Assessing the antioxidant and pro-oxidant activity of phenolic compounds by means of their copper
2011-Antioxidant, cytotoxic, antitumor, and protective DNA=FX=抗氧化细胞毒等

Pharmacognosy Res. 2011 Jul-Sep; 3(3): 160–165.PMCID: PMC3193615 doi: 10.4103/0974-8490.85000Copyright : © Pharmacognosy ResearchAntioxidant, cytotoxic, antitumor, and protective DNA damage metabolites from the red sea brown alga Sargassum spSeif-Eldin N. Ayyad, Saleh T. Ezmirly, Salim A. Basaif, Walied M. Alarif,1 Adel F. Badria,2 and Farid A. Badria3Department of Chemistry, Faculty of Science, King Abdul Aziz University, P. O. 80203, Jeddah 21589, KSA1Department of Marine Chemsitry, Faculty of Marine Sciences, King Abdulaziz University, Jeddah 21589, P. O. 80207, KSA2Tissue Engineering Lab., Faculty of Dentistry, Alexandria University, Alexandria, Egypt3Department of Pharmacognosy, Faculty of Pharmacy, Mansoura University, Mansoura 35516, Egypt Address for correspondence: Dr. Seif-Eldin N Ayyad, Faculty of Sciences, King Abdulaziz University, Jeddah, Saudi Arabia. E-mail: snayyad2@Received February 1, 2011; Revised April 6, 2011; Accepted September 16, 2011.This is an open-access article distributed under the terms of the Creative CommonsAttribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.AbstractBackground:Macroalgae can be viewed as a potential antioxidant and anti-inflammatory sources owing to their capability of producing compounds for its protection from environmental factors such as heat, pollution, stress, oxygen concentration, and UV radiations.Objective:To isolate major compounds which are mainly responsible for the pharmacological activity of brown alga under investigation, Sargassum sp.Materials and Methods:Algal material was air dried, extracted with a mixture of organic solvents, and fractionated with different adsorbents. The structures of obtained pure compounds were elucidated with different spectroscopic techniques, and two pure materialswere tested for protection of DNA from damage, antioxidant, antitumor, and cytotoxicity.Results:Four pure compounds were obtained, of which fucosterol (1) and fucoxanthin (4) were tested; it was found that fucoxanthin has strong antioxidant and cytotoxicity against breast cancer (MCF-7) with IC50 = 11.5 μg/ml.Conclusion:The naturally highly conjugated safe compound fucoxanthin could be used as antioxidant and as an antitumor compound.Keywords: Antioxidant, cytotoxicity, fucoxanthin, Sargassum spOther Sections▼AbstractINTRODUCTIONMATERIALS AND METHODSRESULTS AND DISCUSSIONREFERENCESThe genus Sargassum belongs to brown algae of class Phaeophyceae in the order Fucales. Numerous species are distributed throughout the tropical and subtropical oceans of the world, where they generally inhabit shallow water and coral reefs. However, the genus may be well known for its planktonic (free-floating) species.[1] It was clear from the literatures that more than 62 species of the genus Sargassum has been investigated,[2] and indicated its productivity with impressive diversity of natural compounds. For instances, the secondary metabolites contain different structural classes such as plastoquinones,[3–5] chromanols,[6] chromenes,[7,8] steroids,[9] and glycerides.[10] The publications showed that, Sargassum metabolites have wide range of biological activities, which include antibiotic,anti-HIV, anticoagulant, anticonvulsant, anti-inflammatory, antineoplastic, and antitumor.[11–14]In continuation of our search program, which interested in the isolation of secondary metabolites from marine sources, especially macroalgae collected from Red Sea,[15–17] a marine macroalgae, identified as Sargassum sp., collected from El- Shuaiba lagoon 80 km south of Jeddah, was investigated. The total extract (Pet. ether: Chloroform: Methanol [1 : 1 : 1]) had been fractionated using differentchromatographic techniques and afforded four metabolites; fucosterol (1), saringosterone (2), saringosterol (3), and fucoxanthin (4). 1 and 4 had been tested toward the Bleomycin-dependent DNA damage, cytotoxicity against HepG2 (human hepatocellular liver carcinoma cell line), WI 38 (Skin carcinoma cell line), Vero (cell line was initiated from the kidney of a normal adult African green monkey), MCF-7 (breast cancer cell lines), antitumor, and antioxidant using2,2‘-azino-bis-3-ethylbenzthiazoline-6-sulfonic acid (ABTS).Other Sections▼AbstractINTRODUCTIONMATERIALS AND METHODSRESULTS AND DISCUSSIONREFERENCESApparatus and materialChromatographic material: Aluminum oxide type 60-120 mesh was used for column chromatography. Thin-layer chromatography (TLC) silica gel GF 254 was used for TLC. Preparative thin-layer chromatography (PTLC) was performed on aluminum oxide plates (20 × 20 cm) of 250-mm thickness. Electron impact mass spectra were determined at 70 eV on a Kratos MS-25 instrument. 1D and 2D NMR (Nuclear Magnetic Resonance) spectra were recorded on Bruker AVANCE III WM 600 MHz spectrometers and 13C NMR at 150 MHz Chemical shift s are given in δ (ppm) relative to TMS (Tetramethyl silane) as internal standard. The Infra Red (IR) spectra were recorded on a Perkin Elmer spectrometer model 100. The spray reagent used is 50%-sulfuric acid in methanol as spraying reagent. The chromatoplate was heated after spraying at 100 to 105°C until the spots attained maximum color intensity. The alga was described as Sargassum (Family Sargassaceae) and was collected by hand from El- Shuaiba lagoon 80 km south of Jeddah, Saudi Arabia, in the Red Sea, in June 2010.Extraction of Sargassum spThe air-dried algal material (350 g) was extracted by equal volume of mixture of Pet. ether, chloroform, and methanol (2 × 6 l, 24 hours for each batch) at room temperature. The extract was concentrated under reduced pressure to obtain 10 g residue. This material was chromatographed on a column of Silica gel.Column chromatographySilica gel column (500 g, 80 × 2.5 cm) was used for resolution. The residue (10 g) was homogenized with small amount of silica gel (50 g) and poured on to the top of the column which was packed in Pet. ether (40: 60). The eluent were used successively (Pet. ether- Ether, Pet. ether- Ethyl acetate). Fractions were collected (50 ml); Pet. ether- Ether mixture of increasing polarity by diethyl ether and then ethyl acetate and followed by TLC using silica-gel chromatoplates, appropriate solvent system and 50% sulfuric acid in methanol as spraying reagent. If the material was not pure, preparative TLC was applied using the appropriate solvent system and the adsorbent aluminum oxide for purification.Isolated compoundsThe fraction A eluted by Pet. ether-Ether (6 : 4) was collected and rechromatographed over PTLC of silica gel using Pet. Ether-ethyl acetate (8 : 2) to give three compounds: 1 , 2, and 3. The fraction B eluted by Pet. ether-Ethyl acetate (6 : 4) was collected and rechromatographed on Sephadex LH-20 using a mixture of MeOH-CHCl3 (9 : 1) and then finally purified by preparative TLC of silica gel using chloroform methanol (9.5 : 0.5) to afford a pure compound 4.Fucosterol (1), (24E)-Stigmasta-5, 24(28)-diene-3β-ol: white solid (30 mg, 0.008% dry wt) m.p. 133-135°C. IR (cm-1): 3480 (OH), 1945 (C = C-H), 1640 (C = C), 1370 (gem. Di-Me); EI-MS m/z: 412 [M, C29H48O] + , 397 [M-CH3]+, 379[M-CH3-H2O]+, 314 (100) [M-C6H10O]+. 1H NMR (CDCl3) δ (ppm) 0.86- 0.88 (d, J = 6.6 Hz, 6H, Me-26 and Me-27), 0.91 (d, J = 6.6 Hz, 3H, Me-21), 0.68 (s, 3H,Me-18), 1.00 (s, 3H, Me-19), 1.59 (d, J = 6.6 Hz, 3H, Me-29), 3.53 (m, H, H-3), 5.35 (m, H, H-6), 5.11 (q, H, H-28), 2.22 (sep, J = 6.6 Hz, H, H-25). 13C NMR (CDCl3) δ (ppm) (39.75, C-1), (35.20, C-2), (71.80, C-3), (42.33, C-4), (140.74,C-5), (121.70, C-6), (36.14, C-7), (35.78, C-8), (50.10, C-9), (39.72, C-10), (28.23, C-11), (42.28, C-12), (42.30, C-13), (56.74, C-14), (31.64, C-15), (34.78, C-16), (56.72, C-17), (11.85, C-18), (19.40, C-19), (39.50, C-20), (24.32, C-21), (37.23,C-22), (31.90, C-23), (146.00, C-24), (33.90, C-25), (22.50, C-26), (22.23, C-27),(115.94, C-28), (18.90, C-29).Saringosterone (2), 24-vinyl cholest -4-ene -3-one: a colorless oil (10 mg, 0.003% dry wt). IR (cm-1): 3420 (OH), 1675 (C = O), 1630 (C = C); EIMS m/z (rel. int.): 426 (12) [M, C29H46O2]+, 383 (21) [M+-C3H7], 313 (30), 271(35), 269 (100). HREIMS: m/z 426.3413 (calcd. 426.3349) C29H46O2; 1H-NMR (CDCl3) δ 5.76 (dd, J = 17, 12 Hz, H, H-28), 5.73 (br s, H, H-4), 5.27 (d, J = 12 Hz, H, H-29), 5.15 (d, J = 17 Hz, H, H-29), 1.16 (s, 3H, Me-19), 0.95 (d, J = 6.5 Hz, 3H, Me-21), 0.86 (d, J = 7.0 Hz, 3H, Me-26), 0.84 (d, J = 7.0 Hz, 3H, Me-27), 0.72 (s, 3H, Me-18).13C-NMR δ (36.54, C-1), (34.65, C-2), (200.35, C-3), (124.43, C-4), (172.35, C-5), (33.64, C-6), (32.65, C-7), (36.32, C-8), (54.41, C-9), (39.25, C-10), (21.65, C-11), (40.22, C-12), (43.08, C-13), (56.26, C-14), (24.85, C-15), (28.86, C-16), (56.55, C-17), (12.63, C-18), (17.34, C-19), (36.84, C-20), (18.35, C-21), (32.54, C-22), (28.95, C-23), (89.76, C-24), (29.05, C-25), (19.46, C-26), (18.07, C-27), (137.78, C-28), (117.12, C-29).Saringosterol (3), 24-vinyl cholest-5-ene-3β, 24-diol, saringosterol: colorless oil (5 mg, 0.001% dry wt.). IR(cm-1): 3445 (OH), 1644 (C=C); EIMS m/z (rel. int.): 428 (12) [M, C29H48O2]+, 410 (6) [M+-H 2O], 314 (40), 273(20), 271 (100), 255 (28), 228 (22), 213 (40), 145 (64). 