溴芬酸钠结构式
溴芬酸钠结构式

溴芬酸钠结构式溴芬酸钠是一种非处方药物,它以其出色的退热、镇痛和抗炎作用而被广泛应用于临床。
溴芬酸钠的结构式可以用化学方程式来表示:C14H9BrNaO3。
在这个方程式中,C代表碳,H代表氢,Br代表溴,Na代表钠,O代表氧。
这种化合物以其特殊的化学结构成为了医学界研究的焦点。
溴芬酸钠作为一种非类固醇抗炎药,对于治疗关节炎、风湿病、痛经、头痛、牙痛等疾病具有显著的疗效。
它通过抑制体内炎症介质的生成和释放,减轻炎症反应,从而达到镇痛和消炎的作用。
此外,溴芬酸钠还可以调节血浆PGE2(前列腺素E2)及其前体酶,抑制PGE2合成,进而抑制炎症细胞的迁移,减少关节或组织的红肿和疼痛。
溴芬酸钠有多种给药途径,常见的有口服片剂和口腔溶液剂。
口服片剂在服用后会快速被吸收,并在体内达到最高浓度。
而口腔溶液剂的吸收速度相对较快,更适用于那些需要迅速缓解疼痛和发热的患者。
口服溶液的好处是对于那些难以吞咽固体剂量的人来说,更容易接受。
然而,尽管溴芬酸钠在临床上被广泛应用,但也存在着一些潜在的副作用和禁忌症。
长期或大剂量使用可能导致胃肠道反应,如恶心、呕吐、腹痛等。
此外,阿斯匹林过敏者、哮喘患者、已知对非类固醇抗炎药过敏的患者以及孕妇禁用。
因此,在使用溴芬酸钠前,应该咨询医生的建议,并按照医生的指示正确使用。
总之,溴芬酸钠作为一种常用的非处方药物,在临床上具有重要的应用价值。
通过抑制炎症反应,减轻疼痛和发热,它为患者提供了快速、有效的治疗选择。
然而,正确的使用也是非常重要的,患者在使用前应了解药物的禁忌症和副作用,以避免不必要的风险。
同时,对于那些有特殊禁忌症或潜在风险的患者,应该在医生的指导下使用。
只有正确使用药物,才能让溴芬酸钠发挥出最好的治疗效果,让患者享受到更好的生活质量。
溴芬酸钠杂质汇总
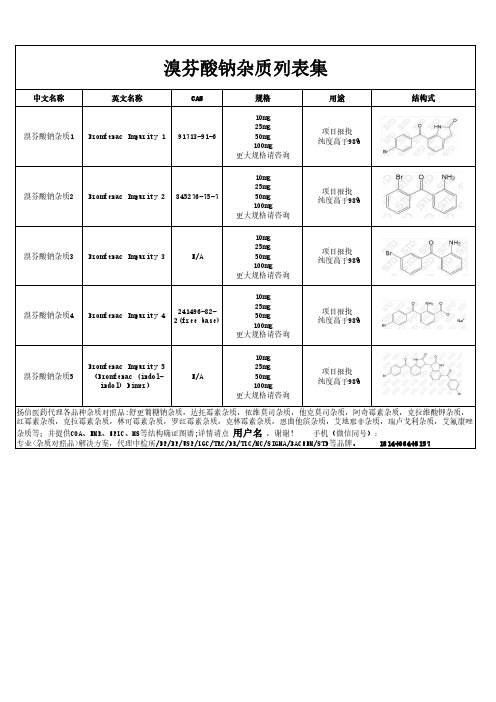
项目报批 纯度高于98%
溴芬酸钠杂质3 Bromfenac Impurity 3
N/A
10mg 25mg 50mg 100mg 更大规格请咨询
项目报批 纯度高于98%
溴芬酸钠杂质4
241496-82Bromfenac Impurity 4 2(free base)
10mg 25mg 50mg 100mg 更大规格请咨询
溴芬酸钠杂质列表集
中文名称
英文名称
CAS
溴芬酸钠杂质1 Bromfenac Impurity 1 91713-91-6
规格
10mg 25mg 50mg 100mg 更大规格请咨询
用途
项目报批 纯度高于98%
结构式
溴芬酸钠杂质2 Bromfenac Impurity 2 845276-75-7
10mg 25mg 50mg 100mg 更大规格请咨询
常用化学药物结构式总汇
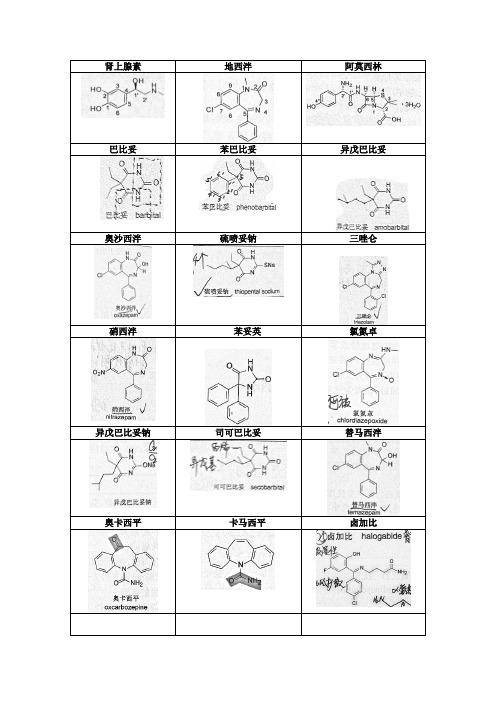
肾上腺素地西泮阿莫西林巴比妥苯巴比妥异戊巴比妥奥沙西泮硫喷妥钠三唑仑硝西泮苯妥英氯氮卓异戊巴比妥钠司可巴比妥替马西泮奥卡西平卡马西平卤加比氯丙嗪异丙嗪氟哌啶醇氯氮平盐酸丙咪嗪阿米替林盐酸氟西汀盐酸吗啡吗啡阿扑吗啡可待因喷他佐辛哌替啶美沙酮盐酸哌替啶盐酸美沙酮喷他佐辛可可碱茶碱咖啡因氯贝胆碱毛果芸香碱溴新斯的明硫酸阿托品去甲肾上腺素异丙肾上腺素肾上腺素多巴胺麻黄碱去氧肾上腺素可乐定沙丁胺醇多巴酚丁胺,多巴胺阿司咪唑盐酸普鲁卡因盐酸利多卡因盐酸达克罗宁阿替洛尔美托洛尔盐酸普萘洛尔维拉帕米硝苯地平盐酸地尔硫卓奎尼丁美西律氟卡尼卡托普利氯沙坦硝酸甘油多巴酚丁胺匹莫苯洛伐他汀吉非贝齐酚妥拉明妥拉唑啉酚苄明哌唑嗪吲哚拉明可乐定肼屈嗪胍乙啶利血平西咪替丁雷尼替丁奥美拉唑昂丹司琼盐酸地芬尼多西沙必利甲氧氯普胺多潘立酮联苯双酯水飞蓟宾阿司匹林水杨酰胺双水杨酯贝诺酯对乙酰氨基酚甲芬那酸吡罗昔康双氯芬酸钠布洛芬羟布宗保泰松吲哚美辛萘普生盐酸氮芥环磷酰胺塞替派替派卡莫司汀白消安顺铂氟尿嘧啶阿糖胞苷巯嘌呤甲氨蝶呤盐酸多柔比星盐酸米托蒽醌羟基喜树碱青霉素钠青霉素G 