation (Qp)center of the bc 1complex,a finding that has helped further our understanding of the structure and function of this part of the respiratory chain.[22]Antimycin,an antifungal antibiotic commonly found in streptomycetes that is toxic to mammals,binds to the other center of the bc 1complex,the Qn center.The crystal structure of the bc 1complex from bovine heart tissue was recently solved by the Deisenhofer group,and the binding sites for myxothiazole and antimycin A were identified.[23]The first patent application for strobilurin A was submitted in collaboration with Hoechst [26]and described the synthesis of strobilurin A,which seemed like it might find use as an antimycotic agent in human medicine.However,the com-pound compared poorly with other antimycotics on the market,and this patent was later relinquished.Several total syntheses of the natural strobilurins and oudemansins have been described and discussed in review articles.[17b,24]3.Lead Structure Optimization in Rival Industrial Research Programs3.1.First Structural VariantsWith their rare combination of favorable molecular and biological properties,the two natural products strobilurin A and oudemansin A were immediately seized upon as candi-dates for model compounds on which to base further synthetic modifications.[24]A clear attraction was the simplicity of their structures,which is a rarity in bioactive natural products.Moreover,the presence of the b -methoxyacrylate group in both natural products gave us a clue that these were not merely solitary active substances in isolation,but were instead part of a group of active compounds.Secondly,their antifungal activity in laboratory tests proved to be attractively high,which together with their ªnovelºmode of actionÐinhibition of respiration combined with low toxicity towards mammalian cellsÐtherefore gave us a powerful boost.Consequently,syntheses of molecular variants of strobilur-in A were underway as early as 1977in the research group of one of us (W.S.).The goal was easily accessible analogues with antibiotic activity that was similar or enhanced as far as possible.[25]The b -methoxyacrylate group,whose heteroatoms are capable of intermolecular,polar interactions,was regarded as essential (i.e.,as the pharmacophore)and was initially modified merely for the usual comparison purposes.The other regions of the molecule were judged to hold greater promise for structural variation.Such considerations gave rise not only to simple phenyl derivatives such as 1±3,but also to norstrobilurin A (5)and the ªshortenedºanalogue 6(Scheme 2).[24,25]Although such compounds showed a lower level of activity than the naturally occurring model compound in laboratory tests,some did show detectable,albeit weak,activity against Penicillium notatum as a representative fungal species both inREVIEWSStrobilurinsScheme 2.Structure and activity of simple strobilurin analogues.Activities were determined on the basis of the inhibition of respiration of and the agar diffusion test with Penicillium notatum .[24,25]testing for inhibition of respiration and in the agar diffusion test.From these results we were able to deduce some initial important structure ±activity relationships.[24,25]The E con-figuration of the pharmacophore as in 1is essential,as the Z isomer 2was inactive.Substituents in the aryl ring such as in 3appeared to be ªallowedº;even a C/N exchange in the enol ether group,which provided the oxime ether 4,did not result in any appreciable reduction in activity.A loss of activity was observed for the more linear (9E )-norstrobilurin A (5)and in the similarly shaped variant 6;lacking in both compounds is the characteristic conformation of strobilurin A and oude-mansin A,in which twisting of the central single bond results in an almost right-angled orientation of the b -methoxyacry-late pharmacophore relative to the phenyl-substituted side chain (Figure 2).In the variants 1±4the side chain is missing completely.In parallel to these variations of the lead structure,collaborations were sought with industrial partners with the aim of probing the usefulness of this class of active substances for the optimization of structure and activity under the viewpoint of potential applications as agricultural