美国环保局 EPA 试验 方法 9066Phenolics (Colorimetric, Automated 4-AAP with Distillation)
epa毒理参数和筛选值

epa毒理参数和筛选值一、概述EPA(美国环保署)毒理参数和筛选值是用于评估化学品毒性的重要标准。
这些参数和值是根据大量的科学研究和实践经验得出的,用于指导化学品的安全使用和管理。
本文将介绍EPA毒理参数和筛选值的基本概念、目的和用途。
二、定义与范围EPA毒理参数和筛选值通常涉及化学品的生物累积性、毒性效应、暴露评估等方面。
这些参数和值适用于各种环境介质(如水、空气、土壤等)和生物体,包括人类和其他动物。
这些参数和值的范围广泛,包括急性毒性、慢性毒性、生态毒性、致畸毒性等。
三、评估方法评估化学品毒性通常采用实验方法,包括动物实验和人体研究。
实验过程中,需要确定合适的剂量范围和暴露时间,以模拟实际环境中的暴露情况。
实验结果将用于计算化学品对生物体的毒性效应,并据此得出相应的毒理参数和筛选值。
四、毒理参数与筛选值的差异毒理参数是指化学品对生物体造成危害的综合能力,通常由一组实验结果得出。
而筛选值是指针对特定目标(如特定组织或器官)或特定生物群体的化学品毒性参数的较低阈值,用于初步判断化学品是否可能对生物体造成危害。
五、应用与影响EPA毒理参数和筛选值对于环境保护和公共健康至关重要。
它们为化学品的风险评估和管理提供了依据,有助于制定合理的政策和管理措施,确保公共安全和生态环境不受损害。
此外,这些参数和值也为科研人员提供了研究化学品毒性的基础数据,有助于推动毒理学研究的发展。
六、结论EPA毒理参数和筛选值是评估化学品毒性的重要标准,涵盖了广泛的化学品和环境介质。
通过实验方法和科学研究,这些参数和值被用来评估化学品的综合毒性,并确定较低的阈值用于初步判断化学品是否可能对生物体造成危害。
这些参数和值对于环境保护和公共健康至关重要,为化学品的风险评估和管理提供了依据,有助于制定合理的政策和管理措施,确保公共安全和生态环境不受损害。
七、建议与展望为了更好地应对化学品对环境和人类健康的威胁,建议加强毒理学研究,提高毒理参数和筛选值的准确性和适用性。
epa标准限值

epa标准限值EPA(美国环境保护局)标准限值是为了保护环境和公共健康而设定的一系列规定和标准。
这些限值通常涉及到大气、水体和土壤中的污染物浓度,以及工业和交通等活动中的排放标准。
下面将对EPA标准限值的背景、种类以及其在不同领域的应用进行详细介绍。
1. 背景:美国环境保护局成立于1970年,其任务之一是通过制定标准限值来防止和控制环境污染。
这些标准限值是根据科学研究和公众健康风险评估制定的,目的是确保空气、水和土壤的质量达到安全水平。
2. 种类:EPA标准限值主要分为空气质量标准(Air Quality Standards)和水质标准(Water Quality Standards)两大类。
•空气质量标准:空气质量标准规定了大气中特定污染物的最大允许浓度,以及评估和监测这些浓度的方法。
常见的空气污染物包括颗粒物(PM2.5和PM10)、臭氧(O3)、二氧化硫(SO2)、一氧化碳(CO)等。
这些标准通过监测站点的数据来评估,并在不同地区设置不同的标准以适应当地气候和人口密度。
•水质标准:水质标准涉及到表面水体(如河流、湖泊)和地下水中的各种物质的浓度限制。
这些物质包括溶解氧、营养物质(如氮和磷)、重金属(如铅、汞)以及各种有机化合物。
水质标准的设定旨在确保水体适合饮用、游泳和支持水生生物。
3. 应用领域:•工业排放:EPA标准限值对工业排放物的控制至关重要。
通过规定特定污染物的排放限值,EPA确保工业活动对环境的影响处于可接受的范围,从而减少空气、水体和土壤的污染。
•交通排放:车辆排放是空气污染的重要来源之一。
EPA通过制定车辆排放标准,要求汽车制造商采用更清洁的燃烧技术,从而减少尾气中的有害物质,改善空气质量。
•废物处理:EPA标准限值也适用于废弃物的处理和处置。
这包括垃圾填埋场和工业废物处理设施,规定了废物中允许的污染物浓度,以及如何安全地处置废物。
总的来说,EPA标准限值在维护环境和公共健康方面发挥着关键作用。
美国VOCs监测的标准规范
美国VOCs监测的标准规范文章导读美国EPA关于VOCs分析方法有3种:环境空气、室内空气和固定污染源。
而EPA也颁布了一系列仪器性能要求标准。
本文从上述两个方面介绍了美国关于VOCs 监测的标准规范。
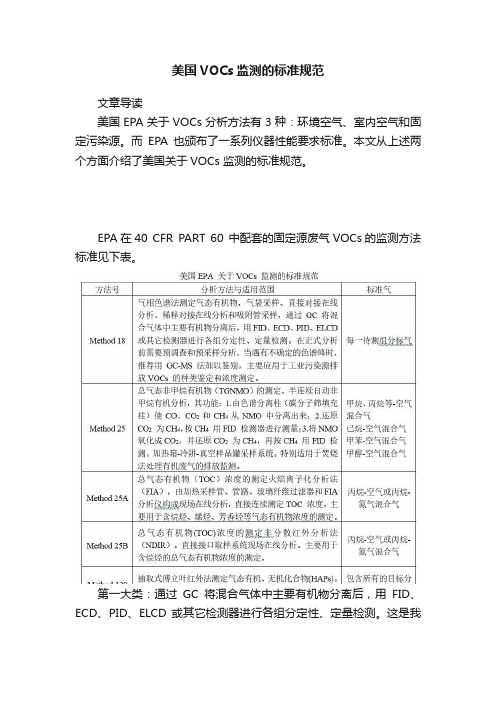
EPA在40 CFR PART 60 中配套的固定源废气VOCs的监测方法标准见下表。
第一大类:通过GC将混合气体中主要有机物分离后,用FID、ECD、PID、ELCD 或其它检测器进行各组分定性、定量检测。
这是我们国内常用的GC-FID在线系统,PID也运营较多,因价格差异和效果差异巨大,一般监管单位目前在固定源上的VOCs在线监测要求均为GC-FID原理。
第二大类:总气态有机物(TOC)浓度的测定火焰离子化分析法(FIA),由加热采样管、管路、玻璃纤维过滤器和FIA 分析仪构成现场在线分析,直接连续测定TOC 浓度。
此法在国内固定源VOCs监测运营不多。
第三大类:总气态有机物(TOC)浓度的测定非分散红外分析法(NDIR)。
直接接口取样系统现场在线分析。
主要用于含烷烃的总气态有机物浓度的测定。
NDIR主要为日本技术,国内有代理商。
第四大类:抽取式傅立叶红外法(FTIR)测定气态有机、无机化合物(HAPs)。
明确水蒸气和二氧化碳是红外波段最普遍的干扰。
主要运用在厂界监测,溯源,及目前国内发展的所谓“空间立体监测”。
值得一直提的是,EPA颁布了一系列仪器性能要求标准(PerformanceSpecification, PS),其中PS8是污染源VOCs在线监测仪器的总纲,PS8A是用FID原理监测总烃仪器的技术要求,PS9针对气相色谱法监测VOCs的仪器,PS15针对傅立叶红外法监测VOCs的仪器,见下表。
素材来源:VOCs减排工作站编辑:VOCs前沿。
EPA方法索引

EPA方法索引和相关标准品EPA 是美国国家环境保护局(U.S Environmental Protection Agency) 的英文缩写。
它的主要任务是保护人类健康和自然环境。
EPA 制定了一系列标准分析方法用于环境监测领域。