苯唑西林钠阿莫西林氨苄西林羧苄西林磺苄西林头孢氨苄头孢羟基苄头孢拉定头孢克洛头孢他啶头孢噻肟钠克拉维酸钾舒巴坦钠舒它西林氨曲南四环素氯霉素氧氟沙星左氟沙星环丙沙星诺氟沙星盐酸环丙沙星异烟肼对氨基水杨酸钠磺胺嘧啶磺胺磺胺醋酰甲氧苄啶硝酸益康唑氟康唑盐酸金刚烷胺利巴韦林齐多夫定阿昔洛韦阿苯达唑吡喹酮奎宁青蒿素甲苯磺丁脲格列本脲盐酸二甲双胍依他尼酸乙酰唑胺螺内酯氨苯碟啶前列腺素米索前列醇甾烷雌甾烷孕甾烷雌二醇炔雌醇己烯雌酚枸橼酸他莫昔芬丙酸睾酮炔诺酮醋酸甲羟孕酮左炔诺孕酮米非司酮黄体酮氢化可的松醋酸地塞米松维生素A醋酸酯维生素E醋酸酯维生素C。
各种溴酸的化学式

各种溴酸的化学式溴酸(Bromic Acid)是一种含有溴元素的无机化合物,化学式为HBrO3。
溴酸可以形成多种不同的溴酸盐,其中最常见的是溴酸钠(NaBrO3)和溴酸钾(KBrO3)。
溴酸是一种强氧化剂,在化学实验和工业生产中有广泛的应用。
溴酸的化学式为HBrO3,其中H表示氢原子,Br表示溴原子,O表示氧原子。
溴酸的结构中,溴原子与氧原子和氢原子形成共价键,氧原子与氢原子之间也形成共价键。
溴酸的分子式中,溴原子的电子排布为2-8-18-7,氧原子的电子排布为2-6,氢原子的电子排布为1。
溴酸的性质与溴酸盐有关。
溴酸盐是溴酸和金属离子形成的盐类化合物,常见的溴酸盐有溴酸钠和溴酸钾。
溴酸盐的化学式中,金属离子与溴酸根离子(BrO3-)结合,形成稳定的晶体结构。
溴酸盐的溶解度与温度有关,通常在室温下溶解度较小,但在高温下溶解度会增大。
溴酸具有强氧化性质,可以氧化许多有机和无机物质。
在实验室中,溴酸常用作氧化试剂,可以用来氧化硫酸铁(Ⅱ)(FeSO4)制备硫酸铁(Ⅲ)(Fe2(SO4)3),也可以氧化亚硫酸钠(Na2SO3)生成硫酸钠(Na2SO4)。
溴酸还可以用于制备其他溴化物,如溴化钾(KBr)和溴化铵(NH4Br)。
溴酸盐在工业上也有广泛的应用。
溴酸钾常用作消防草酸盐(聚溴二苯醚)的抑制剂,可以减少聚溴二苯醚在高温下的自燃性。
溴酸钾还可以用作消毒剂,在饮用水处理和游泳池水处理中起到杀菌作用。
溴酸盐还可以用于制备其他溴化物,如溴酸铵(NH4BrO3)和溴酸钙(Ca(BrO3)2)。
总结起来,溴酸是一种含有溴元素的无机化合物,化学式为HBrO3。
溴酸可以形成多种溴酸盐,常见的有溴酸钠和溴酸钾。
溴酸具有强氧化性质,在实验室和工业生产中有广泛的应用。
溴酸和溴酸盐在化学反应和材料制备中起着重要的作用。
药物化学复习资料(化学结构式)
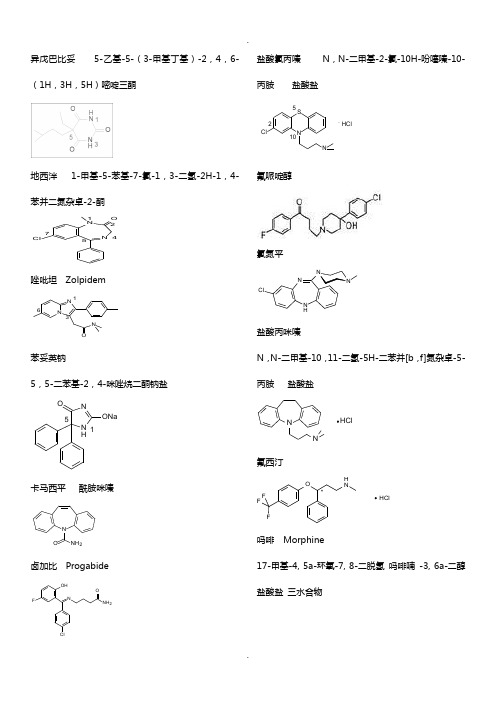
