临床研究专业术语缩略语中英对照表
常用医学检验缩略语中英文词典jian

常用医学检验缩略语中英文词典Aabsolute temperature 凯尔文温度;绝对温度absorbance 吸光率;吸光度;光吸收值A1angiotensin1 血管紧张素1A2angiotensin2 血管紧张素2A3angiotensin3 血管紧张素3AAaggregated albumen 聚集白蛋白AAAatomic absorption analysis原子吸收分析ABCabsolute basophil count 绝对嗜硷细胞计数;aspiration biopsy cytology 抽吸活检细胞学,针吸活检细胞学Absabsorbance 光密度;吸光度Abscabscissa 横座标adenylate cyclase 腺苷酸环化酶Acadherent cell 贴壁细胞〔分〕;粘附细胞anticomplementary抗补体的AcAcacetoacetate 乙酰乙酸盐(或脂)AcCoAAcetyl coenzyme 乙酰辅酶AACCRamylase creatinine clearance ratio 淀粉酶肌酐清除率比AcCTactivating coagulation time 活化凝血时间ACEacetylcholinesterase 乙酰胆碱酯酶angiotensin converting enzyme 血管紧张素转化酶AcGaccelerator globulin (Factor V)促凝血球蛋白,加速素(第五因子)Achacetylcholine 乙酰胆碱ACHadrenal cortical hormone 肾上腺皮质激素AChEacetyl-cholinesterase 乙酰胆碱酯酶ACPacid phosphatase 酸性磷酸酶ACRabsolute catabolic 绝对分解代谢率ACTactivated coagulation time 活化凝血时间ACTGactivated clotting time grout 活化凝血时间组ACTHAdrenoeorticotropic hormone 促肾上腺皮质激素ADabsorbed dose 吸收剂量average deviation 平均偏差ADCCAntibody mediated complement independent cytotoxicity 补体介导抗体独立细胞毒性Antibody-dependent cell-mediated cytotoxicity 细胞中介细胞毒依存性抗体ADHalcohol dehydrogenase 乙醇脱氢酶antidiuretic hormone 抗利尿激素ADLactivities of daily living 日常生活活动Antibody dependent lymphocyte 抗体依赖性淋巴细胞ADPAdenosine diphosphate 二磷酸腺苷ADSantibody deficiency syndrome 抗体缺乏综合征ADTadenosine triposphate 三磷酸腺苷AESanti-eosinophil sera 抗嗜酸性细胞血清AETabsorption-equivalent thickness 吸收当量浓度AFCantibody-forming cells 抗体形成细胞AFCPantibody-forming cells precursor 抗体形成细胞前体AFPalpha fetoprotein 〔alpha-fetoprotein;alphafetoprotein〕甲胎蛋白Agantigen 抗原AGagranulocytosis 粒细胞缺乏症analytical grade 分析等级antiglobulin抗球蛋白A/Galbumin/globulin ratio 白蛋白球蛋白比率AGBantigen-antibody test 抗原抗体试验AGGagammaglobulinemia 无丙种球蛋白血症;血丙种球蛋白缺乏AGLacute granulocytic leukemia 急性粒细胞性白血病AGTantiglobulin test 库姆斯试验;血清抗球蛋白试验(检血中抗体) AGTTabnormal glucose tolerance test 异常葡萄糖耐量试验AHacute hepatitis 急性肝炎AHDantihyaluronidase 抗透明质酸酶auto-immune haemolytic disease 自身免疫溶血性疾病AHFAntihemophilic factor 抗血友病因子(凝血因子5)AHGAntihemophilic globulin 抗血友病球蛋白AHLSantihuman-lymphocyte serum 抗人淋巴细胞血清AHPacute hemorrhagic pancreatitis 急性出血性胰腺炎AHSantihuman serum 抗人血清AHTantihyaluronidase titration enzyme 抗透明质酸酶滴定酶AHTCGanti-human T cells globulin 抗人T细胞球蛋白AICFauto-immune complement fixation 自身免疫补体结合反应AIECadvanced ion exchange cellulose 高级离子交换纤维素AIHAautoimmune haemolytic anemia 自身免疫溶血性贫血AIHDacquired immune hemolytic disease 获得性免疫溶血性贫血autoimmune haemolytic 自身免疫溶血性疾病AKPAlkaline phosphatase 碱性磷酸酶alanine 丙氨酸antilymphocyte antibody 抗淋巴细胞抗体ALGantilymphocyte globulin 抗淋巴细胞球蛋白ALHanterior lobe hormone 垂体前叶素ALLacute lymphoblastic leukemia 急性成淋巴细胞性白血病acute lymphocytic leukemia 急性淋巴细胞性白血病acute lymphoid pneumonia 急性类淋巴细胞性肺炎AlpAlkaline phosphatase 碱性磷酸酶ALSantilymphocyte serum 抗人淋巴细胞血清aldolase 醛缩酶ALTalanine aminotransferase 丙氨酸氨基转移酶AMAantimitochondrial antibodies 抗线粒体抗体anti-myocardial antibody 抗心肌抗体AMLacute monocytic leukemia 急性单核细胞性白血病acute myeloblastic leukemia 急性原始粒细胞性白血病acute myelocytic leukemia 急性髓细胞性白血病acute myelogenous leukemia 急性髓细胞性白血病AMMLacute monomyelocytic leukemia 急性髓单核细胞性白血病acute myelomonocytic leukemia 急性粒-单核细胞性白血病AMoLacute monocytic leukemia 急性单核细胞性白血病AMP-5adenosine-5-phosphoric acid 5-磷酸腺苷AMRaverage minimum requirement 平均最小需要量AMSamylase 淀粉酶atomic mass unit 原子质量单位AMVavian myeloblastosis virus 鸟髓母细胞过多症病毒Amyamylase 淀粉酶ANAEa-naphthyl-acetate esterase 乙酸-1-奈酯酯酶agglutination negative absorption positive 凝集阴性吸收阳性ANCabsolute neutrophil count 绝对中性白细胞计数ANLLacute non-lymphocytic leukemia 急性非淋巴细胞白血病ANLacute nonlymphoblastic leukemia 急性非淋巴母细胞性白血病acute nonlymphocytic leukemia 急性非淋巴细胞性白血病ACHRAcetylcholine receptor 乙酰胆碱受体anti-DNase Banti-deoxyribonuclease 抗DNA酶B; 抗脱氧核糖核酸酶抗Anti-HBcantibody to hepatitis core antigenB 抗乙型肝炎核心抗原抗体Anti-HBeantibody to hepatitis-e antigenB 抗乙型肝炎e抗原抗体Anti-HBsantibody to hepatitis surface antigenB 抗乙型肝炎表面抗原抗体Anti-HGHantihuman growth hormone 抗人生长激素ANTRapparent net transfer 明显纯转化率APAlkaline Phosphatase 碱性磷酸酶acid Phosphatase 酸性磷酸酶APGLalkaline phosphatase activity of the granular leukocytes 白细胞碱性磷酸酶活性APHanterior pituitary hormone 垂体前叶激素APHPanti-pseudomonas human plasma 抗假单胞菌属人血浆APLacute promyelocytic leukemia 急性早幼粒细胞白血病APPacute phase protein 急性期蛋白apparatus 器械;装置APPFantiplatelet plasma factor 血小板血浆因子APPsacute phase protein 急性期蛋白APRanterior pituitary reaction 垂体前叶反应APTTActivated pactiai thrombopilastin time 活化部分凝血激酶时间AQLacceptance quality level 质量合格标准ARachievement ratio 成功率analytic reagent 分析试剂ARCantigen reactive cell 抗原反应细胞ARFCactive rosette-forming T cell 活性玫瑰花结形成T细胞autorosette-forming cell 自身玫瑰花结形成细胞Argarginine 精氨酸ARSantirabies serum 抗狂犬病血清ASantiserum 抗血清antiserum antistreptolysin 抗链球菌溶血素抗血清ASA5-Aminosalicvlic acid 5-氨基水扬酸ASKantistreptokinase 抗链激酶ASLO (ASO & ASTO)antistreptolysin-o 抗链球菌溶血素oASOantistreptolysin-o 抗链球菌溶血素oASOTantistreptolysin titer 抗链球菌溶血素o效价ASTaspartate aminotransferase 天冬氨酸氨基转移酶antibiotic susceptibility test 抗生素敏感试验ATGantitetanus globulin 抗破伤风球蛋白antithymocyte globulin 抗胸腺细胞球蛋白AtLadult T-cell leukemia/lymphoma 成人T细胞性白血病/淋巴瘤ATPAdenosine triphosphate 三磷酸腺苷ATPaseAdenosine triphosphatase ATP酶;三磷酸腺苷酶ATSanti hemocyte serum 抗血细胞血清ATTaspirin tolerance time 阿司匹林耐受时间at wt atomic weight 原子量AuAustralia antigen 澳大利亚抗原;乙型肝炎抗原AUantitoxin unit 抗毒素单位AU-HAAAustralia-hepatitis associated antigen 澳大利亚肝炎相关抗原AuLacute undifferentiated leukemia 急性未分化性白血病AVCautomatic volume control 自动容量调节AVHacute viral hepatitis 急性病毒性肝炎AVIair velocity index 换气速度系数;气速指数AWatomic weight 原子量TopBBACblood alcohol concentration 血液酒精浓度bacterial antigen complex 细菌抗原复合物BA-EDTAbile salt EDTA solution 胆盐乙二胺四乙酸溶液BALBritish anti-lewisite 二巯基丙醇BAPblood agar plate 血琼脂平板BARB-adrenergic receptor beta-肾上腺素能受体Basbasophile 嗜碱细胞BATbotulism antitoxin 肉毒中毒抗毒素BBbank 血库blood buffer[base] 血液缓冲[碱]buffer base 缓冲碱body burden 身体负荷BBBblood buffer base 血液缓冲碱BBTbasal body temperature 基础体温(测定排卵期)blood bank technologist 血库技师BCblood center 血液中心blood count 血细胞计数blood culture 血培养bactericidal concentration 杀菌浓度B-cellbone marrow derived cell 骨髓衍化细胞BCGbacillus Calmette-Guerin 卡介苗,结核菌苗ballistocardiogram 心脏射血容量描记图BCMblood clotting mechanism 血液凝固机理BCPbromcresol purple 溴甲酚紫biomedical computer program 生物医学计算机程序BCP-Dbromcresol purple deoxycholate culture medium 溴甲酚紫脱氧胆酸盐培养基BDTbasophil degranulation test 嗜碱细胞脱粒试验BEblood flow 血流量bouillon filtrate 肉汤过滤液blastogenic factor 母细胞化因子base excess 碱过剩BESbalanced electrolyte solution 平衡电解质溶液B/F(bound/free) ratio 结合游离比率BFMblood flowmeter 血流量计BFPbiologi folse-positive[reaction] 生物学假阳性[反应]BFRbiologi folse-positive reaction 生物假阳性反应BGblood glucose 血糖blood group 血型BGSAblood granulocyte specific activity 血液粒细胞比活性BHPblood hydrostatic pressure 血液流体静力压BHSbeta-hemolytic streptococcus beta-溶血链球菌BIbacteriological index 细菌学指数BLbioluminescence 生物发光BlCblood culture 血液培养BlT (&BLT)blood type 血型BMBachelor of Medicine 医学士bone marrow 骨髓body mass 体质basal metabolism 基础代谢BMGbenign monoclonal gammopathy 良性单克隆丙种球蛋白病BMHRbasal metabolism heart rate 基础代谢心率BMNbone marrow necrosis 骨髓坏死BMRbasal metabolic rate 基础代谢率BODbiochemical oxygen demand 生化需氧量biological oxygen demand 生物需氧量BOMbiological oxygen monitor 生物控制氧量BPbiological parent 生物起源blood pressure 血压boiling point 沸点BPBbromophenol blue 溴酚蓝BRDbilirubin direct 直接胆红素BRPbacterial receptor protein 菌受体蛋白BRTbilirubin total 总胆红素BSblood sugar 血糖BSAbody surface area 体表面积bovine serum albumin 牛血清白蛋白BSPbromsulphalein (test) 溴磺酞钠试验(检肝功能);BSRblood sedimentation rate 红细胞沉降率,血沉BSSbalanced salt solution 平衡盐溶液buffered saline solution 缓冲盐水溶液BTbleeding time 出血时间BTBbromothymol blue 溴麝酚蓝,溴百里酚蓝BUAblood uric acid 血尿酸BUNblood urea nitrogen 血尿素氮BVblood vessel 血管BWbody water 体液body weight 体重blood wassermann 血液梅毒补体结合反应,血液华氏反应TopCC1complement 补体C3complement3 补体3CAcarbonic anhydrase 碳酸酐酶;碳酸脱水酶cellulose acetate 醋酸纤维素;乙酸纤维素centrifugal analyzer 离心分析仪cold agglutinin 冷凝集素common antigen 共同抗原CAHchronic active hepatitis 慢性活动性肝炎CAHDcoronary atheroselerotic heart disease 冠状动脉粥样硬化性心脏病CAMPCyclic adenosine monophosphate 环腺苷一磷酸CAPcatabolite gene-activator 蛋白分解代谢基因激活剂cell agar plate 细胞琼脂平板cystine aminopeptidase 胱氨酸氨肽酶Carbcarbohydrate 