主要包括:EPA T01~T14 系列标准分析方法——空气中有毒有机物分析方法EPA IP1~IP10 系列标准分析方法——室内空气污染物的分析测定方法EPA 200 系列标准分析方法———金属的分析方法EPA 500 系列标准分析方法——饮用水中有机物的分析方法EPA 600 系列标准分析方法——城市和工业废水中有机化合物的分析方法SW -846 系列标准分析方法——固体废弃物试验分析评价手册1300 系列是毒性试验方法3000 系列是金属元素的提取方法3500 系列是半(非) 挥发性有机物的提取方法3600 系列是净化、分离方法5000 系列是挥发性有机物的提取方法6000 系列是测定金属的新方法7000 系列是原子吸收法测定金属元素8000 系列是有机物分析方法9000 系列是常规项目分析方法其中,500系列,600系列和8000系列是环境种有机物分析最常用的方法。
EPA 600系列方法是美国为贯彻“净水法”(CW A) 、“全国水体污染物排放消除制度”(NPDES) 和“许可证制度”,严格控制点源排放,保护地表水,使其免受城市和工业废水中有机物的污染而制定的。
EPA 500 系列方法是为执行“安全饮用水法”(SDW A) 和“国家一级饮用水法案”(National Primary Drinking Water Regulations) ,确保饮用水及饮用水源的质量而制订的。
EPA 500 系列是针对比较洁净的水样(饮用水、地下水、地表水) 开发的,有些方法仅用试剂水和饮用水验证过SW-846 系列集中贯彻了“资源保护回收法”和“陆地处置限制法规”的精神,包含了固体废弃物采样和分析试验的全部方法, 是在EPA200 ~EPA 600 系列的基础上发展起来的。
美国EPA200种潜在致癌物的危害等级

美国EPA200种潜在致癌物的危害等级致癌性是筛选优先污染物的重要依据之一,下表列出了美国EPA公布的200种致癌剂的危害等级。
其中的参数含义为:1、证据的充分程度(Degree of Evidence)化学品对人体的致癌性证据之充分程度可以分为下列几类。
(1)证据充分,指致癌剂和人体癌症之间有因果关系。
(2)证据有限,指能提供一些可信的致癌性证据,但证据尚有限,还需作进一步补充。
(3)证据不充分,可能有3种情况,①能获取的致癌性数据很少;②与证据有关的研究尚不能排除偶然性、误差及混淆等情况;③研究结果无致癌性证据。
根据动物实验取得的致癌性证据的充分程度可分4级。
1级,致癌性证据充分。
2级,致癌性证据有限。
3级,致癌性证据不充分。
4级,无致癌性证据。
2、IARC标准分组国际癌症研究所(International Agency for research on cancer,简称IARC)将人类的肿瘤风险分为3组。
1组:列在此组内的化学品属致癌物,流行病学和暴露实验均已肯定,基致癌证据是充分的。
2组:化学品可能对人体有致癌性。
其中有的对人体的致癌性证据几乎是“充分的”,另一类的证据不够充分。
证据程度较高的为A组,较低的为B组。
例如,2A指对人体的致癌性至少存在着有限证据。
当动物证据充分而人体数据不充分时,归入2B。
3组:列在本组中的化学品对人类没有致癌性。
3、潜力因素值F(Potency Factor Estimate)潜力因素值F定义为1/ED10。
ED10等于10%终身致癌风险的致癌剂剂量。
F可以和致癌性的确认证据一起,用来划分化学品潜在致癌性的危险等级。
4、潜力因素分组(Potency factor Grouping)由于潜力因素值F可表示致癌危险性的相对大小,因而,可将潜在致癌剂的相对潜力因素分为4组。
潜力因素最高的化学品分在1组,中等潜力因素的为2组,低潜力因素的为3组,最低潜力因素的为4组。
美国环保局 EPA 试验 方法美国环保局 EPA 试验 方法 9045dSoil and Waste pH

METHOD 9045DSOIL AND WASTE pH1.0SCOPE AND APPLICATION1.1This method is an electrometric procedure for measuring pH in soils and waste samples. Wastes may be solids, sludges, or non-aqueous liquids. If water is present, it must constitute less than 20% of the total volume of the sample.2.0SUMMARY OF METHOD2.1The sample is mixed with reagent water, and the pH of the resulting aqueous solution is measured.3.0INTERFERENCES3.1Samples with very low or very high pH may give incorrect readings on the meter. For samples with a true pH of >10, the measured pH may be incorrectly low. This error can be minimized by using a low-sodium-error electrode. Strong acid solutions, with a true pH of <1, may give incorrectly high pH measurements.3.2Temperature fluctuations will cause measurement errors.3.3Errors will occur when the electrodes become coated. If an electrode becomes coated with an oily material that will not rinse free, the electrode can (1) be cleaned with an ultrasonic bath, or (2) be washed with detergent, rinsed several times with water, placed in 1:10 HCl so that the lower third of the electrode is submerged, and then thoroughly rinsed with water, or (3) be cleaned per the manufacturer's instructions.4.0APPARATUS