化学专业英语试卷
化学英语试题及答案

一、考点、热点回忆一、固体物质的颜色A 白色固体:氧化钙 CaO 、氢氧化钙Ca(OH)2 、碳酸钠Na 2CO 3、碳酸钙CaCO 3、氢氧化钠NaOH 、B 黄色固体:硫粉SC 红色固体:红磷P 、氧化铁Fe 2O 3、铜CuD 蓝色固体:胆矾E 黑色固体:柴炭C 、氧化铜CuO 、二氧化锰MnO 2、四氧化三铁Fe 3O 4、F 绿色固体:碱式碳酸铜Cu 2(OH)2CO 3G 紫黑色固体:高锰酸钾KMnO 4H 无色固体:冰,干冰,金刚石I 银白色固体:银,铁,镁,铝,汞等金属二、生成沉淀的颜色A 白色沉淀:不溶于水也不溶于稀硝酸:氯化银AgCl 、硫酸钡BaSO 4不溶于水但溶于稀硝酸或稀盐酸:氢氧化镁Mg(OH)2、碳酸钙CaCO 3、碳酸钡BaCO 3B 红褐色沉淀:氢氧化铁Fe(OH)3C 蓝色沉淀:氢氧化铜 Cu(OH)23、 溶液的颜色A 蓝色溶液Cu 2+:硫酸铜CuSO 4、硝酸铜Cu(NO 3)2 、氯化铜CuCl 2等B 黄色溶液Fe 3+:氯化铁FeCl 3、硫酸铁Fe 2(SO 4)3、硝酸铁Fe(NO 3)3等C 浅绿色溶液Fe 2+:氯化亚铁FeCl 2、硫酸亚铁FeSO 4、硝酸亚铁Fe(NO 3)2等D 无色液体:水,双氧水E 紫红色溶液:高锰酸钾溶液F 紫色溶液:石蕊溶液 4、气体的颜色A 红棕色气体:二氧化氮B 黄绿色气体:氯气C 无色气体:氧气,氮气,氢气,二氧化碳,一氧化碳,二氧化硫,氯化氢气体等大多数气体。
二、典型例题1.A 、B 、C 、D 、E 、F 、G 为常见的物质,其中B 、E 、G 属于单质,反映②是炼铁工业中的要紧反映,以下图是它们之间的彼此转化关系。
请回答:红色固体无色气体 浅绿色溶液样品 A 溶液 B 滤 液 甲 沉 淀 乙 沉 淀 C 滤 液 ① 水 溶解 ③ 过量 BaCl 2溶液 过滤 ② 过量 NaOH 溶液 过滤 ④ 过量 Na 2CO 3溶液 过滤 丙 沉 淀 D 滤 液 ⑤ 过量 盐酸 加热蒸发 固体丁(1)A 物质的化学式 。
大学化学化工专业《英语》期末考试试卷含参考答案

大学化学化工专业《英语》期末考试试卷含参考答案1. state-of-the-industry 中文:工业发展水平(1分)2. alkyl ether sulfate中文:烷基醚硫酸盐(酯)(1.5分)3. W/O 英文: water in oil,(oil emulsion) ;中文:油乳胶(油包水)(1.5分)4. 2,6-Dimethy-2,7-octadien-6-ol 画出结构式:(4分)5. The inherent tendency of the whole or a part of a molecule to pass out of or not to penetrate into a water phase.英文: Hydrophoby ;中文:疏水性(亲油性) (1.5分) 6. A substance which, when introduced in a liquid, increases its wetting tendency.英文: Wetting agent ;中文:润湿剂 (1.5分)7. The process by which soil is dislodged from the substrate and bought into a state of solution or dispersion.英文: Detergency ;中文:去污性(力) (1.5分)8. An attribute which is related to benefit not directly but through association or suggestion.英文: Signal attribute ;中文:信号属性 (1.5分) 9. A colorless gas with a characteristic pungent odor, consisting of nitrogen and hydrogen.英文: ammonia ;中文:氨气 (2分)10. A chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom.英文: Carbon dioxide ;中文:二氧化碳 (2分)11. A chemical element with atomic number 9, it is the lightest halogen.英文: Fluorine ;中文:氟 (2分)12. KH2PO4 Potassium dihydrogen phosphate (2分)13. ZnSO4·7H2O Zinc sulfate hept(a)hydrate (2分)14.3-methyl-2-ethyl(-1-)butene (3-methyl-2-ethyl but-1-ene) (3.5分)15.4-(1-ethyl-butayl)-5-hydroxy-2-hexayne-1-al (7.5分) 16. A good example of such a versatile attribute is fragrance. (2分)译文:这样一个多功能属性的好例子就是香味。
化学专业《专业英语》试卷(A 卷)

化学与环境学院2013/2014学年第(1)学期期末考试试卷《专业英语》试卷(A 卷)专业年级班级姓名学号一、单词短语中译英(每小题1分,共10分)1.无机化学_____________2.二氧化碳_____________3.配体_____________4.同分异构体___________5.氯化钠_____________6.酸和碱__________7.摘要___________8.离子键_____________ 9.甲苯_____________10.电子_____________二、单词短语英译中(每小题1分,共15分)(1) carbonate__________ (2) monoxide__________ (3) amino acid__________(4) aromatic__________ (5) protein__________ (6) covalent bond_________(7) raw material__________ (8) chemical shift _________ (9) solute __________(10) oxygen__________ (11) monatomic__________ (12) polymer__________(13) pesticide__________ (14) detergent__________ (15) donor__________(16) nuclear magnetic resonance __________ (17) alkali metal__________(18) geochemistry__________ (19) electrode__________ (20) alkene__________二、阅读理解:(本题共3篇短文,15个小题,根据短文内容从各题的A,B,C,D四个选项中选出一个最佳答案,并将答案代码填入题后的括号内。
化学英语试题及答案
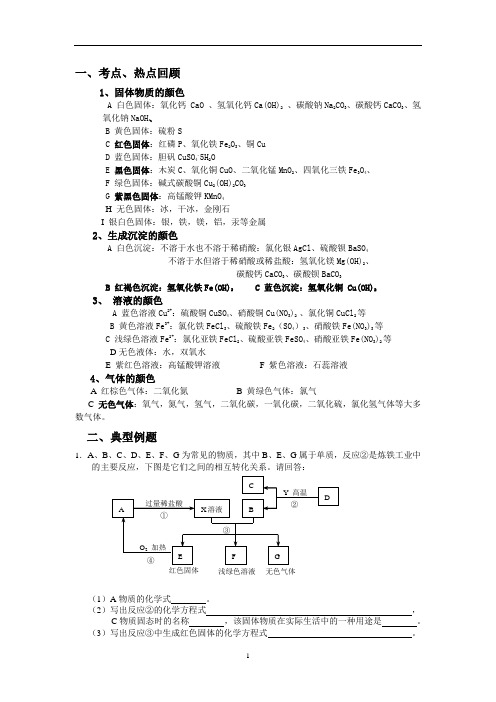
一、考点、热点回顾1、固体物质的颜色A 白色固体:氧化钙 CaO 、氢氧化钙Ca(OH)2 、碳酸钠Na 2CO 3、碳酸钙CaCO 3、氢氧化钠NaOH 、B 黄色固体:硫粉SC 红色固体:红磷P 、氧化铁Fe 2O 3、铜CuD 蓝色固体:胆矾CuSO 4.5H 2OE 黑色固体:木炭C 、氧化铜CuO 、二氧化锰MnO 2、四氧化三铁Fe 3O 4、F 绿色固体:碱式碳酸铜Cu 2(OH)2CO 3G 紫黑色固体:高锰酸钾KMnO 4H 无色固体:冰,干冰,金刚石I 银白色固体:银,铁,镁,铝,汞等金属2、生成沉淀的颜色A 白色沉淀:不溶于水也不溶于稀硝酸:氯化银AgCl 、硫酸钡BaSO 4不溶于水但溶于稀硝酸或稀盐酸:氢氧化镁Mg(OH)2、碳酸钙CaCO 3、碳酸钡BaCO 3B 红褐色沉淀:氢氧化铁Fe(OH)3C 蓝色沉淀:氢氧化铜 Cu(OH)23、 溶液的颜色A 蓝色溶液Cu 2+:硫酸铜CuSO 4、硝酸铜Cu(NO 3)2 、氯化铜CuCl 2等B 黄色溶液Fe 3+:氯化铁FeCl 3、硫酸铁Fe 2(SO 4)3、硝酸铁Fe(NO 3)3等C 浅绿色溶液Fe 2+:氯化亚铁FeCl 2、硫酸亚铁FeSO 4、硝酸亚铁Fe(NO 3)2等D 无色液体:水,双氧水E 紫红色溶液:高锰酸钾溶液F 紫色溶液:石蕊溶液 4、气体的颜色A 红棕色气体:二氧化氮B 黄绿色气体:氯气C 无色气体:氧气,氮气,氢气,二氧化碳,一氧化碳,二氧化硫,氯化氢气体等大多数气体。
二、典型例题1.A 、B 、C 、D 、E 、F 、G 为常见的物质,其中B 、E 、G 属于单质,反应②是炼铁工业中的主要反应,下图是它们之间的相互转化关系。
请回答:(1)A 物质的化学式 。
(2)写出反应②的化学方程式 ,C 物质固态时的名称 ,该固体物质在实际生活中的一种用途是 。
(3)写出反应③中生成红色固体的化学方程式 。
化学化工专业英语试卷

黄淮学院化学化工系2012-2013学年度第二学期期末考试《专业英语》A 卷1. octane :2. decyl :3. butanol :4. hexene :5. heptyl aldehyde :6. oxyacid :7. aliphatic compound : 8. oxidation reaction : 9. organic chemistry : 10.transition element : 11. orbital electron: 12.solubilizer : 13.quantative analysis : 14.negative charge : 15.carbonate : 16.surfactant : 17.docosane hectane : 18.alkane :19.sodium hydroxide : 20.chloride : 1. 酮:2.乙氧基化物:3. 键角:4. 热力学函数:5. 同系列:6. 甲苯:7. 构象异构:8. 14烷:9. CH 3CH 2CH 2CH 2CH 2-: 10.电离能: 1.Progress in the so-called “active ingredients ”, which enhance and extent this cosmetic effect measurably, is large enough area to merit another separate aritcle.2.In contrast to inorganic compounds, the molecular attraction of organic compounds is weak, so organic compounds are usually volatile and possess low melting points.3.Benzene can undergo the typical substitution reactions of halogenation,一、 Write the Chinese name for each of the following (每小题1分,共20分)二、 Write the English name for each of the following(每小题1分,共10分)三、 Translate the following sentences into Chinese (每小题3分,共30分)nitration, sulphonation and Friedel-Crafts reaction.4.Evaporation is conducted by vaporizing a portion of the solvent to produce a concentrated solution or thick liquor.5.The presence of a substituent group in benzene exerts a profound control over both orientation and the ease of introduction of the entering substituent.6.The functional group of a ketone consists of a carbon atom connected by a double bond to an oxygen atom.7.At equilibrium, these two rate are equal; cupric ion is still reacting with ammonia molecules to form the complex, and the complex is still decomposing, but just as much cupric ammonia complex is being decomposed in unit time as is being formed.8.The reaction of an acid chloride with an amine is used commercially in the manufacture of the very important range of semi-synthetic penicilings,first produced by the Beechan Group in 1959.9.Thus satisfactory binding propertise are essential for trouble-free compression and the production of good quality cakes over long manufacturing periods.10.The synthesis of organic compounds involves conversion of availablesubstances of known structure, through a sequence of particular, controlled chemical reactions, into other compounds bearing a desired molecular structure.The active ingredients were identified in the unsaponifiable fraction of this