fungicides or clinical antimycotics.The response from industry to our overtures was,owing to other more pressing priorities,for the most part low-key.In 1982Zeneca (then still part of ICI)requested a sample of oudemansin,which they obtained from one of us (T.A.).Interest shown by BASF in 1983within the framework of a publicly funded joint project [27]in collabo-ration with several university research groups led to a sample of strobilurin A being provided for in vivo greenhouse testing on plants infected with fungal pathogens.The test results were disappointing:The activity of strobilurin A wassurprisinglyFigure puter-generated space-filling and stick models of strobil-urin A (left)and the enol ether stilbene 7a (right).Carbon:black,oxygen:red,hydrogen:white.(We are grateful to Dr.W.Altenhofen,BASF,for the calculations and the computer graphics.)weak,being barely perceptible against only two out of six phytopathogenic fungal species at high application concen-trations.[28]This was contrary to expectations after the very good results in agar diffusion tests in the laboratory,and the question was posed as to what might be the possible causes.Together with Schirmer,who at the time was responsible at BASF crop-protection research for collaboration with uni-versity research teams,we arrived at the hypothesis that the triene system of strobilurin A might have been the cause of a rapid photolytic and/or oxidative breakdown of the natural product,leading to a rapid loss of activity under the conditions of the greenhouse tests.The apparent plausibility of this hypothesis led us to dispense for the time being with verification through analytical experimentation.A different approach appeared more attractive:The idea we came up with was to stabilize the triene system with an aromatic bridge and,in the hope of improved in vivo activity,to synthesize and carry out tests on the ªenol ether stilbeneº7a .The synthesis was swiftly accomplished in the Steglich research group (Scheme 3).[29a]As it turned out,we hit the bull s-eye with 7a .Not only did it exhibit an activity against P .notatum in the inhibition of respiration test that was at least ten times higher than that of strobilurin A,[29b,c]its potency and spectrum of activity in in vivo greenhouse tests in plants infected with fungal pathogens fulfilled our ambitious expectations.Good (`15%infection)to very good activities (`5%infection)at application concentrations of 63ppm,and in some cases even at 16ppm,were recorded for the following fungal diseases:powdery mildew of wheat (Erysiphe graminis ),wheat brown rust (Puccinia recondita ),rice blast disease (PyriculariaREVIEWS H.Sauter etal.Scheme3.Synthesis of the enol ether stilbene7a.[24,29a]AIBN 2,2'-azobisisobutyronitrile,NBS N-bromosuccinimide.oryzae),barley net blotch(Drechslera teres),grapevine downy mildew(Plasmopara viticola),and late blight(Phytophthora infestans).[30]The bridging by the central arene subunit in the enol ether stilbene7a did not result in a reduction of affinity for the mitochondrial target,it was if anything even increased,and clearly bestowed on the moleculeÐas hopedÐthe required stability.Thus,compared with strobilurin A,7a represented a modified,more potent,and overall more promising lead structure.3.2.A Further Generation of Strobilurin VariantsGuided by this result,and with BASF broadening their involvement in the project,we turned our attention to the synthesis of some further variants of7,for example8±12 (Scheme4).In laboratory tests these compounds all showed activities comparable to that of strobilurin A,and exceeded this distinctly in the greenhouse tests.Some of the new derivatives exhibited even higher activity than the enol ether stilbene7a,depending on the test fungus.