异戊巴比妥 5-乙基-5-(3-甲基丁基)-2,4,6-(1H ,3H ,5H )嘧啶三酮地西泮 1-甲基-5-苯基-7-氯-1,3-二氢-2H-1,4-苯并二氮杂卓-2-酮NNO Cl12457唑吡坦 ZolpidemNN ON 136苯妥英钠5,5-二苯基-2,4-咪唑烷二酮钠盐N H NO ONa15卡马西平 酰胺咪嗪N ONH 2卤加比 ProgabideOHFNClNH 2O盐酸氯丙嗪 N ,N-二甲基-2-氯-10H-吩噻嗪-10-丙胺 盐酸盐.HClNSClN2510氟哌啶醇氯氮平NNNN HCl盐酸丙咪嗪N ,N-二甲基-10,11-二氢-5H-二苯并[b ,f]氮杂卓-5-丙胺 盐酸盐NNHCl氟西汀OH NFFF HCl*吗啡 Morphine17-甲基-4, 5a-环氧-7, 8-二脱氢 吗啡喃 -3, 6a-二醇盐酸盐 三水合物OOHN HO134567891011121314151617. HCl . 3H 2O盐酸哌替啶1-甲基-4-苯基-4-哌啶甲酸乙酯盐酸盐NOO . HCl盐酸美沙酮 NO. HCl喷他佐辛NHOH咖啡因 Caffeine1,3,7-三甲基-3,7-二氢-1H - 嘌呤 -2,6-二酮一水合物N NNNO O. H 2O 137吡拉西坦2-(2-氧代-吡咯烷-1-基)乙酰胺NH 2NOO氯贝胆碱 Bethanechol ChlorideOO H 2NN +(CH 3)3 Cl -CH 3毛果芸香碱NN OH 3CCH 3O溴新斯的明 Neostigmine BromideN +(CH 3)3 Br -ON OH 3CCH 3多奈哌齐硫酸阿托品 Atropine Sulphate. H 2SO 4 . H 2ON OOHOCH 32溴丙胺太林Br -OOO NH 3CCH 3CH 3CH 3CH 3+哌仑西平苯磺阿曲库铵++N H 3CO H 3COOCH 3H 3COO O N OOOCH 3OCH 3OCH 3OCH 3CH 3H 3C SOO O -. 2泮库溴铵1,1¢-[3a ,17b-双-(乙酰氧基)-5a-雄甾烷-2b ,16b-二基]双-[1-甲基哌啶鎓]二溴化物++H NHHHONCH 3CH 3H 3COH 3C H 3C OH 3C O. 2Br -肾上腺素 EpinephrineHO H NOHHOCH 3麻黄碱 EphedrineCH 3H NOHCH 3沙丁胺醇 SalbutamolOHH NCH 3CH 3HOCH 3HO马来酸氯苯那敏N ,N-二甲基-g-(4-氯苯基)-2-吡啶丙胺顺丁烯二酸盐,又名扑尔敏.NClNO OOH OH氯雷他定4-(8-氯-5,6-二氢-11H-苯并[5,6]-环庚烷[1,2-b]吡啶-11-亚基-1-羧酸乙酯NN OOCl盐酸西替利嗪2-[4-[(4-氯苯基)苯基甲基]-1-哌嗪基]乙氧基乙酸二盐酸盐.2HClOOOHClNN咪唑斯汀 Mizolastine2-〔〔1-〔1-〔(4-氟苯基)甲基〕-1H-苯并咪唑-2-基〕哌啶基-4-基〕甲基氨基〕嘧啶-4(3H )-酮盐酸普鲁卡因 Procaine Hydrochloride 4-氨基苯甲酸-2-(二乙氨基)乙酯盐酸盐盐酸利多卡因 Lidocaine HydrochlorideN-(2,6-二甲苯基)-2-(二乙氨基)乙酰胺盐酸盐一水合物盐酸达克罗宁盐酸普萘洛尔Propranolol 1-异丙氨基-3-(1-萘氧基)-2-丙醇盐酸盐硝苯地平Nifedipine盐酸地尔硫卓DiltiazemHydrochlorideHCl硫酸喹尼丁(9S )-6′-甲氧基-脱氧辛可宁-9-醇硫酸盐二水合物2H 2SO 42H 2O1盐酸胺碘酮(2-丁基-3-苯并呋喃基)[4-[2-(二乙氨基)乙氧基]-3,5-二碘苯基]甲酮盐酸盐HClOOOIIN123451,3,2,1,2,卡托普利 1-[(2S )-2-甲基-3-巯基-1-氧代丙基]-L-脯氨酸N OOH1氯沙坦2-丁基-4-氯-1-[[2′-(1H -四唑-5-基)[1,1′-联苯]-4-基]甲基]-1H-咪唑-5-甲醇4'3'2'1'432145154321NHN N NNN OHCl硝酸甘油1,2,3-丙三醇三硝酸酯地高辛洛伐他汀 Lovastatin吉非贝齐Gemfibrozil氯吡格雷Clopidogrel(S )-a-(2-氯苯基)-6,7-二氢噻吩并[3,2-C]吡啶-5(4H )-乙酸甲酯32??????????