vegetable product. After solvent extraction and drying, the pure unsaponifiables are obtained in the form of a waxy solid. This waxy solid is then redissolved in untreated shea butter to increase the unsaponifiable content and thus lead to the unsaponifiable shea butter concentrate. Used in cosmetics at levels of up to 2%,it provides excellent protection against sunlight and skin dryness.Another example is the extract of the kola nut, known for its anti-irritant properties. As available in the market, it has an objectionable color and odor . At Estee Lauder, we analyzed and separated its constituents, identified the individual components with anti-irritant properties, and recombined them in the most effective ratio. In the process , objectionable color and odor were removed and possible allergens(过敏原)eliminated. All this indicates that cosmetics formulated with plant extracts today can be more effective and , at the same time, more elegant than 10 or 20 years ago.四、Translate the following paragraph into Chinese(本大题共1个小题,共25分)采用一种简单、可靠并且有效的气相色谱法,来同时测定草药鱼腥草与鱼腥草注射液中8种活性组分的含量。
04级化学专业《专业英语》试卷

04级化学专业《专业英语》试卷绍兴文理学院 06 学年第二学期化学专业 04 级《专业英语》试卷(答题卷)I. Write the formula for each of the following chemicals: (30 points)1) silver nitrate 2) ferric oxide3) potassium sulfate 4) ammonium chloride5) magnesium hydroxide 6) sodium phosphate7) silicon dioxide 8) zinc sulfide9) lithium bromide 10) calcium carbonate11) lead acetate 12) carbon tetrachloride13) cyclohexane 14) meta-diethyl benzene15) dimethylamine 16) 3-methyl-1-butyne17) meta-nitrotoluene 18) 2-bromo-5-phenyl-3-octene19) 3-hexanone 20) N,N-dimethyl acetamide21) p-phenylbenzamide 22) benzoyl chloride23) ethylene glycol 24) methyl n-propyl ether25) 1,3-pentadiene 26) methyl formate27) o-phthalic anhydride 28) propionic acid29) formaldehyde 30) p-methoxybenzaldehydeII. Give the English name for each of the following compounds (15 points):1) H2SO42) Al2O33) KH2PO44) SO35) CH2=CH-CH36) (CH3)3CCl7) CF3COOH 8) H2NCH2CH2NH29) (CH3)2CHOH 10) p-F-C6H4-OH11) CH3-CH(CH3)-CH2-CO-CH3 12) CH3-CH=CH-CHO13) CH3-CH(OH)-COOH 14) CH3CO-CH2COOC2H515) CH3(CH2)3C≡NIII. For the following descriptions or definitions, determine true or false for each statement based on principles in chemistry (10 points):1) The oxidized and reduced species that appear in an ion-electron equation are may be non-reactants.2) Standard enthalpy of formation is the heat of formation of one mole of a compound by combination of its elements in their standard states at a specified temperature.3) Enantiomers are pairs of molecules with the same formula that rotate plane-polarized light in opposite directions.4) A nucleophile is an electron deficient atom or group that will bond with an atom that has a available electron pair5) Theoretical yield is the maximum amount of a product that can be formed according to a ba lanced chemical equation IV. (5 points) Answer the following questions in ENGLISH:Nitrous oxide, N2O, undergoes decomposition when heated to give N2 and O2.2 N2O (g) → 2 N2 (g) + O2 (g)What is the molar composition of the gaseous mixture produced? Compare this composition to that of air and predict whether the mixture will support combustion or not?V. (5 points) Fill in the blanks in the following paragraph with the appropriate words or phrases listed at the end of paragraph: The rules that govern the naming of chemical compounds are known collectively as chemical ________________. In a simple way, the name of a cation consists of the name of the element, the _____________on the ion as a Roman numeral in parenthesis, and the word “ion”. The name of a monatomic _______________ (e.g., Cl-) consists of the name of the element with the ending“ide”, followed by the word “ion”. A binary compound is one containing atoms or ions of only two ________________. Salts are _________________ formed between cations and anions of acids. For binary molecular compounds, prefixes are used to indicate the number of each element present.anion elements nomenclature ionic compounds chargeVI. (5 points) Fill in the blanks in the following paragraph with the appropriate words or phrases listed as follows:The geometry around the C=C double bond in an __________ plays an important role in the chemistry of these compounds. The presence of the bond restricts rotation around a C=C double bond. There is no way to rotate one end of this bond relative to the other without breaking the bond. Because the _____________ is relatively strong (270 kJ/mol), rotation around the C=C double bond cannot occur at room temperature. Alkenes therefore form stereoisomers that differ in the way substituents are arranged around the C=C double bond. The ____________ in which similar substituents are on the same side of the double bond is called cis; whereas that with similar substituents are across from each other, is called trans. The cis isomer of 2-butene, for example, has both _____________ groups on the same side of the double bond. In the trans isomer the CH3 groups are on the ______________ sides of the double bond.opposite alkene methyl bond energy isomerVII. (10 points) Fill in the blanks in the following with the appropriate words or phrases listed at the end of the paragraph: So far, we have built a small repertoire of reactions that can be used to convert one functional group to another. We have briefly discussed converting alkenes to alkanes; alkanes to alkyl halides; alkyl halides to alcohols; alcohols to ethers, aldehydes, orketones; and aldehydes to carboxylic acids. We have also shown how carboxylic acids can be converted into esters and amides. We have yet to encounter a reaction, however, that addresses a basic question: How do we make C-C bonds? One answer resulted from the work that Francois A.Grignard started as part of his Ph.D. research at the turn of the last century.Grignard noted that alkyl ____________ react with magnesium metal in diethyl ether to form compounds that contain a metal-carbon bond. Methyl bromide, for example, forms methylmagnesium bromide.Et2OCH3Br + Mg →CH3MgBrBecause carbon is considerably more _______________ than magnesium, the metal-carbon bond in this compound has a significant amount of ionic character. Grignard reagents such as CH3MgBr are best thought of as hybrids of ionic and ___________ Lewis structures.CH3-Mg-Br ?