AND MATERIALS4.1pH meter with means for temperature compensation.4.2Glass electrode.4.3Reference electrode -- A silver-silver chloride or other reference electrode of constant potential may be used.NOTE:Combination electrodes incorporating both measuring and referenced functions are convenient to use and are available with solid, gel-type filling materials that requireminimal maintenance.4.4Beaker -- 50-mL.4.5Thermometer and/or temperature sensor for automatic compensation.4.6Analytical balance -- capable of weighing 0.1 g.5.0REAGENTS5.1Reagent grade chemicals shall be used in all tests. Unless otherwise indicated, it is intended that all reagents shall conform to the specifications of the Committee on Analytical Reagents of the American Chemical Society, where such specifications are available. Other grades may be used, provided it is first ascertained that the reagent is of sufficiently high purity to permit its use without lessening the accuracy of the determination.5.2Reagent water. All references to water in this method refer to reagent water, as defined in Chapter One.5.3Primary standard buffer salts are available from the National Institute of Standards and Technology (NIST) and should be used in situations where extreme accuracy is necessary. Preparation of reference solutions from these salts requires some special precautions and handling, such as low-conductivity dilution water, drying ovens, and carbon-dioxide-free purge gas. These solutions should be replaced at least once each month.5.4Secondary standard buffers may be prepared from NIST salts or purchased as solutions from commercial vendors. These commercially available solutions, which have been validated by comparison with NIST standards, are recommended for routine use.6.0SAMPLE PRESERVATION AND HANDLINGSamples should be analyzed as soon as possible.7.0PROCEDURE7.1Calibration7.1.1Because of the wide variety of pH meters and accessories, detailedoperating procedures cannot be incorporated into this method. Each analyst must beacquainted with the operation of each system and familiar with all instrument functions.Special attention to care of the electrodes is recommended.7.1.2Each instrument/electrode system must be calibrated at a minimum oftwo points that bracket the expected pH of the samples and are approximately three pH units or more apart. Repeat adjustments on successive portions of the two buffersolutions until readings are within 0.05 pH units of the buffer solution value. If anaccurate pH reading based on the conventional pH scale [0 to 14 at 25 E C] is required, the analyst should control sample temperature at 25 ± 1 E C when sample pH approaches the alkaline end of the scale (e.g., a pH of 11 or above).7.2Sample preparation and pH measurement of soils:7.2.1To 20 g of soil in a 50-mL