华法林钠OO NaOO利血平Reserpine 11,17a-二甲氧基-18b-[(3,4,5-三甲氧基苯甲酰)氧]-3b ,20a-育亨烷-16b-甲酸甲酯• 西咪替丁 Cimetidine N ’-甲基-N ”-[2-[[(5-甲基-1H -咪唑- 4-基)-甲基]硫代]-乙基]-N -氰基胍雷尼替丁Ranitidine N ’-甲基-N- [2-[ [ [ 5- (二甲氨甲基)-2-呋喃基] 甲基] 硫代] 乙基] -2-硝基-1,1-乙烯二胺盐酸盐奥美拉唑(R, S )5-甲氧基-2- (4-甲氧基-3,5-二甲基-吡啶-2-基-甲基氧硫基) 苯并咪唑昂丹司琼(消旋)1,2,3,9-四氢-9-甲基-3-[(2-甲基-1H-咪唑-1-基)甲基]-4H -咔唑-4-酮西沙必利 (±)顺式-4-氨基-5-氯-N-[1-[3-(4-氟苯氧基)丙基]-3-甲氧基-4-哌啶基]-2-甲氧基苯甲酰胺西沙必利甲氧氯普胺 MetoclopramideN-[(2-二乙氨基)乙基]-4-氨基-2-甲氧基-5-氯-苯甲酰胺多潘立酮Domperidone 5-氯-1-[1-[3-(2,3-二氢-2-氧代-1H -苯并咪唑-1-基)丙基]-4-哌啶]-2,3-二氢-1H -苯并咪唑-2-酮联苯双酯Bifendate对乙酰氨基酚(Paracetamol ) 扑热息痛N -(4-羟基苯基)乙酰胺CH 3N HOHO阿司匹林 Aspirin 2-(乙酰氧基)苯甲酸; 乙酰水杨酸OO HOC H 3O吲哚美辛 lndomethacin2-甲基-1-(4-氯苯甲酰基)-5-甲氧基-1H -吲哚-3-乙酸NH 3COOHO CH 3OCl双氯芬酸钠 Diclofenac Sodium 2-[( 2,6-二氯苯基 ) 氨基 ] 苯乙酸钠 又名双氯灭痛。
溴酚蓝

用途说明
用途说明
配置溴酚蓝指示液。用途:变色范围:pH2.8-4.6(黄-蓝),酸碱指示剂;非水滴定用指示剂,蛋白电泳 染色;病毒化验等 。
配置方法
配置方法
方法1:1%的溴酚蓝 将托盘天平上称取1g溴酚蓝定溶于100ml无水乙醇中,转移入滴瓶中,贴标签备用。 方法2:0.05%的溴酚蓝 配western blot用的2SDS上样缓冲液需要用到0.1%的溴酚蓝,2-DE中溴酚蓝一般也是配成0.1%母液。实际 上,溴酚蓝在水中的溶解度不高,直接将0.1g溴酚蓝溶于100ml水是有困难的。下面是其较合适的配制方法: 0.05%的溴酚蓝配制方法:取溴酚蓝0.1g,加0.05mol/L氢氧化钠溶液3.0ml使溶解,再加水稀释至200ml, 即得。变色范围pH2.8~4.6(黄→蓝绿)。只是不知道加入NaOH会不会影响2-DE实验。估计影响不大,因为指示 剂用量毕竟很少。 方法3:1%溴酚蓝 加1g水溶性钠型溴酚蓝于100ml水中,搅拌或涡旋混合直到完全溶解。溴酚蓝其钠盐易溶解在水里。
是一种pH指示剂,在pH 3.0~4.6范围,颜色由黄变蓝。常用做电泳指示染料,凝胶中电泳迁移速度在小分子 核酸或蛋白质区域。
性状描述
性状描述
浅黄色到棕黄色粉末;易溶于氢氧化钠溶液,溶于甲醇、乙醇和苯,微溶于水(约0.4g/100ml);最大吸收 波长422nm
物理参数
物理参数
熔点:273℃(dec.)(lit.)