[30]In the design of strobilurin variants a conceptual subdivi-sion of the enol ether stilbene lead structure into three molecular regions proved useful:side chain,arene bridge,and pharmacophore(Scheme5).Through variation of the side chain we succeeded in obtaining compounds of type7,[31]8,[32] 9and10,[33]and11,[34]each the subject of separate patent applications.3.3.Initial Field Trials at BASFThe next step was the biological evaluation of preselected candidates under outdoor conditions.In the course of optimization of a lead structure the transition from green-house tests to field trials demands particularly close attention. Three aspects are of prime importance here:*The quantities of active substance required,and hence the outlay on their synthesis,rise dramatically.For in vitro testing and other laboratory tests a few milligrams are normally sufficient.Even for an extensive series of dilutions in the greenhouse tests the amount of active material required is only about100mg in total.Thousands of individual structural variants can be tested per year as a matter of routine without undue difficulty.In contrast,with application of100±1000g haÀ1,preliminary field trialsÐnormally in small plots of one square meterÐrequire between1and10g per indication depending on the plant species,fungal pathogen, and number of treatments,that is,up to100g of each active substance must be supplied.Additional amounts of active substance are also required for the development of formula-tions suitable for application under outdoor conditions,for example as emulsion concentrates,suspension concentrates, or water-dispersible powders orgranules.Scheme4.Strobilurin variants based on the enol ether stilbene7a.[24,31±34]Scheme5.Structurally modified regions of the enol ether stilbene lead structure.REVIEWS Strobilurins*For these and other practical reasons the number of compounds that can be tested under outdoor conditions is restricted to barely more than one hundred per year.This means that compounds that fail to make it to the field trial stage are essentially dropped as individual candidates in the optimization process.*Years of experience have shown the model tests in the greenhouse to be of only limited value as a predictor of the all-important activities in field trials under real-life conditions. Clearly discrepancies[35a]are possible in the sense of false positive and false negative greenhouse results,[35b]particularly when an attempt is made to assess the order of potency within individual families of active compounds.[35c,36]This special situation is evident from the values shown in the bottom line of Table1.Here,the rank correlation coefficients[39]between the field trial and greenhouse test results range from usable values(0.74for powdery mildew of wheat,0.6for late blight) to values of little prognostic usefulness(for exampleÀ0.3for net blotch disease).In this respect the selection of theªrightºcandidates for the field trials,which due to seasonal growing cycles are only possible at certain times of the year,[37]becomes the critical rate-determining step,and hence also the decisive step for the overall success[36]of the lead structure optimization process (see Figure4,Section4.4).Of the roughly100strobilurin derivatives synthesized up to December1985at BASF,a first batch of15compounds was selected for small plot trials in the1986season and tested in various indications according to the results obtained in the earlier greenhouse tests.Table1summarizes these com-pounds and the results obtained.[38]In field trials against powdery mildew of wheat a clear disparity is evident between the good results with the four dihydrostilbenes8and the lack of activity of the stilbenes7. This seems plausible in view of the known photolability of stilbenes,which can undergo E3Z isomerization and sub-sequent facile photocyclization to dihydrophenanthrenes followed by further reactions such as oxidative aromatiza-tion.[40]Overall the rank correlation with the greenhouse test results of the family of compounds tested is good(r s 0.74), though the inferiority of the stilbenes7in the greenhouse tests is not so pronounced as under the more severe photochemical conditions in the field trials.Particularly noteworthy was the activity of8d against powdery mildew of wheat in field trials. The activity of8d at maximum infection pressure(100% infection in the untreated plots),albeit at higher rates of application(1000g),exceeded that of the standard reference substance,CorbelÐa most encouraging result.In tests against net blotch disease even the three stilbenes7 tested here proved to have some activity at a lower infection pressure(35%).By contrast,the sole dihydrostilbene tested, 8d,again showed distinctly higher activity,exceedingÐalbeit