beaker, add 20 mL of reagent water, cover, andcontinuously stir the suspension for 5 min. Additional dilutions are allowed if working with hygroscopic soils and salts or other problematic matrices.7.2.2Let the soil suspension stand for about 1 hr to allow most of thesuspended clay to settle out from the suspension or filter or centrifuge off the aqueousphase for pH measurement.7.2.3Adjust the electrodes in the clamps of the electrode holder so that, uponlowering the electrodes into the beaker, the glass electrode will be immersed just deep enough into the clear supernatant solution to establish a good electrical contact through the ground-glass joint or the fiber-capillary hole. Insert the electrodes into the samplesolution in this manner. For combination electrodes, immerse just below the suspension.7.2.4If the sample temperature differs by more than 2 E C from the buffersolution, the measured pH values must be corrected.7.2.5Report the results as "soil pH measured in water at E C" where " E C" isthe temperature at which the test was conducted.7.3Sample preparation and pH measurement of waste materials7.3.1To 20 g of waste sample in a 50-mL beaker, add 20 mL of reagent water,cover, and continuously stir the suspension for 5 min. Additional dilutions are allowed if working with hygroscopic wastes and salts or other problematic matrices.7.3.2Let the waste suspension stand for about 15 min to allow most of thesuspended waste to settle out from the suspension or filter or centrifuge off aqueousphase for pH measurement.NOTE:If the waste is hygroscopic and absorbs all the reagent water, begin theexperiment again using 20 g of waste and 40 mL of reagent water.NOTE:If the supernatant is multiphasic, decant the oily phase and measure the pH of the aqueous phase. The electrode may need to be cleaned (Step 3.3) if itbecomes coated with an oily material.7.3.3Adjust the electrodes in the clamps of the electrode holder so that, uponlowering the electrodes into the beaker, the glass electrode will be immersed just deep enough into the clear supernatant to establish good electrical contact through the ground-glass joint or the fiber-capillary hole. Insert the electrode into the sample solution in this manner. For combination electrodes, immerse just below the suspension.7.3.4If the sample temperature differs by more than 2 E C from the buffersolution, the measured pH values must be corrected.7.3.5Report the results as "waste pH measured in water at E C" where " E C"is the temperature at which the test was conducted.8.0QUALITY CONTROL8.1Refer to Chapter One for the appropriate QC protocols.8.2Electrodes must be thoroughly rinsed between samples.9.0METHOD PERFORMANCE9.1No data provided.10.0REFERENCES1.Black, Charles Allen; Methods of Soil Analysis; American Society of Agronomy:Madison, WI, 1973.2.National Bureau of Standards, Standard Reference Material Catalog, 1986-87, SpecialPublication 260.METHOD 9045D SOIL AND WASTE pH。