溴酚蓝
一种电泳指示染料
目录
01 性状描述
03 分子结构数据
02 物理参数 04 计算化学数据
05 贮存方法
07 用途说明
目录
06 合成方法 08 配置方法
基本信息
溴酚蓝,是一种有机化合物,分子式为C19H10Br4O5S,分子量为669.961,浅黄色到棕黄色粉末;易溶于氢 氧化钠溶液,溶于甲醇、乙醇和苯,微溶于水(约0.4g/100ml),最大吸收波长422nm折射率:123.26 2、摩尔体积(cm3/mol):304.5 3、等张比容(90.2K):893.7 4、表面张力(dyne/cm):74.1 5、极化率(10-24cm3):48.86
溴芬酸钠结构式

溴芬酸钠结构式
溴芬酸钠是一种非常常见的药物,被广泛用于缓解疼痛和消炎。
其化学结构式为C14H10Br2NNaO2。
溴芬酸钠是一种非处方药,属于非甾体抗炎药(NSAIDs)的一种。
它通过抑制炎症介质的产生,从而减轻疼痛和炎症的症状。
它被广泛应用于治疗各种疼痛和炎症性疾病,例如头痛、牙痛、关节炎、风湿痛等。
溴芬酸钠的化学结构由苯环和溴代苯环组成,中间连接有氮原子和一个钠离子。
这种结构使得它具有较强的生物活性和药理活性。
它通过与特定的受体结合,抑制炎症反应并减轻疼痛。
溴芬酸钠作为NSAIDs的一种,与其他NSAIDs相比具有一些特殊的优点。
首先,它可以迅速缓解疼痛和炎症,作用快速,效果明显。
其次,它具有较低的胃肠道刺激性,不会引起胃溃疡和出血等不良反应。
此外,它还具有较长的持续时间,一次使用可维持较长时间的疗效。
虽然溴芬酸钠是一种非处方药,但仍然需要注意一些使用注意事项。
首先,不宜长期或大剂量使用,以免出现不良反应。
其次,对于孕妇、哺乳期妇女、儿童、年老体弱者等特殊人群,应在医生的指导下使用。
同时,注意与其他药物的药物相互作用,避免不良反应的发生。
总之,溴芬酸钠是一种非常常用的药物,可以有效缓解疼痛和消炎。
然而,使用时应注意使用注意事项,以免出现不良反应。
在需要使用时,最好在医生的指导下使用,以确保安全有效。
溴芬酸钠英文说明书

____________________________________________________________________________________________________ __________________________________________________________________________________________________HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use Bromday (bromfenac ophthalmicsolution) 0.09% safely and effectively.See full prescribing information for Bromday.Bromday (bromfenac ophthalmic solution) 0.09% Initial U.S. Approval: 1997 ------------INDICATIONS AND USAGE---------Bromday is a nonsteroidal anti-inflammatory drug (NSAID) indicated for the treatment of postoperativeinflammation and reduction of ocular pain in patientswho have undergone cataract extraction(1). ---------DOSAGE AND ADMINISTRATION---Instill one drop into the affected eye(s) once daily beginning 1 day prior to surgery, continued on the day of surgery and through the first 14 days post-surgery (2.1). ------DOSAGE FORMS AND STRENGTHS --Topical ophthalmic solution: bromfenac 0.09% (3) -------WARNINGS AND PRECAUTIONS-----•Sulfite Allergic Reactions (5.1) •Slow or Delayed Healing (5.2) •Potential for cross-sensitivity (5.3) •Increase bleeding of ocular tissues (5.4) •Corneal effects including keratitis (5.5) • Contact Lens Wear (5.6) -------------ADVERSE REACTIONS-------------The most commonly reported adverse reactions in 27% of patients were abnormal sensation in eye, conjunctival hyperemia and eye irritation (including burning/stinging) (6.1). To report SUSPECTED ADVERSE REACTIONS,contact ISTA Pharmaceuticals, Inc. at 1-877-788-2020, or FDA at 1-800-FDA-1088 or /medwatch. See 17 for PATIENT COUNSELING INFORMATION Revised: 9/2010FULL PRESCRIBING INFORMATION: CONTENTS* 1 INDICATIONS AND USAGE 2 DOSAGE AND ADMINISTRATION 2.1 Recommended Dosing 2.2 Use with Other Topical Ophthalmic Medications 3 DOSAGE FORMS AND STRENGTHS 4 CONTRAINDICATIONS 5 WARNINGS AND PRECAUTIONS 5.1 Sulfite Allergic Reactions 5.2 Slow or Delayed Healing 5.3 Potential for Cross-Sensitivity 5.4 Increased Bleeding Time 5.5 Keratitis and Corneal Reactions 5.6 Contact Lens Wear 6 ADVERSE REACTIONS 6.1 Clinical Trial Experience 6.2 Post-Marketing Experience 8 USE IN SPECIFIC POPULATIONS 8.1 Pregnancy 8.3 Nursing Mothers 8.4 Pediatric Use 8.5 Geriatric Use 11 DESCRIPTION12 CLINICAL PHARMACOLOGY 12.1 Mechanism of Action 12.3 Pharmacokinetics 13 NONCLINICAL TOXICOLOGY 13.1 Carcinogenesis, Mutagenesis andImpairment of Fertility 14 CLINICAL STUDIES 14.1 Ocular Inflammation and Pain 16 HOW SUPPLIED/STORAGE AND HANDLING 17 PATIENT COUNSELING INFORMATION 17.1 Slowed or Delayed Healing17.2 Sterility of Dropper Tip 17.3 Concomitant Use of Contact Lenses17.4 Concomitant Topical Ocular Therapy*Sections or subsections omitted from the full prescribinginformation are not listed.FULL PRESCRIBING INFORMATION1 INDICATIONSANDUSAGE Bromday (bromfenac ophthalmic solution)0.09% is indicated for the treatment ofpostoperative inflammation and reduction ofocular pain in patients who have undergonecataract surgery.2 DOSAGEANDADMINISTRATION 2.1 RecommendedDosingFor the treatment of postoperativeinflammation in patients