糖类;碳水化合物CAScoronary atherosclerotic score 冠状动脉粥样硬化斑cold-agglutination syndrome 冷凝集综合征CATcold agglutination test 冷凝集试验computer-aided tomography 计算机辅助X线断层摄影术CBFcerebral blood flow 脑血流量clinical blood flow meter 临床血流量计CBGCorticotropin binding globulin 促肾上腺皮质激素结合球蛋白CBPAcompetitive protein binding assay 竞争蛋白结合试验CBVcirculating blood volume 循环血容量coxsackie B virus 柯萨奇B病毒CCcardiac catheterization 心导管术cubic centimeter 立方厘米creatinine clearance 肌酸酐清除率CCAchick cell agglutination 鸡细胞凝集(亚洲流感疫苗)combinding cholalic-acid 结合胆酸chronic cold agglutinin 慢性冷凝集素CCADchronic cold agglutinin disease 慢性冷凝集素病CCATconglutination complemen tabsorption test 胶固补体吸附试验CCBVcentral circulating blood volume 中心循环血量度CCDcarbonate compensation depth 碳酸盐代偿深度CCFTcephalin-cholesterol flocculation 脑磷酯胆固醇絮凝CCIchronic coronary insufficiency 慢性冠状动脉供血不足creatinine clearance index 肌酐清除指数CCKcholecystokinin 胆囊收缩素CCSconstant current source 恒定电源CDACongenital dyserythropoietic anemia 先天性红细胞生成异常性贫血CDCAchenodeoxycholic acid 鹅去氧胆酸CDMcystine-deficient medium 缺乏胱氨酸培养基CDNAComplementary deoxyribonucleic acid 互补脱氧核糖核酸CDRcomplementary determining region互补决定区CEconstant error 常数误差cytopathic effect 细胞病性效应CEACarcinoembryonic antigen 癌胚抗原CEFcentrifugation extractable fluid 可提取的离心液CEHcholesterol ester hydrolase 胆固醇酯水解酶CEIDcrossed electroimmunodiffusion 交叉电泳免疫扩散CEPcongenital erythropoietic porphyria 先天性红细胞生成性卟啉症Ceph Floccephalin flocculation test 脑磷酯絮凝试验CFcentrifugal force 离引力Christmas factor 凝血因子IX抗血友病因子Bcomplement fixation 补体结合CFAcomplement-fixing antibody 补体结合抗体CFHchorionic gonadotrophic hormone 绒毛膜促性腺激素CFIchemotaxis factor inhibitor 趋化性因子抑制物CFTcapillary fragility test 毛细血管脆性试验CFUcolony forming unit 菌落形成单元CFU-Ccolony forming unit culture 菌落形成单元培养物CGchorionic gonadotropin 绒毛膜促性激素CGDconstitutional growth delay 体格生长延迟CGlchronic granulocytic leukemia 慢性粒细胞白血病cGMPcyclic guanosine monophosphate 环鸟苷酸CGPcholine glycerophosphatide 甘油磷脂胆碱CgRTCongo red test 刚果红试验CGTchorionic gonadotropin 绒[毛]膜促性腺激素CGTTcortisone glucose tolerance test 皮质素葡萄糖耐量试验CHcholine acetylase 胆碱乙酰酶congenital hypothyroidism 先天性甲状腺功能减退症CH50fifty percent hemolytic unit of complement 50%补体溶血单位ChAcholine acetylase 胆碱乙酰酶Che CHEcholinesterase 乙酰胆碱酯酶;胆碱酯酶ChFEchemotactic factor for eosinophils 嗜酸性细胞趋化因子ChFLchemotactic factor for lymphocytes 淋巴细胞趋化因子ChFNchemotactic factor for neutrophils 中性粒细胞趋化因子Chjcholestatic jaundice 胆汁郁积性黄疸Chlcholesterol 胆固醇CHOcholesterol 胆固醇CarbohydrateChrchromatography 色谱法Clchemical ionization 化学电离CIECountercurrent immunoelectrophoresis 对流免疫电泳CIEPCounter immunoelectrophoresis 对流免疫电泳CIFcolony inhibitory factor 菌落抑制因子competent inducing factor 活性诱发因子CIgcold-insoluble globulin 冷不溶性球蛋白CIHcarbohydrate-induced hyperglyceridemia 糖诱发高甘油脂血症Certificate of Industrial Health 工业卫生证书CIRAchromatographic in-frared analyzer 色谱红外线分析器CKcreatine kinase 肌酸激酶CLchemiluminescence 化学荧光,化学发光cytotoxicity lymphocyte 细胞毒性淋巴细胞CLASclinical laboratory automation system 临床实验室自动化系统CLLchronic lymphatic leukemia 慢性淋巴性白血病chronic lymphocytic leukemia 慢性淋巴细胞性白血病CLSLchronic lymphosarcomatous leukemia 慢性淋巴肉瘤性白血病CLTclinical laboratory technology 临床实验室技术学clotting time 凝固时间CMCcarboxymethyl cellulose 羧甲基纤维素chronic mucocutaneous candidiasis 慢性皮肤粘膜念珠菌病critical micellar concentration 临界胶态分子团浓度CMIcarbohydrate metabolism index 碳水化合物代谢指数cellular mediated immunity 细胞介导免疫CMIRcell mediated immune response 细胞介导免疫反应CMLchronic myelocytic leukemia 慢性粒细胞白血病CMPCytidine monophosphate 胞嘧啶核苷一磷酸CMRcarbon-13 magnetic resonance spectroscopy 碳-13磁性共振分光镜CMTcardiolipin microflocculation test 心肌磷脂微量絮凝试验(梅毒) CMULPcarboxylate-modified uniform latex particle 羧化物均匀乳胶微粒CMVCytomegalovirus 巨细胞病毒CNSHAcongenital nonspherocytic hemolytic anemia 先天性非球形红细胞溶血性贫血Co-2coenzyme-Ⅱ(triphosphopyridine nucleotide) 辅酶ⅡCo-Acoenzyme-A 辅酶ACoASHcoenzyme A 辅酶ACO2CPcarbon dioxide combining power 二氧化碳结合CODchemical oxygen demand 化学需氧量COGTTcortisone glucose tolerance test 皮质素葡萄糖耐量试验COHbCarboxyhemoglobin 碳氧血红蛋白COMTcatechol-o-methyltransferase 儿茶酚-邻-甲基转移酶COPcollold(al) osmotic pressure 胶体渗透压CPClinical Pathologv 临床病理学chemically Pure 化学纯度CpAcirculating platelet aggregates 循环血小板聚集物CpAHpara-aminohippurate clearance 对氨基马尿酸清除率CPK (CK)Creatine phosphokinase 肌酸磷酸激酶CPK-1brain CPK isoenzyme (CPK-BB) 脑CPK同功酶(CK-BB)CPK-2hybrid muscle CPK isoenzyme (CPK-MB) 混合肌CPK同功酶(CK-MB)CPK-3muscle CPK isoenzyme (CPK-MM) 肌CPK同功酶(CK-MM)cpscount(or cycles) per second 每秒数目或转速CPSCarbomyl-phosphate synthethase 羧基磷酸盐合成酶CRcreatinine 肌酸肝,肌酐CRBCchicken red blood cell 鸡红细胞CRFcase report form 病历报告形式chronic renal failure 慢性肾衰竭Corticotropin releasing factor 促皮质素释放因子coagulase reacting factor 凝固酶反应因子CRNantibody complement requiring neutralizing antibody 需要补体的中和抗体CRPC reactive protein C反应蛋白CRPAC reactive protein antiserum C反应蛋白抗血清Cr-SRBCcr-labelled sheep red blood cell 铬标记绵羊红细胞CRTclot retraction time 血块凝缩时间complex reaction time 复合反应时间CScolorimetric solution 比色液concentrated strength 浓缩度control serum 对照血清CSAcolony stimulating activity 菌落刺激活性cell surface antigen 细胞表面抗原CSRcorrected sedimentation rate 校正[红细胞]沉降率cortisol secretion rate 皮质醇分泌率CTcoagulation time 凝血时间calcitionin 降钙素cholesterol total 胆固醇总数Chlamydia trachomatis {拉}沙眼衣原体computerized tomography 计算机体层摄影术connective tissue 结缔组织contraction time 收缩时间CTPCyclophosphamide 胞嘧啶核苷三磷酸CTTcarbohydrate tolerance test 糖耐量试验CTUcelsius thermal unit 摄氏热量单位CUclinical unit 临床单位color unit 色单位CVcorpuscular volume 红细胞容积conduction velocity 传导速度Coxsackie virus 柯萨奇病毒coefficient of variation 变异系数TopDDAAOd-amino acid oxidase d-氨基酸氧化酶(右旋)dADPdeoxyadenosine diphosphate 脱氧腺苷二磷酸dAMPdeoxyadenosine monophosphate 脱氧腺苷二一磷酸DAOdiamine oxidase 二胺氧化酶DAPdirect agglutination pregnancy test 妊娠直接凝集试验DARRdirect antiglobulin rosetting reaction 直接抗球蛋白玫瑰花结反应DARTdata acquisition right time 数据获取正确时间dATPdeoxyadenosine triphosphate 脱氧腺苷三磷酸DBdirect bilirubin 直接胆红素dye baseline 染料基线dry bulb temperature 干球温度DBCLdilute blood clot lysis (method) 稀释[性]血块溶解[法]DBHdopamine beta-hydroxylase 多巴胺β-羟化酶DBSGuide to biomedical Standards 生物医学标准指南despeciated bovine serum 去种特异性牛血清DCdifferential count [白细胞]分类计数direct Coombs'(test) 直接库姆斯[试验],直接抗球蛋白试验DCAdeoxycholate-citrate agar 去氧胆酸盐-柠檬酸盐琼脂DCRdielectrophoresis collection rate 电解质电泳收集率direct cortical response 直接皮质反应DCSdisease control serum 疾病检验血清,疾病对照血清DCTPAdesoxycorticosterone triphenylacetate 三苯醋酸去氧皮质酮dependent drainage 体位引流法,重力引流法differential diagnosis 鉴别诊断DEdisc electrophoresis 盘状电泳DEAEdiethylaminoethanol 二乙氨基乙醇DESDiethylstilbestrol 己烯雌酚DFdengue fever 登革热degree of freedom 自由度dilution factor 稀释因素DFSdifferential fluorescent staining 鉴别荧光染色DFTdialysable free thyroxine 可透析的游离甲状腺素DGdeoxyguanosine 脱氧鸟苷酸DHADihydroxyacetone 二羟基丙酮DHEADehydroepiandosterone 脱氢表雄酮DHLYd-hydroxylysine d-羟赖氨酸DHTDihydrotachysterol 双氢速甾醇DIdecontamination index 去污指数〔核〕dry ice 干冰;固态二氧化碳dye index 染料指数DICdisseminated inravascular coagulation 弥散性血管内凝血,播散性血管内凝血Diph-Tetdiphtheria-tetanus 白喉-破伤风Diph-Toxdiphtheria-toxoid 白喉类毒素DKPdibasic potassium phosphate 磷酸氢二钾DKSdeoxyketo steroid 去氧类固醇DLDonath-langsteiner test 多-兰二氏试验(测阵发性寒冷性血红蛋白尿)limit of detection 测定范围diffusing capacity of the lungs for carbon monoxide 肺一氧化碳弥散量DLEdiscoid lupus erythematosus 盘状红斑狼疮disseminated lupus erythematosus 播散性红斑狼疮diabetes mellitus 糖尿病DNratio of urinary dextrose to nitrogen 尿中葡萄糖与氮的比率DNADeoxyribonucleic acid 脱氧核糖核酸DNA/GDeoxyribonucleic acid content per genome 脱氧核糖核酸含量/染色体组DNAPDeoxyribonucleic acid polymerase 脱氧核糖核酸聚合酶DNasedeoxyribonuclease 脱氧核糖核酸酶;DNA酶DNPdeoxy-ribonucleoprotein 脱氧核糖核蛋白dinitrophenol 二硝基苯酚DOdiamine oxidase 二胺氧化酶dissolved oxygen 溶解氧DOCdeoxycorticosterone 脱氧皮质[甾]酮DOCADesoxycorticosterone acetate 醋酸去氧皮质酮(盐皮质激素制剂)DOGdeoxyglucose 去氧葡糖〔抗病毒药〕DOPA3,4-dihydroxyphenylalanine 多巴;3,4-二羟苯丙氨酸DPCdendritic phagocytic cell 树突状吞噬细胞DPN (NAD)dermatosis papulosa nigra 黑色丘疹性二磷酸吡啶核苷酸;辅酶ⅠDPNHdinitro-phenyl-hydrazine二硝基苯肼DSDown's syndrome 唐氏综合征;先天愚型donor's serum 供体血清degree of substitution 置换程度dilute strength 稀释程度D/Sdextrose-saline solution 葡萄糖盐溶液DSAdisease-数字减影血管造影术susceptible antigen 疾病敏感性抗原discrete sample analyzer 不连续样品分析器,离散标本分析器Doppler spectrum analyzer 多普勒频谱分析器digital