1H NMR (CDCl3) d 5.73 (dd, J=17, 12 Hz, H, H-28), 5.34 (br s, H, H-6), 5.28 (d, J = 12 Hz, H, H-29), 5.17 (d, J = 17 Hz, H, H-29), 3.53 (m, H, H-3), 1.02 (s, 3H, Me-19), 0.97 (d, J = 6.6 Hz, 3H, Me-21), 0.88 (d, J = 6.6 Hz, 3H, Me-26], 0.86 (d, J = 6.6 Hz, 3H, Me-27), 0.67 (s, 3H, Me-18], 13C-NMR d (37.17,C-1), (32.54, C-2), (72.43, C-3), (37.94, C-4), (141.43, C-5), (122.31, C-6), (32.35, C-7), (36.84, C-8), (50.74, C-9), (37.93, C-10), (21.75, C-11), (40.41,C-12), (42.97, C-13), (57.43, C-14), (24.95, C-15), (29.06, C-16), (56.55, C-17), (12.54, C-18), (17.32, C-19), (36.54, C-20), (18.35, C-21), (32.33, C-22), (28.95, C-23), (89.85, C-24), (29.19, C-25), (20.06, C-26), (19.53, C-27), (137.82, C-28), (117.04, C-29).Fucoxanthin (4): red residue (60 mg, 0.017% dry wt.). IR (cm-1): 3439 (OH), 2361 and 2332 (sp-hybrid carbon [allenic]), 1721 and 1253 (ester), and 1654 and 1605(polyene); HRFAB: 658.4224 (calcd. 658.4233) [C42H58O6], 460(5), 391(5),307(50), 154(100), 1H-NMR (CDCl3) d 7.15(d, J = 11.0 Hz, H, H-10), 6.75 (m, H, H-15), 6.66 (d, J = 15.0 Hz, H, H-12), 6.63 (m, H, H-15’), 6.60 (m, H, H-11’), 6.57 (m, H, H-11), 6.41 (d, J = 11.5 Hz, H, H-14), 6.35 (d, J = 15.0 Hz, H, H-12’), 6.27 (d, J = 11.5 Hz, H, H-14’), 6.13 (d, J = 11.0 Hz, H, H-10’), 6.05(br s, H, H-8’), 5.38 (m, H, H-3’), 3.81(m, H, H-3), 3.65 and 2.60 (qAB, J = 18.5 Hz, 2H, H-7), 2.33 and 1.78 (m, 2H, H-4), 2.27 and 1.51 (m, 2H, H-4’), 2.04 (s, 3H, Ac), 2.01 and 1.41(m, 2H, H-2’), 1.99 (s, 6H, H-20, H-20’), 1.95 (s, 3H, H-19), 1.81 (s, 3H, H-19’), 1.47 and 1.37 (m, 2H, H-2), 1.38 (3H, s, H-18’), 1.35 (s, 3H, H-17’), 1.22(s, 3H, H-18), 1.07 (s, 3H, H-16’), 1.03 (s, 3H, H-17), 0.96 (s, 3H, H-16). 13C-NMR (CDCl 3) δ (35.18, C-1), (47.11, C-2), (64.34, C-3), (41.68, C-4), (66.19, C-5), (67.11, C-6), (40.83, C-7), (197.87, C-8), (134.54, C-9), (139.14, C-10), (123.39, C-11), (145.05, C-12), (136.67, C-13), (135.44, C-14), (132.19, C-15), (28.16, C-16), (25.06, C-17), (21.46, C-18), (11.85, C-19), (12.80, C-20), (35.79, C-1’), (45.45, C-2’), (68.03,C-3’), (45.25, C-4’), (72.71, C-5’), (117.52, C-6’), 202.36, C-7’), (103.39, C-8’), (132.19, C-9’), (128.54, C-10’), (125.71, C-11’), (138.09, C-12’), (137.11, C-13’), (132.52, C-14’), (129.44, C-15’), (31.31, C-16’), (32.14, C-17’), (29.22, C-18’), (14.04, C-19’), (12.94, C-20’), (170.45, Ac-1), (21.18, Ac-2).Biological evaluation of the isolated compoundsDNA (Calf Thymus type1), bleomycin sulfate, butylated hydroxyanisole, thiobarbituric acid (TBA), ethylenediaminetetraacetic acid (EDTA), and ascorbic acid were obtained from sigma. 2,2’-azo-bis-(2-amidinopropane) dihydrochloride and ABTS were purchased from Wako Co., USA.Cell viability and Bleomycin-dependent DNA damage assay in negative control (Fucoxanthin on MCF-7)The reaction mixture contained DNA (0.5 mg/ml), bleomycin sulfate (0.05 mg/ml), MgCl2 (5 mM), FeCl3 (50 mM), and samples to be tested in a conc. of 0.1 mg/ml.L-ascorbic acid was used as positive control. The mixture was incubated at 37°C for 1 hour. The reaction was terminated by addition of 0.05 ml EDTA (0.1 M). Thecolor was developed by adding 0.5 ml TBA (1% w/v) and 0.5 ml HCl (25% v/v), followed by heating at 80°C for 10 minutes. After centrifugation, the extent of DNA damage was measured by increase in absorbance at 532 nm.[18,19] The viability of the cells was determined by the microscopical examination[20] using a hemocytometer and using trypan blue stain (stains only the dead cells). Antioxidant activity screening assay2,2’-azino-bis-3-ethylbenzthiazoline-6-sulfonic acid methodFor each of the investigated compounds, 2 ml of ABTS solution (60 μM) was added to 3 ml MnO2 solution (25 mg/ml), all prepared in 5 ml aqueous phosphate buffer solution (pH, 7; 0.1 M). The mixture was shaken, centrifuged, filtered, and the absorbance of the resulting green-blue solution (ABTS radical solution) at λ 734 nm was adjusted to approximately ca. 0.5. Then, 50μl of (2 mM) solution of the tested compound in spectroscopic grade MeOH/phosphate buffer (1: 1) was added. The absorbance was measured and the reduction in color intensity was expressed as inhibition percentage. L-ascorbic acid was used as standard antioxidant (positive control). Blank sample was run without ABTS and using MeOH/phosphate buffer (1: 1) instead of tested compounds. Negative control was run with ABTS and MeOH/phosphate buffer (1: 1) only.[21]Cytotoxicity and antitumor assaySamples were prepared for assay by dissolving in 50 μl of DMSO (Dimethyl Sulfoxide), and diluting aliquots into sterile culture medium at 0.4 mg/ml. These solutions were sub-diluted to 0.02 mg/ml in sterile medium and the two solutions were used as stocks to test samples at 100, 50, 20, 10, 5, 2, and 1 mg/ml in triplicate in the wells of microtiter plates. The compounds were assayed in triplicate on monolayers grown in Dulbecco's modified Eagle's medium supplemented with 10% (v/v) calf serum (HyClone Laboratories, Ogden, UT), 60 mg/ml Penicillin G, and 100 mg/ml streptomycin sulfate maintained at 37°C in a humidified atmosphere containing about 15% (v/v) CO 2 in air. All medium components were obtained from Sigma Chemical Co., St. Louis, MO, unless otherwise indicated. Cell stockswere maintained at 34°C in culture flasks filled with medium supplemented with 1% (v/v) calf serum. Subcultures of cells for screening were grown in the wells of microtiter trays (Falcon Microtest III 96-wells trays, Becton Dickinson Labware, Lincolin Park, NJ) by suspending cells in medium following trypsin-EDTA treatment, counting the suspension with a hemocytometer, diluting in medium containing 10% calf serum to 2 × 104 cells per 200 ml culture, aliquoting into each well of a tray, and culturing until confluent. Microtiter trays with confluent monolayer cultures of cells were inverted, the medium shaken out, and replaced with serial dilutions of sterile compounds in triplicate in 100 l medium followed by titered virus in 100 ml medium containing 10% (v/v) calf serum in each well. In each tray, the last row of wells was reserved for controls that were not treated with compounds. Trays were cultured for 96 hours. Trays were inverted onto a paper towel pad, the remaining cells rinsed carefully with medium, and fixed with 3.7% (v/v) formaldehyde in saline for at least 20 minutes. The fixed cells were rinsed with water, and examined visually. The cytotoxic activity is identified as confluent, relatively unaltered monolayers of stained cells treated with the investigated compounds. Cytotoxicity was estimated as the concentration that caused approximately 50% loss of the monolayer. 5-fluorouracil was used as a positive control.[22]Other Sections▼AbstractINTRODUCTIONMATERIALS AND METHODSRESULTS AND DISCUSSIONREFERENCESCompound 1 showed in its EI MS a molecular ion at m/z 412,(Liebermann-burchard reaction gave the typical slow reacting green color of a Δ5 sterol) which[23] together with 13C NMR data suggested a molecular formula of C29 H48O. The loss of part of the side chain (C7H14) is characteristic of sterols[23] witha Δ24(28). 1H NMR spectroscopic data were typical of sterols, in which a sign al at δ0.88 (6H, d, J= 6.6 Hz) attributable to the two methyls of an isopropyl group, a signal at δ 0.91 (3H, d, J= 6.6 Hz) and two signals at δ 1.00 (3H, s) and at δ 0.68 (3H, s) assigned to two methyl groups attached to two quaternary carbons areassigned to C-26, C-27, C-21, C-19 and C-18, respectively. In addition, a doublet at δ 1.59 ppm (J= 6.6 Hz) for the proton at C-29 (δc18.70), a quartet δ 5.18 ppm (J = 6.6 Hz) at C-28 proton (δc115.54), a multiplet at δ 5.35 for C-6 proton (δc 121.70), and a multiplet at 3.53 for C-3 proton (δc 71.80) were observed. 13C NMR of compound 1 indicated 29 carbons, of which two double bonds and a secondary alcohol (cf exp.) were present. The DEPT spectra showed that compound 1 contains six methyl, ten methylene, nine methane, and four quaternary carbons. The stereochemistry at the side chain double bond can be explained by observing the1HNMR spectra at the C-25 proton which resonates at δ 2.32 ppm (δc 36.50) in case of (E) isomer (Frost, ward, 1968). The position of the ethylidene group was proved from HMQC, HMBC correlations, and COSY experiment. These data are in agreement with literature[24] which can allow us to assign compound 1 as fucosterol [Figure 1].Compound 2 showed in its EI MS a molecular ion at m/z 426, which together with 13C-NMR and HREI MS suggested a molecular formula of C29H46O2 (m/z426.3413; calcd. 