[CH3-] [Mg2+] [Br-]Grignard reagents are our first source of carbanions (literally, "anions of carbon"). The Lewis structure of the CH3- ion suggests that carbanions can be Lewis bases, or electron-pair _____________. Grignard reagents such as methylmagnesium bromide are therefore sources of a nucleophile that can attack the + end of the C=O double bond in aldehydes and ketones. Thus the most important aspect of the chemistry of Grignard reagents is the ease with which this reaction allows us to couple alkyl chains. Isopropylmagnesium bromide, for example, can be used to graft an isopropyl group onto the ____________ chain of an appropriate ketone.electronegative hydrocarbon covalent donors halidesVIII. (10 points) Fill in the blanks in the following with the appropriate words or phrases listed at the end of the paragraphs: Why do some solids dissolve in water? The sugar we use to sweeten coffee or tea is a molecular solid, in which the individual molecules are held together by relatively weak ________________________. When sugar dissolves in water, the weak bonds between the individual sucrose molecules are broken, and these C12H22O11 molecules are released into ________________. It takes energy to break the bonds between the C12H22O11 molecules in sucrose. It also takes energy to break the hydrogen bonds in water that must be disrupted to insert one of these sucrose molecules into solution. Sugar dissolves in water because energy is give n off when the slightly polar sucrose molecules form intermolecular bonds with the polar water molecules. The weak bonds that form between the solute and the solvent compensate for the energy needed to disrupt the structure of both the pure solute and the solvent. In the case of sugar and water, this process works so well that up to 1800 grams of sucrose can dissolve in a liter of water.Ionic solids(or salts) contain positive and negative ions, which are held together by the strong force of __________________ between particles with opposite charges. When one of these solids dissolves in water, the ions that form the solid are released into solution, where they become associated with the ___________________solvent molecules.H2ONaCl(s) →Na+(aq) + Cl-(aq)We can generally assume that salts ________________ into their ions when they dissolve in water. Ionic compounds dissolve in water if the energy given off when the ions interact with watermolecules compensates for the energy needed to break the ionic bonds in the solid and the energy required to separate the water molecules so that the ions can be inserted into solution.dissociate intermolecular forces attraction polar solutionIX. (10 points) Fill in the blanks in the following with the appropriate words or phrases listed at the end of the paragraphs:A soap bubble is simply a very thin sheet of water sandwiched between two layers of soap molecules, also called _____________________. These molecules are called amphiphilic. This means that part of this molecule is attracted to water, which is hydrophilic, and another part is repelled from water, which is _____________________.When such a molecule is put in water, as many as possible will crowd to the surface, so that the heads can stay in the water, while the tails stick out into the ___________. This is why soap-like molecules are called surfactants, since they mostly affect the _________ of water. It is these molecules that make soap bubbles stable.Some bubbles result from gas being trapped in solution, such as CO2 in soda and champagne, or N2 in the blood of deep sea divers. Much study goes into understanding bubbles dissolved in gases, as well as how the formation of large surfaces from surfactants used to construct bubbles are affected by different factors, e.g., temperature, surface __________ , nucleation sites, etc..air, pressure, hydrophobic, surface, surfactant molecules。
化学英语考试卷及答案
第1页,共4页第2页,共4页任课教师签名:刘生桂 命题教师签名:刘生桂 系主任签名: 主管院长签名:湛江师范学院2010年- 2011学年度第1学期期末考试试题(考试时间: 120 分钟)考试科目:化学专业英语一 词汇题 (20分)1 无机化学 Inorganic chemistry 11 结晶 crystal2 配位化学 coordination chemistry 12 室温 room temperature3 酸 acid 13 回流 reflux 4碱 alkali 14 克 gram5 氧气 oxygen gas 15 臭氧 ozon6 氮气 nitrogen gas 16 摘要 summary7 烧杯 beaker 17 结果 result8 试管 test tube 18 蒸发 evaporation9 酒精灯 spirit lamp 19 蒸馏 distillation 10 纯化 purification 20 甲烷 methane二 翻译题 (共60分)1 if you decide to go into agriculture, you will need to know about fertilizersandpesticides,as well as animal nutrition. Even if you enter some profession thatseems to have no connection with chemistry, such as law, you will find a knowledge of chemistry very wyers freguently have to deal with patents that concern chemical inventions.Some members of the U.S. Congress have had extensive chemical training,which gives them a great advantage in discussions of enviromental pollution, nuclear energy,the regulations of the Food and Drug Administration, and in other legislation that concerns scientific matters.如果你决定进入农业,你需要知道化肥和杀虫剂,以及动物营养。
2020-2021某大学《化学专业英语》期末课程考试试卷(含答案)
《化学专业英语》期末课程考试试卷考试所需时间:120分钟适用专业:应用化学总分:100分PartⅠ、Choice(28×1.5=42)1 How many neutrons are present in an atom of tin that has the atomic number 50 and a mass number of 119?A. 50B.69C. 119D. 1692. Isotopes of an element differ in their ____.A. atomic numbersB. electron configurationsC. number of protonsD. masses3. Atomic masses for elements shown on the periodic table are not expressed as whole numbers because __A. the number of protons in an atom of an element variesB. atoms may gain or lose electrons during a chemical reactionC. they represent weighted averages of the isotopes of that atomD. scientists cannot measure the masses of atoms with great precision4. Group 17 elements, the halogens, are the most reactive of the nonmetals because they ____.A. are the farthest to the right of the periodic tableB. require only one electron to form the stable configurations of the noble gasesC. have the largest atomic radiiD. have the greatest ionization energies5.In the modem periodic table, elements are ordered ac-cording to ____.A. decreasing atomic massB. Mendeleev's original designC. increasing atomic numberD. when they were discovered6.The energy it takes to remove an electron from an atom as you move left to right across the period, from Na through to Cl, ___.A. generally increasesB. generally decreasesC. does not changeD. varies unpredictably7.Which of the following is the correct formula for iron (III) sulfate? ____.A. Fe3SO4C. Fe3(SO4)2B. Fe2(SO4)3D. 3FeSO48. The electroneutrality principle ____.A. states that the number of cations equals the number of anionsB. is demonstrated in any polyatomic ionC. states that the net charge on a binary ionic com-pound is zeroD. all of the above9. The correct name for NH4NO3is ____.A. ammonium carbonateB. ammonium hydroxideC. ammonium acetateD. ammonium nitrate10. When an acid reacts with a metal, ____A.the hydronium ion concentration increasesB.the metal forms anionsC.water is producedD.the pH value decreases11.Which of the following is a binary acid ?A. H2O B. H3PO4C. H2SO4D. HCl12.Which of the following solutions would have a pH value greater than 7 ?A.