spectrum analyzer 数字光谱分析器DSFdifferential spectrofluorometer 鉴别诊断荧光分光光度计DSPdibasic sodium phosphate 磷酸氢二钠DTdye test 染料试验differently tested 鉴别试验;鉴别检验合格的DTNdiphtheria toxin normal 标准白喉毒素DTPdiphtheria/tetanus/poliomyelitis 白喉/破伤风/脊髓灰质炎DUdensity(optical) unknown 未知光密度TopEE1estrone 雌酮E2estradiol 雌二醇E3estriol 雌三醇EAelectric affinity 电亲合力early antigen 早期抗原erythrocyte antibody 红细胞抗体EACethyl acetate 乙酸乙酯ethyl acrylate 丙烯酸乙酯electroanalytical chemistry 电分析化学EAC-Rosetteerythrocyte antibody complement rosette 红细胞抗体补体玫瑰花结EACXerythrocyte-antibody-complement X 红细胞-抗体-补体X (X表示补体某一成分或某些成分) EAHLGequine antihuman lymph oblast globulin 马抗人淋巴母细胞球蛋白EAHLSequine antihuman lymph oblast serum 马抗人淋巴母细胞血清EAPerythrocyte acid phosphatase 红细胞酸性磷酸酶EALelemental analysis system 成分分析系统EBclementary body 1,血小板2,原生小体Epstein-Barr virus EB 病毒,非洲淋巴细胞瘤病毒EBFerythroblastosis fetalis 胎儿成红细胞增多病EBPestradiol binding protein 雌二醇结合蛋白exclusion chromatography 分离色谱法E/Cestrogen/creatinine)ratio 雌激素与肌酸酐比率ECAenterobacterial common antigen 肠杆菌共同抗原ECBVeffective circulating blood volume 有效循环血容量ECFeffective capillary flow 有效毛细血管流量endogenous eytotoxic factor 内源性细胞毒(性)因子extracellular fluid 细胞外液ECFAeosinphilic chemotactic factor of anaphylaxis 嗜峻性细胞趋化因子Echoenteric cytopathogenic human orohan virus 人肠道细胞病变孤儿病毒,埃可病毒ECLTeuglobulin clot lysis time 优球蛋白血块溶解时间ECPerythoid committed precursor cell 红系定向前驱细胞extracellular common protein 细胞外普通蛋白ECVextraxellular volume 细胞外容量ECWextracellular water细胞外水,细胞外液Edestradiol 雌二醇(卵泡刺激素)EDTAEthylene diamine tetraacetate 依地酸,乙二胺四乙酸盐European Dialysis and Transplant Association 欧洲透析与移植协会EEOelectroendosmosis 电内渗现象EFelectrofocusing 电聚焦elongation factor 延伸因子encephalitogenic factor 致脑炎因子erythrocytic fragmentation 红细胞破裂excreted factor 分泌因子EFReffective filtration rate 有效滤过率EFVextracellular fluid volume 细胞外液量EGGequine gamma globulin 马丙种球蛋白EGOTerythrocyte glutamic oxaloacetic transaminase 红细胞谷(氨酸)草(酰乙酸)转氨酶EGRerythrocyte glutathione reductase 红细胞谷胱甘肽还原酶eHoxidation-reduction potential 氧化还原电位EHAAepidemic hepatitis associated antigen 流行性肝炎相关抗原EHBFexercise hyperemia blood flow 运动性充血血流量estimated hepatic blood flow 估量肝血流量extrahepatic blood flow 肝外血流量EHFEbola hemorrhagic fever 埃波拉出血热epidemic hemorrhagic fever 流行性出血热EHLeffective half-life 有效半衰期endogenous hyperlipidemia 内源性高脂血症EIAenzyme imunoassay 酶免疫测定EIDegg infective dose 卵感染剂量electroimmunodiffusion 电泳免疫扩散EIFerythropoietic inhibiting factor 红细胞生成的抑制因子PERNVextraincidence rate in non-vaccinated group 未接种人群额外发病率EIRVextraincidence rate in vaccinated group 接种疫苗组额外发病率EKerythrokinase 红细胞激酶ELBearly labelled bilirubin 早期标记胆红素ELFextremely low frequency 极低频率enzyme-linked fluorescent assay 酶联荧光测定ELISAenzyme-linked immunosorbant assay 酶结合免疫吸附测定ELMIAenzyme-linked monoclonal antibody inhibition assay 酶联单克隆抗体抑制测定ELTeuglobulin lysis time 优球蛋白溶解时间EMelectron microscopy 电子显微镜术erythrocyte mass 红细胞总量E of Merror of measurement 实验误差EMAEthyimalonic-abipic aciduria 乙基丙二酸-脂肪酸尿症early membrane antigen 早期膜抗原EMBeosin-methylene blue culture medium 曙红甲蓝琼脂培养基EMPEPerythrocyte membrane protein electrophoretic pattern 红细胞漠蛋白电泳型ENAextractable nuclear antigen 可抽出性核抗原epidural narcotic analgesia 硬膜外麻醉性镇痛EPendotoxin protein 内毒素蛋白epithelial cell 上皮细胞endogenous pyrogen 内源性致热原electric potential 电位electrophoresis 电泳EPPestrogen / progestogen preparation 雌激素/孕激素制剂erythropoietic protoporphyria 红细胞生成性原卟啉症ERerythrocyte receptor 红细胞受体estrogen receptor 雌激素受体equivalent roentgen unit 当量伦琴单位ERBFeffective renal blood flow 有效肾血流量erythrocyte responsive cell 红细细胞生成性效应细胞ESelectron spectrometer 电子分光计enzyme substrate 酶解物ESEelectrostatic unit 静电单位erythropoietic stimulating factor 红细胞生成的刺激因子ESIenzyme substrate inhibitor complex 酶底物抑制复合物ESRelectron spin resonance 电子自旋共振erythrocyte sedimentation rate 红细胞沉降率,血沉ETeffective temperature 有效温度exchange transfusion 交换输血;换血ETKMevery test known to man 人所知的每一种试验ETReffective thyroxine ratio 有效甲状腺素ETTextrathyroid thyroxine 甲状腺外甲状腺素EUenzyme unit 酶单位EUVextreme ultraviolet spectroscopy 紫外线分光光度计EYAegg yolk agar medium 蛋黄琼脂培养基TopFFAflameless atomic absorption 无焰原予吸收fatty acid 脂肪酸fluorescent antibody 荧光抗体FAAfatty acid acceptor 脂肪酸受体FACfluorescent affinity chromatography 荧光亲和色谱法FAMAfluorescent antibody to membrane antigen 抗膜抗原荧光抗体Fellow of the American Medical Association 美国医学会会员FANAfluorescent antinuclear antibody 荧光抗核抗体FATfluorescent antibody test 荧光抗体试验FBEfull blood examination 全血检查FBGfasting blood glucose 空腹血糖fibrinogen breakdown product 纤维蛋白原降解产物folate-binding protein 叶酸结合蛋白FBSfasting blood sugar 空腹血糖fetal blood sampling 胎儿血样fetal bovine serum 牛胎血清FCfunctional classfication 功能分类FCRflow cytosorting 流式细胞分类术FDfibrinogen derivative 纤维蛋白原衍生物fluorescent densitometer 荧光密度计fatal dose 致死量FDPFibrin degradation prodncts 纤维蛋白降解产物freeze-dried plasma 冷冻干燥血浆FEfatty ester 脂肪酸酯FECfree erythrocyte coproporphyrin 游离红细胞粪卟啉FECFVfunctional extracellular fluid volume 功能性细胞外液容量FECPfree erythrocyte coproporphyrin 游离红细胞粪卟啉Fe Diron deficiency anemia 缺铁性贫血FEPfree erythrocyte protoporphyrin 游离红细胞原卟啉FFfat-free diet 无脂膳食father factor 父传因子fertility factor,F factor 生育因子fixing fluid 固定液FFIfree from infection 无感染的FFMfat-free mass 脱脂物质FGfibrinogen 纤维蛋白原FHfamilial hypercholesterolemia 家族性高胆固醇血症fulminant hepatitis 暴发性肝炎flame ionization detector 火焰离子化检测器FITCfluorescein isothiocyanate 异硫氰酸荧光素FIVformalin-inactivated vaccine 福尔马林灭活疫苗FLfluorescein 荧光素flammable liquid 可燃液体FMfibrin monomer 纤维蛋白单聚体flow meter 流量计FMHfat-mobilizing hormone 动员脂肪激素FNfunction 机能false negative 假阴性FPCfish protein concentrate 浓缩鱼蛋白FPGfasting plasma glucose 空腹血糖FPMfilter paper microscopic test 滤纸显微镜试验〔检查梅毒的絮凝试验FRflocculation reacion 絮凝反应,絮状反应filtration rate 滤过率fixed ratio 固定比率FRCFunctional residual capacity 功能残气量FreeHBfree hemoglobin 游离血红蛋白FRFFollicle stimulating hormone releasing factor 促卵泡激素释放因子fried friedman test for pregnancy 弗里德曼妊娠试验frig refrigerator 冰箱,冷藏箱FSAfetal sulfoglucoprotein antigen 胎儿硫糖蛋白抗原fluorosilicic acid 氟硅酸fsar fiat secunndum {拉} 依常规操作FSFfibrin stabilizing factor 纤维蛋白稳定因子,八因子FSHfollicle-stimulating hormone 促卵泡激素FSHRFfollicle-stimulating hormone releasing factor 促卵泡激素释放因子FSHRHfollicle- stimulating hormone releasing hormone 促卵泡激素释放激素FSPfibrinogen-split products 纤维蛋白原裂解产物fibrinolytic-split products 溶纤维蛋白裂解产物FERfeedback shift register 反馈转变定位FTferritin 铁蛋白fluid toxoid 液体类毒素fragility test (红细胞)脆性试验free thyroxine 游离甲状腺素filtration time 滤过时间FTAFluorescent treponemal antibody 荧光梅毒密螺旋体抗体FTA-AbsFluorescent treponemal antibody absorption test syphilis 荧光梅毒密螺旋体抗体吸收试验FTIFree thyroxine index 游离甲状腺素指数Fufecal urobilinogen 粪尿胆素原FVCforced vital capacity 用力肺活量(最大肺活量)FVEforced expiratory volume 用力呼气量FWFelix-Weil reaction 裴-外二氏反应〔检斑疹伤寒的血清凝集反应〕Folin-Wu method 福-吴二氏法(检肌酐、葡萄酸糖、非蛋白氮)FWRFelix-Weil reaction 斐-外二氏反应FYFYDuffy blood group negative human erythrocyte 达菲氏血型阴性人红细胞FYIFor Your Information 供参考TopGGAgastric analysis 胃液分析granulocyte agglutinin 粒细胞凝集素glucuronic acid 葡萄糖醛酸GABAgamma-aminobutyric acid γ-氨基丁酸GABHSAminobutyric acid 氨酪酸GADglutamic acid decarboxylase 谷氨酸脱羟酶GAPglyceraldehyde Phosphate 磷酸甘油醛GAPDglyceraldehyde phosphate dehydrogenase 磷酸甘油醛脱氢酶GARGGgoat antibody to rabbit gamma globulin 山羊抗兔丙种球蛋白抗体GATgelatin agglatination test 明胶凝集试验GCgroup specific component of blood 血型特异性成分ganglion cells 神经节细胞gas chromatograph 气相色谱法goblet cell 杯状细胞granular casts 颗粒管型glass capillary 玻璃毛细管gonococcus 淋病奈瑟氏菌GCFgenetics citation factor 遗传学传递因子GCFTgonorrhea complement fixation test 淋病补体结合试验GChgranulocytic chalone 粒细胞抑素GCMgeneral cytochemical methods 普通细跑化学方法GCMSgas chromatography mass spectroscopy 气相色谱物质分光镜检查GDgel diffusion 凝胶扩散Gaucher's disease 高歇氏病;家族性脾性贫血GDHglutamic acid dehydrogenase 谷氨酸脱氢酶glycerophosphate dehydroge nase 磷酸甘油脱氢酶growth and development hormone 生长发育激素GEgelelectrophoresis 凝胶电泳G/Egranulocyte / erythroid ratio 粒细胞红细胞比率GEFgel electrofocusing 凝胶电聚焦,凝胶等电点聚焦gonadotropin enhancing factor 促性腺激素增强因子。
临床英语术语缩写表格