426.3349). The double doublet at δ 5.75 (J = 18, 13 Hz), doublet at 5.29 (J = 13 Hz), and doublet at 5.17 (J = 18 Hz) in the 1H-NMR and signals at δc 137.79 and 117.10 in the 13C NMR were attributable to terminal vinyl protons, three methyl doublets at 0.96, 0.87, 0.85 in the 1H-NMR spectrum. These data, together with the presence of a fragment at m/z 271 in the mass spectrum due to the loss of the side-chain C10H19O, suggest a stigmastane skeleton with unsaturation at C-28. The IR spectrum showed strong bands at 3420 and 1675 cm-1. The above data, together with the presence of signals at δc 200.38, 172.38, 124.42, and 89.75 in the 13C NMR spectrum and a signal at δ 5.74 in the 1H NMR spectrum, suggested the presence of α,β -unsaturated carbonyl group and a tertiary hydroxyl function in the molecule. As the compound contains two double bonds and two oxygen atoms, one as carbonyl and the second as tertiary hydroxyl, also has seven degrees of unsaturation revealed by mass spectrometry, it must be a tetracyclic product. The structure of known compound 2 [Figure 1] was established by comparison of itsdata (cf exp.) with literature.[25]Compound 3, its structure was established by comparing their physical and spectral data (cf exp.) with those in the literature[25,26] as 24-vinyl cholest -5-ene -3β,24-diol.Compound 4 was isolated as a bright orange solid and the IR showed the presence of the hydroxyl (3439 cm-1), sp-hybrid carbon (allenic) (2361, 2332 cm-1), ester (1721, 1253 cm-1), and polyene (1654, 1605 cm-1) groups. Its molecular formula was deduced as C42H58O6 based on HRFAB-MS analysis [M+], m/z: 658.4224 (Calcd. for C42H58O6, 658. 4233), and NMR spectroscopic data (cf exp.). The 1H and 13C NMR spectra of active compound revealed signals assignable to polyene having acetyl, conjugated ketone, two quaternary geminal dimethyls, two quaternary geminal methyls of oxygen, four olefinic methyls, and allene functionalities. The physicochemical features outlined above suggested that the active compound was a carotenoid in which one of the hydroxyl groups was acetylated. This suggestion was further supported by the UV spectrum [448(ε560,000), 248 (24,000)]. From detailed comparison of the data for the active compound with those of fucoxanthin [Figure 1], the active compound was in agreement with an authentic fucoxanthin in all aspects.[27–29]The data obtained from Table 1 indicate that fucoxanthin (4) have some protective activity to DNA by certain mechanism . The isolated compounds were screened for their antitumor activity against MCF-7. The viability of the cells used in control experiments exceeded 95%; compound 4 proved to have cytotoxic activity sustained for 48 hours after adding the compound to MCF-7, as shown in Table 1. Compounds 1 and 4 were tested for antioxidant activity, where fucoxanthin (4) exhibited the highest antioxidant activity by 72% inhibition [Table 2]. On the other hand, compound 1 exhibited a weak to moderate activity.Compounds 1 and 4 displayed significant antioxidant and anticancer activities [Table 3] against HepG2 (human hepatocellular liver carcinoma cell line), WI 38 (Skin carcinoma cell line), Vero (cell line was initiated from the kidney of a normaladult African green monkey), and MCF-7 (breast cancer cell lines). Fucoxanthin reduced the viability of MCF-7 cells with IC50 (μg/ml) = 11.5.The authors would like to acknowledge SABIC, the Saudi Arabian Company for Basic Industries, for the financial support of this work (SP-11-2) through the collaboration with the Deanship of Scientific Research (DSR) at King Abdul-Aziz University.Source of Support: SABIC, the Saudi Arabian Company for Basic Industries, through the collaboration with the Deanship of Scientifi c Research (DSR) at King Abdul-Aziz UniversityConflict of Interest: None declared.Other Sections▼AbstractINTRODUCTIONMATERIALS AND METHODSRESULTS AND DISCUSSIONREFERENCES1. Blunt JW, Munro MH. Christchurch, New Zealand: Department of Chemistry, University of Canterbury; 2007. Marin Literature Database.2. Blunt JW, Copp BR, Hu WP, Munro MH, Northcote PT, Prinsep MR. Marine natural products. Nat Prod Rep. 2008;25:35–94. [PubMed: 18250897]3. Segawa M, Shirahama H. New Plastoquinones from the Brown Alga Sargassum sagamianum var. yezoense. Chemical Letters. 1987;1:1365–66.4. Mori J, Iwashima M, Wakasugi H, Saito H, Matsunaga T, Ogasawara M, et al. New plastoquinones isolated from the brown alga, Sargassum micracanthum. Chem Pharm Bull (Tokyo) 2005;53:1159–63. [PubMed: 16141587]5. Ishitsuka M, Kusumi T, Nomura Y, Konno T, Kakisawa H. New geranylgeranylbenzoquinone derivatives from Sargassum tortile. Chemical Letters. 1979;8:1269–72.6. Kato T, Kumanireng AS, Ichinose I, Kitahara Y, Kakinuma Y, Kato Y. Structure and synthesis of the active component from a marine alga, Sargassum tortile, which induces the settling of swimming larvae of Coryne uchidai. Chemical Letters. 1975;4:335–8.7. Jang KH, Lee BH, Choi BW, Lee HS, Shin J. Chromenes from the brown alga Sargassum siliquastrum. J Nat Prod. 2005;68:716–23. [PubMed: 15921416]8. Kikuchi T, Mori Y, Yokoi T, Nakazawa S, Kuroda H, Masada Y, et al. Structure of sargatriol, a new isoprenoid chromenol from a marine alga, Sargassum tortile. Chem Pharm Bull (Tokyo) 1975;23:690–2.9. Tang H, Yi Y, Yao X, Zhou D, Lu T, Jiang Y. Studies on bioactive steroid constituents from Sargassum carpophyllum. Chin Pham J. 2002;37:262–5.10. Tang H, Yi Y, Yao X, Zhang S, Zou Z, Li L. Glycerides from marine brown algae Sargassum carpophyllum. Chinese Journal of Marine Drugs. 2002;21:5–9.11. Ayyad S-EN, Abdel-Halim OB, Shier WT, Hoye TR. Cytotoxic hydroazulene diterpenes from brown alga Cystoseira myrica. Z Naturforsch C. 2003;58:33–8. [PubMed: 12622222]12. Lincolon RA, Strupinski K, Walker JM. Bioactive compounds from algae. Life Chem Rep.1991;8:97–183.13. Ayyad S-EN, Slama MO, Mokhtar AH, Anter AF. Cytotoxic bicyclic diterpene from the brown alga Sargassum crispum. Boll Chim Farm. 2001;140:155–9. [PubMed: 11486605]14. Banais B, Francisco C, Gonzalez E, Fenical W. Diterpenoid metabolites from the marine alga Cystoseira elegans. Tetrahedron. 1983;39:629–38.15. Alarif WM, Ayyad S-EN, Al-lihaibi SS. Acyclic Diterpenoid from the Red Alga Gracilaria Foliifera. Rev Latinoamer Quím. 2010;38:52–8.16. Alarif WM, Abou-Elnaga Z Sh, Ayyad S-EN, Al-lihaibi SS. Insecticidal Metabolites from the Green Alga Caulerpa racemosa. CLEAN – Soil, Air and Water. 2010;38:548–57.17. Ayyad S-EN, Makki MS, Al-kayal NS, Basaif SA, El-Foty KO, Asiri AM, et al. Cytotoxic and Protective DNA Damage of Three New Diterpenoids from the Brown Alga Dictoyota dichotoma. Eur J Med Chem. 2011;46:175–82. [PubMed: 21130542]18. Gutterdge J, Rowley D, Halliwell B. Superoxide dependent formation of hydroxyl radicals in the presence of iron salts.Detection of free ion in biological systems using bleomycin-dependent degradation of DNA. Biochem J. 1981;199:263–5. [PMCID: PMC1163361] [PubMed: 6175315]19. Abdel-Wahab BF, El-Ahl AA, Badria FA. Synthesis of new 2-naphthyl ethers and their protective activities against DNA damage induced by bleomycin-iron. Chem Pharm Bull (Tokyo) 2009;57:1348–51. [PubMed: 19952442]20. Badria F, Ameen M, Akl MR. Evaluation of Cytotoxic Compounds from Calligonum comosum L. growing in Egypt. Z. 2007;62:656–60.21. El-Gazzar AB, Youssef MM, Abu-Hashem AA, Badria FA. Design and synthesis of azolopyrimidoquinolines, pyrimidoquinazolines as anti-oxidant, anti-inflammatory and analgesic activities. Eur J Med Chem. 2009;44:609–24. [PubMed: 18462840]22. El-Subbagh HI, Abu-Zaid SM, Mahran MA, Badria FA, Al-obaid AM. Synthesis and biological evaluation of certain alpha, beta-unsaturated ketones and their corresponding fused pyridines as antiviral and cytotoxic agents. J Med Chem. 2000;43:2915–21. [PubMed: 10956199]23. Gibbons GF, Goad LJ, Goodwin TW. The identification of 28- isofucosterol in the marine green algae Enteromorpha intestinalis and Ulva lactuca. Phytochemistry. 1968;7:983–8.24. Goad JL, Akihisa T. 1st ed. London: Blackie Academic and Professional; 1977. Analysis of Sterols; pp. 382–3.25. Ayyad S-EN, Sowellim SZ, el-Hosini MS, Abo-Atia A. The structural determination of a new steroidal metabolite from the brown alga Sargassum asperifolium. Z Naturforsch C. 2003;58:333–6. [PubMed: 12872924]26. Tsuda K, Hayatsu R, Kashida Y, Akagi S. Steroid studies IV. Studies on the constitution of sargasterol. J Am Chem Soc. 1958;80:921–5.27. Haugan JA, Englert G, Glinz E, Liaaen-Jensen S. Algal carotenoids. 48. Structural assignments of geometrical isomers of fucoxanthin. Acta Chem Scand. 1992;46:389–95.28. Yan X, Chuda Y, Suzuki M, Nagata T. Fucoxanthin as the major antioxidant in Hizikia fusiformis, acommon edible seaweed. Biosci Biotechnol Biochem. 1999;63:605–7. [PubMed: 10227153]29. Ayyad S-EN, Diab M. NMR Application of Mosher's Method: The absolute configurations of fucoxanthin from Turbinaria triquatra. Alex J Pharm Sci. 2002;16:27–30.Figures and TablesFigure 1Structure of compounds 1-4Table 1Cell viability and bleomycin-dependent DNA damage assay of fucoxanthin (4)Table 2Antioxidant activity of tested compounds by ABTS methodTable 3Cytotoxicity of tested compounds on different cancer cell linesArticles from Pharmacognosy Research are provided here courtesy ofMedknow Publications。
《化学生物学实验课件:抗氧化活性测定》
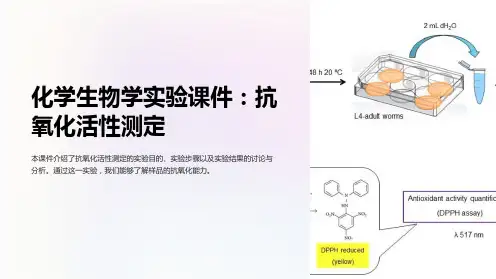
DPPH方法的原理
2
菜或其他植物材料。
了解DPPH自由基的工作原理,其用
于测定抗氧化活性。
3
抗氧化活性测定的步骤
将样品与DPPH反应,并测定吸收度 变化,以计算抗氧化能力。
实验结果
实验结果显示,不同样品的抗氧化活性不同。某些样品表现出较高的抗氧化能力,可能对健康有益。
讨论与分析
通过对实验结果的讨论与分析,我们可以得出结论,如何根据抗氧化活性来 评估样品的营养价值和潜在功效。
2. Chen, L. et al. (2018). Evaluation of antioxidant activity of plant extracts: A comparative study. Journal of Agricultural and Food Chemistry, 66(16), 4073-4080.