at double the rate of applicationÐthe level of the standard Desmel.In tests against glume blotch on wheat8d,the front runner against powdery mildew of wheat,again showed high activity, this time,however,closely followed by stilbene7e.The tests against wheat brown rust were not especially fruitful,owing to the particularly low infection pressure(18%)and the relatively weak activity of the five dihydrostilbenes tested. In tests against late blight at a high infection pressure,the dihydrostilbene8a proved to be the best,although two of the stilbenes also showed measurable activity.In the case of activity against apple scab stilbene7a was in first place and on the level of the standard.In summary,from a structure±activity viewpoint the photolabile stilbenes were distinctly inferior to the dihydrostilbenes under outdoor conditions.The former did,however,show some activity in tests in which the interval between the application of the active substance and the evaluation of the test was relatively brief(applications against glume blotch,late blight,and apple scab),that is 8days compared with about30days in the other tests.For the structure types9and10only a single example of each underwent field trials,hence a full,experimentally-based assessment of these structure types was not possible at that time. The first results from these small-plot trials under outdoor conditions,which were of decisive importance to the assess-ment of the overall structure type,were obtained in May1986. Even at this early stage some results were highly encouraging, for example the first indication that the activities of8a,8b, and8d were very good and of the same order as that of the standard reference material Corbel.In addition,initial results from tests against net blotch disease in barley,for example after treatment with8b,gave rise to high expectations and much euphoria.Not only had our good fortune seemingly seen us through the birth of this new class of fungicidal active substances,we were able to look ahead to the further development of the baby full of hope and confidence.In the midst of our elation at this breakthrough we came face to face with a most unpleasant surprise.At the end of April1986a comprehensive patent application had been published by ICI(now Zeneca),[41]which was brought to our attention a short time later.The first priority date was October19,1984.[42]The basic formula I of what was an extremely broad and general claim is shown in Scheme6.R1 and R2represent a wide variety of substituents ranging from hydrogen toªoptionally substitutedºalkyl,aryl,cycloalkyl-alkyl,and other groups.[43]Even richer in variants were the definitions for X,Y,and Z.On the other hand,the actual core element of these structures was defined in subclaim7[41] (formula XII in Scheme6).Additional subclaims were sub-mitted for structures of type13.As individual compounds ªourºenol ether stilbene7a and the hydrogenated variant8a that had already attracted attention in our field trials,had been the subjects of specific claims.What was striking were the parallels with our ideas and concepts that lay at the heart of this application.All the BASF applications for the enol ether strobilurins[31±34]were hit by this patentÐa disaster that set us back all the more at the time,as our field trial results had led us to the brink of verifying the great potential of the strobilurins,not to mention their potential practical applica-tions.Back in the laboratory we had some hard questions to ask ourselves.How could such a thing had happened?Had we been caught napping?Had indiscretions,espionage even, played a part?The answer was soon in coming and was plain to see:All this had taken place in the context of a fair contest between rival research groups.。
农用杀菌剂作用靶标的研究进展

农用杀菌剂作用靶标的研究进展
朱家鑫;姜明宏;刘根炎
【期刊名称】《化学与生物工程》
【年(卷),期】2024(41)5
【摘要】农用杀菌剂作为防治植物病害的主要手段,如今已发展出多个作用靶标。
从抑制病原微生物结构的合成、抑制病原微生物的呼吸作用、抑制病原微生物代谢物质的合成等3种作用机制综述了农用杀菌剂作用靶标的研究进展,为设计和开发新型作用机制的农用杀菌剂提供了思路。
【总页数】10页(P9-18)
【作者】朱家鑫;姜明宏;刘根炎
【作者单位】武汉工程大学化工与制药学院绿色化工过程教育部重点实验室新型反应器与绿色化学工艺湖北省重点实验室
【正文语种】中文
【中图分类】TQ455
【相关文献】
1.Strobilurin类杀菌剂作用靶标的研究进展
2.农用杀菌剂作用机理的研究进展
3.甲氧基丙烯酸酯类杀菌剂特异性靶标及其研究进展
4.重要内吸性杀菌剂的作用靶标及其登记应用和抗药性现状
因版权原因,仅展示原文概要,查看原文内容请购买。
世界杀菌剂新品种的开发进展及发展趋势