.临床研究常用术语缩写表编号术语缩写英文全称 / 中文全称ADR Adverse drug reaction/ 不良反响AE Adverse Event/ 不良事件ASV Accompanied Site Visit/ 陪伴访视BD 业务拓展 Business DevelopmentBS 生物统计 BiostatisticsCCF Central Clinical File 申办方临床研究文件夹CD Controlled Documents/ 控制文件CDA Confidentiality Disclosure Agreement/ 保密协议CDC Center for Disease Control/ 疾病控制中心CSDs Clinical Study Documents 临床研究文件CEC Central Ethics Committee/ 中心伦理委员会Co-I Coordinating Investigator负责协调不一样中心参加多中心临床试验研究者的研究者COF Change Order Form/ 工作范围更改申请表CIF Central Investigator ’s File 申办者—研究者文件夹(中心研究者文件夹)CM Clinical Monitoring / Operations/ 临床监查 / 营运CMA Clinical Monitoring Associate/ 临床研究监查助理CR Complete Response 康复Clinical Research Associate (equivalent to Clinical StudyCRA Monitor)临床监查员CRC Clinical Research Coordinator/ 临床研究协调员CRF Case Report Form or Case Record Form/ 病例报告表CRO Contract Research Organization/ 合同研究组织CSDs Clinical Study Documents/ 临床研究文件CSR Clinical Study Report/ 临床研究报告Clinical Trial Assistant (equivalent to Clinical ResearchCTA Assistant)临床研究助理CTA Clinical Trial Agreement/ 临床试验协议CTA Clinical Trial Application/ 临床试验申请CTS Clinical Trial Supplies/ 临床试验用品CTX Clinical Trial Exemption/ 临床试验免责CV Curriculum Vitae/ 简历DCF Data Clarification Form / 数据澄清表DCR Data Clarification Report (see DCF)/ 数据澄清报告DCRF Data Clarification and Resolution Form (see DCF)/ 数据澄清和解决表编号术语缩写英文全称 / 中文全称DM Data Management/ 数据管理DMP Data Management Plan/ 数据管理计划书DQF Data Query Form/ 数据疑问表DS Data Source/ 数据源EC Ethics Committee / 伦理委员会eCRF Electronic Case Report Form/ 电子病历报告表EDC Electronic Data Capture/ 电子数据收集EOS End of Study/ 研究结束EU European Union/ 欧盟FAS Full Analysis Set/ 全剖析集FDA Food and Drug Administration/ 美国食品药品管理局FM Approved Standard Form/ 赞同的标准表格GCP Good Clinical Practice/ 临床试验质量管理规范GLP Good Laboratory Practice / 实验室质量管理规范GMP Good Manufacturing Practice/ 药品生产质量管理规范GRP Good Research Practice/ 科学研发质量管理规范GSP Good Statistical Practice/ 统计质量管理规范HCO Head of Clinical Operations 临床营运总监IB Investigator's Brochure/ 研究者手册IC Informed Consent/ 知情赞同ICF Informed Consent Form (also see IC)/ 知情赞同书ICH International Conference on Harmonization/ 国际协调会议International Conference on Harmonisation Tripartite ICH-GCP Guideline on Good Clinical Practice国际协调会议药品临床试验质量管理规范指南IDB Investigational Drug Brochure / 试验药物手册IEC Independent Ethic Committee/ 独立伦理委员会IND Investigational New Drug (US FDA)/研究用新药IP Investigational Product/ 研究用产品IRAEs Immediately Reportable Adverse Events/ 立刻上报的不良事件IRB Institutional Review Board. / 机构审察委员会ITT Intention to treat/ 意愿性治疗ISA Investigator Study Agreement/ 研究者合同ISF Investigational Site File 研究者文件夹LM Line Manager/ 直线经理LOI Letter of Intent/ 意愿书MOH Ministry of Health/ 卫生部MSA Master Services Agreement/ 主服务协议MTD Maximum Tolerated Dose/ 最大耐受剂量MW Medical Writing/ 医学写作NA Not Available/ 不行用NCE New Chemical Entity/ 新化学实体编号术语缩写英文全称 / 中文全称NCS Not Clinically Significant/ 无临床意义ND Not Done/ 未做NDA New Drug Application./ 新药上市申请OD Other Documents/ 其余文件OP Operating Procedure/ 操作规程OOS Out Of Scope/ 超工作范围OS Overall Survival/ 整体生计期OTL Operational Team Lead/ 营运团队负责人PD Protocol Deviation/ 方案偏离PI Principle Investigator / 主要研究者PIN Personal Identification Number/ 个人确认密码PK Pharmacokinetics/ 药物代谢动力学PM Project Manager/ 项目经理PMF Project Managerment File/项目管理文件夹PMI Periodic Maintenance Inspection/ 按期保护检查PMS Post-Marketing Surveillance/ 上市后药物检测PP Project Plan/ 项目计划PP Per Protocol/ 切合方案集PR Patient Recruitment/ 患者招募QA Quality Assurance/ 质量管理QC Quality Control/ 质量控制RA Regulatory Authorities/ 监察管理部门RM Remote Monitoring/ 远程监查On-Site Monitoring/ 现场监查 =On-Target Monitoring/ 目标化监查SAE Serious Adverse Event / 严重不良事件SC Study Coordinator/ 研究协调员SCV Site Close-out Visit/ 中心封闭访视SSV Site Selection Visit/ 中心挑选访视SMV Site Monitoring Visit/ 中心监察访视SVR Site Visit Report/ 中心访视报告SD Source Data/ 源数据SDV Source Data Verification/ 原始数据核查SFDA State Food and Drug Administration/ 国家食品药品监察管理局SIC Subject Identification Code/ 受试者辨别代码SIF Site Information Form/ 中心信息表SIV Site Initiation Visit/ 中心启动访视SOP Standard Operating Procedure/ 标准操作规程SOW Scope of Work/ 工作范围Sub-I Subinvestigator 次要研究者SUSAR Suspected Unexpected Serious Adverse Reaction 可疑的非预期的严重不良反响TP Template/ 模版编号术语缩写 英文全称 / 中文全称 TMF Trial Master File/试验主文档UADR Unexpected Adverse Drug Reaction/ 非预期药物不良反响 UADE Unanticipated adverse drug effect/ 非预期的不良反响UAE Unexpected adverse event/ 非预期的不良事件WIWork Instruction/工作指南SOP 种类缩写表OP 操作规程 WI 工作指南 TP 模板 TemplateFM 赞同的标准表格 Approved Standard Forms OD 其余文件 Other Documents业务部门缩写表 BS 生物统计 BD 业务拓展CM 临床监查 DM 数据管理 Data ManagementIT 信息技术 Information TechnologyMS 医学科学服务 Medical Science Service PM 项目管理 Project Management QA 质量保证 Quality Assurance RM 记录管理 Records Management RA 注册事务 Regulatory Affairs SM SOP 管理 SOP ManagementST 研究中心管理服务 TR 培训 Training试验主文档: (TMF ) Trial Master FilePMF 项目管理文件夹 Project Management File CCF 申办方临床研究文件夹 Central Clinical File CIF 申办方 - 研究者文件夹 Central Investigator File ISF 研究者文件夹 Investigator Site FileBSF 生物统计学文件夹 Biostatistics Study FileDMSF 数据管理研究文件夹 Data Management Study FileSite Management Service / 营运 Clinical Monitoring/Operation Business Development Biostatistics/ Functional Area Abbreviation Table: Work Instructions Operating Procedures(1).临床研究常用术语缩写表和解决表内容总结(1).临床研究常用术语缩写表和解决表。
临床试验中英对照词汇表englishvocabularyofclinicaltrials
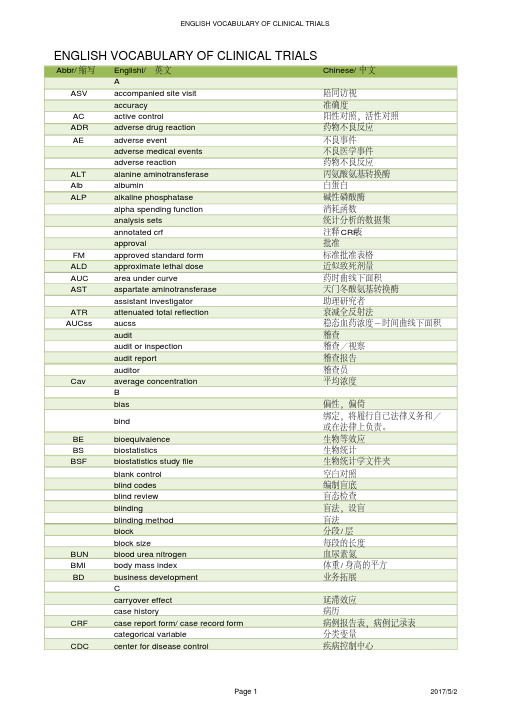
Abbr/缩写Englishi/英文Chinese/中文AASV accompanied site visit陪同访视accuracy准确度AC active control阳性对照,活性对照ADR adverse drug reaction药物不良反应AE adverse event不良事件adverse medical events不良医学事件adverse reaction药物不良反应ALT alanine aminotransferase丙氨酸氨基转换酶Alb albumin白蛋白ALP alkaline phosphatase碱性磷酸酶alpha spending function消耗函数analysis sets统计分析的数据集annotated crf注释CRF表approval批准FM approved standard form标准批准表格ALD approximate lethal dose近似致死剂量AUC area under curve药时曲线下面积AST aspartate aminotransferase天门冬酸氨基转换酶assistant investigator助理研究者ATR attenuated total reflection衰减全反射法AUCss aucss稳态血药浓度-时间曲线下面积audit稽查audit or inspection稽查/视察audit report稽查报告auditor稽查员Cav average concentration平均浓度Bbias偏性,偏倚bind 绑定,将履行自己法律义务和/或在法律上负责。
BE bioequivalence生物等效应BS biostatistics生物统计BSF biostatistics study file生物统计学文件夹blank control空白对照blind codes编制盲底blind review盲态检查blinding盲法,设盲blinding method盲法block分段/层block size每段的长度BUN blood urea nitrogen血尿素氮BMI body mass index体重/身高的平方BD business development业务拓展Ccarryover effect延滞效应case history病历CRF case report form/ case record form病例报告表,病例记录表categorical variable分类变量CDC center for disease control疾病控制中心ENGLISH VOCABULARY OF CLINICAL TRIALSCDE center for drug evaluation 国家食品药品监督管理局药品审评中心CCF central clinical file申办方临床研究文件夹CEC central ethics committee中心伦理委员会CIF central investigator's file中心研究者文件夹CDA certified data analyst数据分析师COF change order form工作变更申请表CMC chemistry manufacturing and controls临床前药学研究CMO chief media officer首席医学官CFDA china food and drug administration国家食品药品监督管理总局CD circular dichroism圆二色谱CL clearance清除率clinical auditor临床稽查员CDMS clinical data management system临床数据管理系统clinical director临床总监clinical equivalence临床等效应CMA clinical monitoring associate临床研究监查助理CM clinical monitoring/operations临床监查/运营CPA clinical project assistant临床项目助理CRA clinical research associate临床研究监察员,临床研究助理CRC clinical research coordinator临床协调员clinical study临床研究CSDs clinical study documents临床研究文件CSR clinical study report临床研究报告CTMS clinical trail management system临床试验管理系统clinical trial临床试验CTA clinical trial agreement临床试验协议CTA clinical trial application临床试验申请CTA clinical trial assistant临床研究助理CTX clinical trial exemption临床试验免责CTP clinical trial protocol临床试验方案CTS clinical trial supplies临床试验用品clinical trial/ study report临床试验报告Cmax cmax,maximum concentration峰浓度CI co-inveatigator合作研究者CTD common technical document国际药品注册申请技术文件格式comparison对照CR complete response完全缓解compliance依从性composite variable复合变量CATD computer-assisted trial design计算机辅助试验设计confidence interval可信区间confidence level置信水平CDA confidentiality disclosure agreement保密协议consistency test一致性检验CRO contract research organization合同研究组织contract/ agreement协议/合同control group对照组CD controlled documents控制文件coordinating committee协调委员会COI coordinating investigator协调研究者CAPA corrective action & preventive action纠正/预防措施CIOMS council for international organizations ofmedical sciences国际医学科学组织理事会cov close out visits关闭访视crea肌酐cross-over study交叉设计Css css, steady-state concentration稳浓度cure痊愈CV curriculum