化学生物学实验课件:抗 氧化活性测定
本课件介绍了抗氧化活性测定的实验目的、实验步骤以及实验结果的讨论与 分析。通过这一实验,我们能够了解样品的抗氧化能力。
实验目的
通过抗氧化活性测定实验,我们的目的是评估不同样品的抗氧化能力,从而 了解其潜在的健康益处。
实验步骤
1蔬
结论
本实验验证了不同样品的抗氧化活性差异,并提供了评估样品健康益处的方法。
参考文献
1. Smith, A. et al. (2015). Antioxidant activity measurement: methodologies and limitations. Journal of Food Science, 80(4), R981-R993.
抗氧化剂抗氧化能力测定法

抗氧化剂抗氧化能力测定法English Answer:Antioxidant Capacity Assay Methods.Antioxidant capacity assays are widely used to evaluate the ability of a substance to inhibit oxidation. Various methods have been developed to measure antioxidant capacity, each with its own advantages and limitations. Here are some commonly used methods:(1) DPPH Assay (2,2-diphenyl-1-picrylhydrazyl)。
The DPPH assay is a simple and widely used method to measure the radical scavenging capacity of antioxidants. In this assay, DPPH, a stable free radical, is reduced to its non-radical form by antioxidants, leading to a color change from purple to yellow. The reduction rate and extent are proportional to the antioxidant capacity of the sample.(2) FRAP Assay (Ferric Reducing Antioxidant Power)。
The FRAP assay measures the ability of antioxidants to reduce ferric ions (Fe3+) to ferrous ions (Fe2+). Antioxidants reduce Fe3+ to Fe2+ in the presence of a chromogenic substrate, resulting in a color change that can be quantified spectrophotometrically. The FRAP assay is commonly used to assess the reducing power of antioxidants.(3) ABTS Assay (2,2'-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid))。
英类黄酮类黄酮的相互作用及其对它们的抗氧化活性的影响

Flavonoid–flavonoid interaction and its effect on their antioxidant activityMaria Hidalgo a ,Concepción Sánchez-Moreno b ,Sonia de Pascual-Teresa a,*a Department of Metabolism and Nutrition,Instituto del Frío,Spanish National Research Council (CSIC),JoséAntonio Novais 10,Ciudad Universitaria,E-28040Madrid,SpainbDepartment of Plant Food Science and Technology,Instituto del Frío,Spanish National Research Council (CSIC),JoséAntonio Novais 10,Ciudad Universitaria,E-28040Madrid,Spaina r t i c l e i n f o Article history:Received 19October 2009Received in revised form 11December 2009Accepted 24December 2009Keywords:Antioxidant activity Flavonoids Interactions Synergism Antagonisma b s t r a c tFlavonoids are polyphenols widely distributed in fruits and vegetables,and have been shown to be good antioxidants in different models.However,studies undertaken with the aim of predetermining the anti-oxidant power of a given food on the basis of its flavonoid content have,in most cases,failed due to dif-ferences between the theoretical and the calculated antioxidant power of that given product.In the present work,the antioxidant activity of eleven flavonoids (cyanidin-3-O-glucoside,malvidin-3-O-gluco-side,delphinidin-3-O-glucoside,peonidin-3-O-glucoside,pelargonidin-3-O-glucoside,catechin,epicate-chin,kaempferol,myricetin,quercetin and quercetin-3-b -glucoside)was measured by two different in vitro tests:DPPH Åradical scavenging activity and ferric reducing antioxidant power (FRAP).In order to evaluate the effect of flavonoid interactions on their antioxidant power we compared the capacity of individual flavonoids with that obtained by mixing them with another flavonoid.The majority of DPPH Åscavenging activities in these combinations promoted antagonistic effects,except for some synergistic interactions such as kaempferol paired with myricetin.In the FRAP assay,the interaction between epicat-echin and quercetin-3-b -glucoside showed the highest synergistic effect,whereas myricetin with quer-cetin resulted in an antagonistic effect.It can be concluded,therefore,that there are synergistic and antagonistic interactions between flavo-noids that may explain the results obtained when measuring the antioxidant effect of whole food extracts.The present results may also assist in the future design of functional foods or ingredients based on their antioxidant activity.Ó2010Elsevier Ltd.All rights reserved.1.IntroductionFlavonoids are among the most studied phytochemicals found in plant foods and include a large number of different molecules which may result in diverse biological activities.Numerous studies have focused on determining flavonoid antioxidant activity,many of which have used pure compounds,calculating their individual antioxidant power and performing structure–activity relationship studies (Plumb,de Pascual-Teresa,Santos-Buelga,Cheynier,&Wil-liamson,1998;Rice-Evans,Miller,&Paganga,1996).In other stud-ies the antioxidant power of a given food sample,mainly oils (Mateos,Domínguez,Espartero,&Cert,2003),fruits and vegetables (García-Alonso,Rimbach,Rivas-Gonzalo,&de Pascual-Teresa,2004;Plumb et al.,1996)or tea and wine (Leung et al.,2001;Sán-chez-Moreno,Larrauri,&Saura-Calixto,1999)has been character-ised in depth and,in some cases,the correlation between flavonoid composition and antioxidant power has been examined (Fernán-dez-Pachón,Villano,García-Parrilla,&Troncoso,2004).When in 1936Szent-Györgyi (Rusznyák &Szent-Györgyi,1936)reported the presence of what he first called ‘‘vitamin P”in citrus fruits,he had already hypothesized that flavonoids and vitamin C worked synergistically to strengthen capillaries (Rusznyák &Szent-Györgyi,1936).Subsequently,some studies showed that biological interactions took place between flavonoids and some vitamins in in vitro and in vivo models.Lotito and Fraga (1998)de-scribed the protective effect of catechin against a -tocopherol depletion in plasma.More recently,Kadoma,Ishihara,Okada,and Fujisawa (2006)demonstrated that there was a synergic antioxi-dant effect between d -tocopherol and epicatechin and epigalloca-techin gallate in an in vitro model.Frank et al.(2006)showed that the inclusion of quercetin,catechin or epicatechin in the diet of rats gave rise to an increase in a -tocopherol concentrations in blood plasma and liver.Many studies on the antioxidant potential of flavonoids in fruits,vegetables,wine or tea have concluded that it is impossible to predict the antioxidant power of a given product by studying just one type of flavonoid or other kind of antioxidants contained in the product,such as vitamin C or E.In some cases the possible existence of synergic or antagonistic effects between the various antioxidants present in plant foods and derived products has been0308-8146/$-see front matter Ó2010Elsevier Ltd.All rights reserved.doi:10.1016/j.foodchem.2009.12.097*Corresponding author.Tel.:+34915492300;fax:+34915493627.E-mail address:soniapt@if.csic.es (S.de Pascual-Teresa).Food Chemistry 121(2010)691–696Contents lists available at ScienceDirectFood Chemistryj o u r n a l h o m e p a g e :/locate/foodchempostulated(García-Alonso et al.,2004;Vinson,Su,Zubik,&Bose, 2001).However until now very few studies have focused on the assessment offlavonoid–flavonoid interactions in terms of antiox-idant activity.Heo,Kim,Chung,and Kim(2007)did notfind any synergistic effect between the assayedflavonoids by using the ABTS method and expressing results as a vitamin C equivalent. However,Pinelo,Manzocco,Nuñez,and Nicoli(2004)found an antagonistic effect when phenols interacted at three different tem-peratures using the DPPH method and several studies showed a synergistic antioxidant effect offlavonoids on free-radical-initiated peroxidation of linoleic acid(Rossetto et al.,2002).An antioxidant effect was observed by Pignatelli et al.(2000)with theflavonoids quercetin and catechin,indicating that these components of red wine act synergistically to inhibit platelet adhesion to collagen and collagen-induced platelet aggregation by virtue of their antiox-idant effect.In general,all results for isolatedflavonoids indicate high anti-oxidant activity.Although the in vitro antioxidant properties for isolated polyphenols have already been well documented(Rive-ro-Pérez,Muñiz,&González-Sanjosé,2008),in this paper we will extend this knowledge to include the antioxidant properties pro-duced by the interaction of twoflavonoids,in terms of synergistic or antagonistic effects.To accomplish this objective we have measuredflavonoid anti-oxidant activity andflavonoid–flavonoid interactions by employ-ing the following two methods:scavenging of the stable2,2-diphenyl-1-picrylhydrazyl(DPPHÅ)radical and ferric reducing anti-oxidant power(FRAP).In particular,we have compared the antiox-idant capacity of a system containing a mixture of twoflavonoids with that of each singleflavonoid measured individually,in order to better understand the global antioxidant capacity offlavonoid rich products such as red wine or fruit juices.Theflavonoids stud-ied were:cyanidin-3-O-glucoside chloride,malvidin-3-O-gluco-side chloride,delphinidin-3-O-glucoside chloride,peonidin-3-O-glucoside chloride,pelargonidin-3-glucoside chloride,(+)catechin, (À)epicatechin,kaempferol,myricetin,quercetin and quercetin-3-b-glucoside.2.Materials and methods2.1.Chemicals6-Hidroxy-2,5,7,8,-tetramethylchroman-2-carboxylic acid97%, 2,2-diphenyl-1-picrylhydrazyl radical(DPPHÅ),2,4,6-tris(2-pyri-dyl)-s-triazine(TPTZ),iron(III)chloride hexahydrate,acetate buf-fer saline,myricetin,(+)catechin,(À)epicatechin and quercetin dihydrate were purchased from Sigma–Aldrich Química S.A.