世界杀菌剂新品种的开发进展及发展趋势2006-12-13世界需要粮食,农业需要农药.要保证农作物的增产丰收,除杀虫、除草、灭鼠外,对病害的防治也是重要手段.杀菌剂与杀虫剂和除草剂相比,其市场额和品种相对较少,并且杀菌剂市场波动较大.但是,80年代以来,世界杀菌剂新品种的开发仍取得很大进展,如三唑类、酰胺类、嘧啶胺类、甲氧基丙烯酸酯类等.现将近20年来世界杀菌剂新品种的开发进展及发展趋势介绍如下:一、开发进展及特点1. 三唑类自1973年拜耳公司推出第一个商品化具有手性碳的杀菌剂三唑酮之后,三唑类杀菌剂的发展特别引人注目.其发展之快,数量之多,是以往任何杀菌剂所无法比拟的.目前,这类杀菌剂已有约40个品种商品化,其中近年来开发的品种有7个.近期开发的化合物特点是除对白粉病、锈病、黑星病等有活性外,对网斑病、灰霉病、眼纹病等多种病害亦有很好的活性,持效期长.另一特点是与常用的三唑类杀菌剂相比分子结构变化较大,且大多含氟.环氧菌唑对一系列禾谷类作物病害如立枯病、白粉病、眼纹病等十多种病害有很好的防治作用,不仅具有很好的保护、治疗和铲除活性,而且具有内吸和较佳的残留活性,使用剂量为75~125g/hm2.氟喹唑主要用于防治由担子菌钢、半知菌类和子囊菌纲真菌引起的多种病害,可有效地防治苹果上的主要病害如苹果黑病和苹果白粉病,对白粉病菌、链核盘菌、尾孢霉属、茎点霉属、壳针孢属、埋核盘菌属、柄锈菌属、驼孢锈菌属和核盘菌属等真菌引起的病害均有良好的防治效果.使用剂量为100~400g/hm2.意大利Isagro公司开发的氟醚唑属第二代三唑类杀菌剂,具有优良的广谱活性,持效期长达4~6周,使用剂量低,通常为25~100g/hm2.硅氟唑是由日本三共化学公司开发的含硅、含氟三唑类杀菌剂,具有很广的杀菌谱,其对子囊菌类、担子菌类及众多不完全菌类均有很高的抗菌活性.使用剂量为50~100g/hm2,商品名为Mongazit、Patchikoron、Sanlit.羟菌唑是由美国氰胺公司开发的一种新型、广谱内吸性杀菌剂,兼具优良的保护及治疗作用,其作用机理虽与其它三唑类杀菌剂一样,但活性谱则差别较大.主要用于禾谷作物防治矮形锈病、叶锈病、黄锈病、冠锈病、白粉病、颖枯病以及壳针孢、穗镰刀菌等引起的病害.既可茎叶处理又可作种子处理,商品名为Caramba.茎叶处理30~90g/hm2,持效期5~6周.种子处理:~7.5g/100kg种子.罗纳普朗克公司开发的环菌唑对种传病害有特效.主要用于防治禾谷类、玉米、豆科、果树等作物中镰孢酶属、柄锈菌属、麦类核腔菌属、黑粉菌属、腥黑粉菌属、白粉菌属、圆核腔菌、壳针孢属、柱隔孢属等引起的病害如白粉病、锈病、黑星病、网斑病、灰霉病等.可种子处理、也可茎叶喷雾,持效期长达4~6周.种子处理时用量为2.5g/100kg种子,茎叶喷雾时用量为60g/hm2.从化学结构上看,环菌唑加氢即得羟菌唑.丙硫菌唑是由拜耳作物科学公司研制的新型广谱三唑硫酮类杀菌剂,几乎对所有麦类病害都有很好的防效,还能防治油菜和花生的土传病害以及主要叶面病害.使用剂量为200g/hm2,在此剂量下,活性优于或等于常规杀菌剂如氟环唑、戊唑醇、嘧菌环胺等,且对作物具有良好的安全性,商品名为Proline、Input.三唑类杀菌剂与其他内吸性杀菌剂具有不同的作用机制,它通过阻碍真菌麦角甾醇的生物合成而影响真菌细胞壁的形成,对危害作物生长的多数真菌病害均有良好防治效果.多数三唑类杀菌剂具有高效、广谱、长效、强内吸性以及立体选择性等活性特点.三唑类杀菌剂同时还具有一定的植物生长调节活性如多效唑、抑芽唑和烯效唑等,它通过抑制植物体内赤霉素的合成,消除植物顶端优势,具有增产、早熟、抗倒、抗逆等多种功能.另一方面,三唑类杀菌剂是内吸治疗型杀菌剂,作用机制和作用位点单一,长期频繁的使用,病害已产生了较严重的抗药性,不少品种由于抗性问题已失去了原有的高效性.如三唑酮防治草莓白粉病,用量少防效低,用量大则易产生药害,抑制草莓生长,导致减产.此外,三唑类杀菌剂只对真菌起作用,对细菌及病毒无活性.植物病害往往是多种病害同时发生,因此使用三唑类杀菌剂需要配合其它杀菌剂或防病毒剂才能有良好的综合防效.近年来,三唑类杀菌剂由于自身的抗性和活性问题已开始受到strobilurin类杀菌剂的强烈冲击,但这类杀菌剂在世界农药工业中仍占有重要地位,如戊唑醇、氟硅唑和丙环唑1999年的销售额分别达到、和亿美元,戊唑醇和环氧菌唑2002年的销售额分别为和亿美元.2. 酰胺类杀菌剂酰胺类化合物作为杀菌剂已有几十年的历史,至今已有30多个品种商品化,其中80年代以后开发的占一半以上.下面主要介绍近年来开发的新品种.罗门哈斯公司开发的噻氟酰胺是琥珀酸酯脱氢酶抑制剂,即在菌三羧酸循环中抑制琥珀酸酯脱氢酶的合成.对丝核菌属、柄锈菌属、黑粉菌属、腥黑粉菌属、伏革菌属和核腔菌属等致病真菌有活性.对担子菌纲真菌引起的病害如立枯病等有特效.既可用于水稻、禾谷类作物和草坪等的茎叶处理使用剂量为125~250g/hm2,又可用于禾谷类作物和非禾谷类作物拌种处理7~30g/100kg种子,商品名为Greatam、Pulsor、Beton.日本拜耳公司开发的环丙酰菌胺是一种环丙烷羧酰胺内吸性杀菌剂,其作用机理与现有杀菌剂不同,无杀菌活性,不抑制病原菌丝的生长,以预防为主,治疗活性较弱.主要用于稻田防治稻瘟病,用药量为75~400g/hm2,商品名为Win、Winadmire、Solazas、Arcado、Protega.环酰菌胺是拜耳公司开发的另一个保护性杀菌剂,由于具有良好的环境相容性,对授粉昆虫和动物无毒害作用,已被美国环保局划为减少危害农药.该品种主要用于防治葡萄、桔柑、桃树、草莓和蔬菜等作物上的各种灰霉病及念株菌引起的病害,且与已有杀菌剂苯并咪唑类、酰亚胺类、三唑类、嘧啶胺类、N-苯基氨基甲酸酯类等无交互抗性.用药量为370~1000g/hm2,商品名为Teldor、Password、Elevate.呋吡菌胺是日本住友化学公司开发的吡唑酰胺类杀菌剂.其抑制真菌线粒体中的琥珀酸的氧化作用,从而避免立枯丝核菌丝体分离,而对真菌线粒体还原型烟酰胺腺嘌呤二核苷酸NADH的氧化作用无影响,其具有优异的预防治疗效果,对担子菌纲的大多数病菌绢病等有特效.大田防治水稻纹枯病的剂量为450~600g/hm2,商品名为Limber.噻唑菌胺是由韩国LG生命科学公司开发的新型噻唑酰胺类杀菌剂,能有效地抑制马铃薯晚疫病菌菌丝体的生长和孢子的形成,主要用于防治卵菌纲病害,使用剂量为200~250g/hm2,它的可湿性粉剂25%WP已在韩国上市,商品名为Guardian.硅噻菌胺是由孟山都公司开发的含硅的噻酚酰胺类杀菌剂.具体作用机理尚不清楚,与三唑类、甲氧基丙烯酸酯类的作用机理不同,研究表明其是能量抑制剂,可能是ATP抑制剂.具有良好的保护活性,残效期长.主要作种子处理,用于小麦全蚀病的防治,使用剂量为5~40g/kg种子.氰菌胺是由日本农药株式会社与巴斯夫公司共同研制开发的新颖内吸性杀菌剂,属于黑色素生物合成抑制剂,对水稻稻瘟病防效优异,且持效期较长.茎叶处理用量为200~400g/hm2,灌施剂量为2100~2800g/hm2,商品名为Achieve、Achi-Bu、Helmet.此外,住友化学公司开发的双氯氰菌胺、安万特公司开发的氟酰菌胺、捷利康公司开发的环啶菌胺、三井化学公司开发的penthiopyrad等品种也属于酰胺类杀菌剂.酰胺类杀菌剂的作用机理比较复杂,许多品种之间互不相同.酰胺类杀菌剂在世界杀菌剂市场中仍占有相当重要的地位.如甲霜灵、恶霜灵、苯霜灵和甲呋酰胺等苯酰胺类杀菌剂中,仅高效甲霜灵2002的销售额就达到亿美元.它们作为防治霜霉目真菌的专用药剂,具有显着的保护、治疗和铲除作用,广泛应用于马铃薯和番茄晚疫病的防治.然而,由于苯酰胺类杀菌剂对病菌作用位点单一只对卵菌类有高效,一旦作用位点发生突变,药剂即不能在其位点发挥作用,因而导致病菌易产生抗药性.据报道,由于抗药性产生而导致药效降低的事例已屡见不鲜.但同时也应该看到,近年来一些具有独特作用机理的酰胺类杀菌剂新品种的开发成功,使这类杀菌剂呈现出美好的发展前景.3. 嘧啶胺类嘧啶胺类化合物是90年代初开发的一类重要杀菌剂,对灰葡萄孢菌所致的各种病害有特效.目前有4个品种商品化:甲基嘧菌胺、嘧菌胺、环丙嘧菌胺和氟嘧菌胺.艾格福公司开发的甲基嘧菌胺具有保护、叶片穿透及根部内吸活性,在田间药效试验中,对葡萄、草霉、番茄、洋葱、菜豆、豌豆、黄瓜、茄子及观赏作物的灰霉病以及苹果黑星病有优异的防效,使用剂量为200~800g/hm2.日本组合化学工业公司和石原化学工业公司共同开发的嘧菌胺对苹果和梨上黑星病菌,黄瓜、葡萄、草莓和番茄上的灰葡萄孢菌有很好的防效,使用剂量为~1.0kg/hm2,商品名为Frupica.诺华公司开发的环丙嘧菌胺主要用于大麦、小麦、葡萄、草莓、果树、蔬菜、观赏作物等防治灰霉病、白粉病、黑星病、网斑病、颖枯病以及小麦眼纹病等.叶面喷雾或种子处理,也可作大麦种衣剂用药.日本宇部兴产公司和日产公司共同开发的氟嘧菌胺主要用于防治小麦、大麦和观赏作物的白粉病和锈病等.嘧啶胺类杀菌剂的作用机制独特,该类药剂在离体条件下对病菌的抗菌性很弱,但用于寄主植物上却表现很好的防治效果,该类药剂能抑制病菌甲硫氨酸的生物合成和细胞壁降解酶的分泌,从而影响病菌侵入寄主植物.如甲基嘧菌胺和嘧菌胺的作用机理是抑制病原菌蛋白质分泌,包括降低一些水解酶水平,据推测这些酶与病原菌进入寄主植物并引起寄主组织的坏死有关.环丙嘧菌胺是蛋氨酸生物合成的抑制剂,同三唑类、咪唑类、吗啉类、二羧酰亚类、苯基吡咯类杀菌剂无交互抗性,对敏感或抗性病原菌均有优异的活性.4. 甲氧基丙烯酸酯类甲氧基丙烯酸酯strobilurin类杀菌剂来源于具有杀菌活性的天然抗生素strobilurin A,自1969年Mugikek等发现其杀菌活性.经过二十多年的结构优化,终使此类杀菌剂开发成功,在杀菌剂开发史上树立了继三唑类杀菌剂之后又一个新的里程碑.strobilurin类杀菌剂首例上市时间为1996年,到目前为止已有8个品种商品化:嘧菌酯、醚菌酯、肟菌酯、苯氧菌胺、啶氧菌酯、唑菌胺酯、氟嘧菌酯和烯肟菌酯.