vitea履历DDCRF data clarification and resolution form数据澄清和解决表DCF data clarification form数据澄清表DCR data clarification report数据澄清报告DM data management数据管理DMP data management plan数据管理计划书DMSF data management study file数据管理研究文件夹DQF data query form数据疑问表database建立数据库database go live数据库批准启动DF degree of fluctuation波动度/系统descriptive statistical analysis描述性统计分析dichotomies二分类DSC differential scanning calorimetry示差扫描量热法DFS disease free survival无病生存期diviation偏差documentation记录/文件DLT dose limting toxicity剂量限制毒性dose-reaction relation剂量-反应关系double blinding双盲double dummy双模拟double dummy technique双盲双模拟技术double-blinding双盲dro out脱落drug-oriented药物源性Eeffectiveness疗效eCRF electronic case report form电子病历报告表EDC electronic data capture电子数据采集系统EDP electronic data processing电子数据处理系统emergency envelope应急信件EOS end of study研究结束end point终点endpoint criteria/ measurement终点指标equivalence等效性essential documentation必须文件EC ethics committee伦理委员会ESH-ESC european soceity of hypertension-europeansociety of cardilogy欧洲高血压-心脏协会EU european union欧盟EBM evidence-based medicine循证医学excellent显效exclusion criteria排除标准Ffactorial design析因设计failure无效,失败final point终点fixed-dose procedure固定剂量法FDA food and drug administration 食品药品管理局/美国食品药品管理局forced titration强制滴定FAS full analysis set全分析集GGC-FTIR gas chromatography-fourier transforminfrared spectroscopy气相色谱-傅利叶红外联用GC-MS gas chromatography-mass spectrometer气相色谱-质谱联用generic drug通用名药global assessment variable全局评价变量GLU glucose葡萄糖GAP good agricultural practice中药材种植管理规范GCP good clinical practice药物临床试验质量管理规范GLP good laboratory practice药品实验室管理规范GMP good manufacturing practice药品生产质量管理规范GLP good non-clinical laboratory practice药物非临床研究质量管理规范GSP good supply practice药品经营质量管理规范GUP good use practice药品使用质量管理规范group sequential design成组序贯设计HHCO head of clinical operations临床运营总监HEV health economic evaluation健康经济学评价HCT historial control trial历史对照研究HIM hospital intensive monitoring医院集中监测hypothesis test假设检验IIRAEs immediately reportable adverse events立即上报的不良反应improvement好转inclusion criteria入选标准IEC independent ethics committee独立伦理委员会information gathering信息收集IT information technology信息技术IC informed consent知情同意ICF informed consent form知情同意书IR infrared radiation红外吸收光谱initial meeting启动会议inspection视察/检查institution inspection机构检查IRB institutional review board机构伦理委员会ICH intennational conference on hamonization oftechnical requirements for registration ofpharmaceuticals for human use人用药品注册技术要求国际技术协调会/国际协调会议ICH-GCP intennational conference on hamonizationtripartite guideline on good clinicalpractice国际协调会议药品临床试验质量管理规范指南intention to treat意向性治疗ITT intention-to –treat意向性分析IVRS interactive voice response system互动式语音应答系统interim analysis期中分析IDB investigational drug brochure试验药物手册IND investigational new drug新药临床研究IP investigational product研究用产品investigator研究者ISF investigator site file研究者文件夹ISA investigator study agreement研究者合同IB investigator's brochure研究者手册IXRS ixsystems交互式互动服务提供商JKKPS karnofsky performance status行为状态评分LLOCF last observation carry forward最近一次观察的结转LD50lethal dose, 50%半数致死剂量LOI letter of intent意向书LOQ limit of quantitation定量限LM line manager直线经理LC-MS liquid chromatography -mass spectrometry液相色谱-质谱联用logic check逻辑检查lost of follow up失访Mmarketing approval/ authorization上市许可证masking设盲MRT mass rapid transit平均滞留时间MS mass spectrometry质谱MS-MS mass spectrometry-mass spectrometry质谱-质谱联用MSA master services agreement主服务协议matched pair匹配配对MTD maximal tolerable dose最大耐受剂量MedDRA meddra ICH国际医学用语词典medical director医学总监MW medical writing医学写作MOH ministry of health卫生部missing value缺失值mixed effect model混合效应模式monitor监查员monitoring监查monitoring report监查报告multi-center trial多中心试验NNCE new chemical entity新化学实体NDA new drug application新药上市申请NOAEL no observed adverse effect level未见不良反应剂量水平non-clinical study非临床研究non-inferiority非劣效性non-parametric statistics非参数统计方法NRCCT non-randomized concurrent controlled trial非随机同期对照试验 NA not available不可用NCS not clinically significant无临床意义ND not done未做NMR nuclear magnetic resonance核磁共振Oobedience依从性ODR odr旋光光谱offsite archive异地存档on-site monitoring现场监查on-target monitoring目标化监查open-blinding非盲open-label非盲OP operation procedure操作规程OTL opperational team lead运营团队负责人optional titration随意滴定original medical record原始医疗记录OD other documents其他文件OOS out of scope超出工作范围outcome结果outcome assessment结果指标评价outcome measurement结果指标outlier离群值OS overall survival总生存时间Pparallel group design平行组设计parameter estimation参数估计parametric statistics参数统计方法PR partial response部分缓解patient file病人档案patient history病历PR patient recruitment患者招募patient-oriented人源性PP per protocol符合方案集PMI periodic maintenance inspection定期维护检查PIN personal identification number个人确认密码PK phamacokinetics药物代谢动力学PD pharmacodynamics药效学;药代动力学PKAS pharmacokinetics analysis set药代动力学分析集placebo安慰剂placebo control安慰剂对照polytomies多分类PMS post-marketing study上市后临床试验PMS post-marketing surveillance上市后药物检测power检验效能PSV pre –study visit启动前访视precision精密度preclinical study临床前研究primary endpoint主要终点primary variable主要变量PI principal investigator主要研究者PL product license产品许可证PD progressive disease病情进展PMF project management file项目管理文件夹PM project maneger项目经理protocol试验方案protocol amendment方案补正PD protocol deviation方案偏离PFDA pure food and drug administration (美国)食品及药物管理局(正式名称为Food and Drug Administration,略作FDA)QQA quality assurance品质保证QAU quality assurance unit质量保证部门QC quality control质量控制query form应用疑问表query list应用疑问表Rrandomization随机化RCT randomized controlled trial随机对照试验range check范围检查rating scale量表reconciliation和解RM records management记录管理RA regulatory affairs注册事务RA regulatory authorities监督管理部门RSD relative standard deviation日内和日间相对标准差RM remote monitoring远程监查replication可重复RECIST response evaluation criteria in solid实体瘤疗效反应的评价标准RMV routine monitoring visits例行监查run in准备期SSAS safety analysis set安全性评价的数据集safety evaluation安全性评价sample size样本量scale of ordered categorical ratings有序分类指标SOW scope of work工作范围secondary variable次要变量sequence试验次序SAR serious adverse reaction严重不良反应seriousness严重性severity严重程度SAE severity adverse event严重不良事件significant level检验水准simple randomization简单随机single blinding单盲site audit试验机构稽查SCV site close-out visit中心关闭访视SIF site information form中心信息表SIV site intiation visits启动访视SMV site monitoring visit中心监查方式SSV site selection visit中心筛选访视SVR site visit report中心访视报告SD source data原始数据SD source document原始文件SDV soutce data verification原始数据核查specificity特异性sponsor申办者sponsor-investigator申办研究者SD stable disease病情稳定standard curve标准曲线SD standard deviation标准差SOP standard operating procedure标准操作规程SFDA state food and drug administration 中国国家食品药品监督管理局的旧称statistic统计量statistical analysis plan统计参数计划书statistical model统计模型statistical tables统计分析表stratified分层study aud研究稽查SC study coordinator研究协调员subgroup亚组Sub-I subinvestigator次要研究者SI sub-investigator助理研究者subject受试者subject diary受试者日记subject enrollment受试者入选subject enrollment log受试者入选表SIC subject identification code受试者识别代码subject recruitment受试者招募subject screening log受试者筛选表superiority检验survival analysis生存分析SUSAR suspected unexpected sar疑似非预期严重不良反应sxrd单晶X-射线衍射system audit系统稽查Ttarget variable目标变量TP template模板MRHD the maximum recommended human dose最高推荐人用剂量TG thermogravimetric analysis热重分析T1/2time 1/2消除半衰期TTP time-to-progression 疾病进展时间tlc、hplc制备色谱tmax达峰时间TP total protein总蛋白T-BIL total-bilirubin总胆红素T-CHO total-cholesterol总胆固醇TR training培训transformation变量变换treatment group试验组trial error试验误差TMF trial master file试验主文件夹trial objective试验目的trial site临床试验中心triple blinding三盲two one-side test双单侧检验UUV-VIS ultraviolet–visible spectroscopy紫外可见吸收光谱UADE unanticipated adverse drug effect非预期不良反应unblinding揭盲/破盲UADR unexpected adverse drug reaction非预期药物不良反应 UAE unexpected adverse event非预期不良事件Vvariability变异variable变量visual analogy scale直观类比打分法visual check人工检查vulnerable subject弱势受试者Wwash-out清洗期washout period洗脱期well-being福利,健康WI work instruction工作指南XYZ。
临床监查员专业术语、缩略语中英对照表
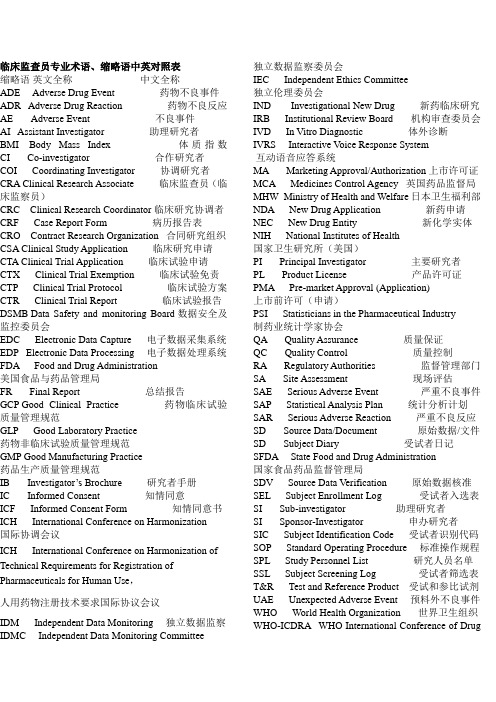