美国EPA 关于空气自动监测系统性能指标的规定和测试方法
美国EPA关于大气自动监测系统性能指标的规定和测试方法引言环境空气污染的自动监测方法有多种,一般采用湿法和干法两种。
湿法是基于化学量理论的库仑法和电导法等测量原理,需使用大量试剂,存在试剂调整和废液处理等问题,操作比较繁琐,故障率较高,维护工作量较大;干法是基于物理光谱测量理论,使样品始终保持在气体状态,没有试剂的损耗,维护工作量较小。
比如SO2测量采用紫外荧光法,NOx测量采用化学发光法,O3测量采用紫外光度法,CO测量采用气体过滤相关分析法等,目前我国绝大部分空气自动监测采用的是该方法。
干法测量以欧美为主。
美国开展空气自动监测已有30年的历史,在空气自动监测方面积累了丰富的经验,并制定了详细的规范。
其中物理光谱法作为美国EPA的推荐方法,得到了广泛的应用。
湿法测量以日本为主,但自1996年起日本在法定的测量方法中增加了干式测量法。
利用物质的光谱特性进行污染物的分析已成为自动监测仪器发展的必然趋势。
我国在环境空气质量监测和质量保证方面的规定都参考了美国国家环保署(EPA)的规定。
目前,大气自动监测和空气质量日报工作在我国大部分省市已广泛开展,自动监测仪器监测数据的准确可靠是日报工作中的基础。
为使监测人员了解美国EPA关于空气自动监测的相关规定,特将其有关SO2、NO2、O3、CO自动监测仪器的性能指标规定和测试方法作简要说明,以供参考。
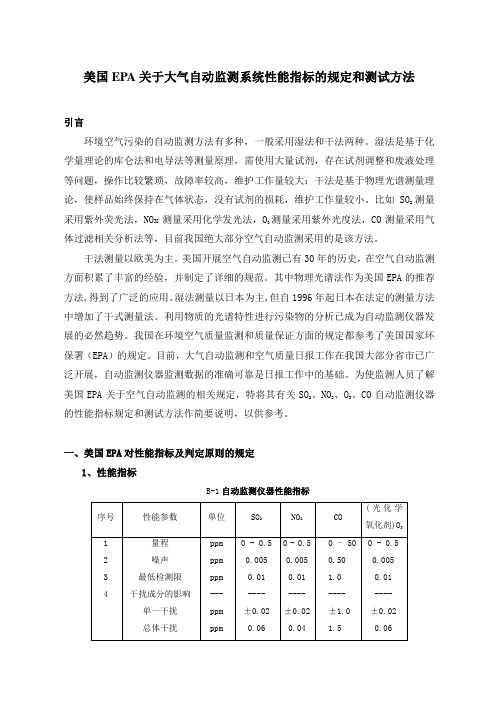
一、美国EPA对性能指标及判定原则的规定1、性能指标B-1自动监测仪器性能指标M/0.02447,M是该气体的摩尔质量。
2、判定原则对于每个性能指标(量程除外),测试程序从开始起要重复7次,得到7组测试结果。
每组结果要和表B-1中的规定指标相比较,高于或超出规定指标的值是一个超标值。
每个参数的7个结果说明如下:(1)0次超标:被测的参数合格;(2)3次或更多次超标:该参数不合格;(3)1次或2次超标:再重复测试该参数 8次,得到共15个测试结果。
将此15个测试结果说明如下:a:1次或2次超标:通过测试;b:3次以上:该参数不合格。
epa测试标准

epa测试标准
EPA(美国环保局)的测试标准是针对各种污染物和产品制定的,旨在确保产品符合相关法规和标准,以保护环境和人类健康。
以下是一些EPA的测试标准:
污染物排放限制:EPA制定了一系列污染物排放限制,要求企业、工厂和机构遵守。
这些限制涵盖了空气、水和土壤中的污染物,如二氧化硫、氮氧化物、挥发性有机化合物等。
消费品安全标准:EPA负责制定和执行消费品安全标准,以确保消费品不会对人类健康造成危害。
这些标准涵盖了家电、家具、儿童用品、玩具、化妆品等产品。
农药注册与评价:EPA负责评价农药的安全性和有效性,以确保农药的使用不会对人类健康和环境造成危害。
化学物质管理:EPA负责管理和限制化学物质的生产和使用,以确保这些物质不会对环境和人类健康造成危害。
环保设备与系统性能测试:EPA还制定了各种环保设备与系统性能测试标准,以确保这些设备能够有效地降低污染物的排放。
以上是一些EPA的测试标准,具体标准可能因产品、污染物和法规而有所不同。
在进行相关测试时,建议咨询专业的环保机构或实验室,以确保测试结果的准确性和可靠性。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
9066 1CD-ROM Revision 0 Date September 1986METHOD 9066PHENOLICS (COLORIMETRIC, AUTOMATED 4-AAP WITH DISTILLATION)1.0SCOPE AND APPLICATION1.1This method is applicable to the analysis of ground water and of drinking, surface, and saline waters.1.2The method is capable of measuring phenolic materials from 2 to 500ug/L in the aqueous phase using phenol as a standard.2.0SUMMARY OF METHOD2.1This automated method is based on the distillation of phenol and subsequent reaction of the distillate with alkaline ferricyanide (K Fe(CN)) and 364-amino-antipyrine (4-AAP) to form a red complex which is measured at 505 or 520 nm.3.0INTERFERENCES3.1Interferences from sulfur compounds are eliminated by acidifying the sample to a pH of <4.0 with H SO and aerating briefly by stirring.243.2Oxidizing agents such as chlorine, detected by the liberation of iodine upon acidification in the presence of potassium iodide, are removed immediately after sampling by the addition of an excess of ferrous ammonium sulfate (5.5). If chlorine is not removed, the phenolic compounds may be partially oxidized and the results may be low.3.3Background contamination from plastic tubing and sample containers is