(Ma-drid,Spain).Quercetin-3-b-glucoside and kaempferol96%from Fluka,Sigma–Aldrich Química S.A.(Madrid,Spain).Cyanidin-3-O-glucoside chloride,pelargonidin-3-O-glucoside chloride,malvi-din-3-O-glucoside chloride,delphinidin-3-O-glucoside chloride and peonidin-3-O-glucoside chloride were purchased from Extra-synthese(Lyon,France).Methanol(HPLC grade)from Lab-Scan (Dublin,Ireland).Ethanol absolute99%,HCl37%and dimethyl sulf-oxide(DMSO)were obtained from Panreac(Barcelona,Spain).2.2.DPPHÅradical scavenging capacity assayThe DPPHÅmethod reported by Brand-Williams,Cuvelier,and Berset(1995)was used but with some modifications.DPPHÅis a stable radical widely used to monitor the free radical scavenging abilities(the ability of a compound to donate an electron)of vari-ous antioxidants.The DPPHÅradical has a deep violet colour due to its impaired electron,and radical scavenging can be followed spec-trophotometrically by the loss of absorbance at517nm,as the pale yellow nonradical form is produced.To optimise the conditions used to run the DPPHÅassay in microplates we modified the DPPHÅconcentration,the time of reaction and the range of concentrations used for theflavonoids.As a result,wefinally set the DPPHÅcon-centration at100l M,the time of reaction at1h and the antioxi-dant concentration between100and500l M.Briefly,10l l of individualflavonoid or5l l of each of the paired flavonoids were placed in a96-well microplate,in order to give the same level offlavonoid concentration,then290l l of100l M DPPHÅin methanol was added,mixed well,and after1h of incuba-tion in the dark absorbance was measured at517nm using a microplate reader(Power Wave XS,BIOTEK).All samples were run in triplicate.The results were expressed as EC50(l M),and ob-tained by plotting the remaining percentage of DPPHÅagainst the flavonoid concentration to obtain the amount of antioxidant neces-sary to decrease the initial DPPHÅconcentration by50%.2.3.Ferric reducing antioxidant power(FRAP)assayThe total antioxidant potential of a sample was also determined using the ferric reducing antioxidant power(FRAP)assay by Benzie and Strain(1996).This method is based on reducing the power of an antioxidant compound.A potential antioxidant will reduce the ferric ion(Fe3+)to the ferrous ion(Fe2+)at low pH;the latter forms a blue complex(Fe2+/TPTZ),measured at593nm.The FRAP reagent was freshly prepared by mixing together0.3M acetate buffer(pH 3.6),10mM TPTZ in40mM HCl and20mM FeCl3in a proportion 10:1:1(v/v/v),respectively.Testedflavonoids were prepared at 100l M and200l M in DMSO.The assay was carried out by placing 10l l individualflavonoid at100l M or5l l at200l M for each of the pairedflavonoid in a96-well microplate and then adding 290l l of FRAP reagent.In a second FRAP assay,we selected com-binations offlavonoids in different concentration ratios(3:1,2:1 and1:1)using afixedfinal concentration(200l M).After15min of incubation at37°C and shaking,absorbance was read at 593nm.All samples were run in triplicate.Results were compared with a standard curve prepared daily with different concentrations of Trolox and were expressed as l M trolox equivalents(TE).2.4.Statistical analysisThe results were reported as means±standard deviation(SD)of at least three measurements,each performed in triplicate.One-way analysis of variance(ANOVA)was used to compare the means, and the least significant difference(LSD)test showed the values statistically different.Differences were considered significant at P<0.05.All statistical analyses were performed with Statgraphics Plus5.1(Statistical Graphics Corporation,Inc.,Rockville,MD,USA).3.Results and discussionIt has been suggested thatflavonoids have several potential health benefits due,in part,to their antioxidant activity,and re-cently,research on natural antioxidants,includingflavonoids,has increased actively in variousfields.According to Moon and Shi-bamoto(2009),in order to study the antioxidant activity of these compounds,it is important to choose an adequate assay based on the chemistry of the compound of interest.Some assays are concerned with electron or radical scavenging,including the2,2-diphenyl-1-picrylhydrazyl(DPPH)assay or the2,20-azinobis(3-eth-ylbenzothiazoline-6-sulfonic acid)(ABTS)assay whereas other as-says are focused on reducing oxidising ability such as the ferric reducing antioxidant power(FRAP)assay or the Ferrous Oxida-tion-Xylenon Orange(FOX)assay.692M.Hidalgo et al./Food Chemistry121(2010)691–696M.Hidalgo et al./Food Chemistry121(2010)691–696693 Table1Antioxidant activity of singleflavonoids and in combination measured by DPPHÅand FRAP methods.Samples EC50(l M)Difference in DPPH antioxidant activity(%)FRAP a Difference in FRAP antioxidant activity(%) Peonidin-3-O-glucoside473.9±9.7209.7±13.3P+CY494.0±39.6À2.2±7.2516.9±27.7À7.8±0.9bP+DP516.4±1.6À19.0±1.7b446.9±28.5À5.2±0.9bP+MV544.4±9.0À13.0±0.7371.9±45.3À8.1±2.8bP+PG591.4±18.4À4.1±2.4315.5±15.7À5.3±1.8P+CAT642.3±17.8À37.2±2.4b332.1±9.9À3.3±3.1P+ECAT550.4±6.0À26.5±2.7b327.6±18.5À4.4±1.6bP+K592.4±15.0À0.3±1.8369.9±33.0À5.6±0.9bP+MY413.9±7.8À10.3±0.7b499.7±39.0À4.9±3.3P+Q523.9±1.9À41.3±2.4b594.0±40.00.1±1.3P+Q-3-G715.1±26.6À79.4±8.8b370.4±20.1À1.4±0.7Cyanidin-3-O-glucoside509.8±35.2377.0±9.4CY+DP433.3±15.2À3.4±6.2565.1±26.8À4.7±1.6CY+MV481.1±30.2 3.9±4.3585.0±40.60.7±8.1CY+PG547.1±46.0 1.3±6.6594.3±26.10.0±7.0CY+CAT495.5±32.7À41.1±7.7b599.4±26.2 1.1±7.1CY+ECAT468.2±28.1À5.3±4.0585.3±5.0À2.0±2.9CY+K517.5±26.4 2.0±8.4617.4±11.1À0.3±5.7CY+MY298.5±21.719.6±11.7b784.5±21.6 1.0±6.3CY+Q410.1±19.3À16.4±14.1711.5±25.3 5.4±6.0CY+Q-3-G522.1±25.8À45.6±19.5b622.5±17.913.1±9.9bDelphinidin-3-O-glucoside397.2±7.7266.0±23.6DP+MV484.3±46.6À9.5±9.6438.4±19.0À2.9±2.6DP+PG547.3±44.1À4.7±7.8411.1±39.4À0.4±4.9DP+CAT502.6±18.7À29.0±3.5b419.9±12.4 5.2±3.7DP+ECAT495.4±0.1À29.6±6.1b433.0±7.57.1±2.9DP+K505.1±45.67.9±7.8447.7±22.4À0.6±3.3DP+MY356.4±33.9À2.0±5.6641.7±39.6À0.4±3.9DP+Q417.6±25.0À28.4±7.8b659.5±33.6 2.2±5.4DP+Q-3-G460.0±3.0À35.5±0.6b476.0±13.6 2.9±13.0Malvidin-3-O-glucoside482.2±37.4208.6±19.6MV+PG589.7±18.3À11.8±7.8353.1±25.4 1.2±7.2MV+CAT655.1±47.3À45.1±4.5b329.1±15.8 2.4±4.1MV+ECAT429.6±18.9À7.3±5.0330.3±17.0 2.8±2.1MV+K759.8±2.3À20.5±7.6b364.7±18.1À0.2±3.0MV+MY386.1±28.4À0.2±4.5b536.9±35.60.4±3.8MV+Q581.0±3.4À51.0±2.7b592.7±39.29.3±3.9bMV+Q-3-G764.1±9.8À88.1±2.4b368.8±22.111.0±4.4bPelargonidin-3-O-glucoside651.0±16.2137.0±11.8PG+CAT814.9±36.3À52.2±2.4b283.5±25.3À0.1±4.9PG+ECAT781.0±46.1À50.7±9.7b293.9±27.8 1.7±4.7PG+K855.6±31.1À24.6±3.7b313.0±22.00.9±2.6PG+MY475.5±15.2À1.9±3.0519.3±35.80.7±5.6PG+Q594.7±47.8À31.8±10.2b567.7±35.913.2±1.0bPG+Q-3-G840.1±24.3À76.0±6.1b323.1±19.5 6.4±6.4(+)Catechin380.6±15.4141.1±8.1CAT+ECAT462.9±31.9À14.1±4.6b274.3±13.3À2.3±3.0CAT+K539.8±56.3 2.4±9.1311.2±11.5À0.4±2.5CAT+MY377.1±16.2À10.0±3.6527.2±34.4 2.5±3.6CAT+Q357.1±11.8À7.8±5.9560.3±14.3 6.7±3.3bCAT+Q-3-G360.6±43.1À4.2±9.7337.5±12.216.0±6.5b(À)Epicatechin369.4±26.4148.2±12.9ECAT+K492.6±40.57.2±3.9326.6±15.70.8±2.7ECAT+MY342.7±25.3À7.8±3.3557.7±47.411.6±4.1bECAT+Q318.6±20.0À2.6±7.0571.0±35.98.8±4.5bECAT+Q-3-G361.9±10.7À7.3±12.6362.1±15.522.4±5.6bKaempferol699.9±30.2182.2±8.3K+MY381.3±16.221.7±2.9b466.3±3070.0±3.9K+Q382.8±13.619.9±1.0b583.4±22.67.9±2.4K+Q-3-G450.2±24.013.6±1.7b356.6±15.1 6.0±5.9Myricetin274.0±5.4330.5±27.7MY+Q382.8±13.6À17.9±5.8b618.0±35.0À4.9±1.6bMY+Q-3-G450.2±24.0À20.8±3.7b571.3±30.612.6±2.8bQuercetin256.2±9.2368.2±13.9Q+Q-3-G321.8±12.5À16.2±8.7b591.4±3.413.3±5.5bQuercetin-3-b-glucoside291.5±21.5161.0±9.0Peonidin-3-O-glucoside(P),cyanidin-3-O-glucoside(CY),delphinidin-3-O-glucoside(DP),malvidin-3-O-glucoside(MV),pelargonidin-3-O-glucoside(PG),catechin(CAT), epicatechin(ECAT),kaempferol(K),myricetin(MY),quercetin(Q),and quercetin-3-b-glucoside(Q-3-G).a Results expressed:as micromolar trolox equivalents(TE).b Within columns indicates a statistically different value from the comparison between experimental results,obtained for a solution of twoflavonoids,with theoretical results, calculated by summing the effects of individual compounds measured separately.In order to determine the synergistic/antagonistic effect for DPPH and FRAP the following equations were used:Difference in DPPH antioxidant activity=100À[mixture EC50Â200/(A EC50+B EC50)];where mixture EC50is the result obtained experimentally for the mixture of twoflavonoids(A and B)in the experimental conditions described in the methodological section,and A EC50and B EC50values are the EC50measured individually for each compound.Difference in FRAP antioxidant activity=[mixture FRAP valueÂ100/(A FRAP value+B FRAP value)]À100;where mixture FRAP value is the result obtained experimentally for the mixture of twoflavonoids(A and B)in the experimental conditions described in the methodological section,and