临床监查员专业术语、缩略语中英对照表缩略语英文全称中文全称ADE Adverse Drug Event 药物不良事件ADR Adverse Drug Reaction 药物不良反应AE Adverse Event 不良事件AI Assistant Investigator 助理研究者BMI Body Mass Index 体质指数CI Co-investigator 合作研究者COI Coordinating Investigator 协调研究者CRA Clinical Research Associate 临床监查员(临床监察员)CRC Clinical Research Coordinator 临床研究协调者CRF Case Report Form 病历报告表CRO Contract Research Organization 合同研究组织CSA Clinical Study Application 临床研究申请CTA Clinical Trial Application 临床试验申请CTX Clinical Trial Exemption 临床试验免责CTP Clinical Trial Protocol 临床试验方案CTR Clinical Trial Report 临床试验报告DSMB Data Safety and monitoring Board 数据安全及监控委员会EDC Electronic Data Capture 电子数据采集系统EDP Electronic Data Processing 电子数据处理系统FDA Food and Drug Administration美国食品与药品管理局FR Final Report 总结报告GCP Good Clinical Practice 药物临床试验质量管理规范GLP Good Laboratory Practice药物非临床试验质量管理规范GMP Good Manufacturing Practice药品生产质量管理规范IB Investigat or’s Brochure研究者手册IC Informed Consent 知情同意ICF Informed Consent Form 知情同意书ICH International Conference on Harmonization 国际协调会议ICH International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use,人用药物注册技术要求国际协议会议IDM Independent Data Monitoring 独立数据监察IDMC Independent Data Monitoring Committee 独立数据监察委员会IEC Independent Ethics Committee独立伦理委员会IND Investigational New Drug 新药临床研究IRB Institutional Review Board 机构审查委员会IVD In Vitro Diagnostic 体外诊断IVRS Interactive V oice Response System互动语音应答系统MA Marketing Approval/Authorization 上市许可证MCA Medicines Control Agency 英国药品监督局MHW Ministry of Health and Welfare日本卫生福利部NDA New Drug Application 新药申请NEC New Drug Entity 新化学实体NIH National Institutes of Health国家卫生研究所(美国)PI Principal Investigator 主要研究者PL Product License 产品许可证PMA Pre-market Approval (Application)上市前许可(申请)PSI Statisticians in the Pharmaceutical Industry制药业统计学家协会QA Quality Assurance 质量保证QC Quality Control 质量控制RA Regulatory Authorities 监督管理部门SA Site Assessment 现场评估SAE Serious Adverse Event 严重不良事件SAP Statistical Analysis Plan 统计分析计划SAR Serious Adverse Reaction 严重不良反应SD Source Data/Document 原始数据/文件SD Subject Diary 受试者日记SFDA State Food and Drug Administration国家食品药品监督管理局SDV Source Data Verification 原始数据核准SEL Subject Enrollment Log 受试者入选表SI Sub-investigator 助理研究者SI Sponsor-Investigator 申办研究者SIC Subject Identification Code 受试者识别代码SOP Standard Operating Procedure 标准操作规程SPL Study Personnel List 研究人员名单SSL Subject Screening Log 受试者筛选表T&R Test and Reference Product 受试和参比试剂UAE Unexpected Adverse Event 预料外不良事件WHO World Health Organization 世界卫生组织WHO-ICDRA WHO International Conference of DrugRegulatory Authorities WHO国际药品管理当局会议Active Control 阳性对照、活性对照Audit 稽查Audit Report 稽查报告Auditor 稽查员Blank Control 空白对照Blinding/masking 盲法/设盲Case History 病历Clinical study 临床研究Clinical Trial 临床试验Clinical Trial Report 临床试验报告Compliance 依从性Coordinating Committee 协调委员会Cross-over Study 交叉研究Double Blinding 双盲Endpoint Criteria/measurement 终点指标Essential Documentation 必需文件 Exclusion Criteria 排除标准 Inclusion Criteria 入选表准 Information Gathering 信息收集 Initial Meeting 启动会议Inspection 检察/视察Institution Inspection 机构检察Investigational Product 试验药物 Investigator 研究者Monitor 监查员(监察员) Monitoring 监查(监察) Monitoring Plan 监查计划(监察计划) Monitoring Report 监查报告(监察报告) Multi-center Trial 多中心试验Non-clinical Study 非临床研究 Original Medical Record 原始医疗记录 Outcome Assessment 结果评价 Patient File 病人档案 Patient History 病历 Placebo 安慰剂 Placebo Control 安慰剂对照 Preclinical Study 临床前研究 Protocol 试验方案 Protocol Amendments 修正案 Randomization 随机 Reference Product 参比制剂 Sample Size 样本量、样本大小 Seriousness 严重性 Severity 严重程度 Single Blinding 单盲 Sponsor 申办者 Study Audit 研究稽查Subject 受试者Subject Enrollment 受试者入选Subject Enrollment Log 受试者入选表Subject Identification Code List 受试者识别代码表 Subject Recruitment 受试者招募Study Site 研究中心Subject Screening Log 受试者筛选表System Audit 系统稽查Test Product 受试制剂Trial Initial Meeting 试验启动会议Trial Master File 试验总档案Trial Objective 试验目的Triple Blinding 三盲Wash-out 洗脱Wash-out Period 洗脱期。
-临床试验常用的英文缩写

-临床试验常用的英文缩写专业术语缩略语英文全称中文全称DCF data clarification form 数据澄清表,用于纸质query SDV source data verification 原始数据核对ADE Adverse Drug Event 药物不良事件ADR Adverse Drug Reaction 药物不良反应AE Adverse Event 不良事件AI Assistant Investigator 助理研究者BMI Body Mass Index 体质指数CI Co-investigator 合作研究者COI Coordinating Investigator 协调研究者CRA Clinical Research Associate 临床监查员(临床监察员)CRC Clinical Research Coordinator 临床研究协调者CRF Case Report Form 病历报告表CRO Contract Research Organization 合同研究组织CSA Clinical Study Application 临床研究申请CTA Clinical Trial Application 临床试验申请CTX Clinical Trial Exemption 临床试验免责CTP Clinical Trial Protocol 临床试验方案CTR Clinical Trial Report 临床试验报告DSMB Data Safety and monitoring Board 数据安全及监控委员会EDC Electronic Data Capture 电子数据采集系统EDP Electronic Data Processing 电子数据处理系统FDA Food and Drug Administration 美国食品与药品管理局FR Final Report 总结报告GCP Good Clinical Practice 药物临床试验质量管理规范GLP Good Laboratory Practice 药物非临床试验质量管理规范GMP Good Manufacturing Practice 药品生产质量管理规范IB Investigator’s Brochure研究者手册IC Informed Consent 知情同意ICF Informed Consent Form 知情同意书ICH International Conference on Harmonization 国际协调会议IDM Independent Data Monitoring 独立数据监察IDMC Independent Data Monitoring Committee 独立数据监察委员会IEC Independent Ethics Committee 独立伦理委员会IND Investigational New Drug 新药临床研究IRB Institutional Review Board 机构审查委员会IVD In Vitro Diagnostic 体外诊断IVRS Interactive Voice Response System 互动语音应答系统MA Marketing Approval/Authorization 上市许可证MCA Medicines Control Agency 英国药品监督局MHW Ministry of Health and Welfare 日本卫生福利部NDA New Drug Application 新药申请NEC New Drug Entity 新化学实体NIH National Institutes of Health 国家卫生研究所(美国)PI Principal Investigator 主要研究者PL Product License 产品许可证PMA Pre-market Approval (Application) 上市前许可(申请)PSI Statisticians in the Pharmaceutical Industry 制药业统计学家协会QA Quality Assurance 质量保证QC Quality Control 质量控制RA Regulatory Authorities 监督管理部门SA Site Assessment 现场评估SAE Serious Adverse Event 严重不良事件SAP Statistical Analysis Plan 统计分析计划SAR Serious Adverse Reaction 严重不良反应SD Source Data/Document 原始数据/文件SD Subject Diary 受试者日记SFDA State Food and Drug Administration 国家食品药品监督管理局SDV Source Data Verification 原始数据核准SEL Subject Enrollment Log 受试者入选表SI Sub-investigator 助理研究者SI Sponsor-Investigator 申办研究者SIC Subject Identification Code 受试者识别代码SOP Standard Operating Procedure 标准操作规程SPL Study Personnel List 研究人员名单SSL Subject Screening Log 受试者筛选表T&R Test and Reference Product 受试和参比试剂UAE Unexpected Adverse Event 预料外不良事件WHO World Health Organization 世界卫生组织WHO-ICDRA WHO International Conference of Drug Regulatory Authorities WHO国际药品管理当局会议Active Control 阳性对照、活性对照Audit 稽查Audit Report 稽查报告Auditor 稽查员Blank Control 空白对照Blinding/masking 盲法/设盲Case History 病历Clinical study 临床研究Clinical Trial 临床试验Clinical Trial Report 临床试验报告Compliance 依从性Coordinating Committee 协调委员会Cross-over Study 交叉研究Double Blinding 双盲Endpoint Criteria/measurement 终点指标Essential Documentation 必需文件Exclusion Criteria 排除标准Inclusion Criteria 入选表准Information Gathering 信息收集Initial Meeting 启动会议Inspection 检察/视察Institution Inspection 机构检察Investigational Product 试验药物Investigator 研究者Monitor 监查员(监察员)Monitoring 监查(监察)Monitoring Plan 监查计划(监察计划)Monitoring Report 监查报告(监察报告)Multi-center Trial 多中心试验Non-clinical Study 非临床研究Original Medical Record 原始医疗记录Outcome Assessment 结果评价Patient File 病人档案Patient History 病历Placebo 安慰剂Placebo Control 安慰剂对照Preclinical Study 临床前研究Protocol 试验方案Protocol Amendments 修正案Randomization 随机Reference Product 参比制剂Sample Size 样本量、样本大小 Seriousness 严重性Severity 严重程度Single Blinding 单盲Sponsor 申办者Study Audit 研究稽查Subject 受试者Subject Enrollment 受试者入选Subject Enrollment Log 受试者入选表Subject Identification Code List 受试者识别代码表Subject Recruitment 受试者招募Study Site 研究中心Subject Screening Log 受试者筛选表System Audit 系统稽查Test Product 受试制剂Trial Initial Meeting 试验启动会议Trial Master File 试验总档案Trial Objective 试验目的Triple Blinding 三盲Wash-out 洗脱Wash-out Period 洗脱期。
临床试验常用的英文缩写