[OH-]=2.4×10-2MB.[H3O+]=1.53×10-4MC.0.0001M HClD.[OH-]=4.41×10-11M13.If the empirical formula of a compound is known, thenA. its true formula is also knownB. its percentage composition can be calculatedC. the arrangement of its atoms is also knownD. the percentage water in the compound can be determined14.The units for molar mass are ___A. g/mol C. g/atomsB. atoms/mol D. mol/g15.Which of the following compounds has the highest percentage composition of oxygen?A. CH4O C. H2OB CO2 D. Na2CO316.Pressure can be measured in ___A. grams C. pascalsB. meters D. liters17.A sample of oxygen gas has a volume of 150 mL when its pressure is 0.947 atm. If the pressure is increased to 0. 987 atm and the temperature remains constant, the new gas volume will be ___A. 140 mL C. 200 mLB. 160 mL D. 240 mL18.A sample of neon gas occupies a volume of 752 mL at 25℃. What volume will the gas occupy at 500℃ if the pressure remains constant? ___A. 694 mL C. 815 mLB. 752 mL D. 955 mL19.Potatoes will cook faster at sea level than at higher altitudes because the water used to cook them willA. be boiling more rapidlyB. boil at a lower temperatureC. increase in temperature while boilingD. boil at a higher temperature20.If the temperature outside is 26℃,then the temperature would be ____ kelvins.A. 26 C. 299B. 273 D. -24721. If the empirical formula of a compound is known, thenA. its true formula is also knownB. its percentage composition can be calculatedC. the arrangement of its atoms is also knownD. the percentage water in the compound can be determined22.Examine the following skeletal structure:OHOThe correct chemical formula for this compound isA. C2H4O2C. C5H8O2B. C5H4O2D. CHO23.Identify the following reactions as either reduction or oxidation. Indicate whether they occur at the cathode or anode. A. Ra(s) →Ra2+(aq) + 2e-B. Hg22+(aq) + 2e-→2Hg(l)C. Pb(s) + SO42-(aq) →PbSO4(s) + 2e-D. O2(g) + 2H2O(l) + 4e-→4OH-(aq)24.In the following reaction, which species is being reduced?2K+Br2→2K++2Br-A. K only C. both K and Br2B. Br2only D. neither K nor Br225.The electrode at which reduction occurs is ____.A. always the anodeB. always the cathodeC. either the anode or the cathodeD. always the half-cell26. Sulfuric acid, H2SO4, or a similar substance is added to water that is to be electrolyzed in order to ____.A. react with the waterB. keep the electrode cleanC. provide adequate conductivityD. supply energy27.If an exothermic reaction has reached equilibrium, increasing the temperature willA. favor the forward reactionB. favor the reverse reactionC. favor both the forward and reverse reactionD. have no effect on the equilibrium28 Consider the following reaction:COBr2(g) →CO(g) + Br2(g)At 73°C , the Keqvalue for this reaction is 0.190. This Keqvalue indicates that ____.A. the reverse reaction is favoredB. the forward reaction is favoredC. the reaction has reached equilibriumD. the concentrations of CO(g) and Br2(g) are greater than theconcentration of Br2(Part Ⅱ common skill (20×0.5=10)1.write out the English speaking of the following symbol s(1).Mg(OH)2(2)↓→+++3223CaCOCaCO (3).log n x(4).nX(5).−−→−∆,Cu (6). X -8 (7). 1235(8). 3:2 (9). ± (10).100℃2.write out the chemical Chinese meaning of the followingabbreviation(1).alc. (2).amt. (3).A ·P (4).app. (5).contg. (6).C ·P (7).detn. (8).fig. (9).L-R (10).resp.Part Ⅲ Write out IUPAC naming of the following organic matter in English ( 1.5×8=12)CH 3CH 3CH 3CH 3⑴ C H 3CH 3CH 3CH 3⑵CH 2CH 3⑶C H 3CH 3H 3OH ⑷CH 3O CH 3⑸CH 3CH 3O⑹CH 3OHO ⑺CH 32O⑻Part Ⅳ write out English name of the following chemical elements(1×10)H B C N O F Si S K ClPart Ⅴ Translate the following passages into Chinese (2×8+1×10=26)Passage one : Certain membranes made of an animal bladder, a slice ofvegetable tissue, or a piece of parchment, act as a barrier between twosolutions, and simultaneously allow specific types of molecules. These are called semipermeable membranes. Semipermeable membranes that allow passage of solvent molecules but do not allow passage of solute molecules or ions are called osmotic membranes. If a NaCl solution is separated from pure water by an osmotic membrane, H 2O molecules spontaneously penetrate the membrane from both directions; however, passage across the membrane from the pure water side is faster than passage across the membrane from the solution side. The net result is exactly like that illustrated already previously and involves a net transfer of H 2O from the pure water side of the membrane to the solution side of the membrane. The passage of solvent molecules from a region with little or no dissolved solute, through an osmotic membrane, to a region with more dissolved solute is called osmosis.Passage two: As in ionic bonding and covalent bonding, outer shell electrons are responsible foe bonding between metal atoms. However, it is unreasonable to assume that ionic bonds occur between metal atoms since all the atoms are alike and no single atom would give up electrons to another atom. Covalent bonding between metal atoms is almost as unreasonable because not enough outer shell electrons are available for as many shared-pair bonds as each metal atom seems to form. Instead, a metallic lattice consists of a regular array of positive ions immersed in a cloud of highly mobile outer shell electrons. Metals have relatively low ionization energies or relatively loose holds on their outer shell electrons. These electrons are free to move throughout the metallic lattice. Metallic bonding results from attraction between the positive ions and the cloud of negative electrons. Such attractive forces are weaker than ionic or covalent bonding forces. Thus, many metals are soft and fairly low melting. Potassium is soft enough to be cut with a knife and melt at 