A FRAP value and B FRAP vale are the FRAP values measured individually for each compound.In both methods,positive values are considered synergistic and negative values antagonistic effects.Therefore,the correct evaluation of the antioxidant activity of several commercialflavonoids requires the use of more than one method,consequently,we used two methods based on fundamen-tally different approaches such as the DPPHÅand FRAP methods, which are highly sensitive assays with reproducible results.3.1.DPPHÅradical scavenging capacityThe results obtained experimentally for the differentflavonoid combinations were compared with theoretical values calculated by adding up the effects of both individual compounds analysed separately.In this way,any statistically significant effect resulting from these combinations could be established whether synergistic or antagonistic.As a consequence,it was possible to calculate per-centagewise variations in antioxidant activity for each combina-tion of twoflavonoids and compare the results with those calculated for their theoretical activity.The results obtained are summarised in Table1which clearly indicates that the majority of the mixtures showed a loss of antioxidant capacity compared with their theoretical values.After comparing the EC50values of individual compounds,flavo-nols such as quercetin or myricetin,with two and three hydroxyl groups respectively in B-rings,showed the highest antioxidant activity.The present results are in accordance with those of other authors(Cao,Sofic,&Prior,1997)who demonstrated that the number and pattern of hydroxyl substitutions on the B-ring are associated with the highest antioxidant activity.Moreover,it seems that the combination of catechol moiety with a double bond at C2–C3and a hydroxyl group in position3makes it an extremely active scavenger(Van Acker et al.,1996).In contrast,our study revealed that pelargonidin-3-glucoside and kaempferol,with only one hydroxyl group in the B-ring,were the individual compounds with lowest antioxidant capacity.How-ever,when kaempferol was paired with myricetin,quercetin or quercetin-3-glucoside we obtained a statistically significant in-crease in antioxidant activity,so it can be described as having a synergistic effect(Fig.1).Furthermore,in these combinations,an increase in antioxidant activity of about20%was achieved com-pared with their theoretical values.In the same way,in the cyani-din-3-glucoside and myricetin-3-glucoside combination a synergistic effect was also found.However,when we paired pelargonidin-3-glucoside,another compound with low antioxidant activity,with compounds such as catechin,epicatechin,kaempferol,quercetin or quercetin-3-glu-coside,the results seemed to indicate very weak DPPHÅscavenging activities,revealing statistically significant antagonism when real values are compared with theoretical ones.The structural differences existing between kaempferol and pelargonidin-3-glucoside could be responsible for the opposite ef-fect of interaction in these mixtures,and even though kaempferol has only one hydroxyl group in the B-ring as does pelargonidin,it does contain the2,3-double bond in the C-ring and the4-oxo func-tion.On the other hand,pelargonidin has a glucoside residue at C3 and an oxonium ion(O+)in the C-ring.Apart from the foregoing,antagonistic effects were also found in the interactions of peonidin-3-glucoside,delphinidin-3-gluco-side,malvidin-3-glucoside,catechin,epicatechin,myricetin,quer-cetin or quercetin-3-glucoside.In the majority of cases,these interactions tended to have an antagonistic effect on radical scavenging capacity.It is important to note that the strongest antagonistic reaction was found when quercetin-3-glucoside was paired with thefive anthocyanins assayed in this study,which resulted in a consider-able loss of antioxidant activity.Anthocyanins,frequently found in coloured fruit and vegetables,are a group offlavonoids with an exceptionally good individual scavenging capacity,a fact that has already been confirmed in published research(Borkowski, Szymusiak,Gliszczynska-Swiglo,Rietjens,&Tyrakowska,2005; García-Alonso et al.,2005).Previous studies have asserted that anthocyanin antioxidant capacity depends on the aglycon moiety and the glycoside forms,thus explaining why the number of sugar residues at the C3position seems to be associated with lower activ-ity(Rice-Evans et al.,1996).However,other authors concluded that anthocyanin glycosilation increases antioxidant capacity (Wang,Cao,&Prior,1997;Yoshiki,Okubo,&Igarashi,1995).On the other hand,theflavonoid quercetin which is an antioxidant occurring mainly in foods as a3-or40-glucoside,and also as quer-cetin-3-rutinoside(commonly found in black tea)can be trans-formed into quercetin-3-glucoside by splitting the rhamnose molecule,consequently thisflavonoid is widely distributed in fresh fruits and vegetables as well as in processed food products. Although,anthocyanins and quercetin-3-glucoside exert good anti-oxidant power individually,our results indicate that when these flavonoids are paired,an interaction takes place affecting their to-tal antioxidant capacity.The tendency offlavonoids to combine when they are mixed together is well-known,hence it is possible that in these interactions a hydrogen-bonding betweenflavonoids may occur,decreasing the availability of the hydroxyl groups, which may in turn reduce the possibility of interaction with the radical DPPHÅ.The importance of these antagonistic interactions cannot be overstated as anthocyanins and quercetin-3-glucoside are usually present together in many fruits and vegetables as well as in red wine and fruit juices.Moreover,they are also essential components in some of the functional ingredients and foods that have been pro-posed over the past few years(Blando,Gerardi,&Nicoletti,2004; Gonzalez-Molina,Moreno,&Garcia-Viguera,2008).From the foregoing it is evident that the significant differences in antioxidant activity can be explained byflavonoid interaction mechanisms.In conclusion,these data support the hypothesis that flavonoid interactions reduce in part,the total antioxidant activity found in food matrices,and in general,in plant foods and fruit ex-tracts,juices and other derived products.3.2.Ferric reducing antioxidant power(FRAP)After assaying all the possibleflavonoid combinations in pairs, we obtained a preliminary evaluation of the antioxidant capacity of theflavonoids,and in this way were able to compare the exper-imental results obtained for each of the pairedflavonoids with the-oretical values calculated by adding up the effect of individual compounds measured separately.We were then able to establish which of these combinations produced a statistically significant ef-694M.Hidalgo et al./Food Chemistry121(2010)691–696fect,either synergistically or antagonistically,with respect to their theoretical values(Table1).Finally,in order to reveal the contribu-tion of eachflavonoid to the antioxidant activity in the mixture, different concentration ratios were assayed.Therefore,the values summarised in Table2,show the differences in antioxidant activity found between theoretical and real values offlavonoid combina-tions in different concentration ratios.As can be observed,the interaction among epicatechin and quercetin-3-glucoside showed the highest antioxidant activity in every ratio.The other combina-tions with high synergistic effects were epicatechin with myricetin, quercetin with quercetin-3-glucoside and catechin with quercetin-3-glucoside.This last interaction is shown in Fig.2where the real FRAP value was higher than that calculated theoretically for each flavonoid assayed in different concentration ratios.In the case of the combination of peonidina-3-glucoside a significant synergistic effect was only found when the two compounds were mixed in a isomolar ratio wile no effect was found at all the other ratios assayed.Likewise,there were other interactions that enhanced the anti-oxidant activity in solution,indicating a synergistic effect,as for in-stance in the case of pelargonidin with quercetin,myricetin with quercetin-3-glucoside,cyanidin-3-glucoside with quercetin-3-glu-coside,malvidin-3-glucoside with quercetin-3-glucoside and quer-cetin,or quercetin with catechin and epicatechin.In view of these results,it appears that theflavonols quercetin and quercetin-3-glucoside trigger a noticeable increase in antioxi-dant activity when mixed in solution with anotherflavonoid.The comparison of quercetin and quercetin-3-glucoside demonstrated that blocking the3-hydroxyl group in the C-ring of quercetin while retaining the30,40-dihydroxy structure in the B-ring did not de-crease the antioxidant power of this compound using the FRAP method.Furthermore,in contrast with what was shown in the DPPHÅassay,quercetin-3-glucoside promoted the most important synergistic effects in the majority of the interactions.Apart from this,we also found that there