eliminated by filling the wash receptacle by siphon (using Kel-F tubing) and using glass tubes for the samples and standards.4.0APPARATUS AND MATERIALS4.1Automated continuous-flow analytical instrument:4.1.1Sampler : Equipped with continuous mixer.4.1.2Manifold .4.1.3Proportioning pump II or III .4.1.4Heating bath with distillation coil .4.1.5Distillation head .9066 2CD-ROM Revision 0 Date September 19864.1.6Colorimeter: Equipped with a 50 mm flowcell and 505 or 520nm filter.4.1.7Recorder .5.0REAGENTS5.1ASTM Type II water (ASTM D1193): Water should be monitored for impurities.5.2Distillation reagent: Add 100 mL of concentrated phosphoric acid (85% H PO ) to 800 mL of Type II water, cool and dilute to 1 liter.345.3Buffered potassium ferricyanide: Dissolve 2.0 g potassium ferricyanide, 3.1 g boric acid, and 3.75 g potassium chloride in 800 mL of Type II water. Adjust to pH of 10.3 with 1 N sodium hydroxide (5.3) and dilute to 1liter. Add 0.5 mL of Brij-35 (available from Technicon). (Brij-35 is a wetting agent and is a proprietary Technicon product.) Prepare fresh weekly.5.4Sodium hydroxide (1 N): Dissolve 40 g NaOH in 500 mL of Type II water, cool and dilute to 1 liter.5.54-Aminoantipyrine: Dissolve 0.65 g of 4-aminoantipyrine in 800 mL of Type II water and dilute to 1 liter. Prepare fresh each day.5.6Ferrous ammonium sulfate: Dissolve 1.1 g ferrous ammonium sulfate in 500 mL Type II water containing 1 mL H SO and dilute to 1 liter with freshly 24boiled and cooled Type II water.5.7Stock phenol: Dissolve 1.00 g phenol in 500 mL of Type II water and dilute to 1,000 mL. Add 0.5 mL concentrated H SO as preservative (1.0 mL =241.0 mg phenol).CAUTION: This solution is toxic.5.8Standard phenol solution A: Dilute 10.0 mL of stock phenol solution (5.6) to 1,000 mL (1.0 mL = 0.01 mg phenol).5.9Standard phenol solution B: Dilute 100.0 mL of standard phenol solution A (5.8) to 1,000 mL with Type II water (1.0 mL = 0.001 mg phenol).5.10Standard phenol solution C: Dilute 100.0 mL of standard phenol solution B (5.9) to 1,000 mL with Type II water (1.0 mL = 0.0001 mg phenol).5.11Using standard solution A, B, or C, prepare the following standards in 100-mL volumetric flasks. Each standard should be preserved by adding 2 drops of concentrated H SO to 100.0 mL:249066 3CD-ROM Revision 0 Date September 1986Standard Solution (mL) Concentration (ug/L)Solution C1.01.02.02.03.03.0 5.05.0Solution B1.010.0 2.020.0 5.050.0 10.0100.0Solution A2.0200.0 3.0300.0 5.0500.06.0SAMPLE COLLECTION, PRESERVATION, AND HANDLING6.1All samples must have been collected using a sampling plan that addresses the considerations discussed in Chapter Nine of this manual.6.2Biological degradation is inhibited by the acidification to a pH <4with H SO . The sample should be kept at 4E C and analyzed within 28 days