68.7℃. On the other hand, some of the transition metals, where significant covalent character is superimposed on the metallic lattice, are hard and high melting.Tungsten is very hard and melts at about 3410℃Passage three: Major branches of Chemistry .The body of knowledge about chemicals and chemical reactions is so vast that for convenience chemists have divided the study of chemistry into several major branches: l. Analytical chemistry: The study of what types of elements and compounds are present in a sample of matter — called qualitative analysis — and how much of each element and compound is present in a sample of matter — called quantitative analysis.2. Physical chemistry: The study of the scientific laws and theories that attempt to describe and explain the structure of matter, the chemical bonds that hold matter together, the changes that matter undergoes, and the energy involved in these changes.3. Organic chemistry: The study of the properties and reactions of hydrocarbons, compounds containing only the elements carbon and hydrogen, and other compounds derived from hydrocarbons that contain one or more other elements such as oxygen, nitrogen, sulfur, phosphorus, and chlorine. About4.9 million of the 5 million officially identified compounds are classified as organic compounds—explaining why an entire branch of chemistry is devoted to studying these compounds4. Inorganic chemistry: The study of all elements and the properties and reactions of the compounds not classified as organic compounds.5. Biochemistry: The study of the properties and reactions of compounds found in living organisms and those that are important to living organisms.These branches make it easier to study chemistry. Real chemistry, however, almost always involves a blend of information and ideas from most — if not all — of these branches. This book is concerned with general chemistry — a survey and introduction to all the major branches of chemistry except biochemistry.2020-2021《化学专业英语》期末课程考试试卷答案PartⅠ、Choice(28×1.5=42 points)(按顺序填入答案)1—5题 ADCAC 6—10题 ABBCB11—15题 CABAC 16—20题 CACDC 21—25题 CACBA 26—28题 CBA Part Ⅱ1.(1) Magnesium hydroxide(2) Nitrogen reacts with hydrogen to form ammonia at high temperatureand pressure with the presence of a catalyst.(3) Log x to the base n.(4) The nth root of x.(5) Calcium carbonate when heated produces calcium oxide and carbondioxide.(6) X to the minus eighth (power).(7) Five over one hundred and twenty_three.(8) The ratio of two to three.(9) Plus and minus.(10) One (a) hundred degrees Centigrade.2. (1)醇(2)量(3)分析纯(4)装置(5)含有。
化学化工英语试题及答案
化学化工英语试题及答案一、选择题(每题2分,共20分)1. Which of the following is a chemical element?A. WaterB. OxygenC. HydrogenD. Carbon答案:B, C, D2. The chemical formula for table salt is:A. NaOHB. NaClC. HClD. NaHCO3答案:B3. What is the process called when a substance changes from a solid to a liquid?A. SublimationB. VaporizationC. MeltingD. Condensation答案:C4. In the periodic table, which group contains alkali metals?A. Group 1B. Group 2C. Group 17D. Group 18答案:A5. What is the name of the process where a substance decomposes into two or more substances due to heat?A. CombustionB. OxidationC. ReductionD. Decomposition答案:D6. Which of the following is a physical property of a substance?A. ColorB. TasteC. SolubilityD. Reactivity答案:A7. What is the term for a compound that releases hydrogen ions (H+) when dissolved in water?A. BaseB. AcidC. SaltD. Neutral答案:B8. The law of conservation of mass states that in a chemical reaction:A. Mass is lostB. Mass is gainedC. Mass remains constantD. Mass can be converted into energy答案:C9. Which of the following is a type of chemical bond?A. Ionic bondB. Covalent bondC. Hydrogen bondD. All of the above答案:D10. What is the name of the process where a substance absorbs energy and changes from a liquid to a gas?A. MeltingB. VaporizationC. SublimationD. Condensation答案:B二、填空题(每题2分,共20分)1. The symbol for the element iron is ________.答案:Fe2. The pH scale ranges from ________ to ________.答案:0 to 143. A compound that produces a basic solution when dissolvedin water is called a ________.答案:base4. The smallest particle of an element that retains its chemical properties is called a ________.答案:atom5. The process of separating a mixture into its individual components is known as ________.答案:separation6. The study of the composition, structure, and properties of matter is called ________.答案:chemistry7. The process of a substance changing from a gas to a liquid is called ________.答案:condensation8. A(n) ________ reaction is a type of chemical reactionwhere two or more substances combine to form a single product. 答案:synthesis9. The volume of a gas at constant temperature and pressureis directly proportional to the number of ________.答案:moles10. The process of converting a solid directly into a gas without passing through the liquid phase is known as ________. 答案:sublimation三、简答题(每题10分,共30分)1. Explain what is meant by the term "stoichiometry" in chemistry.答案:Stoichiometry is the calculation of the relative quantities of reactants and products in a chemical reaction.It is based on the law of conservation of mass and involvesthe use of balanced chemical equations and the molar massesof substances to determine the amounts of reactants needed to produce a certain amount of product or the amounts ofproducts formed from a given amount of reactant.2. Describe the difference between a physical change and a chemical change.答案:A physical change is a change in the state or form of a substance without altering its chemical composition. Examples include melting, freezing, and boiling. A chemical change, on the other hand, involves a change in the chemical composition of a substance, resulting in the formation of new substances. Examples include combustion and rusting.3. What are the three main types of chemical bonds, and givean example of each.答案:The three main types of chemical bonds are ionic bonds, covalent bonds, and metallic bonds. An ionic bond is formed when electrons are transferred from one atom to another, resulting in the formation of oppositely charged ions. An example is the bond between sodium (Na) and chloride (Cl) in table salt (NaCl). A covalent bond is formed when two atoms share electrons, as seen in water (H2O) where hydrogen atoms share electrons with oxygen. Metallic bonds occur in metals, where a "sea" of delocalized electrons is shared among positively charged metal ions, as in sodium metal。