was an important antagonistic interaction when we paired myricetin with quercetin, in a ratio1:1.The activity obtained was significantly lower than the sum of the individual values and as a result,this antagonistic effect represented a decrease of antioxidant activity of about9%.In addition to the aforementioned interaction,we also found another two antagonistic effects,although in these cases,the de-crease in antioxidant activity was less important.In thefirst case, antagonism took place when mixing peonidin-3-glucoside and malvidin-3-glucoside,corresponding with anthocyanins,which contain the30-OMe and the30-and50-OMe groups in the B-ring, respectively.In the second case,it occurred between peonidin-3-glucoside and delphinidin-3-glucoside.In agreement with other authors(Rice-Evans et al.,1996),the insertion of methoxyl groups in the B-ring does not enhance antioxidant capacity when the study is carried out with individual standards,however other stud-ies(Rivero-Pérez et al.,2008)showed that methoxyl derivates in-creased antioxidant efficiency when interactions among pigments were considered.These results indicate thatflavonoid interactions,in general, increase antioxidant activity when assayed by the FRAP method. Although the values obtained from each method showed significative differences,these differences could probably be ex-plained on the basis of the chemical nature and reactivity of theTable2Difference in antioxidant activity between real and theoretical values for selected combination in different ratios as measured by FRAP method.Combinations Molar ratio3:12:11:11:21:3CAT+Q28.14±13.726.4±9.034.1±10.636.7±3.133.7±11.2 CAT+Q-3-G32.7±8.448.6±14.049.4±8.247.7±9.151.1±6.7 CY+Q-3-G19.9±8.623.6±6.535.7±3.333.0±4.523.3±5.4 ECAT+MY45.0±2.245.0±2.252.9±7.348.4±1.745.8±5.3 ECAT+Q-3-G56.3±5.756.0±7.667.7±9.367.7±9.655.7±5.4 ECAT+Q26.9±2.522.0±1.325.8±1.418.3±1,719.1±9.6 MV+Q18.2±1.614.2±7.020.5±5.010.7±2.18.7±6.7 MV+Q-3-G27.1±4.137.0±5.535.3±2.230.8±6.427.8±5.6 MY+QÀ30.8±5.7À53.7±5.6À60.4±10.0À52.1±8.5À43.7±8.3 MY+Q-3-G38.7±9.336.6±8.435.1±2.035.4±4.038.3±3.0 P+CY 2.3±6.1 1.7±8.410.5±6.4À0.7±9.7À0.8±3.9 P+DP 4.9±3.2À5.2±5.9À5.2±1.6À2.0±4.9À0.5±1.1 P+ECAT8.3±4.28.8±2.69.7±4.411.8±3.59.5±3.4 P+K12.8±1.8À0.5±1.90.1±4.60.9±6.413.4±1.0 P+MV 1.2±4.5À5.3±0.3À9.3±1.8À6.8±1.8À7.5±1.9 PG+Q23.8±5.222.4±3.237.9±5.713.0±2.327.4±4.3 Q+Q-3-G33.4±10.535.6±5.537.8±7.719.2±5.613.4±12.8Peonidin-3-O-glucoside(P),cyanidin-3-O-glucoside(CY),delphinidin-3-O-glucoside(DP),malvidin-3-O-glucoside(MV),pelargonidin-3-O-glucoside(PG),catechin(CAT), epicatechin(ECAT),kaempferol(K),myricetin(MY),quercetin(Q),and quercetin-3-b-glucoside(Q-3-G).Synergistic and antagonistic effect of the combinations assayed in different ratios were calculated by obtaining the differences between the real value,obtained experi-mentally,and the theoretical value obtained as the sum of the added concentration of both compound by using the following equation:Difference in FRAP antioxidant activity=mixture FRAP valueÀ[(A FRAP valueÂA concentration)+(B FRAP valueÂB concentration).Positive values are synergisms and negative values antagonisms.M.Hidalgo et al./Food Chemistry121(2010)691–696695。
脑出血后血肿周围水肿形成机制的研究进展

山东医药2021年第61卷第2期脑出血后血肿周围水肿形成机制的研究进展范敬争,姜玉艳天津医科大学总医院,天津300052摘要:血肿周围水肿的形成是脑出血后二次脑损伤发生的关键因素,与脑出血患者的生存预后紧密相关。
脑出血后,血管源性因素(血块的形成及回缩、血肿周围静水压的下降、血浆蛋白的外渗)、炎症反应、凝血级联反应、红细胞溶解产物、补体成分等参与了脑出血后血肿周围水肿的形成和发展,造成了神经功能进一步恶化,使脑细胞发生不可逆损伤。
因此,抑制脑出血后血肿周围水肿的形成可使脑出血患者获益。
关键词:脑出血;脑水肿;病理机制doi:10.3969/j.issn.1002-266X.2021.02.024中图分类号:R743.34文献标志码:A文章编号:1002-266X(2021)02-0092-03脑出血是急性脑血管病中最严重的一种,是目前中老年人主要致死性疾病之一。
我国是脑出血的高发国家,每年由于各种原因导致的脑出血患者超过150万,且呈逐年增高的趋势[1]。
临床上单纯解决脑出血的占位效应不能明显改善脑出血患者的预后,因此,脑出血后二次脑损伤的产生和发展是影响患者疗效和预后的关键因素。
二次脑损伤包括血肿周围水肿的形成及神经细胞的缺失、变性或死亡,而血肿周围水肿是二次脑损伤发生的关键因素。
动物实验表明,血肿周围水肿量起初增长很轻微,2h后开始增多,3~4d达到高峰,随后水肿缓慢下降,直到出血后7d仍然存在[2]。
脑出血后血肿周围水肿的演变分为三个阶段:第一阶段,发生在出血最初几小时,这个过程包括血块回缩、静水压下降导致血浆渗出到血肿周围形成血管源性水肿,其同时造成血肿周围的脑血流量轻微下降,进而引起血块周围暂时性缺血;第二阶段,发生在出血之后的24~48h,此阶段主要是炎症反应及凝血酶激活引起的细胞毒性水肿,其直接造成血脑屏障破坏、脑组织代谢活性降低及随后的脑血流量下降;第三阶段,发生在出血3d后,此阶段主要是红细胞溶解破坏和血红蛋白毒性引起的延迟期水肿,其所诱导的神经毒性等血液成分同时能造成神经损伤。
光照、加热对虾青素稳定性和抗氧化性的影响

虾青素(astaxanthin,3,3’_二轻基- 4,4’-二丽基 - P ,f 胡萝卜素)属于类胡萝卜素中的一种,其分子 具有高度对称结构,有 4 种几何异构体[|-2],即全反 式 ,9-顺 式 、13-顺 式 和 15-顺 式 异 构 体 ,如 图 1 所 示 。虾青素分子结构中所具有的发色团使其在紫外 -可见光区有独特的吸收区,因而其结晶或溶液在 可见光下具有十分绚丽的紫红色。室 温 条 件 下 ,虾 青素在丙酮、二 氯 甲 烷 、二 甲 亚 砜 、乙醇及其他非极 性溶剂中的溶解性较好[3]。虾青素具有很强的抗
light on astaxanthin were mainly caused by ultraviodative test showed that there was more than
one isomer of astaxanthin possesses antioxidant activity, and the anti-oxidability of the 1 3 - c i s isomers was higher than a l l -
摘 要 :目 的 考 察 光 照 、加 热 情 况 下 虾 青 素 的 稳 定 性 和 抗 氧 化 性 的 变 化 。方 法 配 制 一 定 浓 度 的 虾 青 素 溶 液 , 分 别 经 过 阳 光 直 射 、紫 外 和 加 热 处 理 ,测 定 溶 液 中 各 虾 青 素 异 构 体 的 浓 度 ,并 做 1 ,1 - 二 苯 基 - 2 - 三 硝 基 苯 肼 ( D P P H ) 抗 氧 化 性 试 验 。结 果 在 光 照 和 加 热 条 件 下 ,减 少 的 全 反 式 虾 青 素 转 化 为 其 顺 式 异 构 体 ,且 主 要 转 化 为 1 3 - 顺 式 异 构 体 ,对 应 的 转 化 率 分 别 为 5 0 % 、1 0 0 % 。 通 过 紫 外 照 射 试 验 发 现 ,虾 青 素 各 异 构 体 含 量 变 化 与 光 照 相 近 ,说 明 光 照 对 虾 青 素 的 影 响 主 要 是 由 于 其 中 的 紫 外 光 引 起 的 。1 ,1 - 二 苯 基 - 2 - 三 硝 基 苯 肼 清 除 试 验 表 明 ,虾青素 不 止 一 种 异 构 体 有 活 性 ,且 1 3 - 顺 式 异 构 体 抗 氧 化 性 高 于 全 反 式 。结 论 在 光 照 和 加 热 情 况 下 虾 青 素 容 易 发 生 降 解以及异构体之间的转化。
分析甘草的抗氧化损伤作用与细胞保护机制
分析甘草的抗氧化损伤作用与细胞保护机制甘草(Glycyrrhiza)是一种常见的中草药,被广泛用于中医中药的制剂中。
除了其具有独特的药理作用外,甘草还被发现有抗氧化损伤的作用,并且能够对细胞起到保护作用。
本文将对甘草的抗氧化损伤作用以及其作为细胞保护剂的机制进行详细的分析。
抗氧化损伤作用是指物质对抗氧化性物质引起的氧化反应及其所产生的自由基产生的保护作用。
甘草中丰富的活性成分,如甘草酸、异甘草素、甘草素等,被证明具有较强的抗氧化活性。
这些成分能够清除体内的自由基,减轻或抑制氧化损伤,从而达到抗氧化的效果。
甘草作为抗氧化剂,其作用机制主要体现在以下几个方面。
首先,甘草中的甘草酸具有较强的自由基清除能力。
研究表明,甘草酸能够通过直接捕获自由基,中和其活性,并阻断其对细胞的损伤。
此外,甘草酸还能够增强机体内源性抗氧化酶的活性,例如超氧化物歧化酶和谷胱甘肽过氧化物酶,进一步加强抗氧化防御系统。
其次,甘草中的异甘草素和甘草素也具有抗氧化活性。
这两种成分能够通过增加体内抗氧化酶的合成和活性,提高抗氧化防御能力。
此外,它们还能够调节细胞中的信号通路,抑制氧化应激反应的发生,从而降低氧化损伤的程度。
此外,甘草还含有多种多糖类化合物,如甘草三萜糖、甘露醇二糖和麦芽糖等。
这些化合物在体内可以起到抗氧化的作用。
研究发现,甘草三萜糖能够通过增强细胞内还原型谷胱甘肽的含量,提高细胞的抗氧化能力。
此外,甘露醇二糖和麦芽糖也能够抑制脂质氧化,保护细胞的完整性。
另外,甘草还可以通过调节氧化应激相关的基因表达发挥抗氧化损伤的作用。
研究发现,甘草酸能够抑制多种氧化应激诱导的基因的表达,如氧化还原应激反应、炎症反应和细胞死亡相关的基因。
这些调节作用有助于减轻细胞的氧化损伤,并保护细胞的正常功能。
总之,甘草作为一种中草药,在抗氧化损伤方面具有重要的作用。
其活性成分能够清除自由基,增强抗氧化防御能力,调节氧化应激反应,并保护细胞的完整性。
叶黄素对糖尿病视网膜病变的作用和机制
叶黄素对糖尿病视网膜病变的作用和机制王俐媛;韩悦;张强;郭芳;马乐【摘要】叶黄素(Lutein)是一种含氧的类胡萝卜素.由于人体不能合成,其主要来源为食物.多项研究显示,叶黄素对包括糖尿病视网膜病变在内的年龄相关性退行性眼病具有保护作用.动物和细胞研究提示叶黄素可通过缓解高血糖诱导的氧化应激反应,起到保护视网膜血管内皮细胞,减少视网膜新生血管形成,达到减轻视网膜损伤的效用.本文将阐述叶黄素在预防和控制糖尿病视网膜病的发生发展等方面作用机制和研究现状.%Lutein belongs to the xanthophyll family of carotenoids, which are mainly synthesized from dark green leafy plants.Evidence from several studies has shown that patients with age-related degenerative eye disease including diabetic retinopathy (DR),may benefit from oral luteinintake.Animal and cell researches suggest that lu-tein,through oxidative stress induced by hyperglycemia remission,could protect retinal vascular endothelial cells, reduce formation of retinal neovascularization,and therefore mitigating possible retinal damages.This article re-views the results and possible mechanisms of lutein on DR at cellular and molecular level,offering insights in preven-tion and control measures of DR.【期刊名称】《国外医学(医学地理分册)》【年(卷),期】2015(036)004【总页数】3页(P311-313)【关键词】叶黄素;糖尿病视网膜病变;机制研究【作者】王俐媛;韩悦;张强;郭芳;马乐【作者单位】西安交通大学医学部公共卫生学院,西安 710061;西安交通大学医学部公共卫生学院,西安 710061;西安交通大学医学部公共卫生学院,西安 710061;西安交通大学医学部公共卫生学院,西安 710061;西安交通大学医学部公共卫生学院,西安 710061【正文语种】中文【中图分类】R774糖尿病视网膜病变(diabetic retinopathy,DR)是糖尿病常见的微血管并发症,目前尚无行之有效的治疗手段,致盲率高,目前已成为发达国家的主要致盲原因。
银川2024年小学4年级下册第16次英语第六单元测验卷(有答案)
银川2024年小学4年级下册英语第六单元测验卷(有答案)考试时间:90分钟(总分:110)B卷一、综合题(共计100题共100分)1. 选择题:What do you call a large cat with spots?A. TigerB. LeopardC. CheetahD. Lion答案:B2. 填空题:Look at the _____ (天鹅) swimming gracefully on the lake.3. 听力题:The ______ helps protect the body from bacteria.4. 听力题:I want to ________ my bike.5. 填空题:The first Olympic Games were held in ancient ______ (希腊).6. 听力填空题:I love going hiking during the __________.7. 填空题:The dolphin plays with its _________. (朋友)8. 选择题:What is the capital of Kiribati?a. Tarawab. Kiritimatic. Abemamad. Butaritari答案:a9. 听力题:The atomic mass of an element includes protons and ______.10. 填空题:The jellyfish has a _______ (透明) body.11. 填空题:My favorite _____ is a spinning top.12. 选择题:What is the process of learning called?A. TeachingB. StudyingC. EducatingD. Training答案:B13. 填空题:My grandmother is my favorite _______ because she tells stories.14. 填空题:I enjoy drawing ______ animals.15. 听力题:The chemical formula for table salt is ______.16. 选择题:What is the time when the sun goes down called?A. SunriseB. SunsetC. NoonD. Midnight答案: B17. 选择题:What is the name of the largest land animal?A. GiraffeB. LionC. ElephantD. Hippo答案:CA ____ is a big animal that can carry heavy loads.19. 填空题:My friend loves __________ (帮助别人).20. 选择题:What do flowers need to grow?A. WaterB. SandC. MetalD. Plastic21. 填空题:A _______ (老虎) is often seen in the zoo.22. 填空题:Cacti grow in _______ environments and need little care.23. 听力题:She is _____ (practicing) her guitar.24. 选择题:Which fruit is yellow and curved?A. AppleB. BananaC. OrangeD. Grape答案:B25. 听力题:Oxidizing agents accept _____ during a chemical reaction.26. 