捷利康公司开发的嘧菌酯是第一个商品化的甲氧基丙烯酸酯类杀菌剂,高效、广谱,对几乎所有的真菌钢子囊菌纲、担子菌纲、卵菌纲和半知菌类病害如白粉病、锈病、颖枯病、网斑病、霜霉病、稻瘟病等均有良好的活性.可用于茎叶喷雾、种子处理,也可进行土壤处理,主要用于谷物、水稻、花生、葡萄、马铃薯、果树、蔬菜、咖啡、草坪等.使用剂量为25~400g/hm2,商品名为Abound、Amistar、Heritage、Quadris、Admire.巴斯夫公司开发的醚菌酯具有广谱、持效期长等特点,主要用于蔬菜、小麦、水稻、马铃薯、苹果、梨、南瓜、葡萄、棉花及观赏植物等,对子囊菌纲、担子菌纲、半知菌类和卵菌纲等致病真菌引起的大多数病害都有良好的活性.使用剂量为50~400g/hm2,商品名为Discus、Candit、Allegro、Mentor、Stroby、Cygnus、Sovran.诺华公司开发的肟菌酯不仅杀菌谱广,而且具有优良的保护、治疗、渗透活性,耐雨水冲刷,持效期长等特性.除对白粉病、叶斑病有特效外,对锈病、霜霉病、立枯病、苹果黑星病有良好的活性.主要用于麦类作物小麦、大麦、黑麦和黑小麦及葡萄、苹果、花生、香蕉、蔬菜、水稻等,使用剂量为50~200g/hm2,商品名为Flint、Compass、Stratego、Swifh、Zest、Sphere.日本盐野义制药公司开发的苯氧菌胺具有广谱的杀菌活性.除对稻瘟病有特效外,对白粉病、霜霉病等亦有良好的活性.适宜作物如水稻、小麦、果树和蔬菜等,使用剂量为150~200g/hm2,商品名为Oribright.啶氧菌酯是Zeneca公司继嘧菌酯之后,开发的又一个strobilurin类杀菌剂,具有良好的保护及治疗活性,且持效期长,对环境友好、安全.主要用于防治小麦、大麦、燕麦及黑麦中的叶面病害如叶枯病、叶锈病、颖枯病、褐斑病、白粉病等,与现有strobilurin 类杀菌剂相比,对小麦叶枯病、网斑病和云纹病有更强的治疗效果.该化合物既具有木质内吸性又具有蒸发活性,因而施药后,有效成份能有效再分配及充分传递.使用剂量为250g/hm2,商品名Acanto.唑菌胺酯是BASF公司以N-对氯苯基吡唑基替换了醚菌酯分子结构中的邻甲基苯基,而开发的又一甲氧基丙烯酸酯类广谱杀菌剂.通过叶面喷洒,它能控制子襄菌纲、担纲菌纲、半知菌纲、卵菌纲等大多数病害.对孢子萌发及叶内菌丝体的生长有很强的抑制作用,具有保护和治疗活性.具有渗透性及局部内吸活性,持效期长,耐雨水冲刷.被广泛用于小麦、水稻、花生、葡萄、蔬菜、香蕉、柠檬及草坪的病害防治,用于农作物的使用剂量为50~250g/hm2,用于草坪的剂量为280~560g200g200g恶咪唑类恶咪唑类杀菌剂是目前国外公司研究开发的热点之一,有三个品种报道:商品化的恶唑菌酮和氰唑磺菌胺以及在开发中的咪唑菌酮.恶唑菌酮是由杜邦公司开发的新型恶唑啉二酮类、高效、广谱杀菌剂.具有保护、治疗、铲除、渗透、内吸活性,主要用于防治果树、蔬菜、禾谷类作物中的重要病害如白粉病、锈病、颖枯病、网斑病、霜霉病、晚疫病等.商品名为Equation、Famoxate、Charisma、Tanos.氰唑磺菌胺是由日本石原产业化学公司开发的新型咪唑类杀菌剂.是细胞色素bc1中Qi抑制剂,不同于β-甲氧基丙烯酸酯是细胞色素bc1中Qo抑制剂.对卵菌所有生长阶段均有作用.可用于马铃薯、葡萄、番茄、蔬菜黄瓜、白菜、洋葱、莴苣、草坪中防治霜霉病、疫病如黄瓜霜霉病、葡萄霜霉病、番茄晚疫病、马铃薯晚疫病等.具有很好的保护活性,持效期长,且耐雨水冲刷.即可用于茎叶处理,也可用于土壤处理防治草坪和白菜病害,商品名为Ranman、Docious、Mildicut.咪唑菌酮是由安万特作物科学公司开发的新型咪唑酮类杀菌剂.具有触杀、渗透、内吸活性,又有良好的保护和治疗活性.除对卵菌纲类真菌引起的霜霉病、疫病包括早疫病和晚疫病等有良好的活性外,对果树黑斑病亦有很好的活性.主要用于莴苣、葡萄、马铃薯、西红柿等作物,使用剂量为75~150g/hm2,商品名为Reason、Fenomen、Sereno、Sagaie.恶咪唑类杀菌剂与苯基酰胺类杀菌剂如甲霜灵无交互抗性,均是线粒体呼吸抑制剂,但不同于β-甲氧基丙烯酸酯类杀菌剂.6. 吡咯类吡咯类杀菌剂来源于天然产物硝吡咯菌素,是非内吸性的广谱菌剂,对灰霉病有特效.主要品种有两个:拌种咯和氟咯菌腈,均由瑞士诺华公司开发.拌种咯和氟咯菌腈的活性谱相似,前者主要作种子处理用,后者既可作为叶面杀菌剂,也可作为种子处理剂,且活性高于前者.适宜作物如小麦、大麦、玉米、豌豆、油菜、水稻、观赏作物、硬果、蔬菜、葡萄和草坪等.作为叶面杀菌剂用于防治雪腐镰孢菌、小麦网腥黑腐菌、立枯病菌等,对灰霉病有特效;作为种子处理剂:主要用于谷物和非谷物类作物中防治种传和土传病菌如链格孢属、壳二孢属、曲霉属、镰孢菌属、长蠕孢属、丝核菌属及青霉属菌等.吡咯类杀菌剂的作用机理是通过抑制葡萄糖磷酰化有关的转移,并抑制真菌菌丝体的生长,最终导致病菌死亡.因其作用机理独特,故与现有杀菌剂无交互抗性.7.氨基酸类氨基酸类杀菌剂因其对人类、环境安全,目前亦是世界农药公司研究的热点之一,已有二个品种商品化.苯噻菌胺是日本组合化学公司开发的新型氨基酸类杀菌剂,主要用于葡萄、马铃薯、蔬菜等防治霜霉病、疫病等,使用剂量为25~75g/hm2.拜耳公司开发的异丙菌胺主要用于葡萄、马铃薯、番茄、黄瓜、柑枯、烟草等作物中防治霜霉病、疫病等.其既可用于茎叶处理,也可用于土壤处理防治土传病害.使用剂量为100~300g/hm2.具体的作用机理尚不清楚,研究表明其影响氨基酸的代谢,且与已知杀菌剂作用机理不同,与甲霜灵、霜脲氰等无交互抗性.它是通过抑制孢子囊胚芽管的生长、菌丝体的生长和芽孢形成而发挥对作物的保护、治疗作用.8. 肉桂酸衍生物早在1970年Staples等已报道肉桂酸衍生物3,4-二甲氧基肉桂酸甲酯具有杀菌活性,其中顺式cis-异构体在日本作为农药使用,反式几乎没有活性.20世纪80年代Shell公司在此基础上,成功地研制了杀菌剂烯酰吗琳,同样是顺式有活性,但顺反异构体在光照下可以相互转化,总有效体为80%.虽然文献报道烯酰吗啉具有很好的保护和治疗活性,但实际上治疗活性很差.90年代初,刘长令用氟原子取代烯酰吗啉分子中苯环上的氯原子,发现了活性尤其是治疗活性明显优于烯酰吗啉的新杀菌剂氟吗啉,其顺反异构体均有活性.氟吗啉是沈阳化工研究院开发的丙烯酰胺类杀菌剂.是我国有史以来第一个真正创制的农用杀菌剂、是首次获得中国和美国发明专利的农用创制杀菌剂.具有良好的内吸、保护和治疗活性.对卵菌亚纲病原菌引起的病害霜霉病、晚疫病如黄瓜霜霉病、葡萄霜霉病、马铃薯晚疫病、番茄疫病、辣椒疫病、烟草疫病等有优异的活性.施用浓度为50~200mg/L.作为保护剂使用,浓度为50~100mg/L;作为治疗剂使用,浓度100~200mg/L.氟吗啉于1999年11月投产,中试规模为年产原药20吨.现已列为“十五”攻关项目,进一步进行工艺优化研究、制剂与剂型研究、应用和市场推广研究.“十五”攻关完成后,将实现年产氟吗啉原药200吨的规模化生产.除了烯酰吗啉和氟吗啉外,还有很多类似物,但无商品化品种再出现.烯酰吗啉和氟吗啉都属于肉桂酸衍生物,同时其分子结构中均含吗啉环结构,但它们与一般吗啉类杀菌剂十三吗啉、吗菌啉、丁苯吗啉不同.一般吗啉类杀菌剂主要用于防治由大、小麦白粉病、叶锈病和网惺黑穗病等引起的病害,其作用机制基本上都是抑制菌体内麦角甾醇的生物合成;而烯酰吗啉和氟吗啉的作用机制是干扰细胞壁的形成及抑制孢子萌发,对霜霉属、疫霉属等卵菌引起的病害有特效,对麦类白粉病等没有作用效果,说明这两种杀菌剂的主要作用基团并非吗啉环,而是结构中的其它基团发挥作用.9. 其它类其它类品种主要包括:啶菌恶唑、活化酯、螺环菌胺、苯氧喹啉等.啶菌恶唑是沈阳化工研究院开发的另一个新杀菌剂品种,属于甾醇合成抑制剂,具有独特的作用机制和广谱杀菌活性,且同时具有保护治疗作用,有良好的内吸性,通过根部和叶茎吸收能有效控制叶部病害的发生和危害.该化合物对番茄、黄瓜、葡萄灰霉病,小麦、黄瓜白粉病,黄瓜黑星病,水稻稻瘟病等均有良好的防治效果.使用剂量为200~400g/hm2.与苯并咪唑类杀菌剂无交互抗性.活化酯是诺华公司开发的苯并噻二唑羧酸酯类杀菌剂.它是植物抗病活化剂,几乎没有杀菌活性.多种生物因子和非生物因子可激活植物自身的防卫反应即“系统活化抗性”,从而使植物对多种真菌和细菌产生自我保护作用.其可在水稻、小麦、蔬菜、香蕉、烟草等中作为保护剂使用.主要用于预防白粉病、锈病、霜霉病等,使用剂量为12~30g/hm2,商品名为Bion、Unix Bion.螺环菌胺是拜耳公司开发的甾醇生物合成抑制剂,主要抑制C-14脱甲基化酶的合成.它是一种新型、内吸性的叶面杀菌剂,主要用于防治小麦白粉病和各种锈病;大麦云纹病和条纹病,对白粉病特别有效.作用速度快且持效期长,兼具保护和治疗作用.使用剂量为500~750g/hm2.苯氧喹啉是道农业科学公司开发的喹啉类内吸性杀菌剂.它是一个保护性杀菌剂,没有治疗作用,因此必须在可见症状出现前使用.该杀菌剂对谷物类、葡萄、蛇麻和樱桃等作物的白粉病及灰霉病和稻瘟病防治有特效,叶面施药后,药剂可迅速地渗入到植株组织中,并向顶转移,持效期长达70d.使用剂量为125~250g/hm2,商品名为Fortress、Legend、Arius、Helios.二、发展趋势农作物能否健康生长,除受虫、草害影响外,对病害的防治亦很重要.随着环保观念的加强和可持发展战略的实施,高效、低毒、高活性、低残留已成为农药发展的必然趋势.展望21世纪的杀菌剂工业,将呈现以下特点:1. 作用机理独特、广谱高效的杀菌剂已成为国际上近期的开发重点近年来国外开发的杀菌剂品种主要是内吸性及选择性较好的,大多具有杂环结构,有些引入氟原子以增加杀菌活性.特别是作用机理独特、广谱高效的杀菌剂已成为国际上近期的开发重点,总体有三个方向:①针对病原菌抗性开发的新型杀菌剂,如乙霉威对多菌灵产生抗性的病害灰霉病有特效;②以天然产物为先导化合物开发的具有独特作用机理的新型杀菌剂,如吡咯类和丙烯酸酯类杀菌剂等不仅活性高,且与已知杀菌剂无交互抗性;③为增强作物自身对病害免疫能力的植物激活剂是近年来发展的,具有全新作用机理的一类新颖农药,如新一代植物防病激活剂活化酯具有“系统自动抗病性”.2. 非内吸性杀菌剂在国内外市场上仍将占据较大份额由于内吸性杀菌剂作用点较单一,病原菌的繁殖速度较快,因此抗性产生较快.同除草剂、杀虫剂相比,内吸性杀菌剂的寿命较短;又由于短时期内农业上的转基因技术对杀菌剂工业影响最小对除草剂工业影响最大,因此,新杀菌剂的创制研究显得尤为重要.预计新型的作用机理独特,与现有杀菌剂无交互抗性的内吸广谱杀菌剂的应用会逐渐扩大.但从长远看,由于硫制剂、铜制剂、代森锰锌和百菌清等非内吸性杀菌剂具有成本低、广谱和不易产生抗性的特点,它们在市场上仍将经久不衰,并占据较大份额,如代森锰锌、硫磺和百菌清2002年的销售额分别为、和亿美元.此外,在病害防治中,内吸和非内吸杀菌剂的混用制剂将会占据主力位置,植物活化剂的使用量亦将上升.。