化学专业英语练习题
Final Examination PaperⅠⅠ. Monochoice questions依数性6. The red blood cell will be shrinkable in which solution listed below?()A. 10.0 g·L-1CaCl2·2H2O(Mr=147)B. 12.5g·L-1NaHCO3(Mr=84.0)C. 1.00 g·L-1NaClD. 224g·L-1C3H5O3Na(Mr=112)11. There are four water solutions of the equal volume in which there are equal mass of glucose, CaCl2, NaHCO3and sucrose respectively. Then whose freezing-point is the lowest? ( )[Mr(glucose)180( HAc)60(Na2CO3)106 (CaCl2) 111]A. GlucoseB. HAcC. CaCl2D. Na2CO312. Which choice listed below is isotonic solution? ( )A. 5% glucose solution and 5% sucrose solutionB. 1 mol·L-1 glucose solution and 0.5 mol·L-1 sucrose solutionC. 0.5 mOsmol·L-1 urea solution and 0.5 mOsmol·L-1 NaCl solutionD. 0.5 mol·L-1 MgSO4solution and 0.5 mol·L-1 CaCl2 solution电解质9. We learn that the K a of HF is 3.53×10-4 and the K b of NH3·H2O is 1.79×10-5, then which option following is true?( )A. NH4+is a stronger acid than HFB. NH4+is a weaker acid than HFC. the acidic strength of NH4+and HF are equal.D. can not do the compare13. A solution was prepared by mixing equal volume of 0.10mol·kg-1NH4Cl and 0.10mol·kg-1NH3·H2O, its ionic strength ( I ) is ( ) mol·kg-1.A. 0.05B. 0.075C. 0.10D. 0.1514. 0.10mol NaOH and 0.10mol HAc are dissolved into 1.0L distilled water together, please calculate the pH of this solution( ) (Ka(HAc)=1.74×10-5)(A) 10.28 B. 11.28 C. 8.88 D. 12.2815. There is 1L 0.1mol·L-1H2CO3 solution with the addition of 0.5ml 0.1mol·L-1 HCl solution. Which choice listed below is true? ( )A. pH decrease, dissociation degree of H2CO3 decrease.B. pH decrease, dissociation degree of H2CO3 increase.C. pH increase, dissociation degree of H2CO3 increase.D. pH increase, dissociation degree of H2CO3 decrease.缓冲溶液16.The buffer range of a buffer solution prepared by mixing 500ml 0.4mol·L-1 H2CO3 solution and 200ml 0.4mol·L-1 NaOH is about ( ). (pK a1=6.37; pK a2=10.25 )A. 1.12~3.12B. 6.21~8.21C. 11.32~13.32D. 5.37~7.37.19. If you mix two solutions of equal volume in each option listed below, which option has no buffer action. ()A. 0.2mol·L-1HCl和0.2mol·L-1KClB. 0.02mol·L-1HCl和0.04mol·L-1NH3·H2OC. 0.01mol·L-1KH2PO4和0.2mol·L-1Na2HPO4D. 0.01mol·L-1NaOH和0.02mol·L-1HAc20. The most important ACID resistant in the plasma of human being is ()A. H2PO4-B. HPO42-C. HCO3-D. H2CO321. The color of the solution is orange with the addition of methyl orange indicator. In order tokeep the pH of the solution stable, which buffer system listed below is the best? ( )A. 0.1mol·L-1 HAc — 0.1mol·L-1 NaAc (K a = 1.8×10-5)B. 0.1mol·L-1 NH3·H2O — 0.1mol·L-1 NH4Cl (K b = 1.8×10-5)C. 0.1mol·L-1 NaH2PO4 — 0.02mol·L-1 Na2HPO4(K a = 6.2×10-8)D. 0.1mol·L-1 HCN — 0.02mol·L-1 NaCN (Ka = 4.9×10-10)Ⅱ.Fill the blank. (Please fill your answers into the blanks following).1. When the HCl standard solution is used to titrate a sample solution which containing NaHCO3 and Na2CO3, the indicator is(1) in the first step of the titration,the indicator is (2)in the second step of the titration (pKa1=6.35;pKa2=10.33)2.A 2.05 g sample of white phosphorus was dissolved in 25.0g of carbon disulfide,CS2. The of the carbon disulfide solution was found to be 1.59℃. The molecular weight of the phosphorus is (3) g·mol-1 in solution? The formula of molecular phosphorus is (4) mol·L-1 (boiling-point elevation constant K b of CS2=2.4; Mr(P)=31)Ⅲ.Calculation1. A sample of 0.1276g of an unknown monoprotic acid was dissolved in 25.00 mL of water andtitrated with 0.0633 M NaOH solution. The volume of base required to reach the equivalence point was 18.4 mL. (a) Calculate the molar mass of the acid. (b) After 10.00 mL of base had been added in the titration, the pH was determined to be 5.87. What is the K a of the unknown acid?Final Examination PaperⅡⅠ. Monochoice questions依数性6. The osmotic pressure of a solution prepared by the equal volume of 8.4%(g/ml)NaHCO3 and18%(g/ml)glucose (C6H12O6) is equal to the osmotic pressure of ( ). [Mr(glucose)180 (NaHCO3)84]A. 5.85%(g/ml)NaCl solutionB. 1.5mol·L-1sucrose solutionC. 1mol·L-1glucose solutionD. 1 mol·L-1 CaCl2 solution7. Which of the following statements is a logical deduction ( )A. if a nonvolatile solute is added to water, the boiling point of the solution will be 100℃.B. the addition of a volatile solute will change the boiling point of the water.C. atmospheric pressure will affect the composition of the aqueous solution.D. if a nonvolatile solute is added to water, the freezing point of the solution will be lower thanthat of water.电解质8. Which species is the strongest acid that can exist in aqueous solution? ( )A. NaOHB. Na2CO3C. OH-D. KOH9. A 0.1 mol·L-1 solution of potassium acetate, KC2H3O2, has a lower pH than a 0.1 mol·L-1 solution of potassium cyanide, KCN. From this, you can correctly conclude that ( )A. hydrocyanic acid, HCN, is a weaker acid than acetic acid, HC2H3O2.B. hydrocyanic acid, HCN, is less soluble in water than acetic acid, HC2H3O2.C. the cyanide ion, CN–, is a weaker base than the acetate ion, C2H3O2–.D. acetate ion, C2H3O2, partially dissociates to form hydronium ion, H3O+.10. The factor that does not affect on the activity coefficient has ( )A. ionic concentrationB. charge on the ionC. ionic strengthD. K a or K b11.The pH of mixed solution by 0.10mol·L-1NH3 and 0.10mol/L NaOH is about ( ).(K b=1.8×10-5)A. 9B. 1C. 6D. 1312. The solubility of BaSO4 is not changed when it was dissolved in ( ).A. 1mol·L-1KCl solutionB. 2mol·L-1 Na2SO4 solutionC. pure waterD. no answer13. There is 1L 0.4mol·L-1Na2CO3 solution with the addition of 1.0 ml 0.1mol·L-1 HCl solution. Which choice listed below is true? ( )A. pH decrease, dissociation degree of CO32- decrease.B. pH decrease, dissociation degree of CO32- increase.C. pH increase, dissociation degree of CO32- increase.D. pH increase, dissociation degree of CO32- decrease.14. 