专业术语缩略语英文全称中文全称ADE Adverse Drug Event 药物不良事件ADR Adverse Drug Reaction 药物不良反应AE Adverse Event 不良事件AI Assistant Investigator 助理研究者BMI Body Mass Index 体质指数CI Co-investigator 合作研究者COI Coordinating Investigator 协调研究者CRA Clinical Research Associate 临床监查员(临床监察员)CRC Clinical Research Coordinator 临床研究协调者CRF Case Report Form 病历报告表CRO Contract Research Organization 合同研究组织CSA Clinical Study Application 临床研究申请CTA Clinical Trial Application 临床试验申请CTX Clinical Trial Exemption 临床试验免责CTP Clinical Trial Protocol 临床试验方案CTR Clinical Trial Report 临床试验报告DSMB Data Safety and monitoring Board 数据安全及监控委员会EDC Electronic Data Capture 电子数据采集系统EDP Electronic Data Processing 电子数据处理系统FDA Food and Drug Administration 美国食品与药品管理局FR Final Report 总结报告GCP Good Clinical Practice 药物临床试验质量管理规范GLP Good Laboratory Practice 药物非临床试验质量管理规范GMP Good Manufacturing Practice 药品生产质量管理规范IB Investigator’s Brochure研究者手册IC Informed Consent 知情同意ICF Informed Consent Form 知情同意书ICH International Conference on Harmonization 国际协调会议IDM Independent Data Monitoring 独立数据监察IDMC Independent Data Monitoring Committee 独立数据监察委员会IEC Independent Ethics Committee 独立伦理委员会IND Investigational New Drug 新药临床研究IRB Institutional Review Board 机构审查委员会IVD In Vitro Diagnostic 体外诊断IVRS Interactive Voice Response System 互动语音应答系统MA Marketing Approval/Authorization 上市许可证MCA Medicines Control Agency 英国药品监督局MHW Ministry of Health and Welfare 日本卫生福利部NDA New Drug Application 新药申请NEC New Drug Entity 新化学实体NIH National Institutes of Health 国家卫生研究所(美国)PI Principal Investigator 主要研究者PL Product License 产品许可证PMA Pre-market Approval (Application) 上市前许可(申请)PSI Statisticians in the Pharmaceutical Industry 制药业统计学家协会QA Quality Assurance 质量保证QC Quality Control 质量控制RA Regulatory Authorities 监督管理部门SA Site Assessment 现场评估SAE Serious Adverse Event 严重不良事件SAP Statistical Analysis Plan 统计分析计划SAR Serious Adverse Reaction 严重不良反应SD Source Data/Document 原始数据/文件SD Subject Diary 受试者日记SFDA State Food and Drug Administration 国家食品药品监督管理局SDV Source Data Verification 原始数据核准SEL Subject Enrollment Log 受试者入选表SI Sub-investigator 助理研究者SI Sponsor-Investigator 申办研究者SIC Subject Identification Code 受试者识别代码SOP Standard Operating Procedure 标准操作规程SPL Study Personnel List 研究人员名单SSL Subject Screening Log 受试者筛选表T&R Test and Reference Product 受试和参比试剂UAE Unexpected Adverse Event 预料外不良事件WHO World Health Organization 世界卫生组织WHO-ICDRA WHO International Conference of Drug Regulatory Authorities WHO国际药品管理当局会议Active Control 阳性对照、活性对照Audit Report 稽查报告Auditor 稽查员Blank Control 空白对照Blinding/masking 盲法/设盲Case History 病历Clinical study 临床研究Clinical Trial 临床试验Clinical Trial Report 临床试验报告Compliance 依从性Coordinating Committee 协调委员会Cross-over Study 交叉研究Double Blinding 双盲Endpoint Criteria/measurement 终点指标Essential Documentation 必需文件Exclusion Criteria 排除标准Inclusion Criteria 入选表准Information Gathering 信息收集Initial Meeting 启动会议Inspection 检察/视察Institution Inspection 机构检察Investigational Product 试验药物Investigator 研究者Monitor 监查员(监察员)Monitoring 监查(监察)Monitoring Plan 监查计划(监察计划) Monitoring Report 监查报告(监察报告) Multi-center Trial 多中心试验Non-clinical Study 非临床研究Original Medical Record 原始医疗记录Outcome Assessment 结果评价Patient File 病人档案Patient History 病历Placebo 安慰剂Placebo Control 安慰剂对照Preclinical Study 临床前研究Protocol 试验方案Protocol Amendments 修正案Reference Product 参比制剂Sample Size 样本量、样本大小 Seriousness 严重性Severity 严重程度Single Blinding 单盲Sponsor 申办者Study Audit 研究稽查Subject 受试者Subject Enrollment 受试者入选Subject Enrollment Log 受试者入选表Subject Identification Code List 受试者识别代码表 Subject Recruitment 受试者招募Study Site 研究中心Subject Screening Log 受试者筛选表System Audit 系统稽查Test Product 受试制剂Trial Initial Meeting 试验启动会议Trial Master File 试验总档案Trial Objective 试验目的Triple Blinding 三盲Wash-out 洗脱Wash-out Period 洗脱期。
临床试验与实验室中常见的中英文名词与缩写

临床试验与实验室中常见的中英⽂名词与缩写中国创新药咨询与服务先锋CRO 临床试验以及实验室中常见的英⽂缩写药物临床试验英⽂缩写缩略语英⽂全称中⽂全称ADE Adverse Drug Event 药物不良事件ADR Adverse Drug Reaction 药物不良反应AE Adverse Event 不良事件AI Assistant Investigator 助理研究者BMI Body Mass Index 体质指数CI Co-investigator 合作研究者COI Coordinating Investigator 协调研究者CRC Clinical Research Coordinator 临床研究协调者CRF Case Report Form 病历报告表CRO Contract Research Organization 合同研究组织CSA Clinical Study Application 临床研究申请CTA Clinical Trial Application 临床试验申请CTX Clinical Trial Exemption 临床试验免责CTP Clinical Trial Protocol 临床试验⽅案CTR Clinical Trial Report 临床试验报告DSMB Data Safety and monitoring Board 数据安全及监控委员会EDC Electronic Data Capture 电⼦数据采集系统EDP Electronic Data Processing 电⼦数据处理系统FDA Food and Drug Administration 美国⾷品与药品管理局FR Final Report 总结报告GCP Good Clinical Practice 药物临床试验质量管理规范GCP Good Laboratory Practice 药物⾮临床试验质量管理规范GMP Good Manufacturing Practice 药品⽣产质量管理规范IB Investigator’s Brochure 研究者⼿册IC Informed Consent 知情同意ICF Informed Consent Form 知情同意书ICH International Conference on Harmonization 国际协调会议IDM Independent Data Monitoring 独⽴数据监察IDMC Independent Data Monitoring Committee 独⽴数据监察委员会IEC Independent Ethics Committee 独⽴伦理委员会IND Investigational New Drug 新药临床研究IRB Institutional Review Board 机构审查委员会IVD In Vitro Diagnostic 体外诊断IVRS Interactive Voice Response System 互动语⾳应答系统MA Marketing A pproval/Authorization 上市许可证MCA Medicines Control Agency 英国药品监督局MHW Ministry of Health and Welfare ⽇本卫⽣福利部NDA New Drug Application 新药申请NEC New Drug Entity 新化学实体NIH National Institutes of Health 国家卫⽣研究所(美国)缩略语英⽂全称中⽂全称PI Principal Investigator 主要研究者PL Product License 产品许可证PMA Pre-market Approval (Application) 上市前许可(申请)PSI Statisticians in the Pharmaceutical Industry 制药业统计学家协会QA Quality Assurance 质量保证QC Quality Control 质量控制RA Regulatory Authorities 监督管理部门SA Site Assessment 现场评估SAE Serious Adverse Event 严重不良事件SAP Statistical Analysis Plan 统计分析计划SAR Serious Adverse Reaction 严重不良反应SD Source Data/Document 原始数据/⽂件SD Subject Diary 受试者⽇记SFDA State Food and Drug Administration 国家⾷品药品监督管理局SDV Source Data Verification 原始数据核准SEL Subject Enrollment Log 受试者⼊选表SI Sub-investigator 助理研究者SI Sponsor-Investigator 申办研究者SIC Subject Identification Code 受试者识别代码SOP Standard Operating Procedure 标准操作规程SPL Study Personnel List 研究⼈员名单SSL Subject Screening Log 受试者筛选表T&R Test and Reference Product 受试和参⽐试剂UAE Unexpected Adverse Event 预料外不良事件WHO World Health Organization 世界卫⽣组织WHO-ICDRA WHO International Conference ofWHO 国际药品管理当局会议Drug Regulatory Authorities 药物临床试验英⽂缩写英⽂全称中⽂全称Accuracy 准确度Active control, AC 阳性对照活性对照Adverse drug reaction, ADR 药物不良反应Adverse event, AE 不良事件Adverse medical events 不良医学事件Adverse reaction 药物不良反应Alb ⽩蛋⽩ALD(Approximate Lethal Dose)近似致死剂量ALP 碱性磷酸酶Alpha spending function 消耗函数ALT 丙氨酸氨基转换酶Analysis sets 统计分析的数据集Approval 批准Assistant investigator 助理研究者AST 天门冬酸氨基转换酶ATR 衰减全反射法AUCss 稳态⾎药浓度-时间曲线下⾯积Audit 稽查Audit or inspection 稽查/视察Audit report 稽查报告Auditor 稽查员Bias 偏性偏倚Bioequivalence ⽣物等效应Blank control 空⽩对照Blind codes 编制盲底Blind review 盲态审核Blind review 盲态检查Blinding method 盲法Blinding/masking 盲法/设盲Block 层Block size 每段的长度Carryover effect 延滞效应Case history 病历Case report form/ case record form CRF 病例报告表病例记录表Categorical variable 分类变量Cav 平均浓度CD 圆⼆⾊谱CL 清除率Clinical equivalence 临床等效应Clinical study 临床研究Clinical study report 临床试验的总结报告Clinical trial 临床试验Clinical trial application CTA 临床试验申请Clinical trial exemption CTX 临床试验免责Clinical trial protocol CTP 临床试验⽅案Clinical trial/ study report 临床试验报告Cmax 峰浓度Co-investigator 合作研究者Comparison 对照Compliance 依从性Composite variable 复合变量Computer-assisted trial design CATD 计算机辅助试验设计Confidence interval 可信区间Confidence level 置信⽔平Consistency test ⼀致性检验Contract research organization CRO 合同研究组织Contract/ agreement 协议/合同Control group 对照组Coordinating committee 协调委员会Crea 肌酐CRF(case report form) 病例报告表Crossover design 交叉设计Cross-over Study 交叉研究Css 稳浓度Cure 痊愈Data management 数据管理Database 建⽴数据库Descriptive statistical analysis 描述性统计分析DF 波动系统Dichotomies ⼆分类Diviation 偏差Documentation 记录/⽂件Dose-reaction relation 剂量-反应关系Double dummy 双模拟Double dummy technique 双盲双模拟技术Drop out 脱落DSC 差⽰扫描热量计Effectiveness 疗效Electronic data capture EDC 电⼦数据采集系统Electronic data processing EDP 电⼦数据处理系统Emergency envelope 应急信件End point 终点Endpoint Criteria 终点指标Endpoint criteria/ measurement 终点指标Equivalence 等效性Essential Documentation 必需⽂件Ethics committee 伦理委员会Excellent 显效Exclusion criteria 排除标准Factorial design 析因设计Failure ⽆效失败Final point 终点Fixed-dose procedure 固定剂量法Forced titration 强制滴定Full analysis set 全分析集GC-FTIR ⽓相⾊谱-傅利叶红外联⽤GC-MS ⽓相⾊谱-质谱联⽤Generic drug 通⽤名药Global assessment variable 全局评价变量GLU ⾎糖Good clinical practice, GCP 药物临床试验质量管理规范Good manufacture practice, GMP 药品⽣产质量管理规范Good non-clinical laboratory practice, GLP 药物⾮临床研究质量管理规范Group sequential design 成组序贯设计Health economic evaluation, HEV 健康经济学评价Hypothesis test 假设检验Hypothesis testing 假设检验Improvement 好转Inclusion Criteria ⼊选表准英⽂全称中⽂全称Inclusion criteria ⼊选标准Independent