who haveundergone cataract extraction, one drop of Bromday ophthalmic solution should beapplied to the affected eye(s) once dailybeginning 1 day prior to cataract surgery,continued on the day of surgery, andthrough the first 14 days of thepostoperative period.2.2 Use with Other Topical OphthalmicMedicationsBromday ophthalmic solution may beadministered in conjunction with othertopical ophthalmic medications such asalpha-agonists, beta-blockers, carbonicanhydrase inhibitors, cycloplegics, andmydriatics. Drops should be administeredat least 5 minutes apart.3 DOSAGE FORMS AND STRENGTHSTopical ophthalmic solution: bromfenac 0.09%.4 CONTRAINDICATIONSNone.5 WARNINGSANDPRECAUTIONS 5.1 SulfiteAllergicReactionsContains sodium sulfite, a sulfite that maycause allergic-type reactions includinganaphylactic symptoms and life-threatening orless severe asthmatic episodes in certainsusceptible people. The overall prevalence ofsulfite sensitivity in the general population isunknown and probably low. Sulfite sensitivity isseen more frequently in asthmatic than in non-asthmatic people.5.2 Slow or Delayed HealingAll topical nonsteroidal anti-inflammatorydrugs (NSAIDs) may slow or delay healing.Topical corticosteroids are also known toslow or delay healing. Concomitant use oftopical NSAIDs and topical steroids mayincrease the potential for healing problems.5.3 Potential for Cross-SensitivityThere is the potential for cross-sensitivity toacetylsalicylic acid, phenylacetic acidderivatives, and other NSAIDs. Therefore,caution should be used when treatingindividuals who have previously exhibitedsensitivities to these drugs.5.4 Increased Bleeding TimeWith some NSAIDs, there exists thepotential for increased bleeding time due to interference with platelet aggregation.There have been reports that ocularlyapplied NSAIDs may cause increasedbleeding of ocular tissues (includinghyphemas) in conjunction with ocularsurgery.It is recommended that Bromday ophthalmicsolution be used with caution in patients withknown bleeding tendencies or who arereceiving other medications which may prolong bleeding time.5.5 Keratitis and Corneal ReactionsUse of topical NSAIDs may result in keratitis.In some susceptible patients, continued use oftopical NSAIDs may result in epithelialbreakdown, corneal thinning, corneal erosion,corneal ulceration or corneal perforation. These events may be sight threatening. Patients withevidence of corneal epithelial breakdownshould immediately discontinue use of topicalNSAIDs and should be closely monitored forcorneal health.Post-marketing experience with topicalNSAIDs suggests that patients withcomplicated ocular surgeries, cornealdenervation, corneal epithelial defects,diabetes mellitus, ocular surface diseases(e.g., dry eye syndrome), rheumatoidarthritis, or repeat ocular surgeries within a short period of time may be at increasedrisk for corneal adverse events which may become sight threatening. Topical NSAIDs should be used with caution in thesepatients.Post-marketing experience with topicalNSAIDs also suggests that use more than24 hours prior to surgery or use beyond 14days post surgery may increase patient risk for the occurrence and severity of cornealadverse events.5.6 Contact Lens WearBromday should not be administered whilewearing contact lenses6 ADVERSEREACTIONS6.1 Clinical Trial ExperienceThe most commonly reported adverseexperiences reported following use ofbromfenac after cataract surgery include:abnormal sensation in eye, conjunctivalhyperemia, eye irritation (includingburning/stinging), eye pain, eye pruritus, eyeredness, headache, and iritis. These eventswere reported in 2-7% of patients.6.2 Post-MarketingExperience The following events have been identifiedduring post-marketing use of bromfenacophthalmic solution 0.09% in clinical practice.Because they are reported voluntarily from apopulation of unknown size, estimates offrequency cannot be made. The events, which have been chosen for inclusion