of 24collection.7.0PROCEDURE7.1Set up the manifold as shown in Figure 1.7.2Fill the wash receptacle by siphon. Use Kel-F tubing with a fast flow (1 liter/hr).7.3Allow colorimeter and recorder to warm up for 30 min. Run a baseline with all reagents, feeding Type II water through the sample line. Use polyethylene tubing for sample line. When new tubing is used, about 2 hr may be required to obtain a stable baseline. This 2-hr time period may be necessary to remove the residual phenol from the tubing.7.4Place appropriate phenol standards in sampler in order of decreasing concentration. Complete loading of sampler tray with unknown samples, using glass tubes. If samples have not been preserved as instructed in Paragraph 6.2,add concentrated H SO to 100 mL of sample. Run with sensitivity setting at full 24scale or 500.9066 4CD-ROM Revision 0Date September 19867.5Switch sample from Type II water to sampler and begin analysis.7.6Calculation:7.6.1Prepare a linear standard curve by plotting peak heights ofstandards against concentration values. Compute concentration of samples by comparing sample peak heights with standards.8.0QUALITY CONTROL8.1All quality control data should be maintained and available for easy reference or inspection.8.2Calibration curves must be composed of a minimum of a blank and three standards. A calibration curve should be made for every hour of continuous sample analysis.8.3Dilute samples if they are more concentrated than the highest standard or if they fall on the plateau of a calibration curve.8.4Employ a minimum of one blank per sample batch to determine if contamination has occurred.8.5Verify calibration with an independently prepared check standard every 15 samples.8.6Run one spike duplicate sample for every 10 samples. A duplicate sample is a sample brought through the whole sample preparation and analytical process.9.0METHOD PERFORMANCE9.1In a single laboratory using sewage samples at concentrations of 3.8, 15, 43, and 89 ug/L, the standard deviations were +0.5, +0.6, +0.6, and +1.0 ug/L, respectively. At concentrations of 73, 146, 299, and 447 ug/L, the standard deviations were +1.0, +1.8, +4.2, and +5.3 ug/L, respectively.9.2In a single laboratory using sewage samples at concentrations of 5.3 and 82 ug/L, the recoveries were 78% and 98%, respectively. At concentrations of 168 and 489 ug/L, the recoveries were 97% and 98%, respectively.9066 5CD-ROM Revision 0Date September 198610.0REFERENCES1.Gales, M.E. and R.L. Booth, "Automated 4AAP Phenolic Method," AWWA 68, 540 (1976).2.Standard Methods for the Examination of Water and Wastewater, 14th ed., p. 574, Method 510, (1975).3.Technicon AutoAnalyzer II Methodology, Industrial Method No.127-71W, AA II.9066 6CD-ROM Revision 0Date September 19869066 7CD-ROM Revision 0Date September 1986。