25℃, the Ksp of Ag2CrO4is 1.12×10-12, so the concentration of Ag+ions in the saturate solution of Ag2CrO4 is ( )A. 6.54×10-5mol·L-1B. 1.21×10-5mol·L-1C. 1.21×10-4mol·L-1D. 6.54×10-4mol·L-1缓冲溶液15.The buffer range of a buffer solution in which there are the same concentrations of Na2HPO4 and NaH2PO4 is about. (pK a1=2.12; pK a2=7.21 pK a3=12.32)Which one is wrong? ( ).A. 1.12~3.12B. 6.21~8.21C. 11.32~13.32D. all the choice above16. To determine the content of Mg2+ and Ca2+ ions in tap water, in order to keep the pH =10, which buffer system listed below is the best? ( )A. 0.1mol·L-1 HAc — 0.1mol·L-1 NaAc (K a = 1.8×10-5)B. 0.1mol·L-1 NH3·H2O — 0.1mol·L-1 NH4Cl (K b = 1.8×10-5)C. 0.1mol·L-1 NaH2PO4 — 0.02mol·L-1 Na2HPO4(K a = 6.23×10-8)D. 0.1mol·L-1 H2CO3— 0.02mol·L-1 NaHCO3(Ka = 4.3×10-7)17. The most important ACID resistant in the plasma of human being is ()A. H2PO4-B. HPO42-C. HCO3-D. H2CO318. If you mix two solutions of equal volume in each option listed below, which option has no buffer action. ()A. 0.2mol·L-1HCl and 0.2mol·L-1KClB. 0.02mol·L-1HCl and0.04mol·L-1NH3·H2OC. 0.01mol·L-1KH2PO4and 0.2mol·L-1Na2HPO4D. 0.01mol·L-1NaOH and 0.02mol·L-1HAcⅡ.Fill the blank. (Please fill your answers into the blanks following)1. the theoretical range of color change of a weak basic indicator is (1) which K b is 1.0×10-42. A 1.0 g sample of the protein hemoglobin is dissolved in enough water to make 1 L (kg) of solution. The osmotic pressure of the solution is measured at 25 ℃and found to be 0.1 kPa. The molecular mass of hemoglobin is (2) and the molality of the hemoglobin solution is (3) .Final Examination PaperⅢⅠ. Monochoice questions1.依数性How much is the normal freezing points of the solution in which 21.0g NaCl is dissolved in 135mLof water ? [K f=1.86 K·kg·mol-1 , Mr(NaCl)=58.5] ( )A. -9.89℃B. 19.89℃C. 9.89℃D. 19.89℃2.If you want to have osmosis between two dilute solutions separated by semipermeable membrane, which choice listed below is wrong.()A.Both of the two osmotic pressure are not equal.B. Both of the two osmolarity are not equal.C.Both of the two solutions are not isotonic.D. Both of the two molality are not equal.3.In 500mL normal saline water, the osmolarity of the Cl-ions is ( ) mOsmol·L-1 [Mr(Cl)=35.5]A. 77B. 196C. 154D. 3084.The minimum mass of NaCl that would have to be added to 1.200×103 g H2O so the resulting solution would not freeze outside on a cold day(-10℃) is ( ) (K f=1.86 K·kg·mol-1 , Mr[NaCl]=58.5)A.94.3gB. 188.6gC.282.9gD.377.2g5.电解质There is 1L 0.1mol·L-1 HAc solution with the addition of 0.5mL 0.1mol·L-1 NaCl solution. Which choice listed below is true? ( )A. pH decrease, dissociation degree of HAc decrease.B. pH decrease, dissociation degree of HAc increase.C. pH increase, dissociation degree of HAc increase.D. pH increase, dissociation degree of HAc decrease.6.What is ionic strength ( I ) for the solution that contains 0.10 mol·kg-1NaCN and 0.10 mol·kg-1HCN ( ).A. 0.025mol·kg-1B. 0.050mol·kg-1C. 0.20mol·kg-1D. 0.10mol·kg-17.K sp for SrSO4 is 4.0×10-8 at certain temperature. How much is the solubility of SrSO4 in H2O. ( )A. 4.0×10-8 mol·L-1B. 2.0×10-4 mol·L-1C. 8.0×10-8 mol·L-1D. 1.0×10-4 mol·L-18.Which substance can use as ampholyte in different solvent?( )A. Na NO3B. HAcC. NaClD. Na OH9.缓冲溶液Which option of the following determines the capacity of a buffer ( )A. Conjugate acid-base pairB. Buffer-component ratioC. Buffer rangerD. p K a of the acid component10.The buffer range of a buffer solution prepared by mixing 100mL 0.2mol·L-1H2A solution and 100mL 0.3mol·L-1 NaOH is about ( ). (pK a1=4.00; pK a2=9.00 )A. 3.00~5.00B. 5.00~7.00C. 8.00~10.00D. 9.00~11.0011.If two solutions are mixed in equal volume in each option listed below, which option has no buffer action? ()A. 0.2 mol·L-1 NaOH and 0.2 mol·L-1 KClB. 0.2 mol·L-1 HCl and 0.4 mol·L-1 NH3·H2OC. 0.1 mol·L-1 H3PO4 and 0.1 mol·L-1 Na2HPO4D. 0.1 mol·L-1 NaOH and 0.2 mol·L-1 HAc12.To prepare a buffer of pH 9, which buffer system listed below is the best? ( )A. 0.1 mol·L-1 HAc — 0.1 mol·L-1 NaAc (p K a = 4.75)B. 0.1 mol·L-1 NH3·H2O — 0.1 mol·L-1 NH4Cl (p K b = 4.75)C. 0.1 mol·L-1 H2CO3— 0.15 mol·L-1 NaOH(pK a1=6.37; pK a2=10.25 )D. 0.1 mol·L-1 HCN — 0.02 mol·L-1 NaCN (p K a = 9.5)Ⅱ. Simple answer question1.酸碱滴定Can 0.1000 mol·L-1 formic acid (HCOOH) of 20.00 mL be titrated by 0.1000 mol·L-1 NaOH standard solution directly? Please give reasons. What indicator can be used to signal endpoint (p K a = 3.75)?(5 marks)2.电解质溶液Pivaic acid is a monoprotic weak acid. A 0.100 mol·L-1 solution of pivalic acid has a pH=3.00. What is the pH of 0.100 mol·L-1 sodium pivalate at the same temperature?3. How many significant figures are there in each of the following numbers (assume that each number is a measured value)?3.25 0.0025 0.0203 2.3% 0.900 0.2530 1.3830 2.0 105 pH=3.21Final Examination PaperⅣⅠ. Monochoice questions1.酸碱滴定If you had to do the calculation of (22.83--21.43)/1.4000, what would be the correctresult of significant figure? ( )A. 0.10B. 0.100C. 1.00D. 1.0002.If the K HIn of a weak acidic indicator is 1.0×10-5, what is the color transition pH range of thisindicator? ( )A. 4-6B. 6-8C. 7-9D. 8-103.What result would be if NaOH solution was standardized against potassium hydrogen phthalate, the measured concentrations of the NaOH solution in the cases that the mass of potassium hydrogen phthalate should be 0.3510 g, but was recorded as 0.3570 g by mistake? ( )A. highB. lowC. unchangeD. uncertain4.How many grams of potassium hydrogen phthalate (KHC8H4O4) primary standard substance are required to standardize about 25 mL of 0.1 mol·L-1 NaOH solution? ( ) [Mr(KHC8H4O4)=204 g·mol-1]A. 0.2550gB.0.5100gC. 0.05100gD. 1.0200g5.依数性How much is the normal freezing points of the solution in which 15.4g of urea is dissolved in 66.7 mL of water ? [K f=1.86 K·kg·mol-1 , Mr(CON2H4)=60.0] ( )A. -7.16℃B. 0℃C.-0.25℃D. 1.11℃6.The osmolarity of 1000 mL officinal solution in which it contains NaHCO3 of 6.45g·L -1 and KCl of 5.79 g·L -1 is ( ) mOsmol·L-1. [Mr(NaHCO3)=84, Mr(KCl)=74.5]A. 76.3B. 152.7C. 309D. 6107. A hemoglobin (Hb) solution of 1L is prepared by dissolving 35.0g of Hb into water. If the osmotic pressure of the solution is found to be 1.33kPa at 25℃. The molar mass of hemoglobin is ( ) .A. 537B. 5.37×10-4C. 6.52×104D. 1008.电解质8,9,10,11,12,13,14There is 1L 0.1mol·L-1NaHCO3solution with the addition of0.1mol NaCl solid. Which choice listed below is true? ( )A. pH unchanged, dissociation degree of HCO3 unchange.B. pH decrease, dissociation degree of HCO3 increase.C. pH increase, dissociation degree of HCO3 increase.D. pH increase, dissociation degree of HCO3 decrease.9.what is ionic strength ( I ) for 0.10 mol·kg-1 NaCl solution ( ) mol·kg-1.A. 0.025 mol·kg-1B. 0.050mol·kg-1C. 0.20mol·kg-1D. 0.10mol·kg-1PO ion is 3.3×10-7 10.In a saturated solution of calcium phosphate, the concentration of 34mol·L-1. the K sp of Ca3(PO4)2 is ( )A. 3.3×10-7B. 1.65×10-7C. 9.9×10-21D. 1.3×10-3211.Which substance can use as strong base in glacial acetic acid ?