甲氧基丙烯酸酯类杀菌剂

甲氧基丙烯酸酯类杀菌剂(国外称Strobilurin)是一种仿生杀菌剂,是继苯并咪唑和三唑类之后的一个里程碑式的农用杀菌剂,经过近20年的发展,成为一类非常重要的杀菌剂,在上亿美金销售额的杀菌剂中占有多个就是实证。
这类杀菌剂的先导化合物:嗜球果伞素A(S trobilurin A)和嗜球果伞素B(Strobilurin B)最早是由德国IBWF的T.Anke和Steglich 教授于1977年首次从嗜球果伞(Strobilurus tenacellus,也有译作附胞球果菌)培养液中分离得到的。
这个IBWF(Institute of Biotechnology and Drug Research)就是德国生物技术药物研究所,位于德国凯泽斯劳腾(Kaiserslautern,Germany)大学内。
而实际上,这个Strobilurin A与Musikek等人1969年从霉状小奥德蘑(Oudemansiella mucida)中分离得到的Mucidin极其相似,这个Mucidin具有抗真菌活性。
strobilurin A与mucidin的红外光谱、紫外光谱以及元素组成一致,而旋光不同。
随后Anke等人为了搞清这二者是否为同一物质,进一步研究小奥德蘑(Oudemansiella mucida)并分离到了strobilurin A 外,还得到了结晶状的小奥德蘑素1981年Sedmera等发表了mucidin的结构,将mucidin的构型定为E, E, E 。
而Beck er等人则首次报道了strobilurin A与strobilurin B、oudemansin A结构相似,而且它们的杀菌活性均源于同样的作用机制:通过阻碍细胞色素b和c1这间的电子传递来抑制线粒体呼吸。
1984年Anke和Steglich确定了strobilurin A的立体构型为E, Z, E 。
直到1986年,将mucidin和strobilurin A直接对比才证实了两者的一致性。
甲氧基丙烯酸酯类杀菌剂发展与应用

2.3 吡唑醚菌酯
吡唑醚菌酯 250g/L悬浮 2
剂(巴斯夫、广
东德利)
唑醚·代森 60%WG (巴 2
联
斯夫、广东德利)
烯酰·吡唑 酯
18.7%WG 2
(巴斯夫、广东德 利)
黄瓜白粉病、霜 霉病,白菜炭疽 病、西瓜炭疽病 等
番茄早疫病、晚 疫病等
马铃薯早疫病、 晚疫病、黄瓜霜 霉病、甜瓜霜霉 病
G143A
苹果链格孢 斑点病
苹果
美国 G143A
茄链格孢 早疫病
马铃薯
美国
F129L(苯丙氨 酸突变为亮氨 酸)
抗性情况 高抗
>4347 中抗
3
4
禾本科布氏 白粉菌
白粉病
小麦和大 麦
欧盟
G143A
小麦:欧洲北部和西部法国 南部高抗;匈牙利,奥地利,
捷克和波兰中抗或抵抗
大麦:法国中部、北部,德 国北部,英国和爱尔兰高抗 ;德国其他地区中抵抗;捷 克和波兰仅发现局部地区有 抗性;澳大利亚,匈牙利, 意大利,丹麦和瑞典无抗性
嘧菌酯种子处理剂能够提高种子发芽率及植株生长。 • 2007-2017年在加拿大安大略湖附近,利用嘧菌酯和咯菌腈/
精甲霜灵复配制剂(1g/100Kg+6.25 g/100Kg Seed)作为种 子处理用于防治菜豆炭疽病。 • 近期咯菌腈和两个Strobilurins类杀菌剂嘧菌酯和肟菌酯被引 入种子处理市场以提高种子处理的效力以及防治包括镰刀 菌在内的多种土传病害。 • 2005-2006年,在美国北达科他州东南部城市法戈利用0.02 g a.i./Kg Seed嘧菌酯作为种子处理防治胶粘萼距花(Cuphea viscosissima Jacq.×C.)猝倒病。其效果稍差于精甲霜灵。 • Strobilurins类杀菌剂嘧菌酯和肟菌酯种子处理剂能够用于防 治由禾谷镰孢菌和腐霉菌引起的玉米和大豆病害。
吡唑醚菌酯研究进展

吡唑醚菌酯的研究及应用进展摘要:综述了吡唑醚菌酯的理化性质、作用方式、毒理学、环境归趋与残留分析,介绍了该杀菌剂的合成化学、应用研究及其开发进展。
关键词:吡唑醚菌酯;杀菌剂;综述Abstract: A review on pyraclostrobin summarized its physical & chemical properties, mode of action, toxicology andenvironmental fate, and introduced its synthetic chemistry, application and development progress.Key words: pyraclostrobin; fungicide; review前言吡唑醚菌酯是兼具吡唑结构的甲氧基丙烯酸酯类杀菌剂,1993年由德国巴斯夫公司继肟菌酯后又开发的此类杀菌剂,农药登记名称及商品名:250克/升吡唑醚菌酯乳油。
2001年登记并上市,目前已用于100 多种作物上[1]。
2009年,其销售额达到7.35 亿美元,仅次于嘧菌酯,成为全球第二大杀菌剂[2]。
吡唑醚菌酯广谱、高效、毒性低,对非靶标生物安全,对使用者和环境均安全友好,是strobilurin 类杀菌剂中市场前景较好、专利即将过期的重要产品。
1理化性质吡唑醚菌酯的分子式Ci9Hi8ClN304,分子量387.1。
化学名称为N- (2- (1- (4-氯苯基)-IH-吡唾-3-基氧甲基〕苯基} (N-甲氧基)氨基酸甲酯。
纯品为白色或灰白色体,熔点为(63.7?65.2)°C;蒸汽压2.6*10Pa (201)、6.4*10Pa (25°C); Henry常数25.3*10Pam3/mol (20°C);分配系数logP=3.99(22C);水中溶解度为1.9nig/L(2(rC),甲醇溶解度为lOOg/L;异丙醇中溶解度是37g/L;正辛醇(24g/L)【2】。
4种常用甲氧基丙烯酸酯类杀菌剂的急性毒性评价

4种常用甲氧基丙烯酸酯类杀菌剂的急性毒性评价谭丽超,峰,程燕,单正军(生态环境部南京环境科学研究所,江苏南京210042)Acute Toxicity of Four Commonly Used Strobilurin FungicidesTan Lichao,P Feng,Cheng Yan,Shan Zhengjun(Nanjing Institute of Environmentai Science, MEE,NaniongJoangtu210042,Chona)Abstracr:The acute toxicity of250g/T azoxystrobin SC,50%kresoxim-metUyi WP,15%pyra-0030(X31SC and22.5%picexystrobin SC i birds,honeybees,simwoRn,fish,dlgae,daphnin and eerthwoRns was detewnined.The results indicated that the four strobiluRn fungicides showed low toxic to bees w ith dll the4acute toxicity LD50(48h)of abova100"g a.d/bee,low toxic to birds with LC50(48h)value(W743,>1000,>1500and>1000mg a.i./kg weight,low toxic to silkwomi with LC50(48h)value of430-28,>2000,31.61and345-48mg a.//T,low toxic to erthwowns with LC50(48h)value of19.84,>100,>100and>100mg a.i./kg soil for250g/L azoxystrobin SC,50%kresoxim-methyl WP,15%pyraclostrobin SC and22.5%picexystrobin SC,aetpecicaey.Modeaaieioiccciy,hcgh ioiccciy,eiiaemeioiccciyand hcgh ioiccciyioocth wcih LC50(48h)value W7.46,0.65,0.051and0.12mg a.i./L,high toxicity,high toxicity,c-treme toxicity and extreme toxicity to daphnin with EC50(48h)value of0.59,0.22,0.067and0.018mg a.i./Z,moderate toxicity,low toxicity,low toxicity and high toxicity to algae with EC50(48h)value W0.66,31-96,5-51and0.036mg a.i./Z were obsewed for250//Z azoxystrobin SC,50%k ae to icm-me ihy oWP,15%pyaacootiaobcn SCand22.5%pccoiytiaobcn SC,aetpec-icaeoy.Ln tummaay,a o iheoouatiaobcouacn oungcccdetthowed oowioiccciyioie a etiacaooaganctmt, buicouod cauteapoienicaoactk ioaquaiccecotytiemt.Key worit:strobiluRn;fungicige;non一tarael oryanisms;acute toxicity收稿日期:2020-9-4基金项目:环境保护部标准项目(农药主态风险评价程序与方法,项目编号:2014-72)。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Logo 特点:
※ 本身对病原菌没有明显的杀菌或抑菌活 性,但能诱发植物自身的免疫系统,抵御病 害侵袭。 ※ 一种抗病激活剂可以防治多种病害,植 物一旦产生系统抗病性,其抗病性具有持久 性和广谱性,甚至可以遗传。 ※ 不会对病原菌产生选择压力,不易产生 抗药性。
Logo
乙膦铝
C2H5O H
O P Al
线粒体 核糖体 纺锤体
Logo
杀菌剂对菌体内能生成的影响
1
2 3
对乙酰辅酶A形成的影响
对三羧酸循环影响 对呼吸链的影响
4
5
对脂质氧化的影响
对氧化磷酸化的影响
Logo
对乙酰辅酶A形成的影响
TPP(硫胺素焦磷酸)在丙酮酸脱 羧过程中起转移乙酰基的作用
TPP+(氧化型) 有机硫类克菌丹破坏TPP+ 抑制乙酰辅酶A的形成
对氧化磷酸化的影响
葡萄糖+O2
ADP+Pi
ATP酶
6CO2+6H2O
ATP+H2O
砷、铜、锡、汞等杀菌剂能直接影响 ATP酶的活性,抑制氧化磷酸化反应
Logo 杀菌剂对代谢物质的生物合成及其功能的影响 抑制RNA的生物合成 3种RNA聚合酶,甲霜灵专化性抑制rRNA的合成 干扰核酸代谢
图1 具有天然杀菌活性的β-甲氧基丙烯酸酯衍生物
D. W. Bartlett Pest Manag Sci 2002, 58, 649-662
S N S
OMe N CONH2 MeO myxothiazol A
粘噻唑
图2 粘噻唑的结构式
K. Gerth J Antibiot. 1980, 33,1474-1479
苯来特 十三吗啉 多氧霉素
稻瘟灵
Logo
Logo
破坏菌体细胞膜
脂质 蛋白质 甾醇 盐类的亚单元 每个亚单元由金 属桥和疏水键
1 2 3
有机硫杀菌剂 与膜上的疏水 键或金属桥结 合,生物膜受 破坏
含重金属的杀 菌剂,作用于 细胞膜上的三 磷酸腺苷水解 有机磷杀 酶的-SH基, 菌剂 改变膜的透性
对细胞膜组分 甾醇的破坏
>50 >50 >50 >50 >50 >50 >50 >50 >50 >50 >50 3.1 6.3 3.1 >50
G. Thierbach Antimicrob. Agents Chemother. 1980, 19, 504-507
哪种代谢途径涉及到粘噻唑的生物合成
表4 粘噻唑与放射性同位素标记的氨基酸的结合
标记化合物 L-(35S)Cysteine hydrochloride L-(U-14C) Isoleucine L-(U-14C) Leucine L-(U-14C) Phenylalanine L-(U-14C) Proline L-(U-14C) Threonine DL-(benzene ring U-14C) Tryptophan L-(U-14C) Tyrosine (U-14C) Acetic acid, sodium salt DL-(2-14C Mevalonic acid 加入100ml培养基 中的放射能(μCi) 380 50 50 10 25 50 4 结合 + + + _ _ + _
Logo
1杀菌剂对细胞壁的影响 几丁质-多抗酶素
细胞壁 真菌 微纤维 纤维素-烯酰吗啉 细菌: 多肽多糖-青霉素
无定形物
Logo
杀菌剂对菌体细胞结构和功能的破坏
杀菌剂对细胞壁的影响
环烃类 咪唑类
UDP-N-乙 酰葡萄糖
穿透 细胞质膜
聚 合
细胞壁 形成
有机磷杀菌剂 破坏膜透性
trifloxystrobin
metominostrobin famoxadone fluoxastrobin fenamidone
肟菌酯
苯氧菌胺 恶唑菌酮 氟嘧菌酯 咪唑菌酮
拜耳
盐野义 杜邦 拜耳 安万特
1998
1993 1996 1994 1998
1999
1999 1997 2004 2001
D. W. Bartlett Pest Manag Sci 2002, 58, 649-662
优点及应用
1. 具有安全、高效、广谱的特性
阿米西达防治谱
作物 小 麦 病害名称 白粉病 叶枯病 颖枯病 锈 病 白粉病 叶锈病 网斑病 云纹病 霜霉病 白粉病 黑星病 黑斑病 作物 瓜类 病害名称 白粉病 霜霉病 蔓枯病 炭疽病 叶枯病 早疫病 炭疽病 斑枯病 白粉病 纹枯病 稻瘟病 胡麻叶斑病 立枯病 锈 叶斑病 病
大 麦 葡 萄 梨
番茄 水稻 花生
轮纹病
优点及应用
2.
3.
具有内吸传导性和扩散性
具有保护、治疗和铲除作用
全程的防护作用
阻止病 菌侵入 杀死或抑制 病菌扩展 抑制病 菌产孢
优点及应用
4.
具有全新的作用机制
阿米西达
三唑类
苯并咪 唑类
无交互抗 性
真菌细胞
三:Strobilurin类 杀菌剂作用靶标 的研究进程
Logo
杀菌剂作用机理
抑菌(内吸) 杀菌 对病菌无毒作用
Logo
杀菌剂的作用原理
杀菌剂对细胞壁的影响
Title
破坏菌体细胞膜 杀菌剂对菌体细胞结构 和功能的破坏 破坏菌体内一些细胞器或 其他细胞结构
Title
杀菌剂对菌体内能生成 的影响 杀菌剂对代谢物质的生 物合成及其功能的影响
Title
G. Thierbach Antimicrob. Agents Chemother. 1980, 19, 504-507
表3 粘噻唑对不同细菌的最小抑制浓度(MIC)
实验菌种 MIC (μg/ml)
Gram-negative Acinetobacter calcoaceticus DSM 30006 Agrobacterium tumefaciens DSM 30150 Alcaligenes eutrophus DSM 531 Escherichia coli DMS 613 Pseudomonas aeruginosa GBF 62 Salmonella typhimurium DSM 50912 Serratia marcescens GBF 61 Gram-prositive Arthrobacter simples ATCC6946 Brevibacterium ammoniagenes DSM 20305 Bacillus megaterium GBF 24 Bacillus subtilis ATCC 6051 Micrococcus luteus GBF 38 Mycobacterium sp. GBF 3 Nocardia coralline ATCC 13258 Staphylococcus aureus GBF 16
Logo
2. 影响寄主—病原物关系的化合物
调节寄主植物抗病性
植物受病原物诱导的产生系统的抗病性能,称作系 统 获 得 性 抗 病 性 ( Systemic Acguired Resistance, SAR) 除病原物外,某些化学药品亦具有类似功能,这类 化学物质就是植物抗病激活剂(Plant Activator) 生物因子、物理因子或化学因子 ↓ 激发寄主受体产生防卫信号 ↓ 激活或诱导植物防卫基因表达
表1 商品化的Strobilurin类杀菌剂品种
杀菌剂 Azoxystrobin picoxystrobin pyraclostrobin Kresoxim-methyl 通用名 嘧菌酯 啶氧菌酯 唑菌胺酯 醚菌酯 结构 公司 先正达 先正达 巴斯夫 巴斯夫 公布时间 1992 2000 2000 1992 首次面市时间 1996 2002 2002 1996
糖有氧氧化途径
三羧酸循环
磷酸戊糖途径
克菌丹
对三羧酸循环影响
福美双 克菌丹 硫磺
乙酰辅酶A 草酰乙酸 柠檬酸
2CO2
有机硫代森类 8-羟基喹啉
苹果酸
克菌丹 异柠檬酸
延胡索酸 硫磺
萎锈灵
a-酮戊二酸
琥珀酸
琥珀酰 辅酶A
对呼吸链的影响
鱼腾酮 安密妥½O2 敌苦双 敌克松 联苯酚 Alternative respiration
Strobilurin类杀菌剂
作用靶标的研究进展
1. Strobilurin类杀菌剂简介 2. Strobilurin类杀菌剂的特性 3. Strobilurin类杀菌剂作用靶标的 研究过程 4. 展望
一:Strobilurin类 杀菌剂简介
发
现
1969年Musikek及其合作者首先报道, Strobilurin A 以来,到目前为止文献报道的已 有30多个此类抗生素,但结构简单的有15个, 其中Strobilurin 类11个,Oudemansin类3个, 另一个是Myxothiazol。
Logo
杀菌剂对糖酵解、有氧氧化和磷酸戊糖三条途径的影响
Cu、Hg、克菌丹、百菌清
糖酵解途径 ATP ADP 葡萄糖 6-磷酸葡萄糖 磷酸己糖 异构酶 6-磷酸果糖
e +2 2H H AD P N A N 2H e +2
乳酸
丙酮酸
乙酰辅酶A
3-磷酸葡萄糖酸
3-磷酸甘油醛
1,3-磷酸甘油醛 百菌清
5-磷酸核酮糖
3
有争议: (1)诱导抗病性 (2)直接对病原菌作用
防治卵菌纲病害 产生酚类和黄酮类抗病物质 激发植物坏死斑反应,是侵染点中有大量的酚 类物质积累。另外斑块部位可以使病菌的进一步发 展受阻。
Logo 苯并噻二唑(BTH)类 气巴-嘉基在开发磺酰脲类除草剂时发现,苯并 噻二唑-7-羧酸甲酯有SAR活性 O C S CH3 S 诺华公司商品名Bion N N 30g/ha对小麦白粉病具有持久性抗性。