ethics committee IEC 独⽴伦理委员会Information consent form ICF 知情同意书Information Gathering 信息收集Informed consent IC 知情同意Initial meeting 启动会议Inspection 检察/视察Institution inspection 机构检查Institution review board, IBR 机构审查委员会Intention-to –treat ITT 意向性分析(-统计学)Interactive voice response system IVRS 互动式语⾳应答系统Interim analysis 期中分析International Conference of Harmonization ICH ⼈⽤药品注册技术要求国际技术协调会国际协调会议Investigational Product 试验药物Investigator 研究者Investigator’s brochure, IB 研究者⼿册Last observation carry forward, LOCF 最接近⼀次观察的结转LC-MS 液相⾊谱-质谱联⽤LD50 板数致死剂量LOCF, Last observation carry forward 最近⼀次观察的结转Logic check 逻辑检查LOQ (Limit of Quantization) 定量限Lost of follow up 失访Marketing approval/ authorization 上市许可证Matched pair 匹配配对Missing value 缺失值Mixed effect model 混合效应模式Monitor 监察员Monitoring 监查Monitoring Plan 监察计划Monitoring Report 监察报告MRT 平均滞留时间MS 质谱MS-MS 质谱-质谱联⽤MTD(Maximum Tolerated Dose)最⼤耐受剂量Multi-center Trial 多中⼼试验New chemical entity NCE 新化学实体New drug application NDA 新药申请NMR 核磁共振谱Non-clinical Study ⾮临床研究Non-inferiority ⾮劣效性Non-parametric statistics ⾮参数统计⽅法Obedience 依从性ODR 旋光光谱Open-label ⾮盲Optional titration 随意滴定Original medical record 原始医疗记录Outcome 结果Outcome Assessment 结果评价Outcome assessment 结果指标评价Outcome measurement 结果指标Outlier 离群值Parallel group design 平⾏组设计Parameter estimation 参数估计Parametric statistics 参数统计⽅法Patient file 病⼈档案Patient history 病历Per protocol PP 符合⽅案集Placebo 安慰剂Placebo control 安慰剂对照Polytomies 多分类Power 检验效能Precision 精密度Preclinical study 临床前研究Primary endpoint 主要终点Primary variable 主要变量Principle investigator PI 主要研究者Product license PL 产品许可证Protocol 试验⽅案Protocol Amendments 修正案Quality assurance QA 质量保证Quality assurance unit QAU 质量保证部门Quality control QC 质量控制Query list query form 应⽤疑问表Randomization 随机Range check 范围检查Rating scale 量表Reference Product 参⽐制剂Regulatory authorities RA 监督管理部门Replication 可重复RSD ⽇内和⽇间相对标准差Run in 准备期Safety evaluation 安全性评价Safety set 安全性评价的数据集Sample size 样本量样本⼤⼩Scale of ordered categorical ratings 有序分类指标Secondary variable 次要变量Sequence 试验次序Serious adverse event SAE 严重不良事件Serious adverse reaction SAR 严重不良反应Seriousness 严重性Severity 严重程度Severity 严重程度Significant level 检验⽔准Simple Randomization 简单随机Single blinding 单盲Site audit 试验机构稽查SOP 试验室的标准操作规程Source data SD 原始数据Source data verification SDV 原始数据核准Source document SD 原始⽂件Specificity 特异性Sponsor 申办者Sponsor-investigator 申办研究者Standard curve 标准曲线Standard operating procedure SOP 标准操作规程Statistic 统计量Statistical analysis plan 统计分析计划Statistical model 统计模型Statistical tables 统计分析表Stratified 分层Study Audit 研究稽查Study audit 研究稽查Study Site 研究中⼼Subgroup 亚组Sub-investigator 助理研究者Subject 受试者Subject 受试者Subject diary 受试者⽇记Subject Enrollment 受试者⼊选Subject enrollment log 受试者⼊选表Subject identification code SIC 受试者识别代码Subject Identification Code List 受试者识别代码表Subject Recruitment 受试者招募Subject screening log 受试者筛选表Superiority 检验Survival analysis ⽣存分析SXRD 单晶 X-射线衍射System audit 系统稽查System Audit 系统稽查T1/2 消除半衰期Target variable ⽬标变量T-BIL 总胆红素T-CHO 总胆固醇Test Product 受试制剂TG 热重分析TLC、HPLC 制备⾊谱Tmax 峰时间TP 总蛋⽩Transformation 变量变换Treatment group 试验组Trial error 试验误差Trial Initial Meeting 试验启动会议Trial Master File 试验总档案Trial objective 试验⽬的Trial site 试验场所Triple blinding 三盲Two one-side test 双单侧检验Un-blinding 揭盲Unexpected adverse event UAE 预料外不良事件UV-VIS 紫外-可见吸收光谱Variability 变异Variable 变量Visual analogy scale 直观类⽐打分法Visual check ⼈⼯检查Vulnerable subject 弱势受试者Wash-out 洗脱Washout period 洗脱期实验室检查英⽂缩写英⽂全称中⽂全称⾎常规WBC white blood cell count ⽩细胞计数GR% granulocyte 中性粒细胞百分⽐LY% lymphocyte 淋巴细胞百分⽐MID% 中值细胞百分⽐EOS% eosimophil 嗜酸性粒细胞百分⽐AL% allergy lymphocyte 变异淋巴细胞百分⽐ST% 中性杆状粒细胞百分⽐RBC red blood cell 红细胞计数HGB hemoglobin ⾎红蛋⽩HCT hematocrit 红细胞⽐积红细胞⽐积MCV mean corpusular volume 平均红细胞体积MCH mean corpusular hemoglobin 平均红细胞⾎红蛋⽩含量平均红细胞⾎红蛋⽩浓度MCHC mean corpuscular hemoglobinconcerntrationRDW red blood cell volume distribution width 红细胞分布宽度变异PLT/BPC platelet count/blood platelet count ⾎⼩板计数MPV mean platelet volume 平均⾎⼩板体积PCT plateletocrit ⾎⼩板⽐积PDW platelet distribution width ⾎⼩板分布宽度尿便常规PH acidity 酸碱度NIT nitrite 亚硝酸盐GLU glucose 尿糖SG specific gravity ⽐重PRO protein 尿蛋⽩BLD blood 隐⾎BIL bilirubin 尿胆红素URO urobilinogen 尿胆原WBC white blood cell ⽩细胞addish 计数addish count 艾迪⽒计数/HP high power objective 每⾼倍视野/LP low power objective 每低倍视野OB occult blood test ⼤便隐⾎试验CSF cerebrospinal 脑积夜Pandy pandy 庞⽒试验⽣化检验TB total bilirubin 总胆红素DB direct bilirubin 直接胆红素TP total protein 总蛋⽩ALB albumin ⽩蛋⽩GLOB globulin 球蛋⽩UREA urea 尿素CREA creatinine 肌肝UA uric acid 尿酸GLU glucose ⾎糖ALT alanine amiotransferase 丙氨酸氨基转移酶AST aspartate aminotransferase 门冬氨酸氨基转移酶GGT γ-glutamyl transpeptadase ⾕氨酰转肽酶CK creatine kinase 肌酸肌酶CK-MB creatine kinase-MB 肌酸肌酶同⼯酶LDH lactate dehydrogenase 乳酸脱氢酶α-HBD α-hydroxybutyric dehydrogenase α-羟丁酸脱氢酶AMY serum amylase ⾎淀粉酶TG triglyceride 肝油三脂CHOL cholesterol 胆固醇HDL-c high-density lipoprotein cholesterol ⾼密度脂蛋⽩LDL-c low-density lipoprotein cholesterol 低密度脂蛋⽩VLDL very low-density lipoprotein 极低密度脂蛋⽩Ca serum calcium 钙Mg serum magnesium 镁IP inorganic phosphate ⽆机磷ALP alkaline phosphatase 碱性磷酸酶TBA total biliary acid 总胆汁酸ASO antistreptolysin 抗链球菌溶⾎素O a-AG a-acid glycoprotein a-酸性糖蛋⽩CRP C-reactive protein C 反应蛋⽩RF rheumatoid factor 类风湿因⼦MTP mili-total protein 微量蛋⽩IgG immunoglobin G 免疫球蛋⽩GIgA immunoglobin A 免疫球蛋⽩AIgM immunoglobin M 免疫球蛋⽩MC3 complement C3 补体C3C4 complement C4 补体C4cTNT troponin T 肌钙蛋⽩T MYOG myoglobin 肌红蛋⽩Na sodium 钠K kalium 钾Cl chloride 氯Ga calcium 钙Mg magnesium 镁⼄肝标志物HBV hepatitis B virus ⼄肝病毒HBsAg hepatitis B surface antigen ⼄肝表⾯抗原HBsAb antibody to hepatitis surface antigen ⼄肝表⾯抗体HBcAg hepatitis B core antigen ⼄肝核⼼抗原HBcAb antibody to hepatitis B core antigen ⼄肝核⼼抗体HBeAg hepatitis B e-antigen ⼄肝e 抗原HBeAb antibody to hepatitis B e-antigen ⼄肝e 抗体ELISA enzymelinked immunosorbentassy 酶联免疫吸附试验HAV hepatitis A virus 甲肝病毒HCV hepatitis C virus 丙肝病毒输⾎免疫全套HBV hepatitis B virus ⼄型肝炎病毒HCV hepatitis C virus 丙型肝炎病毒TP treponema pallidum 梅毒螺旋体HIV human immunodeficiency virus ⼈类免疫缺陷病毒。
临床英语术语缩写表
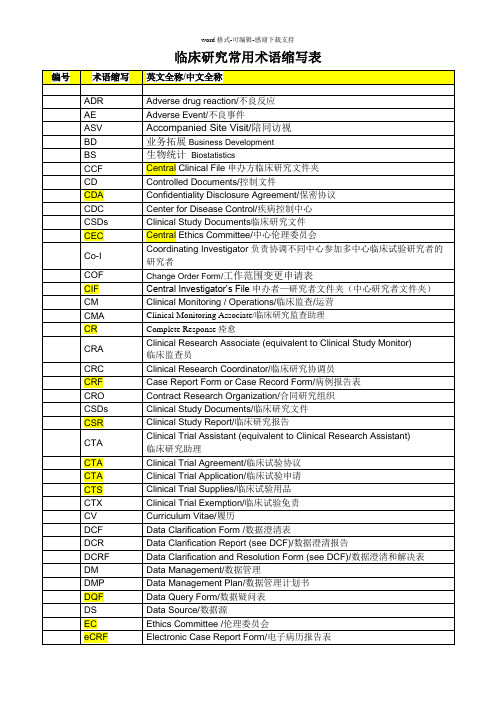
临床研究常用术语缩写表SOP 类型缩写表OP 操作规程Operating Procedures WI 工作指南Work InstructionsTP 模板TemplateFM 批准的标准表格Approved Standard FormsOD 其他文件Other Documents业务部门缩写表/ Functional Area Abbreviation Table:BS 生物统计BiostatisticsBD 业务拓展Business DevelopmentCM 临床监查/运营Clinical Monitoring/OperationDM 数据管理Data ManagementIT 信息技术Information TechnologyMS 医学科学服务Medical Science ServicePM 项目管理Project ManagementQA 质量保证Quality AssuranceRM 记录管理Records ManagementRA 注册事务Regulatory AffairsSM SOP 管理SOP ManagementST 研究中心管理服务Site Management ServiceTR 培训Training试验主文档:(TMF)Trial Master FilePMF 项目管理文件夹Project Management FileCCF 申办方临床研究文件夹Central Clinical FileCIF 申办方-研究者文件夹Central Investigator FileISF 研究者文件夹Investigator Site FileBSF 生物统计学文件夹Biostatistics Study FileDMSF 数据管理研究文件夹Data Management Study File。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
临床研究专业术语缩略语中英对照表为了方便专业人士在临床研究领域的交流和理解,本文提供了临床研究中常见的专业术语的缩略语中英对照表。
这些缩略语旨在简化长句和复杂术语,使他们更易于书写和快速交流。
以下是常见临床研究专业术语及其相应的缩略语及中英文对照:
1. Adverse Event(AE)-- 不良事件
2. Serious Adverse Event(SAE)-- 严重不良事件
3. Placebo(PBO)-- 安慰剂
4. Randomized Controlled Trial(RCT)-- 随机对照试验
5. Informed Consent(IC)--知情同意
6. Institutional Review Board(IRB)-- 伦理审查委员会
7. Case Report Form(CRF)-- 病例报告表
8. Data Monitoring Committee(DMC)-- 数据监测委员会
9. Risk-Benefit Ratio(RBR)-- 风险-效益比
10. Investigational New Drug(IND)-- 新药研究
11. Good Clinical Practice(GCP)-- 良好临床实践
12. Adverse Drug Reaction(ADR)-- 不良药物反应
13. Intent-to-Treat(ITT)-- 治疗意向分析
14. Protocol Violation(PV)-- 方案违规
15. Sponsor-Investigator(SI)-- 研究发起人
16. Standard Operating Procedures(SOPs)-- 标准操作规程
17. Confidentiality Agreement(CA)-- 保密协议
18. Data and Safety Monitoring Board(DSMB)-- 数据和安全监测委
员会
19. Clinical Research Coordinator(CRC)-- 临床研究协调员
20. Declaration of Helsinki(DoH)--《赫尔辛基宣言》
以上是临床研究专业术语的缩略语中英对照表。
这些缩略语在临床
研究的实践和文献中经常出现,掌握它们有助于提高专业沟通的效率
和准确性。
同时,对于学习临床研究的初学者来说,了解这些缩略语
也是迈向专业领域的一大步。
请注意,本表列出的缩略语是根据当前的临床研究实践和常用文献
编制的,随着技术和研究方法的不断发展,新的缩略语也可能会出现。
因此,建议在使用和阅读相关文献时,要随时关注并了解最新的专业
术语和缩略语。
最后,希望本文提供的临床研究专业术语缩略语中英对照表对您在
临床研究领域的学习和工作有所帮助。
通过熟练掌握这些缩略语,您
将能够更加高效地与同行进行交流,并更好地理解相关研究文献和报告。
祝愿您在临床研究的道路上取得不断的进步和成功!。