( )A. HAcB. NH3C. H2OD. H3PO412.A solution is 0.15 mol·L-1in Pb2+and 0.20 mol·L-1in Ag+. If a solid of Na2SO4is added slowly to this solution until the Ag+ starts to precipitate as the sulfate. What is SO42- concentration reached at least at this point? ( ) K sp for PbSO4 = 2.53×10-8, Ag2SO4 =1.20×10-5.A. 1.7×10-8B. 2.53×10-8C. 3.0×10-4D. 1.20×10-513.pH of solution in which 0.2 mol·L-1 H3PO4 solution and 0.2 mol·L-1 Na3PO4 solution are mixed in the same volume is ( D ) (Ka1= 7.5×10-3 , Ka2= 6.3×10-8 , Ka3= 2.2×10-13)A. 12.8B. 1.32C. 2.12D. 7.2114.125.0 mL of 0.40 mol·L-1 propanic acid, HPr, is diluted to 500.0 mL. What will the final pH of the solution be? (K a=1.0×10-5) ( )A. 3B. 11C. 5D. 9缓冲溶液15. The buffer range of a buffer solution prepared by mixing 100ml 0.2mol·L -1 H 3PO 4 solution and 100ml 0.5mol·L -1 NaOH is about ( ). (pK a1=2.16; pK a2=7.21; pK a3=12.32 )A. 1.16~3.16B. 6.21~8.21C. 8.00~10.00D. 11.32~13.3216. The color of the solution is yellow with the addition of methyl orange indicator and red with the addition of methyl red. In order to keep the pH of the solution stable, which buffer system listed below is the best? ( )A. 0.1mol·L -1 HAc — 0.1mol·L -1 NaAc (p K a = 4.75)B. 0.1mol·L -1 NH 3·H 2O — 0.1mol·L -1 NH 4Cl (p K b = 4.75)C. 0.1mol·L -1 NaH 2PO 4 — 0.1mol·L -1 Na 2HPO 4 (pK a2=7.21 )D. 0.1mol·L -1 HCN — 0.1mol·L -1 NaCN (p K a = 9.5)17. Which option has largest increase of pH when add 0.5mL of 0.1 mol·L -1 NaOH in the following solution? ( )A. 0.1 mol·L -1 HAc — 0.1 mol·L -1 NaAc (p K a = 4.75)B. 0.1 mol·L -1 NH 3·H 2O — 0.1 mol·L -1 NH 4Cl (p K b = 4.75)C. 0.1 mol·L -1 H 2CO 3 — 0.15 mol·L -1 NaOH (pK a1=6.37; pK a2=10.25 )D. 0.1 mol·L -1 HCN — 0.02 mol·L -1 NaCN (p K a = 9.5)18. To prepare a buffer of pH 10.5, which buffer system listed below is the best? ( )A. CH 3NH 2·HCl —CH 3NH 2 (p K a =10.65)B. NH 3·H 2O —NH 4Cl (p K a =9.25)C. Na 3PO 4 —Na 2HPO 4 (pK a3 = 12.32)D. H 2CO 3—NaHCO 3 (pK a1=6.37)Ⅱ.Calculation1. 电解质Ethylamine, CH 3CH 2NH 2, has a strong, pungent odor similar to that ammonia. Likeammonia, it is a base. A 0.10 mol·L -1 solution has a pH of 11.86. Calculate the K b for theethylamine, and find K a for its conjugate acid, 323NH CH CH .2. Calculate the osmotic pressure of 0.020mol·L-1 NaCl solution at 25 ℃.3. (1) 0.1mol·L-1 HAc solution 。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
2009 —2010学年第一学期
化学与材料学院(系)07级应用化学专业
《专业英语》期末试卷
1.Write the structural formula or Chinese name for each of the following
(2% for each answer):
(1)barium ion: (2)chlorate ion:
(3)potassium ion: (4)carbonic acid:
(5)ammonium ion: (6)pyrrole:(吡咯)
(7)polystyrene: (聚苯乙烯) (8)p-hydroxybenzoic acid:(对羟基苯甲酸)(9)benzonitrile (苄腈) (10)critical pressure: (临界压力)(11)methanal: (甲醛)(12)buffer solution :(缓冲溶液)(13)alkali burette:(碱式滴定管)(14)extract :(萃取)(15)tetrasulfur dinitride: (S4N2)(16)aldose:(醛醣)(17)sodium dihydrogenphosphate (磷酸二氢钠)
(18)zinc oxide:
(19)6-ethyl-4-methyldecane:
(20)quantitative analysis: (定量分析)
2.Write the English name for each of the following(2% for each answer):
(1)IBr: (2)天平(balance)(3)阴离子(anion) (4)H2SO3
(5)滴液漏斗: (dropping funnel)(6)CuNO3:
(7)AgF: (8)滴定(n.):(titrate)(9)Ca(MnO4)2: (10)辛醇:
(11)十三烷:
(12)(CH3CH2)2Hg: (diethylmercury)
(13)
CH3CHCH CH2
CH3:
(14)
CH3CH2CHCOOH
CH3: (15)
CHO
HO
NH2:(2-amino-5-hydroxybenzaldehyde)
3.Translate the following paragraphs into Chinese:
(1)Pick the size of your separatory (sep.) funnel. You will usually use 125 or 250-mL,
large scale reactions (1–10 g) can require 500-mL or 1-L sizes. Remember that your sep. funnel will contain the solvent and wash liquid which must be thoroughly mixed. (5% for the answer)
参考答案:挑选出你要的分液漏斗的大小。
你通常会使用125毫升或者250毫升的分液漏斗,对于大规模的反应(1-10克)你需要使用500毫升或1 L的分液漏斗。
记住你的分液漏斗将含有溶剂和洗涤液,必需予以彻底的混合。
(2)Dilute the crude reaction mixture with your solvent of choice and transfer to your
chosen sep. funnel. Large amounts of material require large amounts of solvent.
Normal reactions (50-500 mg of product) can be diluted with between 25–100 mL of solvent. (5% for the answer)
参考答案:用你所选用的溶剂稀释反应粗产物的混合物,并且转移到你选择的分液漏斗中。
大量的材料需要大量的溶剂。
正常的反应(50-500毫克的产品)能用25-100毫升溶剂稀释。
(3)Dry the organic layer. After removing your solution from the aqueous phase, a
drying agent is added to remove all traces of water. This is usually MgSO4, more effective and faster, but slightly acidic; or Na2SO4, less effective and slower, but neutral. These compounds bind to any water remaining in the organic solution, forming clumps when they react. A decent amount of drying agent should be added, but as long as some solid is not clumped, no more needs to be added. (6% for the answer)
参考答案:干燥的有机层:从水相中取出溶液后,干燥剂的加入是为了除去遗留下来的水份。
通常使用硫酸镁作干燥剂,因为它非常有效而且吸水很快,但是略带酸性,或者也可以用硫酸钠,只是效益比较和速度比较慢,但是中性的。
这些化合物会和有机溶液中的水相结合,当它们发生反应时就会形成团块。
应该有足够量的干燥剂被加入,但是只要一些固体不会形成团块,就不需要再加入更多的干燥剂。
(4)Distillation is an extremely useful technique that is used to purify reagents and
separate crude product mixtures. There are two varieties of distillation atmospheric pressure and reduced pressure. (4% for the answer)
(5)
参考答案:蒸馏是一个非常有用的技术,用于分离纯化试剂和粗产品的混合物。
一般有两种蒸馏方法:常压蒸馏和减压蒸馏
以上的参考答案由吴明江提供,仅供参考。
4.Read the Abstract from Reactive & Functional Polymers 66 (2006) 1278–1283and
then answer the questions:(中文回答)
Tuthor: Qinghua Zhang, Shouxin Wen, Ziqiang Lei .
Title:Heterogeneous Baeyer-Villiger oxidation of ketones using hydrogen peroxide
as oxidant catalyzed by aminomethyl polystyrene resin-supported tin complex.
Abstract: An aminomethyl polystyrene resin supported tin complex (PS-Sn) catalyst
was prepared by a simple procedure and found to be an active and selective catalyst
for the Baeyer-Villiger (BV) oxidation of cyclic and acyclic ketones including
2-adamantanone, 2-methylcyclohexanone, cyclohexanone, 4-tert-butylcyclohexanone,
4-methylcyclohexanone, 3-methyl-2-pentanone, 4-methyl-2-pentanone, and
cyclopentanone using 30% hydrogen peroxide as oxidant. This polymer-supported tin
complex acts as an efficient and stable heterogeneous catalyst for the Baeyer-Villiger
oxidation of ketones by 30% hydrogen peroxide. The catalyst can be prepared in large
scale in a simple manner and can be recycled.
(1) What oxidant was used in the BV oxidation (3% for the answer)
(2) What do the words “acyclic” and “heterogeneous” mean (3% for the answer)
(3) What are the advantages of the catalyst (4% for the answer)
Heterogeneous 非均相的acyclic 无环的catalyst 催化剂。