填空题:The ________ was a prominent figure in the history of philanthropy.27. 选择题:What is 3 + 5?A. 6B. 7C. 8D. 928. 填空题:The _______ (World Health Organization) focuses on global health issues.The koala loves to eat ________________ (桉树叶).30. 选择题:What color are bananas when they are ripe?A. GreenB. YellowC. RedD. Blue答案: B31. 填空题:The _____ (火烈鸟) stands on one leg while resting.32. 听力题:The chemical reaction that produces energy is called ______.33. 填空题:I like to build models with my ____ kit. (玩具名称)34. 听力题:The _____ (lamp/desk) is bright.35. 填空题:We enjoy ______ (参观) new places.36. 选择题:Which holiday celebrates the New Year?A. ChristmasB. ThanksgivingC. New Year's DayD. Halloween答案: C37. 填空题:My dog loves to play in the _______ (草地).38. 听力题:Oxygen is essential for __________ to occur.39. 填空题:Reading books about _________ (玩具) can spark my _________ (想象力).40. 听力题:A solution with a pH greater than is considered ______.We have a ______ (丰富的) educational system.42. 选择题:Which season comes after winter?A. FallB. SummerC. SpringD. Autumn答案: C. Spring43. 填空题:I like to read ________ (诗歌).44. 填空题:The __________ (历史的启示) inspires growth.45. 听力题:The ______ is the smallest unit of matter.46. 填空题:I enjoy picking _____ (野花) in the fields.47. 填空题:Fish live in the ________________ (水).48. 选择题:What do we call the art of folding paper into decorative shapes?A. OrigamiB. CalligraphyC. PotteryD. Weaving答案:A49. 填空题:The goat can eat almost _________ (任何) plant.50. 填空题:My favorite actress is _______ (名字). 她的表演很 _______ (形容词).51. 听力题:He is riding a ________ bike.52. 选择题:What do we call the person who tells stories?A. AuthorB. ChefC. TeacherD. Doctor答案: A53. 选择题:What is the name of the time zone that includes New York City?A. Pacific TimeB. Eastern TimeC. Central TimeD. Mountain Time答案:B54. 填空题:The __________ (历史的启示性) can inspire action.55. 填空题:The invention of ________ changed how we live daily.56. 选择题:Which planet is known as the Red Planet?A. EarthB. MarsC. JupiterD. Saturn答案: B57. 填空题:The __________ (古代印度) was known for its rich culture.58. 选择题:What do we call a group of sheep?A. HerdB. FlockC. PackD. School答案:B59. 听力题:A rock cycle describes how rocks change from one type to ______.60. 听力题:A _______ can help to demonstrate the principles of energy transfer.The cat is ________ on the sofa.62. 填空题:The _______ (小狼) plays with its siblings in the den.63. 听力题:The chemical symbol for sulfur is ______.64. 选择题:What is the opposite of 'happy'?A. JoyfulB. CheerfulC. SadD. Excited答案:C65. 填空题:The _______ (小山羊) jumps from rock to rock.66. 听力题:They are _____ (fishing) at the lake.67. 选择题:What do we call the liquid used to paint walls?A. InkB. PaintC. DyeD. Stain68. 填空题:The _____ (小狗) loves to chase its tail.69. 听力题:The chemical symbol for aluminum is _______.70. 选择题:What do we call the scientific study of plants?A. BotanyB. ZoologyC. EcologyD. Geography答案: AA _______ change is when the appearance changes, but the substance remains the same. (物理)72. 听力题:When you drop a ball, it falls due to ______.73. 填空题:I have _______ (很多) friends at school.74. 听力题:The ________ (narrative) tells a story.75. 小鸟) builds nests in spring. 填空题:The ___76. 选择题:What do we call the smallest particle of an element?A. AtomB. MoleculeC. CompoundD. Ion77. 填空题:The ________ (历史事件) shaped our nation.78. 填空题:I have a box of ________ (画笔) and ________ (颜料) to decorate my toys. Art is________ (有趣的).79. 选择题:What is the main ingredient in bread?A. SugarB. FlourC. RiceD. Salt答案: B80. 填空题:I have a collection of ________.81. 填空题:The _______ (骆驼) can survive in the desert.We go _____ (surfing) in the ocean.83. 填空题:My favorite animal is a _______ (老虎).84. 选择题:What is the name of the largest land animal?A. RhinocerosB. HippopotamusC. ElephantD. Giraffe85. 填空题:When it rains, I use my ________ (雨伞) and wear my ________ (雨鞋). I still enjoy going outside!86. 选择题:What is the name of the ocean located to the east of Africa?A. Atlantic OceanB. Indian OceanC. Arctic OceanD. Pacific Ocean答案:B87. 听力题:My friend is great at doing ____ (magic) tricks.88. 选择题:What is 14 - 7?A. 5B. 6C. 7D. 889. 填空题:We visit the ______ (文化中心) to learn about traditions.90. 听力题:Acids release hydrogen ions (H⁺) in a _____ (solution).91. 选择题:What do you call a person who repairs cars?A. MechanicB. ElectricianC. PlumberD. Carpenter答案:A92. 填空题:A frog's skin is often ______ (湿润).93. 选择题:Which gas is essential for breathing?A. HeliumB. HydrogenC. OxygenD. Nitrogen答案:C94. 填空题:The scientist studies _____ (化学) processes in the lab.95. 选择题:What is the main ingredient in sushi?A. ChickenB. FishC. RiceD. Vegetable答案:C96. ts are known for their ______, which can be used in crafts. (某些植物因其纤维而闻名,可以用于手工艺。
自由基清除率英语
自由基清除率英语
"自由基清除率"的英语表达可以是"Free Radical Scavenging Rate"或"Antioxidant Activity",具体取决于上下文和描述的背景。
以下是两个表达的例子:
Free Radical Scavenging Rate:
"The herbal extract demonstrated a high free radical scavenging rate, indicating its potential as a natural antioxidant."
中文翻译:该草药提取物表现出较高的自由基清除率,表明其具有作为天然抗氧化剂的潜力。
Antioxidant Activity:
"Researchers measured the antioxidant activity of various compounds and found that vitamin C exhibited significant antioxidant activity."
中文翻译:研究人员测量了各种化合物的抗氧化活性,发现维生素C表现出显著的抗氧化活性。
这两个表达都涉及到评估物质抵抗自由基损伤的能力,其中"自由基清除率"强调了对自由基的清除,而"抗氧化活性"则更广泛地描述了抗氧化作用。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Assessing the antioxidant and pro-oxidant activity of phenolic compounds by means of their copper reducing activity
CRAI method
Received: 20 June 2005 / Revised: 13 October 2005 / Accepted: 24 October 2005 / Published online: 17 January 2006 C Springer-Verlag 2005
Materials and methods
Standard and samples
Phenolic acids (tannic acid, gallic acid, caffeic acid, ferulic acid, chlorogenic acid and p-coumaric acid), ascorbic acid, trolox (6-hydroxy-2,5,7,8,-tetramethylchroman2-carboxylic acid), a water-soluble analogue of vitamin E, TPTZ (2,4,6,-tris-2(pyridyl)-s-triazine), EDTA, thiobarbituric acid, tetraetoxypropane (TEP), linoleic acid, trichloroacetic acid, tween 20, CuCl, CuSO4, FeSO4 and FeCl3 were all from Sigma-Aldrich (St-Louis, MO, USA). NH3 (30%) and H2O2 (33%) were from Panreac (Barcelona, Spain), sodium diethyldithiocarbamate (DDTC) was from Fluka Chemicals (Madrid, Spain). All other reagents were of analytical grade.
Keywords Antioxidant . Pro-oxidant . Phenolic compounds . FRAP . TBARS . CRAI
Introduction
Free radicals play a crucial role in the pathogenesis of several human diseases, such as cancer, rheumatoid arthritis and various neurodegenerative and pulmonary diseases [1]. Antioxidants protect against radicals and are therefore important tools in obtaining and preserving health. In the recent years the study of the antioxidant activity of many natural products has become a growing interest point for the scientific community. It is widely recognized that the
Eur Food Res Technol (2006) 223: 225–231 DOI 10.1007/s00217-005-0181-0
ORIGINAL PAPER
Jose´ A. Rufia´n-Henares · Cristina Delgado-Andrade · Francisco J. Morales
Abstract The antioxidant or pro-oxidant activity of some dietary phenolic compounds has been determined by their reducing activity over Fe3+ and Cu2+. The FRAP method has shown that phenolic acids exert a high reducing activity that give rise to a decrease in linoleic acid oxidaБайду номын сангаасion mediated by Fe3+, measured as TBARS. However, the reducing activity of phenolic compounds over Cu2+ leads to linoleic acid oxidation. Reducing capability against copper has been measured by a new approach called CRAI (Copper Reducing Activity Index), and results agreed with the degree of hydroxylation of the studied compounds. Sensibility and reproducibility of the method have been checked by measuring the inter-day and intra-day variation (1.08 and 1.89%, respectively). Possible pro-oxidant activity of phenolic compounds should be considered when the overall antioxidant activity in Cu naturally rich or Cu-enriched foodstuffs for dietetic purposes has to be evaluated.
226
which are naturally rich in copper, such as chocolate, nuts and meats [15], since copper is mainly present in foodstuffs as Cu2+. In this way, the present work was aimed at studying the copper reducing ability of certain phenolic compounds and other antioxidants such as ascorbic acid and trolox, used as reference, as good models to evaluate the antioxidative status of food samples. In addition, antioxidant or pro-oxidant activity has been measured over linoleic acid oxidation and the classical FRAP method.
A well-established method frequently used to assess the antioxidant properties of natural compounds is the inhibition of lipid peroxidation [9]. The use of commercially accessible individual compounds provides the repeatability of determination in any laboratory. Among individual fatty acids, methyl linoleate and linoleic acid are the most used. These compounds are relatively inexpensive and their oxidation is quite representative of the most essential features of biologically relevant lipid peroxidation [10].