due to eithertheir seriousness, frequency of reporting,possible causal connection to topicalbromfenac ophthalmic solution 0.09% or acombination of these factors, include cornealerosion, corneal perforation, corneal thinning,and epithelial breakdown. [see Warnings andPrecautions (5)]8 USE IN SPECIFIC POPULATIONS 8.1 PregnancyTeratogenic Effects: PregnancyCategory C. Reproduction studiesperformed in rats at oral doses up to 0.9mg/kg/day (1300 times the recommendedhuman ophthalmic dose [RHOD]) and inrabbits at oral doses up to 7.5 mg/kg/day(11,000 times RHOD) revealed noevidence of teratogenicity due tobromfenac. However, 0.9 mg/kg/day in rats caused embryo-fetal lethality, increasedneonatal mortality, and reduced postnatalgrowth. Pregnant rabbits treated with 7.5mg/kg/day caused increased post-implantation loss.There are no adequate and well-controlled studies in pregnant women. Becauseanimal reproduction studies are not always predictive of human response, this drugshould be used during pregnancy only ifthe potential benefit justifies the potentialrisk to the fetus.Nonteratogenic Effects:Because of the known effects of prostaglandin biosynthesis-inhibiting drugs on the fetalcardiovascular system (closure of ductusarteriosus), the use of Bromday ophthalmicsolution during late pregnancy should beavoided.8.3 NursingMothersCaution should be exercised when Bromday is administered to a nursing woman.8.4 PediatricUseSafety and efficacy in pediatric patients below the age of 18 have not been established. 8.5 Geriatric UseThere is no evidence that the efficacy or safetyprofiles for Bromday differ in patients 65 yearsof age and older compared to younger adult patients. 11 DESCRIPTIONBromday (bromfenac ophthalmic solution) 0.09% is a sterile, topical, nonsteroidalanti-inflammatory drug (NSAID) for ophthalmic use. Each mL of Bromday contains 1.035 mg bromfenac sodium(equivalent to 0.9 mg bromfenac free acid).Bromfenac sodium is designated chemically as sodium 2-amino-3-(4bromobenzoyl) phenylacetatesesquihydrate, with an empirical formula ofC 15H 11BrNNaO 3• 1½H 2O. The structuralstructure for bromfenac sodium is: Bromfenac sodium is a yellow to orangecrystalline powder.The molecular weight of bromfenac sodium is 383.17. Bromdayophthalmic solution is supplied as a sterileaqueous 0.09% solution, with a pH of 8.3. The osmolality of Bromday ophthalmic solution is approximately 300 mOsmol/kg. Each mL of Bromday ophthalmic solution contains: Active : bromfenac sodium hydrate 0.1035% Preservative : benzalkonium chloride (0.05 mg/mL) Inactives : boric acid, disodium edetate (0.2 mg/mL), polysorbate 80 (1.5 mg/mL), povidone (20 mg/mL), sodium borate, sodium sulfite anhydrous (2 mg/mL), sodium hydroxide to adjust pH and water for injection, USP. 12 CLINICAL PHARMACOLOGY 12.1 Mechanism of Action Bromfenac is a nonsteroidal anti-inflammatory drug (NSAID) that has anti-inflammatory activity. The mechanism of its action is thought to be due to its ability to block prostaglandin synthesis by inhibiting cyclooxygenase 1 and 2. Prostaglandins have been shown in manyanimal models to be mediators of certain kindsof intraocular inflammation. In studiesperformed in animal eyes, prostaglandins havebeen shown to produce disruption of the blood-aqueous humor barrier, vasodilation, increasedvascular permeability, leukocytosis, and increased intraocular pressure. 12.3 PharmacokineticsThe plasma concentration of bromfenac following ocular administration of 0.09% Bromday (bromfenac ophthalmic solution) in humans is unknown. Based on the maximum proposed dose of one drop to the eye (0.045 mg) and PK information from other routes of administration, the systemic concentration of bromfenac is estimated to be below the limit of quantification (50 ng/mL) at steady-state in humans. 13 NONCLINICAL TOXICOLOGY 13.1 Carcinogenesis, Mutagenesis and Impairment of Fertility Long-term carcinogenicity studies in rats andmice given oral doses of bromfenac up to 0.6 mg/kg/day (900 times the recommended human ophthalmic dose [RHOD] of 1.67 mcg/kg in 60 kg person on a mg/kg/basis, assuming 100% absorbed) and 5 mg/kg/day (7500 times RHOD), respectively revealed no significant increases in tumor incidence. Bromfenac did not show mutagenic potential in various mutagenicity studies, including the reverse mutation, chromosomal aberration, and micronucleus tests.Bromfenac did not impair fertility whenadministered orally to male and female rats atdoses up to 0.9 mg/kg/day and 0.3 mg/kg/day, respectively (1300 and 450 times RHOD,respectively).14 CLINICALSTUDIES14.1 Ocular inflammation and pain following cataract surgeryClinical efficacy was evaluated in threerandomized, double-masked, placebo-controlled trials in which subjects requiring cataract surgery were assigned to Bromday or placebo. Patients were dosed with onedrop per eye starting the day beforesurgery and continuing for 14 days. Theprimary endpoint was clearing of ocularinflammation by day 15. An additionalefficacy endpoint was the number ofpatients who were pain free on day 1 aftercataract surgery.In 2 of the 3 studies, Bromday ophthalmicsolution had statistically significant higherincidence of completely clearinginflammation (46-47% vs. 25-29%) andalso had a statistically significant higherincidence of subjects that were pain free at day 1 post cataract surgery (83-89% vs.51-71%).16 HOWSUPPLIED/STORAGEAND HANDLINGBromday (bromfenac ophthalmic solution)0.09% is supplied in a white LDPE plasticsqueeze bottle with a 15 mm LDPE whitedropper-tip and 15 mm polypropylene gray cap as follows:1.7 mL in 7.5 mL container (NDC 67425-99917) STORAGEStore at 15º – 25ºC (59º – 77ºF).17 PATIENTCOUNSELING INFORMATION17.1 Slowed or Delayed HealingPatients should be advised of the possibilitythat slow or delayed healing may occur whileusing NSAIDs.17.2 Sterility of Dropper TipPatients should be advised to not touchdropper tip to any surface, as this maycontaminate the contents.17.3 Concomitant Use of Contact LensesContact lenses should not be worn during theuse of this product.17.4 Concomitant Topical Ocular TherapyIf more than one topical ophthalmicmedication is being used, the medicinesshould be administered at least 5 minutes apartRx Only©ISTA Pharmaceuticals®, Inc. Manufactured for: ISTA Pharmaceuticals, Inc. Irvine, CA 92618By: Bausch & Lomb IncorporatedTampa, FL 33637Under license from:Senju Pharmaceuticals Co., Ltd.Osaka, Japan 541-0046® and ™ marks owned by ISTA Pharmaceuticals, Inc.。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
溴芬酸钠结构式
1. 溴芬酸钠的基本信息
溴芬酸钠(Sodium bromfenac)是一种非甾体类抗炎药物,化学名称为2-氨基-3-苯基丙酸溴乙酯。
它是一种白色或几乎白色的结晶性粉末,可溶于水。
溴芬酸钠具有较强的抗炎、镇痛和抗血管新生等药理作用。
2. 溴芬酸钠的化学结构式
如上图所示,溴芬酸钠的化学结构式为:C14H10BrNNaO3。
3. 溴芬酸钠的制备方法
3.1 化学合成法
溴芬酸钠可以通过以下步骤合成:
1.取得苯基丙二脱氢乙酮(Phenylpropylidenacetone)作为起始原料。
2.将苯基丙二脱氢乙酮与臭素反应,生成2-苯基丙二脱氢乙亚胺
(Phenylpropylidenimine)。
3.将2-苯基丙二脱氢乙亚胺与溴乙酸酐反应,生成2-苯基丙二脱氢乙酸溴乙
酯(Phenylpropylidenacetic acid bromoethyl ester)。
4.最后,将2-苯基丙二脱氢乙酸溴乙酯与碳酸钠反应,生成溴芬酸钠。
3.2 生物合成法
除了化学合成法外,溴芬酸钠还可以通过生物合成法制备。
这种方法利用微生物或植物细胞内的代谢途径来合成目标化合物。
具体的生物合成方法需要进一步研究和开发。
4. 溴芬酸钠的药理作用
4.1 抗炎作用
溴芬酸钠通过抑制前列腺素合成的关键酶环氧化酶(COX),从而减少炎症反应中前列腺素E2(PGE2)的产生。
它对环氧化酶-1和环氧化酶-2都有较高的选择性抑制作用。
通过抑制炎症介质的合成,溴芬酸钠可以减轻炎症反应引起的疼痛、红肿和局部渗出等症状。
4.2 镇痛作用
溴芬酸钠具有较强的镇痛作用。
它通过抑制前列腺素E2在中枢神经系统中的合成,减少对疼痛刺激的感知和传导。
溴芬酸钠对于轻至中度的急性和慢性疼痛均具有一定的镇痛效果。
4.3 抗血管新生作用
溴芬酸钠还具有抑制血管新生的作用。
它可以通过抑制血管内皮生长因子(VEGF)等促血管生成因子的合成和释放,阻断异常血管生成,从而减少新生血管对组织造成的损害。
5. 溴芬酸钠的临床应用
由于其优良的药理特点,溴芬酸钠被广泛应用于临床治疗中:
•眼科领域:溴芬酸钠眼药水可用于治疗眼部炎症和疼痛,如结膜炎、角膜炎等。
•风湿科领域:溴芬酸钠片剂可用于治疗风湿性关节炎、强直性脊柱炎等风湿性疾病引起的关节疼痛和肿胀。
•外科领域:溴芬酸钠注射液可用于术后镇痛和消除手术创伤引起的组织水肿。
6. 溴芬酸钠的药物相互作用
在使用溴芬酸钠时,需要注意以下药物相互作用:
•抗凝血药物:与溴芬酸钠合用可能增加出血风险,需慎重使用。
•利尿剂:与溴芬酸钠合用可能降低利尿剂的效果。
•胰岛素和口服降血糖药物:与溴芬酸钠合用可能增加低血糖风险,需密切监测血糖。
7. 溴芬酸钠的不良反应
溴芬酸钠在临床应用中可能出现一些不良反应,包括:
•消化系统不良反应:如恶心、呕吐、腹痛、消化不良等。
•眼部不良反应:如视觉模糊、眼部灼热感、泪水增多等。
•过敏反应:如皮疹、荨麻疹、过敏性休克等。
8. 结语
综上所述,溴芬酸钠是一种具有抗炎、镇痛和抗血管新生作用的药物。
它通过抑制前列腺素合成和其他机制发挥药理效果。
在临床上,溴芬酸钠被广泛用于眼科和风湿科领域的治疗,并有望在其他领域得到进一步应用。
在使用时需注意药物相互作用和可能出现的不良反应,以确保安全有效地使用溴芬酸钠。
